Chapter 4 The cardiovascular system
The cardiovascular history
Presenting symptoms (Table 4.1)
Chest pain
The mention of chest pain by a patient tends to provoke more urgent attention than other symptoms. The surprised patient may find himself whisked into an emergency ward with the rapid appearance of worried-looking doctors. This is because ischaemic heart disease, which may be a life-threatening condition, often presents in this manner (Table 4.2). The pain of angina and myocardial infarction tends to be similar in character; it may be due to the accumulation of metabolites from ischaemic muscle following complete or partial obstruction of a coronary artery, leading to stimulation of the cardiac sympathetic nerves.1,2 Patients with cardiac transplants who develop coronary disease in the transplanted heart may not feel angina, presumably because the heart is denervated. Similarly, patients with diabetes are more likely to be diagnosed with ‘silent infarcts’.
| Major symptoms |
| Chest pain or heaviness |
| Dyspnoea: exertional (note degree of exercise necessary), orthopnoea, paroxysmal nocturnal dyspnoea |
| Ankle swelling |
| Palpitations |
| Syncope |
| Intermittent claudication |
| Fatigue |
| Past history |
| History of ischaemic heart disease: myocardial infarction, coronary artery bypass grafting |
| Rheumatic fever, chorea, sexually transmitted disease, recent dental work, thyroid disease |
| Prior medical examination revealing heart disease (e.g. military, school, insurance) |
| Drugs |
| Social history |
| Tobacco and alcohol use |
| Occupation |
| Family history |
| Myocardial infarcts, cardiomyopathy, congenital heart disease, mitral valve prolapse, Marfan’s syndrome |
| Coronary artery disease risk factors |
| Previous coronary disease |
| Smoking |
| Hypertension |
| Hyperlipidaemia |
| Family history of coronary artery disease |
| Diabetes mellitus |
| Obesity and physical inactivity |
| Male sex and advanced age |
| Raised homocysteine levels |
| Functional status in established heart disease |
| Class I—disease present but no symptoms, or angina* or dyspnoea† during unusually intense activity |
| Class II—angina or dyspnoea during ordinary activity |
| Class III—angina or dyspnoea during less than ordinary activity |
| Class IV—angina or dyspnoea at rest |
* Canadian Cardiovascular Society (CCVS) classification.
† New York Heart Association (NYHA) classification.
TABLE 4.2 Causes (differential diagnosis) of chest pain and typical features
| Pain | Causes | Typical features |
| Cardiac pain | Myocardial ischaemia or infarction | Central, tight or heavy; may radiate to the jaw or left arm |
| Vascular pain | Aortic dissection | Very sudden onset, radiates to the back |
| Aortic aneurysm | ||
| Pleuropericardial pain | Pericarditis +/− myocarditis | Pleuritic pain, worse when patient lies down |
| Infective pleurisy | Pleuritic pain | |
| Pneumothorax | Sudden onset, sharp, associated with dyspnoea | |
| Pneumonia | Often pleuritic, associated with fever and dyspnoea | |
| Autoimmune disease | Pleuritic pain | |
| Mesothelioma | Severe and constant | |
| Metastatic tumour | Severe and constant, localised | |
| Chest wall pain | Persistent cough | Worse with movement, chest wall tender |
| Muscular strains | Worse with movement, chest wall tender | |
| Intercostal myositis | Sharp, localised, worse with movement | |
| Thoracic zoster | Severe, follows nerve root distribution, precedes rash | |
| Coxsackie B virus infection | Pleuritic pain | |
| Thoracic nerve compression or infiltration | Follows nerve root distribution | |
| Rib fracture | History of trauma, localised tenderness | |
| Rib tumour, primary or metastatic | Constant, severe, localised | |
| Tietze’s syndrome | Costal cartilage tender | |
| Gastrointestinal pain | Gastro-oesophageal reflux | Not related to exertion, may be worse when patient lies down—common |
| Diffuse oesophageal spasm | Associated with dysphagia | |
| Airway pain | Tracheitis | Pain in throat, breathing painful |
| Central bronchial carcinoma | ||
| Inhaled foreign body | ||
| Other causes | Panic attacks | Often preceded by anxiety, associated with breathlessness and hyperventilation |
| Mediastinal pain | Mediastinitis | |
| Sarcoid adenopathy, lymphoma |
To help determine the cause of chest pain, it is important to ascertain the duration, location, quality, and precipitating and aggravating factors (the four cardinal features), as well as means of relief and accompanying symptoms (the SOCRATES questions; see Chapter 1).3
The term anginaa was coined by Heberden from the Greek and Latin words meaning ‘choking’ or strangling; and the patient may complain of crushing pain, heaviness, discomfort or a choking sensation in the retrosternal area or in the throat. It is best to ask if the patient experiences chest ‘discomfort’ rather than ‘pain’, because angina is often dull and aching in character and may not be perceived as pain.
The pain or discomfort is usually central rather
than left-sided. The patient may dismiss his or her pain as non-cardiac because it is not felt over the heart on the left side. It may radiate to the jaw or to the arms, but very rarely travels below the umbilicus. The severity of the pain varies.
These features constitute typical angina (Table 4.3).4 Although angina typically occurs on exertion, it may also occur at rest or wake a patient from sleep. Ischaemic chest pain is usually unaffected by respiration. The use of sublingual nitrates characteristically brings relief within a couple of minutes, but this is not specific as nitrates may also relieve oesophageal spasm and also have a pronounced placebo effect.
TABLE 4.3 Clinical classification of angina from the European Society of Cardiology
| Typical angina | Meets all 3 of the following characteristics: |
Other causes of retrosternal pain are listed in Table 4.2. Chest pain made worse by inspiration is called pleuritic pain. This may be due to pleurisy (page 110) or pericarditis (page 78). Pleurisy may occur because of inflammation of the pleura as a primary problem (usually due to viral infection), or secondary to pneumonia or pulmonary embolism. Pleuritic pain is not usually brought on by exertion and is often relieved by sitting up and leaning forwards. It is caused by the movement of inflamed pleural or pericardial surfaces on one another.
Massive pulmonary embolism causes pain of very sudden onset which may be retrosternal and associated with collapse, dyspnoea and cyanosis
Table 4.4a Differential diagnosis of chest pain
| Favours angina | Favours pericarditis or pleurisy | Favours oesophageal pain |
| Tight or heavy | Sharp or stabbing | Burning |
| Onset predictable with exertion | Not exertional | Not exertional |
| Relieved by rest | Present at rest | Present at rest |
| Relieved rapidly by nitrates | Unaffected | Unaffected unless spasm |
| Not positional | Worse supine (pericarditis) | Onset may be when supine |
| Not affected by respiration | Worse with respiration | Unaffected by respiration |
| Pericardial or pleural rub |
(page 136). It is often pleuritic, but can be identical to anginal pain, especially if associated with right ventricular ischaemia.
Spontaneous pneumothorax may result in pain and severe dyspnoea (page 132). The pain is sharp and localised to one part of the chest.
Cholecystitis can cause chest pain and be confused with myocardial infarction. Right upper quadrant abdominal tenderness is usually present (page 170).
Dyspnoea
| Favours myocardial infarction (acute coronary syndrome) | Favours angina |
| Onset at rest | Onset with exertion |
| May be severe | Less severe |
| Sweating | No sweating |
| Anxiety (angor) | Mild or no anxiety |
| No relief with nitrates | Rapid relief with nitrates |
| Associated symptoms (nausea and vomiting) | Associated symptoms absent |
| Favours myocardial infarction | Favours aortic dissection |
| Central chest pain | Radiates to back |
| Subacute onset (minutes) | Instantaneous onset |
| May be severe | Very severe |
| Favours myocardial ischaemia | Favours chest wall pain |
| Exertional | Positional |
| Occurs with exertion | Often worse at rest |
| Brief episodes | Prolonged |
| Diffuse | Localised |
| No chest wall tenderness (only discriminates between infarction and chest wall pain) | Chest wall tenderness |
volume change in the lungs, because of a reduction in compliance of the lungs or increased resistance to air flow. Cardiac dyspnoea is typically chronic and occurs with exertion because of failure of the left ventricular output to rise with exercise; this in turn leads to an acute rise in left ventricular end-diastolic pressure, raised pulmonary venous pressure, interstitial fluid leakage and thus reduced lung compliance. However, the dyspnoea of chronic cardiac failure does not correlate well with measurements of pulmonary artery pressures, and clearly the origin of the symptom of cardiac dyspnoea is complicated.5 Left ventricular function may be impaired because of ischaemia (temporary or permanent reduction in myocardial blood supply), previous infarction (damage) or hypertrophy (often related to hypertension). As it becomes more severe, cardiac dyspnoea occurs at rest.
Orthopnoea (from the Greek ortho ‘straight’; see Table 4.5), or dyspnoea that develops when a patient is supine, occurs because in an upright position the patient’s interstitial oedema is redistributed; the lower zones of the lungs become worse and the upper zones better. This allows improved overall blood oxygenation. Patients with severe orthopnoea spend the night sitting up in a chair or propped up on numerous pillows in bed. The absence of orthopnoea suggests that left ventricular failure is unlikely to be the cause of a patient’s dyspnoea (negative likelihood ratio [LR] = 0.046).
TABLE 4.5 Causes of orthopnoea
| Cardiac failure |
| Uncommon causes |
| Massive ascites |
| Pregnancy |
| Bilateral diaphragmatic paralysis |
| Large pleural effusion |
| Severe pneumonia |
Paroxysmalbnocturnal dyspnoea (PND) is severe dyspnoea that wakes the patient from sleep so that he or she is forced to get up gasping for breath. This occurs because of a sudden failure of left ventricular output with an acute rise in pulmonary venous and capillary pressures; this leads to transudation of fluid into the interstitial tissues, which increases the work of breathing. The sequence may be precipitated by resorption of peripheral oedema at night while supine. Acute cardiac dyspnoea may also occur with acute pulmonary oedema or a pulmonary embolus.
Cardiac dyspnoea can be difficult to distinguish from that due to lung disease or other causes (page 109)7. One should inquire particularly about a history of any cardiac disease that could be responsible for the onset of cardiac failure. For example, a patient with a number of known previous myocardial infarctions who develops dyspnoea is more likely to have decreased left ventricular contractility. A patient with a history of hypertension or a very heavy alcohol intake may have hypertensive heart disease or an alcoholic cardiomyopathy. The presence of orthopnoea or paroxysmal nocturnal dyspnoea is more suggestive of cardiac failure than of lung disease.
Ankle swelling
It is important to find out whether the patient is taking a vasodilating drug (e.g. a calcium channel blocker), which can cause peripheral oedema. There are other (more) common causes of ankle oedema than heart failure that also need to be considered (page 71). Oedema that affects the face is more likely to be related to nephrotic syndrome (page 213).
Palpitations
This is not a very precise term. It is usually taken to mean an unexpected awareness of the heartbeat.8 Ask the patient to describe exactly what he or she notices and whether the palpitations are slow or fast, regular or irregular, and how long they last (Questions box 4.2).
Questions box 4.2
Questions to ask the patient with palpitations
! denotes symptoms for the possible diagnosis of an urgent or dangerous problem.
It may be helpful to ask the patient to tap the rate and rhythm of the palpitations with his or her finger. Associated features including pain, dyspnoea or faintness must be inquired about. The awareness of rapid palpitations followed by syncope suggests ventricular tachycardia. These patients usually have a past history of significant heart disease. Any rapid rhythm may precipitate angina in a patient with ischaemic heart disease.
Table 4.6 Causes (differential diagnosis) of dyspnoea, palpitations and oedema
| Favours heart failure | Favours lung disease | |
| History of myocardial infarction | History of smoking | |
| Onset after some exertion (asthma) | ||
| No wheeze | Wheezing | |
| PND | PND absent | |
| Orthopnoea | Orthopnoea absent | |
| Abnormal apex beat | ||
| Third heart sound (S3) | ||
| Mitral regurgitant murmur | ||
| Overexpanded chest | ||
| Pursed-lips breathing | ||
| Early and mid-inspiratory crackles | Fine end-inspiratory crackles | |
| Cough only on lying down | Productive cough | |
| Palpitations differential diagnosis | Ankle oedema differential diagnosis | |
| Feature | Suggests | Favours heart failure |
| Heart misses and thumps | Ectopic beats | History of cardiac failure |
| Worse at rest | Ectopic beats | Other symptoms of heart failure |
| Very fast, regular | SVT (VT) | Jugular venous pressure elevated (+ve LR 9.0*) |
| Instantaneous onset | SVT (VT) | Favours hypoproteinaemia |
| Offset with vagal manoeuvres | SVT | Jugular venous pressure normal |
| Fast and irregular | AF | Oedema pits and refills rapidly, 2–3s† |
| Forceful and regular—not fast | Awareness of sinus rhythm (anxiety) | Favours deep venous thrombosis or cellulitis |
| Unilateral | ||
| Skin erythema | ||
| Calf tenderness | ||
| Severe dizziness or syncope | VT | |
| Pre-existing heart failure | VT | |
| Favours drug-induced oedema | ||
| Patient takes calcium channel blocker | ||
| Favours lymphoedema | ||
| Not worse at end of day | ||
| Not pitting when chronic | ||
| Favours lipoedema | ||
| Not pitting | ||
| Spares foot | ||
| Obese woman | ||
PND = paroxysmal nocturnal dyspnoea.
SVT = supraventricular tachycardia.
VT = ventricular tachycardia.
AF = atrial fibrillation.
* McGee S, Evidence-based clinical diagnosis, 2nd edn. St Louis: Saunders, 2007.
† Khan NA, Rahim SA, Avand SS et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA 2006 Feb 1; 295(5):536–546.
Table 4.7 Differential diagnosis of syncope and dizziness
| Favours vasovagal syncope (most common cause) |
| Onset in teens or 20s |
| Occurs in response to emotional distress, e.g. sight of blood |
| Associated with nausea and clamminess |
| Injury uncommon |
| Unconsciousness brief, no neurological signs on waking |
| Favours orthostatic hypotension |
| Onset when getting up quickly |
| Brief duration |
| Injury uncommon |
| More common when fasted or dehydrated |
| Known low systolic blood pressure |
| Use of antihypertensive medications |
| Favours ‘situational syncope’ |
| Occurs during micturition |
| Occurs with prolonged coughing |
| Favours syncope due to left ventricular outflow obstruction (AS, HCM) |
| Occurs during exertion |
| Favours cardiac arrhythmia |
| Family history of sudden death (Brugada or long QT syndrome) |
| Anti-arrhythmic medication (prolonged QT) |
| History of cardiac disease (ventricular arrhythmias) |
| History of rapid palpitations |
| No warning (heart block—Stokes-Adams attack) |
| Favours vertigo |
| No loss of consciousness |
| Worse when turning head |
| Head or room seems to spin |
| Favours seizure |
| Prodrome—aura |
| Tongue bitten |
| Jerking movements during episode |
| Sleepiness afterwards |
| Head turns during episode |
| Follows emotional stress |
| Cyanosis |
| Muscle pain afterwards |
| Favours metabolic cause of syncope (coma) |
| Hypoglycaemic agents, low blood sugar |
AS = aortic stenosis.
HCM = hypertrophic cardiomyopathy.
Patients may have learned manoeuvres that will return the rhythm to normal. Attacks of supraventricular tachycardia (SVT) may be suddenly terminated by increasing vagal tone with the Valsalva manoeuvre (page 70), carotid massage, by coughing, or by swallowing cold water or ice cubes.
Syncope, presyncope and dizziness
Syncope is a transient loss of consciousness resulting from cerebral anoxia, usually due to inadequate blood flow. Presyncope is a transient sensation of weakness without loss of consciousness. (See Questions box 11.4, page 326.)
Syncope may represent a simple faint or be a symptom of cardiac or neurological disease. One must establish whether the patient actually loses consciousness and under what circumstances the syncope occurs—e.g. on standing for prolonged
periods or standing up suddenly (postural syncope), while passing urine (micturition syncope), on coughing (tussive syncope), or with sudden emotional stress (vasovagal syncope). Find out whether there is any warning, such as dizziness or palpitations, and how long the episodes last. Recovery may be spontaneous or the patient may require attention from bystanders.
If syncope is due to an arrhythmia, there is a sudden loss of consciousness regardless of the patient’s posture; chest pain may also occur if the patient has ischaemic heart disease or aortic stenosis.10 Recovery is equally quick. Exertional syncope may occur with obstruction to left ventricular outflow by aortic stenosis or hypertrophic cardiomyopathy. Profound and sudden bradycardia, usually a result of complete heart block, causes sudden and recurrent syncope (Stokes-Adamsc attacksd). These patients may have a history of atrial fibrillation. Typically they have periods of tachycardia (fast heart rate) as well as periods of bradycardia (slow heart rate). This condition is called the sick sinus syndrome. The patient must be asked about drug treatment that could cause bradycardia, e.g. beta-blockers, digoxin, calcium channel blockers.
It is important to ask about a family history of sudden death. An increasing number of ion channelopathies are being identified as a cause of syncope and sudden death. These inherited conditions include the long QT syndrome and the Brugada syndrome. They are often diagnosed from typical ECG changes. In addition, certain drugs can cause the acquired long QT syndrome (Table 4.8).
| Associated with QT interval prolongation and ventricular arrhythmias |
| Anti-arrhythmics; flecainide, quinidine, sotalol, procainamide, amiodarone |
| Gastric motility promoter; cisapride |
| Antibiotics; clarithromycin, erythromycin |
| Antipsychotics; chlorpromazine, haloperidol |
| Associated with bradycardia |
| Beta-blockers |
| Some calcium channel blockers (verapamil, diltiazem) |
| Digoxin |
| Associated with postural hypotension |
| Most antihypertensive drugs, but especially prazosin and calcium channel blockers |
| Anti-Parkinsonian drugs |
Neurological causes of syncope are associated with a slow recovery and often residual neurological symptoms or signs. Bystanders may also have noticed abnormal movements if the patient has epilepsy. Dizziness that occurs even when the patient is lying down or which is made worse by movements of the head is more likely to be of neurological origin, although recurrent tachyarrhythmias may occasionally cause dizziness in any position. One should attempt to decide whether the dizziness is really vertiginous (where the world seems to be turning around), or whether it is a presyncopal feeling.
Intermittent claudication and peripheral vascular disease
The word claudicatione comes from the Latin meaning to limp. Patients with claudication notice pain in one or both calves, thighs or buttocks when they walk more than a certain distance. This distance is called the ‘claudication distance’. The claudication distance may be shorter when patients walk up hills. A history of claudication suggests peripheral vascular disease with a poor blood supply to the affected muscles. The most important risk factors are smoking, diabetes, hypertension and a history of vascular disease elsewhere in the body, including cerebrovascular disease and ischaemic heart disease. More severe disease causes the feet or legs to feel cold, numb and finally painful at rest. Rest pain is a symptom of severely compromised arterial supply. Remember the six P’s of peripheral vascular disease:
Risk factors for coronary artery disease
An essential part of the cardiac history involves obtaining detailed information about a patient’s risk factors—the patient’s cardiovascular risk factor profile (Questions box 4.4).
Hypercholesterolaemia is the next most important risk factor for ischaemic heart disease. Many patients now know their serum cholesterol levels because widespread testing has become fashionable. The total serum cholesterol is a useful screening test, and levels above 5.2 mmol/L are considered undesirable. Cholesterol measurements (unlike triglyceride measurements) are accurate even when a patient has not been fasting. Patients with established coronary artery disease benefit from lowering of total cholesterol to below 4 mmol/L. An elevated total cholesterol level is even more significant if the high-density lipoprotein (HDL) level is low (less than 1.0 mmol/L). Significant elevation of the triglyceride level is a coronary risk factor in its own right and also adds further to the risk if the total cholesterol is high. If a patient already has coronary disease, hyperlipidaemia is even more important. Control of risk factors for these patients is called ‘secondary prevention’. Patients who have multiple risk factors for ischaemic heart disease (e.g. diabetes and hypertension) should have their cholesterol controlled aggressively. If the patient’s cholesterol is known to be high, it is worth obtaining a dietary history. This can be very trying. It is important to remember that not only foods containing cholesterol but those containing saturated fats contribute to the serum cholesterol level. High alcohol consumption and obesity are associated with hypertriglyceridaemia.
Smoking is probably the next most important risk factor for cardiovascular disease and peripheral vascular disease. Some patients describe themselves as non-smokers even though they stopped smoking only a few hours before. The number of years the patient has smoked and the number of cigarettes smoked per day are both very important (and are recorded as packet-years, page 6). The significance of a history of smoking for a patient who has not smoked for many years is controversial. The risk of symptomatic ischaemic heart disease falls gradually over the years after smoking has been stopped. After about 2 years the risk of myocardial infarction falls to the same level as for those who have never smoked. After 10 years the risk of developing angina falls close to that of non-smokers.
Past history
Patients may recall a diagnosis of rheumatic fever in their childhood, but many were labelled as having ‘growing pains’.11 A patient who was put to bed for a long period as a child may well have had rheumatic fever. A history of rheumatic fever places patients at risk of rheumatic valvular disease.
Hypertension may be caused or exacerbated by aspects of the patient’s activities and diet (Questions box 4.5). A high salt intake, moderate or greater alcohol use, lack of exercise, obesity and kidney disease may all be factors contributing to high blood pressure. Non-steroidal anti-inflammatory drugs cause salt and fluid retention and may also worsen blood pressure. Ask about these, about previous advice to modify these factors, and about any drug treatment of hypertension when interviewing any patient with high blood pressure.
Examination anatomy
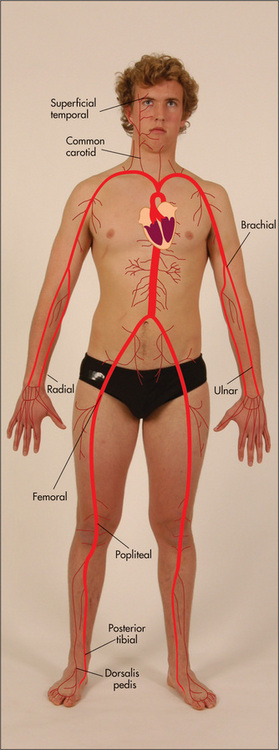
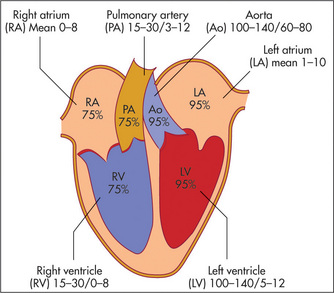
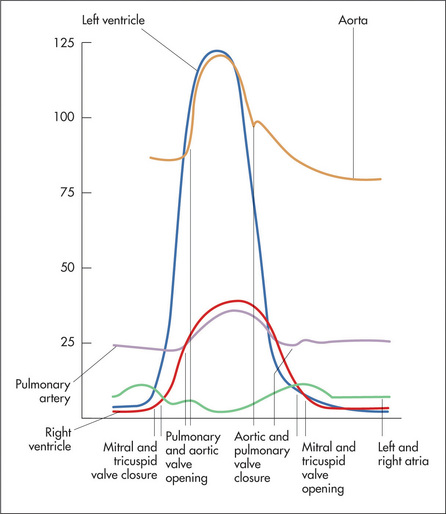
The surface anatomy of the heart and of the cardiac valves (Figure 4.1) and the positions of the palpable arteries (Figure 4.2) must be kept in mind during the examination of the cardiovascular system. In addition the physiology of blood flow through the systemic and pulmonary circuits need to be understood if the cardiac cycle and causes of cardiac murmurs are to be understood (Figure 4.3).
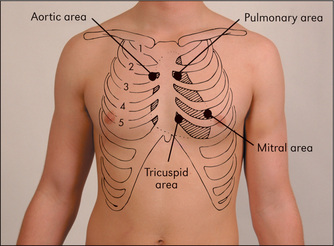
Figure 4.1 The areas best for auscultation do not exactly correlate with the anatomical location of the valves
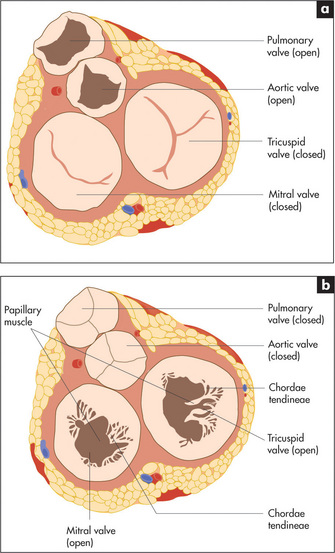
The cardiac valves separate the atria from the ventricles (the atrioventricular or mitral and tricuspid valves) and the ventricles from their corresponding great vessels. Figure 4.4 shows the fibrous skeleton that supports the four valves and their appearance during systole (cardiac contraction) and diastole (cardiac relaxation).
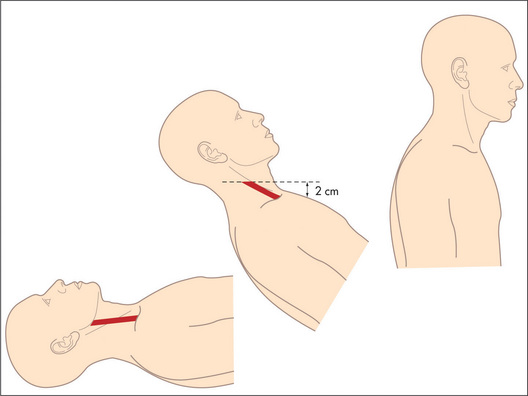
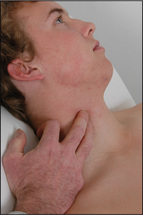
The filling of the right side of the heart from the systemic veins can be assessed by inspection of the jugular veins in the neck (Figure 4.5) and by palpation of the liver. These veins empty into the right atrium.
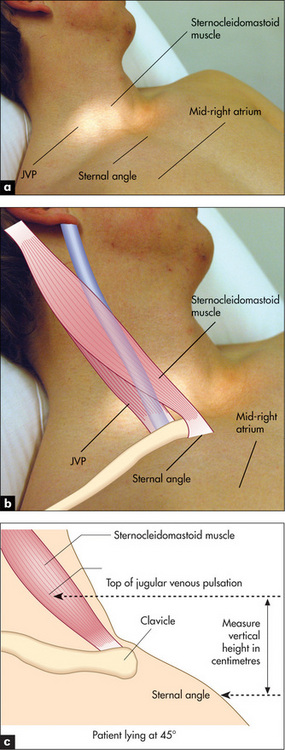
Figure 4.5 The jugular venous pressure (JVP) (a) Assessment of the JVP. The patient should lie at 45 degrees. The relationships between the sternomastoid muscle, the JVP, the sternal angle and the mid-right atrium are shown. (b, c) The anatomy of the neck showing the relative positions of the main vascular structures, clavicle and sternocleidomastoid muscle. See also Figure 4.6.
Figures (b) and (c) adapted from Douglas G, Nicol F, Robertson C, Macleod’s Clinical Examination, 11th edn. Edinburgh: Churchill Livingstone, 2005.
The internal jugular vein is deep in the sternomastoid muscle, while the external jugular vein is lateral to it. Traditionally, use of the external jugular vein to estimate venous pressure is discouraged, but the right internal and external jugular veins usually give consistent readings. The left-sided veins are less accurate because they cross from the left side of the chest before entering the right atrium. Pulsations that occur in the right-sided veins reflect movements of the top of a column of blood that extends directly into the right atrium. This column of blood may be used as a manometer and enables us to observe pressure changes in the right atrium. By convention, the sternal angle is taken as the zero point and the maximum height of pulsations in the internal jugular vein, which are visible above this level when the patient is at 45 degrees, is measured in centimetres. In the average person the centre of the right atrium lies 5 cm below this zero point (Figures 4.5a and 4.6).
The cardiovascular examination
Positioning the patient
It is important to have the patient lying in bed with enough pillows to support him or her at 45 degrees (Figure 4.7). This is the usual position in which the jugular venous pressure (JVP) is assessed. Even a ‘targeted’ cardiovascular examination in an outpatients’ clinic or surgery can only be performed adequately if the patient is lying down and an examination couch should be available. During auscultation, optimal examination requires further positioning of the patient, as discussed later.
General appearance
Look at the general state of health. Does the patient appear to be ill? If he or she looks ill, try to decide why you have formed that impression. Note whether the patient at rest has rapid and laboured respiration, suggesting dyspnoea (see Table 5.6, page 110).
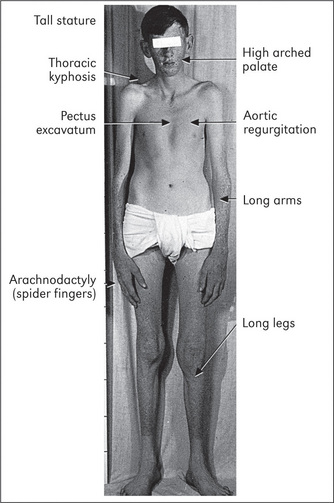
There are also some syndromes that are associated with specific cardiac disease. Marfan’s syndromef (Figure 4.8, page 50), Down syndromeg (page 314) and Turner’s syndromeh (page 314) are important examples.
The hands
Pick up the right hand. Look first at the nails. Now is the time for a decision as to the presence or absence of clubbing. Clubbing is an increase in the soft tissue of the distal part of the fingers or toes. The causes of clubbing are surprisingly varied (Table 4.9). The mechanism is unknown but there are, of course, several theories. One current theory is that platelet-derived growth factor (PDGF), released from megakaryocyte and platelet emboli in the nail beds, causes fibrovascular proliferation. Megakaryocytes and clumps of platelets do not normally reach the arterial circulation. Their large size (up to 50 μm) prevents their passing through the pulmonary capillaries when they are released from the bone marrow. In conditions where platelets may clump in the arterial circulation (infected cardiac valve) or bypass the pulmonary capillaries (right to left shunt associated with congenital heart disease), they can reach the systemic circulation and become trapped in the terminal capillaries of the fingers and toes. Damage to pulmonary capillaries from various lung disorders can have the same effect.
| Common |
| Cardiovascular |
| Cyanotic congenital heart disease |
| Infective endocarditis |
| Respiratory |
| Lung carcinoma (usually not small cell carcinoma) |
| Chronic pulmonary suppuration: |
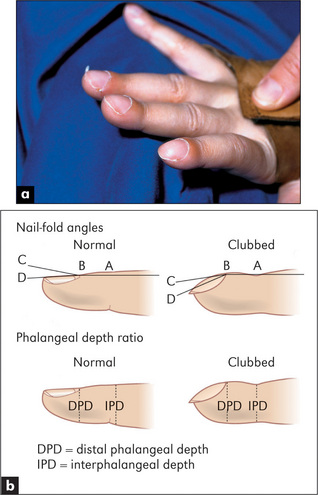
Proper examination for clubbing involves inspecting the fingernails (and toenails) from the side to determine if there is loss of the angle between the nail bed and the finger—the hyponychial angle (Figure 4.9). One accepted measurement is the interphalangeal depth ratio. The anteroposterior (AP) dimension of the finger is measured at the distal interphalangeal joint and compared with the AP diameter at the level of the point where the skin joins the nail. A ratio of more than 1 means clubbing.12,i Eventually, the distal phalanx becomes enlarged, due to soft-tissue swelling. This angle can be measured with a shadowgraph, which projects the silhouette of the finger so that it can be measured with a protractor. It is not in common use. If the angle is greater than 190°, clubbing is generally agreed to be present. Patients hardly ever notice that they have clubbing, even when it is severe. They often express surprise at their doctor’s interest in such an unlikely part of their anatomy.
Before leaving the nails, look for splinter haemorrhages in the nail beds (Figure 4.10). These are linear haemorrhages lying parallel to the long axis of the nail. They are most often due to trauma, particularly in manual workers. However, an important cause is infective endocarditis (page 79), which is a bacterial or other infection of the heart valves or part of the endocardium. In this disease splinter haemorrhages are probably the result of a vasculitis in the nail bed, but this is controversial. Other rare causes of splinter haemorrhages include vasculitis, as in rheumatoid arthritis, polyarteritis nodosa or the antiphospholipid syndrome, sepsis elsewhere in the body, haematological malignancy or profound anaemia.
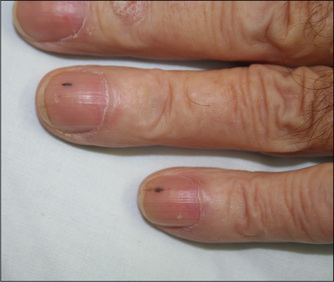
Figure 4.10 Splinter haemorrhages in the fingernails of a patient with staphylococcal aortic valve endocarditis
From Baker T, Nikolić G, O’Connor S, Practical Cardiology, 2nd edn. Sydney: Churchill Livingstone, 2008, with permission.
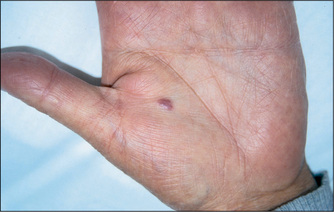
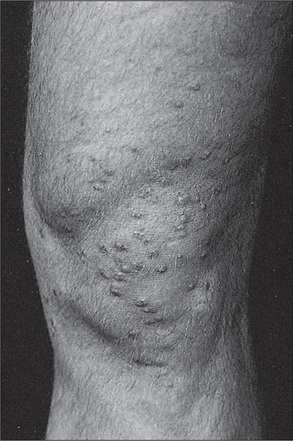
Osler’s nodesj are a rare manifestation of infective endocarditis. These are red, raised, tender palpable nodules on the pulps of the fingers (or toes), or on the thenar or hypothenar eminences. They are reported to have occurred in 50% of patients before antibiotic treatment of endocarditis became available. Currently they are seen in fewer than 5% of patients. Janeway lesionsk (Figure 4.11) are non-tender erythematous maculopapular lesions containing bacteria, which occur very rarely on the palms or pulps of the fingers in patients with infective endocarditis.l
Tendon xanthomata are yellow or orange deposits of lipid in the tendons that occur in type II hyperlipidaemia. These can be seen over the tendons of the hand and arm. Palmar xanthomata, and tuboeruptive xanthomata over the elbows and knees, are characteristic of type III hyperlipidaemia (Figure 4.12).
The arterial pulse
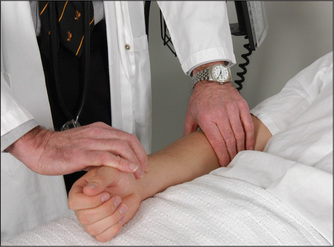
Although the radial pulse is distant from the central arteries, certain useful information may be gained from examining it. The pulse is usually felt just medial to the radius, using the forefinger and middle finger pulps of the examining hand (Figure 4.13). The following observations should be made: (i) rate of pulse, (ii) rhythm and (iii) presence or absence of delay of the femoral pulse compared with the radial pulse (radiofemoral delay). The character and volume of the pulse are better assessed from palpation of the brachial or carotid arteries.
Rate of pulse
Practised observers can estimate the rate quickly. Formal counting over 30 seconds is accurate and requires only simple mathematics to obtain the rate per minute. The normal resting heart rate in adults is usually said to be between 60 and 100 beats per minute but a more sensible range is probably 55 to 95 (95% of normal people). Bradycardia (Greek bradys ‘slow’, kardia ‘heart’) is defined as a heart rate of less than 60 beats per minute. Tachycardia (Greek tachys ‘swift’, kardia ‘heart’) is defined as a heart rate over 100 beats per minute. The causes of bradycardia and tachycardia are listed in Table 4.10.
| Bradycardia | |
| Regular rhythm | Irregular rhythm |
| Physiological (athletes, during sleep: due to increased vagal tone) | Irregularly irregular |
| Drugs (e.g. beta-blockers, digoxin, amiodarone) | Atrial fibrillation (in combination with conduction system disease or AV nodal blocking drugs) due to: |
* This is the difference between the heart rate counted over the praecordium and that observed at the periphery. In beats where diastole is too short for adequate filling of the heart, too small a volume of blood is ejected during systole for a pulse to be appreciated at the wrist.
Rhythm
The rhythm of the pulse can be regular or irregular. An irregular rhythm can be completely irregular with no pattern (irregularly irregular or chaotic rhythm); this is usually due to atrial fibrillation (Table 4.10). In atrial fibrillation coordinated atrial contraction is lost, and chaotic electrical activity occurs with bombardment of the atrioventricular (AV) node with impulses at a rate of over 600 per minute. Only a variable proportion of these is conducted to the ventricles because (fortunately) the AV node is unable to conduct at such high rates. In this way, the ventricles are protected from very rapid rates, but beat irregularly, usually at rates between 150 and 180 per minute (unless the patient is being treated with drugs to slow the heart rate). The pulse also varies in amplitude from beat to beat in atrial fibrillation because of differing diastolic filling times. This type of pulse can occasionally be simulated by frequent irregularly occurring supraventricular or ventricular ectopic beats.
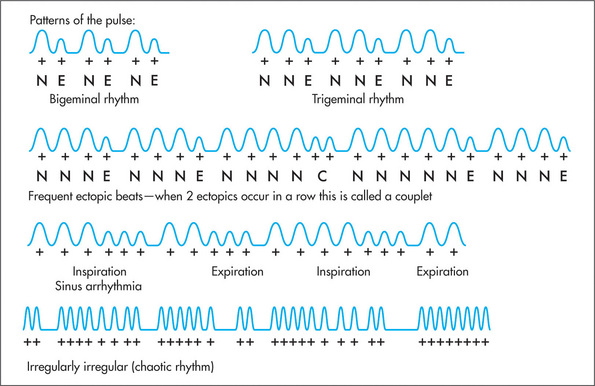
Patterns of irregularity (Figure 4.14) can also occur when patients have frequent ectopic beats. These may arise in the atrium (atrial ectopic beats—AEBs) or in the ventricle (ventricular ectopic beats—VEBs). Ectopic beats quite commonly occur in a fixed ratio to normal beats. When every second beat is an ectopic one, the rhythm is called bigeminy. A bigeminal rhythm caused by ectopic beats has a characteristic pattern: normal pulse, weak pulse, delay, normal pulse, … . Similarly, every third beat may be ectopic—trigeminy. A pattern of irregularity is also detectable in the Wenckebach phenomenon.m Here the AV nodal conduction time increases progressively until a non-conducted atrial systole occurs. Following this, the AV conduction time shortens and the cycle begins again.
Radiofemoral and radial–radial delay
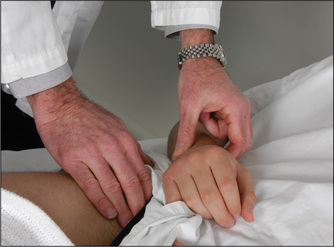
This is an important sign and often neglected. While palpating the radial pulse, the clinician places the fingers of the other hand over the femoral pulse, which is situated below the inguinal ligament, one-third of the way up from the pubic tubercle (Figure 4.15). A noticeable delay in the arrival of the femoral pulse wave suggests the diagnosis of coarctation of the aorta, where a congenital narrowing in the aortic isthmus occurs at the level where the ductus arteriosus joins the descending aorta. This is just distal to the origin of the subclavian artery. This lesion can cause upper limb hypertension.
The blood pressure
Measurement of the arterial blood pressuren is an essential part of the examination of almost any patient. Usually, indirect measurements of the systolic and diastolic pressures are obtained with a sphygmomanometer (Greek sphygmos ‘pulsing’, manos ‘thin’).13 The systolic blood pressure is the peak pressure that occurs in the artery following ventricular systole, and the diastolic blood pressure is the level to which the arterial blood pressure falls during ventricular diastole. Normal blood pressure is defined as a systolic reading of less than 140 mmHg and a diastolic reading of less than 90 mmHg. In some circumstances, lower pressures may be considered normal (e.g. in pregnancy) or desirable (e.g. for diabetics).
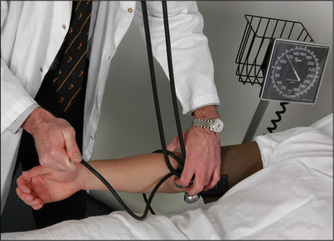
Measuring the blood pressure with the sphygmomanometer
The cuff is wrapped around the upper arm with the bladder centred over the brachial artery (Figure 4.16). This is found in the antecubital fossa, one-third of the way over from the medial epicondyle. For an approximate estimation of the systolic blood pressure, the cuff is fully inflated and then deflated slowly (3–4 mmHg per second) until the radial pulse returns. Then, for a more accurate estimation of the blood pressure, this manoeuvre is repeated with the diaphragm of the stethoscope placed over the brachial artery, slipped underneath the distal end of the cuff’s bladder.
The patient’s brachial artery should be at about the level of the heart which is at the level of the fourth intercostal space at the sternum. If the arm is too high, e.g. at the level of the supraclavicular notch, the blood pressure reading will be about 5 mmHg lower; and if the arm is too low the reading will be higher than is accurate.
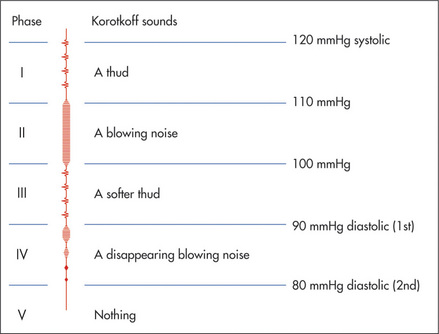
Five different sounds will be heard as the cuff is slowly released (Figure 4.17). These are called the Korotkoffo sounds. The pressure at which a sound is first heard over the artery is the systolic blood pressure (Korotkoff I). As deflation of the cuff continues, the sound increases in intensity (KII), then decreases (KIII), becomes muffled (KIV) and then disappears (KV). Different observers have used KIV and KV to indicate the level of the diastolic pressure. KV is probably the best measure. However, this provides a slight underestimate of the arterial diastolic blood pressure. Although diastolic pressure usually corresponds most closely to KV, in severe aortic regurgitation KIV is a more accurate indication. KV is absent in some normal people and KIV must then be used.
During inspiration, the systolic and diastolic blood pressures normally decrease (because intrathoracic pressure becomes more negative, blood pools in the pulmonary vessels, so left-heart filling is reduced). When this normal reduction in blood pressure with inspiration is exaggerated, it is termed pulsus paradoxus. Kussmaul meant by this that there was a fall in blood pressure and a paradoxical rise in pulse rate. A fall in arterial pulse pressure on inspiration of more than 10 mmHg is abnormal and may occur with constrictive pericarditis, pericardial effusion, or severe asthma. To detect this: lower the cuff pressure slowly until KI sounds are heard intermittently (expiration) and then until KI is audible with every beat. The difference between the two readings represents the level of the pulsus paradoxus.
High blood pressure
This is difficult to define.13 The most helpful definitions of hypertension are based on an estimation of the level associated with an increased risk of vascular disease. There have been many classifications of blood pressure, as what is considered normal or abnormal changes as more information comes to hand. Table 4.11 gives a useful guide to current definitions. If recordings above 140/90 mmHg are considered abnormal, high blood pressure may occur in up to 20% of the adult population.p Blood pressure measured by the patient at home, or by a 24-hour monitor, should be up to 10/5 mmHg less than that measured in the surgery.
| Category | Systolic (mmHg) | Diastolic (mmHg) |
| Optimal | < 120 | < 80 |
| Normal | 120–129 | 80–84 |
| High normal | 130–139 | 85–89 |
| Mild hypertension (grade 1) | 140–159 | 90–99 |
| Moderate hypertension (grade 2) | 160–179 | 100–109 |
| Severe hypertension (grade 3) | > 180 | > 110 |
* Khan NA, Rahim SA, Avand SS et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA 2006 Feb 1; 295(5):536–546.
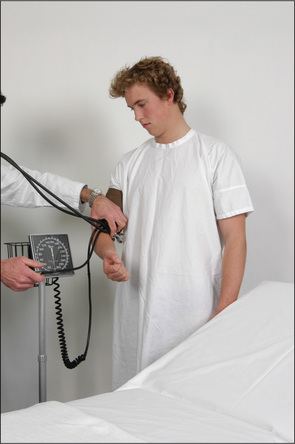
Postural blood pressure
The blood pressure should routinely be taken with the patient both lying down and standing (Figure 4.18).15 A fall of more than 15 mmHg in systolic blood pressure or 10 mmHg in diastolic blood pressure on standing is abnormal and is called postural hypotension (Table 4.12). It may cause dizziness or not be associated with symptoms. The most common cause is the use of antihypertensive drugs, α-adrenergic antagonists in particular.
| Hypovolaemia (e.g. dehydration, bleeding); Hypopituitarism |
| Addison’s* disease |
| Neuropathy—autonomic (e.g. diabetes mellitus), amyloidosis, Shy-Drager syndrome) |
| Drugs (e.g. vasodilators and other antihypertensives, tricyclic antidepressants, diuretics, antipsychotics) |
| Idiopathic orthostatic hypotension (rare progressive degeneration of the autonomic nervous system, usually in elderly men) |
The face
Inspect the sclerae for jaundice (page 25). This can occur with severe congestive cardiac failure and hepatic congestion. Prosthetic heart valve induced haemolysis of red blood cells, due to excessive turbulence, is an uncommon but cardiac cause of jaundice. Xanthelasmata (Figure 4.19) are intracutaneous yellow cholesterol deposits around the eyes and are relatively common. These may be a normal variant or may indicate type II or III hyperlipidaemia, though they are not always associated with hyperlipidaemia.
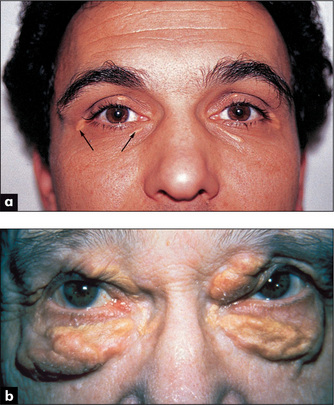
Figure 4.19 Xanthelasmata
Figure b from McDonald FS, ed., Mayo Clinic images in internal medicine, with permission. © Mayo Clinic Scientific Press and CRC Press.
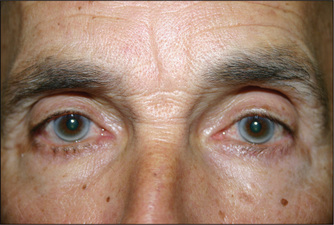
Look at the pupils for an arcus senilis (Figure 4.20). This half or complete grey circle is seen around the outer perimeter of the pupil and is probably associated with some increase in cardiovascular risk.q
The neck
Carotid arteries
The carotids are not only easily accessible, medial to the sternomastoid muscles (Figure 4.21), but provide a great deal of information about the wave form of the aortic pulse, which is affected by many cardiac abnormalities. Never palpate both carotid arteries simultaneously as they provide much of the blood supply to the brain (a vital organ).
Evaluation of the pulse wave form (the amplitude, shape and volume) is important in the diagnosis of various underlying cardiac diseases and in assessing their severity. It takes considerable practice to distinguish the different important types of carotid wave forms (Table 4.13). Auscultation of the carotids may be performed now or in association with auscultation of the praecordium.
| Type of pulse | Cause(s) |
| Anacrotic | Aortic stenosis |
| Small volume, slow uptake, notched wave on upstroke | |
| Plateau | Aortic stenosis |
| Slow upstroke | |
| Bisferiens | Aortic stenosis and regurgitation |
| Anacrotic and collapsing | |
| Collapsing | Aortic regurgitation |
| Hyperdynamic circulation | |
| Patent ductus arteriosus | |
| Peripheral arteriovenous fistula | |
| Arteriosclerotic aorta (elderly patients in particular) | |
| Small volume | Aortic stenosis |
| Pericardial effusion | |
| Alternans | Left ventricular failure |
| Alternating strong and weak beats |
Jugular venous pressure (JVP)—pulsation
Just as the carotid pulse tells us about the aorta and left ventricular function, the jugular venous pressure (JVP) (Figure 4.5, page 47) tells us about right atrial and right ventricular function.16 The positioning of the patient and lighting are important for this examination to be done properly. The patient must be lying down at 45 degrees to the horizontal with his or her head on pillows and in good lighting conditions. This is a difficult examination and there is considerable inter- (and intra-)observer variation in the findings.
When the patient is lying at 45 degrees, the sternal angle is also roughly in line with the base of the neck (Figure 4.5c). This provides a convenient zero point from which to measure the vertical height of the column of blood in the jugular vein. The jugular venous pulsation (movement) can be distinguished from the arterial pulse because: (i) it is visible but not palpable and has a more prominent inward movement than the artery; (ii) it has a complex wave form, usually seen to flicker twice with each cardiac cycle (if the patient is in sinus rhythm); (iii) it moves on respiration—normally the JVP decreases on inspiration; and (iv) it is at first obliterated and then filled from above when light pressure is applied at the base of the neck.
The assessment of the character of JVP is difficult even for experienced clinicians. There are two positive waves in the normal JVP.r The first is called the a wave and coincides with right atrial systole.s It is due to atrial contraction. The a wave also coincides with the first heart sound and precedes the carotid pulsation. The second impulse is called the v wave and is due to atrial filling, in the period when the tricuspid valve remains closed during ventricular systole. Between the a and v waves there is a trough caused by atrial relaxation. This is called the x descent. It is interrupted by the c point, which is due to transmitted carotid pulsation and coincides with tricuspid valve closure; it is not usually visible. Following the v wave, the tricuspid valve opens and rapid ventricular filling occurs; this results in the y descent (Figure 4.22).
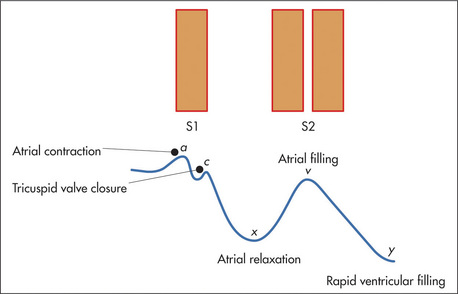
Figure 4.22 The jugular venous pressure, and its relationship to the first (S1) and second (S2) heart sounds
In Table 4.14, characteristic changes in the JVP are described. Any condition in which right ventricular filling is limited (e.g. constrictive pericarditis, cardiac tamponade or right ventricular infarction) can cause elevation of the venous pressure, which is more marked on inspiration when venous return to the heart increases. This rise in the JVP on inspiration, called Kussmaul’st sign, is the opposite of what normally happens. This sign is best elicited with the patient sitting up at 90 degrees and breathing quietly through the mouth.
| Causes of an elevated central venous pressure |
| Right ventricular failure |
| Tricuspid stenosis or regurgitation |
| Pericardial effusion or constrictive pericarditis |
| Superior vena caval obstruction |
| Fluid overload |
| Hyperdynamic circulation |
| Wave form |
| Causes of a dominantawave |
| Tricuspid stenosis (also causing a slow y descent) |
| Pulmonary stenosis |
| Pulmonary hypertension |
| Causes of cannonawaves |
| Complete heart block |
| Paroxysmal nodal tachycardia with retrograde atrial conduction |
| Ventricular tachycardia with retrograde atrial conduction or atrioventricular dissociation |
| Cause of a dominantvwave |
| Tricuspid regurgitation |
| xdescent |
| Absent: atrial fibrillation |
| Exaggerated: acute cardiac tamponade, constrictive pericarditis |
| ydescent |
| Sharp: severe tricuspid regurgitation, constrictive pericarditis |
| Slow: tricuspid stenosis, right atrial myxoma |
The abdominojugular reflux test (hepatojugular reflux) is a way of testing for right or left ventricular failure or reduced right ventricular compliance.17 Pressure exerted over the middle of the abdomen for 10 seconds will increase venous return to the right atrium. The JVP normally rises transiently following this manoeuvre.u If there is right ventricular failure or left atrial pressures are elevated (left ventricular failure), it may remain elevated (>4cm) for the duration of the compression—a positive hepatojugular reflux. The sudden fall in the JVP (>4 cm) as the pressure is released may be easier to see than the initial rise. It is not necessary to compress the liver and so the older name, hepatojugular reflux, is not so appropriate. It is important that the patient be relaxed, breathe through the mouth and not perform a Valsalva manoeuvre. The examiner should press firmly with the palm over the middle of the abdomen. It is not necessary to apply pressure for more than 10 seconds.
The praecordium
Now at last the examiner has reached the praecordium.
Inspection
Skeletal abnormalities such as pectus excavatum (funnel chest, page 121) or kyphoscoliosis (Greek kyphos ‘hunchbacked’, skolios ‘curved’), a curvature of the vertebral column (page 121), may be present. Skeletal abnormalities such as these, which may be part of Marfan’s syndrome, can cause distortion of the position of the heart and great vessels in the chest and thus alter the position of the apex beat. Severe deformity can interfere with pulmonary function and cause pulmonary hypertension (page 81).
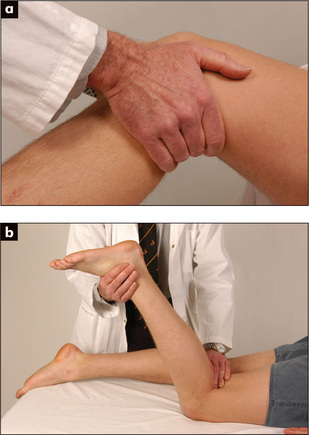
Look for the apex beat. Its normal position is in the fifth left intercostal space, 1 cm medial to the midclavicular line (Figure 4.23). It is due primarily to recoil of the heart as blood is expelled in systole. There may be other visible pulsations—for example, over the pulmonary artery in cases of severe pulmonary hypertension.
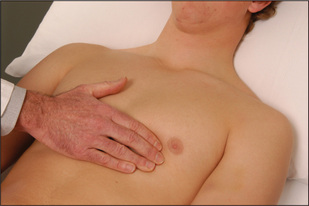
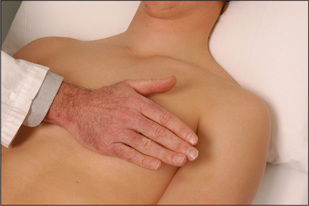
Palpation
The apex beat must be palpated (Figures 4.23 and 4.24).18 It is important to count down the number of interspaces. The first palpable interspace is the second. It lies just below the manubriosternal angle. The position of the apex beat is defined as the most lateral and inferior point at which the palpating fingers are raised with each systole. The normal apex is felt over an area the size of a 20 cent (50 p) coin (Figure 4.23). Use firm pressure with the tips of the fingers into the rib interspaces. The heel of the examiner’s hand is lifted off the patient’s sternum. Note that the apex beat is palpable in only about 50% of adults.
It is worth noting that the palpable apex beat is not the anatomical apex of the heart but a point above it. At the time the apex beat is palpable,v the heart is assuming a more spherical shape and the apex is twisting away from the chest wall. The area above the apex, however, is moving closer to the chest and is palpable. If the apex beat is displaced laterally or inferiorly, or both, this usually indicates enlargement,18 but may sometimes be due to chest wall deformity, or pleural or pulmonary disease (page 121).
The character of the apex beat may provide the examiner with vital diagnostic clues. The normal apex beat gently lifts the palpating fingers. There are a number of types of abnormal apex beats. The pressure loaded (heaving, hyperdynamic or systolic overloaded) apex beat is a forceful and sustained impulse. This occurs with aortic stenosis or hypertension. The volume loaded (thrusting) apex beat is a displaced, diffuse, non-sustained impulse. This occurs most commonly in advanced mitral regurgitation or dilated cardiomyopathy. The dyskinetic apex beat is an uncoordinated impulse felt over a larger area than normal in the praecordium and is usually due to left ventricular dysfunction (e.g. in anterior myocardial infarction). The double impulse apex beat, where two distinct impulses are felt with each systole, is characteristic of hypertrophic cardiomyopathy (page 91). The tapping apex beat will be felt when the first heart sound is actually palpable (heart sounds are not palpable in health) and indicates mitral or very rarely tricuspid stenosis. The character, but not the position, of the apex beat may be more easily assessed when the patient lies on the left side.
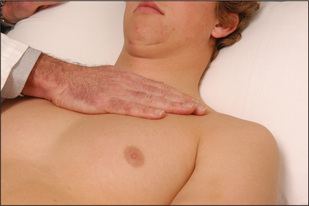
Other praecordial impulses may be palpable in patients with heart disease. A parasternal impulse may be felt when the heel of the hand is rested just to the left of the sternum with the fingers lifted slightly off the chest (Figure 4.25). Normally no impulse or a slight inward impulse is felt. In cases of right ventricular enlargement or severe left atrial enlargement, where the right ventricle is pushed anteriorly, the heel of the hand is lifted off the chest wall with each systole. Palpation with the fingers over the pulmonary area may reveal the palpable tap of pulmonary valve closure (palpable P2) in cases of pulmonary hypertension (Figure 4.26).
Turbulent blood flow, which causes cardiac murmurs on auscultation, may sometimes be palpable. These palpable murmurs are called thrills. The praecordium should be systematically palpated for thrills with the flat of the hand, first over the apex and left sternal edge, and then over the base of the heart (this is the upper part of the chest and includes the aortic and pulmonary areas) (Figure 4.26).
Apical thrills can be more easily felt with the patient rolled over to the left side (the left lateral position) as this brings the apex closer to the chest wall. Thrills may also be palpable over the base of the heart. These may be maximal over the pulmonary or aortic areas, depending on the underlying cause, and are best felt with the patient sitting up, leaning forwards and in full expiration. In this position the base of the heart is moved closer to the chest wall. A thrill that coincides in time with the apex beat is called a systolic thrill; one that does not coincide with the apex beat is called a diastolic thrill.
Percussion
It is possible to define the cardiac outline by means of percussionw but this is not routine (page 124).19 Percussion is most accurate when performed in the fifth intercostal space. The patient should lie supine and the examiner percusses from the anterior axillary line towards the sternum. The point at which the percussion note becomes dull represents the left heart border. A distance of more than 10.5 cm between the border of the heart and the middle of the sternum indicates cardiomegaly. The sign is not useful in the presence of lung disease.
Auscultation
Now at last the stethoscope is required.20 However, in some cases the diagnosis should already be fairly clear. In the viva voce examination, the examiners will occasionally stop a candidate before auscultation and ask for an opinion.
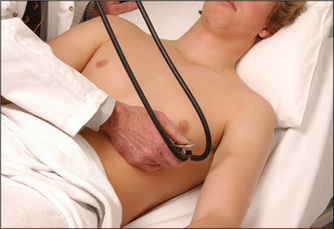
Auscultation of the heart begins in the mitral area with the bell of the stethoscope (Figures 4.1, page 45, and 4.27). The bell is designed as a resonating chamber and is particularly efficient in amplifying low-pitched sounds, such as the diastolic murmur of mitral stenosis or a third heart sound. It must be applied to the chest wall lightly, because forceful application will stretch the skin under the bell so that it forms a diaphragm. Some modern stethoscopes do not have a separate bell; the effect of a bell is produced when the diaphragm is placed lightly on the chest, and of a diaphragm when it is pushed more firmly.
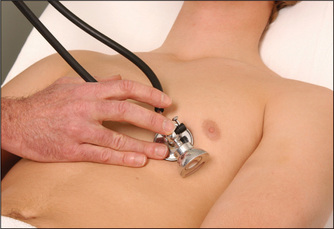
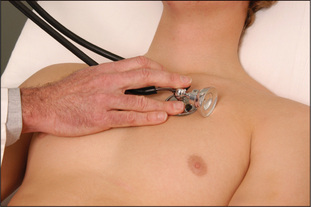
Next, listen in the mitral area with the diaphragm of the stethoscope (Figure 4.28), which best reproduces higher-pitched sounds, such as the systolic murmur of mitral regurgitation or a fourth heart sound. Then place the stethoscope in the tricuspid area (fifth left intercostal space) and listen. Next inch up the left sternal edge to the pulmonary (second left intercostal space) and aortic (second right intercostal space) areas (Figures 4.29 and 4.30), listening carefully in each position with the diaphragm.
It can be difficult to decide which heart sound is which. Palpation of the carotid pulse will indicate the timing of systole and enable the heart sounds to be more easily distinguished. It is obviously crucial to define systole and diastole during auscultation so that cardiac murmurs and abnormal sounds can be placed in the correct part of the cardiac cycle. Students are often asked to time a cardiac murmur; this is not a request to measure its length, but rather to say in which part of the cardiac cycle it occurs. Even the experts can mistake a murmur if they do not time it. It is important, during auscultation, to concentrate separately on the components of the cardiac cycle. The clinician should attempt to identify each and listen for abnormalities. There can be more than 12 components to identify in patients with heart disease. An understanding of the cardiac cycle is helpful when interpreting the auscultatory findings (Figure 4.31).
Abnormalities of the heart sounds
Alterations in intensity
The first heart sound (S1) is loud when the mitral or tricuspid valve cusps remain wide open at the end of diastole and shut forcefully with the onset of ventricular systole. This occurs in mitral stenosis because the narrowed valve orifice limits ventricular filling so that there is no diminution in flow towards the end of diastole. The normal mitral valve cusps drift back towards the closed position at the end of diastole as ventricular filling slows down. Other causes of a loud S1 are related to reduced diastolic filling time (e.g. tachycardia or any cause of a short atrioventricular conduction time).
The second heart sound (S2) will have a loud aortic component (A2) in patients with systemic hypertension. This results in forceful aortic valve closure secondary to high aortic pressures. Congenital aortic stenosis is another cause, because the valve is mobile but narrowed, and closes suddenly at the end of systole. The pulmonary component of the second heart sound (P2) is traditionally said to be loud in pulmonary hypertension, where the valve closure is forceful because of the high pulmonary pressure. In fact, a palpable P2 correlates better with raised pulmonary pressures than a loud P2.21
Splitting
In the case of fixed splitting of the second heart sound, there is no respiratory variation (as is normal) and splitting tends to be wide. This is caused by an atrial septal defect where equalisation of volume loads between the two atria occurs through the defect. This results in the atria acting as a common chamber.
Extra heart sounds
The third heart sound (S3) is a low-pitched (20–70 Hz) mid-diastolic sound that is best appreciated by listening for a triple rhythm.22 Its low pitch makes it more easily heard with the bell of the stethoscope. It has been likened (rather accurately) to the galloping of a horse and is often called a gallop rhythm. Its cadence is similar to that of the word ‘Kentucky’. It is more likely to be appreciated if the clinician listens not to the individual heart sounds but to the rhythm of the heart. It is probably caused by tautening of the mitral or tricuspid papillary muscles at the end of rapid diastolic filling, when blood flow temporarily stops. A physiological left ventricular S3 sometimes occurs in children and young people and is due to very rapid diastolic filling. A pathological S3 is due to reduced ventricular compliance, so that a filling sound is produced even when diastolic filling is not especially rapid. It is strongly associated with increased atrial pressure.
A left ventricular S3 is louder at the apex than at the left sternal edge, and is louder on expiration. It can be associated with an increased cardiac output, as occurs in pregnancy and thyrotoxicosis. Otherwise, it is an important sign of left ventricular failure and dilatation, but may also occur in aortic regurgitation, mitral regurgitation, ventricular septal defect and patent ductus arteriosus.23
The fourth heart sound (S4) is a late diastolic sound pitched slightly higher than the S3.24 The cadence of an S4 is similar to that of the word ‘Tennessee’. Again, this is responsible for the impression of a triple (gallop) rhythm. It is due to a high-pressure atrial wave reflected back from a poorly compliant ventricle. It does not occur if the patient is in atrial fibrillation, because the sound depends on effective atrial contraction, which is lost when the atria fibrillate. Its low pitch means that unlike a split first heart sound it disappears if the bell of the stethoscope is pressed firmly onto the chest.
Additional sounds
A non-ejection systolic click is a high-pitched sound heard during systole and is best appreciated at the mitral area. It is a common finding. It may be followed by a systolic murmur. The click may be due to prolapse of one or more redundant mitral valve leaflets during systole. Non-ejection clicks may also be heard in patients with atrial septal defects or Ebstein’s anomaly (page 89).
An atrial myxoma is a very rare tumour which may occur in either atrium. During atrial systole a loosely pedunculated tumour may be propelled into the mitral or tricuspid valve orifice causing an early diastolic plopping sound, a tumour plop. This sound is only rarely heard even in patients with a myxoma (about 10%).
A diastolic pericardial knock may occur when there is sudden cessation of ventricular filling because of constrictive pericardial disease.25
Prosthetic heart valves produce characteristic sounds (page 90). Rarely, a right ventricular pacemaker produces a late diastolic high-pitched click due to contraction of the chest wall muscles (the pacemaker sound).26
Murmurs of the heart
In deciding the origin of a cardiac murmur, a number of different features must be considered. These are: timing, the area of greatest intensity, the loudness and pitch, associated features (peripheral signs), and the effect of dynamic manoeuvres, including respiration and the Valsalva manoeuvre (Figure 4.32). The presence of a characteristic murmur is very reliable for the diagnosis of certain valvular abnormalities, but for others less so.
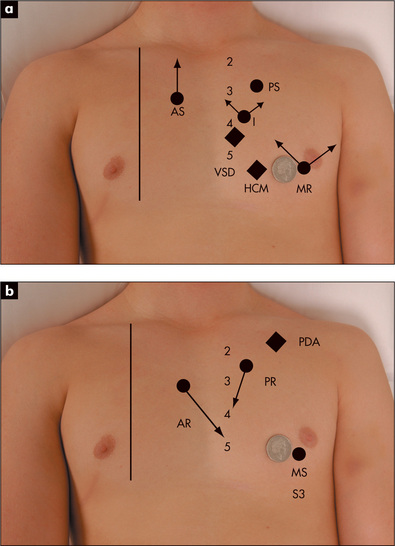
Figure 4.32 Sites of maximum intensity and radiation of murmurs and heart sounds
Timing (Table 4.15)
| Timing | Lesion |
| Pansystolic | Mitral regurgitation |
| Tricuspid regurgitation | |
| Ventricular septal defect | |
| Aortopulmonary shunts | |
| Midsystolic | Aortic stenosis |
| Pulmonary stenosis | |
| Hypertrophic cardiomyopathy | |
| Pulmonary flow murmur of an atrial septal defect | |
| Late systolic | Mitral valve prolapse |
| Papillary muscle dysfunction (due usually to ischaemia or hypertrophic cardiomyopathy) | |
| Early diastolic | Aortic regurgitation |
| Pulmonary regurgitation | |
| Mid-diastolic | Mitral stenosis |
| Tricuspid stenosis | |
| Atrial myxoma | |
| Austin Flint* murmur of aortic regurgitation | |
| Carey Coombs† murmur of acute rheumatic fever | |
| Presystolic | Mitral stenosis |
| Tricuspid stenosis | |
| Atrial myxoma | |
| Continuous | Patent ductus arteriosus |
| Arteriovenous fistula (coronary artery, pulmonary, systemic) | |
| Aortopulmonary connection (e.g. congenital, Blalock‡ shunt) | |
| Venous hum (usually best heard over right supraclavicular fossa and abolished by ipsilateral internal jugular vein compression) | |
| Rupture of sinus of Valsalva into right ventricle or atrium | |
| ‘Mammary souffle’ (in late pregnancy or early postpartum period) |
Note: The combined murmurs of aortic stenosis and aortic regurgitation, or mitral stenosis and mitral regurgitation, may sound as if they fill the entire cardiac cycle, but are not continuous murmurs by definition.
† Carey F Coombs (b. 1879), Bristol physician.
‡ Alfred Blalock (1899–1965), Baltimore physician.
The pansystolic murmur extends throughout systole, beginning with the first heart sound, then going right up to the second heart sound. Its loudness and pitch vary during systole. Pansystolic murmurs occur when a ventricle leaks to a lower pressure chamber or vessel. As there is a pressure difference from the moment the ventricle begins to contract (S1), blood flow and the murmur both begin at the first heart sound and continue until the pressures equalise (S2). Causes of pansystolic murmurs include mitral regurgitation,x tricuspid regurgitation and ventricular septal defect.
With an ejection (mid)systolic murmur, the murmur does not begin right at the first heart sound; its intensity is greatest in midsystole or later, and wanes again late in systole. This is described as a crescendo-decrescendo murmur. These murmurs are usually caused by turbulent flow through the aortic or pulmonary valve orifices or by greatly increased flow through a normal-sized orifice or outflow tract.
As the name implies, continuous murmurs extend throughout systole and diastole. They are produced when a communication exists between two parts of the circulation with a permanent pressure gradient so that blood flow occurs continuously. They can usually be distinguished from combined systolic and diastolic murmurs (due, for example, to aortic stenosis and aortic regurgitation), but this may sometimes be difficult. The causes are presented in Table 4.15.
A pericardial friction rub is a superficial scratching sound; there may be up to three distinct components occurring at any time during the cardiac cycle. They are not confined to systole or diastole. A rub is caused by movement of inflamed pericardial surfaces; it is a result of pericarditis. The sound can vary with respiration and posture; it is often louder when the patient is sitting up and breathing out. It tends to come and go, and is often absent by the time students can be found to come and listen for it. It has been likened to the crunching sound made when walking on snow.
A mediastinal crunch (Hamman’s signy) is a crunching sound heard in time with the heartbeat but with systolic and diastolic components. It is caused by the presence of air in the mediastinum, and once heard it is not forgotten. It is very often present after cardiac surgery and may occur associated with a pneumothorax or after aspiration of a pericardial effusion.
Area of greatest intensity
Although the place on the praecordium where a murmur is heard most easily is a guide to its origin, this is not a particularly reliable physical sign. For example, mitral regurgitation murmurs (GOOD SIGNS GUIDE 4.1) are usually loudest at the apex, over the mitral area, and tend to radiate towards the axillae, but they may be heard widely over the praecordium and even right up into the aortic area or over the back. Conduction of an ejection murmur up into the carotid arteries strongly suggests that this arises from the aortic valve.
GOOD SIGNS GUIDE 4.1 Characteristic murmurs and valvular heart disease
| Sign | Positive LR | Negative LR |
| Characteristic systolic murmur | ||
| Aortic stenosis | 3.3 | 0.1 |
| Mild mitral regurgitation or worse (moderate or severe) | 5.4 | 0.4 |
| Mild tricuspid regurgitation or worse | 14.6 | 0.8 |
| Moderate to severe tricuspid regurgitation | 10.1 | 0.4 |
| Characteristic diastolic murmur | ||
| Mild aortic regurgitation or worse | 9.9 | 0.3 |
| Pulmonary regurgitation | 17.4 | NS |
NS = not significant.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
Loudness and pitch
Unfortunately, the loudness of the murmur is not always helpful in deciding the severity of the valve lesion. For example, in the severest forms of valve stenosis, murmurs may be soft. However, murmurs are usually graded according to loudness. Cardiologists most often use a classification with six grades (Levine’s grading system):27
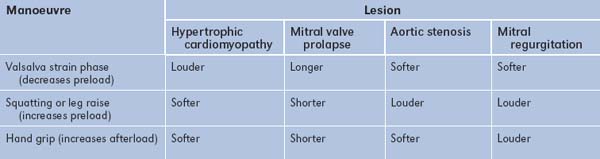
Dynamic manoeuvres (GOOD SIGNS GUIDE 4.2)
All patients with a newly diagnosed murmur should undergo dynamic manoeuvre testing (Table 4.16).28
| Sign | Positive LR | Negative LR |
| Louder on inspiration—right-sided murmur | 7.8 | 0.2 |
| Louder with Valsalva strain—hypertrophic cardiomyopathy | 14.0 | 0.3 |
| Louder squatting to standing—hypertrophic cardiomyopathy | 6.0 | 0.1 |
| Softer with isometric handgrip—hypertrophic cardiomyopathy | 3.6 | 0.1 |
| Louder with isometric handgrip—mitral regurgitation | 5.8 | 0.3 |
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
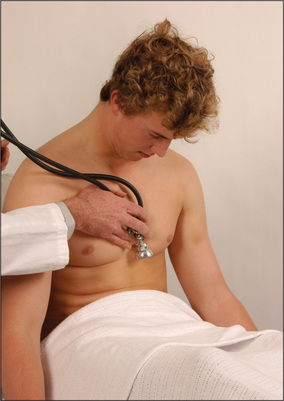
Figure 4.33 Dynamic auscultation for aortic regurgitation or a pericardial friction rub; patient in deep expiration
Auscultation of the neck
This is often performed as a part of dynamic auscultation for valvular heart disease, but certain aspects of the examination may be considered here. Abnormal sounds heard over the arteries are called bruits. These sounds are low-pitched and may be more easily heard with the bell of the stethoscope. Carotid artery bruits are most easily heard over the anterior part of the sternomastoid muscle above the medial end of the clavicle. Ask the patient to stop breathing for a brief period to remove the competing noise of breath sounds. It may be prudent to ask the patient not to speak. The amplified voice is often painfully loud when heard through the stethoscope.
A systolic bruit may be a conducted sound from the heart. The murmur of aortic stenosis is always audible in the neck and a soft carotid bruit is sometimes audible in patients with severe mitral regurgitation or pulmonary stenosis. A bruit due to carotid stenosis will not be audible over the base of the heart. Move the stethoscope from point to point onto the chest wall; if the bruit disappears, it is likely the sound arises from the carotid. It is not possible to exclude a carotid bruit in a patient with a murmur of aortic stenosis that radiates to the neck. Carotid artery stenosis is an important cause of a carotid bruit. More severe stenosis is associated with a noise that is longer and of increased pitch. Total obstruction of the vessel leads to disappearance of the bruit. It is not possible to make a diagnosis of significant (>60% obstruction) carotid stenosis clinically. The bottom line is that a carotid bruit poorly predicts significant carotid stenosis or stroke risk.Thyrotoxicosis can result in a systolic bruit (page 303) due to the increased vascularity of the gland.
A continuous noise is sometimes audible at the base of the neck. This is usually a venous hum, a result of audible venous flow. It disappears if light pressure is applied to the neck just above the stethoscope. Occasionally a loud machinery murmur (page 93) or severe aortic regurgitation (page 86) may cause a similar sound. Haemodialysis patients frequently have an audible bruit transmitted from their arterio-venous fistula.
The back
It is now time to leave the praecordium. Percussion and auscultation of the lung bases (Chapter 5) are also part of the cardiovascular examination. Signs of cardiac failure may be detected in the lungs; in particular late or pan-inspiratory crackles or a pleural effusion may be present. The murmur associated with coarctation of the aorta may be prominent over the upper back.
The abdomen
Lay the patient down flat (on one pillow) and examine the abdomen (Chapter 6). You are looking particularly for an enlarged tender liver which may be found when the hepatic veins are congested in the presence of right heart failure. Distension of the liver capsule is said to be the cause of liver tenderness in these patients. When tricuspid regurgitation is present the liver may be pulsatile, as the right ventricular systolic pressure wave is transmitted to the hepatic veins. Test for hepatojugular reflux.17,29Ascites may occur with severe right heart failure. Splenomegaly, if present, may indicate infective endocarditis.
Feel for the pulsation of the abdominal aorta, to the left of the middle line. It is often palpable in normal thin people but the possibility of an abdominal aortic aneurysm should always be considered when the aorta’s pulsations are palpable and expansile (page 173).30,31
The lower limbs (Table 4.17)
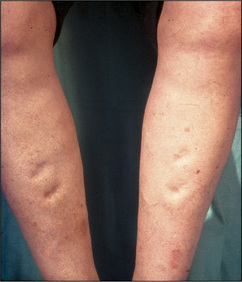
Palpate behind the medial malleolus of the tibia and the distal shaft of the tibia for oedema by compressing the area for at least 15 seconds with the thumb. This latter area is often tender in normal people, and gentleness is necessary. Oedema may be pitting (the skin is indented and only slowly refills—Figure 4.34) or non-pitting. Oedema due to hypoalbuminaemia often refills more quickly.
| 1 Inspection—anterior and lateral surfaces, sole of foot, between toes |
Pitting oedema occurs in cardiac failure unless the condition has been present for a long time and secondary changes in the lymphatic vessels have occurred. If oedema is present, note its upper level (e.g. ‘pitting oedema to mid-calf’ or ‘pitting oedema to mid-thigh’). Severe oedema can involve the skin of the abdominal wall and the scrotum as well as the lower limbs. Causes of oedema are listed in Table 4.18.
| Pitting lower limb oedema |
| Cardiac: congestive cardiac failure, constrictive pericarditis |
| Drugs: calcium antagonists |
| Hepatic: cirrhosis causing hypoalbuminaemia |
| Renal: nephrotic syndrome causing hypoalbuminaemia |
| Gastrointestinal tract: malabsorption, starvation, protein-losing enteropathy causing hypoalbuminaemia |
| Beri-beri (wet) |
| Cyclical oedema |
| Pitting unilateral lower limb oedema |
| Deep venous thrombosis |
| Compression of large veins by tumour or lymph nodes |
| Non-pitting lower limb oedema |
| Hypothyroidism |
| Lymphoedema |
* William Milroy (1855–1914), Professor of Medicine, University of Nebraska, described the disease in 1928.
Look for evidence of Achillesaa tendon xanthomata due to hyperlipidaemia. Also look for cyanosis and clubbing of the toes (this may occur without finger clubbing in a patient with a patent ductus arteriosus, because a rise in pulmonary artery pressures, sufficient to reverse the direction of flow in the shunt, has occurred).
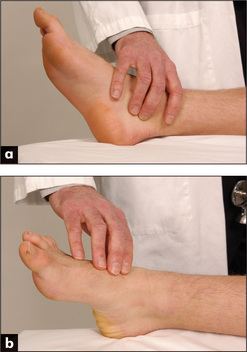
Peripheral vascular disease
Examine both femoral arteries by palpating and then auscultating them. A bruit may be heard if the artery is narrowed. Next palpate the following pulses: popliteal (behind the knee—Figure 4.35a: if this is difficult to feel when the patient is supine, try the method shown in Figure 4.35b), posterior tibial (under the medial malleolus, Figure 4.36a) and dorsalis pedis (on the forefoot, Figure 4.36b) on both sides.32
Patients with exertional calf pain (intermittent claudication) are likely to have disease of the peripheral arteries. More severe disease can lead to pain even at rest and to ischaemic changes in the legs and feet (GOOD SIGNS GUIDE 4.3). Look for atrophic skin and loss of hair, colour changes of the feet (blue or red) and ulcers at the lower end of the tibia.4 Venous and diabetic ulcers can be distinguished from arterial ulcers (Figures 4.37–4.39).
| Sign | Positive LR | Negative LR |
| Sores or ulcers on feet | 7.0 | NS |
| Feet pale, red or blue | 2.8 | 0.7 |
| Atrophic skin | 1.7 | NS |
| Absent hair | 1.7 | NS |
| One foot cooler | 6.1 | 0.9 |
| Absent femoral pulse | 6.1 | NS |
| Absent dorsalis pedis and posterior tibial pulses | 14.9 | 0.3 |
| Limb bruit present | 7.3 | 0.7 |
| Capillary refill time >5 seconds | 1.9 | NS |
| Capillary refill >20 seconds | 3.6 | NS |
NS = not significant.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
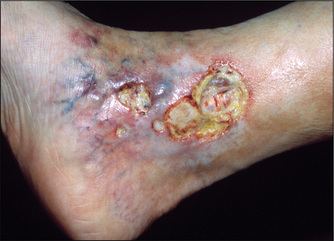
Figure 4.37 Venous ulcer
This venous ulcer has an irregular margin, pale surrounding neo-epithelium (new skin), and a pink base of granulation tissue. There is often a history of deep venous thrombosis. The skin is warm and oedema is often present. (See Table 4.19.)
From McDonald FS, ed., Mayo Clinic images in internal medicine, with permission. © Mayo Clinic Scientific Press and CRC Press.
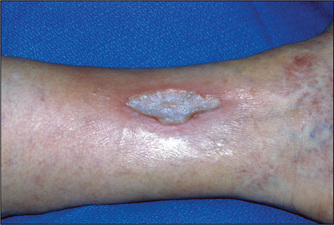
Figure 4.38 Arterial ulcer
This arterial ulcer has a regular margin and ‘punched out’ appearance. The surrounding skin is cold. The peripheral pulses are absent. (See Table 4.19.)
From McDonald FS, ed., Mayo Clinic images in internal medicine, with permission. © Mayo Clinic Scientific Press and CRC Press.
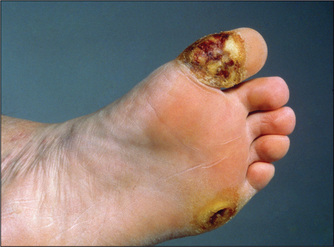
Figure 4.39 Diabetic (neuropathic) ulcer
Neuropathic ulcers are painless and are associated with reduced sensation in the surrounding skin. (See Table 4.19.)
From McDonald FS, ed., Mayo Clinic images in internal medicine, with permission. © Mayo Clinic Scientific Press and CRC Press.
Look for reduced capillary return (compress the toenails—the return of the normal red colour is slow).33 In such cases, perform Buerger’s testbb to help confirm your diagnosis: elevate the legs to 45 degrees (pallor is rapid if there is a poor arterial supply), then place them dependent at 90 degrees over the edge of the bed (cyanosis occurs if the arterial supply is impaired). Normally there is no change in colour in either position.
Acute arterial occlusion
It can be the result of embolism, thrombosis or injury. Peripheral arterial embolism usually arises from thrombus in the heart, where it is often secondary to (i) myocardial infarction, (ii) dilated cardiomyopathy, (iii) atrial fibrillation or (iv) infective endocarditis.34
Deep venous thrombosis
Deep venous thrombosis is a difficult clinical diagnosis (GOOD SIGNS GUIDE 4.4).35 The patient may complain of calf pain. On examination, the clinician should look for swelling of the calf and the thigh, and dilated superficial veins. Feel then for increased warmth and squeeze the calf (gently) to determine if the area is tender. Homans’ signcc (pain in the calf when the foot is sharply dorsiflexed) is of limited diagnostic value and is theoretically dangerous because of the possibility of dislodgment of loose thrombus.
| Sign | Positive LR | Negative LR |
| Asymmetrical calf swelling >2 cm difference | 2.1 | 0.6 |
| Thigh swelling | 2.5 | 0.6 |
| Superficial venous dilatation | 1.9 | NS |
| Tenderness and erythema | NS | NS |
| Asymmetrical skin warmth | 1.4 | NS |
| Homans’ sign | NS | NS |
NS = not significant.
From McGee S, Evidence-based physical diagnosis, 2nd edn. St Louis: Saunders, 2007.
The causes of thrombosis were described by Virchowdd in 1856 under three broad headings (the famous Virchow’s triad): (i) changes in the vessel wall, (ii) changes in blood flow, and (iii) changes in the constitution of the blood. Deep venous thrombosis is usually caused by prolonged immobilisation, cardiac failure (stasis) or trauma (vessel wall damage), but may also result from occult neoplasm, disseminated intravascular coagulation, the contraceptive pill, pregnancy and a number of inherited defects of coagulation (the thrombophilias: e.g. Factor V Leiden, anti-thrombin III deficiency).
Varicose veins
If a patient complains of ‘varicose veins’, ask him or her to stand with the legs fully exposed.36Inspect the front of the whole leg for tortuous, dilated branches of the long saphenous vein (below the femoral vein in the groin to the medial side of the lower leg). Then inspect the back of the calf for varicosities of the short saphenous vein (from the popliteal fossa to the back of the calf and lateral malleolus). Look to see if the leg is inflamed, swollen or pigmented (subcutaneous haemosiderin deposition secondary to venous stasis).
Trendelenburg test:ee with the patient lying down, the leg is elevated. Firm pressure is placed on the saphenous opening in the groin, and the patient is instructed to stand. The sign is positive if the veins stay empty until the groin pressure is released (incompetence at the saphenofemoral valve). If the veins fill despite groin pressure, the incompetent valves are in the thigh or calf, and Perthes’ testff is performed.
The differential diagnosis of leg ulcers is summarised in Table 4.19.
| 1 Venous stasis ulcer—most common (Figure 4.37) |
| Site: around malleoli |
| Character: irregular margin, granulation tissue in the floor. Surrounding tissue inflammation and oedema |
| Associated pigmentation, stasis eczema |
| 2 Ischaemic ulcer (Figure 4.38) |