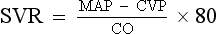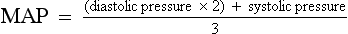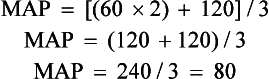The Cardiovascular System
SYSTEMWIDE ELEMENTS
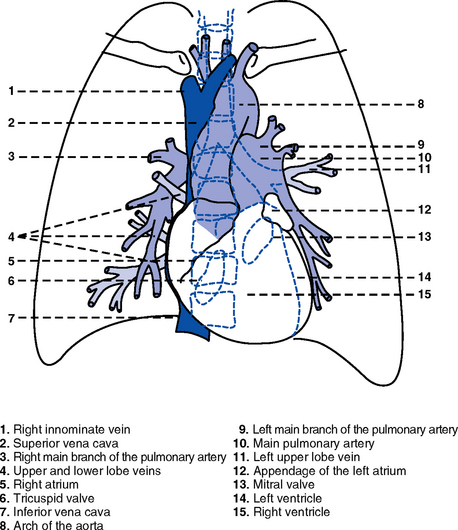
1. Heart (Figures 3-1 and 3-2)
a. The heart lies in the mediastinum, on and to the left of the midline
b. Its long axis is oriented from the right shoulder blade to the left upper quadrant of the abdomen
c. The base (top wide area) of the heart (atria and great vessels) is located diagonally at the second intercostal space, right and left sternal borders
d. The apex, or tip, of the heart (junction of the ventricles and ventricular septum) is usually located at the fifth intercostal space, on the left midclavicular line
a. Pericardium: Fibrous sac surrounding the heart and containing small amounts (15 to 50 ml) of pericardial fluid. This lubricated space protects the heart from friction, allowing it to easily change volume and size during contractions. The pericardium also keeps heart muscle anchored within the mediastinum.
b. Epicardium: Outer surface of the heart (includes epicardial coronary arteries, autonomic nerves, adipose tissue, lymphatics)
c. Myocardium: Muscular, contractile portion of the heart. Muscle fibers wrap around the heart in multiple, interlacing layers.
d. Endocardium: Inner surface of the heart
e. Papillary muscles: Myocardial structures extending into the ventricular chambers and attaching to the chordae tendineae
f. Chordae tendineae: Strong tendinous attachments from the papillary muscles to the tricuspid and mitral valves; prevent prolapse of the valves into the atria during systole
a. Atria: Thin-walled, low-pressure chambers
i. Right and left atria act as reservoirs of blood for their respective ventricles
ii. Right atrium (RA), located above and to the right of the right ventricle, receives systemic venous blood via the superior vena cava and inferior vena cava, and venous blood from the heart via the coronary sinus
iii. Left atrium (LA), superior and posterior to the other chambers, receives oxygenated blood returning to the heart from the lungs via the pulmonary veins
iv. When the mitral and tricuspid valves open, there is rapid filling of blood passively from atria into ventricles (about 80% to 85% of total filling)
v. At the end of diastole, atrial contraction (“atrial kick”) forcefully adds 15% to 20% more to the ventricular volume
b. Ventricles: Major “pumps” of the heart
i. Right ventricle (RV) is anterior under the sternum
(a) Thin-walled, low-pressure system
(b) Contracts and propels deoxygenated blood into the pulmonary circulation via the pulmonary artery (the only artery in the body that carries deoxygenated blood)
ii. Left ventricle (LV) is the main “pump”: Conical (ellipsoid) structure behind and to the left of the RV
(a) Thick-walled, high-pressure system
(b) Squeezes and ejects blood into the systemic circulation via the aorta during ventricular systole
iii. Interventricular septum is functionally more a part of the LV than of the RV. It forms the anterior wall of the LV. Its curved shape protrudes into the RV cavity.
a. Atrioventricular (AV) valves
i. Location and structure: Situated between the atria and the ventricles (tricuspid valve on the right, mitral valve on the left)
(a) Tricuspid valve is composed of three leaflets: The large anterior leaflet, and the two smaller posterior and septal leaflets
(b) Mitral valve is composed of two leaflets: The long, narrow posterior (mural) leaflet (like a toilet seat) and an oval anterior (aortic) leaflet (like a toilet lid)
ii. Function: These are one-way “check” valves that permit unidirectional blood flow from the atria to the ventricles during ventricular diastole and prevent retrograde flow during ventricular systole
(a) Pulmonary valve is situated between the RV and the pulmonary artery. It consists of three semilunar cusps that attach to the wall of the pulmonary trunk.
(b) Aortic valve is situated between the LV and aorta. It consists of three slightly thicker valve cusps, the bases of which attach to a valve annulus (fibrous ring).
ii. Function: Permit unidirectional blood flow from the outflow tract during ventricular systole and prevent retrograde blood flow during ventricular diastole
(a) With ventricular systole, the valves open when the respective ventricle contracts and pressure is greater in the ventricle than in the artery
(b) After ventricular systole, pressure in the artery exceeds pressure in the ventricles. This and retrograde blood flow cause the valve to close.
(c) Second heart sound (S2) is produced when the aortic (A2) and pulmonic (P2) valves close. A2 is normally the initial and major component of S2.
5. Coronary vasculature (Figure 3-3)
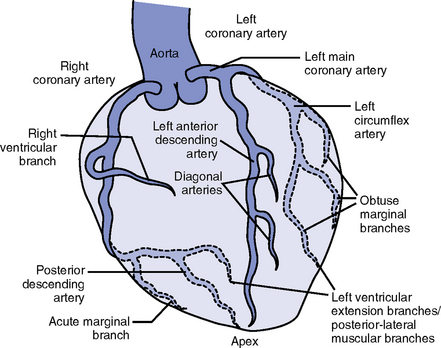
FIGURE 3-3 Coronary artery anatomy.
i. Two main arteries branch off at the base of the aorta, supplying blood to the heart
ii. Right coronary artery (RCA)
(a) Originates behind the right coronary cusp of the aortic valve
iii. Left coronary artery (LCA)
(a) Left main coronary artery (LMCA): Branches into the left anterior descending and circumflex arteries
(b) Left anterior descending (LAD) artery
(1) Supplies the anterior two thirds of the interventricular septum and the anterior wall of the LV
(2) Branches include diagonals (two to six other diagonals may be present), septal perforators (three to five other perforators may be present)
(c) Left circumflex (LCX) artery also branches from the LMCA
c) Inferior-posterior LV in 10% of hearts
d) Lateral posterior surface of the LV via the obtuse marginal branches (OMBs)
e) Posterior descending artery arises from the LCX artery in 15% of hearts (see description under RCA)
(2) Branches of the LCX include the OMBs (may be one to three), which supply the lateral wall of the LV and occasionally the posterior lateral muscular branch
iv. Coronary collaterals: Potential vascular connections between the RCA and LCA exist
i. Return deoxygenated blood to the RA, mostly through the coronary sinus
ii. Follow paths similar to those of the arteries; have no valves
i. Coronary vascular reserve: Coronary circulation has the ability to increase flow to meet added needs up to approximately six times normal
ii. Coronary blood flow is about 70 to 90 ml/min
iii. The heart uses most of the oxygen available in the coronary circulation; little oxygen reserve exists
iv. Most of coronary blood flow is in diastole, because in systole, coronary artery blood flow usually decreases due to ventricular compression and contraction
6. Neurologic control of the heart
a. Autonomic nervous system: Influences contractility, depolarization-repolarization, and rate of conductivity
i. Sympathetic stimulation: Norepinephrine release is the main impetus of stimulation to the heart; its two effects include the following:
ii. Parasympathetic stimulation: Occurs via the tenth cranial (vagus) nerve. Acetylcholine release is the main parasympathetic impetus to cardiac effects.
iii. Ventricles have mainly sympathetic innervation and only sparse vagal innervation
iv. Parasympathetic influences normally predominate in the conducting system (SA node, AV node)
b. Chemoreceptors: Afferent receptors located in the carotid and aortic bodies. Sensitive to changes in partial pressure of oxygen, partial pressure of carbon dioxide, and pH, causing changes in heart rate and respiratory rate via stimulation of vasomotor center in the medulla.
c. Baroreceptors: Stretch receptors in the heart and blood vessels that respond to pressure and volume changes
7. Cardiac muscle microanatomy and contractile properties: See Box 3-1 for key elements
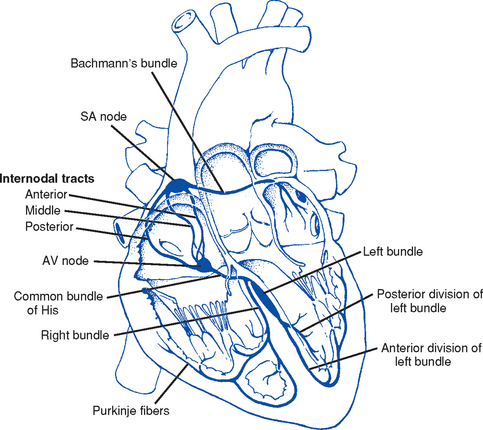
8. Anatomy of the cardiac conduction system (Figure 3-4)
i. Normal pacemaker of the heart, possessing the fastest inherent rate of automaticity (approximately 70 beats/min)
ii. Located in the right superior wall of the RA at the junction of the superior vena cava and the RA
b. Internodal atrial conduction
i. Impulse is conducted from the SA node through the RA and LA musculature to the AV node
ii. Although the atria do not have specialized high-speed conduction tracts comparable to the ventricular bundles and fascicles, there are preferred conduction pathways (e.g., Bachmann’s bundle, which conducts impulses from the SA node to the LA)
i. Delays the impulse from the atria before it goes to the ventricles. This allows time for both ventricles to fill before ventricular systole.
ii. Inherent rate of automaticity is approximately 40 beats/min
iii. Located in the right interatrial septum, above the tricuspid valve’s septal leaflet
d. Bundle of His: Arises from the AV node and conducts the impulse to the bundle branch system. The bundle of His is close to the annulus of the tricuspid valve.
e. Bundle branch system: Pathways that arise from the bundle of His and branch at the top of the interventricular septum
i. Right bundle branch is the smaller, direct continuation of the bundle of His. It transmits the impulse down the right side of the interventricular septum to the RV myocardium.
ii. Left bundle branch is the larger branch from the bundle of His. It transmits the impulse to the septum and the LV. The left bundle branch divides into three parts:
i. Arises from the distal portion of the bundle branches, forming networks on the ventricle’s endocardial surface
ii. Transmits the impulse into the subendocardial and myocardial layers of both ventricles
iii. Provides for depolarization of the myocardium (from endocardium to epicardium)
iv. Ventricles have their own inherent rate of automaticity of approximately 20 to 30 beats/min
a. Electrophysiologic properties of cardiac muscle cells
i. Excitability: Ability to depolarize and form an action potential when sufficiently stimulated
ii. Automaticity: Ability to generate an impulse without an outside stimulus
iii. Conductivity: Ability to conduct an electrical impulse to neighboring cells, spreading the impulse throughout the heart to achieve total depolarization
iv. Refractoriness: Temporary inability of the depolarized cell to become excited and generate another action potential
b. Resting membrane potential (RMP): Electrical charge of cardiac muscle cell at rest. Cell ions consist primarily of sodium, potassium, and calcium.
i. Sodium ion concentration is greater outside the cell
c. Depolarization: Change in the electrical charge of a stimulated cell from negative to positive by the flow of ions across the cell membrane. Sodium moves into the cell, potassium moves out.
d. Repolarization: Recovery or recharging of a cell’s normal polarity. Sodium moves back out of the cell, potassium moves into the cell. The cell recovers its negative charge.
e. Threshold potential: The electric voltage level at which cardiac cells become activated and produce an action potential, which leads to muscular contraction
f. Stimulation of myocardial cells
i. Stimulus may be chemical, electrical, or mechanical
ii. When the cell is stimulated, the electrical charge inside the cell becomes less negative, and depolarization occurs
iii. When the threshold potential is reached, changes occur in the membrane
iv. SA and AV nodes achieve threshold potential first
v. Cell membrane permeability is altered, and specialized channels in the membrane open, which allows the entry of sodium and calcium ions into the cell
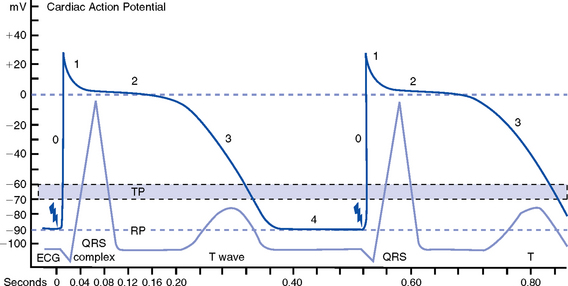
g. Action potential: As cardiac cells reverse polarity, the electrical impulse generated during that event creates an energy stimulus that travels across the cell membrane—a high-speed, short-lived, self-reproducing current (heart only). This is represented on an action potential curve (Figure 3-5).
i. Phase 0—Depolarization: A quick upstroke (several milliseconds) representing the initial phase of excitation
ii. Phase 1—Initial phase of repolarization
iii. Phase 2—Plateau phase of repolarization: Slow inward current of calcium (and, to a lesser extent, sodium); potassium diffuses out of the cells
iv. Phase 3—Last phase of repolarization: Outward current of potassium increases, and the slow, inward current of sodium and calcium decreases. Cells rapidly repolarize, returning to normal RMP.
h. Cardiac pacemaker cells (SA and AV nodes) action potential
i. Pacer cells, having increased automaticity, spontaneously depolarize in phase 4 without a stimulus. Other cells of the heart, having repolarized, require another stimulus to become depolarized.
(a) Rate of automaticity may be altered by increasing or decreasing the slope of phase 4
(b) Increasing the slope speeds heart rate; decreasing the slope slows heart rate
ii. Spontaneous depolarization of pacer cells is caused by a steady influx of sodium and efflux of potassium
i. Refractoriness of heart muscle
i. Absolute refractory period (effective refractory period): Another stimulus to the cell will not produce another action potential (phases 0, 1, and 2 and part of phase 3 of the action potential curve)
ii. Relative refractory period: Only a very strong stimulus can initiate an action potential and cause depolarization (latter part of phase 3)
iii. Supernormal period: Weak stimulus (one that would not normally elicit an action potential) can evoke an action potential and cause depolarization (at the end of phase 3)
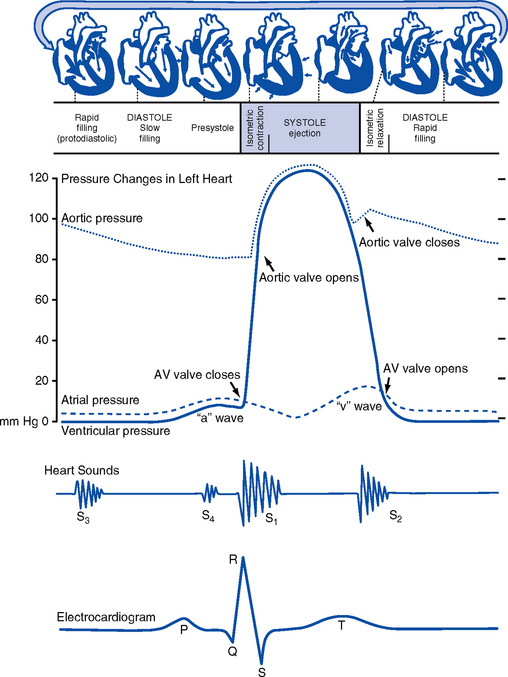
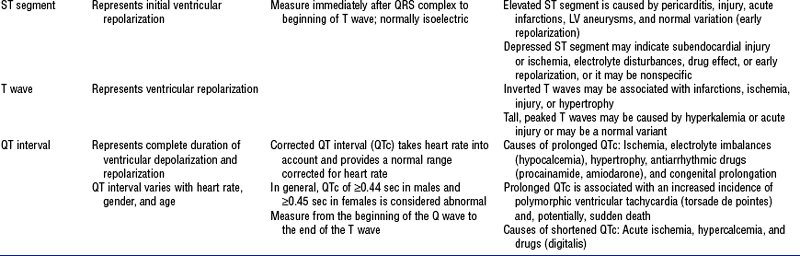
10. Events in the cardiac cycle (Figure 3-6)
a. Ventricular systole: Contraction and emptying of the ventricles
i. QRS complex: Represents ventricular depolarization (an electrical event)
ii. First phase of ventricular contraction (systole) is called isovolumetric contraction. Pressure increases, but no blood is ejected until LV pressure exceeds aortic pressure (and opens the aortic valve).
iii. As pressure rises in the ventricles, the AV valves close, producing the first heart sound (S1, composed of mitral [M1] and tricuspid [T1] components)
iv. The “c” wave of the atrial pressure curve is produced when the AV valves are pushed backward toward the atria as ventricular pressure builds
v. When LV pressure exceeds the pressure in the aorta, the aortic valve opens (comparable events in the RV occur with the pulmonic valve)
vi. Blood is rapidly ejected into the aorta (systolic ejection)
vii. LV pressures decrease, falling below the pressure in the aorta, ventricular ejection stops, and the aortic valve closes. (Comparable events occur in the pulmonary artery, closing the pulmonic valve.)
viii. Closing of the aortic and pulmonic valves produces the second heart sound (S2, composed of aortic [A2] and pulmonic [P2] components)
ix. Aortic valve closure is represented by the dicrotic notch in the aortic pressure waveform
x. Repolarization of the ventricles occurs at this time and produces the T wave on the electrocardiogram (ECG)
xi. After the aortic valve closes, pressure in the LV falls rapidly (isovolumetric relaxation phase); no blood enters the ventricle
xii. LA “v” wave is produced by rapid filling of the atria during ventricular systole, against closed AV valves. This marks the end of systole.
b. Ventricular diastole: Filling phase of the ventricles
i. When pressure is lower in the ventricles than in the atria, the AV valves reopen, which initiates the early rapid filling phase of the ventricles during diastole. This marks the start of diastole.
ii. Pressure in the atria is higher than diastolic pressure in the ventricles, so blood flows from the atria into the ventricles
iii. “a” wave: Atrial pressure rises with atrial contraction
iv. P wave (ECG): In late diastole, represents atrial depolarization (an electrical event)
11. Variables affecting LV function and cardiac output (CO)
a. CO: Amount of blood ejected by the LV in 1 minute
i. CO is the product of stroke volume (SV) and heart rate (HR):
ii. SV is the amount of blood ejected by the LV with each contraction, or the difference between left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV): SV = LVEDV – LVESV; (60 to 130 ml)
iii. Normal resting CO = 4 to 8 L/min
iv. CO is determined by preload, afterload, contractility, and heart rate
b. Preload: The degree to which muscle fibers are lengthened (stretched) prior to contraction
i. In the intact heart, preload is secondary to the volume (size) of the chamber. This is determined by the amount of blood filling the chamber.
ii. Increases in preload increase the CO as described by the Frank-Starling law of the heart
iii. Muscle fibers can reach a point of stretch beyond which contraction is no longer enhanced, and further increases in preload do not yield any further increase in CO
c. Afterload: Initial resistance that must be overcome by the ventricles to develop force and contract, opening the semilunar valves and propelling blood into the systemic and pulmonary circulatory systems (systolic contraction)
i. Factors affecting afterload include arterial resistance (wall stress and thickness), aortic impedance, and blood viscosity
ii. Systemic vascular resistance (SVR) is used as a rough estimate of afterload
iii. To calculate SVR: Mean arterial pressure (MAP) minus central venous pressure (CVP); this number is divided by CO; the resulting value then is multiplied by 80 and converts into dynes/sec/cm−5 (1 dyne is the force that gives a mass of 1 g an acceleration of 1 cm/sec2):
iv. Normal SVR = 900 to 1400 dynes/sec/cm−5
v. Excessive afterload: Increases LV stroke work, decreases SV, increases myocardial oxygen demands, and may result in LV failure
d. Contractility (inotropic state): Heart’s contractile strength
i. There is no way to measure contractility directly. Contractile state can be assessed indirectly through its effects on CO or with noninvasive imaging.
ii. Factors increasing the contractile state of the myocardium include
(a) Use of positive inotropic drugs (e.g., digitalis, milrinone, epinephrine, dobutamine)
iii. Factors decreasing the contractile state of the myocardium include
i. CI is CO corrected for differences in body size (CO of 4 L/min may be adequate for a 100-lb woman but inadequate for a 200-lb man)
ii. Based on body surface area (BSA) as estimated from a height and weight nomogram: CI = CO/BSA
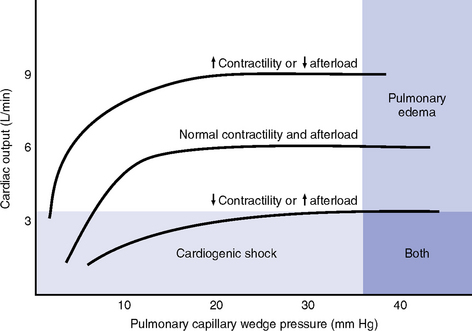
h. Ventricular function curve: Shows how to relate the contributions of preload, afterload, and contractility (but not heart rate) to ventricular function (Figure 3-7)
a. Major functions: Provides tissues with blood, nutrients, and hormones and removes metabolic wastes
b. Resistance to flow: Depends on diameter of vessels (especially arterioles), viscosity of blood, and elastic recoil in vessel walls
c. Circulating blood volume: There is approximately 5 L of total circulating blood volume in the adult body
d. Major components of the vascular system
(a) Strong, compliant, elastic-walled vessels that branch off the aorta, carry blood away from the heart, and distribute it to capillary beds throughout the body
(c) Able to stretch during systole and recoil during diastole because of elastic fibers in the arterial wall
(a) Control systemic vascular resistance and thus arterial pressure
(b) Have strong smooth muscle walls innervated by the autonomic nervous system
(1) Adrenergic (stimulatory) system: Releases two neurotransmitters (epinephrine, norepinephrine). Epinephrine stimulates β-receptors (increases heart rate, increases contractility, dilates arterioles). Norepinephrine stimulates α-receptors (vasoconstriction).
(2) Cholinergic (inhibitory) system: Releases acetylcholine (decreases heart rate; releases nitric oxide, causing vasodilatation).
(a) Tissue bed exchange of oxygen and carbon dioxide and solutes between blood and tissues; site of fluid volume transfer between plasma and interstitium
(b) Gas exchange caused by diffusion. Diffusion of a substance is from an area of high concentration to an area of low concentration until equilibrium is established.
(1) Increased capillary hydrostatic pressure moves fluid from the vessel into the interstitium
(2) Greater capillary osmotic pressure moves fluid from the interstitium into the vessels
(3) Plasma protein concentration in the capillaries provides the osmotic gradient
(4) Retains fluid in the intravascular space
(5) Prevents edema formation in the interstitium
(6) Albumin accounts for 75% of total plasma osmotic pressure; fibrinogen accounts for a small amount
(7) Serum albumin level is a good indicator of a patient’s colloid osmotic pressure
(a) Stores about 65% of total blood volume
(b) Receives blood from capillaries
(c) Conducts blood back to the heart within a low-pressure system
(d) No muscle layer: Veins are compressed by the contraction of surrounding skeletal muscle
(e) Valves in the veins prevent reverse blood flow
(f) Venous pressure in the lower extremities is normally 20 mm Hg or less
13. Control of peripheral blood flow
a. Autoregulation: Ability of the tissues to control their own blood flow (vasodilatation, vasoconstriction)
i. Coronary blood flow remains fairly constant over a wide range of blood pressures
ii. As coronary perfusion pressure drops below 50 mm Hg, autoregulatory ability becomes impaired
b. Autonomic regulation of vessels
i. Vasoconstriction occurs when norepinephrine is released by stimulation of the sympathetic nervous system (adrenergic effect)
ii. Vasodilatation occurs when acetylcholine is released by stimulation of the parasympathetic nervous system (cholinergic effect) or by inhibition of vasoconstriction
c. Stretch receptors: Baroreceptors (pressoreceptors) keep MAP constant
i. Receptor sites in the arteries (aortic arch, carotid sinus, pulmonary arteries, and atria)
ii. Action with increased blood pressure
(a) Respond to stretching of arterial walls
(b) Impulse transmitted from the aortic arch via the vagus nerve to the medulla
(c) Parasympathetic nervous system stimulated, sympathetic nervous system inhibited
(d) Result: Decreased heart rate and contractility, dilation of peripheral vessels, decreased SVR, decreased blood pressure
i. Renin-angiotensin-aldosterone system also helps control arterial pressure (see Chapter 5)
ii. Renin is a protease secreted by the kidneys; converts angiotensinogen to angiotensin I
iii. Renin release from the kidneys is affected as follows:
(a) Decreased blood pressure (i.e., hemorrhage, dehydration, diuretics, sodium depletion) → increases in renin secretion
(b) Rise in sympathetic output (β stimulation) → increases in renin secretion
(c) Fall in sodium concentration → increases in renin secretion (decreased volume)
iv. Angiotensin I is converted to angiotensin II. (These effects are blocked by angiotensin-converting enzyme [ACE] inhibitors.)
v. Angiotensin II, the most potent vasoconstrictor known, is produced when increased renin secretion stimulates its formation
(a) Effects of angiotensin II include the following:
(1) Arteriolar constriction, which increases systolic and diastolic pressures
(2) Stimulation of the adrenal cortex to secrete aldosterone, which causes sodium and water retention
(3) Increase in extracellular fluid volume, which shuts off the stimulus that initiated renin secretion so that blood pressure is maintained at the normal level
(b) Effects of angiotensin II are blocked at its receptors by angiotensin II receptor blockers (ARBs)
b. Pulse pressure: Difference between systolic and diastolic pressures
i. Function of SV and arterial capacitance
ii. Normal pulse pressure: 30 to 40 mm Hg
iii. Changes in SV (with exercise, shock, heart failure) are reflected in similar changes in pulse pressure
c. MAP: Average arterial pressure during the cardiac cycle; dependent on mean arterial blood volume and elasticity of the arterial wall
Patient Assessment
a. Main complaint: Patient’s explanation for seeking medical assistance
b. History of present illness: Ascertain the following:
ii. Onset: Date, time of day, duration, course, precipitating factors
iii. Signs and symptoms: Exacerbations, remissions
(a) Discomfort: Character, location, radiation, quality, duration, factors that aggravate or produce, factors that alleviate
(b) Fatigue: With or without activity
(c) Edema: Location, degree, duration
(d) Syncope and presyncope: Onset (presyncopal warning or sudden event), time and circumstances of occurrence (postural, nonpostural, activity), provocative events (cough, micturition, head movement)
(e) Dyspnea: Orthopnea, paroxysmal nocturnal dyspnea, dyspnea on exertion (determine how much exercise it takes to elicit in number of blocks, flights of stairs, etc.)
(f) Palpitations: Nature, length, associated symptoms
(h) Claudication: Hip or calf? How many blocks can you walk?
c. Medical history: Identify all previous illnesses, injuries, and surgical procedures
i. Patient’s assessment of general health for last several years
ii. Risk factors: Hypertension, hypercholesteremia, smoking, family history of cardiac disease, diabetes
iii. Last medical examination, hospitalizations, prior relevant cardiac tests (e.g., echocardiography, catheterization)
iv. Heart history: Coronary artery disease (CAD), angina, myocardial infarctions (MIs), hypertension, valvular disease, arrhythmias, trauma, peripheral vascular disease, congenital heart defects, heart murmurs, rheumatic fever, cerebrovascular accident (CVA), transient ischemic attacks
i. State of health or cause of death and age at death of immediate family members
ii. Hereditary, familial diseases pertaining to cardiovascular system
i. Present and past work experiences
ii. Level of activity, exercise
iii. Smoking and drinking habits (present, past)
v. Nutrition: Foods eaten, meals per day, who prepares meals
f. Medication history: Identify all prescribed or over-the-counter medications. Determine why and how often the patient is taking drug(s), dosages, any side effects, compliance issues.
g. Allergies: Medications, foods (i.e., shellfish), environmental substances, iodine (potential reaction to contrast medium used during cardiac catheterization procedures)
2. Nursing examination of patient
i. General overall appearance: Skin and mucous membranes
(e) Edema: Found in dependent areas; pitting versus nonpitting (extremities and sacrum)
(a) Pulses: Palpate bilaterally
(1) Check rate, rhythm, character, and volume
(2) Describe pulses, using scale of 0 to 3
(3) Common sites for palpation of arteries
(4) Describe pulse characteristics
a) Normal pulse character: Smooth, rounded
b) Pulse deficit: Inability to palpate all contractions of the heart
1) Premature or rapid contractions may not generate a peripheral pulse
2) Determine by comparing radial pulse with auscultated apical pulse; record difference in rates
c) Pulsus parvus et tardus: Small (parvus) pulse with delayed (tardus) slow upstroke and prolonged downstroke. Noted in
1) Aortic stenosis: Parvus and tardus
2) Mitral stenosis: Only parvus
d) Pulsus alternans: Pulse waves alternate, every other beat is weaker; caused by impaired myocardium; noted in severe LV failure
(1) Sphygmomanometer: Key points
b) Positioning of cuff: No less than 2.5 cm from the antecubital fossa
c) Falsely low measurement: Cuff too large for arm, arm above heart level, inability to accurately hear first Korotkoff sound
d) Falsely high measurement: Cuff too small for arm, loose cuff not centered over brachial artery, arm below heart level
(2) Take blood pressure in both arms. More than a 10- to 15-mm Hg difference in systolic pressures indicates diminished arterial flow on the side with the lower reading (obstruction, dissection)
(3) Orthostatic blood pressure drop: Assess at-risk patients
a) Check blood pressure supine, sitting, standing
b) Fall of more than 20 mm Hg of systolic pressure signifies orthostatic hypotension
(4) Pulsus paradoxus: Exaggeration of the normal physiologic response to inspiration (blood pressure lower on inspiration than on expiration)
a) Examine with the patient breathing normally
b) Inflate sphygmomanometer until no Korotkoff sounds are heard; slowly deflate cuff until Korotkoff sounds first heard on expiration; note pressure reading
c) Continue to deflate cuff until sounds heard during both expiration and inspiration; note reading
d) Subtract second reading from first to determine pulsus paradoxus
e) Normally, on inspiration, the difference between inspiration and expiration is less than 11 mm Hg. With pulsus paradoxus, fall in blood pressure on inspiration is 11 mm Hg or greater.
(a) Neck veins give important clues regarding fluid status
(1) Jugular veins reflect RA and RV filling pressures
(2) Internal jugular veins are harder to visualize than external jugular veins, but they more accurately reflect pressure and volume changes in the RA (central venous pressure)
(3) Check for distention and pulsation
a) Elevate the head of the bed until jugular waves can be seen
b) Shine a bright light tangentially to illuminate vessels, if not obvious
(4) Determine jugular venous pressure
a) The sternal angle (angle of Louis) is roughly 5 cm above the atrium (when the patient is upright or lying down)
b) Measure the distance in centimeters from the sternal notch to the top of the distended neck vein
c) The value obtained plus the 5 cm provides a rough estimate of central venous pressure
(b) Check for hepatojugular reflux
(1) Place the patient at a 45-degree angle
(2) Compress the upper right abdomen for 30 to 45 seconds (causes additional venous return from liver to heart)
(3) If hepatojugular reflux is present, the jugular pulses become more pronounced, and the level of filling of neck veins will rise (signifies inability of the right side of the heart to deal with the added volume)
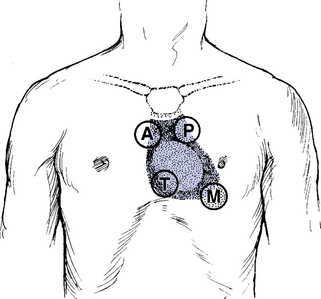
(a) Palpate three areas: Base, apex, and left sternal border; check for
(1) Pulsations (e.g., the point of maximal intensity [PMI]; the patient must be supine)
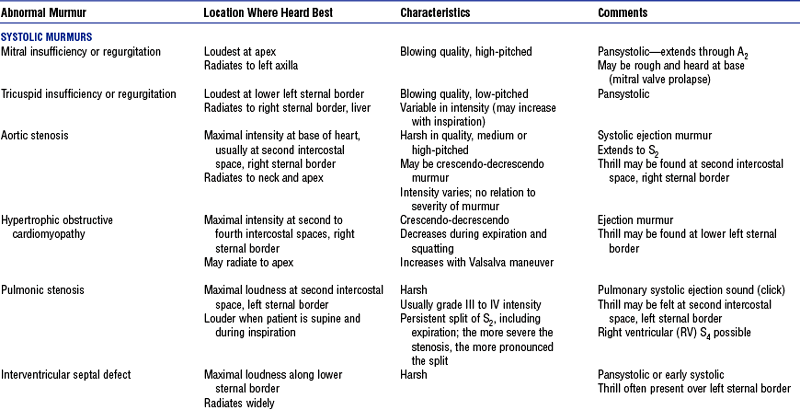
(2) Thrills (palpable vibrations, analogous to the sensation felt on the throat of a purring cat) signify turbulence or murmur loud enough to feel (aortic stenosis, mitral stenosis, PDA, ventricular septal defect [VSD])
(3) Left peristernal lift: Suggests RV dilatation
(4) Apical impulse (PMI in the normal heart): Not always easy to palpate
a) Normally located at the fifth left intercostal space, midclavicular line, and is approximately 2 cm in size
b) PMI that can be palpated over two or more intercostal areas signifies diffuse PMI resulting from
1) LV dilatation (LV volume overload)
2) Aortic insufficiency, mitral regurgitation, dilated cardiomyopathy
c) A forceful, sustained apical impulse indicates LV hypertrophy
d) Nonsustained but forceful apical impulses are created by high-output states (fever, anemia, anxiety, hyperthyroidism)
a) Bell: Use to hear low-pitched sounds such as ventricular filling sounds (S3 and S4) and filling rumble of mitral and tricuspid stenosis
b) When using the bell of the stethoscope, press only hard enough to create a seal; otherwise, underlying skin functions as a diaphragm and low-pitched sounds will not be heard
c) Diaphragm: Use to hear high-pitched sounds such as heart sounds S1 and S2, ejection clicks, opening snaps, and most murmurs
d) Usual listening positions: Supine, left lateral decubitus position, sitting up, and leaning forward
(2) Origin of heart sounds: Opening and closing of valves (see Figure 3-6) and rapid acceleration or deceleration of blood produce either low- or high-pitched sounds
a) First heart sound (S1): Produced by mitral and tricuspid valve closure
1) Marks the onset of ventricular systole
2) LV depolarizes and contracts before the RV
4) Component parts of S1 may be split (mitral component [M1] before tricuspid component [T1])
b) Second heart sound (S2): Produced by aortic and pulmonic valve closure
1) Listen with diaphragm at the pulmonic area
2) Both component parts of S2 may be heard: Aortic component [A2] before pulmonary component [P2]. Normal P2 is heard only at pulmonic area.
d) Physiologic (normal) split of S2 (A2P2)
1) P2 is delayed on inspiration when the RV is slower to contract than the LV
2) Delay due to increased volume loading of the RV in inspiration caused by increased venous return to the heart
3) RV ejection of blood is prolonged and delays pulmonic valve closure, prolonging the time from aortic closure (A2) to pulmonic closure (P2). A resulting split occurs between A2 and P2 during quiet inspiration.
4) A2 precedes P2 and is generally louder
5) Split of S2 is heard only over the pulmonic area
6) Best heard in quiet respiration when the patient is sitting or standing
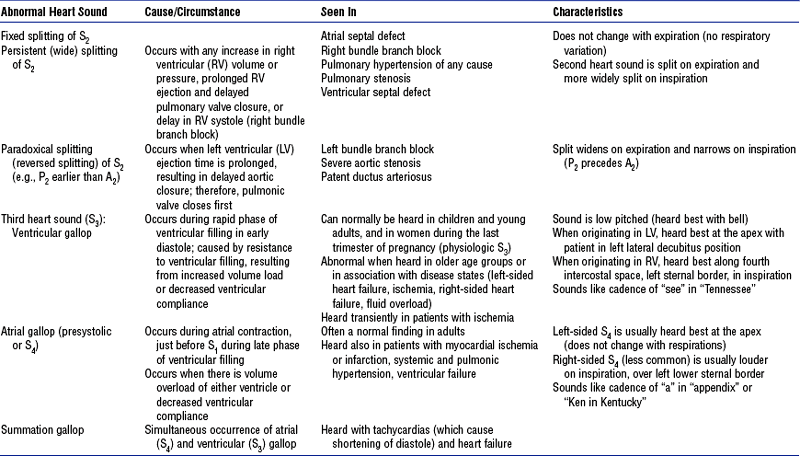
(4) Abnormal heart sounds: See Table 3-1
a) Ejection clicks: Sharp, high-pitched sounds just after S1; caused by tensioning of the great vessels as they distend in early systole
1) Are like leather rubbing or new snow crunching
2) Should be heard with the diaphragm in full expiration (are loudest when the patient is leaning forward)
3) Often have three components (ventricular systole, ventricular filling, and atrial systole)
c) Opening snaps: Sounds produced by a stenotic mitral valve snapping into the open position
d) Prosthetic valves: Crisp, sometimes metallic clicking, with both opening and closure heard
vi. Abdominal examination: Note the following
vii. Extremities examination: Note the following
(a) Edema: Indicates right-sided heart failure, venous stasis, venous insufficiency
(b) Color, temperature changes: Indicate arterial insufficiency (especially if asymmetrically cool)
(c) Skin condition: Petechiae, jaundice
(d) Hair loss: Indicates arterial disease
(e) Ulcerations: Indicates stasis, ischemia
(f) Peripheral pulses: Check for bruits
(g) Motor and sensory function: Numbness, foot drop (in advanced peripheral ischemia)
(h) Clubbing of nail beds: Cyanotic congenital heart defects
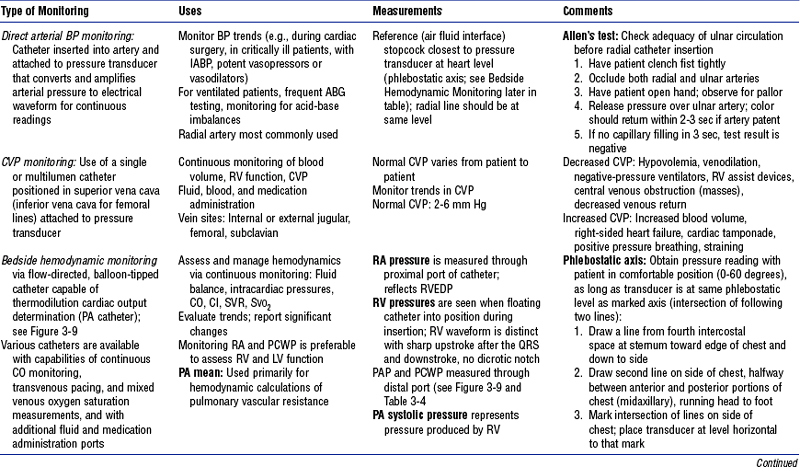
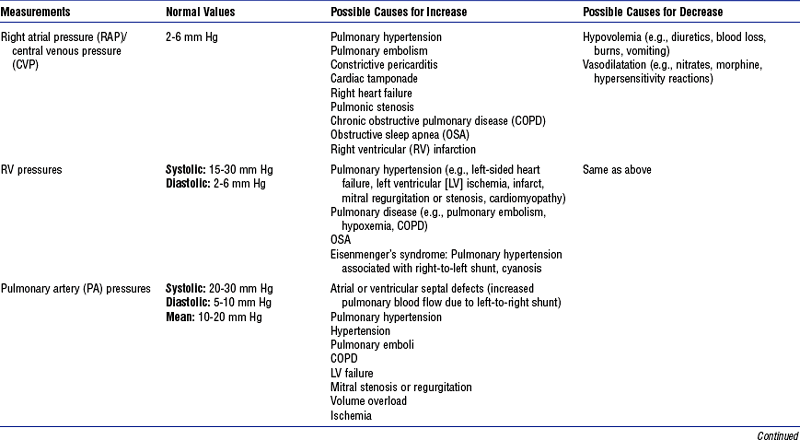
b. Monitoring data (Figure 3-9)
i. See Table 3-3 for types of bedside monitoring and Table 3-4 for hemodynamic pressures
ii. Complications of bedside hemodynamic monitoring
iii. To prevent complications associated with these monitoring catheters, ensure the following:
(a) Balloon is deflated after wedge pressure is obtained, to prevent pulmonary infarction
(b) Catheter has not wedged when it is deflated or has not slipped back into the RV (risk of ventricular tachycardia [VT])
(c) Only 0.8 to 1.5 ml of air is used to inflate the balloon (with 3-ml syringe): Risks of balloon overinflation include possible rupture, emboli, infarctions
(d) Catheter is inserted under sterile technique (use catheter guard to cover catheter)
(e) Introducer sheath is sutured to the skin to prevent catheter migration, which can cause ventricular ectopy; possible perforation of RA, RV, or pulmonary artery; or pulmonary infarction
(f) Pressurized, heparinized drip is used to maintain patency and to prevent both clot formation at the end of the catheter and possible embolization
(g) Electrical equipment is well grounded and operating correctly (to prevent electrically induced ventricular fibrillation [VF])
3. Appraisal of patient characteristics
i. Level 1—Minimally resilient: Mr A., an 82-year-old male with severe aortic stenosis in rapid atrial fibrillation and recovering from a CVA
ii. Level 3—Moderately resilient: Mrs. B., a 71-year-old obese female with history of insulin-dependent diabetes and hypertension. She is admitted with a blood pressure of 195/110 mm Hg, sinus tachycardia, atypical chest pain, and abdominal discomfort.
iii. Level 5—Highly resilient: Mr. C., a 45-year-old male with a history of hypertension, admitted complaining of left-sided chest and shoulder pain. Cardiac catheterization results are normal.
i. Level 1—Highly vulnerable: Mr A., whose family reports that he lost his wife (of 60 years) 4 days earlier when she suddenly collapsed at home. Results of his transesophageal echocardiography show several large clots in the LA.
ii. Level 3—Moderately vulnerable: Mr. D., a 53-year-old male with a history of sudden cardiac death (requiring implantation of a cardiac defibrillator), ulcerative colitis, pericardial effusion, and steroid-induced diabetes. He is anemic, with tarry stools. His wife is also very anxious and requires a great deal of attention.
iii. Level 5—Minimally vulnerable: Mrs. E., 48 years old, admitted with acute pericarditis, responding well to an oral nonsteroidal antiinflammatory drug [NSAID]; her pain is resolving, and her condition is stable
i. Level 1—Minimally stable: Mr. F., a 47-year-old male with history of scleroderma, Raynaud’s disease, and severe pulmonary hypertension. He is presently on intravenous (IV) dobutamine. He has 2+ edema bilaterally in his lower extremities. His lungs have crackles in the bases. He is being evaluated for epoprostenol (Flolan) home therapy to assist in improving his quality of life. He is not eligible for heart transplantation.
ii. Level 3—Moderately stable: Mrs. G., a 78-year-old female with a history of ischemic cardiomyopathy, severe mitral regurgitation, hyperlipidemia, and three-vessel disease. Complained of chest pain at home, relieved with nitroglycerin.
iii. Level 5—Highly stable: Mr. H., a 65-year-old male with a history of third-degree AV block, who received a permanent pacemaker 2 days earlier. His incision site is healing well, and he has minimal discomfort. The pacemaker is functioning appropriately.
i. Level 1—Highly complex: Miss P., a 27-year-old female with a history of open heart transplantation 7 years ago and MI 3 years ago. Has had numerous coronary interventions, including recent placements of stents to her LCA. She has a history of a permanent pacemaker, asthma, hypertension, heart failure, and chronic renal insufficiency. She is admitted with a large pericardial effusion.
ii. Level 3—Moderately complex: Mr. S., 63 years old, with a history of open heart transplantation 10 years ago, hospitalized 1 month ago for endocarditis. Admitted from skilled care with an infected right Hickman catheter, an elevated temperature, and a heart rate of 105 beats/min with frequent premature ventricular contractions, and is on several IV inotropic agents; urinary output is marginal.
iii. Level 5—Minimally complex: Mrs. V., who had acute-onset atrial tachycardia and was given IV medications, after which her heart rhythm converted into sinus rhythm. She is up in her room ready for instructions before going home.
i. Level 1—Few resources: Mrs. B., who lives alone and whose family is gone. It is physically hard for her to leave her apartment. She has a small income and cannot afford the combination of drugs prescribed for her hypertension, diabetes, and hyperlipidemia.
ii. Level 3—Moderate resources: Mr. L., 60 years old, with a history of coronary artery bypass graft 3 years ago, who experienced chest discomfort while at his cardiac rehabilitation group. His wife and family are supportive and involved in his care.
iii. Level 5—Many resources: Mrs. R., a 65-year-old woman with MI with ST-segment elevation, who had a percutaneous coronary intervention (PCI) and was given thrombolytics 90 minutes after the onset of symptoms. She is married to a staff physician and her daughter is a nurse.
i. Level 1—No participation: Mrs. Q., a 68-year-old obese female who experienced cardiac arrest on a medical floor and has a history of end-stage renal disease, diabetes, and hypertension. She is unconscious and has a full code status.
ii. Level 3—Moderate level of participation: Miss P., who, although she is a patient with a highly complex condition, is very interested in her diagnosis, medication, and therapy. She attempts to watch her intake and diet. Her parents are also involved in all care aspects. They do, however, verbalize some inaccurate medical information leading to erroneous conclusions.
iii. Level 5—Full participation: Mrs. M., a 40-year-old female with pulmonary hypertension who is awaiting heart transplantation. She is independent in her care activities. She and her husband take full advantage of educational resources and talk with transplant patients.
g. Participation in decision making
i. Level 1—No participation: Mr. T., 18 years old, homeless, victim of a stab wound to the chest, intubated, unconscious, brought to the operating room for emergency thoracotomy
ii. Level 3—Moderate level of participation: Mr. O., a 60-year-old male admitted with chest pain due to a reoccluded coronary lesion, who decided he did not need to continue his clopidogrel after discharge from the hospital
iii. Level 5—Full participation: Mr. F. (in a highly unstable condition), who with his family is actively involved in end-of-life decision making. In conferences with the physicians and nurses, he and his family have requested that he not be intubated or resuscitated if the situation presents. They want Mr. F. to have only comfort measures.
i. Level 1—Not predictable: Mrs. G., who received an automatic implantable cardiac defibrillator during her hospitalization. The day after her discharge home, her defibrillator discharged eight times throughout the evening and night. She is readmitted to reevaluate medical therapy.
ii. Level 3—Moderately predictable: Mr. H., 49 years old, who was admitted for another MI (had his first MI when he was 37 years old) and received brachytherapy and a stent to the CX artery. Eptifibatide (Integrelin) is infusing. Vital sign are stable.
iii. Level 5—Highly predictable: Mr. X., who has a small VSD that does not require surgery. Staff stressed the importance of good dental care and prophylactic antibiotics because of the high risk of infectious endocarditis.
i. Cardiac troponin T and troponin I: Group of compounds that bind to tropomyosin and help with excitation-contraction in muscle
(a) Most sensitive cardiac markers; very specific, mark injury to myocytes (not just cell death)
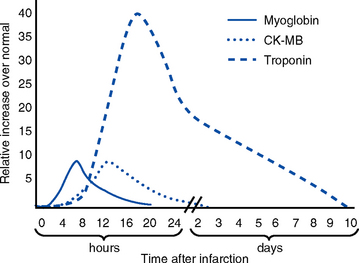
(b) Levels rise 4 to 6 hours after the onset of ischemic symptoms; peak at 18 to 24 hours after MI; fall slowly, over up to 2 weeks (Figure 3-10)
(c) Cleared by kidneys, so levels are elevated in chronic renal failure
(d) Rare for the value to be more than 0.1 ng/ml in a normal individual
(e) Facilitate quicker decision making in identification, risk stratification, and treatment of patients
(f) Predictor of high risk for subsequent cardiac events in acute coronary syndromes, postoperative vascular surgery; prognostic indicator for pulmonary embolus
ii. Creatine kinase (CK): CK-MB isoenzyme
(a) Enzyme associated with adenosine triphosphate conversion in contractile muscle tissue; found in the heart, brain, and skeletal tissues
(b) MB isoenzyme levels very sensitive for cardiac tissue; not as sensitive in early MI less than 6 hours after onset
(c) Level rises 4 to 8 hours after onset, peaks 12 to 24 hours after MI, returns to normal in approximately 24 to 48 hours
(d) CK-MB concentration of more than 5% of total CK indicates myocardial necrosis
iii. Myoglobin: Heme-containing protein
(a) Very sensitive marker but not cardiac specific; can be elevated with cardiopulmonary resuscitation (CPR), falls, and injections
(b) Level peaks 8 hours after infarct, then rapidly returns to normal in 18 to 24 hours
(c) Useful in the emergency department for early MI detection, ruling out of MI, reperfusion monitoring
iv. Brain natriuretic peptide (BNP)
(a) Released with myocardial stretch
(b) Level correlates with LV dysfunction
(c) Used to evaluate heart failure (both LV and RV) and pulmonary emboli and to differentiate cardiac from pulmonary causes of pulmonary distress and dyspnea
(d) Higher levels indicate poor prognosis
(e) Falsely low results can occur in obese patients due to clearance in adipose tissue (adipose tissue removes BNP from the circulation)
(f) Falsely high results can occur in the elderly, hypertensive individuals, females, and patients being given nesiritide
(b) Independent predictor of future cardiovascular risk
(c) Elevations may also indicate an acute infection, inflammatory disease processes, possible uremia
(a) Partial thromboplastin time (PTT): Used for monitoring heparin levels
(b) International normalized ratio (INR): Used for measuring the effectiveness of anticoagulant therapy
(1) For atrial fibrillation, therapeutic range for INR is 2.0 to 3.0
(2) For prosthetic valves, therapeutic range for INR is 2.5 to 3.5
(c) Activated clotting time (ACT): Bedside test done to measure the time required for blood coagulation
vii. Complete blood cell count (CBC), hemoglobin (Hb) level, hematocrit (HCT)
viii. Electrolyte levels, blood urea nitrogen (BUN) level, creatinine level, glucose level
ix. Fasting serum lipid profile: Normal levels
(a) Total cholesterol level: Goal is less than 200 mg/dl
(b) High-density lipoprotein (HDL): Goal is HDL level above 40 mg/dl
(c) Low-density lipoprotein (LDL): Level of less than 100 mg/dl is optimal if CAD is present (otherwise, 130 mg/dl is the goal)
(d) Triglycerides: Desired level is less than 150 mg/dl; 150 to 199 mg/dl is borderline; 200 to 499 mg/dl is high; more than 500 mg/dl is very high
(e) Lipoprotein(a): Marker for CAD, not usually followed; levels of more than 30 mg/dl associated with CAD
x. Homocysteine level: To identify folic acid–responsive hyperlipidemia. Normal level is 5 to 15 μmol/L. Elevated levels considered an independent risk factor for CAD.
i. Chest radiograph is used to visualize
(a) Cardiac size, position, chamber size
(b) Abnormalities of the heart, great vessels, lungs, pleura, and ribs
ii. Magnetic resonance imaging (MRI): Safe diagnostic technique involving no ionizing radiation. Used with contrast to produce a magnetic resonance angiogram (MRA).
(a) Provides a three-dimensional view of cardiovascular structure
(b) Creates a computer-assisted image, measures tissue proton density
(c) MRI is used to determine or identify
(d) Safe alternative to radiography for children and pregnant women
(e) As a magnetic device, MRI machine interferes with pacemaker function
(f) Not used for patients with prosthetic metallic devices (valves, prosthetic joints). Can displace or damage such devices due to powerful magnet
iii. Ultrafast (electron beam) computed tomography (EBCT): Form of computed tomography (CT) in which a rapid electron beam is used for high-speed imaging
(a) Provides two-dimensional image of cardiovascular structures
iv. Myocardial imaging: Radioisotope is injected into a peripheral vein and its cardiac uptake can be imaged. Methods include
(a) Multiple-gated acquisition (MUGA) scan
(1) Used to measure the EF of the LV
(2) Very accurate unless the patient has an irregular rhythm, because multiple images cannot be “gated” (superimposed) on ECG
(b) Myocardial scintigraphy (perfusion imaging)
(1) Involves the use of thallium-201 or technetium Tc 99m sestamibi to identify ischemia, infarct, and myocardial viability
(2) Normal myocardium takes up the isotope from the blood
(3) Decreased myocardial perfusion results in decreased uptake; ischemic sites show normal uptake at rest and decreased uptake on exercise. Infarcted sites show no uptake at all.
(4) Pharmacologic agents (dipyridamole, adenosine) are used to simulate the effects of exercise (potentially to induce ischemia) in patients who are unable to exercise
(5) Perfusion imaging is more sensitive and specific (80% to 85%) than exercise ECG–stress testing (70%)
(c) Infarct-avid imaging (myocardial infarct indicators)
(1) Technetium Tc 99m pyrophosphate is injected into a peripheral vein
(2) Infarcted areas of the heart show increased levels of radioactivity as “hot spots.” These appear within 4 hours of infarction, may not peak until 12 to 24 hours later, and remain positive for 2 to 7 days.
(3) Limited usefulness in acute MI; useful when ECG changes are not definitive or when enzyme levels have already returned to normal
v. Cardiac catheterization and angiography: High-quality coronary images produced by x-ray digital imaging. Radiopaque contrast medium is injected into the coronary arteries for visualization; recordings are made on digital media. Still photographs may be produced for patient records.
(1) Right-sided heart catheterization performed via the right or left femoral or brachial vein with the catheter advanced into the RA and then past the tricuspid valve through the RV into the pulmonary artery to record pressures, perform angiography, determine CO and resistances, and define anatomy
(2) Left-sided heart catheterization performed in a retrograde manner via the femoral or brachial artery (or transseptal approach through the RA and intraatrial septum); catheter is advanced into the LV to determine pressure
(1) Technique: Radiopaque contrast medium is injected into the LV cavity
a) Evaluate ventricular wall motion and chamber size
1) Identify akinetic areas and wall motion abnormalities
2) Detect hypokinetic areas: Weaker than normal contractions in systole
3) Detect dyskinetic areas: Areas bulge outward during systole instead of contracting
b) Assess function, determining
c) Detect ventricular aneurysms
d) Evaluate mitral, aortic valves
e) Demonstrate ventricular-level left-to-right intracardiac shunts (VSD)
(1) Radiopaque contrast material injected into the ostia of the LCA and RCA, allowing multiple views and recordings of coronary arterial circulation
a) To assess the extent of significant CAD by identifying the presence and severity of lesions
b) To guide therapeutic options in ischemic heart disease
c) To assess possible coronary arterial spasm
d) To administer intracoronary thrombolytics and drugs
e) To perform transcatheter interventional procedures: PCI, coronary stent placement, rotational atherectomy, and percutaneous transluminal coronary angioplasty (PTCA)
(f) Complications of diagnostic cardiac catheterization
(1) Death: Approximately 0.11% incidence (most frequently seen in left main disease)
(2) MI: Approximately 0.05% incidence
(3) Neurologic events (stroke): Approximately 0.07% incidence
(4) Acute renal failure or oliguria caused by contrast medium and inadequate hydration
(5) Transient cardiac arrhythmias, bradycardia, conduction disturbances
(6) Hemorrhage or hematoma at insertion site
(7) Allergic reactions to contrast medium
(8) Arterial perforation, thrombosis, embolus, and dissection
(9) Hypovolemia (due to diuresis from contrast medium and nothing-by-mouth [NPO] status)
i. ECG: Records the electrical activity of the heart
(1) Arrhythmias and conduction defects
(5) Hypertrophy of the ventricles and enlargement of the atria
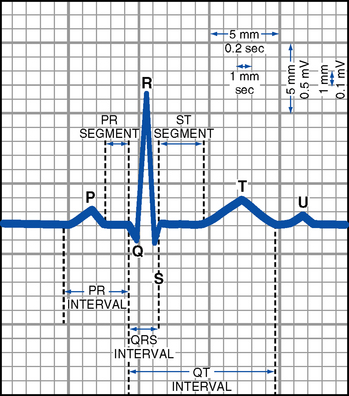
FIGURE 3-11 Normal electrocardiogram complex.
(c) Deflections: Waves of the ECG recording are either above or below the isoelectric line
(1) Positive deflections occur when the heart’s depolarization wave moves toward the positive electrode of the recording lead
(2) Negative deflections occur when the heart’s depolarization wave moves away from the positive electrode of the recording lead
(3) Biphasic deflections occur when the heart’s depolarization wave is moving both toward and away from the positive electrode. If the wave of depolarization is perpendicular to the positive electrode, waves may be small or absent.
(d) ECG waves: Representation, measurement, abnormalities—see Table 3-5, Figure 3-11
TABLE 3-5
Electrocardiogram (ECG) Waves and Intervals: Description, Characteristics, and Abnormalities
(e) 12-Lead ECG (Box 3-3 and Figure 3-12)
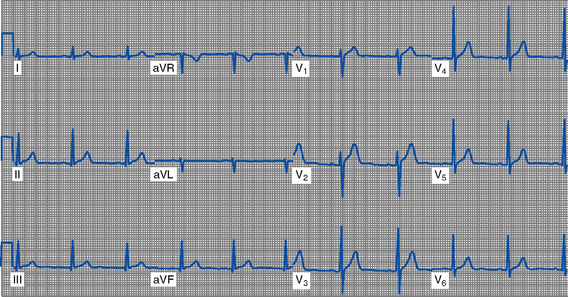
FIGURE 3-12 Twelve-lead electrocardiogram (sinus rhythm).
(1) Standard limb leads: Two electrodes of opposing polarity (positive and negative) are used to record electrical activity. These bipolar electrodes are placed on the patient’s arms and/or legs.
(2) Augmented limb leads: Electrical activity is recorded between one positive electrode (unipolar) and the electrical sum of other two standard limb electrodes. The wave’s amplitude is augmented (enhanced voltage) for ease of visualization.
(3) Precordial (chest) V leads: Record electrical activity between one positive electrode (unipolar) and the electrical sum of the three standard limb electrodes.
(f) Miscellaneous monitoring leads
(1) Modified chest lead (MCL1)
a) Electrode placement: Similar to lead V1
b) Typical pattern of PQRST is negative
c) Helps differentiate ventricular arrhythmias from supraventricular arrhythmias with right bundle branch block (RBBB) aberrancy
1) LV origin of VT is likely with Rsr′ pattern: left peak is taller than right peak of the “rabbit ears”
2) Aberrant conduction of SVT is likely if the first beat of a run of ectopic beats has an rsR′ pattern: right peak of “rabbit ears” is taller
d) Differentiation between RV and LV ectopy is possible
e) RBBB and left bundle branch block (LBBB) may be differentiated
(2) Lewis lead: Used for finding P wave; arm leads are positioned along the sternal border until P waves become evident
(3) Transvenous leads or esophageal pill leads (a small gelatin capsule enclosing an electrode and attached to thin wire) are helpful in looking for P waves or differentiating supraventricular from ventricular rhythms
(g) See Box 3-4 for ECG and rhythm assessment checklist
(h) See Table 3-6 for descriptions of cardiac arrhythmias and conduction defects
TABLE 3-6
Cardiac Arrhythmias and Conduction Defects
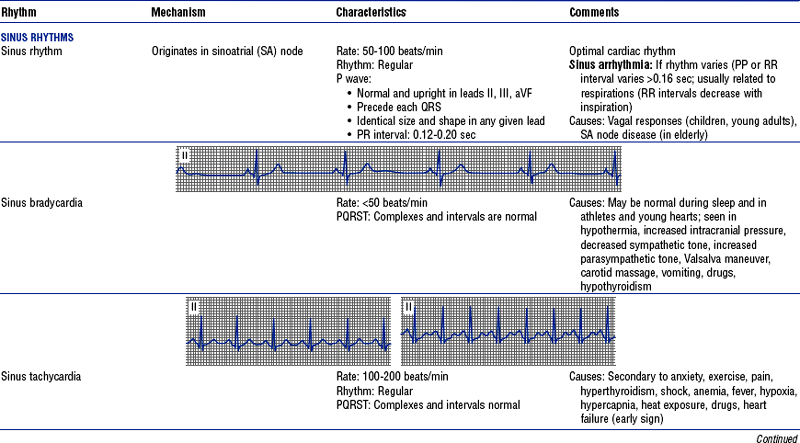
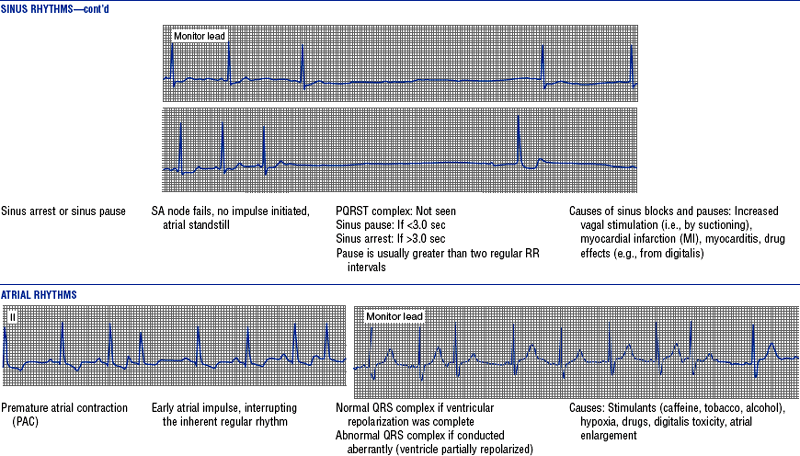
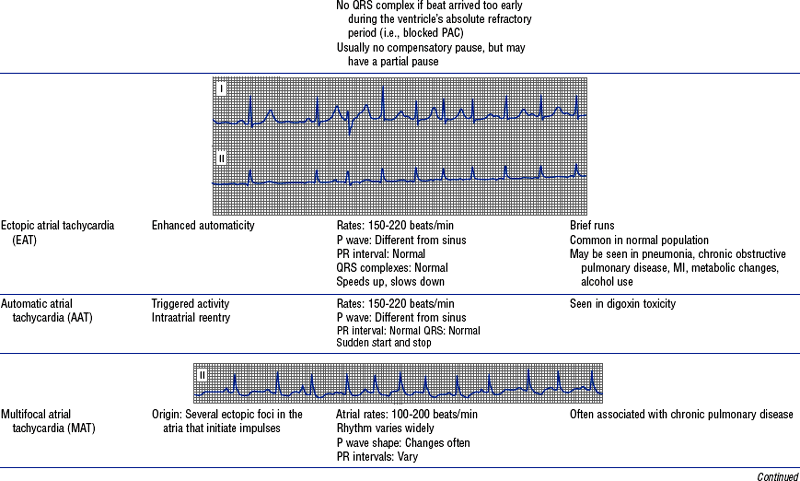
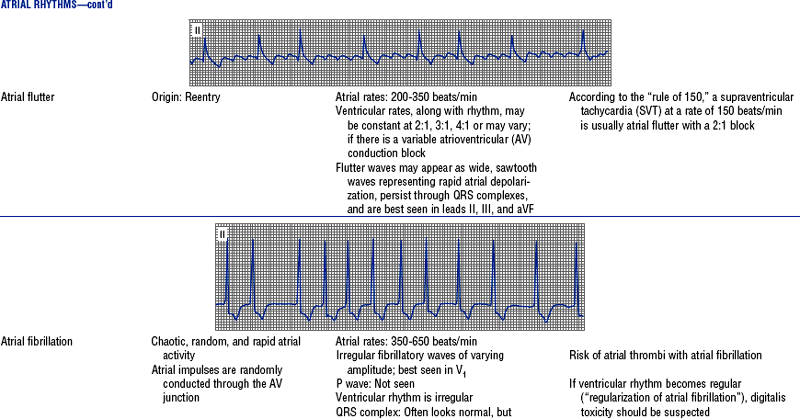
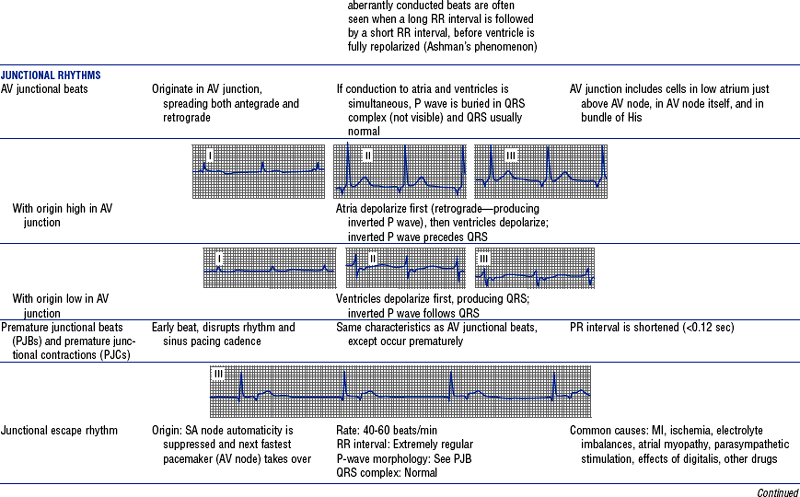
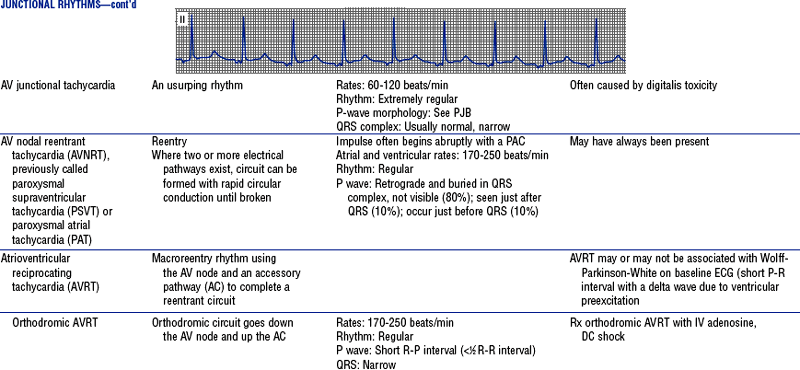
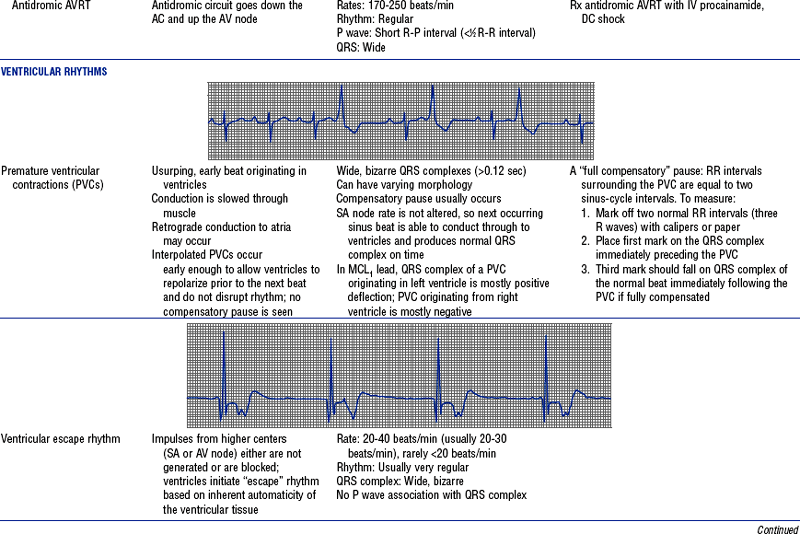
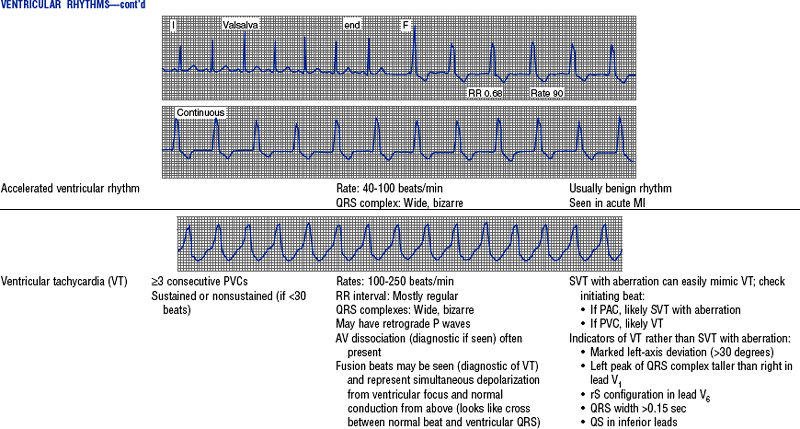
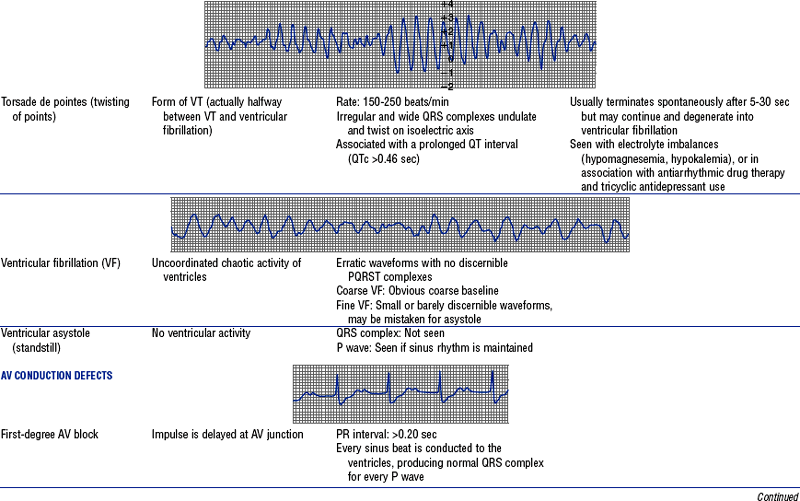
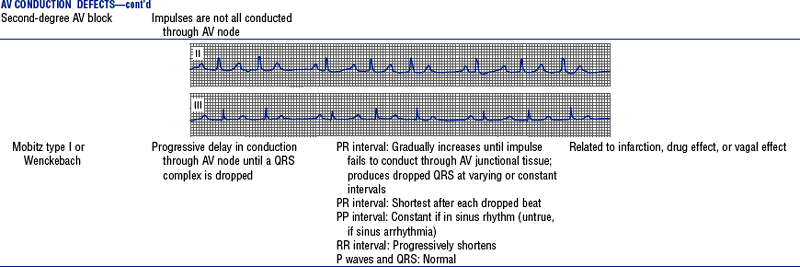
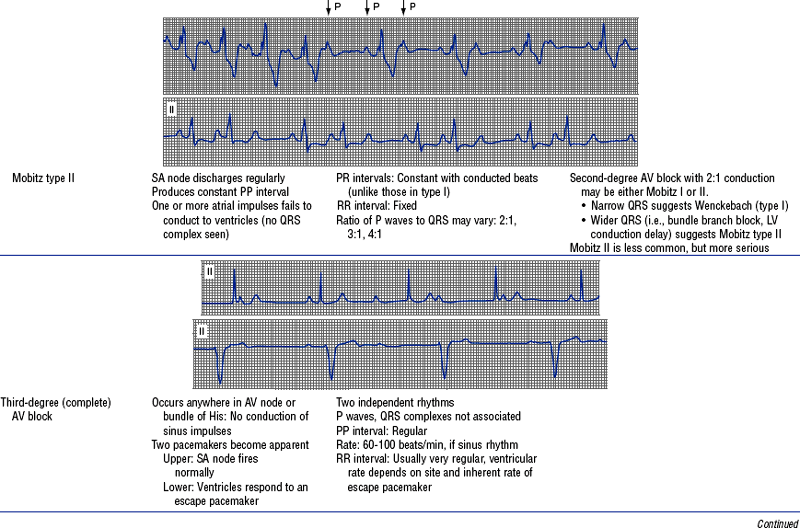
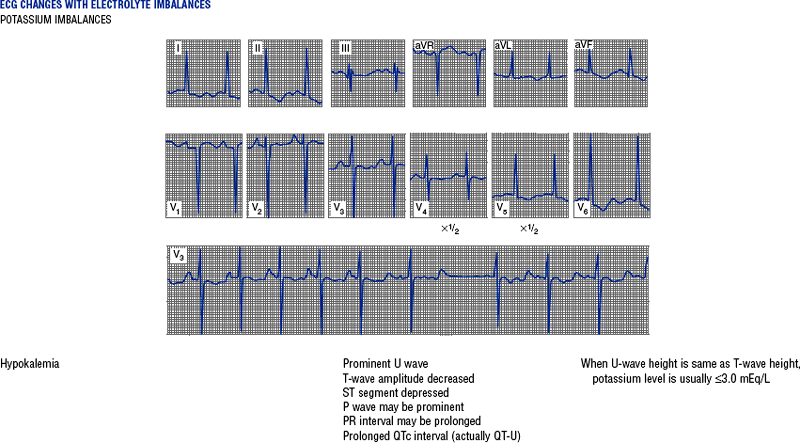
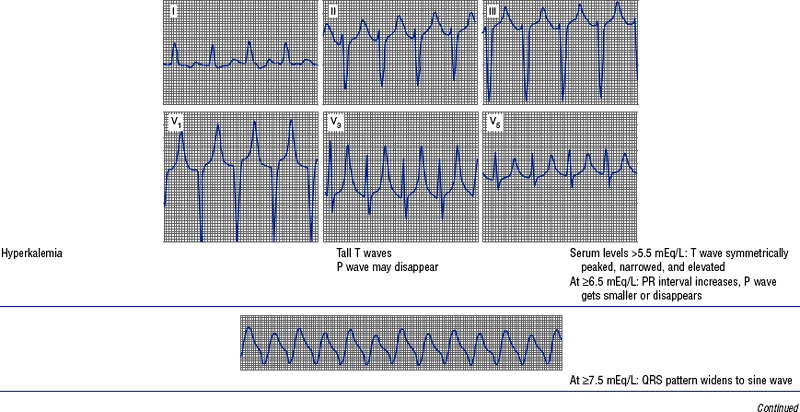
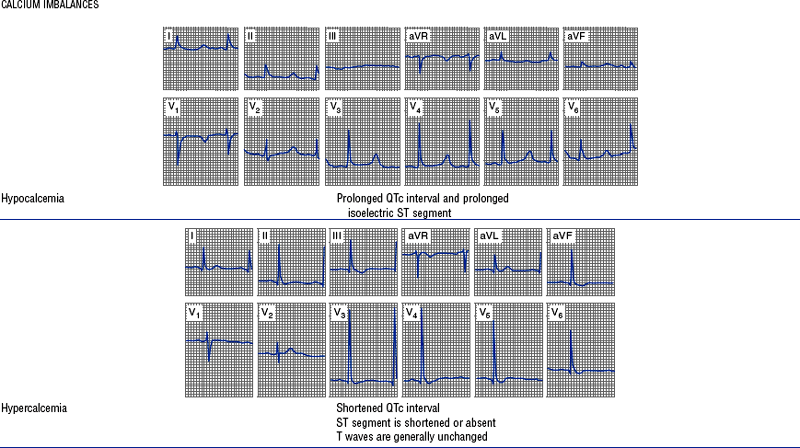
Figures from Chou T, Knilans TK: Electrocardiography in clinical practice, ed 4, Philadelphia, 1996, Saunders.
ii. Echocardiography: One of the most important noninvasive tools
(a) High-frequency ultrasonic vibrations are emitted via a transducer on the patient’s chest or in the esophagus. The returning echo of sound waves is received. An image is created and recorded for interpretation.
(1) Chamber size and function, including
(2) Valvular morphology and function
(3) Intracardiac masses, including tumors, thrombi, and vegetations
(c) M mode: Used to measure intracardiac dimensions; measures chamber size and wall thickness
(d) Two-dimensional echocardiography provides real-time imagery of the heart and its structures using a two-dimensional ultrasonic beam
(e) Doppler echocardiography is used to demonstrate the velocity and direction of blood flow through the heart and great vessels
(f) Color flow imaging: Doppler signals are processed to depict real-time velocities superimposed on a two-dimensional echocardiogram. Red represents flow toward the transducer; blue represents flow away from the transducer. Lighter shades mean higher velocity. Used to evaluate shunts, regurgitation, stenosis.
(1) Bubble echo (agitated saline): Used to detect congenital or acquired shunts, patent foramen ovale
(2) Enhancing agents (e.g., Optison): Used to enhance the LV endocardial border and help identify myocardial infarct or ischemia
(h) Stress echocardiography: Images obtained before, during, and after exercise or pharmacologic stress (states of increased myocardial oxygen demand)
(1) Methods used include treadmill (most common) and pharmacologic stressing (dobutamine, dipyridamole, adenosine)
(2) Ischemia results in a region of hypokinesis (decreased wall motion)
(3) Evaluates extent and location of CAD and ischemic mitral regurgitation
(i) Transesophageal echocardiography (TEE): Transducer is placed in the esophagus
(1) Capable of exquisite definition of cardiac structure and function because of the proximity of the transducer to the heart
(2) Used in the operating room when the adequacy of valvular repair is to be evaluated and when more detail is needed (in cases of prosthetic valves or suspected patent foramen ovale, LA thrombus, or aortic dissection; in patients with poor acoustic penetration of the ultrasonic signal from the chest, such as patients with chronic obstructive pulmonary disease or a heavy build)
iii. Intravascular ultrasonography (IVUS): A small ultrasonic transducer attached to a catheter tip is threaded into the coronary artery over a guidewire. It provides high-resolution images of the inside of the artery. Invaluable in the catheterization laboratory for interventional procedures. Assesses the following:
iv. Exercise electrocardiography (exercise stress testing)
(a) ECG is taken during exercise
(b) Indications for exercise testing
(3) To perform a functional assessment in patients known to have CAD (such as patients who have had an MI or angioplasty, or are undergoing coronary bypass surgery) to assess risk, severity, and prognosis
(4) To evaluate the effectiveness of revascularization or medical therapy for CAD
(5) To evaluate arrhythmias, especially exercise-induced VT
(6) To evaluate patients with rate-responsive pacemakers
(7) To screen persons entering physical fitness programs or high-risk professions (e.g., airline pilots) for CAD
(c) Contraindications to exercise testing
(2) Unstable (preinfarction) angina (on effort)
(3) Uncompensated heart failure (HF)
(5) Uncontrolled, severe hypertension
(6) Severe illness such as fulminant infection, asthma, renal failure
(1) Patient must be able to exercise to at least 85% of the maximum predicted heart rate to have a successful test
(2) Resting ECG must have normal ST segments at baseline
(3) Test is insensitive to single-vessel disease (will miss 40% of such cases, giving a false-negative result)
(4) False-positive results are frequent in patients at low risk for CAD, patients taking digoxin, and patients with LV hypertrophy
v. Long-term ambulatory monitoring
(1) Holter monitor: ECG continuously recorded over a 24- to 48-hour period. Tape scanned and analyzed.
(2) Cardiac event monitors (e.g., King of Hearts monitor): Looping event recorder kept with the patient for up to 30 days. Patient records ECG tracings when symptoms occur, then transmits over telephone. ECG then forwarded and evaluated by the physician.
(3) Implanted loop recorders: Cardiac monitors implanted in the patient to identify suspected rhythms that have not otherwise been seen
(b) Used in documenting the following:
(1) Arrhythmias not demonstrated by resting or exercise ECG, especially with symptoms of palpitations, syncope
(2) Efficacy of surgical and medical therapy for arrhythmias
(c) Records at least two leads: I and V5 simultaneously
(d) Diary is kept by the patient to note symptoms (chest pain, palpitations, syncope) and activities during recording to correlate with rhythm
vi. Electrophysiologic studies (EPS): Series of programmed electrical stimuli are applied within the heart to the endothelium through electrodes in the cardiac chambers, under fluoroscopic guidance. Used to induce cardiac arrhythmias.
(a) Purpose: To reproduce arrhythmias in a controlled environment to assess the best mode of therapy for their control (e.g., medications, pacemaker, ablation)
(1) Patients with ventricular or supraventricular tachyarrhythmias
(2) Patients at high risk for sudden cardiac death
(3) Patients with unexplained recurrent syncopal episodes with suspected cardiac cause
(4) Patients who have survived a cardiac arrest without identified cause
Patient Care
1. Decreased cardiac output (CO)
a. Description of problem: Decreased CO can be due to either mechanical or electrical cardiac dysfunction. Findings can include the following:
i. Changes in the patient’s hemodynamics: Blood pressure, heart rate, CO
ii. ECG changes or arrhythmias
iv. Weakness, fatigue, dizziness
v. Shortness of breath, dyspnea, crackles
vi. Cold and clammy skin, cyanosis, pallor
vii. Decreased peripheral pulses
viii. Decreased or absent urinary output (oliguria, anuria)
i. Work of the heart is decreased by decreasing myocardial oxygen demands
ii. Myocardial oxygen supply is increased (minimizing ischemia, size of infarct)
iii. Hemodynamics are normal: CO is adequate
iv. Heart rate is controlled and arrhythmias are eliminated
c. Collaborating professionals on health care team: Nurse, physician, pharmacist, and physical therapist
i. Monitor heart rate, rhythm, and patient responses (e.g., blood pressure, mental status, diaphoresis, pain, shortness of breath)
ii. Assess blood pressure at regular intervals and with changes in the patient’s condition. Discuss with the physician what drug is to be administered for significant changes in blood pressure.
iii. Watch the patient’s oxygenation by frequently checking pulse oximetry readings and assessing breath sounds, respirations, and circulation. Administer oxygen as needed.
iv. Assess changes in the patient’s neurologic status. Observe for central ner-vous system disturbances (confusion, restlessness, agitation, dizziness).
v. Administer fluids as ordered to maintain left ventricular end-diastolic pressure (LVEDP)
vii. Monitor intake and output (urine output should average at least 30 ml/hr), daily weights
viii. Check presence and quality of peripheral pulses
ix. Check for other signs of perfusion deficits: Cool skin, sluggish capillary refilling
x. Monitor hemodynamic pressures, CO readings if the patient has a right-sided heart catheter
(a) Notify the physician of significant hemodynamic changes
(b) Collaborate with the physician regarding medication needs, management of hemodynamic status
(c) Be aware of the rationale for current therapy, parameters, and goals of care
xi. Have the patient notify the nurse immediately at the onset of chest discomfort and other associated symptoms of distress. Place the patient in a semi-Fowler position or position of comfort. Stress the importance of early recognition and treatment of problems.
xii. Watch for and identify any ECG changes
(a) Document the rhythm strip (at least once a shift)
(b) Obtain a 12-lead ECG if a new arrhythmia is noted
(c) Determine the patient’s response to arrhythmia, verbally and through vital signs
(d) Administer appropriate emergency drugs if the patient’s cardiac rhythm becomes significantly bradycardic or tachycardic, per unit protocols
(e) Have emergency equipment readily available and fully stocked
(f) Be knowledgeable about and prepared to use emergency equipment (defibrillator, transcutaneous pacer, transvenous pacer)
(g) Administer CPR and call code if the patient is pulseless, in VF, or in asystole
a. Description of problem: Acute pain due to ischemia. Findings can include the following:
i. Patient communication of discomfort: Description of quality (on a scale of 1 to 10, with 10 being the worst), intensity, location, radiation, timing, and aggravating and alleviating factors (movement, deep inspiration, positioning)
c. Collaborating professionals on health care team: Nurse, physician, physician assistant, advanced registered nurse practitioner, pharmacist, occupational and physical therapists, dietitian, social worker, home health aide
i. Have the patient notify the nurse immediately at the onset of chest discomfort and other associated symptoms of distress. Stress the importance of early recognition and treatment of chest discomfort.
ii. Administer oxygen per unit protocol
iii. Check vital signs, monitor ECG
iv. Do a 12-lead ECG immediately
v. Ensure that the patient has a patent IV line
vi. Administer and titrate medications to alleviate angina
viii. Collaborate with the physician on medication needs (types, dosages, frequency, route) and titrations and adjustments of medications, depending on the patient response
ix. Monitor the patient’s pain
(a) Assess quality, duration, intensity, frequency of the pain
(b) Assess the effectiveness of medications
(c) Look for trends and drug interactions, and identify other possible comfort measures
x. Provide other comfort interventions as appropriate (e.g., back rub, repositioning, special mattresses)
xi. Alert the physician if pain continues so that further actions can be determined. Cardiac pain means the myocardium is in jeopardy, and immediate interventions are needed.
a. Description of problem: Due to cardiac dysfunction, the patient may exhibit the following clinical findings, reflecting a decreased tolerance for the activities of daily living
ii. Dyspnea with progressively less exertion
iv. Discomfort in chest, neck, jaw, shoulder, arm
i. Cause is identified and treated
ii. Patient engages in progressive ambulation
iii. No complications of bed rest are present (skin intact, breath sounds clear, no signs of deep venous thromboembolism)
iv. Patient is educated regarding the need for routine exercise and weight control (as needed)
c. Collaborating professionals on health care team: Physical and occupational therapists; cardiac rehabilitation nurse for activity plans in hospital and after discharge; social worker to assist with home equipment acquisition, home visits, nursing home placement (temporary or permanent); dietitian
i. Assess and document the patient’s response to progressive ambulation, including monitoring of heart rate and rhythm, respiration, and blood pressure
ii. Assist the patient with initial increases in ambulation
iii. Administer pain medication, as needed, before planned ambulation (if the patient is pain free, progression in ambulation will be more successful)
iv. Plan rest periods between various treatments, visits, and ambulation
v. Teach the patient how to progress safely: Instruct in correct positioning and efficient use of body for each step
vi. Ensure that the patient is instructed with regard to the availability and use of special equipment to assist in ambulation (walkers, canes, wheelchairs), if needed
vii. If the patient becomes unstable (ischemic pain, vital signs beyond set limits, arrhythmias), help the patient back to bed and immediately evaluate the need for oxygen, medications (e.g., nitrates, antiarrhythmics), ECG, notification of the physician, emergency equipment
viii. Arrange consultations with other health professionals, as appropriate
ix. Encourage the patient and family to openly ventilate feelings and ask questions regarding the ability to ambulate and lifestyle resumption and changes
i. Patient has verbalized and demonstrated an understanding of activity capacity and limitations
ii. Exercise program is incorporated into the patient’s lifestyle. Weight control goals are met, if appropriate.
iii. Activity plan includes a support system and community services, as needed, to achieve the desired lifestyle
4. Inadequate knowledge of cardiac diagnosis, medications, or treatment
i. Patient and/or family verbalizations indicate a lack of knowledge or inappropriate or incorrect information
ii. Patient may have been noncompliant with the recommended regimen, which resulted in incorrect or inappropriate care at home
iii. Patient is easily agitated, hostile, worried, suspicious (because of misinformation, misunderstanding, or misinterpretation)
iv. Patient and/or family is unable to plan realistic goals or home care
i. Patient (and family) verbalizes an understanding of the diagnosis, medications, treatment, and follow-up care
c. Collaborating professionals on health care team: See Acute Chest Pain
ii. Assess readiness to learn (the patient is alert, pain free, not sleep deprived; information is not given immediately after sedatives are administered)
iii. Determine the best methods for the patient to learn (group, one to one, videos)
iv. Reinforce learning with the use of printed materials related to the disease, discharge instructions, procedures, and medications
v. Instruction sheets should be available in languages other than English
vi. Arrange for an interpreter if the patient does not understand English (staff, family member, interpretation services)
vii. Document teaching and the patient’s response
viii. Arrange appropriate consults: Cardiac rehabilitation; dietary, occupational, and physical therapy; home health; social work
ix. Schedule practice and return demonstrations of psychomotor skills
i. Patient or significant other verbalizes an understanding of the medical condition, medications, necessary home care, follow-up, and diet and any other lifestyle changes
ii. Patient or significant other knows where to seek assistance for information and help
iii. Patient or significant other demonstrates proper techniques for various applicable home self-care procedures (low-molecular-weight heparin injections, pulse monitoring, wound care, IV therapy)
SPECIFIC PATIENT HEALTH PROBLEMS
a. Injury (due to LDL cholesterol, toxins, infections, or mechanical causes) occurs to the endothelial cells in the intima of the coronary arteries, altering cell structure
b. Platelets adhere and aggregate at the site of injury, and macrophages migrate to the area as a result of injury. Smooth muscle cells and macrophage foam cells enter the intimal layer. These accumulations promote the development over time of a fatty fibrous plaque, or “fatty streak.” Migration of LDL into the subintimal space results in “lipid core.”
c. This plaque is a pearly white accumulation in the intimal lining, consisting mostly of smooth muscle cells but also collagen-producing fibroblasts and macrophages. These deposits protrude into the lumen, obstructing blood flow.
d. Progressive narrowing of the vessel occurs
e. This process tends to occur at vessel bifurcations and at the proximal end of the artery
f. The fatty fibrous plaque can rupture and form either a mural thrombus or an occlusive thrombus
i. A mural thrombus can partially or totally obstruct the artery. The disrupted plaque and mural thrombus can develop into a more fibrotic, stenotic lesion, which changes the plaque’s geometry.
ii. An acute, labile, occlusive thrombus can totally obstruct the artery and create clinical complications (MI, unstable angina, sudden cardiac death)
iii. The ruptured plaque, caused by endothelial injury and exposure to blood flow, activates platelet and fibrin formation, enhancing thrombus formation
g. Coronary blood flow may be further diminished by vasoconstriction (resulting from the release of vasoactive agents [thromboxane A2, angiotensin II], impaired vasodilatation, and platelet activation)
a. Heredity: Familial component for premature heart disease; MI or sudden cardiac death in father, mother, or siblings
b. Age: CAD is more prevalent among middle-aged and older persons (males older than 45 years of age; females older than 55 years of age)
c. Gender: CAD is more prevalent among men
i. Before age 55, prevalence is three to four times higher among men than among women (before menopause)
ii. After age 55, prevalence rates slowly equalize for both sexes; at age 75, rates are close to equal
i. Enhances atherogenic progression; decreases HDL cholesterol level; influences thrombus formation, plaque instability, arrhythmias
ii. Dose and duration dependent: Risk of death from CAD is two to six times higher among smokers than among nonsmokers
e. Hyperlipidemia: High levels of triglycerides, LDL, and very-low-density lipoproteins are associated with an increased risk of CAD
i. Triglycerides: Higher than 200 mg/dl
ii. LDL: Higher than 130 mg/dl
iii. HDL: Lower than 40 mg/dl (HDL level higher than 60 mg/dl is a negative risk factor)
i. Contributes to direct vascular injury, along with the effects of increased wall stress and oxygen demands
ii. Approximately 30% of American adults have hypertension (the rate is up to three times higher in African Americans than in the general population). Women have a higher incidence of hypertensive heart disease.
iii. Defined as systolic blood pressure higher than 140 and/or diastolic pressure higher than 90 mm Hg (Chobanian, Bakris, Black, et al, 2003)
g. LV hypertrophy: Heart’s response to chronic pressure overloads; associated with increased risk for cardiovascular events
h. Thrombogenic risk factors: Deficiencies in serum coagulation inhibitors (antithrombin III, protein C, and protein S), elevated plasma fibrinogen level, enhanced platelet aggregation
i. Diabetes mellitus: Patients with diabetes mellitus are twice as likely to develop CAD as persons without diabetes
j. Obesity: Positively associated with an increased rate of CAD; also contributes to the development of hypertension and diabetes
i. Fat distribution plays a role (abdominal or central obesity carries a higher risk)
ii. Metabolic syndrome: Recognized syndrome (also referred to as insulin resistance syndrome) associated with higher risk and identified by the presence of three or more of the following:
k. Sedentary lifestyle; studies show a positive relationship between inactivity and CAD, mainly resulting from its aggravation of other risk factors
b. Physical examination (see Patient Assessment section): CAD may be asymptomatic and may be diagnosed due to abnormal findings on tests (stress testing, echocardiography)
b. Exercise ECG stress testing: Used to rule out ischemia, to evaluate chest pain symptoms, to stratify the risk of known CAD, to detect arrhythmias, and to assess the efficacy of treatment
c. Myocardial perfusion imaging (with exercise or pharmacologic stress): Used to detect inadequate myocardial perfusion (ischemia) or the absence of perfusion (infarction) by assessing the degree of uptake in the myocardium of a radioactive tracer
i. Exercise stress echocardiography: Ultrasound images taken during or after treadmill or bicycle exercise
ii. Pharmacologic stress echocardiography: Dobutamine or adenosine is used to increase myocardial oxygen requirements (similar to exercise demands)
e. EBCT: Done with an ultrafast CT scanner. Measures coronary calcium on artery walls and calculates score, which, if elevated, is associated with increased possibility of CAD
f. Cardiac catheterization and coronary angiography: Used to define cardiac function and coronary anatomy to guide therapy
g. IVUS: Catheter-tipped two-dimensional ultrasonic probe is used to define intracoronary anatomy
h. Intracardiac echocardiography (ICE): Experimental; used to define intracoronary anatomy
a. Modifiable risk factors are under control and/or improved
b. Risk factor modification is incorporated into the patient’s lifestyle
6. Collaborating professionals on health care team: Physician, nurse, dietitian, pharmacist, community service worker (i.e., smoking cessation programs)
a. Anticipated patient trajectory: Primary prevention is the key to decreasing the morbidity and mortality of CAD
(a) Nutritional status, dietary habits should be assessed.
(b) Education should be provided on the rationale for compliance with a cholesterol-controlled diet if lipid levels are above the goals.
(c) Information should be provided to the patient and family on low-fat, low-cholesterol foods. Referrals should be given to hospital and community resources for follow-up and reinforcement.
(d) Diet should include the use of monounsaturated and polyunsaturated fats (olive, sunflower, and corn oils; soft oleomargarine) and avoidance of trans-fatty acids, which should be less than 7% of total calories. Other dietary additions can include fish and ω-3 fatty acids, fiber, and flaxseed.
(e) Goals for weight should be discussed. Weight and height should be measured. Body mass index (BMI) should be determined.
(a) Antiplatelet agents (such as aspirin [acetylsalicylic acid], 81 mg/day) should be included in the patient’s health regimen, if not contraindicated
(b) Lipid-lowering agents will often be necessary; the patient should be aware of their uses and side effects, and follow-up requirements
(c) Antihypertensive agents may be necessary (diuretics, ACE inhibitors, β-blockers)
(a) An adequate support system to assist in changing to heart-healthy behaviors and lifestyle is important
(b) Complementary and alternative medical methods to assist with stress reduction include massage therapy, yoga, regular exercise, postural therapy, and acupuncture
(a) Provide the patient with information regarding the risk factors for CAD
(b) Encourage the patient and significant others to quit smoking; provide information and help regarding risks, methods for stopping, and nicotine replacement
(c) Provide information on lipid-lowering diets that have 30% or less fat, with less than 7% saturated fat
(d) Goals for lipid management
(1) LDL level of less than 130 mg/dl (less than 100 mg/dl if CAD is present)
(e) If the patient is hypertensive
(1) Blood pressure goal: 140/90 mm Hg or lower
(2) Modifications include weight control, routine exercise, moderate alcohol consumption, and sodium restriction
(f) Weight control should be discussed with the patient and a plan for weight loss developed (especially for patients who are more than 120% of their ideal weight for height). Hypertensive patients and/or patients with elevated glucose or triglyceride levels should receive information on achieving ideal body weight.
(g) Encourage exercise and physical activity (after risks are assessed, often after exercise testing in patients over 40 years of age)
(1) Goal of 30 minutes, three to four times weekly (minimum); preferably 30 to 60 minutes of moderate exercise, including walking, cycling, jogging, swimming
(2) Other opportunities for increased physical activity should be explored
(h) Increased emphasis is being placed on education regarding women and cardiovascular disease and on more aggressive medical management for women along with the inclusion of more female patients in clinical trials
b. Potential complications: See the following sections for each of the possible sequelae of CAD