CHAPTER 15 Targeting Pain Generators
Spinal pain is very common and exacts a significant toll for the individual and society. Low back pain has a lifetime prevalence from 54% to 80%, an annual prevalence of 15% to 45%, and a point prevalence of 30%.1 Low back pain is the second most common reason for disability among adults in the United States with approximately 150 million work days lost per year.2,3 Spinal pain is the second most common reason for outpatient generalist physician visits, the third most common reason for surgical interventions, and the fifth most common reason for hospitalization.4,5 The cost to care for patients with low back pain is more than $100 billion per year.6 The prevalence of individuals seeking care for low back pain also seems to be increasing significantly. From 1992-2006, the prevalence of patients presenting with chronic impairing low back pain increased from 4% to 10%.7 Cervical pain exacts a significant toll in terms of individual morbidity and socioeconomic burden. Targeting pain generators through precision diagnostic methods is the first step toward appropriate and effective treatments for chronic spinal pain.
Much of the epidemiologic data on low back pain are nonspecific, meaning that a cause cannot be found in most cases. Despite evidence to the contrary in the 21st century, these older, inaccurate epidemiologic studies continue to be quoted by current authors. One of the oldest epidemiologic studies commonly quoted was published more than 40 years ago by Dillane and colleagues8 and was based on a retrospective practice audit of data gathered more than 50 years ago. Dillane and colleagues8 reported that they could not detect a cause for low back pain in approximately 80% of female and 90% of male patients with acute back syndrome. These authors did not report the use of any x-ray studies and apparently relied solely on history and physical examination.8 They diagnosed approximately 11% of male patients and approximately 4% of female patients with low back “strain.” Until more recently, the only tools to diagnose the etiology of low back pain have been history, physical examination, and sometimes x-ray or computed tomography (CT) scan.
In 1982, Nachemson9 reviewed the literature on chronic low back pain. In perhaps the most frequently quoted epidemiologic study on the cause of chronic low back pain, Nachemson9 reported that in only 15% of cases could a pathoanatomic explanation be found for patients with chronic low back pain (>3 months). Nachemson9 stated, “probably very little can be done at our present state of ignorance to treat these patients and to improve their natural histories.” Low back pain is a symptom, not a diagnosis, in the same way that abdominal pain is a symptom and not a diagnosis. In acute cases of low back pain, this nonspecific diagnosis usually suffices because most cases of acute, first-time low back pain resolve with minimal intervention; however, when low back pain becomes chronic, recurrent, and disabling, the clinician must diagnose the source of the pain so that an appropriate treatment plan may be devised.
When a source of pain is not obvious, diagnosis often depends on who makes the diagnosis and sets the reference standards by which the diagnosis is “proven.” Who is right? For that matter, can anyone reliably diagnose the cause of chronic benign spinal pain? Many authors argue that chronic benign spinal pain is largely due to exaggerated functional complaints and irreversible central nervous system sensitization,10 making pain self-perpetuating and diagnosis all but impossible. These contentions are not often supported by primary studies,11 however, and authors and clinicians question this diagnosis.12,13
Interventionalists developed and refined precision, fluoroscopically guided diagnostic interventional spine procedures in the 1980s and 1990s14 to diagnose and treat nonspecific spinal pain better. Fluoroscopically guided block procedures are now considered the reference standard to confirm a tissue diagnosis. Out of the previous era of “ignorance,” many diagnostic protocols have been validated and standardized.15,16 Using the results of precision-guided diagnostic procedures, surgeons identify spinal segments for fusion at various stages of the degenerative cascade.17 Most surgeons still depend on an accurate diagnosis of a specific pain generator to select appropriate therapy18 because surgical results for chronic benign pain syndromes without a reversible anatomic cause are generally poor.19
The debate continues regarding diagnostic injections as new research emerges, along with better treatment options. Spinal pain is a complex interaction of many biopsychosocial factors. Chronic spinal pain may originate from one or more spinal levels and different anatomic structures in the anterior, middle, and posterior columns. Spinal pain also varies over time. Pain can be caused by abnormal mechanical stress on normal tissue affected by structural deformity, normal mechanical stress on injured tissues, minor stress on chronically inflamed and sensitized tissues, damaged nerves, and a varying combination of all of these. Chronic pain causes a greater or lesser degree of central sensitization and together with a multitude of functional factors, including the requirement for copious amounts of opiates, often makes accurate diagnosis difficult. Nevertheless, it can be argued that most chronic axial spinal pain is due to accumulated repetitive strain or low-grade trauma,20 acute injuries to the major underlying structures and their supporting ligaments, or both. Ongoing stimulation from these peripheral structures to a greater or lesser extent maintains a state of peripheral and central sensitization. In time, adaptive responses within the posterior, middle, and anterior columns may attenuate, exacerbate, or cause new sources of pain.
Despite this complexity, specific tissue pain generators can be hypothesized based on history, physical examination, imaging studies, and response to directed treatment. Interventional procedures are used to test the hypothesis that pain is related to a structural abnormality hypothesized by clinical and imaging findings. (The word hypothesis is used loosely here; arguably one only can confirm a clinical impression using diagnostic blocks. A hypothesis is confirmed using a study protocol that can show approximately <5% probability that the findings are due to chance). Foremost, interventional procedures are perhaps best used to refute one’s hypothesis that a particular structure is painful. That is, diagnosis is made through the process of systematically excluding various tissue causes of axial and extremity pain in the posterior, middle, and anterior columns.19
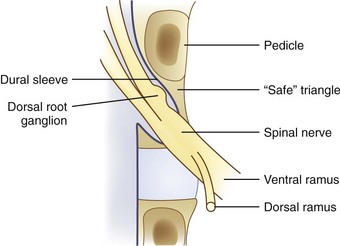
This chapter presents primarily evidenced-based standards and some expert opinions for confirming or refuting one’s hypothesis that a particular structure, structures, or segments are a source of spinal pain.15 A discussion of pain resulting from “red flag” conditions, such as fracture, tumor, infection, systemic diseases, or referred from nonspinal structures, is not included; likewise, “yellow flag” conditions (psychosocial factors) are not discussed in detail. Evaluation of the anterior column using provocative discography is discussed elsewhere, so the discussion in this chapter is focused on diagnosis of pain originating from the posterior and middle columns, in particular, pain originating from the zygapophyseal joint and sacroiliac joint in the posterior column and from the nerve root, dorsal root ganglion, and dura in the middle column. The diagnostic use of injection procedures is explored and not their therapeutic value other than the diagnostic value of response or nonresponse to treatment. Finally and most importantly, this chapter is not a systematic review, and the interested reader is referred to numerous systematic reviews on the diagnostic value of spine injections.1,15,16,20–26
Diagnostic Analgesic Injections as Reference Standard
The belief that chronic benign spinal pain is difficult to diagnose is supported by the low specificity and sensitivity of the history, physical examination, and various imaging modalities as the reference standard for diagnosing chronic benign spinal pain11,27,28 and a bias that chronic pain is to a greater rather than lesser extent a neuropathic process with central sensitization.19 If one uses interventional diagnosis with precision fluoroscopically guided procedures as a reference standard for identifying pain, however, one can arrive at a tissue diagnosis in approximately 70% to 80% of cases.29,30 Which approach is right? Truth usually lies somewhere in between.
The rationale for facet blocks is based on the anatomic fact that the innervation of facet joints (medial branches) is known and that zygapophyseal joints are capable of causing pain.15 Local anesthetic blocks rely on the specificity of anesthetizing a single or limited number of structures or nerves and on the patient’s capacity to distinguish clearly a reduction in preblock pain after anesthetizing one or more structures. Injection of a limited volume of local anesthetic into a zygapophyseal joint or its nerve supply is relatively specific for anesthetizing a joint and its capsule. Similarly, local anesthetic injected into the disc should anesthetize nociceptors within radial annular fissures that communicate with the nucleus. When anesthetizing the nerve root within the middle column, the block is less specific for axial pain relief because several structures may be partially blocked (e.g., dorsal root ganglion, ventral rami, sinuvertebral nerve, posterior longitudinal ligament).
Anesthetizing a structure does not reveal the cause of pain; anesthetizing the nerve supply simply relieves pain. This is an important concept. The cause of pain should dictate the type of treatment, and the treatment is only as good or bad as its success in eliminating or modulating the cause. If the cause of pain in the case of a specific zygapophyseal joint is synovial inflammation, one would expect short-term to intermediate pain relief after the intra-articular injection of corticosteroids. If a patient’s pain is due to mechanical or neuropathic causes, there is no reason that corticosteroids would be effective other than the expected duration of the local anesthetic. There is no reason that there should be longer term pain relief except for the expected rate of placebo response or reported pain relief secondary to spontaneous pain regression.31 That is, relief of pain during the local anesthetic phase does not distinguish irreversible neuropathic pain from reversible nociceptive pain. In the case of chronic radicular pain, significant relief of pain for several weeks after the injection of corticosteroids would suggest that there is a reversible structural cause.32
Testing Protocols for Diagnostic Injections
As essential as precision technique is in the performance of diagnostic blocks, so is standardized assessment. Standardized diagnostic block evaluation sheets should be filled out for each patient; detailed postprocedure assessment protocols and sample evaluation instruments are available in the International Spine Intervention Society Practice Guidelines.15 Preprocedural and postprocedural evaluation should be performed by unbiased personnel and checked by the physician. It is recommended that the patient fill out a body pain diagram with pain scores (visual analog scale [VAS]) before and after the procedure. Additionally, the patient should rate current pain levels with various movements (e.g., lumbar flexion, extension, side-bending, sitting, and standing).
Confounding Factors
Sedation
An important, potentially confounding factor when performing diagnostic blocks concerns the use of sedation. Logically, one would assume that administration of opiates and sedatives before a diagnostic block would increase the false-positive rate; however, Manchikanti and colleagues33 found that this proportion of patients was relatively small, and there was no difference with use of saline, opiate, or sedative. In a randomized study of 60 patients, Manchikanti and colleagues33 titrated medication to relaxation using saline, midazolam, or fentanyl. They found that only 50% of patients receiving sodium were relaxed, whereas 100% of patients receiving either fentanyl or midazolam were relaxed. In all groups, 10% of the patients reported greater than 80% pain relief with active motion testing. Even so, typically, one limits or omits sedation before a diagnostic injection, however.
Biopsychosocial Factors
More recently, authors have reiterated the importance of shifting the concept of “backache.” Kikuchi34 recommended changing the term spinal disorder to biopsychosocial pain syndrome and the term morphologic abnormality to mechanical, functional disorder. According to Kikuchi,34 morphologic and structural abnormalities do not always explain all of a patient’s pain, and chronic backache should not be seen as an isolated spinal disease. A significant amount of scholarship has been devoted to enumerating the psychosocial factors associated with spinal pain. In a classic study comparing workers with symptomatic disc herniation (requiring surgery) versus asymptomatic workers, significant differences were found in three areas: presence of nerve root compromise, psychosocial factors (depression, anxiety, marital status, self-control), and work perception (satisfaction, job loss, occupational stress, intensity of concentration).35 Of the risk factors, two of three were functional, not morphologic.
Although the long-term results of treatment for chronic axial back pain may be influenced by various psychosocial factors,36,37 the possible effect of psychosocial factors in determining the patient’s tested perception of pain and functional improvement with treatment does not indicate that the diagnosis was incorrect. In many cases, when a “biologic” pain generator can be correctly identified and treated, the psychosocial distress resolves. If it is true that psychological variables determine whether a patient admits relief on various testing instruments, does the evidence of physiologic distress noted on test scores reverse when the patient’s chronic pain is relieved?
Wallis and colleagues38 studied 17 patients after whiplash injury with a single symptomatic cervical zygapophyseal joint who were enrolled in a randomized controlled trial of percutaneous radiofrequency neurotomy. At 3 months after the procedure, all patients whose pain was relieved had complete resolution of preoperative psychological distress; in contrast, all but one of the patients who did not experience pain relief continued to experience psychological distress. Manchikanti and colleagues39 found no correlation between somatization disorder and inappropriate Waddell signs and symptoms to response in pain relief after a comparative double block protocol for diagnosing facet pain. Derby and colleagues40 found no difference in response to pressure-controlled disc stimulation between patients with abnormal psychometric Distress Risk Assessment Method scores and asymptomatic volunteers.
Posterior Compartment: Zygapophyseal Joint and Sacroiliac Joint
Zygapophyseal Joint
Each spinal segment is composed of a three-joint complex: the intervertebral discs and two posterolateral facet joints.41 Facet joints can also be called zygapophyseal joints. The word apophysis is Greek, meaning an “offshoot” or a “bony protruberance.”42 Anatomically, the zygapophyseal joint is an outgrowth of the vertebral body. The zygapophyseal or facet joints are formed by the articulation of the inferior articular process of one vertebra with the superior articular process of the adjoining vertebra. Zygapophyseal joints have classic synovial joint features: hyaline cartilage surfaces, a synovial membrane, and a surrounding joint capsule.43 Facet joints have varied morphology and function based on their location within the spine. Although the intervertebral disc is loaded primarily in flexion, the zygapophyseal joints are loaded in extension and lumbar rotation.44,45 The orientation of the facet joint varies based on the requirements of regional spine function. Lumbar facets are situated sagittally to limit axial rotation and loading, whereas the cervical and thoracic facets are oriented coronally to limit shearing forces on the disc. Innervation is via the medial branches of the dorsal ramus in most locations.
Pathophysiology of Facet Pain
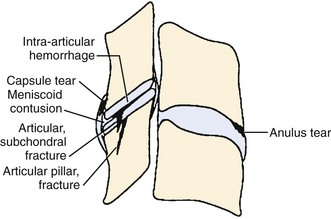
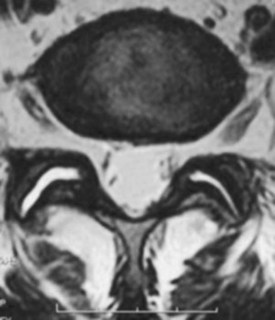
Traumatic injury to lumbar and cervical zygapophyseal joints is common and probably occurs to a lesser extent to the thoracic joints. In the setting of trauma, there is a clear pathophysiologic difference between facet joints and nontraumatic controls. Zygapophyseal joint sections from autopsy specimens of individuals with a past history of trauma but dying of natural causes show significant age-related, gender-related, and trauma-related changes in the bone, cartilage, and soft tissues, including subchondral sclerosis, fibrillation and splitting of cartilage, and cartilage length differences, versus subjects with no history of trauma.46 Histologic sections of the lumbar zygapophyseal joints of mostly motor vehicle accident victims revealed fractures of the superior articular process, central infarctions of the subchondral bone plate, and capsule tears including the ligamentum flavum.47,48 Most tissue sections (77%) show soft tissue injuries, and approximately 30% (11 of 33) show fractures and infarctions. Sections from the cervical spine in trauma victims show similar injuries to the zygapophyseal joint articular cartilage and annular lesions in the intervertebral discs and cartilaginous endplates (Fig. 15–1).49 In the lumbar and cervical spines, lesions were found exclusively in the trauma patients and in none of the patients in the control group.46,49,50
Many of these pathoanatomic findings are occult on routine x-ray, CT scan, and magnetic resonance imaging (MRI) but may be the cause of ongoing neck pain in survivors of motor vehicle accidents or other significant trauma. In a small study with short-term follow-up (approximately 3 months), Eisenstein and Parry51 examined zygapophyseal joints in 12 patients who underwent successful fusion for zygapophyseal joint mediated pain (diagnosed by provocation arthrography, intra-articular blocks, and negative discography) versus controls and found histologic changes similar to changes of chondromalacia patellae and osteoarthritis of large joints. The most frequent finding was focal full-thickness cartilage necrosis or loss of cartilage with exposure of subchondral bone; osteophyte formation was absent in all specimens.51 Degenerative histologic findings alone do not make a definitive diagnosis of facet syndrome, however. Ziv and colleagues52 reported a high proportion of coarsely fibrillated or ulcerated (or both) facets in fresh cadaveric spines from young adults (30 to 50 years old); such degeneration remains constant throughout adulthood.
Traumatic and repetitive injury leading to painful “facet arthritis”48 may cause pain because zygapophyseal joints and their capsules are heavily innervated structures subject to high stress and strain during spinal loading.53 Joints comprise free and encapsulated nerve endings containing substance P and calcitonin gene-related peptide.54–56 Substance P, calcitonin gene-related peptide, and immunoreactive sensory and autonomic nerves are found in zygapophyseal joint synovial membranes.57 Facet capsules contain low-threshold mechanoreceptors, mechanically sensitive nociceptors, and silent nociceptors.58 These low-threshold and high-threshold mechanoreceptors fire when the joint capsule is stretched or compressed, and their firing can be suppressed by injected lidocaine and hydrocortisone.59 In animal models, induced inflammation decreases the threshold of nerves within the joint capsules and causes elevated baseline discharge rates.59 In animal models of knee arthritis, acute inflammation sensitizes fine articular afferents, which become active at rest and respond more vigorously to routine painless joint range of motion.60
Excessive stretching damages the zygapophyseal joint capsules and causes axonal swelling, retraction balls, and inflammation.58 The result is hyperexcitability and spontaneous firing, which are synonymous with neuropathic pain. Capsular injury during a whiplash injury may cause persistent neck pain secondary to chronic capsular overstretching. Animal studies suggest that facet capsule strains comparable to strains previously reported for whiplash kinematics and subcatastrophic failures of this ligament activate nociceptors within the capsule.61–65 In addition, chronic capsular loading in animals may cause central inflammation resulting in mechanical hyperalgesia and in some cases centrally maintained pain.56,63,66,67
Animal studies showing central sensitization are consistent with the widespread hypersensitivity documented in whiplash patients. Although focal sensitization to mechanical stimuli may be found 3 months after whiplash injury, which mostly resolves by 6 months, some patients develop persistent pain with symptoms that are consistent with chronic neuropathic pain.68 Patients with persistent pain at approximately 6 months show signs of more widespread hyperalgesia69 and hypersensitivity to cutaneous and muscular stimulation in neck and lower limb consistent with central hypersensitivity.10,70
If chronic pain originating from injury to the zygapophyseal joints is due to capsular stretch and maintained by central hypersensitivity, local anesthetic with or without corticosteroid should suppress nociceptive input for the duration of the local anesthetic effect and in some cases (e.g., similar to a sympathetically maintained pain state) for days to weeks.71 If most of a patient’s pain is due to mechanical and central causes, there would be no reason why local anesthetic and corticosteroid would be more effective than local anesthetic alone.72–74 Former and latter logical outcomes are supported in prospective and randomized controlled trials,74 although alleviating pain with medial branch blocks was shown in one study to relieve pain for an average of several months.75,76
Decreasing peripheral input 6 months or longer by heat ablation of medial branches relieves pain77; the pain typically returns within the expected time it takes for the medial branches to regenerate. Such prolonged relief of pain, if accompanied by resolution of widespread hypersensitivity, would imply that central hypersensitivity is reversible when the peripheral source of input is interrupted. That is, if there is a concern that persistent central hypersensitivity would lead to failure of a proposed localized or segmental stabilization procedure, resolution of widespread and local hypersensitivity after medial branch neurotomy might predict that decrease in nociceptive input by surgical stabilization would be successful. Zygapophyseal joint pain often occurs at more than one segment, however, and one must identify adjacent or skipped level sources of zygapophyseal joint pain, especially if one is considering surgical fusion or arthroplasty.
When cadaveric lumbar spines are anteriorly fixated at one level, motion is transferred to adjacent segments causing increased capsular stretch in the adjacent facet joints.78 In extension, cervical arthroplasty models exhibit significant increases of facet force at the treated level. In the fusion model, the facet forces decrease at the treated segment and increase at the adjacent segment.79 Failure to recognize symptomatic pathology at an adjacent level or the same level may lead to early or late return of pain. This is not a failure of the diagnostic blocks; it is a failure to obtain a thorough diagnosis.
Rationale for Control Blocks in Diagnostic Facet Intra-articular and Medial Branch Blocks
Can a diagnosis of facet syndrome be made without injections? The diagnosis of zygapophyseal joint pain is typically hypothesized based on clinical findings and imaging studies. Most clinicians rely on a variety of favorite criteria, such as localized unilateral pain that is worse in extension, pain worse in the morning and better with gentle movement, concordant pain provoked with palpation approximately 1 cm lateral to the midline over the zygapophyseal joints, and imaging studies showing signs of facet degeneration. A “facet syndrome”80 diagnosed by clinical findings has not been substantiated, however, if one uses as a reference standard the relief of pain after placebo-controlled anesthetic blocks.23,27,81 The current best evidence has not found any individual clinical finding or cluster of findings that can predict response to the reference standard of pain relief after local anesthetic block of the medial branches or intra-articular zygapophyseal joint block.
The purpose of diagnostic facet blocks is to establish the diagnosis or rule it out, similar to a liver biopsy of a suspicious lesion. Diagnostic facet blocks are a tertiary intervention in patients with chronic pain that has not resolved with time and conservative care. The current standard for the diagnosis of facet mediated pain is the use of controlled differential (double) blocks to confirm or refute one’s hypothesis that the facet joint is a pain generator. Because of the high false-positive rates of single diagnostic blocks, a single block does not constitute a diagnosis, and control blocks are essential to decrease the incidence of false-positive responses.82 The reported false-positive rate of a single diagnostic block ranges from 17% to 63%.83 In a retrospective review of 438 patients using a double block paradigm requiring 80% relief, the false-positive rates for a single diagnostic block were 45%, 42%, and 45% for the cervical, thoracic, and lumbar regions.83
Although Cohen and colleagues84 showed that treatment results after medial branch neurotomies were not changed by requiring a placebo control, the current published standard of interventional societies requires a confirmatory block before making a diagnosis of zygapophyseal joint mediated pain. The best-studied double block protocol requires a difference in pain relief duration based on a shorter or longer acting medication—typically, greater than 1 hour for lidocaine and greater than 2 hours for bupivacaine.85,86 Because the goal is to have a placebo control and because consent and patient compliance issues hinder using a saline block control, one may argue that any prior diagnostic injection in which the patient reports no relief is a valid control block, especially because the patient and the physician were anticipating relief. Because many insurance companies in the United States no longer authorize or consider double blocks medically necessary, the occurrence of a previous “negative” block evaluation could be the negative control.
When the treatment is relatively benign, convincing relief with physician and staff testing after facet or medial branch injections in an older patient with clinical symptoms consistent with zygapophyseal joint pain may not justify confirmatory injections.84 A young patient who has little or no facet abnormalities, who is on a significant dose of narcotics, and who reports less than convincing approximately 80% relief should undergo a second confirmatory injection before considering interventional or surgical treatment based on the block results.
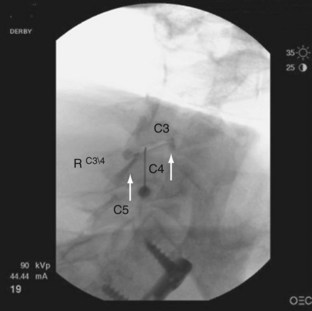
The rationale for how many and which levels to test is debated. Levels are typically chosen based on known pain referral patterns, prevalence studies, and localized manual palpation. Using a comparative double block control, Manchukonda and colleagues83 found that most often two joints were symptomatic in the lumbar spine and three adjacent joints were symptomatic in the thoracic and cervical spine. The most logistically efficient and least costly method is to rule out facet pain globally on the side or sides of the patient’s pain and at the proximity of approximately two to three adjacent levels. One would inject (in the case of lumbar spine) the L2-5 medial branches to block the L3-4, L4-5, and L5-S1 zygapophyseal joints (Fig. 15–2 shows medial branch anatomy of the lumbar spine; Fig. 15–3 shows a lumbar medial branch block). If no relief occurred and there was no evidence that the higher joints were involved, and if one is confident that the facets were denervated, one can eliminate facet pain from the diagnosis. If the result is positive, one can perform more selected denervation on a confirmatory injection. Although this could be argued to be the most efficient method, many third-party payers in the United States limit injections to two levels per session.
Diagnostic Accuracy
Kaplan and colleagues87 reported that medial branch blocks may fail because of venous uptake. Venous uptake occurred in 7 of 20 (35%) medial branch blocks. If venous uptake was encountered, repositioning of the needle resulted in joint anesthesia only 50% of the time. When venous uptake was encountered, the subjects were brought back for a later injection. These findings stress the importance of using contrast medium for medial branch blocks and carefully observing the flow pattern. Kaplan and colleagues87 also found that in 11% of cases they were unable to anesthetize the joint, even in the absence of venous uptake. Medial branch blocks in the lumbar spine would have an 11% false-negative rate; this may have been due to anomalous or collateral facet innervation or insufficient volume of local anesthetic reaching the target nerve.
Rarely discussed is the consistency with which one may expect a longer duration of action of bupivacaine versus lidocaine. Although bupivacaine has a longer duration of action than lidocaine, the mass of drug reaching the nerve is the most important variable, and one cannot guarantee that the same amount will be available on consecutive sessions. In addition, most patients are not kept in the recovery area for the duration of local anesthetic to be evaluated, and the duration depends on a patient’s self-reporting, which may or may not be consistent between injections. Lord and colleagues86 showed that using a double block comparative standard, 65% of patients failed to recognize the difference in duration of pain relief but did accurately distinguish a separate placebo-controlled block with saline.
The crucial factors for specificity of the diagnosis of zygapophyseal joint pain are accurate targeting of the intended structure under fluoroscopy, confirmed by contrast medium, and the delivery of the appropriate volume of local anesthetic. Intra-articular joint blocks are specific, unless there is a medial capsular tear or injection of excessive volumes (>1 mL injected into a lumbar zygapophyseal joint or 0.3 mL injected into a cervical zygapophyseal joint), which may rupture the capsule and spread medially into the epidural space.88,89 Many early studies of facet intra-articular blocks used 2 to 8 mL per injection. When reviewing negative studies regarding facet injections and the systematic reviews that still quote these studies, the discerning reader should check the total volume of injectant used.89,90 Destouet and colleagues89 found that volumes of injectant of 0.5 to 1.5 mL commonly ruptured the superior recess of the capsule and extravasated; in later studies, Destouet and Murphy91 aspirated the 0.5 to 1.5 mL of contrast dye before adding local anesthetic and steroid. Cadaveric studies performed with variable volumes of methylene blue injected into facet joint (1 to 4 mL) showed that the dye extended not as expected into the paraspinal tissues but rather into the epidural space and around the spinal nerves.92 Moran and colleagues92 described the facet capsule as thick dorsally, whereas anteriorly the facet synovial membrane is contiguous with the ligamentum flavum, and the adipose tissue in the superior recess is in direct contact with the adipose tissue around the spinal nerve. Randomized controlled studies validate the specificity of medial branch blocks for relief of zygapophyseal joint mediated pain.93
To maintain the specificity of medial branch block, a low volume of local anesthetic is used to anesthetize a specific cervical93 or lumbar medial branch.87,88 If volumes greater than 0.5 mL are used, the close proximity of the lumbar lateral and intermediate branch to the medial branch potentially might increase false-positive rates by blocking paraspinal soft tissues (ligaments or muscles or both).20,94 In addition, volumes greater than 0.5 mL on the superior edge of the lumbar transverse process may spread onto the dorsal and ventral spinal nerves.88 Decreasing input into the central nervous system by anesthetizing any structure or nerve could relieve pain by decreasing or modulating central input. Although this is a probable confounding factor, the reference typically cited is the study by North and colleagues,95 which reported an unacceptably high false-positive rate for diagnostic blocks in patients with low back pain. The conclusion reached by North and colleagues95 was that diagnostic blocks had limited specificity. Their protocol clearly lacked diagnostic specificity, however. They used an excessive 3 mL volume of bupivacaine for the medial branch blocks. Because 0.5 mL of local anesthetic placed too close to the superior edge flows onto the exiting root, the reported decrease in sciatic pain was more likely due to anesthetizing the spinal nerve and not a false-positive effect of neuromodulation.88
Because patients in the study by North and colleagues95 reported an average relief of 75% to 80% after anesthetizing the sciatic nerve, operators must be aware that blocking this nerve anywhere along its course may result in the report of pain relief. As previously mentioned, relief of pain for the duration of the local anesthetic effect cannot distinguish between reversible inflammatory or compressive causes of pain and nonreversible neuropathic causes of pain.
Although North and colleagues95 found that 3 mL of local anesthetic placed into muscles at several levels has relatively minimal effect on sciatic pain, some authors question whether anesthetizing the needle track increases false-positive responses.20 Patients probably report more pain relief when the needle track is anesthetized. Rather than a false-positive response, one would expect better pain relief when patients are not experiencing lingering needle-related pain at the same time as pain relief resulting from anesthetizing the zygapophyseal joints.
Lumbar Spine: Facet Syndrome
History
In 1911, Goldthwait96 reported that facet joint asymmetry could cause lumbago, sciatica, and paraplegia. Ghormley97 first coined the term facet syndrome as a cause of referred pain and the sciatica resulting from direct nerve root compression by the facet. Badgley97a first described the facet joint as an independent source of referred pain in greater detail. Mooney and Robertson80 described “facet syndrome” referral patterns by injection of hypertonic saline into the lumbar facets of patients with positive diagnostic blocks. Subsequently, Mooney and Robertson80 were the first to use x-ray–guided intra-articular injections with local anesthetic and corticosteroid; they reported complete pain relief in approximately one fifth of patients presenting with low back and leg pain. Dreyfuss and colleagues88 were the first to describe an effective lumbar medial branch block technique. Using this technique, Kaplan and colleagues87 showed that pain resulting from facet capsular distention could be successfully blocked in approximately 90% of cases by a medial branch block with 2% lidocaine versus saline.
Lumbar Zygapophyseal Joint Pain
Studies reporting the prevalence of zygapophyseal joint pain report figures ranging from 15% to 52%. In 1994, Schwarzer and colleagues98 established the prevalence of zygapophyseal joint pain using a double block comparative protocol in 96 patients with a mean age of 38 years and mean duration of low back pain of 16 months mostly secondary to work-related injuries and trauma presenting to two tertiary U.S. clinics. (Fig. 15–4 shows a lumbar intra-articular zygapophyseal joint block.) Schwarzer and colleagues98 found that the combination of discogenic pain and zygapophyseal joint pain is uncommon. In a group of 176 patients, 47% had initial relief with a screening lidocaine block, but only 15% had 50% or greater relief with a confirmatory block.
Manchikanti and colleagues,99 also using a double block protocol but requiring 75% pain relief and a differential response, reported an even higher initial response of 81 of 120 (67.5%) to lidocaine medial branch blocks and a much greater percentage (45%) of the total reporting longer 75% relief after confirmatory bupivacaine medial branch blocks. This patient group was older, however, than Schwarzer’s group with a mean age of 47 years and with a longer mean duration of low back pain of 47 months. The false-positive rate for one block was 41%. In a later study, Manchikanti and colleagues100 revised the prevalence of zygapophyseal joint pain downward from 45% to 27% (95% confidence interval 22% to 32%). Schwarzer and colleagues101 studied an older group of patients with a mean age of 57 years referred to an Australian rheumatology clinic with low back pain for an average of 7 years. A diagnosis of zygapophyseal joint pain was made in 40% (95% confidence interval 27% to 53%). Requiring 90% relief of original pain, the prevalence was 32%, and requiring 100% relief, the prevalence was 11%. Manchikanti and colleagues100 reported an even higher prevalence of 52% zygapophyseal joint pain in a group of patients 65 years old or older.
No consistent history, physical examination, or imaging findings correlated to positive block responses have been found. In the early 1980s, uncontrolled single, variable injectant volume, intra-articular zygapophyseal joint injections were used as the reference standard for identifying lumbar zygapophyseal joint pain, and these authors reported correlations with various history or physical examination findings.51,102 Some studies103,104 reported that a cluster of five of seven features (Revel criteria) could predict a 75% decrease in pain after a single intra-articular block. The seven items in the cluster are age older than 65 years, pain well relieved by recumbency, no exacerbation of pain with coughing and sneezing, no exacerbation of pain with forward flexion, no exacerbation of pain with extension, no exacerbation of pain with rising from flexion, and no exacerbation of pain with the extension-rotation test. Subsequent well-conducted studies did not replicate these studies.28,105 As mentioned, most of these earlier studies used single medial branch blocks, which have been reported to have 25% and 38% false-positive rates for the diagnosis of zygapophyseal joint pain.106,107
Newer studies of clinical correlations refined the technique with an appropriate injectant volume and a confirmatory double block paradigm with either a second intra-articular injection or a medial branch confirmatory injection with bupivacaine lasting longer than the pain relief after a prior lidocaine block.28,92,101,106,108,109 These studies did not find any clinical correlates with history or physical examination. In particular, extension and rotation were not predictive of response. A systematic review of all published studies comparing clinical outcome after local anesthetic blocks and clinical signs and symptoms found no consistent clinical features with a high specificity.11 The review found several clinical features with a high sensitivity, however, and these features may be cautiously used to exclude the diagnosis of facet mediated pain. These features include pain not increased with cough, pain not relieved with recumbency, and pain that can be centralized.11 There are no consistent reproducible history or physical examination criteria that predict a positive response to a facet block. History and physical examination are better at ruling out facet mediated pain than diagnosing facet pain.
The current best evidence also shows that radiologic imaging, with a few more recent exceptions, does not correlate with response to zygapophyseal joint blocks. The conflicting evidence that radiologic imaging may predict outcome from uncontrolled lumbar zygapophyseal joint blocks may be partially due to lack of rigor in the reference standard used to define a positive response in earlier studies.20 In 1979, Carrera110 reported that 73% (n = 63) of patients describing pain relief after uncontrolled intra-articular injection of 2 to 4 mL of local anesthetic had CT evidence of lumbar facet disease versus 13% who had no evidence of disease. It is well accepted, however, that injectant volume should not exceed 1 mL; otherwise, the injection loses specificity, with a leak of local anesthetic around the nerve root and along vertebral levels within the epidural space.92
A large study by Jackson and colleagues111 of 390 patients found no relationship between imaging and pain relief after uncontrolled intra-articular lumbar zygapophyseal joint injections. Supporting the findings by Jackson and colleagues,111 Schwarzer and colleagues,112 in the only study using placebo-controlled injection, found no correlation between CT findings and a positive response comparing local anesthetic with saline blocks in 63 patients when more stringent criteria of controlled injections were used as the reference standard. Similarly, Cohen and colleagues113 found no relationship in 192 patients between MRI findings of zygapophyseal joint hypertrophy or degeneration and response to medial branch neurotomies based on positive response to a single medial branch block. Kawaguchi and colleagues114 likewise found no significant relationship between low back pain symptoms and radiographic abnormalities in a group of 106 patients with rheumatoid arthritis.
The intriguing bright spot on the horizon is the finding that where MRI or single photon emission computed tomography (SPECT) shows imaging findings consistent with either “inflammation” or “edema,” a stronger correlation emerges (Fig. 15–5). Although not confirming the diagnosis of zygapophyseal joint pain with a reference standard, Friedrich and colleagues115 more recently found that an estimated 14% (21 of 145) of patients with low back pain had MRI evidence of facet joint edema, and follow-up MRI scan showed “almost perfect” agreement between change in pain and a reduction in intensity of edema on sagittal short tau inversion recovery (STIR) images. Radionuclide bone scintigraphy detects bone areas with synovial changes (inflammation or hyperemia) or increased osteoblast activity and degenerative regions with a high degree of remodeling. Osteophytes in process of growing show a high degree of bone scan activity. As mentioned earlier, a positive lumbar SPECT scan predicts a statistically significant reduction in pain after facet blocks.31
Zygapophyseal Joint Pain Referral Maps
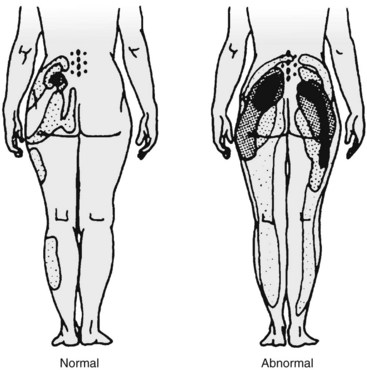
Pain referral patterns have been studied using stimulation of patients during provocative diagnostic injections116 by injection of hypertonic solutions into normal and abnormal subjects80 or by electrical stimulation of medial branches.80,117 Most studies showed distinct but overlapping referral areas; it is likely that the pain referral patterns obtained in normal volunteers are smaller because of less sensitization. There are also limits to the referral maps; Mooney and Robertson80 reported on lumbar facet referral maps in normal volunteers and subjects with a positive diagnostic facet block (Fig. 15–6). Under fluoroscopic guidance, they injected contrast dye (unspecified volume) followed by 3 to 5 mL of hypertonic saline. Some of the distal extremity pain seen in the diagrams may be due to excessive volume of saline with irritation of the sciatic nerve roots. Given the lack of sensitivity and specificity of history, physical examination, and imaging and until more research is performed with finite injectant volumes (in patients with confirmed dual positive blocks), these referral maps can be used as a starting point to guide selection of levels to be injected.
Predictive Value
Historically, lacking robust studies, guideline and systematic review articles have been relegated to quoting studies with methodologic flaws as implied evidence that one need not diagnose facet mediated pain before surgery.22,94 There is no reason that a variable amount of relief after uncontrolled, variable volume, intra-articular zygapophyseal joint blocks should predict fusion outcomes using surgical fusion techniques from the 1980s in a group of patients being operated on for various unknown or unstated reasons. Jackson118 correlated relief after spinal fusion in 36 patients from 1980-1988 to results of a single intra-articular facet injection with 1.5 mL of local anesthetic and an unknown volume of contrast dye. Of patients, 85% had “some improvement” with an average relief after injection of 29%. The authors found no relationship between fusion surgery performed for unstated reasons and a “favorable response” to facet injection. The surgeries were presumably performed not because the authors believed the patients’ symptoms were due to their zygapophyseal joints. The surgical results based on their “mean pain and functional assessment scores” also seemed to improve by significantly less than 50%, suggesting poor patient selection.
An important historical study, published by Esses and Moro in 1993,119 is often quoted to refute the therapeutic utility of zygapophyseal joint blocks; however, it warrants a careful, critical review. Esses and Moro119 concluded that single intra-articular diagnostic facet joint injections “should not be used in determining treatment because they are not predictive of either surgical or nonsurgical success.” This study had significant methodologic shortcomings, which limit the validity of the authors’ conclusions. First, the study was retrospective with patients surveyed by telephone approximately 5 years after surgery. Second, 1.5 mL of local anesthetic was injected into the facets, and no mention is made of the volume of contrast dye needed to confirm needle position; the injections likely were nonspecific because of facet capsule rupture from excessive volume (>1 mL). Third, the patient population was markedly heterogeneous with significant confounding factors: an average duration of back pain of 8 years and approximately 40% of patients with a history of prior surgeries, including failed fusions. More than 50% of patients underwent three-level, four-level, or five-level fusions, which are known to have a poorer outcome than single-level or two-level fusions.
Fourth, of the 82 patients who underwent surgery, 36 (44%) had 0% relief from facet injections. Almost one half of the patients undergoing surgery had no relief from diagnostic blocks. Eight of 19 (42%) of the patients with complete relief after facet injections declined surgery, leaving only 11 of 82 (13%) patients who underwent surgery who had 100% relief from facet blocks. The remaining 35 of 82 patients (43%) had “partial but significant relief” (the exact percentage relief is not reported). Fifth, 30 of 82 (37%) patients had prior surgeries (laminectomy, discectomy, and fusion). It is well known that patients with failed back surgery syndrome often fare poorly with repeat surgery. Also, during the 1980s, diagnosis of the etiology of failed back surgery syndrome was elusive and might not be corrected by a posterior arthrodesis. For failed back surgery syndrome, facet joint pain comprises only 3% of cases; the most common diagnoses are foraminal stenosis (25% to 29%), painful disc (20% to 22%), pseudarthrosis (14%), neuropathic pain (10%), recurrent disc herniation (7% to 12%), and sacroiliac joint pain (2%).120
Next, Esses and Moro119 did not match the surgery to specific facet levels blocked. Patients had either one-level or two-level facet blocks, yet the following posterior fusions were performed: 20, single-level; 3, two-level; 10, three-level; 4, four-level; and 12, five-level or greater, including thoracic spine (wherein facets were never blocked). Finally, significant questions arise regarding the efficacy of the surgical intervention because there was no significant difference between surgical and nonsurgical outcomes. As reported, only approximately one third of patients in either the surgical or the nonsurgical group had a good outcome. Because of methodologic flaws and limitations of the Esses and Moro study,119 facet intra-articular injections cannot be impugned as either predictive or nonpredictive of surgical success.
In another observational study, Lovely and Rastogi121 required a “positive response” to intra-articular injection of greater than 70% relief after bupivacaine facet block for 6 hours and required a confirmatory response on two subsequent injections. Of 28 patients, 23 had a good to excellent outcome after fusion surgery; however, large volumes of 3 to 5 mL were used during the blocks, making interpretation difficult. At present (2008), there is no research regarding the utility of cervical or thoracic facet blocks as presurgical screening tests.
By comparison, when a specific treatment is directed at a cause of pain originating from the zygapophyseal joint, accurate diagnostic testing does matter. In a more recent study, researchers reported that when a putative inflammatory cause of lumbar facet pain was confirmed using a positive SPECT scan, a positive response (a significant reduction in pain) was clearly predicted with intra-articular and pericapsular steroids at 1 and 3 months compared with subjects with negative scans or routine care.31
In regard to the therapeutic utility of double, controlled differential facet blocks, there is a clear and direct relationship between relief of pain after controlled medial branch blocks with a well-validated treatment for zygapophyseal joint pain, medial branch neurotomies. In a study by Dreyfuss and colleagues,122 patients who obtained greater than or equal to 80% relief from medial branch blocks were selected to undergo lumbar radiofrequency neurotomy. At 12 months, 60% of the patients obtained at least 90% relief of pain, and 87% obtained at least 60% relief. Dreyfuss and colleagues122 concluded that lumbar medial branch neurotomy is an effective means of reducing pain in patients carefully selected on the basis of controlled diagnostic blocks. The most recent, high-quality study available on radiofrequency is a randomized controlled trial evaluating radiofrequency neurotomy in patients with chronic low back pain.123 The trial used three positive blocks in the inclusion criteria and a “sham radiofrequency” procedure for comparison; statistically significant reduction in pain and improvement in various quality of life variables were obtained. In another study, when the diagnosis is confirmed by relief of pain for greater than 3 months after medial branch neurotomies, repeat neurotomies are successful in greater than 75% in lumbar and cervical spine.124,125
Cervical Spine Facet Syndrome
History
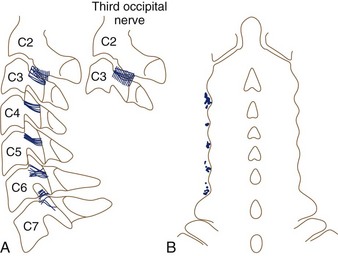
The cervical facet joints are known to be sources of neck and extremity pain and headache. In 1940, Hadden126 described pain from zygapophyseal joints causing headache. In the 1970s, Macnab127 described pain arising from the facet joints after whiplash injury. Bogduk and Marsland128 devised a technique to block the third occipital nerve, which relieved neck pain and headache stemming from the C2-3 facet joint in 70% of patients. Headache arising from C0-1 or C1-2 joints has also been described.129,130 Bogduk and Marsland131 were also the first to describe medial branch blocks for all cervical spine levels. They studied patients presenting with idiopathic neck pain and reported that medial branch block and intra-articular blocks provided complete, temporary relief of pain for 70% of patients.
Cervical Zygapophyseal Joint Pain
Based on the confirmatory block paradigm, the cervical facet joints are a common source of chronic neck pain; the prevalence of cervical facet syndrome is greater than the prevalence of lumbar facet syndrome. Cervical discogenic pain shares referral patterns with facet pain, but it is far less common.132 Based on comparative blocks of cervical facet joints causing chronic neck pain with either associated headache or shoulder pain, the C2-3 (36%) and C5-6 facet joints (35%) were the most common pain generators.133 After whiplash injury, Level I prospective clinical studies provide evidence that facet joints are the most common source of chronic pain.134,135 Cervicogenic headache stemming from the C2-3 facet after whiplash has a 53% prevalence.134
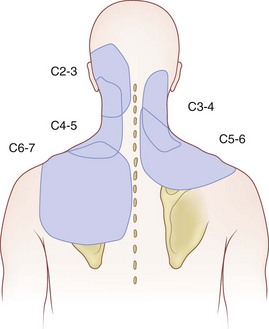
Often neglected are C0-1 and C1-2 joints in evaluation of upper neck pain and headache. Dreyfuss and colleagues129 studied the referral patterns for the atlantoaxial and lateral atlantoaxial joints. In 2002, Aprill and colleagues136 failed to confirm the null hypothesis that lateral atlantoaxial joints are not a common source of occipital headache. These investigators found that of 34 patients presenting with symptoms and signs of atlantoaxial joint pain, 21 obtained complete relief of headache after diagnostic injection of local anesthetic. Pain referral patterns have been defined in C2-3 through C7-T1 facets (Fig. 15–7).15 Innervation of the cervical facet joints is well described (Fig. 15–8).15 The cervical zygapophyseal joints can be blocked either by medial branch blocks or with intra-articular injections (Fig. 15–9).
Prevalence rates for pain originating from cervical facets range from 36% to 60%. The false-positive rate for a single, uncontrolled block is 27% (95% confidence interval 15% to 38%).137 The following prevalence rates (mean [95% confidence interval]) are reported from studies using either a double block or a triple block paradigm (normal saline as a placebo): 54% (40% to 68%),134 36% (27% to 45%),138 60% (33% to 64%),135 and 60% (50% to 70%).139 Manchikanti and colleagues100 restudied the prevalence of cervical zygapophyseal joint pain in a larger group of patients and found a similar 55% (95% confidence interval 49% to 61%) prevalence. The most recent study by Manchikanti’s group in 2007,83 of 438 patients requiring 80% relief of pain for 2 hours’ duration with lidocaine and 3 hours’ duration with bupivacaine, reported a prevalence of 39%. Corroborating the high prevalence of cervical zygapophyseal joint pain, Yin and Bogduk139a in a private practice clinic audit found a 55% prevalence of cervical zygapophyseal joint pain using a strict double comparative block protocol.
Similar to lumbar zygapophyseal joint pain, there are no high-quality studies showing a particular set of clinical features that can predict results of diagnostic cervical facet or medial branch blocks.140 With diagnosis by medial branch blocks, one exceptionally skilled manipulative therapist was able to identify all 15 subjects with diagnostic block–proven symptomatic zygapophyseal joints and specify the correct symptomatic segment. None of the five patients with asymptomatic joints was misdiagnosed as having symptomatic zygapophyseal joints.141 A later follow-up study by the same group failed to confirm the apparent high specificity and sensitivity, however, and reported a high sensitivity but low specificity and concluded that manual examination of the cervical spine lacks validity for the diagnosis of cervical zygapophyseal joint pain. In the study by Aprill and colleagues136 of C1-2 facet pain as a source of occipital headache, only 60% of the patients shared clinical criteria that predicted a positive response to the block.
Advanced imaging has not been correlated with positive responses to diagnostic blocks. Hechelhammer and colleagues142 found no relationship between short-term pain relief after cervical intra-articular and pericapsular injection of local anesthetic and corticosteroid and the degree of osteoarthritis graded on a CT scan.
Thoracic Spine
The prevalence of patients who complain of chronic upper back or mid-back pain ranges from 3% to 22%.30,143,144 One survey study of 35- to 45-year-olds estimated the prevalence of thoracic pain to be 15%.145 Thoracic zygapophyseal joint pain referral patterns have been reported (Fig. 15–10).146,147 Thoracic medial branch anatomy has also been described (Fig. 15–11).148
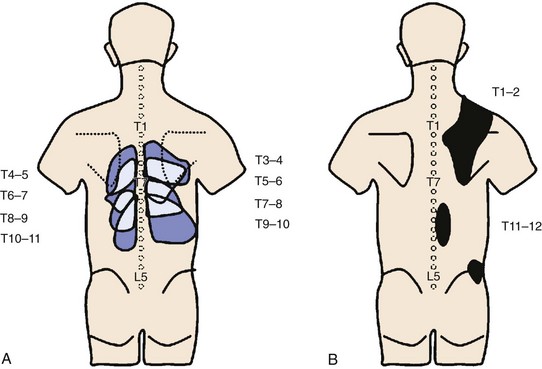
FIGURE 15–10 Maps of referred pain patterns in segments indicated. A, Based on Dreyfuss et al146 in normal volunteers. B, Based on Fukui et al147 in patients with single positive facet block.
(From Bogduk N [ed]: Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, International Spine Intervention Society, 2004.)
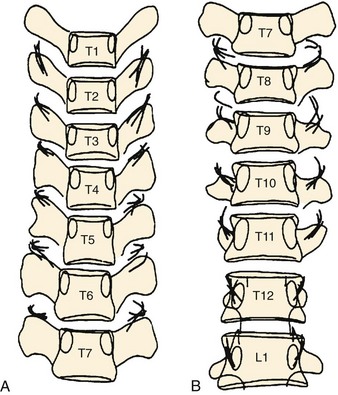
FIGURE 15–11 A and B, Composite sketch of work by Chua and Bogduk148 with radiographs of cadaveric thoracic spines. Medial branches of thoracic dorsal rami marked with wires to depict location with respect to transverse processes. Note middle thoracic levels, where medial branches are within intertransverse space versus crossing transverse process.
(From Bogduk N [ed]: Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, International Spine Intervention Society, 2004.)
There are no pathognomonic clinical or radiographic findings by which thoracic zygapophyseal joint pain may be diagnosed.149 As with the cervical and thoracic spine, diagnosis is by suspicion and, at a minimum, the pain pattern should correlate with established pain referral maps.15 The methods physicians apply clinically to the diagnosis and treatment of thoracic facet joint pain rest largely on research done in the lumbar and cervical spine. This is not an entirely unreasonable approach based on what clinicians know in general regarding facet anatomy and innervation; however, more research is needed.
Investigators have mapped out the referral patterns for the thoracic joints. These findings are often used as a starting point to select which thoracic facets to block.146,147 Dreyfuss and colleagues146 mapped out thoracic facet joint referral patterns in normal volunteers and found that capsular distention did not provoke pain in 27.5% of volunteers. Fukui and colleagues147 mapped out referral patterns in patients with suspected thoracic zygapophyseal joint pain who had a positive response to local anesthetic in C7-T1 to T2-3 and T11-12 facet joints. There was considerable overlap between the C7-T1 and T2-3 thoracic joints, and pain maps from these joints are not considered reliable enough to identify the symptomatic segmental level. Dreyfuss and colleagues146 studied nine asymptomatic volunteers who underwent 40 provocative thoracic facet injections from T3-4 to T10-11. Referral patterns were consistently unilateral. The area of the most intense pain for segments from T2-3 to T11-12 was one level inferior and lateral. Significant overlap occurred over three to five levels. The researchers found that needle position can be confirmed with 0.1 to 0.3 mL of contrast dye, and adequate blocks can be achieved with a volume of 0.5 to 0.6 mL. Normally, thoracic zygapophyseal joints cannot hold more than 0.75 mL (Fig. 15–12 shows a typical thoracic zygapophyseal joint block).15
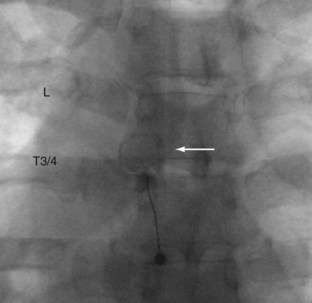
FIGURE 15–12 Left T3-4 zygapophyseal joint intra-articular injection. Note circular zygapophyseal joint arthrogram (arrow).
(Courtesy of Richard Derby, MD.)
One research group has performed the three studies in the literature using a controlled, double block paradigm, requiring 75% to 80% relief based on the duration of the local anesthetic used.150–152 Combining all three studies with patients presenting with chronic middle or upper spinal pain (n = 183), using dual blocks obtains a 40% prevalence of thoracic facet syndrome, with a false-positive rate of 42% if using a single block paradigm.26
What is the predictive value of a positive dual block? In other words, how well do patients fare who have positive dual blocks and undergo therapeutic intervention? Research is limited in this regard. One systematic review26 reported that only therapeutic thoracic medial branch blocks received a 1A or 1B/strong recommendation. Manchikanti and colleagues153,154 performed two studies. In the first study, 55 consecutive patients were studied; greater than 70% of patients had statistically significant relief (defined as >50% relief) at 3, 6, and 12 months. Most patients received four injections of bupivacaine with or without 1 mL of sarapin and 1 mg of methylprednisolone per milliliter of solution with 1 to 1.5 mL of solution injected per nerve. In the second study of 48 patients with positive dual blocks, 24 patients received bupivacaine, and 20 patients received bupivacaine plus betamethasone. Statistically significant (>50%) pain relief was reported in both groups at all time points up to 1 year. In the systematic review of radiofrequency neurotomy, only two studies were on thoracic medial branch neurotomy; however, both were of low quality and failed to meet inclusion criteria for the review because of lack of diagnosis by controlled blocks, small patient sample, and other methodologic shortcomings.26 More research is needed in regard to diagnosis and treatment of thoracic pain so that the evidence can be graded and systematically reviewed, the caveat being that a lack of evidence is not equivalent to no evidence.
Summary
Infection may occur after any interventional procedure. Various infections are reported after zygapophyseal joint injections, including paraspinal abscess,155 facet abscess,156 osteomyelitis,157 and epidural abscess.158 In addition to infections, subdural injections or injection into the spinal cord may occur. A case of transient tetraplegia159 was reported during a cervical facet injection performed without fluoroscopy and most likely was an accidental subdural injection of local anesthetic. Even when using fluoroscopy there is a risk of accidental subdural injection or potential spinal cord injection. The danger is especially real when performing cervical intra-articular injection using a lateral technique. Using this technique, the needle is passed laterally using a lateral fluoroscopy view. If the anteroposterior view is not periodically checked, one may not recognize passage of the needle through the facet and dura and then into the cord. In a thin individual, the cord may be reached with a 1-inch needle. Keeping the needle directly over the inferior or superior facet and touching the bone before entering the joint helps the interventionalist avoid accidentally entering the spinal canal.
Sacroiliac Joint
With the gradual acceptance of local anesthetic block relief after fluoroscopy-guided sacroiliac joint blocks as the reference standard for diagnosis, there is a renewed interest in the sacroiliac joint as a legitimate source of chronic pain.160 The degree of impact on health is the same as that of radiculopathy as evidenced by statistically similar scores in health-related quality of life testing instruments between patients with a diagnosis of sacroiliac joint pain and patients with a diagnosis of radiculopathy.161
Similar to zygapophyseal joint and discogenic pain, the diagnosis of sacroiliac joint pain depends on the reference standard used (and the particular population studied) to confirm the diagnosis. Society guidelines most often require a placebo control or differential blockade with 50% to 90% relief.1,15,162 Typically, a differential duration of reported pain relief of lidocaine (approximately 1 hour) compared with bupivacaine (approximately 4 hours) is required. Although concordant provocation of pain during joint arthrography has been used as an additional requirement, the high percentage of asymptomatic patients reporting pain during sacroiliac joint injection implies that provocation has a high false-positive potential. Currently, using the dual block paradigm, the best estimates of prevalence of sacroiliac joint pain range from 10% to 38%. For single, uncontrolled sacroiliac joint injections, the false-positive rate is 20% to 54%.163–167
Pathophysiology
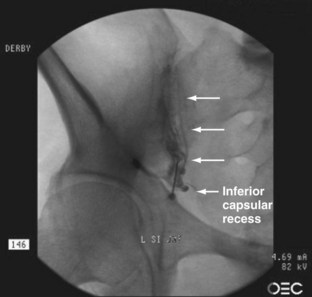
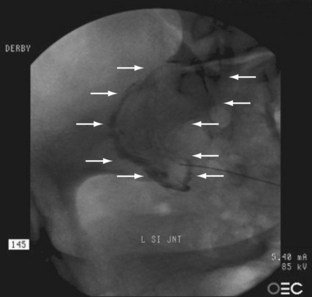
The sacroiliac joint has long been recognized as a synovial, fluid-filled diarthrodial joint between the sacrum and ilia with thick 6-mm sacral cartilage and thinner, approximately 1-mm iliac cartilage (Fig. 15–13). The joint is auricular or C-shaped with the convex side of the “C” facing anteriorly and inferiorly (Fig. 15–14).168 Although the anterior portion is no more than a thickened capsule, the posterior capsule blends into the extensive, thick posterior ligamentous structures, which bind the sacrum to the spine and bilaterally to the ilia. After puberty, the iliac surface develops a convex ridge, and the sacral surface develops a corresponding concave depression. These articular surfaces allow slight movement between the contiguous bony surfaces.
Although early in life gliding motions in all directions are permitted, by the middle of the 2nd decade of life the joints develop prominent ridges centrally along the entire length of the iliac surface and a corresponding groove along the sacral surface. Bowen and Cassidy168 believed that this interdigitation of the joint surfaces restricts motion to a sagittal rotation or posterosuperior-anteroinferior “nodding” along the crest of the interdigitations. The motion is complex, however, and usually limited to less than 4 degrees of rotation and less than 1.6 mm of translation. Significant motion occurs only after severing the interosseous ligament.169 It is unclear whether a type or degree of sacroiliac joint motion causes pain in older individuals. Beyond the 6th decade, cadaveric specimens commonly show a central region of ossification of the interosseous sacroiliac ligament and the presence of ridges and depressions, which likely result in little to no movement of the sacroiliac joint in these older individuals.170 Although restricted by para-articular osteophyte formation, intra-articular bony ankylosis may be rare.168
Several investigators have studied the innervation of the sacroiliac joint. Nakagawa171 reported innervation from the ventral rami of L4 and L5; the superior gluteal nerve; and the dorsal rami of L5, S1, and S2. An anatomic dissection of the innervation of the sacroiliac joint was performed by Yin and colleagues172 for the purpose of defining the exact position of the nerves for “sensory stimulation–guided sacroiliac joint radiofrequency neurotomy.” These authors dissected cadavers and placed small-gauge wires adjacent to the lateral branch nerves entering the joint and over the dorsal sacrum to the dorsal sacral foramen from S1 to S3. In 1998, Willard173 reported dissection of 10 cadavers that revealed that the S1 and S2 lateral branches provide the primary innervation of the sacroiliac joint and associated dorsal ligaments. Occasional contribution was found by S3 but not S4. Predominant innervation from lateral branches of S1 was also reported by Grob and colleagues.174 These authors found dorsal nerves derived from S1-4 exclusively innervated the sacroiliac joint and associated ligaments. Nerves were distributed to superficial and deep dorsal sacroiliac ligaments and to the sacrotuberous and sacrospinous ligaments. Emerging from the sacral foramen, the nerves course laterally, sandwiched between superficial and deep portions of the sacroiliac ligaments. There is a great variability in the location and number of lateral branch nerves side to side and between individuals.172 Currently, the standard for blocking the sacroiliac joint is to block the L5 dorsal ramus and S1-3 lateral branches.
Berthelot and colleagues174a used the term sacroiliac joint lato-sensu to describe pain from the sacroiliac joint that may be emanating from adjoining ligaments rather than simply the synovial joint. These ligaments include the iliolumbar ligaments, dorsal and ventral sacroiliac ligaments, and sacrospinous and sacrotuberous ligaments. The prevalence of pain originating from these structures has received little formal study, and there is no validated technique to diagnose ligamentous pain. Nevertheless, sacroiliac joint ligamentous pain is proclaimed as a frequent primary source of low back and buttock pain by orthopaedists.175 More importantly, a negative response to a sacroiliac joint injection does not mean that pain does not originate from the iliolumbar ligament and sacroiliac joint ligaments. A more recent histologic study found calcitonin gene-related peptide and substance P immunoreactive nerve fibers in the normal sacroiliac joint anterior capsular ligament and interosseous ligament. The authors of the study opined that diagnostic infiltration techniques for sacroiliac joint pain should employ extra-articular and intra-articular approaches.176
In a comparative study, Murakami and colleagues177 performed periarticular injections in 25 patients and intra-articular injections in another 25 patients. Periarticular injections relieved on average 92% pain in 100% of the injected patients compared with only 9 of 25 patients receiving intra-articular injections. All 16 patients not receiving relief by intra-articular injections were improved after periarticular injections. The presence of other structural abnormalities does not rule out the sacroiliac joint as a primary source of pain. Weksler and colleagues178 studied 55 patients with herniated discs with axial and referred leg pain, without objective neurologic deficits but with positive sacroiliac provocation tests. Using intra-articular injection of local anesthetic as the reference standard, the mean baseline VAS pain score decreased 30 minutes after injection from 7.8 to 1.3. In 46 patients 8 weeks after injection, VAS scores ranged from 0 to 3.
The question of whether fusion surgery leads to increased stress on the sacroiliac joint and may be a cause of failed back surgery syndrome was first raised by Frymoyer and colleagues,179 although their method of assessing sacroiliac joint pathology yielded a negative result; in 1978, Frymoyer and colleagues179 evaluated patients with radiographs (no diagnostic blocks) 10 years after posterior fusion versus postdiscectomy and found no significant difference in radiographic abnormalities; they opined that sacroiliac pain was “noncontributory” to persistent low back pain after surgery. In their subject population, they believed that the graft donor site was a more common pain generator. Fusion to the sacrum might be expected to stress the sacroiliac joints and lead to late failures or to early failures owing to undiagnosed sacroiliac joint pain. Ha and colleagues180 prospectively examined 37 patients undergoing posterolateral lumbar and lumbosacral fusions; 22 patients had a floating fusion, and 10 patients had a lumbosacral fusion. CT scans of the sacroiliac joint were performed before surgery and at 2 weeks, 1 year, and 5 years after surgery and compared with 34 matched controls. The incidence of sacroiliac joint degeneration was 75% in the fusion group versus 38.2% in the control group and greater in patients fused to the sacrum. Both groups reported significant improvements in VAS and Oswestry Disability Index scores, and there was no difference in scores between the two groups.
More recent research has shown that the sacroiliac joint can be a significant source of pain after fusion. Biomechanical models seem to support these conclusions. Ivanov and colleagues181 performed a finite element study with lumbosacral models and fusion constructs and found that fusion to the sacrum increased motion and stresses at the sacroiliac joint. Cadaveric studies show that disruption of the ventral band of the iliolumbar ligament significantly increases sacroiliac joint mobility.182 Ebraheim and colleagues183 evaluated the prevalence of sacroiliac joint disruption by CT scan in 24 patients after fusion with persistent “donor site pain” after posterior superior iliac crest graft harvesting. They found a high prevalence of persistent sacroiliac joint pain in patients with inner table disruption. Patients with violation of the synovial portion of the sacroiliac joint had severe degenerative changes on CT versus mild to moderate degeneration with inner table disruption only. It seems that the original hypothesis by Frymoyer and colleagues179 that sacroiliac joint dysfunction was the cause of donor site pain may have been correct.
What is the evidence for using diagnostic blocks as the reference standard? Diagnosis of sacroiliac joint pain has been reported by researchers using single and dual blocks; with these methods, prevalence rates of sacroiliac joint pain after lumbar fusion range from 27% to 35%. Maigne and Planchon184 studied 40 patients after fusion with continued pain using 75% pain relief after a single sacroiliac joint intra-articular injection as the “gold standard.” They reported a 35% rate of positive blocks. The only characteristic that distinguished the positive from the negative responders was a different distribution of postoperative pain compared with preoperative pain. A pain-free interval of 3 months after surgery was significant; however, increased uptake in the sacroiliac joint on bone scintigraphy or posterior iliac bone graft harvesting was not significant.
Katz and colleagues185 studied 34 patients after lumbosacral fusion with continued pain thought to be due to sacroiliac joint with intra-articular injections of local anesthetic and corticosteroids. Eleven patients (32%) had greater than 75% pain relief with local anesthetic and a minimum of 10 days’ continued pain relief (with steroid) and were considered to have definite sacroiliac joint pain. Another 10 patients (29%) had greater than 75% relief with local anesthetic but no long-term relief. There was no correlation between the donor site and pain side. Irwin and colleagues163 used dual comparative sacroiliac joint blocks as the reference standard to define sacroiliac joint pain and found that the 27% positive responders tended to be older. They found no statistical relationship between age, body mass index, and gender.
Diagnostic Accuracy of Clinical History and Physical Examination for Sacroiliac Pain
The diagnostic utility of history and accepted sacroiliac joint physical examination tests was first rigorously examined by Dreyfuss and colleagues in 1996.186 Their study was designed to determine if any single or combination of 12 history and physical examination findings could predict intra-articular sacroiliac joint pain as judged against a single positive intra-articular sacroiliac joint block with greater than 90% pain relief. In 85 patients, there were 45 positive blocks. None of the 12 physical examination tests or the presence of 5 to 12 positive tests or any combination of these 12 tests correlated with the presence of sacroiliac joint pain. One important historical feature was notable, however: only 2 of 45 patients drew pain above the L5 level, suggesting that pain below L5 is more likely to be of sacroiliac joint origin. Maigne and colleagues187 reached a similar conclusion using dual comparative blocks: no single provocation test reached statistical significance in the 10 patients (18.5%) who had temporary pain relief on the confirmatory injection.
Although no single provocative maneuver has been shown to be of diagnostic value, using the dual block paradigm, several studies have obtained highly acceptable sensitivity (85% to 91%) and specificity (78% to 79%) rates by combining three or more sacroiliac joint pain provocation tests for diagnosis by physical examination.11,164,165,167,188 There is some slight variation in the tests used by various authors, but in summary they include the following provocation tests: thigh thrust, distraction test, Gaenslen test, Patrick sign, compression test, midline sacral thrust test, and heel drop test. Specificity increased to 87% if the patient’s pain did not centralize or could not be made to move toward the spinal midline (which is typical of discogenic pain).189 When three or more provocation tests (distraction, compression, thigh thrust, Patrick sign, Gaenslen test) are negative, the likelihood of sacroiliac joint pain is very low (6% to 15%); when all provocation tests are negative, the sacroiliac joint was never the source of pain.164,165,167,189
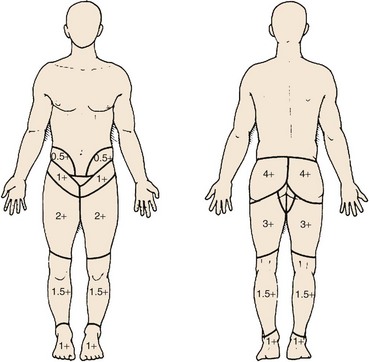
In terms of pain referral maps, Slipman and colleagues190 and Dreyfuss and colleagues186 concluded that of all alleged signs of sacroiliac joint pain, maximum pain below L5 coupled with pointing to the posterior superior iliac spine or tenderness just medial to the posterior superior iliac spine (sacral sulcus tenderness) has the highest positive predictive value of 60%; if these do not exist, the likelihood of sacroiliac joint pain is less than 10%. Although the maximal sacroiliac joint pain is below L5, pain can be referred into the entire lower extremity, with 94% of patients reporting buttock pain, 48% reporting thigh pain, and 28% reporting lower leg pain (Fig. 15–15).187,191,192 Referral to the lower extremity is possible from sacroiliac joint pain and cannot reliably be distinguished from other pain sources (e.g., S1 radiculopathy).191,193
Lastly, although pain referral patterns between responders and nonresponders are similar, Fortin and colleagues194 described an area of pain approximately 3 × 10 cm just inferior to the posterior superior iliac spine that was common in all their subjects with sacroiliac joint pain. More recently, Murakami and colleagues195 studied the specificity and sensitivity of the “Fortin” point with periarticular injections. Labeled the one finger test, 18 of 38 patients pointed to a location of pain at the posterior superior iliac spine or within 2 cm of the posterior superior iliac spine, which had a positive effect with periarticular sacroiliac joint block. The authors recommended that sacroiliac joint pain should be considered in patients who can point to their pain using one finger in the vicinity of the posterior superior iliac spine.
Systematic reviews report various conclusions regarding the specificity of the physical examination and sacroiliac joint block to diagnose sacroiliac joint pain based on the authors’ assessment of the diagnostic accuracy of diagnostic sacroiliac joint blocks. The review by Berthelot and colleagues196 concluded that sacroiliac joint blocks and sacroiliac joint maneuvers were unreliable for diagnosing sacroiliac joint pain. In contrast, Hansen and colleagues197 concluded in their review that there was moderate evidence for the specificity and validity of diagnostic sacroiliac joint injection and limited evidence for the accuracy of provocative maneuvers. Using a comparative double block reference standard, the most recent meta-analysis and systematic review concluded that the pooled data of the thigh thrust test, compression test, and three or more positive stress tests showed discriminative power for diagnosing sacroiliac joint pain.198
Diagnostic Accuracy of Imaging
No imaging studies consistently provide findings that are helpful to diagnose primary sacroiliac joint pain. CT, MRI, and bone scan are done predominantly to exclude other causes of pain rather than to diagnose mechanical sacroiliac joint pain. Among patients referred to a low back pain clinic with a variety of pathologies, Hodge and Bessette199 found a high percentage (75%) of patients with sacroiliac joint arthritis shown on CT scan. Although these authors did not confirm the diagnosis with sacroiliac joint injections, they opined that sacroiliac joint arthritis should be considered a possible diagnosis.
There is limited diagnostic value of CT scan in mechanical sacroiliac joint disease as defined by pain relief after sacroiliac joint blocks under CT scan guidance. Comparing the CT scans of patients diagnosed with sacroiliac joint pain using image-guided analgesic sacroiliac joint blocks with a matched control group of asymptomatic patients, Elgafy and colleagues200 reported that an abnormal sacroiliac joint CT scan had a sensitivity of 57% and a specificity of 69%. Although sacroiliac joint scintigraphy can detect early sacroiliitis,201 stress fractures, infection, and tumors, the sensitivity of bone scans for detecting mechanical sacroiliac joint pain is poor (range 12% to 46%).202,203 Patients with a positive bone scan are likely to have mechanical or arthritic sacroiliac joint pain with a reported specificity of 90% to 100%.190,203
Diagnostic Accuracy of Sacroiliac Joint Injections
The current standard for diagnosing sacroiliac joint pain is pain relief after dual controlled sacroiliac joint injections, owing to the high false-positive rate of single blocks.162 When blocking the sacroiliac joint or lateral branches of the sacroiliac joint, imaging guidance must be used. The success of “blind” intra-articular injection is only 22%.204 A positive response should include approximately 70% relief for 1 to 2 hours of relief after a lidocaine block and 3 to 4 hours of relief after a confirmatory block with bupivacaine. Although the reference standard is reasonable, there are several caveats for the diagnosis of mechanical pain originating within the sacroiliac joint. Patients may exhibit extra-articular or periarticular sacroiliac joint pain or perhaps both. As noted earlier, Murakami and colleagues177 relieved a significant amount of sacroiliac joint pain with periarticular injections. In a retrospective review of 120 patients, subjects who received intra-articular and periarticular injections had superior pain relief compared with subjects receiving intra-articular injections alone.205
False-positive results may occur secondary to leak of contrast dye through capsular tears, which may be present even in asymptomatic individuals. Extracapsular flow is present in 61% of sacroiliac joint intra-articular injections in patients.206 Of sacroiliac joint intra-articular injections, 27% show extravasation that communicates with nearby neural structures, including dorsal sacral foramina extravasation, superior recess extravasation at the sacral ala level to the fifth lumbar epiradicular sheath, and ventral extravasation to the lumbosacral plexus.206 Patients who have postblock extremity numbness are usually considered to have a leak, and the block is typically repeated at a different session.
Dreyfuss and colleagues193 used a double-blind randomized controlled trial to assess ability of single-site, single-depth L5 dorsal ramus and S1-3 lateral branch blocks to anesthetize the sacroiliac joint in 19 volunteers, using sacroiliac joint fluid distraction before and after blocks to determine effectiveness. The authors reported that only 40% of the volunteers did not feel distention after the blocks. The poor results prompted a cadaveric study of multisite, multidepth blocks to anesthetize the joint. L5 dorsal ramus block was performed at the standard location of the S1 superior articular process and the sacral ala; S1-2 lateral branches were blocked (right side) at the 2:30 o’clock, 4:00 o’clock, and 5:30 o’clock positions; and S3 lateral branch was blocked at the right 2:30 o’clock and 4:00 o’clock positions. The lateral branch blocks were performed 8 to 10 mm lateral to the posterior sacral foramen. A 0.2 mL volume of green dye was injected on the dorsal sacral plate, and an additional 0.2 mL was injected 2 to 3 mm above the sacral plate.
Dissection revealed S1-3 lateral branch nerves were stained in 91% (31 of 34) of cases. Employing the same protocol on 20 volunteer subjects using intraosseous ligament probing and capsular distention, Dreyfuss and colleagues193 found that 86% of the sham local anesthetic injection subjects retained the ability to feel capsular distention, leading the authors to conclude that lateral branch blocks do not reliably block the intra-articular portion of the joint and that intra-articular blocks do not reliably block the extra-articular ligaments. One may conclude that to evaluate fully intra-articular and extra-articular pain sources, dorsal ramus and lateral branch blocks and intra-articular injections should be done. The caveat is the nerve blocks were successful in 70% of cases leaving a potential 30% false-negative cases. Injecting larger volumes or injecting the ligaments directly may potentially reduce the false-negative results with the risk of increasing false-positive results secondary to leak of local anesthetic through the posterior foramen.
Predictive Value
Surgical fusion outcomes for mechanical sacroiliac joint pain are reported for only a few small case series audits of initial outcomes after several “new” techniques for fusing the sacroiliac joint.207–210 Published case series use pain relief after image-guided analgesic sacroiliac joint injections as the reference standard for diagnosing sacroiliac joint pain. Although Schutz and Grob208 reported an 82% unacceptable outcome after bilateral sacroiliac joint fusion in 17 patients based on results from sacroiliac joint anesthetic block, three other studies using novel techniques reported more favorable results for mostly unilateral fusions. Al-Khayer and colleagues209 reported an approximate 50% decrease in VAS and a 14-point decrease in Oswestry Disability Index in nine patients at 2 years after percutaneous sacroiliac joint arthrodesis using a Hollow Modular Anchorage screw (Aesculap, Sheffield, UK). Using percutaneously inserted fusion cages and bone morphogenetic protein, Wise and Dall207 reported an average back pain VAS improvement of 4.9 and leg pain VAS improvement of 2.4 in 13 patients at 6 months. Finally, Ziran and colleagues,210 using CT-guided sacroiliac joint blocks as a reference standard, percutaneously fused 17 patients with recalcitrant sacroiliac joint pain and found a statistically significant correlation (P < .02) between final postoperative pain scores and preinjection and postinjection pain scores.
Evidence has been limited based on observation studies assessing the outcome of various treatments for sacroiliac joint pain. Cohen and colleagues211 selected patients for various types of radiofrequency neurotomy of the L4 medial branch, L5 dorsal branch, and S1-3 lateral branches using the reference standard of a single sacroiliac joint intra-articular block with greater than or equal to 75% relief of pain for 2 hours after injection of 2 mL of bupivacaine. Of 18 patients, 13 obtained satisfactory relief of pain with average scores reduced by 60%, 50%, and 57% at 1 month, 3 months, and 6 months. Only two patients in the placebo group obtained relief; pain scores of the placebo subjects were unchanged from baseline. Yin and colleagues172 used dual injection into the sacroiliac joint intraosseous ligament to diagnose sacroiliac joint pain. Of patients, 64% reported a minimum of 60% subjective pain relief for a minimum of 6 months after sensory stimulation–guided sacral lateral branch radiofrequency neurotomy.
Summary
Sacroiliac joint pain is a significant cause of chronic low back pain that is diagnosable and treatable with precision injection techniques. Prevalence of sacroiliac joint pain, based on a dual differential block protocol, ranges from 10% to 38%; for single, uncontrolled blocks, the false-positive rate is 54%.163–167 The sacroiliac joint as a pain generator is no longer disputed. Current research also suggests that the sacroiliac joint is a significant source of persistent pain after lumbar fusion and may be a cause of graft donor site pain.183–185 Although motion is limited and complex, the joint is known to rotate less than 4 degrees and to translate less than 1.6 mm. Anatomic studies have elucidated the innervation to the joint, with most practitioners directing diagnostic and therapeutic interventions to the L5 dorsal ramus and S1-3 lateral branches.171,173 Sacroiliac joint pain is now thought to emanate from the joint itself and extra-articular ligamentous sources. Interventionalists are just beginning to diagnose and treat putative extra-articular pain generators.
In contrast to the history and physical examination for zygapophyseal joint pain, certain diagnostic features for sacroiliac joint pain have been validated by controlled blocks. Maximal pain below L5 coupled with pointing to the posterior superior iliac spine has a predictive value of 60%.186,190 Although no single physical examination test has been shown to be of diagnostic value, using the dual block paradigm, several studies have shown high sensitivity (85% to 91%) and specificity (78% to 79%) by combining three or more provocative sacroiliac joint maneuvers.165,167 Specificity increases to 87% if the patient’s pain cannot be centralized.212 Diagnostic imaging of sacroiliac joint pain has not been shown to be helpful, other than excluding nonmechanical causes of sacroiliac joint pain. The sensitivity of bone scans for detecting sacroiliac joint pain is poor (range 12% to 46%).202,203 CT scan of the sacrum in a patient with persistent low back or buttock pain after lumbar fusion may be useful, particularly if the synovial joint has been violated. In these patients, severe degenerative changes were found on CT scan.183
If pain persists, new techniques have also been described for blocking the interosseous sacral ligaments.205 Regarding the predictive value of diagnostic sacroiliac joint injection for sacroiliac joint arthrodesis, some case studies show poor results for arthrodesis; other studies using novel techniques report better results.207–210 Neurotomy of sacroiliac joint lateral branches after diagnostic block has shown promising results in an observational study.213 Other researchers have also shown promising results with periarticular blockade.177
Middle Compartment: Selective Nerve Root Blocks
Despite the growing sophistication of modern imaging, the source of extremity pain is not always clearly apparent. Extremity pain may also be referred from the hip, buttock, or shoulder secondary to intrinsic pathology in these structures. Radicular pain can be secondary to entrapment by bone, ligament, or disc or result from leakage of noxious cytokines from either the disc or an inflamed zygapophyseal joint without evidence of compression. Segmental instability, albeit difficult to detect or prove, may cause repetitive dynamic irritation of the dorsal root ganglion leading to chronic dorsal root ganglion hypersensitivity. Advanced MRI often shows multilevel degenerative pathology, abnormalities on the side opposite the patient’s symptoms, or abnormalities that are asymptomatic.214–216 Except for the most profound structural abnormalities, MRI provides morphologic information only; significant correlations must be made by the clinician.217 Confounding the diagnosis further, pain patterns often do not follow classic referral pain distributions.218,219
Selective injection of local anesthetic around the spinal nerve within or near the intervertebral foramen has long been used to help surgeons confirm or refute their hypothesis that a particular root is the source of pain. Selective nerve root blocks are distinguished from transforaminal epidural steroid injections. With a selective nerve root block, a small volume of contrast medium, approximately 0.5 mL, is injected just to outline the exiting spinal nerve and ventral and dorsal roots (Figs. 15-16 through 15-18); then the same volume of local anesthetic is injected to maintain specificity. Greater volumes of local anesthetic spread to adjacent levels. With selective nerve root blocks, relief of pain does not determine the cause of pain. Greater or lesser relief of pain may occur even if the cause of pain is peripheral entrapment or if the blocked nerve innervates a painful structure such as the hip. Relief of pain for the duration of the local anesthetic may occur even if the root has irreversible damage.
Diagnostic Accuracy of Selective Nerve Root Blocks
Diagnostic accuracy and ultimately utility depend on the degree to which a technically satisfactory block of a nerve stops the nociceptive input into the spinal canal and the degree that pain caused by any lesion within the nerve at or distal to the injection site is relieved. A greater or lesser degree of pain relief caused by a lesion affecting the nerve proximal to the injection site should also be taken into account when determining value.95 Ideally, blocking an unaffected nerve would not relieve any pain. The degree to which these goals are accomplished constitutes the diagnostic accuracy as measured by sensitivity, specificity, and predictive value. Understanding these variables guides the clinician in terms of either accepting or discarding the block results or whether even to consider obtaining the information.
To study the diagnostic accuracy, one would select cases of acute or subacute monoradiculopathy caused by an obvious single-level lesion verified by imaging studies, intraoperative findings, and relief after surgical intervention. The most common “gold standard” lesion would be L4-5 paracentral herniation irritating the traversing L5 root.220 Blinding the patient, the symptomatic root and presumably at least one unaffected root would be blocked at different sessions, and the data would be prospectively collected. The lesion would be confirmed at surgery and by postsurgical pain relief.
Although many prior studies retrospectively studied the ability of provocation and relief of pain to predict structural nerve entrapment and surgical outcome, only two studies examined injections performed on symptomatic roots and presumed asymptomatic roots with the expressed goal of defining sensitivity and specificity, and both studied only the value of lumbar injections.220,221 From these two studies, particularly the more recent study by Yeom and colleagues,220 one may estimate the diagnostic value of lumbar diagnostic root blocks.
Assessment of Effect
Only the study by Yeom and colleagues220 determined the optimal cutoff level in the percent relief of pain reported by a patient after a procedure needed to qualify for a positive response. Using receiver operator curves, Yeom and colleagues220 chose a cutoff of 70% subjective relief of pain after a lumbar transforaminal block as the best value to provide optimal accuracy but stated that this level could be adjusted depending on the importance of avoiding false-negative versus false-positive results.
Although provocation of concordant pain was frequently used in the past and perhaps is useful information, more recent studies use techniques to avoid creating pain during injection.220,222 Pain referral patterns obtained by electrical stimulation may be considered as supplementary proof or nonproof.222
Sensitivity
The most likely causes of low sensitivity or a high rate of false-negative injections are inadequate blocks owing to poor spread around the root, failure to reach pathologic site, dilutional effects with inadequate mass of anesthetic reaching the root, or poor diffusion because of scarring.220 van Akkerveeken221 calculated 100% sensitivity in 46 patients using 0.2 to 0.5 mL of 0.5% bupivacaine (with provocation) and reported 100% of pain relief at 1 hour. Yeom and colleagues,220 using 1 mL of 2% lidocaine without considering provocation, calculated a lower sensitivity of 57% (27 of 47) in all patients, increasing to 71% (25 of 35) when injections with inadequate spread were excluded. The causes of the inadequate blocks were spread of injectant into adjacent tissues in 4 of 10 patients, block by huge herniation in 4 of 10 patients, and intraepiradicular sheath injection in 2 of 10 patients. Although Yeom and colleagues220 had no explanation in the remaining 10 cases, these false-negative results might be explained by a paracentral herniated disc, which, although affecting primarily the traversing root, may also cause chemical irritation of the exiting root.
In addition, Dooley and colleagues223 found that the most common reason for typical pain provocation during lumbar block with incomplete pain relief is multilevel pathology. The most probable cause in obvious cases is an inadequate block performed at a location distal to the structural entrapment. Diagnostic injections are often performed in patients with long-standing chronic pain and patients with prior surgery who may have intraneural and extraneural scarring. In such cases, local anesthetic may not penetrate the nerve effectively, and incomplete relief would be expected. Using a more concentrated anesthetic or an anesthetic that preferentially blocks nociceptors (e.g., bupivacaine) may reduce these false-negative responses.
Specificity
Because surgery is often less effective in patients with equivocal structural pathology, in patients with atypical, long-standing pain or prior surgery, one would ideally want to have minimal or no pain relief after the block of an asymptomatic root. van Akkerveeken221 used a 0.2- to 0.5-mL volume of 0.5% bupivacaine and required 100% pain relief for 1 hour. He reported a specificity of “around” 90%.221 In the lumbar spine, using 1 mL of 2% lidocaine and a cutoff value of equal or greater than 70% pain relief, Yeom and colleagues220 calculated 86% (50 of 58) specificity, which increased to 91% (43 of 47) specificity after excluding 7 patients with overflow of local anesthetic. Although this overflow was thought to be a probable cause of false-positive blocks in 4 of 11 cases, 7 of 11 cases were true-negatives, indicating that the estimated overflow when using 1 mL is about 20% (10 of 47) and with a potentially clinically observable effect in less than 10%.
Furman and colleagues224 showed that even after injecting only 0.5 mL, the contrast pattern indicated nonselective flow in 30% of lumbar injections. The mass of drug overflowing at these low volumes may not be significant and is consistent with van Akkerveeken’s higher, approximately 90% specificity. North and colleagues95 reported an average 50% relief of sciatic pain when blocking the medial branches at several levels using a 3-mL volume, which would spread into the neuroforamen and epidural space, making the putative medial branch block nonspecific.88 Nevertheless, convergence may be an alternative explanation of less than 50% pain relief in some cases, and a nonspecific “placebo” response may explain some or most false-positive responses.
Predictive Value
Many, mostly retrospective, observational studies describe in variable levels of detail the predictive value of lumbar root blocks. One retrospective study included the surgical predictive value of cervical and lumbar injections.222 Another prospective, diagnostic cervical selective root block study compared the diagnostic value of imaging with the short-term surgical predictive value of the test.225 No studies to date support the use of diagnostic thoracic selective root injections, although this is primarily because the thoracic spine is not often studied because of the low prevalence of herniated thoracic discs.
In the only prospective outcome study, van Akkerveeken,221 in his doctoral thesis, presented a series of studies correlating the value of selective root blocks to diagnosis of various lumbar entrapment syndromes and later summarized the data in a journal publication in 1993. A positive response was provocation of concordant pain and “disappearance” of leg pain after 0.2 to 0.5 mL of 0.5% bupivacaine. He studied patients with radiologic signs of nerve root entrapment but without localizing neurologic signs who subsequently underwent surgical decompression. Excluding the patients who had positive blocks and refused surgery, van Akkerveeken221 reported a positive predictive value of 95% with a 95% confidence interval of 77% to 100%.
History
Spine surgeons began using diagnostic root blocks in the late 1960s to help locate sources of radicular pain not well visualized with myelography.219,226–229 Provocation of symptoms, pattern of the neurogram, and relief of pain were used to identify hidden pathology that was later confirmed or refuted during surgical exploration. A high degree of correlation was found between “positive” blocks and surgical findings. In addition, some early studies began reporting the surgical outcome based on selective nerve root block findings.227 The routine use of CT and MRI improved the identification of structural causes of root compression, and some surgeons began using root blocks in difficult cases where provocation and relief of pain helped to determine the operated level.223,230–232 Surgeons noted that although MRI and CT improved visualization of pathology, imaging did not correlate with cause of pain, and it did not correlate the abnormal anatomy with actual symptoms.223
Structural confirmation of suspected pathology and subsequent pain relief after surgery were reported in mostly retrospective case series. These studies also reported selective nerve root blocks were better able to identify a symptomatic root compared with CT and MRI in “difficult” cases.217,222,230,231,233 Of particular note was a finding that although outcome of patients diagnosed with various nerve root entrapment syndromes was excellent, patients diagnosed with scarring or arachnoiditis had very poor outcomes.223,231 All studies reported “successful” surgery to a greater or lesser extent in approximately 90% to 95% of patients following pain relief after selective nerve injections, if patients having prior surgery, scarring, and arachnoiditis were excluded. In the two studies that evaluated surgical outcome on patients with less than approximately 95% relief after injection, surgical results were modest to poor.222,223
In an observational study in 1971, Macnab226 analyzed the causes of nerve root involvement in 68 patients who had undergone a “negative exploration” for presumed radicular pain caused by a herniated disc. Various pathologies were described, including migration of a disc fragment into the intervertebral foramen, nerve root kinking by the pedicle, articular process impingement, and extraforaminal lateral disc herniation. In the case of pedicular kinking, Macnab226 described a technique of placing a 25-gauge needle into the intervertebral foramen and injecting 0.5 to 1 mL of oil-soluble contrast material. The provocation of concordant pain by striking the nerve with the needle, the characteristic contrast outline of the “kinked” nerve root within the foramen, and subsequent relief of pain after injection of 1 mL of 2% lidocaine were used to establish the diagnosis and led to “excellent results” in the six studied patients. Macnab226 also described two patients with an undiscovered extraforaminal lateral disc herniation who underwent successful operation after relief of pain with a selective nerve root block. Likewise, Schutz and colleagues228 in 1973 described the use of selective root blocks in 23 patients. In 13 of 15 patients who underwent surgery, the positive results of the selective nerve root blocks were confirmed.
Using a selective nerve root block technique similar to Macnab, Tajima and colleagues229 in 1977 described various contrast patterns after injection of 2 mL of water-soluble contrast media, including cutoff patterns of contrast flow within the foramen and lateral recess indicating stenosis or block by a herniated disc. Provocation and pain relief after injection of 3 mL of 1% lidocaine confirmed the diagnosis, which was later proven during surgical exploration in this small case series. Kikuchi and colleagues219 published a larger case series comprising 332 patients in 1982, in which they performed nerve root infiltration in all patients and correlated the resulting neurogram with anatomic findings of cadaveric dissections. In most patients, pain was relieved by injection at a single level. The cadaveric studies revealed the following causes of atypical pain: congenital or acquired abnormalities of nerve and nerve roots, sensory rootlets communicating with adjacent nerves, conjoined nerves, and the common occurrence of the furcal nerve exiting much more commonly at L4 than L5 level and giving branches to the lumbosacral trunk and femoral and obturator nerves. Kikuchi and colleagues219 also described the descent of the vertebral pedicle associated with disc collapse, degenerative changes of the articular facet, and compression of nerve at different sites.
Krempen and Smith227 in 1974 were the first to report surgical outcomes based on provocation and pain relief after injection of the nerve root with 1 mL of 1% lidocaine. They also described and included radiographs of neurogram patterns of extraforaminal disc herniations, pedicle kinking, articular process impingement, and scar tissue. These authors used the injections to diagnose pain in 21 patients with prior lumbar laminectomies and commented that most patients were able to pinpoint the level of the lesion to either of two injected levels. Of the 16 operated patients, 3 had excellent results, 9 had good results, and 4 had moderate results. The technique involved inserting an 18-gauge spinal needle 4 cm above the transverse process and approximately 6 cm from midline, directed downward and medially to strike the nerve. In the 1980s, Haueisen and colleagues233 used Krempen and Smith’s technique of spinal nerve injections to diagnose pain in difficult-to-diagnose patients, including 57% who had previous lumbar surgery. Of 63 operated patients, Haueisen and colleagues233 confirmed compression of the suspected nerve root in 93% of the cases; at an average follow-up of 20 months, 73% of patients had no pain, slight pain, or some pain. Myelography and electromyelography aided in correct diagnosis of the lesion in only 24% and 38% of the cases.
Dooley and colleagues223 used provocation and relief of pain after selective nerve root block to review retrospectively the results of 63 patients undergoing operations based on positive pain reproduction and pain relief after injection of 1 mL of 1% lidocaine correlated with surgical findings and outcome. The authors presented results according to whether the patients had full or incomplete pain relief and whether pain was reproduced. Of patients with reproduction and full pain relief, 45 of 46 had an anatomic diagnosis made at the time of surgery. Eight patients had herniated nucleus pulposus, and all were relieved of leg pain at follow-up. At follow-up, 17 patients had bony entrapment, and 14 (82%) were asymptomatic. Only 1 of 11 patients found to have arachnoiditis was pain-free at follow-up, although 5 of 7 patients found to have periradicular adhesions but without intraneural scarring were asymptomatic at follow-up. Patients with reproduction but incomplete relief included one patient who was diabetic with probable neuropathy causing failed surgery; the other three patients had pathology at other levels, and only one of the three had a satisfactory surgical outcome. In patients who had no reproduction and incomplete relief, only 5 of 14 cases were relieved of symptoms, and the authors recommended that patients with this group of responses should undergo careful reevaluation.
In 1988, Jonsson and colleagues225 reported total relief of pain in 51% of patients undergoing diagnostic lumbar “root anesthesia” in 100 cases of sciatic pain with normal findings on myelography or CT or MRI or minor inconsistent abnormalities (n = 40) or multilevel involvement (n = 9). The patients experiencing pain relief underwent surgical root decompression with short-term surgical outcome comparable to conventional surgery in more obvious cases.
In 1990, Stanley and colleagues230 likewise reported outcome based on response to injection in which they included only positive and negative responses. Positive responses required pain provocation and relief of pain with 1 mL of 1% lidocaine; a negative response was defined as nonconcordant pain and only partial relief or no relief of pain. At least two roots were studied in every patient. Of 20 patients with positive responses, 19 underwent operation, and Stanley and colleagues230 found that “nerve root infiltration” identified the symptomatic level in 18 of 19 cases compared with CT scan and myelogram, which identified the correct level in 14 of 19 cases and 12 of 19 cases. Patients with negative blocks were not offered operation. The 2 of 16 patients with prior spinal surgery who had a “positive” response underwent successful surgery.
In 1989, Herron231 reported the use of root blocks with pain provocation and pain relief after 1 mL of 0.5% bupivacaine. A positive response included reproduction of pain and at least 75% pain relief. Herron231 divided outcomes into good, fair, and poor. For a good outcome, he required 75% pain relief and return to previous work status with minimal medications and minimal or no restrictions of physical activities. In the previously unoperated disc herniation group, 15 of 18 patients had good results, and 3 had fair results. In nine patients, the imaging studies were positive at two levels, but surgery was performed only at one symptomatic level identified with a root block. There were seven good results and two fair results. In patients with previous unoperated spinal stenosis, 19% had a poor outcome versus 52% poor outcomes in patients with prior stenosis surgery. Herron231 noted that in most patients with radiculopathy, selective nerve root blocks are not needed because the level was readily apparent on clinical examination and imaging studies; however, root blocks were useful for patients with equivocal findings, previous surgery, and multilevel structural pathology.
Porter and colleagues232 used CT-guided root blocks employing a two-needle technique to place an inner needle adjacent to the target nerve. In contrast to previous authors, these authors did not include provocation and injected 1.5 mL of 0.5% bupivacaine. Porter and colleagues232 reported that of the 18 patients undergoing surgery, 78% had a good outcome; 2 patients had unsuccessful surgeries.
The study in 2005 by Sasso and colleagues222 is the most comprehensive, albeit retrospective, evaluation of the value of selective nerve root injections to predict lumbar and cervical surgical outcomes. Sasso and colleagues222 studied 101 patients culled from an institutional database from 1996-1999. Injections were performed by placing the needle tip just below the superior pedicle without intentional pain provocation. Additionally, a stimulating electrode to locate the needle close to the exiting nerve was employed. The authors noted the neurogram. A volume of 0.5 to 0.75 mL of 2% lidocaine was injected requiring greater than 95% pain relief during postblock provocative testing for a positive result. Confirmatory injections were performed when pain relief was 80% to 95%. Surgical follow-up was at a mean of 16.2 months with 18 patients undergoing cervical surgery and 83 patients undergoing lumbar surgery.
Finally, Derby and colleagues32 in 1992 reported the correlation between immediate leg pain relief after lumbar block and 1-year surgical outcomes. The authors segregated 78 patients undergoing epidural injections with a minimum of 80% immediate postblock leg pain relief into two dichotomous groups including patients with 50% or greater subjective leg pain relief lasting for 1 week or longer and patients with duration of extremity pain lasting 1 year or longer. Regardless of immediate pain relief, 85% of patients who had pain for less than 1 year had a positive surgical result defined as 50% or greater pain relief at 1 year regardless of immediate pain relief. More importantly, and by far the largest group (38 of 71), 95% of the patients who did not respond to the block had a poor surgical outcome. Derby and colleagues32 opined that the poor outcome might be explained in some cases by an inadequate structural correction, inadequate stabilization, or functional reasons, but most failures probably represented irreversible changes in the neural structures. Although unstudied and so unconfirmed, the results by Derby and colleagues32 are consistent with findings reported by Kumar and colleagues234 that outcome after spinal cord stimulation in patients with failed back surgery syndrome was superior to revision surgery.
Technical Considerations
Techniques used by diagnostic lumbar studies place a needle varying in size from 18-gauge to 25-gauge into the foramen. Although older studies located the root by producing paresthesias,219,223,226,229,231–233 more recent studies use a standard International Spine Intervention Society technique of placing the needle tip just below the pedicle at the approximate 6:00 o’clock position without purposefully provoking pain.15 The transforaminal lumbar technique used by Macnab in the 1960s is similar to the current technique and the technique often used by many “older” interventionalists, including the senior author.32,235 The needle is first advanced to contact the transverse process beginning approximately 6 cm from the midline, parallel to the transverse process and at an angle of approximately 30 degrees. The needle is advanced into the foramen at a position that would be approximately 6:00 o’clock below the pedicle.223,226
Another older described selective nerve root block technique used in the lumbar spine starts with needle insertion approximately 6 cm from the midline and approximately 2 to 3 cm above the transverse process and directs the needle into the foramen at a cephalad-caudad angle to contact the ventral root at approximately the midpoint between the upper and lower pedicles and slightly lateral to the foramen.219,229,233 A stimulating electrode can also be used to verify close proximity of the needle tip to the nerve.222 Although this technique has been referred to as a selective nerve root block, it is actually a selective ventral ramus block or, if the dorsal root ganglion is outside the foramen, a dorsal root ganglion block.
All prior lumbar studies except one232 used approximately 1 mL of contrast dye to outline the nerve. Most studies and guidelines recommend visualizing contrast spread using live fluoroscopy during injection. In the cervical spine, some authors advocate observing contrast flow in an anteroposterior view using digital subtraction fluoroscopy to be better able to recognize potential injection into an artery coursing medially toward the spinal cord.236,237
Some prior studies used a volume varying from 0.3 to 0.5 mL of 0.5% bupivacaine221 to 1.5 mL of 0.5% bupivacaine in the lumbar spine,232 but most injected 1 mL of either 1% or 2% lidocaine. In the only diagnostic article that evaluated cervical injections, Sasso and colleagues222 used 0.5 to 0.75 mL of 2% lidocaine but varied their volume depending on the observed contrast dye distribution. No studies have evaluated the diagnostic value of thoracic injections.
The authors recommend limiting the volume to 0.3 to 1 mL in the lumbar spine and 0.3 to 0.5 mL in cervical and thoracic spine. Although many prior studies used 1% to 2% lidocaine, the authors recommend a higher concentration to ensure adequate block.220,222 At a minimum, 2% lidocaine should be used; however, an equal combination of 4% lidocaine with 0.75% bupivacaine or 0.5% bupivacaine alone can be used. The volume can be adjusted between the lower and upper limit depending on the contrast flow pattern.
Summary
Patients with clinically significant radicular pain unresponsive to conservative care and medications may be offered a therapeutic injection including local anesthetic and corticosteroids. The injection can be performed using an interlaminar, transforaminal, or combined approach and can be performed at all suspected levels using volumes of injectant that cover all suspected symptomatic levels. If the patient has convincing pain relief for 1 week or longer, it is likely that the cause of pain is reversible and secondary to inflammation.32 More importantly, if the patient reports minimal or very short-term relief of extremity pain, the pain has been present for greater than 1 year, and the offending pathology is unconvincing, the pain may be neuropathic or referred somatic pain.32 If pain relief is satisfactory and lasts several weeks or longer, one may use additional therapeutic injections to facilitate conservative care, and there may be no need to proceed with exactly identifying the symptomatic level. When pain is recurring or poorly responsive to therapeutic injections and the clinical and imaging studies are inconclusive or indicate more than one potential pain level, diagnostic transforaminal injections may be considered. Table 15–1 summarizes indications for diagnostic selective nerve root blocks.
TABLE 15–1 Indications for Diagnostic Selective Nerve Root Blocks
|
3 Patients with chronic radicular pain present for ≥1 year, resistant to usual care and being considered for surgery
|
A low volume of a concentrated anesthetic solution should be used that is limited to 0.3 mL or less in the cervical spine, 0.5 mL or less in the thoracic spine, and 1 mL or less in the lumbar spine.224 One might consider using an equal mixture of 4% lidocaine and 0.75% bupivacaine or 0.5% bupivacaine alone. If performing a therapeutic injection, 0.5 mL of nonparticulate corticosteroid (e.g., 5 mg dexamethasone) may be injected in the cervical and thoracic spine approximately 1 to 2 minutes after local anesthetic injection, and either nonparticulate or a longer acting depot preparation may be injected in the lumbar spine (e.g., approximately 20 mg of triamcinolone acetonide or 3 mg of betamethasone).
The immediate results and the patient’s longer term pain relief are used to counsel the patient on his or her chances of obtaining relief of extremity pain after a surgical procedure. Patients who have immediate pain relief after one level block of approximately 70% or greater and pain less than 1 year’s duration have an 85% or greater chance of a satisfactory result.220–223,230,235 If the patient has had prior surgery, one might want to lower the patient’s expectation from 85% to perhaps approximately 70% or less depending on how convincing the structural pathology appears on MRI or CT.231,233 Patients who have unconvincing structural pathology, radicular pain greater than 1 year’s duration, relief of less than approximately 70% of pain after block, less than approximately 1 week of therapeutic pain relief, and especially evidence of intraradicular or extraradicular scarring should be referred for possible spinal cord stimulation or other nonoperative treatment.32,220,221,223,231
Patients with clear structural nerve entrapment with radicular pain less than approximately 1 year’s duration, with no immediate or delayed longer duration relief, may be offered surgery, but the patient should be counseled that there is an approximately 60% chance of a good outcome.222 If the duration of the patient’s pain is greater than 1 year, perhaps the patient should be told that there is an approximately 60% chance of having partial pain relief but that the pain relief would likely be less than 50%.32,222 Even if the same patient with more chronic radicular pain had immediate pain relief but no longer term relief, and especially if there was suspected neuropathic pain and a prior surgery, the patient should be counseled that the chances of a good outcome are no greater than approximately 50%.231 Finally, the authors emphasize that relief of pain does not determine the cause of the pain, and if a patient’s root pain is neuropathic, decompression with or without stabilization would most likely not provide satisfactory relief of pain.238
Pitfalls regarding selective nerve root blocks include complications of the procedure. Although complications after transforaminal injections are mostly minor,239–241 there are growing concerns regarding the safety of cervical transforaminal injections242,243 and to a lesser extent thoracic and lumbar injections based on published and unpublished cases of neurologic damage after the injection of local anesthetic and depot corticosteroids into the neuroforamen.244–248 Reported and unreported complications mostly involve the use of particulate corticosteroids that are alleged to have been injected into the vertebral or radicular artery. Some unpublished legal cases are, however, consistent with direct injection into the cord. Although legal cases claim injury was secondary to injection of particulate corticosteroids into a lumbar or thoracic radicular artery, to the authors’ knowledge there has been no reported case of neurologic damage secondary to arterial injection using nonparticulate corticosteroids; nonparticulate corticosteroids are now recommended when performing cervical transforaminal injections.249 Spinal cord injection is rare and is easily preventable by using a shorter needle, always advancing the needle over bone (superior articular process), checking an anteroposterior fluoroscopy view before injection, and titrating patient sedation appropriately.
Although current techniques strive to avoid contacting the nerve, contact does occasionally occur, and probing for paresthesias was a common technique in the past. Lasting effects are probably uncommon, and none of the prior reviewed diagnostic block studies reported any complications. Injecting local anesthetic or contrast dye directly into the dorsal root ganglion, nerve, or epiradicular sheath may cause a flare in pain, however, lasting several days to several weeks.220 Permanent injury is probably rare and to the authors’ knowledge unreported.
A needle placed too far medially can pierce the nerve root sleeve surrounded by the dura contiguous with the subarachnoid space. Injection may cause a high spinal block, which may necessitate resuscitation if injected in the cervical spine and may potentially lead to some degree of cord or root irritation secondary to added preservatives if depot steroids are injected. Puncture of the dura may also cause a low-pressure cerebrospinal fluid headache, which usually resolves spontaneously or can be treated with a routine blood patch. Slipman and colleagues218 reported a case of recalcitrant headache cured after transforaminal blood patch. Infection may occur, but is rare. If the patient has a foraminal disc protrusion, inadvertently passing a needle into the disc may occasionally occur and could lead to a disc space infection.250 If the operator knows that disc injection has occurred, use of a small amount of intradiscal and intravenous antibiotics should be considered (as would be the routine with discography).
Pearls and Pitfalls
Key Points
1 Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539-1550.
This is a systematic review of the evidence for identifying the source of chronic low back pain.
2 Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591-614.
3 Manchukonda R, Manchikanti KN, Cash KA, et al. Facet joint pain in chronic spinal pain: An evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539-545.
4 Dreyfuss P, Dreyer SJ, Cole A, et al. Sacroiliac pain. J Am Acad Orthop Surg. 2004;12:255-265.
5 Bogduk N. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004.
1 Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: Evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
2 CDC: Prevalence of disabilities and associated health conditions among adults—United States 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120-125.
3 Guo HR, Tanaka S, Halperin WE, et al. Back pain prevalence in US industry and estimates of lost workdays. Am J Public Health (1971). 1999;89:1029-1035.
4 Cypress BK. Characteristics of physician visits for back symptoms: A national perspective. Am J Public Health (1971. 1983;73:389-395.
5 Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976). 1995;20:1668-1673.
6 Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21-24.
7 Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251-258.
8 Dillane JB, Fry J, Kalton G. Acute back syndrome: A study from general practice. BMJ. 1966;2:82-84.
9 Nachemson AL. The natural course of low back pain. In: White A, Gordon SL, editors. Symposium on Idiopathic Low Back Pain. St Louis: Mosby; 1982:46-51.
10 Sheather-Reid RB, Cohen ML. Psychophysical evidence for a neuropathic component of chronic neck pain. Pain. 1998;75:341-347.
11 Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539-1550.
12 Fritz JM, George S. The use of a classification approach to identify subgroups of patients with acute low back pain: Interrater reliability and short-term treatment outcomes. Spine (Phila Pa 1976). 2000;25:106-114.
13 Kent P, Keating J. Do primary-care clinicians think that nonspecific low back pain is one condition? Spine (Phila Pa 1976). 2004;29:1022-1031.
14 Derby R. Diagnostic block procedures. Spine (Phila Pa 1976). 1986;1:47-64.
15 Bogduk N. Practice Guidelines: Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2004.
16 Boswell MV, Colson JD, Sehgal N, et al. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10:229-253.
17 Boden S, Wiesel SW, Laws ER, et al. The Aging Spine. Philadelphia: WB Saunders; 1991.
18 Berven S, Tay BB, Colman W, et al. The lumbar zygapophyseal (facet) joints: A role in the pathogenesis of spinal pain syndromes and degenerative spondylolisthesis. Semin Neurol. 2002;22:187-196.
19 Wetzel FT. Chronic benign cervical pain syndromes: Surgical considerations. Spine (Phila Pa 1976). 1992;17(10 Suppl):S367-S374.
20 Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591-614.
21 Cohen SP. Sacroiliac joint pain: A comprehensive review of anatomy, diagnosis and treatment. Anesth Analg. 2005;101:1440-1453.
22 Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 13: Injection therapies, low-back pain, and lumbar fusion. J Neurosurg Spine. 2005;2:707-715.
23 Boswell MV, Singh V, Staats PS, et al. Accuracy of precision diagnostic blocks in the diagnosis of chronic spinal pain of facet or zygapophysial joint origin. Pain Physician. 2003;6:449-456.
24 Dreyfuss P, Dreyer SJ, Cole A, et al. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12:255-265.
25 Dutta S, Lee M, Falco FJ, et al. Systematic assessment of diagnostic accuracy therapeutic utility of lumbar facet interventions. Pain Physician. 2009;12:437-460.
26 Atluri S, Datta S, Falco FJ, et al. Systematic review of diagnostic utility and therapeutic effectiveness of thoracic facet joint interventions. Pain Physician. 2008;11:611-629.
27 Sehgal N, Dunbar EE, Shah RV, et al. Systematic review of diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: An update. Pain Physician. 2007;10:213-228.
28 Manchikanti L, Pampati V, Fellows B, et al. The inability of the clinical picture to characterize pain from facet joints. Pain Physician. 2000;3:158-166.
29 Yin W, Bogduk N. The nature of neck pain in a private pain clinic in the United States. Pain Med. 2008;9:196-203.
30 Manchikanti L, Pampati V, Rivera J, et al. Role of facet joints in chronic low back pain in the elderly: A controlled comparative prevalence study. Pain Pract. 2001;1:332-337.
31 Pneumaticos SG, Chatziioannou SN, Hipp JA, et al. Low back pain: Prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology. 2006;238:693-698.
32 Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine (Phila Pa 1976). 1992;17(6 Suppl):S176-S183.
33 Manchikanti L, Boswell MV, Manchukonda R, et al. Influence of prior opioid exposure on diagnostic facet joint nerve blocks. J Opioid Manag. 2008;4:351-360.
34 Kikuchi S. New concept for backache: Biopsychosocial pain syndrome. Eur Spine J. 2008;17(Suppl 4):421-427.
35 Boos N, Rieder R, Schade V, et al. 1995 Volvo Award in clinical sciences: The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine (Phila Pa 1976). 1995;20:2613-2625.
36 Lilius G, Laasonen EM, Myllynen P, et al. Lumbar facet joint syndrome: A randomised clinical trial. J Bone Joint Surg Br. 1989;71:681-684.
37 Lilius G, Harilainen A, Laasonen EM, et al. Chronic unilateral low-back pain: Predictors of outcome of facet joint injections. Spine (Phila Pa 1976). 1990;15:780-782.
38 Wallis BJ, Lord SM, Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: A randomised, double-blind, placebo-controlled trial. Pain. 1997;73:15-22.
39 Manchikanti L, Pampati V, Fellows B, et al. Influence of psychological factors on the ability to diagnose chronic low back pain of facet joint origin. Pain Physician. 2001;4:349-357.
40 Derby R, Lee S-H, Chen Y, et al. The influence of psychologic factors on diskography in patients with chronic axial low back pain. Arch Phys Med Rehabil. 2008;89:1300-1304.
41 Kirkaldy-Willis WH, Farfan FH. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110-123.
42 . Apophysis. Available at http://en.wikipedia.org/wiki/Apophysis Accessed April 13, 2009
43 Bogduk N. Clinical Anatomy of the Lumbar Spine, 4th ed. London: Elsevier; 2005.
44 Andersson GB, Ortengren R, Nachemson AL. Intradiskal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop Relat Res. 1977;129:156-164.
45 Adams MA, Hutton WC. The mechanical function of the lumbar apophyseal joints. Spine (Phila Pa 1976). 1983;8:327-330.
46 Uhrenholt L, Hauge E, Charles AV, et al. Degenerative and traumatic changes in the lower cervical spine facet joints. Scand J Rheumatol. 2008;37:375-384.
47 Taylor JR, Twomey LT, Corker M. Bone and soft tissue injuries in post-mortem lumbar spines. Paraplegia. 1990;28:119-129.
48 Twomey LT, Taylor JR, Taylor MM. Unsuspected damage to lumbar zygapophyseal (facet) joints after motor-vehicle accidents. Med J Aust. 1989;151:210-212. 215
49 Taylor JR, Twomey LT. Acute injuries to cervical joints: An autopsy study of neck sprain. Spine (Phila Pa 1976). 1993;18:1115-1122.
50 Uhrenholt L, Grunnet-Nilsson N, Hartvigsen J. Cervical spine lesions after road traffic accidents: A systematic review. Spine (Phila Pa 1976). 2002;27:1934-1941. discussion 1940
51 Eisenstein SM, Parry CR. The lumbar facet arthrosis syndrome: Clinical presentation and articular surface changes. J Bone Joint Surg Br. 1987;69:3-7.
52 Ziv I, Maroudas C, Robin G, et al. Human facet cartilage: Swelling and some physicochemical characteristics as a function of age. Part 2: Age changes in some biophysical parameters of human facet joint cartilage. Spine (Phila Pa 1976). 1993;18:136-146.
53 Kallakuri S, Singh A, Chen C, et al. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine (Phila Pa 1976). 2004;29:1182-1186.
54 Giles LG, Taylor JR. Innervation of lumbar zygapophyseal joint synovial folds. Acta Orthop Scand. 1987;58:43-46.
55 Giles LG, Taylor JR. Human zygapophyseal joint capsule and synovial fold innervation. Br J Rheumatol. 1987;26:93-98.
56 Kallakuri S, Singh A, Lu Y, et al. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556-563.
57 Ahmed M, Bjurholm A, Kreicbergs A, et al. Sensory and autonomic innervation of the facet joint in the rat lumbar spine. Spine (Phila Pa 1976). 1993;18:2121-2126.
58 Cavanaugh JM, Lu Y, Chen C, et al. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(Suppl 2):63-67.
59 Cavanaugh JM, Ozaktay AC, Yamashita HT, et al. Lumbar facet pain: Biomechanics, neuroanatomy and neurophysiology. J Biomech. 1996;29:1117-1129.
60 Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54:1109-1122.
61 Lu Y, Chen C, Kallakuri S, et al. Neural response of cervical facet joint capsule to stretch: A study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49-65.
62 Lu Y, Chen C, Kallakuri S, et al. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23:779-787.
63 Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: Behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383-1393.
64 Lee KE, Davis MB, Mejilla RM, et al. In vivo cervical facet capsule distraction: Mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373-395.
65 Winkelstein BA, Santos DG. An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine (Phila Pa 1976). 2008;33:856-862.
66 Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686-688.
67 Quinn KP, Lee KE, Ahaghotu CC, et al. Structural changes in the cervical facet capsular ligament: Potential contributions to pain following subfailure loading. Stapp Car Crash J. 2007;51:169-187.
68 Kasch H, Stengaard-Pedersen K, Arendt-Nielsen L, et al. Pain thresholds and tenderness in neck and head following acute whiplash injury: A prospective study. Cephalalgia. 2001;21:189-197.
69 Sterling M, Jull G, Vicenzino B, et al. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104:509-517.
70 Curatolo M, Petersen-Felix S, Arendt-Nielsen L, et al. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17:306-315.
71 Manchikanti L, Manchikanti KN, Manchukonda R, et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: Preliminary report of a randomized, double-blind controlled trial: Clinical trial NCT00355914. Pain Physician. 2007;10:425-440.
72 Bogduk N. A narrative review of intra-articular corticosteroid injections for low back pain. Pain Med. 2005;6:287-296.
73 Barnsley L, Lord SM, Wallis BJ, et al. Lack of effect of intraarticular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med. 1994;330:1047-1050.
74 Carette S, Marcoux S, Truchon R, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325:1002-1007.
75 Manchikanti L, Singh V, Falco FJ, et al. Cervical medial branch blocks for chronic cervical facet joint pain: A randomized, double-blind, controlled trial with one-year follow-up. Spine (Phila Pa 1976). 2008;33:1813-1820.
76 Manchikanti L, Singh V, Falco FJ, et al. Lumbar facet joint nerve blocks in managing chronic facet joint pain: One-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain Physician. 2008;11:121-132.
77 Manchikanti L, Singh V, Vilims BD, et al. Medial branch neurotomy in management of chronic spinal pain: Systematic review of the evidence. Pain Physician. 2002;5:405-418.
78 Little JS, Ianuzzi A, Chiu JB, et al. Human lumbar facet joint capsule strains: II. Alteration of strains subsequent to anterior interbody fixation. Spine J. 2004;4:153-162.
79 Chang UK, Kim DH, Lee MC, et al. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7:33-39.
80 Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149-156.
81 Sehgal N, Shah RV, McKenzie-Brown AM, et al. Diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: A systematic review of evidence. Pain Physician. 2005;8:211-224.
82 Bogduk N. Evidence-informed management of chronic low back pain with facet injections and radiofrequency neurotomy. Spine J. 2008;8:56-64.
83 Manchukonda R, Manchikanti KN, Cash KA, et al. Facet joint pain in chronic spinal pain: An evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539-545.
84 Cohen SP, Stojanovic MP, Crooks M, et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: A multicenter analysis. Spine J. 2008;8:498-504.
85 Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55:99-106.
86 Lord SM, Barnsley L, Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11:208-213.
87 Kaplan M, Dreyfuss P, Halbrook B, et al. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint: A physiologic challenge. Spine (Phila Pa 1976). 1998;23:1847-1852.
88 Dreyfuss P, Schwarzer AC, Lau P, et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks: A computed tomography study. Spine (Phila Pa 1976). 1997;22:895-902.
89 Destouet JM, Gilula LA, Murphy WA, et al. Lumbar facet joint injection: Indication, technique, clinical correlation, and preliminary results. Radiology. 1982;145:321-325.
90 Carrera GF. Lumbar facet joint injection in low back pain and sciatica: Description of technique. Radiology. 1980;137:661-664.
91 Destouet JM, Murphy WA. Lumbar facet block indications and technique. Orthop Rev. 1985. 14
92 Moran R, O’Connell D, Walsh MG. The diagnostic value of facet joint injections. Spine (Phila Pa 1976). 1988;13:1407-1410.
93 Barnsley L, Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Reg Anesth. 1993;18:343-350.
94 Cohen SP, Hurley RW. The ability of diagnostic spinal injections to predict surgical outcomes. Anesth Analg. 2007;105:1756-1775.
95 North RB, Kidd DH, Zahurak M, et al. Specificity of diagnostic nerve blocks: A prospective, randomized study of sciatica due to lumbosacral spine disease. Pain. 1996;65:77-85.
96 Goldthwait JE. The lumbosacral articulation: An explanation of many cases of lumbago, sciatica and paraplegia. Boston Med Surg J. 1911;164:356-372.
97 Ghormley RK. Low back pain with special reference to the articular facets, with presentation of an operative procedure. JAMA. 1933;101:1773-1777.
97a. Badgley CE. Pain of spinal origin. J Mich State Med Soc. 1947;46:812.
98 Schwarzer AC, Aprill CN, Derby R, et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Phila Pa 1976). 1994;19:801-806.
99 Manchikanti L, Pampati V, Fellows B, et al. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician. 1999;2:59-64.
100 Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
101 Schwarzer AC, Wang SC, Bogduk N, et al. Prevalence and clinical features of lumbar zygapophysial joint pain: A study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54:100-106.
102 Fairbank JC, Park WM, McCall IW, et al. Apophyseal injection of local anesthetic as a diagnostic aid in primary low-back pain syndromes. Spine (Phila Pa 1976). 1981;6:598-605.
103 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: Identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73:824-828.
104 Revel M, Poiraudeau S, Auleley GR, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia: Proposed criteria to identify patients with painful facet joints. Spine (Phila Pa 1976). 1998;23:1972-1976. discussion 1977
105 Laslett M, Oberg B, Aprill CN, et al. Zygapophysial joint blocks in chronic low back pain: A test of Revel’s model as a screening test. BMC Musculoskelet Disord. 2004;5:43.
106 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
107 Manchikanti L, Pampati V, Fellows B, et al. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain. 2000;4:337-344.
108 Schwarzer AC, Derby R, Aprill CN, et al. Pain from the lumbar zygapophysial joints: A test of two models. J Spinal Disord. 1994;7:331-336.
109 Anand S, Butt MS. Patients’ response to facet joint injection. Acta Orthop Belg. 2007;73:230-233.
110 Carrera GF. Lumbar facet arthrography and injection in low back pain. Wisc Med J. 1979;78:35-37.
111 Jackson RP, Jacobs RR, Montesano PX. 1988 Volvo award in clinical sciences: Facet joint injection in low-back pain: A prospective statistical study. Spine (Phila Pa 1976). 1988;13:966-971.
112 Schwarzer AC, Wang SC, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20:907-912.
113 Cohen SP, Hurley RW, Christo PJ, et al. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23:45-52.
114 Kawaguchi Y, Matsuno H, Kanamori M, et al. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech. 2003;16:38-43.
115 Friedrich KM, Nemec S, Peloschek P, et al. The prevalence of lumbar facet joint edema in patients with low back pain. Skeletal Radiol. 2007;36:755-760.
116 Marks R. Distribution of pain provoked from lumbar facet joints and related structures during diagnostic spinal infiltration. Pain. 1989;39:37-40.
117 Windsor RE, King FJ, Roman SJ, et al. Electrical stimulation induced lumbar medial branch referral patterns. Pain Physician. 2002;5:347-353.
118 Jackson RP. The facet syndrome: Myth or reality? Clin Orthop Relat Res. 1992;279:110-121.
119 Esses SI, Moro JK. The value of facet joint blocks in patient selection for lumbar fusion. Spine (Phila Pa 1976). 1993;18:185-190.
120 Schofferman J, Reynolds J, Herzog R, et al. Failed back surgery: Etiology and diagnostic evaluation. Spine J. 2003;3:400-403.
121 Lovely TJ, Rastogi P. The value of provocative facet blocking as a predictor of success in lumbar spine fusion. J Spinal Disord. 1997;10:512-517.
122 Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976). 2000;25:1270-1277.
123 Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: A randomized double-blind trial. Spine (Phila Pa 1976). 2008;33:1291-1297. discussion 1298
124 Husted DS, Orton D, Schofferman J, et al. Effectiveness of repeated radiofrequency neurotomy for cervical facet joint pain. J Spinal Disord Tech. 2008;21:406-408.
125 Schofferman J, Kine G. Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine (Phila Pa 1976). 2004;29:2471-2473.
126 Hadden SB. Neurologic headache and facial pain. Arch Neurol. 1940;43:405.
127 Macnab I. The whiplash syndrome. Clin Neurosurg. 1973;20:232-241.
128 Bogduk N, Marsland A. On the concept of third occipital headache. J Neurol Neurosurg Psychiatry. 1986;49:775-780.
129 Dreyfuss P, Michaelsen M, Fletcher D. Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine (Phila Pa 1976). 1994;19:1125-1131.
130 Dreyfuss P, Rogers J, Dreyer S, et al. Atlanto-occipital joint pain: A report of three cases and description of an intraarticular joint block technique. Reg Anesth. 1994;19:344-351.
131 Bogduk N, Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine (Phila Pa 1976). 1988;13:610-617.
132 Bogduk N, Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
133 Cooper G, Bailey B, Bogduk N. Cervical zygapophysial joint pain maps. Pain Med. 2007;8:344-353.
134 Barnsley L, Lord SM, Wallis BJ, et al. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine (Phila Pa 1976). 1995;20:20-25. discussion 26
135 Lord SM, Barnsley L, Wallis BJ, et al. Chronic cervical zygapophysial joint pain after whiplash: A placebo-controlled prevalence study. Spine (Phila Pa 1976). 1996;21:1737-1744. discussion 1744
136 Aprill C, Axinn MJ, Bogduk N. Occipital headaches stemming from the lateral atlanto-axial (C1-2) joint. Cephalalgia. 2002;22:15-22.
137 Barnsley L, Lord S, Wallis B, et al. False-positive rates of cervical zygapophysial joint blocks. Clin J Pain. 1993;9:124-130.
138 Speldewinde GC, Bashford GM, Davidson IR. Diagnostic cervical zygapophyseal joint blocks for chronic cervical pain. Med J Aust. 2001;174:174-176.
139 Manchikanti L, Singh V, Rivera J, et al. Prevalence of cervical facet joint pain in chronic neck pain. Pain Physician. 2002;5:243-249.
139a. Yin W, Bogduk N. The nature of neck pain in a private pain clinic in the United States. Pain Med. 2008;9:196-203.
140 Kirpalani D, Mitra R. Cervical facet joint dysfunction: A review. Arch Phys Med Rehabil. 2008;89:770-774.
141 Jull G, Bogduk N, Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148:233-236.
142 Hechelhammer L, Pfirrmann CW, Zanetti M, et al. Imaging findings predicting the outcome of cervical facet joint blocks. Eur Radiol. 2007;17:959-964.
143 Manchikanti L, Pampati V. Research designs in interventional pain management: Is randomization superior, desirable or essential? Pain Physician. 2002;5:275-284.
144 Stolker RJ, Vervest AC, Groen GJ. Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir. 1993;122:82-90.
145 Linton SJ, Hellsing AL, Halldan K. A population-based study of spinal pain among 35-45-year-old individuals: Prevalence, sick leave, and health care use. Spine (Phila Pa 1976). 1998;23:1457-1463.
146 Dreyfuss P, Tibiletti C, Dreyer SJ. Thoracic zygapophyseal joint pain patterns: A study in normal volunteers. Spine (Phila Pa 1976). 1994;19:807-811.
147 Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. 1997;22:332-336.
148 Chua WH, Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir (Wien). 1995;136:140-144.
149 Dreyfuss P, Tibiletti C, Dreyer S, et al. Thoracic zygapophyseal joint pain: A review and description of an intra-articular block technique. Pain Digest. 1994;4:46-54.
150 Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
151 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the prevalence of facet joint pain in chronic thoracic pain. Pain Physician. 2002;5:354-359.
152 Manchukonda R, Manchikanti KN, Cash KA, et al. Facet joint pain in chronic spinal pain: An evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539-545.
153 Manchikanti L, Manchikanti KN, Manchukonda R, et al. Evaluation of therapeutic thoracic medial branch block effectiveness in chronic thoracic pain: A prospective outcome study with minimum 1-year follow-up. Pain Physician. 2006;9:97-105.
154 Manchikanti L, Singh V, Falco FJ, et al. Effectiveness of thoracic medial branch blocks in managing chronic pain: A preliminary report of a randomized, double-blind controlled trial: Clinical Trial NCT00355706. Pain Physician. 2008;11:491-504.
155 Cook NJ, Hanrahan P, Song S. Paraspinal abscess following facet joint injection. Clin Rheumatol. 1999;18:52-53.
156 Coscia MF, Trammell TR. Pyogenic lumbar facet joint arthritis with intradural extension: A case report. J Spinal Disord Tech. 2002;15:526-528.
157 Arun R, Al-Nammari SS, Mehdian SM. Multilevel vertebral osteomyelitis and facet joint infection following epidural catheterisation. Acta Orthop Belg. 2007;73:665-669.
158 Alcock E, Regaard A, Browne J. Facet joint injection: A rare form cause of epidural abscess formation. Pain. 2003;103:209-210.
159 Heckmann JG, Maihafner C, Lanz S, et al. Transient tetraplegia after cervical facet joint injection for chronic neck pain administered without imaging guidance. Clin Neurol Neurosurg. 2006;108:709-711.
160 Dreyfuss P, Dreyer SJ, Cole A, et al. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12:255-265.
161 Cheng MB, Ferrante FM. Health-related quality of life in sacroiliac syndrome: A comparison to lumbosacral radiculopathy. Reg Anesth Pain Med. 2006;31:422-427.
162 Rupert MP, Lee M, Manchikanti L, et al. Evaluation of sacroiliac joint interventions: A systematic appraisal of the literature. Pain Physician. 2009;12:399-418.
163 Irwin RW, Watson T, Minick RP, et al. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86:37-44.
164 Laslett M, Aprill CN, McDonald B, et al. Diagnosis of sacroiliac joint pain: Validity of individual provocation tests and composites of tests. Manual Ther. 2005;10:207-218.
165 Laslett M, Young SB, Aprill CN, et al. Diagnosing painful sacroiliac joints: A validity study of a McKenzie evaluation and sacroiliac provocation tests. Aust J Physiother. 2003;49:89-97.
166 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4:308-316.
167 van der Wurff P, Buijs EJ, Groen GJ. A multitest regimen of pain provocation tests as an aid to reduce unnecessary minimally invasive sacroiliac joint procedures. Arch Phys Med Rehabil. 2006;87:10-14.
168 Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine (Phila Pa 1976). 1981;6:620-628.
169 Simonian PT, Routt MLJr, Harrington RM, et al. Anterior versus posterior provisional fixation in the unstable pelvis: A biomechanical comparison. Clin Orthop Relat Res. 1995;310:245-251.
170 Rosatelli AL, Agur AM, Chhaya S. Anatomy of the interosseous region of the sacroiliac joint. J Orthop Sports Phys Ther. 2006;36:200-208.
171 Nakagawa T. [Study on the distribution of nerve filaments over the iliosacral joint and its adjacent region in the Japanese]. Nippon Seikeigeka Gakkai Zasshi. 1966;40:419-430.
172 Yin W, Willard F, Carreiro J, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: Technique based on neuroanatomy of the dorsal sacral plexus. Spine (Phila Pa 1976). 2003;28:2419-2425.
173 Willard F: S1-S4 dorsal rami and divisions. Presented at Third World Conference on Low Back and Pelvic Pain, Vienna, Austria, 1998.
174 Grob KR, Neuhuber WL, Kissling RO. [Innervation of the sacroiliac joint of the human]. Z Rheumatol. 1995;54:117-122.
174a. Berthelot JM, Labat JJ, Le Gorff B, et al. Provocative sacroiliac joint maneuvers and sacroiliac joint block are unreliable for diagnosing sacroiliac joint pain. Joint Bone Spine. 2006;73:17-23.
175 Dorman T, Ravin T. Diagnosis and Injection Techniques in Orthopedic Medicine. Baltimore: Williams & Wilkins; 1999.
176 Szadek KM, Hoogland PV, Zuurmond WW, et al. Nociceptive nerve fibers in the sacroiliac joint in humans. Reg Anesth Pain Med. 2008;33:36-43.
177 Murakami E, Tanaka Y, Aizawa T, et al. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: Prospective comparative study. J Orthop Sci. 2007;12:274-280.
178 Weksler N, Velan GJ, Semionov M, et al. The role of sacroiliac joint dysfunction in the genesis of low back pain: The obvious is not always right. Arch Orthop Trauma Surg. 2007;127:885-888.
179 Frymoyer JW, Howe J, Kuhlmann D. The longterm effects of fusion on the sacroiliac joints and ilium. Clin Orthop Relat Res. 1978;134:196-201.
180 Ha KY, Lee JS, Kim KW. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: A prospective cohort study over five-year follow-up. Spine (Phila Pa 1976). 2008;33:1192-1198.
181 Ivanov AA, Kiapour A, Ebraheim NA, et al. Lumbar fusion leads to increases in angular motion and stress across sacroiliac joint: A finite element study. Spine (Phila Pa 1976). 2009;34:E162-E169.
182 Pool-Goudzwaard A, Hoek van Dijke G, Mulder P, et al. The iliolumbar ligament: Its influence on stability of the sacroiliac joint. Clin Biomech (Bristol, Avon). 2003;18:99-105.
183 Ebraheim NA, Elgafy H, Semaan HB. Computed tomographic findings in patients with persistent sacroiliac pain after posterior iliac graft harvesting. Spine (Phila Pa 1976). 2000;25:2047-2051.
184 Maigne JY, Planchon CA. Sacroiliac joint pain after lumbar fusion: A study with anesthetic blocks. Eur Spine J. 2005;14:654-658.
185 Katz V, Schofferman J, Reynolds J. The sacroiliac joint: A potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16:96-99.
186 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21:2594-2602.
187 Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976). 1996;21:1889-1892.
188 Young S, Aprill C, Laslett M. Correlation of clinical examination characteristics with three sources of chronic low back pain. Spine J. 2003;3:460-465.
189 Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16:142-152.
190 Slipman CW, Sterenfeld EB, Chou LH, et al. The predictive value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79:288-292.
191 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20:31-37.
192 Slipman CW, Jackson HB, Lipetz JS, et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81:334-338.
193 Dreyfuss P, Snyder BD, Park K, et al. The ability of single site, single depth sacral lateral branch blocks to anesthetize the sacroiliac joint complex. Pain Med. 2008;9:844-850.
194 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: Pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19:1475-1482.
195 Murakami E, Aizawa T, Noguchi K, et al. Diagram specific to sacroiliac joint pain site indicated by one-finger test. J Orthop Sci. 2008;13:492-497.
196 Berthelot J-M, Labat J-J, Le Goff BT, et al. Provocative sacroiliac joint maneuvers and sacroiliac joint block are unreliable for diagnosing sacroiliac joint pain. Joint Bone Spine. 2006;73:17-23.
197 Hansen HC, McKenzie-Brown AM, Cohen SP, et al. Sacroiliac joint interventions: A systematic review. Pain Physician. 2007;10:165-184.
198 Szadek KM, van der Wurff P, van Tulder MW, et al. Diagnostic validity of criteria for sacroiliac joint pain: A systematic review. J Pain. 2009;10:354-368.
199 Hodge JC, Bessette B. The incidence of sacroiliac joint disease in patients with low-back pain. Can Assoc Radiol J. 1999;50:321-323.
200 Elgafy H, Semaan HB, Ebraheim NA, et al. Computed tomography findings in patients with sacroiliac pain. Clin Orthop Relat Res. 2001;382:112-118.
201 Kacar G, Kacar C, Karayalcin B, et al. Quantitative sacroiliac joint scintigraphy in normal subjects and patients with sacroiliitis. Ann Nucl Med. 1998;12:169-173.
202 Slipman CW, Sterenfeld EB, Chou LH, et al. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine (Phila Pa 1976). 1996;21:2251-2254.
203 Maigne JY, Boulahdour H, Chatellier G. Value of quantitative radionuclide bone scanning in the diagnosis of sacroiliac joint syndrome in 32 patients with low back pain. Eur Spine J. 1998;7:328-331.
204 Rosenberg JM, Quint TJ, de Rosayro AM. Computerized tomographic localization of clinically-guided sacroiliac joint injections. Clin J Pain. 2000;16:18-21.
205 Borowsky CD, Fagen G. Sources of sacroiliac region pain: Insights gained from a study comparing standard intra-articular injection with a technique combining intra- and peri-articular injection. Arch Phys Med Rehabil. 2008;89:2048-2056.
206 Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20:1429-1434.
207 Wise CL, Dall BE. Minimally invasive sacroiliac arthrodesis: Outcomes of a new technique. J Spinal Disord Tech. 2008;21:579-584.
208 Schutz U, Grob D. Poor outcome following bilateral sacroiliac joint fusion for degenerative sacroiliac joint syndrome. Acta Orthop Belg. 2006;72:296-308.
209 Al-Khayer A, Hegarty J, Hahn D, et al. Percutaneous sacroiliac joint arthrodesis: A novel technique. J Spinal Disord Tech. 2008;21:359-363.
210 Ziran BH, Heckman D, Smith WR. CT-guided stabilization for chronic sacroiliac pain: A preliminary report. J Trauma. 2007;63:90-96.
211 Cohen SP, Hurley RW, Buckenmaier CC3rd, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109:279-288.
212 Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16:142-152.
213 Cohen SP, Abdi S. Lateral branch blocks as a treatment for sacroiliac joint pain: A pilot study. Reg Anesth Pain Med. 2003;28:113-119.
214 Benzel EC, Hart BL, Ball PA, et al. Magnetic resonance imaging for the evaluation of patients with occult cervical spine injury. J Neurosurg. 1996;85:824-829.
215 Lehto IJ, Tertti MO, Komu ME, et al. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49-53.
216 Siivola SM, Levoska S, Tervonen O, et al. MRI changes of cervical spine in asymptomatic and symptomatic young adults. Eur Spine J. 2002;11:358-363.
217 Anderberg L, Annertz M, Brandt L, et al. Selective diagnostic cervical nerve root block—correlation with clinical symptoms and MRI-pathology. Acta Neurochir (Wien). 2004;146:559-565. discussion 565
218 Slipman CW, Plastaras CT, Palmitier RA, et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: Are dynatomal maps identical to dermatomal maps? Spine (Phila Pa 1976). 1998;23:2235-2242.
219 Kikuchi S, Hasue M, Nishiyama K, et al. Anatomic and clinical studies of radicular symptoms. Spine (Phila Pa 1976). 1984;9:23-30.
220 Yeom JS, Lee JW, Park KW, et al. Value of diagnostic lumbar selective nerve root block: A prospective controlled study. AJNR Am J Neuroradiol. 2008;29:1017-1023.
221 van Akkerveeken PF. The diagnostic value of nerve root sheath infiltration. Acta Orthop Scand Suppl. 1993;251:61-63.
222 Sasso RC, Macadaeg K, Nordmann D, et al. Selective nerve root injections can predict surgical outcome for lumbar and cervical radiculopathy: Comparison to magnetic resonance imaging. J Spinal Disord Tech. 2005;18:471-478.
223 Dooley JF, McBroom RJ, Taguchi T, et al. Nerve root infiltration in the diagnosis of radicular pain. Spine (Phila Pa 1976). 1988;13:79-83.
224 Furman MB, Lee TS, Mehta A, et al. Contrast flow selectivity during transforaminal lumbosacral epidural steroid injections. Pain Physician. 2008;11:855-861.
225 Jonsson B, Stromqvist B, Annertz M, et al. Diagnostic lumbar nerve root block. J Spinal Disord. 1988;1:232-235.
226 Macnab I. Negative disc exploration: An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891-903.
227 Krempen JF, Smith BS. Nerve-root injection: A method for evaluating the etiology of sciatica. J Bone Joint Surg Am. 1974;56:1435-1444.
228 Schutz H, Lougheed WM, Wortzman G, et al. Intervertebral nerve-root in the investigation of chronic lumbar disc disease. Can J Surg. 1973;16:217-221.
229 Tajima T, Furukawa K, Kuramochi E. Selective lumbosacral radiculography and block. Spine (Phila Pa 1976). 1980;5:68-77.
230 Stanley D, McLaren MI, Euinton HA, et al. A prospective study of nerve root infiltration in the diagnosis of sciatica: A comparison with radiculography, computed tomography, and operative findings. Spine (Phila Pa 1976). 1990;15:540-543.
231 Herron LD. Selective nerve root block in patient selection for lumbar surgery: Surgical results. J Spinal Disord. 1989;2:75-79.
232 Porter DG, Valentine AR, Bradford R. A retrospective study to assess the results of CT-directed peri-neural root infiltration in a cohort of 56 patients with low back pain and sciatica. Br J Neurosurg. 1999;13:290-293.
233 Haueisen DC, Smith BS, Myers SR, et al. The diagnostic accuracy of spinal nerve injection studies: Their role in the evaluation of recurrent sciatica. Clin Orthop Relat Res. 1985;198:179-183.
234 Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
235 White AH, Derby R, Wynne G. Epidural injections for the diagnosis and treatment of low-back pain. Spine (Phila Pa 1976). 1980;5:78-86.
236 Jasper JF. Role of digital subtraction fluoroscopic imaging in detecting intravascular injections. Pain Physician. 2003;6:369-372.
237 Baker R, Dreyfuss P, Mercer S, et al. Cervical transforaminal injection of corticosteroids into a radicular artery: A possible mechanism for spinal cord injury. Pain. 2003;103:211-215.
238 North RB, Kidd DH, Campbell JN, et al. Dorsal root ganglionectomy for failed back surgery syndrome: A 5-year follow-up study. J Neurosurg. 1991;74:236-242.
239 Derby R, Lee SH, Kim BJ, et al. Complications following cervical epidural steroid injections by expert interventionalists in 2003. Pain Physician. 2004;7:445-449.
240 Pobiel RS, Schellhas KP, Eklund JA, et al. Selective cervical nerve root blockade: Prospective study of immediate and longer term complications. AJNR Am J Neuroradiol. 2009;30:507-511.
241 Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86:277-283.
242 Scanlon GC, Moeller-Bertram T, Romanowsky SM, et al. Cervical transforaminal epidural steroid injections: More dangerous than we think? Spine (Phila Pa 1976). 2007;32:1249-1256.
243 Provenzano DA, Fanciullo G. Cervical transforaminal epidural steroid injections: Should we be performing them? Reg Anesth Pain Med. 2007;32:168. author reply 169-170
244 Lee JH, Lee JK, Seo BR, et al. Spinal cord injury produced by direct damage during cervical transforaminal epidural injection. Reg Anesth Pain Med. 2008;33:377-379.
245 Ruppen W, Hugli R, Reuss S, et al. Neurological symptoms after cervical transforaminal injection with steroids in a patient with hypoplasia of the vertebral artery. Acta Anaesthesiol Scand. 2008;52:165-166.
246 Muro K, O’Shaughnessy B, Ganju A. Infarction of the cervical spinal cord following multilevel transforaminal epidural steroid injection: Case report and review of the literature. J Spinal Cord Med. 2007;30:385-388.
247 Suresh S, Berman J, Connell DA. Cerebellar and brainstem infarction as a complication of CT-guided transforaminal cervical nerve root block. Skeletal Radiol. 2007;36:449-452.
248 Tiso RL, Cutler T, Catania JA, et al. Adverse central nervous system sequelae after selective transforaminal block: The role of corticosteroids. Spine J. 2004;4:468-474.
249 Derby R, Lee SH, Date ES, et al. Size and aggregation of corticosteroids used for epidural injections. Pain Med. 2008;9:227-234.
250 Hooten WM, Mizerak A, Carns PE, et al. Discitis after lumbar epidural corticosteroid injection: A case report and analysis of the case report literature. Pain Med. 2006;7:46-51.