147 Tetanus
 Epidemiology
Epidemiology
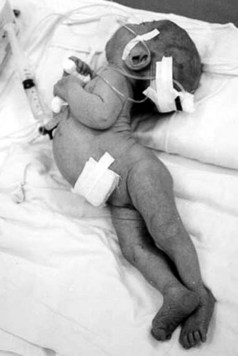
Tetanus is now rare in the Western world, with only 28 cases in the United States in 2007,1 but it is still a common problem in developing countries, where 80% of cases occur in Africa and Southeast Asia. Immunization programs targeting infants and pregnant women have coincided with a decline in the incidence of tetanus over recent years, but the estimated global incidence remains high, with an estimated 290,000 people dying from the disease in 2006.2 In developing countries, neonatal deaths account for a large proportion of cases, and maternal and neonatal tetanus are responsible for an estimated 180,000 deaths/year (Figure 147-1).3 In developed countries, the elderly are particularly at risk, owing to missed boosters and reduced antibody levels.4 Drug users are also at risk from injection site contamination, especially those using subcutaneous administration methods. Up to 18% of infections in the United States occur in injecting drug users.
 Pathophysiology
Pathophysiology
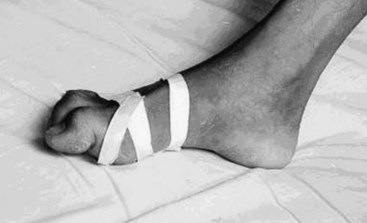
Tetanus is caused by a potent neurotoxin from the gram-positive bacterium, C. tetani (Figure 147-2). C. tetani is a ubiquitous organism capable of surviving in the environment as highly resistant spores and has been isolated from soil, street dust, and human and animal feces.5 Once in a suitable anaerobic environment, these spores germinate, the bacteria multiply, and toxin is released. The most common sources of infection are minor lacerations to the limbs or the umbilical stump in neonates (Figure 147-3).6 In 20% of cases, no source of infection can be found.7 More unusual entry sites include dental infection, ear piercing, unsterile surgery, or injections.
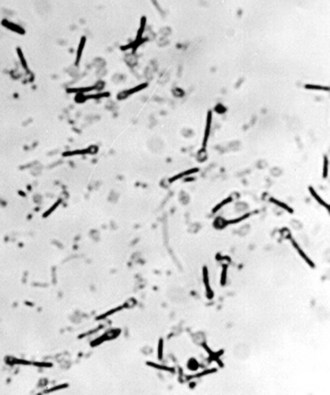
Figure 147-2 Clostridium tetani: a gram-positive bacillus with terminal spores.
(Courtesy J. Campbell, Oxford University Clinical Research Unit, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.)
Tetanus toxin is preferentially taken up by motor nerves, either locally or after circulation in the bloodstream. It enters the nerve and is transported by a specific component of the neuronal retrograde transportation system up the axon. It crosses the synapse and enters the γ-aminobutyric acid (GABA) G presynaptic nerve terminal.8 The toxin is a zinc-dependent endopeptidase and cleaves vesicle-associated membrane protein II (VAMP II, or synaptobrevin) at a single peptide bond. This molecule is essential for synaptic release of neurotransmitters, and cleavage disrupts synaptic transmission. The toxin preferentially affects the GABA inhibitory interneurons afferent to motor nerves in the spinal cord and brainstem. By preventing inhibitory discharge, unrestricted motor nerve activity occurs, resulting in the increased muscle tone and spasms characteristic of tetanus. In severe forms of tetanus, the autonomic nervous system is also affected, perhaps as a result of toxin action within the brainstem, giving rise to marked cardiovascular instability.9,10
 Clinical Features
Clinical Features
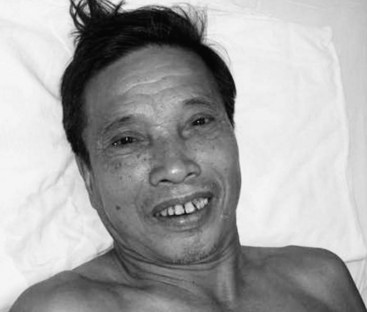
Two main forms of tetanus exist. The majority of tetanus cases are generalized, affecting all muscle groups. However, a milder form—localized tetanus—also exists, affecting muscle groups in the immediate vicinity of a wound. A subgroup of localized tetanus—cephalic tetanus—is also recognized and is associated with a higher mortality,11 perhaps owing to early laryngospasm or autonomic disturbance resulting from brainstem involvement. Cephalic tetanus is often associated with lower motor neuron palsies affecting the third or seventh cranial nerves (Figure 147-4). Localized tetanus of any variety may progress to the generalized form.

Figure 147-4 Cephalic tetanus associated with lower motor neuron palsy of seventh cranial nerve on left side of face.
After infection, a period of time known as the incubation period elapses before symptoms arise; this period is usually between 4 and 14 days. Initial symptoms include muscle stiffness, with muscle groups with short neuronal pathways affected first; hence, trismus and back pain are present in most cases on admission. Involvement of the facial and pharyngeal muscles produce the characteristic “risus sardonicus” and dysphagia (Figure 147-5). Increased tone in the muscles of the trunk results in opisthotonus. Muscle groups adjacent to the initial site of infection are often particularly severely affected, producing an asymmetric picture.
The time from the first symptom to the first spasm is termed the period of onset. Both the period of onset and incubation period have prognostic significance, with shorter times being associated with more severe disease (<48 hours for period of onset and <7 days for incubation period).12 Spasms may be spontaneous but can also be provoked by physical or emotional stimuli. Laryngospasm can occur early in the disease process, often in isolation, resulting in acute upper airway obstruction. Respiration may also be affected by spasms involving the chest muscles. Without facilities for mechanical ventilation, respiratory failure due to muscle spasm is the most common cause of death.5 Hypoxia is common in tetanus,13 either due to spasms or difficulties clearing the copious bronchial secretions and aspiration.
Muscle spasms are usually most severe during the first and second weeks of illness but may persist for 3 to 4 weeks, after which rigidity may remain for several more weeks. In severe tetanus, autonomic disturbance usually appears during the second week. Signs of sympathetic overactivity usually predominate, evident as periods of tachycardia and hypertension. Severe tetanus is associated with a hyperkinetic circulation, particularly if muscle spasms are poorly controlled.14 Changes in blood pressure are mainly due to changes in systemic vascular resistance, with little alteration in the cardiac index.15 Circulating catecholamines, in particular epinephrine, are increased in patients with tetanus compared to others with similar-severity critical illness.16
Acute renal failure is a recognized complication of tetanus, with dehydration, rhabdomyolysis due to spasms, and autonomic disturbance all contributing.17,18 Other complications include tendon avulsions, vertebral fractures secondary to muscle spasm, gastrointestinal bleeding, venous thrombosis, and thromboembolism (Table 147-1).19
| System | Complication |
|---|---|
| Cardiovascular | Hyper/hypotension |
| Tachy/bradycardia | |
| Arrhythmias | |
| Ischemia | |
| Venous thrombosis/thromboembolism | |
| Respiratory | Type I and type II respiratory failure |
| Acute respiratory distress syndrome (ARDS) | |
| Aspiration pneumonia | |
| Ventilator-associated pneumonia | |
| Other | Acute renal failure |
| Gastrointestinal bleeding | |
| Sepsis | |
| Vertebral fractures | |
| Bed sores |
 Management
Management
Antitoxin should be administered to neutralize any unbound toxin. Standard therapy is human immune globulin (HIG) (3000-6000 International Units intramuscularly as a single dose (or if unavailable, equine antitoxin, 500 units/kg). However, a recent meta-analysis and a randomized controlled trial have indicated intrathecal administration of 50 to 1500 International Units of human immune globulin may result in better outcome.20 Tetanus infection does not result in immunity; therefore, all patients should be actively immunized with a full primary immunization course.
Sedation with benzodiazepines is the standard therapy for tetanus; they inhibit endogenous antagonists of GABAA receptors and may counteract the effects of tetanus toxin. IV diazepam or midazolam is usually used, and doses up to 200 mg/d are frequently required.21 Phenobarbitone and chlorpromazine have historically been used to provide adjunct sedation, and their use may be beneficial in patients with autonomic disturbance. Alternatively, if spasms are not sufficiently controlled using benzodiazepines, nondepolarizing muscle relaxants and intermittent positive-pressure ventilation (IPPV) are indicated. Cardiovascularly inert drugs should be used if possible, and pancuronium should be avoided owing to its sympathomimetic side effects.22
Autonomic instability is difficult to treat. Rapid fluctuations in blood pressure mean drugs with short half-lives are desirable. The use of beta-blockers is controversial because their use has been associated with episodes of profound hypotension.23 Esmolol may confer some advantage in this setting, but little data have been published to support its use. Conventional therapy consists of heavy sedation using high-dose benzodiazepines, morphine, and/or chlorpromazine.24 Other treatments reported include clonidine and epidural bupivacaine, but data supporting their use are limited.25 In addition to hypertension, hypotensive episodes may also occur, and if they are unresponsive to volume expansion, inotropes are required.
Recent interest has focused on the use of IV magnesium sulfate to control spasms and treat autonomic dysfunction, either as an adjunct to sedation or as a first-line agent. Doses of 1 to 3 g/h have been used to achieve serum concentrations of 2 to 4 mmol/L. A case series of 40 patients used magnesium as a first-line therapy in place of benzodiazepines26 and reported adequate spasm control in 38 of 40 patients, with 17 of the 24 patients aged younger than 60 years avoiding IPPV. However, these results were not born out by a larger randomized controlled trial of 195 patients which found that ventilation rates did not differ between those receiving magnesium (serum concentrations 2-4 mmol/L) and placebo, although requirements for nondepolarizing neuromuscular blocking agents and other sedatives was reduced.27 Concerns still exist regarding the safety of magnesium in sites without facilities for mechanical ventilation, and on the basis of published data, its routine use in this setting cannot yet be endorsed.
Patients with severe tetanus often require 2 or 3 weeks of mechanical ventilation until spasms subside, and nosocomial infection, particularly pneumonia, is an important problem. In one series, the incidence of ventilator-associated pneumonia in tetanus patients was reported to be 52.6%, with autonomic disturbance an independent risk factor (RR, 31.65; 95% CI, 2.68-373.74).28
 Outcome
Outcome
Outcome in tetanus depends on the severity of the disease and the facilities available for treatment. Adverse prognostic factors are given in Table 147-2. If the disease is not treated, mortality from tetanus is greater than 60% and higher in neonates. Even with treatment, adult mortality rates of 10% to 45% are reported, and up to 65% in neonates.3,6,29 Few studies have been performed investigating the long-term effects of tetanus on patients who survive, but it appears that recovery is complete in most, although some persistent electroencephalographic abnormalities and difficulties in balance, speech, and memory have been reported.30,31
| Age | <7 Days, Premature Birth, or >70 Years |
|---|---|
| Incubation period | <7 days (<6 days in neonate) |
| Period of onset | <48 hours |
| Portal of entry | Umbilicus, uterus, burns, open fractures, postoperative, intramuscular injections |
| Spasms | Present |
| Temperature | >38.5°C |
| Heart rate | >140 beats/min (adult) |
| >150 beats/min (neonate) |
From Thwaites CL, Yen LM, Glover C et al. Predicting the clinical outcome of tetanus: the tetanus severity score. Trop Med Int Health 2006;11:279–87; and from Vakil BJ. Table ronde: propositions pour une classification internationale. In: 4th International Conference on Tetanus, Dakar, 1975, p. 3349–67.
Key Points
Caleo M, Schiavo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54:593-599.
Detailed description of actions of tetanus toxin and pathophysiology of tetanus.
Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947-1959.
Report of global epidemiology and public health initiatives.
Miranda-Filho Dde B, Ximenes RA, Barone AA, Vaz VL, Vieira AG, Albuquerque VM. Randomised controlled trial of tetanus treatment with antitetanus immunoglobulin by the intrathecal or intramuscular route. BMJ. 2004;328:615-617.
Brunelte GW, Kozarsky PE, Magill AJ, et al. CDC yellow book. Atlanta: Centers for Disease Control and Prevention; 2010.
Current recommendations for travelers and immunization schedules.
Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for the treatment of severe tetanus: a randomised controlled trial. Lancet. 2006;368:1436-1443.
1 Centers for Disease Control and Prevention. Summary of notifiable diseases–United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;56:1-94.
2 Brunette GW, Kozarsky PE, Magill AJ, et al. CDC yellow book. CDC Health Information for International Travel 2010. Atlanta: Centers for Disease Control and Prevention; 2010.
3 Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370(9603):1947-1959.
4 McQuillan GM, Kruszon-Moran D, Deforest A, et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660-666.
5 Udwadia FE. Tetanus. New York: Oxford University Press; 1994.
6 Chukwubike OA, God’spower AE. A 10-year review of outcome of management of tetanus in adults at a Nigerian tertiary hospital. Ann Afr Med. 2009 Jul-Sep;8(3):168-172.
7 Thwaites CL, Yen LM, Nga NT, et al. Impact of improved vaccination programme and intensive care facilities on incidence and outcome of tetanus in southern Vietnam, 1993-2002. Trans R Soc Trop Med Hyg. 2004;98(11):671-677.
8 Caleo M, Schiavo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54(5):593-599.
9 Kerr JH, Corbett JL, Prys-Roberts C, et al. Involvement of the sympathetic nervous system in tetanus: Studies on 82 cases. Lancet. 1968;2:236-241.
10 Sykora M, Diedler J, Veltkamp R, et al. Autonomic impairment in tetanus: delayed baroreflex involvement. J Neurol Sci. 2008;270(1-2):201-204.
11 Jagoda A, Riggio S, Burguieres T. Cephalic tetanus: A case report and review of the literature. Am J Emerg Med. 1988;6:128-130.
12 Thwaites CL, Yen LM, Glover C, et al. Predicting the clinical outcome of tetanus: the tetanus severity score. Trop Med Int Health. 2006;11(3):279-287.
13 Femi-Pearse D. Blood gas tensions, acid-base status, and spirometry in tetanus. Am Rev Respir Dis. 1974;110:390-394.
14 Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87:477-487.
15 De Michele DJ, Taveira Da Silva AM. Cardiovascular findings in a patient with severe tetanus. Crit Care Med. 1983;11:828-829.
16 Thwaites CL, Yen LM, Cordon SM, et al. Urinary catecholamine excretion in tetanus. Anaesthesia. 2006;61(4):355-359.
17 Daher EF, Abdulkader RC, Motti E, et al. Prospective study of tetanus-induced acute renal dysfunction: role of adrenergic overactivity. Am J Trop Med Hyg. 1997;57:610-614.
18 Cook TM. Severe anuric renal failure in a patient with tetanus. Br J Anaesth. 2002;88:741-742.
19 Januszkiewicz J. Compression fractures of vertebrae in tetanus: Prospective study of 50 cases. In 4th International Conference on Tetanus, Dakar, 1975, pp 285-90.
20 Kabura L, Ilibagiza D, Menten J, et al. Intrathecal vs. intramuscular administration of human antitetanus immunoglobulin or equine tetanus antitoxin in the treatment of tetanus: a meta-analysis. Trop Med Int Health. 2006;11(7):1075-1081.
21 Okoromah CN, Lessi FE. Diazepam for treating tetanus (Cochrane Review). In: The Cochrane Library. Chichester, UK: John Wiley & Sons Ltd.; 2004.
22 Fassoulaki A, Eforakopoulou M. Vecuronium in the management of tetanus: Is it the muscle relaxant of choice? Acta Anaesth Belg. 1988;39:75-78.
23 Buchanan N, Smit L, Cane RD, et al. Sympathetic overactivity in tetanus: Fatality associated with propranolol. BMJ. 1978;2:254-255.
24 Buchanan N, Cane RD, Wolfson G, et al. Autonomic dysfunction in tetanus: The effects of a variety of therapeutic agents, with special reference to morphine. Intensive Care Med. 1979;5:65-68.
25 Bhagwanjee S, Bosenberg AT, Muckart DJ. Management of sympathetic overactivity in tetanus with epidural bupivacaine and sufentanil: experience with 11 patients. Crit Care Med. 1999;27:1721-1725.
26 Attygalle D, Rodrigo N. Magnesium as first line therapy in the management of tetanus: A prospective study of 40 patients. Anaesthesia. 2002;57:811-817.
27 Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for the treatment of severe tetanus: a randomised controlled trial. Lancet. 2006;368(9545):1436-1443.
28 Cavalcante NJ, Sandeville ML, Medeiros EA. Incidence of and risk factors for nosocomial pneumonia in patients with tetanus. Clin Infect Dis. 2001;33:1842-1846.
29 Fetuga BM, Ogunlesi TA, Adekanmbi F, et al. Neonatal tetanus in the babies of Nigerian mothers immunised against tetanus. Trop Doct. 2009 Jul;39(3):135-137.
30 Luisto M, Seppalainen AM. Electroencephalography in tetanus. Acta Neurol Scand. 1989;80:157-161.
31 Luisto M. Outcome and neurological sequelae of patients after tetanus. Acta Neurol Scand. 1989;80:504-511.