The Abdominal Wall and Peritoneal Cavity
Peritoneal Cavity
Overview: The peritoneum is a thin serosal membrane of mesodermal origin that comprises a single layer of mesothelial cells resting on a basement membrane.1 It is divided into visceral and parietal components, and the space between the two components constitutes the peritoneal cavity. The layer covering the abdominal viscera, omentum, and the mesenteries is designated visceral, whereas the layer covering the abdominal walls, undersurface of the diaphragm, anterior surface of the retroperitoneal viscera, and the pelvis is designated as parietal. The peritoneum is continuous in males, whereas in females it is discontinuous at the ostia of the oviducts to allow communication between the peritoneal cavity and extraperitoneal pelvis.
The mesothelial cells produce a small amount of sterile fluid within the peritoneal cavity that is continuously circulated by the movement of the diaphragm and peristalsis of bowel, and the fluid provides a frictionless surface over which the viscera can move, a site for fluid transport, and local bacterial defense.1 Peritoneal fluid predominantly flows up the right paracolic gutter into the right supramesocolic compartment, and 90% of the fluid is cleared by the subphrenic lymphatics to the supradiaphragmatic nodes.2 Areas of relative stasis include 1) the rectouterine pouch or cul-de-sac (pouch of Douglas) in females, 2) the rectovesical region in males, 3) the right lower abdomen at the end of the small bowel mesentery, 4) the left lower abdomen along the sigmoid mesocolon, 5) the right paracolic gutter, and 6) the right subhepatic/subdiaphragmatic space (Morison pouch).2
Entities Affecting the Peritoneum
Overview: The term pneumoperitoneum refers to the presence of air within the peritoneal cavity. Benign postoperative pneumoperitoneum is a separate entity that results from accumulation of free air following abdominal surgery. Usually, free peritoneal air clears more rapidly in children than in adults, but the timing can be variable. The timing of clearance usually depends upon the amount of air initially trapped after surgery, which in most cases is related to the patient’s body habitus; obese patients trap less air than thin patients.3 Several studies have demonstrated clearing of free air in 68% to 90% of children postoperatively by 24 hours, but free air can be seen for as long as 6 to 7 days postoperatively in 2% to 3% of cases.3
Etiology: Free intraperitoneal air is most commonly a consequence of gastrointestinal (GI) tract perforation.4 In the neonate, this usually results from intestinal obstruction, necrotizing enterocolitis, or spontaneous gastric or bowel perforation, usually at the ileocecal region (Box 87-1).5 Necrotizing enterocolitis is the most common cause of pneumoperitoneum in the neonatal intensive care unit.6
Free intraperitoneal gas sometimes results from mediastinal extension in newborns supported by mechanical ventilation. Rarely, nasogastric or nasoduodenal tubes may perforate the bowel. The position of the tube is often a clue to the perforation (e-Fig. 87-1).
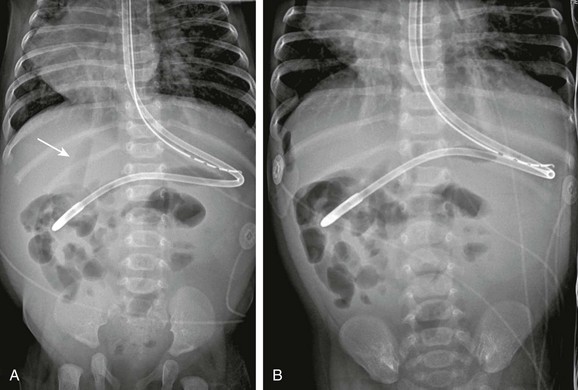
e-Figure 87-1 Perforation by enteric tube.
A, This 3-month-old infant with dextrocardia and heterotaxy underwent imaging for abdominal distension. The feeding tube is more lateral than expected, and a concerning lucency overlies the liver (arrow). B, The left-side-down decubitus view shows gas collecting adjacent to the liver. The patient was found to have a duodenal perforation at surgery.
In children beyond the neonatal period, perforated peptic ulcers and inflammatory bowel disease (IBD) are other causes of pneumoperitoneum; it should be noted that pneumoperitoneum is rarely found with appendiceal perforation, because the omentum usually seals off the perforation very quickly.7 Trauma, both accidental and nonaccidental, may also result in pneumoperitoneum.
Clinical Presentation: Pneumoperitoneum may be suspected clinically because of the history of an underlying disease that predisposes to bowel perforation, detection of acute abdominal distension with increased tympany on physical examination, clinical deterioration of the patient, and occasional fortuitous discovery on imaging examinations of the chest or abdomen.
Imaging: A single, supine abdominal radiograph is usually the most common imaging study requested for patients with suspected abdominal pathology. The cited overall detection rate of free intraperitoneal air on supine imaging ranges from 56% to 59%; detection rates as high as 80.4% have been quoted on supine abdominal radiographs; and the rate is 78.7% on supine chest radiograph.4 It is important to be familiar with the various signs of intraperitoneal free air on supine radiographs, because this may be the only initial study requested by the patient’s primary care giver.
Once suspected, the diagnosis can be confirmed on horizontal-beam plain radiographs. Upright radiographs will show air collecting between the diaphragm and the liver on the right and between the diaphragm and the liver, spleen, stomach, or colon on the left (Figs. 87-2 and 87-3). Young children and those too ill to sit or stand can be examined in the decubitus position. The decubitus view should be obtained with the right side up to allow the liver to fall away from the wall of the peritoneal cavity; this allows visualization of free peritoneal air between the liver and the abdominal wall. Both techniques are considered equally effective, and the choice usually depends upon the patient’s age and clinical status and the preference of the radiologist.4
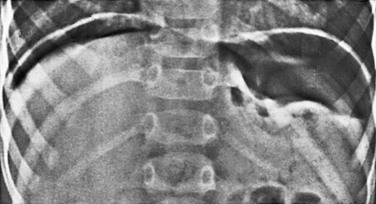
Figure 87-2 Free intraperitoneal air.
An upright examination of the abdomen in a 2-year-old boy with perforated gastric ulcer. Air is easily demonstrated between the diaphragm and the liver on the right side and between the diaphragm and the spleen and stomach on the left.
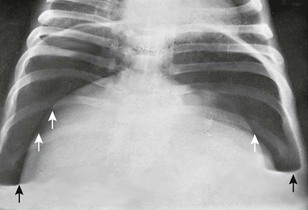
Figure 87-3 Pyopneumoperitoneum.
Massive amount of free air in the abdomen below the diaphragm (white arrows) with air-fluid levels (black arrows) on an upright film.
If decubitus or upright imaging is difficult to obtain, a supine view using a horizontal-beam technique can be utilized. On the horizontal-beam supine radiograph, free peritoneal air may collect between the anterior surface of the liver and the anterior abdominal wall (Fig. 87-4), but small amounts of free air may be more difficult to detect, particularly if located over loops of bowel.
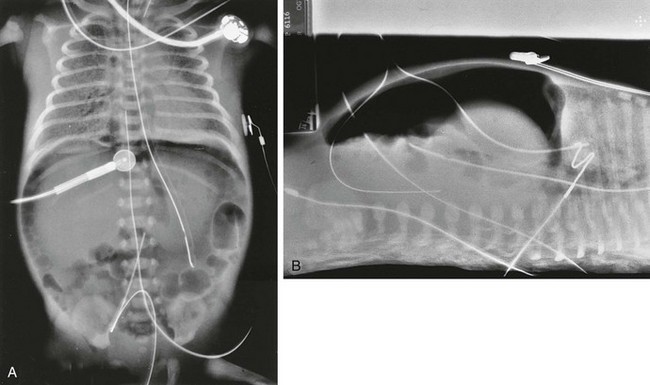
Figure 87-4 Pneumoperitoneum.
A, Anteroposterior view of the chest and abdomen in a newborn with pulmonary interstitial emphysema, pneumomediastinum, and pneumoperitoneum. A large amount of gas is in the peritoneal cavity, the Rigler sign is positive, and density of the liver is decreased compared with the extraperitoneal soft tissues. The falciform ligament per se is not visualized because of the obscuring umbilical venous catheter. B, Cross-table lateral view of the same patient demonstrates large pneumoperitoneum without air-fluid levels, suggesting that the air has dissected into the peritoneum from the chest.
Tension pneumomediastinum can dissect along the retroperitoneum and the subadventitial layer of the mesenteric vessels and can rupture freely into the peritoneal cavity. The latter is usually suspected because of the history of assisted ventilation and the presence of pneumomediastinum on the chest radiograph. A ruptured viscus permits both air and fluid to escape into the peritoneal cavity, causing abnormal extraluminal air-fluid levels (compares Figs. 87-3 and 87-4). Dissecting pneumomediastinum results in only air within the peritoneal space, thus a significant air-fluid level is not identified in the peritoneal space on horizontal-beam examination. The differentiation may remain difficult in patients with preexistent ascites, therefore correlation with chest radiographs, clinical history, and physical findings may be required to make the distinction.
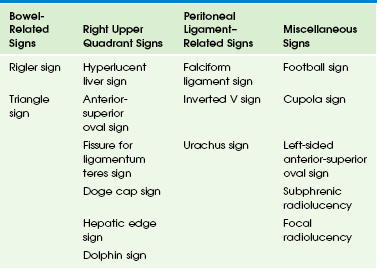
Multiple signs related to pneumoperitoneum are described on plain radiographs (Table 87-1) based on the location and volume of air and its relationship to adjacent structures. The lucency caused by the free air rising to an anterior position in the abdomen is most easily detected when it projects over the liver, thus many right upper quadrant signs of free air are described.8 The normal liver is uniform in radiodensity and is typically denser than the heart. Air overlying the liver on a supine radiograph decreases the radiodensity of that portion of the liver over which it lies.
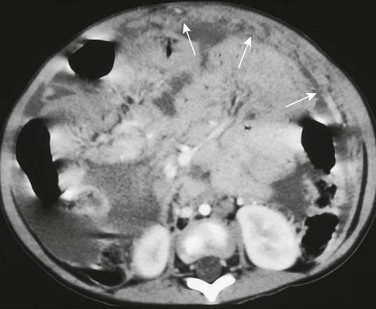
Small amounts of free air may appear only as subtle, localized collections in the right upper quadrant on supine radiographs. Linear collections represent air in the right subhepatic space, known as the hepatic edge sign, whereas triangular collections are seen with air in the Morison pouch (e-Fig. 87-5); this is known as the Doge cap sign, because it resembles the cap worn by the Venetian Doge.9,10 A linear collection of air may also be located in the fissure of the ligamentum teres.11
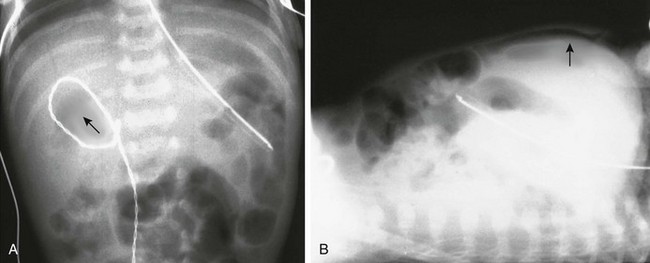
e-Figure 87-5 Pneumoperitoneum.
A, Supine view of the abdomen in an infant with free air resulting from necrotizing enterocolitis. A small collection of gas is noted in the Morison pouch (arrow). B, Same patient as in A in the supine position, using a horizontal-beam technique. Air is demonstrated between the liver and anterior abdominal wall (arrow).
The Rigler sign refers to visualization of the outer edge of the bowel wall caused by the presence of air on both sides of the wall (e-Fig. 87-6). The telltale triangle sign is a triangular focus of extraluminal air seen on the cross-table lateral view of the abdomen, created by the external surface walls of adjacent bowel loops and the anterior abdominal wall, as the air collects at the highest point in the peritoneal space.12
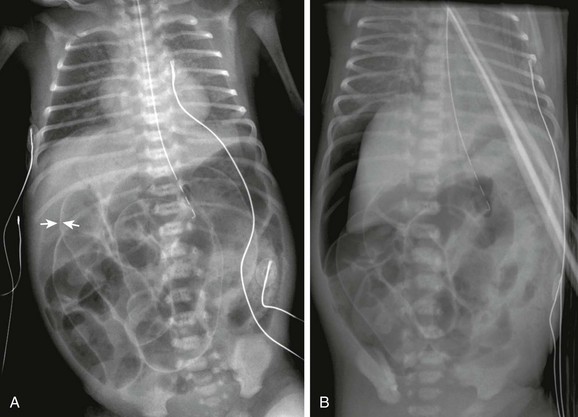
e-Figure 87-6 Pneumoperitoneum with Rigler sign.
A, Supine radiograph in a 6-day-old 33-week-gestation premature infant shows a large amount of free intraperitoneal gas with right upper quadrant lucency. Rigler sign is present, and air outlines both sides of the bowel loops (arrows); subtle signs of portal venous gas are also present. B, Left-side-down decubitus view of the same infant shows a large amount of free intraperitoneal gas rising to outline the liver. Rigler sign is again evident.
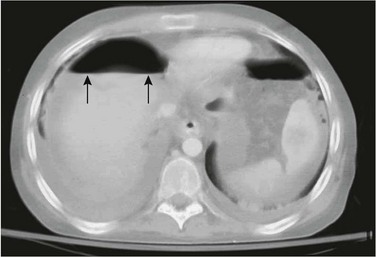
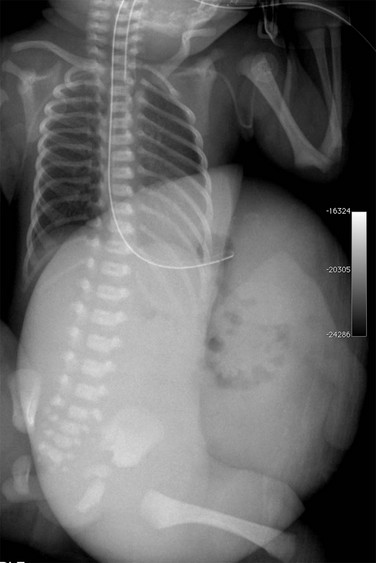
A sufficiently large amount of free air can be seen as a large, ovoid lucency overlying the abdominal contents (Fig. 87-7). As the liver falls away from the anterior peritoneal surface in the supine position, free peritoneal air can dissect along both sides of the falciform ligament, which attaches the liver to the anterior abdominal wall. The ligament, when outlined by free peritoneal air, appears as a very thin, vertical, opaque line (e-Fig. 87-8). The distension of the flanks caused by the free intraperitoneal air and the outline of the falciform ligament centrally are the elements of the well-known football sign, so termed because of its similarity to a football; the falciform ligament represents the central thread in the ball (see Fig. 87-7). A less commonly encountered sign of pneumoperitoneum is the inverted V sign, caused by air outlining the medial umbilical folds in the pelvis.13
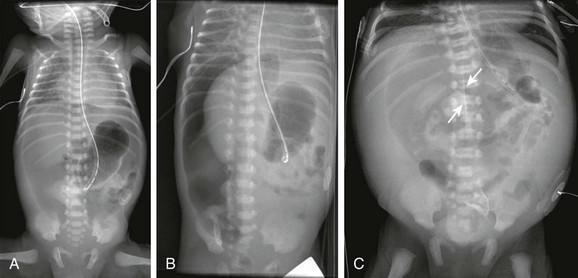
Figure 87-7 Pneumoperitoneum with “football sign.”
A, Supine radiograph in a 5-day-old 30-week-gestation premature infant shows a large lucency over the entire abdomen. B, Decubitus view in the same infant confirms the large pneumoperitoneum. Multiple intestinal perforations were found at surgery. C, Another patient with pneumoperitoneum demonstrates the classic football sign on abdominal imaging. Gas outlines the falciform ligament (arrows), and a large lucency overlies the upper abdomen centrally as the gas accumulates anteriorly. At surgery, this patient was found to have a colonic perforation.
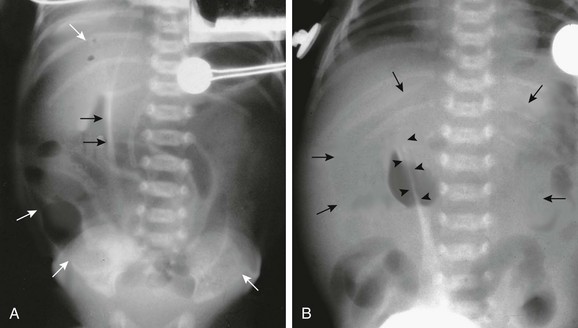
e-Figure 87-8 Pneumoperitoneum with football sign.
A, Supine view of the abdomen in an infant with perforated viscus. A large oval lucency (white arrows) overlies the entire abdomen, coupled with the dense line of the falciform ligament (black arrows), outlined by air on both sides—the so-called football sign. B, Supine view of the abdomen in another infant with free peritoneal air reveals a smaller oval lucency (arrows) and the falciform ligament surrounded by air (arrowheads).
Apart from plain radiographs, ultrasound (US) can also be used in the detection of pneumoperitoneum by detecting gas over the liver, with a reported sensitivity of 93%, specificity of 64%, and accuracy of 90%.14 However, we do not believe that US should be considered definitive in diagnosing or excluding a pneumoperitoneum without associated extensive expertise and experience, and US findings should be confirmed by appropriate radiographic evaluation.
Although not typically performed for assessment of pneumoperitoneum, computed tomography (CT) is an extremely sensitive method to identify small amounts of intraperitoneal or extraperitoneal air and intraperitoneal air-fluid levels. CT is superior to upright radiography in demonstrating free intraperitoneal air,15 and it can be optimized by reviewing abdominal images using lung-window parameters (e-Fig. 87-9 and Fig. 87-10).
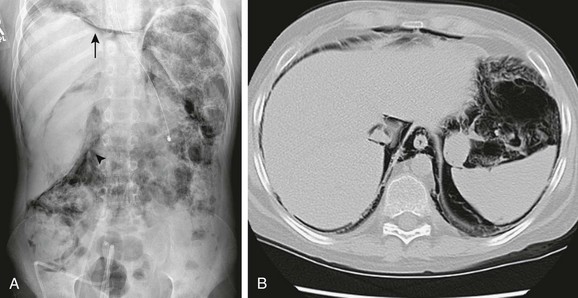
Figure 87-10 Pneumatosis intestinalis.
A, Radiograph of the abdomen and pelvis in a patient who underwent bone marrow transplantation for refractory recurrent neuroblastoma. Abundant pneumatosis intestinalis has resulted in pneumoperitoneum (arrow) and pneumoretroperitoneum (arrowhead). B, Computed tomographic image through the upper abdomen viewed with lung windows confirms the pneumoperitoneum, pneumoretroperitoneum, and pneumomediastinum. Note absence of air-fluid levels.
Mimics and Potential Pitfalls
A pseudo-Rigler sign occurs when two loops of dilated air-filled bowel lie adjacent to one another. The line seen in the pseudo-Rigler sign is thicker than with free peritoneal air, because it represents a double thickness of bowel wall (from the two adjacent bowel loops), whereas the line in patients with free peritoneal air—a true Rigler sign—represents a single bowel wall. However, this is not always a reliable differentiation, because the underlying disease-causing perforation may lead to a thickened bowel wall. In equivocal cases, a horizontal-film radiograph should be obtained for clarification.16
Ascites
Overview: It is normal for a small amount of fluid to be present in the peritoneal cavity. This is more common in females, and it may be seen incidentally on cross-sectional imaging. Ascites refers to abnormal or pathologic accumulation of fluid within the peritoneal cavity.
Etiology: Pathologic intraperitoneal fluid collections stem from a variety of causes and most commonly result from sequestration of fluid from the splanchnic vascular bed. Other causes of pathologic intraperitoneal fluid include hemoperitoneum, urinary ascites, bile, pancreatic juices, chylous fluid, and cerebrospinal fluid (CSF). Transudative ascites is most commonly found in patients with hepatobiliary disease, especially cirrhosis; heart failure; hyponatremia; renal failure; peritonitis; and Budd-Chiari syndrome. Exudative ascites can occur secondary to peritoneal infections and peritoneal metastases. Perforation of the GI tract results in the escape of both air and fluid into the peritoneal cavity. In children, the most common causes for ascites are hepatic, renal, and cardiac disease.17
The lesser sac, Morison pouch, paracolic gutters, pelvis, and recesses formed by many of the peritoneal ligaments are all sites where fluid can collect (Fig. 87-11). Typically, small amounts of ascites collect in the pelvis when the patient is supine. As the amount of fluid increases, it moves cephalad along the paracolic gutters into the subhepatic spaces and Morison pouch, and it can sometimes be identified in the fossa of the ligamentum teres (e-Fig. 87-12). Ascites eventually spreads through the peritoneal cavity and into the mesenteric recesses (Fig. 87-13); in cases of inflammation, loculations may occur. Encysted collections of CSF may be seen adjacent to the tip of a ventriculoperitoneal shunt tube (“CSF pseudocyst”), usually as a result of an inflammatory response around the shunt tube tip (Fig. 87-14).
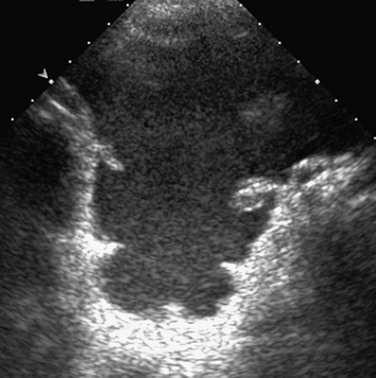
Figure 87-11 Complex ascites.
Transverse ultrasound image through the deep pelvis in a 6-year-old patient with epithelioid sarcoma demonstrates abundant complex ascites. The thickening of the lateral peritoneal surface indicates peritoneal disease.
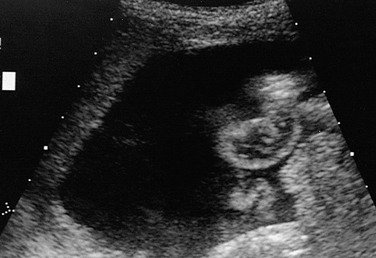
Figure 87-13 Ascites.
Transverse sonogram through the left lower quadrant demonstrates a massive ascitic fluid collection that outlines thick-walled loops of intestine in a patient with graft-versus-host disease following bone marrow transplantation.
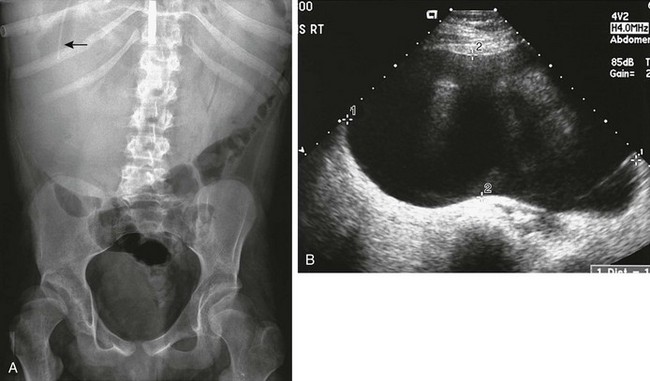
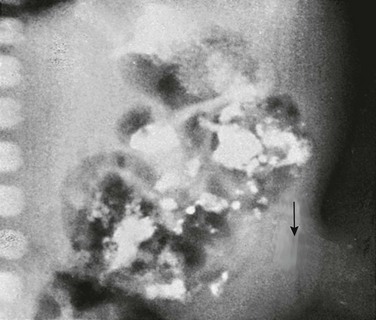
Figure 87-14 Cerebrospinal fluid pseudocyst.
A, Abdominal radiograph demonstrates a large soft tissue mass that occupies the entire upper abdomen, especially the right abdomen. The tip of a ventriculoperitoneal shunt is seen in the right upper quadrant (arrow). B, Transverse image through the entire upper abdomen confirms a large cystic mass surrounding the tip of the shunt.
Clinical Presentation: The clinical hallmark of ascites is abdominal distension, which in itself is a nonspecific sign. The clinical findings are in part governed by the underlying etiology. Early satiety and dyspnea can be seen with increasing accumulation of fluid within the abdominal cavity.17
Abdominal compartment syndrome is an uncommon sequela of acute accumulation of large-volume ascites and may arise secondary to collection of any material that leads to intraabdominal hypertension. The increased pressure in the confined space leads to progressive organ failure with significant associated mortality. It is most commonly described after trauma but can also be seen in the setting of surgery and other entities, such as pancreatitis. The diagnosis is usually made at the bedside with measurement of intravesical pressure.18 The criteria for diagnosis of abdominal compartment syndrome include elevation of intraabdominal pressure to 20 mm Hg or higher, coupled with impaired function of at least one organ; typically, it affects respiratory or renal function.19 On CT this syndrome can be suggested by a ratio of anteroposterior to transverse diameters of the abdomen exceeding 0.81 (Fig. 87-15).19 However, abdominal measurements on a single CT scan may be nonspecific, because an increased anteroposterior abdominal dimension may be seen with chronic ascites. Other findings on imaging include an elevated diaphragm, the presence of hemoperitoneum and increasing girth on serial examinations, attenuated inferior vena cava or renal veins, and shock bowel. Although not specific, a combination of these findings in the appropriate clinical setting or worsening of these findings on sequential imaging studies should raise the possibility of abdominal compartment syndrome.18
Imaging: Diagnosis of ascites is usually made based on clinical history, physical examination, and aspirated fluid analysis. Imaging is usually performed to confirm the clinical ascites, estimate its volume, and identify loculations, septations, or internal echoes or to assist in sampling or draining of ascitic fluid.
Abdominal radiographs are only sensitive to large amounts of intraperitoneal fluid. In such cases, the gas-filled bowel loops will appear centrally located within the abdomen (e-Fig. 87-16). Separation of bowel loops may also occur as a result of ascites, but this appearance can be simulated by large amounts of intraluminal fluid or by thick-walled bowel loops (e-Fig. 87-17).20
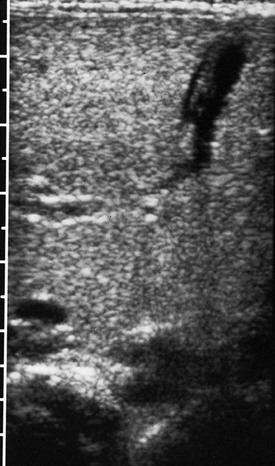
e-Figure 87-12 Ascites.
Oblique sonogram through the liver demonstrates ascitic fluid in the fissure of the ligamentum teres. The ligament and the obliterated umbilical vein can be seen within the fluid collection.
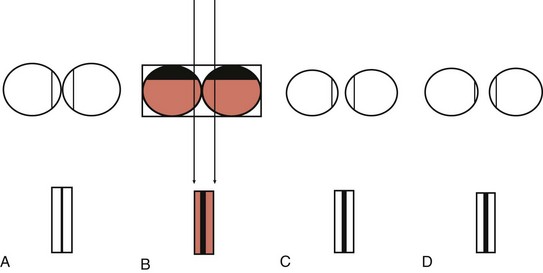
e-Figure 87-16 Pseudoseparation and true separation of bowel loops.
The x-ray beam detects gas with intervening fluid density. A, Adjacent bowel loops filled with air cast a shadow of a normal, thin bowel wall. B, Adjacent loops filled largely with dependent fluid and a small amount of air cast a shadow equivalent to fluid-wall-wall-fluid, which appears as a thick line, simulating separation of bowel loops and falsely suggesting the presence of ascites. C, Thick-walled bowel loops filled with gas cast a shadow of two thick adjacent walls, again falsely simulating ascites. D, If the gas-filled loops are separated by ascites, loops of gas-filled bowel cast a thick shadow, equivalent to two bowel walls and intervening ascitic fluid between the intraluminal air columns. Note that the thickness of the shadow cast is the same in B, C, and D.
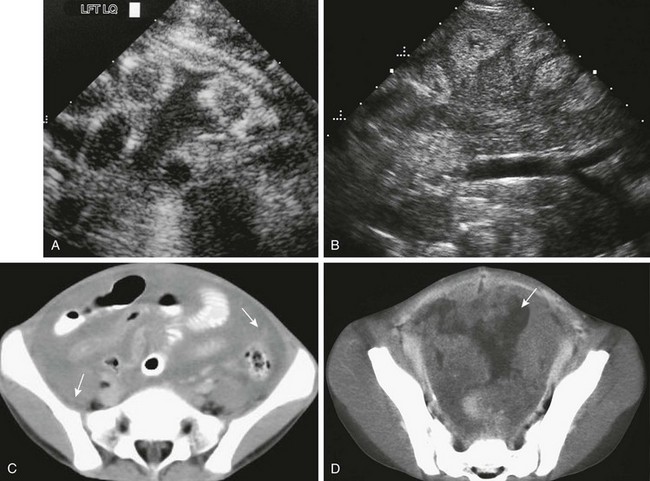
e-Figure 87-17 Complex peritoneal fluid.
A, Transverse sonogram through the left lower quadrant of a 1-year-old boy with disseminated intravascular coagulation and thrombocytopenia following surgery for congenital heart disease. A fluid collection separates multiple loops of bowel. However, the fluid is not anechoic, as in simple ascites, but contains some internal echoes. Paracentesis revealed a hematocrit level within the ascitic fluid. B, Peritoneal mesothelioma in a 7-year-old boy who had been treated at age 3 for non-Hodgkin lymphoma; treatment included whole-abdomen radiation therapy. Coronal ultrasound through the midabdomen shows echogenic material surrounding, separating, and displacing small bowel loops, which are also hyperechoic. C, Axial computed tomographic (CT) image through the same area shows ascites and peritoneal soft tissue densities and masses (arrows). D, Axial CT through the pelvis shows intense peritoneal enhancement and numerous soft tissue masses (arrow).
e-Figure 87-18 Abdominal compartment syndrome in a 6-year-old with abdominal epithelioid sarcoma.
The ratio of the anteroposterior to transverse diameters of the midabdomen was abnormally increased, at 0.84.
US is an extremely sensitive imaging modality for ascites.21 Simple ascites usually appears as anechoic fluid and can be seen in the various peritoneal recesses.22 Septations, loculations, and internal echoes usually suggest complex fluid, which can be seen in the setting of blood, chyle, inflammatory cells, or peritoneal metastases (e-Fig. 87-18). Ascites occasionally will pass through the esophageal hiatus or through patent pleuroperitoneal canals to present as intrathoracic fluid.23
CT is equally sensitive to detect ascites, although it does not visualize internal septations, which are easily seen on US. Because of radiation exposure, CT is not the first-line modality in the evaluation of ascites. Additional information related to the cause of ascites may be discernible, depending on the underlying condition.24
Treatment: The course, prognosis, and treatment of ascites depend entirely on the cause. Drainage of the ascitic fluid can usually provide symptomatic relief, however, in most cases treatment is aimed at the underlying disorder. The treatment for abdominal compartment syndrome includes emergent drainage or decompressive laparotomy. The mortality rate in abdominal compartment syndrome remains high, at approximately 60% to 70%.18
Peritonitis
Overview: Peritonitis is a generalized or loacalized inflammatory process that affects the peritoneum. Acute generalized peritonitis is usually of infectious etiology and can be further subclassified as primary and secondary. Primary peritonitis, also called spontaneous bacterial peritonitis, is a primary infection of the peritoneal cavity that does not result from intraabdominal pathology. Secondary peritonitis results from secondary infection of the peritoneum, usually from urogenital or GI sources, particularly perforation.25 Abscesses may develop locally and at sites where fluid is likely to accumulate, which are at times distant from the perforation site. The subhepatic and subphrenic spaces are common distant sites for abscess formation.
Etiology: Primary peritonitis may occur spontaneously in patients without underlying pathology, and it is usually seen in association with postnecrotic cirrhosis and nephrotic syndrome.25 Access of organisms to the peritoneal cavity through the fallopian tubes is another putative cause of this condition, supported by the increased occurrence in patients with intrauterine contraceptive devices.25
Secondary peritonitis in children is most commonly caused by a perforated appendix. Other causes of bowel perforation that could potentially result in secondary peritonitis include inflammatory bowel disease, incarcerated hernias, complications of Meckel diverticulum, midgut volvulus, intussusception, hemolytic uremic syndrome, necrotizing enterocolitis, typhlitis, and traumatic perforation.17 Peritoneal dialysis is another cause of peritonitis in children and is the most common cause of dialysis failure.26
Granulomatous peritonitis is usually associated with infectious etiologies such as tuberculosis, histoplasmosis, or pneumocystosis, most often in immunocompromised hosts. Tuberculosis that involves the peritoneum occurs in approximately 4% of patients with tuberculosis27 but is reported to occur in 10% of children aged less than 10 years.28 Noninfectious causes for granulomatous peritonitis include foreign material, such as talc and barium, meconium, bowel contents, bile, or gallstones.2 Meconium peritonitis is a sterile peritonitis that results from prenatal perforation of the bowel; this is discussed in Chapter 103.
Clinical Presentation: Patients usually come to medical attention with fever (≥39.5° C), diffuse abdominal pain, nausea, and vomiting. Signs of peritoneal inflammation can be elicited on physical examination; these signs include rebound tenderness, abdominal wall rigidity, and decreased or absent bowel sounds from a paralytic ileus.17 Patients with infectious granulomatous peritonitis secondary to histoplasmosis or pneumocystosis are nearly always immunocompromised.2
Imaging: Abdominal radiographs in patients with peritonitis often show a nonspecific, adynamic ileus pattern with dilated bowel, multiple intraluminal air-fluid levels, and evidence of ascites; in addition, the properitoneal fat plane may be obliterated (e-Fig. 87-19). US may demonstrate intraperitoneal fluid with internal echoes and septations, and abscesses are identified as focal collections of mixed echogenicity.
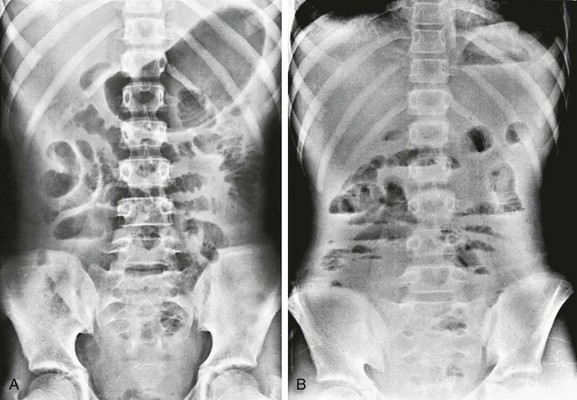
e-Figure 87-19 Peritonitis.
A, Supine abdominal radiograph in a teenage patient with purulent peritonitis shows gas-filled loops of small intestine that are mildly dilated and separated from one another. B, Upright view of the same patient shows multiple scattered air-fluid levels throughout the peritoneal cavity.
CT demonstrates enhancement of the peritoneal lining with associated dense ascites (Fig. 87-20). Abscesses appear as focal fluid collections with relatively high attenuation values and densely enhancing walls (Fig. 87-21). On magnetic resonance imaging (MRI), abscesses in the peritoneum show similar findings as elsewhere, with high T2 signal and dense peripheral enhancement. Both gallium citrate– and indium-labeled white blood cells have been used as scintigraphic agents in the diagnosis of abscesses, although these are often unnecessary.
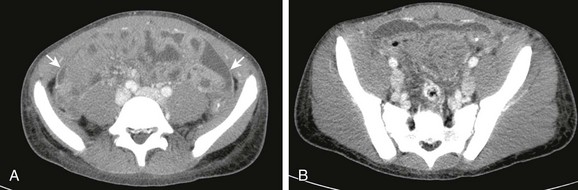
Figure 87-20 Peritonitis.
A, Postcontrast computed tomographic image in a 14-year-old boy with perforation occurring after severe retching following a Heller myotomy shows low-attenuation fluid within the pelvis and between bowel loops. The peritoneal lining is enhancing (arrows) consistent with a clinical diagnosis of peritonitis. B, Image caudal to A shows similar findings.
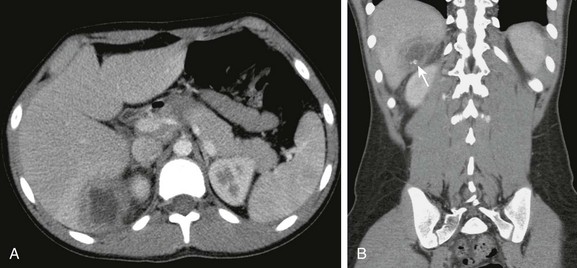
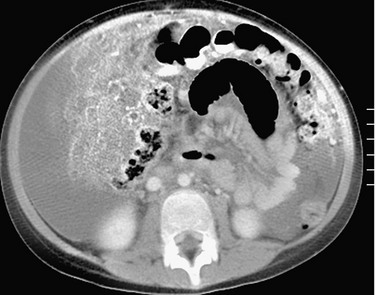
Figure 87-21 Ruptured appendicitis with intraabdominal abscess.
A, Postcontrast computed tomographic scan in a patient with perforated appendicitis shows an irregular, thick-walled, peripherally enhancing abscess adjacent to the right lobe of the liver. B, Coronal reformat reveals the location of the mass in the subhepatic/parahepatic region. In addition, two hyperdense foci are seen (arrow) within the mass, most consistent with appendicoliths.
Granulomatous peritonitis is secondary to a spectrum of lesions, as discussed above. Peritoneal tuberculosis has been subdivided into three overlapping subtypes—a wet type, a fibrotic variety, and a dry, plastic type—with decreasing ascites and increasing soft tissue components along the spectrum.2,29 The wet type is the most common and is characterized by ascites, which on CT is often (but not always) of high density2,28,29 with free or localized fluid collections. Dry plastic or fibrotic fixed patterns are characterized by a relative lack of ascites and a variable amount of peritoneal and omental nodules and masses, peritoneal adhesions, and fibrotic fixation of the small bowel and mesentery as the predominant features.2 Omental involvement is usually seen as diffuse, infiltrating, ill-defined enhancing lesions that produce a “smudged” appearance of the omentum (Fig. 87-22). The findings in tuberculosis are indistinguishable from those in histoplasmosis.2 Although no single CT feature is diagnostic of peritoneal tuberculosis, additional imaging features that help in diagnosis include concomitant central, low-attenuation lymph node enlargement, miliary microabscesses in the liver or spleen, splenic or lymph node calcification, and inflammation that involves the terminal ileum and cecum.29,30
Treatment: The treatment of peritonitis includes correction of the underlying etiology and supportive therapy. General supportive measures include vigorous intravenous rehydration and correction of electrolyte disturbances and infection control. Early control of the infection can be achieved medically, operatively, and through image-guided percutaneous interventions.
Abdominal Wall and Peritoneal Calcification
Overview: Abdominal wall calcification is uncommon in infants and children. The etiology depends upon the location of the calcification—whether it is skin, muscles, soft tissue, or peritoneum—and on the age of the patient. Most causes of intraabdominal calcification are related to specific organs, and these are discussed in the appropriate chapters.
Etiology: Fat necrosis is of one of the causes of abdominal wall calcification in neonates and infants.31 Although there are many causes of fat necrosis, the majority are associated with neonatal asphyxia, sepsis, gestational diabetes, and hypothermia.32 Older children with hypothermia, hepatic failure, and renal failure may also experience subcutaneous fat necrosis.31
Abdominal wall calcification may be seen in fibrodysplasia ossificans progressiva and in myositis ossificans, but these lesions are more common in the thoracic wall than in the abdominal wall. Calcifications secondary to dermatomyositis are more likely to be in the extremities than in the trunk, but calcifications can be found in the abdominal wall. Subcutaneous hemangiomas may contain phleboliths. Abdominal wall calcification in infants has also been described following subcutaneous emphysema and in prune-belly syndrome.33
The most common cause of peritoneal calcification in the neonate is meconium peritonitis,34 discussed in Chapter 103. Peritoneal calcification in older children is rare. Intestinal perforation with subsequent peritonitis may cause calcification, as can granulomatous tuberculous peritonitis. Other causes include peritoneal dialysis, calcification along surgical scars, hyperparathyroidism, and peritoneal malignancies such as ovarian adenocarcinoma.34
Clinical Presentation: Subcutaneous fat necrosis appears clinically as firm, erythematous plaques. In addition to the underlying condition, patients may develop hypercalcemia, particularly when involvement is extensive.35
Imaging: Most abdominal wall and peritoneal calcifications are detected fortuitously on plain radiographs and/or CT scans obtained for other clinical indications. Correlation with any underlying predisposing disease, location, and shape of the calcification may help identify the underlying etiology. Peritoneal calcification associated with calcified lymph nodes is significantly more likely to be associated with malignancy, and a sheetlike appearance of peritoneal calcification was associated significantly more frequently with benign disease.34
Anterior Abdominal Wall Abnormalities
Overview: Anterior abdominal wall defects encompass a variety of conditions, most commonly omphalocele and gastroschisis. Omphalocele refers to a defect larger than 4 cm that occurs in the midline and typically contains both gut and the liver and may or may not include other abdominal organs covered by a bilayer consisting of the peritoneum as the inner layer and the amnion as the outer layer, with Wharton’s jelly in between. It is differentiated from the less common umbilical cord hernia in that the latter is less than 4 cm, does not contain liver, has a normal abdominal wall, and has few associated anomalies. Gastroschisis has no covering membrane and contains only gut, although a gonad may occasionally protrude through the defect, located adjacent to the umbilical cord insertion, typically on the right. Umbilical hernia, unlike the above defects, is covered by skin, tends to become apparent several weeks after birth, and is not associated with malrotation.36
Overview: Omphaloceles constitute the second most common anterior abdominal wall defect, with a prevalence of approximately 1 to 5 in 10,000 live births; it is more common in boys.36,37
Etiology: An omphalocele forms when fusion fails in the lateral folds of the body wall, the rectus muscles fail to meet in the midline, and the herniated bowel fails to return into the peritoneal cavity, with the umbilical cord inserting into the covering membrane (Fig. 87-23 and e-Fig. 87-24).36,37 The herniated viscera are covered by an outer layer of amnion and Wharton jelly and an inner membrane of parietal peritoneum.37 Involvement of the cephalic folds results in the spectrum of pentalogy of Cantrell/ectopia cordis: midline supraumbilical abdominal wall defect, pericardial and diaphragmatic defects, cardiac or ventricular diverticulum herniation through the defect, congenital heart disease, and sternal clefts (Fig. 87-25).37,38 Failure of caudal fold development results in cloacal exstrophy.36,37
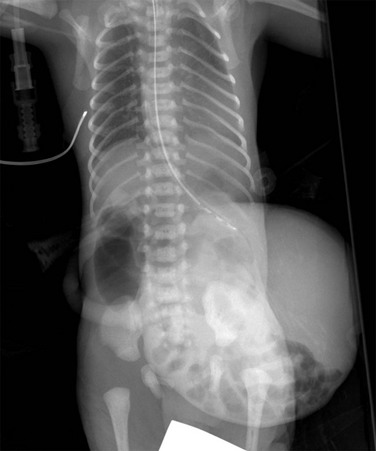
Figure 87-23 Omphalocele.
Frontal view of the chest and abdomen in a neonate shows a large anterior wall defect covered by a membrane and containing the liver, which is well visualized adjacent to air-filled and mildly distended bowel loops. The umbilical cord inserts on the omphalocele.
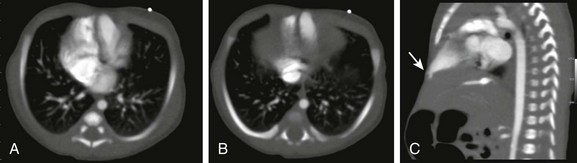
Figure 87-25 Pentalogy of Cantrell.
Neonate with an inferior sternal defect and protrusion of anterior abdominal wall with deficient epigastric abdominal wall musculature. A and B, Axial images from a contrast-enhanced computed tomographic scan show a midline heart with a left ventricular diverticulum. C, Sagittal reformat demonstrates the herniating left ventricular diverticulum to better advantage (arrow). (Courtesy Marta Hernanz-Schulman, MD, Nashville, TN.)
Clinical Presentation: Associated anomalies are present in 50% to 70% of cases. Chromosomal anomalies, especially trisomies, occur in 40% to 60%.39 Absence of liver in the sac, smaller defects, and abnormal amounts of amniotic fluid are associated with an increased incidence of other anomalies, and 50% of these patients have congenital heart disease. Omphaloceles also are associated with Beckwith-Wiedemann syndrome and omphalocele–bladder exstrophy–imperforate anus–spinal defect complex.39 Intrauterine growth retardation and prematurity commonly coexist.37
Imaging: The diagnosis is usually made on antenatal US. Antenatal evaluation includes assessment of the umbilical cord insertion, presence or absence of a covering membrane, contents of the omphalocele, and coexisting anomalies.40 Multiple associated anomalies indicate a poorer prognosis, as do oligohydramnios and polyhydramnios.
Gastroschisis
Overview: The prevalence of gastroschisis has been rising over the past three decades, particularly in infants born to young mothers, for reasons that remain unclear,36,37,41 with a prevalence of approximately 2 to 5 per 10,000 live births.42 Gastroschisis is not usually associated with chromosomal abnormalities or congenital anomalies outside of the GI tract. Complex gastroschisis results when associated GI complications are present.43
Etiology: The etiology of gastroschisis remains uncertain, but it is postulated to result from ischemia to the paraumbilical abdominal wall and results in its involution. The defect occurs much more commonly on the right and may be related to mistiming of the involution of the right omphalomesenteric artery and right umbilical vein, with herniation of the bowel through the defect.37 A vascular genesis of the defect is supported by reported incidence in cases of maternal use of vasoactive compounds, such as cocaine and cigarettes.44
Clinical Presentation: Infants with gastroschisis are typically born prematurely. At birth the bowel may appear normal, but soon after, it may be covered with fibrinous exudates.36 The maternal α-fetoprotein level is elevated, because the lack of covering membrane allows the bowel loops to be exposed to the amniotic fluid. The incidence of associated anomalies is approximately 10% to 20%, most involve the GI tract,45 and include antenatal perforation, necrosis, atresia, and volvulus, sometimes resulting in congenitally short bowel.36,46
Imaging: On prenatal US, gastroschisis is characterized by a normal umbilical insertion; a defect located adjacent to the umbilicus, most commonly on the right; and absence of a covering sac (e-Fig. 87-26 and Fig. 87-27). Dilatation of the bowel loops on prenatal imaging has been found to correlate with complex gastroschisis, with poorer survival and increased morbidity.
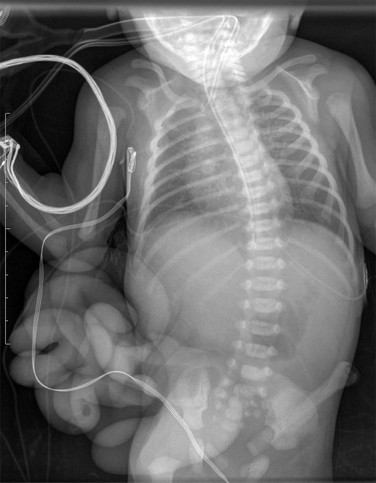
Figure 87-27 Gastroschisis.
Frontal view of the chest and abdomen of a newborn shows bowel loops outside the abdominal cavity. The bowel loops are not covered by a membrane, and the umbilical cord inserts into the anterior abdominal wall to the left of the defect.
Treatment: In the immediate postnatal period, close attention to heat preservation and fluid balance is important, because the large surface area of the exposed bowel leads to heat and fluid loss. Rapid coverage of the exposed bowel is a priority and may be accomplished with a Silastic silo bag. The defect itself may need to be surgically enlarged, if the bowel is at risk of vascular compromise.37 Whenever possible, surgery as soon as possible after delivery is advocated.36
Omphalomesenteric Duct and Urachal Remnants
Omphalomesenteric Duct Remanant
Overview: Omphalomesenteric duct remnant refers to a variety of conditions that range from a Meckel diverticulum to ligamentous and umbilical abnormalities (Fig. 87-28).
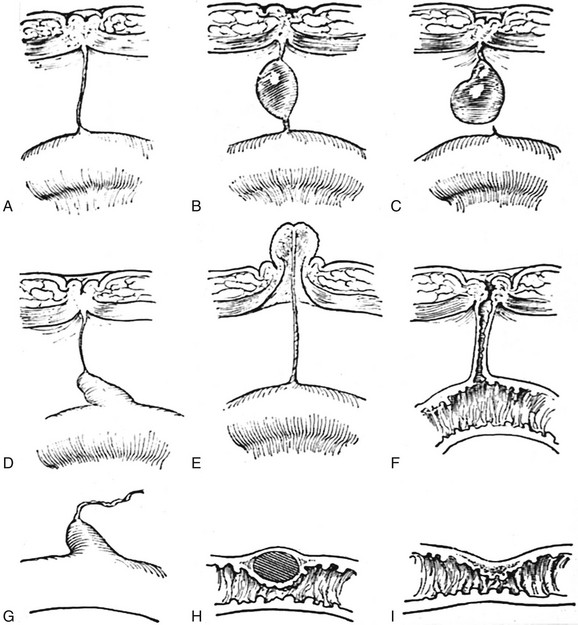
Figure 87-28 Variants of omphalomesenteric duct remnants.
A, Persistent cord between the ileal wall and the closed umbilicus. B, Cyst in the same cord. C, Cyst anchored at the umbilical end of the cord but free at the ileal end. D, Meckel diverticulum attached to the closed umbilicus by a closed cord. E, Everted mucocele of the umbilicus with the cord attached to the ileal wall. F, Fecal fistula open at both the umbilical and ileal ends. G, Meckel diverticulum open at the ileal end but blind at the umbilical end, which is unattached. H, Intramural cystic diverticulum. I, Local stenosis of the ileum at the site of the mouth of the omphalomesenteric duct. (From Cullen TS. Embryology, anatomy and diseases of the umbilicus. Philadelphia: Saunders; 1916)
Etiology: The omphalomesenteric duct, also called the vitelline duct, connects the primitive midgut, the future ileum, to the yolk sac remnant; normally, it obliterates during the fifth to seventh week of embryonic development. However, if this process does not proceed normally, a spectrum of abnormalities may ensue. Closure of the duct with failure of complete involution results in a ligamentous remnant. The spectrum of abnormalities has been subdivided into three general categories.47 In a type 1 abnormality, the entire duct is open (Fig. 87-29). A type 2 abnormality refers to a duct that is open at one end but ends blindly at the other; when open at the ileal end, it represents a Meckel diverticulum (see Chapter 103). A type 3 abnormality refers to focal patency along the course of the duct.
Clinical Presentation: Patients with a type 1 abnormality will come to medical attention with fecal material draining from the umbilicus. Patients in whom the duct is open only along the umbilical end are seen with discharge from the umbilicus that consists of secretions produced by the lining of the tract. A type 3 abnormality, or vitelline duct cyst, is often asymptomatic but may become infected, in which case it can manifest with acute symptoms. Omphalomesenteric duct remnants or attachments may also lead to mechanical bowel obstruction.
Imaging: In a type 1 abnormality, contrast injected through the umbilicus passes directly into the ileum. In a type 2 defect, contrast injection reveals a blind-ending sinus tract of varying length. Imaging over the palpable abnormality in a type 3 defect may demonstrate a simple or complex cystic mass. These may also occur as incidental findings in asymptomatic patients.
Urachal Remnant
Overview: The urachus connects the bladder and the allantois; when normally obliterated, it becomes the median umbilical ligament. In a manner analogous to the omphalomesenteric duct, the urachus can be open throughout its course from the bladder to the skin surface at the umbilicus. Alternatively, a portion of the urachus can be open as a blind-ending sinus, from either the dome of the bladder, as a vesicourachal diverticulum, or from the umbilicus, as an umbilicourachal sinus; this is seen in approximately 15% of cases. If only the midportion remains patent, it is known as a urachal cyst, seen in approximately 30% of cases.49
Etiology: Urachal remnants result from failure of obliteration of a portion or of the entire embryonic urachus.
Clinical Presentation: In an infant with a fully patent urachus, leaking of urine is typically noticed in the neonatal period. Other urologic abnormalities—such as hypospadias, posterior urethral valves, or renal ectopia—may be present in a few cases.49 Umbilicourachal sinuses may have intermittent discharge. Vesicourachal diverticula, although typically asymptomatic, may serve as a reservoir for stone formation or for development of malignancy in adulthood.49,50
Imaging: The patent urachus may be seen as a fluid-filled tubular structure that extends from the anterior-superior aspect of the bladder to the umbilicus on US or CT. Voiding cystourethrograms may demonstrate the patent connection to the umbilicus, or injection of the umbilical sinus tract may demonstrate the lesion. The vesicourachal diverticulum appears as an extension of the bladder at its anterosuperior portion and is often found in patients being imaged for prune-belly syndrome.49 An urachal cyst is seen as a well-circumscribed cyst in the anterior abdominal wall on US or CT, with mural thickening and increased enhancement if complicated by infection (Fig. 87-30).51
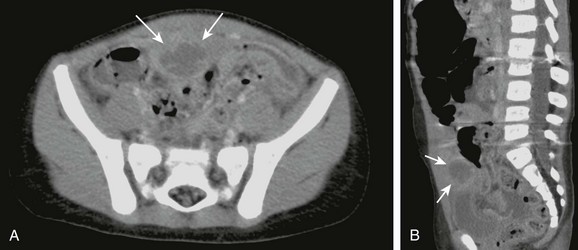
Figure 87-30 Infected urachal cyst.
A, Contrast-enhanced computed tomographic scan of the abdomen and pelvis shows a thick-walled cyst (arrows) with surrounding inflammation just superior to the urinary bladder. This infected urachal cyst was later removed intraoperatively. B, Midline sagittal reformat shows its location with respect to the bladder and umbilicus to better advantage.
Groin and Pelvic Hernias
Overview: Inguinal hernias may be direct or indirect, depending on their relationship to the inferior epigastric vessels. Direct inguinal hernias, with the hernia sac medial to the epigastric vessels, are acquired, rather than congenital, and are uncommon in children. Indirect inguinal hernias, with the hernia sac lateral to the epigastric vessels, are the most common form of inferior abdominal wall herniation. The true incidence of inguinal hernias is difficult to determine, but it ranges from 0.8% to 4.4% in children and is more common in boys.52 Premature infants are at increased risk of developing inguinal hernia, with an approximate 30% incidence.53 Patients with bladder exstrophy, Ehlers-Danlos syndrome, or prune-belly syndrome also have an increased incidence of inguinal hernia.52
Etiology: The processus vaginalis normally closes between the thirty-sixth and fortieth weeks of gestation. However, if it remains open, as in indirect inguinal hernia, abdominal contents can herniate through the inguinal ring into the scrotum in boys (Fig. 87-31). In girls, abdominal contents or ovaries can herniate through the canal of Nuck into the labia majora (Fig. 87-32; see also Fig. 87-31).
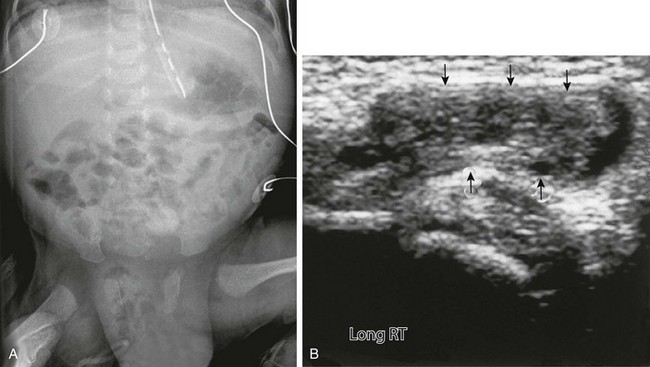
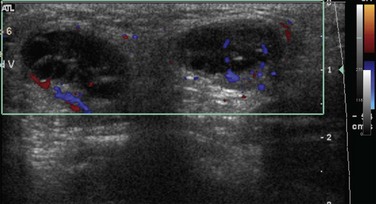
Figure 87-31 Inguinal hernia.
A, Anteroposterior view of the abdomen and pelvis in a newborn boy shows an enlarged scrotum, with multiple air-filled loops of intestine within it, secondary to indirect right inguinal hernia. B, Ultrasound in the inguinal region of an infant girl with a labial mass confirms a herniated ovary (arrows) in the canal of Nuck.
Clinical Presentation: Most inguinal hernias in children are asymptomatic, but incarceration or strangulation can occur (e-Fig. 87-33) and can lead to intestinal obstruction. Asymptomatic patients usually come to medical attention with an intermittent painless bulge in the inguinal region, scrota, or labia that is noticed when the abdominal pressure rises, such as during crying, straining, or coughing.52,53 If the bowel loop becomes entrapped or incarcerated in the hernia, the patient may exhibit signs and symptoms of bowel obstruction, such as abdominal distension or vomiting. If the hernia is not reduced, the blood supply to the trapped bowel loops may be compromised, leading to bowel necrosis and perforation. Incarceration occurs most frequently in the first 6 months of life.53
Imaging: Inguinal hernias may be seen on radiographs as a loop of bowel in the scrotum (see Fig. 87-31, A), but they are often obscured by gonadal shielding. Herniating bowel loops may be an incidental finding on small bowel series, and contrast enema with reflux into the small intestine in infants with intestinal obstruction may show a pinched-off loop at the entrance to the inguinal canal.
In patients with equivocal clinical findings, US is highly accurate (95%) in demonstrating an inguinal hernia (Fig. 87-34; also see Fig. 87-31, B).54 If the bowel loops are filled with fluid, US can identify the intestinal wall surrounding the intraluminal fluid and may show bubbles of air or peristalsis, and color Doppler US may show blood flow in the wall of the herniated bowel loop. These findings help differentiate a hernia from a hydrocele.
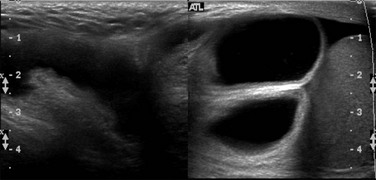
Figure 87-34 Inguinal hernia.
Composite oblique ultrasound image along the inguinal canal from a scrotal sonogram demonstrates an inguinal hernia that contains moderately distended fluid-filled bowel loops. During real time imaging, the herniated bowel loops demonstrated peristalsis. The normal right testis is seen lower in the scrotal sac.
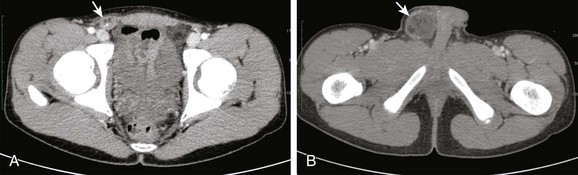
e-Figure 87-33 Incarcerated inguinal hernia.
A and B, Sequential axial images from a contrast-enhanced computed tomographic scan of the abdomen and pelvis demonstrates an incarcerated right inguinal hernia (arrows) that contains omentum, with adjacent inflammatory changes (arrows).
Although hernias may be seen with CT (see Fig. 87-30) and MRI,55,56 these studies are rarely indicated as first-line modalities for diagnosing hernias; usually, they are incidental findings when these examinations are performed for other reasons.
Benign and Malignant Neoplasms
Overview: Abdominal cystic lesions include lymphatic malformations; lesions related to the bowel, such as duplication cysts; and cysts related to the urogenital system, such as urachal cysts. The term mesenteric cyst has been previously applied to cysts of variable histopathologic origin.58,59 More recent literature classifies such “mesenteric cysts” based on histopathologic features, such as lymphatic, mesothelial, enteric, urogenital, or dermoid cysts or mature cystic teratomas and pseudocysts.60,61 This section will discuss lymphatic and mesothelial cystic peritoneal lesions.
Lymphatic Malformations
Overview: Lymphatic malformations, formerly known as lymphangiomas, are the most common type of vasculolymphatic malformation to affect the peritoneal cavity and mesentery.62 Lymphatic malformations have an endothelial lining with walls that contain smooth muscle and lymphatic spaces.60,63
Etiology: Lymphatic malformations do not have a clear origin and have been variously postulated to be developmental, congenital, or neoplastic.64–66 A prevalent hypotheses suggests that they result from the proliferation of abnormal lymphatic channels that do not communicate with the systemic lymphatics.67
Clinical Presentation: Lymphatic malformations may be encountered incidentally, either as a palpable abdominal mass—which, if sufficiently large, could be mistaken for ascites—or during imaging for another reason. When symptomatic, lymphatic malformations may manifest with small bowel obstruction secondary to acute enlargement of the cyst or to segmental volvulus of adjacent bowel loops, with symptoms that include abdominal distension, pain, vomiting, and peritonitis or septic shock.59,62,67,68
Imaging: On US, lymphatic malformations are typically seen as thin-walled, fluid-filled, single or multiple cysts, often with thin septations.11 In the presence of hemorrhage or infection, internal echoes may be detected. Ascites, often chylous, can also be present.62 Despite the internal echoes, Doppler interrogation will reveal no internal flow within the echogenic but cystic component, differentiating it from a solid mass. On real time visualization, movement of the internal echoes can be seen and documented on video clip.
CT shows cystic or multicystic lesions with thin or imperceptible walls, and internal septations may enhance as a result of the presence of vascular structures. The CT attenuation of the cyst contents may be lower than water because of their chylous nature, but usually they are homogenous.62,64
On MRI, lymphatic malformations are typically of low signal on T1- and high signal on T2-weighted sequences.69 Internal hemorrhage may be manifested as increased signal intensity on both T1- and T2-weighted sequences,69 and internal septations are seen as linear structures that may enhance; the peripheral rim and septations may enhance, but the majority of the lesion does not enhance and should not enhance centrally.69 MRI may aid in distinguishing large lymphangiomatous masses that have hemorrhaged from complex intraperitoneal fluid (Fig. 87-35). Cross-sectional imaging is useful to assess the entire extent of the lesion and to define the relationship with adjacent structures.
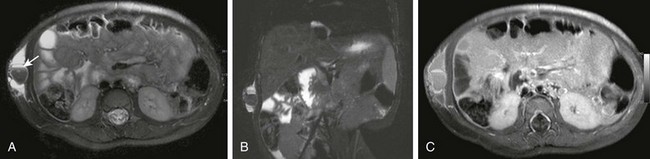
Figure 87-35 Abdominal wall lymphatic malformation.
A 16-month-old girl with 2 weeks of localized swelling in the right flank. A and B, Axial and coronal T2-weighted magnetic resonance images with fat saturation show a multiloculated cystic lesion within the subcutaneous tissues of the abdominal wall with some mass effect upon the underlying musculature. Most of the locules are hyperintense on T2, with a large T2 hypointensity within and a fluid level (arrow) consistent with a hematocrit level. C, After the administration of contrast, T1-weighted, fat-suppressed image shows that the septations enhance, but the internal contents do not, which is typical of a lymphatic malformation. No solid components were detected.
Treatment: Lymphatic malformations may be treated with sclerotherapy or surgical resection. The decision largely depends on the location and size of the lesion. Staged sclerotherapy and/or surgical resection may be required, particularly with multicentric lesions or lesions that extend into the retroperitoneum. The prognosis is generally good, with a low recurrence rate.67
Cysts of Mesothelial Origin
Overview: Cysts of mesothelial origin include simple mesothelial cysts, benign cystic mesotheliomas, and malignant cystic mesothelioma. Simple cysts are unilocular and are usually 1 to 5 cm in size, whereas benign cystic mesotheliomas are large and multilocular.60 Mesothelial cysts are lined by flat cuboidal or columnar cells without lymphatic structures; this distinguishes these lesions from lymphatic malformations.60,63
Benign cystic mesothelioma is a rare, usually multilocular peritoneal lesion that most commonly arises from the peritoneal surface of the pelvis. This lesion has many alternative names that include peritoneal inclusion cyst, multilocular inclusion cyst, and benign multicystic mesothelioma.1 Although most commonly found in middle-aged women, it can also occur in children.1,70–72
Etiology: The cause of mesothelial cysts remains unclear, although theories postulate developmental, neoplastic, and reactive etiologies. The reactive hypothesis suggests that a stimulus produces chronic irritation that can lead to reactive and loculated proliferation of mesothelial cells, resulting in a cystic fluid collection with mesothelial lining.72
Clinical Presentation: Similar to lymphatic malformations, mesothelial cysts can be detected incidentally as asymptomatic masses,63,68 or they may present with acute abdominal symptoms that can mimic appendicitis.58,59,73
Other Benign Lesions
Overview: Lipoblastoma is a rare, benign, mesenchymal fat-containing tumor seen almost exclusively in infants and children. Multiple series support that these tumors affect children under 8 years of age, with 70% to 90% affecting children under 3 years of age, with a slight male predilection.74–79 The most common sites involved are the subcutaneous and superficial soft tissue of the neck and extremities.74,76,77 Approximately 7% of lipoblastomas occur in the abdomen.80 Lipoblastoma differs from lipoblastomatosis in that it is a well-circumscribed, encapsulated lesion, whereas lipoblastomatosis is a diffuse, infiltrating process.74,80
Etiology: Lipoblastomas arise from embryonal white fat, in contradistinction to hibernomas, which arise from brown fat.79 The etiology of the lesions is not known, but lipoblastomas demonstrate chromosomal abnormalities with deletions or abnormal sequencing. The vast majority of cases have characteristic cytogenetic abnormality containing 8q11-13 clonal chromosomal rearrangement that affects PLAG1.81–83 Distinction from liposarcoma, particularly the myxoid variety, may be difficult: histologic criteria are based on a uniform growth pattern, absence of nuclear atypia, and cytogenetics; clinical criteria include patient age, because liposarcomas in children younger than 10 years are very rare.78,83
Clinical Presentation: Clinical manifestations depend upon mass size and location and its effect on adjacent structures. Lipoblastomas frequently manifest as rapidly growing painless masses. Abdominal lipoblastomas may result in symptoms of vomiting, anorexia, abdominal pain, and diarrhea secondary to mass effect upon adjacent structures.80
Imaging: Imaging features of lipoblastoma reflect the underlying pathology, and they vary depending upon the extent of underlying myxoid stroma versus adipose tissue.83 Abdominal radiographs may demonstrate radiolucency,84 although US may be the first imaging to confirm the presence of a mass and to assess its characteristics and location. A lipoblastoma is seen as a homogenously hyperechoic solid lesion, but mixed echogenicity and fluid-filled spaces can be present.83,85 On CT and MRI, lipoblastoma appears as a lobular, fat-containing, well-circumscribed mass, often with internal septations (Fig. 87-36), but the amount of fat will depend on the maturity of the cells that make up the tumor, with the proportion of mature adipocytes positively correlating with fat attenuation and signal characteristics respectively on CT and MRI.83 If fat is the predominant component, the lipoblastoma is usually indistinguishable from a lipoma, with the diagnosis suggested based on patient age. If myxoid stroma is the predominant component, as seen in very young children, imaging will reflect decreased fat85 and a variable amount of contrast enhancement.83
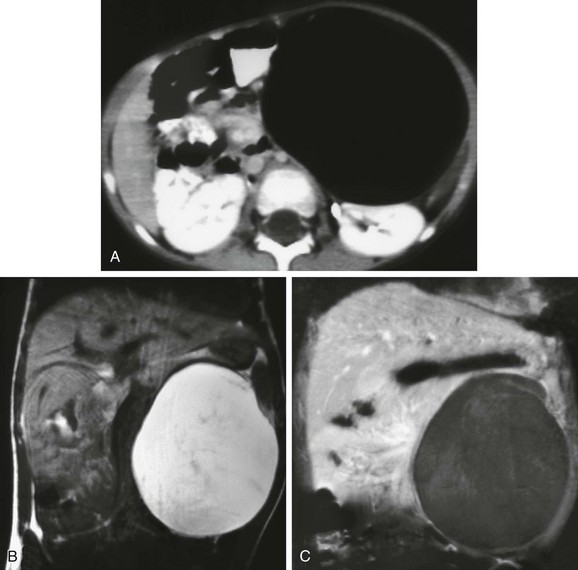
Figure 87-36 Lipoblastoma.
Encapsulated left upper quadrant lipoblastoma attached to the pancreatic tail of a 2-year-old girl. A, Contrast-enhanced computed tomographic image through the midabdomen shows a large fatty mass. Coronal magnetic resonance T1-weighted sequences without (B) and with (C) fat saturation depict the fatty nature of the entire mass as well as some internal septations.
Differential diagnosis includes liposarcoma, which may be difficult to distinguish based solely on imaging findings.78,83 Benign cystic teratomas also contain fat, but these may be distinguished from lipoblastoma by calcification or ossification.83
Treatment and Follow-up: Treatment for lipoblastoma is complete surgical resection, but the lesions may locally recur in up to 9% to 25% of cases. Recurrences are more often seen with lipoblastomatosis, likely related to incomplete resection. Current management suggests that extensive surgery is not justified to achieve complete resection, because the lesions have no malignant potential, and spontaneous regression and maturation into lipoma may occur.83,86,87
Desmoid Tumors
Overview: Desmoid tumors are histologically benign but locally aggressive, nonmetastasizing tumors that belong to a group of disorders known as fibromatoses.88,89 Approximately 37% to 50% of desmoids arise in the abdominal region.90 In the abdominal wall, desmoid tumors arise from the aponeuroses of fascia and near surgical scars.89
These tumors can occur sporadically or in association with familial adenomatous polyposis (FAP) and Gardner syndrome, and they occur in as many as 20% of affected patients.89,91 They can also occur in FAP patients after prophylactic colectomy and are one of the leading causes of death after colectomy in these patients.92,93
Etiology: The etiology of desmoid tumors is unknown but is thought to be multifactorial.90 Their development within the setting of the polyposis syndromes suggests a genetic correlation. Desmoid tumors tend to develop after trauma and often arise 6 to 30 months after colectomy in FAP patients91 or after chemotherapy or radiation treatment.88 There is also evidence of hormonal influence, and some tumors express estrogen receptors.88 These lesions are more common in women, with a ratio as high as 4 : 1.90
Clinical Presentation: Abdominal desmoids may be asymptomatic. However, as they enlarge, they may infiltrate adjacent structures and lead to symptoms related to compression of bowel, vascular, or other retroperitoneal structures.90
Imaging: Desmoid tumors vary in imaging characteristics, depending upon the amounts of collagen, proliferating fibroblasts, fibrosis, and vascularity. The fibrous and cellular composition of desmoid tumors varies with the stage of their evaluation.
On US, the margins may be ill defined or irregular, and the echogenicity is variable. CT density and margins likewise vary. On MR, desmoid tumors typically demonstrate decreased signal intensity on T1- and variable signal intensity on T2-weighted sequences compared with muscle. An association has been found between increased tumor cellularity, as shown on T2-weighted images, and a tendency to rapid tumor growth.89 Enhancement patterns are variable (Fig. 87-37). MRI is a useful method for staging desmoid tumors, assessing stage of activity, and detecting recurrence.
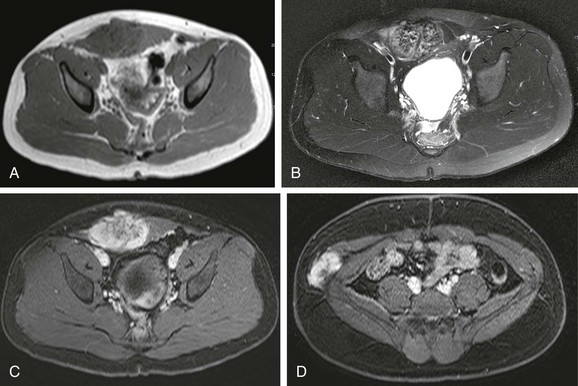
Figure 87-37 Desmoid tumor.
A 16-year-old girl had an abdominal wall mass for 1 year. A, Axial T1-weighted magnetic resonance image shows a relatively well-defined rounded mass within the right rectus muscle. B, Axial T2-weighted image with fat saturation shows a heterogenous appearance to the mass. C, T1-weighted fat saturation after administration of contrast shows avid enhancement, especially when compared with adjacent muscle. D, An additional lesion with similar imaging characteristics is also identified more laterally in the abdominal wall.
Treatment: Surgical resection has been the mainstay of treatment; however, many abdominal desmoids are discovered when they are no longer easily resectable, particularly with sufficiently wide margins to prevent recurrence, thus risking significant morbidity and mortality.93 The goal of resection is negative margins; positive margins or incomplete resection are treated with adjuvant radiotherapy.73,91,94 Other systemic therapies have been tried, including steroids, antiestrogen agents such as tamoxifen, nonsteroidal antiinflammatory agents such as sulindac, and chemotherapeutic agents such as vinblastine and methotrexate.91,94
Pseudomyxoma Peritonei
Overview: Pseudomyxoma peritonei occurs when the omental and peritoneal surfaces are caked with copious amounts of mucinous or gelatinous material.2
Etiology: Pseudomyxoma peritonei arises from an appendiceal lesion in most patients, through rupture of an appendiceal mucinous adenoma into the peritoneal cavity. Abundant amounts of mucus, which may or may not contain epithelial cells, spreads through peritoneal pathways and accumulates in spaces such as the cul-de-sac, Morison pouch, and paracolic gutters.2,95 Pseudomyxoma peritonei may also originate in, or may secondarily involve, the ovary.95
Clinical Presentation: Pseudomyxoma peritonei is more common in adults and affects women more than men.95 Patients typically present with abdominal pain and weight loss, despite increasing abdominal size.2
Imaging: Imaging findings are generally similar to those seen in massive ascites. US reveals ascitic fluid that demonstrates nonmobile internal echoes.2 Septations and occasionally solid-appearing masses can be seen. On CT the mucinous material is typically low density and accumulates in dependent areas as discussed above. Scalloping of visceral surfaces, particularly the liver, is an important imaging sign to differentiate pseudomyxoma peritonei from ascites.2 On imaging it is difficult to differentiate the benign form of pseudomyxoma peritonei from peritoneal mucinous carcinomatosis. Adenopathy, pleural involvement, enhancing masses, and invasion of visceral organs favors a malignant process.2
Treatment: The treatment for the benign forms of pseudomyxoma peritonei consists mainly of surgical evacuation and appendectomy, with the potential need for resection of other involved structures. Malignant causes need additional treatment, such as intraperitoneal instillation of chemotherapeutic agents and radiotherapy.96,97
Malignant Lesions
Peritoneal Metastases and Carcinomatosis
Overview: Peritoneal metastatic spread is rare in pediatric patients. Germ cell tumors or carcinoma of the colon account for up to 47% of cases of peritoneal carcinomatosis in pediatric patients.98 Other tumors that can be seen with diffuse peritoneal dissemination include Wilms tumors, neuroblastoma, teratoma, desmoplastic small round cell tumor (DSRCT), and non-Hodgkin lymphoma.98,99
Etiology: Malignancy disseminates throughout the peritoneum by intraperitoneal seeding, direct invasion, hematogenous spread, or lymphatic dissemination.2 Peritoneal metastases frequently occur in the presence of other sites of metastatic disease. Seeding may occur at the time of operative intervention through tumor rupture and intraperitoneal spillage, with rare instances of intraabdominal spread of intracranial disease via a ventriculoperitoneal shunt.
Malignant cells migrate through the normal circulation of the peritoneal fluid. The adhesion of tumor cells to mesothelial cells is believed to be mediated through the expression of intercellular adhesion molecules.2,100 Ascites results when disseminated disease interferes with the absorptive capacity of the mesothelial lining of the peritoneum.100
Clinical Presentation: Peritoneal metastases may initially be asymptomatic; with progression of disease, patients come to medical attention with abdominal enlargement, ascites, nausea, and abdominal pain.98,99 Approximately 20% are seen with bowel obstruction and 50% with ascites.100,101 CT may demonstrate solitary or multiple masses, diffuse peritoneal thickening, peritoneal enhancement, and tumor studding of the peritoneal surfaces.98 Diffuse caking is a feature of peritoneal metastases from rhabdomyosarcoma, non-Hodgkin lymphoma, and germ cell tumors (e-Figs. 87-38 and 87-39).98
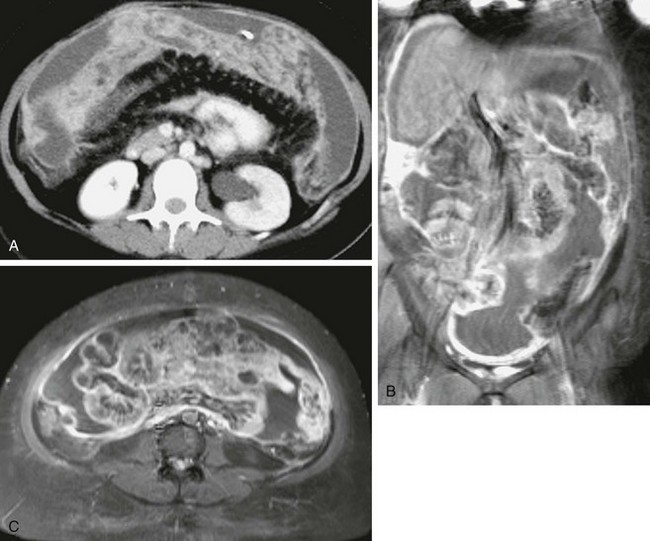
e-Figure 87-38 Peritoneal mesothelioma.
An 18-year-old patient with a history of posterior fossa medulloblastoma is seen with new onset of hemorrhagic ascites. A, Contrast-enhanced computed tomographic image through the midabdomen demonstrates abundant ascites and abnormal enhancement of the peritoneal surfaces. Note diffuse, enhancing omental caking. Coronal (B) and axial (C) contrast-enhanced T1-weighted magnetic resonance images of the abdomen and pelvis demonstrate the thickened, intensely enhancing peritoneal surfaces and abundant ascites.
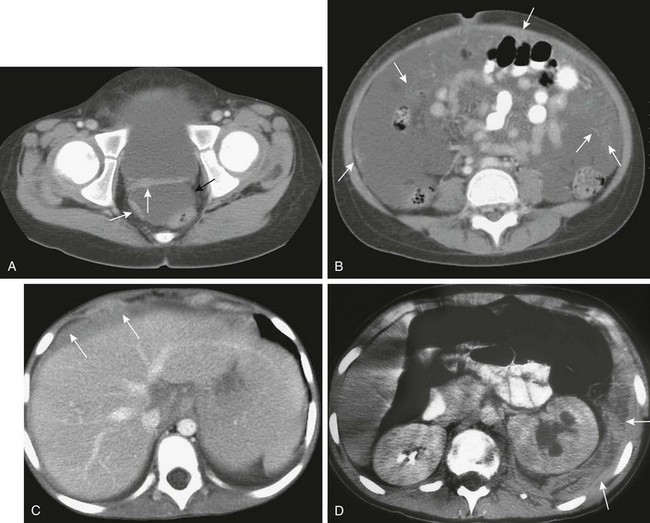
e-Figure 87-39 Peritoneal metastases may have a varied appearance.
A, Axial contrast-enhanced computed tomographic (CT) image of the deep pelvis shows a moderate amount of pelvic ascites (black arrow), resulting from the peritoneal metastases evidenced by abnormal thickening and enhancement of the peritoneal surface (white arrows) in a patient with history of desmoplastic small round cell tumor (DSRCT) and recurrent ascites. B, Contrast-enhanced CT through the midabdomen in a patient with epithelioid sarcoma shows more extensive peritoneal disease and complex ascites. Note tiny masses studding the peritoneum (arrows) and numerous strands of solid disease throughout the ascites (arrows). Contrast-enhanced CT images in a patient with Burkitt lymphoma. C, Patient with metastatic melanoma. D, Diffuse thickening of the peritoneal surface (arrows in C and D). E, Transverse ultrasound image shows metastatic disease manifested as a discrete mass (arrow) surrounded by ascitic fluid. F, Contrast-enhanced CT image shows a cystic peritoneal metastasis (arrow) in a patient with neuroblastoma.
MRI detection of peritoneal implants may be improved over CT because of the inherent tissue contrast and multiplanar capabilities.2 Peritoneal implants tend to be hypointense on T1- and hyperintense on T2-weighted sequences, with variable enhancement.100 Delayed images obtained 10 to 15 minutes after gadolinium administration may show lesions better, because they are slow to enhance.2,100 However, respiratory and bowel motion may degrade identification of peritoneal and serosal lesions. Positron emission tomography (PET) or CT scan may also help to identify peritoneal carcinomatosis with greater confidence, depending on the size of the lesions.2
Desmoplastic Small Round Cell Tumor
Overview: DSRCT is a rare and aggressive abdominal malignancy that belongs to the small, round, blue cell tumor family.101–103 Intraperitoneal involvement is most common, and less common sites include paratesticular, pleural, lung, ovary, sinus, central nervous system, kidney, and stomach.102,104,105
Etiology: The etiology of DSRCT is unknown; it has been hypothesized that the tumor originates from the mesothelial, submesothelial, or subserosal mesenchyma.100 Pathologically DSRCTs are part of the small, blue, round cell family that includes Ewing sarcoma, neuroblastoma, Wilms tumor, rhabdomyosarcoma, and primitive neuroectodermal tumors. Immunocytochemical staining is usually required to distinguish DSRCTs from other small round cell tumors.106
Clinical Presentation: DSCRTs typically affect adolescents and young adults aged 15 to 25 years, and there is a strong male predilection with ratios that range from 3 : 1 to 9 : 1. Patients generally come to medical attention with vague abdominal pain and/or distension, and palpable abdominal masses may be present on physical exam.101,104
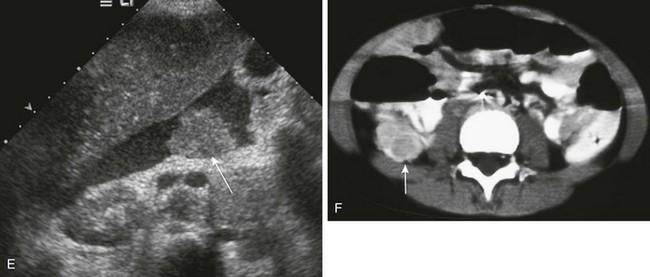
Imaging: On CT imaging, DSRCT is usually seen as multiple, scattered peritoneal masses without an apparent primary parenchymal source. The masses are generally of low attenuation and have a variable degree of central necrosis or hemorrhage with mild to moderate contrast enhancement.102,107 US and MRI findings for DSRCT are variable and nonspecific (e-Fig. 87-40). The utility of fluorodeoxyglucose PET/CT is still evolving and is currently used to evaluate metastatic progression and response to systemic therapy.108,109
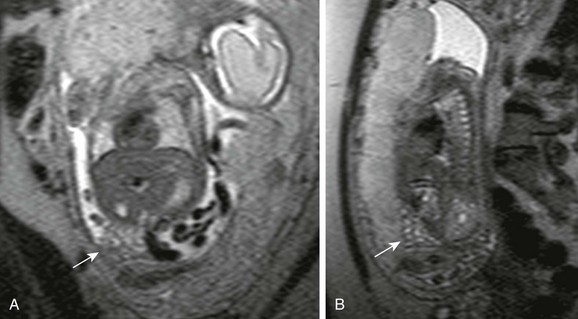
e-Figure 87-40 Desmoplastic small round cell tumor in a 14 year-old boy with 2-month history of fatigue, poor appetite, and anemia.
A, Axial contrast-enhanced pelvic computed tomographic image shows a well-defined midline pelvic mass that is of homogenous intermediate density. B, Corresponding axial T2-weighted magnetic resonance image shows the mass to have diffusely increased signal with mild heterogeneity. C, The appearance in a short-tau inversion recovery sequence is similar to B. D, T1-weighted coronal image shows the mass to be isointense with muscle. E, T1-weighted fat-saturated coronal image with contrast demonstrates mild heterogenous enhancement of the lesion.
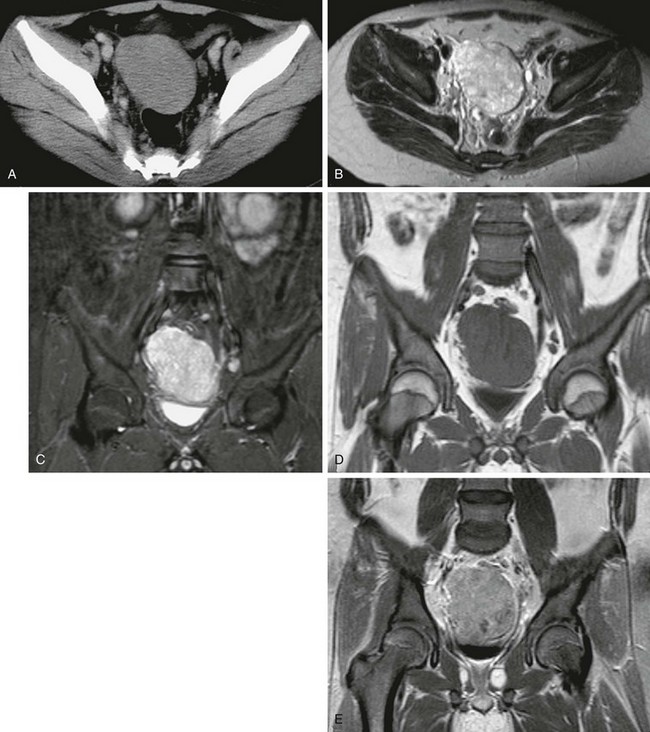
Tumor spread by direct peritoneal seeding results in multiple omental and mesenteric masses. Liver metastases, both intrahepatic (hematogenous) and serosal, are seen in up to 50% of patients (Fig. 87-41, B).104,107 Other hematogenous metastatic lesions are less common. In addition, hydronephrosis and small-bowel obstruction may result from mass effect. Multiple studies have found a correlation between retroperitoneal lymphadenopathy and ascites with a dominant or large pelvic mass (see Fig. 87-41).1,104,110 Although imaging features are usually nonspecific, the combination of a dominant pelvic mass, scattered small peritoneal masses, retroperitoneal lymphadenopathy, and ascites is somewhat characteristic for this entity. Primary differential diagnostic considerations include peritoneal carcinomatosis, rhabdomyosarcoma, neuroblastoma, lymphoma, and germ cell tumors.104,110,111
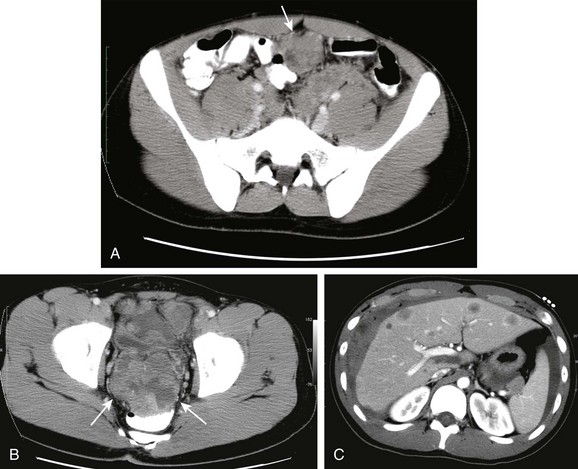
Figure 87-41 Desmoplastic small round cell tumor.
A and B, Contrast-enhanced computed tomographic images in a 17-year-old boy show multiple heterogenously enhancing pelvic masses (arrows) with a dominant mass seen within the rectovesical pouch. C, Metastatic spread and peritoneal seeding are evident as multiple enhancing nodules within the liver with a large, heterogenous, peritoneal subcapsular mass indenting the hepatic surface.
Treatment: Treatment generally includes a combination of chemotherapy and surgical resection. Chemotherapy may be useful for presurgical treatment, to reduce tumor bulk prior to resection, because improved outcomes have been reported in patients with complete resection.102,103,112 However, DSRCT is an aggressive malignancy that has a poor prognosis despite treatment, with a reported 29% survival at 3 years.112
Miscellaneous Tumors
Rhabdomyosarcoma comprises about half of pediatric soft tissue sarcomas but is less common in the trunk (Fig. 87-42).113 Abdominal wall and other truncal rhabdomyosarcomas have a poorer prognosis when compared with other sites.113 The truncal lesions are more likely to be of the embryonal than the alveolar type, tend to present with advanced disease, and are more likely to be greater than 5 cm at presentation. The ability to undergo gross total resection influences the prognosis, and gross total resection should be the goal of therapy.
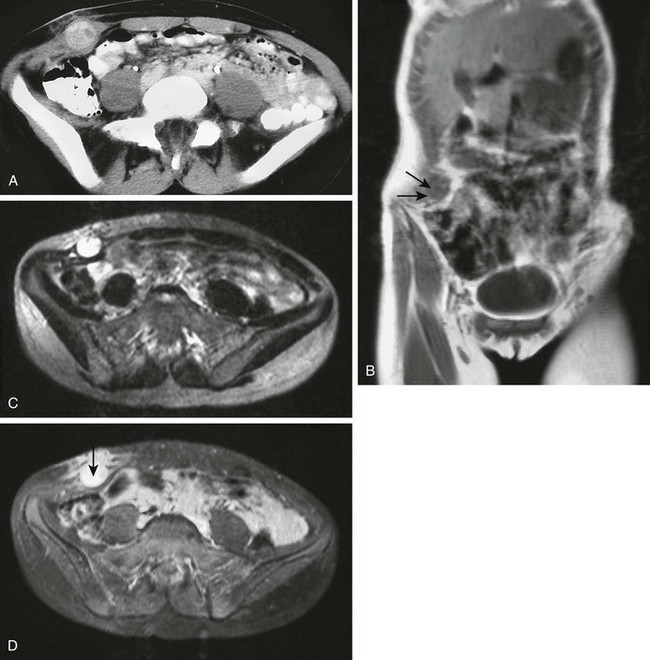
Figure 87-42 Embryonal rhabdomyosarcoma.
A 13-year-old girl with a firm subcutaneous mass in the right lower abdominal wall. A, Contrast-enhanced computed tomographic image of the lower abdomen shows a heterogenously enhancing mass that causes focal enlargement of the right lateral oblique muscles. Edema is evident within the subcutaneous fat overlying the mass. Local infiltration by tumor cannot be excluded on this image. B, Oblique, coronal, noncontrast T1-weighted MR image shows the 3-cm round tissue mass within the right lateral oblique muscles (arrows). C, On an axial T2-weighted magnetic resonance image, the mass exhibits intense signal (as it did on short-tau inversion recovery sequence, not shown). D, Intense enhancement is seen with intravneous contrast administration in the soft tissue mass and in the overlying subcutaneous fat (arrow). This finding warrants concern about tumor infiltration.
Synovial sarcoma is a rare malignancy constituting 5% to 6% of pediatric soft tissue sarcomas, It most often originates in the extremities and near large joints, but it may rarely affect the anterior abdominal wall.114
Leiomyosarcoma often originates in the retroperitoneum, genitourinary, or GI tract and lower extremity.115 Rarely, such a lesion may arise in the omentum (Figs. 87-43 and 87-44).
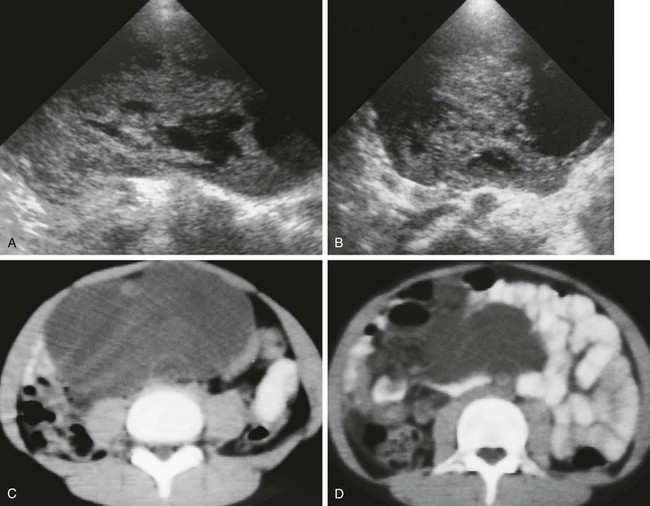
Figure 87-43 Leiomyosarcoma.
Longitudinal (A) and transverse (B) ultrasound images through the midabdomen in a 10-year-old boy show a large, heterogenous, solid and cystic mass. C and D, Computed tomographic images without intravenous contrast show a large, homogenous, low-density, midline soft tissue mass displacing bowel.
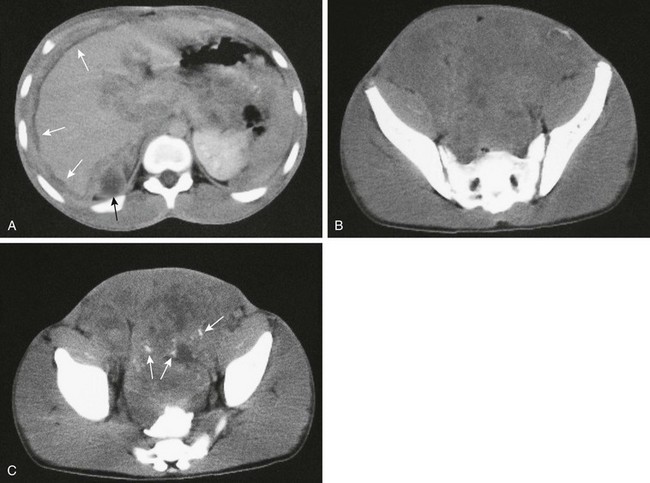
Figure 87-44 Leiomyosarcoma.
A, Contrast-enhanced computed tomographic image in a 16-year-old boy shows extensive peritoneal metastases around the liver (white arrows) and surrounding the pancreas in addition to a cystic peritoneal metastasis (black arrow). B, Lower image in the upper pelvis shows that the mass occupies most of the lower abdomen and pelvis. C, Another image lower in the pelvis shows scattered calcifications (arrows). Bowel contrast is present in the rectum.
Liposarcoma has been briefly discussed; the presence of fat may help to distinguish this tumor from other sarcomas. Although more commonly seen in the retroperitoneum, it is the least frequent soft tissue sarcoma to occur in childhood.116
Fibrosarcoma has two forms in children: the congenital or infantile form occurs in children up to 2 years of age, and the childhood form affects the 10- to 15-year-old age group (Fig. 87-45). The histology of the two forms is similar, but the infantile form has a distinct chromosomal translocation. Infantile fibrosarcoma has been reported to involve the trunk and retroperitoneum in children.115,117 After complete surgical resection, the prognosis is better for the congenital or infantile form than for the childhood/adult form.117
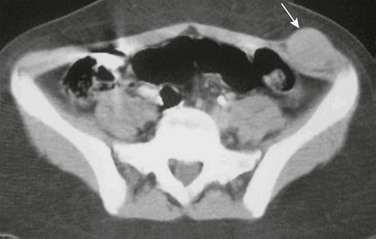
Figure 87-45 Fibrosarcoma.
Contrast-enhanced computed tomographic image through the upper pelvis in a 14-year-old girl shows a well-defined, round 3-cm mass in the left anterior abdominal wall. The mass expands and is isointense with the left lateral oblique muscles (arrow).
Malignant mesenchymomas are very rare soft tissue tumors seen predominantly in adults. These tumors contain at least two distinct histologic sarcoma subtypes and are considered to be high-grade tumors with an overall poor prognosis. The most common sites of primary malignant mesenchymoma are the retroperitoneum or the thigh, but these have been reported in various locations.118 CT findings are of a soft tissue mass with mixed attenuation that often contains areas of necrosis and calcification, with heterogenous enhancement and moderate vascularity (Fig. 87-46). Using MR imaging, these lesions are typically heterogenous on T2-weighted images.119 Treatment includes a combination of surgical incision, radiation therapy, and chemotherapy.
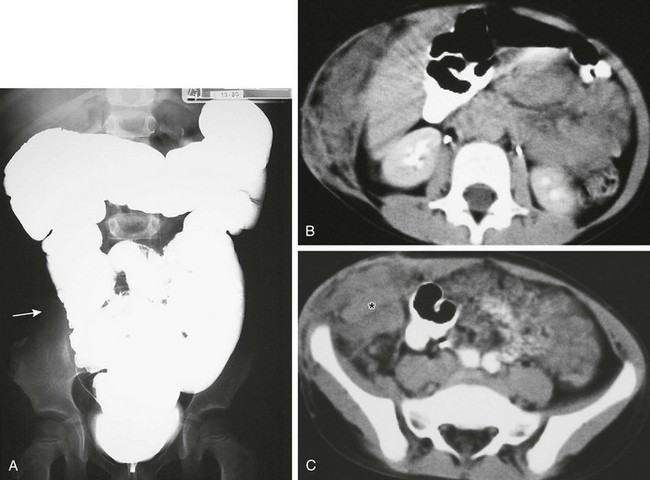
Figure 87-46 Recurrent mesenchymoma in a 5-year-old girl originally diagnosed at age 3 years.
A, Abdominal radiograph from a barium enema demonstrates a normal colon with displacement of the right colon (arrow) from the adjacent abdominal wall mesenchymoma. B and C, Axial computed tomographic images after intravenous and oral contrast administration show expansion of the anterolateral abdominal wall by heterogenous infiltrative soft tissue (asterisk). Extension from the subcutaneous location (B and C) infiltrates the abdomen, with mass effect on adjacent structures, most notably the right colon, as depicted in A.
Malignant mesothelioma of the peritoneum is rare in children and does not seem to be related to radiation or asbestos exposure.1,120 Children with peritoneal malignant mesothelioma come to medical attention with ascites and multiple tumor nodules along the peritoneal surface (see e-Fig. 87-38).120
Agarwal, A, et al. Peritoneal calcification: causes and distinguishing features on CT. AJR Am J Roentgenol. 2004;182(2):441–445.
Bellah, R, et al. Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults. AJR Am J Roentgenol. 2005;184(6):1910–1914.
Chiu, YH, et al. Reappraisal of radiographic signs of pneumoperitoneum at emergency department. Am J Emerg Med. 2009;27(3):320–327.
Ledbetter, DJ. Gastroschisis and omphalocele. Surg Clin North Am. 2006;86(2):249–260. vii
Patel, A, et al. Abdominal compartment syndrome. AJR Am J Roentgenol. 2007;189(5):1037–1043.
References
1. Levy, AD, et al. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28(2):583–607. [quiz 621-622].
2. Levy, AD, Shaw, JC, Sobin, LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29(2):347–373.
3. Wiot, J, et al. Postoperative pneumoperitoneum in children. Radiology. 1967;89:285.
4. Chiu, YH, et al. Reappraisal of radiographic signs of pneumoperitoneum at emergency department. Am J Emerg Med. 2009;27(3):320–327.
5. Miller, RE. Perforated viscus in infants: a new roentgen sign. Radiology. 1960;74:65–67.
6. Leonidas, JC, et al. Pneumoperitoneum in ventilated newborns: a medical or a surgical problem? Am J Dis Child. 1974;128(5):677–680.
7. Greenberg, BE. Spontaneous pneumoperitoneum in appendicitis. Radiology. 1961;77:248–251.
8. Menuck, L, Siemers, PT. Pneumoperitoneum: importance of right upper quadrant features. AJR Am J Roentgenol. 1976;127(5):753–756.
9. Levine, MS, et al. Diagnosis of pneumoperitoneum on supine abdominal radiographs. AJR Am J Roentgenol. 1991;156(4):731–735.
10. Brill, PW, Olson, SR, Winchester, P. Neonatal necrotizing enterocolitis: air in Morison pouch. Radiology. 1990;174(2):469–471.
11. Cho, KC, Baker, SR. Air in the fissure for the ligamentum teres: new sign of intraperitoneal air on plain radiographs. Radiology. 1991;178(2):489–492.
12. Seibert, JJ, Parvey, LS. The telltale triangle: use of the supine cross table lateral radiograph of the abdomen in early detection of pneumoperitoneum. Pediatr Radiol. 1977;5(4):209–210.
13. Bray, JF. The “inverted V” sign of pneumoperitoneum. Radiology. 1984;151(1):45–46.
14. Chen, SC, et al. Selective use of ultrasonography for the detection of pneumoperitoneum. Acad Emerg Med. 2002;9(6):643–645.
15. Stapakis, JC, Thickman, D. Diagnosis of pneumoperitoneum: abdominal CT vs. upright chest film. J Comput Assist Tomogr. 1992;16(5):713–716.
16. Ly, JQ. The Rigler sign. Radiology. 2003;228(3):706–707.
17. Kliegman, RM, Stanton, B. Nelson Textbook of Pediatrics, 19th ed. Philadelphia, PA: Elsevier Saunders; 2011.
18. Patel, A, et al. Abdominal compartment syndrome. AJR Am J Roentgenol. 2007;189(5):1037–1043.
19. Hendrick, JM, et al. Abdominal compartment syndrome in a newly diagnosed patient with Burkitt lymphoma. Pediatr Radiol. 2006;36(3):254–257.
20. Hoffman, RB, Wankmuller, R, Rigler, LG. Pseudoseparation of bowel loops: a fallacious sign of intraperitoneal fluid. Radiology. 1966;87(5):845–847.
21. Thoeni, RF. The role of imaging in patients with ascites. AJR Am J Roentgenol. 1995;165(1):16–18.
22. Colli, A, et al. Thickening of the gallbladder wall in ascites. J Clin Ultrasound. 1991;19(6):357–359.
23. Pandolfo, I, et al. Mediastinal pseudotumor due to passage of ascites through the esophageal hiatus. Gastrointest Radiol. 1989;14(3):209–211.
24. Dinkel, E, et al. Sonographic evidence of intraperitoneal fluid. An experimental study and its clinical implications. Pediatr Radiol. 1984;14(5):299–303.
25. Johnson, CC, Baldessarre, J, Levison, ME. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis. 1997;24(6):1035–1045. [quiz 1046-1047].
26. Chadha, V, Schaefer, FS, Warady, BA. Dialysis-associated peritonitis in children. Pediatr Nephrol. 2010;25(3):425–440.
27. Na-ChiangMai, W, et al. CT findings of tuberculous peritonitis. Singapore Med J. 2008;49(6):488–491.
28. Andronikou, S, Welman, CJ, Kader, E. The CT features of abdominal tuberculosis in children. Pediatr Radiol. 2002;32(2):75–81.
29. Burrill, J, et al. Tuberculosis: a radiologic review. Radiographics. 2007;27(5):1255–1273.
30. Ha, HK, et al. CT differentiation of tuberculous peritonitis and peritoneal carcinomatosis. AJR Am J Roentgenol. 1996;167(3):743–748.
31. Duhn, R, Schoen, EJ, Siu, M. Subcutaneous fat necrosis with extensive calcification after hypothermia in two newborn infants. Pediatrics. 1968;41(3):661–664.
32. Hogeling, M, et al. Extensive subcutaneous fat necrosis of the newborn associated with therapeutic hypothermia. Pediatr Dermatol. 2012;29(1):59–63.
33. Naidech, HJ, Chawla, HS. Soft-tissue calcification after subcutaneous emphysema in a neonate. AJR Am J Roentgenol. 1982;139(2):374–376.
34. Agarwal, A, et al. Peritoneal calcification: causes and distinguishing features on CT. AJR Am J Roentgenol. 2004;182(2):441–445.
35. Scales, JW, et al. An infant with firm, fixed plaques. Arch Dermatol. 1998;134(4):425–426.
36. Klein, M. Congenital defects of the abdominal wall. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
37. Ledbetter, DJ. Gastroschisis and omphalocele. Surg Clin North Am. 2006;86(2):249–260. [vii].
38. Cantrell, JR, Haller, JA, Ravitch, MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet. 1958;107(5):602–614.
39. Nicolaides, KH, et al. Fetal gastro-intestinal and abdominal wall defects: associated malformations and chromosomal abnormalities. Fetal Diagn Ther. 1992;7(2):102–115.
40. Bair, JH, et al. Fetal omphalocele and gastroschisis: a review of 24 cases. AJR Am J Roentgenol. 1986;147(5):1047–1051.
41. Salihu, HM, et al. Omphalocele and gastroschisis in the State of New York, 1992-1999. Birth Defects Res A Clin Mol Teratol. 2003;67(9):630–636.
42. Laughon, M, et al. Rising birth prevalence of gastroschisis. J Perinatol. 2003;23(4):291–293.
43. Kuleva, M, et al. Is complex gastroschisis predictable by prenatal ultrasound? BJOG. 2012;119(1):102–109.
44. Fogata, ML, et al. Prenatal diagnosis of complicated abdominal wall defects. Curr Probl Diagn Radiol. 1999;28(4):101–128.
45. Molik, KA, et al. Gastroschisis: a plea for risk categorization. J Pediatr Surg. 2001;36(1):51–55.
46. Snyder, CL, et al. Management of intestinal atresia in patients with gastroschisis. J Pediatr Surg. 2001;36(10):1542–1545.
47. DiSantis, DJ, Siegel, MJ, Katz, ME. Simplified approach to umbilical remnant abnormalities. Radiographics. 1991;11(1):59–66.
48. Clley, R. Disorders of the umbilicus. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
49. Yu, JS, et al. Urachal remnant diseases: spectrum of CT and US findings. Radiographics. 2001;21(2):451–461.
50. Snyder, CL. Current management of umbilical abnormalities and related anomalies. Semin Pediatr Surg. 2007;16(1):41–49.
51. Nagasaki, A, Handa, N, Kawanami, T. Diagnosis of urachal anomalies in infancy and childhood by contrast fistulography, ultrasound and CT. Pediatr Radiol. 1991;21(5):321–323.
52. Kapur, P, Caty, MG, Glick, PL. Pediatric hernias and hydroceles. Pediatr Clin North Am. 1998;45(4):773–789.
53. Glick, PL, Boulanger, S. Inguinal hernias and hydroceles. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
54. Chou, TY, et al. Inguinal hernia in children: US versus exploratory surgery and intraoperative contralateral laparoscopy. Radiology. 1996;201(2):385–388.
55. Burkhardt, JH, et al. Diagnosis of inguinal region hernias with axial CT: the lateral crescent sign and other key findings. Radiographics. 2011;31(2):E1–E12.
56. van den Berg, JC. Inguinal hernias: MRI and ultrasound. Semin Ultrasound CT MR. 2002;23(2):156–173.
57. Brandt, ML. Pediatric hernias. Surg Clin North Am. 2008;88(1):27–43. [vii-viii].
58. Hebra, A, et al. Mesenteric, omental, and retroperitoneal cysts in children: a clinical study of 22 cases. South Med J. 1993;86(2):173–176.
59. Bliss, DP, Jr., et al. Mesenteric cysts in children. Surgery. 1994;115(5):571–577.
60. de Perrot, M, et al. Mesenteric cysts. Toward less confusion? Dig Surg. 2000;17(4):323–328.
61. Tan, JJ, Tan, KK, Chew, SP. Mesenteric cysts: an institution experience over 14 years and review of literature. World J Surg. 2009;33(9):1961–1965.
62. Konen, O, et al. Childhood abdominal cystic lymphangioma. Pediatr Radiol. 2002;32(2):88–94.
63. Takiff, H, et al. Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch Surg. 1985;120(11):1266–1269.
64. Pickhardt, PJ, Bhalla, S. Primary neoplasms of peritoneal and sub-peritoneal origin: CT findings. Radiographics. 2005;25(4):983–995.
65. Levine, C. Primary disorders of the lymphatic vessels–a unified concept. J Pediatr Surg. 1989;24(3):233–240.
66. de Perrot, M, et al. Abdominal lymphangioma in adults and children. Br J Surg. 1998;85(3):395–397.
67. Ricketts, R. Mesenteric and Omental Cysts. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
68. Kosir, MA, Sonnino, RE, Gauderer, MW. Pediatric abdominal lymphangiomas: a plea for early recognition. J Pediatr Surg. 1991;26(11):1309–1313.
69. Kim, JS, et al. Maximizing time-resolved MRA for differentiation of hemangiomas, vascular malformations and vascularized tumors. Pediatr Radiol. 2012;42(7):775–784.
70. Terry, NE, Fowler, CL. Benign cystic mesothelioma in a child. J Pediatr Surg. 2009;44(5):e9–e11.
71. Stojsic, Z, et al. Benign cystic mesothelioma of the peritoneum in a male child. J Pediatr Surg. 2012;47(10):e45–e49.
72. Shakya, VC, et al. Benign cystic mesothelioma of the peritoneum in a child-case report and review of the literature. J Pediatr Surg. 2011;46(4):e23–e26.
73. Coran, AG, Adzick, NS. Pediatric surgery, 7th ed. Philadelphia, PA: Elsevier Mosby; 2012.
74. Chung, EB, Enzinger, FM. Benign lipoblastomatosis. An analysis of 35 cases. Cancer. 1973;32(2):482–492.
75. Collins, MH, Chatten, J. Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 25 tumors. Am J Surg Pathol. 1997;21(10):1131–1137.
76. Stringel, G, et al. Lipoblastoma in infants and children. J Pediatr Surg. 1982;17(3):277–280.
77. Jung, SM, et al. Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 16 cases in Taiwan. Pediatr Surg Int. 2005;21(10):809–812.
78. Mentzel, T, Calonje, E, Fletcher, CD. Lipoblastoma and lipoblastomatosis: a clinicopathological study of 14 cases. Histopathology. 1993;23(6):527–533.
79. Hicks, J, et al. Lipoblastoma and lipoblastomatosis in infancy and childhood: histopathologic, ultrastructural, and cytogenetic features. Ultrastruct Pathol. 2001;25(4):321–333.
80. Cudnik, R, et al. Mesenteric lipoblastoma: a rare location in children. J Pediatr Surg. 2008;43(12):e5–e7.
81. Hibbard, MK, et al. PLAG1 fusion oncogenes in lipoblastoma. Cancer Res. 2000;60(17):4869–4872.
82. Brandal, P, Bjerkehagen, B, Heim, S. Rearrangement of chromosomal region 8q11-13 in lipomatous tumours: correlation with lipoblastoma morphology. J Pathol. 2006;208(3):388–394.
83. Moholkar, S, Sebire, NJ, Roebuck, DJ. Radiological-pathological correlation in lipoblastoma and lipoblastomatosis. Pediatr Radiol. 2006;36(8):851–856.
84. Murphey, MD, et al. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433–1466.
85. Mo, YH, et al. Mesenteric lipoblastoma: case report. Pediatr Radiol. 2003;33(1):37–40.
86. Jones, KB, et al. Unusual presentation of lipoblastoma as a skin dimple of the thigh. A report of three cases. J Bone Joint Surg Am. 2004;86-A(5):1040–1046.
87. Mognato, G, et al. Is surgical treatment of lipoblastoma always necessary? J Pediatr Surg. 2000;35(10):1511–1513.
88. Rosoff, PM, Larrier, N, Rice, HE. Intra-abdominal desmoid tumor after successful treatment for Hodgkin disease. Pediatr Blood Cancer. 2005;45(5):728–731.
89. Healy, JC, et al. MR appearances of desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol. 1997;169(2):465–472.
90. Shields, CJ, et al. Desmoid tumours. Eur J Surg Oncol. 2001;27(8):701–706.
91. Lelli, JJ. Polypoid diseases of the gastrointestinal tract. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
92. Arvanitis, ML, et al. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990;33(8):639–642.
93. Quintini, C, et al. Mortality of intra-abdominal desmoid tumors in patients with familial adenomatous polyposis: a single center review of 154 patients. Ann Surg. 2012;255(3):511–516.
94. Jordan, A. Other soft tissue tumors. In: Coran A, et al, eds. Coran: Pediatric Surgery. Philadelphia: Elsevier, 2012.
95. van Ruth, S, et al. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol. 2003;29(8):682–688.
96. Jurgeleit, HC. Pseudomyxoma peritonei. A localized, benign variant of appendiceal origin. Dis Colon Rectum. 1986;29(7):469–470.
97. Qu, ZB, Liu, LX. Management of pseudomyxoma peritonei. World J Gastroenterol. 2006;12(38):6124–6127.
98. Kaste, SC, et al. Peritoneal metastases in children with cancer. Cancer. 1998;83(2):385–390.
99. Andronikou, S, Moon, A, Ussher, R. Peritoneal metastatic disease in a child after excision of a solid pseudopapillary tumour of the pancreas: a unique case. Pediatr Radiol. 2003;33(4):269–271.
100. Huh, WW, et al. Peritoneal sarcomatosis in pediatric malignancies. Pediatr Blood Cancer. 2013;60(1):12–17.
101. Gerald, WL, Rosai, J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9(2):177–183.
102. Kim, YS, et al. Retroperitoneal desmoplastic small round cell tumor: pediatric patient treated with multimodal therapy. World J Gastroenterol. 2009;15(33):4212–4214.
103. Mingo, L, Seguel, F, Rollan, V. Intraabdominal desmoplastic small round cell tumour. Pediatr Surg Int. 2005;21(4):279–281.
104. Bellah, R, et al. Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults. AJR Am J Roentgenol. 2005;184(6):1910–1914.
105. Egloff, AM, et al. Desmoplastic small round cell tumor of the kidney in a pediatric patient: sonographic and multiphase CT findings. AJR Am J Roentgenol. 2005;185(5):1347–1349.
106. Leuschner, I, Radig, K, Harms, D. Desmoplastic small round cell tumor. Semin Diagn Pathol. 1996;13(3):204–212.
107. Pickhardt, PJ, et al. Desmoplastic small round cell tumor of the abdomen: radiologic-histopathologic correlation. Radiology. 1999;210(3):633–638.
108. Kushner, BH, et al. Solitary relapse of desmoplastic small round cell tumor detected by positron emission tomography/computed tomography. J Clin Oncol. 2008;26(30):4995–4996.
109. Ben-Sellem, D, et al. Desmoplastic small round cell tumor: impact of F-FDG PET induced treatment strategy in a patient with long-term outcome. Rare Tumors. 2009;1(1):e19.
110. Jeong, YJ, et al. Neoplastic and nonneoplastic conditions of serosal membrane origin: CT findings. Radiographics. 2008;28(3):801–817. [discussion 817-818; quiz 912].
111. Hiralal, et al. Desmoplastic round cell tumour of the abdomen. Singapore Med J. 2007;48(1):e19–e21.
112. Quaglia, MP, Brennan, MF. The clinical approach to desmoplastic small round cell tumor. Surg Oncol. 2000;9(2):77–81.
113. Chui, CH, et al. Predictors of outcome in children and adolescents with rhabdomyosarcoma of the trunk–the St Jude Children’s Research Hospital experience. J Pediatr Surg. 2005;40(11):1691–1695.
114. Fetsch, JF, Meis, JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469–477.
115. Kransdorf, MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(1):129–134.
116. Marcus, KC, et al. Childhood soft tissue sarcoma: a 20-year experience. J Pediatr. 1997;131(4):603–607.
117. Stein-Wexler, R. Pediatric soft tissue sarcomas. Semin Ultrasound CT MR. 2011;32(5):470–488.
118. Brady, MS, et al. Malignant mesenchymoma. Cancer. 1996;77(3):467–473.
119. Suzuki, S, et al. Retroperitoneal malignant mesenchymoma: imaging findings in five cases. Abdom Imaging. 1999;24(1):92–97.
120. Moran, CA, Albores-Saavedra, J, Suster, S. Primary peritoneal mesotheliomas in children: a clinicopathological and immunohistochemical study of eight cases. Histopathology. 2008;52(7):824–830.