CHAPTER 102 The Abdominal Aorta
TECHNIQUES
CT Techniques
Using MDCT, a helical volume is acquired with a detector collimation of between 0.5 and 1.5 mm, and reconstructed with a similar thickness using a matrix size of 512 × 512 or greater. The use of tube current modulation techniques (e.g., automated exposure control) reduces radiation exposure compared to fixed-tube current techniques. For MDCT angiography, increased vascular enhancement can be achieved by reducing the kVp to 100 keV (or 80 keV in smaller patients) because this increases photon attenuation by iodinated contrast and moves the mean energy closer to the k-edge of iodine (33.2).1
MRI Techniques
Coils and Patient Position
MR Pulse Sequences
Precontrast Sequences
Steady-State Free Precession: Steady-state free precession sequences (also termed balanced-FFE, true-FISP, and FIESTA) are rapid, have high intrinsic contrast resolution, and have been shown to be accurate in evaluating renal artery stenosis,2 aneurysm sac contents (Figure 102-1),3 thoracic aortic dissection, and aneurysm.4 These sequences can be performed with breath-hold techniques or free breathing with navigator gating, and they provide an overview of the aneurysm sac and surrounding contents.
CONTRAST MEDIA
Contrast Media Dose
For abdominal aortic CTA, doses of up to 150 mL of nonionic contrast delivered at 3 to 6 mL per second have been routinely used in the past. However, with rapid acquisition techniques, doses as low as 50 mLs have been used, especially in smaller patients.5 For MRA, a typical gadolinium-chelate contrast agent dose is 0.2 mmol/kg injected at 2 mL/sec.
MRA: K-Space
By using time-resolved magnetic resonance imaging (TR-MRA) techniques, the dynamics of blood flow in the abdominal vessels can be demonstrated in a way similar to conventional angiography. In TR-MRA, there is over-sampling of central k-space, which is acquired every 2 to 8 seconds for 1 to 3 minutes after gadolinium injection, without the need of a timing bolus. Time-resolved MRA has been shown to be an effective means of classifying endoleaks following endovascular repair.6
ABDOMINAL AORTIC ANEURYSM
Abdominal aortic aneurysm (AAA) is enlargement of the abdominal aorta above a diameter of 3 cm.7
Prevalence and Epidemiology
Abdominal aortic aneurysms are nearly five times more common in men than in women and almost twice as common in people of European descent than African Americans. The prevalence of aneurysm is also increased in those with a family history of AAA, and is strongly related to a history of smoking.8 Other risk factors include age, coronary artery disease or another manifestation of atherosclerosis, high cholesterol, and hypertension. In a large ultrasound screening study,9 an abdominal aortic aneurysm was detected in approximately 5% of males older than 65 years. Ruptured AAA occurs in 1% to 3% of men per year aged 65 years or more, and mortality is 70% to 95%. Left untreated, AAA leads to death in about one third of patients.8
Etiology and Pathophysiology
Although the exact etiology of AAA remains unclear, it appears that degradation of elastin, collagen, and other structural proteins in the aortic wall is a major factor.10 Atherosclerosis is considered to play an important role in the etiology, likely via chronic inflammation in the aortic wall. An imbalance of T-helper and T-suppressor lymphocytes leads to a proliferation of B-lymphocytes. Elastin-derived peptides (EDPs), which are breakdown products of medial elastin, are thought to be the initiating and propagating antigen in this process. Chronic inflammation leads to excess matrix metalloproteinases and degradation of medial elastin and collagen.11 However, the etiology is unquestionably multifactorial. Current research suggests that genetic, environmental, hemodynamic, and immunologic factors all contribute to the development of aneurysms.
Clinical Manifestations of Disease
Most AAAs are asymptomatic and are discovered incidentally on routine physical examination or during other imaging studies. Patients with ruptured AAAs often present with an abrupt onset of back pain as well as abdominal pain and tenderness. Patients may present critically ill. Most will have a pulsatile abdominal mass. Rupture has a high mortality rate, with 25% mortality prior to arriving at the hospital and an overall 30-day survival rate of approximately 10%.12
The rate of aneurysm growth increases with increasing diameter in concordance with Laplace law. Aneurysms smaller than 4 cm grow at 2 to 4 mm/year, those measuring 4 to 5 cm grow at 2 to 5 mm per year, and aneurysms >5 cm grow at 3 to 7 mm per year.13 The risk of rupture increases with aneurysm size and rate of aneurysm expansion. In the U.K. Small Aneurysm Trial,14 aneurysms with a cross-sectional diameter of 5 to 5.9 cm in size had an annual risk of rupture of 6.5%. Elective AAA repair carries a 4% to 6% mortality. Recommendations10 suggest elective repair of aneurysms greater than 5.5 cm in males, and greater than 4.5 to 5 cm in women. Aneurysm length is not thought to be associated with rupture risk.15
Imaging Indications and Algorithm
The U.S. Preventive Services Task Force (USPSTF)16 recommends screening for AAA in men aged 65 to 75 years of age who have ever smoked. It can also be considered in patients with a strong family history of AAA. Ultrasound is the modality of choice for screening in most patients. Its advantages include the absence of intravenous contrast, absence of ionizing radiation, and relatively low cost.
For asymptomatic and smaller aneurysms, ultrasound surveillance is recommended (Table 102-1). For aneurysms >4.5 cm, CT or MRI offer the advantages of greater measurement accuracy, better depiction of the suprarenal aorta and branch vessels, and superior reproducibility versus ultrasonography.
| Diameter up to | Re-image aorta in |
|---|---|
| <3.5 cm | 3 years |
| <4.0 cm | 2 years |
| <4.5 cm | 1 year |
| <5 cm | 6 months |
| 5-5.5 cm | 3-6 months* |
* Also consider referral to a vascular surgeon.
Derived from Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation 2005; 111(6):816-828.
Imaging Techniques and Findings
Radiography
The presence of calcification in the abdominal aortic wall, although commonly present, is not invariable. Moreover, a tortuous and calcified aorta may mimic an AAA. A lateral radiograph (Fig. 102-2) may depict aortic calcification more clearly. However, radiographs are unreliable for diameter measurements of the aortic wall and are not recommended for diagnosis or surveillance.17
Ultrasonography
Examination of the abdominal aorta is an essential component of a complete abdominal ultrasound study, and should be examined in all patients presenting with acute abdominal pain. Abdominal aortic aneurysms should be detectable by sonography in up to 100% of patients.18
The aorta appears on ultrasound as a hypoechoic tubular structure with echogenic walls (Fig. 102-3A). The anterior and posterior walls of the aneurysm are usually better seen than are the lateral walls. Mural thrombus (see Fig. 102-3B) has low to medium echogenicity and is attached to the margins of the aortic wall. At times, mural thrombus will have a lamellated appearance. Thrombus that appears to “flutter” during the cardiac cycle may be at risk of embolization.
In suspected aneurysm rupture, a bedside ultrasonogram may be helpful for those patients who are too unstable for CT, or if CT is not readily available. Ultrasound scanning may assist in determining the aneurysm size and the presence of retroperitoneal or intraperitoneal fluid (Fig. 102-4), although the role of ultrasonography in identifying impending rupture is limited.19
CT and MRI
Compared to ultrasonography, CT and MRI (Fig. 102-5) provide superior depiction of the extent and shape of the aneurysm, involvement of the renal, mesenteric, and iliac arteries, and the suprarenal abdominal and thoracic aorta. On average, ultrasound underestimates aneurysm size by 3 to 9 mm compared to CT angiography.20 Due to their excellent contrast resolution and multiplanar capabilities, CT and MR angiography are now the mainstay of aneurysm characterization prior to endovascular or surgical repair. CT is the modality of choice for the evaluation of suspected rupture of the abdominal aorta19; usually it can be performed within minutes, and has clear benefits in showing alternative causes of acute abdominal pain. Contrast-enhanced CT provides information about the lumen size, location, extent, relationship to branch vessels, presence of active contrast extravasation, and complications secondary to aneurysm rupture.
Signs of Rupture, Impending Rupture, and Contained Rupture
Noncontrast CT is useful in demonstrating the presence of an abdominal aortic aneurysm, maximum aneurysm size, and the presence of retroperitoneal hemorrhage. A high attenuating crescent within the wall of aneurysm (Figs. 102-6 and 102-7) is a sign of impending or frank aneurysm rupture.21 A high attenuation crescent is denser than the psoas muscles (on enhanced CT) and the lumen (on nonenhanced CT scans).
The most common finding in aneurysm rupture is a retroperitoneal hematoma adjacent to the abdominal aortic aneurysm (see Figs. 102-4, 102-7, and 102-8). This blood usually tracks into the pararenal and perirenal spaces (Fig. 102-9). Active extravasation (Figs. 102-9 and 102-10) is frequently visualized on contrast-enhanced CT images. The “draped aorta sign,” where the abdominal aorta is closely applied to the spine with lateral “draping” of the aneurysm around the vertebral body (see Fig. 102-4), has been described as a finding of a deficient posterior wall of the aorta and a contained leak.22 Other sites of rupture include the bowel (most commonly the duodenum), and inferior vena cava (Fig. 102-11).23 Signs of AAA rupture, impending rupture, and contained rupture are (1) periaortic and retroperitoneal hemorrhage; (2) contrast extravasation; (3) high attenuating crescent sign; and (4) draped aorta sign.
FDG-PET
Recent evidence suggests that increased aortic wall metabolism, as measured by FDG-PET, may suggest increased rupture risk. Increased metabolism may represent increased activation of inflammatory cells in the aortic wall, which leads to increased degradation of elastin and collagen in the aneurysm wall.24 Pilot studies examining the role of FDG-PET-CT in asymptomatic and symptomatic AAAs,25 have demonstrated increased activity in those with symptomatic AAAs, and increased activity in focal areas of increased inflammation and collagen degradation.
Treatment Options
Medical
Medical therapy is usually instituted in patients with smaller aneurysms not treated surgically or endovascularly. Smoking cessation is paramount because smoking plays a major role in aneurysm growth.26,27 Although the effect of hypertension and dyslipidemia on aneurysm growth and rupture are unknown, treating these conditions may prolong survival. Statin therapy has been shown to reduce mortality and slow aneurysm growth.26 β-blockers may slow the expansion rate of aneurysms.28
INFLAMMATORY AORTIC ANEURYSM
Definition
Inflammatory abdominal aortic aneurysms are defined by the presence of a thickened aneurysm wall, marked peri-aneurysmal and retroperitoneal fibrosis, and dense adhesions of adjacent abdominal organs.11
Prevalence and Epidemiology
IAAAs constitute approximately 3% to 10% of abdominal aortic aneurysms. Male sex and smoking are both strong risk factors, with a male-to-female ratio ranging from 6 : 1 to 30 : 1. From 77% to 100% of patients smoke.11 Other risk factors include northern European descent and autoimmune disease.
Imaging Techniques and Findings
Ultrasound
Sonographic signs include aortic dilation, thickened and echogenic aortic wall, and a hypoechoic periaortic cuff measuring 1 to 1.5 cm.29
CT and MRI
MDCT and MRI are the mainstays in the investigation of suspected inflammatory abdominal aortic aneurysms. CT accurately distinguishes IAAA from AAA in 93.7% of cases.30 Signs include a thickened, calcified aortic wall surrounded by a low density soft tissue mass (Figs. 102-12 and 102-13). There is relative sparing of the posterior wall of the aorta. The inflammatory soft tissue surrounding the aorta undergoes contrast enhancement in both CT and MRI (Fig. 102-14). On CT, periaortic inflammatory soft tissue generally has lower density than acute blood, helping differentiation from acute AAA rupture. CT and MRI are useful to assess associated effects on adjacent structures, such as ureteric encasement, hydronephrosis, inferior vena cava narrowing, and bowel involvement. Ureteric involvement is present in approximately 25% of cases (see Fig. 102-13) at presentation.11
Treatment Options
Endovascular
Endovascular repair of AAAs has shown promising early and mid-term results and is appealing in high-risk patients, especially when technical difficulties are anticipated due to periaortic inflammation and fibrosis. Only small case series have been reported. In a meta-analysis of 46 patients treated with endovascular repair,32 there was no peri-procedural mortality. The primary technical success rate of 95.6%, was associated with medium term regression in aneurysmal sac size, reduced periaortic fibrosis, and improvement in renal impairment.32
MYCOTIC AORTIC ANEURYSM
Prevalence and Epidemiology
Mycotic AAAs are rare, representing 0.7% to 2.6% of all aortic aneurysms.33 They are associated with immunosuppression and chronic comorbid conditions, such as diabetes or renal failure.34
Clinical Manifestations of Disease
Most patients have nonspecific symptoms including fever and pain. Diagnosis is challenging until late in the disease. Even with recent advances, a high rate of aneurysm rupture has been reported (50% to 85%).34
Imaging Findings
CT and MRI
Approximately two thirds of infected aortic aneurysms involve the abdominal aorta, although most also involve the suprarenal or thoraco-abdominal aorta. Aneurysms are mostly saccular (95%) (Fig. 102-15) and may have an irregular contour. In the largest published series,33 about 50% of patients had a periaortic soft tissue mass, soft tissue stranding, and/or fluid surrounding the aneurysm. One of the characteristic features of infected aortic aneurysm is rapid progression. Periaortic gas, adjacent vertebral body destruction, and psoas muscle abscesses are also signs of infection and additional clues of a mycotic AAA.
Treatment Options
Medical
Parenteral antimicrobial therapy is mandatory in all mycotic aneurysms. Patients are treated for at least 6 weeks and until inflammatory markers normalize.35 Some suggest treating patients with antibiotics for life.36 Initial treatment is empiric. After microorganism susceptibility testing has been performed, more specific agents can be started.
Surgical/Interventional
In cases of suprarenal mycotic aortic aneurysms, in situ repair or reconstruction is the preferred surgery.37 In-situ bypass reconstruction is the procedure of choice in infected infrarenal aortic aneurysms. Critically ill patients who cannot tolerate surgery can be treated with endovascular repair until definitive intervention can be performed.38,39
COMPLICATIONS OF ENDOVASCULAR AORTIC ANEURYSM REPAIR
Prevalence and Epidemiology
Early endoleaks, according to the EUROSTAR registry, occur in 18% of patients. These are usually graft related, and seal spontaneously. A meta-analysis of endoleaks following EVAR revealed a total of 24% of patients experiencing endoleaks, with the majority (66%) present immediately after stent graft placement. These most commonly arose from the distal stent attachment site, and were persistent.40
Etiology and Pathophysiology
Endoleaks are classified according to the site and cause of the leak (Table 102-2). Deficient sealing at the proximal or distal end of the stent graft causes type I endoleaks. Primary type I endoleaks are usually caused by difficult anatomy (e.g., angulated aneurysm neck), a noncircular landing zone, malpositioning, or underdilation of the stent graft. Secondary type I leaks may be caused by aneurysm remodeling, stent graft migration, or progressive dilation of the neck.41 Systemic blood pressure occurs within the aortic sac in type 1 endoleaks, and there is persistent tension on the aortic wall.
| I |
Adapted from Golzarian J, Valenti D. Endoleakage after endovascular treatment of abdominal aortic aneurysms: Diagnosis, significance and treatment. Eur Radiol 2006; 16(12):2849-2857.
Type II endoleaks are due to retrograde filling of the aneurysm sac from branch arteries, usually the lumbar arteries or inferior mesenteric artery (Fig. 102-16). About 40% of type II endoleaks will seal spontaneously, and the risk of aneurysm expansion and rupture with type II endoleaks is lower than with types I or III.
Imaging Indications and Algorithm
Due to the absence of long-term clinical data on stent-graft performance, it is generally accepted that life-long imaging surveillance is necessary.42 However, the ideal surveillance strategy has been widely debated. The Society of Interventional Radiology Device Forum,43 recommends four views of the abdomen (AP, lateral, and oblique) performed after placement of the graft, and every 6 months for at least 2 years. In addition, imaging with CTA, MRI, or ultrasound should be considered at baseline. If no complications are present, surveillance should be repeated every 6 months for 2 years, and then yearly.43 Some investigators42,44 have used AP and lateral radiographs and CTA at less frequent intervals.
Imaging Techniques and Findings
Radiography
Radiography plays a useful role in the evaluation for stent graft expansion; migration, kinking, dislocation, and hook or tent fracture can be identified.45 Although multiplanar reformations on CT allow characterization of many of these complications, plain radiographs have the advantage because they are less susceptible to artifact from metallic prostheses.
Stent migration usually involves caudal migration of the proximal end, whereas the distal end of the stent is more likely to move cranially. Care should be taken to avoid parallax error when assessing for migration.45 Kinking or deformity of the stent often accompanies migration.
Ultrasound
Techniques used for endoleak surveillance include color duplex ultrasound (CDU) and contrast enhanced ultrasound (CEUS) using a micro-bubble agent. Overall, studies have shown a greater sensitivity for endoleak detection when contrast-enhanced ultrasound is used. Reported sensitivities vary widely, ranging from 12% to 100%, likely because sonography for endoleaks is operator and experience dependent.44
MRI
MRI is particularly suitable for the surveillance of nitinol stents, which have fewer artifacts on MRI. Stainless steel stents and elgiloy stents can cause extensive artifacts on MRI such that they may preclude proper visualization of the abdominal aortic lumen.42 Studies have shown MRI to be at least as sensitive for endoleak detection as CT. MRI is typically performed using dynamic gadolinium-enhanced MRA (Fig. 102-17) using timing similar to CTA.
Treatment Options
Type V endoleaks (endotension): Treatment of endotension is controversial. After careful exclusion of overt type I through III endoleaks, surgical revision should at least be considered.46,47 Secondary endografting is more appropriate in critically sick patients who are poor surgical candidates.
TRAUMATIC ABDOMINAL AORTIC INJURY
Prevalence and Epidemiology
Abdominal aortic injuries are rare, and represent only 4% to 6% of aortic injuries. These injuries are commonly associated with high-speed motor vehicle accidents and have been linked to steering wheel injury to the lower abdomen and the use of lap-belt restraints.48
Imaging Techniques and Findings
CT and MRI
On CT, the direct signs of abdominal aortic injuries include intimal flaps, irregular aortic morphology, focal enlargement of the aorta, and contrast extravasation.49 Periaortic retroperitoneal hematoma may also be present. Abdominal aortic injuries are typically limited in length. The most commonly injured sites are at the level of the inferior mesenteric artery, renal arteries, and between the IMA and bifurcation (Fig. 102-18).50 Given long acquisition times, MRI is not appropriate for imaging of aortic trauma in the acute setting.
Treatment Options
Treatment is necessary, except for those with minimal intimal disruptions. Surgical treatment is associated with an overall mortality of 27%. Because of the high incidence of associated bowel injury, surgical placement of a prosthetic graft at the time of initial laparotomy risks graft infection. Endoluminal treatment of aortic injuries using uncovered stents provides excellent results with inframesenteric aortic dissections.50 For those cases of contained transection, both covered and uncovered stents have been employed successfully.
ACUTE AORTIC OCCLUSION
Acute occlusion of the abdominal aorta is a vascular emergency, usually resulting from a saddle embolus or thrombosis. It has a high mortality rate (75%) with conservative treatment.51
Etiology and Pathophysiology
Acute aortic occlusion may result from an aortic saddle embolus, thrombosis of preexisting atheromatous plaque, thrombosis of a small abdominal aortic aneurysm, or thrombosis related to aortic dissection. The high mortality likely results from the absence of collateral pathways at the time of presentation and common comorbid conditions such as cardiac disease (61%).51
Clinical Manifestations of Disease
The absence of both femoral pulses in a patient without significant atherosclerosis risk factors is highly suggestive of the diagnosis.49 Patients may present with paralysis mimicking acute spinal cord compression, and a subsequent delay in diagnosis may increase mortality. Coexistent cardiac arrhythmias suggest a saddle embolus.
Imaging Indications and Algorithm
When acute aortic occlusion is suspected, CT is the most appropriate imaging modality given its wide availability and quick acquisition. If iodinated contrast is contraindicated (contrast allergy), MRI and ultrasound (Fig. 102-19) can also be used.
CT and MRI
On CT, acute aortic occlusion is manifested by the absence of contrast enhancement of the aorta distal to the occlusive site (Figs. 102-20 and 102-21). Collateral vessels will be largely absent. Secondary ischemia in bowel or solid organs may also be present due to global decreased perfusion or to small cardiac emboli. Cardiac sources of emboli are sometimes seen (Fig. 102-20). When present, abdominal aortic aneurysms are well evaluated by CT.
TAKAYASU ARTERITIS
Etiology and Pathogenesis
The exact etiology of TA remains unclear, although infections, autoimmune and genetic factors may play a role. T cells and natural killer cells, which infiltrate the vessel wall, play a role in vascular injury; antiendothelial antibodies have also been reported.52 Moreover, certain human leukocyte antigen (HLA) alleles, such as HLA-B52, HLA-B39, and HLA-DRB1*1301 have been associated with the disease in certain ethnic groups and in certain clinical situations.52
Prevalence and Epidemiology
Takayasu disease predominantly affects young women, with a mean age of diagnosis of 35 years, although nearly 20% of cases present after the age of 40.53 It has been most commonly reported in people of Asian descent.
Clinical Manifestations
Three clinical phases are described in TA: an early “prepulseless” systemic phase, a later occlusive phase, and a final “burned out” phase. The phases usually overlap. Most patients present during the occlusive phase of disease. During the “prepulseless” phase, diagnosis is difficult because patients have nonspecific signs including fever, weight loss, and arthralgias. In the occlusive phase, ischemic complications dominate: claudication, angina, visual impairment, and syncope. The American College of Rheumatology has developed classification criteria for Takayasu disease.54
Imaging Indications and Algorithm
Ultrasound
Characteristic long segments of smooth, homogeneous, mildly echogenic circumferential wall thickening are present. This has been called the “macaroni phenomenon.”55 Unlike atherosclerosis, the wall is not irregular, and there are few, if any, calcifications.
CT and MRI
Both the vessel wall and lumen are well evaluated on CT and MRI. Therefore, these techniques have advantages for the detection of early disease. Concentric arterial wall thickening is seen early on. With continued inflammation, vascular stenoses, occlusions, and aneurysms develop (Fig. 102-22).
Not only does MRI enable assessment of vessel wall involvement, it is ideal for serial follow-up in young patients. Patients can be comprehensively evaluated with MRI without the risks of ionizing radiation. The pulmonary arteries and extracranial arteries, as well as the aorta, can be evaluated. Findings described with MRI and MRA include increased T2 signal around inflamed vessels, aortic wall thickening, vascular dilation, multifocal stenoses, and collateral vessels.56
PET
18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) and 18FDG-PET co-registered with contrast enhanced CT have recently been suggested as a means of evaluating the early changes of TA, and showing the distribution of inflammatory activity in the aorta and its branches,57 with high diagnostic accuracy.58 The uptake pattern in the early stages of disease is linear and continuous.58 Uptake becomes more patchy in later disease.
Treatment Options
Medical
Glucocorticoids are the primary treatment for TA, usually arresting disease progression and systemic inflammation. Arterial stenosis may reverse unless there has been associated fibrosis of the aortic wall.59 In cases refractory to steroids, trials of methotrexate, azathioprine, or other immunomodulators can be initiated.
GIANT CELL ARTERITIS (TEMPORAL ARTERITIS)
Prevalence and Epidemiology
The prevalence of giant cell arteritis in the United States is approximately 160,000 cases (approximately 0.06%), which is likely an underestimate given that many patients have subclinical disease.60 Affected patients tend to be older than 50 years of age.
Etiology and Pathophysiology
Alhough both the humoral and cellular immune system are involved in the pathogenesis of giant cell arteritis, cell-mediated processes are paramount.61 The initial activating factor is unknown; autoantigens, infectious agents, and toxins have been implicated. Transmural inflammation of the arteries results in eventual luminal occlusion through intimal hyperplasia of branch vessels of the aorta. However, in the aorta, inflammation results in aneurysm formation, dissection, and rupture.
Differential Diagnosis
Other vasculitides should be the main consideration if there is multisystemic inflammation.
Imaging Techniques and Findings
CT and MRI
Abdominal aortic aneurysms, dissections, and rupture can be detected with CT or MRI. On CT, associated mural aortic thickening (Fig. 102-23) (possibly representing inflammatory tissue proliferation) may also be present.62 On MRI, high mural signal on fluid-sensitive sequences and mural wall enhancement within the aorta may represent active inflammation.63
PET
FDG-PET is a sensitive marker for large vessel vasculitis and may be a contributory tool in the diagnosis and follow up of giant cell arteritis. Increased uptake may be present in the walls of the aorta and branch vessels, most often in a linear continuous pattern.58 The degree of FDG-PET uptake in the aortic wall may correlate with the degree of aortic inflammation.58 In addition, increased FDG uptake may correlate with aortic enlargement.64
Treatment Options
Surgical and Interventional
The natural history of giant cell arteritis is not known. Currently, treatment recommendations are based on criteria for aortic repair in atherosclerotic abdominal aortic aneurysms. Abdominal aortic aneurysms larger than 5.5 cm that are growing rapidly or demonstrating dissection, are repaired. Open repair has been the intervention of choice. Endovascular repair may be a viable option in the treatment of abdominal aortic aneurysms related to giant cell arteritis.65
AORTOENTERIC FISTULAS
Prevalence and Epidemiology
Approximately 80% of aortoenteric fistulas arise in the duodenum, and about 60% of these arise in the third part.66 Outside the duodenum, the jejunum, sigmoid colon, stomach, and ileum can be involved. Aorta-duodenal fistulas following surgical repair of a prior aortic aneurysm (secondary) are more common than de novo aorta-duodenal fistulas from an abdominal aortic aneurysm (primary). Aorta-duodenal fistulas occur approximately 0.4% to 2.4% of the time following aortic aneurysm surgery. They can manifest soon after surgery or years later.
Imaging Indications and Algorithms
CT
There may be significant overlap in the CT appearance of periaortic infection and aortoenteric fistulas. In both cases, ectopic gas (Fig. 102-24), periaortic fluid, periaortic soft tissue thickening, bowel wall thickening, periaortic fat plane obliteration, and pseudoaneurysms may be present.67 To definitively diagnose aortoenteric fistula, blood or contrast leaking into bowel must be visualized. The presence of a secondary aortoenteric fistula can also be inferred if the aortic graft is seen within bowel lumen.68
Treatment Options
Surgical treatment and antibiotic treatment are paramount. In cases of secondary fistulas, the infected graft is removed and extra-anatomic bypass is placed. Infected graft removal and in situ graft replacement has also been proposed as an alternative. Primary fistulas can be treated similarly. Endovascular repair may be a temporizing step in patients too ill for immediate surgery.69
ABDOMINAL AORTIC DISSECTION
In a review of the literature by Farber and colleagues,70 about 40% of spontaneous abdominal aortic dissections were associated with AAA. Abdominal aortic dissection may be the result of atheromatous disease; conversely, the dissection may cause aneurysm formation due to a weakened wall. Abdominal aortic dissections generally originate at or below the level of the renal arteries (Fig. 102-25).
They may be iatrogenic (e.g., as a complication of vascular catheterization), traumatic, or spontaneous. Like thoracic dissection, isolated aortic dissection is associated with hypertension.71 Marfan syndrome has not been associated with abdominal aortic dissection. Patients usually present with back pain, peripheral ischemia, and signs of distal embolization but may be asymptomatic. A pulsatile abdominal mass is usually present.
Because of its low incidence, the natural history is unknown, although rupture has been reported in up to 25% of patients in one study.70 In uncomplicated cases, medical management typically consists of blood pressure control and vasodilators. Treatment may include open or endovascular repair in patients with major aortic branch occlusion, aortic expansion, extension of the dissection, and aortic rupture.
KEY POINTS
 Inflammatory abdominal aortic aneurysm
Inflammatory abdominal aortic aneurysm
 Traumatic abdominal aortic injury
Traumatic abdominal aortic injury
 Acute aortic occlusion
Acute aortic occlusion
 Takayasu arteritis
Takayasu arteritis
Gotway MB, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184(6):1945-1950.
Hellmann DB, Grand DJ, Freischlag JA. Inflammatory abdominal aortic aneurysm. JAMA. 2007;297(4):395-400.
Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816-828.
Rakita D, et al. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics. 2007;27(2):497-507.
Sakalihasan N, Hustinx R, Limet R. Contribution of PET scanning to the evaluation of abdominal aortic aneurysm. Semin Vasc Surg. 2004;17(2):144-153.
, 2005 Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243(3):641-655.
1 Kalva SP, et al. Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr. 2006;30(3):391-397.
2 Maki JH, et al. Steady-state free precession MRA of the renal arteries: breath-hold and navigator-gated techniques vs. CE-MRA. J Magn Reson Imaging. 2007;26(4):966-973.
3 Schwope RB, et al. MR angiography for patient surveillance after endovascular repair of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188(4):W334-W340.
4 Pereles FS, et al. Thoracic aortic dissection and aneurysm: evaluation with nonenhanced true FISP MR angiography in less than 4 minutes. Radiology. 2002;223(1):270-274.
5 Cohen EI, et al. Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair. J Magn Reson Imaging. 2008;27(3):500-503.
6 Kubo S, et al. Thoracoabdominal-aortoiliac MDCT angiography using reduced dose of contrast material. AJR Am J Roentgenol. 2006;187(2):548-554.
7 Johnston KW, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452-458.
8 Lederle FA, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441-449.
9 Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325(7373):1135.
10 Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816-828.
11 Tang T, et al. Inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2005;29(4):353-362.
12 Brown PM, et al. Selective management of abdominal aortic aneurysms in a prospective measurement program. J Vasc Surg. 1996;23(2):213-220.
13 Hallin A, Bergqvist D, Holmberg L. Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2001;22(3):197-204.
14 Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352(9141):1649-1655.
15 Siegel CL, et al. Abdominal aortic aneurysm morphology: CT features in patients with ruptured and nonruptured aneurysms. AJR Am J Roentgenol. 1994;163(5):1123-1129.
16 U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
17 Julius Grollman, M.A.B., Thomas Casciani, Antoinette S. Expert Panel on Vascular Imaging: Gomes, Stephen R. Holtzman, Joseph F. Polak, David Sacks, William Stanford, Michael Jaff, Gregory L. Moneta. American College of Radiology ACR Appropriateness Criteria: Pulsatile Abdominal Mass. 2005 2005 [cited 2008 December 2, 2008]; Available from http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/Vascular/PulsatileAbdominalMassDoc13.aspx
18 Rumack CM, Wilson SR, Charboneau JW. Diagnostic Ultrasound, 3rd ed. St. Louis: Elsevier Mosby; 2005. 2 v. (xxiv, 2080, xxxiv)
19 Rakita D, et al. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics. 2007;27(2):497-507.
20 Sprouse LR2nd, et al. Comparison of abdominal aortic aneurysm diameter measurements obtained with ultrasound and computed tomography: Is there a difference? J Vasc Surg. 2003;38(3):466-471.
21 Mehard WB, Heiken JP, Sicard GA. High-attenuating crescent in abdominal aortic aneurysm wall at CT: a sign of acute or impending rupture. Radiology. 1994;192(2):359-362.
22 Halliday KE, al-Kutoubi A. Draped aorta: CT sign of contained leak of aortic aneurysms. Radiology. 1996;199(1):41-43.
23 Schwartz SA, et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188(1):W57-W62.
24 Sakalihasan N, Hustinx R, Limet R. Contribution of PET scanning to the evaluation of abdominal aortic aneurysm. Semin Vasc Surg. 2004;17(2):144-153.
25 Reeps C, et al. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008;48(2):417-423.
26 Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003;348(19):1895-1901.
27 Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
28 Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg. 1994;19(4):727-731.
29 Cullenward MJ, et al. Inflammatory aortic aneurysm (periaortic fibrosis): radiologic imaging. Radiology. 1986;159(1):75-82.
30 Iino M, et al. Sensitivity and specificity of CT in the diagnosis of inflammatory abdominal aortic aneurysms. J Comput Assist Tomogr. 2002;26(6):1006-1012.
31 Hellmann DB, Grand DJ, Freischlag JA. Inflammatory abdominal aortic aneurysm. JAMA. 2007;297(4):395-400.
32 Puchner S, et al. Endovascular therapy of inflammatory aortic aneurysms: a meta-analysis. J Endovasc Ther. 2005;12(5):560-567.
33 Macedo TA, et al. Infected aortic aneurysms: imaging findings. Radiology. 2004;231(1):250-257.
34 Oderich GS, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34(5):900-908.
35 Cina CS, et al. Ruptured mycotic thoracoabdominal aortic aneurysms: a report of three cases and a systematic review. J Vasc Surg. 2001;33(4):861-867.
36 Katz SG, Andros G, Kohl RD. Salmonella infections of the abdominal aorta. Surg Gynecol Obstet. 1992;175(2):102-106.
37 Chan FY, et al. In situ prosthetic graft replacement for mycotic aneurysm of the aorta. Ann Thorac Surg. 1989;47(2):193-203.
38 Corso JE, Kasirajan K, Milner R. Endovascular management of ruptured, mycotic abdominal aortic aneurysm. Am Surg. 2005;71(6):515-517.
39 Koeppel TA, et al. Mycotic aneurysm of the abdominal aorta with retroperitoneal abscess: successful endovascular repair. J Vasc Surg. 2004;40(1):164-166.
40 Schurink GW, Aarts NJ, van Bockel JH. Endoleak after stent-graft treatment of abdominal aortic aneurysm: a meta-analysis of clinical studies. Br J Surg. 1999;86(5):581-587.
41 Golzarian J, Valenti D. Endoleakage after endovascular treatment of abdominal aortic aneurysms: diagnosis, significance and treatment. Eur Radiol. 2006;16(12):2849-2857.
42 Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243(3):641-655.
43 Geller SC. Imaging guidelines for abdominal aortic aneurysm repair with endovascular stent grafts. J Vasc Interv Radiol. 2003;14(9 Pt 2):S263-S264.
44 Manning BJ, O’Neill SM, Haider SN, et al. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg. 2008;49(1):60-65.
45 Fearn S, et al. Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance. J Endovasc Ther. 2003;10(5):894-901.
46 Kougias P, et al. Successful treatment of endotension and aneurysm sac enlargement with endovascular stent graft reinforcement. J Vasc Surg. 2007;46(1):124-127.
47 Veith FJ, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35(5):1029-1035.
48 Roth SM, et al. Blunt injury of the abdominal aorta: a review. J Trauma. 1997;42(4):748-755.
49 Bhalla S, Menias CO, Heiken JP. CT of acute abdominal aortic disorders. Radiol Clin North Am. 2003;41(6):1153-1169.
50 Gunn M, Campbell M, Hoffer EK. Traumatic abdominal aortic injury treated by endovascular stent placement. Emerg Radiol. 2007;13(6):329-331.
51 Surowiec SM, et al. Acute occlusion of the abdominal aorta. Am J Surg. 1998;176(2):193-197.
52 Maffei S, et al. Takayasu’s arteritis: a review of the literature. Intern Emerg Med. 2006;1(2):105-112.
53 Maksimowicz-McKinnon K, Hoffman GS. Takayasu arteritis: what is the long-term prognosis? Rheum Dis Clin North Am. 2007;33(4):777-786. vi
54 Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129-1134.
55 Schmidt WA, Gromnica-Ihle E. What is the best approach to diagnosing large-vessel vasculitis? Best Pract Res Clin Rheumatol. 2005;19(2):223-242.
56 Gotway MB, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184(6):1945-1950.
57 Kobayashi Y, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46(6):917-922.
58 Walter MA. [(18)F]fluorodeoxyglucose PET in large vessel vasculitis. Radiol Clin North Am. 2007;45(4):735-744. viii
59 Kerr GS. Takayasu’s arteritis. Rheum Dis Clin North Am. 1995;21(4):1041-1058.
60 Anchishkin AI, Khodyreva V, Kurilov EN. [An organizational model for the activities of microbiology laboratories]. Zh Mikrobiol Epidemiol Immunobiol. 1991;5:79.
61 Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160-169.
62 Agard C, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum. 2008;59(5):670-676.
63 Narvaez J, et al. Giant cell arteritis and polymyalgia rheumatica: usefulness of vascular magnetic resonance imaging studies in the diagnosis of aortitis. Rheumatology (Oxford). 2005;44(4):479-483.
64 Blockmans D, et al. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatology (Oxford). 2008;47(8):1179-1184.
65 Baril DT, et al. Endovascular treatment of complicated aortic aneurysms in patients with underlying arteriopathies. Ann Vasc Surg. 2006;20(4):464-471.
66 Lemos DW, et al. Primary aortoduodenal fistula: a case report and review of the literature. J Vasc Surg. 2003;37(3):686-689.
67 Low RN, et al. Aortoenteric fistula and perigraft infection: evaluation with CT. Radiology. 1990;175(1):157-162.
68 Hagspiel KD, et al. Diagnosis of aortoenteric fistulas with CT angiography. J Vasc Interv Radiol. 2007;18(4):497-504.
69 Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005;92(2):143-152.
70 Farber A, et al. Spontaneous infrarenal abdominal aortic dissection presenting as claudication: case report and review of the literature. Ann Vasc Surg. 2004;18(1):4-10.
71 Trimarchi S, et al. Acute abdominal aortic dissection: insight from the International Registry of Acute Aortic Dissection (IRAD). J Vasc Surg. 2007;46(5):913-919.

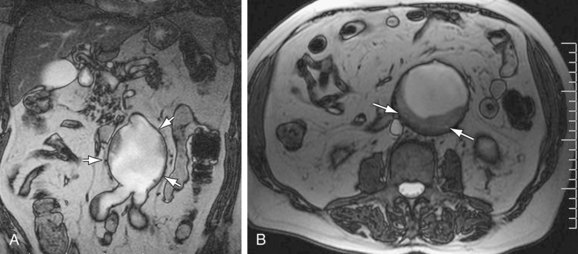
 Figure 102-1
Figure 102-1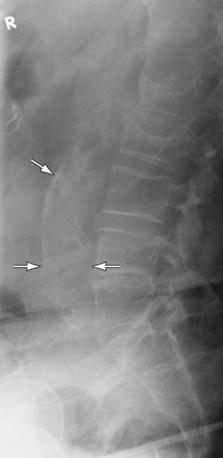
 Figure 102-2
Figure 102-2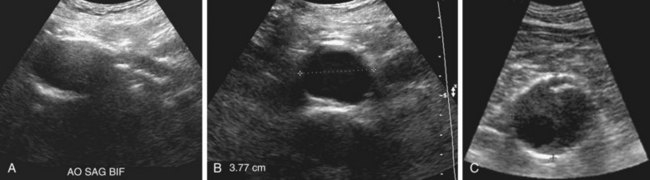
 Figure 102-3
Figure 102-3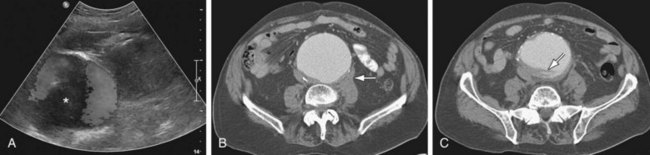
 Figure 102-4
Figure 102-4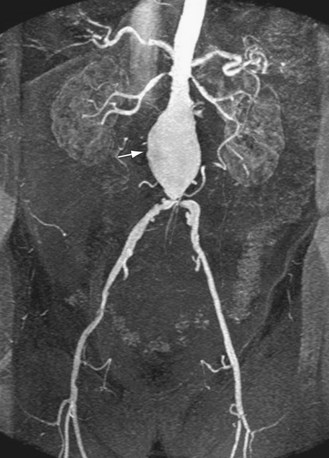
 Figure 102-5
Figure 102-5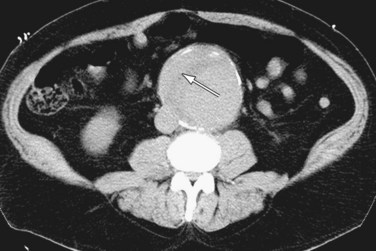
 Figure 102-6
Figure 102-6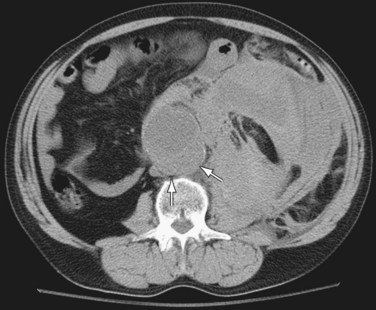
 Figure 102-7
Figure 102-7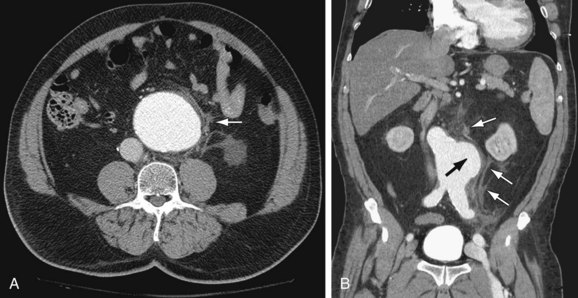
 Figure 102-8
Figure 102-8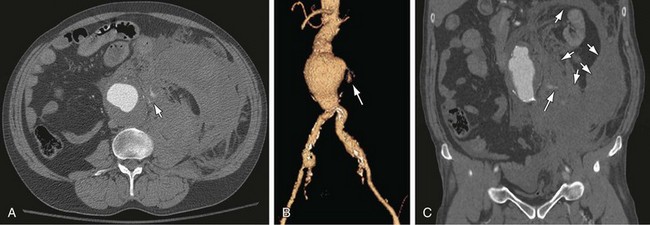
 Figure 102-9
Figure 102-9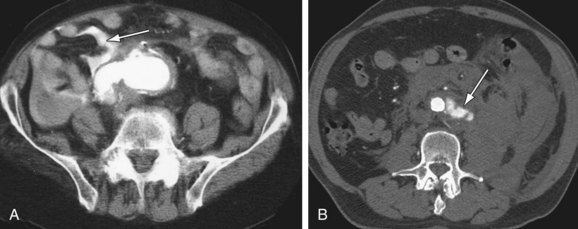
 Figure 102-10
Figure 102-10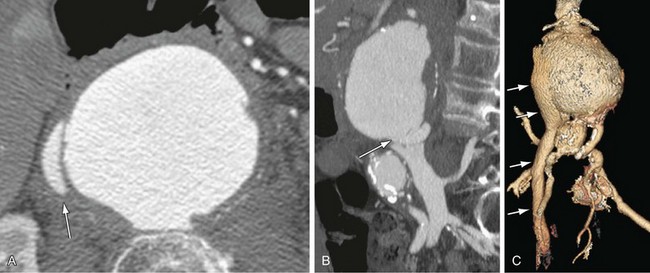
 Figure 102-11
Figure 102-11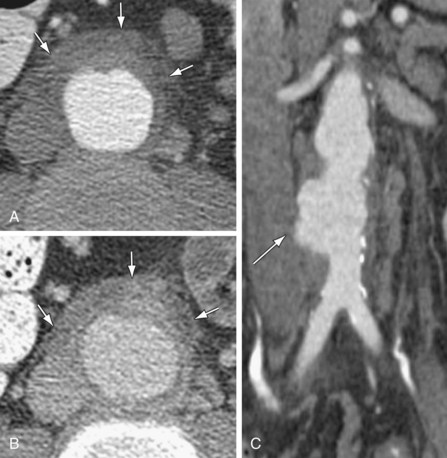
 Figure 102-12
Figure 102-12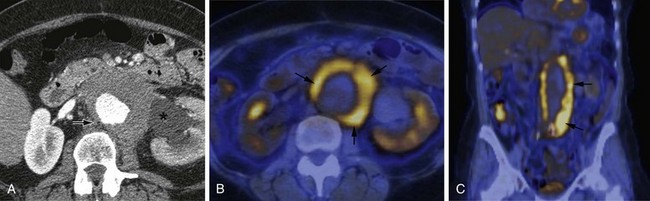
 Figure 102-13
Figure 102-13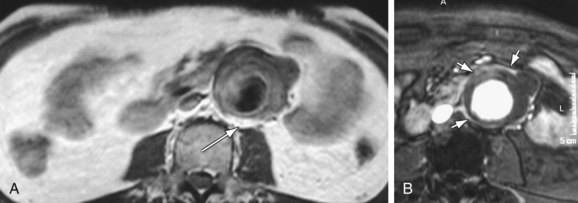
 Figure 102-14
Figure 102-14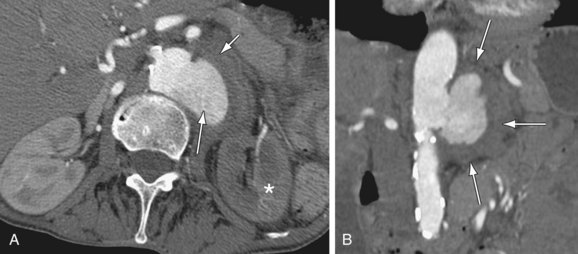
 Figure 102-15
Figure 102-15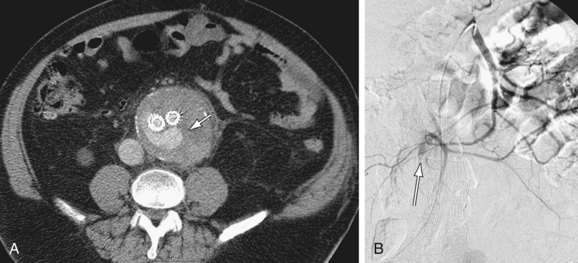
 Figure 102-16
Figure 102-16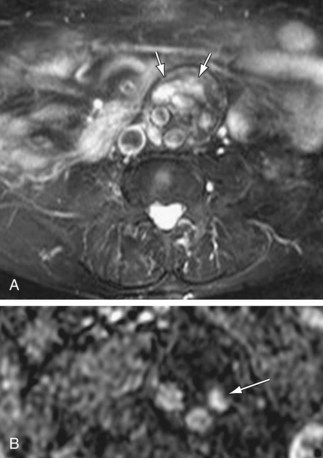
 Figure 102-17
Figure 102-17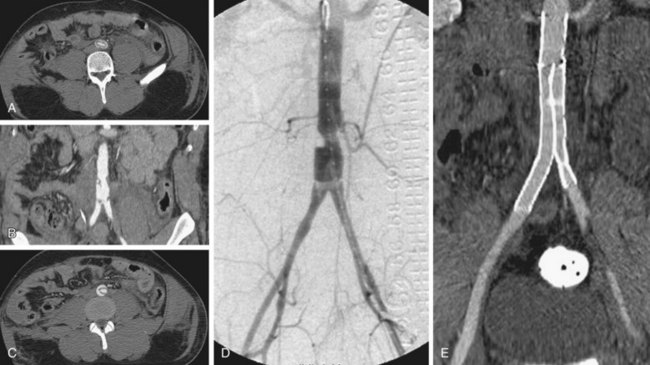
 Figure 102-18
Figure 102-18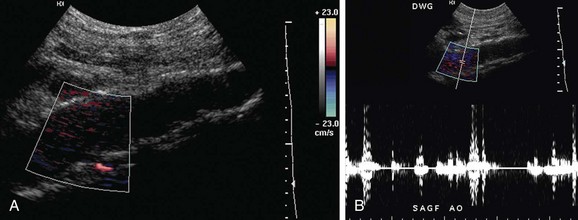
 Figure 102-19
Figure 102-19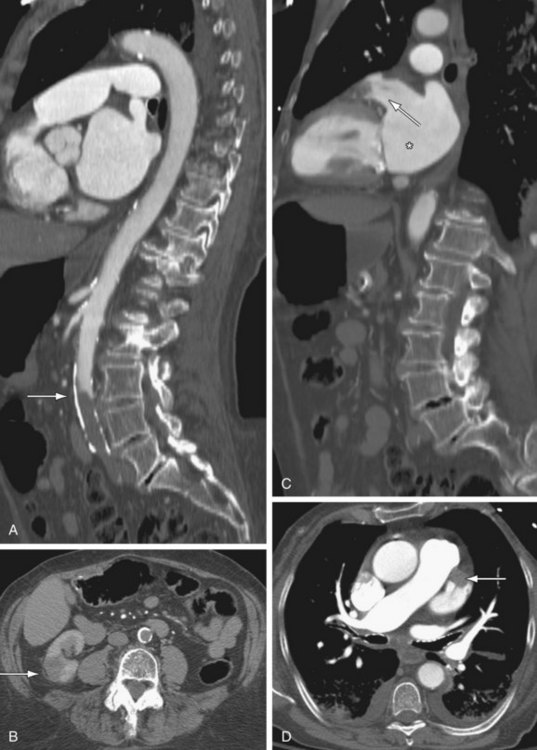
 Figure 102-20
Figure 102-20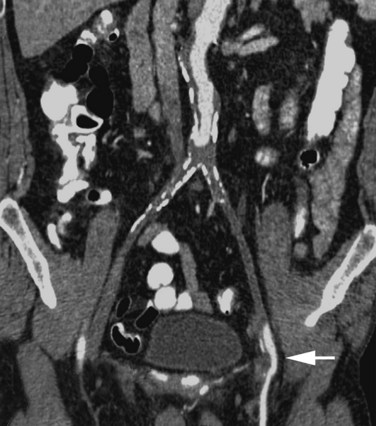
 Figure 102-21
Figure 102-21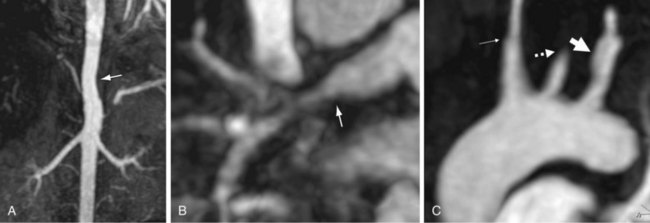
 Figure 102-22
Figure 102-22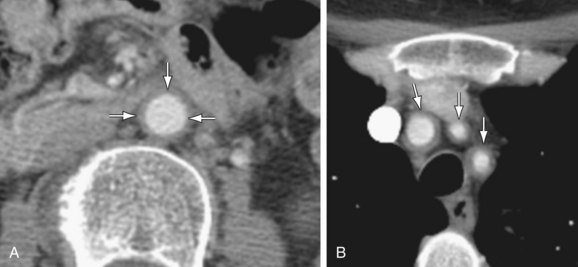
 Figure 102-23
Figure 102-23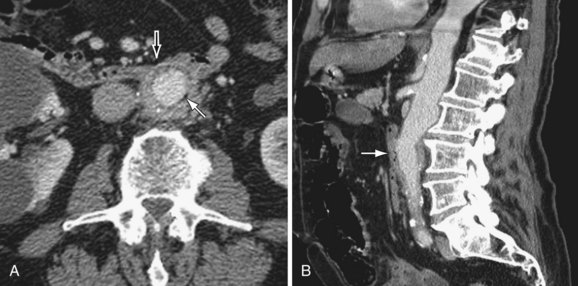
 Figure 102-24
Figure 102-24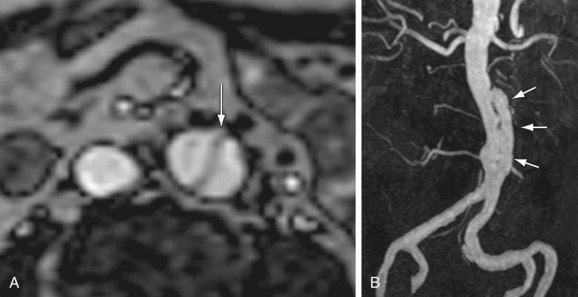
 Figure 102-25
Figure 102-25




