The Aorta and Inferior Vena Cava
The Aorta and Inferior Vena Cava
Abdominal Doppler examinations are performed on vessels which lie more deeply than the peripheral vessels and this has several consequences. First, lower-frequency transducers are used and this limits the size of Doppler shift which will be obtained for a given velocity. Second, longer-pulse repetition intervals are required to allow the sound to travel the greater distances; this also limits the size of Doppler shift which can be measured as a result of the Nyquist limit (see Chapter 1). Operators should therefore seek to minimise the scan depth and use the highest-frequency transducer compatible with adequate visualisation.
The Aorta
ANATOMY
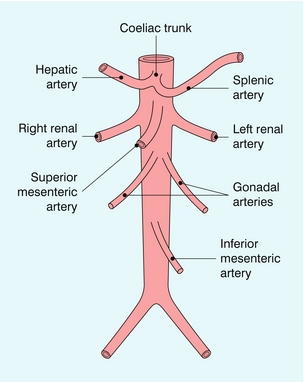
The abdominal aorta gives branches to the abdominal organs and to the abdominal wall. The parietal branches to the abdominal wall are not usually large enough to be seen regularly using colour Doppler and will not be considered further. The visceral branches (Fig. 6-1) supply the liver, kidneys, adrenal glands, gonads, spleen, bowel and pancreas. The vessels to the adrenals and gonads are also usually too small to be seen reliably on ultrasound; the renal, hepatic and iliac arteries are covered elsewhere in greater detail.
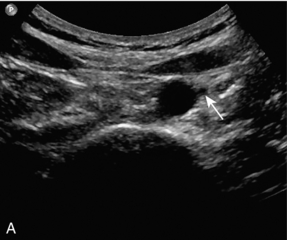
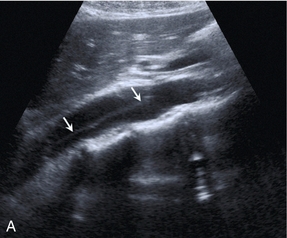
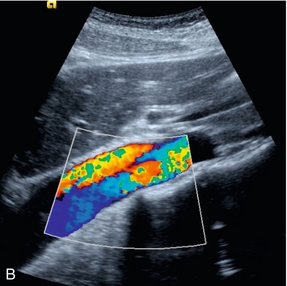
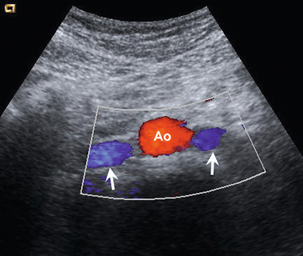
The splanchnic arteries supply the bowel and associated organs. The coeliac trunk (Fig. 6-2) arises from the anterior aspect of the aorta just after it has entered the abdomen. The trunk is only about 1 cm long and divides into three branches: the common hepatic artery, the splenic artery and the left gastric artery. The common hepatic artery passes to the right over the head of the pancreas, where it gives off the gastroduodenal artery which can be seen passing inferiorly between the head of the pancreas and the margin of the duodenum; the other branches from this segment of the hepatic artery are not usually apparent on ultrasound. The artery ascends in the lesser omentum as the proper hepatic artery, in company with the portal vein and common bile duct, to the porta of the liver, where it divides into right and left hepatic arteries. The splenic artery passes to the left and runs along the superior margin of the body of the pancreas to the hilum of the spleen. It has a tortuous course and an arterial loop may be mistaken for a small cyst in the pancreas if the situation is not recognised; colour Doppler allows quick identification of the true nature of the ‘cyst’. The right gastric artery arises from the splenic artery but is not usually seen on ultrasound.
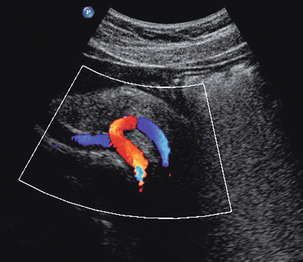
FIGURE 6-2 Transverse view of the coeliac axis showing the splenic artery on the right and the hepatic artery on the left.
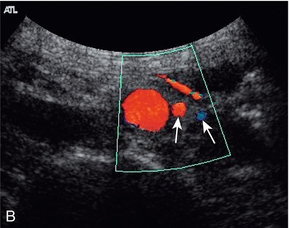
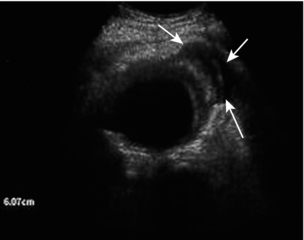
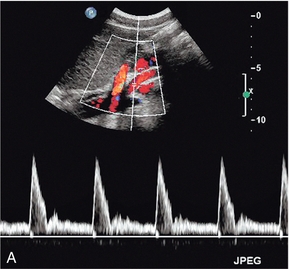
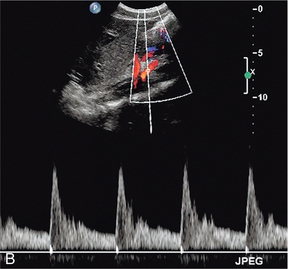
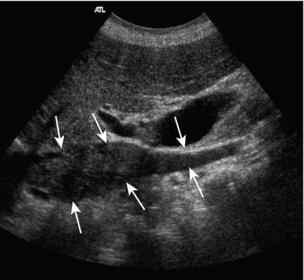
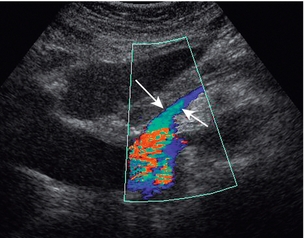
The superior mesenteric artery (Fig. 6-3) arises 1–2 cm below the coeliac trunk and supplies the small bowel and colon to the distal transverse colon. The superior mesenteric vein is seen on the right side of the upper portion of the artery and can be followed to its confluence with the splenic vein, forming the portal vein. The individual branches of the superior mesenteric artery are not usually seen clearly on ultrasound. The inferior mesenteric artery (Fig. 6-4) arises from the anterior aorta about 3–4 cm above the bifurcation and runs inferiorly to the left side of the aorta. The inferior mesenteric vein may be seen on the left of the artery but diverges as it passes up to join the splenic vein.
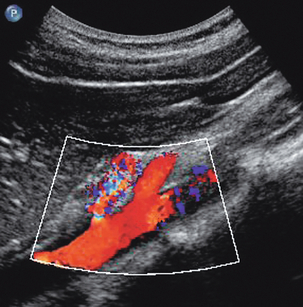
FIGURE 6-3 Longitudinal scan showing the origin of the coeliac axis superiorly and the superior mesenteric artery just below this. Colour Doppler shows aliasing and a tissue bruit around a stenotic coeliac axis origin.
SCANNING TECHNIQUE
Splanchnic Arteries
The coeliac trunk and its main branches are examined using colour and spectral Doppler. The main trunk is short but it is directed towards the transducer so that an excellent Doppler angle is achieved. The proximal hepatic and splenic arteries, together with the superior mesenteric artery, are often orientated almost at right angles to the transducer with an anterior approach (Fig. 6-2), so that some experimentation with points of access is required to get acceptable Doppler angles. The hepatic artery is followed to the right and the gastroduodenal artery can be identified beside the head of the pancreas. The proper hepatic artery is traced towards the porta where it divides into the right and left hepatic arteries. The origin of the superior mesenteric artery is examined (Fig. 6-3) and the vessel traced as far distally as it remains visible. Firm pressure with the transducer may help in displacing bowel gas from in front of the vessel, but care must be taken not to compress the artery and produce a spuriously high Doppler shift. Colour Doppler is used to identify any abnormal areas of flow, including ‘visible bruits’, or tissue vibrations, which may be seen in cases of severe stenosis (Fig. 6-3). Power Doppler is of less value in the abdomen than in peripheral vessels as arterial pulsation, respiratory movement and bowel gas motion can all cause marked motion artefacts which obscure the signal from the vessel.
The inferior mesenteric artery is sometimes difficult to locate. It can be found by scanning transversely up from the bifurcation and it may be identified just to the left of the aorta, 2–4 cm above the bifurcation (Fig. 6-4).
NORMAL AND ABNORMAL FINDINGS
Aorta
The calibre of the normal aorta varies with the age, sex and build of the patient, being larger in men, older patients and tall patients. The calibre also varies with the level in the abdomen. Goldberg et al. found an average diameter of 22 mm above the renal arteries, 18 mm just below the renal arteries and 15 mm above the bifurcation.1 The normal Doppler waveform in the aorta also varies with location. In the upper aorta there is a narrow, well-defined systolic complex with forward flow during diastole; below the renal arteries the diastolic flow is much reduced and above the bifurcation it is absent, or reversed diastolic flow may occur, with a waveform similar to that seen in the lower limb arteries (Fig. 6-5).2
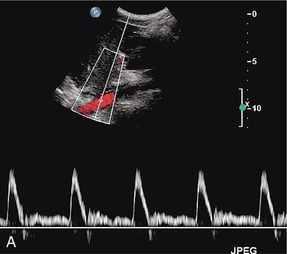
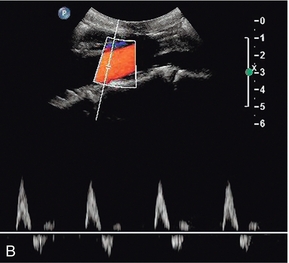
FIGURE 6-5 (A) The aortic waveform in the upper abdomen showing diastolic flow. (B) The aortic waveform above the bifurcation with absent diastolic flow and a waveform similar to that seen in the lower limb arteries.
The main abnormalities affecting the aorta are atheroma, aneurysm, dissection and para-aortic masses. Atheroma can affect the aorta and produce stenosis (Fig. 6-6), or occlusion; aortic disease, unless severe is usually overshadowed clinically by symptoms arising from the peripheral or the coronary arteries. Sometimes there is uncertainty as to whether aortic disease seen on arteriography is clinically significant. In these cases velocity ratios taken from above and at the stenosis can be used to assess the degree of haemodynamic compromise. However, accurate criteria are not as fully developed for aortic stenosis, unlike the situation for carotid and peripheral Doppler examinations; however, in one study3 a peak systolic velocity ratio of 2.8 correlated (86% sensitivity, 84% specificity) with aortoiliac stenoses of > 50% diameter reduction and a ratio of 5.0 showed some correlation (65% sensitivity, 91% specificity) with stenoses > 75%. If the stenotic area is clearly seen, a direct measurement of diameter or area stenosis may be obtained; colour or power Doppler is of value in defining the margins of the residual lumen.
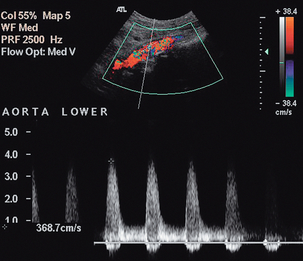
FIGURE 6-6 Colour Doppler image of a stenosis in the middle aortic segment showing significant turbulence and a peak systolic velocity of 3.7 m/s.
An aneurysm of the abdominal aorta can be defined as an increase of the anteroposterior diameter over 3 cm, or a localised increase of 1.5 times the diameter of the adjacent normal aorta. Aneurysms may extend into the abdomen from the thoracic aorta, or may arise within the abdominal aorta, usually affecting the infrarenal segment. Aneurysms are nearly always true aneurysms secondary to degeneration of the connective tissue in the vessel wall. Occasionally mycotic aneurysms secondary to infection, or pseudoaneurysms secondary to trauma, may be seen. Ultrasound diagnosis is normally straightforward; the cardinal measurement is the anteroposterior (AP) diameter, which is best obtained by scanning transversely with the ultrasound beam at right angles to the long axis of the vessel, in order to ensure a true anteroposterior measurement. The technique of diameter measurement has been amended since the advent of screening programmes so that the AP diameter of the aneurysm is measured between the inner aspects of the aneurysm wall in the antero-posterior dimension (the inner to inner method, ITI) (Fig. 6-7); and not the previous measurement using the outer aspects of the wall. In terms of a screening programme, the ITI method has been shown to be more reproducible and it is therefore preferred.4 It is also important to locate the upper and lower margins of the aneurysmal segment, particularly in relation to the renal arteries. If these cannot be identified with certainty it should be remembered that the main renal arteries usually arise from the aorta 1–2 cm below the superior mesenteric artery, and this vessel can therefore be used as an approximate marker for the renal vessels. The proximal common iliac arteries should also be checked.
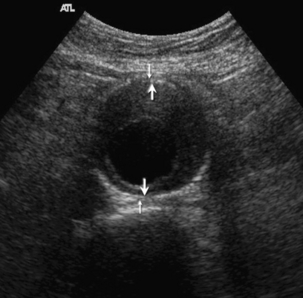
FIGURE 6-7 Transverse view of an aneurysm. The smaller arrows show the outer to outer measuring points. The larger arrows show the inner to inner (ITI) measurement used by screening programmes.
The main complications from aneurysms are leakage or rupture; occasionally an aortocaval or aortoduodenal fistula may develop (Fig. 6-8); high-volume pulsatile flow is seen in the inferior vena cava on Doppler in cases of caval fistula. Ultrasound does not have a major role in the diagnosis of these conditions as CT or angiography provide more comprehensive information if the patient’s condition allows time for imaging before surgery. FAST (Focused Assessment with Sonography in Trauma) scanning in the Emergency Department may be used for initial identification of an aneurysm in a patient with abdominal symptoms which may be related to a leaking aneurysm. If an aneurysm is seen, the patient can then be transferred directly for emergency surgery or appropriate imaging if the clinical status allows time for this.
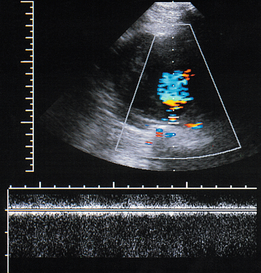
FIGURE 6-8 Colour Doppler image of an aortocaval fistula in a patient with an aneurysm. The spectral Doppler gate is on the fistula and the spectral display shows a turbulent signal which is largely off the spectral range at these settings.
Occasionally ultrasound may show a para-aortic haematoma in a patient if an aneurysm has not been suspected (Fig. 6-9); and rarely, a leaking, ruptured aneurysm may be seen on ultrasound (Fig. 6-10).
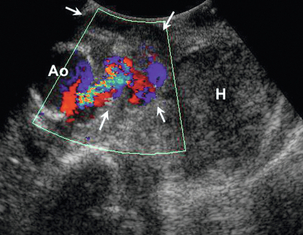
FIGURE 6-10 A leaking aneurysm of the aorta. Colour Doppler shows the leak from the aorta (Ao) into a partly thrombosed pseudo-aneurysm (arrows), with a further, large haematoma (H) seen lateral to this.
Screening for aortic aneurysms in men over 60–65 is beneficial in terms of reducing mortality;5,6 approximately 4% of men and 1% of women over 65 have been shown to have an aorta more than 3 cm in diameter. The NHS Abdominal Aortic Aneurysm Screening Programme offers ultrasound scans to men over 65; if a diameter greater than 3 cm is found then a follow-up scan is offered at 1 year if the diameter is 3–4.4 cm and in 3 months if the diameter is 4.5–5.4 cm. If the diameter is more than 5.5 cm the patient is referred for vascular surgery assessment. Growth of a smaller aneurysm by > 1 cm in a year, or the development of tenderness in patients with smaller aneurysms also leads to vascular surgery referral.
Ultrasound can also be used in the follow up of patients who have had endovascular aneurysm repair (EVAR). Although contrast-enhanced CT is considered the gold standard technique for demonstrating leaks,7 Doppler ultrasound with echo-enhancing agents has been shown to be a useful method for assessment and may show leaks that have not been apparent on CT.8,9 Ultrasound can be used to monitor the size of the aneurysmal sac: following a successful EVAR the sac may or may not shrink but an increase in size of more than 0.5 cm indicates an endoleak. Ultrasound is also useful for identifying graft limb occlusions or stenoses. However, ultrasound is less good for detecting structural problems with the stent graft, such as stent distortion or fracture.10 Unenhanced colour Doppler is less reliable when compared with CT angiography and digital subtraction arteriography. One suggested strategy is to alternate CT and ultrasound in the follow-up of stent graft patients10 as this could result in substantial cost reduction, reduced radiation exposure and reduction in the risk of contrast-induced nephrotoxicity.
Dissection of the abdominal aorta nearly always results from the extension of a dissection of the thoracic aorta extending into the abdomen (Fig. 6-11). Rarely, it may originate in the abdominal aorta, or result from trauma. The aorta is usually dilated to some extent but dissection can occur in the presence of a normal-calibre aorta. The flap may be visible depending on its orientation in relation to the ultrasound beam, and if a dissection is suspected the aorta should be examined from several different approaches in an effort to show the flap. Spectral and colour Doppler will show the presence and character of any flow in the true and false lumens and, even if a flap is not visible, the different flows in the two channels may be apparent on Doppler; reversed flow may be seen in the non-dominant channel due to compression in systole; if one of the channels is thrombosed then the appearances can be a little confusing. Doppler can also be used to assess blood flow in the major branches supplying the bowel, liver, kidneys and lower limbs.11 Clevert et al.12 reported on the role of ultrasound in the assessment of a series of 68 dissections, 25 of which involved the aortic and iliac segments. For the 13 aortic dissections the sensitivity for colour Doppler was 85%, power Doppler 85% and B-flow 98%; for the 12 iliac dissections, the sensitivity for colour Doppler was 67%, power Doppler 75% and 98% for B-flow when compared with the reference techniques (a mix of CT, magnetic resonance angiography and digital subtraction angiography).
Splanchnic Arteries
Blood flow in the superior and inferior mesenteric arteries varies depending on whether the patient is fasting, or has recently eaten. In the fasting state the flow is consistent with a relatively high-resistance vascular bed with low diastolic flow. Following the ingestion of food there is a reduction in the peripheral resistance of the mesenteric vessels, resulting in increased diastolic flow, together with an increase in peak systolic velocity (Fig. 6-12).
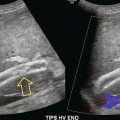
The main indication for examination of blood flow in the splanchnic arteries is the investigation of possible intestinal ischaemia. One population study13 showed a prevalence of 17.4% for mesenteric artery stenosis in a population with a mean age of 77 years. Of the patients with mesenteric artery stenosis, 86% had isolated coeliac artery stenosis (Fig. 6-3), 7% had combined coeliac and superior mesenteric artery stenosis, 5% had isolated superior mesenteric artery stenosis and 2% had coeliac axis occlusion; however none of those affected had symptoms of intestinal ischaemia. The usual indication for ultrasound examination is possible subacute or chronic ischaemia; significant acute ischaemia presents as an acute abdomen and is managed accordingly. The splanchnic circulation is capable of developing multiple collateral channels and this makes the assessment of possible gut ischaemia difficult. The demonstration of stenosis of two of the three splanchnic arteries is strongly suggestive of the diagnosis and, in the appropriate clinical situation, the demonstration of severe stenosis in one vessel with occlusion of another is also supportive of the diagnosis. Colour Doppler is of value in identifying the abnormal segment (Fig. 6-3), although care must be taken not to mistake a high shift resulting from disease with the high shift from normal velocity flow, which is seen due to the low Doppler angle resulting from the orientation of the coeliac trunk and proximal superior mesenteric artery to the ultrasound beam. In addition, colour Doppler may show a visible tissue bruit if there is a significant stenosis. The proximal 2–3 cm of the vessels is the most common site for disease and a peak velocity of more than 2.8 m/s in the superior mesenteric artery correlates well with a stenosis of more than 70% diameter reduction with a sensitivity of 92% and a specificity of 96%; whereas the equivalent peak systolic velocity for the coeliac axis is 2 m/s (87% sensitivity, 80% specificity).14 Indirect signs of intestinal ischaemia include oedema of the mucosa and bowel wall and also reduced peristalsis. In severe cases gas bubbles may be seen in the portal vein flow; these produce a characteristic popping sound on spectral Doppler (Fig. 6-13).
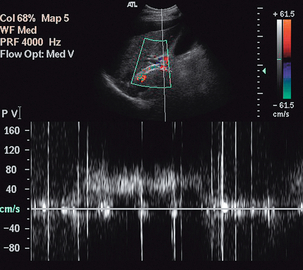
FIGURE 6-13 The spectral display from the portal vein of a patient with severe ischaemia and a transjugular intrahepatic portosystemic shunt (TIPS) showing the characteristic high-intensity echoes from bubbles of gas in the blood.
The problems associated with the diagnosis of mesenteric ischaemia are illustrated by the fact that 18% of patients over 60 years without symptoms of mesenteric ischaemia have been shown to have significant disease on Doppler.13,15 This emphasises the need to assess the findings in the light of the clinical situation.
OTHER APPLICATIONS
Blood flow in the coeliac and mesenteric arteries is also responsive to a variety of pharmacological agents such as glucagon and somatostatin, or pathological states such as cirrhosis and Crohn’s disease.16 Doppler ultrasound can show flow changes associated with these situations and may hold some promise for the future in the assessment of disease activity, or response to treatment.
The Inferior Vena Cava
ANATOMY
The inferior vena cava is formed by the confluence of the common iliac veins at the level of the 5th lumbar vertebra and runs cranially to the right of the midline. It passes through the diaphragm at the level of the 8th thoracic vertebra and enters the right atrium. In the embryo there is a complex arrangement of venous sinuses which form during embryogenesis, and several of these contribute to the inferior vena cava; this means that there are many variations of anatomy which may be seen. The most common variation is that the common iliac veins continue cranially as paired ‘venae cavae’ (Fig. 6-14), with the left component crossing to join the right side at the level of the left renal vein. Many other variations have been recorded; these are more easily assessed using contrast-enhanced CT than ultrasound, but may cause some confusion if they are seen during an ultrasound examination and not recognised.
NORMAL AND ABNORMAL FINDINGS
Flow in the inferior vena cava is slow and varies with both respiration and cardiac pulsation (Fig. 6-15). On inspiration the diaphragm descends. This results in negative pressure in the chest and increased pressure in the abdomen, and blood therefore flows from the abdomen to the chest; the reverse occurs on expiration. Superimposed on this is the more rapid periodicity resulting from cardiac activity; this is seen particularly in the upper abdomen. The prominence of the caval waveform also depends on the degree of hydration of the patient. A dehydrated patient’s cava will be narrow and difficult to see below the renal veins, whereas in fluid overload the cava is dilated and there is cardiac periodicity detectable down into the iliac veins.
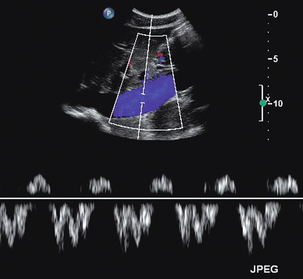
FIGURE 6-15 The inferior vena cava in the upper abdomen showing the variations in flow which occur with respiration and cardiac activity; the various components of the waveform in the inferior vena cava and hepatic veins are described in detail in Chapter 8 and Figure 8-12.
One of the most common indications for specific examination of the inferior vena cava is to assess whether thrombus from a pelvic or lower limb deep vein thrombosis has extended up into the cava. Thrombus may fill the lumen of the cava and even produce some expansion of the vessel; alternatively, a tongue of thrombus may be seen lying free in the lumen, extending up towards the right atrium (Fig. 6-16).
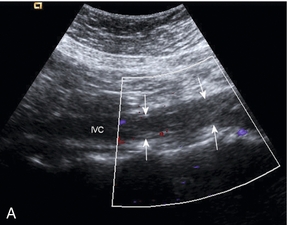
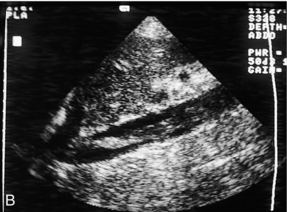
FIGURE 6-16 (A) Thrombus from an iliac DVT extending into the lower IVC (arrows) (B) A tongue of thrombus extending into the upper inferior vena cava in a patient with deep vein thrombosis.
Caval filters are inserted in some patients who are at risk of pulmonary embolus from more distal thrombus. There are several types but all are inserted in the mid- or lower cava, below the renal veins. Identifying a metallic, echogenic structure within the inferior vena cava above the level of the renal veins may indicate migration of the filter. There is a small risk that thrombus may extend past the filter, or develop at the site of the filter. Colour Doppler is a quick and easy method for confirming patency of the cava around and above the filter.17 The metal struts of the filter can be recognised within the lumen of the cava and colour Doppler, or power Doppler, will show blood flow past the level of the filter (Fig. 6-17).
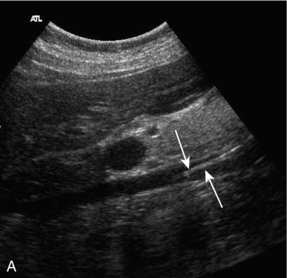
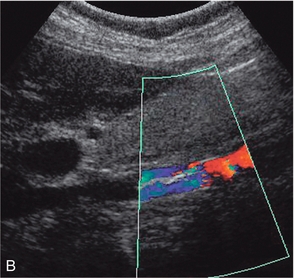
FIGURE 6-17 (A) Real time image of an IVC containing a filter in the lower segment; (B) colour Doppler image of the same patient with a caval filter. The change in colour reflects the changing Doppler angle as the blood flows through the sector scan.
Renal tumours and hepatocellular carcinoma are two tumours which have a tendency to invade venous channels and, as a result, tumour thrombus may extend into the cava (Fig. 6-18) from the renal or hepatic vein.18 Compromise of the venous drainage of the liver or kidneys is shown by loss of the normal cardiac and respiratory periodicity of the venous waveform and the tumour thrombus may be clearly seen as it extends into the caval lumen. Some tumours in the retroperitoneum may compress or directly invade the inferior vena cava causing obstruction to the venous return from the lower abdomen and legs. Although the caudal segments of the inferior vena cava and the iliac veins usually remain patent, they will often be dilated, flow will be sluggish or reversed, the flow profile will be flat, and there will be an absence of the normal Valsalva response. Rarely, intrinsic tumours, usually mesenchymal in origin such as fibrosarcomas or leiomyosarcomas, may develop in the caval wall; lipomas have also been reported.19
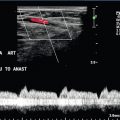
Following liver transplantation the cava should be assessed to ensure satisfactory flow. The appearances will depend on the type of anastomosis performed. In the past, the segment of donor cava attached to the new liver replaced the equivalent segment of native cava, which had been removed with the diseased liver. Many surgeons now perform a ‘piggyback’ technique, where the native cava is left in place, the inferior end of the donor caval segment is oversewn and the upper end anastomosed to the native cava. This results in a postoperative appearance which can be confusing if it is not recognised, as there will appear to be two cavae associated with the transplanted liver (Fig. 6-19).
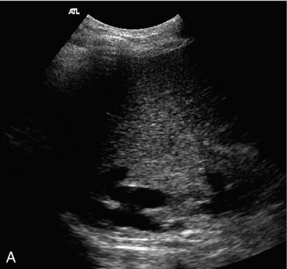
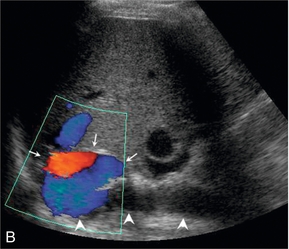
FIGURE 6-19 (A) A real time image of the upper caval region in a liver transplant patient with a ‘piggyback’ caval anastomosis, showing the native cava posteriorly and the donor cava just anterior to it; (B) colour Doppler image showing the donor cava (arrows) and native cava (arrowheads).
Other postoperative problems which may occur in relation to the cava following transplantation include compression, if the new liver is relatively large; distortion of the cava may also occur if there is relative twisting of the caval channel as a result of fitting the donor liver into the native abdomen. In the longer term stenosis may develop at the sites of anastomosis. Liver transplantation is covered further in Chapter 7.
Fistulae involving the cava may rarely occur spontaneously, often secondary to an aortic aneurysm (Fig. 6-8), or they may be surgically created as is the case with portocaval shunts. In the case of aortocaval fistulae, colour Doppler may show a visible tissue bruit with pulsatile flow in the cava above the level of the fistula; sometimes the fistula itself is difficult to identify. Surgical portocaval shunts are usually side-to-side shunts in the upper abdomen at the level where the proximal main portal vein passes close in front of the cava (Fig. 6-20). A tissue bruit may be apparent and the shunt is more easily identified if the liver can be used as a window through to the point of anastomosis; turning the patient up onto the left side may facilitate visualisation. However, these are rarely performed now, having been replaced by transjugular intrahepatic portosystemic shunts (TIPS) (see Chapter 7).
REFERENCES
1. Goldberg, B. B., Ostrum, B. J., Isard, H. J. Ultrasonographic aortography. J Am Med Assoc. 1996; 198:353–358.
2. Taylor, K. J. W., Burns, P. N., Woodcock, J. P., et al. Blood flow in deep abdominal and pelvic vessels: ultrasonic pulsed Doppler analysis. Radiology. 1985; 154:487–493.
3. De Smet, A. A., Kitslaar, P. J. A duplex criterion for aortoiliac stenosis. Eur J Vasc Surg. 1990; 4:275–278.
4. Hartshorne, T. C., McCollum, C. N., Earnshaw, J. J., et al. Ultrasound measurement of aortic diameter in a national screening programme. Eur J Vasc Endovasc Surg. 2011; 42:195–199.
5. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005; 142:198–202.
6. Cosford, P. A., Leng, G. C., Thomas, J., Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. Issue 2, 2007, doi: 10. 1002/14651858. CD002945. pub2. [Art. No. : CD002945].
7. Raman, K. G., Missig-Carroll, N., Richardson, T., et al. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg. 2003; 38:645–651.
8. Napoli, V., Bargellini, I., Sardella, S. G., et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004; 233:217–225.
9. Bendick, P. J., Bove, P. G., Long, G. W., et al. Efficacy of ultrasound contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg. 2003; 37:381–385.
10. Thurnher, S., Cejna, M. Imaging of aortic stent-grafts and endoleaks. Radiol Clin North Am. 2002; 40:799–833.
11. Thomas, E. A., Dubbins, P. A. Duplex ultrasound of the abdominal aorta – a neglected tool in aortic dissection. Clin Radiol. 1990; 42:330–334.
12. Clevert, D. A., Rupp, N., Reiser, M., et al. Improved diagnosis of vascular dissection by ultrasound B-flow: a comparison with color-coded Doppler and power Doppler sonography. Eur Radiol. 2005; 15:342–347.
13. Hansen, K. J., Wilson, D. B., Craven, T. E., et al. Mesenteric artery disease in the elderly. J Vasc Surg. 2004; 40:45–52.
14. Moneta, G. L. Screening for mesenteric vascular insufficiency and follow-up of mesenteric artery bypass procedures. Semin Vasc Surg. 2001; 14:186–192.
15. Roobottom, C. A., Dubbins, P. A. Significant disease of the coeliac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. Am J Radiol. 1993; 161:985–988.
16. Perko, M. J. Duplex ultrasound for assessment of superior mesenteric artery blood flow. Eur J Vasc Endovasc Surg. 2001; 21:106–117.
17. Smart, L. M., Redhead, D. N., Allan, P. L., et al. Follow-up study of Gunther and LGM inferior vena caval filters. J Intervent Radiol. 1992; 7:115–118.
18. Bissada, N. K., Yakout, H. H., Babanouri, A., et al. Long-term experience with management of renal cell carcinoma involving the inferior vena cava. Urology. 2003; 61:89–92.
19. Grassi, R., Di Mizio, R., Barberi, A., et al. Case report. Ultrasound and CT findings in lipoma of the inferior vena cava. Br J Radiol. 2002; 75:69–71.