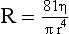Chapter 3
 The Carotid and Vertebral Arteries; Transcranial Colour Doppler
The Carotid and Vertebral Arteries; Transcranial Colour Doppler
The Carotid and Vertebral Arteries
CAROTID DISEASE AND STROKE
Stroke is a major cause of morbidity and mortality. In the United Kingdom, there are approximately 150 000 first strokes each year and some 53 000 deaths a year as a result of stroke; in the United States the equivalent figures are 795 000 strokes and 137 000 deaths each year. There are significant direct and indirect costs for society, in addition to the impact on the individuals and their families.1
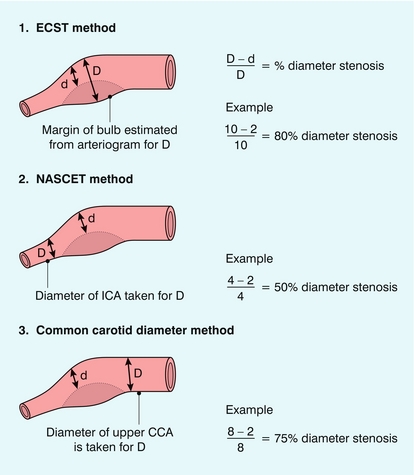
ECST & NASCET
Two major trials have shown that endarterectomy for symptomatic patients with significant stenoses confers a significant advantage over medical management in terms of reducing morbidity and mortality.1,2 The European Carotid Surgery Trial (ECST)2 data showed that, during the follow-up period, there was an 18.6% reduction in the risk of ipsilateral major stroke for the surgical patients with stenoses > 80% diameter reduction. The equivalent figure for the North American Symptomatic Carotid Endarterectomy Trial (NASCET)3 study was a 17% reduction in the risk of ipsilateral stroke if surgery was undertaken in patients with > 70% diameter reduction. The risk of surgically related death or stroke was 7% in ECST and 5.4% in NASCET and because of this small but real risk, endarterectomy for lesser degrees of stenosis, or in asymptomatic patients with stenosis, needs to be considered more carefully.
The two major trials used different methods for assessing the degree of carotid stenosis on arteriograms (Fig. 3-1), which resulted in a stenosis measured at 80% diameter reduction in ECST corresponding to a 50% diameter reduction by NASCET measurements. The ECST data were therefore reviewed using the NASCET criteria and this showed a similar 18.7% risk reduction in patients with a stenosis of 70–99% diameter reduction using the NASCET criteria.4
For symptomatic patients with less severe degrees of stenosis, endarterectomy is of marginal benefit, conferring an absolute risk reduction of 4.6% for patients with a 50–70% stenosis (NASCET) but no benefit for lesser degrees of stenosis.5,6 For patients with asymptomatic stenoses > 60% (NASCET), the absolute risk reduction from endarterectomy was only 3% over 3 years.7
The American Academy of Neurology reviewed the evidence from the various trials and has provided recommendations for the management of patients with carotid stenosis8 (Box 3-1).
INDICATIONS FOR CAROTID ULTRASOUND
Ultrasound of the extracranial cerebral circulation is used predominantly in the assessment of patients with symptoms which might arise from disease in the carotid arteries, such as amaurosis fugax and transient ischaemic attacks (TIA), in order to identify those patients with significant carotid stenosis who will benefit from surgery. There are also other indications for which ultrasound of the neck vessels is of value and the main indications for ultrasound of the carotids are shown in Box 3-2.
Cerebral Ischaemic Symptoms
1. Those without significant disease.
2. Those with mild disease (< 50% diameter reduction), who will benefit from medical therapy if they are symptomatic.
3. Those with more severe disease (50–70% diameter stenosis), who will be treated medically and may be followed to assess progression of disease, particularly if they are symptomatic.
4. Those patients with severe disease (> 70% diameter reduction) who will benefit from surgery if they are symptomatic.
5. Those patients with a complete occlusion, who are therefore not candidates for surgery.
The relationship between the presence of carotid artery disease and the development of cerebral ischaemic symptoms is not straightforward and detailed discussion of this subject is beyond the scope of this book. However, patients who have suffered from temporary ischaemic symptoms, such as TIA, reversible ischaemic neurological deficits, or amaurosis fugax, are significantly more likely to suffer a stroke than asymptomatic subjects: 36% of patients who have a TIA will have an infarct within 5 years of the TIA, compared with an annual stroke rate of 1% for asymptomatic, elderly individuals.9 Therefore it is reasonable to investigate patients with reversible ischaemic cerebral symptoms in order to identify those with a 70% or greater stenosis who will benefit from endarterectomy. Those with lesser degrees of stenosis can be treated medically and followed up; those who progress to more than 70% diameter stenosis can then be considered for surgery.
The situation regarding the examination of patients with asymptomatic carotid bruits is also complex. The authors, along with many people, would wish to know the status of their arteries if they were found to have an asymptomatic carotid bruit. However, a Cochrane Review of surgery for asymptomatic stenosis7 concluded that ‘for most people with a narrowing of the carotid artery which is not causing any symptoms a surgical operation carries a risk and has little benefit’. The review found that the absolute risk reduction from surgery on patients with > 60% stenosis (NASCET) was only 1% a year, for centres with a surgical complication rate of < 3%. More recent studies have concluded that as ‘best medical therapy’ continues to improve, surgery for asymptomatic carotid stenosis is no longer indicated.10,11 Ultrasound will therefore have a role in the identification of those patients who might be considered for endarterectomy, in those centres which offer surgery. However, if there is a policy not to offer surgery to asymptomatic patients, then it might be argued that an ultrasound examination is unnecessary.
Patients at Risk of Perioperative Stroke
Arterial disease is usually a generalised process, although it affects different arterial territories to varying degrees. Therefore, patients undergoing surgery for conditions such as coronary artery disease, peripheral arterial disease and aortic aneurysms may also have significant carotid disease; there is concern that perioperative morbidity from strokes can be increased in these patients as a result of emboli or inadequate perfusion. Diabetics can also have severe arterial disease and are at risk from perioperative strokes when undergoing major surgery. A review of carotid artery disease and stroke during coronary artery bypass surgery12 showed that patients without carotid disease had a < 2% stroke rate and this only rose to 3% in patients with an asymptomatic stenosis > 50%; it also drew attention to the role of aortic arch disease in the aetiology of strokes in coronary artery bypass graft patients. For patients with symptoms, ultrasound assessment of the carotids is valuable to allow decisions on modification to surgical bypass technique, or whether carotid endarterectomy should also be considered in addition to the surgery for the primary condition. However, this decision would depend on the relative urgency of the primary condition and many centres, whilst taking note of the carotid disease, will proceed with the main operation and consider subsequent endarterectomy in symptomatic patients.
Postendarterectomy Patients
1. Early occlusion due to thrombosis, occurring within the first 24–48 hours after the operation.
2. Stenosis developing over 12–18 months due to neointimal hyperplasia.
3. Recurrence of atheroma over a period of several years, resulting in restenosis.
Colour Doppler ultrasound provides a rapid and straightforward method for diagnosis of these complications.
Routine follow-up of asymptomatic patients is not justified by the pick-up rate for developing significant recurrent stenoses,13 but any patient suffering symptoms related to the operated side should be examined by colour Doppler in the first instance.
Pulsatile Masses
Colour Doppler ultrasound provides a rapid technique for the assessment of pulsatile neck lumps. There is a variety of causes for these; the main ones are listed in Box 3-3.
Carotid Dissection
Dissection of the carotid artery may develop from a variety of causes.14
1. It may occur spontaneously, usually consequent upon atheromatous change.
2. It may result from the extension of an aortic arch dissection.
3. It may develop following trauma to the neck, such as occurs in whiplash injuries.
4. As a result of iatrogenic causes, such as carotid catheterisation.
Colour Doppler can be used to identify different flow patterns on either side of the flap, or the presence of a thrombosed channel, and monitor subsequent progress.
Epidemiological Studies and Monitoring of Therapy
The carotids provide a convenient window for the assessment of the whole arterial system. Patterns of development of atheroma vary in different arterial circulations. It was initially thought that changes in the carotids might allow some prediction of severity of disease in other vessels, such as the coronary arteries; in addition, their examination could also provide a method for assessing the rate of progression, or regression of disease, if treatment regimens were being investigated, or epidemiological studies were being performed. However, more recent consideration suggests that the value of using carotid disease as a marker of atherosclerosis lies more in refining the subject’s classification based on other risk factors.15,16
ANATOMY AND SCANNING TECHNIQUE
The main steps in the examination are given in Box 3-4. The patient lies supine, with their neck a little extended by placing a pillow under their shoulders. The patient should be comfortable and excessive extension of the neck should be avoided. In addition, some patients with carotid or veretebral disease may find that neck extension compromises the flow of blood to the cerebral circulation, so if the patient appears to be asleep it is worth checking that they have not lost consciousness. Some patients may not be able to lie supine; if this is the case they can usually be examined adequately in a sitting position.
A high-frequency transducer (7–14 MHz) is used17 and the examination starts with a transverse scan of the carotid artery from as low in the neck as possible, to as high in the neck as possible behind the angle of the mandible. This approach will allow the depth and course of the vessels to be ascertained, together with the level of the bifurcation and the orientation of its branches (Fig. 3-2). In addition, areas of major disease will be identified and can be noted for further assessment.
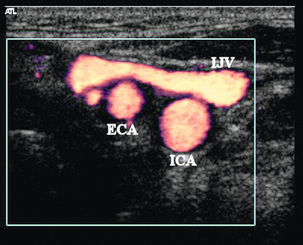
FIGURE 3-2 The left carotid bifurcation on transverse scanning from a lateral approach using power Doppler. The external carotid artery (ECA) and a small branch vessel lie anteriorly with the internal jugular vein (IJV) lying laterally, the internal carotid artery (ICA) lies behind the ECA.
Identification of the Internal and External Carotid Arteries
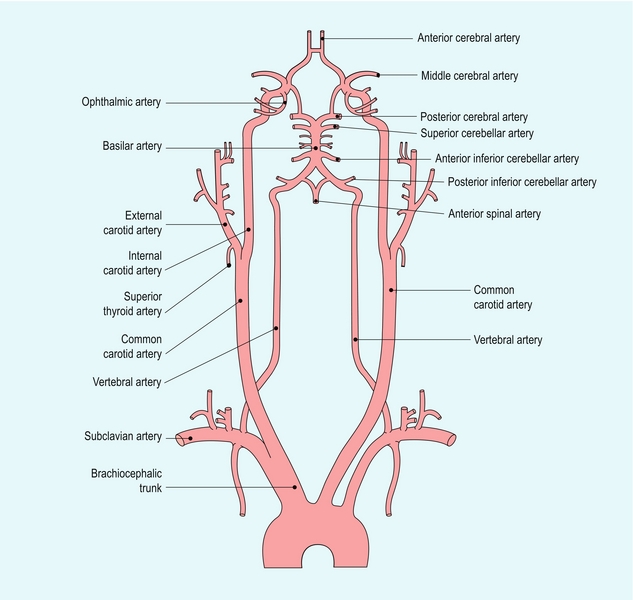
The common carotid artery on the right arises from the brachiocephalic artery behind the right sternoclavicular joint (Fig. 3-3), where the origin can usually be seen on ultrasound. On the left it usually arises directly from the aorta, so that its origin on the left cannot be seen on scanning from the neck. The level of the carotid bifurcation is usually at about the level of the upper border of the laryngeal cartilage but it may vary considerably. The two branches of the common carotid artery are the internal carotid artery and the external carotid artery. It is essential that they are identified positively, otherwise there is the possibility that disease in one vessel will be mistakenly attributed to the other, which may lead to further inappropriate investigations. The external carotid artery is usually the easier of the two branches at the bifurcation to recognise positively and the criteria to look for are listed in Box 3-5.
The external carotid artery has branches just above the bifurcation (Fig. 3-4); the superior thyroid, ascending pharyngeal and lingual arteries may all arise from the external carotid artery below, or around, the level of the angle of the mandible.
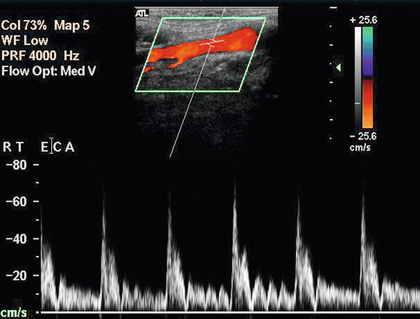
FIGURE 3-4 The external carotid artery showing a branch arising just above the bifurcation and fluctuations induced in the spectrum by tapping the superficial temporal artery at the level of the zygoma.
The external carotid artery is nearly always the more anterior of the two branches. In one study it lay anteromedial to the internal carotid artery in 48.5% of bifurcations studied, anterior in 34.5% and anterolateral in 13%; other positions accounted for only 4% of vessels.18
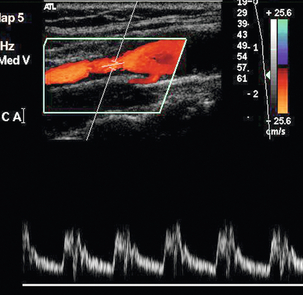
The external carotid artery supplies the relatively high-resistance vascular bed of the facial muscles, pharynx, tongue and scalp. Therefore the external carotid artery has relatively less diastolic flow, which makes it appear more pulsatile on colour Doppler and to have a characteristic waveform on spectral Doppler with relatively low diastolic flow (Fig. 3-5A). In addition the dichrotic notch of the pulse wave is usually more prominent in the external carotid artery spectrum than in the internal carotid artery spectrum.
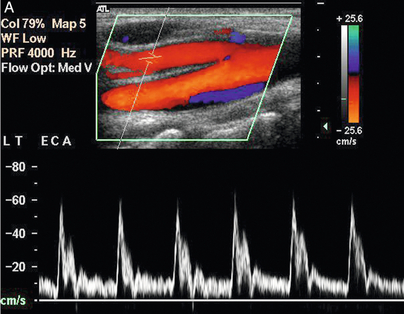
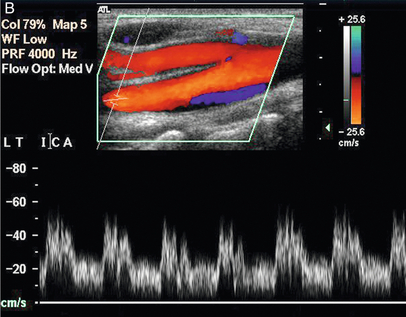
FIGURE 3-5 (A) The external carotid artery and the characteristic spectrum seen in normal vessels. There is relatively low diastolic flow and a prominent dichrotic notch compared with the spectrum from the internal carotid artery. (B) The internal carotid artery and its characteristic spectrum with more flow throughout diastole. The normal area of reversed flow in the carotid bulb is clearly visible.
The superficial temporal artery is one of the terminal branches of the external carotid artery, and if this is tapped by a finger as it passes over the zygoma it will produce rapid, clear fluctuations in the waveform in the external carotid artery, whereas there is generally little or no effect in the ipsilateral common carotid artery or internal carotid artery (Fig. 3-4).
Once the external carotid artery has been identified positively then it can be assumed that the other large vessel arising from the carotid bifurcation is the internal carotid artery. This vessel is nearly always the more posterior of the two branches and tends to run deeply and more posteriorly. It does not have visible branches at this level but the bulge of the carotid bulb is usually apparent in subjects without severe disease. Colour Doppler will show the normal area of reversed flow in the carotid bulb, sometimes referred to as the boundary layer separation zone. The spectrum from the internal carotid artery is less pulsatile and more sustained than that of the external carotid artery, with relatively high diastolic flow (Fig. 3-5B).
Diseased vessels may be more difficult to distinguish as plaques can obscure visual details; local and remote disease can lead to alterations in the normal patterns of flow, so that distinction on the basis of the appearance of the waveform may be impossible; ‘internalisation’ of external carotid artery flow may be seen in patients with a severe stenosis or occlusion affecting the internal carotid artery and in whom external–internal collaterals around the meninges and cerebral circulation result in increased diastolic flow in the external carotid artery (Fig. 3-6). In addition some high bifurcations may be very difficult to see well enough to allow reliable assessment; in this situation scanning transversely with colour Doppler switched on may allow localisation of the internal and external carotid arteries, so that some spectral information can be acquired.
Standard Velocity Measurements
Once the bifurcation and its branches have been identified and assuming that no areas of significant disease are present, it is good practice to take peak systolic velocity measurements from the common carotid artery, the internal carotid artery and the external carotid artery in order to have a record of the examination. These are obtained using spectral Doppler from the upper common carotid artery 2–3 cm below the bifurcation; the internal carotid artery from 1 to 2 cm above the bulb, or as high as possible, in order to allow the normal bulbar turbulence to settle; and from the lower external carotid artery. For routine measurements the sample volume is set at about one-third of the total diameter and placed in the centre of the vessel in order to avoid the natural turbulence at the edge of the lumen and ‘wall thump’ from inclusion of the vessel wall in the sample volume. The Doppler angle is kept as low as possible, ideally in the range 45–60°; it is good practice to try to keep to a specific angle, such as 55° or 60°, in order to improve the reproducibility of results between examinations. For tight stenoses it is better to reduce the size of the sample volume, as this allows the area of the peak systolic flow signal to be better defined. Colour Doppler allows more accurate assessment of the direction of flow in a stenosis as this may not always be parallel to the walls of the vessel (Fig. 3-7). The precise final location of the sample volume is chosen using a combination of the audible Doppler signal and the spectrum so that the clearest, highest-frequency audible signal and the best spectral trace are obtained; in stenoses, the sample volume should be moved through the length of the stenotic segment in order to locate the peak signal. The gain for the spectral display is set to use the full grey scale without over- or under-saturation.
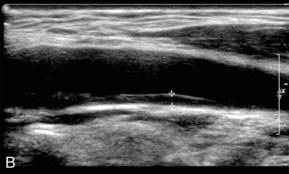
Measuring the Intima-Medial Thickness
This is not always required but should it need to be measured, for instance as part of a population survey, then it can be measured on an image of the distal common carotid wall where the echoes from the intima-media complex are most easily distinguished. The machine settings should be set to give a clear, uncluttered image of the vessel wall and the position of the transducer adjusted to show the characteristic double-line appearance of the vessel wall (Fig. 3-8). The image should be magnified as much as possible to make the measurement easier to perform. The intima-medial thickness (IMT) is best demonstrated in the upper common carotid artery on the posterior wall 1–2 cm below the bifurcation where the vessel is usually at right angles to the ultrasound beam. The internal carotid artery is more difficult to assess as the vessel slopes obliquely away from the transducer face in many cases. A minimum of three measurements over a 1 cm segment of the upper CCA are taken. Automated edge detection systems are available on some ultrasound systems but with careful attention to detail it is possible to measure the IMT manually with satisfactory, reproducible accuracy. The precise upper limit of the normal range is a matter of some discussion. IMT is known to vary with age, gender and race16 but values of less than 0.8 mm correlate well with lack of coronary artery disease, whereas an increasing thickness above this level is associated with increasingly severe coronary artery disease, an increased risk of myocardial infarction and also stroke.19
The Vertebral Arteries
Once both carotids have been examined, the vertebral arteries are assessed. The vertebral artery on each side is the first branch of the subclavian artery (Fig. 3-3). It passes posteriorly and upwards to the vertebral foramen in the transverse process of the sixth cervical vertebra (V1 segment) (Fig. 3-9), and from there it passes upwards in the vertebral canal to the level of the axis (C2) (V2 segment). It emerges from the vertebral canal at C2, passing behind the lateral mass of the atlas (C1) to enter the skull through the foramen magnum (V3 segment) and runs anterior to the brain stem (V4 segment) to join with the vessel from the other side in front of the brain stem to form the basilar artery.20
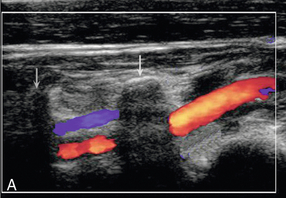
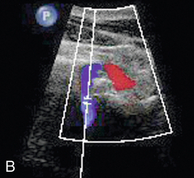
FIGURE 3-9 (A) The vertebral artery passing from its origin (V1 segment) to enter the vertebral canal and then running cranially (V2 segment) between the vertebral transverse processes (arrows). (B) The vertebral artery looping round the atlas (V3 segment).
There may be marked variation in the size of the vertebral arteries and their relative contribution to basilar artery flow; when there is disparity of size, the left artery is usually the larger of the two and in 7–10% of individuals there are significant segments of hypoplasia, which result in the artery not being visible.21 Clear visualisation of the vein but not the artery suggests that the artery may be either thrombosed or congenitally absent.
ASSESSMENT OF DISEASE
Measurement of the Degree of Stenosis
Direct Visualisation and Measurement
If the stenosis and plaque can be seen clearly then it is possible to measure the calibre of the residual lumen and the original calibre of the vessel. Diameter reduction ratios, or area reduction ratios, are the usual methods for describing the reduction in vessel calibre; percentage residual lumen can also be used. Diameter measurements are generally a little quicker to perform but are slightly less representative of the stenosis, as they do not take account of variations in plaque thickness around the circumference of the vessel and there is the potential to underestimate, or overestimate, the degree of stenosis (Fig. 3-10). Care must be taken to examine a diseased segment of vessel in both transverse and longitudinal views so that the distribution of plaque can be clearly assessed and the most appropriate diameter measurement can be made; this is usually the shortest diameter. Measuring stenoses by area reduction, although more time consuming, overcomes this problem with the eccentricity of the plaque being taken into account as the luminal and vessel areas are measured.
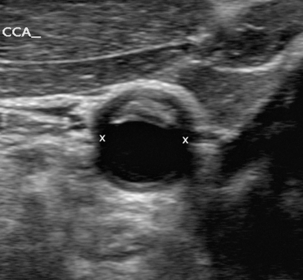
FIGURE 3-10 Transverse view of a plaque in the common carotid artery with asymmetrically located plaque. Note that an inappropriate longitudinal scan plane (x–x) could result in a significant underestimation of the degree of stenosis.
It is important that the type of measurement used is clearly defined as either a diameter reduction or an area reduction, because significant misunderstandings may occur in the interpretation of the results. For a given stenosis, a 50% diameter reduction corresponds to a 70% area reduction, so that misinterpretation of a 70% area stenosis as a diameter reduction will result in a significant overestimation in the assessment of calibre reduction, possibly leading to unwarranted surgery (Fig. 3-11). It is good practice always to define the value of a stenosis as either an area or a diameter reduction and this is essential if the measurement is in a different form from that normally used.
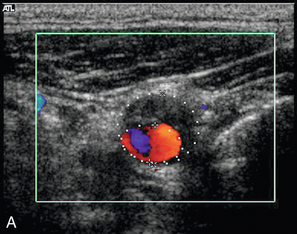
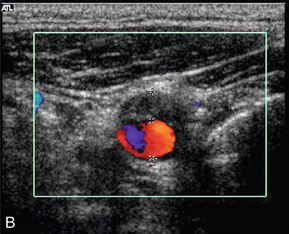
FIGURE 3-11 A stenosis measured with (A) an area-reduction calculation (measured as 62%) and (B) a diameter-reduction calculation (measured as 40%).
If the patient is being considered for endarterectomy, in addition to measuring the degree of stenosis, it is important to assess the level of the bifurcation, the length of the stenosed segment and the diameter of the internal carotid artery above the stenosis. The reason for this is that high bifurcations (< 1.5 cm from the angle of the mandible), high distal extent of the stenotic segment (> 2 cm above the bifurcation) and a small internal carotid artery (< 0.5 cm), or a kinked internal carotid artery, can complicate surgery and prior warning will allow the appropriate surgical technique to be used. Ultrasound can predict these features satisfactorily and supplemental arteriography is not usually necessary.22
Doppler Criteria
In many cases the region of the stenosis is not seen clearly due to complex plaque structure and calcification. In these cases direct measurement cannot be used to quantify the degree of stenosis and Doppler criteria must be used. Over the years, much work has been done correlating Doppler findings with degrees of stenosis found on arteriography, or at surgery. It has been shown that carefully obtained Doppler criteria correspond well with the degree of stenosis, and values which allow the severity of internal carotid artery stenoses to be predicted have been developed. However, the literature can be confusing, with apparently widely varying velocities being quoted for specific levels of stenosis in the early days of duplex ultrasound: one study suggesting that a peak systolic velocity of > 1.3 m/s was appropriate for diagnosis of a diameter stenosis of 60% or greater;23 whilst another study proposed a velocity of 2.25 m/s for a 70% stenosis.24 This apparent lack of consensus emphasises the fact that there was variation from one department to another depending on the equipment and technique used. Each department must therefore develop and audit criteria which they find work in their environment and complement the clinical criteria and practices used in their institution.
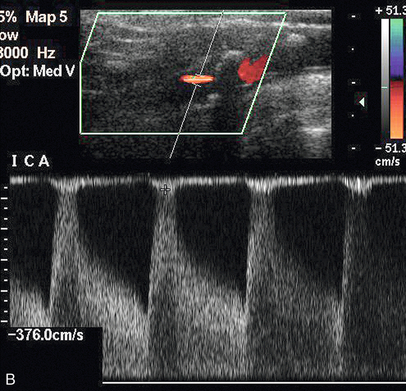
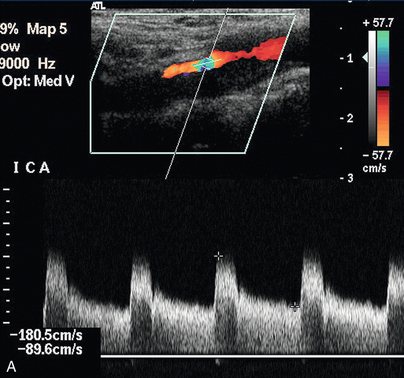
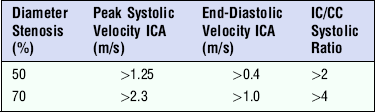
The peak systolic velocity, end-diastolic velocity and the ratio of peak systolic velocities in the internal and common carotid arteries (IC/CC systolic ratio) are the most useful measurements in general practice25,26 (Fig. 3-12). Spectral broadening and filling in of the window under the spectrum are subjective, difficult to quantify and can be affected significantly by gain control settings; however, they do indicate abnormal flow if they are present. The IC/CC diastolic ratio can also be measured but this does not usually add to the information obtained from the three main criteria. The main levels which need to be distinguished are 50% diameter reduction, where blood flow starts to decline, and 70% diameter reduction, which is the level strongly associated with clinical symptoms and for which surgery will be considered. The values for the criteria which the authors have found to be useful in their practice to predict these levels of stenosis in the internal carotid artery are shown in Table 3-1 and are based on those reported by Grant et al.25 and Oates et al.26 It is important to remember that the peak systolic and diastolic values refer only to the internal carotid artery, not to the common carotid artery or external carotid artery. Furthermore, it should be borne in mind that physiological variations due to heart rate, cardiac output and contralateral occlusion may affect the velocities in a vessel, potentially leading to a false diagnosis of a pathologically high velocity, although these cases should be clarified by the use of the velocity ratios. It should also be remembered that peak velocities decline with very high degrees of stenosis (> 90% diameter stenosis)27 as discussed in Chapter 2.
TABLE 3-1
Diagnostic Criteria for Doppler Diagnosis of Stenoses of 50% and 70%
ICA, internal carotid artery; IC/CC, internal carotid/common carotid.
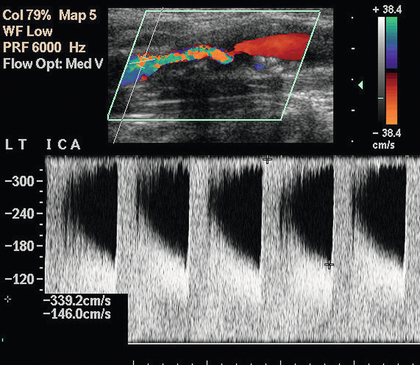
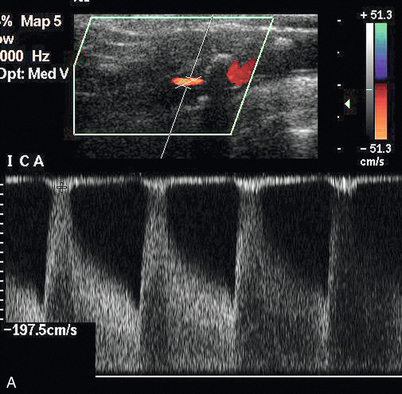
FIGURE 3-12 A stenosis of the internal carotid artery showing a peak systolic velocity in excess of 3.4 m/s and an end-diastolic velocity of 1.5 m/s. There is aliasing of the colour Doppler display.
Attempts have been made to use colour or power Doppler criteria to assess the severity of stenoses.28,29 Direct measurement of the residual lumen based on power or colour Doppler can be made but it is essential that care is taken with the gain settings to ensure that there is not any over- or underestimation of the residual lumen; this type of direct measurement will always be less accurate than measurements made on a good B-mode image but, for more severe degrees of stenosis (> 50% diameter reduction), they can provide additional confirmatory information. For cases where a diagnosis must be made between ‘subtotal’, or ‘near’ occlusion and complete occlusion, then careful setting up of the colour and power Doppler modes is essential for reliable distinction (see below).
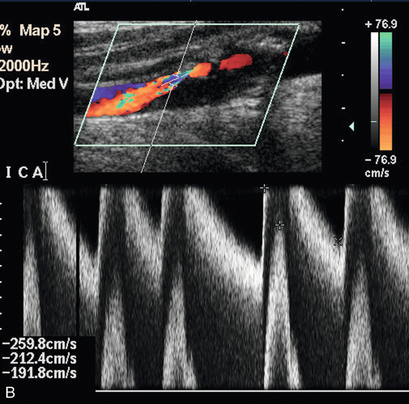
In addition to the direct measurement of the residual lumen demonstrated by the colour map, a cursor with angle correction can be placed over the colour map and used to provide an estimate of the mean velocity in the underlying pixel. This allows the mean velocities in a stenotic segment to be estimated. However, these correlate less well with the degree of stenosis than peak systolic or diastolic velocities. Although experienced operators can often get a good idea of the severity of a stenosis from the overall appearances and the colour map changes, it is always better to use the colour map to identify areas of abnormal flow and use this to position the sample volume for spectral Doppler analysis as it may sometimes be difficult to grade stenoses using colour findings alone (Fig. 3-13).
Effects of Disease Elsewhere
The velocities and flow characteristics seen in any given section of a vessel depend not only on local conditions but also on conditions elsewhere along the vessel, in other vessels connected to that vascular territory (Fig. 3-14A & B) and to other factors, such as heart rate, cardiac output and blood pressure (Box 3-6). The best example of this is vertebral or subclavian steal, where a proximal occlusion of the subclavian artery results in reversed flow in the ipsilateral vertebral artery.
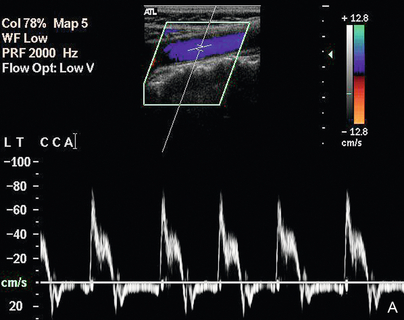
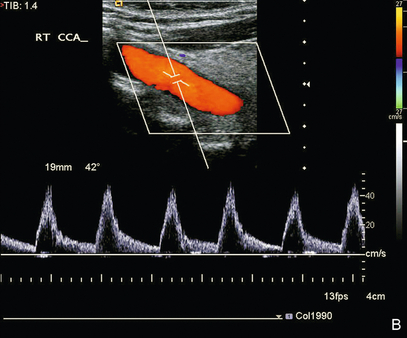
FIGURE 3-14 Common carotid waveforms (A) in a patient with aortic valve incompetence showing reversed diastolic flow on both colour and spectral Doppler; (B) in a patient with aortic valve stenosis showing delayed systolic acceleration.
In patients with bilateral severe carotid stenoses, when one side is stented or operated upon, the improvement in flow up the operated vessel will reduce flow up the contralateral carotid and this may result in a decrease in peak velocities on the untreated side that might lead to this stenosis being down-graded in severity. In one series of patients with bilateral significant stenoses on duplex scanning,30 the stenosis on the non-operated side was reclassified as non-haemodynamically significant in 20% of cases. It is therefore necessary for these patients to be reassessed prior to any management decisions relating to the untreated artery.
Carotid Occlusion
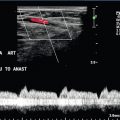
Occlusion can affect the internal carotid artery (Fig. 3-15) or common carotid artery separately, or together. Occlusion of the common carotid artery does not always result in occlusion of the internal carotid artery, as sufficient blood flow may be provided by retrograde flow down the ipsilateral external carotid artery to maintain patency of the internal carotid artery. This pattern of abnormal flow may be quite confusing if it is not recognised but it is of clinical importance, as these patients can still suffer ischaemic events in the relevant internal carotid artery territory.31 The opposite pattern of flow may be seen in a small number of patients with common carotid artery occlusion with reverse flow in the internal carotid artery on the side of the common carotid artery occlusion (a carotid steal phenomenon) and antegrade flow in the ipsilateral external carotid artery. If the lower margin of the occluded segment is above the level of the bifurcation then a characteristic pattern of forward and reverse flow (‘stump thump’) is seen in the patent residual lumen of the internal carotid artery (Fig. 3-15B).
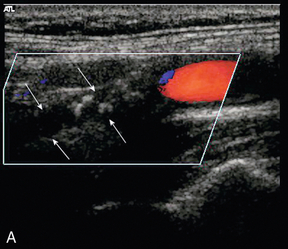
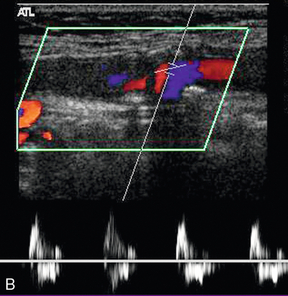
FIGURE 3-15 (A) An occluded internal carotid artery, the occluded lumen is indicated by the arrows. (B) characteristic low-velocity forward and reverse flow in the ICA just below an occlusion (‘stump thump’)
If an occlusion of the internal carotid artery is suspected it is essential that care is taken to ensure that the Doppler settings on the machine are appropriate for locating any low-velocity, small-volume flow that may be present in a narrow residual lumen in the segment under investigation. Colour and power Doppler assessments of the vessel should be carried out with the system set for maximum sensitivity in order to identify any small residual lumen (‘string sign’ or pseudo-occlusion) (Fig. 3-16) or adjacent vessels that might confuse the situation.32 Spectral Doppler should also be performed carefully at maximum sensitivity, although care must be taken to identify and exclude any waveforms arising from adjacent vessels. Conversely, colour Doppler or power Doppler may show the location of a residual channel in a vessel that was otherwise thought to be occluded; echo-enhancing agents are of value if there is any persisting uncertainty.
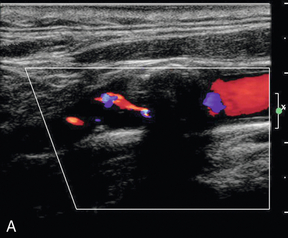
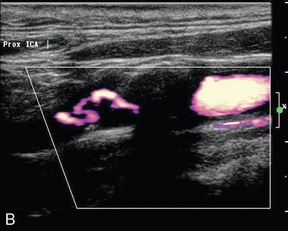
FIGURE 3-16 A thin residual channel on colour Doppler (A) and on power Doppler (B) which is more sensitive for weaker, slower signals and shows the tortuous residual lumen more clearly.
Occlusion of the internal carotid artery results in the reduction of diastolic flow in the ipsilateral common carotid artery, so that the common carotid artery waveform becomes more like that seen in the external carotid artery. Reduction of common carotid artery end-diastolic flow may therefore be an initial clue to the presence of an internal carotid artery occlusion, or a very severe stenosis.33 Care should be taken, however, as the development of collateral channels between the external carotid artery and internal carotid artery circulations in the orbit and meninges can result in ‘internalisation’ of the external carotid artery flow with relatively high diastolic flow in the external carotid artery; this results in a mistaken impression of a patent internal carotid artery, if it is not recognised. The ‘temporal tap’ manoeuvre can usually clarify the situation. Occlusion of the carotid on one side may result in increased flow through the contralateral carotid vessels, as discussed earlier.
Recanalisation of an occluded carotid artery is not as rare as might be imagined.34 One series followed eight patients with internal carotid artery occlusion with serial 6-monthly ultrasounds for a period of up to 8 years, all of the eight patients showed evidence of spontaneous recanalisation occurring between 6 and 96 months.35
Plaque Characterisation
Much effort has been expended in attempting to classify atheroma and plaques on ultrasound, particularly in the carotids, where high-resolution ultrasound gives good images of many plaques. Steffen et al.36 proposed a classification of plaque which takes account of the different types of plaque and its components (Fig. 3-17). Types 1 and 2 were predominant in symptomatic arteries, whereas types 3 and 4 were more common in asymptomatic patients; these findings have been supported by other groups.37–39 These studies suggest that the more friable, lipid-containing, soft plaques are more likely to result in plaque disruption and produce symptoms than firmer, more fibrous and coherent plaques.
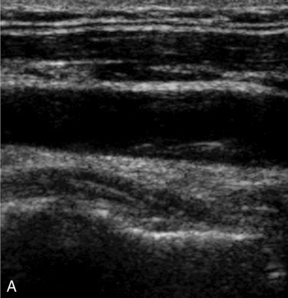
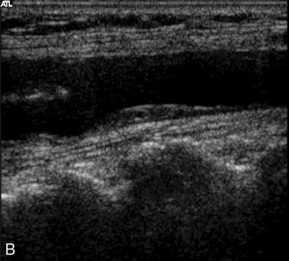
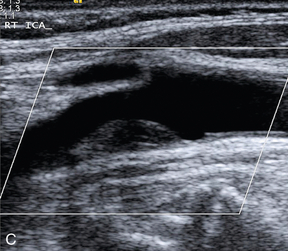
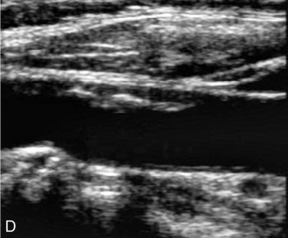
FIGURE 3-17 Different types of plaque seen on ultrasound. (A) Type 1, dominantly echolucent with a thin echogenic cap. (B) Type 2, substantially echolucent lesions with small areas of echogenicity. (C) Type 3, dominantly echogenic lesions with small areas of echolucency of < 25%. (D) Type 4, uniformly echogenic lesions.
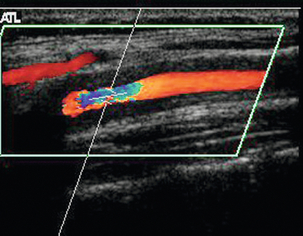
The value of ultrasound in predicting the complications associated with plaques is more difficult to define. These complications include intraplaque haemorrhage, surface ulceration and adherent thrombus. The presence of intraplaque haemorrhage has been inferred from the presence of hypoechoic areas within the plaque. However, it is also possible that many of these areas are aggregates of lipid or necrosis, rather than areas of haemorrhage. Sometimes an ulcer in the plaque can be clearly seen (Fig. 3-18), but many plaques are irregular without being ulcerated and, conversely, an ulcerated plaque may not be identified on ultrasound. Thrombus adherent to the surface is suggested by an anechoic or hypoechoic area adjacent to the plaque surface on colour or power Doppler. It is important that the system is set up appropriately, otherwise the lack of colour on the image may be due to technical factors, rather than the presence of thrombus.
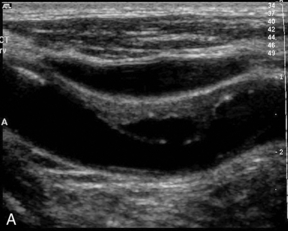
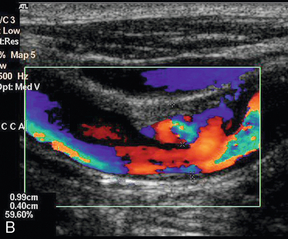
FIGURE 3-18 An ulcerated plaque. A thin rim is seen on the B-scan image (A) but colour Doppler (B) shows flow within the plaque.
Some studies have shown a good correlation between the ultrasound appearances and those found at operation,40,41 but the results from others have been less satisfactory, with poor prediction of ulceration or haemorrhage.42,43 Another study from the Seattle group showed that there was no significant difference in plaque constituents between the endarterectomy specimens removed from symptomatic and asymptomatic patients with high-grade stenoses.44 Visualisation of plaque type in high-grade (> 60% diameter reduction) stenoses is usually inadequate to identify reliably plaque characteristics and the Seattle study would suggest that the degree of stenosis is the more important factor in this group of patients. However, if there is a good view of the diseased segment, the plaque can be described in terms of its type (1–4), extent (focal, diffuse, circumferential) and any obvious associated complications (ulceration, thrombus, haemorrhage). If visualisation is moderate or poor then discretion is necessary and only those features which are clearly seen should be noted. For example, it may be difficult to distinguish between a plaque ulcer and a gap between two adjacent plaques, or to decide if a colour void associated with the plaque surface is really due to adherent thrombus or to technical factors. Ulceration should only be diagnosed if the plaque and the ulcer are clearly seen, otherwise plaques should be described as smooth or irregular. It should be remembered that many diseased segments are not clearly seen due to the presence of calcification, which makes any attempt at plaque characterisation very difficult, or impossible.
Takayasu’s arteritis is a condition of unknown aetiology which can affect the carotid arteries amongst other vessels. A cell-mediated auto-immune reaction is strongly implicated with granulomatous infiltration of the vessel wall leading to fibrosis and scarring, occasionally ectasia or aneurysm formation may be seen. The appearances on ultrasound reflect these changes with diffuse, homogeneous mural thickening (Fig. 3-19) with or without significant stenosis, dilatation or aneurysm formation.45
Pulsatile Masses
The main causes of pulsatile neck masses are given in Box 3-3. Normal but prominent carotids and ectatic carotid or subclavian arteries are easily identified using colour Doppler and do not usually require any further investigation.
Aneurysms of the carotid arteries can also be identified as they are in continuity with the artery. The majority arise following surgery but they may also occur following trauma, including whiplash neck injuries and biopsy of cervical masses. Pseudoaneurysms may also be seen. The flow in the aneurysm may be seen with colour Doppler, unless there is thrombosis of the lumen of the dilated segment. In some cases of aneurysm of the common carotid artery, it may be difficult to identify the internal carotid artery above the dilatation and care must be taken to establish whether it is patent or not; flow in the ipsilateral ophthalmic artery is not necessarily evidence of patency, as this may come from collateral filling via the circle of Willis. Aneurysms of the upper internal carotid artery may be difficult to identify with certainty, or the findings may be misinterpreted as a straightforward stenosis or dissection.46
Lymph nodes and other masses adjacent to the carotids will transmit pulsations and require distinction from intrinsic vascular lesions. This is not usually a problem, but occasionally a deposit will surround the carotid artery and, unless there is a previous history of malignancy, diagnosis can be difficult. Adherence to the carotid sheath can be assessed by gentle palpation and getting the patient to swallow so that relative movement between the mass and the carotid can be assessed.47 Colour Doppler ultrasound also has an important role in defining the solid nature of a neck lump prior to biopsy and excluding a vascular lesion, such as an aneurysm.
Carotid body tumours are rare but can be diagnosed easily with ultrasound. Characteristically there is a hypoechoic mass between the two branches at the bifurcation, spreading them apart in a ‘wine glass’ deformity (Fig. 3-20). Colour Doppler shows a highly vascular lesion and the external carotid usually shows a low-resistance pattern of flow on spectral Doppler.48
Dissection of the Carotid Arteries
The ultrasound findings in this condition can vary considerably. The vessel may be occluded completely; show a smooth tapering stenosis, with or without a recognisable haematoma/thrombosed false lumen being visible (Fig. 3-21); or a membrane with a double lumen may be seen with variable flow patterns in the two channels on either side.49,50 Recanalisation of the occluded vessel is a recognised occurrence and occurs in up to 60% of cases.51 Dissection of the vertebral arteries may also occur and is usually manifest as absent flow in the affected artery.49
Vertebral Arteries
Frequently only cursory attention is paid to the vertebral arteries, unless there are specific signs and symptoms pointing to a posterior fossa problem, when a more detailed examination of the vessels is required.20 As noted previously, there are several variations of anatomy and size of the vertebral arteries which may affect the ultrasound findings; a further issue to be considered is that disease affecting one artery may be compensated by the other side, or collaterals from the circle of Willis, so that blood flow to the posterior fossa circulation is maintained and local, unilateral vertebral artery disease is of little clinical significance. Nevertheless, some basic patterns of flow can be identified:
1. No flow detected in the region of the relevant vertebral artery means that the artery is either hypoplastic, absent, or thrombosed. Visualisation of the artery on B-mode without demonstrable flow within it on colour/power and spectral Doppler with the system set to maximum sensitivity suggests that it is thrombosed, or dissected.
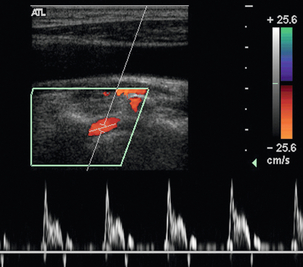
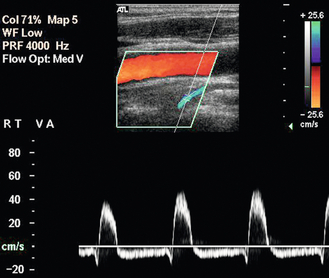
2. Demonstration of a focal area of increased velocity at the origin of the vertebral artery (Fig. 3-22A), or at some point along its course between the subclavian artery and the foramen magnum, is consistent with a focal stenosis, the significance of which will depend on the clinical situation and the state of the contralateral vertebral artery. Visualisation of the vertebral artery is normally inadequate to make a reliable direct diameter reduction measurement feasible.
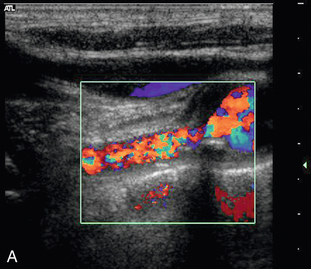
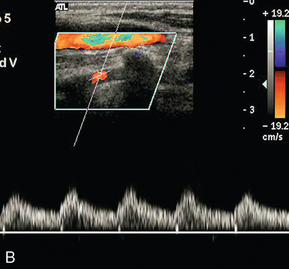
FIGURE 3-22 (A) A stenosis at the origin of the vertebral artery with marked turbulence shown on colour Doppler. (B) Spectral Doppler in the V2 segment shows a ‘tardus-parvus’ waveform with delayed systolic acceleration.
3. A ‘tardus-parvus’ waveform suggests a proximal stenosis; this should be sought by careful examination of the artery from the subclavian artery up to the foramen magnum (Fig. 3-22B).
4. The proximal vertebral artery waveform shows reduced or absent diastolic flow, implying a distal stenosis/occlusion (Fig. 3-23).
5. Reversed flow is consistent with subclavian steal syndrome. This occurs when there is an occlusion or tight stenosis of the proximal subclavian artery at its origin and blood supply to the arm is maintained by reversal of blood flow down the ipsilateral vertebral artery (Fig. 3-24).
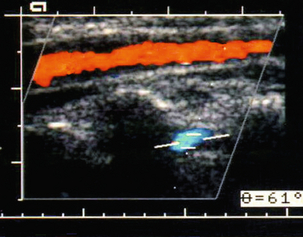
FIGURE 3-24 Reversed flow in the vertebral artery in a patient with a proximal left subclavian artery stenosis. The vertebral artery is the opposite colour (blue) from the common carotid artery.
6. A biphasic vertebral artery waveform may be seen in patients with a developing steal situation from slightly less severe subclavian stenoses (Fig. 3-25) and, in some patients, reversed flow may only occur with the arm in certain positions, or after a period of exercise; therefore scanning after a period of arm exercise, such as elbow flexions holding a book, or some other relatively heavy object, should be considered if a steal syndrome is suspected. Alternatively a pressure cuff can be inflated to occlude blood flow to the arm and then released after 2–3 min. The resulting reactive hyperaemia in the arm produces an increased demand for blood and reversal of flow in the relevant vertebral artery.
Reporting Carotid Ultrasound Examinations
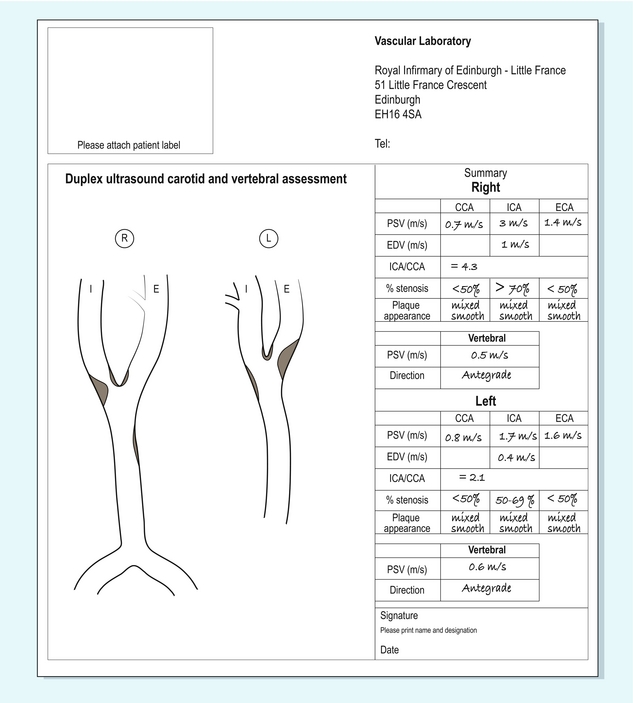
Unlike an arteriogram, or magnetic resonance angiography (MRA) examination, there are no easily interpretable images from a Doppler examination as the outcome is a mixture of image assessment, Doppler parameters and clinical judgement. It is therefore sensible to standardise the information given in the report of a Doppler examination, to ensure that all necessary data are recorded.17 The easiest way to do this is to record the data onto a standardised form, which can then be used in reaching management decisions about the patient, or comparing the Doppler examination with any associated MRA, computed tomography angiography (CTA), or arteriogram. The precise details will vary from centre to centre, depending on local preferences. The form used in our institution is shown in Figure 3-26. The main information which should be noted is given in Box 3-7.
PROBLEMS AND PITFALLS IN CAROTID ULTRASOUND
Problems and pitfalls can arise from a variety of sources. These can be divided into those resulting from poor or faulty technique and those arising from pathological or physiological causes (Box 3-8). Technical aspects of setting up the system are discussed elsewhere (see Appendix) but other aspects which can lead to problems should be considered.
Long and Eccentric Lesions
Eccentric lesions may cause problems if the exact disposition of plaque around the circumference of the vessel is not appreciated (Fig. 3-10). Care should be taken to examine areas of disease transversely, as well as longitudinally, as discussed earlier. Another problem with eccentric lesions is that the high-velocity jet may emerge from the stenosis at an unusual angle that is not parallel to the vessel wall, as many people would assume; colour Doppler is useful in identifying these oblique jets and allows for a more accurate angle correction to be achieved (Fig. 3-7).26
Tortuous Vessels
Sharp twists in the course of a vessel result in changes to the pattern of blood flow in the lumen: blood on the outer margin flows faster than blood on the inside of the bend (Fig. 2-5); in addition, turbulence is generated, even if plaques are not present. Furthermore, assessing the angle of flow for calculation of velocity can be difficult, so that accurate, representative velocities are hard to define, especially if there is associated disease. Whenever possible, flow characteristics should be assessed as far as possible from the curved segment but if a measurement has to be made in a curved segment, then the angle correction should be aligned to be parallel to the wall at that point.26
High-Grade Stenoses
In critical stenoses (> 85% diameter reduction; see Chapter 2) velocity decreases; in addition, there is only a small volume of blood flowing through the narrow residual lumen. The signal strength is therefore poor and the Doppler shift is lower than might be expected. It is important to ensure that the machine is set up appropriately to detect these weaker, lower Doppler shifts, if they are not to be missed and a false diagnosis of occlusion reached (Fig. 3-16). The low flow distal to a very severe stenosis results in relative narrowing of the internal carotid artery lumen above the stenosis and this should be borne in mind when reporting these cases prior to carotid endarterectomy. The higher sensitivity of power Doppler and the increase in signal strength obtained with echo-enhancing agents are two developments which both individually and together improve the success of ultrasound in discriminating between an occlusion and a small residual lumen.52
Lesions at the Bifurcation
These can cause problems as a significant amount of plaque may be present in the bulb but, because of the relative dilatation of the lumen which is normally present here, this does not result in any narrowing of the flow channel between the common carotid artery and the internal carotid artery. Although this will not have any effect on the overall flow in the vessel, this disease may provide a source of emboli and may not be recognised if spectral criteria alone are used to identify stenotic segments, as there will not be a significant increase in velocity.26
Disease Elsewhere in the Vascular Tree
Vascular ultrasound allows direct assessment of much of the extracranial cerebral circulation from the clavicular region into the upper cervical area and disease in these segments can be measured directly. However, changes in vessels remote from those under examination may affect the findings at any given point. As discussed previously, contralateral occlusion results in increased flow through the patent carotid with higher than normal velocities as a result of this increased flow. In patients with bilateral high-grade stenoses, an improvement in blood flow following surgery or stenting on one side will lead to a decrease in blood flow in the remaining stenosed vessel, which will reduce peak systolic velocities and may lead to a reclassification of the degree of stenosis.30 Aortic valve disease (Fig. 3-14) or a common carotid artery origin stenosis on the left will not be visualised directly on carotid examination; the presence of disease in these areas can only be inferred from damping of the waveform with, or without, turbulence. Similarly disease more distal in the carotid siphon region will not be detected by cervical carotid examination.
CAROTID STENTS
Insertion of stents into stenotic segments of the common and internal carotid arteries is becoming increasingly recognised as an alternative to endarterectomy for treatment of significant extracranial carotid disease. Insertion of a stent affects the biomechanical properties of the arterial wall resulting primarily in increased stiffness; this means that velocity measurements from a non-stenotic stented segment will be higher than those from an equivalent unstented artery. Lal et al.53 have proposed that a normal velocity through a stented segment can be up to 1.5 m/s; a 50% in stent restenosis is indicated by velocities ≥ 2.2 m/s; and an 80% in stent restenosis by velocities ≥ 3.4 m/s. In patients with bilateral severe stenoses, stenting or operating on one side should result in a change in blood flow in the non-operated artery so, as discussed previously, this means that a careful review of the current situation should be made before any conclusions are drawn or decisions made in relation to the other side.30
ACCURACY IN RELATION TO OTHER TECHNIQUES
Arteriography
Many studies have shown that spectral Doppler and colour Doppler have satisfactory accuracy in the diagnosis and assessment of disease when compared with arteriography. Some care must be taken when considering studies comparing the two techniques, as different types of angiography are used as the gold standard: direct carotid injection, arch injection, digital subtraction arteriography (DSA) and venous DSA have all been used. Different methods of measuring the degree of stenosis are also used (Fig. 3-1).54 These will result in different estimations of the degree of stenosis for a bifurcation lesion depending on whether the diameter of the residual lumen is compared with the diameter of the common carotid artery, the diameter of the internal carotid artery, the estimated diameter at the bulb as calculated from calcification in the wall, or from the alignment of the vessels. There is also a degree of variation between different observers in the estimation of stenosis on arteriography, which may be up to 20%.55 In addition, angiography has some intrinsic procedural risks arising from catheterisation and exposure to contrast and radiation. The risk of minor stroke from carotid arteriography has been reported to range from 1.3 to 4.5% and in the ACAS the combined neurological morbidity/mortality from arteriography in patients with haemodynamically significant stenoses was 1.2%.56 A review of techniques used in the investigation of stroke patients reported a mortality of 1% and a disabling stroke rate of 4% from intra-arterial angiography.57 In many centres, arteriography is now reserved for further assessment of complex cases, rather than as a primary diagnostic tool.
The overall performance of Doppler ultrasound compared with arteriography is good. Cardullo et al.58 reviewed 16 spectral Doppler studies with 2146 Doppler–arteriogram comparisons; non-colour duplex Doppler had an overall sensitivity of 96%, specificity of 86%, positive predictive value of 89%, negative predictive value of 94% and accuracy of 91% for the diagnosis of a diameter stenosis greater than 50%. Subsequently, further studies have confirmed the value of colour Doppler with similar or better levels of accuracy and also its value in improving diagnostic confidence, clarifying difficult situations and reducing examination times. In particular the difficult distinction between a critical stenosis and a complete occlusion can be achieved in nearly all cases with the use of colour Doppler.59
Magnetic Resonance Angiography and Computed Tomography Angiography
The two main MRA techniques are ‘time of flight’ (TOF) and contrast-enhanced MRA (CEMRA): they both ‘image’ the blood and infer the wall characteristics from the shape of the blood flowing in the vessel, whereas colour Doppler provides information on the wall of the vessel and any plaque, as well as the flowing blood. CEMRA is more recently developed and appears to have advantages in terms of sensitivity and specificity when compared with other ‘non-invasive’ techniques for carotid assessment (Table 3-2).57 MRA is still limited to some extent by signal voids at relatively high velocities and artefacts can occur with patient movements such as swallowing. Earlier problems from signals arising in plaque haemorrhage and lipid deposits are less intrusive with modern equipment, sequences and processing algorithms. Another disadvantage of CTA and MRA, apart from exposure of the patient to contrast agents and radiation (CTA), is the significant amount of postprocessing and image manipulation required for an accurate assessment of the examination to be achieved. Although general data processing capacity and speeds have increased with modern computers, there is still a need to review several MR sequences, or undertake further workstation review of CTA and MRA exams in order to get a clear picture of the vessels, which add to the complexity of the examination and the time taken to reach a diagnosis.
TABLE 3-2
Sensitivities and Specificities of Non-Invasive Imaging Tests for Different Degrees of Stenosis
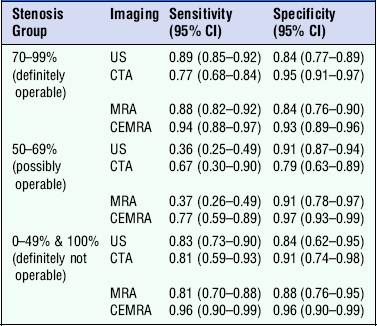
From Wardlaw et al.57
Several authors have suggested that Doppler is satisfactory for initial screening of patients for carotid disease and for identification of patients requiring surgery in most cases but that CTA or MRA are useful additional tests which can be used instead of angiography if the ultrasound is inadequate or indeterminate, or if clinical uncertainty persists.60–63
Compared with arteriography, CTA and MRA, Doppler ultrasound is relatively cheap, rapid, non-invasive and accurate for the diagnosis of extracranial carotid disease. Whilst it will not provide information on siphon disease or aortic arch disease, this is not usually a significant problem in most patients in relation to the decision to perform an endarterectomy. If patients are being considered for stenting, then they will need an assessment of the aortic arch and carotid origins, normally by MRA or CTA. If a policy to operate on ultrasound alone is to be implemented then it is good practice to have a second operator scan the patient to confirm the assessment of the degree of stenosis57 and the department/laboratory must ensure that scanning protocols and results are continuously reviewed, audited and validated with care taken to identify patients who will benefit from further imaging.
Transcranial Doppler of the Cerebral Circulation
The use of ultrasound to assess intracranial structures is not a new phenomenon. One of the first clinical applications of ultrasound as a diagnostic technique was in the assessment of midline intracranial structures with A-scan equipment. More recently, high-quality imaging and Doppler studies have been obtained in neonates through the patent fontanelles, or through the relatively thin bone of the neonatal skull. Transcranial Doppler was first described by Aaslid in 198264 and the technique of pulsed transcranial Doppler has subsequently been developed in many centres. This technique provides useful information on the direction and velocity of blood flow and the changes which may occur in these with various physiological, pharmacological or pathological conditions; it can also be used to monitor the intracranial circulation during carotid and other vascular surgical procedures.65 However, it is a difficult technique to learn and to perform reliably as the vessels must be located without any imaging information.
Modern ultrasound equipment can now be configured to get some imaging detail and colour Doppler information from within the adult skull in many cases; best results are obtained with dedicated transducers and software. This allows localisation and positive identification of the major arteries and specific segments of these. The main problem is the bone of the skull vault, where it has been estimated that the attenuation can vary from 15–25 dB to 40–60 dB depending on the type and thickness of the bone for a single passage across the skull vault.66 As the sound pulse has to pass through the skull on both the inward and outward segments of its passage, there is therefore considerable loss of energy. Power Doppler is of value in locating the vessels and the advent of intravascular ultrasound contrast agents has improved the signal-to-noise ratio significantly; it also makes the location of intracranial vessels more straightforward.67
TECHNIQUE
There are three potential sites of access for transcranial examinations in the adult: the transtemporal window, the suboccipital approach and the transorbital approach. The transtemporal window is used for assessment of the internal carotid arteries, the middle, anterior and posterior cerebral arteries. The window is located by applying liberal amounts of acoustic coupling gel to the hair and skin of the temporal region in front and above the external auditory meatus. Slowly moving the transducer around will allow the point of best transmission to be identified. In some 10% of subjects it may not be possible to get worthwhile images and Doppler signals;68 there tends to be greater attenuation in older patients, females and Afro-Caribbean patients.69
The pituitary fossa and suprasellar cistern are the most recognisable structures in the transverse plane. Once these have been identified, colour Doppler can be used to locate the main arteries and the direction of flow in these vessels (Fig. 3-27). The ipsilateral middle cerebral artery is seen passing peripherally from the end of the internal carotid artery. It cannot often be seen in its entirety in a single scan plane and the transducer position must be varied to follow it out to the Sylvian fissure, where it turns posteriorly. The origin of the contralateral middle cerebral artery can also usually be identified. The proximal segments of the anterior cerebral arteries are also seen from the transtemporal approach. The direction of flow in the ipsilateral anterior cerebral artery is normally away from the transducer and flow is towards the transducer in the contralateral vessel. This arrangement will be altered if there is occlusion of the ipsilateral internal carotid artery and collateral flow through the anterior communicating artery is present. In this situation, flow in the anterior cerebral artery on the side of the occluded carotid will be reversed towards the transducer. The posterior cerebral arteries can be seen arising from the basilar artery and passing around the cerebral peduncles.
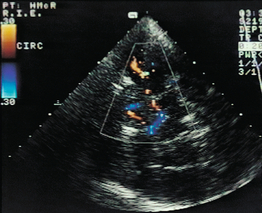
FIGURE 3-27 The anterior and middle cerebral arteries on transcranial colour Doppler ultrasound through the transtemporal window.
The suboccipital window is located by scanning transversely in the midline under the occipital bone. It is often better to position the transducer slightly to one side or the other of the midline as the nuchal ligament can interfere with the clarity of the image. The vertebral arteries can be seen passing around the atlas (Fig. 3-9B) and into the foramen magnum. The point where they join to form the basilar artery may be seen if it lies low enough in relation to the foramen magnum.
The major cerebral veins and venous sinuses are more difficult to demonstrate due to their anatomical locations and slow flow within them, but contrast agents have been reported to improve this situation.67
INDICATIONS
Although still largely a research tool, the technique does have several potential applications. These were reviewed in 2004 by the American Academy of Neurology (Box 3-9).70 In particular the ability to bring the ultrasound machine to the patient allows the technique to be used to monitor cerebral blood flow in a variety of cerebrovascular disorders, such as subarachnoid haemorrhage and stroke.
REFERENCES
1. Stroke Statistics. British Heart Foundation and The Stroke Association, 2009. www.stroke.org.uk
2. European Carotid Surgery Trialists’ (ECST) Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–1387.
3. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Benefit of carotid endarterectomy in patients with moderate or severe stenosis. New Engl J Med. 1998; 339:1415–1425.
4. Rothwell, P. M., Gutnikov, S. A., Warlow, C. P. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003; 34:514–523.
5. Rerkasem, K., Rothwell, P. M. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 4, 2011.
6. Rothwell, P. M., Eliasziw, M., Gutnikov, S. A., et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003; 361:107–116.
7. Chambers, B. R., Donnan, G. A. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev. 4, 2005.
8. Chaturvedi, S., Bruno, A., Feasby, T., et al. Carotid endarterectomy – An evidence based review. Neurology. 2005; 65:794–801.
9. Zwiebel, W. J. Duplex sonography of the cerebral arteries: efficacy, limitations and indications. AJR Am J Roentgenol. 1992; 158:29–36.
10. Abbott, A. L. Medical (non-surgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009; 40:e573–83.
11. Marquardt, L., Geraghty, O. C., Mehta, Z., et al. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical therapy: a prospective, population based study. Stroke. 2010; 41:e11–17.
12. Naylor, A. R., Mehta, Z., Rothwell, P. M., et al. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. 2002; 23:283–294.
13. Naylor, A. R., Merrick, M. V., Sandercock, P. A. G., et al. Serial imaging of the carotid bifurcation and cerebrovascular reserve after carotid endarterectomy. Br J Surg. 1993; 80:1278–1282.
14. Bassi, P., Lattuada, P., Gomitoni, A. Cervical cerebral artery dissection: a multicenter prospective study (preliminary report). Neurol Sci. 2003; 24(Suppl. 1):S4–S7.
15. Touboul, P. -J., Hennerici, M. G., Meairs, S., et al. Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc Dis. 2007; 23:75–80.
16. Hien-Tu, N. -T., Benzaquen, B. S. Screening for subclinical coronary artery disease measuring carotid intima media thickness. Am J Cardiol. 2009; 104:1383–1388.
17. Tahmasebpour, H. R., Buckley, A. R., Cooperberg, P. L., et al. Sonographic examination of the carotid arteries. Radiographics. 2005; 25:1561–1575.
18. Trigaux, J. P., Delchambre, F., Van Beers, B. Anatomical variations of the carotid bifurcation: implications for digital subtraction angiography and ultrasound. Br J Radiol. 1990; 63:181–185.
19. Simon, A., Megnien, J. -L., Gilles, C. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010; 30:182–185.
20. Buckenham, T. M., Wright, I. A. Ultrasound of the extracranial vertebral artery. Br J Radiol. 2004; 77:15–20.
21. Berguer, R., Kieffer, E. The aortic arch and its branches: anatomy and blood flow. In: Kieffer E., Berguer R., eds. Surgery of the arteries to the head. New York: Springer Verlag; 1992:5–31.
22. Wain, R. A., Lyon, R. T., Veith, F. J., et al. Accuracy of duplex ultrasound in evaluating carotid anatomy before endarterectomy. J Vasc Surg. 1998; 27:235–242.
23. Bluth, E. I., Stavros, A. T., Marich, K. W., et al. Carotid duplex sonography: a multicentre recommendation for standardized imaging and Doppler criteria. Radiographics. 1988; 8:487–506.
24. Robinson, M. L., Sacks, D., Perlmutter, G. S., et al. Diagnostic criteria for carotid duplex sonography. AJR Am J Roentgenol. 1988; 151:1045–1049.
25. Grant, E. G., Benson, C. B., Moneta, G. L., et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis – Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003; 229:340–346.
26. Oates, C. P., Naylor, A. R., Hartshorne, T., et al. Joint recommendations for reporting carotid ultrasound investigations in the United Kingdom. Eur J Vasc Endovasc Surg. 2009; 37:251–261.
27. Spencer, M. O., Reid, J. M. Quantitation of carotid stenosis with continuous wave (CW) Doppler ultrasound. Stroke. 1979; 10:326–330.
28. Erickson, S. J., Mewissen, M. W., Foley, W. D., et al. Stenosis of the internal carotid artery: assessment using colour Doppler imaging compared with angiography. AJR Am J Roentgenol. 1989; 152:1299–1305.
29. Steinke, W., Meairs, S., Ries, S., et al. Sonographic assessment of carotid artery stenosis. Comparison of power Doppler imaging and color Doppler flow imaging. Stoke. 1996; 27:91–94.
30. Sachar, R., Yadav, J. S., Roffi, M., et al. Severe bilateral carotid stenosis: the impact of ipsilateral stenting on Doppler-defined contralateral stenosis. J Am Coll Cardiol. 2004; 43:1358–1362.
31. Belkin, M., Mackey, W. C., Pessin, M. S., et al. Common carotid artery occlusion with patent internal and external carotid arteries: diagnosis and surgical management. J Vasc Surg. 1993; 17:1019–1027.
32. Krieghauser, J. S., Patel, M. D., Nelson, K. D. Carotid pseudostring sign from vasa vasorum collaterals. J Ultrasound Med. 2003; 22:959–963.
33. Androulakis, A. E., Labropoulos, N., Allan, R., et al. The role of common carotid artery end-diastolic velocity in near or total internal carotid artery occlusion. Eur J Vasc Endovasc Surg. 1996; 11:140–147.
34. Meves, S. H., Muhs, A., Federlein, J., et al. Recanalisation of acute symptomatic occlusions of the internal carotid artery. J Neurol. 2002; 249:188–192.
35. Camporese, G., Verlato, F., Salmistraro, G., et al. Spontaneous recanalization of internal carotid artery occlusion evaluated with color flow imaging and contrast arteriography. Int Angiol. 2003; 22:64–71.
36. Steffen, C. M., Gray-Weale, A. C., Byrne, K. E., et al. Carotid artery atheroma: ultrasound appearances in symptomatic and asymptomatic vessels. Aust N Z J Surg. 1989; 59:529–534.
37. Gronholdt, M. L., Nordestgaard, B. G., Schroeder, T. V., et al. Ultrasonic lucent plaques predict future strokes. Circulation. 2001; 104:68–73.
38. Golledge, J., Cuming, R., Ellis, M., et al. Carotid plaque characteristics and presenting symptom. Br J Surg. 1997; 84:1697–1701.
39. Geroulakos, G., Ramaswami, G., Niclaides, A., et al. Characterization of symptomatic and asymptomatic carotid plaques using high resolution real-time ultrasonography. Br J Surg. 1993; 80:1274–1277.
40. O’Donnell, T. F., Erdoes, L., Mackey, W. C., et al. Correlation of B-mode ultrasound imaging and arteriography with pathologic findings at carotid endarterectomy. Arch Surg. 1985; 120:443–449.
41. Bluth, E. I., Kay, D., Merritt, C. R. B., et al. Sonographic characterization of carotid plaque: detection of haemorrhage. AJR Am J Roentgenol. 1986; 146:1061–1065.
42. Ratliff, D. A., Gallagher, P. J., Hames, T. K., et al. Characterisation of carotid artery disease: comparison of duplex scanning with histology. Ultrasound Med Biol. 1985; 11:835–840.
43. O’Leary, D. H., Holen, J., Ricotta, J. J., et al. Carotid bifurcation disease: prediction of ulceration with B-mode US. Radiology. 1987; 162:523–525.
44. Hatsukami, T. S., Ferguson, M. S., Beach, K. W., et al. Carotid plaque morphology and clinical events. Stroke. 1997; 28:95–100.
45. Gotway, M. B., Araoz, P. A., Macedo, T. A., et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005; 184:1945–1950.
46. Rosset, E., Albertini, J. -N., Magnan, P. E., et al. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg. 2000; 31:713–723.
47. Mann, W. J., Beck, A., Schreiber, J., et al. Ultrasonography for evaluation of the carotid artery in head and neck cancers. Laryngoscope. 1994; 104:885–888.
48. Barry, R., Pienaar, A., Pienaar, C. Duplex Doppler evaluation of suspected lesions at the carotid bifurcation. Ann Vasc Surg. 1993; 7:140–144.
49. de Bray, J. M., Lhoste, P., Dubaz, F., et al. Ultrasonic features of extracranial carotid dissections: 47 cases studied by angiography. J Ultrasound Med. 1994; 13:659–664.
50. Logason, K., Hardemark, H. G., Bärlin, T., et al. Duplex scan findings in patients with spontaneous cervical artery dissections. Eur J Vasc Endovasc Surg. 2002; 23:295–298.
51. Steinke, W., Rautenberg, W., Schwartz, A., et al. Non-invasive monitoring of internal carotid artery dissection. Stroke. 1994; 25:998–1005.
52. Sitzer, M., Fürst, G., Siebler, M., et al. Usefulness of an intravenous contrast medium in the characterization of high-grade internal carotid stenosis with colour Doppler-assisted duplex imaging. Stroke. 1994; 25:385–389.
53. Lal, B. K., Hobson, R. W., 2nd., Tofighi, B., et al. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008; 47:63–73.
54. Rothwell, P. M., Gibson, R. J., Slattery, J., et al. Prognostic value and reproducibility of measurements of carotid stenosis: a comparison of three methods on 1001 angiograms. Stroke. 1994; 25:2440–2444.
55. Chikos, P. M., Fisher, L. D., Hirsch, J. H., et al. Observer variability in evaluating extracranial carotid stenoses. Stroke. 1983; 14:885–892.
56. Executive Committee for the Asymptomatic Carotid Atherosclerosis (ACAS) Study. Endarterectomy for asymptomatic carotid artery stenosis. J Am Med Assoc. 1995; 273:1421–1428.
57. Wardlaw, J. M., Chappell, F. M., Stevenson, M., et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess. 10(30), 2006.
58. Cardullo, P. A., Cutler, B. S., Brownell Wheeler, H. Detection of carotid disease by duplex ultrasound. J Diagn Med Sonogr. 1986; 2:63–73.
59. Lee, D. H., Gao, F. Q., Rankin, R. N., et al. Duplex and color Doppler flow sonography of occlusion and near occlusion of the carotid artery. Am J Neuroradiol. 1996; 17:1267–1274.
60. Cinat, M. E., Casalme, C., Wilson, S. E., et al. Computed tomography angiography validates duplex sonographic evaluation of carotid stenosis. Am Surg. 2003; 69:842–847.
61. Back, M. R., Rogers, G. A., Wilson, J. S., et al. Magnetic resonance angiography minimizes the need for arteriography after inadequate carotid duplex ultrasound scanning. J Vasc Surg. 2003; 38:422–430.
62. Herzig, R., Burval, S., Krupka, B., et al. Comparison of ultrasonography, CT angiography and digital subtraction angiography in severe carotid stenoses. Eur J Neurol. 2004; 11:774–781.
63. Buskens, E., Nederkoorn, P. J., Buijs-Van Der Woude, T., et al. Imaging of carotid arteries in symptomatic patients: cost-effectiveness of diagnostic strategies. Radiology. 2004; 233:101–112.
64. Aaslid, R., Markwalder, T. M., Nornes, H. Non-invasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982; 57:769–774.
65. Doblar, D. D. Intraoperative transcranial ultrasonic monitoring for cardiac and vascular surgery. Semin Cardiothorac Vasc Anesth. 2004; 8:127–145.
66. White, D. N., Curry, G. R., Stevenson, R. J. The acoustic characteristics of the skull. Ultrasound Med Biol. 1978; 4:225–252.
67. Bauer, A., Becker, G., Krone, A., et al. Transcranial duplex sonography using ultrasound contrast enhancers. Clinl Radiol. 1996; 51(Suppl. 1):19–23.
68. Ringelstein, E. B., Kahlscheuer, E., Niggemeyer, E., et al. Transcranial Doppler sonography: anatomical landmarks and normal velocity values. Ultrasound Med Biol. 1990; 16:745–761.
69. Halsey, J. H. Effect of emitted power on waveform intensity in transcranial Doppler. Stroke. 1990; 21:1573–1578.
70. Sloan, M. A., Alexandrov, A. V., Tegeler, C. H., et al. Assessment: Transcranial Doppler ultrasonography. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004; 62:1468–1481.