Cardiovascular system
A Ablation procedures
Maze and mini-maze procedures
The MAZE procedure is offered to patients at high risk for stroke who have unsuccessful attempts at pharmacologic treatment. It is an “open-heart” cardiac surgery procedure intended to eliminate atrial fibrillation (AF). The name refers to the series of incisions arranged in a mazelike pattern in the atria. The Cox MAZE III procedure is now considered to be the “gold standard” for effective surgical cure of AF. It may be performed concomitant with mitral valve repair or replacement for patients who also have mitral valve disease. MAZE is performed using pulmonary vein isolation and a number of incisions in the right and left atria. These incisions or cryoablations ultimately form scar tissue, thereby mechanically interrupting transmission of triggering impulses of AF. For open MAZE, it is necessary for the patient to have a sternotomy and cardiopulmonary bypass (CPB). All of the monitoring and medication necessary for CPB is required for the MAZE procedure. In addition to the lesions made in the atria, the left atrial appendage is often removed because it is thought to be a culprit in the stasis of blood flow, thereby increasing the possibility of thrombus formation and stroke. The term mini-MAZE is still sometimes used to describe an open-heart procedure requiring CPB, but it more commonly refers to minimally invasive epicardial procedures not requiring CPB.
The mini-MAZE procedure is performed using thoracoscopy on a beating heart. “Keyhole” incisions are used for the mini-MAZE, and the patient is placed in the lateral position. Routine monitoring with the addition of an arterial line and one-lung ventilation are adjuncts of this procedure.
Pulmonary vein isolation and catheter ablation for persistent atrial fibrillation
Surgical management of rate-related cardiac rhythm anomalies has historically been provided by catheter ablation of the offending right atrial conduction pathways. For conditions such as Wolff-Parkinson-White syndrome, right atrial flutter, and supraventricular tachycardia, catheter ablation to permanently block impulses has been performed by groin catheterization similar to cardiac angiography. Until recently, AF has not been addressed in this manner.
Research on the contributing triggers for AF has illuminated the possibility that pulmonary vein isolation and ablation of offending fibers is an alternative when chemical means fail. Catheter ablation for AF has been performed with patients under general anesthesia. The procedure involves groin catheterization, continuing with a catheter puncture across the atrial septal wall and advancement of the catheter into the left atrium. A lesion is created with the catheter tip and using a specified energy. The lesion then prevents transmission of offending impulses that trigger the AF.
General anesthesia management includes an endotracheal tube, an arterial line, and resuscitative drugs. Hemodynamic instability, including multiple arrhythmias and blood pressure swings, may occur during this procedure. These are often short lived and resolve spontaneously without any intervention or with very little intervention on the part of the anesthesia provider.
B Automatic internal cardioverter defibrillator
Definition
Automatic implantable cardioverter defibrillators are surgically implanted to prevent sudden cardiac death from malignant ventricular tachyarrhythmias. These are self-contained diagnostic devices that continuously monitor the patient’s heart rate and electrocardiographic activity. They sense potentially lethal ventricular arrhythmias and treat them with electrical discharges. Whereas pacemakers use low-energy impulses measured in microjoules, these defibrillators release an electrical discharge of approximately 30 J after sensing periods of fibrillation lasting approximately 20 seconds. Most devices now can be programmed to reconfirm ventricular tachycardia or ventricular fibrillation after charging to prevent inappropriate shock therapy.
Indications
Patients considered for implantation are those who have had minimal success with standard antiarrhythmic drug therapy. The majority of patients have severe coronary artery disease with reduced left ventricular (LV) function, ischemic cardiomyopathy, or idiopathic cardiomyopathy.
Anesthetic technique
1. Anesthetic management is best handled with monitored anesthesia care, but a brief period of general anesthesia might be used during defibrillator testing and programming of the device.
2. Prolonged periods of asystole are at times encountered and can cause cerebral and myocardial ischemia.
3. Vasoactive drugs are helpful for blood pressure stabilization.
4. If ventricular tachycardia occurs before clinical induction, lidocaine treatment should be avoided because it may result in the inability to induce ventricular tachycardia on demand during testing.
5. Standard monitoring should include electrocardiography (ECG) leads II and V5 and an arterial line for continuous blood pressure assessment.
6. Intravenous (IV) sedation or general anesthesia may be used for these patients. Because of the stress associated with testing and the amount of sedation necessary for the procedure, some practitioners advocate that general anesthesia with a controlled airway is the best choice.
C Surgery for coronary artery disease
Coronary artery disease is the predominant cause of death in patients in the fourth and fifth decades of life and the most common cause of premature death in men ages 35 to 45 years. Annually, approximately 1.5 million individuals endure some level of myocardial insult. The most recent data show that more than 1,285,000 inpatient angioplasty procedures, 427,000 inpatient bypass procedures, 1,471,000 inpatient diagnostic cardiac catheterizations, 68,000 inpatient implantable defibrillators, and 170,000 inpatient pacemaker procedures are performed in the United States each year.
Coronary artery disease alters coronary blood flow, decreases coronary reserve, and increases the incidence of coronary artery vasospasm. Risk factors associated with the progression of coronary artery disease include age, gender, genetic predisposition, obesity, hyperlipidemia, hypertension, stress, diabetes mellitus, and smoking. Exacerbating the effects of coronary artery disease are combinations of peripheral vascular disease, carotid disease, and a compromised pulmonary system.
Patients with atherosclerotic coronary disease become symptomatic when 75% of the coronary vessel is occluded, resulting in a decrease in coronary blood flow. Ischemia depresses myocardial function and causes severe chest pain referred to as angina pectoris. In addition to pain, cells are subject to increased irritability and become increasingly vulnerable to fibrillation, alterations in the conduction pathways, and thrombus formation.
Anesthesic technique
The goals of anesthetic management for coronary revascularization are directed toward producing analgesia, amnesia, and muscle relaxation; abolishing autonomic reflexes; maintaining physiologic homeostasis; and providing myocardial and cerebral protection. The avenues available to accomplish these goals include an effective preoperative evaluation, administration of modest doses of sedation and pain medication before any attempt at line placement is made; and use of O2 in the preoperative setting. Administration of a balanced anesthetic with opioid, inhalation agents, sedative–hypnotics, and muscle relaxant provides a stable hemodynamic state for the difficult cardiovascular patient. The inhalation agents offer the additional advantage of anesthetic preconditioning, which is cardioprotective.
Preoperative assessment
A thorough preoperative assessment of the patient should include a comprehensive review of systems, airway status, and laboratory data; physical examination; review of surgical history; and review of current medications. Actual reports of diagnostic procedures such as cardiac catheterization, echocardiography, and Doppler studies should be reviewed by the anesthesia provider.
Hemodynamic status
Evaluation of cardiovascular status includes a discussion with the patient regarding his or her functional status.
Cardiac catheterization may be used for diagnostic assessment, electrophysiologic evaluation, or direct intervention for patients in cardiogenic shock. The catheterization evaluation provides information about pressures and oxygen saturations of the four chambers of the heart, pulmonary artery (PA) pressure, systemic pressure, body surface area, cardiac index (CI) (L/min/m2), stroke index (mL/beat/m2), LV fraction (EF), degree of stenosis in coronary vessels, and coronary dominance. The normal values are listed in the table on pg. 241.
| Notation | Range (mmHg) | |
| Central venous pressure | CVP | 0–8 |
| Right atrial pressure | RAP | 0–8 |
| Right ventricular end-systolic pressure | RVESP | 15–25 |
| Right ventricular end-diastolic pressure | RVEDP | 0–8 |
| Pulmonary artery pressure (systolic) | PAP systolic | 15–25 |
| Pulmonary artery pressure (diastolic) | PAP diastolic | 8–15 |
| Pulmonary artery pressure (mean) | PAP | 10–20 |
| Pulmonary capillary wedge pressure; pulmonary artery occlusion pressure | PCWP; PAOP | 6–12 |
An EF of 50% or greater in a patient with normal valve function is acceptable. If the patient has mitral regurgitation, an EF of 50% to 55% is considered to indicate LV dysfunction. An EF of less than 50% reflects a moderate reduction of ventricular function. Poor cardiac function relates to an EF below 30% and may stem from ventricular hypokinesis, akinesis, or dyskinesis.
Echocardiography is used to evaluate ventricular function by measuring wall motion during systole. It can permit a qualitative and quantitative assessment and reflects the four types of abnormal wall functions described previously.
Single-photon emission computed tomography (SPECT) is another diagnostic tool used in the preparation of patients for cardiac surgery. It is a noninvasive procedure that makes use of a radionuclide tracer to provide a three-dimensional picture of heart structures and function. It is capable of producing a measurement of rate and volume of blood flow, size and location of blockages or narrowing of vessels, and more accurate diagnosis of heart disease in women.
Laboratory data
Laboratory examinations for patients with ischemic heart disease involving two or more associated risk factors (diabetes, obesity, family history, and smoking) should include a complete blood count; electrolytes; cardiac enzymes (including enzyme fraction for creatinine phosphokinase), serum creatinine, cholesterol; and coagulation screening profile.
Coagulation function studies are used to monitor patients receiving heparin therapy and warfarin products. Platelet inhibitors are often part of the drug regimens of patients who need cardiac surgery. These agents are associated with the risk of excess bleeding. However, no evidence suggests that surgery is contraindicated in this situation. It is recommended that 24 to 48 hours elapse before surgery is performed after these drugs are discontinued. The platelet inhibitors (glucoprotein IIb/IIIa receptor inhibitors) in use at this time include abciximab (ReoPro), eptifibatide (Integrilin), and tirofiban (Aggrastat). It should be emphasized that long-term heparin therapy results in prolonged bleeding times and may affect calculations of loading doses of heparin required for CPB. Other issues related to the cause and treatment of adverse bleeding are discussed later in this chapter.
Long-term use of medications
Prevention of rebound hypertension and reduction of perioperative hemodynamic stress are primary considerations for continuation of antihypertensive therapy until the morning of surgery. Potential drug interactions with anesthetic agents should be anticipated and evaluated, and treatment should proceed accordingly.
Calcium channel blockers are used widely to control hypertension, angina, and arrhythmias in patients with cardiovascular disease. Their continued use up to the day of surgery is a common practice and provides the advantage of controlling dysrhythmias and preventing coronary spasm. Potential hazards associated with continued therapy include a reduction in patient responses to inotropes and vasopressors and atrioventricular (AV) conduction problems.
β-Adrenergic receptor–blocking agents play an important role in the therapy of cardiovascular patients and must be continued up to and during the preinduction period. β-Blockers allow for reduction in myocardial oxygen consumption by providing an overall decrease in sympathetic stimulation and catecholamine release. Because the heart rate is slowed, diastolic filling is improved. These drugs are helpful in controlling anginal symptoms, hypertension, tachycardia, and myocardial ischemia. Bronchospasm and decreased inotropic response to β stimulants in conjunction with greater vasoconstriction in response to sympathomimetics are potential disadvantages to continuation of β-blockade up to the time of surgery.
Digitalis therapy may be continued until the morning of surgery if it is used to treat rapid ventricular response to AF or flutter; otherwise, it may be discontinued because other inotropes with greater efficacy may be given preoperatively if needed. If potassium is used, its effects must be carefully monitored.
It is recommended that antidysrhythmics be continued until the day of surgery except for disopyramide, encainide, and flecainide, which should be discontinued except in the presence of the most life-threatening dysrhythmias. These agents have been associated with increased mortality, and postbypass myocardial infarction has been noted with their continuance. Disopyramide has been noted to cause difficulty in termination of CPB.
Antidepressants provide no advantage if continued up to the day of surgery and may interact negatively with sympathomimetics. However, as noted previously, it is important to give sedation and anxiolysis to these patients during the preoperative phase.
Anesthetic technique
Electrocardiography and noninvasive blood pressure
Leads II and V5 can help in the diagnosis of dysrhythmias, ischemia, conduction defects, and electrolyte disturbances. None of the standard leads can detect posterior wall ischemia. The noninvasive blood pressure cuff must be placed on the same side as the arterial line to allow for correlation of blood pressure.
Radial arterial line
Sternal retraction may play a role in distorting the radial artery waveform. The right radial artery is usually selected in cases in which the left internal mammary artery is dissected for anastomosis and because radial arterial line monitoring may show a false low number because of compression of the left subclavian artery at the retractor. The brachial artery is contraindicated because it is an end artery of the arm.
Other arterial line sites
Use of the brachial artery for monitoring is most commonly dismissed because it provides the bulk of circulation for the lower arm and is considered an end artery. The femoral artery is superficial and offers access to the central arterial tree. It also provides appropriate access if intraaortic balloon pump (IABP) placement is necessary. However, if the femoral artery is used, it should be noted that an alternative site may become necessary if use of IABP is instituted.
1. Central venous pressure (CVP): Use of the right internal jugular (IJ) vein is recommended because cannulation of the left IJ vein increases the risk of laceration of the left brachiocephalic vein. CVP lines may be used for monitoring, to provide a central line for fluid and drugs, and in situations in which a PA catheter is not used.
2. PA catheter: The PA catheter was historically used for all coronary artery bypass graft (CABG) procedures, but it is associated with complications and now has a more narrow range of uses. It is indicated for use in high-risk patients with an EF of less than 40%.
3. Transesophageal echocardiography (TEE): TEE allows continuous monitoring of the chambers of the heart, ascending and descending aorta, valvular function, chamber filling, and wall contractility and motion. Detection of gaseous or particulate emboli, identification of intracardiac shunting, diagnosis of aortic dissection, evaluation of saphenous vein graft flow, and confirmation of LV dimension (filling) during weaning are other potential applications for this monitoring method. TEE may predict or suggest myocardial ischemia as defined by regional wall motion abnormalities, valve replacement procedures, cardiac aneurysms, intracardiac tumors, aortic dissection, and repair of complex congenital lesions. Relative contraindications to the use of TEE include dysphagia, mediastinal radiation, upper gastrointestinal surgery or bleeding, esophageal stricture, tumor, varices, and recent chest trauma. In conjunction with the increased use of TEE, certain complications have been reported to occur during open-heart surgery. Cardiac arrhythmias, bronchospasm, and esophageal laceration are rare. The common cardiac formulas are listed in the table on pg. 244.
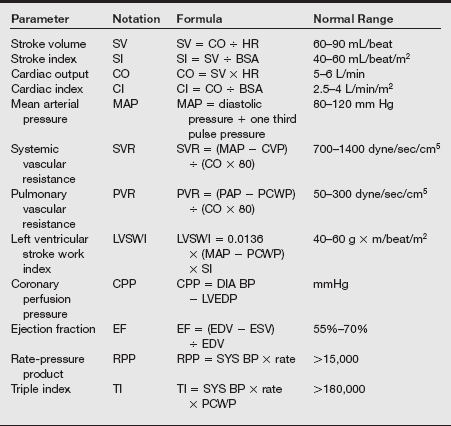
BSA, Body surface area; CVP, cardioventricular pacing; DIA BP, diastolic blood pressure; EDV, end-diastolic volume; ESV, end-systolic volume; HR, heart rate; LVEDP, left ventricular end-diastolic pressure; PAP, peak airway pressure; PCWP, pulmonary capillary wedge pressure; SI, stroke index; SYS BP, systolic blood pressure.
4. Monitoring core temperature: Accurate monitoring of core temperature is essential to control target hypothermia as well as to reestablish normothermia. The most accurate indicator of core temperature is at the thermistor of the PA catheter. Brain temperature is reflected in nasopharyngeal measurement, but a lag time occurs on rewarming. The probe should be inserted before heparinization to a depth of 7 to 10 cm through the nares. Tympanic temperature may also lag behind brain rewarmed temperature and is no better for monitoring of this parameter. Bladder or rectal temperature measurement is today considered inaccurate when renal and splanchnic blood flow is decreased. Brain temperature should not drop below 20° C because profound hypothermia (15° to 20° C) appears to cause a loss of cerebral autoregulation.
5. Cerebral monitoring: In addition to the monitoring of brain temperature, electrophysiologic monitoring is often used. Electroencephalography (EEG) is not an effective method for monitoring subtle changes, but any asymmetric EEG activity is considered a problem. Bispectral analysis monitoring may correlate with the depth of anesthesia; it is actually a derived parameter to assess the degree of wakefulness.
Perfusion principles
The goal of CPB is to provide a motionless heart in a bloodless field while the vital organs continue to be adequately oxygenated. The CPB pump provides respiration (oxygenation and elimination of CO2), circulation (maintenance of perfusion pressure), and regulation of temperature (hypothermia to preserve myocardium). Initiation of CPB subjects the circulating blood of the patient to significant physiologic and physical changes.
Anesthetic and perfusion management must address the impact of low flow indices, reduced metabolic requirements, changing viscosity of the patient’s circulating volume, and postoperative inflammatory response. Multiple factors interact to create a substantially new environment for physiologic homeostasis.
Hemodynamic abnormalities that occur during CPB include endothelial dysfunction (“total body systemic inflammatory response”), which causes symptoms similar to those in patients with sepsis or trauma. Other abnormalities include persistent heparin effect, platelet dysfunction or loss, coagulopathy, fibrinolysis, and hypothermia.
Rapid recirculation of the total blood volume during CPB subjects blood and tissue components to a foreign environment that invites cellular trauma. The patient experiences tremendous alterations in core temperature, hematocrit (in the form of hemodilution), the coagulation cascade, and perfusion pressures (nonpulsatile perfusion).
As a result of excessive hemodilution, the platelet count decreases rapidly to 50% of the preoperative level but usually remains above 100,000 per microliter. Bleeding time is greatly prolonged, and platelet aggregation and function are impaired. Reductions occur in the plasma concentrations of coagulation factors II, V, VII, IX, X, and XII and are attributed to hemodilution.
Extracorporeal circuit
The CPB (pump) circuit consists of separate disposable components bioengineered to interface with perfusion pumps, fluid-based thermoregulating systems, air-oxygen blenders, anesthetic vaporizers, pressure transducers, temperature monitors, and in-line oxygen and blood-gas analyzers. The pump components include venous cannulas from the right atrium or vena cava, which are usually fenestrated at the tip and reinforced. Venous tubing includes the venous return for the blood drained to the machine from the LV.
The venous drainage to the venous reservoir depends on gravity, patient intravascular volume, and the position of and resistance from the venous cannula. The table height can affect venous drainage to the pump. Drainage collects in the venous reservoir, where air bubbles are removed and drainage from other reservoirs is mixed together. If a low volume is allowed here, air can be entrained into the arterial circulation.
Blood suctioned from the heart, pericardium, and pleural spaces drains to the cardiotomy reservoir. The CPB circuit pushes blood forward and returns blood under pressure to the patient by means of either rollers (most common) or a centrifugal (vortex) pump.
Prime
A significant factor is the amount of crystalloid solution required to prime the tubing, reservoir, filters, and oxygenator. Establishment of an air-free circuit is essential for unimpaired fluid volume transport and prevention of air embolism.
Most circuits require at least 2000 mL of a solution such as Normosol, Plasmalyte A, or Isolyte S, with pH and electrolytes closely matching the composition of whole blood. Added to this base solution are heparin, sodium bicarbonate, mannitol, hetastarch, albumin, and possibly corticosteroids or antihyperfibrinolytic agents. This addition can result in priming volumes in excess of 2000 mL, which, when transfused to the patient at the onset of CPB, can cause a hemodilutional bolus of 30% to 50% of the patient’s circulating blood volume.
Vascular transport
The heart and lungs are isolated and bypassed from systemic blood flow. This function is accomplished by right atrial or vena caval cannulation with subsequent diversion of venous blood that is returning to the heart.
The venoatrial cannulas are connected to polyvinyl chloride tubing that extends from the surgical field to the venous reservoir situated at a level well below the patient’s heart to facilitate gravity exsanguination.
Blood from the reservoir is propelled by roller or centrifugal pump to the oxygenator, where it becomes arterialized by interfacing with a membrane oxygenator.
A heat exchanger mounted on the oxygenator provides for control of blood temperature. Oxygenated blood passes through an arterial filter and an in-line arterial gas monitoring device.
Aortic cannula placement is distal to the sinus of Valsalva and proximal to the brachiocephalic (innominate) artery. The arterial line pressure of the extracorporeal circulation (ECC) depends on flow and resistance but usually is maintained below 300 mmHg.
Myocardial protection techniques
Injury to the myocardium is a complex occurrence and may result from numerous physiologic events. Tachycardia, hypertension or hypotension, and ventricular distention can all play a role in the events that produce an oxygen supply–demand imbalance.
Contractile function deteriorates rapidly after the initial insult of ischemia. Rapid cardioplegia-induced cardiac arrest, decompression of the ventricles, and hypothermia are the underlying techniques used to ensure myocardial protection during CPB.
The duration of aortic cross-clamping time, collateral coronary blood supply, frequency of cardioplegia delivery, and composition of cardioplegia are factors that influence the extent of reperfusion injury. Intermittent doses of cold crystalloid cardioplegia help to maintain cardiac arrest, hypothermia, and pH; counteract edema; wash out metabolite; and provide oxygen and substrate for aerobic metabolism.
Administration of inhalation anesthetics has been shown to produce protection against myocardial ischemia and reperfusion injury. This phenomenon is termed anesthetic-induced preconditioning (APC) and derives from positive effects on mitochondria, potassium adenosine triphosphate channels, reactive oxygen species, calcium overload, and inflammation. APC reduces myocardial necrosis and improves postoperative cardiac performance.
Cardioplegia
Cardioplegia is a potassium solution administered into the coronary circulation to provide diastolic arrest. It is composed of potassium (15–30 mEq/L), calcium to prevent ischemic contracture (stone heart), albumin or mannitol for osmolarity correction, and glucose or simple amino acids as a metabolic substrate.
The cardioplegia delivers oxygen and nutrients, removes waste products, and cools or rewarms the heart. It is administered in an antegrade manner into the aortic root, from which it distributes to the coronaries and into the myocardium. It may also be administered in a retrograde fashion into the coronary sinus, from which it distributes through veins, venules, and capillaries of the myocardium.
The cardioplegia composition is blood or crystalloid based. Blood-based cardioplegia is oxygenated blood that is diluted with fluid at a 4:1 ratio. It has a hematocrit of 16% to 18% and is given at 4° to 14° C.
Crystalloid-based solutions do not contain hemoglobin; therefore, they deliver dissolved O2 only. Because of this, crystalloid solutions can be used only with myocardial hypothermic techniques. Intracellular cardioplegia has a low sodium content to produce loss of membrane potential by eliminating the sodium gradient across the membrane.
Extracellular solutions produce diastolic arrest by depolarization of the membrane with high potassium concentrations.
Anticoagulation
Initiation of CPB requires systemic heparinization to establish a safe level of anticoagulation. The currently accepted regimen is 300 units of heparin per kilogram of patient weight.
The heparin dose is usually calculated to maintain an activated clotting time (ACT) of 400 sec (the normal range is 130 sec or less).
Heparin is administered IV through the central venous port. Its peak effect occurs within 2 minutes, and verification is based on the ACT, which should be established 5 to 10 minutes after administration.
Special circumstances such as long-term heparinization, antithrombin III deficiency, heparin-induced thrombocytopenia (HIT), and excessive hemodilution may cause “heparin resistance,” which alters the algorithm for calculating the loading dose.
Management of a patient with heparin-associated thrombocytopenia and thrombosis (HATT) presents a particular challenge. HIT is evident after exposure to heparin because the platelet count suddenly falls. The onset can be as soon as 2 days or as long as 5 days after institution of heparin therapy. Surgery should be postponed if at all possible, and heparin must be eliminated from the patient’s medication regimen until the platelets are normal and do not aggregate in response to heparin. A polysulfated glycosaminoglycan (danaparoid) as well as a thrombin inhibitor (hirudin) have been used safely for CPB.
Prophylaxis and treatment of coagulopathy
Antifibrinolytics
Patients for CABG procedures on CPB receive an antifibrinolytic. First-time patients are treated with aminocaproic acid.
Aminocaproic acid and tranexamic acid
Aminocaproic acid (Amicar) was initially proposed for the treatment of fibrinolysis associated with prostate and cardiac surgery. Tranexamic acid is considered to be more potent than aminocaproic acid. Antifibrinolytics are hemostatic agents given as an IV loading dose and then by continuous infusion before CPB.
The loading dose of aminocaproic acid is 100 to 150 mg/kg followed by an infusion dose of 10 to 15 mg/kg/hr. The dose of tranexamic acid is 10 to 15 mg/kg loading with an infusion of 1 to 1.5 mg/kg/hr. The drug has renal excretion and a plasma half-life of approximately 80 minutes. These drugs have proven effective in reducing bleeding after bypass.
Desmopressin
Desmopressin acetate (DDAVP) is a synthetic analog of vasopressin, which releases a variety of hemostatically active substances from the vascular endothelium. It is administered in doses of 0.3 mcg/kg intravenously, intranasally, or subcutaneously.
It has a half-life of 55 minutes (with clinical effects lasting from 5 to 6 hours) and results in an approximately fourfold increase in circulating levels of factor VIII, prostacyclin, tissue plasminogen activator, and von Willebrand factor.
The overall effect of desmopressin is hemostatic. DDAVP has also been used to treat uremia, cirrhosis, platelet disorders, and mild or moderate cases of hemophilia A (von Willebrand disease). Current evidence does not support the broad administration of DDAVP to cardiac surgical patients as prophylaxis for bleeding.
Pharmacologic approaches to blood pressure control
Blood pressure control during the perioperative phase may be accomplished with the use of pharmacologic agents independently or in combination. Vasodilators such as hydralazine, nitroglycerin, and nitroprusside are useful for control of blood pressure and improving peripheral blood flow.
α-Adrenergic agonists (e.g., clonidine) reduce stress-mediated neurohumoral responses to induction and CPB. They decrease heart rate and blood pressure and have sedative and antinociceptive characteristics, which may reduce opioid requirements without respiratory depression. They can be used independently or in conjunction with IV induction agents and opioids; they help to reduce the amount of agent required.
Careful use of β-blockers can decrease heart rate, contractility, and blood pressure, which works to reduce oxygen use. These drugs increase the duration of diastole to allow for a more complete oxygenation of the LV. They act synergistically with nitroglycerin and blunt tachycardia and decrease ischemia of the myocardium. They have the ability to reduce catecholamine-induced ventricular arrhythmias. The disadvantage associated with β-blockers is that they may precipitate bradyarrhythmias, heart block, or bronchospasm. β-Blockers available in IV form for use during cardiac surgery include esmolol, labetalol, metoprolol, and propranolol. Reversal of the effects of β-blockers can be achieved through use of β-agonists (isoproterenol) and cardiac pacing (unless emergent CPB initiation is possible).
Vasodilator therapy includes direct vasodilators (hydralazine, nitroglycerin, or nitroprusside), α-adrenergic blockers (labetalol, phentolamine), angiotensin-converting enzyme inhibitors (enalaprilat IV), central α-agonists (clonidine), or calcium channel blockers (nicardipine IV, verapamil, or diltiazem). Disadvantages include a slow onset of action or long duration of action, reflex tachycardias, and toxicity reactions. The drug therapy is to be selected individually for each patient and situation and administered judiciously for the desired effect.
Vasopressor therapy includes agents with selective direct effects (methoxamine, phenylephrine), α1-agonist mixed agents (dopamine, ephedrine, epinephrine, noradrenaline), or vasopressin (direct peripheral vasoconstriction with no β-adrenergic effects).
Other drugs that work indirectly to increase blood pressure include the positive inotropic drugs (e.g., dobutamine, dopamine, and milrinone). Calcium reverses hypotension associated with the use of halogenated agents, calcium channel blockers, hypocalcemia, β-blockers, and CPB. When administered intravenously by central line, it can increase blood pressure as well as reverse the cardiac effects of toxicity resulting from hyperkalemia.
Preoperative period
Considerations during the preoperative period include sedation and monitoring of the patient while placement of appropriate invasive monitoring lines ensues.
All equipment should be available and invasive lines inserted before the patient is brought to the operating room.
Invasive monitors are useful during induction and should be placed before induction except in emergency situations such as ruptured aneurysms, cardiac tamponade, or ventricle rupture.
Induction
Hemodynamic alterations occur at induction of anesthesia. The induction plan should take into consideration LV function. No single technique has been demonstrated as superior with regard to the prevention of postoperative myocardial infarction by intraoperative ischemia. It is important to control heart rate because this parameter is most likely to produce myocardial ischemia.
On induction, myocardial ischemia can be detected through the ECG, TEE, or PA wedge pressure readings.
Intraoperative events that are known to precipitate ischemia are listed in the following box.
Methods for diminishing the incidence of ischemia include therapeutic interventions such as preoxygenation before induction, reduction of wall tension with nitroglycerin, control of heart rate with β-adrenergic blocking agents, reduction of the work of the myocardium through control of myocardial depression with increased anesthetic levels, and maintenance of coronary perfusion pressure through the use of a nonchronotropic agent such as phenylephrine.
Many cardiac patients have low circulating blood volume because of hypertensive vasoconstriction. A reduced plasma volume may necessitate fluid loading or prophylactic treatment with pressors such as phenylephrine before induction. Hypovolemia should be anticipated and can be monitored by observation of blood pressure alterations and CVP.
High or low cardiac output (CO) has a significant effect on the pharmacokinetics of anesthetic agents. The anesthetic ideally should not interfere with heart rate or metabolic demand for oxygen. Patients with disease of the left main coronary artery are more susceptible to insult during induction. Hemodynamic changes in these patients precipitate extension of already present ischemic effects.
A slow, methodic, balanced technique with combinations of midazolam, fentanyl or sufentanil, etomidate, thiopental, or propofol and a nondepolarizing muscle relaxant reduces hemodynamic changes and meets the goals of diminishing workload on the myocardium. The common opioid doses for maintenance during CPB are listed in the box on pg. 250.
Intraoperative period
Incision to bypass: After induction, the skin incision and sternal split constitute two very stimulating steps in the process of preparation for CPB.
A patient in whom anesthesia is adequate will show minimal response to these steps, thereby reducing the need for additional adjuncts. A continuous opioid infusion with “background” volatile agent or continuous infusion of propofol may help to maintain blood pressure and ensure amnesia as well as provide a smooth transition toward CPB.
During sternotomy, for approximately 15 to 20 seconds, the lungs must be deflated to prevent laceration or puncture. In most cases, the left internal mammary artery is dissected and mobilized for anastomosis to the left anterior descending artery. This requires placement of an internal mammary artery retractor on the left side of the operating table. When there is a left radial arterial line, careful attention to positioning of the wrist ensures that the arterial waveform is not damped. When only saphenous vein grafts are to be harvested, the pace of the operation may increase significantly, and cannulation of the aorta and right atrium may occur earlier.
Once the internal mammary and saphenous grafts are mobilized or harvested, the patient requires heparinization before cannulation of the aorta and right atrium. Bolus administration of heparin is administered in a central line and may decrease arterial pressure 10% to 20%. Anticoagulation is measured with ACT approximately 3 to 5 minutes after heparin administration. The ACT should be approximately 400 seconds before it is safe to institute CPB. The following box is a checklist for CPB.
Aortic cannulation is associated with a hypertensive response, probably because of direct stimulation of sympathetic nerves in the aortic arch. Reduction of mean arterial pressure (MAP) assists aortic cannulation and prevents laceration of the aorta. As a result of manipulation of the heart during cannula placement, venous cannulation (right atrium) may lead to fluctuations in arterial pressure. Right atrial cannulation drains the superior and inferior venae cavae; ventricular tachyarrhythmias may occur, and CO and blood pressure may decrease. If additional cannulation of the coronary sinus for retrograde cardioplegia delivery is required, severe hypotension may ensue and necessitate administration of volume by the perfusionist via the aortic cannula.
After the patient has been placed on extracorporeal support and adequate perfusion flow and pressure have been achieved, most surgeons prefer to stop ventilation to deflate the lungs and optimize surgical conditions. Some surgeons prefer to continue ventilation until the myocardium is motionless. CPB should not commence unless all of the parameters for institution of bypass have been addressed by the anesthesia provider.
Initiation of bypass: Multiple events occur simultaneously and can cause a significant drop in blood pressure at the initiation of CPB. Hemodilution decreases blood viscosity and dilutes catecholamines in the plasma, contributing to the drop in pressure. Rapid cooling of the patient occurs to a target temperature of 28° C. When the target temperature is reached or spontaneous hypothermic fibrillation of the heart occurs, the aorta is cross-clamped. At times, the myocardial arrest may require retrograde administration of cardioplegia via the coronary sinus.
Cerebral and renal protection is important during CPB. Techniques for cerebral protection include metabolic suppression, which can be accomplished with hypothermia, burst suppression, use of calcium channel blockers to reduce the incidence of vasospasm, and decrease in intraoperative bleeding attained through use of antifibrinolytics. The perfusionist also plays a central role in providing cerebral protection. As core temperature is lowered, the pH rises, placing the patient in an alkalotic state. The alpha-stat system represents alkalotic management of cerebral perfusion, but pH-stat relies on hypercarbia to manage CBF. The essential difference is that alpha-stat management represents CBF that is not dependent on MAP and does not mandate the addition of exogenous CO2 to maintain pH in the normal range.
Renal protection is best maintained by the preservation of renal blood flow and monitoring of urine production. Risk factors for renal dysfunction after CPB include prolonged bypass time (>3 hours) and low CO. Osmotic diuretics, low-dose dopamine, and fenoldopam are used during CPB in patients at risk for development of acute renal failure. Prevention of renal insufficiency postoperatively includes control of hypertension, control of hyperglycemia, reduction of “pump” time, maintenance of fluids, and the use of medications that promote urinary output. Urinary output is considered satisfactory if it measures 1 mL/kg/hr during CPB.
Bypass: During cardiac bypass, anesthesia is maintained with an opioid drip as well as the addition of volatile agent on the perfusion circuit.
Controversy exists regarding an acceptable mean blood pressure range during CPB. Keep in mind that CBF is autoregulated, as is flow to other organs. Because of hypothermia, the lower limit of autoregulation is further decreased. This fact, coupled with the presence of high perfusion pressures, can result in an increase in the possibility of emboli and bleeding on the surgical field; the use of 50 to 70 mmHg is promoted as a practical norm in most facilities.
ACT is checked every 20 to 30 minutes by the perfusionist and is maintained at greater than 400 seconds with the addition of heparin to the pump as necessary. Because of hemodilution, the hematocrit frequently falls to approximately 20 g/dL, which is an acceptable level in most patients. Hypokalemia can be problematic, and the perfusionist checks electrolytes frequently during the pump run. Fluids are kept to a minimum during the bypass phase, and the perfusionist often makes adjustments.
It is important to have open communications with the perfusionist, surgeon, and anesthesia provider during CPB so that coagulation, pressure maintenance, and adjustment of electrolyte imbalances are carefully regulated.
Weaning from bypass: When a patient is being weaned from CPB, considerations should include the ventricular function of the heart before bypass and the length of time the aorta was cross-clamped. Below is a checklist for weaning from bypass.
If the ventricle was in good condition before bypass and the cross-clamp time was less than 60 minutes, the initiation of inotropes is probably not necessary. Otherwise, an inotrope should be chosen in consultation with the surgeon. Additional parameters that should be verified include patient temperature, heart rhythm, monitor status, and adequacy of perfusion.
During weaning, the perfusionist partially occludes the venous line to increase right atrial pressure, blood flows into the right ventricle and out through the PA, and pressures become pulsatile.
The rate of rewarming must be limited to 1° C per 3 to 5 minutes to prevent formation of gaseous emboli in the circulatory system or the ECC. Rewarming begins slightly before removal of the aortic cross-clamp, when the last distal anastomosis begins in multiple graft procedures, or when the valve sutures have been placed during valve replacement procedures. The temperature gradient between arterial and venous blood should be maintained below 10° C, and the time frame for rewarming is usually 30 minutes. Laboratory values, including arterial blood gases, electrolytes, and hematocrit, should be obtained. Patient ventilation is reinstituted.
An infusion of calcium chloride (1 g/100 mL 5% dextrose in water [D5W]) is administered via a central line. After most of the calcium chloride has been given, a small test dose of protamine, usually 10 mg, is administered to test for an unexpected reaction before infusion. If the patient remains stable, protamine administration is initiated slowly over approximately 20 minutes to avoid hypotension. Protamine is given in a calculated dose that is approximately a 1:1 ratio of the initial heparin dose. When one-third of the dose of protamine has been given, the perfusionist should be told to stop collecting blood via suction from the operative field because this would clot the pump. Small increments of phenylephrine may be administered to maintain blood pressure within desired ranges. The surgeon can restart bypass throughout the weaning process as necessary. Communication is vital during the weaning process. The possibility always exists that the patient will have to be returned to bypass.
Notify the surgeon when the protamine has been completely administered and recheck the ACT. Recheck CO and pressures to establish postoperative baselines.
When bypass is completely discontinued, check ACT values and institute treatment if they are elevated. Bypass may have to be reinstituted if severe hypotension, excessive bleeding, or a persistently low CO is present. If the protamine is completely administered and a return to bypass is required, heparin (300 units/kg) may be readministered. At times, an IABP may be required.
In anticipation of the increased metabolic uptake associated with this phase of the operation, administration of amnestic agents and muscle relaxants to maintain appropriate anesthetic levels should be instituted. When the aortic cross-clamp is removed, reperfusion to the myocardium allows the heart to rewarm and flushes residual cardioplegic solution and accumulated metabolic byproducts out of the coronary vessels. Hypotension should be anticipated. Plasma levels of atrial natriuretic factor have been shown to decrease with the onset of aortic cross-clamping and either decrease or increase significantly when the cross-clamp is removed.
Sweating during the rewarming phase of CPB represents a normal thermoregulatory response that can be associated with cutaneous dilation that is caused by elevated skin temperatures. Postoperative shivering should be avoided to prevent increased oxygen demand and carbon dioxide production.
Protamine administration: Protamine sulfate is the drug of choice for heparin reversal. It inactivates heparin by binding with it to form an inert salt. At the conclusion of CPB, the residual amount of heparin is assessed and appropriately neutralized.
Protamine is initiated after a test dose of 1 mg in 100 mL over 10 minutes before heparinization. It binds and inhibits the anticoagulation effects to circulating heparin.
Adverse reactions to protamine include histamine-releasing reactions, true anaphylaxis mediated by a specific antiprotamine antibody, or reactions in which release of thromboxane leads to pulmonary vasoconstriction or bronchoconstriction. In the presence of increased risk factors, heparinase I may be given.
Complications of cardiac bypass
Separation from cardiopulmonary bypass
Preexisting ventricular dysfunction or myocardial insult associated with CPB complicates the process of weaning the patient from extracorporeal support. Separation from bypass presents a tremendous challenge in providing appropriate pharmacologic and mechanical support sufficient for the recovery of ventricular function. The major pharmacologic interventions include the use of both inotropic and vasodilator treatment of systolic or diastolic dysfunction.
Tachycardia and arrhythmias must be prevented, arterial blood pressure maintained, and myocardial contractility promoted while constraints on oxygen demand are maintained. The ideal regimen for pharmacologic therapy includes drugs that have rapid onset and termination, have a neutralizing effect on ischemia, and are nontoxic to the myocardium.
Catecholamines have variable effects on heart rate, rhythm, and metabolism. Milrinone, epinephrine, norepinephrine, dobutamine, dopamine, and isoproterenol are selected based on targeted functions. Dopamine increases pulmonary vascular resistance, PA pressure, and LV filling pressures; however, chronic heart failure can lead to a depletion of neurotransmitters, making indirect-acting catecholamines such as dopamine less effective. The impact of high-dose dopamine on renal perfusion cannot be ignored, and low (renal dose) levels may be required if concern for α-adrenergic–mediated constriction of renal or tissue beds exists.
Calcium may be beneficial on termination of CPB and should be administered just before weaning when ionized calcium levels are deficient and inotropic assistance is required.
Phosphodiesterase inhibitors such as milrinone may be helpful in providing prophylactic inotropic support in anticipation of ventricular failure. In conjunction with decreased LV wall tension, milrinone promotes cardiac function without increasing myocardial oxygen demand. Milrinone is similar to dobutamine in its effects on myocardial contractility, myocardial oxygen consumption rate, systemic vascular resistance (SVR), and pulmonary vascular resistance; however, patients are less susceptible to biochemical changes in neurohumoral regulation, which may reduce the efficacy of β-agonists.
Blood from the pump is sequestered into the cell saver device and concentrated to be returned to the patient via IV infusion after separation from CPB. This assists in bolstering the blood pressure without the administration of large amounts of crystalloid or pressors. In addition, colloid may be included to decrease the incidence of hypotension. Monitoring devices should be recalibrated before separation from the CPB, and the lungs should be expanded and mechanical ventilation instituted before weaning. This is done to assess the possibility of atelectasis, pneumothorax, and hydrothorax.
Pacing
Usually during rewarming, fibrillation of the myocardium occurs with a gradual progression to normal sinus rhythm. If defibrillation does not occur spontaneously, antiarrhythmic therapy, electrical cardioversion, or both are used. Control of heart rate and rhythm may be effected through the use of atrial, ventricular, AV sequential, or overdrive pacing in addition to necessary antiarrhythmic drugs. Epicardial electrode placement on the wall of the atrium is routine for cardiac surgery. Pacemakers allow for rapid adjustment of decreased CO when the conduction pathway is damaged or highly irritable.
Atrial contraction determines CO by controlling the volume of blood ejected into the ventricle. Atrial volume, ventricular volume and compliance, and the pattern of atrial contraction influence ventricular filling. If ventricular pacing is used alone, CO may decrease because of loss of the atrial “kick,” but AV pacing alters the AV interval in relation to the PR interval, thereby improving CO.
Left ventricular dysfunction
Left ventricular dysfunction may be indicated by a rise in PA pressure in conjunction with depressed systemic arterial blood pressure. The causes of LV failure can be varied and may include preoperative markers such as a diminished LV EF and LV hypokinesis or akinesis.
Transesophageal echocardiography is an invaluable tool for confirming and isolating hypodynamic ventricular wall motion. If depressed contractility results from lack of appropriate inotropic and pharmacologic support, this should be remedied immediately. In some cases, the myocardium may be “stunned” and may require additional support through the reengagement of CPB, resting of the heart, and examination of the anastomosis for leaks and the grafts for air emboli or kinking.
In the event that cardiac depression remains unresolved or worsens, a mechanical assist device such as an IABP may be percutaneously introduced to provide diastolic augmentation and decreased afterload.
Right ventricular dysfunction
An inflammatory mediated response from the ECC or acute anaphylactic reaction caused by protamine sulfate or blood product transfusion may lead to increased pulmonary vascular resistance, resulting in depressed right ventricular function.
Pharmacologic intervention to reduce pulmonary vasoconstriction includes nitric oxide–based vasodilators and β2-adrenergic agonists. For cases in which conventional treatment fails, intervention may include the use of prostaglandin E1.
Left atrial injection of norepinephrine has been demonstrated to increase systemic pressures while avoiding the first-pass effects on the pulmonary vascular system. When right ventricular failure is unrelated to pulmonary vascular resistance, phosphodiesterase inhibitors may be beneficial for resolving the condition while avoiding the vasodilatory effects of prostaglandins.
Failure to wean
The quality of the surgical correction and the quality of myocardial preservation are important determinants of the success in weaning from CPB. Failure to wean can be attributed to multiple factors, including heart block or ventricular dysfunction resulting from hyperkalemia, interruption of coronary flow (because of air, fat, or particulate emboli), extended CPB and aortic cross-clamp times, and arrhythmias associated with reperfusion injury.
Failure to wean on the primary attempt may lead to significant damage or distention of the heart. Systemic hypotension may promote metabolic acidosis and organ damage or failure. Additional inotropic support may be required for returning to bypass under these conditions and may result in the additional administration of blood products as a result of excessive hemodilution. If return to CPB becomes necessary, an additional heparin bolus may be required.
These situations are always emergent and require extreme caution in ensuring that the patient is being ventilated and adequately anticoagulated and that anatomic reconnection to ECC is achieved before bypass is reengaged. Preparations should include an avenue for mechanical support (e.g., IABP, ventricular assist device) if the need arises.
Intraaortic balloon pump
In the event of LV failure or myocardial hypokinesia resulting from CPB or ischemic insult, insertion of an intraaortic balloon may be necessary to provide diastolic counterpulsation for the patient.
The intraaortic balloon is a distensible polyurethane catheter that is percutaneously inserted through the femoral artery using the Seldinger technique for large-diameter catheter placement. The tip of the catheter allows aortic pressure monitoring and is threaded to the descending thoracic aorta with the tip at the distal aortic arch. The balloon (size 34, 40, or 50 mL) is inflated with helium or carbon dioxide gas. Balloon deflation can be triggered by the R wave of the ECG or by the arterial pressure waveform, atrial pacing mode, AV sequential pacing mode, or internal asynchronous timing (not recommended).
Inflation of the intraaortic balloon is timed to occur during diastole, forcing blood into the coronary arteries and periphery. It deflates during systole to promote ventricular ejection. Diastolic augmentation is achieved during inflation and results in increased coronary perfusion pressure as well as increased flow to the great vessels arising from the aorta. In most instances, this diastolic augmentation results in pressures greater than the patient’s systolic pressure. Afterload reduction results when the balloon is rapidly deflated before ventricular ejection, reducing ventricular wall tension and therefore myocardial oxygen demand.
Ventricular assist devices
The placement of a ventricular assist device is an option when termination of bypass cannot be tolerated by the patient and no other options to ensure survival exist. This effort in most cases represents a bridge to cardiac transplantation or allows for additional resting time to promote recovery of severely compromised cardiac contractile function. Most institutions have established protocols and criteria for considering a patient as a candidate for this device. Age, pulmonary function, and organ system viability are factors in this selection process.
Cardiopulmonary bypass is reinstituted to prepare the circuit and cannulation sites for transfer from the ECC to a centrifugal assist device. Cannulation depends on which ventricle requires support and represents a mechanically assisted atrial-aortic shunt of blood flow to circumvent the impaired ventricle. This is a simple circuit with no oxygenator or heating element component; therefore, during the transfer from the ECC to the mechanical assist device, the patient must be ventilated. Appropriate pharmacologic support is essential.
Extubation
The current trend is toward early extubation of the postoperative cardiac surgical patient. Controversy surrounds the efficacy of extubation within 2 to 4 hours of closure. However, “fast tracking,” a term used to describe early extubation and discharge from the intensive care unit, has become popular as a cost-effective technique associated with this major surgical procedure. The patient population for fast-track cardiac anesthesia must be a “less sick” group to prevent precipitation of hypertensive episodes and increased postoperative myocardial ischemia. Ideally, fast-track candidates are younger than 70 years of age; have normal ventricular and valvular function and an uncomplicated surgery; and are free of renal, neurologic, and coagulation disorders in the immediate postoperative period.
The selection of agents for fast tracking starts in the preinduction phase with agents that have a short duration of action. Lower doses of opioids are supplemented with low-dose inhalation agents and propofol infusions. α-Agonists are used as adjuncts because of their ability to blunt neurohumoral stress responses. Postoperative analgesia can be accomplished with the use of low-dose morphine, nonsteroidal anti-inflammatory drugs, patient-controlled analgesia, and thoracic epidurals with short-acting opioids.
Extubation criteria include a warm patient, low-dose or no inotropic drugs or vasoactive drips, no balloon pump, and bleeding less than 100 mL/hr. The patient must be awake, pain free, and hemodynamically stable and must meet all conventional metabolic criteria for extubation. Regardless of the anesthetic technique used, the key to optimizing patient recovery is postoperative pain management, which facilitates early mobility and nutritional intake.
D Minimally invasive coronary artery bypass techniques
Port-access coronary artery bypass grafting
To minimize postoperative pain and to speed recovery, some cardiovascular surgeons have used a port access method of coronary artery bypass (PACAB). In these procedures, multiple ports are placed in the chest wall for video-assisted surgery in addition to performance of a mini-thoracotomy in some patients. The Heartport system used during PACAB necessitates the femoral artery approach and uses an endoaortic balloon occlusion for instillation of cardioplegia. One-lung ventilation and extensive monitoring are required during the internal mammary artery (IMA) dissection. The indirect visualization of the heart through echocardiography, video (endoscopy), and fluoroscopy makes the procedure somewhat cumbersome and tedious for the surgeon.
Anesthetic techniques include all monitors and considerations for CPB as well as the need for one-lung ventilation. Patient benefits from this port-access technique include less postoperative pain, a reduced intensive care unit stay, an accelerated recovery time, improved postoperative pulmonary function, and a reduced need for inpatient cardiac rehabilitation. Aortic atherosclerotic disease is a contraindication for PACAB procedure. Heartport has a long bypass run for single vessel CABG, which maximizes the risk of stroke even though the sternotomy is eliminated.
Procedures that benefit from port access include multivessel CABG, mitral valve procedures, aortic valve replacements, and some congenital heart defect procedures. Concerns remain regarding aortic dissection as well as stroke and embolism because the surgeon does not have direct access to the surgical field and cannot directly suction air from the heart. Patient selection is an important factor in safety of the procedure.
Minimally invasive direct coronary artery bypass
Minimally invasive direct coronary artery bypass (MIDCAB) follows the basics of conventional CABG procedures but does not require CPB, cardioplegia, or a large incision. Through a small incision (10–12 cm) and under direct vision, the graft is anastomosed while the heart is still beating. This procedure is beneficial to the patient because of the smaller incision, the absence of CPB and its inherent complications, and the reduced need for blood transfusions. The disadvantages include the fact that it is limited to use for only one or two arteries and that one-lung ventilation often is requested by the surgeon. Because the heart continues to beat, the anastomoses are difficult to suture, and significant ischemia may occur, precipitating hemodynamic compromise of the patient. The perfusionist and CPB equipment must be immediately available for urgent conversion to coronary bypass is necessary. Anesthetic management is closely related to that for off-pump coronary artery bypass procedures with normal sternotomies.
Off-pump coronary artery bypass
Surgical techniques in this area, along with advances in equipment, allow multivessel procedures with median sternotomy to be performed. Because CPB is not used, hearts of patients undergoing off-pump coronary artery bypass (OPCAB) are normothermic, and maintenance of coronary perfusion and hemodynamic stability are absolutely necessary. Communication between the surgeon and the anesthesia provider is of paramount importance. The anesthesia provider is a crucial member of the team who should be as observant of the surgical field as the surgeon. Unlike CPB cases, during OPCAB procedures, the grafting phase requires involvement and vigilance on the part of the anesthesia provider.
The patients undergoing OPCAB are anesthetized with the intent of “early” extubation. A modified fast-track approach that avoids ischemia while facilitating early extubation is desirable. The choice of an anesthetic must take into consideration that a slow heart rate facilitates the surgical procedures and reduces myocardial oxygen demand. A narcotic–oxygen–muscle relaxant technique facilitates minute-to-minute control of hemodynamics. Maintenance of the systolic pressure promotes hemodynamic stability when the heart position is changed during exposure of the different vessels. It is recommended that the systolic pressure be maintained above 100 mmHg. Prudent volume loading and positioning of the patient in the Trendelenburg position with a right rotation promote recovery of blood pressure when retraction is used in exposure of the posterior pericardium.
Extensive invasive monitoring is indicated, along with a large-bore peripheral IV and a right IJ triple-lumen catheter capable of handling continuous thermodilution CO and transvenous pacing. Multiple central ports must be available for continuous infusion of various vasoactive medications. Temperature monitoring is vital. It is necessary to maintain fluids and any other drips or instillations at warm temperatures, to use a forced air-warming device on the head and neck, and to maintain a warm room temperature. If the grafts are completely arterial, as is often the case in OPCAB procedures, the anesthesia provider should investigate the possibility of placing a forced air warmer on the patient’s lower extremities.
The muscle relaxant chosen should be one without histamine-releasing effects. Some anesthesia providers insist that a neuromuscular blocker such as pancuronium, which independently causes tachycardia, should be avoided. However, when potent narcotics such as sufentanil are used, the synergistic effects of these two drugs should be considered and used. A target heart rate of not greater than 70 beats/min can be achieved with the addition of an esmolol drip. Because surgical manipulation itself precipitates arrhythmias, antiarrhythmic medications (lidocaine, magnesium, or amiodarone) for treatment of these problems must be readily available. In addition to drugs, it is appropriate to let the surgeon know what impact surgical manipulations have on the myocardium and to ask the surgeon to stop temporarily, if possible, when the situation warrants it. If bradycardia becomes a problem, treatment with medications as well as epicardial or transvenous pacing may be necessary.
The use of antifibrinolytics is controversial because some surgeons are concerned about graft thrombosis associated with the use of these agents. Anticoagulant therapy is facility specific but usually directed at a target ACT of 300 seconds and incomplete coagulation reversal. When protamine is given, it is usually at a reduced dose to achieve an anticoagulation level 25% to 50% above the control ACT. When instilling protamine, the ACT should be checked one-third and two-thirds of the way through the dose to avoid overshooting the target ACT. In off-pump procedures, the coagulation system is normal because it has not been exposed to the ECC and its effects; therefore, the possibility of pulmonary embolus, graft clotting, and so on exists just as in other major vascular procedures.
For OPCAB patients to be extubated, they must be warm, awake, pain free, and receiving no or low-dose inotropes and vasoactive drugs; no balloon pump must be in use; bleeding must be less than 100 mL/hr; and patients must be hemodynamically stable and meet conventional metabolic and mechanical criteria for extubation. The table on pg. 260 shows some monitoring approaches for OPCAB and MIDCAB.
Monitoring Approaches for OPCAB and MIDCAB
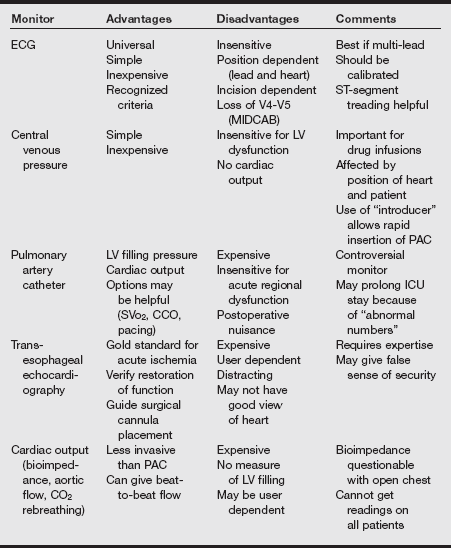
CCO, Continuous cardiac output; ICU, intensive care unit; LV, left ventricular, MIDCAB, minimally invasive direct coronary artery bypass grafting; OPCAB, off-pump coronary artery bypass grafting; PAC, pulmonary artery catheter, SVo2, mixed venous oxygen saturation.
From Hensley FA, Martin DE, Gravlee GP, eds. A Practical Approach to Cardiac Anesthesia. 2008. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
E Pacemakers
Indications
1. Sick sinus syndrome or symptomatic bradycardia.
a. AV block including third-degree heart block and type 2 second-degree heart block
b. Fascicular block including symptomatic bifascicular block and trifascicular block
3. Temporary pacemakers are indicated for same reasons as permanent pacemakers. They may serve as a bridge until permanent pacing is available or until the cardiac condition stabilizes, such as after coronary artery bypass grafting.
Preoperative assessment
1. Assess the patient’s current cardiac status and symptoms.
2. Determine the original reason for the pacemaker.
3. Determine the type of pacemaker and the settings. If unknown, the specific model number and manufacturer may be obtained by radiography of the pace generator.
4. Determine the location of the generator. Usually it is located in the upper chest, but occasionally it may be located in the abdomen.
5. Pacemaker wires may become easily dislodged in the first 6 weeks after placement. Central line placement may need to be done under fluoroscopy to avoid lead disruption.
6. Magnetic resonance imaging (MRI) is contraindicated in patients with pacemakers because of the potential for pacemakers to dysfunction unless the pacemaker is MRI compatible.
Perioperative management
Although most pacemakers now are resistant to electromagnetic interference (EMI) from the use of electrocautery, it can occasionally occur. The pacemaker output may be inhibited, or the pacemaker may be reset to a preset pacing mode (DOO or VOO).
If EMI occurs, a doughnut magnet can be applied directly over the pacemaker, which will cause it to convert to an asynchronous mode or a committed mode. Most pacemakers will automatically convert to an asynchronous mode when prolonged EMI is detected, so the magnet intervention is rarely needed.
Heart rate should be monitored with a precordial or esophageal stethoscope, pulse oximeter, or arterial line or by palpating the pulse during electrocautery.
Postoperatively, pacemaker function is not routinely checked unless interference was detected or the surgical procedure involved primary insertion or generator change.
Pacemaker insertion
Insertion sites can be the subclavian vein or through the cephalic vein in the deltopectoral groove. Leads are placed through the subclavian vein through the tricuspid valve into the right atrium or dual chamber leads may be placed with one in the right atrium and one in the right ventricle. Anesthetic requirements are usually local anesthesia administered by the surgeon and IV sedation.
Common complications during placement include dysrhythmias. Less common potential complications include pneumothorax, dislodging of electrodes, and cardiac tamponade.
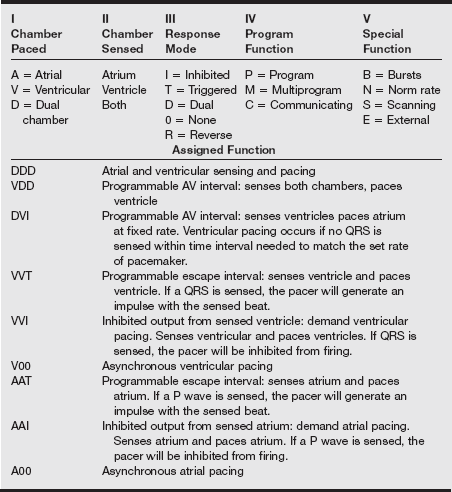
Pacemaker types (definitions and nomenclature)
Unipolar pacing involves a negative electrode in the atrium or ventricle and a positive ground far from the heart. These are rarely used and are more prone to interference from electrocautery and other devices such as microwaves.
Bipolar pacing involves placement of both electrodes in the chamber being paced or sensed. Asynchronous generators simply provide electrical impulses without sensing. Synchronous generators have both pulse-sensing and generating circuits. A five-letter code describes the functions and settings of the pacemaker (see the table on pg. 262).
Automatic internal cardiac defibrillator
Internal cardiac defibrillators (ICDs) are used in populations that are at high risk of sudden death from cardiac tachydysrhythmias.
Internal cardiac defibrillators consist of two defibrillating electrodes or patches and separate electrodes that are used for pacing and sensing. The patches are placed near the pericardium. The generator is implanted in either the abdomen or chest wall.
All ICDs are extremely sensitive to EMI used in electrocautery. EMI is detected as ventricular fibrillation by the device and delivers a shock to the patient. Magnet application will cause the device to suspend detection, but it will not interfere with the pacing function. Intraoperatively, if ventricular fibrillation occurs, the magnet can simply be removed, and the device can deliver a shock, usually within 10 seconds. External defibrillators should always be readily available in case of failure.
The ICD can be turned off manually for the duration of the operation before the patient is taken into the operating room, and it can be turned back on in the postanesthesia care unit. Personnel trained in the use of an external cardiac defibrillator should be readily available while the device is turned off.
If only bipolar cautery is used, the device can usually remain on.
F Valvular heart disease
Aortic stenosis
Disease of the aortic valve may present as aortic valvular stenosis, insufficiency, or a combination of both. Valvular heart disease is usually caused by rheumatic disease, but it may also occur secondary to calcific degeneration in elderly patients. Endocarditis and congenital abnormalities of the bicuspid valve account for most of the remainder. It is rarely possible to repair the aortic valve; therefore, most conditions require valve replacement.
Pathophysiology
Chronic obstruction to LV ejection results in concentric LV hypertrophy and myocardium that is highly susceptible to ischemia (even in the absence of coronary artery disease). Aortic stenosis is severe when the valve area is less than 0.6 cm2 and the pressure gradient is greater than 70 torr.
Hemodynamic goals
Left ventricular filling is dependent on atrial contractions, heart rate, and normal intravascular volume. Decreases in SVR are dangerous because of the fixed ventricular ejection; decreased SVR results in decreased blood pressure, coronary perfusion pressures, and resultant ischemia. Extreme and rapid increase in SVR increases LW workload and further decrease stroke volume through the stenotic aortic valve.
Dysrhythmias
Dysrhythmias should be aggressively treated. Because the ventricle is stiff, atrial contraction is critical for ventricular filling and stroke volume.
Anesthetic technique
1. Usually a high-dose narcotic technique is used: fentanyl, etomidate, and a muscle relaxant.
2. Avoid anesthetic agents that reduce vascular tone. Vasopressors should be available for induction.
3. Maintain intravascular volume and sinus rhythm.
4. Maintain heart rate; avoid decreased SVR and blood pressure.
5. External cardiac massage is not effective in these patients. Ventricular tachycardia and fibrillation are usually fatal.
Aortic regurgitation
Pathophysiology
The incompetent aortic valve results in a decrease in forward LV stroke volume because part of the ejected LV volume regurgitates back into the LV from the aorta, resulting in chronic volume overload of the LV and eccentric hypertrophy.
Aortic regurgitation causes a decrease in aortic diastolic blood pressure and decreased coronary artery perfusion pressures, resulting in subendocardial ischemia and angina (even in the absence of coronary artery disease). The magnitude of regurgitation is dependent on the duration of flow and the pressure gradient across the valve. Regurgitation can be reduced by increasing heart rate and decreasing SVR.
In chronic aortic regurgitation, as end-diastolic volume increases, stroke volume increases so that the EF is well maintained until LV failure occurs. When failure occurs, CO decreases, end-diastolic volume increases, and pulmonary edema results.
In acute aortic regurgitation, the sudden increase in LV volume without ventricular hypertrophy results in sudden cardiac failure.
Mitral stenosis
Pathophysiology
Increased left atrial pressure and volume overload occur as a result of the narrowed mitral orifice. Persistent increases in the left atrial pressure are reflected back through the pulmonary circulation, leading to right ventricular hypertrophy and failure, tricuspid regurgitation, and perivascular edema in the lungs. The left atrial enlargement predisposes the patient to formation of thrombi and systemic emboli, especially with the development of AF.
Anesthetic technique
Tachycardia results in inadequate LV filling and concomitant hypotension. Continued preoperative administration of digitalis and β-antagonists, the selection of anesthetics with a minimal propensity to increase heart rate, and achievement of an anesthetic depth sufficient to suppress sympathetic nervous system responses are recommended.
Induction agents should be administered slowly to avoid drug-induced reductions in SVR and resultant hypotension in the presence of a fixed LV stroke volume. Avoid ketamine because of the increase in heart rate associated with this drug.
Preoxygenation and brief laryngoscopy reduce the potential for hypoxia, hypercarbia, and acidosis. (These potentiate pulmonary vasoconstriction, which will potentiate right-sided heart failure.) Avoid increases in heart rate; avoid decreases in myocardial contractility, SVR, and blood pressure.
Mitral regurgitation
Pathophysiology
Chronic volume overload of the left atrium occurs, resulting in a decreased LV stroke volume because of part of the stroke volume’s regurgitation through the incompetent valve. The increase in left atrial pressure results in elevated pulmonary pressures and right-sided heart failure. LV hypertrophy results to compensate for the decreased CO. PA capillary wedge pressure overestimates LV end-diastolic pressure. The amount of regurgitation depends on the size of the valve orifice, the heart rate, and the pressure gradient across the valve.
Mild increases in heart rate improve LV stroke volume. Bradycardia results in acute volume overload of the left atrium.
The drug of choice for hypotension is ephedrine.
The pressure gradient across the valve is determined by the compliance of the LV and the impedance to LV ejection into the aorta. Reducing SVR can improve forward flow.
Anesthetic technique
1. Select agents that promote vasodilation and increase heart rate.
2. Avoid myocardial depression, which will decrease CO.
3. Barbiturates, benzodiazepines, etomidate, and succinylcholine are good choices.
4. Maintain normocarbia and oxygen saturation to prevent increases in pulmonary hypertension associated with pulmonary vasoconstriction.