Chapter 6 The cardiovascular system
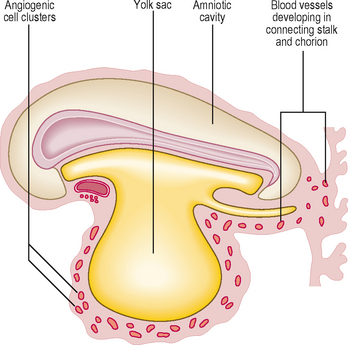
One of the first systems to develop in the embryo is the cardiovascular system because of the need to transport oxygen to embryonic cells. In the early embryo, nutrients are derived from trophoblastic digestion of the uterine mucosa, then via diffusion from the contents of the yolk sac. This soon becomes inadequate for the needs of the rapidly growing embryo. As the number of cells in the embryo increase most of the cells lose contact with a surface for diffusion to occur. The initial components of the cardiovascular system appear as angiogenic cell clusters in the extra-embryonic mesoderm lining the yolk sac (Fig. 6.1). These give rise to channels that extend into the embryo. The early embryonic blood vessels that appear around the neural plate in a horseshoe-shaped arrangement arise from the unsegmented mesoderm, which forms rostral to the prochordal plate. These clusters merge to form the cardiogenic area of the embryo, and the cells form tube-like structures, which initially become the paired heart tubes. The paired heart tubes bulge into the midline portion of the intra-embryonic coelom, which is the future pericardial cavity. The dorsal aortae develop on either side of the midline and connect with the heart tubes.
Heart tube formation
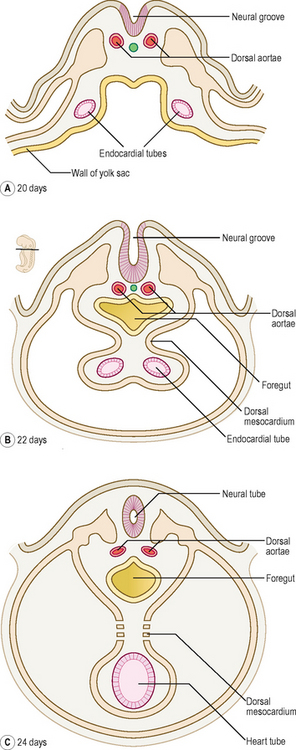
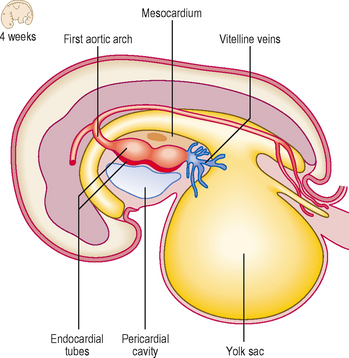
With the longitudinal and lateral folding of the embryo the heart tubes fuse to form a single tube, which is carried around to the region of the future thorax (Figs 6.1 and 6.2). The heart tube itself comprises an inner endothelial lining and an outer myocardial layer. The outer surface of the heart tube becomes invested in mesodermal tissue, which becomes the future visceral pericardium, or epicardium. This tube is retained within the pericardial cavity by a dorsal mesocardium which suspends the tube in much the same way as the gut tube is suspended in the peritoneal cavity. The heart tube is attached at its proximal and distal ends by the future ‘great’ arterial and venous vessels leaving and entering the heart respectively (Fig. 6.3). Soon, however, the dorsal mesocardium breaks down, leaving the heart tube attached merely at the margins of the pericardium. The heart tube itself differentiates during this time, to produce a thicker myocardium.
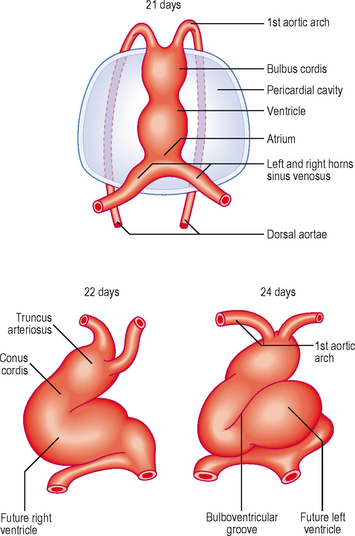
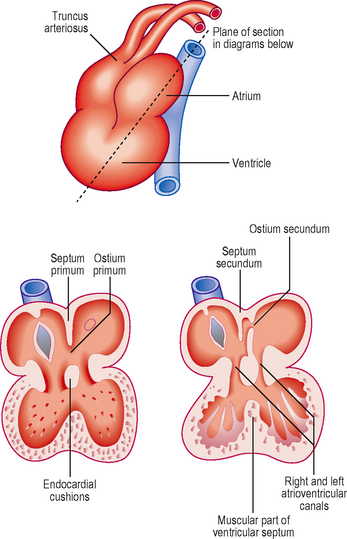
The heart tube continues to elongate in the pericardial cavity and develops a series of expansions. By day 23 it is too long to be accommodated in the volume available as a straight tube, thus it bends. This forms the cardiac loop (Fig. 6.4). The heart tube comprises an atrial portion, which initially is a single chamber, and a ventricular portion: the connection between the two becomes the narrow atrioventricular canal. In this region the atrioventricular valves will form later from structures called endocardial cushions. These are swellings of mesenchymal tissue, covered by endocardium. The canal connects the common atrium and primitive ventricle. Between the primitive ventricle and the arterial outflow is the bulbus cordis, which becomes the right ventricle. The left ventricle arises from the original primitive ventricle. The bends in the heart tube then occur at set places: the bulboventricular groove and the atrioventricular groove. The bulbus cordis becomes the conus cordis that leads from both future ventricles and is the outflow tract which leads on to the truncus arteriosus (Fig. 6.4). The truncus arteriosus continues to form the proximal parts of the aorta and pulmonary trunk. The proximal third of the bulbus cordis and the future right ventricle become trabeculated, whereas the conus remains smooth-walled, being associated with the outflow tracts.
Septation of the heart tube
In the atria, septation begins at about the fourth week. Initially, in the common atrium, a ridge develops in its roof. This is the septum primum (or primary septum) and it grows towards the endocardial cushions of the atrioventricular canal (Fig. 6.5). The opening that persists between the septum and the cushion is known as the ostium primum (or primary foramen). With further growth the ostium primum is closed, as it reaches the endocardial cushions. Before this closure occurs, however, small holes appear in the septum, which merge to form the ostium secundum (secondary foramen) (Fig. 6.5). By this means blood is able to flow between the two atria, an important feature of the fetal circulation (see later). Whilst the septum primum develops, another septum begins to form immediately to the right of the septum primum, the septum secundum (secondary septum). This grows over the septum primum, but never completely divides the atria, and leaves the opening of the foramen ovale (Fig. 6.5). The septum primum filling the foramen becomes the valve of the foramen ovale. This constitutes a vital mechanism for enabling circulation of blood from the right side of the heart, which contains oxygenated blood from the placenta, to pass into the systemic circulation, without passing through the pulmonary circulation. The pressure of blood on the right side of the heart is thus sufficiently high to force open the flap valve and allow the blood into the left atrium. After birth, air breathing commences and the pulmonary circulation begins, causing pressure to rise on the left side of the heart. The effect of this is to close the flap valve. In most people this results in the formation of a complete septum, the interatrial septum. In up to 20% of individuals, however, this is an incomplete seal, and may be opened with a probe, hence probe patency of the foramen ovale. Despite this, it is still a functional septum, allowing no passage of blood from the right to the left side. After birth, with the closure of the foramen ovale, the resulting central depression in the interatrial septum is known as the fossa ovalis.
The heart tube can loop to the left rather than the right, resulting in the anomaly of dextrocardia in which the heart comes to lie on the right hand side of the thorax, not the left. The condition may accompany situs inversus, a condition in which there is a complete mirror-image reversal of the internal organs of the thorax and abdomen. Such a condition is compatible with normal functioning, though there is an increased risk of volvulus (see Chapter 7) in the gastrointestinal tract.
Septum formation in the ventricle
The ventricles increase in size and internal volume due to growth of the myocardium. The ventricular wall becomes remodelled, leading to trabeculation. Tissue develops from the floor of the ventricles, midway between the right and left sides, and grows towards the endocardial cushions. This is the muscular component of the interventricular septum (Fig. 6.6). However, it never reaches the cushions, leaving a small gap. This gap is filled by two components: further growth of the endocardial cushions themselves, and downgrowths from the septum that divides the truncus arteriosus (see below). These two components constitute the membranous part of the septum. This part of the septum accommodates the atrioventricular bundle of the conducting system of the heart. Thus, the only myocardial connection between the atria and ventricles is this conducting tissue. This arrangement ensures the sequential contraction of the atria followed by the ventricles, because these two pairs of chambers are otherwise electrically isolated from one another.
Septum formation in the outflow tracts of the heart
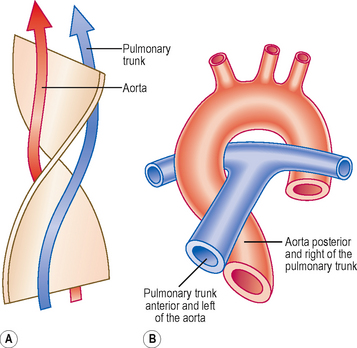
Initially, the truncus arteriosus is a structure with a single lumen. However, a septum develops, dividing the future ascending aorta and the pulmonary trunk. In the fifth week a pair of ridges appears opposite each other within the conus cordis and truncus arteriosus, the right and left truncoconal swellings (Figs 6.6 and 6.7). The two ridges approach one another and form the aorticopulmonary septum, separating the two major outflow tracts of the two ventricles. The septum develops as a spiral structure, which accounts for the spiralling around each other of the pulmonary trunk and ascending aorta (Fig. 6.7).
Development of the venous drainage into the heart
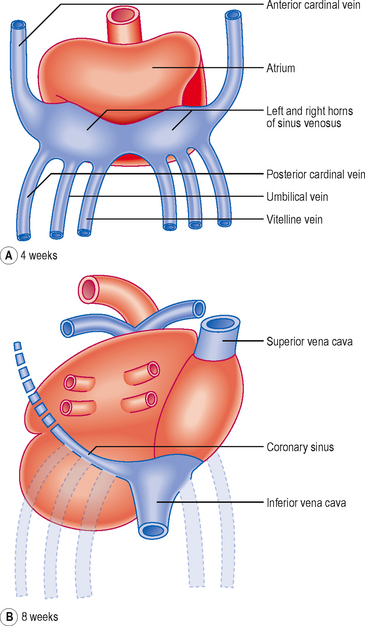
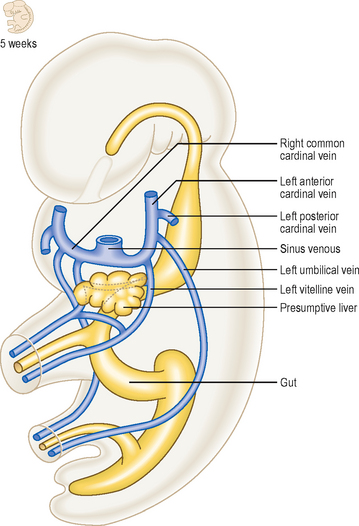
 The common cardinal veins into which drain the anterior and posterior cardinal veins: by these two veins all the blood from the body of the embryo drains to the atria; and
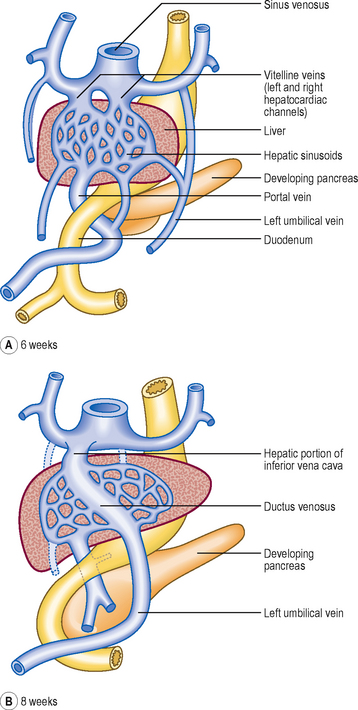
The common cardinal veins into which drain the anterior and posterior cardinal veins: by these two veins all the blood from the body of the embryo drains to the atria; and The vitelline veins and the umbilical veins, which drain blood from the yolk sac and the placenta respectively (Fig. 6.8).
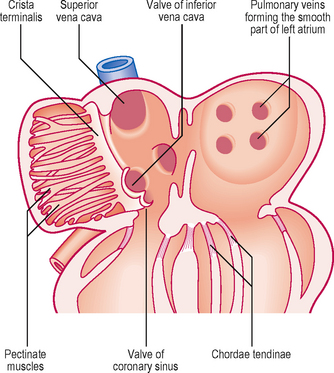
The vitelline veins and the umbilical veins, which drain blood from the yolk sac and the placenta respectively (Fig. 6.8).The umbilical veins carry oxygenated blood to the heart and the vitelline veins drain the derivatives of the gut tube. However, the original symmetry of the horns of the sinus venosus is soon lost because of venous blood shunts from the left to the right side of the body. Whereas the right sinus horn becomes the most proximal portion of the inferior and superior venae cavae, the left largely disappears aside from the part that becomes the coronary sinus (Fig. 6.8). As the right sinus horn and venae cavae enlarge, they are gradually drawn into the posterior wall of the atrium. This is the sinus venarum, and represents the smooth-walled portion of the atrium, whereas the primitive atrium is displaced anteriorly as a muscular pouch, the right auricle (Fig. 6.9). The smooth and rough parts are separated by a ridge or crest called the crista terminalis (corresponding to the sulcus terminalis, a groove on the exterior of the heart). From this crest a parallel array of pectinate muscles projects into the auricular portion of the chamber. In the fetal heart the lower part of the crista terminalis forms the valve of the inferior vena cava, and this is arranged so as to direct blood through the foramen ovale into the left atrium. This valve disappears after birth.
The pulmonary veins develop in situ between the lungs and the heart, and enter the left atrium. Gradually, the single opening of the pulmonary vein changes as further parts of the two sets of pairs of veins are drawn into the wall of the left atrium: this component forms the smooth-walled component (Fig. 6.9).
Valve formation in the atrioventricular canal and truncus arteriosus
There are two swellings of mesenchyme in the wall of the atrioventricular canal that form the endocardial cushions. These appear at the end of the fourth week at the superior and inferior borders of the atrioventricular canal. Soon after two further swellings arise on the right and left sides of the canal; thus there are four projections into the canal. Subsequent growth causes the superior and inferior cushions to meet and fuse, forming right and left atrioventricular canals. The two atrioventricular valves arise from the margins of the atrioventricular canals. Remodelling occurs, which results in the formation of the two flap valves. The bicuspid or mitral valve on the left has two leaflets, whereas the tricuspid valve on the right has three leaflets. As a consequence of the remodelling, parts of the walls of the ventricles become so thin that the muscular cords connecting the valves with the wall are replaced by connective tissue, the chordae tendinae (Fig. 6.9).
Development of the arterial system
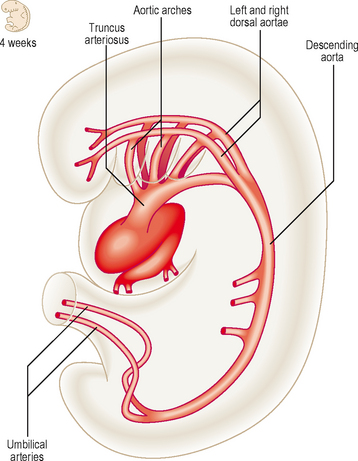
The arterial system of the embryo includes the aortic arches and the paired dorsal aortae. These two aortic vessels unite to form the descending aorta which passes the length of the embryo, supplying visceral and somatic structures as it does so. The umbilical arteries from the placenta drain the embryo of deoxygenated blood (Fig. 6.10).
Aortic arches
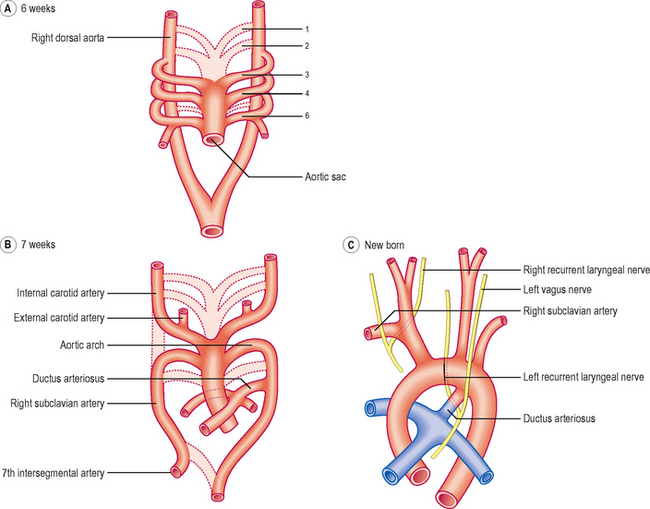
The outflow channel of the heart is the dilated distal part of the truncus arteriosus, the aortic sac. This sac contributes vessels to the pharyngeal arches that develop in the future neck region of the embryo from about the fourth week onwards. Within each arch the artery develops and meets with the branch from the aortic sac. By this means five or six pairs of aortic arches are formed. The aortic arches join the dorsal aorta on each side. Whereas initially there is symmetry in development (Fig. 6.11A), changes soon result in an asymmetrical pattern. The aortic sac divides into a right and a left dorsal aorta. The two vessels continue into the body of the embryo where they fuse just inferior to the heart, continuing as a single vessel (Figs 6.10 and 6.11A).
The pattern of the subsequent development of the aortic arches is remarkable for two reasons: (1) large portions of the original aortic arches disappear and (2) there are asymmetries for some arch pairs. The first pair largely disappears except for the small part that persists as the maxillary artery. The second pair also largely disappears. The third pair forms the common carotid arteries, and the external carotid and proximal parts of the internal carotid arteries (Fig. 6.11B). The distal part of the internal carotid arteries form from the dorsal aortae. The fate of the fourth pair is different on the two sides. On the right side the proximal portion of the right subclavian artery forms, whereas on the left side, the fourth arch contributes part of the arch of the aorta. The fifth pair never makes any significant appearance. The sixth pair also exhibits asymmetric development. The proximal part of the sixth arch pair forms the pulmonary arteries with the pulmonary trunk forming from the truncus arteriosus. On the left side the distal part of the sixth arch persists in fetal life as the ductus arteriosus, an important bypass channel for the oxygenated blood from the placenta to avoid passing into the lungs (Fig. 6.11C). Thus, this oxygenated blood passes into the systemic circulation more rapidly than it would otherwise do. On the right side the sixth arch forms the distal portion of the right subclavian artery. The left subclavian artery forms from the seventh intersegmental artery, a branch of the dorsal aorta to the developing upper limb of the embryo. Along with these changes to the arch pattern, the portion of the dorsal aorta on the right side between the seventh intersegmental artery and the start of the fused dorsal aorta disappears (Fig. 6.11B). Whilst these changes take place, the position of the heart, and therefore the arches too, alters so that the heart pushes into the future thoracic cavity. Consequently, the carotid and brachiocephalic vessels undergo elongation, and the recurrent laryngeal nerves are similarly lengthened, though their course is different on the two sides. This is because of the different arrangements of the sixth arch pairs. On the left side the nerve hooks under the ductus arteriosus, whereas on the right side the distal part of the sixth arch disappears and hence there is no impediment to the recurrent laryngeal nerve; thus it rides up to hook under the right subclavian artery (Fig. 6.11C).
Development of the venous system
By the fifth week of development there are three major sets of veins: the vitelline, umbilical and cardinal veins. The vitelline veins drain the gut tube, the umbilical veins bring oxygenated blood from the placenta, and the cardinal veins drain the head and body wall (Fig. 6.12). The proximal portions of both umbilical veins disappear, as does the distal part of the right umbilical vein. The distal left umbilical vein persists and carries the oxygenated blood from the placenta to the liver (Fig. 6.13). Here there is a shunt to avoid much of the blood having to enter the liver. The ductus venosus passes across the future visceral surface of the liver from the left umbilical vein to the hepatic portion of the inferior vena cava. Also draining into this is the future hepatic portal vein (Fig. 6.13A). The fate of the vitelline veins is related to the drainage of the blood from the liver and the fate of the sinus venosus. The growth of the liver interrupts the veins which become incorporated into the liver sinusoids. Blood drains from the liver into the sinus venosus via right and left hepatocardiac channels, which also derive from the vitelline veins. The sinus venosus undergoes a left to right shunt so that whereas originally there is an equal volume of blood draining into the sinus via the left and right hepatocardiac channels, the left channel disappears. Thus, all the blood drains via the right channel, and this forms the hepatic portion of the inferior vena cava (Fig. 6.13).
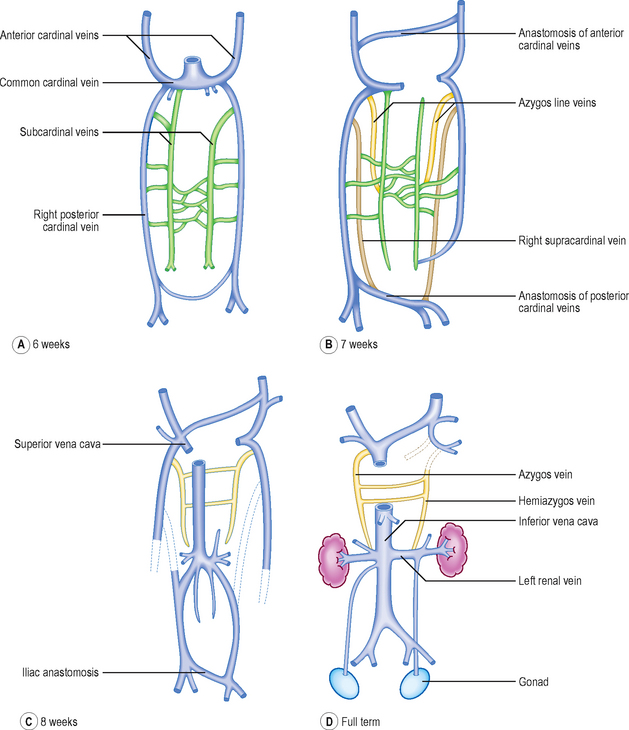
The cardinal veins drain blood from the body wall of the embryo. They do this as anterior and posterior cardinal veins, draining into the sinus venosus at the common cardinal vein. During the fifth week of development additional longitudinal venous channels form in the trunk of the embryo. Initially, these are paired and include the subcardinal, supracardinal and azygos line veins (Fig. 6.14A, B). Over the next 2 weeks a progressive asymmetry arises, resulting in a right-sided dominance. As a consequence some channels enlarge whilst others regress. This changing pattern results in the formation of the inferior vena cava as a single vessel that includes portions of the subcardinal and supracardinal veins. The azygos line veins persist as the azygos system of veins. There is also a left to right shunt in the anterior cardinal veins so that the symmetrical pattern is lost, favouring the development of the right and left brachiocephalic veins and their convergence as the superior vena cava. Similarly, at the distal end of the inferior vena cava, the posterior cardinal veins undergo a left to right shunt forming the common iliac veins, draining into the single inferior vena cava. In this way, the left-sided components of the venous system disappear in favour of the right-sided developments, most of which are centred on the formation of the inferior vena cava (Fig. 6.14D).
Changes in the vascular system at birth
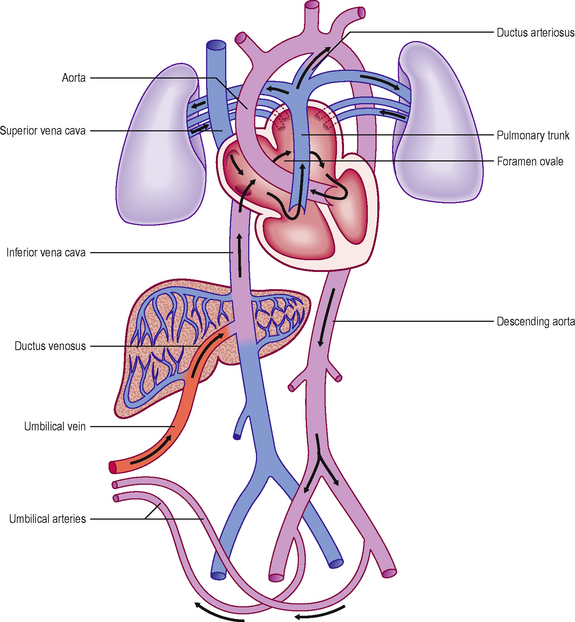
Prior to birth the fetus acquires its oxygen via the placenta. To facilitate this process there are a number of differences in the morphology and organization of the vascular system and heart (Fig. 6.15). After birth, with the start of lung breathing, changes occur in the cardiovascular system. The principal change in the heart is the closure of the foramen ovale. This occurs because of the change in pressures in the two atria. Once lung breathing begins, blood passes into the pulmonary circulation and hence pressure falls in the right atrium. Coincidentally, pressure rises in the left atrium with the larger volume of blood returning from the lungs. Thus, the septum primum and septum secundum are pressed together, causing a functional closure of the foramen ovale that later seals anatomically. The ductus arteriosus is redundant after the start of lung breathing and becomes the ligamentum arteriosum. Initially, closure of the ductus arteriosus is stimulated by bradykinin from the lungs after their first inflation, leading to smooth muscle contraction in its wall, and later by replacement of its epithelial lining tissue with fibrous connective tissue. In the fetus the umbilical arteries bring deoxygenated blood back to the placenta. These close after birth, resulting in the formation of the medial umbilical ligaments. The left (and not the right) umbilical vein brings oxygenated blood from the placenta to the fetus. After birth the vein becomes the ligamentum teres (hepatis), and is found in the free border of the peritoneal ligament, the falciform ligament. The channel responsible for short circuiting the circulation of the liver, the ductus venosus, also degenerates after birth to form the ligamentum venosum, and lies in the fissure on the visceral surface of the liver, at the root of the lesser omentum.
 The cardiovascular system is one of the first body systems to develop, in order to carry oxygen and nutrients around the embryo.
The cardiovascular system is one of the first body systems to develop, in order to carry oxygen and nutrients around the embryo. The heart tube develops from unsegmented mesoderm at the rostral end of the embryo, and during longitudinal and lateral folding it is carried round to its future thoracic position, within the future pericardial cavity.
The heart tube develops from unsegmented mesoderm at the rostral end of the embryo, and during longitudinal and lateral folding it is carried round to its future thoracic position, within the future pericardial cavity. The tube undergoes septation, separating the atria, ventricles and great vessels. Atrial septation involves the development of two septa: the septum primum and the septum secundum. These septa are not complete, enclosing the foramen ovale. This closes at birth as a consequence of the differential pressures on the two sides.
The tube undergoes septation, separating the atria, ventricles and great vessels. Atrial septation involves the development of two septa: the septum primum and the septum secundum. These septa are not complete, enclosing the foramen ovale. This closes at birth as a consequence of the differential pressures on the two sides. In the ventricles a muscular upgrowth from the floor of the ventricles plus a membranous component from the endocardial cushions forms the interventricular septum.
In the ventricles a muscular upgrowth from the floor of the ventricles plus a membranous component from the endocardial cushions forms the interventricular septum. The division of the truncus arteriosus is accomplished by the appearance of the spiral aortico-pulmonary septum, which separates the pulmonary trunk from the ascending aorta. The arterial system develops as two dorsal aortae which fuse after the arch, just caudal to the heart.
The division of the truncus arteriosus is accomplished by the appearance of the spiral aortico-pulmonary septum, which separates the pulmonary trunk from the ascending aorta. The arterial system develops as two dorsal aortae which fuse after the arch, just caudal to the heart. The truncus arteriosus gives rise initially to five or six pairs of aortic arches, which feed into the two dorsal aortae. The symmetry of this pattern is lost as the adult pattern of the arches develops.
The truncus arteriosus gives rise initially to five or six pairs of aortic arches, which feed into the two dorsal aortae. The symmetry of this pattern is lost as the adult pattern of the arches develops. The veins of the embryo drain into the sinus venosus, which also loses its initially symmetric pattern. Draining into the sinus are the umbilical and vitelline veins, draining respectively the placenta and yolk sac. The body of the embryo is drained via the anterior and posterior cardinal veins, via the common cardinal vein into the sinus venosus.
The veins of the embryo drain into the sinus venosus, which also loses its initially symmetric pattern. Draining into the sinus are the umbilical and vitelline veins, draining respectively the placenta and yolk sac. The body of the embryo is drained via the anterior and posterior cardinal veins, via the common cardinal vein into the sinus venosus. After birth changes occur in the morphology and organization of the cardiovascular system as a result of lung breathing. Thus, the umbilical arteries become the medial umbilical ligaments, the left umbilical vein (the right disappears) becomes the ligamentum teres hepatis, the ductus venosus becomes the ligamentum venosum and the ductus arteriosum becomes the ligamentum arteriosum. The foramen ovale between the two atria closes, leaving the fossa ovalis in the interatrial septum.
After birth changes occur in the morphology and organization of the cardiovascular system as a result of lung breathing. Thus, the umbilical arteries become the medial umbilical ligaments, the left umbilical vein (the right disappears) becomes the ligamentum teres hepatis, the ductus venosus becomes the ligamentum venosum and the ductus arteriosum becomes the ligamentum arteriosum. The foramen ovale between the two atria closes, leaving the fossa ovalis in the interatrial septum.
 Clinical box
Clinical box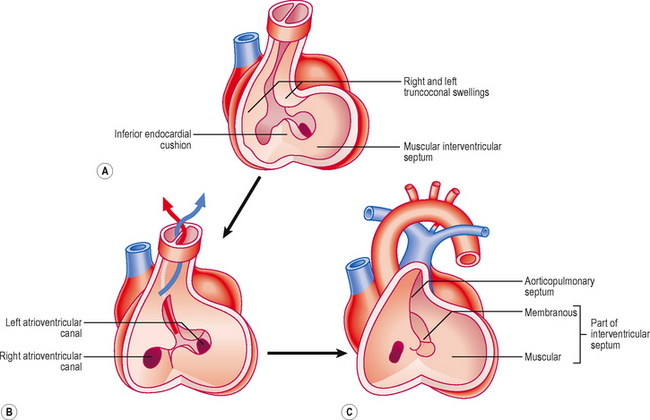
 Clinical box
Clinical box