Chapter 12. The ankle and foot
SUMMARY
This chapter confines itself to common lesions in the ankle and foot arising from arthritis, trauma or overuse and begins with a presentation of the relevant anatomy and palpation techniques to aid their identification. Points from the history are considered and a logical sequence of objective examination is given, followed by discussion of lesions and suggestions for treatment and management.
ANATOMY
Inert structures
The inferior tibiofibular joint is the articulation between the fibular notch on the lateral aspect of the tibia and the distal end of the fibula. It is considered to be a syndesmosis because the firm union of the two bones is largely due to the interosseous membrane. Anterior and posterior ligaments reinforce the joint.
The deep part of the posterior ligament is the inferior transverse tibiofibular ligament which passes under the posterior ligament from the tibia into the malleolar fossa of the fibular. It is a thickened band of yellow elastic fibres forming part of the articulating surface of the ankle joint.
The firm union of the inferior tibiofibular joint is a major factor in the inherent stability of the ankle joint mortise. When the ankle joint is in dorsiflexion, the close packed position, the elastic nature of the inferior tibiofibular joint ligament allows the joint to yield and separate, accommodating the wider anterior aspect of the trochlear surface of the talus. In plantarflexion the ligament recoils as the narrower posterior aspect of the talus moves into the mortise, approximating the malleoli to maintain a pinch-like grip on the talus.
Dorsiflexion and plantarflexion of the ankle will induce small accessory movements in the inferior tibiofibular joint which in turn affect the superior tibiofibular joint. Injuries of the syndesmosis usually involve forced dorsiflexion, causing widening or diastasis of the ankle mortise (Edwards & DeLee 1984, Boytim et al 1991, Marder 1994). However, isolated injuries are uncommon and damage usually occurs in association with other major ligamentous disruption and fracture.
The ankle joint (talocrural joint) is a uniaxial, synovial hinge joint between the mortise, formed by the distal ends of the tibia and fibula, including both malleoli, and the dome of the talus. Its function is complex, with the talocrural, subtalar and inferior tibiofibular joints working in concert to allow coordinated movement of the rear foot (Hertel 2002). It bears more weight per unit area than any other joint, and any malalignment or instability may lead to degenerative changes (Sartoris 1994). The joint surfaces are covered with hyaline cartilage and surrounded by a fibrous capsule which attaches to the margins of the articulating surfaces. The capsule is lined with synovium and reinforced by strong collateral ligaments. The congruity of the articular surfaces during loading of the joint, the static ligamentous control and the dynamic control of the musculotendinous units all contribute to the stability of the ankle joint (Hertel 2002).
The collateral ligaments are roughly triangular in their attachments, radiating downwards from the malleoli to a wide base. Each has anterior and posterior components which link with the talus, and a central component that links with the calcaneus.
Movement at the ankle joint occurs about a transverse axis in a sagittal plane and amounts to approximately 20° of dorsiflexion and 35° of plantarflexion, allowing the foot to adjust to the surface on which it is placed. Dorsiflexion achieves the close packed position of the ankle joint and no movement of the talus in the mortise should be possible in this position. In full plantarflexion, the loose packed position, a small amount of side-to-side movement of the talus should be possible.
The medial collateral (deltoid) ligament forms a strong multiligamentous complex spreading out in a fan shape over the medial aspect of the ankle joint. It forms a continuous line of attachment from the navicular in front, along the sustentaculum tali to the talus behind. The origins and insertions of the various parts of the ligament are contiguous and therefore not clearly demarcated, but it is generally accepted to have deep and superficial fibres. The superficial fibres cross the ankle and subtalar joints, while the deep fibres cross the ankle joint (Boss & Hintermann 2002).
As the medial collateral ligament offers such strong support to the medial aspect of the ankle joint, traumatic injuries more commonly cause fracture and disruption of the syndesmosis rather than ligamentous injury. Most strain on the ligament occurs with a dorsiflexion and eversion stress. Biomechanical problems in the foot can, however, lead to a gradual onset overuse lesion of the medial collateral ligament and treatment should be directed to the cause.
The lateral collateral ligament consists of three separate bands which leave the ligament deficient in its support of the lateral aspect of the ankle joint. Consequently forced inversion of the plantarflexed foot commonly affects the lateral collateral ligament (Liu & Jason 1994, Lee & Maleski 2002).
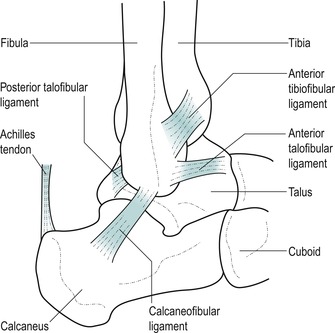
The components of the lateral collateral ligament are as follows (Fig. 12.1):
• The anterior talofibular ligament is an integral part of the capsule of the ankle joint. It arises from the anterior border and tip of the lateral malleolus and passes deeply and anteromedially across the ankle joint to the neck of the talus in the sinus tarsi. It is a wide, flattened band approximately the width of the patient’s index finger. In the anatomical position, the ligament runs almost parallel to the transverse axis of the foot, while in plantarflexion it runs more parallel to the vertical axis of the leg. In this position of plantarflexion, the ligament is most likely to be sprained particularly if the foot is inverted (Kannus & Renstrom 1991, Kumai et al 2002). It is the most vulnerable of the three components to a forced plantarflexion and inversion injury (Colville et al 1990, Hertel 2002)
• The calcaneofibular ligament is a narrow cord, separate from the capsule of the ankle joint. It arises from the apex of the lateral malleolus and passes obliquely inferoposteriorly, under the peroneal tendons, to attach to the calcaneus just behind the peroneal tubercle. Here it is closely related to the overlying peroneal sheath containing the tendons of peroneus longus and brevis (Marder 1994, Miller & Bosco 2002). This component of the ligament crosses both the ankle and subtalar joints and is vulnerable to varus stress; it is most taut in dorsiflexion and is most likely to be sprained with forced inversion in dorsiflexion (Colville et al 1990)
• The posterior talofibular ligament is a strong, thick band arising from the posterior border of the lateral malleolus and passing horizontally and posteromedially to the posterior aspect of the talus. Clinically, it is rare to find involvement of this ligamentous component.
 |
| Figure 12.1
Lateral collateral ligament of the ankle.
|
The contribution of the individual lateral ligaments to ankle joint stability is not constant, but depends on the position of the ankle and foot in space. Ligamentous stabilization is most critical in the unloaded ankle as this is when injury tends to occur as initial contact is made with the ground during the gait cycle. During weight-bearing, stability is maintained by the bony congruency of the mortise. The anterior talofibular ligament has the weakest tensile strength with a load to failure two to three and a half times lower than that for the calcaneofibular ligament which is rarely injured in isolation (Boruta et al 1990, Miller & Bosco 2002).
The tibiofibular syndesmosis is established by the interosseous ligament, the posterior inferior tibiofibular ligament and the anterior inferior tibiofibular ligament, which is the weakest of the three. Post-traumatic antero-lateral laxity following injury to the anterior talofibular ligament may lead to anterior extrusion of the talar dome in dorsiflexion, resulting in impingement of the anterior inferior tibiofibular ligament (Bekerom & Raven 2007).
The foot consists of 26 bones and 57 joints which enable it to act as a rigid structure for weight-bearing, e.g. pointing in ballet, or to be converted into a flexible structure for mobility, e.g. gait activities (Nordin & Frankel 2001). The foot supports body weight and controls posture by maintaining the centre of gravity. It assists propulsion and lift, as well as restraining gait activities and acting as a shock absorber. To provide this variety of functions, arches have developed together with the joints, ligaments and muscles, all contributing to functional activities. The main joints and ligaments of the arches, of clinical concern in orthopaedic medicine, will be described briefly here.
The subtalar (talocalcaneal) joint is a synovial joint between the talus and underlying calcaneus, which works in conjunction with the ankle and mid-tarsal joints to form a functional component. These joints together allow the foot to adapt to the surface of stance and to act as a shock absorber. They allow adjustment of the arches of the foot and, in conjunction with muscle activity, provide spring and propulsion to gait. During walking, the subtalar joint adapts to the side-to-side slope of the ground, accompanied by secondary rotation of the tibia (Evans 1990).
The subtalar joint consists of separate anterior and posterior joint cavities divided by the sinus tarsi (a narrow tunnel running obliquely forwards and laterally between the talus and the calcaneus). Interosseous and cervical ligaments stabilize the subtalar joint and form a barrier between the two joint capsules; they have been described as the ‘cruciate ligaments of the subtalar joint’ (Hertel 2002). This division of the subtalar joint has implications for injection of the joint which will be discussed below.
Movements at the subtalar joint are complex due to the shape of the articulating facets, which allow a degree of play to occur simultaneously in three planes. The calcaneus ‘pitches’, ‘turns’ and ‘rolls’ (Kapandji 1987) under the talus, enabling these accessory movements to contribute to the functional movements of the joint. This results in triplanar motion of the talus around a single oblique axis. The subtalar joint, therefore, is a uniaxial joint with one degree of freedom of movement, supination, which achieves a varus position of the calcaneus, and pronation, which achieves a valgus position of the calcaneus (Levangie & Norkin 2001).
The mid-tarsal (transverse tarsal) joints consist of the calcaneocuboid joint laterally and the talocalcaneo-navicular joint medially. These joints adapt the posture of the foot, keeping the sole of the foot in contact with the ground whatever the slope of the surface or the position of the leg. Together they act as a shock absorber as well as providing elasticity and spring to gait.
The calcaneocuboid joint is supported by the dorsal calcaneocuboid ligament, which is a capsular ligament that runs along the dorsolateral aspect of the joint, and the plantar calcaneocuboid (short plantar) and the long plantar ligaments (Levangie & Norkin 2001, Palastanga et al 2006). The dorsal calcaneocuboid ligament is sometimes involved in an inversion sprain of the ankle as it resists inversion and adduction of the mid-tarsal joint.
The talocalcaneonavicular joint is supported by the spring ligament (otherwise known as the plantar calcaneo-navicular ligament) which spans the gap between the sustentaculum tali on the calcaneus and the navicular below the talar head. Consequently, this joint can be visualized as a ball and socket joint with the facet on the head and lower surface of the neck of the talus as the ball. The osseoligamentous socket is formed by the navicular anteriorly, the sustentaculum tali and calcaneus posteriorly and the spring ligament, and supports the head of the talus.
Movements at the mid-tarsal joint (calcaneocuboid and talocalcaneonavicular joint complex) do not occur in isolation (Levangie & Norkin 2001, Palastanga et al 2006). Accessory movements at the mid-tarsal joints consist of dorsiflexion and plantarflexion, abduction and adduction, and inversion and eversion.
A series of arches, ligaments and muscles satisfies the requirements of the foot to be able to provide both strength and mobility.
• The medial and lateral longitudinal arches are supported posteriorly by the calcaneus and anteriorly by the metatarsal heads. The talus forms the summit of the arch. The medial longitudinal arch is the larger of the two and has a dynamic role for gait. It consists of the calcaneus, talus, navicular, the cuneiforms and three medial metatarsals. It absorbs and transmits weight backwards through the calcaneus and forwards to the metatarsal heads, while providing elasticity for propulsion. The lateral longitudinal arch has a static role for weight-bearing. It is lower than the medial, making contact with the ground throughout its length to support load in standing. The main supporting mechanism for the longitudinal arches is the plantar fascia. Acting as a cable between the heel and the toes, it locks the joints of the foot and prevents the arches from collapsing during weight-bearing.
• The transverse arch is formed by the distal row of tarsal bones and the bases of the metatarsals, and acts to support and transmit body weight. As body weight is applied, the metatarsal bones separate and flatten slightly.
The arches of the foot depend on ligamentous and muscular support. The important ligaments are the long and short plantar ligaments, plantar aponeurosis and the spring ligament (plantar calcaneonavicular ligament). The intrinsic muscles of the foot maintain the arches, together with some of the long muscles of the leg. Tibialis anterior supports the medial arch, while peroneus longus supports the lateral arch.
The plantar fascia is a strong aponeurosis on the plantar surface of the foot which, together with the mid-tarsal ligaments and intrinsic and extrinsic muscles, withstands loading during weight-bearing. It consists of three variably developed components known as central, medial and lateral cords. The central cord is biomechanically the most important, arising from the medial side of the medial calcaneal tuberosity and consisting of longitudinally arranged fibres of collagen and elastin. The fibres pass distally into the forefoot, widening and thinning before dividing into five distinct bands that extend into the toes (Karr 1994, Yu 2000). At its insertion into the calcaneal tuberosity (the enthesis) it has a thickened ‘cuff’ similar in appearance to the rotator cuff of the shoulder (Yu 2000). This strong connecting cable passing between the pillars of the longitudinal arch has very little ability to lengthen, but under loading gives slightly to act as a shock absorber.
Contractile structures
The numerous tendons crossing the ankle joint complex contribute to dynamic stability as well as producing movement of the various joints. The lateral muscles have a role in controlling supination and the anterior muscles probably slow the plantarflexion component of supination, acting to prevent injury to the lateral ligaments (Hertel 2002). As tendons cross the ankle joint, they change their direction from running vertically to horizontally. Several retinacula prevent the tendons from bowstringing under activity, while synovial sheaths protect the tendons as they pass under the retinacula.
The talus provides attachment for ligaments and many tendons pass over it, although it does not itself give insertion to any contractile unit.
All anterior muscles of the lower leg are supplied by the deep peroneal nerve and their principal function is dorsiflexion of the ankle and extension of the toes.
Tibialis anterior (L4–L5) takes origin from the upper two-thirds of the lateral tibial shaft and adjacent interosseous membrane. It becomes tendinous in its lower third, passing across the anteromedial aspect of the ankle joint to insert into the medial aspect of the medial cuneiform and the base of the first metatarsal. Its function is to dorsiflex the ankle during the swing-through phase of gait and to invert the foot. It raises the medial longitudinal arch and works in conjunction with other muscles to counteract gravity and to control foot placement. This tendon runs a relatively straight course and is not a common cause of pain. However, hill running or irritation from tight-fitting boots, for example, may cause lesions of tibialis anterior (Frey & Shereff 1988, Chandnani & Bradley 1994).
Extensor hallucis longus (L5, S1) takes origin from the middle of the anteromedial border of the fibula and passes downwards and medially to a tendon which inserts into the base of the distal phalanx of the hallux. Functionally it dorsiflexes the ankle and extends the hallux to enable the big toe and foot to clear the ground during the swing-through phase.
Extensor digitorum longus (L5, S1) takes origin from the upper two-thirds of the anterior aspect of the fibula and passes downwards under the extensor retinacula, dividing into four tendons which insert into the dorsal digital expansions of the lateral four toes. Functionally, it assists dorsiflexion of the ankle and extends the lateral four toes, to clear the ground during the swing-through phase.
Peroneus tertius (L5, S1), a divorced part of extensor digitorum longus, arises from the lower anterolateral aspect of the fibula and inserts into the base of the fifth metatarsal. It functions as a weak dorsiflexor of the ankle and an evertor of the foot.
The peroneal muscles pass under two rectinacula to reach the lateral side of the foot. The principal function of the lateral muscles is to evert the foot, controlling side-to-side movements in standing and acting as the primary lateral dynamic stabilizers of the ankle (Frey & Shereff 1988). Peroneus longus, together with tibialis anterior, forms a stirrup for the foot, maintaining and supporting the arches. Both peroneus longus and brevis are supplied by the superficial peroneal nerve.
Peroneus longus is the main evertor of the foot and draws the medial side of the foot down, as in plantarflexion and eversion. Peroneus brevis also produces eversion and plantarflexion (Palastanga et al 2006).
Peroneus longus (L5, S1–S2), the more superficial of the two peroneal tendons, arises from the head and upper lateral two-thirds of the fibula. It becomes tendinous just above the ankle and passes behind the lateral malleolus in a sheath common to it and peroneus brevis. Continuing on, it crosses the lateral surface of the calcaneus, passing below the peroneal tubercle, where it leaves peroneus brevis. It occupies a groove on the lateral and plantar surfaces of the cuboid and crosses the sole of the foot obliquely to insert into the lateral side of the base of the first metatarsal and adjacent medial cuneiform.
Peroneus brevis (L5, S1–S2) arises from the lower lateral surface of the fibula. It lies in front of peroneus longus as the tendons pass behind the lateral malleolus in the common sheath. It crosses the calcaneus above the peroneal tubercle to insert into the base of the fifth metatarsal.
Generally, the more superficial posterior muscles are concerned mainly with plantarflexion of the ankle, while the deep muscles flex the toes. Both groups are supplied by the tibial nerve.
Gastrocnemius (S1–S2) is the most superficial of the posterior muscles and gives the calf its characteristic shape. It has two heads of origin from the appropriate posterior femoral condyle. The medial head is larger, extends more distally and is a common site for muscle belly injuries. The two muscle heads come together in a broad tendon which is joined on the anterolateral side by soleus, forming the Achilles tendon. Functionally, gastrocnemius flexes the knee and plantarflexes the ankle.
Soleus (S1–S2) lies deep to gastrocnemius, arising from the upper posterior aspect of the fibula and soleal line of the tibia. The muscle fibres blend into a membranous tendon which lies deep to the gastrocnemius, allowing both muscles to function individually. These tendon fibres then merge with the Achilles tendon.
Plantaris (S1–S2) arises from the posterior aspect of the lateral supracondylar ridge of the femur and descends medially to blend with the Achilles tendon. Its function is to assist gastrocnemius.
Gastrocnemius, soleus and plantaris form a functional group known as triceps surae; as well as functioning together they also have independent functions. Gastrocnemius works with plantaris to flex the knee and plantarflex the foot, providing propulsion to the push-off phase of gait. Soleus works continuously as a postural muscle through its slow-twitch muscle fibres which maintain the upright posture (McMinn et al 1995). In standing, the ankle is in a loose packed position with the centre of gravity falling anterior to the joint. Soleus counteracts the tendency for body weight to move forwards over the stationary foot (Standring 2009).
The Achilles tendon is a long tendon which receives the fibres of gastrocnemius and soleus. It is about 15 cm long (Standring 2009) and its insertion into the calcaneus is cushioned by two bursae: the retrocalcaneal bursa on its deep surface and the subcutaneous Achilles bursa on its superficial surface (Smart et al 1980). The tendon fibres twist as they pass down to their insertion into the middle of the posterior surface of the calcaneus. This twist in the tendon fibres is understood to be responsible for the tendon’s elastic properties, the stored energy providing propulsion to lift the heel during walking, running and jumping activities (Norris 2004). The tendon has a zone of relatively poor vascularity 2–6 cm above its insertion and is prone to overuse, degeneration and rupture, particularly at this site (Smart et al 1980, Chandnani & Bradley 1994).
Tibialis posterior (L4–L5) arises from the upper postero-lateral surface of the tibia. It passes behind the medial malleolus in its own sheath, crosses the deltoid ligament and inserts into the tuberosity of the navicular. It sends tendinous slips onto every tarsal bone except the talus. Functionally it is the main invertor of the foot, working with tibialis anterior. It gives support to the arches of the foot through its many tendinous insertions. It decelerates subtalar pronation after heel contact (Blake et al 1994). Activities which involve rapid changes in direction (e.g. soccer, tennis, hockey) place increased stress on tibialis posterior and make it vulnerable to injury in these situations (Frey & Shereff 1988).
Flexor digitorum longus (S2–S3) arises from the middle of the posterior surface of the tibia and passes downwards, becoming tendinous above the ankle joint. Behind the medial malleolus it is medial to tibialis posterior and occupies the groove under the sustentaculum tali. Dividing into four tendons, it inserts into the base of the distal phalanx of the lateral four toes. Functionally, flexor digitorum longus works with the lumbricals to keep the pads of the toes in contact with the ground, increasing the weight-bearing surface. It also acts to assist plantarflexion during the toe-off phase of gait, and repeated push-off activity may cause injury to this tendon (Frey & Shereff 1988).
The lumbricals (S2–S3) arise from flexor digitorum longus tendons and insert into the dorsal digital expansions. They flex the metatarsophalangeal joints and extend the interphalangeal joints; this counteracts the clawing tendency of the flexor digitorum longus. Together they maintain the medial arch.
Flexor hallucis longus (S2–S3) arises from the lower posterior surface of the fibula and passes behind the medial malleolus and under the sustentaculum tali to insert into the base of the distal phalanx of the big toe. It functions to provide the final thrust for toe-off and is important in supporting the medial longitudinal arch. Activities which involve repeatedly pushing off from the forefoot (e.g. ballet) may cause lesions in this tendon (Frey & Shereff 1988).
A GUIDE TO SURFACE MARKING AND PALPATION
Medial aspect (Fig. 12.2)
Palpate the short, thick, medial tibial malleolus, appreciating that the apex, anterior and posterior borders are all subcutaneous. Compare this with the longer, slender, lateral fibular malleolus which tends to project further distally and lie slightly more posteriorly.
 |
| Figure 12.2
Medial aspect of the ankle.
|
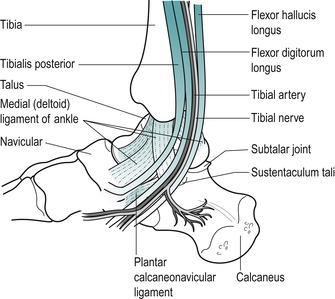
It is difficult to palpate the tendons lying behind the medial malleolus, but from medial to lateral they are tibialis posterior, flexor digitorum longus and flexor hallucis longus ( Tom, Dick and Harry). You can palpate the posterior tibial pulse by applying light pressure behind the medial malleolus, approximately halfway between it and the Achilles tendon.
Consider the triangular medial (deltoid) collateral ligament which fans down from the medial malleolus to attach by a broad base from the navicular in front, to the talus behind.
Palpate the sustentaculum tali lying approximately one thumb’s width directly below the medial malleolus, where it feels like a horizontal, bony shelf.
Move directly forwards from the sustentaculum tali to the next palpable bony bump, the tuberosity of the navicular. This gives insertion to tibialis posterior and lies at the level of the lip of a slip-on shoe. Move directly forwards from the navicular to palpate the medial cuneiform and the base of the first metatarsal.
Anterior aspect (Fig. 12.3)
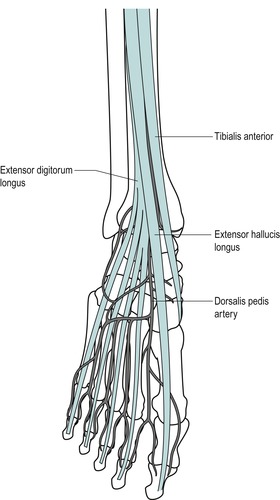
Place the ankle joint into plantarflexion and inversion, making the talus both visible and palpable anterior to the lateral malleolus. Palpate along from the anterior aspect of the lateral malleolus and the lower margin of the tibia to the anterior aspect of the medial malleolus to identify the ankle joint line.
 |
| Figure 12.3
Anterior aspect of the ankle.
|
Move to the front of the lateral malleolus and feel the depression on the lateral side of the talus; this marks the entrance to the sinus tarsi (the narrow tunnel which runs between the talus and calcaneus in front of the subtalar joint).
Palpate the tibialis anterior, extensor hallucis longus, extensor digitorum longus and peroneus tertius tendons (from medial to lateral) as they cross the ankle joint anteriorly.
Palpate the dorsalis pedis pulse approximately halfway between the malleoli on the dorsum of the foot, just lateral to the tendon of extensor hallucis longus.
Posterior aspect (Fig. 12.4)
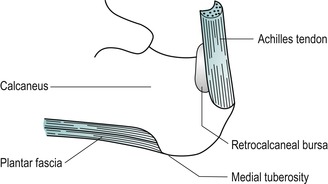
Palpate the calcaneus, the largest of the tarsal bones, which forms the bony prominence of the heel.
 |
| Figure 12.4
Posterior structures of the ankle.
|
Palpate the medial tuberosity of the calcaneus, which can be located at the posteromedial edge of the plantar surface of the calcaneus; deep palpation may be necessary. This marks the insertion of the middle cord of the plantar fascia (Fig. 12.5).
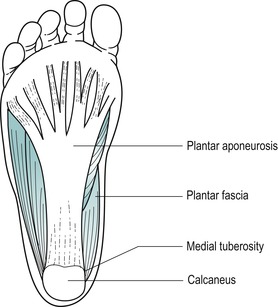 |
| Figure 12.5
Plantar fascia.
|
Locate the insertion of the Achilles tendon into the middle third of the posterior surface of the calcaneus. Palpate the Achilles tendon, appreciating its thickness, and follow it up to the two fleshy bellies of the gastrocnemius; the medial belly should be felt to extend further distally than the lateral.
Lateral aspect (Fig. 12.6)
Palpate the lateral malleolus. Move approximately one finger’s width below it and slightly anteriorly to locate the peroneal tubercle. This tubercle varies in size and position so may not be obvious. It divides the tendons of peroneus longus and brevis.
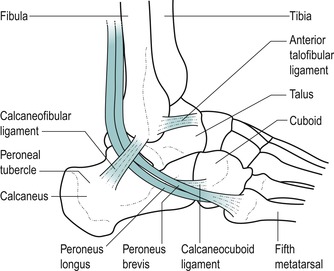 |
| Figure 12.6
Structures of the lateral aspect of the ankle.
|
Consider the individual components of the lateral collateral ligament which take origin from the lateral malleolus. The anterior talofibular ligament is approximately the width of an index finger and it passes deeply, anteromedially to the talus. Its fibres run roughly parallel to the sole of the foot and it may be palpated in the region of the sinus tarsi. The calcaneofibular ligament passes obliquely downwards and backwards, under the peroneal tendons. The posterior talofibular ligament passes horizontally backwards to attach to the posterior talus; it is difficult to palpate.
Palpate the base of the fifth metatarsal and appreciate its prominent tubercle which gives attachment to peroneus brevis. Placing a thumb vertically behind the base of the fifth metatarsal will indicate the approximate position of the calcaneocuboid joint line. Placing another thumb or finger transversely across the tip of the thumb forms a T and the cross-bar of the T is resting over the dorsal aspect of the joint, indicating the approximate position of the dorsal calcaneocuboid ligament.
Position your hand to resist eversion of the foot, to aid identification of the two lateral tendons, peroneus longus and brevis. Behind the lateral malleolus, peroneus brevis lies in front of longus. They divide at the peroneal tubercle, with brevis running above the tubercle and longus below.
COMMENTARY ON THE EXAMINATION
Observation
Before proceeding with the history, a general observation of the patient’s face, posture and gait will alert the examiner to abnormalities, particularly of the gait pattern. A limp usually indicates abnormal weight-bearing, but a ‘short leg’ can produce a limp through functional discrepancies of hyperpronation and a flattened medial arch (Kannus 1992).
History (subjective examination)
The age, occupation, sports, hobbies and lifestyle of the patient may give an indication of the cause of the lesion and alert the examiner to possible biomechanical or postural problems.
The site and spread of pain help to localize the lesion, but, as a peripheral joint, pain is usually well localized. The presence of paraesthesia in the foot or pain in the calf or shin could suggest a more proximal lesion.
The onset of the symptoms may be sudden, due to trauma, or gradual, associated with overuse or arthritis. If the onset is traumatic in nature, the mechanism of injury should be established, particularly to give an indication of the ligaments involved. A ‘snap’ or ‘popping’ sensation at onset may indicate rupture of tendon, ligament or a fracture.
A minor injury is indicated by minimal pain and localized swelling, with the ability to continue weight-bearing activities. More severe injuries will produce diffuse swelling and an inability to weight-bear, suggesting ligamentous rupture or fracture.
The duration of symptoms indicates the stage of the lesion in the inflammatory process. A history of recurrent episodes indicates possible instability, requiring in-depth biomechanical assessment.
The symptoms and behaviour need to be considered. The behaviour of the pain indicates the nature of the lesion: mechanical lesions are eased by rest and aggravated by activity and weight-bearing.
Other symptoms described by the patient could include the ankle giving way; this is a symptom of ligamentous instability or possible loose body in the joint. A clicking or snapping sensation on the lateral aspect of the ankle could be due to disruption of the peroneal retinaculum, allowing subluxation of the tendons.
An indication of past medical history, other joint involvement and medications will aid diagnosis and establish whether contraindications to treatment techniques exist. Rheumatoid arthritis may affect the small joints of the foot. As well as past medical history, establish any ongoing conditions and treatment. Explore other previous or current musculoskeletal problems with previous episodes of the current complaint, any treatment given and the outcome of treatment.
Inspection
This should be conducted in both weight-bearing and non-weight-bearing postures.
Bony deformity and functional abnormalities, of the medial arch in particular, will usually manifest themselves in the weight-bearing position.
Check the height of the medial and longitudinal arches. Pes planus is a structural flat foot which is visible in both weight-bearing and non-weight-bearing positions, while a functional flat foot is only observed on weight-bearing.
The presence of functional flat foot can be generally estimated by looking for a difference in the height of the medial longitudinal arch in the non-weight-bearing and weight-bearing positions. Estimate the distance between the tuberosity of the navicular and the ground, first in a non-weight-bearing sitting position and secondly in the standing weight-bearing position (Evans 1990).
Observe the position of the Achilles tendon from behind for any deviations in the normal straight alignment, which would indicate postural deformity. Also notice if there is any angulation of the forefoot relative to the hindfoot.
Although it is important to note postural abnormalities of the foot, this should be kept relevant to the patient’s presenting signs and symptoms. A detailed biomechanical assessment is not necessary for uncomplicated lesions, but recurrent symptoms or failure to resolve the patient’s symptoms may require referral to a podiatrist or physio-therapy specialist in lower limb biomechanics.
A bony enlargement of the posterior superior calcaneus may be observed at the back of the heel, known as Haglund’s deformity, which may also be associated with bursitis and soft tissue swellings called ‘pump bumps’ (Stephens 1994) (see page 352).
It is worth inspecting the patient’s shoes for abnormal areas of weight-bearing. This is particularly relevant to athletes and should include their training shoes. In a normal gait pattern, wear is seen on the lateral side of the heel and the medial side of the forefoot (Kannus 1992). Camber running or worn-out or incorrect training shoes can alter angles of contact between the foot and the ground and be an external cause of overuse (Evans 1990). Advice will need to be given regarding the type of shoe best suited to the patient’s activities (Anthony 1987). Abnormal callus formation indicates abnormal weight-bearing.
Colour changes such as cyanosis, erythema or pallor may indicate circulatory involvement and a change in colour in transferring from the weight-bearing to the non-weight-bearing position should be further investigated by palpating for the presence of arterial pulses (Figure 12.7 and Figure 12.8). Bruising is often associated with a recently sprained ankle or gastrocnemius muscle belly lesion and tracks peripherally. A severe sprain of the ankle can cause traction injury of the superficial peroneal nerve and vascular structures. Consideration should be given to these possibilities if changes in skin colour persist (Acus & Flanagan 1991).
 |
| Figure 12.7
Palpation for dorsalis pedis pulse.
|
 |
| Figure 12.8
Palpation for posterior tibial artery.
|
Muscle wasting may be seen in the calf or peroneal muscles.
Swelling and any bruising on the lateral aspect of the ankle may be diffuse, indicating a moderate or major ligamentous lesion. Often a rounded mottled egg-shell-like swelling lies in front of the lateral malleolus: this is known as the signe de la coquille d’œuf (Litt 1992).
Minor swelling may be indicated by loss of the hollows behind the malleoli. Local swellings and ganglia should also be noted.
Palpation
As peripheral joints, the ankle and foot are palpated for signs of activity. The presence of heat is assessed (Fig. 12.9) and synovial thickening is palpated most easily along the anterior joint line (Fig. 12.10). Swelling is usually observed, but it is also possible to palpate for swelling, particularly around the malleoli. If the history indicates major ligament sprain, the malleoli and talus can be palpated for any focal areas of tenderness which may indicate a fracture (Lee & Maleski 2002).
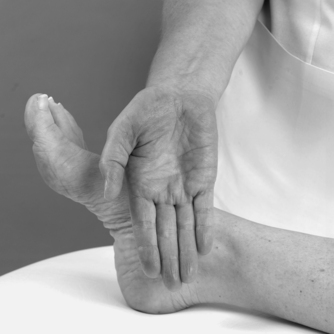 |
| Figure 12.9
Palpation for heat.
|
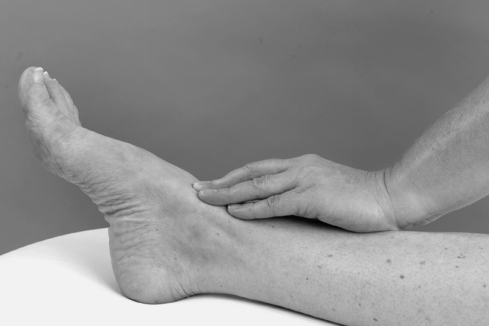 |
| Figure 12.10
Palpation for synovial thickening.
|
State at rest
Before any movements are performed, the state at rest is established to provide a baseline for subsequent comparison.
Examination by selective tension (objective examination)
The suggested sequence for the objective examination will now be given, followed by a commentary including the reasoning in performing the movements and the significance of the possible findings. Comparison should always be made with the other side.
Ankle joint
• Passive dorsiflexion (Fig. 12.11)
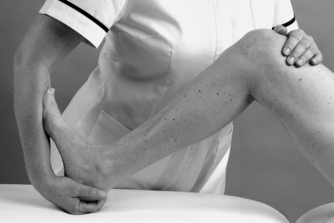 |
| Figure 12.11
Passive ankle dorsiflexion.
|
• Passive plantarflexion (Fig. 12.12)
 |
| Figure 12.12
Passive ankle plantarflexion.
|
Subtalar joint
• Passive varus of the calcaneus to produce supination (Fig. 12.13)
 |
| Figure 12.13
Hand position for varus and valgus stress, subtalar joint: valgus stress shown.
|
• Passive valgus of the calcaneus to produce pronation (Fig. 12.13)
Mid-tarsal joints
• Passive dorsiflexion and plantarflexion (Figure 12.14 and Figure 12.15)
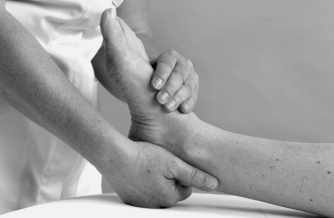 |
| Figure 12.14
Passive mid-tarsal dorsiflexion.
|
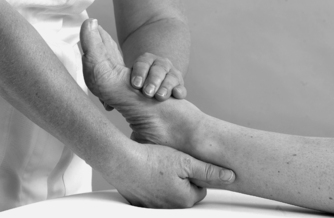 |
| Figure 12.15
Passive mid-tarsal plantarflexion.
|
• Passive abduction and adduction (Figure 12.16 and Figure 12.17)
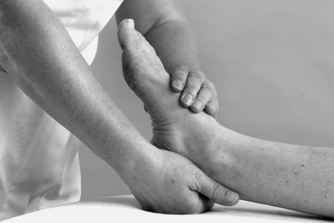 |
| Figure 12.16
Passive mid-tarsal abduction.
|
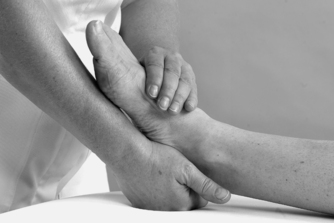 |
| Figure 12.17
Passive mid-tarsal adduction.
|
• Passive eversion and inversion (Figure 12.18 and Figure 12.19)
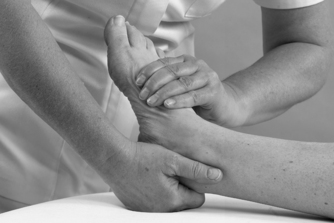 |
| Figure 12.18
Passive mid-tarsal eversion.
|
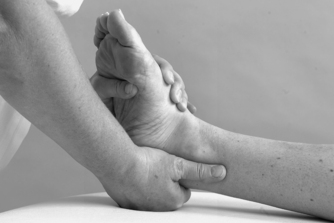 |
| Figure 12.19
Passive mid-tarsal inversion.
|
Gross ligament tests
• Passive inversion in plantarflexion for lateral collateral ligament (Fig. 12.20)
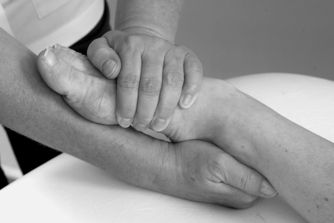 |
| Figure 12.20
Passive inversion in plantarflexion for lateral collateral ligament.
|
• Passive eversion for medial (deltoid) ligament (Fig. 12.21)
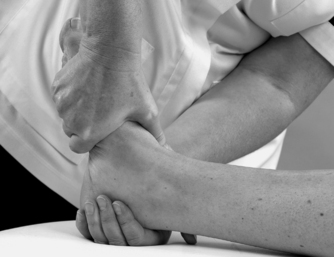 |
| Figure 12.21
Passive eversion for medial (deltoid) ligament.
|
Contractile structures
• Resisted dorsiflexion (Fig. 12.22)
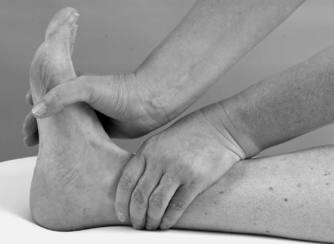 |
| Figure 12.22
Resisted dorsiflexion.
|
• Resisted plantarflexion (Fig. 12.23)
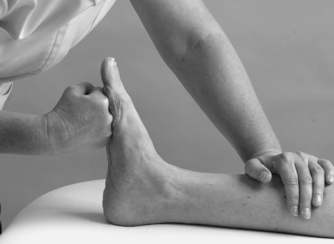 |
| Figure 12.23
Resisted plantarflexion.
|
• Resisted inversion (Fig. 12.24)
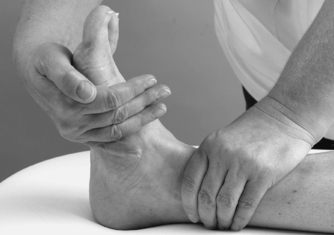 |
| Figure 12.24
Resisted inversion.
|
• Resisted eversion (Fig. 12.25)
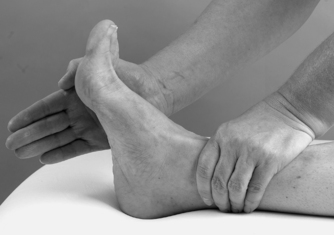 |
| Figure 12.25
Resisted eversion.
|
Accessory ligament tests
• Drawer test for the anterior talofibular ligament (Fig. 12.26)
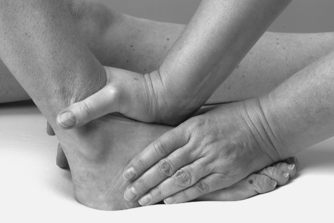 |
| Figure 12.26
Drawer test for the anterior talofibular ligament.
|
• Talar tilt test for the calcaneofibular ligament and integrity of the mortise (Fig. 12.27)
 |
| Figure 12.27
Talar tilt test for the calcaneofibular ligament and integrity of the mortise.
|
• Test for the dorsal calcaneocuboid ligament (Fig. 12.28)
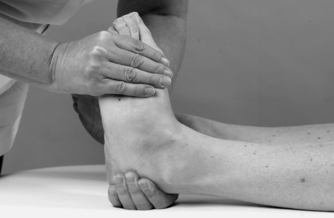 |
| Figure 12.28
Test for the dorsal calcaneocuboid ligament.
|
Toes
• Passive and resisted testing of the toes is not performed routinely, but is included if appropriate
Palpation
• Once a diagnosis has been made, the structure at fault is palpated for the exact site of the lesion
The objective examination is carried out in non-weight-bearing with the patient positioned comfortably in supine lying. The joints are examined first, assessing the range of movement, pain and end-feel. Passive dorsiflexion normally has a hard end-feel and to achieve end range it must be performed with the knee in flexion to take the tension off the gastrocnemius muscle complex, which spans both the knee and ankle joints. Passive plantarflexion normally has a firm elastic end-feel due to tension in the tissues on the dorsal aspect of the foot. The presence of the capsular pattern should be noted.
• Increasing limitation of supination.
• Eventual fixation of the joint in pronation.
• Limitation of adduction and inversion.
• Forefoot fixes in abduction and eversion.
• Gross limitation of extension.
• Some limitation of flexion.
• More limitation of flexion than extension.
• Joints fix in extension.
Although individual passive movements can be produced at the ankle joint, it is difficult to produce isolated passive movements at the subtalar and mid-tarsal joints. To assess the small range of movement available at the subtalar joint, grasp the calcaneus with both hands. Flex the knee to allow relaxation of the gastrocnemius complex and push the ankle joint into the close packed position. The varus and valgus stress is then applied to the calcaneus through the heels of both hands, using body weight. The amount of passive movement available at the subtalar joint is limited to a few degrees of supination and pronation and the normal end-feel is hard for both.
To assess movements occurring at the mid-tarsal joints, pull down on the calcaneus to place the ankle joint into dorsiflexion. Place fingers and thumb on either side of the first metatarsal, around the narrow, lateral aspect of the foot, and move the mid-tarsal joint through its range of passive movements, plantarflexion and dorsiflexion, abduction and adduction, inversion and eversion. These movements are minimal, and difficult to produce in isolation.
Assessing passive movements of the toes is not part of the routine ankle examination, but the movements can be applied as necessary to assess for the presence of the capsular pattern.
The most common injury to the ankle involves the lateral ligaments, but other injuries include lesions of the contractile units, subluxation of the peroneal tendons (through partial or complete rupture of the retinaculae), damage to the subtalar and mid-tarsal joints and injury to the various neurovascular structures crossing the region (Marder 1994, Lee & Maleski 2002). Anterolateral impingement is rare, occurring in only 3% of ankle sprains. It can arise from a meniscoid lesion, synovitis or impingement of the distal fascicle of the anterior inferior tibiofibular ligament (Bekerom & Raven 2007). Fractures and dislocations at the ankle are outside the scope of this book.
The main ligaments are tested by two gross composite movements: passive inversion in plantarflexion for the lateral collateral ligament and passive eversion in dorsiflexion for the medial collateral ligament. Should it be necessary, the ligaments can be assessed individually, and accessory tests can be applied for joint instability (see below and p. 334).
The contractile structures are assessed by resisted tests for pain and power. The joints are placed in the mid-position and maximum resistance applied. Resisted dorsiflexion tests mainly tibialis anterior and resisted plantarflexion tests gastrocnemius and the Achilles tendon. Testing the gastrocnemius group in lying may not produce symptoms and the test may need to be repeated in standing against the resistance of body weight. Resisted inversion tests mainly tibialis posterior and resisted eversion tests the peroneal muscles.
Accessory ligament and instability tests are applied if appropriate. The gross ligament test of passive inversion mainly tests the anterior talofibular ligament which crosses the ankle joint and is taut in plantarflexion. It is the most common ligamentous injury found at the ankle. It is further assessed by the drawer test which assesses its integrity (Marder 1994). Flex the leg to allow the foot to rest on the couch. Position yourself to view movement of the fibula on the lateral side of the ankle. Place one hand over the talus to fix it and the other just above the ankle joint. Apply pressure backwards against the fibula and tibia, comparing the degree of posterior movement of the fibula with the other side. Increased posterior movement of the fibula is a positive sign and indicates laxity or rupture of the anterior talofibular ligament. There is no standardized score for assessing abnormal displacement on the anterior drawer test, with some texts suggesting a grading system of 1–3 in excessive movement relative to the contralateral ankle. A ‘suction sign’ or dimple may be observed on the lateral aspect of the ankle if the anterior talofibular ligament is ruptured (Lee & Maleski 2002, Hattam & Smeatham 2010).
In a cadaver study, Beumer et al (2003) were unable to demonstrate significant displacement during various clinical tests, including the anterior drawer test, suggesting that syndesmotic injury is unlikely to be detected by such tests. However, the mean age of the 17 cadaver specimens was 78.4 years which may have had a bearing on ankle mobility and this was not addressed in the study. They conclude that pain rather than increased displacement should be considered as the outcome measure of these tests, but admit that how increased displacement relates to pain has not been demonstrated. Fujii et al (2000) similarly used elderly cadavers to assess the accuracy of the anterior drawer and talar tilt tests and, although both tests demonstrated reasonable displacement of the hindfoot, neither was sufficient accurately to diagnose specific ligament involvement. It was concluded that these conventional stress tests are not sensitive due to a substantial amount of difference between movement of the cadaver specimens, examiner agreement and positions of the ankle for the tests. To our knowledge, these ligament tests have not been evaluated in vivo.
The calcaneofibular ligament crosses both the ankle joint and the subtalar joint. Injury to this ligament does not often occur in isolation, but is usually in conjunction with the anterior talofibular ligament. The calcaneofibular ligament resists varus stresses to the calcaneus in dorsiflexion. Combined rupture of these ligaments results in an increased talar tilt (Boruta et al 1990, Wilkerson 1992, Marder 1994). With the knee straight, passively dorsiflex the ankle (this allows it to fall just short of the close packed position) and apply a strong varus stress to the calcaneus; compare the range of movement with the other side. This test may also be graded 1–3 relative to the contralateral ankle and again dimpling may be observed (Lee & Maleski 2002).
Disruption of the inferior tibiofibular joint may be due to a forced dorsiflexion injury which can cause widening (diastasis) of the ankle mortise (Marder 1994). In clinical practice, such an injury would usually only occur in conjunction with other major ligamentous damage and possible fracture, but the talar tilt test can also be applied to test the integrity of the mortise. Apply the talar tilt test as above (it can only be applied accurately if the lateral collateral ligaments are intact). If there is any widening of the mortise, pain and excessive movement will be appreciated. A ‘click’ may be felt as the talus tilts excessively in the enlarged mortise (Cyriax & Cyriax 1993, Hattam & Smeatham 2010).
The dorsal calcaneocuboid ligament crosses the calcaneocuboid joint, part of the mid-tarsal complex, and may be involved in a lateral collateral ligament sprain or injured in isolation. To assess its involvement, apply passive adduction and inversion of the mid-tarsal joints looking for increased pain.
CAPSULAR LESIONS
The presence of a capsular pattern at any of the joints in the ankle and foot indicates arthritis. The history will have established the cause of the arthritis.
Osteoarthrosis is uncommon at the ankle unless predisposed by fracture, repetitive postural overuse or instability caused by recurrent sprain. An alteration in lower limb biomechanics may predispose any of the joints to degenerative osteoarthrosis. Localized degenerative osteo-arthrosis of the subtalar joint may follow fractures of the talus or calcaneus (Evans 1990). Occasionally, a lateral ligament sprain produces a traumatic arthritis in the subtalar and mid-tarsal joints and, if unresolved, the joint can be injected with corticosteroid. Rheumatoid arthritis is more common in the smaller joints of the foot. Arthritis and gout of the first metatarsophalangeal joint cause a loss of the functional range of extension essential to gait activities. Corticosteroid injection may be given for symptomatic osteoarthrosis or the principles of mobilization may be applied. Rheumatoid arthritis may respond to corticosteroid injection.
Position the patient in supine with the foot resting on the couch. This places the ankle in a degree of plantarflexion and opens the joint. Several needle entry points are possible and it may be necessary to palpate for a suitable opening over either malleolus, or between the tibialis anterior and extensor hallucis longus tendons, which avoids the dorsalis pedis artery (Fig. 12.29). Having selected a needle entry point, give the injection as a bolus (Fig. 12.30). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
• Increasing limitation of supination.
• Eventual fixation of the joint in pronation.
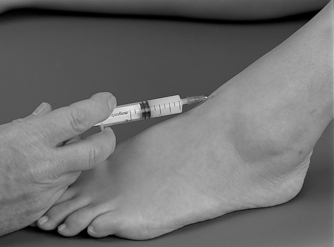 |
| Figure 12.29
Injection of the ankle joint.
|
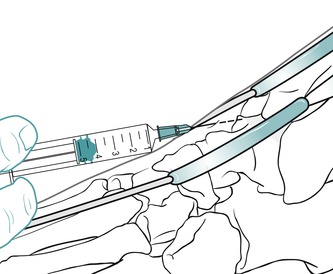 |
| Figure 12.30
Injection of the ankle joint showing direction of approach and needle position.
|
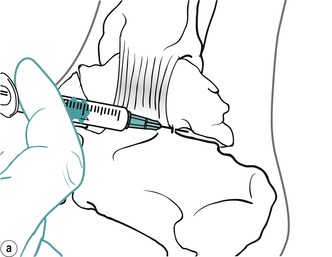
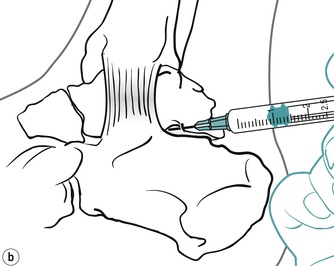
The subtalar joint is divided into two compartments by an interosseous ligament. Locate the joint line immediately above the sustentaculum tali and insert the needle approximately halfway along the joint line, angling it posteriorly to inject the posterior compartment first (Figure 12.31 and Figure 12.32). Then withdraw the needle a little and reinsert it anteriorly to inject the anterior compartment (Figure 12.31 and Figure 12.32). Give the injection as a bolus. The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
• Limitation of adduction and inversion.
• Forefoot fixes in abduction and eversion.
Suggested needle size: 23G × 1 in (0.6 × 25 mm) blue needle
Dose: 10–20 mg triamcinolone acetonide in a total volume of 1.5 mL
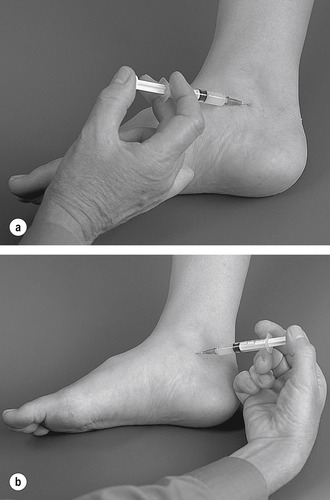 |
| Figure 12.31
Injection of the subtalar joint.
|
 |
 |
| Figure 12.32
Injection of the subtalar joint showing direction of approach and needle position.
|
The injection site will depend on which of the joints are affected, the talocalcaneonavicular joint, calcaneocuboid joint or both. Locate the appropriate joint line dorsally by palpation, avoiding the tendons and the dorsalis pedis artery if injecting the talocalcaneonavicular joint medially. (Figure 12.33 and Figure 12.34 demonstrate injection of the calcaneocuboid joint.) Insert the needle and, once intracapsular, give the injection as a bolus. The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
• Gross limitation of extension.
• Some limitation of flexion.
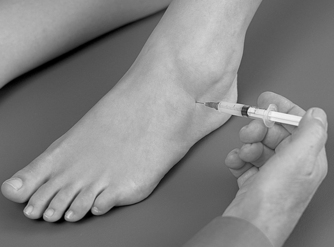 |
| Figure 12.33
Injection of the midtarsal, calcaneocuboid, joint.
|
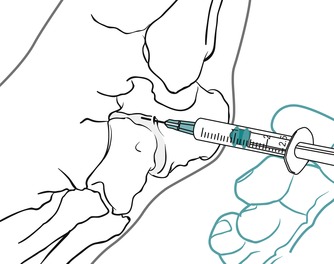 |
| Figure 12.34
Injection of the midtarsal, calcaneocuboid, joint showing direction of approach and needle position.
|
Identify the joint line dorsally by palpation and distract it to allow easier access; choose a point of entry to one side of the extensor tendon (Fig. 12.35). The injection is given as a bolus (Fig. 12.36). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
• More limitation of flexion than extension.
• Joints fix in extension.
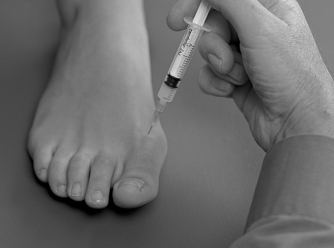 |
| Figure 12.35
Injection of the first metatarsophalangeal joint.
|
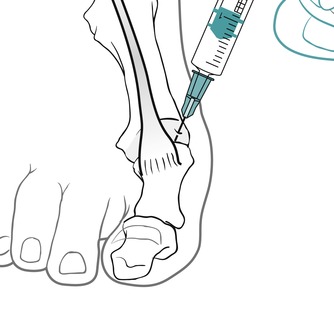 |
| Figure 12.36
Injection of the first metatarsophalangeal joint showing direction of approach and needle position.
|
Identify the joint line dorsally by palpation. Insert the needle avoiding the extensor tendons, and give the injection as a bolus (Figure 12.37 and Figure 12.38). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
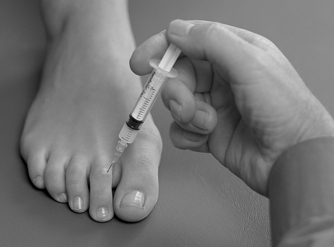 |
| Figure 12.37
Injection of the interphalangeal joint.
|
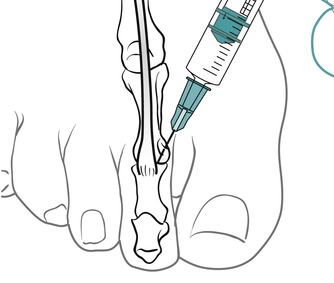 |
| Figure 12.38
Injection of the interphalangeal joint showing direction of approach and needle position.
|
NON-CAPSULAR LESIONS
Lateral collateral ligament sprain
Sprain of the lateral collateral ligament is the most common ankle injury, accounting for approximately 85–90% of ankle sprains (Stanley 1991, Liu & Jason 1994). The anterior talofibular ligament is the weakest of the ligaments in the lateral ligament complex and is involved in nearly all lateral ligaments sprains. The calcaneofibular ligament is involved in 50–75% of all lateral ligament injuries and the posterior talofibular ligament in less than 10% (Ferran Maffulli 2006).
The basic mechanism for lateral ligament sprain is a forced inversion injury (Sartoris 1994, Kerkhoffs et al 2002). Injury occurs commonly in sport, when a player may land on another player’s foot, stumble on uneven ground; or, apart from sport, the patient may simply have tripped or slipped. The most common predisposition factor to suffering a lateral ligament injury is a history of at least one previous strain and an estimated 55% of patients do not seek treatment for a sprained ankle (Hertel 2002).
Diagnosis of sprain can be made on clinical grounds and X-ray investigation is only necessary if there is clear clinical evidence of fracture, e.g. marked bony tenderness to palpation (Auletta et al 1991, Litt 1992). The so-called ‘Ottawa ankle rules’ have also been developed as a guideline to help to decide whether an X-ray is necessary and are based on the identification of bone tenderness in specific areas (Patel & Subramanian 2007). The fibular end of the ligament is more likely to be involved in avulsion fractures than the talar end, which is protected by greater bone density (Kumai et al 2002). Forced inversion may cause avulsion of lateral structures or undisplaced fracture of the lateral malleolus, while impactive forces may stress medial structures (Sartoris 1994).
Lateral collateral ligament sprains most commonly involve the anterior talofibular ligament, with more severe injuries also involving other ligaments and structures on the lateral side of the ankle (Litt 1992, Liu & Jason 1994, Hertel 2002). Lateral collateral ligament sprains are graded according to the severity of the signs and symptoms (Boruta et al 1990, Stanley 1991, Litt 1992, Wilkerson 1992, Liu & Jason 1994, Kerkhoffs et al 2002).
Grade l
This is mild stretching of the ligament and surrounding structures. The patient presents with mild swelling and tenderness over the ligament, little or no haemorrhage, some limitation of movement and some difficulty in weight-bearing. On examination, no clinical instability is noted and prognosis is good. The injury takes approximately 8–10 days to resolve with early mobilization.
Grade ll
This is partial rupture of the anterior talofibular ligament with mild instability of the joint. The patient presents with moderate to severe swelling, bruising, pain, local tenderness, a limited range of movement and great difficulty in weight-bearing. There is limitation of movement in the capsular pattern and a mild degree of ligamentous instability may be detected clinically. Prognosis remains good, the injury taking approximately 15–21 days to resolve with early mobilization.
Grade lll
This is complete rupture of the anterior talofibular and calcaneofibular ligaments and lateral capsule with gross instability of the joint. The patient presents with diffuse swelling and marked evidence of haemorrhaging. There is severe pain and tenderness, loss of movement and great difficulty with weight-bearing. It may be difficult to assess for ligamentous laxity clinically as this may be masked by the swelling and the protective reflex muscle spasm produced by the pain.
Both operative and non-operative treatment options could be considered, but with conservative management prognosis is still good, providing the patient is not required to function at high levels of performance (Stanley 1991, Karlsson & Lansingeer 1992, Liu & Jason 1994). Recurrent sprains of the ankle with clinical laxity may need stress X-ray to measure the laxity before proceeding to eventual surgical repair. In general, surgical intervention for grade III sprains is only considered in the élite athlete, or where recurrent injury has failed to respond to conservative management and is producing functional disability. Otherwise, all grades of sprain are treated conservatively (Karlsson & Lansingeer 1992, Liu & Jason 1994, Ogilvie-Harris & Gilbart 1995).
Early mobilization is the key to the restoration of function in all grades of ligamentous sprain, giving a better overall result and a faster rate of recovery (Stanley 1991, Ogilvie-Harris & Gilbart 1995, Shrier 1995). Non-steroidal anti-inflammatory drugs and ice, used early on in the inflammatory phase, seem to achieve an earlier recovery, but do not alter the overall outcome (Ogilvie-Harris & Gilbart 1995). Corticosteroid injection is not recommended for acute ankle sprains since collagen synthesis may be suppressed (Boruta et al 1990).
Kerkhoffs et al (2002) present a review of randomized controlled trials in the literature to evaluate the effectiveness of immobilization as the treatment for acute sprained ankle in adults. Overall, results were better with functional treatment rather than immobilization, with a higher percentage of patients returning to work and sport, a reduced incidence of persistent swelling, limited movement and instability and higher patient satisfaction rates. Functional treatment is therefore the treatment of choice for acute sprained ankle.
Treatment is directed at all components involved in the sprain and depends on the stage reached in the inflammatory process.
Acute lateral collateral ligament sprain
A complete examination of the ankle and foot is not usually possible following an acute lateral collateral ligament sprain as the swelling and muscle spasm prevent movement. The history will indicate the mechanism of the injury and the amount of heat and swelling will give an indication of the severity. Any movement, active or passive, particularly into inversion, will be painful and pain is experienced on weight-bearing leading to an antalgic gait. The principles of treatment for acute ligament sprain are applied.
Protection, rest, ice, compression and elevation (PRICE) are applied immediately after the onset. Ice applied during the first 3 days after injury has been shown to shorten the recovery period. It is generally applied for 15–20 min three times daily (Hocutt et al 1982, Stanley 1991, Swain & Holt 1993).
Gentle transverse frictions are begun as early as possible, according to the irritability of the lesion, together with Grade A mobilization, aiming to maintain mobility. Treatment is delivered on a daily basis during the early acute phase and the patient is encouraged to maintain a normal heel–toe gait, possibly with the aid of crutches. Ankle supports or tape may be applied to provide compression during the early acute phase (O’Hara et al 1992).
From days 3 to 5 onwards, again dependent upon irritability, there should be sufficient tensile strength in the wound to allow an increasing depth of transverse frictions and a greater range of Grade A mobilization to be applied. Treatment continues until a full range of pain-free movement is attained.
Depending on the severity of the injury, the patient should be relatively pain-free and walking normally within 8–21 days, if seen within a day or two from the onset. Functional rehabilitation should involve muscle balance, peroneal strengthening and proprioceptive work to re-establish normal balance and coordination (Cornwall & Murrell 1991, Karlsson & Lansingeer 1992). Progression to running, sprinting, jumping, figure-of-eight running, twisting and turning follows, depending on the functional requirements of the patient.
Malliou et al (2004) studied young footballers and confirmed that a specific balance training programme can improve proprioception not only as part of rehabilitation after injury but also to improve balance to prevent injury in normals. Specific balance training includes wobble board exercises, closed chain exercises, core stability and sport specific manoeuvres (Laskowski et al 1997). The lateral ligaments require the support of the peroneal tendons to resist inversion stresses; rehabilitation aims to restore muscle strength and to re-establish the protective reflexes (Boruta et al 1990).
Transverse frictions to the lateral collateral ligament components (Cyriax 1984, Cyriax & Cyriax 1993)
The anterior talofibular ligament is usually involved at its fibular end, but palpation will establish the exact site of the lesion which may also be at the talar insertion or across the joint line.
Stand on the patient’s good side, placing an index finger reinforced by the middle finger onto the anterior edge of the lateral malleolus. Direct the transverse frictions back against the malleolus and sweep transversely across the fibres (Fig. 12.39). In the acute case begin the transverse frictions very gently to gain some analgesia. Progress to apply the transverse frictions more deeply and, once on the ligament, apply approximately six effective sweeps to achieve movement. The transverse frictions are followed immediately by Grade A mobilization together with gait correction.
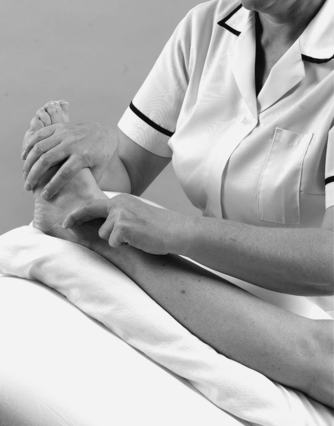 |
| Figure 12.39
Transverse frictions to the anterior talofibular ligament, acute sprain.
|
If the calcaneofibular ligament is involved, the transverse frictions are directed up under the apex of the lateral malleolus and immediately followed by Grade A mobilization.
Treatment of any involvement of the peroneal tendons will be covered under contractile lesions.
Chronic lateral collateral ligament sprain
There is a history of a past sprained ankle which may have resolved without treatment. The patient complains of pain and some swelling on the lateral side of the ankle after exertion. Symptoms of recurrent giving way may indicate mechanical instability (ankle movement beyond the physiological limit which occurs due to anatomical changes such as ligamentous laxity) or functional instability (a subjective feeling of instability which occurs due to neuromuscular and proprioceptive deficits which provide dynamic control) (Tropp 2002). Provocative tests such as the drawer and talar tilt tests, as described above, can provide an indication of mechanical instability.
The treatment approach for residual chronic ankle instability should be based on mechanical correction and functional rehabilitation, with an emphasis on proprioceptive re-education, muscle balance, postural control and taping may be appropriate for sporting activities. As well as chronic lateral ligament sprain and instability, differential diagnosis of continuing lateral ankle pain following inversion injury includes osteochondral fracture, ruptured peroneal tendon, synovitis and ligamentous impingement of the anterior aspect of the ankle, which is uncommon (Bekerom & Raven 2007).
The importance of continuing rehabilitation for several months after the symptoms of acute ligamentous injury have subsided is paramount in preventing chronic recurrence (Hertel 2002). Surgery is only considered if rehabilitation fails (Liu & Jason 1994).
On examination there is usually pain on full passive inversion, indicating involvement of the anterior talo-fibular ligament. Pain on passive dorsiflexion with a varus stress to the calcaneus (talar tilt test) indicates involvement of the calcaneofibular ligament. Pain on passive adduction and inversion applied to the mid-tarsal joints indicates involvement of the dorsal calcaneocuboid ligament.
The ligament fibres have become adherent to the underlying bone and the principle of treatment is to rupture the unwanted adhesions with one Grade C manipulation at each session, once the ligament has been prepared by deep transverse frictions. Following manipulative rupture, the patient is instructed to mobilize vigorously, in order to maintain the movement gained through manipulation. Functional or structural instability is addressed by proprioceptive exercises and balance work (Lentell et al 1990). Treatment is usually successful and requires approximately one to three sessions.
Transverse frictions to the lateral collateral ligament components involved in a chronic sprain (Cyriax 1984, Cyriax & Cyriax 1993)
Transverse frictions to the anterior talofibular (Fig. 12.40) and calcaneofibular ligament (if involved) (Fig. 12.41) are applied as described above. If the dorsal calcaneo-cuboid ligament is involved, the transverse frictions are applied from a position on the patient’s good side; They are delivered by the index finger, reinforced by the middle finger, directed onto the ligament, which is located over the dorsal aspect of the calcaneocuboid joint line (Fig. 12.42). The transverse frictions are applied to all involved ligaments to gain the analgesic effect, prior to the Grade C manipulation, which follows immediately.
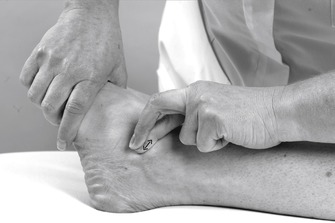 |
| Figure 12.40
Transverse frictions to the anterior talofibular ligament, chronic sprain.
|
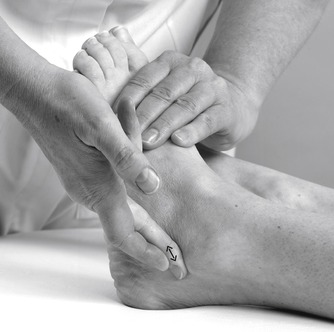 |
| Figure 12.41
Transverse frictions to the dorsal calcaneofibular ligament.
|
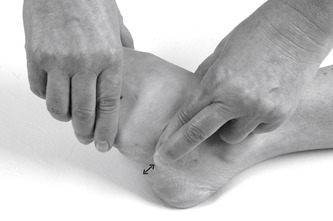 |
| Figure 12.42
Transverse frictions to the calcaneocuboid ligament.
|
Grade C manipulation for chronic lateral collateral ligament sprain (Cyriax 1984, Cyriax & Cyriax 1993)
With the patient lying supine, stand at the foot of the couch. The treatment described is for the left ankle:
• Grasp the patient’s left calcaneus with your left hand and apply a varus movement to pull the subtalar joint into supination (Fig. 12.43). Keep your thumb alongside the palm of your hand to avoid pressing against the lower leg which will tend to block the movement and is uncomfortable for the patient.
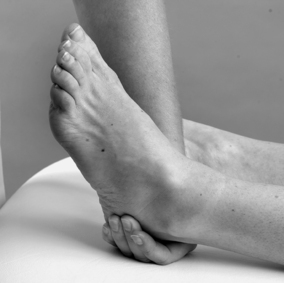 |
| Figure 12.43
Grade C manipulation: calcaneum grasped and placed into varus.
|
• With a ‘flipper’ grip, wrap your right hand around the base of the first metatarsal (Fig. 12.44), with the heel of the hand well down alongside the lateral border of the foot, and pull the foot into maximum plantarflexion (Fig. 12.45a). Be careful not to put your thumb round onto the sole of the foot, as this is uncomfortable for the patient and makes the technique less efficient.
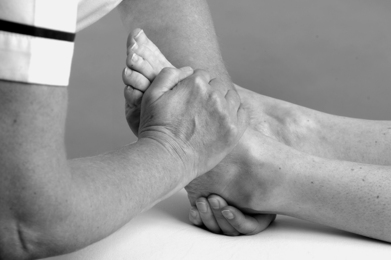 |
| Figure 12.44
Grade C manipulation: ‘flipper’ grip handhold.
|
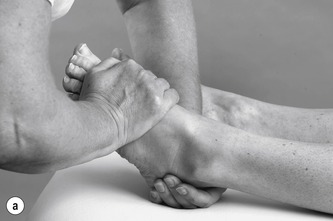 |
 |
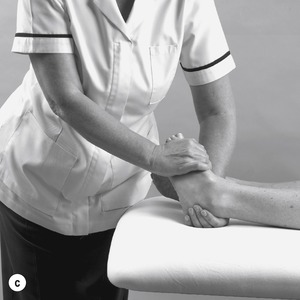 |
| Figure 12.45
(a) Pulling foot into maximum plantarflexion. (b,c) Pulling foot into inversion and adduction.
|
• Maintain the maximum plantarflexion while you step to the left, turning your body to the left through 90° to achieve maximum inversion (Fig. 12.45b, c).
• This step and turn also automatically pulls the forefoot into maximum adduction and inversion, taking up the slack (Fig. 12.46).
 |
| Figure 12.46
Grade C manipulation: body turned pulling the forefoot into maximum adduction and inversion before application of the final thrust.
|
• Apply a minimal amplitude, high velocity thrust by a sharp adduction movement of your right arm.
Medial collateral ligament sprain
Eversion sprains of the ankle represent 5–15% of all ankle injuries and are not common, as the medial collateral ligaments are 20–50% stronger than the lateral ligaments. The mechanism of injury involved is eversion with possible damage to the syndesmosis, or fracture of the malleoli, as well as injury to the medial ligament (Roberts et al 1995, Lee & Maleski 2002).
The treatment principles are applied as for the acute and chronic stages of the lateral collateral ligament if the injury is traumatic. The various directions of the ligament fibres need to be borne in mind, to be able to apply the frictions transversely. A Grade C manipulation is not applied in the chronic stage, due to the multidirectional nature of the fibres.
A secondary medial ligament sprain may develop through postural overuse. This is especially evident with flattening of the medial arch in the elderly. A biomechanical assessment of the foot may be indicated, together with orthotic correction to address the cause.
Retrocalcaneal bursa
The retrocalcaneal bursa is described as saddle-shaped, horseshoe-shaped or shaped like an inverted boomerang. It lies between the distal end of the Achilles tendon, near its insertion, and the superoposterior surface of the calcaneus (Stephens 1994, Bottger et al 1998). It rests against the Achilles fat pad superiorly and blends with the Achilles tendon posteriorly (Frey et al 1992).
Objectively it is difficult to diagnose a retrocalcaneal bursitis differentially from an Achilles tendinopathy, with which it may coexist. It presents with posterior heel pain and a muddle of signs which may include pain on passive dorsiflexion which squeezes the inflamed bursa under the stretched Achilles tendon and/or passive plantarflexion which may squeeze the bursa between the Achilles tendon and the calcaneus. Swelling and tenderness to palpation may be present, just anterior to the insertion of the Achilles tendon. Retrocalcaneal bursitis may be a manifestation of rheumatoid arthritis or one of the spondyloarthropathies such as Reiter’s disease (Hutson 1990, Frey et al 1992, Baxter 1994).
Frey et al (1992) demonstrated the existence of the retro-calcaneal bursa on X-ray using an injection of contrast medium. The normal bursa contains approximately 1 mL of fluid. Patients suffering from retrocalcaneal bursitis accepted less of the contrast medium than normal subjects, leading the authors to propose that this was due to inflammatory fluid, thickened oedematous bursal walls, hypertrophic synovial infoldings and pain. Bottger et al (1998) demonstrated the normal retrocalcaneal bursa to be 1 mm in an anterior–posterior dimension, 6 mm in the transverse dimension and 3 mm in the craniocaudal dimension; bursal dimensions greater than these were seen in symptomatic subjects.
Treatment consists of a local corticosteroid injection.
Suggested needle size: 23G × 1 in (0.6 × 25 mm) blue needle
Dose: 10 mg triamcinolone acetonide in a total volume of 0.75 mL
Position the patient in the prone position with the foot in a degree of plantarflexion. Palpate for the tender area anterior to the distal end of the Achilles tendon and insert the needle from either the medial or lateral aspect, running parallel to the anterior aspect of the Achilles tendon (Figure 12.47 and Figure 12.48). Give the injection as a bolus if possible, or pepper it if you feel the resistance of the synovial folds. The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
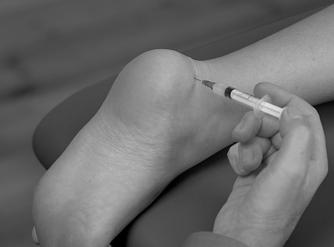 |
| Figure 12.47
Injection of the retrocalcaneal bursa.
|
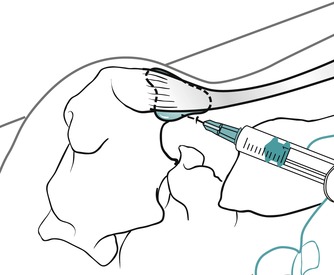 |
| Figure 12.48
Injection of the retrocalcaneal bursa showing direction of approach and needle position.
|
Subcutaneous Achilles bursa
The subcutaneous Achilles bursa lies between the skin and insertion of the Achilles tendon. It may be an adventitious bursa developing as a result of external friction (Gibbon & Cassar-Pullicino 1994). Inflammation of this bursa presents with subcutaneous swelling and tenderness over the heel and may be due to abnormal pressure from heel counters. Treatment consists of relative rest and advising the patient about footwear, with particular regard to the height and rigidity of the heel counter (Stephens 1994).
Plantar fasciitis
Plantar fasciitis produces a typical history of a gradual onset of pain felt over the medial plantar aspect of the heel at the enthesis of the central cord into the calcaneal tuberosity (Yu 2000). Its particular characteristic is pain under the heel when the foot is first put to the floor in the morning, easing after a few steps have been taken (Kibler et al 1991, Karr 1994). It is worse after prolonged periods of standing and on initial exercise, easing as the foot warms up.
Plantar fasciitis usually occurs in middle age, with obesity as a predisposing factor (Gibbon & Cassar-Pullicino 1994). It may be precipitated by an alteration in footwear, e.g. wearing flip-flops or other similar unsupporting shoes, or seen as a relatively common injury in the running athlete, where it constitutes approximately 10% of running injuries seen (Kibler et al 1991).
Postural foot deformity such as pes planus and overpronation lowers the medial longitudinal arch and may overstretch the plantar fascia. Tightness of the Achilles tendon limits dorsiflexion and may contribute to the overpronation (Evans 1990, Karr 1994). Due to the constant-length phenomenon, extension of the toes increases the height of the longitudinal arch – the ‘windlass’ effect of the plantar fascia (Canoso 1981, Sellman 1994) – so that activities involving long-term tip-toe standing, e.g. high heels, may excessively stress the plantar fascia. It may also be associated with rheumatoid arthritis or the spondyloarthropathies.
Diagnosis is made on the typical history and the absence of other findings on examination of the foot and ankle. Passive extension of the toes, with the foot in dorsiflexion, may reproduce the pain by its ‘windlass’ effect on the plantar fascia and tenderness to palpation is usually found over the medial calcaneal tuberosity.
The mechanism of the lesion is thought to be repetitive microtrauma through overloading of the longitudinal arch, which produces focal tears and fascial and perifascial inflammation at the insertion of the plantar fascia at the bone–fascia interface (the enthesis) (Kibler et al 1991, Gibbon & Cassar-Pullicino 1994, Karr 1994). It may be a traction injury occurring through repeated intrinsic muscle contraction against a stretched plantar fascia during the push-off phase of gait, the plantar fascia acting as an aponeurotic attachment for the first layer of the plantar muscles. Both mechanisms could possibly lead to the development of calcaneal spurs (Gibbon & Cassar-Pullicino 1994). Cole et al (2005) note that calcaneal spurs are present in 50% of patients with plantar fasciitis and in up to 19% of patients without, suggesting that the presence or absence of heel spurs is not helpful in diagnosis.
Differential diagnosis should exclude fat pad syndrome which tends to occur acutely following a fall onto the heel or chronically through poor heel cushioning (Brukner & Khan 2007). The patient will experience pain on palpation of the heel and treatment is aimed at addressing the cause, advice on footwear and activity modification.
Plantar fasciitis usually responds to conservative management and Thomas et al (2001) state that 90–95% of cases get better in under 1 year without surgery. However, if symptoms do persist, surgery may be an option, with careful consideration paid to maintaining the load-bearing capacity of the longitudinal arch (Kulthanan 1992, Kim & Voloshin 1995). Tissue examined at the time of surgery has shown a hypercellular inflammatory response, reactive fibrosis and degenerative areas (Kibler et al 1991).
The chronic nature of the condition produces changes in the strength of the plantarflexors with loss of range of dorsiflexion and there may be alterations in the length of the stride (Kibler et al 1991, Chandler & Kibler 1993). All components of dysfunction should be considered when planning a rehabilitation programme.
Treatment of plantar fasciitis is local corticosteroid injection or transverse frictions. Either treatment is combined with rest from overuse activities and a full rehabilitation programme which may include intrinsic muscle exercises and correction of foot posture, if appropriate. DiGiovanni et al (2006) advocate specific stretches to the plantar fascia combining dorsiflexion of the metatarsophalangeal and ankle joints. They conclude that stretches applied in this way are more effective than Achilles tendon stretches in the management of chronic plantar fasciitis.
Although unusual, cases of spontaneous rupture of the plantar fascia have been reported, particularly among athletes. It usually occurs during rapid acceleration as the foot forcibly pushes against the ground, or may occur over time in association with sustained activity such as walking. The patient may report specific injury and a snapping sensation together with the appearance of a painful lump. The plantar fascia may be tender to palpation and bruising may be evident. Occasionally infection may occur, but this is usually associated with systemic disease such as diabetes mellitus with heel ulcerations, or extension from a surrounding soft tissue infection or through penetration by a foreign object. The calcaneus is the most commonly infected tarsal bone (Yu 2000).
An ultrasound scan is sometimes performed prior to injection to assess the degenerative state of the fascia prior to injection, as a safeguard against possible rupture. More research is needed to assess whether this should be performed more routinely.
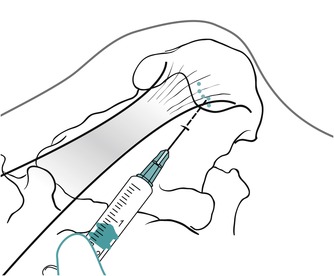
Position the patient in prone lying with the knee flexed and the lower leg resting on a pillow. Hold the foot in dorsiflexion to apply some tension to the plantar fascia. Insert the needle at the medial border of the heel, anterior to the point of tenderness, and angled posteriorly towards the site of tenderness (Figure 12.49 and Figure 12.50). Deliver the injection to the origin of the plantar fascia by a peppering technique, with the needle point in contact with bone at the anterior edge of the medial tubercle. The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
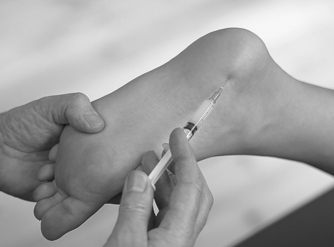 |
| Figure 12.49
Injection of the plantar fascia.
|
 |
| Figure 12.50
Injection of the plantar fascia showing direction of approach and needle position.
|
Dasgupta & Bowles (1995) conducted bone scans on 15 patients with a diagnosis of plantar fasciitis, to localize the inflammatory focus. In 80% of patients, an area was localized to the medial aspect of the plantar surface of the calcaneus. An accurately placed injection into this area abolished the pain and tenderness. An accurate injection technique is important to prevent the need for repeated injection, as cases of plantar fascia rupture associated with corticosteroid injection have been reported (Sellman 1994).
Transverse frictions to the plantar fascia
Position the patient in supine-lying with the foot held in dorsiflexion, supported on a pillow, the leg in some lateral rotation. Standing on the affected side, direct the pressure posteromedially against the origin of the plantar fascia, either with one thumb alone or with one thumb reinforced by the other, and friction transversely across the fibres (Fig. 12.51). Maintain the transverse frictions for 10 min after achieving the analgesic effect. It is important to assess foot posture and to instruct the patient in intrinsic foot exercises. Care should be taken when teaching intrinsic foot exercises to avoid any clawing of the toes, which reinforces the activity of the long plantar flexors. Relative rest is advised where functional movements may continue, but no overuse or stretching until the structure is pain-free on resisted testing.
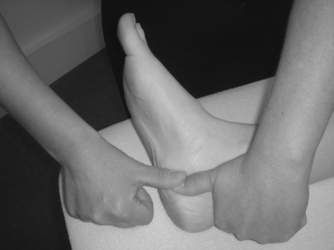 |
| Figure 12.51
Transverse frictions to the plantar fascia.
|
Other techniques including taping and supports may be applied, and a heel raise or cushion may help to reduce the stretch or pressure on the plantar fascia.
Loose bodies
Although relatively rare, loose bodies can occur in the ankle or subtalar joints. They may be due to degenerative changes in the joint or be associated with fragmented spurs on the tibial or talar side, or by avulsion from the dome of the talus or either malleolus (Scranton et al 2000). The patient presents with a history of twinging pain with giving way or a momentary inability to weight-bear; this symptom may also be indicative of mechanical ankle instability due to ligamentous laxity. On examination, a non-capsular pattern may be present. The principle of treatment for a loose body is applied – strong traction and Grade A mobilization. The direction selected for the mobilization is not important.
Loose-body mobilization technique for ankle joint (Cyriax 1984, Cyriax & Cyriax 1993)
Position the patient in supine with the foot level with the end of the couch. Grasp the calcaneus and hold it to act as a fulcrum. Grasp the dorsum of the foot with the web of the other hand and lean back to apply strong traction. Allow the traction to establish, then apply a circumduction movement with the hand placed around the dorsum of the foot (Fig. 12.52).
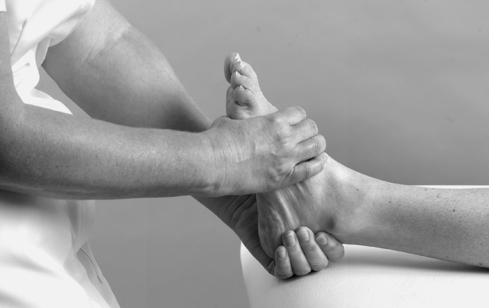 |
| Figure 12.52
Loose-body mobilization technique for ankle joint.
|
Loose-body mobilization technique for subtalar joint (Cyriax 1984)
Position the patient prone with the foot just off the end of the couch. Cross your thumbs over the posterosuperior aspect of the calcaneus and wrap your hands around the talus anteriorly. Lean back to apply strong traction and apply a varus and valgus movement to the calcaneus with the heels of your hands by rotating your body from side to side (Fig. 12.53).
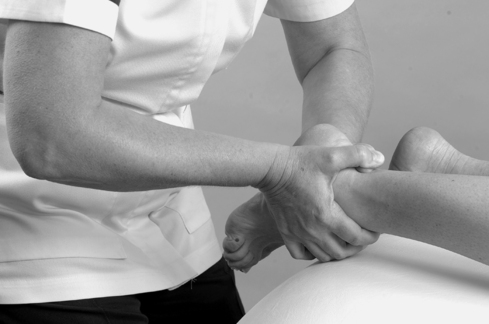 |
| Figure 12.53
Loose-body mobilization technique for subtalar joint.
|
CONTRACTILE LESIONS
The following contractile lesions have been chosen for discussion since they occur relatively commonly in clinical practice. However, the principles of diagnosis and treatment can be applied to any other lesion.
Peroneal tendinopathy
Peroneal tendinopathy may be a chronic overuse injury, e.g. walking or training on unaccustomed surfaces, predisposed by altered foot biomechanics, or have an acute onset as tenosynovitis if the tendons are involved in an inversion sprain of the ankle. A few cases of rupture of the peroneus longus tendon secondary to repetitive inversion injury or forced eversion injury against resistance have been reported, presenting as chronic lateral ankle instability (Patterson & Cox 1999).
On examination pain is felt on the lateral side of the ankle on resisted eversion of the foot. Acute tenosynovitis may also produce pain on passive inversion. The exact site of the lesion is determined by palpation and may be at the musculotendinous junction, the tendons above, behind or below the malleolus, or at the insertion of peroneus brevis into the base of the fifth metatarsal. Disruption of the retinacula may cause snapping of the peroneal tendons due to subluxation or dislocation which may be obvious. However, to confirm this the ankle should be actively dorsiflexed and everted against resistance, which dynamically recreates the subluxation or dislocation of the tendons (Lee & Maleski 2002).
Transverse frictions are applied with the tendons on the stretch if the lesion involves the tendons in their common sheath behind or below the malleolus. If the lesion lies in the common sheath below the malleolus, corticosteroid injection can be applied as an alternative treatment modality.
Transverse frictions to the peroneal tendons (Cyriax 1984, Cyriax & Cyriax 1993)
Musculotendinous junction
Locate the site of the lesion and apply the transverse frictions with the index finger, reinforced by the middle finger (Fig. 12.54). Maintain downward pressure as the frictions are applied transversely across the fibres.
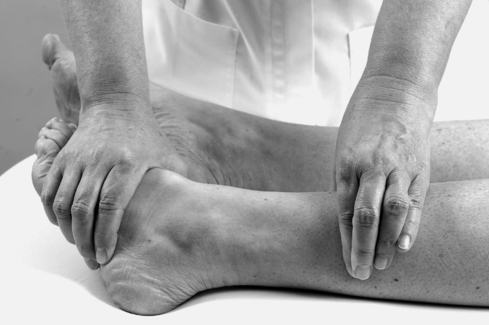 |
| Figure 12.54
Transverse frictions to the peroneal tendons, musculotendinous junction.
|
Above the malleolus
Three fingers are required to cover the extent of the lesion (Fig. 12.55). Maintain downward pressure over the tendons as the transverse frictions are applied.
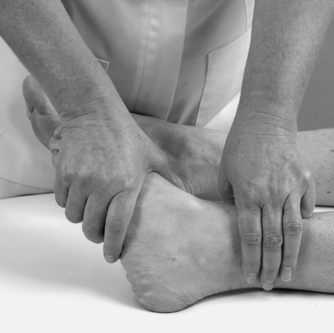 |
| Figure 12.55
Transverse frictions to the peroneal tendons, above the malleolus.
|
Behind the malleolus
Here the tendons run in a common sheath so they must be put on a stretch. Use the middle finger reinforced by the index and apply the transverse frictions by pronation and supination of the forearm (Fig. 12.56).
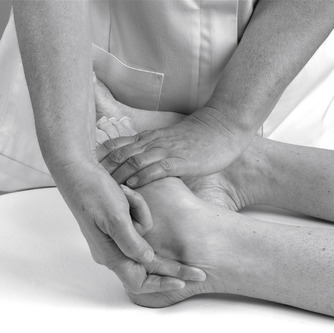 |
| Figure 12.56
Transverse frictions to the peroneal tendons, behind the malleolus.
|
Below the malleolus
Two fingers are required to cover the extent of the lesion (Fig. 12.57). The tendons here continue in their common sheath, therefore they are treated on the stretch. Maintain downward pressure over the tendons as the transverse frictions are applied.
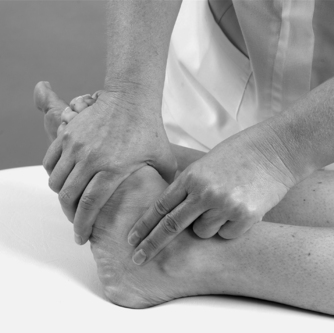 |
| Figure 12.57
Transverse frictions to the peroneal tendons, below the malleolus.
|
At the insertion of peroneus brevis into the base of the fifth metatarsal
An index finger, reinforced by the middle, is applied to the insertion and the frictions are delivered transversely across the fibres (Fig. 12.58).
 |
| Figure 12.58
Transverse frictions to the insertion of peroneus brevis into the base of the fifth metatarsal.
|
Acute lesions are frictioned gently for approximately six effective transverse sweeps after achieving the ana-lgesic effect. Chronic lesions require deep transverse frictions for approximately 10 min after achieving the analgesic effect. Relative rest is advised where functional movements may continue, but no overuse or stretching until the structure is pain-free on resisted testing.
Locate the peroneal tubercle, which indicates the distal end of the common sheath. Insert the needle into the sheath at the point of diversion of the tendons, aiming the needle towards the malleolus, parallel to the tendons (Fig. 12.59). Give the injection as a bolus into the sheath (Fig. 12.60). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
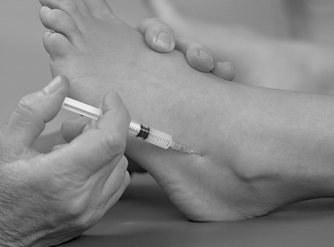 |
| Figure 12.59
Injection technique for tenosynovitis of the peroneal tendons in the common sheath.
|
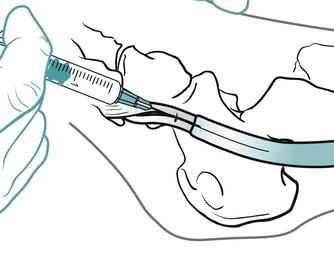 |
| Figure 12.60
Injection technique for tenosynovitis of the peroneal tendons in the common sheath showing direction of approach and needle position.
|
Locate the base of the fifth metatarsal and mark the tender point. Deliver the injection by a peppering technique at the teno-osseous junction (Figure 12.61 and Figure 12.62). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
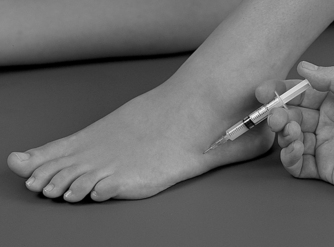 |
| Figure 12.61
Injection technique at the teno-osseous junction of peroneus brevis: base of the fifth metatarsal.
|
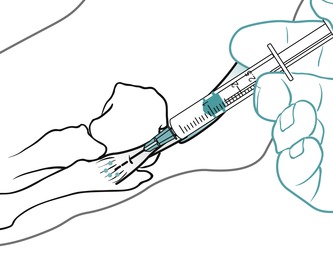 |
| Figure 12.62
Injection technique at the teno-osseous junction of peroneus brevis: base of the fifth metatarsal showing direction of approach and needle position.
|
The peroneal muscles act as dynamic stabilizers of the ankle joint and contract eccentrically to prevent inversion sprains (Shrier 1995). If the peroneal tendinopathy or tenosynovitis is the result of an inversion sprain, proprioception is most likely to have been altered; this may lead to functional instability and recurrent giving way. Rehabilitation must include peroneal strengthening and proprioceptive re-education, once the tendinopathy has been managed appropriately.
Achilles tendinopathy
The Achilles tendon is the longest tendon in the body; it is up to 1.5 cm wide and less than 1 cm in anteroposterior thickness (Chandnani & Bradley 1994). It has the capacity to withstand high tensional forces with forces of 12.5 times body weight recorded (Alfredson & Lorentzon 2000). It consists of approximately 95% type I collagen fibres and elastin embedded in a matrix of proteoglycans and water. These fibres adopt a wavy ‘crimp’ configuration at rest and the primary response to tendon and fibre elongation is straightening out of the collagen fibre crimp. It is not surrounded by a true synovial sheath, but by a paratenon, a thin gliding membrane, which permits free movement of the tendon within the surrounding tissues. The blood supply is from the musculotendinous junction, the teno-osseous junction and the surrounding paratenon (Paavola et al 2002).
The Achilles tendon is a common site for injury and rupture. Symptoms include posterior heel pain with stiffness before, during and after exercise. The tendon is usually sore and thickened, impairing gait (Alfredson et al 2002, Koenig et al 2004). Chronic symptoms are usually of gradual onset (Ohberg & Alfredson 2004).
Achilles tendinopathy can involve a range of lesions at different sites in the tendon. Lesions of the Achilles tendon usually occur about 2–6 cm proximal to its insertion, which is the zone considered to be of relatively poor vascularity (Lagergren & Lindholm 1958, Smart et al 1980, Chandnani & Bradley 1994). Lesions can also exist at the musculotendinous and teno-osseous junctions and the peritenon itself can be involved.
Differential diagnosis should include retrocalcaneal or superficial bursitis, impingement between the posterior part of the calcaneus and the Achilles tendon (Haglund’s deformity), calcification or avulsion fracture, or occasionally mixed pathology (Alfredson & Lorentzon 2000). Ohberg & Alfredson (2003) used ultrasound and colour Doppler to scan patients with chronic heel pain and identified thickened retrocalcaneal bursae, calcifications, bone spurs and loose fragments in the adjacent tissues.
An abnormally large bony bump (exostosis), known as Haglund’s deformity, may be present on the supero-posterior surface of the calcaneus. Soft tissue swellings are often associated with Haglund’s deformity and are known as ‘pump bumps’ (Stephens 1994). The deformity itself may be asymptomatic but the prominence predisposes to mechanical irritation of the retrocalcaneal bursa and strain on the Achilles tendon insertion. Treatment can include NSAIDs, heel raise or change of footwear to avoid friction, intrabursal corticosteroid injection or surgery for resistant cases.
A combination of pain in the Achilles tendon, swelling and impaired performance, together with pain on resisted plantarflexion, provides a clinical diagnosis of Achilles tendinopathy (Paavola et al 2002). Lesions of the Achilles tendon can be a tendonitis, an early acute condition which may be reversible, responding to conservative treatment, or a tendinosis, i.e. focal degenerative lesions within the tendon itself which may be irreversible (Williams 1986, Mahler & Fritschy 1992, Paavola et al 2002). Partial or complete rupture may also exist. The condition has been considered to be a continuum of a degenerative process which may eventually progress to total rupture (Fox et al 1975, Read & Motto 1992).
Overuse has been suggested as a cause of Achilles tendinopathy, especially amongst athletes, although not just in the more elite group. Symptoms are common in recreational athletes within the 35–50 age group, particularly in those who participate in middle- or long-distance running, but they can be caused by less strenuous activities or may even develop without an obvious cause (Alfredson et al 2002, Ohberg Alfredson 2004). There is a higher incidence in older postmenopausal women (J. L. Cook, conference lecture 2008). The condition also occurs in the general population, often in those with a sedentary lifestyle (Alfredson & Lorentzon 2000, Paavola et al 2002). Koenig et al (2004) note that malalignment of the rear foot leading to functional overpronation has been proposed as a cause of Achilles tendinopathy, which supports the value of assessing the biomechanics of the lower limb as part of the objective examination of the ankle and foot. Achilles pain or spontaneous rupture can be associated with spondyloarthropathies (Jebaraj & Rao 2006), a further consideration in causative factors.
Several other aetiological mechanisms are suggested in the various texts for Achilles tendinopathy: incorrect or worn-out footwear, pressure from heel counters, one-off incidents of direct trauma, inappropriate or changes in training surfaces, excessive training, training errors or indirect trauma, muscle weakness and/or imbalance, reduced flexibility, joint stiffness, male gender, obesity or leg length discrepancy.
Frey & Shereff (1988) suggest that since the fibres of gastrocnemius and soleus do not insert into the Achilles tendon in a parallel direction, abnormal shear stresses may be set up, creating an area of weakness and making it susceptible to injury. September et al (2007) have examined the genetic component in tendon and ligament injuries and blood group O or the A/O ratio appears to be associated with Achilles peritendinitis and tendon rupture in some studies. Variation in genes that encode structural proteins including various types of collagen, proteoglycans and glycoproteins may also be involved in Achilles tendon injuries. These findings are interesting but are probably not as relevant as the causative factors that can be identified and addressed as part of rehabilitation.
Leadbetter (cited in Paavola et al 2002) suggests a mechanical theory for tendon degeneration, called the ‘tendinosis cycle’, as the tendon continues to fail to adapt to excessive changes in load either during a single incident or over a prolonged period of time. The vascularization of tendons is relatively sparse compared to muscles and there are relatively few cells available for oxidative metabolism, resulting in a low circulatory and metabolic response to loading (Alfredson et al 2002).
When the tendon is subjected to 4–8% repeated strain (or possibly to 12% (Wang et al 2006)), it is unable to bear further tension. Injury occurs as the tendon tissue becomes fatigued and it is overwhelmed by repetitive microtrauma and thus unable to repair the fibre damage. The structure of the tendon is therefore disrupted and collagen fibres begin to slide past one another, weakening and breaking the collagen cross-links. Alfredson & Lorentzon (2000) emphasize that no inflammatory cells are seen histologically in tendinosis, but increased amounts of interfibrillar gel and glycosaminoglycans with changes in the collagen fibre structure. However, inflammation may affect the paratendinous structures leading to scar tissue development and this will need to be addressed during the treatment programme (Brukner & Khan 2007).
Rees et al (2006) put forward mechanical, vascular and neural theories to explain the aetiology of tendinopathy suggesting that pain may arise from a combination of factors rather than from one alone. They propose that underuse rather than overuse can be responsible and that tendinopathy is a failure of healing with the damaged tendon being subject to fibroplasias resulting in scar tissue formation and a weakened tendon. Both chronic pain and rupture occur most frequently 3 cm above the calcaneal insertion, an area that has been shown to be hypovascular in normal tendons (Pufe et al 2001, Alfredson et al 2002).
Pufe et al (2001) have hypothesized that the lack of vascularity compromises the nutrition required by tendon cells, making it more difficult for the cells to synthesize the extracellular matrix required for repair and remodelling of the fatigue damaged tendon (Tasto et al 2003). Alfredson et al (2002) have a different view, however, and note that blood flow is evenly distributed in the normal Achilles tendon under resting conditions and that the flow is unaltered by age and exercise. If that is so, factors other than peritendinous flow will need to be investigated to account for the increased incidence of lesions of the mid-portion of the Achilles tendon in middle-aged individuals.
Patients present with a history of a gradual onset of pain felt locally at the back of the heel. They may or may not recall the causative factors. In the early phase of the condition pain usually follows strenuous activity, whereas in the more chronic condition pain occurs during all activities and can be present at rest (Paavola et al 2002). The pain is worse when the foot is first put to the floor in the morning, easing after several steps (the different location of the pain distinguishes it from plantar fasciitis). Pain is increased by activity and the tendon itself may show some thickening. It is tender to palpation. Crepitus may be present, due to movement of the tendon within the paratenon which may be filled with fibrin exudate (Paavola et al 2002).
Khan & Cook (2000) initiated the debate of ‘Where does the pain comes from’ in overuse tendon injuries. The traditional inflammatory cause of pain was challenged and the ‘myth’ of tendonitis was dispelled for ‘not withstanding scrutiny’ (Khan et al 2002). Collagen fibre injury was cited as an obvious component and the biochemical irritant model of pain was put forward. Neovascularization and growth of new nerve fibres were later proposed as the cause of the pain.
This has been supported by the pain relief achieved by sclerosant injections that appear to destroy new capillary and nerve growth (Ohberg & Alfredson 2003) and eccentric exercises have also been shown to abolish neovascularization with an accompanying decrease in pain (Ohberg & Alfredson 2004). This does lead to a tension in the aims of treatment techniques however, since Tasto et al (2003) suggest that any treatment modality that stimulates local blood supply and addresses the deficit of angiogenesis may be beneficial in the treatment of tendinosis. More work needs to be done to resolve this apparent conundrum.
On examination, pain is felt on resisted plantarflexion, which should be performed against gravity with body-weight resistance to reproduce the symptoms in minor or chronic lesions. If the history indicates Achilles tendinopathy, but pain cannot be reproduced on examination, the patient may have to perform some sort of provocative exercise to produce symptoms before assessment to allow accurate diagnosis.
Rupture of the Achilles tendon rarely occurs if the tendon is healthy, but may occur if there is existing tendinosis with fibrous degeneration (Smart et al 1980). Rupture, whether partial or complete, may occur through indirect violent trauma, e.g. push-off with knee extension during weight-bearing as in sprinting, unexpected forced dorsiflexion, e.g. missing a step or stumbling, or forced dorsiflexion of the plantarflexed foot, as in falling from a height. It is accompanied by a sudden onset of pain (Smart et al 1980, Mahler & Fritschy 1992). Partial rupture and tendinosis may coexist, however, or possibly be regarded as the same condition (Alfredson & Lorentzon 2000).
Total rupture presents with a history of intense pain at the time of injury, as if being kicked or shot in the tendon, but little pain following injury. Function is disrupted and the patient is unable to tip-toe stand. A gap may be palpable immediately after injury, and the Thompson’s test (also known as Simmond’s test) may be positive. Position the patient in prone lying with the foot hanging off the couch and squeeze the bulk of the calf muscle. If intact, the Achilles tendon plantarflexes the foot (Hattam & Smeatham 2010). Matles test is a further aid to diagnosis of rupture, particularly if chronic. The patient is placed in prone lying and requested to flex the knee to 90°. Normally the foot should be slightly plantarflexed in this position and the test is positive if the foot falls into a neutral or a dorsiflexed position (Frey & Shereff 1988, Lee & Maleski 2002).
X-ray can be used to confirm complete rupture of the Achilles tendon. Kager’s triangle is a triangular space filled with fatty tissue bordered posteriorly by the inner contour of the Achilles tendon, anteriorly by the deep flexor tendons and inferiorly by the upper border of the calcaneus (Grisogono 1989, Cetti & Andersen 1993). In total rupture, Kager’s triangle loses its normal contours on the X-ray picture (Smart et al 1980). Ultrasound and magnetic resonance imaging have since become much more commonly used to confirm diagnosis.
The condition is treated by immobilization in plaster, usually after surgical repair, followed by early mobilization to achieve rapid recovery and to return to normal strength (Saw et al 1993).
Treatment of Achilles tendinopathy
Conservative treatment is advocated by most authors as the initial treatment strategy. Alfredson & Lorentzon (2000) noted that sparse scientific evidence exists to support the various treatments on offer but studies have been performed since to develop the evidence on the basis of the pathology involved, the appropriate treatments to apply and their effect on both the pain and the pathological process. Common treatment objectives are to limit tissue injury and to stimulate a healing response (Tasto et al 2003) with surgery being reserved for those cases where more conservative strategies have failed.
Brukner & Khan (2007) discuss the favourable outcome of sclerosing injections to close down new vessels that have become established in tendinosis. There is level 2 evidence to support the application of nitric oxide donor therapy (glyceryl trinitrate (GTN)) patches and the role of corticosteroid injection is discussed below.
Electrophysical agents are commonly used by physio-therapists including ultrasound, which has been shown to increase protein synthesis in tendons, although this may not necessarily improve the clinical outcome in tendinosis. Extracorporeal shock wave therapy has become more widely used in the treatment of tendinopathy but the outcome of studies has been variable and the mechanism remains unclear. Kader et al (2005) explain that icing reduces the metabolic rate of tendon and decreases the extravasation of blood and protein from the new capillaries in tendinosis that is suspected as a cause of the pain.
Frictions are discussed below as an appropriate technique to apply for pain relief and to mobilize the tissue structure. As with ultrasound, they have been shown to stimulate protein output of tendon cells (Davidson et al 1997) but, as mentioned above, greater amounts of collagen and ground substance may not necessarily be beneficial to pain or pathology (Brukner & Khan 2007). Frictions are commonly used by physiotherapists but evidence to support their effectiveness is mainly anecdotal (J. Kerr, unpublished work 2006). Clinical experience should not be ignored but further research would be valuable to gain evidence to underpin the extensive use of this technique.
Treatment should be accompanied by education of the patient in activity modification, particularly addressing the causative factors. A full explanation should be given of the prognosis and expected recovery time, which may well be in excess of 3 months (Brukner & Khan 2007). Return to sporting activity should be gradual and heel raises may be necessary to reduce the load on the tendon.
Alfredson et al (1998) and Alfredson & Lorentzon (2000) report on a specially designed heavy load eccentric calf muscle training programme over a 12-week period as a treatment for chronic Achilles tendinopathy. All 15 patients included in the study had no pain during running and jogging after the 12-week training period and had returned to their pre-injury activity level. At a 2-year follow-up one patient was shown to have experienced a recurrence of symptoms and progressed to surgery while the rest remained pain-free. The authors could not fully explain the good results, but suggested that it could be due to loading-induced hypertrophy and increased tensile strength of the tendon, or to a stretching effect and lengthening of the muscle–tendon unit. A further explanation suggested that as the training programme was painful to perform, with severe pain being experienced during the early stage of the programme, pain perception within the tendon may have been altered.
Further support for the inclusion of eccentric training in the management of chronic Achilles tendinopathy is suggested by Herrington & McCulloch (2007). Conventional treatment, comprising frictions and ultrasound, was compared to conventional treatment plus eccentric training over a 12-week period in a pilot study with 25 participants in two groups. Both groups showed significant improvement but the group including the eccentric training improved more. Brukner & Khan (2007) provide a word of caution suggesting that eccentric exercises can potentially cause damage if performed inappropriately or excessively; careful instruction and monitoring of the patient are therefore necessary.
Alfredson & Cook (2007) have devised a treatment algorithm for managing Achilles tendinopathy and they discuss the conservative treatments that are available. Surgical treatment requires extensive post-surgical rehabilitation and is reserved for those with tendons that have failed to respond to conservative treatment. Procedures range from percutaneous tenotomy to open procedures where tendon pathology is removed.
For acute injuries, the usual PRICE regime is adopted as necessary and the choice of electrotherapy modalities or cryotherapy is at the discretion of the therapist. The orthopaedic medicine approach involves the application of transverse frictions, but this is integrated into the programme of care for the patient. Other mobilization techniques such as specific soft tissue mobilizations (Hunter 1998) can also be included in this programme.
Transverse frictions to the Achilles tendon (Cyriax 1984, Cyriax & Cyriax 1993)
The most common sites for Achilles tendinopathy are the anterior aspect, the sides of the tendon and the insertion into the calcaneus. The affected site is identified by palpation.
Anterior aspect of the tendon
Position the patient in prone-lying with the foot plantarflexed on a pillow. Push the relaxed Achilles tendon laterally with a finger, placing your middle finger reinforced by the index against the exposed anterolateral surface of the tendon (Fig. 12.63). Apply the transverse frictions by a pronation and supination movement of your forearm. Repeat the same procedure with the tendon pushed medially to gain access to the anteromedial side (Fig. 12.64).
 |
| Figure 12.63
Transverse frictions to the anterolateral aspect of the Achilles tendon.
|
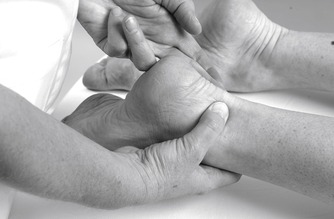 |
| Figure 12.64
Transverse frictions to the anteromedial aspect of the Achilles tendon.
|
Sides of the tendon
Position the patient in prone-lying with the foot resting on a pillow over the edge of the couch. Rest your leg against the patient’s foot to place it in a degree of dorsiflexion, applying enough tension to the Achilles tendon to stabilize it for treatment. Grasp the tendon between your fingers and thumb, depending on the extent of the lesion, and friction transversely across the fibres (Fig. 12.65).
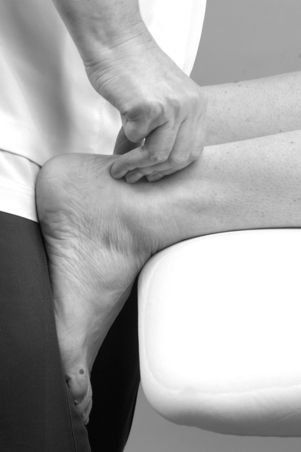 |
| Figure 12.65
Transverse frictions to the sides of the Achilles tendon.
|
Insertion of the Achilles tendon into the calcaneus
Position the patient in prone-lying, with the head of the couch slightly elevated, preferably with a pillow under the foot to relax it into plantarflexion. This puts you into a good position and allows you to hold the calcaneus firmly as you apply transverse frictions to the insertion of the tendon into the middle third of the calcaneus. Make a ring formed by the index fingers, one reinforced by the other, and the thumbs. Rest your index fingers on the calcaneus and wrap your thumbs around the heel (Fig. 12.66). Direct the pressure through your index fingers down onto the insertion and impart the transverse frictions transversely across the fibres.
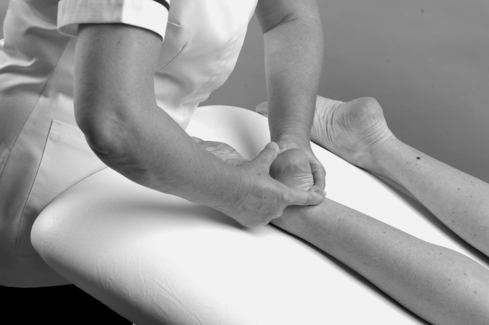 |
| Figure 12.66
Transverse frictions to the insertion of the Achilles tendon into the calcaneus.
|
Relative rest is advised where functional movements may continue, but no overuse or stretching until the structure is pain-free on resisted testing. The ‘heel drop’ eccentric exercise programme is not as effective for lesions at the Achilles insertion as for ‘mid-portion’ or ‘non-insertional tendinopathy’ (Thomas et al 2001, Brukner & Khan 2007, J. L. Cook, conference lecture 2008).
Injection of the Achilles tendon
Controversy exists over the benefits and risks of cortico-steroid injections and their relationship to tendon rupture (Kleinman & Gross 1983, Mahler & Fritschy 1992, Read & Motto 1992). Paavola et al (2002) suggest that the deleterious effects of peritendinous injections on human tendon properties are based solely on uncontrolled case studies, and that no well-controlled prospective clinical trials have been conducted to date. Leadbetter (2005) also challenges studies that link corticosteroid injection with increasing tendon degeneration and rupture, asking why any apparent degenerative changes should be associated with the effects of injection rather than to other forms of microtrauma and the natural outcome of tendinopathy. He does make the personal observation, however, that following spontaneous rupture delayed recovery and healing appear to be more prevalent in patients with a previous history of Achilles tendon injection; timescales are not mentioned.
Corticosteroid injections tend to be used for chronic long-standing lesions in which it is difficult to assess clinically the degree of degeneration present in the tendon and to qualify whether the complication of rupture did or did not occur due to injection or the underlying degeneration. Uncomplicated early acute tendonitis may respond well to an early corticosteroid injection to relieve the inflammation if this is the true pathology, while tendinosis, i.e. focal degeneration of the tendon, may not benefit from corticosteroid injection. It seems that studies based on animal experiments show that intratendinous injections cause a reduction in the tensile strength of the Achilles tendon, while studies relating to peritendinous injections have not shown these adverse effects (Kleinman & Gross 1983, Mahler & Fritschy 1992, Read & Motto 1992).
At the time of writing, the consensus appears to be that at least an ultrasound scan is required prior to peritendinous injection, to assess the general health of the tendon and to establish the extent of degeneration. Injection would be avoided if the degeneration is advanced.
Suggested needle size: 21G × 1½ in (0.8 × 40 mm) green needle
Dose: 20 mg triamcinolone acetonide in a total volume of 2 mL
Position the patient prone on the couch with the ankle resting in dorsiflexion. Two insertions are made, one on each side of the tendon, renewing the needle in between. Advance the needle to its full length adjacent to the tendon and inject half of the solution as a bolus on each side, as the needle is withdrawn (Figure 12.67 and Figure 12.68). The patient is advised to maintain a period of relative rest for approximately 2 weeks following injection.
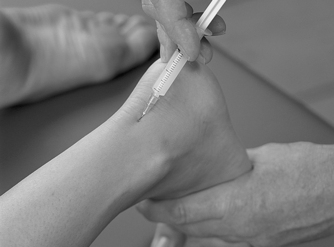 |
| Figure 12.67
Peritendinous injection of the Achilles tendon.
|
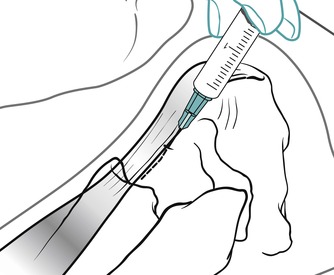 |
| Figure 12.68
Peritendinous injection of the Achilles tendon showing direction of approach and needle position.
|
Gastrocnemius muscle belly
Strain of the gastrocnemius muscle belly often has an acute onset following a sudden sprinting action such as accelerating from a stationary position with the ankle in dorsiflexion, or lunging forward as in squash or tennis. Sudden eccentric overstretching of the muscle, such as missing your step on the kerb, is another mechanism (Brukner & Khan 2007). The lesion is sometimes called ‘tennis leg’ (Cyriax 1982). Patients feel as though they have been kicked or hit in the calf; the pain is acute and weight-bearing is difficult. The limb becomes swollen and bruising develops within 24–48 h. The diagnosis is conclusive from the history and palpation often localizes the lesion to the medial head of the gastrocnemius muscle belly or to the musculotendinous junction where a defect may be felt. Pain is reproduced by resisted plantarflexion and on passive dorsiflexion.
Deep vein thrombosis may be associated with calf injuries and may be difficult to exclude. Taylor (2002) suggests that the force required to damage a muscle or the compressive effects of oedema may be sufficient to cause intravascular damage or microtears leading to roughening of the vessel which may lead to platelet aggregation and thrombus formation. Diagnosis is confirmed in the presence of constant pain, tenderness, heat, swelling and a positive Homan’s sign involving pain on passive overpressure of dorsiflexion with the knee in extension (Brukner & Khan 2007). Imaging may also be used to confirm the diagnosis. Risk factors should be considered that may give rise to the combination of factors leading to thrombus formation as described in the so-called Virchow’s triad, comprising alterations in blood flow, vascular endothelial injury and changes in normal blood flow or consistency. As well as recent trauma, risk factors may include recent surgery or immobilization, recent long haul travel and oestrogen use, as well as family history or previous vascular problems (Taylor 2002).
A chronic strain of the gastrocnemius muscle belly may be the result of a past acute lesion or chronic overuse.
Treatment of acute gastrocnemius muscle belly lesion
Apply the principles of treatment for acute lesions, with protection, rest, ice, compression and elevation (PRICE) being applied as soon as possible after the onset and for 2–3 days after injury. Gentle transverse frictions are given to the full extent of the lesion, at a depth judged to be appropriate for the irritability present, with the muscle belly in a relaxed position (Fig. 12.69). This imitates the normal function of the muscle belly fibres, which is to broaden as the muscle contracts. Transverse frictions are followed immediately by Grade A mobilizations. The patient is taught to maintain a normal heel–toe gait, with the aid of crutches if necessary. A heel-raise takes the pressure off the muscle belly and can be gradually reduced as movement is regained.
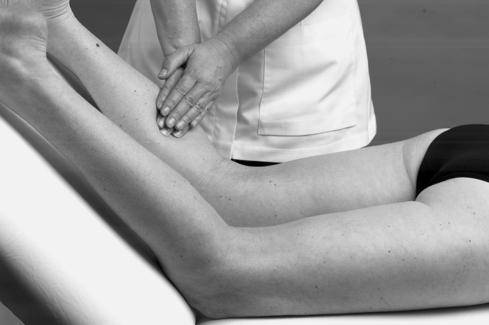 |
| Figure 12.69
Transverse frictions for acute and chronic gastrocnemius muscle belly lesion.
|
Treatment of chronic gastrocnemius muscle belly lesion
Deep transverse frictions are applied with the muscle belly in a relaxed position (Fig. 12.69), to facilitate the broadening of the fibres, having first gained analgesia with a more gently graded technique. Vigorous Grade A exercises are performed. The patient is treated until normal painless function is restored. Should the muscle need to be stretched, it is applied once the muscle is pain-free on resisted testing.
Treatment of gastrocnemius musculotendinous junction
If the lesion is in the musculotendinous junction it may be treated with transverse frictions. Locate the tender site and apply the principles with regard to depth, sweep and extent of the lesion and with consideration for the irritability of the lesion (Fig. 12.70).
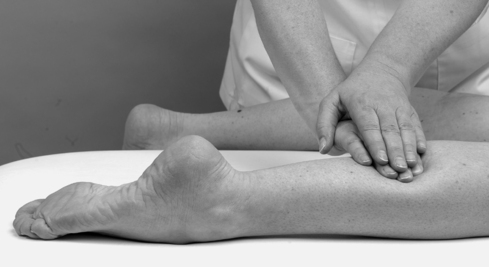 |
| Figure 12.70
Transverse frictions to the gastrocnemius musculotendinous junction.
|
Tibialis posterior
The patient presents with a history of pain felt on the medial aspect of the ankle and the causative factors may not be known. It is often due to the repetitive microtrauma of overuse associated with overpronated foot postures and loss of the medial longitudinal arch. On inspection oedema may be present, thickening of the tendon along its course behind and below the medial malleolus may be obvious and heat may be felt on palpation. The positive sign is pain on resisted inversion and palpation for tenderness localizes the lesion. The lesion may be above, behind or below the medial malleolus or at the tendon insertion. Transverse frictions are applied with the tendon on the stretch in tenosynovitis, as the tendon is enclosed within a synovial sheath for its course around the malleolus. Alternatively, corticosteroid injection can be applied (apply the principles given above for the peroneal tendons).
REFERENCES
Acus, R.W.; Flanagan, J.P., Perineural fibrosis of superficial peroneal nerve complicating ankle sprain: a case report, Foot Ankle 11 (1991) 233–235.
Alfredson, H.; Cook, J., A treatment algorithm for managing Achilles tendinopathy: new treatment options, Br. J. Sports Med. 41 (2007) 211–216.
Alfredson, H.; Lorentzon, R., Chronic Achilles tendinosis: recommendations for treatment and prevention, Sports Med. 29 (2) ( 2000) 135–146.
Alfredson, H.; Pietilä, T.; Jonsson, P.; et al., Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis, Am. J. Sports Med. 26 (3) ( 1998) 360–366.
Alfredson, H.; Bjur, D.; Thorsen, K.; et al., High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique, J. Orthop. Res. 20 (5) ( 2002) 934–938.
Anthony, R.J., The functional anatomy of the running training shoe, Chiropodist ( 1987) 451–459.
Auletta, A.G.; Conway, W.F.; Hayes, C.W.; et al., Indications for radiography in patients with acute ankle injuries: role of the physical examination, Am. J. Roentgenol. 157 (1991) 789–791.
Baxter, D.E., The heel in sport, Clin. Sports Med. 13 (1994) 683–693.
Bekerom, M.P.J.; Raven, E.E.J., The distal fascicle of the anterior inferior tibiofibular ligament (AITFL) as a cause of tibiotalar impingement syndrome: a current concepts review, Knee Surg. Sports Traumatol. Arthrosc. 15 (4) ( 2007) 465–471.
Beumer, A.; van Hemert, W.L.; Swierstra, B.A.; et al., A biomechanical evaluation of clinical stress tests for syndesmotic ankle instability., Foot Ankle Int. 24 (4) ( 2003) 358–363.
Blake, R.L.; Anderson, K.; Ferguson, H., Posterior tibial tendinopathy, J. Am. Podiat. Med. Assoc. 84 (1994) 141–149.
Boruta, P.M.; Bishop, J.O.; Braly, W.G.; et al., Acute lateral ankle ligament injuries: a literature review, Foot Ankle 11 (1990) 107–113.
Boss, A.P.; Hintermann, B., Anatomical study of the medial ankle ligament complex., Foot Ankle Int. 23 (6) ( 2002) 547–553.
Bottger, B.A.; Schweitzer, M.E.; El-Noueam, K.I.; et al., MR imaging of the normal and abnormal retrocalcaneal bursae, Am. J. Roentgenol. 170 (1998) 1239–1241.
Boytim, M.J.; Fischer, D.A.; Neumann, L., Syndesmotic ankle sprains, Am. J. Sports Med. 19 (1991) 294–298.
Brukner, P.; Khan, K., Clinical Sports Med. 3rd edn. ( 2007)McGraw-Hill, Sydney.
Canoso, J.J., Bursae, tendons and ligaments, Clin. Rheum. Dis. 7 (1981) 189–221.
Cetti, R.; Andersen, I., Roentgenographic diagnosis of ruptured Achilles tendons, Clin. Orthop. Relat. Res. 286 (1993) 215–221.
Chandler, T.J.; Kibler, W.B., A biomechanical approach to the prevention, treatment and rehabilitation of plantar fasciitis, Sports Med. 15 (1993) 344–352.
Chandnani, V.P.; Bradley, Y.C., Achilles tendon and miscellaneous lesions, MRI Clin. N. Am. 2 (1994) 89–96.
Cole, C.; Seto, C.; Gazewood, J., Plantar fasciitis: evidence-based review of diagnosis and therapy, Am. Fam. Phys. 72 (11) ( 2005) 2237–2242.
Colville, M.; Marder, R.; Boyle, J.; et al., Strain measurements in lateral ankle ligaments, Am. J. Sports Med. 18 (2) ( 1990) 196–200.
Cornwall, M.W.; Murrell, P., Postural sway following inversion sprain of the ankle, J. Am. Podiat. Med. Assoc. 81 (1991) 243–247.
Cyriax, J., 8th edn.Textbook of Orthopaedic Medicine. vol. 1 ( 1982)Baillière Tindall, London.
Cyriax, J., 11th edn.Textbook of Orthopaedic Medicine. vol. 2 ( 1984)Baillière Tindall, London.
Cyriax, J.; Cyriax, P., Cyriax’s Illustrated Manual of Orthopaedic Medicine. ( 1993)Butterworth Heinemann, Oxford.
Dasgupta, B.; Bowles, J., Scintigraphic localization of steroid injection site in plantar fasciitis, Lancet 346 (1995) 1400–1401.
Davidson, C.; Ganion, L.R.; Gehlsen, G.; et al., Rat tendon morphological and functional changes resulting from soft tissue mobilization, Med. Sci. Sports Exerc. 31 (1997) 1509–1515.
DiGiovanni, B.F.; Nawoczenski, D.A.; Malay, D.P.; et al., Plantar fascia – specific stretching exercise improves outcomes in patients with chronic plantar fasciitis, J. Bone Joint Surg. 88-A (2006) 1775–1781.
Edwards, G.S.; DeLee, J.C., Ankle diastasis without fracture, Foot Ankle 4 (1984) 305–312.
Evans, P., Clinical biomechanics of the subtalar joint., Physiotherapy 76 (1990) 47–81.
Ferran, N.; Maffulli, N., Epidemiology of sprains of the lateral ankle complex., Foot Ankle Clin. 11 (2006) 659–662.
Fox, J.M.; Blanzina, M.E.; Jobe, F.W.; et al., Degeneration and rupture of the Achilles tendon, Clin. Orthop. Relat. Res. 107 (1975) 221–224.
Frey, C.; Shereff, M.J., Tendon injuries about the ankle in athletes, Clin. Sports Med. 7 (1988) 103–118.
Frey, C.; Rosenberg, Z.; Shereff, M.J.; et al., The retrocalcaneal bursa: anatomy and bursography, Foot Ankle 13 (1992) 203–207.
Fujii, T.; Luo, Z.; Kitaoka, H.B.; et al., The manual stress test may not be sufficient to differentiate ankle ligament injuries, Clin. Biomech. 15 (2000) 619–623.
Gibbon, W.W.; Cassar-Pullicino, V.N., Heel pain, Ann. Rheum. Dis. 53 (1994) 344–348.
Grisogono, V., Physiotherapy treatment for Achilles tendon injuries, Physiotherapy 75 (1989) 562–572.
Hattam, P.; Smeatham, A., Special Tests in Musculoskeletal Examination. ( 2010)Churchill Livingstone, Edinburgh.
Herrington, L.; McCulloch, R., The role of eccentric training in the management of Achilles tendinopathy: a pilot study, Phys. Ther. Sport 8 (2007) 191–196.
Hertel, J., Functional anatomy, pathomechanics, and pathophysiology of lateral ankle ligament instability, J. Athl. Train. 37 (4) ( 2002) 364–375.
Hocutt, J.E.; Jaffe, R.; Rylander, C.R.; et al., Cryotherapy in ankle sprains, Am. J. Sports Med. 10 (1982) 316–319.
Hunter, G., Specific soft tissue mobilization in the management of soft tissue dysfunction, Man. Ther. 3 (1) ( 1998) 2–11.
Hutson, M.A., Sports Injuries – Recognition and Management. ( 1990)Oxford Medical Publications, Oxford.
Jebaraj, I.; Rao, A., Achilles tendon enthesopathy in ochronosis, J. Postgrad. Med. 52 (2006) 47–48.
Kader, D.; Maffulli, N.; Leadbetter, W.B.; et al., Achilles tendinopathy, In: (Editors: Maffulli, N.; Renström, P.; Leadbetter, W.B.) Tendon Injuries: Basic Science and Clinical Medicine ( 2005)Springer-Verlag, London, pp. 201–208.
Kannus, P.; Renstrom, P., Treatment for acute tears of the lateral ligaments of the ankle, J. Bone Joint Surg. 73A (1991) 305–312.
Kannus, V.P., Evaluation of abnormal biomechanics of the foot and ankle in athletes, Br. J. Sports Med. 26 (1992) 83–89.
Kapandji, I.A., fifth edn.The Physiology of the Joints: Lower Limb. vol. 2 ( 1987)Churchill Livingstone, Edinburgh.
Karlsson, J.; Lansingeer, O., Lateral instability of the ankle joint, Clin. Orthop. Clin. Res. 276 (1992) 253–261.
Karr, S.D., Subcalcaneal heel pain, Orthop. Clin. N. Am. 25 (1994) 161–175.
Kerkhoffs, G.M., Rowe, B.H., Assendelft, W.J., et al., 2002. Immobilization and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Review. In: Cochrane Library, Issue 2, Update Software, Oxford.
Khan, K.M.; Cook, J.L., Overuse tendon injuries: where does the pain come from?Sports Med. Arthroscopy Rev. 8 (2000) 17–31.
Khan, K.M.; Cook, J.L.; Kannus, P.; et al., Time to abandon the “tendinitis” myth, Br. Med. J. 324 (2002) 626–627.
Kibler, W.B.; Goldberg, C.; Chandler, T.J., Functional biomechanical deficits in running athletes with plantar fasciitis, Am. J. Sports Med. 19 (1991) 66–71.
Kim, W.; Voloshin, A.S., Role of plantar fascia in the load bearing capacity of the human foot, J. Biomech. 28 (1995) 1025–1033.
Kleinman, M.; Gross, A.E., Achilles tendon rupture following steroid injection, J. Bone Joint Surg. 65A (1983) 1345–1347.
Koenig, M.; Torp-Pederson, S.; Ovistgaard, E.; et al., Preliminary results of Doppler guided intratendinous glucocorticoid injection for Achilles tendonitis in five patients, Scand. J. Med. Sci. Sports 14 (2) ( 2004) 100–106.
Kulthanan, T., Operative treatment of plantar fasciitis, J. Med. Assoc. Thai. 75 (1992) 337–340.
Kumai, T.; Takakura, Y.; Rufai, A.; et al., The functional anatomy of the human anterior talofibular ligament in relation to ankle sprains, J. Anat. 200 (5) ( 2002) 457–465.
Lagergren, C.; Lindholm, A., Vascular distribution in the Achilles tendon, Acta Chi. Scand. 116 (1958) 491–495.
Laskowski, E.R.; Newcomer-Aney, K.; Smith, J., Refining rehabilitation with proprioception training: expediting return to play, Phys. Sportsmed. 25 (10) ( 1997) 89–102.
Leadbetter, W.B., Anti-inflammatory therapy in tendinopathy: the role of non-steroidal drugs and corticosteroid injections, In: (Editors: Maffulli, N.; Renström, P.; Leadbetter, W.B.) Tendon Injuries: Basic Science and Clinical Medicine ( 2005)Springer-Verlag, London, pp. 211–232.
Lee, T.K.; Maleski, R., Physical examination of the ankle for ankle pathology, Clin. Podiat. Med. Surg. 19 (2002) 251–269.
Lentell, G.L.; Latzman, L.L.; Walters, M.R., The relationship between muscle function and ankle stability, J. Orthop. Sports Phys. Ther. 11 (1990) 605–611.
Levangie, P.; Norkin, C., Joint Structure and Function. 3rd edn. ( 2001)F A Davis Co., Philadelphia.
Litt, J.C.B., The sprained ankle: diagnosis and management of lateral ligament injuries, Aust. Fam. Phys. 21 (1992) 447–457.
Liu, S.H.; Jason, W.J., Lateral ankle sprains and instability problems, Clin. Sports Med. 13 (1994) 793–809.
Malliou, P.; Gioftsidou, A.; Pafis, G.; et al., Proprioceptive training (balance exercises) reduces lower extremity injuries in young soccer players, J. Back Musculoskeletal Rehabil. 17 (2004) 101–104.
McMinn, R.M.H.; Gaddum-Rosse, P.; Hutchings, R.T.; et al., Functional and Clinical Anatomy. ( 1995)Mosby, London.
Mahler, F.; Fritschy, D., Partial and complete ruptures of the Achilles tendon and local corticosteroid injections, Br. J. Sports Med. 26 (1992) 7–13.
Marder, R.A., Current methods for the evaluation of ankle ligament injuries, J. Bone Joint Surg. 76A (1994) 1103–1111.
Miller, C.A.; Bosco, J.A., Lateral ankle and subtalar instability, Bull. Hosp. Joint Dis. 60 (3) ( 2002) 143–149.
Nordin, M.; Frankel, V.H., Basic Biomechanics of the Musculoskeletal System. 3rd edn. ( 2001)Lippincott, Williams & Wilkins, Philadelphia.
Norris, C.M., Sports Injuries: Diagnosis and Management for Physiotherapists. 2nd edn. ( 2004)Butterworth Heinemann, Oxford.
Ogilvie-Harris, D.J.; Gilbart, M., Treatment modalities for soft tissue injuries of the ankle: a critical review., Clin. J. Sports Med. 5 (1995) 175–186.
O’Hara, J.; Valle-Jones, J.C.; Walsh, H.; et al., Controlled trial of an ankle support Malleotrain in acute ankle injuries, Br. J. Sports Med. 26 (1992) 139–142.
Ohberg, I.; Alfredson, H., Sclerosing therapy in chronic Achilles tendon insertional pain – results of a pilot study, Knee Surg. Sports Traumatol. Arthrosc. 11 (5) ( 2003) 339–343.
Ohberg, I.; Alfredson, H., Effects on neovascularisation behind the good results with eccentric training in chronic mid portion Achilles tendinosis?Knee Surg. Sports Traumatol. Arthrosc. 12 (5) ( 2004) 465–470.
Paavola, M.; Kannus, P.; Järvinen, T.A.; et al., Current concepts review: achilles tendinopathy, J. Bone Joint Surg. 84A (11) ( 2002) 2062–2076.
Palastanga, N.; Field, D.; Soames, R., Anatomy and Human Movement. 5th edn. ( 2006)Butterworth-Heinemann, Edinburgh.
Patel, V.M.; Subramanian, A., Ottawa ankle rules and the use of radiography in acute ankle injuries, CME Orthop. 4 (3) ( 2007) 65–67.
Patterson, M.H.; Cox, W.K., Peroneus longus tendon rupture as a cause of chronic lateral ankle pain, Clin. Orthop. Relat. Res. 365 (1999) 163–166.
Pufe, T.; Peterson, W.; Tillmann, B.; et al., The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons, Virchows Arch. 439 (4) ( 2001) 579–585.
Read, M.T.; Motto, S.G., Tendo Achillis pain: steroids and outcome, Br. J. Sports Med. 26 (1992) 15–21.
Rees, J.; Wilson, A.; Wolman, R., Current concepts in the management of tendon disorders, Rheumatology 45 (2006) 508–521.
Roberts, C.S.; DeMaio, M.; Larkin, J.J.; et al., Eversion ankle sprains, Sports Med. Rehabil. Se. 18 (1995) 299–304.
Sartoris, D.J., Diagnosis of ankle injuries: the essentials, J. Foot Ankle Surg. 33 (1994) 102–107.
Saw, Y.; Baltzopoulos, V.; Lim, A.; et al., Early mobilization after operative repair of ruptured Achilles tendon, Int. J. Care Injured 24 (1993) 479–484.
Scranton, P.E.; McDermott, J.E.; Rogers, J.V., The relationship between chronic ankle instability and variations in mortise anatomy and impingement spurs, Foot Ankle Int. 21 (8) ( 2000) 657–664.
Sellman, J.R., Plantar fascia rupture associated with corticosteroid injection, Foot Ankle Int. 15 (1994) 376–381.
September, A.V.; Schwellnus, M.P.; Collins, M., Tendon and ligament injuries: the genetic component, Br. J. Sports Med. 41 (4) ( 2007) 241–246.
Shrier, I., Treatment of lateral collateral sprains of the ankle: a critical appraisal of the literature., Clin. J. Sports Med. 5 (1995) 187–195.
Smart, G.W.; Taunton, J.E.; Clement, D.B., Achilles tendon disorders in runners – a review, Med. Sci. Sports Exerc. 12 (1980) 231–243.
Standring, S., Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th edn. ( 2009)Churchill Livingstone, Edinburgh.
Stanley, K.L., Ankle sprains are always more than ‘just a sprain’, Postgrad. Med. 89 (1991) 251–255.
Stephens, M.M., Haglund’s deformity and retrocalcaneal bursitis, Orthop. Clin. N. Am. 25 (1994) 41–46.
Swain, R.A.; Holt, W.S., Ankle injuries: tips from sports medicine physicians, Postgrad. Med. 93 (1993) 91–100.
Tasto, J.; Cummings, J.; Medlock, V.; et al., The tendon treatment centre: new horizons in the treatment of tendinosis, Arthroscopy 19 (2003) 213–223.
Taylor, A., Deep vein thrombosis following calf strain: a case study, Phys. Ther. Sport 3 (2002) 110–113.
Thomas, J.; Christensen, J.C.; Kravitz, S.R.; et al., The diagnosis and treatment of heel pain, J. Foot Ankle Surg. 40 (5) ( 2001) 29–340.
Tropp, H., Commentary: functional ankle instability revisited, J. Athl. Train. 37 (4) ( 2002) 512–515.
Wang, J.; Losifidus, M.; Fu, F., Biomechancial basis for tendinopathy, Clin. Orthop. Relat. Res. 443 (2006) 320–332.
Wilkerson, L.A., Ankle injuries in athletes, Prim. Care 19 (1992) 377–392.
Williams, J.G.P., Achilles tendon lesions in sport, Sports Med. 3 (1986) 114–115.
Yu, J.S., Pathologic and post-operative conditions of the plantar fascia: review of MR imaging appearances, Skelet. Radiol. 29 (2000) 491–501.