Chapter 2
The Cell Biology of Neurons and Glia
Axonal Transport as a Research Tool
Classification of Neurons and Groups of Neurons
Electrical Properties of Neurons
Neurons as Information Receivers
Neurons as Information Transmitters
Disorders of Neurotransmitter Metabolism
Structural Support and Response to Injury
Influence on Neurotransmission
Astrocytes at the Blood-Brain Barrier
Control of Local Blood Flow Within the Central Nervous System
Tumors of the Central Nervous System
The number of cells in the adult human central nervous system (CNS) has been estimated at 100 billion. All arise from a relatively small population of precursors, yet a diversity of cell types is seen in the adult. Their most basic classification is as neurons and glia (glial cells).
OVERVIEW
Nerve cells (neurons) manipulate information. Doing so involves changes in the bioelectrical or biochemical properties of the cell, and these changes require a vast expenditure of energy for each cell. The nervous system, compared with other organs, is the greatest consumer of oxygen and glucose. These energy requirements arise directly from the metabolic demand placed on cells, which have large surface areas and concentrate biomolecules and ions against an energy gradient. Along with maintaining its metabolism, each neuron (1) receives information from either the environment or other nerve cells, (2) processes information, and (3) sends information to other neurons or effector tissues.
Glial cells control the CNS environment within which neurons function. They shuttle nutritive molecules from blood vessels to neurons, remove waste products, and maintain the electrochemical surroundings of neurons. Glia also communicate directly with nearby neurons through glial receptors and release mechanisms for certain neurotransmitters. During nervous system development, glia guide neuronal migration and promote synapse formation.
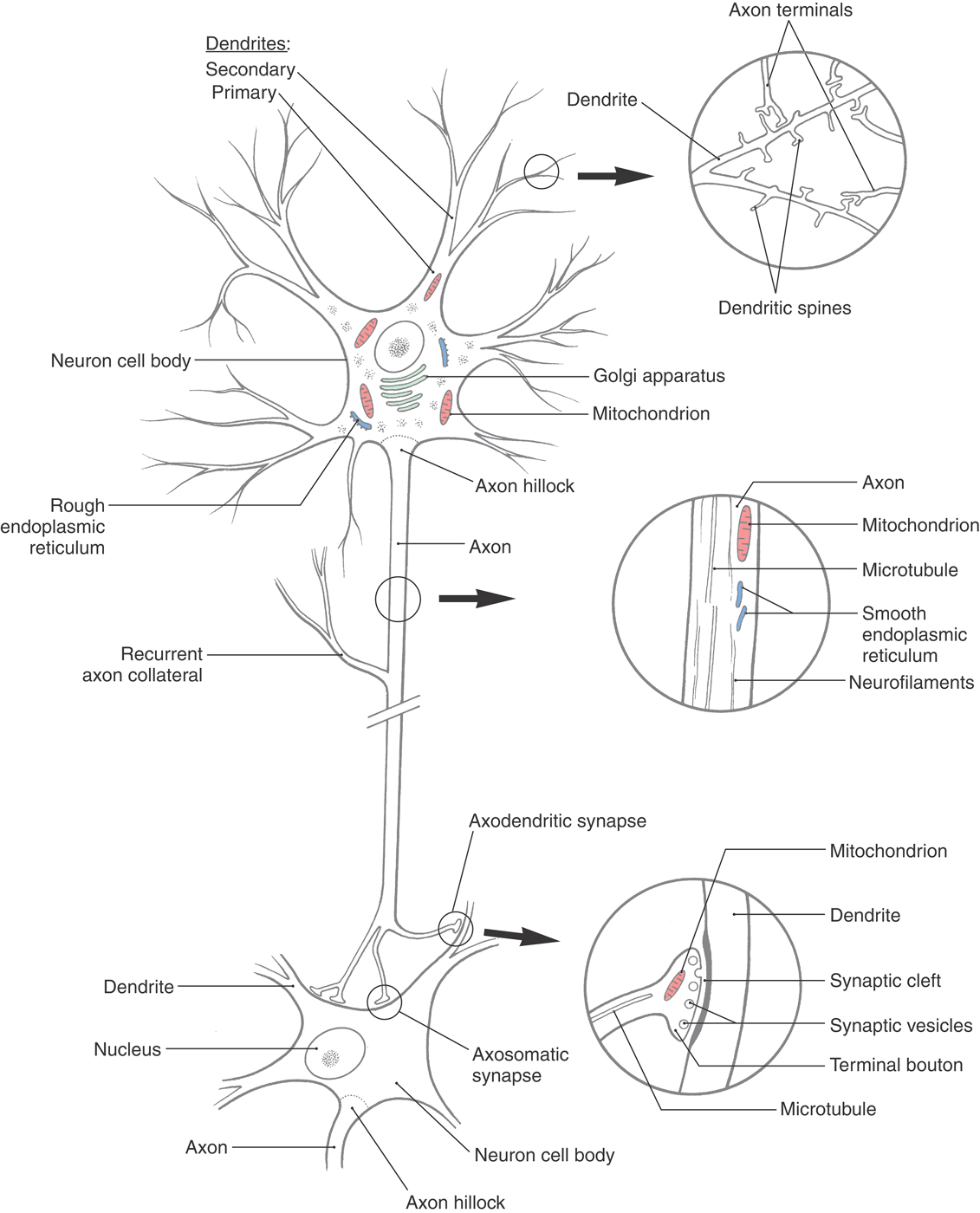
For neurons to carry out the three tasks of receiving, processing, and sending information, they must have specialized structures that contribute to each of these functions. The main components of a neuron are shown in Figure 2-1. In addition, specialized mechanisms and structures are required to solve some special problems specific to neuron function. Two such problems are immediately apparent. First, the mix of ions inside neurons is different from the mix outside the cell. Maintaining this difference requires extraordinary amounts of energy because ions must be pumped against electrical and diffusion gradients. The large surface area of neurons compounds this problem. Second, those neurons that send information over long distances must have a way to supply these distant sites with macromolecules and energy. For the cell biology of neurons to be fully appreciated, it is important to see the biochemical, anatomic, and physiologic properties of neurons as part of an integrated whole, the machinery that permits the neuron to do its specialized functions. In the following sections, we examine how specializations in neuronal architecture and chemistry contribute to meeting these special demands.
STRUCTURE OF NEURONS
The archetypical neuron is bounded by a continuous plasma membrane and consists of a cell body, or soma, from which dendrites and an axon arise (Figs. 2-1 and 2-2). The cell body contains the nucleus surrounded by a mass of cytoplasm that includes the organelles necessary for protein synthesis and metabolic maintenance. Most neurons (multipolar neurons) have several dendrites extending from the cell body (Figs. 2-1 and 2-2). These are usually relatively short processes that taper from a thick base and, in doing so, branch extensively. In contrast, there is a single axon, which is a relatively long process (extending from a few millimeters to more than a meter) with a uniform diameter. The axon has few if any branches along most of its length, branching extensively only near the distal end (the terminal arbor) (Figs. 2-1 and 2-2). In most neurons, information normally flows from the dendrites to the cell body to the axon and its terminals, then to the next neuron or an effector tissue such as muscle. These components of the neuron are described in the order in which information is processed.
Dendrites
Dendrites usually branch extensively in the vicinity of the cell body, giving the appearance of a tree or bush (Figs. 2-1 to 2-3A). They receive signals either from other neurons through contacts (synapses) made on their surfaces or from the environment via specialized receptors. Information travels from distal to proximal (tip to base) along dendrites to converge at the cell body.
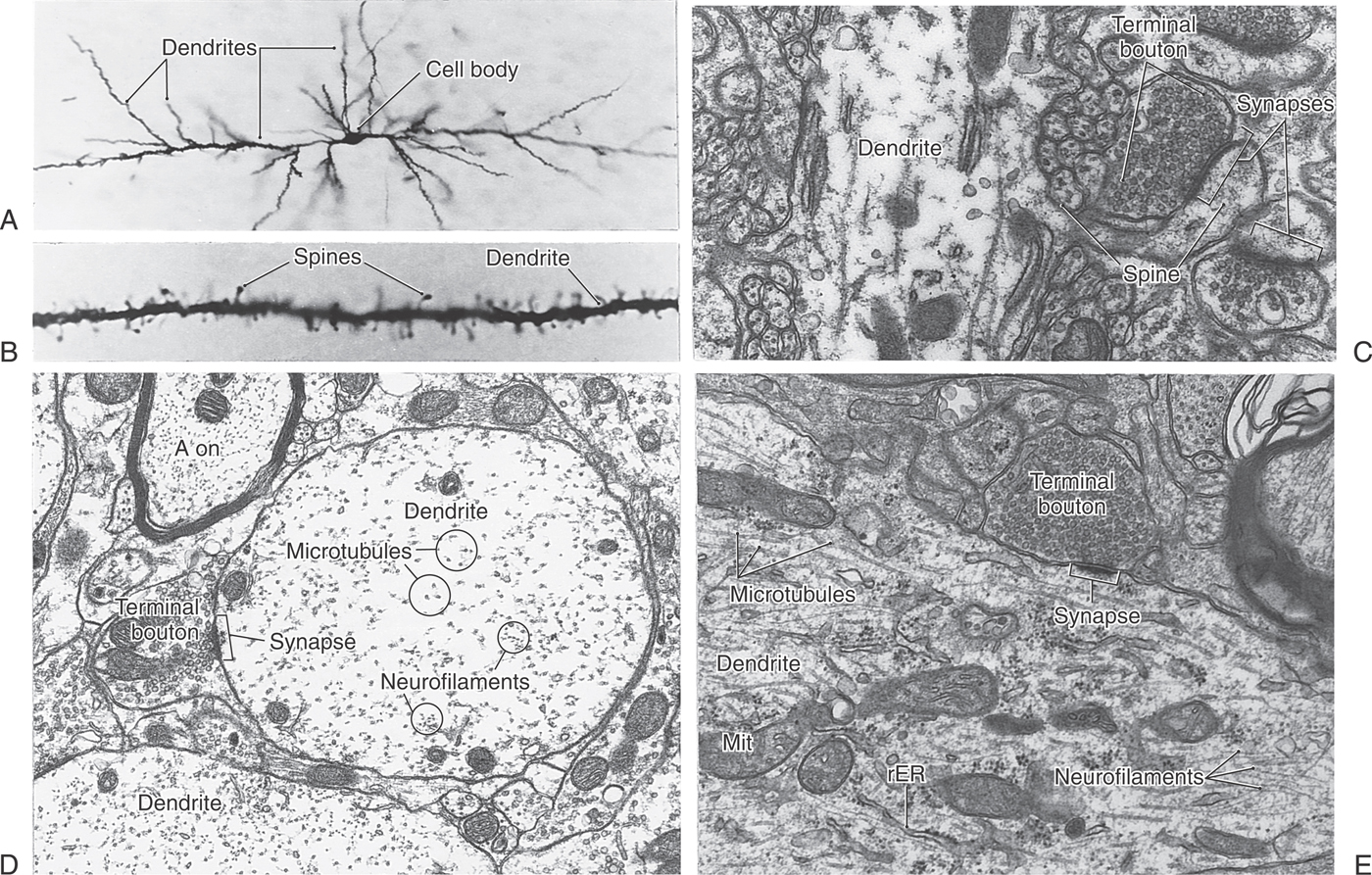
Small bud-like extensions (dendritic spines) of a variety of shapes are frequently seen on the more distal branches of the dendritic tree (Figs. 2-1 and 2-3B, C). These are sites of synaptic contacts (discussed later). The branches of dendrites increase in thickness as they anastomose in their course toward the cell body.
The only organelles seen in thin, distal dendritic branches are cytoskeletal elements, that is, microtubules and neurofilaments (the type of intermediate filament that occurs only in neurons). In addition to cytoskeletal elements, thicker dendrites contain mitochondria, some saccules of endoplasmic reticulum, and collections of polyribosomes and free ribosomes (Figs. 2-1 and 2-3D, E). In many nerve cells, the distal dendrites collect into large, trunk-like primary dendrites that contain the same organelles as the cell body. The microtubular and neurofilamentous skeletons of the dendritic tree are continuous throughout its extent and help maintain its branched structure.
Cell Body
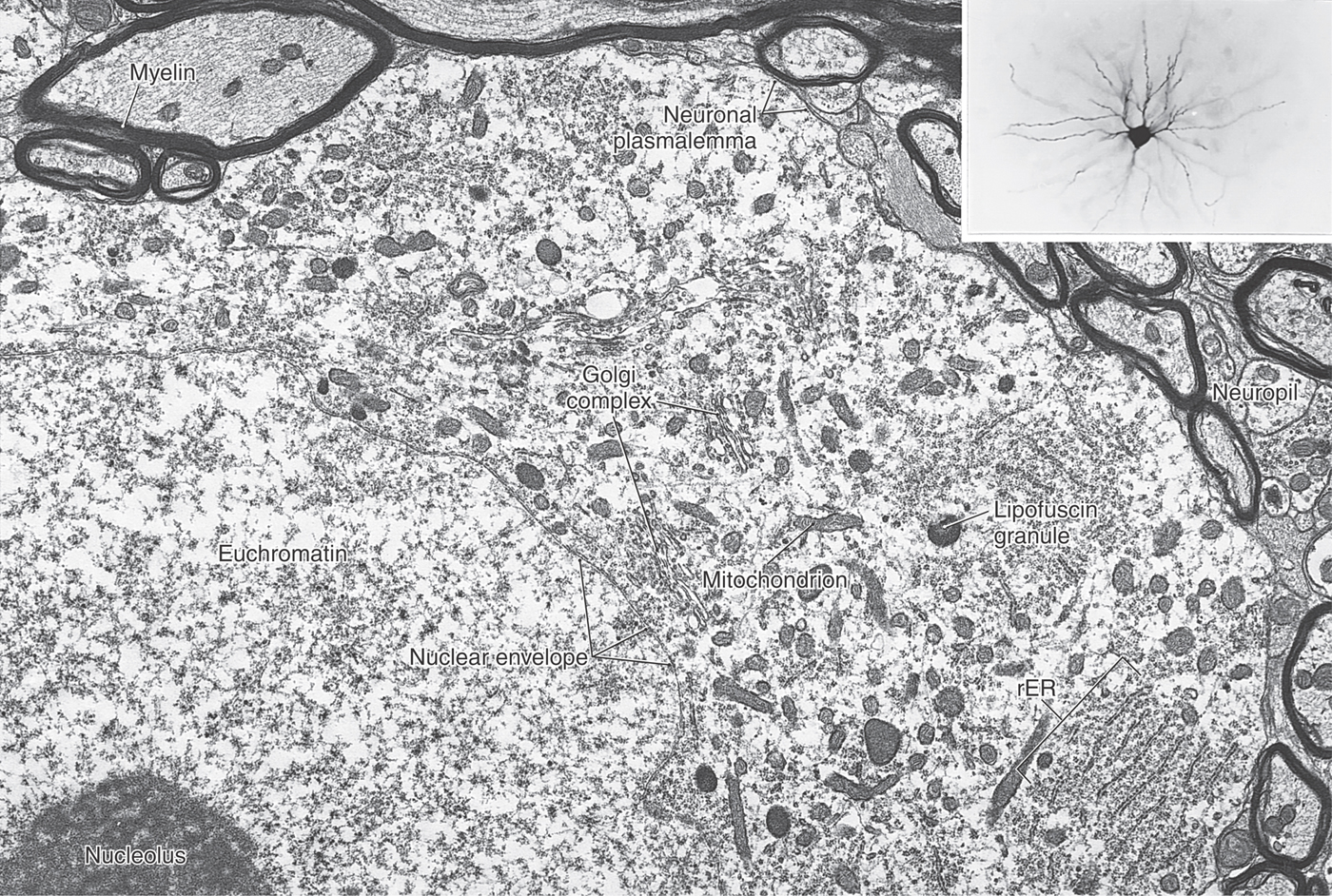
The cell body of a neuron is also called the soma (plural, somata) or perikaryon (plural, perikarya) (Figs. 2-2 and 2-4). The perikaryon is the metabolic center of the nerve cell. Abundant mitochondria reflect the high energy consumption of the cell. Active protein synthesis is indicated by the large size of the nucleus and its content of diffuse chromatin (euchromatin) and at least one prominent nucleolus (the site of ribosomal RNA synthesis). In the cytoplasm, ribosomes are abundant, and the rough endoplasmic reticulum (rER) and Golgi complex are extensive (Fig. 2-1). The rER is basophilic (binds basic dyes) as a result of the large amount of ribosomal RNA attached to the endoplasmic membrane. These extensive, stacked layers of rER are seen as patches of basophilic staining (called Nissl substance) in histologic preparations of nerve cells.
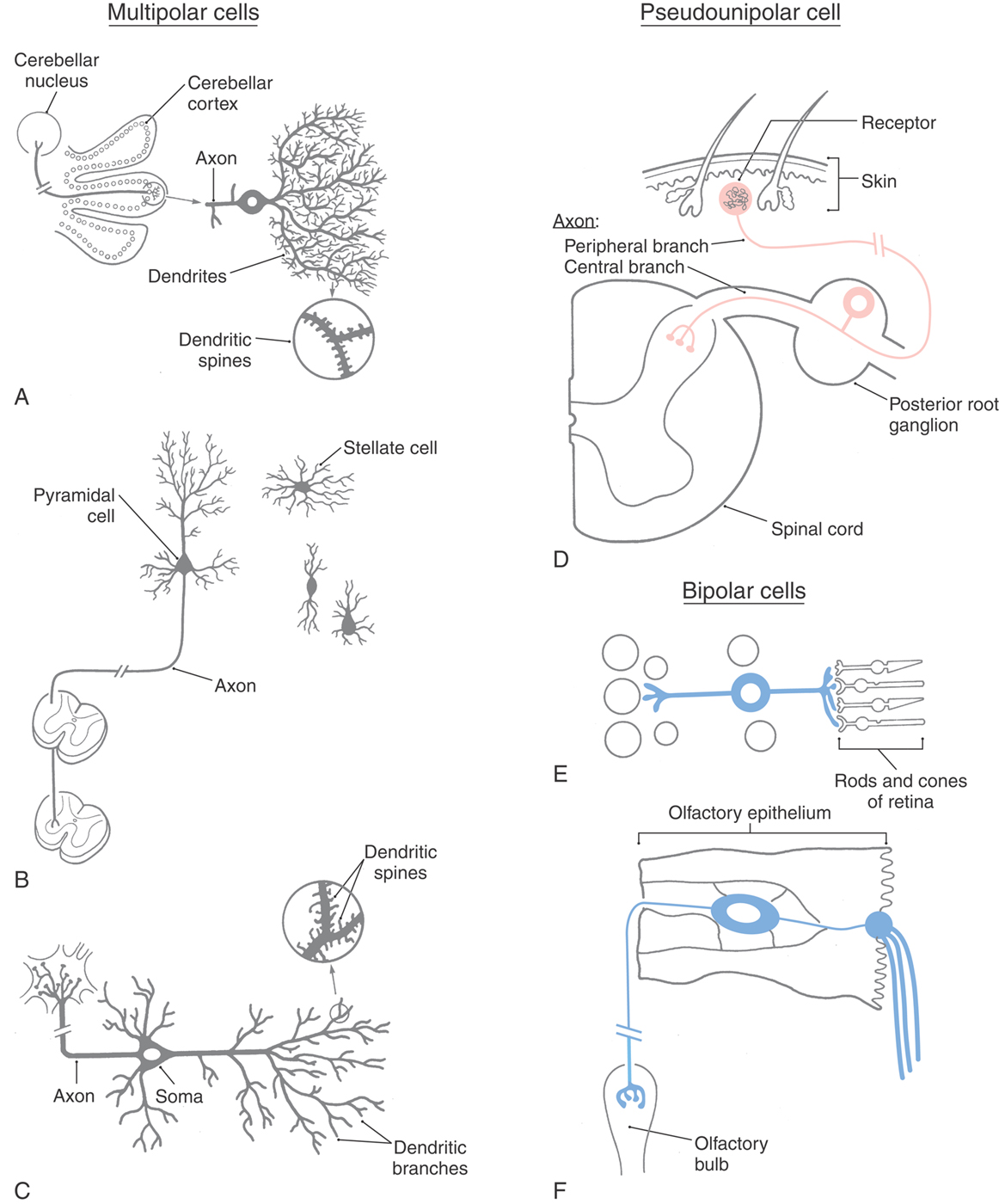
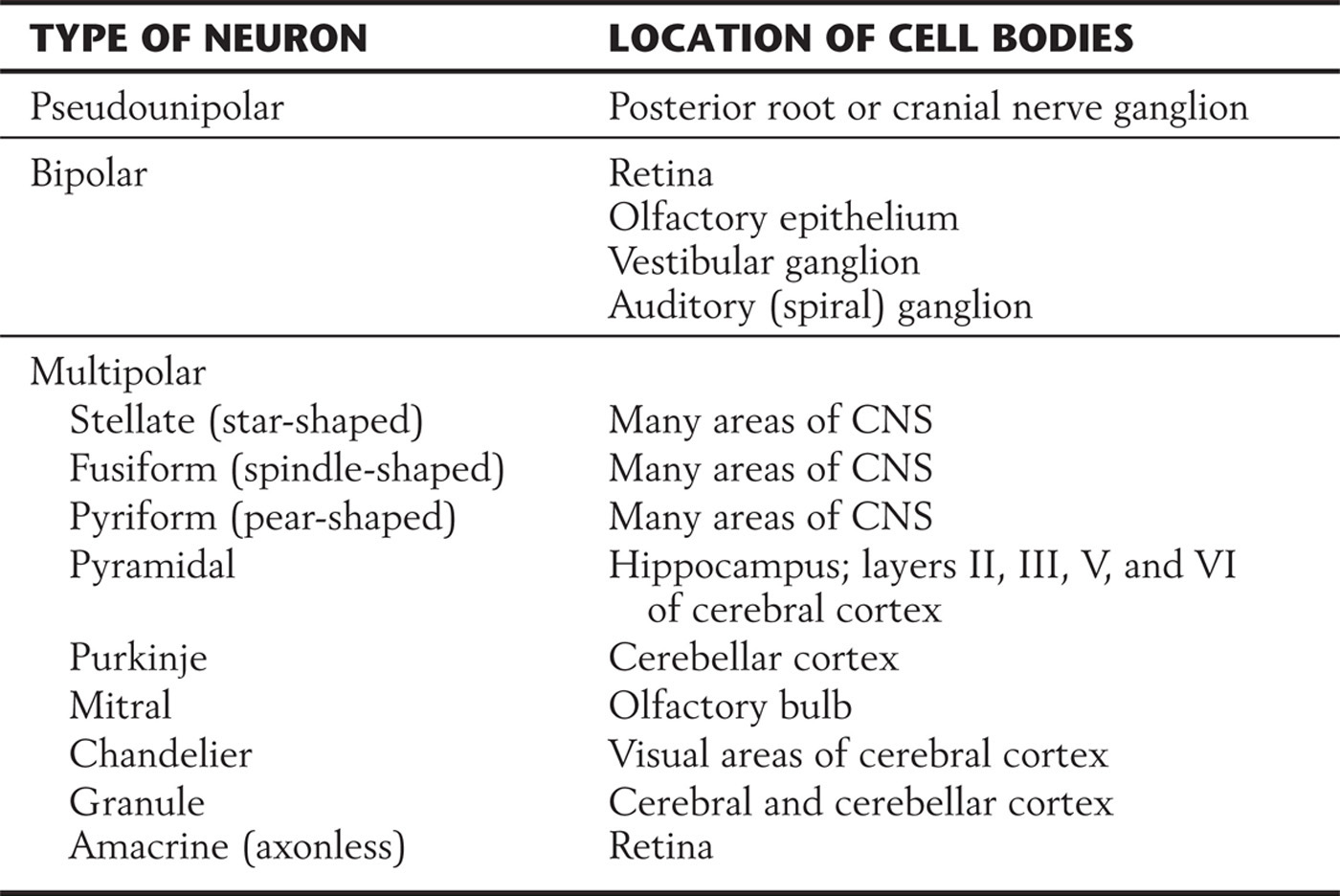
Neurons are classified into three general types on the basis of the shape of the cell body and the pattern of processes emerging from it. These types are the multipolar, pseudounipolar, and bipolar cells (Table 2-1; see also Fig. 2-2).
Table 2-1 A Few of the Neuronal Types Found in the Nervous System
CNS, central nervous system.
The cell bodies of multipolar neurons vary widely in shape, so their profiles in tissue sections may appear fusiform, flask shaped, triangular, polygonal, or stellate (Fig. 2-2A–C). Variations of a stellate polygon are most common. This shape results from the presence of multiple, tapering dendrites that emerge from the cell body. The cell body also emits a single axon that generally appears thin relative to the dendrites because the axon does not taper (discussed in the following section). More than 99% of all neurons are multipolar neurons, and the different kinds of these have characteristic patterns of processes, some of which are listed in Table 2-1.
The pseudounipolar (or unipolar) neuron has a spherical cell body with a centrally placed (concentric) nucleus. The cell body emits a single process that courses only a short distance before bifurcating into a long peripheral branch and a long central branch (Fig. 2-2D). The peripheral branch courses as part of a peripheral nerve to convey sensory information from a somatic or visceral structure, such as the skin, skeletal muscle, or wall of intestine. The distal end of the peripheral process is dendrite-like in the sense that its terminal branches receive information either by functioning as sensory receptors or by contacting other structures that function as receptors. The central branch courses as part of a nerve root to convey the sensory information to the CNS. In effect, the distal and central processes function together as a single axon. The cell bodies of pseudounipolar cells are found primarily in the sensory ganglia of cranial and spinal nerves.
Bipolar neurons have a round or oval perikaryon, with a single process emanating from each end of the cell body (Fig. 2-2E, F). They are commonly found in structures associated with the special senses. In the retina, bipolar cells are interposed between receptor cells and the neurons that send long axons from the retina to the thalamus (output cells). In the olfactory system, they function as both the receptors and the output neurons, with their axons projecting to the olfactory bulb; and in the vestibular and auditory systems, they are the output cells that send information to the brainstem.
Unless special staining methods are used, the cell body of a neuron has the appearance of being the entire cell when it is viewed in histologic sections. However, the volume of the cell body of a neuron constitutes only a small fraction, often less than 1%, of the volume of the axon and dendrites even though the cell body synthesizes and continually replaces all structural molecules of these processes.
Axons and Axon Terminals
The axon arises from the cell body at a small elevation called the axon hillock. The proximal part of the axon, adjacent to the axon hillock, is the initial segment. The cytoplasm of the axon (axoplasm) contains dense bundles of microtubules and neurofilaments (Figs. 2-1 and 2-5A, B). These function as structural elements, and the microtubules also play key roles in the transport of metabolites and organelles along the axon. Axons are typically devoid of ribosomes, a feature that distinguishes them from dendrites at the ultrastructural level.
In contrast to dendrites, axons may extend for long distances before branching and terminating. An extreme example is the length (about 1.5 meters) of the axon of a sensory neuron that conveys touch information from a toe of a tall individual. The axon of such a neuron accounts for approximately 99.8% of the total volume of the neuron. The surface area of an axon can be several thousand times the surface area of the parent cell body. Axons are sometimes referred to as nerve fibers, although strictly speaking, a nerve fiber includes both the axon and a sheath that is provided by support cells (described in a subsequent section).
Axons in the CNS often end in fine branches known as terminal arbors (Fig. 2-5C). In most neurons, each axon terminal is capped with small terminal boutons (boutons terminaux, terminal buttons) (Figs. 2-1 and 2-3C, E). These correspond to functional points of contact (synapses) between nerve cells. In some cells, boutons are found along the length of the axon, where they are called boutons en passant. Other axons contain swellings, or varicosities, that are not button-like but still can represent points of cell-to-cell information transfer.
The site at which an axon terminal communicates with a second neuron, or with an effector tissue, is called a synapse (from the Greek word meaning “to clasp”). In general, the synapse can be defined as a contact between part of one neuron (usually its axon) and the dendrites, cell body, or axon of a second neuron. The contact can also be made with an effector cell such as a skeletal muscle fiber. Synapses are considered later in this chapter in the section Neurons as Information Transmitters.
Axonal Transport
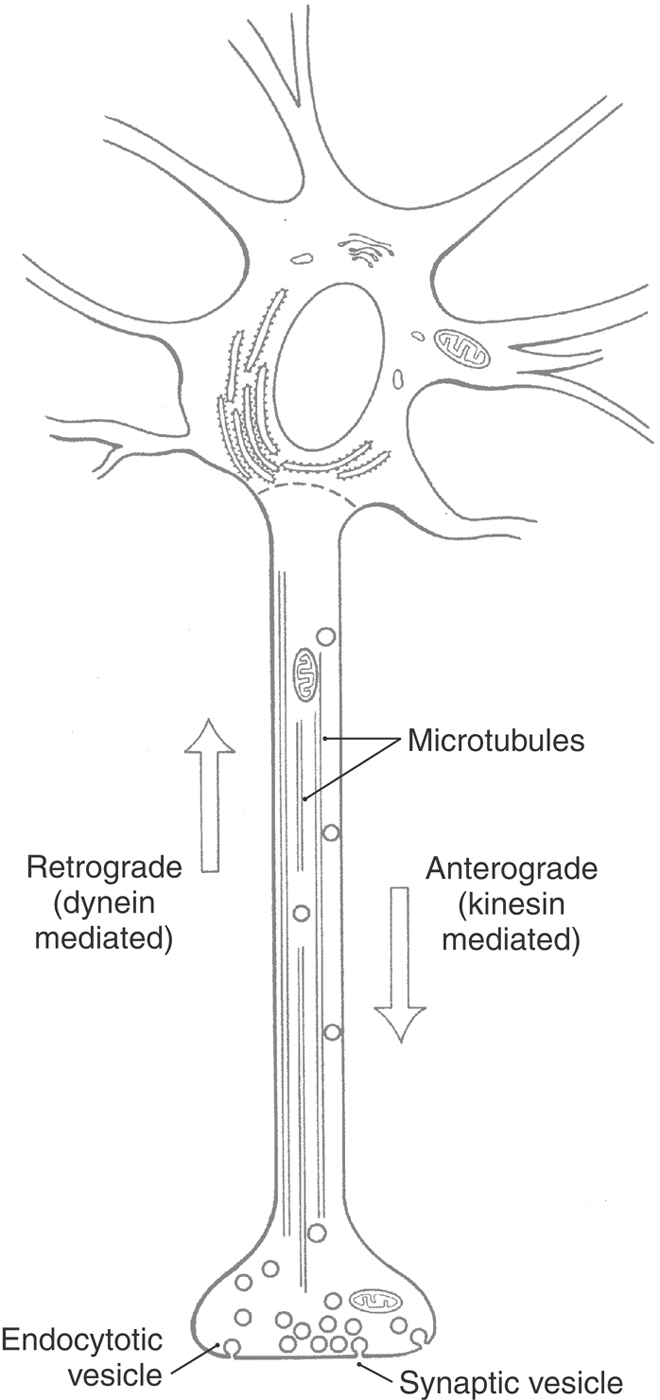
Nerve cells have an elaborate transport system that moves organelles and macromolecules between the cell body and the axon and its terminals. Transport in the axon occurs in both directions (Table 2-2; Fig. 2-6). Axonal transport from the cell body toward the terminals is called anterograde or orthograde; transport from the terminals toward the cell body is called retrograde.
Table 2-2 Characteristics of Axonal Transport
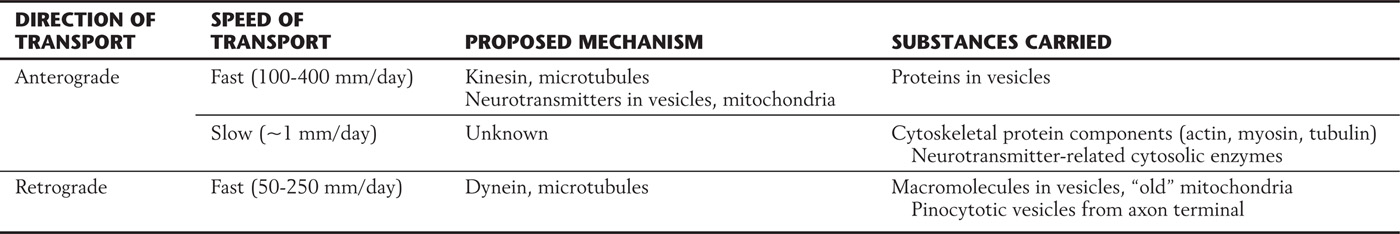
Figure 2-6. Anterograde and retrograde axonal transport.
Anterograde axonal transport is classified into fast and slow components. Fast transport, at speeds of up to 400 mm/day, is based on the action of a protein called kinesin. Kinesin, an adenosine triphosphatase (ATPase), moves macromolecule-containing vesicles and mitochondria along microtubules in much the same manner as a small insect crawling along a straw. Slow transport carries important structural and metabolic components from the cell body to axon terminals; its mechanism is less well understood.
Retrograde axonal transport allows the neuron to respond to molecules, for example, growth factors, that are taken up near the axon terminal by either pinocytosis or receptor-mediated endocytosis. In addition, this form of transport functions in the continual recycling of components of the axon terminal. Retrograde transport along axonal microtubules is driven by the protein dynein rather than by kinesin.
Axonal transport is important in the pathogenesis of some human neurologic diseases. The rabies virus replicates in muscle tissue at the site of a bite by a rabid animal and is then transported in a retrograde direction to the cell bodies of neurons innervating the muscle. The neurons produce and shed copies of the rabies virus, which in turn are taken up by the terminals of adjacent cells. In this way, the infection becomes distributed throughout the CNS, causing the behavioral changes associated with this disease. From the CNS, the virus travels to the salivary glands by means of anterograde axonal transport in neurons innervating these glands. The infected salivary glands, in turn, shed the virus in the saliva.
The toxin produced by the bacterium Clostridium tetani is also transported in a retrograde direction in nerve cells whose axons terminate at the site of infection. Tetanus toxin is released from the nerve cell body and taken up by the terminals of neighboring neurons. However, unlike the rabies virus, which is replicated in the cell body, the tetanus toxin is diluted as it passes from cell to cell. In spite of this dilution effect, patients infected with C. tetani may have a range of neurologic deficits.
Axonal Transport as a Research Tool
The ability of neurons to transport intracellular materials is exploited in investigations of neuronal connections. For example, when the enzyme horseradish peroxidase (HRP) or a fluorescent substance is injected into regions containing axon terminals, it is taken up by these processes and transported in a retrograde direction to the cell body. After histologic preparation, the cell bodies containing these retrograde tracers can be visualized. The presence of the label in a cell body indicates that the neuron has axon terminals at the site of injection.
Tracer studies can also exploit the anterograde transport system of neurons. For example, if radioactively labeled amino acids are injected into a group of neuronal cell bodies, they will be incorporated into neuronal proteins and transported in an anterograde direction. The axons containing the labeled proteins can then be detected by autoradiography. Another commonly used anterograde tracer is HRP conjugated to the glycoprotein-binding molecule (lectin) wheat germ agglutinin (WGA-HRP). Anterograde tracers are used to identify the distribution patterns of axons arising from a specific population of neuronal cell bodies.
The fact that the cell body is the trophic center of the neuron provides two other methods of studying connections in the nervous system. If the cell body is destroyed, the axon undergoes anterograde (Wallerian) degeneration. These degenerated axons can be visualized when neural tissue is impregnated with silver nitrate. Variations on this method make it possible to conduct studies on human material obtained at autopsy. Conversely, injury to the axon will result in a set of changes in the cell body that are referred to as chromatolysis. The cell body swells, the nucleus assumes an eccentric position, and the Nissl substance disperses. (This breakup of the dye-binding parts of the cell gives chromatolysis its name.) This technique has also been used in animal experimentation and in human autopsy material.
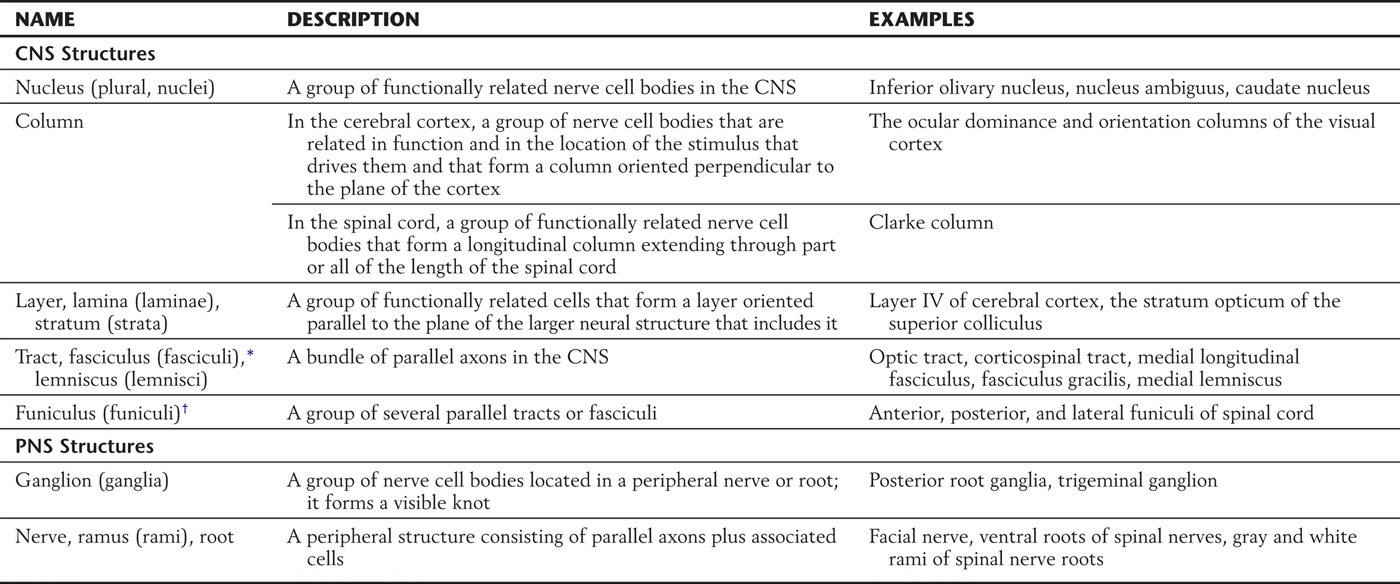
CLASSIFICATION OF NEURONS AND GROUPS OF NEURONS
Functionally related nerve cell bodies and axons are often aggregated to form distinct structures in the nervous system. Table 2-3 lists the main terms used for such structures. In the CNS, a cluster of functionally related nerve cell bodies is most commonly called a nucleus (plural, nuclei); cell bodies that are arranged in a layer may be called a layer, lamina, or stratum, and columnar groups of cell bodies may be called columns. This last term is used for two types of structures. In the cerebral cortex, it refers to a group of cells that are related by function and by the location of the stimulus that drives them. These functional groups form columns oriented perpendicular to the plane of the cortex. The second type of column is found in the spinal cord and refers to a longitudinal group of functionally related cells that extend for part or all of the length of the brainstem or spinal cord.
Table 2-3 Terms Used to Describe Groupings of Neuronal Components
*Latin for “bundle.”
†Latin for “cable.”
CNS, central nervous system; PNS, peripheral nervous system.
Bundles of axons in the CNS are called tracts, fasciculi, or lemnisci. These are typically composed of specific populations of functionally related nerve fibers (as in the corticospinal tract and medial lemniscus). A group of several tracts or fasciculi is called a funiculus or, in certain cases, a system.
In the peripheral nervous system (PNS), collections of cell bodies form a ganglion (plural, ganglia), which may be either sensory (dorsal root, cranial nerve) or motor (visceromotor or autonomic); and axons make up nerves, rami, or roots.
As noted previously, neurons can be classified into multipolar, pseudounipolar, or bipolar neurons on the basis of shape of the cell body and the number and arrangement of processes. Neurons may also be classified on the basis of functional characteristics. A neuron that conducts signals from the periphery toward the CNS is called afferent; one that conducts signals in the opposite direction is called efferent. Neurons with long axons that convey signals to a distant target are called projection neurons, whereas neurons that act locally (because their dendrites and axons are limited to the vicinity of the cell body) are called interneurons or local circuit cells.
Neurotransmitter specificity also can be used to describe neurons and their axons. For example, cells that contain the neurotransmitter dopamine are called dopaminergic neurons. The neurons whose axons form the corticospinal tracts produce the neurotransmitter glutamate and are called glutamatergic.
The distinctions among categories based on shape, projection type, or transmitter type are not as clear as those implied in the preceding discussion. For example, most neurons only vaguely resemble the “ideal” multipolar cell. In addition, neurons may overlap several categories of classification. In practice, references to ganglia, nuclei, and tracts commonly use a blend of these terms. For example, posterior root ganglion cells are pseudounipolar (their shape), sensory (type of input), and afferent (information conveyed toward the CNS), and many are peptidergic (they contain peptides such as substance P).
ELECTRICAL PROPERTIES OF NEURONS
The communicative function of neurons is carried out by fluctuations in their electrical potential. Chapter 3 explains the electrical properties of neurons in depth; at this point, only a brief introduction is needed.
Neurons carry a negative electrical charge relative to the extracellular fluid bathing them. The negative charge is required because there is a preponderance of negatively charged protein molecules within the cell and is the result of the electrochemical gradient of the relatively permanent potassium ion, with more potassium being inside the cell than outside. The uneven distribution of charged particles is maintained by the neuronal plasma membrane, which limits passage of ions, permitting them to cross only when specific ion channels open. The plasma membrane is selectively permeable because certain ions can cross at certain times, but there is not a free exchange across the membrane.
The opening and closing of specific ion channels can be controlled by chemical signals, including neurotransmitters. Channels in some sensory receptor neurons can be controlled by mechanical distortion of the membrane. Still other channels are controlled by voltage changes in the neuron. These allow an explosive feed-forward amplification from a small, chemically induced voltage change to a much larger action potential that occurs with the simultaneous opening of a large number of channels. The small, chemically induced voltage changes are restricted to tiny local areas within the neuron. They result in depolarization of the neuron if positive (sodium) ions enter the cell, reducing its net negative charge, or hyperpolarization if positive (potassium) ions exit, increasing the concentration of negative charge inside the cell.
When the sum of all tiny, local depolarizations and hyperpolarizations reaches a threshold of depolarization at the initial segment of the axon, the voltage-controlled sodium channels open, producing an action potential. The action potential is large enough that it does not remain local but is propagated anterogradely along the entire length of the axon and reaches all of the axon terminals. Arrival of the action potential at the axon terminals causes release of neurotransmitter at synapses, stimulating ion channel opening and local electrical voltage changes in the next neuron in the chain of communication, the postsynaptic neuron.
NEURONS AS INFORMATION RECEIVERS
Neurons collect, transform, and transmit information. Collection of information by the nerve cell occurs when the neuron receives input either from other neurons or directly from the environment. Sensory information enters the nervous system by the latter of the two routes.
Sensory Neural Information
Neurons that receive information from the environment are called primary sensory neurons. These include photoreceptors, chemoreceptors, mechanoreceptors, thermoreceptors, and nociceptors. Further information on these receptor types is found in the chapters describing sensory systems. For most primary sensory neurons, a stimulus results in a graded depolarizing potential, called a generator potential.
The process of converting sensory input into a form interpretable by the nervous system is transduction. Each type of sensory receptor transduces an external physical or chemical stimulus into electrical or chemical changes, which then can be transmitted as signals within the nervous system.
The rod and cone photoreceptors of the retina are specialized for transducing light energy in the form of photons. As few as three photons (possibly even a single photon) can be detected by a trained human observer. As a photon strikes the photoreceptor, it sets in motion a complex chain of events culminating in the closing of a large number of sodium channels that normally are open. As a result, the photoreceptor cell becomes hyperpolarized. This makes the photoreceptor unique among sensory cells in that the membrane potential becomes more negative on application of the stimulus rather than more positive.
In humans, the taste and olfactory receptor cells mediate the two primary types of chemoreception. Both receptor types respond to the presence of specific chemicals dissolved in a solution. Also included in this category are receptors in the hypothalamus, which sense low blood glucose concentration, low oxygen tension, or changes in blood pH; oxygen and pH receptors are also found in the aortic sinus and the carotid body.
Mechanoreceptors transduce various qualities of physical force into electrical signals that are transmitted by sensory neurons. Such receptors are found in the vestibular, auditory, and somatosensory systems.
Other types of sensory receptors include thermoreceptors, which sense temperature changes in the skin and viscera, and nociceptors, which transduce noxious (potentially harmful) stimuli. These receptors mediate what is commonly called pain; this is one of the most common complaints in clinical medicine.
Other Neural Information
Although sensory neurons transduce external stimuli, most nerve cells rely on other neurons for input. In general, the direction of information flow in a neuron is from dendrites to soma to axon, but most cells also receive information at their cell bodies, and many even receive information at the axon terminal. In all these cases, the reception of information is mediated by synapses. Synapses are the points of information transfer from one neuron to another.
NEURONS AS INFORMATION TRANSMITTERS
Synapses
The synapse is the location at which a process of one neuron (usually an axon terminal) communicates with a second neuron or an effector (gland or muscle) cell. In general, there are two broad morphologic categories of synapses, chemical and electrical (or electrotonic). The vast majority of synapses in the mammalian CNS are of the chemical type.
Chemical Synapses
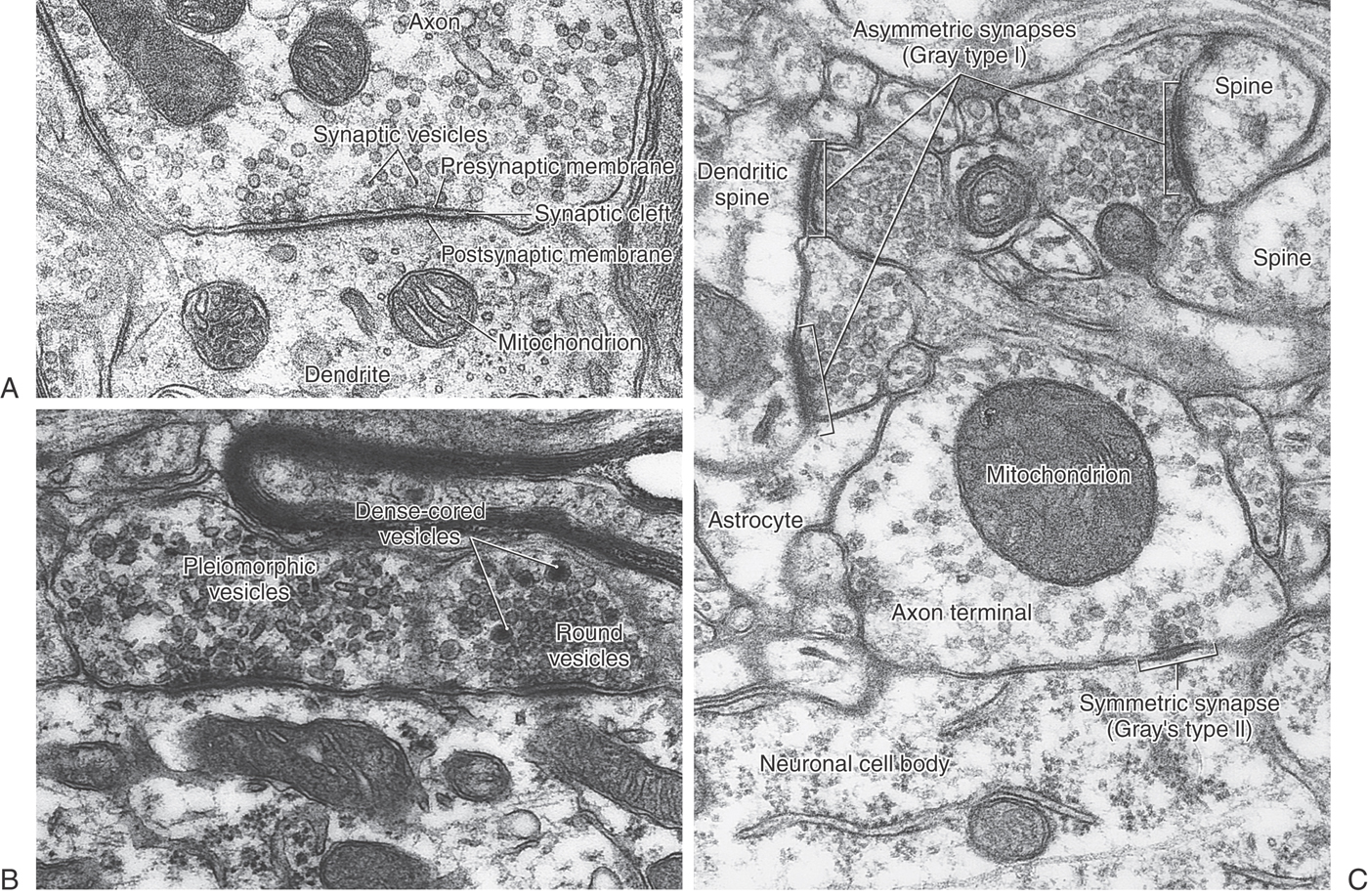
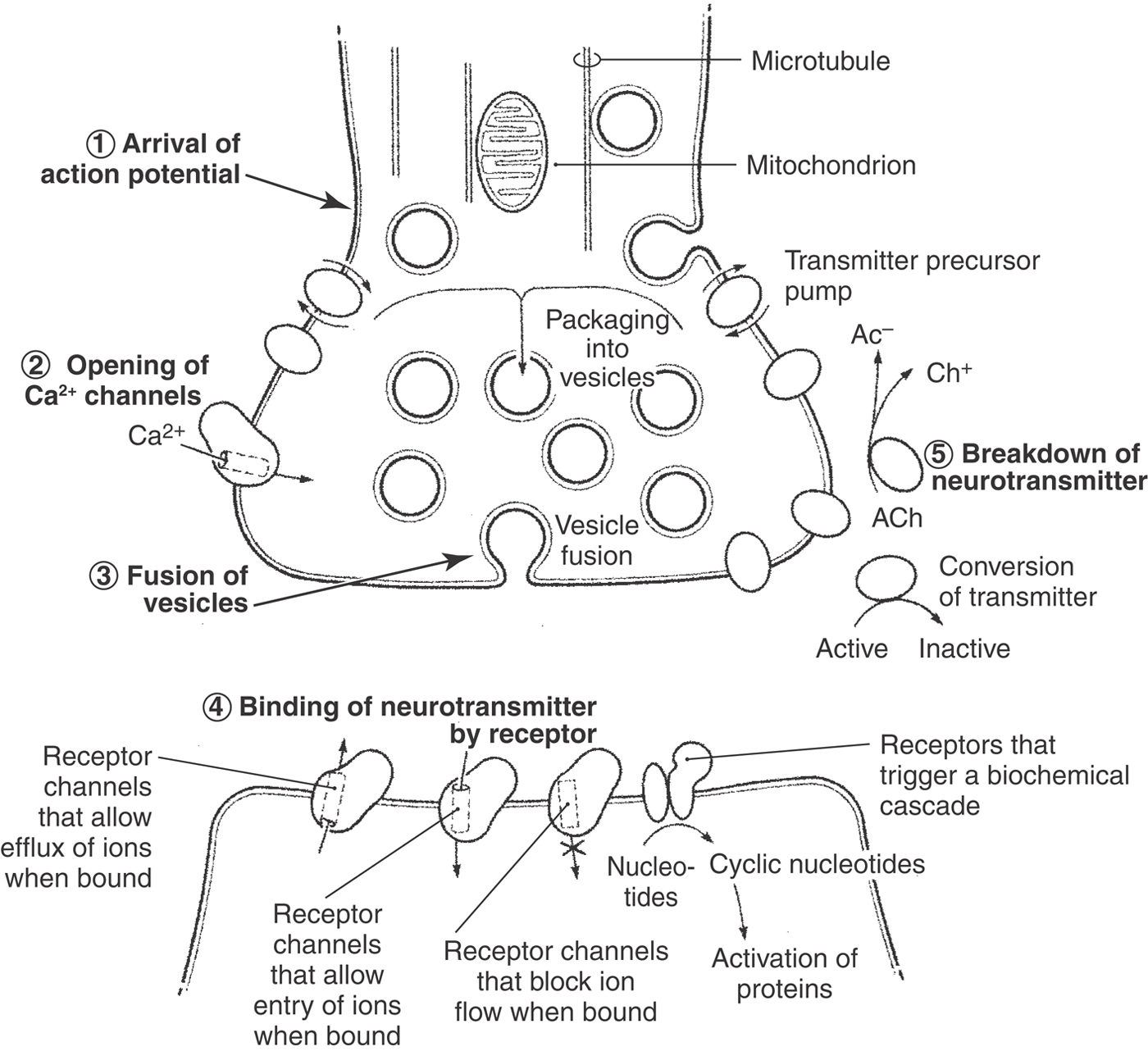
The most common type of CNS synapse comprises an axon terminal of one neuron that is apposed to a dendrite or dendritic spine of a second neuron. The prototypical chemical synapse consists of a presynaptic element, a postsynaptic element, and the intervening space (the synaptic cleft), which is 20 to 50 nm wide (Figs. 2-1 and 2-7). The presynaptic element typically takes the form of an axonal bouton. The bouton contains mitochondria, which supply energy for synaptic function, and also a prominent collection of vesicles, which contain the neurotransmitter that will be released into the synaptic cleft. These vesicles are often aggregated near sites on the presynaptic membrane called active sites (or zones), which are the sites of neurotransmitter release. Directly across the synaptic cleft is the postsynaptic membrane, which often appears thick and dark in electron micrographs (Figs. 2-1 and 2-7). Mitochondria, but not vesicles, are typically present in its vicinity.
As explained in detail in Chapter 4, communication across the synapse is mediated by the neurotransmitter stored in the presynaptic vesicles (Figs. 2-1 and 2-8). Neurotransmitter release is initiated by the arrival of an action potential, which depolarizes the presynaptic terminal. In the terminal, depolarization causes calcium channels to open. The resulting influx of calcium into the cell initiates a sequence of events that cause synaptic vesicles to fuse with the presynaptic plasma membrane and to release their neurotransmitter into the cleft (see Chapter 4 for more detail). The transmitter diffuses across the cleft and binds to specific receptors on the postsynaptic membrane, and this event triggers an electrochemical or biochemical change in the postsynaptic cell. This change represents the information as received by the postsynaptic cell.
Two functional properties of chemical synapses should be noted. First, they are unidirectional; that is, they transmit information only in the direction from the presynaptic cell to the postsynaptic cell. This directionality results because only the presynaptic cell releases the neurotransmitter, and only the postsynaptic cell expresses the receptor protein that will elicit the normal postsynaptic response to the neurotransmitter. Second, the strength of the effect on the postsynaptic membrane is variable and depends partly on the amount of neurotransmitter released into the synapse. Each synaptic vesicle contains a fixed amount of neurotransmitter (called a quantum), so the amount of neurotransmitter released depends on the number of vesicles that fuse with the presynaptic membrane in response to calcium influx.
With light microscopy, chemical synapses are visible only as the terminal boutons of an axon; but in the early years of electron microscopy, two basic morphologic types of synapse became apparent. They were named Gray type I and type II synapses. The characteristics of these synapse types are listed in Table 2-4 and illustrated in Figure 2-7C. At one time, it was believed that type I synapses were excitatory in function and type II synapses were inhibitory. We now know that the excitatory or inhibitory function of a synapse depends on the nature of the receptors present on the postsynaptic membrane and cannot be reliably predicted from the ultrastructural characteristics of the presynaptic bouton. Nevertheless, the scheme is still a useful way to classify chemical synapses. For example, synapses using acetylcholine often have the Gray type I morphology, whereas those using γ-aminobutyric acid (GABA) usually resemble Gray type II synapses.
Table 2-4 Morphologic Characteristics of Gray Type I and Type II Synapses
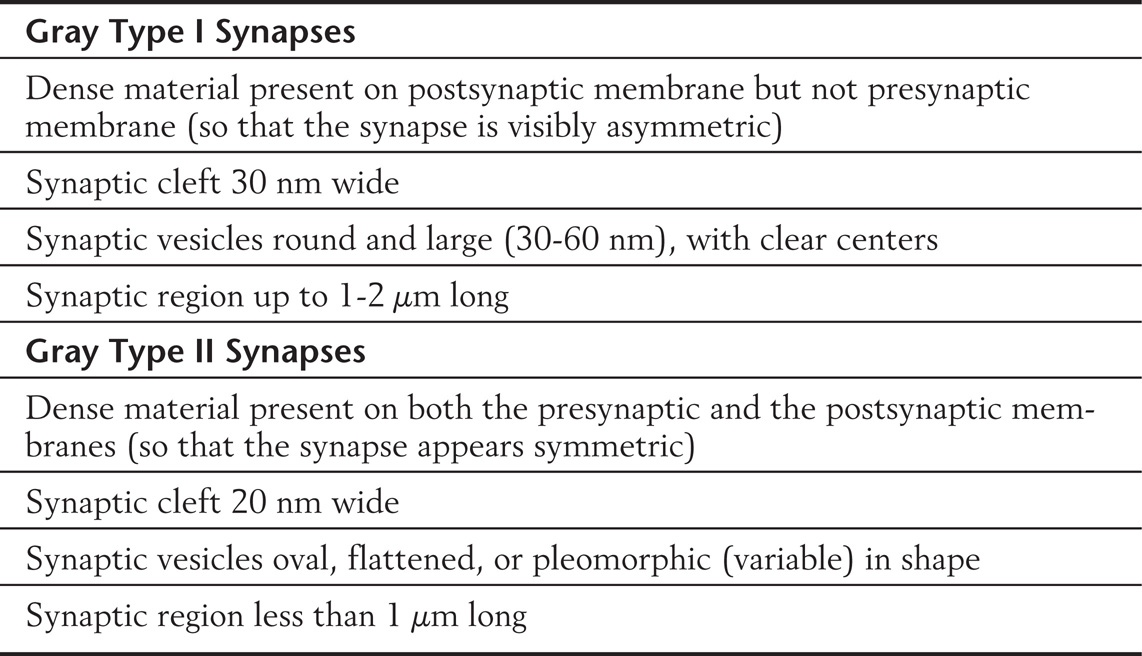
Although the vesicles of Gray type I and type II synapses differ in size and shape, the centers of the vesicles appear clear (electron lucent) in both cases. Vesicles with an electron-dense core are also seen in some synaptic endings; these dense-cored vesicles are generally thought to contain neuropeptides or serotonin as a neurotransmitter. This type of synapse is not included in the Gray classification.
NEUROTRANSMITTERS
As we have seen, neurotransmitters are a means by which information is exchanged among nerve cells as well as between nerve cells and effector cells. Neurotransmitters are considered fully in Chapter 4 and are mentioned here briefly in relation to the structure of a typical neuron.
Some neurotransmitters appear to be consistently either excitatory (e.g., glutamate) or inhibitory (e.g., GABA), but the effect (excitation or inhibition) of a neurotransmitter on a responsive postsynaptic neuron is not due to any inherent property of the signaling molecule itself. Rather, the nature of the specific receptor dictates the response. For example, neurons that respond to the neurotransmitter dopamine can express either of two types of dopamine receptors. Binding of dopamine to one of these, the D1 receptor, results in activation of adenylate cyclase, whereas binding of dopamine to the other, the D2 receptor, results in inhibition of adenylate cyclase activity.
Neurotransmitters may be biogenic amines (e.g., acetylcholine, dopamine, norepinephrine), amino acids (e.g., glutamate, GABA), nucleotides (e.g., adenosine), neuropeptides (e.g., substance P, cholecystokinin, somatostatin), or even gases (e.g., nitric oxide, carbon monoxide). Many of these neurotransmitters are stored in and released from synaptic vesicles in the axon terminal as described previously; but in other cases (e.g., nitric oxide), generation and release of the neurotransmitter does not involve vesicles.
Biogenic amines (acetylcholine) and amino acid neurotransmitters (GABA) are synthesized in the axon terminal, although the enzymes necessary for their synthesis are produced in the cell body and shipped to the terminal by axonal transport. The axon lacks the machinery to synthesize proteins (or membrane lipids) and thus must obtain these materials from the cell body. Thus axonal transport is always necessary to support synaptic function.
Disorders of Neurotransmitter Metabolism
Disorders of neurotransmitter metabolism account for a large variety of neurologic and psychiatric illnesses, but the etiology is not well understood in many cases. This category of diseases is under intensive investigation, and four examples are briefly discussed here.
Parkinson disease affects dopamine-synthesizing neurons located in an area of the brainstem known as the substantia nigra. For unknown reasons, these dopaminergic cells begin dying at an accelerated rate. The loss of dopamine results in a characteristic tremor and inability to properly control movement. Originally, therapy involved administration of supplements of L-dopa, a precursor for dopamine. This treatment increases dopamine synthesis by mass action but loses its effectiveness with time. Currently, therapy involves a combination of L-dopa with carbidopa, which inhibits the enzyme L-aromatic amino acid decarboxylase. Because carbidopa cannot cross the blood-brain barrier (defined later), it decreases the metabolism of L-dopa in peripheral tissues, making more L-dopa available to the CNS for dopamine synthesis in the remaining neurons.
Bipolar disorder affects several million Americans and appears to be caused by imbalances in the phosphatidyl inositol (PI)–linked neurotransmitter systems. An increase in PI turnover is a biochemical change triggered by some subcategories of acetylcholine, serotonin, norepinephrine, and histamine receptors. It is thought that a pathologic imbalance in PI turnover may result in mood changes. The drug lithium carbonate stabilizes PI turnover, thereby stabilizing the patient’s mood.
Alzheimer disease affects more than 1 million Americans. Although the accuracy of diagnosis by psychological testing has improved, a definitive diagnosis can be made only by postmortem microscopic examination of brain tissue. Alzheimer disease is characterized by the degeneration of neurons in basal forebrain nuclei, the loss of synapses in the cerebral cortex and hippocampus, and the presence of pathologic structures called neurofibrillary tangles and senile plaques. Cortical cells normally receive terminals from cholinergic (acetylcholine-releasing) cells in the basal forebrain nuclei. In Alzheimer disease, these terminals are lost, and the activity of choline acetyltransferase (the enzyme responsible for acetylcholine synthesis) in the cortex and hippocampus of diseased patients is extremely low. Other neurotransmitter systems, particularly neuropeptides, are also affected by this disease.
In many cases of myasthenia gravis, the patient’s immune system produces antibodies to the nicotinic acetylcholine receptor, a ligand-gated channel found at the synapse between primary motor neurons and skeletal muscle fibers. Binding of these antibodies to the receptor results in pathologic destruction of the neuromuscular junctions, which in turn causes the muscle weakness characteristic of this disease.
GLIA
Unlike neurons, glial cells do not propagate action potentials. Rather, they provide neurons with structural support and maintain the appropriate microenvironment essential for neuronal function. In addition, one type of glial cell, the astrocyte, modulates synaptic activity in its vicinity by releasing small amounts of neurotransmitters.
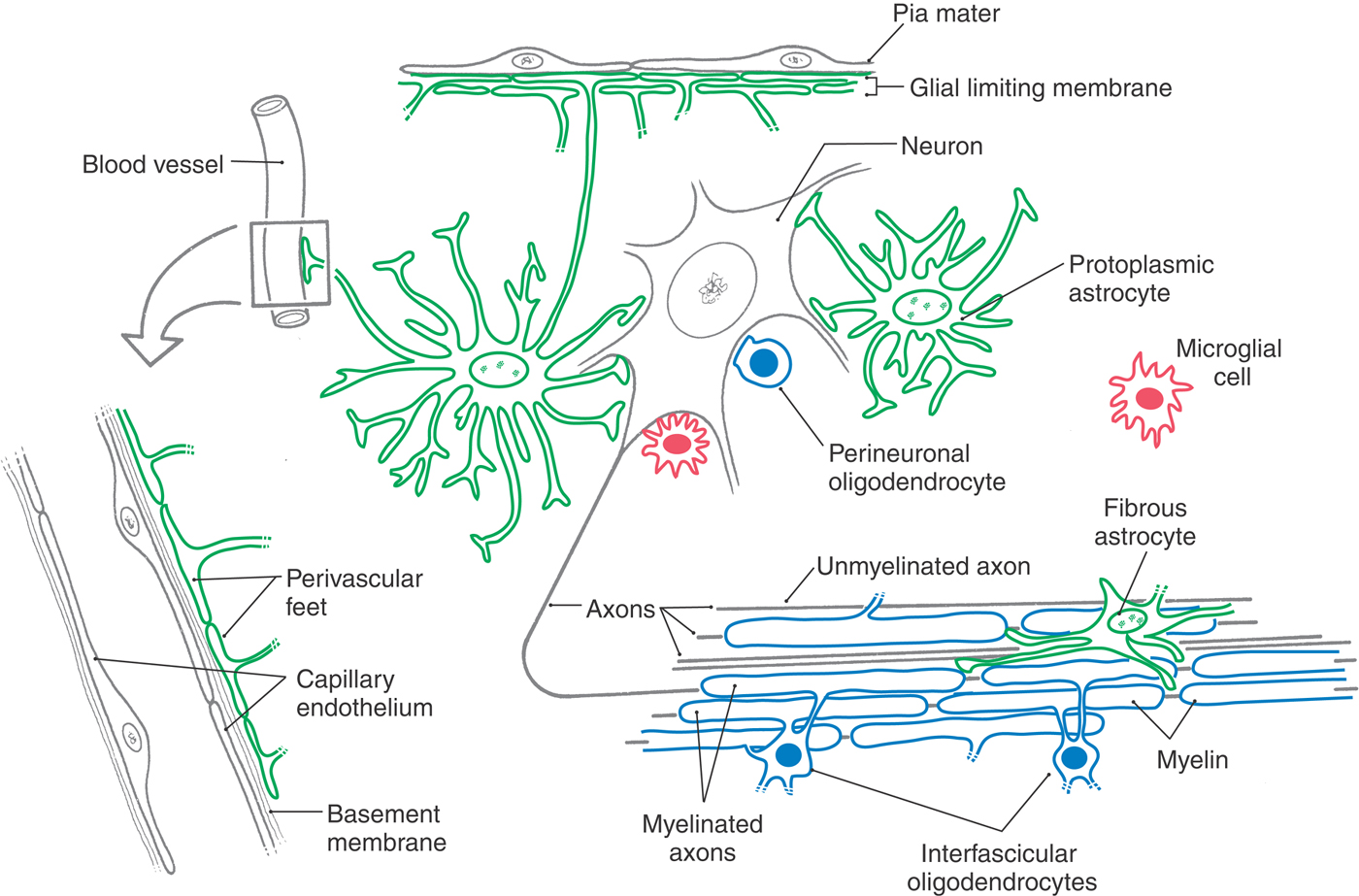
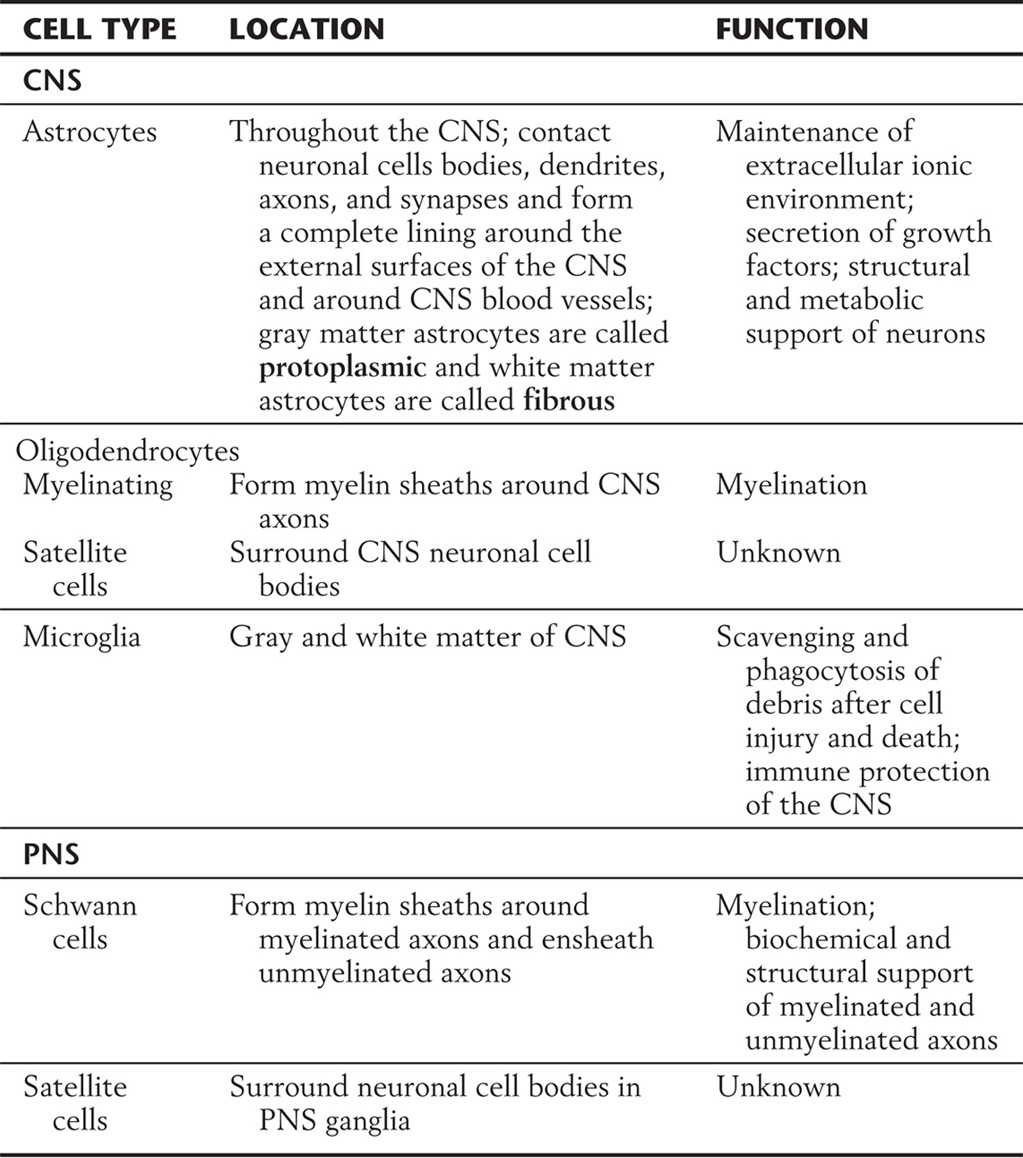
Glia account for most of the cells in the nervous system, and normal brain function requires them. The major types of glial cells in the CNS (Fig. 2-9; Table 2-5) are astrocytes and oligodendrocytes, derived from neuroectoderm, and microglia, derived from mesoderm. The analogous cell types in the PNS are the satellite cells, Schwann cells, and macrophages.
Table 2-5 Types of Glial Cells and Their Locations and Functions
CNS; central nervous system; PNS, peripheral nervous system.
ASTROCYTES
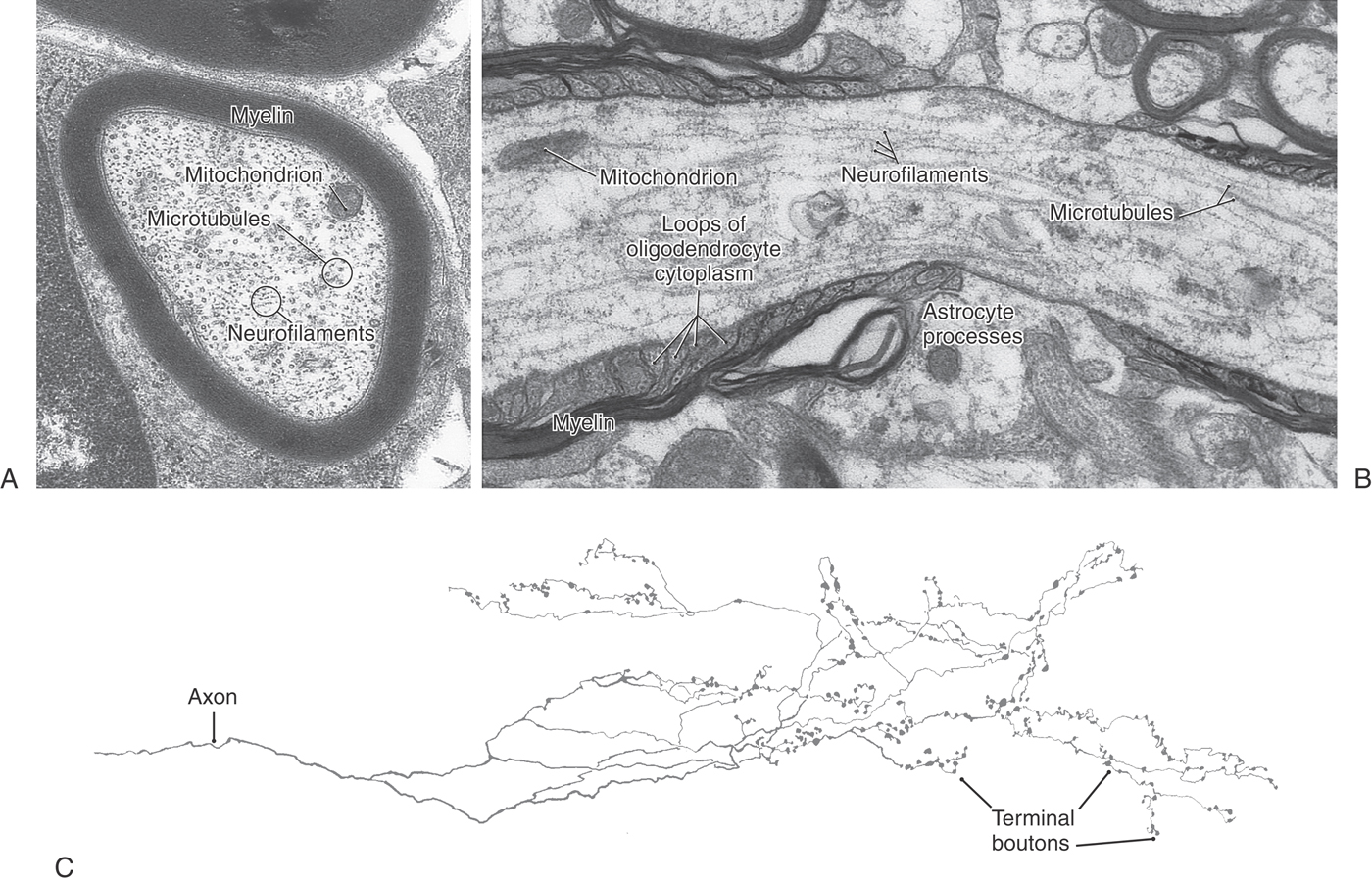
Astrocytes occur throughout the CNS. They are highly branched cells with processes that contact most of the surfaces of neuronal dendrites and cell bodies as well as some axonal surfaces and synapses. Other astrocyte processes end in expansions called end-feet (Fig. 2-9). Astrocyte end-feet join together to completely line the interfaces between the CNS and other tissues. The outer surface of the brain and spinal cord, where it meets the inner surface of the pia mater (the innermost of the meningeal membranes that enclose the CNS), is covered with a coating of several layers of joined end-feet called the glia limitans (or glial limiting membrane). Similarly, every blood vessel in the CNS is jacketed by a layer of end-feet that separates it from the neural tissue.
As shown in Figure 2-9, the astrocytes of gray matter, called protoplasmic astrocytes, differ in shape from the astrocytes of white matter, called fibrous astrocytes because of their greater content of intermediate filaments. Astrocytes can be distinguished immunohistochemically (for purposes of research and diagnosis) by the presence of intermediate filaments with a distinctive marker protein, glial fibrillary acidic protein (GFAP). The GFAP content of protoplasmic astrocytes increases in pathologic conditions.
Structural Support and Response to Injury
During development, astrocytes (in the form of radial glial cells) provide guides for neuronal migration. In the adult brain, astrocytes frame certain clusters of neurons, for example, the columns or barrels of the somatosensory cortex of rodents. In white matter, they also enclose bundles of unmyelinated axons.
When injury to the CNS results in destruction of cells, the space created by the breakdown of debris is filled by proliferation or hypertrophy (or both) of astrocytes, resulting in the formation of an astrocytic scar. That astrocytes retain the ability to proliferate in the mature brain (and thus are more susceptible to events that disrupt the control of cell division) explains why the majority of CNS tumors are of astrocytic origin.
Growth Factors and Cytokines
Current research on astrocytes indicates that they secrete growth factors vital to normal function of some neurons. In disease processes, astrocytes may secrete cytokines and immune mediators such as interleukin (IL)–1, tumor necrosis factor-α , and prostaglandin. Thus astrocytes as well as microglia contribute to the regulation of inflammatory processes in the CNS. In development, astrocytes induce synapse formation through their secretion of thrombospondins. Thrombospondins are a family of extracellular matrix proteins that bind to neuronal surface molecules (calcium channel subunits, integrins, and neuroligin synaptic adhesion proteins). Other astrocyte products, such as cholesterol and lipoproteins, are also thought to enhance synaptic plasticity.
Environmental Modulation
The ionic composition and pH of the extracellular fluid are buffered by astrocytes. These cells have ion channels in their membranes that are different from those in neurons. For example, potassium ions released from neurons during firing of an action potential are cleared from the extracellular space by astrocytes via plasma membrane ion channels. Astrocytes are connected to each other by gap junctions and act as syncytia through which excess potassium ions are shunted to perivascular spaces, restoring balance after heavy local activity. Astrocytes also propagate calcium waves, which spread through gap junctions between astrocytes to cover broad areas. Intracellular calcium levels in astrocytes, as in all cells, regulate secretory activity.
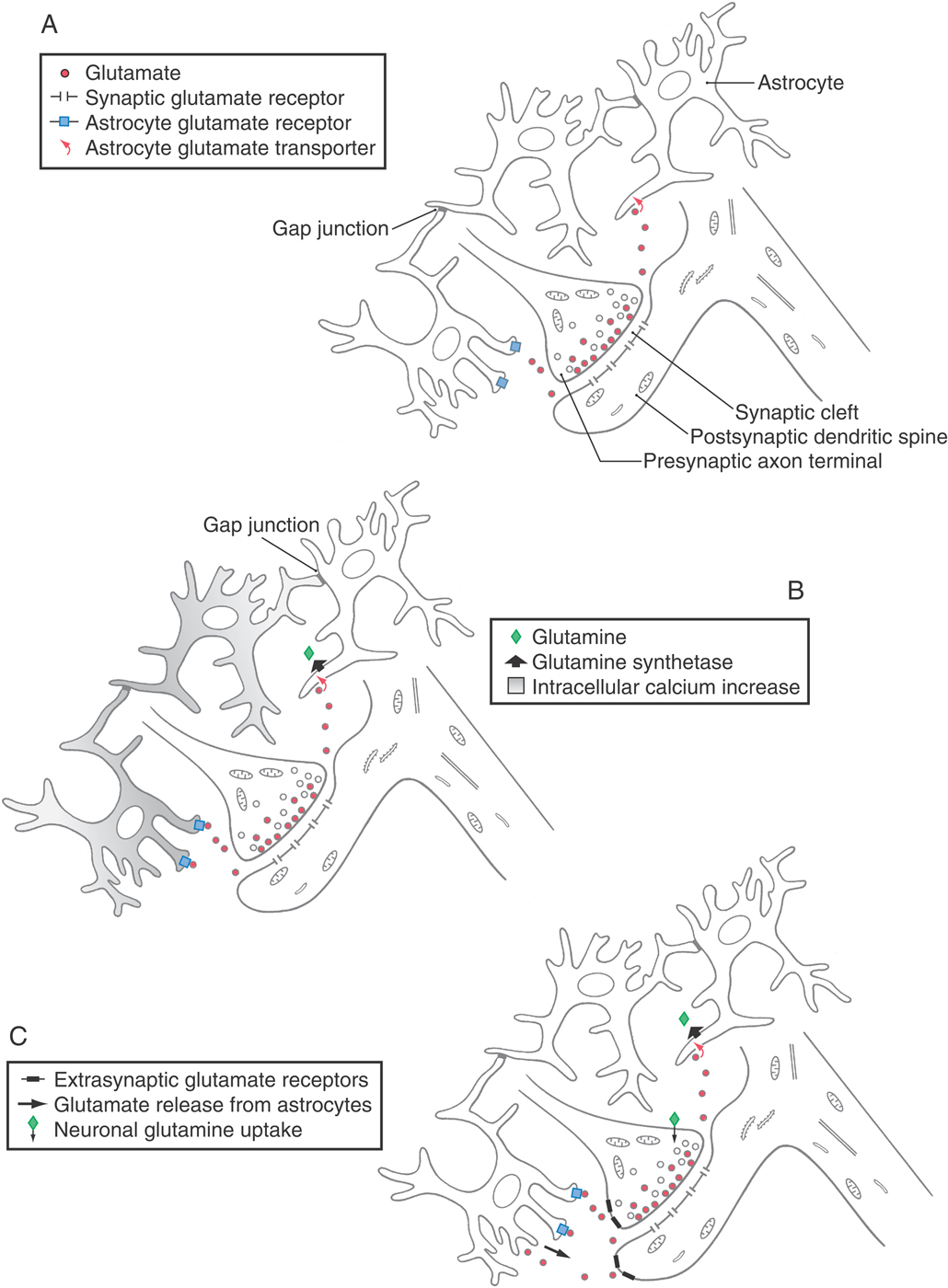
Influence on Neurotransmission
Astrocytes are present at synapses and participate in neurotransmitter metabolism. Their membranes have receptors for some neuroactive substances and uptake systems for others. Astrocytic uptake systems serve to quickly terminate the postsynaptic effect of some neurotransmitters by removing them from the synaptic cleft. For example, the amino acid neurotransmitter glutamate is taken up by astrocytes and is then inactivated by the enzymatic addition of ammonia to produce glutamine (catalyzed by the enzyme glutamine synthetase). Glutamine released from astrocytes can be taken up and reconverted to glutamate in neurons. This astrocytic pathway also detoxifies ammonia in the CNS.
Furthermore, in addition to this metabolic support role, astrocytes’ secretion of the amino acids glutamate and D-serine modulates synaptic efficacy. That is, the strength of individual synapses is adjusted in correlation with their activity patterns, so that a synapse undergoes long-term potentiation or long-term depression. Astrocytes respond to neuronal activity by secreting glutamate or D-serine, which binds to neuronal N-methyl-D-aspartate (NMDA) receptors and modulates synaptic activity. This reciprocal signaling arrangement is illustrated in Figure 2-10. Astrocytes also release other neurotransmitters. The inhibitory neurotransmitter GABA is released from astrocyte anion channels. Its sustained mode of release causes tonic inhibition of synapses in the surrounding area. In contrast, adenosine triphosphate (ATP) is released by astrocytes in response to neuronal signaling. It is metabolized to the neurotransmitter adenosine, which is involved in cellular energy regulation and the sleep-wake cycle.
Regional Heterogeneity
Astrocytes vary biochemically between gray matter (protoplasmic astrocytes) and white matter (fibrous astrocytes) and from one region of the CNS to another. White matter astrocytes differ from gray matter astrocytes in terms of their ion channels, neurotransmitter receptors and uptake systems, and other special properties. For unknown reasons, astrocyte tumors of particular types occur in characteristic distributions rather than with random frequency throughout the CNS. For example, the malignant tumor glioblastoma multiforme develops most frequently in the frontal or temporal lobe of the cerebral cortex.
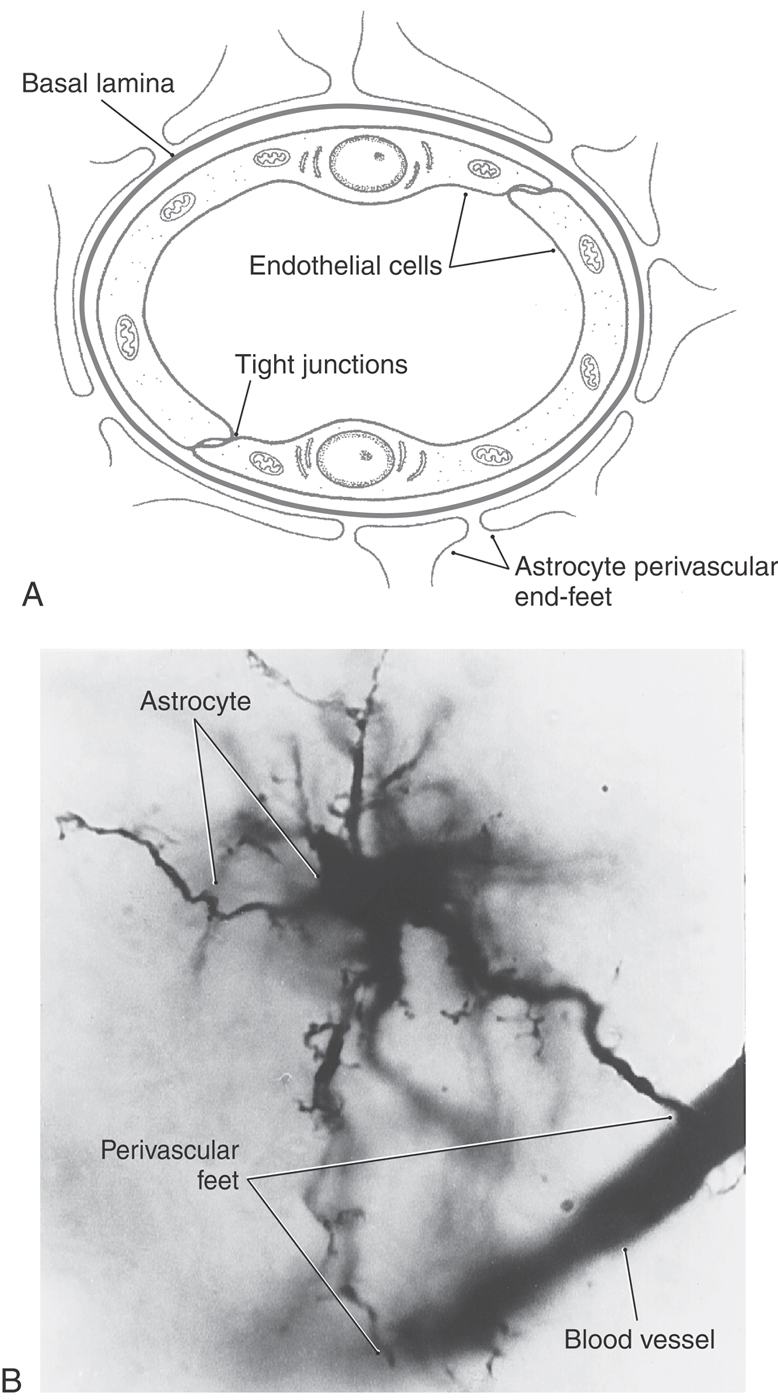
Astrocytes at the Blood-Brain Barrier
In many tissues, solutes can pass freely between the capillary plasma and the interstitial space by diffusing through gaps between endothelial cells. In the CNS, vessels are induced by the surrounding jacket of astrocyte end-feet to form extensive tight junctions, so solutes can reach the neural tissue only by passing through the endothelial cells (Fig. 2-11). The resulting restricted exchange constitutes the blood-brain barrier. In a strict sense, the blood-brain barrier is formed by the tight junctions of the endothelium. However, many people refer to the blood-brain barrier more inclusively as the physical complex of endothelium, basal lamina, and astrocyte end-feet surrounding each CNS vessel (see also Chapter 8). Water, gases, and lipid-soluble small molecules can diffuse across the endothelial cells, but other substances must by carried across by transport systems, and their exchange is highly selective. This selectivity is further enhanced by a reduction in pinocytotic transport. In most tissues of the body, a high level of pinocytotic activity by endothelial cells transports solutes nonspecifically from the blood plasma to the perivascular space. In contrast, endothelial cells of capillaries in most parts of the CNS show little pinocytotic activity. The blood-brain barrier is of major clinical importance because it largely excludes many drugs from the CNS.
CONTROL OF LOCAL BLOOD FLOW WITHIN THE CENTRAL NERVOUS SYSTEM
In a person at rest, 20% of the body’s energy is consumed by the brain, mostly to restore ion concentration gradients across neuronal membranes. Therefore, blood flow increases in regions where neurons are active. This process is called neurovascular coupling, and the increase, functional hyperemia, is the basis of functional magnetic resonance imaging. Clinically, neurovascular coupling is defective in conditions such as migraine, stroke, hypertension, spinal cord injury, and Alzheimer disease. As our understanding of the mechanisms increases, therapeutic interventions may be within reach.
Both astrocytes and neurons cooperate in neurovascular coupling through glutamate signaling. They produce an increase in local blood flow that is at least four times greater than the increase in consumption of oxygen and ATP by the local neurons. In neurons, glutamate acts on NMDA receptors to increase intracellular calcium, to increase the enzyme activity of neuronal nitric oxide synthase, and to release nitric oxide, leading to vasodilation. Both arteriolar smooth muscle and the contractile pericytes of capillaries are relaxed by nitric oxide. In astrocytes, glutamate acts through neuroligin glutamate receptors to increase intracellular calcium, to increase the enzyme activity of phospholipase A2, to increase arachidonic acid in the cell membrane, and to increase production of the arachidonic acid derivatives prostaglandins and epoxyeicosatrienoic acids, leading to vasodilation. The neuronal and astrocytic pathways interact, and both are regulated by oxygen tension. So the exact balance between neuronal and astrocytic control of local blood flow varies according to conditions and region.
In spreading depression, reduced blood flow lasts for hours and may damage neurons. Considering the nitric oxide pathway and its regulation by oxygen tension, it is possible that a negative feedback loop operates in spreading depression, such that reduced oxygen leads to reduced production of nitric oxide, which leads to reduced blood flow (failure of vessels to relax), which leads to even lower availability of oxygen. This pathway and pharmaceutical modulators of it can now be tested experimentally.
OLIGODENDROCYTES
Oligodendrocytes, like astrocytes, occur in both gray matter and white matter (Fig. 2-9). The function of oligodendrocytes is myelination, that is, the provision of an electrochemically insulating sheath around all but the smallest axons in the white matter (Figs. 2-9 and 2-12). Other oligodendrocytes lie adjacent to and surround neuronal cell bodies in the gray matter, but they do not make myelin, and the significance of this arrangement is not well understood.
A myelin sheath is a membranous wrapping around an axon that greatly increases the speed of conduction of action potentials along the axon. Large-diameter axons have thick myelin sheaths and high conduction velocities; smaller diameter axons have thinner myelin sheaths and slower conduction velocities; and the smallest axons are unmyelinated and have the slowest conduction velocities.
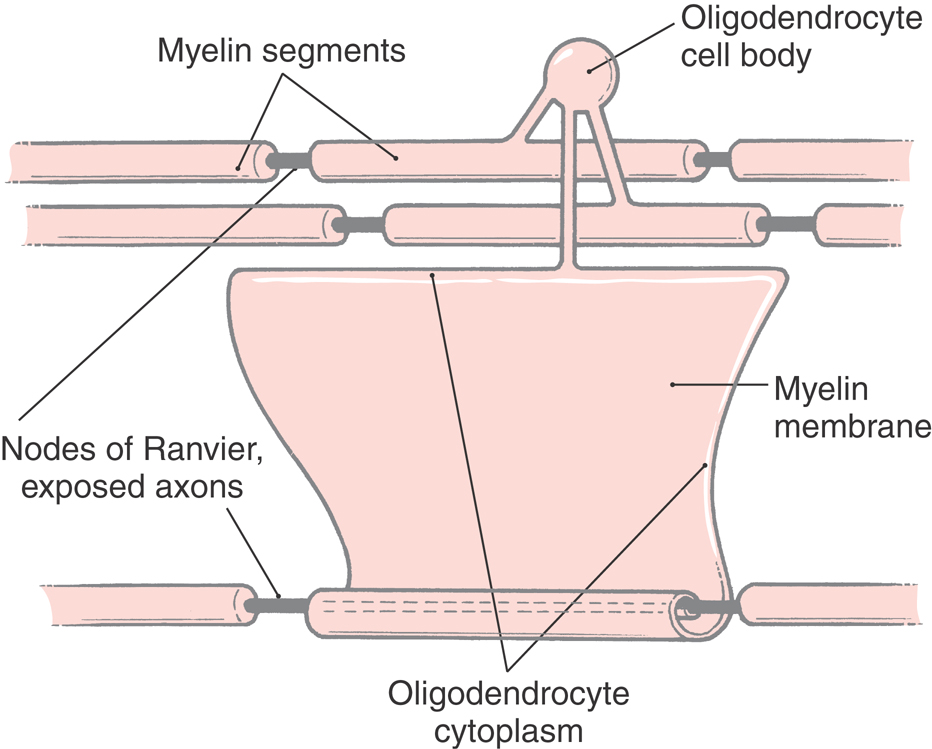
Myelin is formed by a cell-cell interaction in which an axon destined for myelination is recognized by proteins on the oligodendrocyte surface. Developing oligodendrocytes also receive electrochemical signals from active axons. The oligodendrocyte responds by producing a flattened, sheet-like process that wraps repeatedly around the axon (Fig. 2-12). As the layers of membrane accumulate, all cytoplasm is excluded, so that the mature myelin sheath consists of layers of oligodendrocyte plasma membrane firmly pressed together. Cytoplasm remains only in the innermost and outermost turns of the oligodendrocyte process.
The myelin sheath surrounding an axon is not continuous along its entire length. Rather, the axon is covered by a series of myelin segments, each formed by an oligodendroglial cell. The interruptions between segments are called nodes of Ranvier (Fig. 2-5B). Morphologic specializations at the nodes include a dense undercoating of the axonal membrane, as seen at the initial segment of the axon, and contact by an astrocyte process. The rapid ionic exchanges across the axonal membrane essential for generating the action potential and propagating it down the axon occur at the nodes of Ranvier. The depolarization is then passively conducted along the axon (as a graded potential) to the next node. This method, saltatory conduction, is faster and requires much less energy than having ionic exchanges occur continuously along the length of the axon.
The segments of myelin between adjacent nodes of Ranvier are called internodal segments, or internodes. Although the name oligodendrocyte means “cell with few branches,” some of these cells give rise to myelinating processes forming internodal segments on as many as 40 axons.
Myelination occurs in the human CNS from birth through adolescence. Even in adults, there is evidence from imaging studies that white matter changes occur when subjects learn skills like juggling or playing the piano.
In demyelinating diseases, such as multiple sclerosis, groups of oligodendrocytes and their corresponding myelin segments degenerate and are replaced by astrocytic plaques. This loss of myelin results in an interruption of the propagation of the action potential down these axons. Demyelinated axons survive temporarily, and some remyelination is possible by the growth of oligodendrocyte precursor cells that reside in the adult CNS. The particular array of motor, visual, or general sensory losses in a patient with multiple sclerosis reflects the locations of the demyelinating lesions.
MICROGLIA
Microglial cells are the immune effector cells of the CNS, and thus they are the predominant cells involved in CNS inflammation. The precise embryonic origin of the microglial cells has been controversial, but these cells clearly differ from other neural and glial cells of the CNS in that microglia do not arise from neuroectoderm. Rather, they are descendants of myeloid progenitor cells arising from the yolk sac and entering the CNS early in embryonic development. Microglia make up about 1% of the CNS cell population (Fig. 2-9). During health, they are considered to be sessile, or quiescent.
Like astrocytes, each microglial cell spreads its processes to cover a unique territory that does not overlap with that of neighboring microglia. Unlike astrocytes and oligodendrocytes, however, microglia are not connected by gap junctions. In the healthy CNS, the microglial cell body remains fixed in place, but its branched processes continually move in protective surveillance of surrounding tissue. Microglia are versatile in their responses to any threats that may be discovered. In certain disease states, such as viral encephalitis caused by HIV-1, subacute sclerosing panencephalitis, lead encephalopathy, and neurosyphilis, microglia withdraw and reshape their processes to form long rod cells closely apposed to affected neurons. In cases of trauma or severe tissue injury, they become motile, ameboid phagocytes capable of migrating to the site of injury and proliferating. At the injury site, they phagocytose tissue debris.
Metabolic activation of microglia may be even more important to their functioning than the dramatic shape changes observed pathologically. As immune cells, microglia can be stimulated to secrete cytokines, such as interleukins and tumor necrosis factor-α, and other immune mediators, such as arachidonic acid derivatives prostaglandin E2 and platelet-activating factor. Like macrophages, they also secrete growth factors, for example, brain-derived neurotrophic factor. The broad range of microglial products thus includes potentially neurotoxic and neuroprotective mediators of inflammation and tissue repair. Current research is aimed at determining what combinations of these products are triggered by specific stimuli that enter the CNS.
The net effects of microglial activation can be beneficial and protective, as in the case of synaptic stripping. This term is applied to a situation in which the facial nerve (a motor nerve) has been cut peripherally. Microglia then surround the motor neuron cell bodies located in the brainstem and displace or remove all synapses from the surface of the neurons. Once the peripheral axon has grown back and reconnected with its target muscles, new synapses form around the neurons.
In other cases, microglial activation can be harmful. For example, in bacterial meningitis in children, as microglia phagocytose particles of bacteria killed by penicillin, they are stimulated to secrete IL-1β. IL-1β acts on endothelial cells to loosen their tight junctions, allowing leukocytes and blood plasma to enter CNS tissue, escalating the inflammation to a level that can be fatal. When researchers and physicians prevented this secondary inflammation by administering steroids before giving penicillin, microglial cytokine secretion was inhibited, and the survival rate for bacterial meningitis in children vastly improved.
Microglial cells are the CNS cells targeted by human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS). The mechanism by which HIV infection of microglia leads to neuronal damage and dementia is not yet fully understood.
TUMORS OF THE CENTRAL NERVOUS SYSTEM
Primary brain tumors arise from the cells that make up the structure of the brain and spinal cord as well as its coverings. Individual cells belonging to any of the cell populations found in brain tissue or the leptomeninges can give rise to a brain tumor, provided genetic and environmental stimuli favor cell proliferation. However, neurons give rise to tumors only rarely, as would be expected of a cell type that is postmitotic and highly differentiated morphologically.
Glia-Derived Tumors
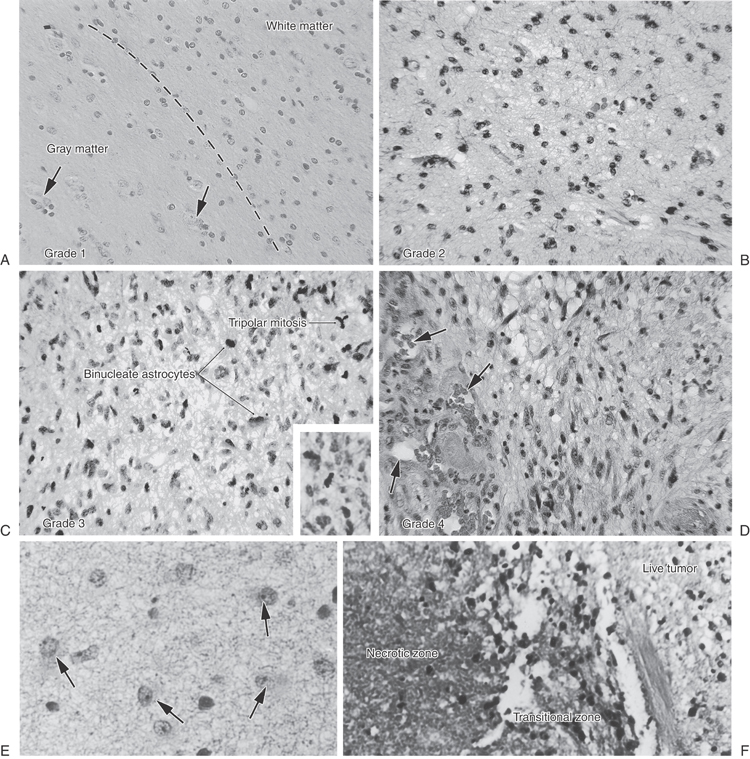
Glial cells are a frequent source of primary brain tumors in adults and children, and of these, astrocytomas are the glial tumors encountered most often. An evaluation of astrocytomas is traditionally made on the basis of how closely or how little the neoplastic cells resemble nonneoplastic astrocytes (degree of differentiation), and this helps predict the outcome for the patient. This grading of astrocytomas is an attempt to better define the biologic aggressiveness of the tumor and to estimate the tumor’s effect on the life span of the patient (prognosis).
Grade 1 astrocytomas, uncommon tumors, resemble differentiated astrocytes that react to an injury within brain tissue (Fig. 2-13A). They usually arise from fibrillary astrocytes in the white matter, which have many stubby processes (Fig. 2-13E). They grow slowly, and gradual enlargement may be the main clue that a neoplasm does exist. On occasion, protoplasmic astrocytes, denizens of gray matter with fewer processes, may form tumors that contain fluid-filled cysts.
The astrocytes of grade 2 astrocytoma, which have prominent processes filled with glial filaments, infiltrate between myelinated axons in white matter and increasingly cluster around neurons in cortical gray matter (Fig. 2-13B). Although they are commonly encountered in adult patients, years may elapse before these tumors are symptomatic. However, if they recur after surgery, these astrocytomas may become more aggressive, transforming into a higher grade. In general, grade 1 and grade 2 astrocytomas are slowly growing masses.
Grade 3 astrocytomas have nuclei that are often enlarged, with increased density of chromatin. Uniformity of nuclear appearance is lost. Mitotic figures, consisting of chromosomes on spindles (Fig. 2-13C), may be frequently noted in tumor cells and are one indicator of rapid cell proliferation. The density of blood vessels is increased. These are rapidly growing, malignant tumors.
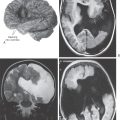
Grade 4 astrocytomas are highly malignant tumors. The astrocytes of these tumors may be spindled, and the elongated nuclei may have many mitotic figures. They can invade the leptomeninges, spreading from one contiguous gyrus to its neighbor. Also known as glioblastoma multiforme (GBM), this astrocytoma subtype characteristically extends from one hemisphere into the other as it marches through the corpus callosum. Complex neovascular structures (Fig. 2-13D) and sharp borders between living and dead tumor tissue (Fig. 2-13F) are intrinsic features of glioblastoma. Unfortunately, this “high-grade” tumor is the most common astrocytoma encountered in middle-aged and elderly adult patients. After diagnosis, the survival time of some patients with a grade 4 tumor (GBM) may be measured in only weeks.
Oligodendroglia, which myelinate axons in the white matter, are also found in the gray matter, where they are neuronal “satellite” cells. These cells can produce slow-growing tumors, located in the lobes of the brain rather than in the diencephalon or in the basal ganglia. The oligodendroglia in these tumors have dark, round nuclei centered within clear cytoplasm, much like the yolk of a fried egg embedded in egg white. Tumor cells form sheets that are subdivided into geometric units by capillary twigs. Hallmarks of oligodendrogliomas are enlarged clusters around neurons (satellitosis) and nodules of tumor cells beneath the pia.
The ventricular spaces of the brain and the central canal of the spinal cord are lined by an epithelium of ependymal cells. Tumors of these cells are termed ependymomas. When ependymomas occur in children or adolescents, they are found in the fourth ventricle. In adults, they are located in the spinal cord, especially at the cervical level. These glial tumors are less infiltrative than astrocytomas. Because they are more circumscribed, they can be more easily dissected away from the surrounding spinal tissues and removed by a neurosurgeon.
Tumors that stem from the last member of the glial family, microglia, were first depicted as forming cellular aggregates around tiny arterioles. The modern interpretation is that these tumors are lymphomas, a large family of neoplasms consisting of bone marrow–derived cells (B lymphocytes) or thymus-derived cells (T lymphocytes). Unlike the lymph nodes in which these tumors predominate, the brain has no lymphatics. Current thinking suggests that the malignant cells reach the CNS by breaching the barrier between blood and brain tissue. Lymphomas of the brain are more frequent in patients who have one of the various states of acquired immunodeficiency (e.g., HIV infection or immunodeficiency induced by medicines required after organ transplantation). In addition, genetic footprints of another virus (Epstein-Barr virus) can frequently be detected when molecular genetic techniques are applied to microscopic sections of lymphomas. Some of the lymphomas can be successfully treated with medicines or radiation.
Tumors in Children
Tumors that primarily affect children may contain cells that function like stem cells. Medulloblastomas arise in the cerebellar hemispheres of children and consist of primitive “blue cells” that are capable of developing along several pathways. The initial cells can mature into members of the glial family or into neurons, all of which may be found in the same tumor. Although the cells still appear as blue cells, electron microscopy or immunohistochemistry can demonstrate astrocytic or neuronal features of the incompletely differentiated cells. Most medulloblastomas retain the characteristic unrestrained growth of embryonal cells, without differentiation. They quickly spread along the surface of the brain and spinal cord and must be treated aggressively.
Benign Primary Brain Tumors
Benign primary brain tumors are often covered by a fibrous, vascularized capsule that discretely demarcates the tumor from surrounding normal brain. As a benign tumor enlarges, it pushes against brain tissue, rather than extending finger-like projections that invade white and gray matter for great distances. Benign primary brain tumors cause problems by compressing normal tissue as they grow. Frequently encountered benign primary tumors of the brain include meningioma (see Chapter 7) and schwannoma.
Metastatic Brain Tumors
Metastatic brain tumors arise from malignant cells that originate outside the nervous system. The growth pattern of metastatic tumors differs from that of primary tumors. Microscopic clumps of malignant cells break away from the initial growths and travel via the bloodstream to the brain. These cell aggregates become lodged at tiny arteriolar branch points, frequently located at the junction of gray and white matter. Using intracellular enzymes that dissolve basement membranes, the malignant cells escape from the confines of the vasculature and start to grow in the brain. Some of the most prevalent malignant tumors that affect men and women frequently metastasize to the brain. Lung carcinoma is the most common primary tumor to secondarily involve the brain. Breast carcinoma may spread to the dura or to the brain substance. Prostate carcinoma can spread to the spinal cord through veins of the Batson venous plexus.
SUPPORTING CELLS OF THE PERIPHERAL NERVOUS SYSTEM
The PNS contains supporting cells called satellite cells and Schwann cells, which are analogous to astrocytes and oligodendrocytes, respectively. Satellite cells surround the cell bodies of neurons in sensory and autonomic ganglia, and Schwann cells ensheath the axons in peripheral nerves (Fig. 2-14).
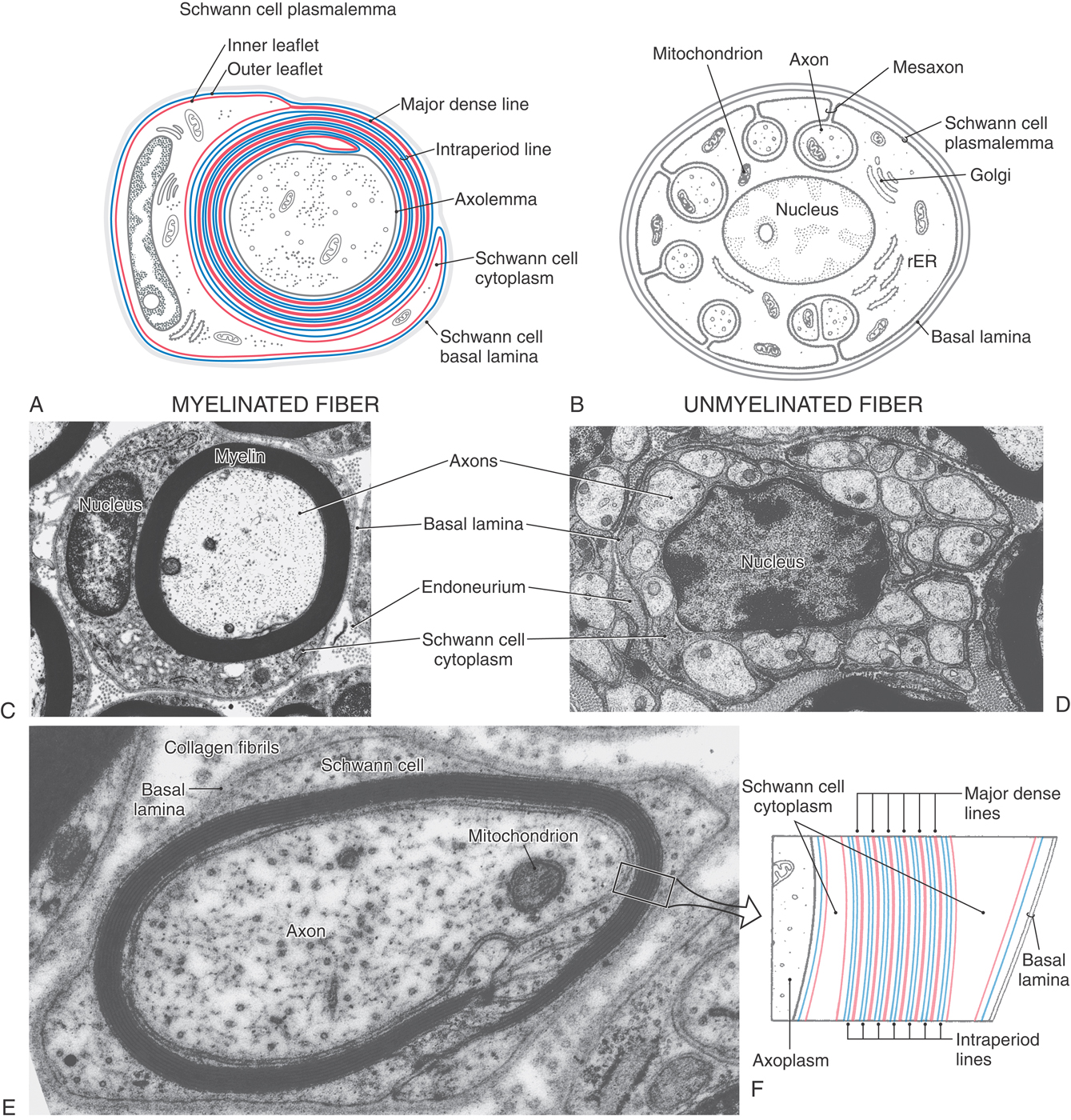
Figure 2-14. Diagrammatic representation of myelinated (A) and unmyelinated (B) fibers in peripheral nerves. Schwann cells ensheath all peripheral nerve axons. Multiple wraps of the plasmalemma of a Schwann cell fuse to form compact myelin (see also Fig. 2-12). A, The inner leaflet of the plasmalemma (red) fuses to form major dense lines, and the outer leaflets (blue) of each adjacent wrap contact each other to form intraperiod lines. B, In an unmyelinated fiber, small axons occupy troughs formed by invaginations of the Schwann cell plasmalemma. rER, rough endoplasmic reticulum. C and D, Electron micrographs of a myelinated fiber (C) and an unmyelinated fiber (D) composed of a single Schwann cell supporting more than 20 axons. E, A small myelinated fiber sectioned through part of a Schmidt-Lanterman cleft reveals the membrane composition of myelin. The layers of the boxed segment of myelin in E are diagrammed in F.
In the PNS, as in the CNS, large- and intermediate-diameter axons have myelin sheaths, and the smallest diameter axons are unmyelinated. Schwann cells produce these myelin sheaths and also envelop the unmyelinated axons. The myelin sheaths are similar to the CNS type, consisting of a tight spiral wrapping of fused plasma membrane. They are also formed similarly by a Schwann cell that is attracted to an axon segment and wraps repeatedly around it to produce a compact sheath (Fig. 2-14). As in the CNS, cytoplasm remains only in the innermost and outermost layers of this wrapping (Fig. 2-14).
Myelination in the PNS differs in the following respects from that in the CNS. Small pockets of cytoplasm known as Schmidt-Lanterman clefts are found at irregular intervals in PNS myelin. A basal lamina covers the external surface of the Schwann cell. The basal lamina is formed by the Schwann cell and may help stabilize it during the process of myelin formation. In addition, each Schwann cell forms the myelin of only a single internode of a PNS axon; in contrast, in the CNS, oligodendrocytes send out numerous processes, each of which forms a myelin internode. Unmyelinated axons in the PNS are enclosed in canals formed by invaginations in Schwann cells (Fig. 2-14). These Schwann cells also are covered by a basal lamina.
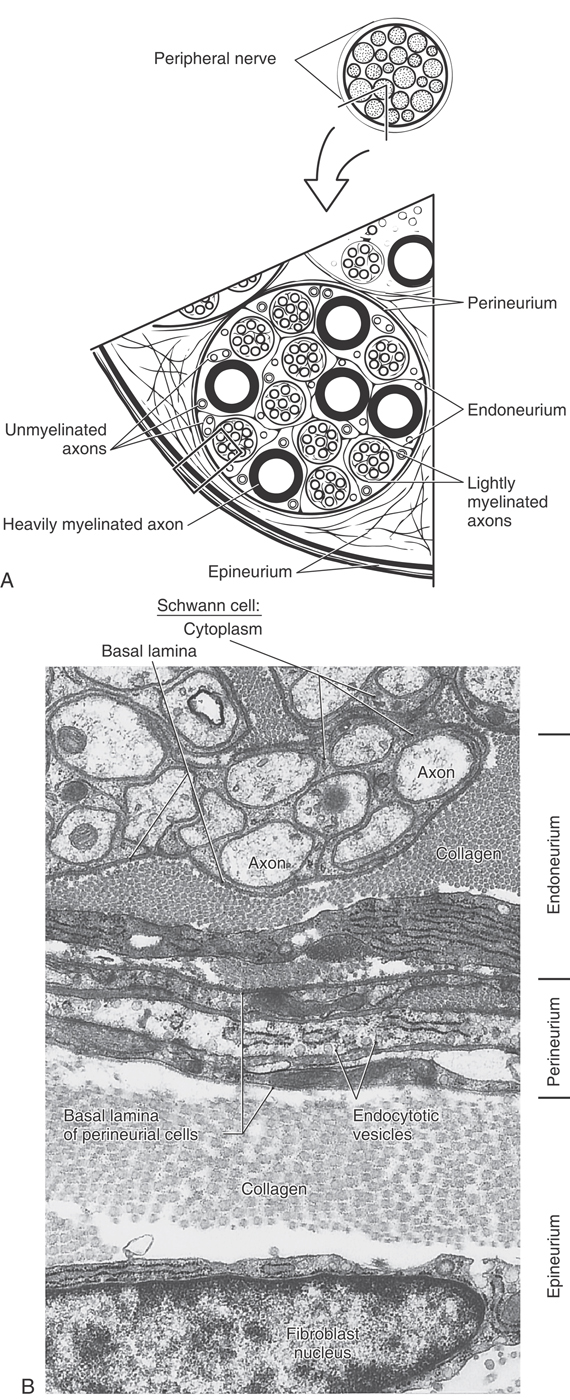
External to the Schwann cell basal lamina, peripheral nerve fibers are covered by three connective tissue sheaths (Fig. 2-15). The innermost of these, the endoneurium, consists of thin type III collagen fibrils and occasional fibroblasts between individual nerve fibers. At the second level, a distinctive sheath, the perineurium, surrounds each group (fascicle) of axons. The perineurium is composed of several concentric layers of flattened fibroblasts, which are unusual because they have a basal lamina and an abundance of pinocytotic vesicles (Fig. 2-15). Perineurial cells also are connected to each other by tight junctions. This arrangement forms a protective blood-nerve barrier against diffusion of substances into peripheral nerve fascicles. Last, the entire peripheral nerve is covered by epineurium, a dense connective tissue sheath of type I collagen and typical fibroblasts.
Tumors of peripheral nerves are usually of Schwann cell origin. The type called a schwannoma arises singly and, because it is encapsulated and does not include nerve fibers, is easily excised. The type known as a neurofibroma is usually multiple. Neurofibromas are generally difficult to remove because they are unencapsulated and infiltrate nerve bundles.
DEGENERATION AND REGENERATION
In the adult mammalian nervous system, neurons lost through disease or trauma are not replaced. Although the adult CNS retains a very small number of neural stem cells, neuronal proliferation is an almost immeasurably rare event outside the olfactory epithelium and hippocampus. In degenerative diseases, such as Parkinson or Alzheimer disease, death of neurons leads to eventual depopulation of the specific groups of neurons affected. However, if axons are damaged but the cell bodies remain intact, regeneration and return of function can occur in some circumstances.
The chance of axonal regeneration is best when a peripheral nerve is compressed or crushed but not severed. In milder lesions in which focal demyelination occurs without axonal degeneration (neurapraxia), there is loss of conduction in the nerve, but recovery is expected. When compression or crushing kills the axons distal to the site of injury, the neuronal cell bodies, which are in the spinal cord or in sensory or autonomic ganglia, usually survive. These cell bodies may undergo chromatolysis in response to the trauma. Days to weeks later, axonal sprouting starts at the point of injury, and the axons grow distally. Meanwhile, in the distal part of the nerve, axons die and are removed by macrophages, but the Schwann cells remain. They lose their myelin but keep their basal lamina. Within these tubes of basal lamina, Schwann cells proliferate, forming cordons called bands of Büngner. These Schwann cells and basal lamina tubes guide the distally growing axonal sprouts. Macrophages, which have been activated by phagocytosis of myelin debris, signal Schwann cells to secrete nerve growth factor, a neurotrophin that promotes axon growth. Regeneration depends on a variety of influences, including neurotrophins and the basal lamina. The growth rate of sprouting axons is about 1 mm/day.
In a compression injury (axonotmesis), the proximal axon sprouts and distal bands of Schwann cells remain in their original orientation, so nerve fibers are lined up just as they were before the injury. Therefore, when the axons regenerate, they will find their original positions within the nerve and are more likely to accurately reconnect with their proper targets.
When a peripheral nerve is severed (neurotmesis) rather than crushed, regeneration is less likely to occur. Sprouting occurs at the proximal end of the axon, and the axon grows, but it may not reach its distal target. As axons grow from the proximal stump toward the distal stump, some may enter appropriate bands of Büngner and may be directed to their correct peripheral targets. These nerve fibers will become functional. Some axons may enter bands of Büngner that lead them to incorrect targets, so normal function does not return. Other axons may fail to enter the Schwann cell tubes, instead ending blindly in connective tissue to form a neuroma. Mechanical or chemical stimulation of these blindly ending sensory axons may be the cause of “phantom pain” in persons with amputated limbs.
In axon tracts of the CNS, little or no regeneration can be expected, and in humans there is no regeneration to a functional state. Basal lamina guides, such as those found in the PNS, are not available. When a CNS axon is severed, the neuron mounts a sprouting response. Astrocytes hypertrophy and proliferate at the site of injury and fill any space left by the injury or by degeneration of the damaged nerve tissue. The responding astrocytes grow in a random orientation and form a scar rather than a pathway. Furthermore, astrocytes may not secrete adequate growth factors to sustain regrowing axons. The astrocytic scar appears to be a barrier rather than a guidance mechanism for axonal sprouts. Moreover, specific molecules present in oligodendrocyte myelin may also inhibit axonal regrowth. The axonal sprouts are eventually retracted, and the loss of function associated with the severed pathway is permanent.
Sources and Additional Reading
Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813.
Kettenmann H, Ransom BR, eds. Neuroglia. New York: Oxford University Press; 1995.
Stevens CF. The neuron. Sci Am. 1979;241:55–65.
Unwin N. Neurotransmitter action: Opening of ligand-gated ion channels. Cell. 1993;72:31–42.