Chapter 21
The Auditory System
Properties of Sound Waves and Hearing
Inner Ear: Structure of the Cochlea
Mechanoelectrical Transduction
Primary Afferent Innervation and Function
Overview of Central Auditory Pathways
Vascular Supply of the Auditory Brainstem and Cortex
Brainstem Auditory Nuclei and Pathways
Lateral Lemniscus and Its Nuclei
Hearing is one of the most important senses. In combination with vision and the ability to speak, it contributes, in a significant way, to the quality of life. In our daily routine, we unconsciously sort out meaningful sounds from background noise, localize the source of sounds, and react (many times in a reflex mode) to unexpected sounds. About 12% of people in the general population experience a diminution or loss of hearing during their lifetime, which in some cases may represent a significant disability.
OVERVIEW
The auditory apparatus is adapted for receiving sound waves at the tympanic membrane and transmitting auditory signals to the central nervous system. Injury to elements of the peripheral apparatus, such as the ear ossicles, may result in conductive deafness. Alternatively, damage to the cochlea or the cochlear portion of the eighth cranial nerve may result in sensorineural (nerve) deafness. When central auditory pathways are injured, the apparent hearing dysfunction (central deafness) is usually combined with other signs and symptoms. Central lesions seldom result in complete deafness in one ear. These three types of hearing losses are considered in more detail when we discuss the external and middle ear, the cochlea and cochlear nerve, and the central auditory pathways, respectively. To understand the neurophysiologic and audiologic methods used in assessing peripheral and central auditory disorders, it is essential to understand the structure and function of the cochlea and central auditory pathways.
PROPERTIES OF SOUND WAVES AND HEARING
Complex sounds are mixtures of pure tones that are either harmonically related, thus having pitch, or that are randomly related and therefore called noise. The cochlear apparatus is designed to analyze sounds by separating complex waveforms into their individual frequency components.
The frequency of audible sounds is measured in cycles per second, or hertz (Hz). A simple sine wave (Fig. 21-1) depicts the cyclic increase and decrease in the compression of air molecules that constitutes a pure tone. The time interval between two peaks is the period, the distance traveled is the wavelength, and the number of cycles per second is the frequency. The intensity is the peak-to-trough amplitude of force at the eardrum.
The normal frequency range for human hearing is 50 to 16,000 Hz. Most human speech takes place in the range of 100 to 8000 Hz, and the most sensitive part of the range is between 1000 and 3000 Hz. Exposure to loud noise can result in selective hearing loss for certain frequencies, and normal aging may reduce the range.
The hearing apparatus is exquisitely sensitive to sound intensity over an enormous dynamic range. Intensity of sound is related to the perception of loudness and is usually measured in units called decibels (dB). Intensity is also related to a measure of sound pressure level at the tympanic membrane. A sound that has 10 times the power of a just-audible sound is said to have a 20-dB sound pressure level. Normal conversational levels of sound are about 50 dB. In our industrial society, we are exposed routinely to noise in the 85- to 100-dB range, such as city traffic, lawn mowers, vacuum cleaners, and even a portable music player’s headphones. Other common examples of noises above 120 dB that may cause varying degrees of discomfort include rock concerts, thunderclaps, firecrackers, and jet engines.
The brain derives the location of a sound by computing differences in the shape, timing, and intensity of the waveforms that reach each ear. The path of the sound is affected by the distance to the ears and by obstacles such as the head (Fig. 21-1). Thus interaural time and intensity differences are related to the angle between the direction in which the head is pointing and the direction of the sound source. Interaural time differences are more important for localizing low-frequency sounds, whereas interaural intensity differences are more important for localizing high-frequency sounds.
PROCESSING OF SOUND: THE EAR
External (Outer) Ear
Sound waves are captured by the external ear (pinna) and channeled through the external auditory meatus to the tympanic membrane (Fig. 21-2A). Resonance features of the pinna and meatus enhance some frequencies more than others in a direction-dependent fashion. For example, sounds coming toward the back of the head are baffled compared with those coming toward the side of the head. Monaural (single-ear) localization depends on such cues, and accuracy in localizing sound is impaired by damage to the pinna.
Middle Ear
The middle ear or tympanic cavity is an air-filled space in the temporal bone that is interposed between the tympanic membrane and the inner ear structures (Fig. 21-2A). Sounds are transmitted across the space from the tympanic membrane to the fluid-filled inner ear by a chain of three bony ossicles: the malleus, incus, and stapes. On one end of this chain, the arm of the malleus is attached to the tympanic membrane, and at the other end, the footplate of the stapes fits into the oval window of the membranous labyrinth of the inner ear. The three bones act as levers to reduce the magnitude of movements of the tympanic membrane while increasing their force at the oval window.
The mechanical stiffness of the ossicle chain acts to compensate for the difference in impedance between air and fluid environments (a function called impedance matching) so that there is optimal transfer of energy between the two media. The stiffness of the ossicle chain can also be modified by two muscles of the middle ear, the tensor tympani and stapedius muscles (middle ear reflex). Diseases such as otosclerosis and otitis media result in conductive hearing loss by affecting the efficiency of the ossicle movement. Otosclerosis, the cause of middle ear conductive hearing loss in about half of cases, may be an inherited disease and is characterized by tissue overgrowth and resultant fixation of the stapes in the oval window. Otitis media is an inflammation of the middle ear and may be accompanied by the accumulation of pus or exudate. In addition, fractures of the temporal bone with direct damage to the ossicles, or indirect damage by bleeding into the middle ear, may result in a conduction deafness.
Conduction Deafness
A conductive deafness is a deficit related to an obstructed, or altered, transformation of sound to the tympanic membrane or through the ossicle chain of the middle ear. For example, damage to the pinna results in a failure of sound waves to be properly conducted to the auditory meatus. In addition, infection involving the auditory canal (otitis externa, sometimes called swimmer’s ear), inflammation of or trauma to the tympanic membrane, and even the excessive accumulation of cerumen (wax) in the auditory canal are other causes of conduction deafness. The deficit experienced by the patient may range from decreased hearing to total deafness in the affected ear. Depending on the cause, conduction deafness may resolve with medication or by removal of the obstruction.
Inner Ear: Structure of the Cochlea
The cochlea is named for its similarity to a conch shell (Fig. 21-2B). Its main elements include the fluid-filled membranous labyrinth, specialized sensory epithelium of the organ of Corti, and neurons of the spiral ganglion with their peripheral and central axonal branches.
The membranous cochlea, the coiled portion of the inner ear, is encased in the osseous cochlea and consists of three spiraling chambers. The cochlea makes approximately two and two-thirds turns from base to apex. Uncoiled, it is about 34 mm long. The base of the cochlear spiral is connected to the saccule of the membranous labyrinth by the ductus reuniens.
The central chamber of the membranous cochlea is the cochlear duct, also called the scala media (Fig. 21-2B). Above it, the scala vestibuli is positioned to communicate with the vestibule, the portion of the membranous inner ear between the oval window and the cochlea. Below, the scala tympani ends at the round window, which separates this space from the middle ear cavity. In cross section, the scala media is bounded by the basilar membrane below, the vestibular or Reissner membrane above, and the stria vascularis externally (Figs. 21-2B and 21-3). The screw-like bony core of the cochlea is the modiolus. A spiral osseous lamina extends outward from the modiolus to join the basilar membrane. The basilar membrane, in turn, is continuous laterally with the spiral ligament. The scala vestibuli and tympani are filled with perilymph. The endolymph, which fills the cochlear duct, is elaborated by the cells and rich capillary bed of the stria vascularis (Fig. 21-3).
Figure 21-3. Cross section through a typical turn of the membranous cochlea.
The organ of Corti is the specialized sensory epithelium resting on the basilar membrane (Fig. 21-3). It is composed of inner and outer hair cells, supporting cells, and the tectorial membrane. The inner hair cells are separated from the outer hair cells by the tunnel of Corti (Fig. 21-3). This tunnel is formed by the filamentous arches of the inner and outer pillar cells and is filled with fluid.
Inner hair cells form a single line spiraling from base to apex, and the outer hair cells form three parallel lines that follow the same course (Fig. 21-4). Once damaged, human hair cells do not regenerate. Research has not yet found a way to augment this. It is uncertain how many of the inner (about 3500) or outer (about 12,000) hair cells must be lost to disease, trauma, or aging before a just-noticeable sensorineural hearing loss ensues. Projecting from the apical surface of each hair cell is a hair bundle consisting of 50 to 150 stereocilia arranged in curving rows (Fig. 21-4). Each hair bundle is polarized so that the longest stereocilia are on the outer border (Fig. 21-4), and the rows of stereocilia are linked by filamentous material at their tips.
The tectorial membrane is a gelatinous arm that extends outward over the sensory epithelium from the limbus of the osseous spiral lamina (Fig. 21-4). The taller stereocilia in each hair bundle are in contact with or embedded in the tectorial membrane. Consequently, movement of the basilar membrane and the organ of Corti will bend the stereocilia against the tectorial membrane and cause a graded depolarization of the hair cells.
The bony modiolus, around which the cochlear duct turns, houses the spiral ganglion (Figs. 21-2 and 21-3). At the edge of the osseous spiral lamina, the peripheral processes of the bipolar cells of this ganglion lose their myelin and pass through perforations to the basilar membrane, where they synapse on the base of the inner and outer hair cells (Fig. 21-4). The central processes of the spiral ganglion cells form the cochlear portion of the vestibulocochlear nerve (cranial nerve VIII). Efferent fibers to the cochlea either spiral along the inner part of the basilar membrane to synapse on inner hair cells or travel radially across the tunnel of Corti to contact outer hair cells (Fig. 21-4).
Mechanoelectrical Transduction
Inner hair cells are extremely sensitive transducers that convert the mechanical force applied to the hair bundle into an electrical signal (Fig. 21-4). Endolymph, like extracellular fluid, has a high concentration of potassium. In contrast, perilymph, like cerebrospinal fluid, has a high concentration of sodium. As indicated in Figure 21-4, the potential difference between the endolymph and the perilymph is +80 mV. This endolymphatic potential appears to be due to the selective secretion and absorption of ions by the stria vascularis. At the same time, ion pumps in the hair cell membrane produce a resting intracellular potential of about −70 mV (Fig. 21-4).
As the basilar membrane moves up in response to fluid movement in the scala tympani, the taller stereocilia are displaced against the tectorial membrane. This causes ion channels at the tips of the stereocilia to open, allowing potassium flow along the electrical gradient to depolarize the cell (Fig. 21-4). The large potential difference between the endolymph and the hair cell interior creates a force of 150 mV that drives potassium into the cell and that increases the range of the cell’s graded electrical response to mechanical displacement. Damage to the stria vascularis results in loss of the endolymphatic potential and failure of mechanoelectrical transduction.
When a hair cell depolarizes, voltage-gated calcium channels at the base of the cell open, and the resulting influx of calcium causes synaptic vesicles to fuse to the cell membrane and to release a neurotransmitter into the synaptic cleft between the hair cell and the cochlear nerve fibers (Fig. 21-4). The transmitter causes depolarization of the afferent fiber, and an action potential is transmitted along the cochlear nerve fiber.
The stimulus-related changes in the electrical potential between the perilymph and the hair cells can be recorded anywhere in the cochlea. The potential varies synchronously with the sound stimulating the ear and is therefore referred to as the cochlear microphonic. This electrical record provides a clinically useful monitor of cochlear function.
Tuning of the Cochlea
The cochlea acts as a frequency filter to separate and analyze individual frequencies from complex sounds. These tuning properties result from anatomic and physiologic characteristics of hair cells and the basilar membrane (Figs. 21-2 and 21-3).
The plunger-like motion of the stapes in the oval window compresses the perilymph. In the fluid medium of the cochlea, this pressure variation imparts motion to the basilar membrane, causing a wave to travel along it (Fig. 21-2C). The basilar membrane is stiffest at its base and becomes progressively more flexible toward its tip. Therefore any given frequency of sound (pure tone) will cause a wave in the basilar membrane that has maximum displacement that is precisely timed and spaced along the membrane. For high tones, this point is close to the base of the cochlea, and for lower frequencies, it is more distal. The response of hair cells to the tone is strongest at the point of greatest displacement. Therefore the position from base to apex along the spiral of the basilar membrane and organ of Corti is directly related to the frequency of the tone that will elicit a response. This relationship of frequency and cochlear position is the basis for the place theory of cochlear tuning. The cochleotopic order and thus tonotopic representation are highly conserved throughout the auditory pathways.
In patients with profound sensorineural hearing loss, some audible sensation may be regained with cochlear implants having a number of fine wire electrodes. Each wire is tuned to a broad frequency band from an electrical receiver, and the wires are implanted so that each stimulates nerve terminals at the appropriate tonotopic point along the cochlear spiral.
Primary Afferent Innervation and Function
The spiral ganglion is made up of two types of bipolar sensory neurons. Type I cells make up 90% to 95% of the cells in the spiral ganglion and have radial branches that synapse with only one or two inner hair cells (Fig. 21-4). As many as 20 or more type I radial fibers converge on each inner hair cell. As a result, type I cochlear nerve fibers respond to a narrow frequency range. In contrast, type II ganglion cells have widely distributed peripheral processes that traverse the tunnel of Corti and synapse with more than 10 outer hair cells (Fig. 21-4). Thus type II cochlear fibers are more sensitive to low-intensity sounds than are type I cells, but they may be less precisely tuned to frequency.
Frequency is coded in the cochlear nerve by the position of afferent fibers along the cochlear spiral. For loud sounds, each afferent fiber responds over a range of frequencies. As the intensity of the sound drops to near threshold, the frequency response range narrows. A tuning curve can be constructed that plots the threshold intensity for each frequency that will elicit a response (Fig. 21-5A). The characteristic frequency is the frequency at which the fiber has the lowest threshold. The discharge pattern of primary afferents over time to pure tone bursts is shown with poststimulus histograms of the number of action potentials summed over many presentations (Fig. 21-5B). Stimulus onset produces an initial high-frequency discharge followed by a lower sustained discharge level that is related to stimulus intensity. When the tone ends, the fiber drops back to a low, spontaneous discharge rate. For low-frequency fibers, the timing of each impulse is phase locked with the stimulus cycle, so that the fiber output preserves the timing information of the signal.
The enormous dynamic range of the human ear to intensity cannot be coded in the response of single nerve fibers. Intensity is coded both by the discharge rate of cochlear nerve fibers and by recruitment of activity in additional afferents as stimulus intensity increases. The discharge rate for the cochlear nerve fibers increases proportionally with intensity over a range of about 40-dB sound pressure level and then plateaus (Fig. 21-5C). At higher stimulus intensities, additional cochlear nerve fibers having sequentially greater thresholds are recruited.
Sensorineural Deafness
Sensorineural deafness (this may sometimes be called nerve deafness) results from damage to the cochlea or to the cochlear root of the vestibulocochlear nerve. The causes of sensorineural deafness are varied and may include repeated exposure to loud noises, treatment with certain antibiotics, infections (such as rubella, mumps, or bacterial meningitis), and tumors at different levels of the neuraxis. When the cause is infectious or inflammatory, it is called labyrinthitis or otitis interna. As is the case with the middle ear, trauma in the form of skull fracture may also result in sensorineural deafness. The deficits experienced by the patient are deafness in the ear on the affected side, varying degrees of tinnitus, a perception of ringing in the ears if the cochlea is damaged, and additional signs and symptoms indicative of damage to the adjacent vestibular root. Repeated exposure to sounds above 85 dB (e.g., industrial noise or loud music) may cause sensorineural hearing loss over time. Immediate sensorineural hearing loss may occur after even a single exposure to a sudden loud blast (e.g., explosion).
WEBER AND RINNE TESTS
As described earlier, conduction deafness and sensorineural deafness may have different causes, and therefore different tests may be used to diagnose these deficits. These tests take advantage of the difference between conduction of sound through air and conduction of sound through bone. To test air conduction, a vibrating tuning fork, usually with a 512-Hz frequency, is held about 1 inch (2.5 cm) from the opening of the auditory canal. Hearing this sound means that the sound waves generated are passing through the external ear and the middle ear; disease or damage in these areas would result in decreased or lost hearing in this ear. To test bone conduction, a vibrating tuning fork is placed directly on the skull. Perceiving these vibrations as sounds means that the sound (vibration) is transmitted directly to the cochlea of the inner ear and bypasses the external ear and the middle ear.
Both the Rinne test and the Weber test use these principles to differentiate conduction deafness from sensorineural deafness. For the Rinne test (bone + air conduction), the tuning fork is placed against the mastoid process. The normal patient perceives the sound in the ear on that side, and after the sound is no longer perceived by bone conduction, the tuning fork is immediately moved to the auditory canal and the sound is again heard (air conduction). If the patient has middle ear disease or deafness, the sound is perceived by bone conduction but not by air conduction; this is a negative Rinne test result. If the patient has sensorineural deafness (cochlea or cochlear nerve damage), the sound is not perceived by bone conduction but is perceived by air conduction; this is a positive Rinne test result.
For the Weber test, the tuning fork is placed on the midline of the skull or forehead. In a patient with normal hearing, the sound (vibration) is perceived about equally in both ears. A patient with sensorineural deafness (e.g., cochlear damage) would perceive the sound of the tuning fork in the normal (opposite) ear, whereas a patient with conduction deafness (canal middle ear obstruction) would perceive the sound of the tuning fork in the ear on the side of the damage.
OVERVIEW OF CENTRAL AUDITORY PATHWAYS
In the major ascending auditory connections from cochlea to cortex, the place code of the cochlea is, as a rule, strictly maintained (Fig. 21-6). Within this tonotopic framework, projections connect similar frequency regions of successive nuclei. Information processing is therefore hierarchical with increasing complexity of feature extraction.
Figure 21-6. Hierarchical order of central auditory pathways.
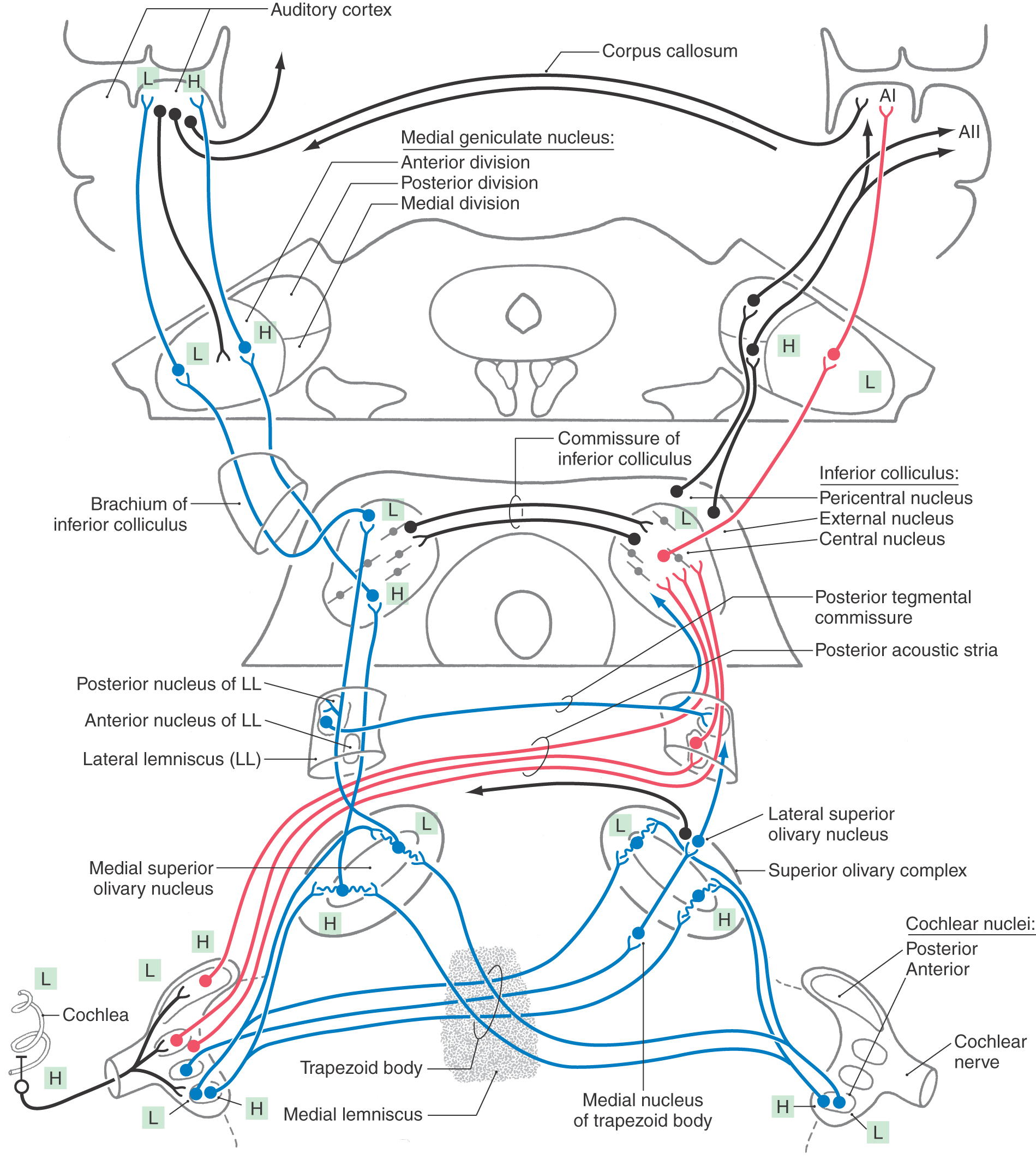
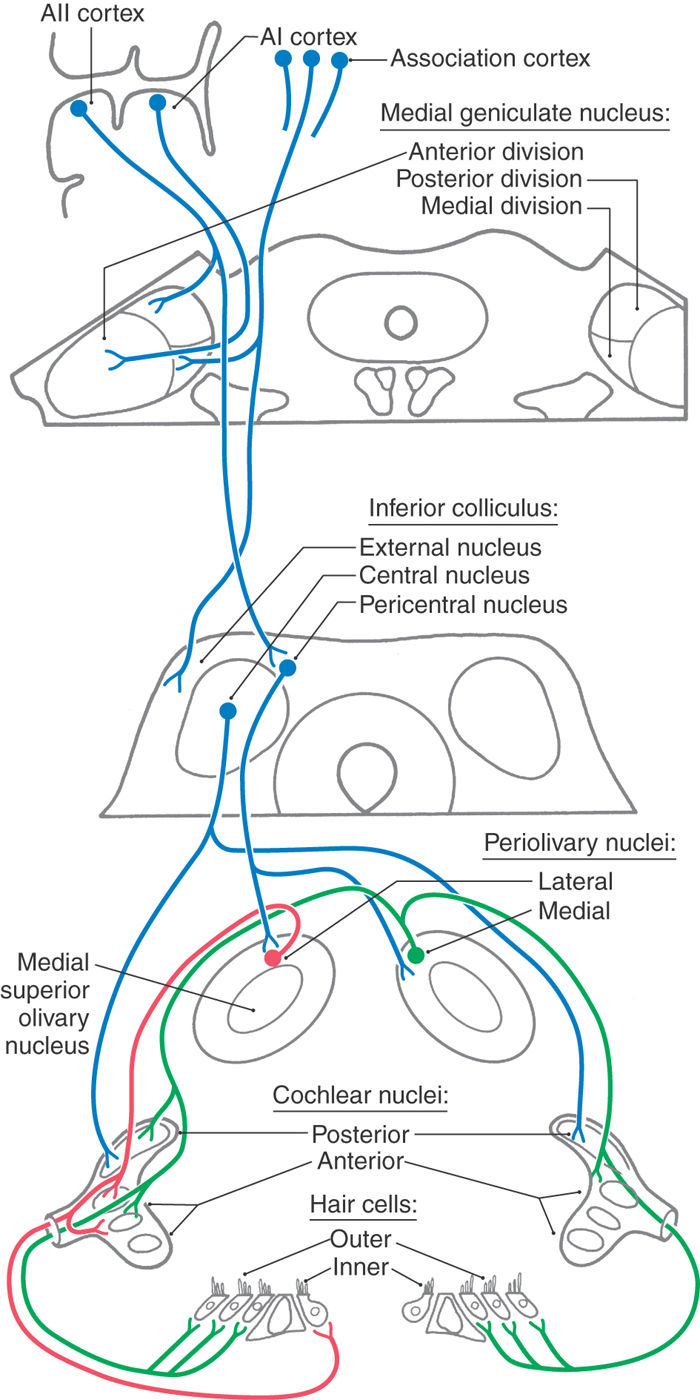
All fibers in the cochlear nerve synapse in the cochlear nuclei. As cochlear information ascends to the auditory cortex, information is distributed through multiple parallel pathways that ultimately converge in the inferior colliculus. The hierarchy of auditory nuclei involved in these parallel pathways includes the cochlear nuclei, nuclei of the superior olive and trapezoid body, nuclei of the lateral lemniscus, and inferior colliculus. Specific fiber bundles that convey this information from one level to the next are the trapezoid body, acoustic stria, and lateral lemniscus. From the midbrain, auditory information is conveyed from the inferior colliculus by its brachium to the medial geniculate nucleus of the thalamus and then through the sublenticular limb of the internal capsule to the auditory cortex. The hierarchy of auditory regions and the fiber bundles that connect one level to the next are summarized in Figures 21-6 and 21-10.
Although fibers conveying auditory input decussate at several levels, this information is routed in one of two orderly ways: (1) monaural information (information about sounds at a single ear) is routed to the contralateral side and (2) binaural information (information about differences between sounds at both ears) is handled by central pathways that receive, compare, and transmit this input. Binaural pathways perform the neural computation needed to localize brief sounds and to extract signal embedded in background noise. Inhibitory as well as excitatory connections are characteristic of these circuits.
Unilateral damage to the cochlear nerve or cochlear nucleus results in monaural deafness. In contrast, unilateral damage at or above the superior olivary complex leaves intact routes from either ear that are conveyed through binaural pathways, so monaural deafness does not occur. The auditory decussations, particularly the trapezoid body, are functionally similar to the optic chiasm in the visual system and have been collectively referred to as a functional acoustic chiasm. Thus central hearing dysfunction may result in inattention to stimuli on the contralateral side or inability to follow conversations in a noisy room (e.g., cocktail effect).
Vascular Supply of the Auditory Brainstem and Cortex
The blood supply to the cochlea and the auditory nuclei of the pons and medulla originates from the basilar artery. The internal auditory (labyrinthine) artery, usually a branch of the anterior inferior cerebellar artery (AICA), supplies the inner ear and the cochlear nuclei. Occlusion of the AICA will result in a monaural hearing loss. This lesion may also damage the emerging fibers of the facial nerve and the pontine gaze center, resulting in monaural deafness combined with ipsilateral facial paralysis and an inability to look toward the side of the lesion.
Vascular lesions higher in the ascending auditory system necessarily interrupt pathways conveying information from both ears. The superior olivary complex and lateral lemniscus are mainly supplied by short circumferential branches of the basilar artery. The superior cerebellar and quadrigeminal arteries supply the inferior colliculus, and the medial geniculate bodies lie in the vascular territory of the thalamogeniculate arteries. The blood supply to the primary auditory and association cortices is via branches of the M2 segment of the middle cerebral artery.
When damage occurs to neural tissue from vascular insults, tumors, or demyelinating diseases such as multiple sclerosis, the effect on conduction time and activity levels in the auditory system can be used in clinical neurophysiology to assist in localization of the pathologic process. Brainstem auditory evoked responses (also called auditory brainstem response, or ABR) are average scalp potentials elicited by a train of clicks and recorded much like an electroencephalogram. A pattern of seven waves occurs in the auditory evoked response, with peaks at regular latencies after the clicks that are correlated with activity levels of the ascending auditory system. The activity from one or more auditory structures may be correlated with a specific wave, as summarized in Figure 21-7; and shifts in latency and amplitude of specific waves may be used to localize the lesion, to assess hearing, or to indicate swelling in response to neurosurgical procedures.
BRAINSTEM AUDITORY NUCLEI AND PATHWAYS
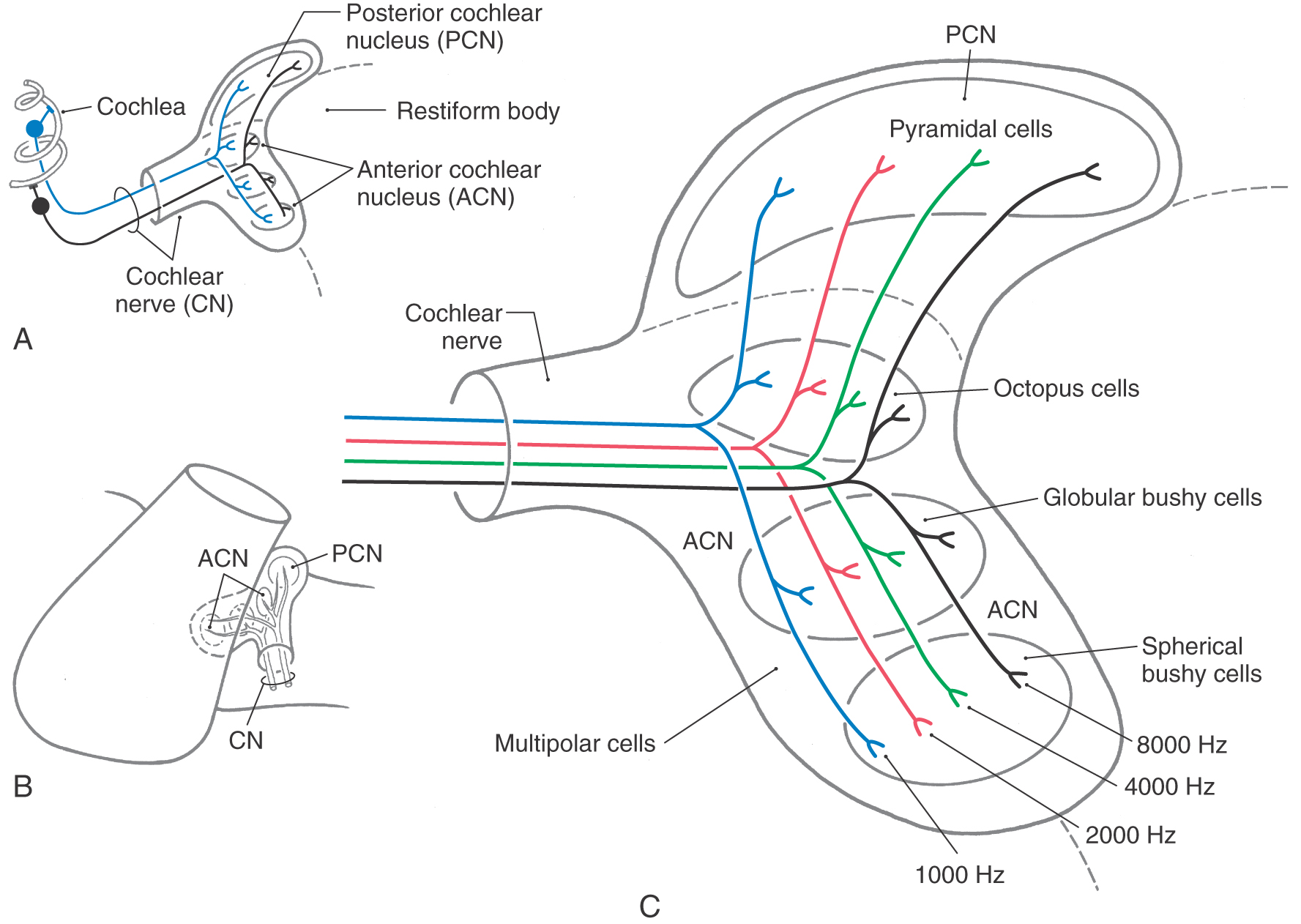
Cochlear Nuclei
The posterior cochlear nucleus (dorsal cochlear nucleus) and the anterior cochlear nucleus (ventral cochlear nucleus) are located lateral and posterior to the restiform body and are partially on the surface of the brainstem at the pontomedullary junction (Fig. 21-8A). The posterior cochlear nucleus drapes over the restiform body just inferior to the pontomedullary junction. At this level, the posterior part of the anterior cochlear nucleus is small in proportion to the posterior cochlear nucleus (Fig. 21-8A, B). The anterior cochlear nucleus extends rostral to the posterior cochlear nucleus (Fig. 21-8C), where it may be covered by the flocculus and by caudal fascicles of the middle cerebellar peduncle.
All cochlear nerve fibers end in the cochlear nuclei on the ipsilateral side (Fig. 21-9). As these fibers enter the brainstem at the cerebellopontine angle, they divide into ascending and descending bundles. Fibers in the ascending bundle synapse in the anterior part of the anterior cochlear nucleus, whereas fibers in the descending bundle synapse in the posterior part of the anterior cochlear nucleus and in the posterior cochlear nucleus.
In the cochlear nuclei, each afferent nerve fiber makes specialized synaptic contacts with several different cell types. Individual fibers and their synaptic contacts distribute along orderly rows so that the resulting order produces distinct tonotopic maps in each division (Fig. 21-9C).
Specific cell types of the cochlear nuclei, in turn, give rise to parallel but separate ascending pathways in the auditory system that analyze and code different sound features while preserving frequency information (see Fig. 21-10 for summary of major cell types, connections, and sound features associated with processing by each). These projections are subdivided into pathways conveying monaural information to the inferior colliculus and those providing input to the superior olivary complex for binaural processing. Most fibers from the anterior cochlear nucleus course anterior to the restiform body part of the inferior cerebellar peduncle to form the trapezoid body (Fig. 21-8B, C). Projections from the posterior cochlear nucleus and some from the anterior cochlear nucleus course posteriorly over the restiform body as the posterior acoustic stria and decussate in the pontine tegmentum before joining the lateral lemniscus.
Superior Olivary Complex
The superior olivary complex is located near the facial motor nucleus in the caudal pons (Figs. 21-8B, C and 21-10). It is the first site in the brainstem where information from both ears converges. This binaural processing is essential for accurate sound localization and the formation of a neural map of the contralateral auditory hemifield.
The medial superior olivary nucleus (MSO), which forms a distinct vertical bar within a diffuse group of periolivary nuclei, is the principal nucleus in the human superior olivary complex (Figs. 21-8B, C and 21-10). The lateral superior olivary nucleus (LSO), located lateral to the MSO, is less distinct.
The trapezoid body is a bundle of myelinated fibers passing anterior to the superior olivary complex and intermingling with fibers of the medial lemniscus as it crosses the midline (Figs. 21-8B and 21-10). Decussating fibers of the trapezoid body end in the contralateral superior olivary complex or ascend in the contralateral lateral lemniscus. The medial nucleus of the trapezoid body lies medial to the MSO. It receives projections from the contralateral anterior cochlear nucleus and gives rise to important local inhibitory circuits within the superior olive.
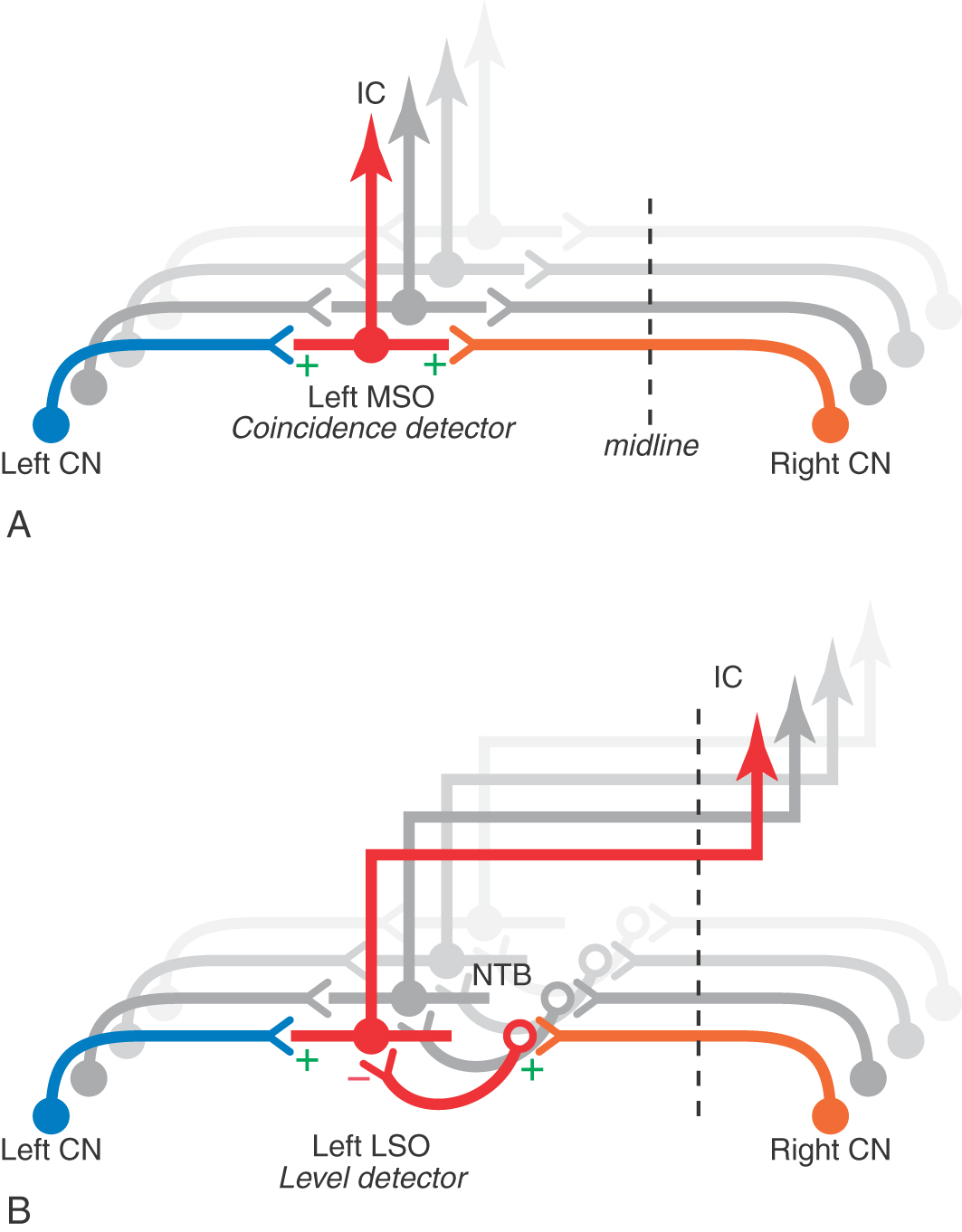
The topographic organization of the afferents to the MSO and the LSO conserves the orderly representation of the cochlea (Fig. 21-10). The MSO receives excitatory input from both ears by way of projections from the ipsilateral and contralateral anterior cochlear nucleus. The excitatory neurotransmitter is probably glutamate or aspartate. The LSO also receives input from both ears. The synapse from the ipsilateral anterior cochlear nucleus is excitatory; however, the pathway from the contralateral ear involves an additional synapse in the trapezoid body nucleus that then inhibits LSO. These local inhibitory circuits use glycine as a neurotransmitter.
The pathways to the MSO from the anterior cochlear nucleus are anatomically arranged so that signals from the contralateral ear arrive close enough in time to those from the ipsilateral ear to summate at each cell only for a sound from a specific point in the contralateral hemifield of space. This mechanism, referred to as coincidence detection, accounts for computation by the MSO of interaural time differences that contribute to sound localization (Fig. 21-11). Coincidence of the signals occurs when the shorter neural conduction time in the path from the ipsilateral cochlear nucleus compensates for the shorter delay of the sound path to the contralateral ear (Figs. 21-10 and 21-11). Additional spatial cues are derived from interaural intensity differences caused by shadowing of sounds by the path from the contralateral side of the head. Interaural level differences are coded by summation of excitatory and inhibitory inputs to LSO neurons (Fig. 21-11).
Ascending projections from the MSO travel largely in the ipsilateral side in the lateral lemniscus and synapse in the central nucleus of the inferior colliculus (Fig. 21-10). These fibers synapse in corresponding frequency regions (low to low, and so on) of the inferior colliculus (Fig. 21-10). The LSO also contributes contralateral excitatory and ipsilateral inhibitory axons to these pathways. Branches of these projections also end in the posterior nucleus of the lateral lemniscus (dorsal nucleus of the lateral lemniscus). This nucleus, in turn, projects to the contralateral inferior colliculus and constitutes an indirect binaural pathway from the superior olive to the inferior colliculus (Fig. 21-10).
Lateral Lemniscus and Its Nuclei
The lateral lemniscus contains axons from second-order neurons in the cochlear nuclei, third-order neurons in the superior olive, and fourth-order neurons in the adjacent nuclei of the lateral lemniscus (Fig. 21-10). It is precisely this heterogeneous collection that prevents a simple correlation of nuclei or tracts with specific wave components of the auditory evoked responses that are widely used to clinically assess the level of brainstem function. The interposition of synaptic delays in each of these components of the lateral lemniscus imparts temporal differences that contribute to at least the second, third, and fourth wave components of the evoked responses.
The larger anterior nucleus of the lateral lemniscus (ventral nucleus of the lateral lemniscus) consists of cells scattered among the ascending fibers of the lateral lemniscus (Fig. 21-10). It extends from the rostral limit of the superior olive to just below the inferior colliculus. These cells project to the inferior colliculus, completing an indirect monaural pathway (Fig. 21-10).
The smaller posterior nucleus of the lateral lemniscus (dorsal nucleus of the lateral lemniscus) is intercalated in the ascending fiber bundles of the lateral lemniscus just caudal to the inferior colliculus (Fig. 21-10). This nucleus receives input mainly from the superior olivary complex. Ascending projections from the posterior nucleus of the lateral lemniscus decussate in the posterior tegmental commissure. These fibers terminate in the contralateral inferior colliculus and, to a lesser degree, in the contralateral posterior nucleus of the lateral lemniscus (Fig. 21-10). This pathway is largely inhibitory, using γ-aminobutyric acid (GABA) as the neurotransmitter. It conveys binaural information and inhibits activity from the opposite hemifield.
Inferior Colliculus
Virtually all ascending auditory pathways terminate in the inferior colliculus (Fig. 21-10). The egg-shaped core of the inferior colliculus, the prominent central nucleus, is nested in a base of afferent fibers formed by fibers of the lateral lemniscus. These fibers are the major source of input to the inferior colliculus. In a shell around the central nucleus, other cells form pericentral and external nuclei (Fig. 21-10). The pericentral nucleus lies posterior to the central nucleus and is traversed by fibers from the commissure of the inferior colliculus. The external nucleus lies lateral and is intersected by fibers that form the brachium of the inferior colliculus.
The central nucleus integrates information from multiple hindbrain auditory sources and in turn projects to the anterior division of the medial geniculate nucleus (Fig. 21-10). It consists of parallel layers of cells with disk-shaped dendritic fields. Afferents from the lateral lemniscus course parallel to these dendritic fields, forming a series of fibrodendritic laminae. Ascending projections diverge and converge in a point-to-plane order in the central nucleus. As a result, each point along the cochlear spiral is represented in an isofrequency lamina. Functionally, cells in the central nucleus are narrowly tuned, with the lowest frequencies represented posterolaterally and higher frequencies anteromedially (Fig. 21-10).
Many cells in the inferior colliculus respond to input from either ear. Among cells with low characteristic frequencies, many are sensitive to interaural time delays, and those with high characteristic frequencies are sensitive to interaural intensity differences. Thus binaural responses of inferior collicular neurons resemble those of the superior olivary neurons, from which they receive a dominant binaural input. These responses are probably further modified by indirect binaural pathways from the posterior nucleus of the lateral lemniscus and by intrinsic circuits in the fibrodendritic laminae. Other cells in the fibrodendritic laminae of the central nucleus are monaural and are mainly excited only by the contralateral ear. Their responses resemble those of cells in the contralateral cochlear nucleus.
Cells in the pericentral and external nuclei are broadly tuned to frequency, and they habituate rapidly to repetitive stimuli. They receive input from the central nucleus and the cerebral cortex and nonauditory input from the spinal cord, posterior column nuclei, and superior colliculus. These nuclei project to the medial geniculate nucleus (Fig. 21-10), superior colliculus, reticular formation, and precerebellar nuclei. Thus the pericentral and external nuclei are probably involved in functions related to attention, multisensory integration, and auditory-motor reflexes (Fig. 21-15).
Medial Geniculate Nucleus
The medial geniculate nucleus forms a small protuberance on the lower caudal surface of the thalamus between the lateral geniculate body and the pulvinar (Fig. 21-10; see also Fig. 15-10). The anterior division of the medial geniculate nucleus receives afferents from the central nucleus of the inferior colliculus and projects to the primary auditory cortex. Isofrequency contours in the anterior division are arranged so that low frequencies are represented laterally and higher frequencies are represented medially (Fig. 21-10). As a result of collicular and thalamic integration, however, most cells are not reliably excited by simple tones and are probably involved in complex feature detection.
The posterior division receives input from the pericentral nucleus of the inferior colliculus and projects to the secondary auditory cortex (Fig. 21-10). These projections are also tonotopically arranged. More broadly tuned and sensitive to habituation, this pathway may convey information about moving or novel stimuli that direct auditory attention.
The medial (magnocellular) division receives afferents from the external nucleus of the inferior colliculus and projects to association areas of the auditory cortex. It contains cells that are broadly tuned to auditory and other sensory stimuli, including vestibular and somesthetic inputs. The medial division projects to temporal and parietal association areas and to the amygdala, putamen, and pallidum. In view of the multisensory convergence that occurs in this pathway, it may be a part of the reticular activating system.
Central Deafness
Central deafness results from damage to the cochlear nuclei or the central pathways that relay auditory information to the auditory cortex. Damage to the cochlear nuclei may cause deafness in the ear on the affected side. On the other hand, central lesions within the brainstem, diencephalon, or auditory cortices may alter the perception of sound but infrequently result in deafness in one ear. In some cases, pontine lesions may result in pontine auditory hallucinosis, such as an orchestra out of tune, buzzing insects, or strands of music. These perceived auditory events are accompanied by more typical symptoms of pontine lesions, such as cranial nerve deficits and long tract signs. A perception of noise or sounds may also be experienced by patients with temporal lobe seizures or a temporal lobe lesion that damages auditory cortices.
AUDITORY AND RELATED ASSOCIATION CORTICES
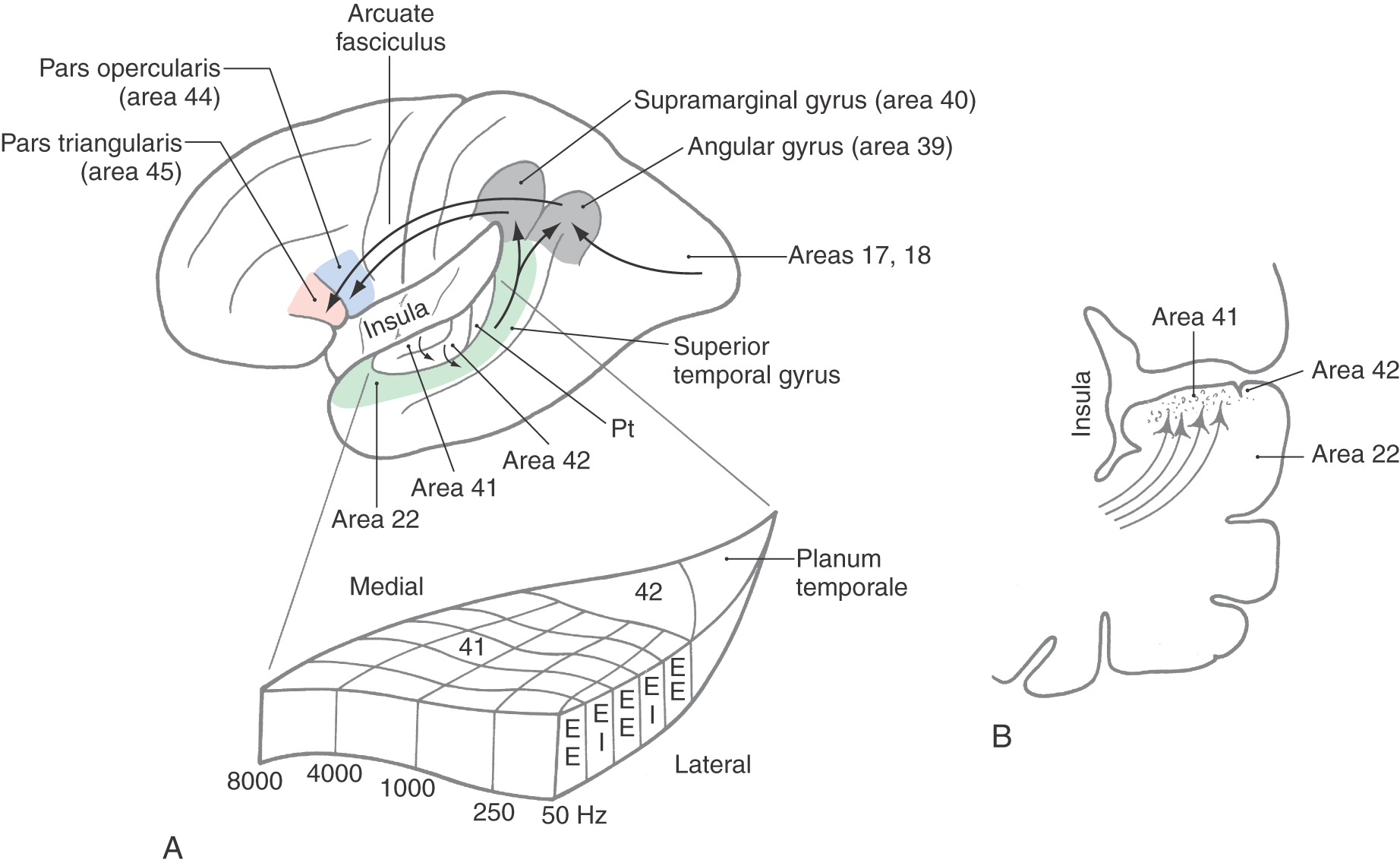
The auditory cortex is located in the transverse gyri of Heschl (Figs. 21-10 and 21-12). Two transverse temporal gyri are buried in the lateral sylvian sulcus, covered by parts of the frontal and parietal opercula and continuous with the superior temporal gyrus. Caudal to the transverse temporal gyri is a smooth area, the planum temporale, which is usually larger on the left side than on the right.
The primary auditory (AI) cortex is located in the first (anterior) transverse temporal gyrus but may extend into the second (posterior) gyrus (Fig. 21-12A). Cytoarchitecturally, the primary auditory cortex corresponds to Brodmann area 41, which encompasses the granular cortex, with its well-developed layer IV containing small granule cells and densely packed small pyramidal cells in layer VI (Fig. 21-12B). Adjacent to the granular cortex in the second transverse gyrus and planum temporale is area 42, which constitutes the secondary auditory (AII) cortex (Fig. 21-12A).
Area 41 is reciprocally connected with the anterior division and area 42 with the posterior division of the medial geniculate body (Fig. 21-13), continuing the persistent parallel pathways of feature processing in the central auditory system. Through the corpus callosum, each auditory cortical area is connected with the reciprocal areas in the other cerebral hemisphere. The tonotopic organization of constituent cells of the cortical layers and incoming afferent fibers extends through the primary auditory cortex as long frequency-specific stripes (Fig. 21-12). The series of stripes so formed have one subcomponent composed of cells excited by stimulation of both ears (EE) alternating with a subcomponent composed of cells excited by the contralateral ear and inhibited by the ipsilateral ear (EI). Perception of auditory events along “what” and “where” paths is common to intracortical connections of auditory cortex with other association parietal and temporal cortices as already described for visual processing (see Chapter 20, Other Visual Cortical Areas) and important for fusion of perception of multisensory events.
An auditory association cortex surrounds the primary auditory area and is located mainly in the posterior portion of the superior temporal gyrus (Fig. 21-12A). It is connected to the primary auditory cortex by the arcuate fasciculus (Fig. 21-12A). Area 22 includes a part of the planum temporale and the posterior portion of the superior temporal gyrus. It receives connections from the primary auditory cortex as well as visual and somesthetic information. This speech receptive area, known as the Wernicke area, may be as much as seven times larger on the left side than on the right. When this area is damaged by occlusion of branches of the middle cerebral artery, an auditory aphasia (Wernicke aphasia) results. In such cases, comprehension of speech sounds is impaired but discrimination of nonverbal sounds is largely unaffected.
The higher association areas of the auditory cortex also extend into the inferior parietal lobule (Fig. 21-12A). This lobule is made up of the angular gyrus (area 39) and supramarginal gyrus (area 40). These two areas are important in aspects of language such as reading and writing and are sometimes included in the Wernicke area.
Brodmann areas 44 and 45 are known as the Broca area for expressive speech and language. They are located in the pars opercularis and pars triangularis of the inferior frontal gyrus (Fig. 21-12A). The major pathway connecting these areas with the primary and association auditory cortex is the arcuate fasciculus (Fig. 21-12). If areas 44 and 45 are damaged along with other motor cortices on the left side by a stroke involving branches of the middle cerebral artery, the result is Broca aphasia. In this disorder, speech is nonfluent, but comprehension of verbal and nonverbal sounds is largely unimpaired.
DESCENDING AUDITORY PATHWAYS
Descending projections make reciprocal connections throughout the auditory pathway. They form feedback loops that provide circuits to modulate information processing from the peripheral level to the cortex (Fig. 21-13). For example, the auditory cortex projects to the medial geniculate nucleus and nuclei of the inferior colliculus. The inferior colliculus projects to the periolivary nuclei, which in turn send olivocochlear efferents to the cochlea. There are also descending projections from the periolivary nuclei to the cochlear nuclei.
Olivocochlear Bundle
The olivocochlear efferent system arises from groups of cells in the periolivary nuclei of the superior olivary complex (Fig. 21-13). These efferent systems travel as the olivocochlear bundle in the vestibular part of the vestibulocochlear nerve. Lateral olivocochlear efferent cells project to the ipsilateral inner hair cells, where they make axoaxonic synapses with type I spiral ganglion afferent fibers (Figs. 21-4 and 21-13). Medial olivocochlear efferent cells have bilateral projections that terminate directly on outer hair cells (Figs. 21-4 and 21-13).
Direct efferent feedback to outer hair cells, in particular, may influence cochlear mechanics and consequently the sensitivity and frequency selectivity of the cochlea. Efferent-induced changes in outer hair cell membrane potentials result in changes in the height of the cells and the stiffness of their stereocilia. These changes modulate basilar membrane motion and thereby influence cochlear function. The tight coupling of the basilar membrane to the tectorial membrane by the outer hair cells enables this efferent mechanism to feed energy back to the cochlea to amplify responses to specific tones. The cochlear amplifier effect is important in selectively tuning the cochlea to important sounds.
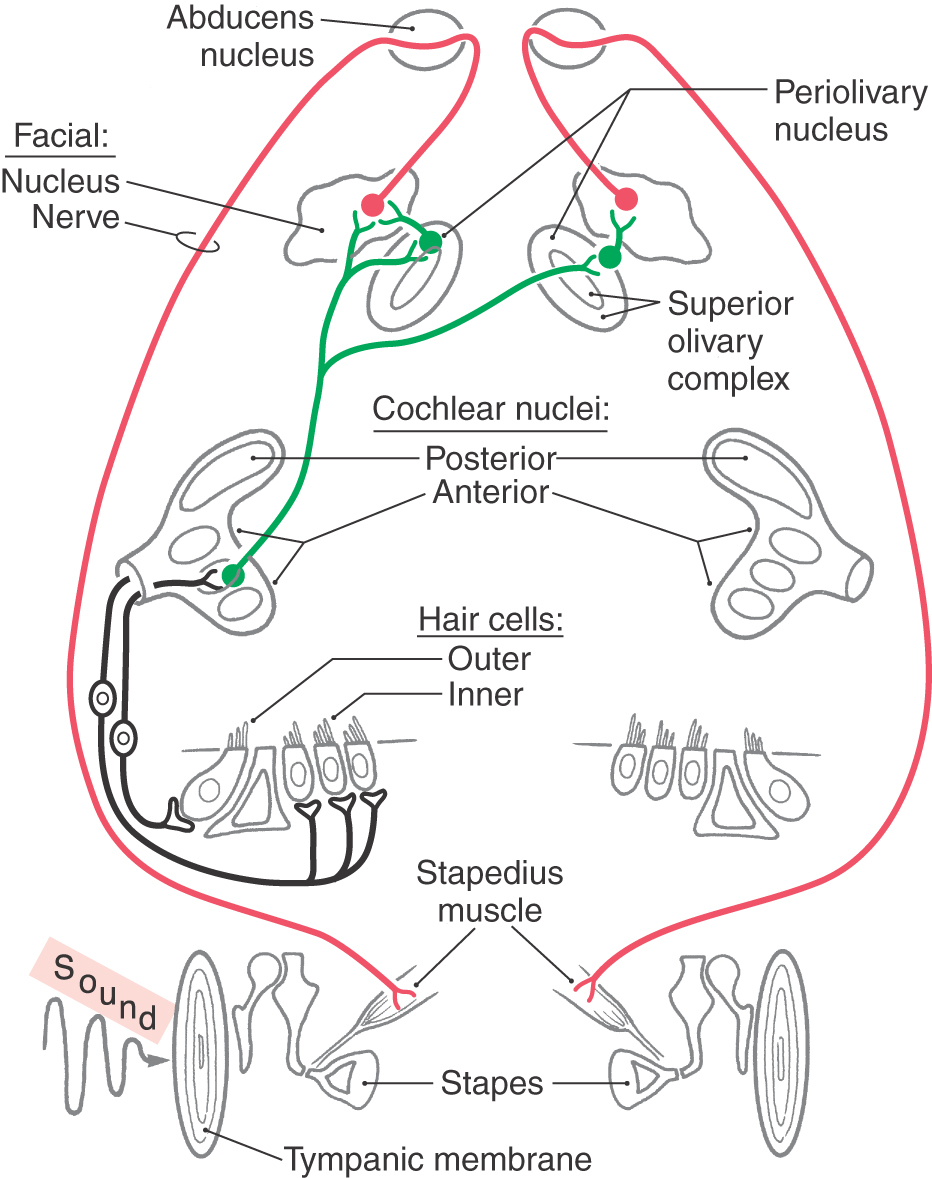
MIDDLE EAR REFLEX
The small striated muscles of the middle ear affect the mechanical impedance of the ossicular chain. These muscles are activated by the middle ear reflex (Fig. 21-14). Because of the timing of the response, the middle ear is more effective in protecting the inner ear from loud sounds that have long duration. It may also serve to dampen the speaker’s own voice.
Figure 21-14. The pathway of the middle ear reflex arc. For simplicity, only the stapedius reflex is shown.
The stapedius muscle is innervated by facial motor neurons, and the tensor tympani muscle is innervated by trigeminal motor neurons. These motor neurons are intimately associated with the caudal end of the superior olivary complex, in the case of the stapedius muscle, and with the rostral end of the superior olivary complex, in the case of the tensor tympani muscle. In these positions, auditory input via axons of neurons in the cochlear nuclei or the superior olivary complex provides the sensory limb of the reflex. The sensory pathways are bilateral, so that stimuli may be presented by earphones to one ear while the device to measure impedance is placed in the ear canal on the other side.
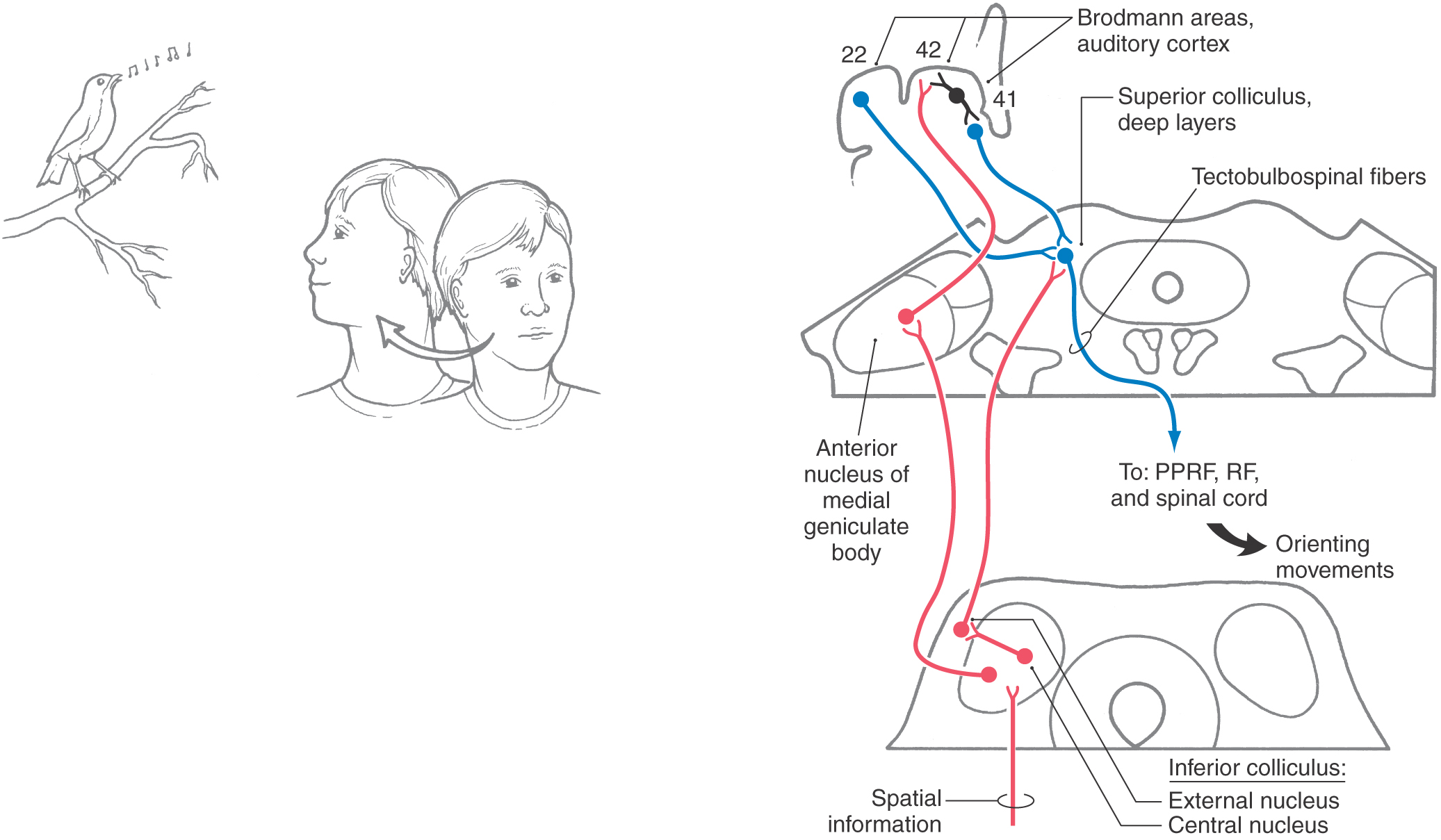
ACOUSTIC STARTLE REFLEX, ORIENTATION, AND ATTENTION
Reflexive and learned responses to sound require sensory-motor integration. In addition to corticocortical interconnections for the dissemination of auditory information, there is also integration of auditory sensory input with motor pathways in the brainstem. Reticulospinal neurons in the region of the lateral lemniscus have dendrites that sample lemniscal activity and are involved in rapid acoustic startle reflex pathways. In addition, the deep layers of the superior colliculus receive auditory information from the inferior colliculus and auditory cortical areas (Fig. 21-15). The deep layers of the superior colliculus integrate auditory, visual, and somesthetic information and project to brainstem and cervical spinal cord nuclei via tectobulbospinal fibers, which are involved in controlling orientation of the head, eyes, and body to sound (Fig. 21-15).
Sources and Additional Reading
Interesting web links related to the auditory system:
Promenade around the Cochlea. Available at http://www.cochlea.org
The Cochlea—graphic tour of the inner ear’s machinery, Available at http://www.vimm.it/cochlea/index.htm
Pickles JO. An Introduction to the Physiology of Hearing. 2nd ed. London: Academic Press; 1988.
Yost WA. Fundamentals of Hearing: An Introduction. San Diego: Academic Press; 1994.

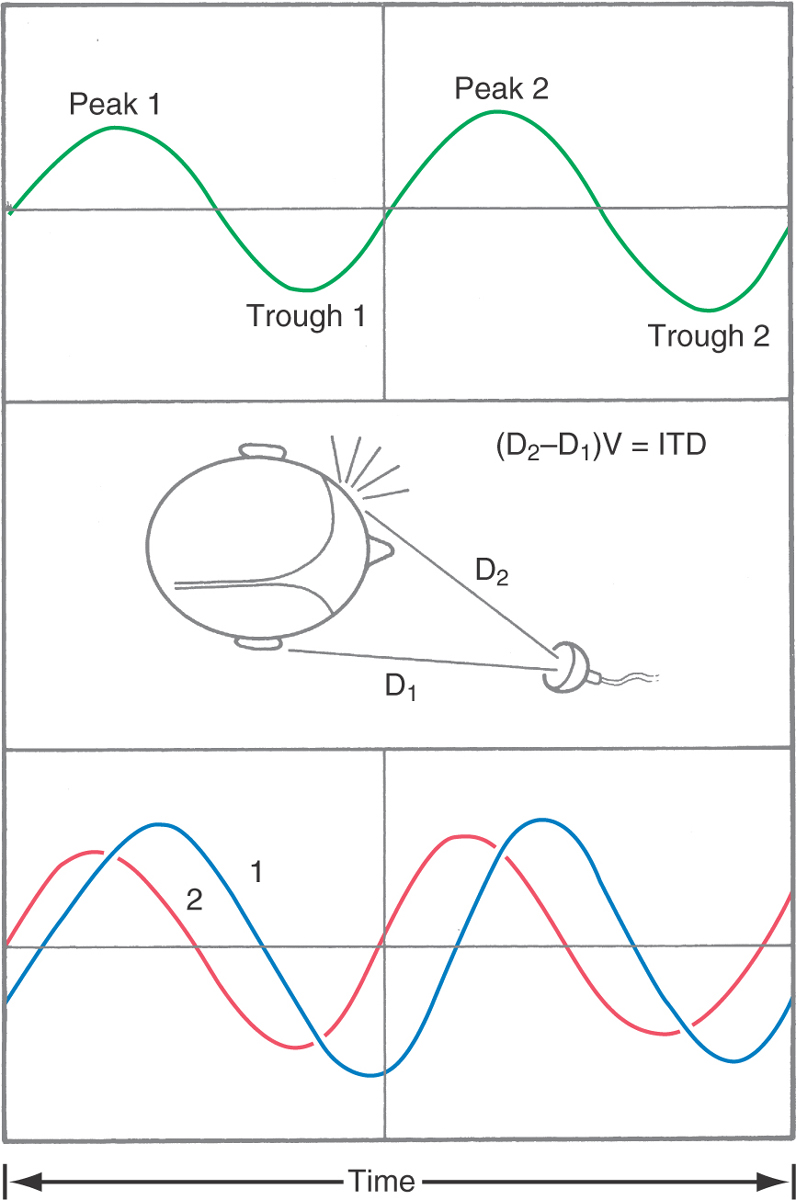
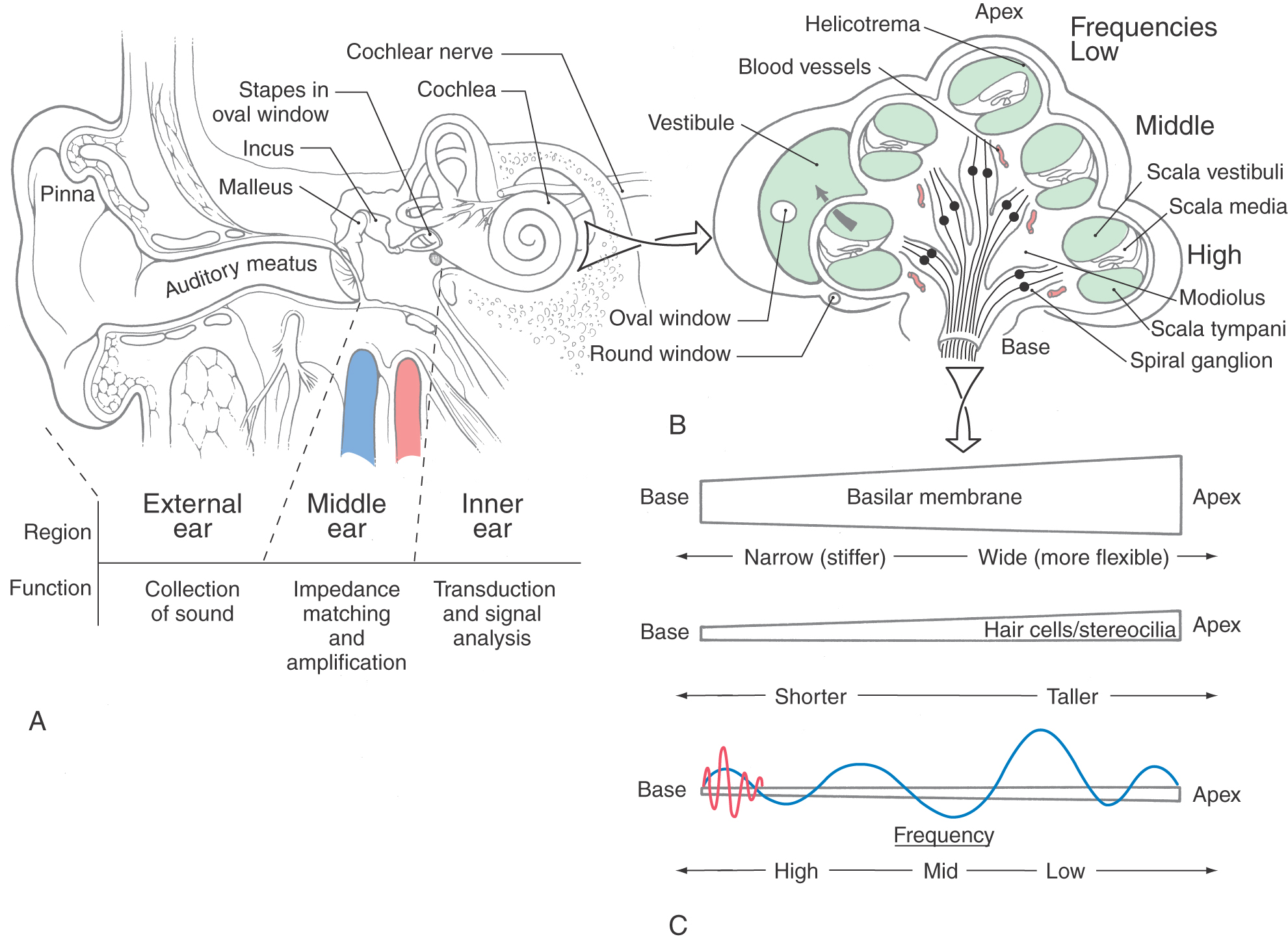
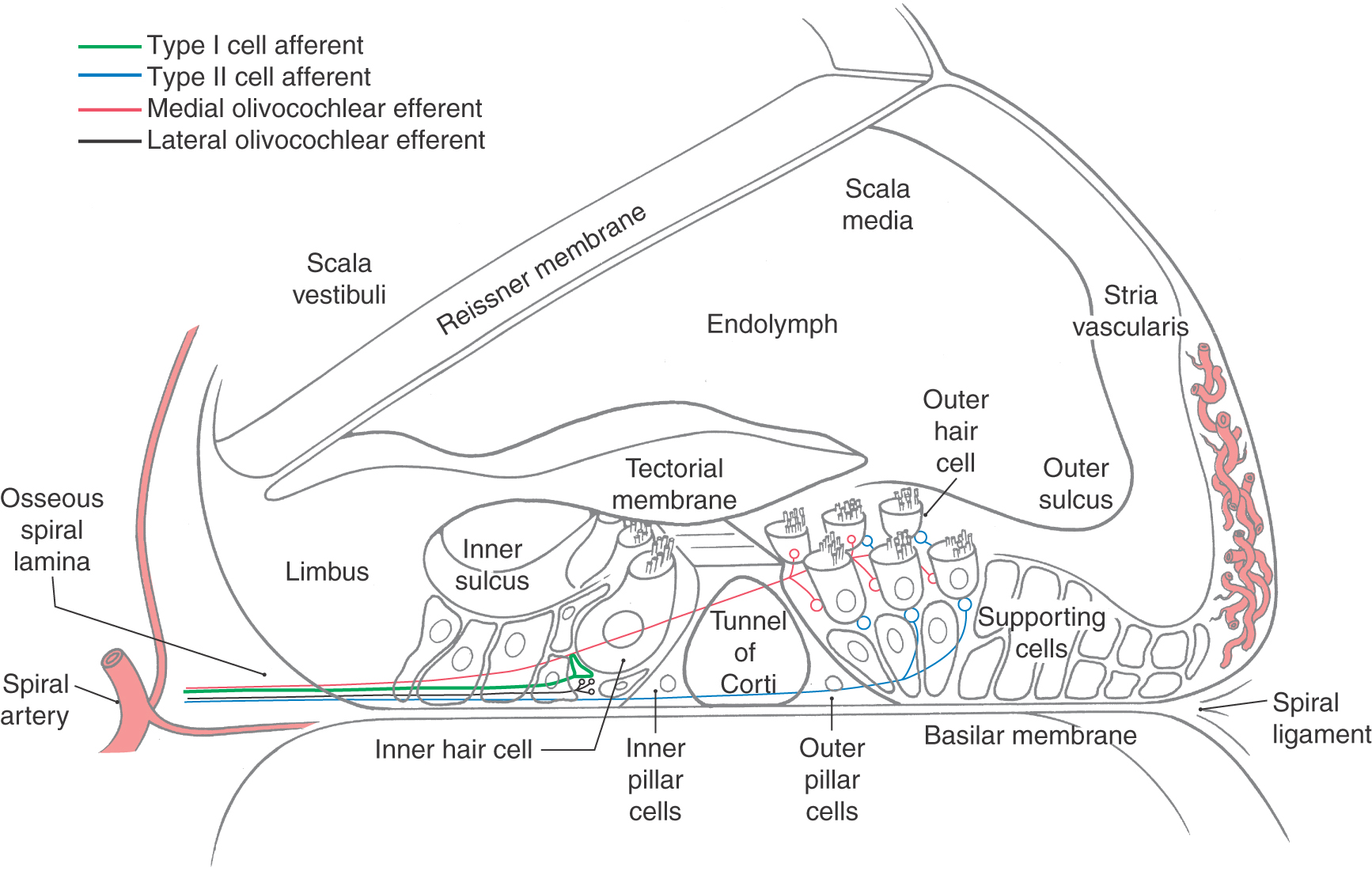
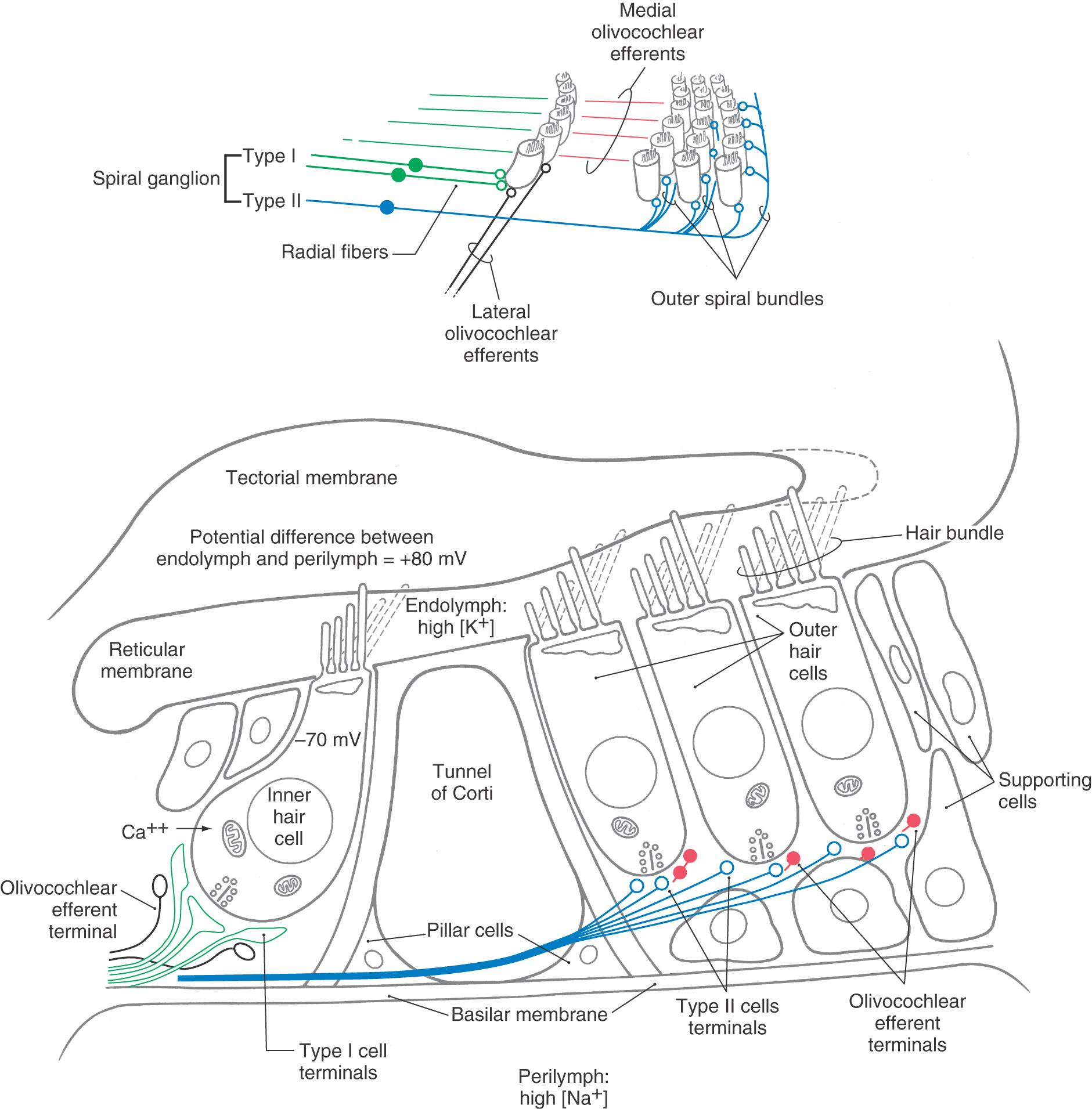
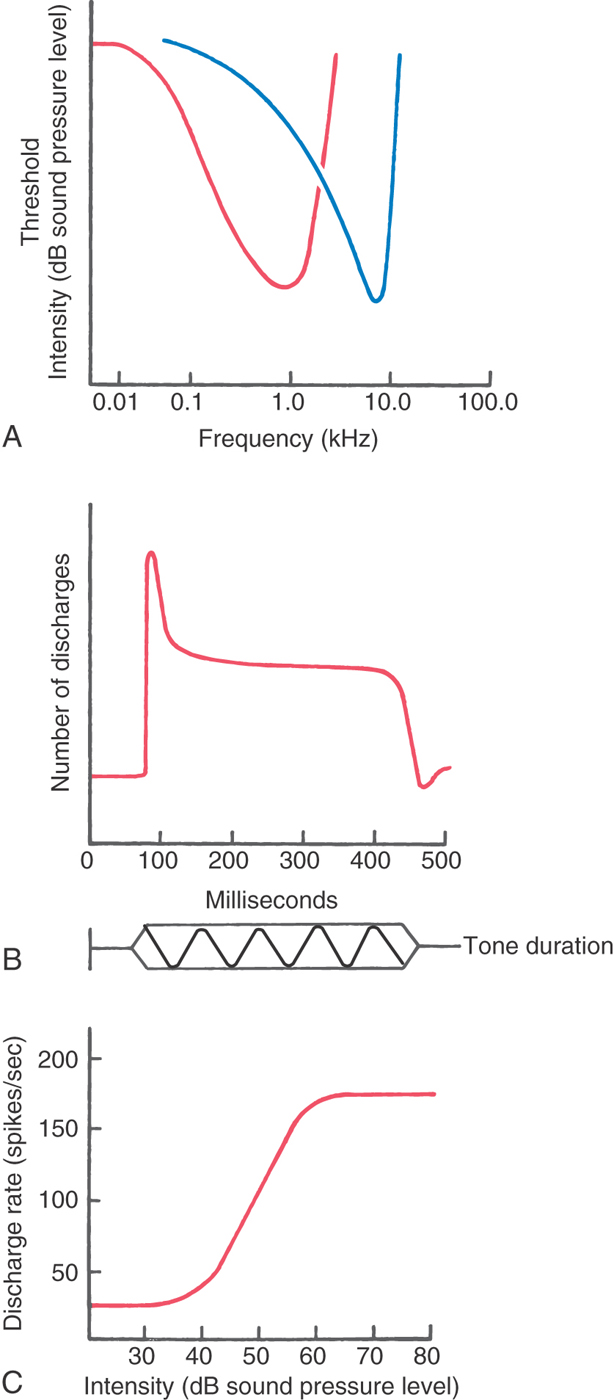
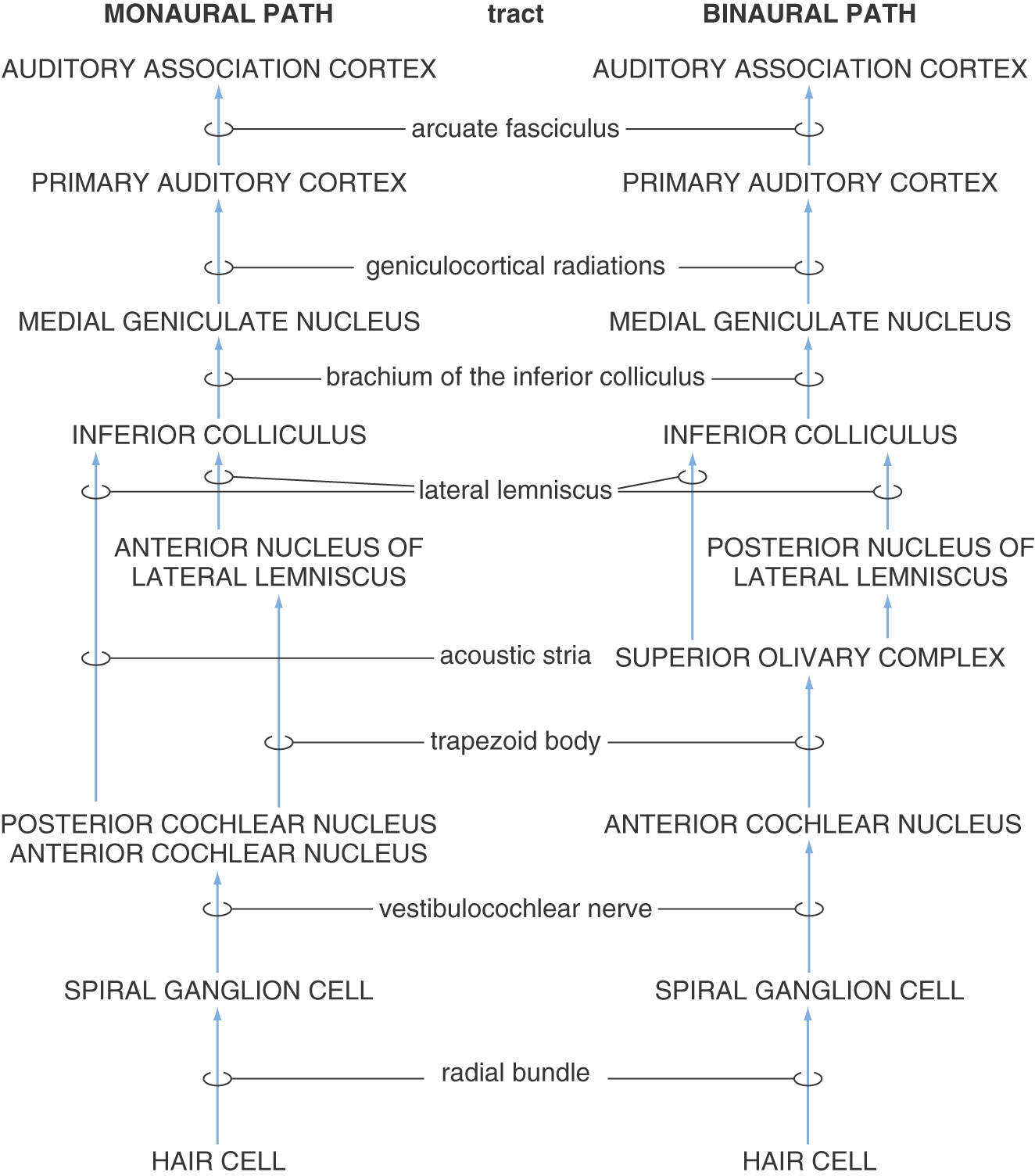
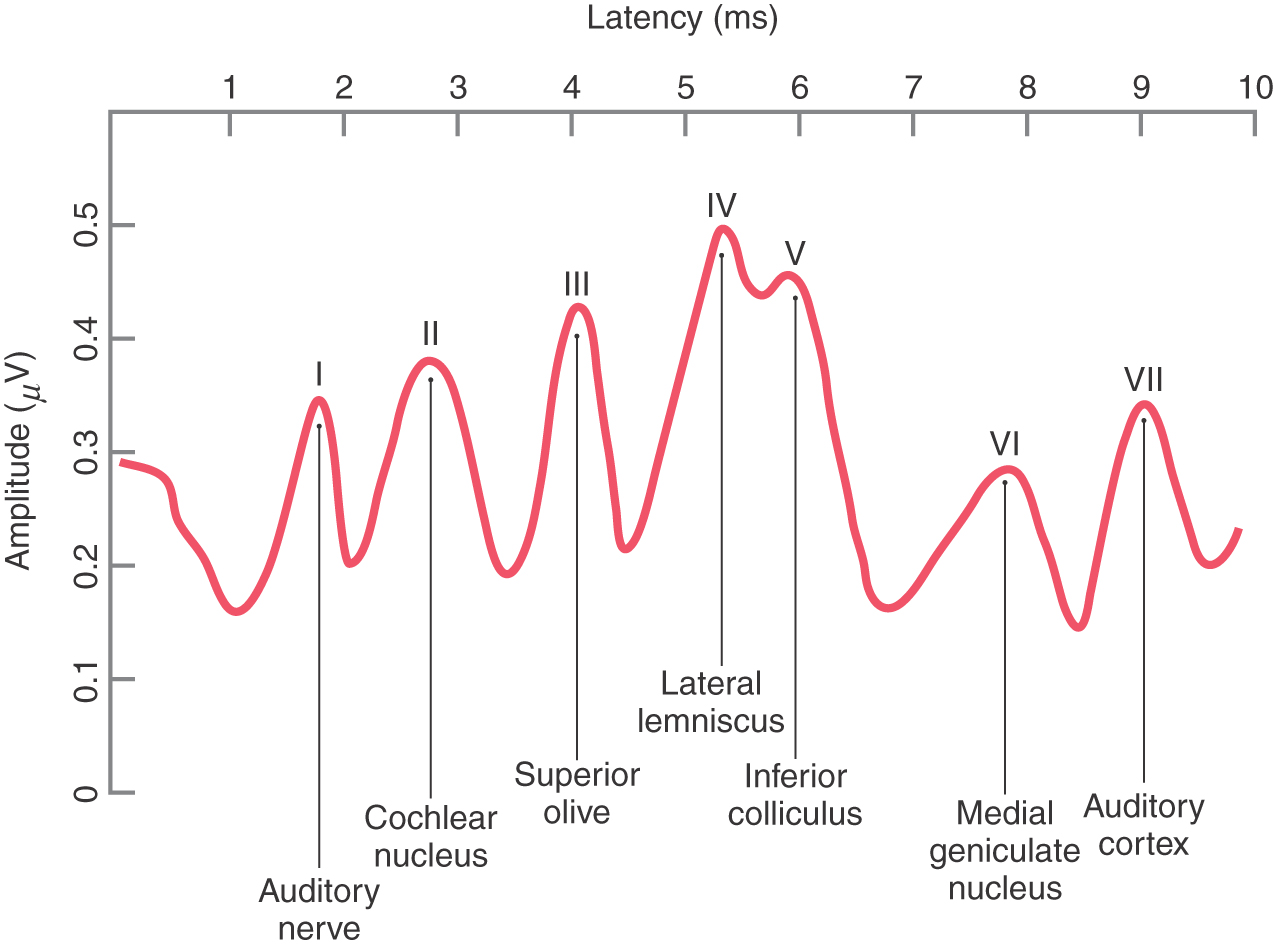
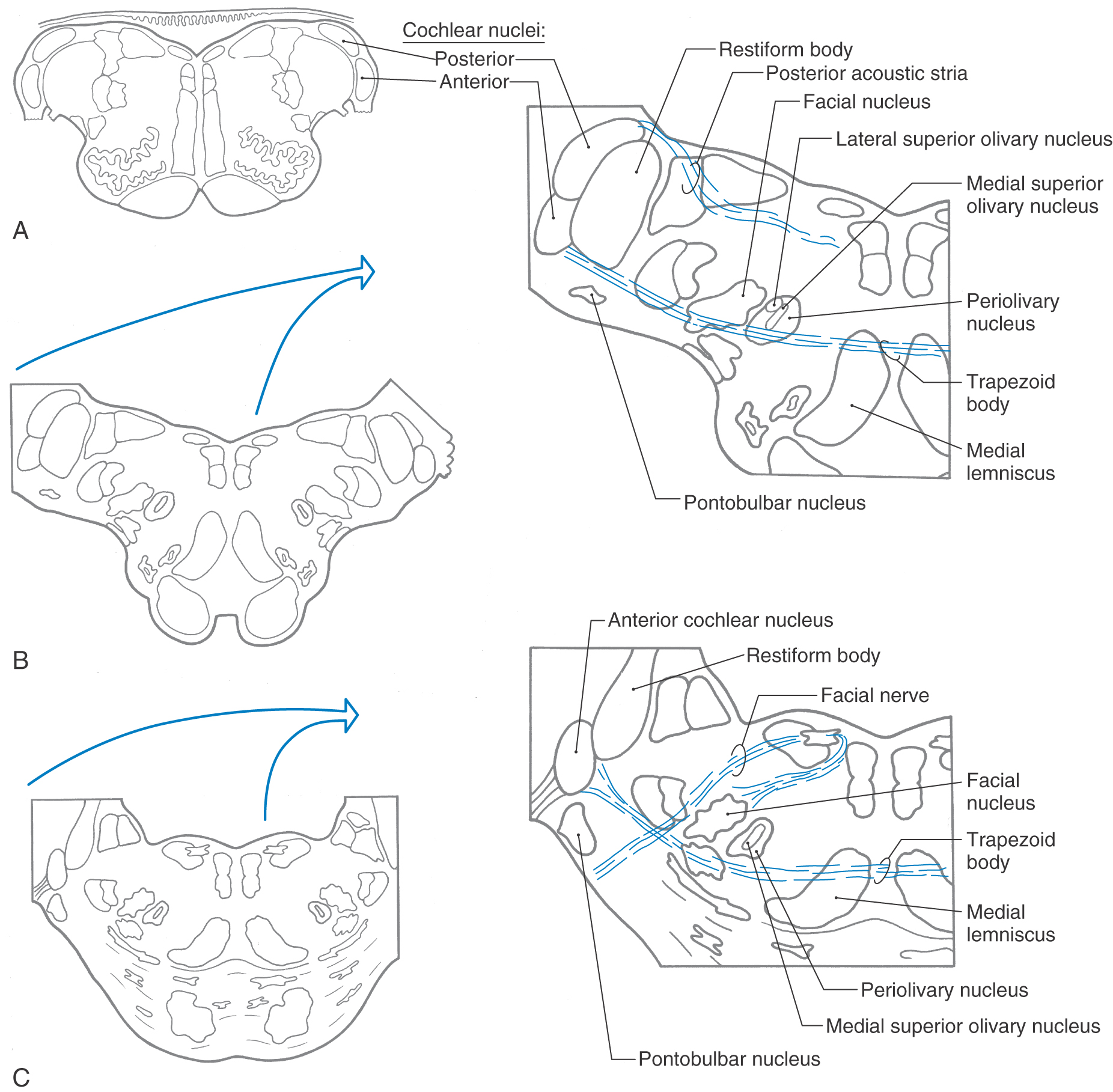
 Figure 21-8.
Figure 21-8.