The Aging Patient
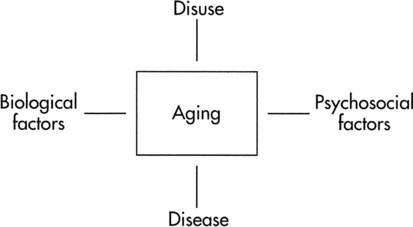
An additional issue is a model of aging that might sort out some of the determinants of aging (Figure 38-1). The model identifies biological factors, disuse, pathology, and psychosocial concerns as determinants of aging. Biological factors address genetics, sex, cellular mechanisms, and metabolic and physiological responses that influence aging. Disuse is implicated in the more sedentary lifestyle led by many older adults, which results in loss of exercise capacity.1 With exercise training, it should be possible to reverse or attenuate capacities that decline as a result of disuse. This chapter focuses on the biological and disuse characteristics of aging. The impact of various pathological conditions is discussed in other chapters. Psychosocial issues related to exercise and aging are not addressed.
Cardiac Changes with Aging
Aerobic Capacity
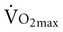 declines with age2,3 at a rate of 0.40 to 0.50 mL/kg/min per year for men and between 0.20 to 0.35 mL/kg/min per year for women (Figure 38-2).4 The reduction is approximately 10% per decade. The decline is faster and greater in men than women; however, men have a larger aerobic capacity overall than women.5,6
declines with age2,3 at a rate of 0.40 to 0.50 mL/kg/min per year for men and between 0.20 to 0.35 mL/kg/min per year for women (Figure 38-2).4 The reduction is approximately 10% per decade. The decline is faster and greater in men than women; however, men have a larger aerobic capacity overall than women.5,6 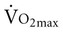 is related to body size, which is generally smaller in women than men.
is related to body size, which is generally smaller in women than men. 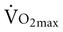 is associated with peripheral vascular reserve in older men but not in women.7 Increases in body weight along with aging result in reduced
is associated with peripheral vascular reserve in older men but not in women.7 Increases in body weight along with aging result in reduced 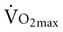 , even if aerobic capacity remains the same, because relative oxygen consumption is related to body weight. Reduced physical activity with aging also contributes to a loss of
, even if aerobic capacity remains the same, because relative oxygen consumption is related to body weight. Reduced physical activity with aging also contributes to a loss of 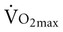 .
.
Considerable disagreement exists regarding the mechanisms that contribute to the decline in 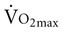 with age. Both cardiac and peripheral changes contribute to this loss. Maximum cardiac output declines with age similarly in men and women.7 A reduction in maximum cardiac output may account for 50% to 100% of the total reduction in
with age. Both cardiac and peripheral changes contribute to this loss. Maximum cardiac output declines with age similarly in men and women.7 A reduction in maximum cardiac output may account for 50% to 100% of the total reduction in 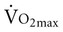 .8–10 McGuire and colleagues (2001)2, however, suggested that reductions in peripheral oxygen extraction were the dominant mechanism responsible for reduced
.8–10 McGuire and colleagues (2001)2, however, suggested that reductions in peripheral oxygen extraction were the dominant mechanism responsible for reduced 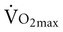 with age. A major component to the decline in maximum cardiac output is decreased maximum heart rate.3,9,11 The decline in heart rate is linearly related to age and occurs in both sedentary and active people.5,12 Discrepancies in the age-related response of maximum stroke volume also exist; studies have reported reduced, preserved, and increased maximum stroke volume with age.2,3,9,13 The decline in
with age. A major component to the decline in maximum cardiac output is decreased maximum heart rate.3,9,11 The decline in heart rate is linearly related to age and occurs in both sedentary and active people.5,12 Discrepancies in the age-related response of maximum stroke volume also exist; studies have reported reduced, preserved, and increased maximum stroke volume with age.2,3,9,13 The decline in 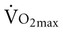 associated with aging is most likely attributable to decreased maximum heart rate, stroke volume, and arteriovenous oxygen content difference, although each component’s contribution varies.8,14
associated with aging is most likely attributable to decreased maximum heart rate, stroke volume, and arteriovenous oxygen content difference, although each component’s contribution varies.8,14
Cardiac Mechanics
Age-related changes in cardiac structure occur in the form of left ventricular wall thickness13,15 and are attributed to an increase in size of cardiac myocytes16 and increased collagen.13 Additional cardiac structural changes include increases in vascular intimal thickness, vascular stiffness, and left arterial dimension.13,17
The diastolic properties (cardiac filling) of the heart alter with age. Diastole requires relaxation of the myocardial fibers, sufficient venous return to rapidly fill the heart, and timing of the atrial contraction to contribute to the end-diastolic volume. Relaxation may be hampered by increased ventricular stiffness, although there is limited evidence of this in humans.14 The period of the isovolumetric myocardial relaxation (the time between aortic valve closing and mitral valve opening) is prolonged with aging.15 Likewise, the peak rate of left ventricular filling during early diastole is progressively reduced so that between the ages of 20 and 80 years, the average rate can be reduced up to 50%.13 Despite the changes in early diastole, the resting left ventricular end-diastolic volume remains the same because of an enhanced left atrial contribution to ventricular filling.13 This is accompanied by an enlarged left atrium and an audible fourth heart sound in most older adults.13,18
Considerable disagreement exists regarding what happens to diastolic function during exercise. End-diastolic volume index (end-diastolic volume normalized for body surface) increases similarly in both young and older men during submaximal exercise; however, only older men remain at these elevated levels during exhaustive exercise.13 Filling pressures during exercise in men increase with age.19 In addition, peak left ventricular diastolic filling rate during submaximal and maximal exercise decreases with aging.20,21 Decreased filling rate is associated with increased ventricular stiffness and prolonged relaxation time.22
Resting measures of systolic and cardiac pump function do not change with aging. The resting end-systolic volume and stroke volume do not change with age. Likewise, ejection fraction at rest (end-diastolic volume − [end-systolic volume/end-diastolic volume]) is similar in healthy older and younger individuals.13
Unlike resting systolic function, the pumping function of the heart changes considerably in response to exercise. Myocardial contractility as measured by the ratio of end-systolic volume to systolic arterial pressure declines during exercise as people age.14 The end-systolic volume index increases, whereas the ejection fraction decreases during exercise.13 Reduced contractile performance is related to decreased response to beta-adrenergic stimulation, changes in the myocardium, increased systolic blood pressure, and ventricular wall abnormalities.8
Exercise Training
For older people who remain active, the rate of decline in 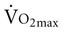 is reduced to 5% per decade, compared with an anticipated decline of 10% per decade in sedentary adults.11 A meta-analysis of 29 studies on endurance training, which included 1030 men and 466 women between the ages of 61 and 78 years, concluded that endurance training increases functional capacity in young older adults.23 Less improvement was seen with increasing age, a shorter length of training, low
is reduced to 5% per decade, compared with an anticipated decline of 10% per decade in sedentary adults.11 A meta-analysis of 29 studies on endurance training, which included 1030 men and 466 women between the ages of 61 and 78 years, concluded that endurance training increases functional capacity in young older adults.23 Less improvement was seen with increasing age, a shorter length of training, low 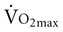 before training, and short duration of exercise sessions. The analysis suggests that a healthy 68-year-old individual who exercises for 30 minutes three times per week for 4 to 6 months can improve
before training, and short duration of exercise sessions. The analysis suggests that a healthy 68-year-old individual who exercises for 30 minutes three times per week for 4 to 6 months can improve 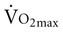 by 14%.23 Similar improvements occur in both men and women.24,25
by 14%.23 Similar improvements occur in both men and women.24,25
The mechanisms underlying improvements in 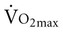 in older adults who engage in endurance training are not clear. One consistent finding, however, is greater extraction of oxygen in the exercising skeletal muscle, which produces a wider arteriovenous oxygen content difference in both older men and women.9,22,26 This implies that adaptations in the peripheral skeletal muscles account for some of the increase in
in older adults who engage in endurance training are not clear. One consistent finding, however, is greater extraction of oxygen in the exercising skeletal muscle, which produces a wider arteriovenous oxygen content difference in both older men and women.9,22,26 This implies that adaptations in the peripheral skeletal muscles account for some of the increase in 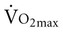 in older adults. The impact of exercise training on maximum cardiac output is uncertain.22 Maximum cardiac output can either remain the same or increase after exercise training, depending on the effect of training on maximum stroke volume and maximum heart rate. Maximum heart rate remains the same in older men regardless of activity level, suggesting that the decline in maximal heart rate depends on factors other than exercise and physical fitness.27 A decrease in response to circulating catecholamines is most often cited as the reason for changes in maximum heart rate with aging.28 Relatively intense endurance training for a year or more can increase peak stroke volume in men.29–31 Adaptations to exercise training in older women have been attributed predominantly to peripheral changes in arteriovenous oxygen content difference rather than central changes in cardiac function.32,33 This apparently occurs despite intensive endurance training over a year-long period. In contrast, a relatively recent study that compared men and women who were masters athletes with healthy sedentary older adults, healthy young persons, and sedentary controls reported that stroke volume for any given filling pressure was greater for masters athletes compared with age-matched sedentary older adults.34
in older adults. The impact of exercise training on maximum cardiac output is uncertain.22 Maximum cardiac output can either remain the same or increase after exercise training, depending on the effect of training on maximum stroke volume and maximum heart rate. Maximum heart rate remains the same in older men regardless of activity level, suggesting that the decline in maximal heart rate depends on factors other than exercise and physical fitness.27 A decrease in response to circulating catecholamines is most often cited as the reason for changes in maximum heart rate with aging.28 Relatively intense endurance training for a year or more can increase peak stroke volume in men.29–31 Adaptations to exercise training in older women have been attributed predominantly to peripheral changes in arteriovenous oxygen content difference rather than central changes in cardiac function.32,33 This apparently occurs despite intensive endurance training over a year-long period. In contrast, a relatively recent study that compared men and women who were masters athletes with healthy sedentary older adults, healthy young persons, and sedentary controls reported that stroke volume for any given filling pressure was greater for masters athletes compared with age-matched sedentary older adults.34
A recent study followed a small group of competitive distance-running men for 10 years with measures of the cardiovascular, pulmonary, and metabolic systems.35 The 5-kilometer race time slowed over the 10 years, along with decreased training mileage, intensity, and frequency. Consistent with this, the peak exercise heart rate and oxygen consumption declined. Another intriguing study indicates that telomere length, a measure of the ability of cells to replicate, is preserved in healthy, older adults who engage in vigorous aerobic exercise.36 The preservation of telomere length is positively related to maximum oxygen consumption, suggesting that exercise reduces cell senescence with aging. Further studies examining the outcomes of prolonged, sustained endurance training in older men and women are needed to characterize the adaptations that occur in women.
The diastolic changes that occur with aging can be reversed with exercise training.20 Levy and colleagues intensively endurance trained 13 rigorously screened older men aged 60 to 82 years (mean age 68 years) and 11 younger men in their 20s, for 6 months. The training produced increased resting, submaximal, and peak filling rates for the older group comparable to the changes observed in the younger group.20 End-diastolic peak volume at rest and exercise also increase after lengthy endurance training.30 Thus training may reduce the age-associated diastolic changes. In contrast, endurance athletes who were followed for 10 years after age 65 showed deterioration in diastolic left ventricular function indices consistent with decreased cardiovascular diastolic function.35 Another study demonstrated that 1 year of vigorous exercise training in sedentary seniors did not improve cardiac stiffening, but improved left ventricular mass and reduced arterial elastance.37 The mechanisms of these responses are uncertain in humans. Studies in rats have shown that exercise training increases calcium uptake in cardiac sarcoplasmic reticulum, decreases relaxation time, reduces the decline of left ventricular pressure, enhances fatty acid oxidation, and increases cytochrome c oxidase levels.20,38,39 All these changes have been associated with reduced diastolic function with aging.
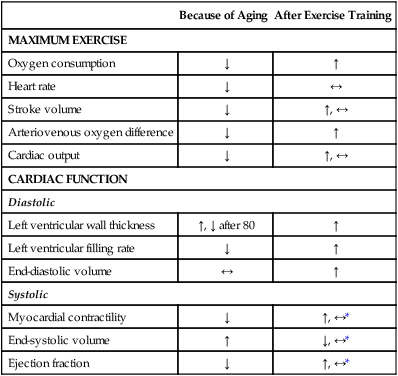
Exercise training can also improve systolic performance in older men as reflected by the increase in exercise stroke volume. Increased peak stroke volume occurs with an increased exercise ejection fraction, a decrease in end-systolic volume, and greater left ventricular wall mass.3,30,31 These changes apparently do not occur in older, estrogen-deficient women.33 Table 38-1 summarizes these changes.
Table 38-1
| Because of Aging | After Exercise Training | |
| MAXIMUM EXERCISE | ||
| Oxygen consumption | ↓ | ↑ |
| Heart rate | ↓ | ↔ |
| Stroke volume | ↓ | ↑, ↔ |
| Arteriovenous oxygen difference | ↓ | ↑ |
| Cardiac output | ↓ | ↑, ↔ |
| CARDIAC FUNCTION | ||
| Diastolic | ||
| Left ventricular wall thickness | ↑, ↓ after 80 | ↑ |
| Left ventricular filling rate | ↓ | ↑ |
| End-diastolic volume | ↔ | ↑ |
| Systolic | ||
| Myocardial contractility | ↓ | ↑, ↔* |
| End-systolic volume | ↑ | ↓, ↔* |
| Ejection fraction | ↓ | ↑, ↔* |
Vascular and Autonomic Changes with Aging
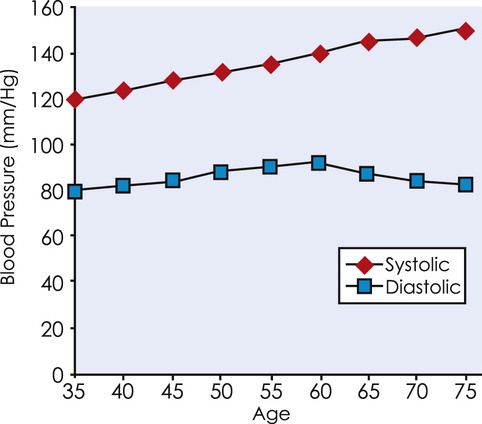
Aging increases arterial stiffness because of a loss of elastin, elastin fractures, and an increase in collagen and calcification.17,40 Sex may influence some of these changes. Some evidence suggests that aortic stiffness may be greater in women after menopause than men.41 Arterial wall thickness and diameter and resting systolic pressure also increase (Figure 38-3).14,17 It is unclear whether peripheral vascular resistance increases in normotensive older adults. Changes in the structure, size, and reactivity of the arteries increase the work of the left ventricle and have been directly implicated in the increase in cardiac myocyte size.
Complaints of dizziness when an older patient moves from supine to standing are frequently encountered by the physical therapist. Postural hypotension, or orthostatic intolerance, generally does not occur in healthy community-dwelling older adults but is common in individuals over 70 years of age who are debilitated and in hospitals or nursing homes.42,43 Orthostatic intolerance is associated with decreased resting diastolic function, possibly decreased stroke volume, extreme inactivity, and, in individuals with hypertension, high systolic blood pressure.44
There are decreases in baroreceptor and cardiopulmonary reflexes with aging.14,45 Arterial and venous dilation are reduced, but vasoconstriction is relatively spared. The heart demonstrates an overall decrease in responsiveness to autonomic stimulation. Older individuals experience higher central venous and mean arterial pressures, but lower forearm blood flow and forearm vascular resistance in response to passive leg raising.45
Response to Exercise
During submaximal and maximal exercise, arterial blood pressure is either unchanged or increased when younger subjects are compared with older subjects.46 There also appears to be impaired peripheral vasodilation in skeletal muscle in response to exercise.9 Redistribution of blood flow during exercise normally involves the shunting of blood from inactive limbs and viscera. There seems to be an enhanced vasoconstriction in inactive muscles during dynamic exercise in healthy older men.47
Exercise Training
Exercise training improves peripheral blood flow in skeletal muscle in 60-year-old men and women.3,48 Exercise training, specifically aerobic training, has been reported to decrease blood pressure.49 Meta-analysis revealed that 4 or more weeks of aerobic training can decrease systolic and diastolic blood pressure50 and mean arterial blood pressure responses in older individuals when compared with sedentary individuals.48 Adults who regularly exercise compared with sedentary individuals have smaller or no age-associated stiffness of the large arteries and have improved vascular endothelial function.51 Aerobic training also reduces the age-related decline in cardiovagal baroreflex sensitivity and partially restores diminished cardiovagal baroreflex sensitivity in older men.52 Aerobic training alters the autonomic nervous system and its control over resting heart rate by enhancing parasympathetic activity and attenuating sympathetic activity.53 During vigorous exercise, the predominant activity of the autonomic nervous system is to increase sympathetic nerve activity, producing an increase in norepinephrine concentrations and increasing blood flow via dilation of the large arteries and constriction of the veins (Table 38-2).13,54 Long-term aerobic training, however, reduces sympathetic nerve activity for a given work rate, thereby lowering exercise heart rate at a given work rate.53 In summary, habitual exercise can improve characteristics of arterial aging, preserve vascular function, and possibly reduce the risk for cardiovascular disease.51
Table 38-2
Vascular and Autonomic Changes with Aging
| Because of Aging | After Exercise Training | |
| Arterial wall thickness | ↑ | ? |
| Systolic blood pressure | ↑ | ↓ |
| Diastolic blood pressure | ↑ and ↔ | ↓ |
| Orthostatic tolerance | ↔ | ? |
| Arterial and venous dilation | ↓ | ? |
| Vasoconstriction | ↔ | ↔ |
| Central venous pressure | ↑ | ? |
↑, Increases; ↓, decreases; ↔, unchanged; ?, insufficient data on older adult subjects.
Pulmonary Changes with Aging
Structure and Function
Two major changes to the pulmonary system associated with aging are decreased elastic recoil and stiffening of the chest wall.8,55 Elastic recoil of the lungs depends on the composition of the connective tissue, the structure of the connective tissue, and alveolar surface tension produced by surfactant.8 Limited evidence suggests that the structure of the connective tissue may be the primary mechanism for age-associated change in elastic recoil. Chest wall stiffness is accompanied by an increase in chest anterioposterior diameter, costal cartilage calcification, narrowing of the intervertebral disks, and changes in the rib to vertebrae articulations.55–57 Postural changes such as kyphosis from osteoporosis limit expansion of the thoracic cage during inspiration and place the diaphragm at a mechanical disadvantage.56
Decreases occur with the alveolar-capillary surface area, the alveolar septal surface area, and the total surface area of lung parenchyma.58 This reduces the alveolar surface area available for gas exchange and increases the amount of physiological dead space.55
Loss of elastic recoil with aging is directly associated with reduced forced expiratory flow.59 Limitations in exhalation are caused by airway narrowing and closure at all lung volumes, thus reducing forced expiratory volume in 1 second (FEV1).8 Additionally, early airway closure also produces an early closing volume and a relative increase in the total residual volume. The combination of reduced elastic recoil and increased chest wall stiffness further increases residual volume and leads to a decrease in forced vital capacity in older individuals.60,61 Flow rates are also lower in women and African-Americans than in white men at any age.8
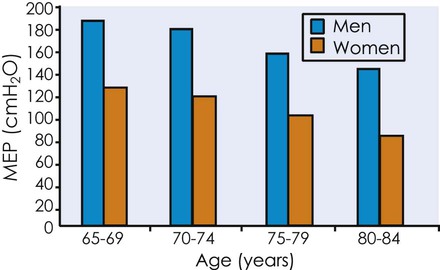
Respiratory muscle strength, as reflected by the ability to create pressure over a range of lung volumes and flow rates, is similar when comparing healthy 30- and 70-year-olds.62 This suggests that respiratory muscle strength does not change with aging; however, transdiaphragmatic pressure is reduced in older compared with younger individuals, suggesting reduced diaphragmatic strength.56 Decline in diaphragmatic strength can contribute to diaphragmatic fatigue and ventilator weaning failure in older individuals.56 Maximal inspiratory and expiratory pressures have been reported to decrease 15% between the sixth and eighth decades (Figure 38-4).63 Perhaps these observed differences are similar to the differences observed with submaximal and maximal cardiac responses with the dynamic pressure measures over a range of volumes and flows considered submaximal.
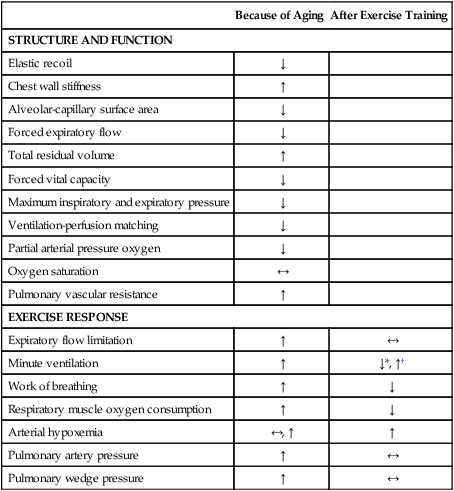
Changes in the surface area result in a decrease in the diffusion capacity of the lung.60,64 Both the loss of surface area and a decrease in pulmonary capillary blood volume contribute to reduced and uneven ventilation-to-perfusion matching in older adults. The resting partial arterial pressure of oxygen declines 5 to 10 mm Hg between the ages of 25 and 75 years.8 These changes do not affect the arterial oxymyoglobin saturation or oxygen content. Parallel changes in the peripheral vasculature, pulmonary vascular resistance, and pulmonary arterial pressure at rest increase (Table 38-3).
Table 38-3
Pulmonary Function Changes with Aging
| Because of Aging | After Exercise Training | |
| STRUCTURE AND FUNCTION | ||
| Elastic recoil | ↓ | |
| Chest wall stiffness | ↑ | |
| Alveolar-capillary surface area | ↓ | |
| Forced expiratory flow | ↓ | |
| Total residual volume | ↑ | |
| Forced vital capacity | ↓ | |
| Maximum inspiratory and expiratory pressure | ↓ | |
| Ventilation-perfusion matching | ↓ | |
| Partial arterial pressure oxygen | ↓ | |
| Oxygen saturation | ↔ | |
| Pulmonary vascular resistance | ↑ | |
| EXERCISE RESPONSE | ||
| Expiratory flow limitation | ↑ | ↔ |
| Minute ventilation | ↑ | ↓*, ↑† |
| Work of breathing | ↑ | ↓ |
| Respiratory muscle oxygen consumption | ↑ | ↓ |
| Arterial hypoxemia | ↔, ↑ | ↑ |
| Pulmonary artery pressure | ↑ | ↔ |
| Pulmonary wedge pressure | ↑ | ↔ |
Responses to Exercise
In addition to the structural and functional changes at rest, several changes occur in breathing during acute exercise. Expiratory flow limitations occur at lower exercise intensities as people age. A normal, healthy 69-year-old will begin to experience flow limitations even in response to moderate exercise.8 Practically, the individual may experience this by having greater difficulty catching his or her breath during exercise.
Normally as people exercise, the tidal volume increases directly with increasing exercise intensity up to about 50% to 60% of the vital capacity. This remains unchanged as people age, although the vital capacity for older adults is reduced.65 Likewise, as exercise and the tidal volume increase, there is a slight drop in the ratio of dead space to tidal volume. This drop is not affected by aging, although the older person will breathe more (have a higher minute ventilation) in response to submaximal exercise.66 Of interest, the short-term modulation of the exercise ventilatory response does not change in healthy older men compared with healthy younger men, despite changes in ventilatory capacity with aging.67
The work of breathing is increased during exercise as people age as a result of several factors. Older adults require an increased ventilatory response to exercise to attain the same alveolar ventilation and PaCO2.66 Combined with the loss of elastic recoil, which causes airway closure at higher lung volumes, expiratory flow limitation occurs at moderate exercise intensities (an expiratory volume of 70 to 100 L·min−1), producing a hyperinflation, a noticeable increase in elastic and flow-resistive work of breathing, and shortness of breath.66 In addition, an increase in the end-expiratory lung volume with increasing exercise results in breathing that occurs at a stiffer point in the lung-volume relationship. The increased stiffness imposes a higher elastic recoil on the ventilatory muscles, requiring greater pressure generated by the inspiratory muscles. The increase in expiratory flow resistance likewise requires greater pressure development by the expiratory muscles. Both the inspiratory and expiratory changes increase the work of breathing. The increase in the work of breathing increases the respiratory muscle oxygen consumption so that the respiratory muscles alone may require 10% to 12% of the total body oxygen consumption during maximal exercise in a sedentary 70-year-old man.8
What is also apparent is that the reserves used to respond to exercise represent a greater percentage of the available capacity. An older individual can exceed 50% of inspiratory muscle capacity even during moderate exercise. This is in contrast to the younger individual who rarely exceeds 50% of the inspiratory muscle capacity with exercise.8 Thus the reserve capacity to generate pleural pressure is reduced in older adults because greater capacity is needed for even moderate exercise.
The variability characteristic of exercise responses in older individuals is particularly evident when considering gas exchange and pulmonary-vascular hemodynamics. In general, most individuals demonstrate only slight changes in arterial blood-gas homeostasis, but some individuals demonstrate arterial hypoxemia with exercise.8 This response may be a factor of fitness. More fit older individuals appear to exhibit progressive arterial hypoxemia and carbon dioxide retention during mild-to-moderate exercise.68,69
Pulmonary artery pressure increases with age at any oxygen consumption or cardiac output during exercise.70 In addition, the maximum pulmonary artery pressure at maximal exercise is reached at substantially lower oxygen consumption and cardiac output levels in older individuals compared with younger people. Pulmonary wedge pressure also increases with age and can exceed 25 mm Hg during peak supine exercise.8 Limited data suggest that these high pressures may in turn induce pulmonary edema during intense exercise in older adults. Pulmonary edema would limit diffusion and may contribute to ventilation-perfusion maldistribution.8
Exercise Training
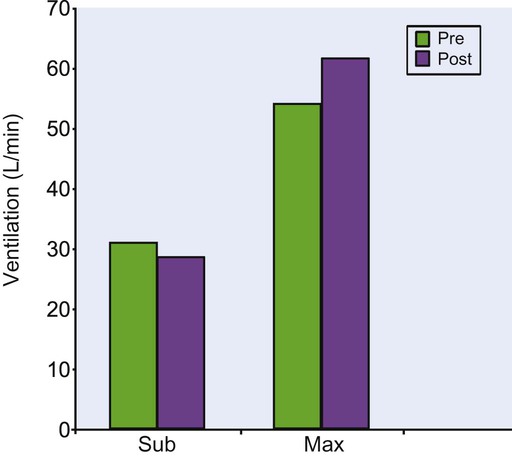
Aerobic training can decrease submaximal minute ventilation.25,71 One study used a walking program with 70-year-old women over a 12-week period and demonstrated a 7.7% drop in submaximal minute ventilation (Figure 38-5).25 Decreased minute ventilation is accompanied by a decrease in carbon dioxide production, respiratory exchange ratio (carbon dioxide production/oxygen consumption), and blood lactate level for any given level of submaximal exercise.25,71 Thus the improved ventilatory efficiency after training may have more to do with improved efficiency in the periphery rather than an impact on the pulmonary system directly. Improved peripheral metabolic efficiency results in the production of less carbon dioxide; therefore the lungs do not have to work as hard to eliminate carbon dioxide. The important functional results of these changes are that the older adults who engage in exercise training will feel less breathlessness, experience a lower perceived exertion, and use a lower proportion of their maximal ventilatory capacity during exercise.72
Exercise training also increases maximum ventilatory responses during maximal exercise.25,72 The 12-week walking program referred to above increased maximum minute ventilation by 14% in 70-year-old women (see Figure 38-5).72 These women walked five days per week at an intensity of 78% of the maximal treadmill heart rate, or 118 beats per minute on average, so even though the program was not very strenuous, it produced substantial changes in maximum ventilation. Exercise training can improve submaximal ventilatory efficiency and increase maximum ventilation in older adults. Table 38-3 summarizes the pulmonary changes seen with aging and exercise.

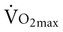 ), or the maximum amount of oxygen used during exercise.
), or the maximum amount of oxygen used during exercise. 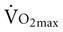 is directly related to cardiac output (the amount of blood pumped by the heart) and the arteriovenous oxygen content difference (the amount of oxygen extracted in the periphery). Cardiac output is the product of heart rate and stroke volume. Aerobic capacity reflects the central cardiac function and the efficiency of the peripheral tissues to extract and use oxygen.
is directly related to cardiac output (the amount of blood pumped by the heart) and the arteriovenous oxygen content difference (the amount of oxygen extracted in the periphery). Cardiac output is the product of heart rate and stroke volume. Aerobic capacity reflects the central cardiac function and the efficiency of the peripheral tissues to extract and use oxygen.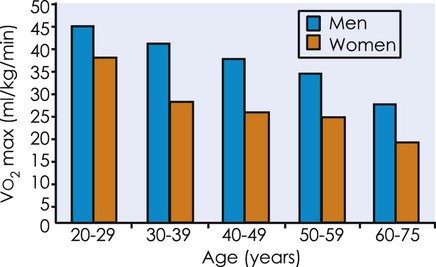
 from 20 to 75 years of age in both men and women.
from 20 to 75 years of age in both men and women.