The adrenal glands
Anatomy
The adrenal glands are retroperitoneal structures weighing approximately 5–7 g that have a characteristic golden colour due to cholesterol in their cortex (see Fig. 3.1). Their position and shape are slightly different on each side. The right gland is pyramidal and lies at the upper pole of the right kidney, between the right crus of the diaphragm and the inferior vena cava. The left adrenal gland is crescentic and is situated on the upper medial aspect of the left kidney. The tail of the pancreas lies anterior and the diaphragm crus is posterior to the left adrenal gland.1 Each gland consists of two distinct parts, which have different structures and functions – the outer cortex and the inner medulla.
Blood supply and lymphatic drainage
The blood supply is derived from branches of three vessels, the inferior phrenic artery, the ipsilateral renal artery and the aorta. These branches often subdivide into a leash of vessels, before entering the gland. Venous return is via a single adrenal vein that drains into the renal vein on the left and directly into the inferior vena cava on the right.1 The right vein is short and an important landmark for the surgeon. The veins drain from the medulla and therefore the venous return from the cortex flows through the medulla. This is relevant as glucocorticoid hormones secreted by the cortex activate phenylethanolamine N-methyltransferase (PNMT), an enzyme involved in the synthesis of catecholamines. Medullary and subcapsular lymphatic plexuses drain into lymphatics that follow the arterial supply to the para-aortic lymph nodes.
Microscopic anatomy
The adrenal cortex is divided into three layers: the outer zona glomerulosa, the zona fasciculata and the innermost zona reticularis. The zona glomerulosa produces mineralocorticoids. It consists of columnar cells, with relatively little cytoplasm compared with their nuclei, organised into clusters. The zona fasciculata is the largest of the cortical zones, occupying approximately 75% of the cortex, that contains poorly staining, polyhedral cells organised in radial columns and is primarily responsible for the production of glucocorticoids. The innermost zona reticularis is characterised by rounded branching cords of cells and produces sex hormones, particularly dehydroepiandrosterone sulphate (DHEAS).2
Embryology
The cortex and medulla have different embryological origins. The adrenal cortex is mesodermal in origin and starts to appear in the fifth week of gestation as two clefts on either side of the embryonic dorsal mesentery that enlarge to form the primitive or foetal cortex. This is surrounded at the seventh week by a second wave of mesothelial cells to form the secondary cortex that eventually becomes the adult adrenal cortex. Concordantly, cells migrating from the neural crest invade the developing adrenal gland to form the adrenal medulla, which is therefore neuroectodermal in origin. The adrenal glands are large at birth, but reduce in size thereafter due to regression of the primary cortex, and do not regain their original size until puberty. The zona glomerulosa starts to appear before delivery, followed by the zona fasciculata and finally the zona reticularis a few months after birth. Neural crest cells, outside the adrenal medulla, are widely present in the embryo, but regress after birth.3
Physiology
Catecholamine synthesis and metabolism
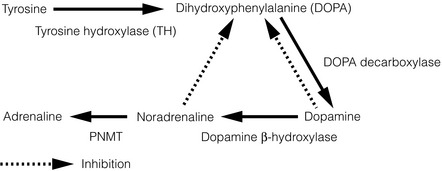
The adrenal medulla synthesises the catecholamines dopamine, noradrenaline (norepinephrine) and adrenaline (epinephrine) from tyrosine by a series of steps via 3,4-dihydroxyphenylalanine (DOPA). The enzyme tyrosine hydroxylase, which controls the rate-limiting step, is largely confined to the central and sympathetic nervous systems, and the adrenal medulla. The final step in the pathway of catecholamine synthesis (to adrenaline) is catalysed by PNMT, which is induced by cortisol as previously discussed (see Fig. 3.2).
After stimulation of the adrenal medulla, catecholamine release occurs by a calcium-dependent process in which secretory granules fuse with the cell membrane (exocytosis). The majority of the released catecholamines are taken back up into the presynaptic terminals of the chromaffin cells and deaminated by monoamine oxidase. The remaining catecholamines enter the systemic circulation, where they have diverse effects and are methylated by carboxy-O-methyltransferase to methoxytyramine, metanephrine and normetanephrine. Vanillylmandelic acid (VMA) is produced in the liver following deamination and methylation of catecholamines, and is excreted along with sulphate-conjugated metanephrines in the urine.4
Catecholamine physiological effects
Basal secretion of catecholamines is low but rises substantially in response to certain stimuli. Catecholamines exert their characteristic effects by binding to cell-membrane-bound α- and β-adrenoreceptors present in most tissues and organs. The physiological effects are typified as the ‘flight or fight’ response, i.e. preparing the organism for optimal performance in times of threat, and include an increase in heart rate, blood pressure and cardiac output, excitation of the central nervous system, increase in blood flow to muscles and decrease in splanchnic perfusion – as well as metabolic effects such as lipolysis, gluconeogenesis and glycogenolysis – to provide substrates for this action to occur.4
Adrenal incidentaloma
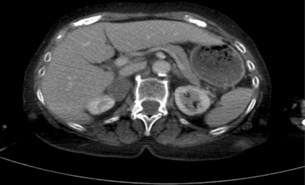
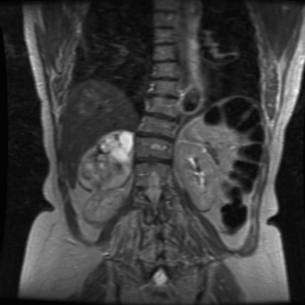
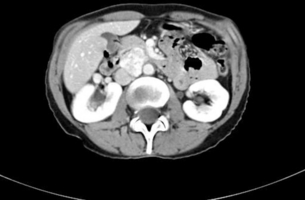
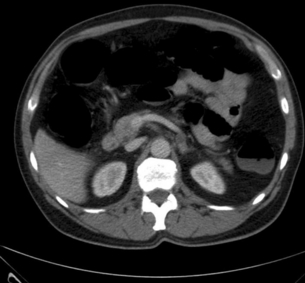
Consider the management of a 65-year-old woman who has an incidental 3-cm right adrenal adenoma on unenhanced computed tomography (CT) scan (see Fig. 3.4) performed following a fall in which she suffers a femoral shaft fracture. There is a recent history of hypertension. After surgery for her fracture a bone mineral density scan shows severe osteoporosis. Biochemical evaluation including 24-hour urinary free cortisol, plasma free metanephrines, plasma aldosterone and renin actitivity are unremarkable, but plasma cortisol fails to suppress after an overnight low-dose dexamethasone test and plasma ACTH is suppressed.
Definition and incidence
Incidentalomas are tumours identified inadvertently during investigation of an unrelated condition. Overall adrenal incidentalomas are present on 4% of abdominal CT scans,5 though their incidence increases with age from < 1% in the third decade to 7% in the eighth decade of life.6 The current widespread use of cross-sectional abdominal imaging has created the clinical dilemma of how to manage these incidentalomas. The objective is to identify those that are potentially malignant, or harmful due to excessive hormonal secretion.
Aetiology
The majority of adrenal incidentalomas are benign non-functioning adenomas. The incidence of clinically significant adrenal incidentaloma varies between studies, but about 15% are found to secrete excess hormones.7 Adrenocortical carcinoma and phaeochromocytoma each account for 5–10%.8 Excluding studies of oncology patients, metastases account for a small (2.5%) proportion of incidentalomas. Lung, breast, ovary, kidney and melanoma are the most common primary tumours that metastasise to the adrenal gland.9 The remaining incidentalomas are due to myleolipoma, haemorrhage, adrenal cortical cyst, ganglioneuroma, neuroblastoma, lymphoma, congenital adrenal hyperplasia, haemangioma, granulomatous disease and other rare diagnoses.7
Biochemistry
Biochemical investigation needs to exclude phaeochromocytoma by measuring fractionated urinary metanephranes or plasma free metanephranes. The diagnosis of Cushing’s syndrome is more difficult to exclude as more subtle forms, so-called subclinical Cushing’s syndrome, occur and 24-hour urinary free cortisol, low-dose (1 mg) overnight dexamethasone suppression test and midnight salivary cortisol may all be required to confirm or exclude the diagnosis. Primary hyperaldosteronism is suggested in hypertensive patients with elevated plasma aldosterone:renin activity ratio >20. Hypokalaemia is present in only half the patients with primary hyperaldosteronism. Serum DHEAS and 17-hydroxyprogesterone are measured to exclude adrenal androgen hypersecretion that occurs in some adrenocortical carcinomas or, when bilateral adrenal masses are present, congenital adrenal hyperplasia.10
Imaging
Adrenal cysts, myelolipoma and haemorrhage have characteristic features on CT enabling diagnosis; differentiating adrenal adenoma from malignant tumours, phaeochromocytoma and other causes of incidentaloma can be more problematic, as some overlap occurs in the appearance of these lesions on cross-sectional imaging.7
Most adrenal adenomas are lipid rich and appear as low-density (or attenuation) masses on unenhanced CT. They are characteristically homogeneous with a regular outline, and adrenal incidentaloma <4 cm in size with these features, and an attenuation value of <10 Hounsfield units on unenhanced CT require no further diagnostic imaging.12
Malignant lesions, phaeochromocytoma and up to 30% of adenomas are lipid poor and have high attenuation on unenhanced CT. Other features that increase the risk of malignancy include heterogeneity, irregular outline and size >4 cm.13 CT with contrast enhancement and washout characterise these lesions. Both adrenocortical carcinomas and medullary tumours show rapid contrast enhancement but adenomas have a rapid washout of contrast, resulting in an attenuation value of <30 Hounsfield units at 10 minutes, in contrast to malignant adrenocortical tumours and phaeochromocytoma where contrast is retained.14
Magnetic resonance imaging (MRI) can distinguish between benign and malignant tumours in 90% of cases. Malignant tumours usually have a higher fluid content than lipid-rich adenomas, which results in high signal intensity on T2-weighted MRI. Loss of signal intensity on out-of-phase MRI with chemical shift imaging, also due to the high lipid content of adenomas, can be used to distinguish benign from malignant tumours.15 Homogeneous enhancement following intravenous gadolinium-enhanced MRI is characteristic of adrenal adenoma.
[18F]Fluorodeoxyglucose (FDG) positron emission tomography (PET) combined with CT is highly accurate at differentiating benign from malignant incidentalomas and is helpful in lesions with an indeterminate appearance on CT or MRI.20 Malignant tumours characteristically have high [18F]FDG uptake compared to adenomas.
Management
The natural history of adrenal incidentalomas is unknown. Although approximately a quarter of incidentalomas increase in size with time, the risk of malignant change is thought to be low. Annual surveillance imaging is often performed though there is limited evidence to recommend the optimum length or frequency of radiological follow-up. To avoid excessive radiation exposure that in itself may be tumour inducing, MRI is the preferred method for serial surveillance. Secretory hyperfunction may also develop during follow-up in up to 9% of incidentalomas, though further data are needed to establish the benefit, length and frequency of follow-up hormonal evaluation.17,18
Although clinically evident Cushing’s syndrome is rare in patients presenting with incidentalomas, more subtle elevations of cortisol secretion, termed ‘subclinical’ Cushing’s syndrome, are detected in 5–20%.19 These patients may exhibit some signs of glucocorticoid excess such as obesity, hypertension, diabetes mellitus or osteoporosis, but lack the characteristic features of the full-blown syndrome. Often one or more of the biochemical tests for Cushing’s syndrome are normal in patients with subclinical Cushing’s syndrome, commonly the urinary free cortisol. The low-dose overnight dexamathasone test or late-night salivary cortisol are more sensitive tests, and progression to full-blown Cushing’s syndrome is unpredictable.17,18,19,21
Efforts to establish evidence-based management of subclinical Cushing’s syndrome are hampered by any agreed diagnostic criteria and the lack of randomised studies comparing medical to surgical management. Uncontrolled surgical series have reported improvements in hypertension, obesity and diabetes mellitus following adrenalectomy in patients with subclinical Cushing’s syndrome, though these studies did not compare surgery with best medical management.10
Adrenocortical carcinoma
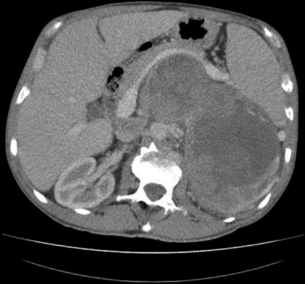
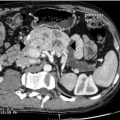
Consider the management of a 50-year-old man who on investigation for abdominal discomfort is found to have a large non-functioning adrenal mass (see Fig. 3.5).What is the differential diagnosis? Staging investigations are negative. What is the surgical approach and which adjuvant therapy is indicated?
Incidence and aetiology
Adrenocortical carcinoma (ACC) is one of the most lethal endocrine tumours but fortunately is rare, with an incidence of 1–2 per million per year. Most ACC is sporadic in origin but it may be associated with a number of rare syndromes, including: multiple endocrine neoplasia type 1 due to mutation in a gene on chromosome 11 encoding the protein menin; Beckwith–Wiedemann syndrome (exophthalmos, macroglossia and nephromegaly) in childhood due to mutation of two genes on chromosome 11 leading to overproduction of insulin-like growth factor (IGF) 2; and Li–Fraumeni syndrome (breast carcinomas, osteosarcomas and brain tumours) due to inactivating mutations of tumour suppressor gene p53 on chromosome 17.22
Clinical features
The majority of ACCs are hormonally functional and present with Cushing’s syndrome, primary hyperaldosteronism, virilisation or feminisation. Secretion of multiple hormones is characteristic of ACC. In our own series, in which the frequency of clinical presentation was quite typical, 57% of ACCs were functioning, 48% presented with Cushing’s syndrome (18% secreted multiple hormones – Cushing’s syndrome and virilisation), 6% presented with pure virilisation and 3% feminisation; 43% were non-functioning, of which 34% had symptoms (abdominal discomfort) and 9% were found incidentally.23
Biochemistry
Biochemical evaluation to establish the steroid secretory profile of ACC should be undertaken as described elsewhere in this chapter. Certain biochemical findings are characteristic of ACC: excess secretion of multiple steroid hormones, particularly glucocortocoids and androgens, elevated DHEAS, raised oestradiol in men and postmenopausal women, and as enzyme function is often defective in ACC, accumulation of steroid precursors such as 17α-hydroxyprogesterone and androstenedione.24 Phaeochromocytoma should also be excluded biochemically, as this is not possible on imaging alone.
Imaging
ACCs typically appear as large (usually >5 cm), heterogeneous lesions with an irregular margin on unenhanced CT. They are denser than lipid-rich adenomas so they have attenuation >10 Hounsfield units on unenhanced CT and display delayed washout of contrast. Local invasion or distant metastases may be apparent on CT. The incidence of malignancy, which increases with size, is 2% in adrenal masses < 4 cm, 6% for those 4–6 cm and 25% in lesions >6 cm.13,25
MRI can be helpful as ACCs have high signal intensity on T2-weighted images, heterogeneous enhancement and delayed washout with gadolinium contrast, and do not exhibit loss of signal intensity on out-of-phase chemical shift imaging seen with lipid-rich adenomas.15 MRI, in addition to an inferior vena cava (IVC) contrast study, is useful if vascular invasion or tumour thrombosis is suspected.
When adrenal tumours cannot be characterised on conventional cross-sectional imaging, [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) with CT has a sensitivity of 100% and specificity of 88% in differentiating malignant from benign lesions. FDG-PET may also be useful to detect recurrent or metastatic disease not seen on CT.13,20
Diagnosis and staging
Percutaneous biopsy is not recommended for the work-up of ACC due to the difficulty in differentiating primary malignant from benign adrenal disease, and the risk of seeding tumour cells along the biopsy track. The only absolute criterion for the diagnosis of ACC is presence of extensive invasion of local structures or metastases. The most widely used algorithm for the diagnosis of ACC was described by Weiss and is based on nine histological features, the presence of three or more indicating malignant potential.26 Ki67 immunohistochemistry may also help differentiate benign from malignant adrenocortical tumours.24
The 2004 International Union Against Cancer TNM stages ACC according to size, presence of lymph node metastases, local invasion and distant metastases, and is based on the original staging system described by MacFarlane in 195827 and Sullivan et al. in 1978.28 The European Network for the Study of Adrenal Tumours (ENSAT) has recently proposed further refinements of this staging system, which is the basis for prognostic and treatment stratification in ACC, as well as enabling comparison of outcomes between treatment centres.29
Treatment
Surgery offers the only potential cure, and prognosis in patients with incompletely resected ACC remains poor, due to lack of response of ACC to systemic therapy. For tumour resection a transabdominal or, if necessary, thoraco-abdominal approach should enable good access to allow vascular control of the aorta, IVC and renal vessels. En bloc resection of the perinephric fat, regional lymph nodes and adjacent organs including kidney, pancreas, liver or spleen maybe required to achieve complete resection. Following surgery, close radiological and biochemical follow-up is undertaken as early identification and re-excision of recurrent disease may improve survival. Palliative surgery may also have a role, particularly in functional tumours.25
Medical
Mitotane potentiates the cytotoxic activity of some chemotherapeutic drugs so combinations can be used in advanced and metastatic disease. Overall, the results of cytotoxic chemotherapy for ACC are disappointing, with the best-reported (partial) response rate being 49%.37 The results of the FIRM-ACT trial to establish the optimum chemotherapy regime should shortly be available. Novel systemic therapies such as IGF receptor and tyrosine kinase inhibitors are currently under investigation.38
Prognosis
The reported overall 5-year survival for ACC varies between 16% and 38% depending on the stage at diagnosis. Median survival following non-curative surgery or diagnosis of metastatic disease is less than 12 months.24
Phaeochromocytoma and paraganglioma
Consider the management of a 60-year-old hypertensive woman investigated for symptoms of palpitations. Twenty-four-hour electrocardiogram and echocardiography are unremarkable and she is commenced on beta-blockers. Subsequently overnight urinary fractionated metanephrines and normetanephrines are found to be markedly elevated. Anatomical and functional localisation studies show a right adrenal phaeochromocytoma (see Fig. 3.9).
Incidence and aetiology
Phaeochromocytoma and extra-adrenal paraganglioma are tumours derived from catecholamine-producing chromaffin cells of neural crest origin arising in either the adrenal medulla (phaeochromocytoma) or extra-adrenal autonomic ganglia (paraganglioma). Phaeochromocytoma has an incidence of 3–8 per million per year and usually presents in middle age, though hereditary forms occur at a younger age. Phaeochromocytoma is very rare in children. Studies suggest 0.05% of all autopsies harbour an undiagnosed phaeochromocytoma and it is probable that this rare tumour is under-recognised in life.39 Traditionally termed the ‘10% tumour’ (10% bilateral, extra-adrenal, familial or malignant), this description has now been challenged by recent advances in genetics and diagnosis.40
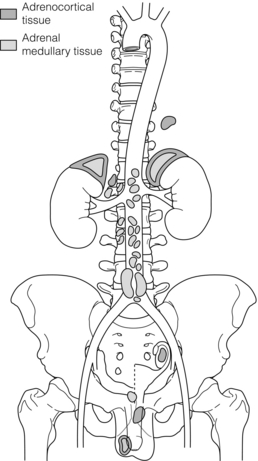
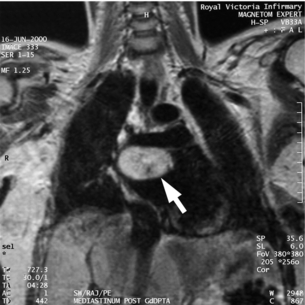
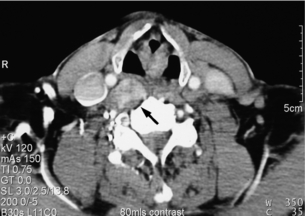
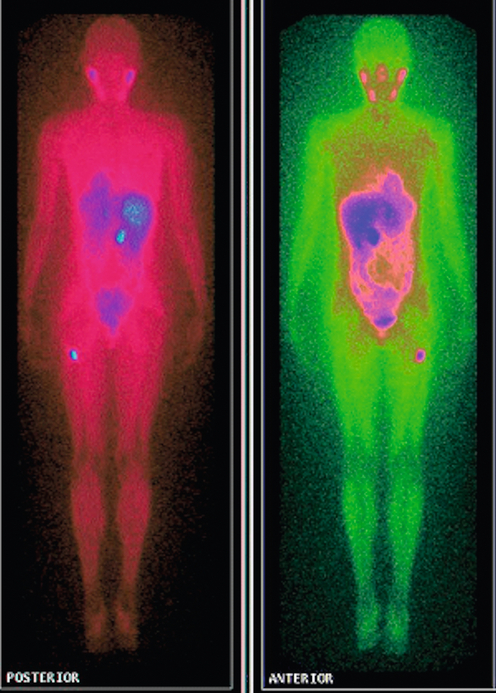
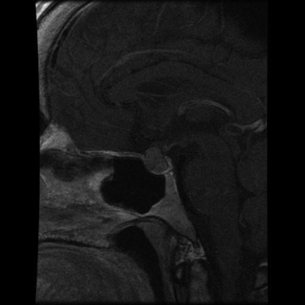
The majority of extra-adrenal sympathetic paragangliomas occur in the abdomen, either arising from sympathetic ganglia in the organ of Zuckerkandl, which is situated along the lower abdominal aorta and its bifurcation, or around the renal hilum (Fig. 3.6). Less common sites for paraganglioma include the urinary bladder or the mediastinum, where they may even arise in the nerves supplying the myocardium (see Fig. 3.7). One-quarter of phaoechromocytomas managed in our unit are extra-adrenal, and a similar high proportion of extra-adrenal tumours are reported from other endocrine surgical centres, though this may reflect referral bias.41 In contrast to sympathetic paraganglioma, those arising from parasympathetic ganglia occur mostly in the head and neck (see Fig 3.8), and rarely produce catecholamines.
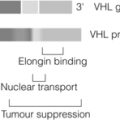
Although the majority of phaeocytochromas are sporadic, it is now thought that up to one-third of patients with phaeocytochromas carry germ-line mutations in predisposing genes, three of which are well known: RET (multiple endocrine neoplasia types 2A and 2B), VHL (von Hippel–Lindau syndrome) and NF1 (neurofibromatosis type 1). The remaining mutations occur in genes (SDHB and C) encoding subunits of the succinate dehydrogenase (SDH) enzyme responsible for the ‘paraganglioma–phaeocytochroma syndrome’.42
Phaeochromocytomas occur in approximately half of patients with multiple endocrine neoplasia type 2 (depending on the codon mutation), in association with medullary thyroid carcinoma (MTC) and hyperparathyroidism in multiple endocrine neoplasia type 2A (MEN2A) and multiple mucosal ganglioneuromas, megacolon and marfanoid habitus in MEN2B. Germ-line mutations in VHL, a tumour suppressor gene that regulates the accumulation of hypoxia-induced proteins and angiogenesis, lead to the rare VHL syndrome, characterised by central nervous system haemangioblastomas, renal cell cancer, cysts of kidney, testis and pancreas, and phaeochromocytoma (in up to a third). The clinical features of NF1 (also known as von Recklinghausen’s disease) include multiple neurofibromas, café-au-lait spots, skin-fold freckling and iris hamartomas. Phaeochromocytoma occurs in < 5% of NF1 patients.43
Hereditary mutations in the SDH genes lead to failure of oxidative phosphorylation, the process by which adenosine triphosphate (ATP) is produced in mitochondria via the citric acid (Kreb’s) cycle. The SDH genes encode succinate dehydrogenase, a key component of aerobic glycolysis, ‘oxidative stress’ and accumulation of pro-oxidants leading to DNA damage. This is thought to underlie the pathogenesis of phaeochromocytoma and paragangliomas.4 Well-recognised genotype–phenotype correlations are seen with SDH mutations and, in contrast to RET, VHL and NF1, patients with mutations in the SDH genes commonly present with paragangliomas. SDHB carriers characteristically present at a young age with solitary malignant abdominal paraganglioma, whereas SDHD carriers are more likely to present with multiple paragangliomas in the head and neck, abdomen and adrenal gland.44
Clinical presentation
The clinical symptoms and signs of phaeochromocytomas are numerous and are due to the paroxysmal excessive secretion of catecholamines. The classic symptoms are headaches, palpitations and sweating, but pallor, nausea, weight loss, tiredness and anxiety commonly occur. The characteristic feature of all presenting symptoms is that they are intermittent or paroxysmal in nature, which makes diagnosis difficult as the patient may be perfectly well between symptomatic episodes. Postural changes, exercise and anxiety often provoke symptoms. Hypertension is the commonest sign, along with diabetes mellitus. ‘Phaeochromocytoma crisis’ due to massive secretion of catecholamines into the circulation may present with sudden death, arrhythmia, heart failure, multi-organ failure or cerebrovascular accident. Anaesthesia, trauma, biopsy, haemorrhage or tumour manipulation during surgery may precipitate these crises. Phaeochromocytoma may also be found incidentally on cross-sectional imaging – approximately 5% of adrenal incidentalomas are phaeochromocytomas. Typically the diagnosis of phaeochromocytoma is delayed by several years as the symptoms overlap many common endocrine, cardiovascular or neurological disorders. The key to diagnosis is staying alert to the possibility of the disease.43
Imaging
Localisation should only be performed once the biochemical diagnosisis is confidently established. The high sensitivity of CT (85–94%) and MRI (93–100%) for the diagnosis of phaeochromocytoma enables accurate anatomical localisation.49 Phaeochromocytomas usually appear as a homogeneous mass with a soft-tissue density of 40–50 Hounsfield units (HU) on unenhanced CT, though larger tumours may appear heterogeneous due to haemorrhage, necrosis, calcification or cyst formation. Concerns about provoking a phaeochromocytoma crisis with ionic contrast medium, necessitating α-adrenergic receptor blockade prior to contrast-enhanced CT, have been addressed as it has been demonstrated that non-ionic contrast does not provoke catecholamine secretion.50
of the relationship to surrounding vessels (see Fig. 3.9) and may be preferred for phaeochromocytoma in the para-aortic and mediastinal regions to assess vessel invasion. MRI is also used to image phaeochromocytoma in children and pregnant women.49
The cost and availability of [18F]DA and [18F]DOPA currently limit their widespread use. Occasionally rapidly growing de-differentiated malignant or metastatic phaeochromocytomas fail to take up any of these radiopharmaceuticals and in these circumstances somatostatin receptor scintigraphy (Octreoscan) or [18 F]fluorodeoxy-D-glucose (FDG) with PET may localise the tumour.39,48,49
Medical management
Once the biochemical diagnosis of phaeochromocytoma is established, pharmacological control of the adverse effects of circulating catecholamines should take priority over localisation studies. Phenoxybenzamine, a long-acting non-competitive and non-selective α-adrenergic receptor antagonist, is the agent most frequently used. Phenoxybenzamine is commenced at a dose of 20 mg twice daily, increased by 10-mg increments with monitoring of arterial blood pressure until postural hypotension occurs. This can usually be accomplished as an outpatient over a 2- to 4-week period. Side-effects of phenoxybenzamine include headache, nasal stuffiness, somnolence and reflex tachycardia.51 Alternatives to phenoxybenzamine have their proponents and include the selective, competitive α1-adrenergic receptor agonists doxazosin52 and urapidil,53 as well as the calcium channel blocker nicardipine.54 Beta-blocking drugs may also be required to counteract tachycardia and arrhythmia, but should not be started until alpha blockade is complete to avoid unopposed α-adrenergic receptor stimulation that may cause hypertensive crisis or pulmonary oedema. The period of preoperative blockade allows intravascular volume expansion to occur and cardiomyopathy secondary to chronic hypertension to resolve. A cautious approach to optimising the patient’s condition for surgery is advised and rapid blockade programmes, although advocated by some, are seldom necessary.51
Prior to operation placement of intra-arterial and central venous catheters is essential to detect and correct potential hypertensive episodes perioperatively. Careful liaison between surgeon and anaesthetist is essential and undue handling of the tumour is to be avoided. Intravenous administration of either the short-acting vasodilator sodium nitroprusside, calcium channel blocker nicardipine or α-adrenergic receptor anatagonist phentolamine are required for intraoperative hypertensive episodes as well as beta-blockers for tachycardia and arrhythmia.51
Once the tumour is devascularised a significant fall in catecholamines will occur, potentially resulting in hypotension; thus, significant volume replacement and/or inotropes may be required preoperatively, and in the high-dependency or intensive care unit postoperatively. In addition, regular monitoring of the blood glucose is advised as the metabolic consequences of removing a phaeochromocytoma include hypoglycaemia due to sudden withdrawal of the lipolytic, glycolytic and glycogenolytic effects of catecholamines that were induced by the phaeochromocytoma.51
Surgical management
Laparoscopic excision of abdominal extra-adrenal paragangliomas is undertaken in our unit, though paragangliomas situated in the para-aortic region are usually approached by open surgery, as they may be intimately related to or invade the aorta. Subtotal adrenalectomy may have a role to avoid the need for lifelong steroid dependence in patients with multiple, bilateral hereditary phaeochromocytomas.57
Case study 4
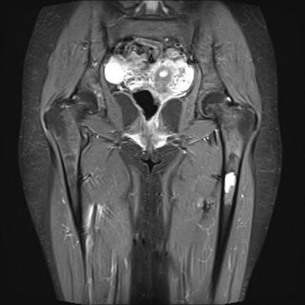
Investigation of abdominal and leg pain in a 60-year-old woman identified a pre-aortic mass (see Fig. 3.10). What is the differential diagnosis based on imaging? Biochemical work-up established the diagnosis of an abdominal paraganglioma. Functional localisation and cross-sectional imaging identify a left femoral metastasis (see Figs 3. 11 and 3.12). What would your management be?
Malignant phaeochromocytoma
The incidence of malignancy is higher in large phaeochromocytomas and extra-adrenal paraganglioma, and SDHB mutation carriers have a particularly high risk of malignancy. There are no histopathological features that reliably distinguish benign from malignant phaeochromocytoma and so malignancy is based on the confirmation of metastatic disease, which usually occurs in lymph nodes, liver, lung or bone.58
Histological scoring systems such as the Phaeochromocytoma of the Adrenal gland Scoring Scale (PASS) have been devised in order to predict malignancy. Ki67 proliferation index may be elevated in malignant phaeochromocytoma.58 Recently, a panel of genes identified by genome-wide expression profiling has been identified that may distinguish benign from malignant tumours, though these results need further validation.59
There are a number of treatment options for metastatic disease, though none is curative. Symptom relief can be achieved by tumour debulking, inhibition of catecholamine synthesis, and α- and β-adrenergic receptor blockade. External beam radiotherapy, chemotherapy, radiofrequency ablation and transcatheter arterial embolisation may have a role. Therapeutic doses of [131I]MIBG can produce symptomatic and hormonal improvement as well as tumour regression or stabilisation.39
Cushing’s syndrome
A 40-year-old woman presents with classic features of Cushing’s syndrome. Biochemical work-up confirms Cushing’s disease and MRI shows a pituitary adenoma (see Fig. 3.15). Trans-sphenoidal surgery to the pituitary fossa is undertaken but the disease persists. Repeat biochemical work-up, including inferior petrosal sinus sampling and MRI, shows an incompletely excised functioning pituitary adenoma. Further trans-sphenoidal surgery still does not control the disease. Consider the further management.
Definition and aetiology
The most common cause of hypercortisolism is exogenous administration of steroids. Endogenous Cushing’s syndrome is more common in women than men and the majority (80%) is ACTH dependent, due to either pituitary (70%) or ectopic (10%) origin; ACTH-independent (or adrenal) Cushing’s is usually due to adrenocortical adenoma or carcinoma and rarely to ACTH-independent macronodular adrenal hyperplasia (AIMAH) or primary pigmented nodular adrenocortical disease (PPNAD).60
Clinical features
The classic features of florid Cushing’s syndrome include centripetal obesity, buffalo hump, moon faces, facial plethora, red–purple striae, hirsutism, loss of libido, menstrual irregularity, depression or psychosis, proximal myopathy and easy bruising (see Figs 3.13 and 3.14). Myopathy, plethora, striae and easy bruising in particular suggest the diagnosis of Cushing’s syndrome.
Biochemical diagnosis
1. Twenty-four-hour urinary free cortisol (UFC). This reflects the average plasma cortisol level, and levels of UFC more than four times greater than normal are diagnostic of Cushing’s syndrome. Twenty-four-hour UFC is less sensitive at diagnosing subclinical Cushing’s syndrome and up to three 24-hour urine samples should be analysed if suspicion of Cushing’s syndrome is high, as glucocorticoid secretion may be intermittent.62
2. Late-night salivary or plasma cortisol. One characteristic of Cushing’s syndrome is loss of the normal circadian rhythm of cortisol secretion and a resting midnight plasma cortisol level of >50 nmol/L is 100% sensitive in Cushing’s patients.63 Plasma midnight cortisol is best performed in a dedicated inpatient investigation unit, as the level of patient stress on an acute medical ward may lead to false-positive results. More conveniently, a late-night saliva sample taken at home may be used, as salivary and plasma cortisol levels are closely correlated. Late-night salivary cortisol levels have high sensitivity and specificity (>92%) for the diagnosis of Cushing’s syndrome.61
3. Low-dose overnight dexamethasone suppression test. Dexamethasone suppression tests work by exploiting loss of negative-feedback loop for cortisol secretion seen in Cushing’s syndrome. Administration of 1–2 mg dexamethasone (a synthetic glucocorticoid that binds to the cortisol receptors in the pituitary inhibiting secretion of ACTH) at midnight should suppress the plasma cortisol level taken at 9 a.m. the following morning. Using a plasma cortisol cut-off of 50 nmol/L, the sensitivity and specificity of the low-dose overnight dexamethasone suppression test for the diagnosis of Cushing’s syndrome is 95% and 80%, respectively.61 Alternatively, a 48-hour 2 mg/day low-dose dexamethasone suppression test may be used, which may have greater specificity than the 1 mg overnight dexamethasone suppression test.
Once the diagnosis of Cushing’s syndrome has been confirmed, its cause must be established, i.e. is it ACTH dependent or not? Measuring plasma ACTH on more than one occasion using a two-site immunometric assay achieves this, as ACTH is suppressed by negative feedback in ACTH-independent (adrenal) Cushing’s syndrome to levels that are undetectable or low. Persistently elevated plasma ACTH levels indicate ACTH-dependent Cushing’s syndrome. Plasma ACTH is usually higher in ectopic ACTH than in pituitary disease.62
ACTH-dependent Cushing’s syndrome
The majority of ACTH-dependent Cushing’s syndrome is pituitary in origin and was first described by Harvey Cushing in 1932, now referred to as Cushing’s disease. Ectopic ACTH production may be due to carcinoid tumours (particularly bronchial), medullary thyroid carcinoma, small-cell lung cancer, neuroendocrine tumours and phaeochromocytoma.64
Differentiating pituitary from ectopic ACTH-dependent Cushing’s syndrome is challenging and is best conducted in specialist endocrinology centres. History and examination may suggest the aetiology. Tumour markers such as urinary 5-hydroxyindoleacetic acid (5-HIAA), serum calcitonin, chromagranin A and gastrointestinal neuroendocrine hormones may be helpful in suspected ectopic ACTH secretion. The high-dose dexamethasone suppression test, corticotropin-releasing hormone test, pituitaryMRI and inferior petrosal sinus sampling may establish the cause of ACTH-dependent disease.60,62
1. High-dose dexamethasone suppression test. This test is based on the fact that pituitary tumours usually retain some negative-feedback control that is totally lost in patients with an ectopic source of ACTH. In a patient with Cushing’s syndrome a reduction in plasma cortisol to < 50% of the baseline value after a single dose of 8 mg or 2 mg dexamethasone, given 6-hourly for 48 hours, suggests pituitary-driven disease. In contrast, no significant drop in plasma cortisol should be seen in ACTH-independent Cushing’s or ectopic ACTH-dependent Cushing’s.
2. Corticotropin-releasing hormone (CRH) test. Cushing’s patients with pituitary-driven disease usually respond to intravenous administration of 100 μg human or ovine CRH by increasing ACTH and cortisol secretion, in contrast to those with adrenal tumours or ectopic ACTH secretion. Occasionally, patients with ectopic ACTH secretion have a positive CRH test, so reducing this test’s specificity.62
3. Inferior petrosal sinus sampling (IPSS). IPSS is an invasive technique that involves radiological placement of a catheter in the inferior petrosal sinus (IPS) to measure ACTH produced from the pituitary gland. Peripheral plasma levels of ACTH are measured simultaneously to detect any concentration gradient. Both sinuses are sampled as ACTH may only be secreted into a single sinus, and CRH should be infused to avoid the possibility that the ACTH is being produced in an episodic fashion. Basal IPS to peripheral plasma ACTH ratio of >2 and CRH-stimulated IPS to peripheral ratio >3 is consistent with Cushing’s disease. Significant vascular and neurological complications are uncommon but have been reported with this technique.65
Imaging
Pituitary MRI is the cross-sectional study of choice for Cushing’s disease (see Fig. 3.15), though an adenoma is found in only 50–60% of patients with the disease as microadenomas are not visible on cross-sectional imaging.66 Furthermore, non-functioning pituitary incidentalomas are present in up to 10% of the population, so interpretation of cross-sectional imaging must be done carefully in conjunction with biochemical tests.67
Cross-sectional imaging of the neck, chest, abdomen and pelvis should be used to look for a cause when ectopic ACTH secretion is suspected. If the primary is not found on CT or MRI then somatostatin receptor scintigraphy (Octreoscan) may have a role, though often the causative lesion remains occult.68
ACTH-independent Cushing’s syndrome
ACTH-independent Cushing’s syndrome is due to adrenal disease, and cross-sectional imaging with CT or MRI scan will usually identify an adrenal lesion. There is an overlap in the appearances of adrenal adenoma and carcinoma on CT imaging, though the risk of malignancy is increased in adrenal lesions that have high attenuation, irregular outline, delayed washout of contrast, are heterogeneous or >4 cm in size.13,14 The higher water content of adrenal carcinoma is exploited in MRI using gadolinium enhancement or chemical shift imaging to differentiate benign from malignant lesions.15 Cushing’s syndrome due to adrenal carcinomas is often characterised by co-secretion of multiple hormones, particularly androgens.
ACTH-independent macronodular adrenal hyperplasia (AIMAH) is an uncommon cause of Cushing’s syndrome characterised by bilateral nodular adrenal hyperplasia that is usually apparent on cross-sectional imaging. A similar appearance is seen in chronic Cushing’s disease when prolonged stimulation of the adrenal glands by high levels of circulating ACTH occurs. Primary pigmented nodular adrenocortical disease (PPNAD) is a rare condition that may be associated with other symptoms of Carney’s complex (see Chapter 4) such as myxomas, blue naevi and pigmented lentigines. The adrenal glands may appear normal on cross-sectional imaging in PPNAD.63
Management
ACTH-dependent Cushing’s syndrome
Pituitary surgery will not be dealt with in detail in this book. Surgical cure rates following selective pituitary adenomectomy via trans-sphenoidal surgery (TSS) are lower for larger adenomas, though a further attempt at TSS may be considered if the primary surgery is unsuccessful (which it may be in up to 40%). Re-operative TSS risks pituitary failure (panhypopituitarism) and so alternative strategies such as external beam irradiation, stereotactic radiosurgery (gamma knife)63 or bilateral laparoscopic adrenalectomy should be considered.69 Radiotherapy to the pituitary has a delayed effect on circulating cortisol levels and also risks panhypopituitarism.
Bilateral adrenalectomy results in permanent adrenal failure and lifelong glucocorticoid and mineralocorticoid replacement to avoid Addisonian crises. The patient should always wear an alert badge and carry a shock pack of glucocorticoids for rapid treatment of stress, such as infection and trauma. The results of bilateral adrenalectomy for the treatment of pituitary ACTH-dependent Cushing’s syndrome are encouraging, with a remission rate of 95%. Quality of life following laparoscopic bilateral adrenalectomy for Cushing’s disease has been shown to be comparable to that following curative TSS.70 Following bilateral adrenalectomy for Cushing’s disease, loss of negative feedback from circulating cortisol may result in hyperpigmentation, enlargement of the ACTH-secreting tumour and increased secretion of ACTH; this is called Nelson’s syndrome and its incidence may be reduced, or at least delayed, with prior radiotherapy to the pituitary.71
Treatment of the primary tumour in Cushing’s syndrome due to ectopic ACTH secretion is preferable but often not possible, and so controlling the symptoms becomes paramount. This may be achieved medically by the use of ketoconazole, metyrapone, aminoglutethimide or mitotane, which inhibit cortisol production or secretion.60 However, even in patients with limited life expectancies, bilateral adrenalectomy may still be the best way to achieve good palliation, particularly if this can be achieved laparoscopically.
Primary hyperaldosteronism
A 30-year-old man with a strong family history of ischaemic heart disease is investigated for hypertension and found to have an elevated plasma aldosterone and suppressed renin activity off antihypertensive medication. CT scan of the abdomen shows a 1.2-cm left adrenal lesion (see Fig. 3.16). Plan the management of this patient.
Definition and aetiology
In 1954 Jerome Conn first described an aldosterone-producing adrenocortical adenoma in a young woman with hypertension; this has since been referred to as Conn’s syndrome. Primary hyperaldosteronism was considered to be uncommon, but is now recognised to occur in 5–13% of hypertensive patients.72 Bilateral adrenal hyperplasia and aldosterone-producing adrenocortical adenoma account for the majority of cases; other rare causes include aldosterone-secreting adrenocortical carcinoma, unilateral adrenal hyperplasia and familial glucocorticoid-suppressible aldosteronism.73
Biochemical diagnosis
Primary hyperaldosteronism should be considered in all hypertensive patients presenting with hypokalaemia, severe or treatment-resistant disease, patients aged < 40 years or those with adrenal incidentaloma.73 Hypertension is usually the only clinical sign. Hypokalaemia is absent in the majority of patients with primary aldosteronism but when present may lead to muscle weakness, cramps, palpitations and polyuria.
Plasma aldosterone concentration (PAC) and plasma renin activity (PRA) should be measured simultaneously in an ambulatory patient suspected of primary aldosteronism. An elevated PAC, suppressed PRA and PAC:PRA ratio >20–30 are consistent with the diagnosis,73 though it is important to note that several drugs that affect the renin–angiotenson–aldosterone axis, including aldosterone antagonists, beta-blockers, calcium-channel blockers and angiotensin-converting enzyme inhibitors, can interfere with the results and so may need to be discontinued prior to biochemical tests.74 Confirmatory tests with the fludrocortisone suppression test or by oral/intravenous saline loading to demonstrate that aldosterone secretion is inappropriate and non-suppressible may be required in borderline cases.72
Imaging
Following biochemical confirmation, localisation with CT scan (or MRI) is undertaken. Aldosterone-producing adrenocortical adenomas are characteristically solitary, homogeneous and < 2 cm in size. Adrenocortical carcinoma usually appears as heterogeneous lesions >4 cm.9 CT in adrenal hyperplasia may be normal or show nodularity. Primary hyperaldosteronism may also be falsely attributed to non-functioning adrenal incidentaloma, which are common in older patients, or to a solitary dominant adrenal nodule seen in patients with bilateral adrenal hyperplasia.6
Aldosterone and cortisol are measured in blood taken from a catheter placed under radiological guidance into the adrenal vein via the inferior vena cava. The side of aldosterone hypersecretion can be established by AVS with a sensitivity of 95% and specificity of 100%. In cases of bilateral adrenal hyperplasia this will also allow the dominant side to be identified.73
Management
When unilateral disease is confirmed on preoperative investigations, minimally invasive surgery is the preferred option for benign tumours, either transperitoneal or retroperitoneal.77 Hypertension and hypokalaemia should be corrected prior to surgery. Postoperatively, hypertension improves in the majority but only resolves completely in about 50%. A family history of hypertension, need for multiple antihypertensive medications, age >50 years and long duration of hypertension predict postoperative antihypertensive requirement.78
Medical management with aldosterone antagonists such as spironolactone or eplerenone can be an effective alternative in those patients who are unfit or decline surgery.79 Spironolactone has a number of side-effects including gynaecomastia, loss of libido, menstrual irregularity and erectile dysfunction that limit its use, though these do not occur with eplerenone.72 The risks of malignancy need to be considered before advocating primary medical management for unilateral disease and the long-term cost of medication is higher than surgery.80 Bilateral adrenal hyperplasia should be managed medically as surgery is unlikely to be curative. Patients with glucocorticoid-remediable aldosteronism should be treated with steroids to suppress ACTH secretion from the pituitary.72
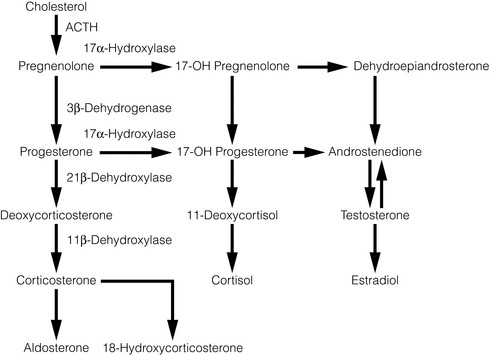
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) includes a group of autosomal recessive disorders characterised by deficiency in end steroid production and overproduction of steroid intermediaries due to an enzyme deficiency in the steroid synthetic pathway from cholesterol. Failure of negative feedback on the pituitary leads to an increase in plasma ACTH levels and adrenal hyperplasia. 21-Hydroxylase deficiency accounts for the majority of cases, which results in glucocorticoid and mineralocorticoid deficiency with overproduction of adrenal androgens leading to ambiguous genitalia in females, salt loss and hyperkalaemia. Medical management of CAH includes replacement of the steroid beyond the enzyme block, which restores the negative-feedback loop, so reducing ACTH and adrenal androgen levels. Patients with CAH in whom medical treatment fails to control hyperandrogenism, or iatrogenic hypercortisolism occurs may benefit from bilateral laparoscopic adrenalectomy.81
Neuroblastoma
Neuroblastoma is a malignant tumour of the developing adrenal medulla or paraspinal autonomic ganglia that has an incidence of 10 per million in children. It is the most common solid tumour in childhood. The median age at diagnosis is 17 months and generally the younger the age at presentation, the better the prognosis. Most present as an abdominal mass, though neuroblastoma may arise in the neck, chest or pelvis. Metastases (bone and bone marrow) are commonly present at diagnosis. Differential diagnosis includes lymphoma and Ewing’s sarcoma. Management tailored to age, stage and tumour biology may include surgery, chemotherapy or immunotherapy. [131I]MIBG is a potential therapeutic agent. Operable tumours are in the minority. Children under the age of 12 months may have good long-term survival rates even when presenting with metastatic disease, as spontaneous remission may occur after resection of the primary tumour.82
Adrenalectomy
The operation should nearly always aim for total excision of the affected adrenal gland. Subtotal adrenalectomy is an acceptable operation in certain circumstances, particularly in hereditary bilateral phaeochromocytoma or to remove benign lesions in solitary adrenal glands, as this may avoid the morbidity of long-term dependence on exogenous steroids. Small, solitary, benign, eccentrically situated adrenal lesions are particularly suitable for subtotal adrenalectomy.83
Open adrenalectomy
Following the first successful adrenalectomy for phaeochromocytoma in 1927 by Charles Mayo, open surgery was the standard approach to the adrenal gland, but it has now been superseded by laparoscopic adrenalectomy for most adrenal tumours.77 Open adrenalectomy, if necessary by a thoraco-abdominal incision, remains the approach of choice for malignant adrenal lesions, as it gives excellent access to enable control of major vessels and en bloc resection of adjacent organs, and minimises the risk of recurrence due to tumour disruption and spillage.
Laparoscopic adrenalectomy
Since laparoscopic adrenalectomy was first described by Michel Gagner in 1992,84 it has become widely adopted by surgeons, anaesthetists and patients, and should now be considered the standard technique for adrenalectomy.
Several factors influence the choice of approach for minimally invasive surgery. The lateral (transabdominal) approach is the most popular as it provides a large working space, easy identification of anatomical landmarks, gravity facilitates mobilisation of surrounding organs (such as the spleen) and, in the event of conversion to an open procedure (which occurs in 5%), there is no need to reposition the patient. The lateral (transabdominal) approach is therefore particularly suitable for larger adrenal tumours, which are technically more difficult to dissect.85
The posterior retroperitoneal approach lends itself to smaller, particularly bilateral tumours, and when intra-abdominal adhesions from previous surgery make the transperitoneal approach difficult.86
Although the laparoscopic approach is preferable for most benign adrenal pathologies, an upper size limit of 10–12 cm is generally advised, due to the technical difficulties of dissecting large tumours and their higher risk of malignancy. Laparoscopy has been advocated for large, potentially malignant adrenal tumours, though increased risk of recurrence has been cited as an indication for open surgery in these circumstances.30–33
Laparoscopic right adrenalectomy
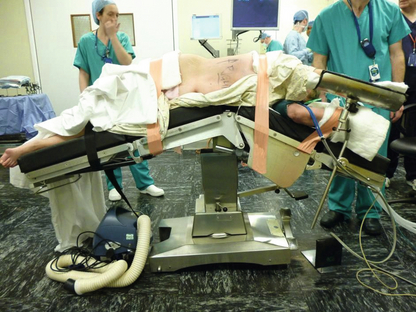
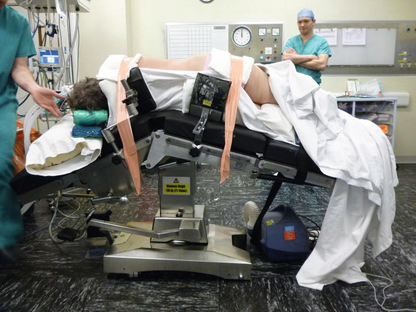
Once the patient has been placed in the correct position (see Figs 3.17 and 3.18), the trocars are introduced in a similar fashion as on the left side. A fourth trocar is more often required on the right to retract the liver. The right triangular ligament of the liver is divided to mobilise the liver, which allows retraction of the liver medially and good exposure of the space between the right adrenal and the inferior vena cava. The peritoneum below the liver and overlying the adrenal gland is then divided above and medial to the right kidney. Once the gland has been identified, it is dissected from the surrounding tissue. It is advisable to dissect the superior pole of the adrenal first, so that the gland remains attached to the kidney and does not migrate further upwards. Again, great care must be taken to ensure that the adrenal vein is identified and controlled prior to division from the vena cava.
Robotic adrenalectomy
Since the first robotic adrenalectomy was performed over a decade ago,87 several centres have now reported their results. The subjective advantages of robotic surgery for the surgeon include the greater range of movement, more degrees of freedom and better visualisation, though it has not been shown whether these translate into better outcomes for the patient. Prospective randomised trials have shown similar outcomes to laparoscopic adrenalectomy but with higher costs and longer operating times for robotic adrenalectomy,88 though operating time may decrease with progression along the learning curve.
References
1. McMinn, R.M.H., Last, R.J. Last’s anatomy: regional and applied, 9th ed. Edinburgh: Churchill Livingstone; 1994. [p. vi].
2. Ross, M.H., Reith, E.J., Romrell, L.J. Histology: a text and atlas, 2nd ed. Baltimore: Williams & Wilkins; 1989.
3. Sadler, T.W., Langman, J., Leland, J., et al. Langman’s medical embryology, 7th ed. Baltimore: Williams & Wilkins; 1995. [p. xi].
4. Pacak, K., Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45(2):65–90. 21615192
5. Bovio, S., Cataldi, A., Reimondo, G., et al, Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. 16699294
6. Kloos, R.T., Gross, M.D., Francis, I.R., et al, Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460–484. 8521790
7. Mantero, F., Terzolo, M., Arnaldi, G., et al, A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637–644. 10690869
8. Terzolo, M., Bovio, S., Pia, A., et al, Management of adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2009;23(2):233–243. 19500766
9. Young, W.F., Jr., Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–610. 17287480
10. Nieman, L.K., Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106–4113. 20823463
11. Mazzaglia, P.J., Monchik, J.M., Limited value of adrenal biopsy in the evaluation of adrenal neoplasm: a decade of experience. Arch Surg. 2009;144(5):465–470. 19451490 This retrospective review of 163 adrenal biopsies demonstrated that only 16% of these showed malignancy in patients with isolated adrenal incidentalomas, compared to 70% of those in patients with a history of non-adrenal primary malignancy. The low negative predictive value and sensitivity of adrenal biopsy limited its value in the work-up of adrenal incidentalomas.
12. Dunnick, N.R., Korobkin, M., Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol. 2002;179(3):559–568. 12185019
13. Boland, G.W., Lee, M.J., Gazelle, G.S., et al, Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998;171(1):201–204. 9648789
14. Pena, C.S., Boland, G.W., Hahn, P.F., et al, Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000;217(3):798–802. 11110946
15. Israel, G.M., Korobkin, M., Wang, C., et al, Comparison of unenhanced CT and chemical shift MRI in evaluating lipid-rich adrenaladenomas. AJR Am J Roentgenol. 2004;183(1):215–219. 15208141
16. Grumbach, M.M., Biller, B.M., Braunstein, G.D., et al, Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003;138(5):424–429. 12614096 This summary from an expert panel convened by the National Institutes of Health sets out current recommendations for the management of adrenal incidentalomas.
17. Barzon, L., Scaroni, C., Sonino, N., et al, Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab. 1999;84(2):520–526. 10022410 This study of 75 patients with adrenal incidentaloma followed up for a median of 4 years showed a risk of mass enlargement or hormonal hyperfunction of 18% and 9.5% at 5 years, though none developed malignancy.
18. Bernini, G.P., Moretti, A., Oriandini, C., et al, Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br J Cancer. 2005;92(6):1104–1109. 15770213
19. Terzolo, M., Bovio, S., Reimondo, G., et al, Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am. 2005;34(2):423–439. 15850851
20. Groussin, L., Bonardel, G., Silvera, S., et al, 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94(5):1713–1722. 19190108
21. Toniato, A., Merante-Boschin, I., Opocher, G., et al, Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg. 2009;249(3):388–391. 19247023 Over a 15-year period, 45 patients with subclinical Cushing’s syndrome were randomised to surgery or conservative management. After mean follow-up of 7 years, greater improvements in diabetes, hypertension, hyperlipidaemia and obesity were observed in the surgical group.
22. Dackiw, A.P., Lee, J.E., Gagel, R.F., et al, Adrenal cortical carcinoma. World J Surg. 2001;25(7):914–926. 11572033
23. Aspinall, S.R., Imisairi, A.H., Bliss, R.D., et al, How is adrenocortical cancer being managed in the UK? Ann R Coll Surg Engl. 2009;91(6):489–493. 19558758
24. Allolio, B., Fassnacht, M., Clinical review. Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91(6):2027–2037. 16551738
25. Schteingart, D.E., Doherty, G.M., Gauger, P.G., et al, Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12(3):667–680. 16172199
26. Weiss, L.M., Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163–169. 6703192
27. MacFarlane, D.A., Cancer of the adrenal cortex; the natural history, prognosis and treatment in a study of fifty-five cases. Ann R Coll Surg Engl. 1958;23(3):155–186. 13571886
28. Sullivan, M., Boileau, M., Hodges, C.V., Adrenal cortical carcinoma. J Urol. 1978;120(6):660–665. 731800
29. Fassnacht, M., Johanssen, S., Quinkler, M., et al, Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. 19025987
30. Miller, B.S., Ammori, J.B., Gauger, P.G., et al, Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34(6):1380–1385. 20372905 This retrospective review of 88 patients who underwent laparoscopic or open surgery for ACC advised against laparoscopy for ACC based on a higher incidence of positive surgical margins and shorter time to recurrence in the laparoscopic group.
31. Palazzo, F.F., Sebag, F., Sierra, M., et al, Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006;30(5):893–898. 16680605
32. Brix, D., Allolio, B., Fenske, W., et al, Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol. 2010;58(4):609–615. 20580485
33. Gonzalez, R.J., Shapiro, S., Sarlis, N., et al, Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 2005;138(6):1078–1086. 16360394
34. Terzolo, M., Angeli, A., Fassnacht, M., et al, Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. 17554118 This multicentre retrospective analysis demonstrated significantly longer recurrence-free survival in 47 patients who received adjuvant mitotane following radical surgery for ACC compared to 122 patients who did not (median time to recurrence 42 months vs. 10–25 months, respectively).
35. Berruti, A., Fassnacht, M., Baudin, E., et al, Adjuvant therapy in patients with adrenocortical carcinoma: a position of an international panel. J Clin Oncol. 2010;28(23):e401–e402 author reply e403. 20567001
36. Fassnacht, M., Hahner, S., Polat, B., et al, Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91(11):4501–4504. 16895957
37. Berruti, A., Terzolo, M., Sperone, P., et al, Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12(3):657–666. 16172198
38. Berruti, A., Ferrero, A., Sperone, P., et al, Emerging drugs for adrenocortical carcinoma. Expert Opin Emerg Drugs. 2008;13(3):497–509. 18764725
39. Eisenhofer, G., Bornstein, S.R., Brouwers, F.M., et al, Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11(3):423–436. 15369446
40. Elder, E.E., Elder, G., Larsson, C., Pheochromocytoma and functional paraganglioma syndrome: no longer the 10% tumor. J Surg Oncol. 2005;89(3):193–201. 15719371
41. Madani, R., Al-Hashmi, M., Bliss, R., et al, Ectopic pheochromocytoma: does the rule often apply? World J Surg. 2007;31(4):849–854. 17372668
42. Gimenez-Roqueplo, A.P., Lehnert, H., Mannelli, M., et al, Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol (Oxf). 2006;65(6):699–705. 17121518
43. Lenders, J.W., Eisenhofer, G., Mannelli, M., et al, Phaeochromocytoma. Lancet. 2005;366(9486):665–675. 16112304
44. Chetty, R., Familial paraganglioma syndromes. J Clin Pathol. 2010;63(6):488–491. 20498024
45. Jimenez, C., Cote, G., Arnold, A., et al, Review: Should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes? J Clin Endocrinol Metab. 2006;91(8):2851–2858. 16735498 This literature review summarises the evidence for genetic testing in patients with sporadic phaeochromocytoma and recommends that this should be undertaken in those presenting < 20 years, or < 50 years with multiple phaeochromocytomas, sympathetic paraganglioma, or with clinical findings or family history suspicious of hereditary disease.
46. Grossman, A., Pacak, K., Sawka, A., et al, Biochemical diagnosis and localization of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sci 2006; 1073:332–347. 17102103 This report summarises the recommendations of an expert panel on the biochemical diagnosis and localisation of phaeochromocytoma from the First International Symposium on Phaeochromocytoma in 2005.
47. Lenders, J.W., Pacak, K., Walther, M.M., et al, Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427–1434. 11903030
48. Pacak, K., Eisenhofer, G., Ahlman, H., et al, Pheochromocytoma: recommendations for clinical practice from the First International Symposium, October 2005. Nat Clin Pract Endocrinol Metab. 2007;3(2):92–102. 17237836
49. Ilias, I., Pacak, K., Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab. 2004;89(2):479–491. 14764749
50. Mukherjee, J.J., Peppercorn, P.D., Reznek, R.H., et al, Pheochromocytoma: effect of nonionic contrast medium in CT on circulating catecholamine levels. Radiology. 1997;202(1):227–231. 8988215
51. Kinney, M.A., Narr, B.J., Warner, M.A., Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2002;16(3):359–369. 12073213
52. Prys-Roberts, C., Farndon, J.R., Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg. 2002;26(8):1037–1042. 12192533
53. Tauzin-Fin, P., Sesay, M., Gosse, P., et al, Effects of perioperative alpha1 block on haemodynamic control during laparoscopic surgery for phaeochromocytoma. Br J Anaesth. 2004;92(4):512–517. 14766711
54. Lebuffe, G., Dosseh, E.D., Tek, G., et al, The effect of calcium channel blockers on outcome following the surgical treatment of phaeochromocytomas and paragangliomas. Anaesthesia. 2005;60(5):439–444. 15819762
55. Parnaby, C.N., Serpell, M.G., Connell, J.M., et al, Perioperative haemodynamic changes in patients undergoing laparoscopic adrenalectomy for phaeochromocytomas and other adrenal tumours. Surgeon. 2010;8(1):9–14. 20222397
56. Shen, W.T., Sturgeon, C., Clark, O.H., et al, Should pheochromocytoma size influence surgical approach? A comparison of 90 malignant and 60 benign pheochromocytomas. Surgery. 2004;136(6):1129–1137. 15657566 In this retrospective study, 90 malignant and 60 benign phaeochromocytomas were compared to determine whether tumour size affected the surgical approach. Laparoscopic adrenalectomy was considered safe in the majority, as tumour size did not discriminate benign from malignant phaeochromocytomas provided there was no evidence of local invasion or metastases.
57. Yip, L., Lee, J.E., Shapiro, S.E., et al, Surgical management of hereditary pheochromocytoma. J Am Coll Surg. 2004;198(4):525–535. 15051000
58. McNicol, A.M., Update on tumours of the adrenal cortex, phaeochromocytoma and extra-adrenal paraganglioma. Histopathology. 2011;58(2):155–168. 20718871
59. Suh, I., Shibru, D., Eisenhofer, G., et al, Candidate genes associated with malignant pheochromocytomas by genome-wide expression profiling. Ann Surg. 2009;250(6):983–990. 19661783
60. Newell-Price, J., Bertagna, X., Grossman, A.B., et al, Cushing’s syndrome. Lancet. 2006;367(9522):1605–1617. 16698415
61. Nieman, L.K., Biller, B.M., Findling, J.W., et al, The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. 18334580
62. Arnaldi, G., Angeli, A., Atkinson, A.B., et al, Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602. 14671138
63. Porterfield, J.R., Thompson, G.B., Young, W.F., Jr., et al, Surgery for Cushing’s syndrome: an historical review and recent ten-year experience. World J Surg. 2008;32(5):659–677. 18196319
64. Aniszewski, J.P., Young, W.F., Jr., Thompson, G.B., et al, Cushing syndrome due to ectopic adrenocorticotropic hormone secretion. World J Surg. 2001;25(7):934–940. 11572035
65. Oldfield, E.H., Doppman, J.L., Nieman, L.K., et al, Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897–905. 1652686
66. Invitti, C., Pecori Giraldi, F., de Martin, M., et al, Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic–Pituitary–Adrenal Axis. J Clin Endocrinol Metab. 1999;84(2):440–448. 10022398
67. Hall, W.A., Luciano, M.G., Doppman, J.L., et al, Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120(10):817–820. 8154641
68. Ilias, I., Torpy, D.J., Pacak, K., et al, Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955–4962. 15914534
69. Chow, J.T., Thompson, G.B., Grant, C.S., et al, Bilateral laparoscopic adrenalectomy for corticotrophin- dependent Cushing’s syndrome: a review of the Mayo Clinic experience. Clin Endocrinol (Oxf). 2008;68(4):513–519. 17970770
70. Thompson, S.K., Hayman, A.V., Ludlam, W.H., et al, Improved quality of life after bilateral laparoscopic adrenalectomy for Cushing’s disease: a 10-year experience. Ann Surg. 2007;245(5):790–794. 17457173
71. Gil-Cardenas, A., Herrera, M.F., Diaz-Polanco, A., et al, Nelson’s syndrome after bilateral adrenalectomy for Cushing’s disease. Surgery. 2007;141(2):147–151. 17263968
72. Young, W.F., Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. 17492946
73. Funder, J.W., Carey, R.M., Fardella, C., et al, Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281. 18552288
74. Seifarth, C., Trenkel, S., Schobel, H., et al, Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol (Oxf). 2002;57(4):457–465. 12354127
75. Nwariaku, F.E., Miller, B.S., Auchus, R., et al, Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg. 2006;141(5):497–503. 16702522 This retrospective study compared the results of adrenal venous sampling and CT in 39 patients with primary hyperaldosteronism and found the results to be concordant in only 54% of cases. It was concluded that CT was unreliable in lateralising the abnormal adrenal gland in primary hyperaldosteronism.
76. Tan, Y.Y., Ogilvie, J.B., Triponez, F., et al, Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J Surg. 2006;30(5):879–887. 16680603
77. Assalia, A., Gagner, M., Laparoscopic adrenalectomy. Br J Surg. 2004;91(10):1259–1274. 15376201 This systematic review sets out the evidence comparing laparoscopic with open adrenalectomy and demonstrates that laparoscopic adrenalectomy is consistently associated with faster recovery and lower morbidity than the open approach.
78. Sawka, A.M., Young, W.F., Thompson, G.B., et al, Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135(4):258–261. 11511140
79. Ghose, R.P., Hall, P.M., Bravo, E.L., Medical management of aldosterone-producing adenomas. Ann Intern Med. 1999;131(2):105–108. 10419425
80. Sywak, M., Pasieka, J.L., Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89(12):1587–1593. 12445071
81. VanWyk, J.J., Ritzen, E.M., The role of bilateral adrenalectomy in the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88(7):2993–2998. 12843131
82. Maris, J.M., Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–2211. 20558371
83. Hardy, R., Lennard, T.W., Subtotal adrenalectomy. Br J Surg. 2008;95(9):1075–1076. 18690616
84. Gagner, M., Lacroix, A., Bolte, E., Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med. 1992;327(14):1033. 1387700
85. Lal, G., Duh, Q.Y., Laparoscopic adrenalectomy – indications and technique. Surg Oncol. 2003;12(2):105–123. 12946482
86. Walz, M.K., Alesina, P.F., Wenger, F.A., et al, Posterior retroperitoneoscopic adrenalectomy – results of 560 procedures in 520 patients. Surgery. 2006;140(6):943–950. 17188142
87. Horgan, S., Vanuno, D., Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 2001;11(6):415–419. 11814134
88. Morino, M., Beninca, G., Giraudo, G., et al, Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc. 2004;18(12):1742–1746. 15809781
89. Berber, E., Tellioglu, G., Harvey, A., et al, Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery. 2009;146(4):621–626. 19789020 This retrospective study found similar perioperative outcomes in 159 patients who underwent lateral laparoscopic transabdominal adrenalectomy or posterior endoscopic retroperitoneal adrenalectomy when selected on the basis of previous abdominal surgery, body mass index, tumour size and bilaterality.
90. Naya, Y., Nagata, M., Ichikawa, T., et al, Laparoscopic adrenalectomy: comparison of transperitoneal and retroperitoneal approaches. BJU Int. 2002;90(3):199–204. 12133053
91. Schreinemakers, J.M., Kiela, G.J., Valk, G.D., et al, Retroperitoneal endoscopic adrenalectomy is safe and effective. Br J Surg. 2010;97(11):1667–1672. 20665481
92. Gagner, M., Pomp, A., Heniford, B.T., et al, Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg. 1997;226(3):238–247. 9339930
93. Henry, J.F., Defechereux, T., Raffaelli, M., et al, Complications of laparoscopic adrenalectomy: results of 169 consecutive procedures. World J Surg. 2000;24(11):1342–1346. 11038204