26 Techniques and Devices for Lead Extraction
This chapter discusses the indications, techniques, and devices for lead extraction in detail, using definitions and information from the 2009 Heart Rhythm Society (HRS) Expert Consensus on Facilities, Training, Indications, and Patient Management for transvenous lead extraction.1
 Definitions
Definitions
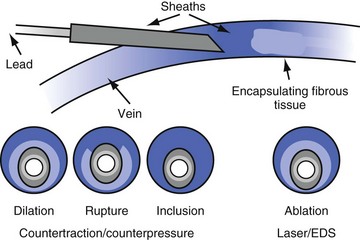
However, with prolongation of the duration of implantation, segments of chronically implanted leads are encased in encapsulating fibrous tissue and may be bound to the vein and/or heart wall, bound to another lead, or both (Figs. 26-1 to 26-4). Removal of chronically implanted leads from these binding sites becomes very difficult and potentially hazardous with traction alone. The tensile strength of encapsulating fibrous tissue is greater than that of the surrounding tissue, and thus leads cannot easily be removed without risking a tear or avulsion of the vein or heart wall. In such cases the removal, separation, and freeing of leads from encapsulating fibrous tissue are defined as lead extraction. As a rule, if one or more of the leads implanted in the patient that require removal are more than 1 year old or require extraction tools for removal, the procedure is labeled as a “lead extraction procedure,” and if not, then a “lead removal without extraction.”
Extraction techniques include traction, countertraction, counterpressure, and tissue disruption, cutting locally with an instrument, laser, or electrosurgery unit. Lead extraction techniques are designed to free the lead from the encapsulating fibrous tissue (countertraction) or to free the encapsulating fibrous tissue (counterpressure) from the vein or heart wall.2–4 Telescoping sheaths are used remotely to apply countertraction and counterpressure at the selected binding sites. Lead extraction procedures are those approaches used to apply the sheaths and remove the lead in a safe and efficacious manner.
Goals and Outcomes
The HRS consensus document, in an effort to unify nomenclature, suggested the following definitions1:
Although there are no published benchmarks, operators should strive for clinical success in 100% of patients with the least possible number of complications. Table 26-1 summarizes current rates of success and complications based on collective studies.
Intraprocedural complications are any complication that occurs during the procedure, as recorded from the time the patient enters the operating or procedure room to the time the patient leaves. This includes all preparation, from administering anesthesia to groin access to closing the incision and reversal of anesthesia. Postprocedural complications are any events that become evident within 30 days after the intraprocedural period. Major complications are any life-threatening complications, those that result in death, or any complication that results in persistent or significant disability or significant surgical intervention. A minor complication is any undesired event related to the procedure that requires medical intervention or “minor procedural intervention” and does not limit, persistently or significantly, the patient’s function or threaten life or cause death (Box 26-1).
Box 26-1
Classification of Implant/Lead Complications
Major
 Indications for Extraction
Indications for Extraction
Considerable divergence of opinion surrounds the indications for lead extraction. However, the 2009 HRS consensus document has unified opinions to a large extent.1 Indications are divided into three major categories: infection, venous access, and broken or nonfunctional leads. Also, emerging indications include implant patients who require magnetic resonance imaging (MRI) scanning for diagnosis (Box 26-2).
Box 26-2
Indications for Transvenous Lead Extraction*
Infection
Class I
Thrombosis or Venous Stenosis
Class I
Leads Affecting Patient: Functional or Nonfunctional Leads
Class I
Class IIa (nonfunctional leads only)
Class IIb
Class III
Magnetic Resonance Imaging–Related Indications
Class IIb
CIED, Cardiac implantable electronic device; ICD, implantable cardioverter-defibrillator.
Data from Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management. Heart Rhythm 6:1085-1104, 2009.
Infection
Infection remains the most common indication for extraction. Complete extraction is indicated in cases of definite CIED infection when there is erosion, pocket infection, lead vegetations, or sepsis (see Chapter 25). Extraction is also recommended in patients with a device and endocarditis or occult gram-positive bacteremia. Extraction is also reasonable in patients with a device and persistent occult gram-negative bacteremia. Antibiotic therapy should be considered adjunctive therapy, and pocket debridement with local relocation is only palliative.
To prevent further serious and potentially lethal complications, all device components, including leads, must be removed to cure the infection. Also, the morbidity associated with a local pocket infection, the lethal sequelae of septicemia, and the potential risk of infected thrombus formation in the heart are well known. Because leaving an infected lead in the body is potentially lethal, the risk of the procedure is clearly less than the risk of lead extraction; that is, the risk of not extracting far exceeds the risk of extracting. The risk of Staphylococcus aureus device infection without extraction was supported by a series of 33 patients from the Duke Medical Center; 10 of 21 patients (47.6%) died without lead extraction, and 2 of 12 (16.7%) died despite lead extraction, and none from lead extraction.5 The safety and efficacy of complete lead extraction, with debridement and delayed reimplantation at a remote anatomic site, were demonstrated in 123 patients at the Cleveland Clinic with device infection. Despite infections with a wide range of bacterial organisms, mostly coagulase-negative staphylococci and S. aureus, extraction was associated with no major complications. Infection recurred only in those four patients who had incomplete extraction or reimplantation concurrent with the extraction.6
Noninfected Systems
When functional or nonfunctional leads pose immediate risk, such as in a patient with life-threatening arrhythmias from a retained lead or fragment, or when the lead design poses a risk of perforation (e.g., Accufix with protrusion of J stylet outside lead), the decision to extract is straightforward (Fig. 26-5). Extraction of functional or nonfunctional leads becomes controversial when there is no immediate threat to the patient, because it is possible to abandon a failed or a nonrequired lead and implant ipsilaterally if the vein is patent, or contralaterally or even transiliac if the ipsilateral vein is occluded. The controversy persists because it is difficult to calculate the risk/benefit ratio of lead extraction versus abandoning such leads. Thus, when considering extraction of noninfected leads, it is important to balance the risk of extraction with the patient’s situation and to factor in the operator’s experience and not just literature data. Some operators may choose to extract only when there is infection; others who are more experienced may elect to extract all leads that are not required, whether functional or not, when the opportunity arises.
Leads Associated with Risk to Patient
In the case of functioning leads, the HRS document specifies that extraction of these leads may be considered when the lead design poses a potential risk in the future, as with the Accufix lead before J-wire fracture or protrusion. Abandoned functional leads that may pose interference risk or those no longer required can be considered for extraction, particularly in the absence of contraindications (see Box 26-2).
Thrombosis or Venous Stenosis
Lead extraction is indicated for patients with clinically significant thromboembolic events with evidence of clot on the lead, as well as in patients with superior vena cava (SVC) or subclavian vein stenosis or occlusion with symptoms. Lead extraction is also recommended in cases of bilateral occlusion for the creation of a conduit or for planned stent deployment (Fig. 26-6). When there is a contraindication to implantation on the contralateral side (e.g., arteriovenous fistula), extraction for creation of a conduit is recommended; otherwise, extraction is considered reasonable.
Creation of a Conduit
Sometimes, when there is severe stenosis with or without symptoms from the obstruction of flow, physicians have initiated balloon venoplasty and stenting without extraction of the leads. This is particularly difficult if either infection or reocclusion occurs, because extraction now becomes impossible without extensive open-heart surgery (Fig. 26-7). A more appropriate approach includes extraction, venoplasty, stenting, and reimplantation through the stent, as reported by Chan et al.7 in a subclavian occlusion that progressed to an SVC occlusion.
Inactive Leads: Functional and Nonfunctional but not in Use
A rationale for extraction of inactive leads is not as straightforward as for active leads. This situation differs from creation of a conduit. For example, if the ipsilateral vein is patent, insertion of a new left ventricular lead should be uneventful. However, the addition of a new ICD lead and abandonment of the original ventricular pacemaker lead creates an inactive, abandoned lead. The disposition of inactive leads is controversial, confusing, and at times emotional. Many questions need to be answered. Why should a lead be removed if it is not causing a problem (e.g., penetration, perforation, arrhythmia), is not dislodged, and is not broken?8 (See Box 26-2.)
 Risks and Outcomes
Risks and Outcomes
The risks associated with lead extraction are tamponade caused by intrapericardial vascular disruption of the SVC or heart; hemothorax from extrapericardial vascular tear outside the pericardial sac and into the thorax; arteriovenous fistula or dissecting hematoma (e.g., aortic arch); and failure to complete the lead extraction (Fig. 26-8). The latter is usually not considered a risk; however, a failed lead extraction may lead to additional procedures or may be a precursor for dangerous situations in the future.
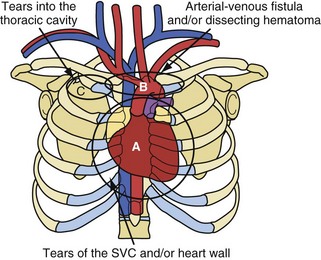
Figure 26-8 Potentially lethal complications from vascular tissue disruption during lead extraction.
Extraction centers from the continental United States and Hawaii voluntarily submitted data for a national registry between December 1988 and December 1999.9–11 The most recent published report, from 1996, included data from 226 centers, 2338 patients, and 3540 leads and demonstrated major complications in 1.4% of the cases (<1% for centers with >300 extraction procedures).10,11 The total U.S. data included 7823 extraction procedures and 12,833 leads (presented in 2000). Multivariate analysis of the data from 1994 through 1999 demonstrated four predictors of major complications (1.6%): (1) implant duration of oldest lead, (2) female gender, (3) ICD lead removal, and (4) use of laser extraction technique. Major complications were (1) death, 0.3%; (2) nonfatal hemopericardium or tamponade, 0.7%; (3) nonfatal hemothorax, 0.2%; (4) transfusion for bleeding/hypotension, 0.1%; (5) pneumothorax requiring a chest tube, 0.1%; and (6) other nonfatal events, 0.2% (including 4 arteriovenous fistulae, 2 pulmonary embolisms, 2 thoracotomies for defibrillator leads trapped in sheaths, 2 respiratory arrests, 2 strokes, 2 cases of renal failure, 1 anoxic encephalopathy, and 1 open-heart retrieval of a device fragment). In the more recent LExICon study of data from 13 centers in the United States and Canada, procedural success was 96.5%, with 97.7% clinical success.12 In LExICon, 1449 patients underwent laser-assisted lead extraction of 2405 leads. Failure to achieve clinical success was associated with body mass index (BMI) of 25 kg/m2 or less and low-extraction-volume centers. Procedural failure was higher in leads implanted for more than 10 years and when performed in low-volume centers. Major adverse events in 20 patients were directly related to the procedure (1.4%), including four deaths (0.28%). Major adverse effects were associated with patients with BMI less than 25 kg/m2. Overall, all-cause in-hospital mortality was 1.86%.
Potentially lethal complications requiring extensive surgical procedures include tear of the vein and heart wall causing tamponade, arterial tears causing arteriovenous fistula or dissecting hematoma; and tears into the thoracic cavity causing a hemothorax (see Fig. 26-8). The procedure-related complications are discussed in detail later. Time and surgeon experience are the two factors related to survival. Low blood pressure and poor tissue perfusion are time-dependent events. Being prepared for a cardiovascular emergency is the only way to meet time constraints. This includes having a cardiovascular surgeon immediately available, along with the proper instrumentation and experienced support personnel. A cardiovascular surgeon has the technical skill to manage these complications but may need direction from the extractor on the proper approach. Once a complication resulting in poor or no perfusion is recognized, the repair should begin immediately. The concern of needlessly subjecting the patient to extensive surgery and morbidity pales in comparison to that of applying the therapy late because of confusion or procrastination. Failure to recognize the complication in a timely fashion or the lack of access to qualified personnel may cause a lethal outcome.
 Clinical Considerations
Clinical Considerations
Patient Information and Preparation
The patient’s blood should be typed and crossmatched for a possible blood transfusion. A current chest radiograph and electrocardiogram (ECG) are mandatory (Fig. 26-9). An echocardiogram is mandatory for two groups of patients before the procedure, even if transesophageal echocardiography (TEE) is routinely available in the procedure room: those with infection, to rule out vegetation in the right atrium; and those with heart failure, to define cardiac function.
Training and Skills
Lead extraction is a fundamental skill that is required to manage device-related complications. Lead extraction, as with lead implantation, is a requisite skill with predictable and expected results. This was not always the case. From its inception in the early 1980s, the procedures evolved rapidly. The management of a device-related complication centered on the lead extraction procedure, overshadowing all other aspects of management. This was because unexpected tears in the SVC and heart wall sometimes occurred without warning, despite the rigid protocols followed. The technology and those rigid protocols have evolved into current procedures. The procedures are less stressful and have predictable results. Predictability allows an extractor to recognize an approach that has a potential for a poor result and change to an approach with a predictably good outcome. The 2009 HRS Expert Consensus document clearly indicates the minimum training needed for competency. Physicians in training should extract a minimum of 40 leads as the primary operator under supervision. To maintain skills a minimum of 20 leads annually should be performed. Supervising physicians should have extracted 75 leads with efficacy and safety consistent with accepted literature.1
The new HRS Expert Consensus document was published after 1-year preparation.1 In the past, meaningful consensus was impossible because of the small number of practitioners and limited data. Now, however, the substantial growth and investment of the international HRS community in transvenous lead extraction permits significant consistency in tools, techniques, procedures, and expected benefits and risks.
 Lead Extraction Techniques
Lead Extraction Techniques
As discussed, segments of chronically implanted leads are encased in encapsulating fibrous tissue and bound to the vein and/or heart wall, bound to another lead, or both. Lead extraction is the removal of chronically implanted leads from these binding sites. Because the tensile strength of encapsulating fibrous tissue is greater than that of the surrounding tissue, leads cannot easily be removed without risking a tear or avulsion of the vein or heart wall. The word ablation best describes the removal, separation, and freeing of leads from encapsulating fibrous tissue. Ablation techniques include traction, countertraction, counterpressure, and tissue disruption, cutting locally with an instrument, laser, or electrosurgery unit. Lead extraction techniques are designed to free the lead from the encapsulating fibrous tissue (countertraction) or to free the encapsulating fibrous tissue (counterpressure) from the vein or heart wall.2 Telescoping sheaths are used remotely to apply countertraction and counterpressure at the selected binding sites. Lead extraction procedures are used to apply the sheaths and remove the lead in a safe and efficacious manner.
Traction, Countertraction, and Counterpressure
Direct Traction
All current lead extraction procedures use some form of traction, or pulling force13 (Fig. 26-10). Pulling on leads was a successful method of extracting leads during the early years of pacing, when leads lacked efficient fixation devices and were implanted for short periods. Traction was applied manually for minutes or applied using various weights or elastic bands for days. Traction proved unsafe and had a high incidence of failure when applied to leads with efficient fixation devices and leads implanted for longer periods. The amount of traction required increases and becomes more dangerous as the duration of the implant and the tensile strength of the fibrous tissue increase. Leads with efficient passive-fixation devices may be difficult to remove 4 to 6 months after implantation. A failed previous attempt to extract a lead frequently damaged the lead, making future extraction attempts more difficult.
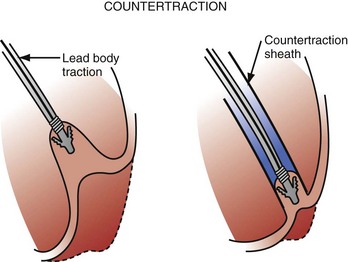
Countertraction
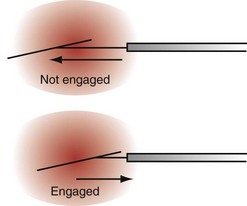
Countertraction is the technique used to free the lead from compliant, encapsulated fibrous tissue. Countertraction was first used to remove a lead from an implantation site in the right ventricle or atrium. Although the technique for extracting leads from the heart wall is discussed first, this is the last step in a normal lead extraction procedure, using any type of sheath. Extraction sheaths free leads from binding sites, proximal to distal. Once the sheath is passed over the lead and down to the implantation site, traction on the lead pulls the site to the sheath (Fig. 26-11). The traction force is countered by the circumference of the sheath. The countertraction sheath focuses the traction force at the tip of the sheath, limiting the excursion of the heart wall. This prevents compliance changes and blockage of the tricuspid valve, with possible perforation, tearing, and avulsion of the heart wall. The countertraction forces are limited by the tensile strength of the lead. At some point, the electrode is freed from the encapsulating fibrous tissue, allowing the heart wall to fall away and the electrode to be pulled out of the sheath.
The way countertraction actually frees the lead is postulated but not known. It is thought that the traction force wedges the lead against the countertraction sheath. The pulling force on the electrode tries to evaginate the encapsulating fibrous tissue. The electrode is then freed either by (1) a plastic deformation of the tissue that allows it to slide out of the encapsulating tissue as the countertraction sheath peels the tissue off the electrode or (2) an actual disruption or bursting of the encapsulating tissue that frees the electrode, or both. For a passive-fixation electrode, the tines are removed intact with the electrode; for an active-fixation electrode, the fixation mechanism is ideally retracted or unscrewed before countertraction is applied. In some cases, continued “unscrewing” of an active-fixation lead results in complete lead removal without the need for countertraction, because of the absence of significant binding at other sites along the lead.14 If the helix will not retract, the electrode and fixation mechanism are removed together. The same scenario likely applies to removal of electrodes from the atrial wall.
Countertraction is also used to free the lead from the encapsulating fibrous tissue at binding sites along the vein and heart wall (see Fig. 26-11). This is possible only if the encapsulating fibrous tissue still has plastic qualities (compliant). The tissue at the binding site is pulled against or into the sheath and is removed by evagination, peeling, or tissue rupture. Countertraction can be performed with either the inner or the outer sheath.
Counterpressure
It is unknown whether the lead is being removed by countertraction or counterpressure (Fig. 26-12). In the past, counterpressure was used to describe the removal of tissue from all sites other than the electrode implantation site. In most cases, removal is still credited to counterpressure; compliant tissue is removed primarily by countertraction, and noncompliant tissue by counterpressure. Not discussed are leads bound to one another by the calcified encapsulating tissue. Separation of the two leads is safe, and the traction force is limited only by the tensile strength of the lead.
Extraction Instruments
Mechanical Sheaths
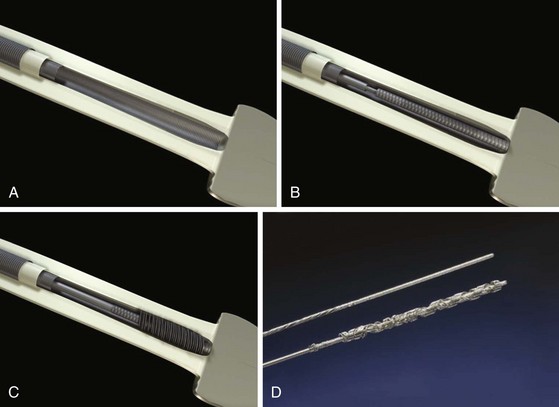
Mechanical sheaths are telescoping sheaths made of Teflon, polypropylene, or stainless steel (Fig. 26-13). These telescoping sheaths are designed to pass over the lead, which acts as a rail guiding the sheaths through the veins and down to the heart wall. Countertraction and counterpressure are applied as the sheaths move down the lead from one binding site to another. The outer sheath also acts as a workstation, facilitating the free movement of the inner sheath and lead by eliminating binding, and it protects the surrounding vascular structures. The leading edges of the sheaths are beveled. The rotation of the beveled tips facilitates maneuvering past obstructions and through the narrow channels along the tortuous paths surrounding the lead body. This is especially true in the superior veins. For the sheaths to pass down the lead in a true course, the lead must be stiff enough to act as a guide rail. The lead is stiffened by pulling it taut. The lead must be stiff enough to resist bending or kinking as the sheaths are passed over it (lead stiffness > sheath stiffness). The telescoping action of the sheaths allows the suppler inner sheath to track over the lead. The larger outer sheath is then advanced using the combination of taut lead and inner sheath as the guide rail. The lead is made taut by traction (pulling on it). A locking stylet is inserted, and a suture is usually tied to the lead, acting as both an extender and a traction handle.
Excimer Laser Sheath
The development of the excimer laser was a milestone for lead extraction (Fig. 26-14). The excimer laser generated a high-energy 308-nanometer laser beam known to disrupt tissue (both cells and hydrated proteins) by an explosive vaporization of intracellular water. The rapid vaporization helped to cool the site. These were appealing properties for lead extraction. Unfortunately, the laser does not ablate heavily mineralized tissue. If the laser could not be maneuvered through this tissue in a grinding manner, counterpressure techniques were required.
The laser sheath technique was evaluated prospectively in two clinical trials. The Pacing Lead Extraction with the Excimer Sheath (PLEXES) trial included only the initial version of the 12F sheath.15 Although there have been substantial improvements in the 12F sheath, including an outer sheath, better mechanical properties to prevent crushing of the optical fibers, lubrication, a flexible distal and a more still proximal segment, and certainly better understanding of how to use the tool, the PLEXES trial was a dramatic success. This randomized clinical trial compared mechanical extraction tools with laser-assisted lead extraction and was used to support the clinical release of this technology. The complete lead removal rate was 94% in the laser group and 64% in the nonlaser group (P = .001). Failed nonlaser extraction was completed with the laser tools 88% of the time. The mean time to achieve a successful lead extraction was significantly reduced for patients randomized to the laser tools: 10.1 ± 11.5 minutes versus 12.9 ± 19.2 minutes for the nonlaser techniques (P < .04). There was only one death, but it was in the laser group; and there were two other potentially life-threatening bleeding episodes in the laser group.
After the trial with the 12F sheaths, a second, nonrandomized cohort trial was done with 14F and 16F sheaths.16 This was particularly important, because implantable defibrillator leads required the 16F sheaths, and many of the bipolar leads (almost all) were better approached with the 14F sheath. In contrast to other, nonlaser sheaths, upsizing of the laser sheath to pass over (include) the fibrosis or calcification is frequently an effective maneuver. In this study, 863 patients underwent extraction of 1285 leads. Expanding the number of research sites from fewer than 10 to 52 gave a broader view of this tool in general practice. The patients treated with the 14F device tended to have older leads than patients in the 12F population; the 16F population, composed mostly of defibrillator patients, was younger, had more recent leads, and was more often male than the 12F population. Clinical success (extraction of entire lead or of lead body minus distal electrode) was observed in 91% to 92% of cases for all device sizes. The overall complication rate was 3.6%, with 0.8% perioperative mortality. The incidence of complications was independent of laser sheath size.
Ultimately, a cohort comparison trial of defibrillator and pacemaker leads extracted with laser assistance was done at the Cleveland Clinic.17 ICD extraction results were compared with the results for a matched cohort of patients undergoing extraction of ventricular pacemaker leads from a national registry and with the experience with pacemaker lead extraction at the Cleveland Clinic. Successful complete extraction of ventricular nonthoracotomy implantable defibrillator leads, in the absence of major complications, was achieved in 96.9% of attempts to extract leads from 161 patients. Clinical success was achieved in 98.1% of patients. There were three major complications, including one death. ICD lead extraction was done at an experienced center with equal risk and no significant difference in procedure or fluoroscopy time.
The total investigational experience with laser sheaths was also reported (October 1995–December 1999), including 2561 pacing and defibrillator leads in 1684 patients at 89 U.S. sites. Of these leads, 90% were completely removed, 3% partially removed, and 7% failures. Major perioperative complications (tamponade, hemothorax, pulmonary embolism, lead migration, death) were observed in 1.9% of patients, with in-hospital deaths in 13 (0.8%). Minor complications were seen in an additional 1.4% of patients. Multivariate analysis showed that implant duration was the only preoperative independent predictor of failure, and female gender was the only multivariate predictor of complications. Success and complications were not dependent on laser sheath size. At follow-up, various extraction-related complications were observed in 2% of patients. The learning curve showed a trend toward fewer complications with experience.18 A similar experience was observed in Europe.19
The LExICon study was the most recent study evaluating the most recent iteration of the laser-assisted lead extraction system.12 In this study, 2405 leads were extracted in 1449 patients from 13 centers. The procedural success was 96.5% with 97.7% clinical success. Major adverse events in 20 patients were directly related to the procedure (1.4%) including four deaths (0.28%). Major adverse effects were associated with patients with BMI less than 25 kg/m2. Overall, all-cause in-hospital mortality was 1.86%.
Electrosurgical Dissection Sheaths
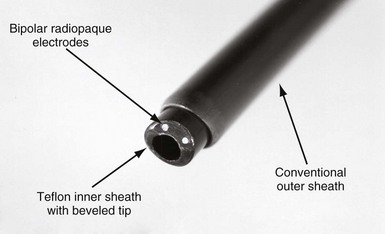
The EDS has two bipolar electrodes positioned at the tip of the bevel (Fig. 26-15). The sheath is connected to an interface plate inserted on a conventional electrosurgery unit (Valley Lab Force V; PEMED, Denver), placed in a bipolar cutting configuration, and activated with a foot switch. The interface plate is attached to the front panel of the electrosurgical unit to ensure that the EDS is connected in a bipolar configuration. The interface also has an attachment to pulse the electrosurgical unit 80 times per minute. A plasma arc is generated between the electrodes. The plasma arc extends out from the electrodes and vaporizes the tissue to a depth of about 1 mm. On continuous discharge, desiccated tissue debris shunts the arc between the electrodes, preventing it from cutting. Also, on continuous discharge, if one of the electrodes touches a conductor coil, a parallel, alternate current (AC) circuit is created consisting of the EDS electrode in contact with the conductor coil, the conductor coil down to an electrode in the heart, and back to the other EDS electrode. An AC current applied to the heart in a unipolar configuration can fibrillate the heart. To ensure cutting and avoid fibrillating the heart, the EDS is operated in a pulsed mode at 80 pulses/min. In the pulsed mode, if a conductor coil is touched, it paces the heart.
Clinical evaluation of the EDS has been formally published only in an observational study from five centers, involving 265 patients with extraction of 459 leads.20 During the investigation, only the 9F and 11F sheaths were used, excluding almost all ICD leads from consideration for extraction. As in all extraction series, some of the leads came out easily and others were more difficult to remove, and the techniques consisted more of an approach than of universal use of one tool to remove all leads. In this case, 542 leads were potentially presented for extraction, but about 15% were removed with direct traction, yielding 459 for which the EDS was employed. The laser tool was used in fewer than 3% of the leads. The average implant duration of the patient’s oldest lead was 8.4 ± 5.0 years; 31% of patients had leads implanted for longer than 10 years. Major complications occurred in 2.6% of patients, including cardiac tamponade in 4 patients (1 surgical repair, 1 after switching to a femoral approach), 1 hemothorax, 1 arteriovenous fistula (surgical repair), and 1 death that was associated with the mechanical removal of an oversized SVC lead for which the EDS was not used. For the 459 leads with attempted removal by the EDS, 99.4% were removed (95.9% completely, 3.5% subtotally with ≤4 cm of lead remaining), and only 0.6% were not removed.
Evolution Sheath
A newer mechanically powered lead extraction sheath set is the Evolution mechanical dilation sheath (Cook Vascular). A rotating inner sheath with a threaded-barrel metal tip is designed to function as a dilating drill (Fig. 26-16). This bores through the encapsulating fibrous tissue as it advances down the lead through the binding sites. The outer sheath is a conventional, beveled plastic sheath. The rotation of the inner sheath is powered by a pistol-grip handle squeezed by the operator (mechanical power). Multiple inner diameter (ID) sizes (7, 9, 11, and 13 French) are available. The initial experience with the Evolution had mixed results.21 There was a high procedural and clinical success rate. The Evolution is particularly useful as a rescue when heavy calcifications are encountered while using the laser sheath. There was a tendency toward wrapping of adjacent leads and also collateral damage to the leads not intended for extraction. The system seems to work best in single-lead systems when collateral damage or wrapping is not an issue. No complications resulted from use of the Evolution.
Locking Stylets
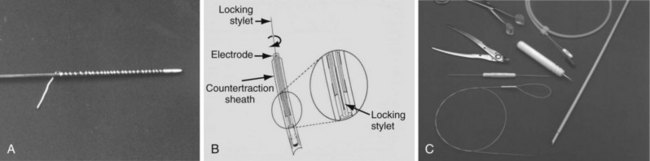
Locking stylets were developed after the mechanical sheaths. From the beginning, the major pacing companies (Medtronic, Cordis, Pacesetter [St. Jude Medical], and Boston Scientific [Guidant, CPI]) all attempted to make a universal locking stylet (one size stylet to fit all leads). These initial attempts were unsuccessful because of breakage of the locking mechanism. The tensile strength was inadequate to withstand the traction forces. Cook Pacemaker (now Cook Vascular) took another approach, abandoning the concept of a universal stylet. Their first-generation locking stylet came in various sizes to fit a variety of conductor coil diameters (Fig. 26-17). The conductor coil had to be measured before selecting the locking stylet. This locking mechanism was a small wire welded to the tip of the stylet and wrapped around the lead. Once the lead was passed down the conductor coil to the electrode, it was rotated counterclockwise, bundling the free wire and causing it to bind against the conductor coil. The greater the traction, the greater was the binding force. The locking stylet bound to the inner conductor coil, ideally at the distal electrode, functioned as a lead extender for applying traction and focused the traction force at the binding site. Focusing the traction force helped maintain the integrity of the lead but did not prevent lead disruption if excessive force was applied or if the lead had poor tensile strength. Also, the locking stylet was conductive, and the heart could be paced during parts of the lead extraction procedure if needed. This was the first effective locking stylet.
Cook’s second-generation locking stylet (Wilkoff Locking Stylet) had a different locking mechanism (Fig. 26-18). These stylets had a small flange at the tip designed to stay flat against the stylet until the preloaded thin cylinder was advanced; the cylinder deflected the flange to lock into the conductor coil. This was an efficient locking stylet that was easy to implant and that could be removed by rotating the stylet, breaking the flange. However, it could not be used if the conductor coil diameter was 0.016 inch or less, and it could not be used with extendable/retractable screw-in leads. Spectranetics made the first near-universal locking stylet (Lead Locking Devices 1, 2, 3 and e) to fit all leads. It used a long wire mesh that bundled and bound the stylet to the conductor coil. This type of locking stylet was efficient and functioned well. Ultimately, the Spectranetics LLD-EZ was developed that works for all but a few leads with extremely small IDs to the inner coil.
Cook’s most recent, third-generation locking stylet (Liberator Locking Stylet) is a true universal locking stylet (one size fits all). It uses a wire coil that is compressed by a reloaded cylinder, expanding the wire coil and binding it against the conductor coil (Fig. 26-19). Both the Liberator and the LLD-EZ are extremely versatile and provide an advantage over the alternative for either advancement down the conductor coil in locking.
Snares
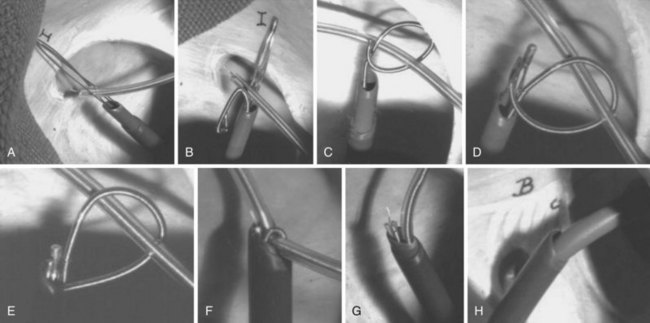
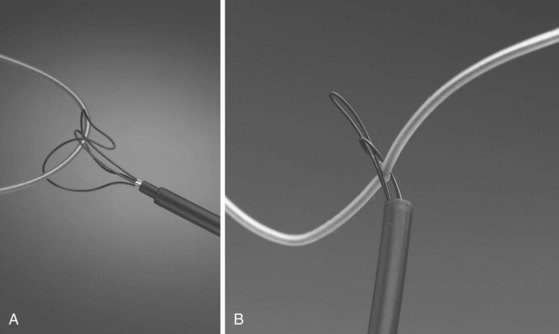
With the Dotter, needle-eye, and gooseneck snares, a reversible loop is created around the lead body to pull the proximal end of the lead out of the superior veins into the IVC, without placing traction on the electrode-myocardial interface (Fig. 26-20). A loop must be created and bound to the lead body. The binding of the loop must be reversible. Irreversibly binding the lead, or inability to remove the loop from around the lead, may result in dangerous traction maneuvers being performed in desperation while trying to extract the lead and snare. Failure to extract the lead subjects the patient to more invasive procedures to remove both lead and snare.
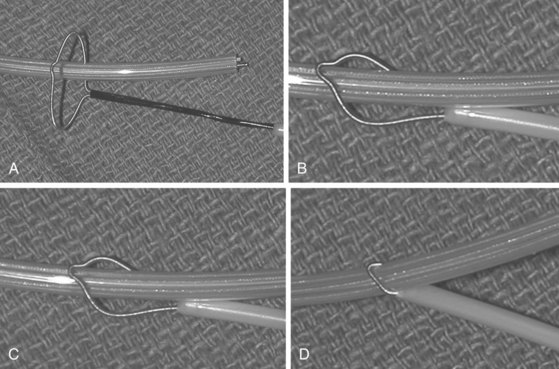
The Cook Needle’s Eye Snare is a more efficient method of grasping the lead in a reversible manner (Fig. 26-21). This snare has a wire loop that is passed over the lead body. A small, wire-loop tongue is then passed over the opposite side of the lead and into the larger wire-loop tongue. Pulling this apparatus into the workstation binds the lead reversibly for safe, indirect traction. Also, the binding forces are more diffuse, resulting in less lead breakage.
The Amplatz Goose Neck Snare is a radiopaque noose that is slipped over the free proximal end of a lead (Fig. 26-22). In situations where the proximal end is floating in the SVC, heart, or a pulmonary vein, the free end can be lassoed and extracted. The gooseneck snare can also be used to grasp the end of a tip-deflecting guidewire or deflectable EP catheter draped over the midportion of the lead. This is an excellent technique to reposition a lead so that the end can be grasped after being pulled down from the subclavian/jugular system, or to grasp the lead reversibly for extraction.
 Lead Extraction Approaches
Lead Extraction Approaches
Transvenous Approaches
Lead Vein Entry Site
Telescoping stainless steel sheaths look dangerous but are safe and effective if properly used (see Fig. 26-13, C). The principle is simple: keep the sheaths “tracking true” over the lead, and apply the force needed to destroy this tissue. A combination of pushing and rotation of the beveled tip is most effective. Once the metal sheaths break into the vein, they are removed and replaced by plastic extraction sheaths. “Tracking true” is a principle used for all sheath maneuvers. All sheath maneuvering must be performed under fluoroscopy, to ensure that the sheaths are tracking over the lead. Any kinking or other deviation of the vein course is dangerous. It creates a false passage that may be extravascular and may damage nearby structures.
Remote Vein Sites
Although most of the leads can be removed from the vein entry site, there are still dangerous situations and lead breakages. In these cases, a remote vein or a transatrial surgical procedure are the only options. In many cases, the femoral vein approach is still the best remote vein approach. However, other remote veins (contralateral external jugular, internal jugular, or axillary-subclavian-brachiocephalic) may be more suitable and easier to use. Some physicians have developed combination approaches, applying mechanical sheaths from both the vein entry site and a remote vein site. This combination constitutes a safe and efficacious approach, as developed by Bongiorni et al.,22 and has become increasingly popular, particularly when the need for reimplantation through the original venous access is no longer required. For cases such as lead breakage, use of a snare to grasp the lead and the subsequent removal of the lead using direct traction or a powered sheath is advantageous. Powered sheaths are not long enough in moderately tall patients for a femoral approach. Consequently, these approaches are confined to the superior veins, via both remote and vein entry sites.
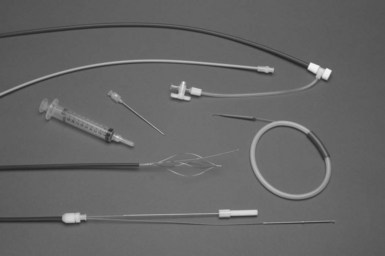
Femoral Approach
As discussed earlier, before the advent of powered sheaths, the femoral approach was used extensively. The indications for its use were failure to extract leads from the superior veins by any technique, lead breakage, and avoidance of the application of excessive force with the mechanical sheaths. The techniques used have not evolved significantly over the past 15 years. The transvenous approach through a femoral vein requires a special sheath set (e.g., Byrd WorkStation) that functions as an introducer, as a workstation for manipulation of snares, and as countertraction sheaths (Fig. 26-23). The set consists of an introducer needle, a guidewire, a 16F workstation, an 11F tapered dilator, an 11F telescoping sheath, a Cook deflection snare, and a Dotter basket snare. The workstation serves many functions. Initially, it acts as a protective sheath. The outer sheath prevents the insertion, withdrawal, and manipulation of the inner sheath and snares from damaging the veins or heart. To prevent clot formation, the workstation has a valve (Check-Flo) to continuously irrigate the sheath. The workstation and snares form a reversible loop to pull the proximal portion of the lead out of the superior veins; the workstation also acts as the outer telescoping countertraction sheath.
Combined Approaches
A combination of the vein entry site approach and a right internal jugular approach is used by some physicians as their primary approach to lead extraction. They use snares and/or a grasping instrument passed through the internal jugular vein into the SVC to pull the lead out of the axillary-subclavian-brachiocephalic veins and, if necessary, to pull the lead into the atrium. Mechanical sheaths are then applied to complete the extraction (to date, powered sheaths are not used).23
Retaining Superior Access with Femoral Snares
Often, the superior access is required for reimplantation, but the subclavian system is occluded. Even so, remarkably, the lead easily slides out without the ability to advance the extraction sheath past the area of occlusion. Reimplantation access is then thwarted. However, the use of a snare, usually an Amplatz Goose Neck, provides traction on the lead during advancement of the extraction sheath to the level of the right atrium, permitting advancement of a guidewire for access through the sheath after the lead removal.24
Surgical Approaches
Transatrial Approach
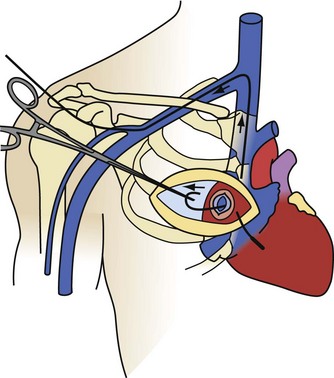
The transatrial approach, first described by Byrd in 1985, is a surgical EP procedure suitable for intracardiac implantation, explantation, and ablation procedures.25 The only disadvantages are the morbidity associated with surgical thoracic pain and the need for a medical electrophysiologist to work with a cardiac surgeon. It is a primary approach for noninfected patients who are candidates for a transatrial lead implantation. Younger patients with occlusion of one brachiocephalic vein or with SVC syndrome have the old leads extracted through a transatrial approach, followed by implantation of new leads. The advantage of the transatrial approach is the ability to remove leads that are not accessible or removable by the SVC or IVC approach. Most of the transatrial extractions are failures of the IVC approach. Rarely, failure of an SVC approach will lead directly to a transatrial approach (e.g., when the workstation cannot be passed through the femoral veins into the heart). Infected patients who are candidates for transatrial lead implants will have the leads extracted by an SVC or IVC approach and the transatrial implantation done after the infection is properly treated.
The transatrial approach is performed as originally described, through a limited surgical incision on the right anterior chest wall (Fig. 26-24). The right atrium is exposed by removal of the third or fourth right costal cartilage (determined by fluoroscopy). The pericardium is opened and suspended, and a pledgeted purse-string suture is placed in the right atrium. If the pericardium has been obliterated from a previous procedure or disease process, a small region of the lateral wall of the right atrium is dissected free. Using fluoroscopy, a pituitary biopsy instrument is inserted through the purse-string. The lead body is grasped in the atrium and pulled out. The lead is then cut, extracting the proximal and distal segments separately. The proximal portion of the lead can usually be pulled out by direct traction. The only limitation to the force employed is the tensile strength of the lead. In occasional cases where the tensile strength is insufficient, telescoping powered sheaths may be required. The distal segment is extracted by inserting a locking stylet, advancing telescoping powered sheaths to the wall, and removing the electrode from the wall using countertraction. This procedure is repeated for each lead to be extracted. On completion of the lead extraction, the atriotomy site is used to insert new leads, or to perform another EP procedure, or the purse-string suture is tightened, tied, and abandoned.
Open-Heart Procedure
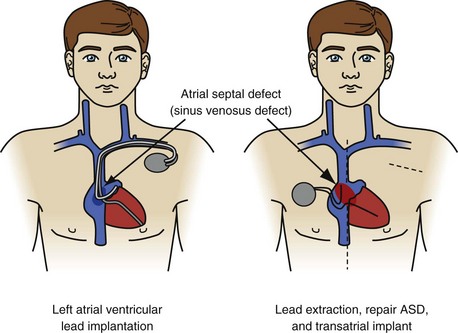
Implantation of leads in the left atrium and ventricle is a notable exception (Fig. 26-25). Leads are implanted into the left ventricle in two ways: through a congenital atrial or ventricular septal defect or retrograde through the aortic valve. All physicians consider the presence of left-sided leads and embolic symptoms an urgent indication for lead removal. Most consider their presence, with the potential for a complication, to be an urgent indication for lead extraction. A few physicians believe that, in the absence of complications (cerebrovascular accident, coronary artery occlusion, infarction of another organ, or sequelae of peripheral embolus), leaving the leads intact is an acceptable option. The rationale is that the extraction procedure is more dangerous than the presence of left-sided leads. The views on lead removal are just as varied. Some believe the leads should be removed; the chambers debrided of vegetation; and congenital defects, if present, repaired with CPB. Others think it is safe to extract the leads using the established right-sided techniques and protecting the brain from emboli by compressing the carotid arteries when necessary. If the second approach has any merit, it would be in removing newly implanted leads. If the newly implanted leads are proved to be free of vegetation and the procedure is monitored using TEE, it is probably safe to extract using direct traction. Specific data are not available on the numbers of physicians using these opinions.
 Special Situations
Special Situations
To evaluate extraction tools and a novel lead design technique for reducing the barriers to extraction of complicated leads, Wilkoff et al.26 used a sheep model with atrial defibrillator leads placed in the CS to the great cardiac vein. The leads, originally designed for atrial defibrillation in the Metrix atrial defibrillator (InControl, Redmond, Wash.), were modified but kept their pigtail configuration for lead stability. Three configurations—one without modification of the defibrillation coil, one with medical adhesive backfill under the defibrillation coil, and one covered with an expanded polytetrafluoroethylene polymer (ePTFE)—were implanted in sheep and were subjected to extraction at either 6 or 14 months. The model proved to be excellent for developing profound fibrosis, and the unmodified leads were almost impossible to remove without hemopericardium. The medical adhesive backfill was much better, and there was almost no trouble removing the ePTFE-covered leads. The study also demonstrated that laser sheaths were dangerous in the CS, because the sheath approximated the size of the vein; a special 7F electrosurgical extraction sheath was relatively much safer to use. During the procedures, the electrosurgical sheath was rotated away from the pericardial and toward the myocardial surface.
Although CS lead extraction has not yet become a clinical issue, implanters should make sound decisions now that promote the extraction of these leads. From the sheep experience, implanters should avoid construction that allows for tissue ingrowth. In some cases, significant ingrowth around CS leads clearly makes extraction more difficult. This is particularly true in patients with the new Starfix (Medtronic) leads, which are designed to increase fibrosis to affix the lead to the intended vein position. The ingrowth of the fibrotic tissue into any lead, but especially in this lead, will make the long-term extraction experience much more challenging and potentially life threatening (Fig. 26-26).
1 Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction. Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management (endorsed by American Heart Association). Heart Rhythm. 2009;6:1085-1104.
2 Byrd CL, Schwartz SJ, Hedin N. Intravascular techniques for extraction of permanent pacemaker leads. J Thorac Cardiovasc Surg. 1991;101:989-997.
3 Byrd CL. Advances in device lead extraction. Curr Cardiol Rep. 2001;3:324.
4 Byrd CL. Extraction of transvenous pacing leads. Am Heart J. 1992;124:1667-1668.
5 Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104:1029-1033.
6 Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133:604-608.
7 Chan AW, Bhatt DL, Wilkoff BL, et al. Percutaneous treatment for pacemaker-associated superior vena cava syndrome. Pacing Clin Electrophysiol. 2002;25:1628-1633.
8 Suga C, Hayes DL, Hyberger LK, et al. Is there an adverse outcome from abandoned pacing leads? J Interv Card Electrophysiol. 2000;4:493-499.
9 Smith HJ, Fearnot NE, Byrd CL, et al. Five-years experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol. 1994;17:2016-2020.
10 Fearnot NE, Smith HJ, Goode LB, et al. Intravascular lead extraction using locking stylets, sheaths, and other techniques. Pacing Clin Electrophysiol. 1990;13:1864-1870.
11 Byrd CL, Schwartz SJ, Hedin NB, et al. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol. 1990;13:1871-1875.
12 Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579-586.
13 Bilgutay AM, Jensen MK, Schmidt WR, et al. Incarceration of transvenous pacemaker electrode: removal by traction. Am Heart J. 1969;77:377-379.
14 Karagoz T, Celiker A, Hallioglu O, Ozme S. Unusual extraction of an active fixation ventricular pacing lead with outer coil fracture in a child. Europace. 2003;5:185-187.
15 Wilkoff BL, Byrd CL, Love CJ, et al. Pacemaker lead extraction with the laser sheath: results of the Pacing Lead Extraction with the Excimer Sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671-1676.
16 Epstein LM, Byrd CL, Wilkoff BL, et al. Initial experience with larger laser sheaths for the removal of transvenous pacemaker and implantable defibrillator leads. Circulation. 1999;100:516-525.
17 Saad EB, Saliba WI, Schweikert RA, et al. Nonthoracotomy implantable defibrillator lead extraction: results and comparison with extraction of pacemaker leads. Pacing Clin Electrophysiol. 2003;26:1944-1950.
18 Byrd CL, Wilkoff BL, Love CJ, et al. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol. 2002;25:804-808.
19 Kennergren C. Excimer laser assisted extraction of permanent pacemaker and ICD leads: present experiences of a European multi-centre study. Eur J Cardiothorac Surg. 1999;15:856-860.
20 Love C, Byrd C, Wilkoff BL, et al. Lead extraction using a bipolar electrosurgical dissection sheath: an interim report. Europace. 2001;3:223-228.
21 Hussein AA, Wilkoff BL, Martin DO, et al. Initial experience with the Evolution mechanical dilator sheath for lead extraction: safety and efficacy. Heart Rhythm. 2010;7:870-873.
22 Bongiorni MG, Di Cori A, Zucchelli G, et al. A modified transvenous single mechanical dilatation technique to remove a chronically implanted active-fixation coronary sinus pacing lead. Pacing Clin Electrophysiol. 2011;34:e66-e69.
23 Bongiorni MG, Giannola G, Arena G, et al. Pacing and implantable cardioverter-defibrillator transvenous lead extraction. Ital Heart J. 2005;6:261-266.
24 Fischer A, Love B, Hansalia R, et al. Transfemoral snaring and stabilization of pacemaker and defibrillator leads to maintain vascular access during lead extraction. Pacing Clin Electrophysiol. 2009;32:336-339.
25 Byrd CL, Schwartz SJ. Transatrial implantation of transvenous pacing leads as an alternative to implantation of epicardial leads. Pacing Clin Electrophysiol. 1990;13:1856-1859.
26 Wilkoff BL, Belott PH, Love CJ, et al. Improved extraction of ePTFE and medical adhesive modified defibrillation leads from the coronary sinus and great cardiac vein. Pacing Clin Electrophysiol. 2005;28:205-211.
27 Byrd CL, Wilkoff BL, Love CJ, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994-1996. U.S. Extraction Database, MED Institute. Pacing Clin Electrophysiol. 1999;22:1348-1357.
28 Kennergren C, Bucknall CA, Butter C, et al. Laser-assisted lead extraction: the European experience. Europace. 2007;9:651-656.
29 Neuzil P, Taborsky M, Rezek Z, et al. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: results of a randomized trial. Europace. 2007;9:98-104.