The bone marrow
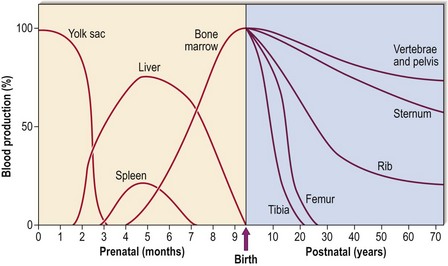
In early fetal life, blood is produced in the mesoderm of the yolk sac. During the second to seventh months the liver and spleen take over. Only in the last 2 months of fetal development does the bone marrow become the predominant site of blood formation. During childhood, marrow in the more peripheral bones becomes gradually replaced by fat, so that in adult life over 70% is located in the pelvis, vertebrae and sternum (Fig 1.1). This explains the sites used for bone marrow sampling (see p. 106).
The structure of the bone marrow
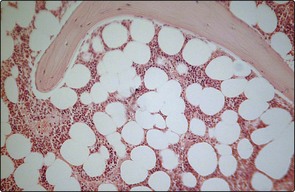
A trephine biopsy allows a two-dimensional view of the bone marrow down the light microscope (Fig 1.2). Haematopoietic cells of varying lineage and maturity are packed between fat spaces and bony trabeculae. Ultrastructural studies reveal clusters of haematopoietic cells surrounding vascular sinuses which allow eventual discharge of mature cells into the blood. Different lineages are compartmentalised; for example, the most immature myeloid precursors lie deep in the marrow parenchyma while more mature forms migrate towards the sinus wall. Lymphocytes tend to surround small radial arteries while erythrocytes form islands around the sinus walls.
Haematopoiesis: the stem cell hierarchy
The first adult haematopoietic stem cells (HSCs) are generated in the aorto-gonad-mesonephros (AGM) region of the embryo. The classical hierarchy diagram (Fig 1.3) where all cells arise in orderly fashion from a HSC is helpful but simplified; in reality, HSCs are groups of cells with diverse potentials depending on transcription factors and the local microenvironment. HSCs are not detectable by microscopic techniques but their existence can be inferred from cell cultures. Culture of these early cells on agar generates groups of more mature and thus recognisable progenitor cells known as colony-forming units (CFUs). For myeloid development the earliest detectable precursor cell creates granulocytes, erythrocytes, monocytes and megakaryocytes and is thus called CFUGEMM. HSCs may also be identified and separated from more committed progenitors by the use of flow cytometry as they have a characteristic immunophenotype.
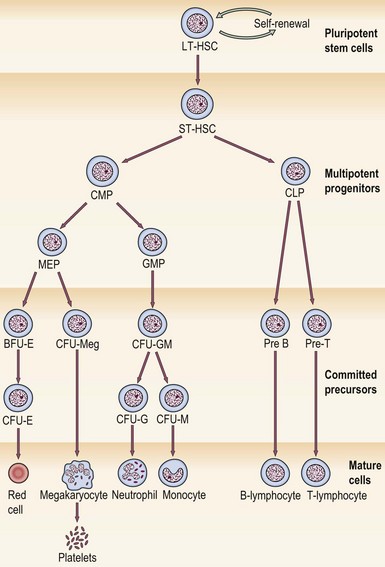
Fig 1.3 The stem cell hierarchy.
LT-HSC, long-term haematopoietic stem cell; ST-HSC, short-term haematopoietic stem cell; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; MEP, megakaryocyte/erythroid progenitor; GMP, granulocyte/macrophage progenitor; CFU, colony-forming unit; BFU, burst-forming unit; G, granulocyte; E, erythroid; M, monocyte; Meg, megakaryocyte.
Regulators of haematopoiesis
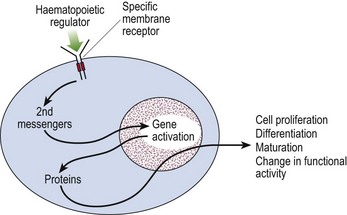
Control of haematopoiesis is mediated via regulatory molecules (or ‘growth factors’ – Table 1.1). These are generally glycoproteins produced by stroma and differentiated blood cells. They may act on more than one cell lineage and frequently show additive and synergistic interactions with each other. Their actions are multiple, including the promotion of proliferation, differentiation and maturation, as well as changing functional activity. Proliferative regulators alter the behaviour of cells by interacting with specific receptors on the cell surface (Fig 1.4).
Table 1.1
Key actions of some haematopoietic regulators
| Growth factor | Key actions |
| Interleukin-1 | Mediates acute phase responses; cofactor for other growth factors |
| Interleukin-2 | Growth factor for activated T-lymphocytes |
| Interleukin-3 | Supports early haematopoiesis by promoting growth of stem cells |
| c-kit ligand (stem cell factor) | Interacts with other factors to stimulate pluripotent stem cells |
| Erythropoietin | Lineage-specific growth factor promoting production of red cells |
| GM-CSF | Growth factor promoting production of neutrophils, monocytes, macrophages, eosinophils, red cells and megakaryocytes |
| G-CSF | Lineage-specific growth factor promoting production of neutrophils |
| M-CSF | Lineage-specific growth factor promoting monocyte and macrophage production |
| Thrombopoietin (Mpl ligand) | Lineage-specific growth factor promoting platelet production |
CSF, colony-stimulating factor; G, granulocyte; M, macrophage.