Chapter 22 Terpenes
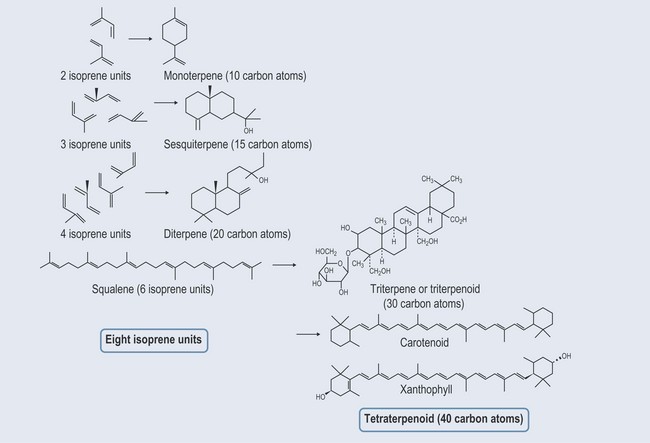
Terpenes are largely found as constituents of essential oils. They are mostly hydro-carbons. The building block is a five-carbon isoprene (CH2=C(CH3)—CH=CH2) unit (Figure 22.1). Terpene hydrocarbons have a molecular formula of (C5H8)n; the n dictates the number of units involved. Terpene hydrocarbons are classified according to the number of isoprene units:
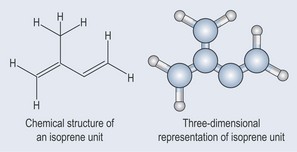
Figure 22.1 The chemical structure of the isoprene building block of terpenes and its three-dimensional representation.
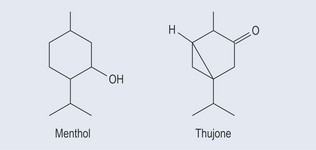
Monoterpenes
General properties of monoterpenes (Figure 22.2):
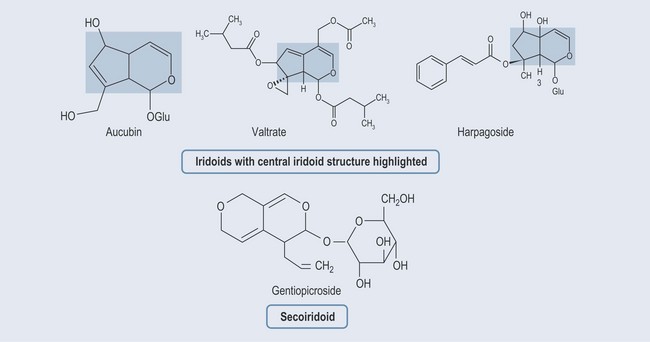
Iridoids
• Examples of Actions
Sesquiterpenes
Sesquiterpenes (Figure 21.5) are found in:
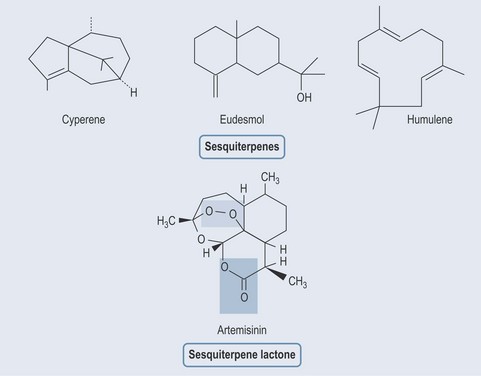
Figure 22.5 Various sesquiterpenes. Artemisinin: green area shows the part of the molecule associated with sequiterpene activity; the purple area shows the part of the molecule that has the antimalarial effect. This is achieved by creating radicals that destroy the malarial parasite. The peroxide group is responsible for this antimalarial effect (Pandey et al 1999).
Diterpenes
Diterpenes (Figure 22.6) are found in:
• Examples of Actions
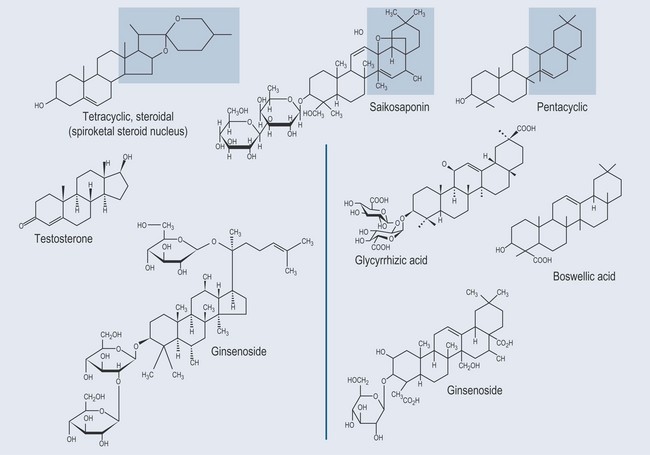
Triterpenes (or Triterpenoids)
The triterpene group has a wide range of effects:
Steroidal triterpenes are found in:
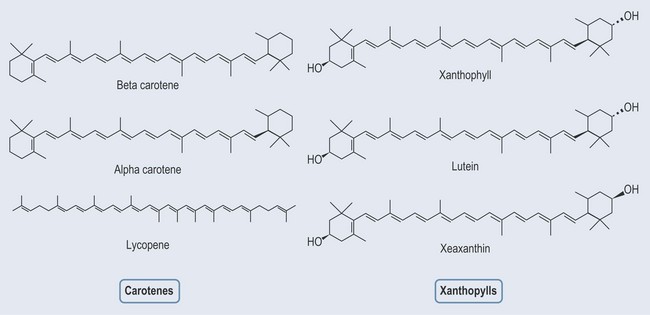
Tetraterpenes
Carotenoids can be divided into:
Carotenes
Carotenes (Figure 22.8; see Chapter 13 ‘Vitamins and minerals’, p. 107) are orange, yellow and red pigments found largely in fruit, vegetables and dark green leafy vegetables. They are components of the pigment systems and are involved in the primary light absorption. Two major types of carotene are:
Beta-carotene is the more common form of the two. Lycopene is the red pigment found in red fruit and tomatoes.