The axilla
current management including sentinel node and lymphoedema
Introduction
While there are aspects of breast surgical oncology that have moved towards a more radical approach, the management of the axilla has tended to become incrementally less invasive. Although it is clear that the status of the axillary lymph nodes continues to be the most significant prognostic marker in the management of breast cancer patients,1 removal of lymph nodes carries no survival benefit.2,3 As such, the purpose of axillary surgery in the context of breast cancer management is primarily for staging and local control.
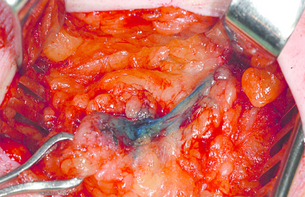
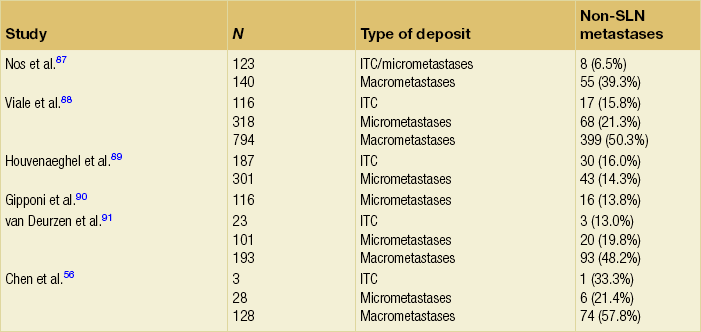
The last several decades have witnessed a metamorphosis in terms of management of the axilla, with significant paradigm shifts occurring within the last several years. Historically, a complete axillary node dissection with removal of level I and II lymph nodes or even levels I–III was standard (Fig. 7.1), and while this operation provided excellent prognostication and local control, it was associated with significant morbidity in terms of lymphoedema and decreased range of movement of the shoulder. Through the pioneering efforts of Cabanas,4 who first coined the term ‘sentinel node’ based on cadaveric studies of penile cancer, and the subsequent groundbreaking work of Donald Morton, who introduced this into the management paradigm in melanoma,5 sentinel node biopsy has revolutionised breast cancer treatment.
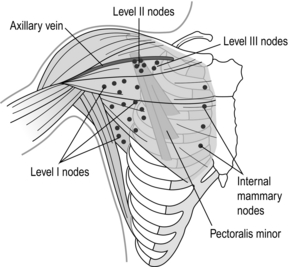
Figure 7.1 Anatomy of the axillary nodes and levels of axillary nodes related to the pectoralis minor. Level I nodes below and lateral, level II nodes under muscle and level III nodes medial to the muscle.
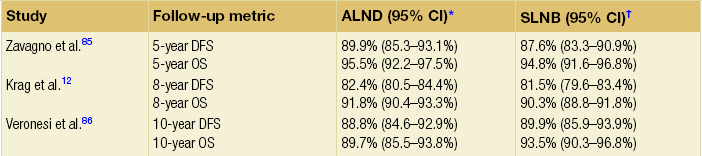
The concept of sentinel node biopsy is that one can map the lymphatic drainage of a tumour to the first draining lymph nodes, and that these nodes will be representative of the lymphatic basin. David Krag and Armando Giuliano6–8 introduced the technique into clinical practice for breast cancer. While initially considered an investigational procedure, large validation studies9–11 have confirmed that sentinel node biopsy is a safe, reliable, minimally invasive procedure that accurately stages the axilla. Subsequent randomised controlled trials12 have corroborated these findings (Table 7.1). The National Surgical Adjuvant Breast and Bowel Project B-32 trial found overall survival, disease-free survival and locoregional recurrence were equivalent between patients who were randomised to sentinel node biopsy followed by routine axillary dissection compared with sentinel node biopsy followed by axillary node dissection only in sentinel node-positive patients.12 Given these trial data and those from numerous other studies, sentinel node biopsy is now considered the standard of care in the management of patients with clinically node negative breast cancer.
Sentinel lymph node biopsy: technique
Considerable debate has surrounded the type of injection material used for sentinel node mapping. Some have advocated the use of blue dyes (such as patent blue V or Isosulphan blue) (Fig. 7.2) alone (severe hypersensitivity reactions with these drugs occur in approximately 1 in every 2000 patients), while others have preferred the use of radioactive tracers (such as technetium-99-labelled sulphur colloid or albumin). Still others have advocated the use of agents together. Numerous retrospective studies have found that while the false-negative rates are similar for the three techniques,7,10,13–15 identification of sentinel nodes is improved with the use of a combined technique.16 A recent prospective trial found that identification rates were 99.1% when dual tracers were used, as opposed to 93.8–95.6% for blue dye and 96.0–96.2% for radioactive tracer.17 An approach that uses radioisotope in all patients and adds blue dye only in those patients where radioisotope uptake into sentinel lymph nodes is not apparent has appeal in that it reduces the number who require blue dye.
While originally many practitioners injected tracers at the site of the tumour, the concept of subareolar injection is appealing, particularly for non-palpable and/or multiple tumours, given the embryologic origins of the lymphatics in Sappey’s plexus. A number of authors have found that subareolar injection identifies the same sentinel node as a peritumoural technique,18,19 has a high identification rate, and the same false-negative rate as other techniques.17,20 Therefore, while the presence of multifocal/multicentric tumours had once been thought to be a relative contraindication to the use of sentinel node biopsy, more recent studies (many of which used a subareolar injection technique, although some utilised multiple peritumoural injections) have found the accuracy, identification and false-negative rates to be similar to smaller unifocal cancers.21
Use of lymphoscintigraphy
Lymphoscintigraphy is often used in melanoma, where drainage patterns can vary widely; however, in breast cancer, where 98–99% of lymphatics drain to the ipsilateral axilla, (Fig. 7.3) lymphoscintigraphy has not been found to be of significant value (uptake of sentinel nodes of radioisotope takes minutes only, allowing injection of isotope after induction of anaesthesia).22,23 In cases of recurrent breast cancer, however, particularly if 10 or more lymph nodes have previously been removed, alternative drainage pathways may be encountered and lymphoscintigraphy is recommended.24 Even in patients who have had prior complete axillary node dissections, Kaur et al. have demonstrated that lymphoscintigraphy can identify drainage in 29% of patients and, of these, drainage may be non-axillary (contralateral and/or internal mammary) in 38% of cases.25 Furthermore, of the non-axillary sentinel nodes biopsied in the recurrent setting, 40% were positive and thus may alter management plans.25
Intraoperative evaluation
Frozen section is the most common technique used, and is associated with a specificity of 99–100% and a sensitivity of 57–74%.26 The sensitivity is far better for macrometastases (84–92%) than micrometastases (17–61%),26 and may be particularly difficult to interpret in patients with invasive lobular carcinomas who have cytologically bland cells and an infiltrative growth pattern. Nonetheless, the overall accuracy of this technique is 83–91%.26
Tew et al. conducted a meta-analysis of 31 studies of touch imprint cytology, and found that specificity and sensitivity of this technique were 94–100% and 34–95% respectively.27 Pooled estimates from 11 studies comparing macro- and micrometastases found that the sensitivity for touch imprint cytology was significantly better for the larger deposits: 81% (95% confidence interval (CI) 74–86%) vs. 22% (95% CI 14–33%) for macro- and micrometastases, respectively.27 On a more global level, the sensitivity of touch imprint cytology is lower than that of frozen section (62% (95% CI 53–70%) vs. 76% (95% CI 65–84%)), while specificity is comparable for both (99%).27 Molecular techniques are available that detect cancer cells in sentinel nodes but these have not found widespread utility.
Sentinel lymph node biopsy: controversial situations
Prophylactic mastectomy
For women at high risk who are undergoing prophylactic mastectomy, some have advocated sentinel node biopsy, as this avoids the need for subsequent axillary surgery if there is an occult breast cancer that is noted on final pathology, and the sentinel node is negative. Others, however, have argued that the risk of there being a cancer with associated lymph node metastasis is so low in the prophylactic setting that routine sentinel node biopsy, despite being of minimal additional morbidity, is not warranted. In a meta-analysis of six papers including 1251 patients who underwent 1343 prophylactic mastectomies, the rate of occult invasive cancer was 1.7% and the rate of positive sentinel nodes was 1.9%.28 Patients with positive sentinel nodes in the prophylactic setting have often been found to have had a locally advanced breast cancer in the originally treated breast so, in these patients, sentinel node biopsy may be warranted when a prophylactic contralateral mastectomy is being performed.28,29
Ductal carcinoma in situ (DCIS)
Some have argued that patients with DCIS who are at high risk of having concomitant invasive disease should have a sentinel node biopsy at the time of their excisional surgery to obviate the need to return to the operating room for a sentinel lymph node biopsy should an invasive focus be found on final pathology. The rate of DCIS on large core biopsy being upgraded to invasive disease on final pathology is reported to be up to 47%.21,30–32 Fewer samples and the use of non-vacuum-assisted devices increase the rates of underestimation of invasive disease.32 Further, clinical factors such as young age, a palpable mass, large tumour size by imaging, high grade, and comedo necrosis are also associated with an increased risk of concomitant invasive disease in patients with core biopsy diagnosis of DCIS.33,34
While some argue that such patients should have a sentinel lymph node biopsy, others prefer to confirm diagnosis of invasive disease before subjecting patients to a potentially unnecessary lymph node evaluation procedure. While sentinel node biopsy is minimally invasive and associated with few risks, it is not innocuous. It is well established that sentinel node biopsy can be performed after breast-conserving surgery has been performed. A recent meta-analysis comparing sentinel node identification and false-negative rates for patients who underwent surgical versus needle biopsy found that rates were comparable (sentinel node identification rates 91.3% vs. 92.8%; false-negative rates 12.3% vs. 9.9%).35 Therefore, prior surgery does not affect the ability to perform this technique accurately and consensus guidelines have accepted that sentinel node biopsy should not be performed routinely in patients with DCIS undergoing breast-conserving surgery. It is accepted that for patients undergoing mastectomy for DCIS, a sentinel lymph node biopsy should be considered at the same time as the mastectomy,36,37 as this technique cannot be performed once the breast is removed and an invasive focus is identified. In those with confirmed DCIS the node positivity rate is low at < 1%.
Neoadjuvant chemotherapy
Some have argued that sentinel node biopsy should be performed prior to neoadjuvant systemic therapy. Studies have demonstrated a high identification (98–100%) and an exceedingly low false-negative rate (0%) in this situation.38 Given that this technique is performed prior to any therapy, there can be no confounding of negative results as a result of therapy, which has resulted in false-negative rates as high as 39% in selected series39 reported in patients who have a sentinel node biopsy following chemotherapy. Others, however, have argued that to do a sentinel node biopsy prior to chemotherapy eliminates the opportunity to spare the 2–35% of patients who have a pathologically complete response in the axilla the morbidity of an axillary dissection.38 Furthermore, to evaluate sentinel nodes after neoadjuvant therapy, when surgery for the primary tumour is planned, may reduce the need for a second operative procedure.
A recent meta-analysis has demonstrated that sentinel node biopsy after neoadjuvant chemotherapy is associated with identification rates of between 71% and 100% (summary estimate 90.9%), false-negative rates between 0% and 39% (summary estimate 10.5%), and accuracy rates between 77% and 100% (summary estimate 94.4%).40 Two groups have performed trials of sentinel lymph node biopsy (SLNB) in the neoadjuvant setting in women with node positive breast cancer whose nodes are cleared by chemotherapy. The issue these trials addressed is whether axillary dissection (AD) can be avoided in responding patients. The ACOSOG Z1071 trial was a single arm study of 756 women (T0-4, N1-2, M0) undergoing neoadjuvant chemotherapy (NAC) with axillary nodal involvement confirmed by ultrasound guided FNA or core biopsy.41 Some patients had the involved nodes clipped. Following completion of NAC, SLNB was performed and there had to be at least 2 SLNs removed before proceeding to AD. The primary endpoint was to determine if the false negative rate (FNR) was <10% in women with N1 disease who had at least 2 sentinel nodes biopsied after NAC. Of 643 patients with a SN identified, 40.3% had a complete pathological response in the axilla. SLNB correctly identified nodal status after NAC in 84% of 695 patients. There was a 12.6% false negative rate (higher than the primary endpoint of 10%). The false negative rate (FNR) was significantly lower (10.8%) if dual tracer with both radiolabelled colloid and blue dye was used, compared with blue dye (4/24 – 16.7%) or radioisotope alone (20/101 – 20%) (p=0.046). The FNR was lower if more than 2 nodes were examined, 9.8% for 3 sentinel nodes, 6.7% for 4 sentinel nodes, but 11% for 5 or more, p=0.004 for trend. Placement of a clip in the positive node at diagnosis also decreased the FNR to 7.4% vs 13.6% without clip placement. An apparent chemotherapy effect in the SNs removed as marked by greater fibrosis or other histopathological changes in the sentinel nodes was also associated with a lower FNR (10.8% vs 13.5% without). Avoiding a high rate of false negatives in patients with node positive disease is essential and the results of this study highlight that technical factors are important for accurate SLNB after NAC. Performance can be improved by the use of dual tracer, examination of a minimum of 2 sentinel lymph nodes and placement of clips in involved nodes at diagnosis. These are the requirements if this technique is to be used after NAC.
The German Breast Group addressed the issue of optimal timing for sentinel lymph node in the prospective German, multi-institutional SENTINA-trial.42 Of 1737 patients entered, 1022 women were clinically node negative and underwent SLNB prior to NAC. The SLN detection rate in this group of women was 99.1 % (1013/1022). Among these, 360 patients had histologically involved nodes and these women underwent a second SLNB followed by AD after NAC. The SLN detection rate in these patients undergoing their second SLNB after NAC was 60.8% (219/360). 592 patients, who presented initially with suspicious axillary nodes converted to a cN0 status after NAC and underwent SLNB, combined with AD. The SLN detection rate in these women was 80.1% (474/592). The difference between the detection rates of these three groups was highly statistically significant (p < 0.001).
Isolated tumour cells and micrometastases
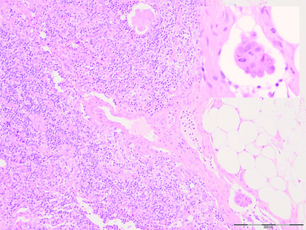
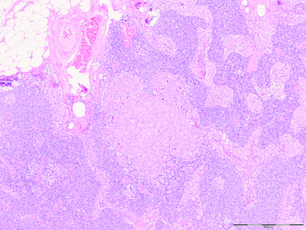
More thorough pathological evaluation of sentinel lymph nodes using serial sectioning and immunohistochemistry has allowed identification of occult metastases in up to 31% of previously ‘node-negative’ patients.43 This stage migration, and the relevance of this previously undetected disease, has been a source of considerable controversy. The American Joint Committee on Cancer classifies sentinel node metastases into three categories: ‘isolated tumour cells’ for metastases < 0.2 mm (which are considered node negative, pN0(i +); Fig. 7.4); ‘micrometastases’ for deposits between 0.2 and 2.0 mm (considered node positive, pN1mi; Fig. 7.5); and ‘macrometastases’, defined as deposits > 2.0 mm (also considered node positive, pN1a; Fig. 7.6).1 These somewhat arbitrary cut-offs have generated debate as to the true implications of both isolated tumour cells and micrometastases.
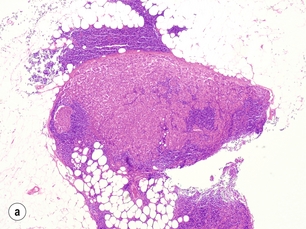
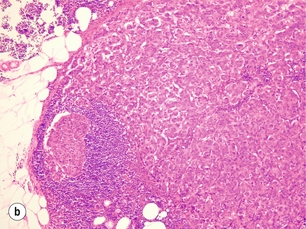
Figure 7.6 Axillary lymph node with an obvious macrometastasis: (left) low power; (right) high power.
In a pooled analysis of 58 studies of patients with metastases < 2 mm, de Boer et al. found that the presence of this small-volume disease in the axillary lymph node was associated with a reduction in survival, which was independent of other prognostic variables (hazard ratio (HR) = 1.44; 95% CI 1.29–1.62).44 Data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial (Fig. 7.7) showed that any occult metastases were associated with a small increased risk of death (HR = 1.40; 95% CI 1.05–1.86), any outcome event (HR = 1.31; 95% CI 1.07–1.60) and distant disease (HR = 1.30; 95% CI 1.02–1.66).45 On subgroup analysis, isolated tumour cells had a smaller increased risk of death, any outcome event and distant disease than micrometastases. The hazard ratios (95% CI) for these three outcomes were 1.27 (1.04–1.54), 1.18 (1.02–1.33) and 1.19 (1.00–1.41), respectively, for isolated tumour cells, and 1.60 (1.32–1.96), 1.38 (1.15–1.60) and 1.41 (1.19–1.68), respectively, for micrometastases.45 The absolute differences in outcomes were, however, very small and the recommendations of the trial committee were that the differences were such that a search for occult metastases by immunohistochemistry on a routine basis when assessing sentinel lymph nodes could not be supported. The ‘Micrometastases and Isolated Tumour Cells: Relevant and Robust, Or Rubbish’ (MIRROR) Study also found that patients with both isolated tumour cells and those with micrometastases who did not receive systemic adjuvant therapy had a higher adjusted event rate than node-negative patients (HR = 1.50, 95% CI 1.15–1.94 and HR = 1.56, 95% CI 1.15–2.13, respectively).46
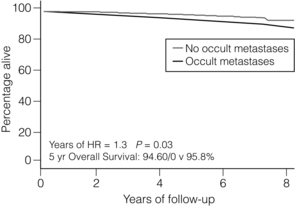
Figure 7.7 Data from NSABP B32 trial comparing overall survival in patients with no metastases vs occult metastases demonstrated on immunohistochemistry.
A number of authors have evaluated the significance of isolated tumour cells and micrometastases in terms of the likelihood of there being other non-sentinel node metastases (Table 7.2). Cserni et al., in a meta-analysis of 25 studies, reported that up to 57% of patients with isolated tumour cells or micrometastases had non-sentinel node involvement.47 The pooled estimates for non-sentinel node involvement vary with tumour burden in the sentinel nodes, and are 20.2% (95% CI 15.5–24.9%) for micrometastases and 9.4% (95% CI 6.2–12.6%) for isolated tumour cells.47 In keeping with these data, van Deurzen et al. found that 12.3% (95% CI 9.5–15.7%) of patients with isolated tumour cells or micrometastases had non-sentinel node involvement.48
Non-sentinel node metastases
Given that a significant proportion of patients with a positive sentinel node will have no further disease in their non-sentinel lymph nodes, a number of authors have developed nomograms and clinical prediction models in an effort to spare some patients with involved sentinel nodes the morbidity of axillary node dissection. Perhaps the best known of these is the Memorial Sloan-Kettering Nomogram developed by Kim van Zee and colleagues. This model predicts the probability of non-sentinel node status based on a number of factors, including pathological tumour size, grade, lymphovascular invasion, multifocality, oestrogen receptor status, the number of positive and negative sentinel lymph nodes, and the method used to identify sentinel lymph node metastases (either by immunohistochemistry, routine haematoxylin–eosin staining, serial sectioning with haematoxylin–eosin, or frozen section).49 A number of other models have also been created, including the Cambridge nomogram,50 the Mayo nomogram,51 the Tenon model,52 the MD Anderson score53 and the Stanford nomogram.54 All of these include some elements that are often available only after the sentinel nodes have been examined histologically, such as lymphovascular invasion and/or size of sentinel node metastases. These elements are clearly important in predicting non-sentinel node metastases, but in order to assist intraoperative decision-making, the Louisville clinical prediction model was developed as a simple clinical prediction model using variables that are available either pre- or intraoperatively, such as tumour size category, number of positive sentinel nodes and number of sentinel nodes removed.55
A recent study by Chen et al. evaluated all of these models in a small independent cohort of 81 patients.56 All models were similar, with areas under the curve ranging from 0.54 (95% CI 0.45–0.63) for the Stanford model to 0.73 (95% CI 0.65–0.81) for the Cambridge. Aside from these two outliers, all other models had areas under the curve ranging from 60% to 68%. The false-negative rate for the prediction models ranged from 0% in the Louisville model to 19.8% in the Tenon model. There is a trade-off, however, between the false-negative rate and the number of patients who fall below the threshold for avoiding axillary dissection – so that models such as the Louisville model that had a very low false-negative rate often were more stringent and allowed fewer patients to avoid an axillary dissection.
Is axillary node dissection necessary in node-positive patients?
While nomograms and models can help to define a cohort of node-positive patients who are at low risk of having non-sentinel node disease, the results of recent randomised controlled trials appear to have rendered such models irrelevant. The American College of Surgeons Oncology Group (ACOSOG) Z-0011 trial randomised 891 sentinel node-positive patients to complete axillary node dissection versus no axillary node dissection.57 All patients received whole-breast tangential irradiation, which will have covered some of the lower two-thirds of the axilla, although no defined characteristics of the axillary radiation dose were specified in the trial and radiation fields used have yet to be reported. The median total number of nodes removed was 17 in the axillary lymph node dissection and two in the sentinel lymph node biopsy group alone. The median number of nodes with histologically demonstrated involvement was one in both groups; however, 27.3% of patients having a subsequent axillary dissection arm had non-sentinel node metastases.
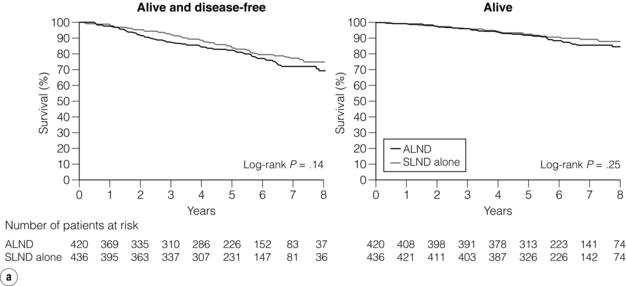
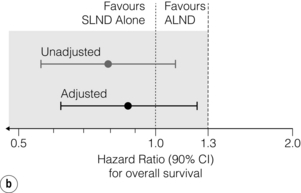
With a median follow-up of 6.3 years, there was no significant difference in axillary recurrence between the patients who had a complete axillary node dissection (0.5%) and those who did not (0.9%). The adjusted hazard ratio for locoregional recurrence comparing complete dissection with sentinel node biopsy alone was 0.825 (95% CI 0.263–2.586, P = 0.7411).55 Rates of locoregional recurrence did not differ between the two arms; local recurrence-free survival at 5 years was 96.7% (95% CI 94.7–98.6%) in the sentinel lymph node biopsy alone group, and 95.7% (95% CI 93.6–97.9%) in the axillary lymph node dissection group (P = 0.28). Disease-free survival did not differ significantly between the two treatment arms; the 5-year disease-free survival was 83.9% (95% CI 80.2–87.9%) for the sentinel lymph node biopsy alone group and 82.8% (95% CI 78.3–86.3%) for the axillary lymph node dissection group (Fig. 7.8). At a median follow-up of 6.3 years, there was no evidence that sentinel lymph node biopsy compared with axillary lymph node dissection resulted in a statistically significant inferior survival (Fig. 7.8). The unadjusted hazard ratio between the two groups was in favour of sentinel lymph node biopsy with a hazard ratio of 0.79 (90% CI 0.56–1.10). The hazard ratio for overall survival, adjusting for adjuvant therapy and age, was 0.87 (90% CI 0.63–1.23). The rates of wound infections, axillary seromas and paraesthesia were significantly higher for the axillary lymph node dissection group (70% vs. 25%, P < 0.001). Lymphoedema was also significantly more common in the axillary lymph node dissection group (P < 0.001), in accordance with other comparisons of sentinel lymph node biopsy and axillary lymph node clearance.
The International Breast Cancer Study Group (IBCSG) 23-01 recently reported as yet unpublished results at the San Antonio Breast Cancer Symposium in December 2011. In this trial, 934 patients with sentinel nodes involved with micrometastases were randomised to complete axillary node dissection compared with no complete axillary node dissection.58 Unlike the ACOSOG Z-0011 trial, only 75% of patients had breast-conserving surgery; 89% of patients in the group randomised to axillary node dissection received adjuvant breast or chest wall radiation therapy, and 92% of patients randomised to no complete axillary dissection received radiation. Five-year disease-free survival was 87.3% for the 464 patients who underwent axillary node dissection and 88.4% for the 467 patients who did not undergo this procedure, with low rates of axillary recurrence in both groups. Locoregional recurrences were seen in five patients (1.1%) of those having no axillary dissection: two in the axilla alone, one in the axilla together with internal mammary nodes, one in the breast and axilla, and one in the internal mammary nodes alone. There was only one patient in the axillary dissection group (0.2%) who developed locoregional recurrence and this was an isolated axillary recurrence. Overall survival was 98% at 5 years in both groups.
The European Organisation of Research and Treatment of Cancer (EORTC) 10981 ‘After Mapping of the Axilla, Radiotherapy or Surgery?’ (AMAROS) trial aims to assess whether surgery or radiation to the axilla produce similar outcomes in sentinel node-positive patients. Between 2001 and 2005, 2000 sentinel node-positive patients were enrolled in the trial, and were randomised to either complete axillary node dissection or axillary radiotherapy, and the trial continues.59 Long-term recurrence and survival data are as yet unpublished, but this trial should provide valuable information regarding the relative utility of dedicated axillary radiation and axillary dissection in providing locoregional control as well as the relative rates of lymphoedema.
Complications of axillary surgery
Much of the significant effort that has gone into predicting non-sentinel node metastases and trials has been aimed at avoiding axillary dissection in the setting of a positive sentinel node and thus avoiding the complications associated with axillary surgery. While even sentinel node biopsy is not innocuous, because of the risk of anaphylaxis associated with blue dye,60 the risks of numbness, lymphoedema and decreased range of motion are significantly less with sentinel node compared with full axillary dissection.61–63 The ‘Axillary Lymphatic Mapping Against Nodal Axillary Clearance’ (ALMANAC) trial demonstrated, in a multicentre randomised controlled trial, that patient-reported quality of life and arm function were significantly better with sentinel node biopsy than axillary node dissection.64 In addition, using both subjective and objective criteria, they found that sentinel node biopsy was associated with less lymphoedema (relative risk (RR) = 0.37; 95% CI 0.23–0.60; 5% vs. 13%) and numbness (RR = 0.37; 95% CI 0.25–0.5; 11% vs. 31%) at 1 year than axillary dissection.64
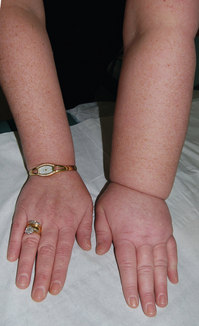
Lymphoedema is one of the most dreaded complications of axillary surgery and/or radiation therapy. The rate of lymphoedema varies in part by the definition that is used, and can range from subclinical swelling that is detected by bioimpedence monitoring or perometer measurements to massive elephantiasis of the upper extremity. In general, however, up to 20% of patients may have some degree of lymphoedema after axillary node dissection. In a meta-analysis of 22 studies, axillary node dissection had a relative risk of 3.07 (95% CI 2.20–4.29) of developing lymphoedema compared to sentinel node biopsy alone.65 Axillary radiation therapy was also associated with a relative risk of 2.97 (95% CI 2.06–4.28) of developing lymphoedema compared to no axillary radiation.65
Given the longevity of breast cancer survivors, the impact of axillary surgery on morbidity and quality of life long term is important. Even in patients undergoing a sentinel node biopsy alone, Liu et al., in a systematic review, demonstrated a prevalence of late morbidity that included pain (7.5–36%), decreased range of motion (0–31%), oedema (0–14%), weakness (11–19%) and sensory disorders (1–66%).66 These effects decreased over time, and were more prevalent in young women.66 Similar findings were noted in the NSABP B-32 trial, which found that while arm morbidity was less in the sentinel node biopsy group (as opposed to those who underwent axillary node dissection), less than 15% of patients in either group reported significant symptoms or limitations beyond 12 months.67 Other prospective trials evaluating the morbidity of sentinel node biopsy compared with axillary node dissection have reported similar findings (Table 7.3).
Table 7.3
Morbidity of sentinel node biopsy vs. axillary node dissection in prospective trials
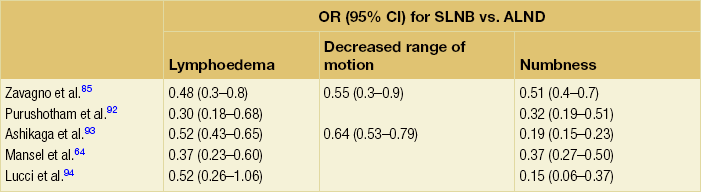
ALND, axillary lymph node; OR, odds ratio; SLNB, sentinel lymph node biopsy.
Some have attempted to reduce morbidity following sentinel node biopsy by adopting a technique in which the lymphatics of the arm are also mapped, and the nodes draining the upper extremity are avoided.68,69 Some have argued that this ‘axillary reverse mapping’ or ARM technique does not reliably identify arm lymph nodes, and that the ARM nodes may harbour metastases or be the same nodes as those draining the breast in a minority of patients.70 As yet, the true reduction in lymphoedema rates associated with this technique remains to be determined, and this procedure remains investigational.
Imaging techniques
A number of authors have investigated various imaging modalities as an alternative or adjunct to surgical staging of the axilla. The most well established of these is ultrasound, which is often combined with fine-needle aspiration for suspicious lymph nodes (Fig. 7.8); however, other techniques such as positron emission tomography and magnetic resonance imaging have also been described.
Ultrasound and fine-needle aspiration
Axillary ultrasound and fine-needle aspiration or core biopsy has been embraced as a means of staging the axilla to avoid sentinel node biopsy in node-positive patients, allowing them to proceed directly to axillary node dissection (Fig. 7.9). Given the findings from the Z-0011 trial, the value of this approach has been questioned as not all node-positive patients may need axillary dissection. Sentinel node biopsy in such patients may still be valuable to determine whether less than three sentinel nodes are involved. A number of studies have found the sensitivity of ultrasound with fine-needle aspiration to range from 21% to 86%, with higher rates being found in patients with more extensive lymph node involvement.71 A recent meta-analysis found the sensitivity and specificity of ultrasound in the detection of lymph node metastases to be 79.6% (95% CI 74.1–84.2) and 98.3% (95% CI 97.2–99.0), respectively.72 Furthermore, the rate of ultrasound-guided needle biopsy yielding an insufficient sample was 4.1% (interquartile range 0–10.9%).72 The role of ultrasound and fine-needle aspiration may therefore need to be redefined. While some may argue that patients who have a positive ultrasound-guided fine-needle aspiration should proceed directly to axillary node dissection given the propensity for extensive disease in this population, others have suggested that patients with limited nodal disease identified in this manner could be treated with sentinel node biopsy. This would give the option of avoiding axillary dissection in some, given the lack of survival benefit of removing lymph nodes, and the widespread use of adjuvant therapy, which may not change even as the number of positive nodes involved increases.
Positron emission tomography (PET)
PET has been explored as a potential means of detecting disease in the axilla, but to date results have been disappointing. In a meta-analysis of 26 studies, Cooper et al. found the sensitivity of PET to be 63% (range 20–100%; 95% CI 52–74%), with a specificity of 94% (range 75–100%; 95% CI 91–96%). The sensitivity was somewhat improved in studies evaluating PET-only (versus PET–computed tomography (CT); 66% vs. 56%, respectively); specificity, however, was improved by PET-CT (versus PET-only; 96% vs. 93%, respectively).73 Sensitivity was 11% for micrometastases (based on five studies of 63 patients) and 57% for macrometastases (based on four studies of 111 patients).73
Magnetic resonance imaging (MRI)
MRI has become popular as a breast imaging technique and its utility in evaluating axillary lymph nodes has also been investigated. In a meta-analysis of nine studies, Harnan et al. found that the ranges of sensitivity and specificity of this technique were 65–100% and 54–100%, respectively.74 Pooled estimates for sensitivity and specificity were 90% for each, but these varied significantly by technique. Ultra-small superparamagnetic iron oxide-enhanced MRI had the highest sensitivity and specificity (98% and 96%, respectively), gadolinium-enhanced MRI performed less well (with a sensitivity and specificity of 88% and 73%, respectively) and magnetic resonance spectroscopy (based on only one study) had sensitivity and specificity of 65% and 100%, respectively.73 At the moment, imaging (whether by ultrasound, PET or MRI) has limited utility without histological confirmation of lymph node metastases.
Management of lymphoedema (see Box 7.1)
Given the need for histological confirmation of lymph node metastases, surgical evaluation of axillary nodes remains a major component of staging for patients with breast cancer. Such surgical excision of nodes, the vast majority of which are normal, carries with it the risk of lymphoedema (Fig. 7.10), albeit in a relatively small proportion of patients. When it does occur, however, there are several techniques for management of this upper extremity swelling.
Complete decongestive therapy
Often considered the primary modality for managing lymphoedema, complete decongestive therapy consists of manual lymph drainage, compression garments/bandaging, lymphatic/decongestive exercises and self-care education.75 Intermittent pneumatic compression is often used as an adjunct to manual drainage.76
Pharmaceutical interventions
While pharmaceutical interventions with diuretics, benzopyrones and flavonoid derivatives have been investigated as potential therapies in the setting of lymphoedema, none have been found to be universally efficacious, and these modalities have been largely abandoned.77
Surgical therapies for lymphoedema
Historic operations, described by Sistrunk78 and Thompson,79 aimed to create a connection between the superficial and deep lymphatic collection systems by resecting subcutaneous soft tissue and deep fascia. While these procedures may have reduced the size of lymphoedematous extremities, they also caused extensive scarring and morbidity without any objective evidence that new lymphatic pathways were created. Hence, these procedures have been abandoned and are of historic interest only. Liposuction has also been proposed as a technique that could reduce the volume of lymphoedematous extremities;80 however, given the risk of injury to residual lymphatics, this technique may actually exacerbate this condition. It is effective in arms with non-pitting swelling with significant excess of fat in the subcutaneous tissue.
Other procedures, aimed at draining excess lymphatic fluid into other lymphatic or venous vessels, have also been described. In particular, some have advocated placing greater omental flaps into the upper extremity while maintaining continuity with the ipsilateral gastroepiploic vessels through a subcutaneous tunnel in the chest.81 The concept behind this procedure is that the omentum has more lymphatic vessels and that excess lymph fluid may be drained through the omental graft, although there is no objective evidence for this. Some have had success with lymphaticolymphatic bypass using lymphatic vessels of the medial thigh as a free composite graft to bypass routes between the upper arm and the supraclavicular region.82 Others have similarly found significant prolonged improvement in lymphoedema using a lymphaticovenular bypass;83 however, these procedures are still anecdotal and not widely adopted.
Finally, microvascular lymph node transfer in which inguinal lymph nodes are transplanted into the axilla as part of a composite soft tissue graft has been suggested.84 Although the hypothesis is that this procedure would sprout new lymphatics that would allow improved lymphatic drainage, there are no data to demonstrate that lymphatics regenerate from these nodes. Therefore, surgical management of lymphoedema remains investigational.
References
1. American Joint Committee on Cancer. Breast. In: Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A., eds. AJCC cancer staging handbook. 7th ed. New York: Springer-Verlag; 2010:417–460.
2. Fisher, B., Jeong, J.H., Anderson, S., et al, Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575. 12192016
3. Veronesi, U., Cascinelli, N., Mariani, L., et al, Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. 12393819
4. Cabanas, R.M., An approach for the treatment of penile carcinoma. Cancer. 1977;39(2):456–466. 837331
5. Morton, D.L., Wen, D.R., Wong, J.H., et al, Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. 1558490
6. Krag, D.N., Weaver, D.L., Alex, J.C., et al, Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2(6):335–339. 8130940
7. Giuliano, A.E., Sentinel lymphadenectomy in primary breast carcinoma: an alternative to routine axillary dissection. J Surg Oncol. 1996;62(2):75–77. 8649044
8. Giuliano, A.E., Kirgan, D.M., Guenther, J.M., et al, Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. 8092905
9. McMasters, K.M., Tuttle, T.M., Carlson, D.J., et al, Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18(13):2560–2566. 10893287 Multi-institutional, prospective validation of the sentinel node biopsy technique.
10. Krag, D., Weaver, D., Ashikaga, T., et al, The sentinel node in breast cancer – a multicenter validation study. N Engl J Med. 1998;339(14):941–946. 9753708 Multi-institutional, prospective validation of the sentinel node biopsy technique.
11. Veronesi, U., Paganelli, G., Galimberti, V., et al, Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864–1867. 9217757 Multi-institutional, prospective validation of the sentinel node biopsy technique.
12. Krag, D.N., Anderson, S.J., Julian, T.B., et al, Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. 20863759 The NSABP B-32 trial randomised patients to sentinel node biopsy followed by routine axillary dissection versus sentinel node biopsy followed by axillary node dissection in node-positive patients only.
13. Veronesi, U., Paganelli, G., Viale, G., et al, Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91(4):368–373. 10050871
14. Tafra, L., Lannin, D.R., Swanson, M.S., et al, Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233(1):51–59. 11141225
15. McMasters, K.M., Wong, S.L., Martin, R.C., et al, Dermal injection of radioactive colloid is superior to peritumoural injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001;233(5):676–687. 11360892
16. Chagpar, A.B., Martin, R.C., Scoggins, C.R., et al, Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery. 2005;138(1):56–63. 16003317 Large cohort study demonstrating that the dual injection technique was superior to either injection of radiotracer or blue dye alone in terms of identification rates.
17. Rodier, J.F., Velten, M., Wilt, M., et al, Prospective multicentric randomized study comparing periareolar and peritumoural injection of radiotracer and blue dye for the detection of sentinel lymph node in breast sparing procedures: FRANSENODE trial. J Clin Oncol. 2007;25(24):3664–3669. 17485709 Prospective randomised trial of periareolar vs. peritumoural injection using dual technique that validates the use of periareolar injection. Further, this study demonstrated that the overall identification rates (either blue or hot nodes) were higher than for either blue or hot nodes alone.
18. Tuttle, T.M., Colbert, M., Christensen, R., et al, Subareolar injection of 99mTc facilitates sentinel lymph node identification. Ann Surg Oncol. 2002;9(1):77–81. 11829434
19. Klimberg, V.S., Rubio, I.T., Henry, R., et al, Subareolar versus peritumoural injection for location of the sentinel lymph node. Ann Surg. 1999;229(6):860–864. 10363900
20. Chagpar, A., Martin, R.C., III., Chao, C., et al, Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg. 2004;139(6):614–618. 15197087
21. Spillane, A.J., Brennan, M.E., Accuracy of sentinel lymph node biopsy in large and multifocal/multicentric breast carcinoma – a systematic review. Eur J Surg Oncol. 2011;37(5):371–385. 21292433
22. McMasters, K.M., Wong, S.L., Tuttle, T.M., et al, Preoperative lymphoscintigraphy for breast cancer does not improve the ability to identify axillary sentinel lymph nodes. Ann Surg. 2000;231(5):724–731. 10767794
23. Mathew, M.A., Saha, A.K., Saleem, T., et al, Pre-operative lymphoscintigraphy before sentinel lymph node biopsy for breast cancer. Breast. 2010;19(1):28–32. 19913418
24. Port, E.R., Garcia-Etienne, C.A., Park, J., et al, Reoperative sentinel lymph node biopsy: a new frontier in the management of ipsilateral breast tumour recurrence. Ann Surg Oncol. 2007;14(8):2209–2214. 17268882
25. Kaur, P., Kiluk, J.V., Meade, T., et al, Sentinel lymph node biopsy in patients with previous ipsilateral complete axillary lymph node dissection. Ann Surg Oncol. 2011;18(3):727–732. 20593244
26. Layfield, D.M., Agrawal, A., Roche, H., Cutress, R.I., Intraoperative assessment of sentinel lymph nodes in breast cancer. Br J Surg. 2011;98(1):4–17. 20812233
27. Tew, K., Irwig, L., Matthews, A., et al, Meta-analysis of sentinel node imprint cytology in breast cancer. Br J Surg. 2005;92(9):1068–1080. 16106479
28. Zhou, W.B., Liu, X.A., Dai, J.C., et al, Meta-analysis of sentinel lymph node biopsy at the time of prophylactic mastectomy of the breast. Can J Surg. 2011;54(5):300–306. 21651834
29. Nasser, S.M., Smith, S.G., Chagpar, A.B., The role of sentinel node biopsy in women undergoing prophylactic mastectomy. J Surg Res. 2010;164(2):188–192. 20869074
30. Dillon, M.F., McDermott, E.W., Quinn, C.M., et al, Predictors of invasive disease in breast cancer when core biopsy demonstrates DCIS only. J Surg Oncol. 2006;93(7):559–563. 16705731
31. Goyal, A., Douglas-Jones, A., Monypenny, I., et al, Is there a role of sentinel lymph node biopsy in ductal carcinoma in situ?: analysis of 587 cases. Breast Cancer Res Treat. 2006;98(3):311–314. 16552627
32. Jackman, R.J., Burbank, F., Parker, S.H., et al, Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001;218(2):497–502. 11161168
33. Yen, T.W., Hunt, K.K., Ross, M.I., et al, Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200(4):516–526. 15804465
34. Shapiro-Wright, H.M., Julian, T.B., Sentinel lymph node biopsy and management of the axilla in ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):145–149. 20956820
35. Javan, H., Gholami, H., Assadi, M., et al, The accuracy of sentinel node biopsy in breast cancer patients with the history of previous surgical biopsy of the primary lesion: systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2012;38(2):95–109. 22138234
36. Lyman, G.H., Giuliano, A.E., Somerfield, M.R., et al, American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. 16157938 ASCO guidelines for sentinel node biopsy.
37. Schwartz, G.F., Giuliano, A.E., Veronesi, U., Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94(10):2542–2551. 12173319 International consensus conference for role of sentinel node biopsy.
38. Chung, A., Giuliano, A., Axillary staging in the neoadjuvant setting. Ann Surg Oncol. 2010;17(9):2401–2410. 20229221
39. Vigario, A., Sapienza, M.T., Sampaio, A.P., et al, Primary chemotherapy effect in sentinel node detection in breast cancer. Clin Nucl Med. 2003;28(7):553–557. 12819407
40. van Deurzen, C.H., Vriens, B.E., Tjan-Heijnen, V.C., et al, Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer. 2009;45(18):3124–3130. 19716287
41. Boughey, J.C., Suman, V.J., Mittendorf, E.A., et al, The role of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4, N1-2) who receive neoadjuvant chemotherapy – results from the ACOSOG Z1071 trial. Cancer Research. December 15;72(24). 2012 Supplement 3. 19716287
42. Kuehn, T., Bauerfeind, I.G.P., Fehm, T., et al, Sentinel Lymph Node Biopsy Before or After Neoadjuvant Chemotherapy – Final Results from the Prospective German, multiinstitutional SENTINA-Trial. Cancer Research. December 15;72(24). 2012 Supplement 3. 19716287
43. Nasser, I.A., Lee, A.K., Bosari, S., et al, Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol. 1993;24(9):950–957. 7504652
44. de Boer, M., van Dijck, J.A., Bult, P., et al, Breast cancer prognosis and occult lymph node metastases, isolated tumour cells, and micrometastases. J Natl Cancer Inst. 2010;102(6):410–425. 20190185
45. Weaver, D.L., Ashikaga, T., Krag, D.N., et al, Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364(5):412–421. 21247310
46. de Boer, M., van Deurzen, C.H., van Dijck, J.A., et al, Micrometastases or isolated tumour cells and the outcome of breast cancer. N Engl J Med. 2009;361(7):653–663. 19675329
47. Cserni, G., Gregori, D., Merletti, F., et al, Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91(10):1245–1252. 15376203
48. van Deurzen, C.H., de Boer, M., Monninkhof, E.M., et al, Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst. 2008;100(22):1574–1580. 19001602
49. Van Zee, K.J., Manasseh, D.M., Bevilacqua, J.L., et al, A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–1151. 14654469
50. Pal, A., Provenzano, E., Duffy, S.W., et al, A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95(3):302–309. 17876750
51. Degnim, A.C., Reynolds, C., Pantvaidya, G., et al, Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg. 2005;190(4):543–550. 16164917
52. Barranger, E., Coutant, C., Flahault, A., et al, An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91(2):113–119. 15868438
53. Hwang, R.F., Krishnamurthy, S., Hunt, K.K., et al, Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10(3):248–254. 12679309
54. Kohrt, H.E., Olshen, R.A., Bermas, H.R., et al, New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer 2008; 8:66. 18315887
55. Chagpar, A.B., Scoggins, C.R., Martin, R.C., et al, Prediction of sentinel lymph node-only disease in women with invasive breast cancer. Am J Surg. 2006;192(6):882–887. 17161113
56. Chen, K., Zhu, L., Jia, W., et al, Validation and comparison of models to predict non-sentinel lymph node metastasis in breast cancer patients. Cancer Sci. 2012;103(2):274–281. 22054165
57. Giuliano, A.E., McCall, L., Beitsch, P., et al, Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. 20739842 Findings of locoregional recurrence between the sentinel node biopsy alone vs. the axillary node dissection arms in the ACOSOG Z-0011 trial for sentinel node- positive patients.
58. Galimberti, V., Cole, B., Zurrida, S., et al. Update of International Breast Cancer Study Group Trial 23-01 to compare axillary dissection versus no axillary dissection in patients with clinically node negative breast cancer and micrometastases in the sentinel node. Cancer Res. 2012; 71(Suppl. 24):102s.
59. Straver, M.E., Meijnen, P., van Tienhoven, G., et al, Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17(7):1854–1861. 20300966
60. Bezu, C., Coutant, C., Salengro, A., et al, Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol. 2011;20(1):e55–e59. 21074413
61. Veronesi, U., Paganelli, G., Viale, G., et al, A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. 12904519
62. Schijven, M.P., Vingerhoets, A.J., Rutten, H.J., et al, Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29(4):341–350. 12711287
63. Haid, A., Koberle-Wuhrer, R., Knauer, M., et al, Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: a comparative evaluation. Breast Cancer Res Treat. 2002;73(1):31–36. 12083629
64. Mansel, R.E., Fallowfield, L., Kissin, M., et al, Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. 16670385 Prospective randomised controlled trial demonstrating the lower morbidity associated with sentinel node biopsy compared to standard axillary node dissection.
65. Tsai, R.J., Dennis, L.K., Lynch, C.F., et al, The risk of developing arm lymphoedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972. 19365624
66. Liu, C.Q., Guo, Y., Shi, J.Y., et al, Late morbidity associated with a tumour-negative sentinel lymph node biopsy in primary breast cancer patients: a systematic review. Eur J Cancer. 2009;45(9):1560–1568. 19285858
67. Land, S.R., Kopec, J.A., Julian, T.B., et al, Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project Phase III Protocol B-32. J Clin Oncol. 2010;28(25):3929–3936. 20679600 Patient-reported outcomes in the NSABP B-32 trial demonstating that sentinel node biopsy is associated with less morbidity than axillary node dissection. Long-term morbidity (beyond 12 months), however, is shown to be minimal.
68. Nos, C., Kaufmann, G., Clough, K.B., et al, Combined axillary reverse mapping (ARM) technique for breast cancer patients requiring axillary dissection. Ann Surg Oncol. 2008;15(9):2550–2555. 18618185
69. Thompson, M., Korourian, S., Henry-Tillman, R., et al, Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14(6):1890–1895. 17479341
70. Noguchi, M., Axillary reverse mapping for breast cancer. Breast Cancer Res Treat. 2010;119(3):529–535. 19842033
71. Mainiero, M.B., Regional lymph node staging in breast cancer: the increasing role of imaging and ultrasound-guided axillary lymph node fine needle aspiration. Radiol Clin North Am. 2010;48(5):989–997. 20868896
72. Houssami, N., Ciatto, S., Turner, R.M., et al, Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254(2):243–251. 21597359
73. Cooper, K.L., Harnan, S., Meng, Y., et al, Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37(3):187–198. 21269795
74. Harnan, S.E., Cooper, K.L., Meng, Y., et al, Magnetic resonance for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011;37(11):928–936. 21855267
75. Mondry, T.E., Riffenburgh, R.H., Johnstone, P.A., Prospective trial of complete decongestive therapy for upper extremity lymphedema after breast cancer therapy. Cancer J. 2004;10(1):42–48. 15000494
76. Szolnoky, G., Lakatos, B., Keskeny, T., et al, Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology. 2009;42(4):188–194. 20218087
77. Cormier, J.N., Askew, R.L., Armer, J.M., Lymphedema: a treatment sequelaeAdvanced therapy of breast disease. Shelton, CT: People’s Medical Publishing House, USA, 2012.
78. Sistrunk, W.E., Contribution to plastic surgery: removal of scars by stages; an open operation for extensive laceration of the anal sphincter; the Kondoleon operation for elephantiasis. Ann Surg. 1927;85(2):185–193. 17865614
79. Thompson, N., The surgical treatment of chronic lymphoedema of the extremities. Surg Clin North Am. 1967;47(2):445–503. 6022236
80. O’Brien, B.M., Khazanchi, R.K., Kumar, P.A., et al, Liposuction in the treatment of lymphoedema; a preliminary report. Br J Plast Surg. 1989;42(5):530–533. 2804517
81. Goldsmith, H.S., De los Santos, R., Beattie, E.J., Jr., Relief of chronic lymphedema by omental transposition. Ann Surg. 1967;166(4):573–585. 6061543
82. Baumeister, R.G., Siuda, S., Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85(1):64–74. 2293739
83. Chang, D.W., Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126(3):752–758. 20811210
84. Becker, C., Assouad, J., Riquet, M., et al, Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243(3):313–315. 16495693
85. Zavagno, G., De Salvo, G.L., Scalco, G., et al, A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247(2):207–213. 18216523
86. Veronesi, U., Viale, G., Paganelli, G., et al, Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600. 20195151
87. Nos, C., Harding-MacKean, C., Freneaux, P., et al, Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. 2003;90(11):1354–1360. 14598414
88. Viale, G., Maiorano, E., Pruneri, G., et al, Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319–325. 15650643
89. Houvenaeghel, G., Nos, C., Mignotte, H., et al, Micrometastases in sentinel lymph node in a multicentric study: predictive factors of nonsentinel lymph node involvement – Groupe des Chirurgiens de la Federation des Centres de Lutte Contre le Cancer. J Clin Oncol. 2006;24(12):1814–1822. 16567771
90. Gipponi, M., Canavese, G., Lionetto, R., et al, The role of axillary lymph node dissection in breast cancer patients with sentinel lymph node micrometastases. Eur J Surg Oncol. 2006;32(2):143–147. 16300921
91. van Deurzen, C.H., van Hillegersberg, R., Hobbelink, M.G., Predictive value of tumour load in breast cancer sentinel lymph nodes for second echelon lymph node metastases. Cell Oncol. 2007;29(6):497–505. 18032826
92. Purushotham, A.D., Upponi, S., Klevesath, M.B., et al, Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23(19):4312–4321. 15994144
93. Ashikaga, T., Krag, D.N., Land, S.R., et al, Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. 20648579
94. Lucci, A., McCall, L.M., Beitsch, P.D., et al, Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. 17485711