Chapter 2 The atom
The smallest unit of pharmacology
What is an Atom?
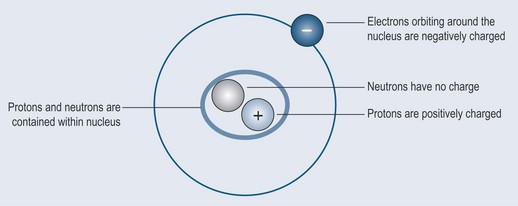
Atoms consist of (Figure 2.1):
The Nucleus
The nucleus is made up of protons and neutrons:
For the level of pharmacology covered in this book, the presence of neutrons is acknowledged here for completeness but they will not be discussed any further.
The Electrons
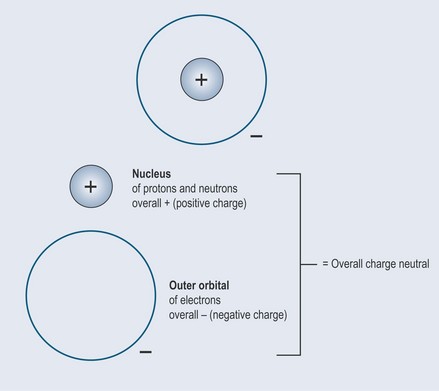
Like moons around a planet, electrons orbit around the nucleus (Figure 2.2). The space in which they move around the nucleus is therefore called an orbital.
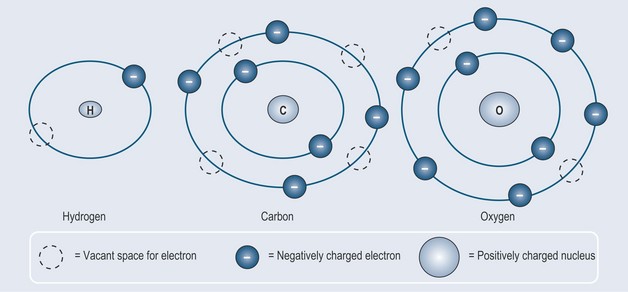
How are Electrons Arranged Around the Nucleus?
Convention dictates that each orbital has a fixed number of available slots that can be filled until they reach capacity. Once an orbital becomes filled, the electrons start to fill the available slots in the next, higher orbital (the next orbital away from the nucleus). In this way, the bigger the atom, the more electrons it will have, and the more orbitals these electrons will need to fill (or occupy) around the nucleus, as can be seen in Figure 2.3.