CHAPTER 93 Syringomyelia
Syringomyelia, or cavitation within the substance of the spinal cord without an ependymal lining, has been recognized for more than 300 years as a pathologic entity. Etienne is credited for the first pathologic description in 1564 in La Dissection du Corps Humain; he described a cystic lesion in the spinal cord that contained a “fluid, reddish, like the fluidity of that of the ventricles.”1
Portal, in 1804, first appreciated and connected the clinical syndrome of an intramedullary cyst with the pathologic changes of the spinal cord.2 Ollivier then coined the term “syringomyelia,” combining the Greek words for “tube or pipe” and “marrow.” He documented a connection between the fourth ventricle and this cystic structure, which he believed to be a congenital anomaly.1,3,4
Those ependymal-lined cavities that appeared to be pathologic dilatations of the central canal were termed hydromyelia. Some authors viewed hydromyelia, in which the central canal was dilated but preserved, and syringomyelia, with or without a connection to the central canal, as stages of a common process. Hence unification of the terms resulted in the concept of syringohydromyelia or hydrosyringomyelia.1–35
In 1973 Barnett published the first English-language monograph on syringomyelia.6 He proposed a classification based on a variety of clinical and experimental observations and studies. The classification scheme consisted of two broad categories: (1) communicating syringomyelia (e.g., Chiari I malformation, Chiari II malformation, basilar arachnoiditis) and (2) noncommunicating syringomyelia (e.g., occurring with spinal dysraphism, spinal cord trauma, spinal cord tumor, spinal arachnoiditis) (Box 93–1).
Recent experimental and clinical work including that of Oldfield and Milhorat and their colleagues7–13 has helped to clarify the pathophysiology and treatment of this complex syndrome.
Etiology, Pathology, Pathophysiology, Prominent Theories
Historical Perspective—Early Theories of Syringomyelia
Although the pathogenesis of syringomyelia has not yet been completely defined, the association between syringomyelia and congenital abnormalities was appreciated long ago. Tamaki and Lubin14 credited Baulmer of establishing this relationship in 1887. Poser noted that Schlesinger’s 1895 monograph stated that there was an associated congenital abnormality in one third of the cases of syringomyelia that he had reviewed.15
Ollivier d’ Angers formulated and Leyden further refined the developmental theory of syringomyelia formation.15,16 These authors stated that syringomyelia must be considered a congenital disorder associated with embryonic maldevelopment, specifically incomplete occlusion of the primitive fold. This improper fusion of the two folds of the primitive medullary groove allowed the abnormal lining of germinal cells to persist, resulting in simple hydromyelia.
Another theory from the congenital viewpoint was that there was a problem inherent in the environment to which the fetus was exposed. Kahler and Pick16 in 1879 theorized that chronic intrauterine inflammation resulted in gliosis and aberrant development of the spinal cord that subsequently led to syrinx formation. In 1910 Haener,16 on the other hand, proposed that events during the act of birth (e.g., trauma) may arouse neural activity in abnormally enclosed tissue, with resultant syrinx formation.
W.J. Gardner—Hydrodynamic Theory “Water Hammer”
In a series of landmark papers, W.J. Gardner expounded his hydrodynamic theory of the pathogenesis of syringomyelia.17–23 Gardner’s theory was the first among three prominent theories to survive up to modern clinical practice. He based his theory on three observations: (1) dye injected into the ventricular system was recovered from the syrinx at operation, (2) fluid withdrawn from the syrinx at operation strongly resembled cerebrospinal fluid (CSF) found in the ventricular system, and (3) experimental hydrocephalus produced by obstruction of the normal outflow of CSF from the fourth ventricle resulted in the formation of syringomyelia that was in communication with the ventricular system.24
The pulse wave effect of the diverted CSF acted as a water hammer, gradually dilating the central canal or dissecting the substance of the spinal cord around the canal and creating a syrinx. From the perspective of Gardner, a congenital hindbrain defect that obstructed the CSF flow from the fourth ventricle to the subarachnoid space was the sine qua non of syringomyelia. Ball and Dayan25 and West and Williams26 questioned Gardner’s theory and the necessity of a direct communication to the fourth ventricle for production of a syrinx. To support this statement, Milhorat and colleagues7,27 demonstrated in large autopsy studies that the majority of syrinxes did not communicate with the fourth ventricle and that the central canal was not patent in most normal adult patients.
B. Williams—Craniospinal Pressure Dissociation Theory
Williams also believed that the cavity enlarged after its initial formation as the result of compression of the lower end of the cavity with the rapid filling of the epidural venous plexus during a cough or sneeze. The fluid in the syrinx was then propelled rostrally, dissecting the central canal or pericentral parenchyma of the spinal cord. Williams applied the term “slosh” to this part of his theory to explain syrinx extension.28
E. Oldfield—Abnormal Pulse Wave Theory
Oldfield and colleagues13 used magnetic resonance imaging (MRI) with and without cardiac gating, intraoperative ultrasonography, and direct intraoperative observation of the exposed hindbrain and documented the downward movement of the cerebellar tonsils during systole. This group interpreted the data as obviating the necessity of a direct communication with the fourth ventricle, as advocated by Gardner. Moreover, they observed that the syringomyelic cord did not enlarge with Valsalva maneuver and that venous pressure had little to do with syrinx elongation, disputing the Williams “suck and slosh” theory. The authors proposed that the abnormal pulse wave in the spinal subarachnoid space, caused by the partial obstruction by the hindbrain, placed pressure on the spinal cord and dissected the central canal, causing the cyst to enlarge.
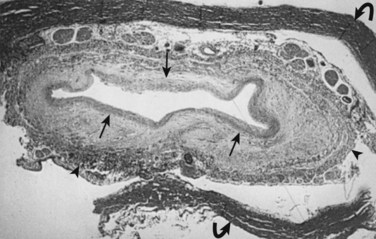
Milhorat and colleagues12 proposed that normal CSF flow was from the spinal subarachnoid space through the parenchyma of the spinal cord into the central canal. The CSF then flowed into the fourth ventricle outlet at the obex. Their theory was supported with a rodent model of syringomyelia. They injected kaolin into the central canal of rats, causing stenosis of the proximal central canal through an inflammatory reaction. A resultant syrinx was formed. The authors suggested that syrinx formation was due to disruption of normal CSF flow by the inflammatory stenosis (Fig. 93–1).
Communicating Syringomyelia
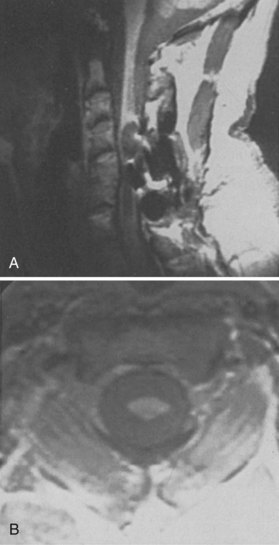
In 1896 Chiari published an addendum to an earlier work in which he described anomalies associated with hydrocephalus. In this latter publication, there were descriptions of patients with hydromyelia. Gardner and Goodall found that a majority of patients undergoing surgical decompression for symptomatic Chiari I malformation had a concurrent syringomyelia (Fig. 93–2).29 Gardner and colleagues30 demonstrated, at operation, communication between the syrinx of the upper cervical cord and the ventricles in patients undergoing suboccipital craniectomy and cervical laminectomy for decompression. Indigo-carmine was injected into the patient’s lateral ventricle, and colored CSF was recovered by direct puncture of the cervical syrinx.
Appleby and colleagues31 established that a “communicating” type of syringomyelia could also be acquired from chronic arachnoiditis involving the basal cisterns and obstructing the outflow of CSF from the fourth ventricle.
Noncommunicating Syringomyelia
Syringomyelia Associated with Spinal Arachnoiditis
The association between spinal arachnoiditis and syringomyelia was first reported by Vulpian in 1861 and by Charcot and Joffroy in 1869. Some authors believed occlusion of blood vessels supplying the cord from profound arachnoid scarring was the underlying pathophysiologic process for intramedullary cavitation.32–34
As previously described, Williams implicated craniospinal pressure dissociation, secondary to obstruction of the subarachnoid space, as the factor responsible for cyst formation and extension in this disease entity.35 In 2004 Chang and colleagues36 explained that the blockage of the spinal subarachnoid CSF pathway produces a relative pressure gradient inside the spinal cord distal to the blockage point that induces CSF leakage into the spinal parenchyma and the formation of syringomyelia. Barnett37 and Milhorat7 considered this type of syringomyelia to be of the “noncommunicating” type because no connection between the cyst and fourth ventricle could be demonstrated., Koyanagi and colleagues38 reviewed a series of 15 patients who underwent various shunting procedures for syrinx treatment caused by spinal arachnoiditis. Although neurologic improvement was found in a decent percentage of patients (60%), many required additional shunting procedures over time due to catheter blockage or failure.
Syringomyelia Associated with Spinal Cord Tumors
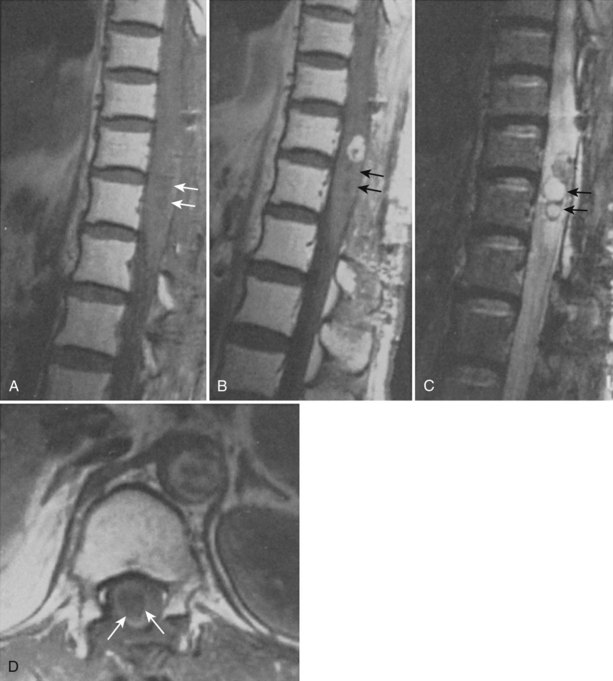
The association between syringomyelia and spinal cord tumors has been well established. Simon in 1875 was the first to report the simultaneous occurrence of syringomyelia and spinal cord tumors.39 Proposed mechanisms for syrinx formation in this environment include (1) edema, (2) blockage of the perivascular spaces with resultant tissue fluid stasis, (3) cavitation secondary to disturbance of blood supply to the spinal cord, and (4) spontaneous hemorrhage or autolysis of the mass.33,40,41 Other authors contend that syringomyelia associated with a spinal cord tumor is due to a direct effect of the neoplasm.15,39,42–44 Some authors believed that this disordered gliosis observed with tumor presence was also the underlying pathophysiologic cause even in cases not associated with an intramedullary neoplasm; this led some physicians to recommend radiation therapy as a rational but extreme form of primary therapy.1,45–48 Today, the use of radiation therapy should be restricted only for primary therapy of a known neoplasm or as an adjunct to surgical resection of tumor (Fig. 93–3).
Spinal CSF Dynamics in the Presence of a Neoplasm
Total or subtotal obstruction of CSF flow by an intramedullary or sometimes extramedullary/intradural tumors may be a significant factor for the development of syringomyelia. The subarachnoid and the extracellular space of the central nervous system should be considered as a single-fluid compartment with no barrier to fluid movement between them. Interference with this normal CSF flow influences extracellular fluid flow out of the spinal cord. The high ratio of syrinx cavities associated with intramedullary tumors may be attributable to their simultaneous influence on the subarachnoid and extracellular spaces. Fluid transudation from tumor vessels, breakdown products of tumor cells, and in some cases active secretion also raise the protein content and thus the viscosity of the extracellular fluid contributing to further aberrances on normal flow dynamics.49 Less commonly, intramedullary cysts can present in association with an extramedullary neurofibroma or meningioma. The removal of the extramedullary mass lesion is usually followed by a spontaneous collapse of the associated syrinx.
Syringomyelia and Spinal Cord Tumors—Clinical Studies
In a surgical series of 100 intramedullary tumors, 45% presented with associated syringes.49 A syrinx was more likely to be found above than below the tumor level. Ependymomas and hemangioblastomas were the most common tumor types to be associated with syringes. Astrocytomas, on the other hand, tended to demonstrate syringes less often. The higher the spinal level, the more likely a syrinx was encountered.
Syringomyelia Associated with Spinal Cord Trauma
The pathophysiologic basis for the formation and extension of syrinxes of the injured spinal cord remains the subject of considerable debate. However, a constant factor in all cases is the alteration of normal CSF flow dynamics. The onset of signs and symptoms of progressive post-traumatic cystic myelopathy ranges from as early as 2 to 3 months following injury to as long as 30 years after injury.50 Approximately 4% to 10% of patients suffering a traumatic spinal cord injury develop progressive spinal cord dysfunction associated with an expanding syrinx.23,51,52
Post-traumatic Syringomyelia—Human Studies
Milhorat’s large autopsy study demonstrated that syrinxes associated with trauma had distinctly different histopathologic findings and were associated with different clinical symptoms when compared with those lesions that were in communication with the fourth ventricle or those cavities that appeared to be isolated dilatations of the central canal.7 These syringes involved the parenchyma of the cord asymmetrically, were not associated with the central canal, and often extended to the pial surface. Examination of pathologic specimens revealed nonreversible damage to spinal nuclei and tracts including focal necrosis, central chromatolysis, and wallerian degeneration.
Post-traumatic Syringomyelia—Mechanisms of Development
The possible factors implicated in the production of the initial cystic lesions in post-traumatic spinal cords included ischemia secondary to arterial and/or venous obstruction, tissue breakdown from lysosomes or other intracellular enzymes, liquefaction of a prior hematoma, mechanical damage from compression of the substance of the cord at the time of initial injury, or tethering by delayed formation of extensive subarachnoid adhesions and/or a bony gibbus.53–61
Similarly, the mechanism for the extension of the syringomyelia remains a matter of controversy leading to several mechanisms being proposed. Some view the rostral and caudal extension of the syrinx, which produces late neurologic symptoms, being a result of a one-way valvelike trapping effect of the subarachnoid space into the cavity.11,62–66 Less frequently observed presentations of post-traumatic cysts include patients with a history of a herniated cervical or thoracic disc with resultant ventral compression from a bony or soft gibbus and disturbance of normal CSF flow. Spinal puncture with injection of an irritative dye (e.g., methylene blue) can cause an ascending arachnoiditis that may develop associated subarachnoid and/or intramedullary cysts. Patients who have subarachnoid hemorrhage may also develop spinal cord tethering with associated intramedullary or subarachnoid cysts.
One author has suggested that post-traumatic syringomyelia may be classified into two types. These authors contend that successful reestablishment of normal CSF flow dynamics by untethering the spinal cord stops the progression of clinical decline and in some cases may return valuable lost function. A preoperative distinction could be made on the basis of the presence (high-pressure type) or absence (low-pressure type) of the flow-void sign on a T2-weighted magnetic resonance image. With a midline myelotomy, fluid within a high-pressure syrinx would pour out, resulting in sustained neurologic improvement. In the low-pressure type, drainage of the syrinx would not collapse the expanded spinal cord and the surgical outcome would be modest at best.67
Clinical Features
Hydrocephalus may be found in 10% to 33% of patients but is more likely related to an associated Chiari malformation.26 This frequent association supports the concept of a hydrodynamic mechanism for the formation of syringomyelia.
Syringomyelia—Physical Examination
The autonomic pathways of the interomediolateral column may be affected, resulting in Horner syndrome, trophic changes of the skin, a neurogenic bladder, and dyshidrosis. Corticospinal tract involvement may give spastic paraparesis. Patients experience a loss of deep tendon reflexes in the upper extremities. A skeletal survey may reveal congenital anomalies including basilar impression and invagination, Klippel-Feil deformity, and spina bifida occurring primarily at C1.68 Developmental scoliosis may occur as a result of cord cavitation and is probably not an unassociated congenital lesion.69 Although discussed extensively in the literature, painless joint destruction (Charcot joints) occur in less than 5% of patients with syringomyelia.70
The clinical course of syringomyelia is usually insidious, worsening over several years to decades. Alternatively, the patient may worsen in an abrupt stepwise fashion punctuated by intervals of clinical stability.71 The prognosis of untreated syringomyelia is compatible with modified but productive survival for decades in 50% of surviving patients.72 The remainder become incapacitated and die as a direct result of the pathologic process. Seki and Fehlings73 designed a rodent model to further elucidate the role of post-traumatic syringomyelia in spinal cord injury.
Diagnosis
Diagnosis of syringomyelia was based on clinical presentation and course in the late 19th and early 20th centuries. This diagnosis represented a difficult task for physicians of that time because the disorder had an insidious onset and a variable clinical course, and modern imaging techniques were unavailable. Intramedullary neoplasms, as well as demyelinating diseases (multiple sclerosis, amyotrophic lateral sclerosis), disorders of metabolism and nutrition (subacute combined degeneration in vitamin B12 deficiency), infectious causes of spinal cord dysfunction (tabes dorsalis from syphilis), and degenerative disease (cervical spondylosis leading to stenosis and cervical myelopathy), could all serve as confusing mimickers of a syringomyelic process.1 In a majority of patients, the classic finding of segmental weakness and atrophy of the hands and arms, with loss of tendon reflexes and segmental dissociated sensory loss, could not be found.74
Imaging and Electrophysiologic Evaluation in Syringomyelia Diagnosis
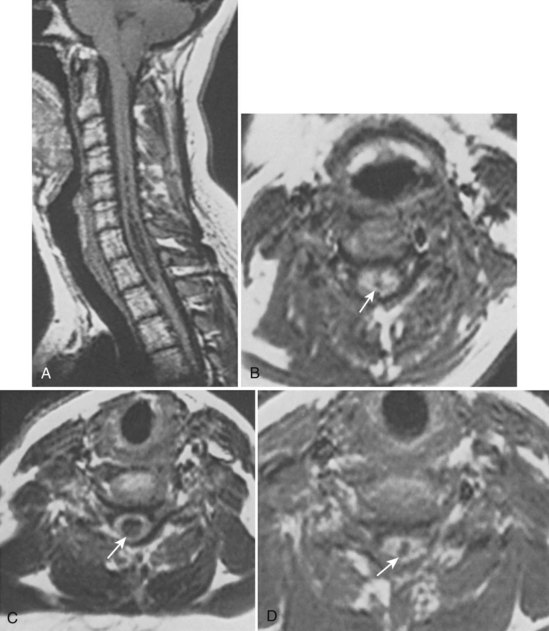
Currently, the diagnostic test of choice for syringomyelia is the MRI.75 The initial MRI examination includes, as a minimum, sagittal and transverse views of the lesion plus the adjacent spinal cord and/or brainstem in T1-weighted images. The addition of T2-weighted and/or mixed proton density images can complement the T1-weighted images (Fig. 93–4). Gadolinium-enhanced images are considered an essential part of the workup to detect tumors and differentiate between scar and disc material, especially in postoperative or post-traumatic cases.76 Inclusion of the entire rostrocaudal extent of each cyst or cysts is important.
In addition to conventional MRI, a new generation of MRI software technology is currently available. Cine MRI is a real-time, motion picture–like analysis of spinal fluid flow dynamics in and around the spinal cord cyst.15 Magnetic resonance angiography (MRA) is another technologic advance that can be helpful in cases of syringomyelia associated with vascular lesions.
Spinal instrumentation, bullet fragments, or the inability of the patient to tolerate the enclosed space of the scanner degrades the ability of the MRI to provide adequate anatomic assessment. In these cases, a myelogram in combination with immediate and delayed high-resolution CT is performed. Difficulty in differentiating myelomalacia or an intramedullary tumor from a confluent syrinx represents a limitation of this technique.77
Roser and colleagues78 have used electrophysiologic monitoring (e.g., the presence of silent periods with analysis of spino-thalamic pathways) to assist in early diagnosis of syringomyelia that will likely become symptomatic and select out patients with benign hydromyelia. In a follow-up clinical study this group reviewed a group of patients with idiopathic syringes (e.g., not caused from Chiari I malformations, tumors, cranio-cervical junction abnormalities, scoliosis, trauma).79 Conduction studies performed included somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), and detection of spino-thalamic pain pathways with silent period studies were all negative in this idiopathic group. The major presenting symptom of these patients was pain (e.g., radicular, neuropathic, musculoskeletal), which prompted the original MRI examination. Over time these patients tend to do better with conservative treatment than those in nonidiopathic groups.80
Treatment
The treatment of syringomyelia continues to be controversial. There is no universally accepted method of intervention or consensus on the benefit of therapy. The first report of successful surgical therapy of syringomyelia was by Abbe and Coley in 1892.81 They performed a three-level laminectomy and opened the dura to expose the cyst and aspirated clear fluid from the lesion, which then collapsed. This report was significant not because of clinical improvement with cyst drainage but because it showed that syringomyelia could be approached surgically without morbidity.
Direct Approach to Noncommunicating Syringomyelia
Methods
If surgery for syringomyelia-associated spinal arachnoiditis and deteriorating neurologic status is attempted, then the reconstruction of an alternative subarachnoid pathway around the area of adhesion is essential.70 A wide laminectomy, intradural exploration with limited dissection of adhesions to establish free communication with the subarachnoid space above and below the area of adhesion, and an expansile duraplasty are performed.
Preoperative drainage of an intramedullary cyst may be accompanied by rapid neurologic improvement, whereas surgical removal of an associated tumor routinely decompresses the syrinx.59 One must also be attentive to delayed development of syringomyelia after spinal surgery because it may account for postoperative neurologic deterioration in some patients.82
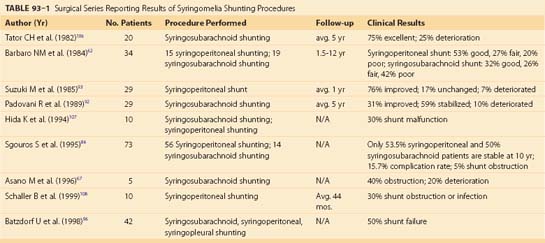
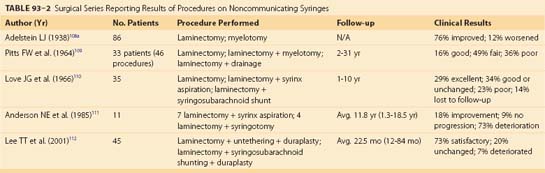
Surgical intervention for a post-traumatic cyst was first undertaken with good results by Freeman in 1955.83 An essential component of the surgery is untethering the spinal cord and nerve root adhesions, as well as reduction of any bony gibbus or soft tissue mass at the site of injury. This may first require a posterior approach for intradural exploration and untethering of the cord and root adhesions, followed by an anterior approach to remove any bony gibbus or soft tissue mass. Others include reconstruction of the subarachnoid space with a surgical meningocele and the addition of a syringosubarachnoid shunt in order to maintain the patency of the cyst in the subarachnoid space.62,84,85 However, shunt failure rates as high as 5% to 50% have been reported (Table 93–1). The introduction of a foreign body in the form of a shunt tube, as well as surgical trauma, may also result in fixation of the cord to the dura by scar tissue and neurologic deterioration, therefore negating a lasting benefit from an untethering procedure.77
Poussepp and Elsberg86,87 have been given credit for development of the midline myelotomy (the “Elsberg-Poussepp operation”) for treatment of spinal cord cysts. Frazier, on the other hand, performed a paramedian myelotomy attempting to marsupialize the syrinx into the subarachnoid space.88
Results
A recent review of surgery, through various approaches and techniques, for syringomyelia found that the reported effectiveness of surgical intervention had not changed in the past 100 years (except for a reduction in the observed mortality rate), with 67% to 76% of patients improving neurologically postoperatively.89,90 Barnett, in contrast, reported that management of patients with arachnoiditis was unrewarding, whether medical or surgical therapy was attempted. He considered both steroids and lysis of adhesions to be ineffective in the treatment of this disorder.
A recent article by Falci and colleagues91 reported on a large surgical series of posttraumatic spinal cord tethering patients (many with syrinxes) at a single center over the course of 10 years. Surgeries were performed by a single surgeon using a consistent technique of spinal cord detethering, expansile duraplasty, and when indicated, cyst shunting. No significant change was noted on preoperative versus postoperative American Spinal Injury Association sensory and motor index scores; however, outcome questionnaires resulted in significant benefits of self-assessment of arrest of functional loss and improvement of motor or sensory complaints.
Although effective in a number of small surgical series, the placement of a syringoperitoneal or syringosubarachnoid shunt is of questionable value.* The optimal treatment and determination of the indications for surgery await further research with relevant experimental models and a well-designed, multicenter clinical trial.89
Tables 93-1 and 93-2 list some of the major surgical series in treating noncommunicating syringomyelia, represented most prominently by post-traumatic syringomyelia, with lysis of adhesions and/or shunting of the syrinx. Overall, they show surgical treatment of post-traumatic syringomyelia to be of modest reward. Many patients show short-term recovery of neurologic function, only to deteriorate again in long-term follow-up.
Indirect Approach to Communicating Syringomyelia
Methods
Gardner94 championed a new approach to the patient with a posterior fossa herniation (Chiari I malformation) on the basis of his hydrodynamic theory of syringomyelia formation. The operation he advocated was a suboccipital craniectomy for decompression of the cerebellar tonsillar herniation and closure of the communication between the syrinx and the fourth ventricle by plugging the obex with a piece of muscle.
Others have questioned the necessity and advisability of using an obex plug. Batzdorf argued that a plug in the obex was not necessary and potentially blocked a drainage path for the syrinx following bony decompression at the skull base and expansile duraplasty.95,96 In addition, he advocated a limited suboccipital craniectomy to prevent downward herniation of the cerebellar tonsils postoperatively.
Tonsillar resection has been proposed as a useful adjunct to foramen magnum decompression to obtain simultaneous restoration of free CSF flow at the foramen of Magendie, hindbrain decompression, and relief of the “piston-like” action of the tonsils. Several important issues aiming at prevention of postoperative arachnoiditis are to be outlined: (1) meticulous dissection of the pia mater covering the tonsils from the arachnoid layer, (2) subpial aspiration of the tonsils using the ultrasonic aspirator and creating a wide aperture in the lower fourth ventricle, and (3) suture of the arachnoid and duraplasty using a pericranial graft.97,98 However, one study found that none of the patients who had coagulation of their tonsils showed clinical improvement, versus 77% of patients without tonsillar manipulation.25
The majority of syringes associated with hindbrain malformations are not communicating but are isolated both rostrally and caudally by central canal stenosis. A posterior decompression alone may not effectively treat the syringes. An additional drainage-type procedure may be effective in these specific cases.9,99–101 A long-term follow-up study (median = 88 months) by Aghakhani and colleagues102 followed 157 surgically treated patients with Chiari I malformation and syringomyelia. More than 90% of the individuals showed improvement or stabilization in both clinical grade and syrinx size following surgery. Clinical worsening was more likely with greater age, increased syrinx size, severe motor deficits, or arachnoiditis at diagnosis.
The main objective in the surgical treatment of Chiari I malformation and related syringomyelia is to restore normal CSF dynamics at the craniovertebral junction by posterior fossa decompression. However, this decompression should be small enough to avoid downward migration of the hindbrain into the craniectomy defect. Current techniques to achieve these goals include not only posterior fossa decompression but also posterior fossa reconstruction.103,104
One study questioned the need for early interventional surgery for incidentally identified syringomyelia associated with Chiari I malformations.105 The long-term clinical courses of patients with asymptomatic, incidentally identified syringomyelia associated with Chiari I malformation were observed to be benign. The study concluded that unless changes in neurologic or MRI findings are detected, early interventional surgery is not necessary.
Results
Table 93–3 lists some of the large surgical series in treating communicating syringomyelia, represented by syringomyelia associated with Chiari I malformations. Overall, these studies show that posterior fossa decompression and/or reconstruction through a variety of techniques leads to effective neurologic improvement or halting of neurologic deterioration in 60% to 90% of cases.
TABLE 93–3 Surgical Series Reporting Result of Procedures on Chiari Malformations/Communicating Syringomyelia
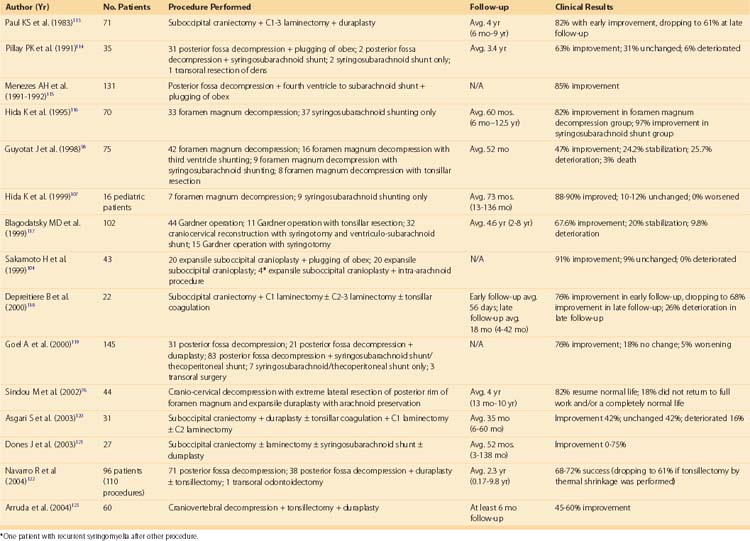
Because substantial differences among the reported methods advocated by groups treating syringomyelia continue, a multicenter clinical trial must be organized before optimal treatment can be established.89
Conclusions
Pearls
Pitfalls
Key Points
1 Gardner WJ. Anatomic anomalies common to myelomeningocele of infancy and syringomyelia of adulthood suggest a common origin. Cleve Clin Q. 1959;26:118-133.
2 Williams B. On the pathogenesis of syringomyelia: a review. J R Soc Med. 1980;73:798-806.
3 Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
4 Falci S, Charlotte I, Lammertse D. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. J Neurosurg Spine. 2009;11:445-460.
5 Schurch B, Wichmann W, Rossier AB. Posttraumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61.
1 Finlayson A. Syringomyelia and related conditions. In: Joynt RJ, editor. Clinical Neurology, Vol 3. Philadelphia: JB Lippincott; 1989:1-17.
2 Williams B. Progress in syringomyelia. Neurol Res. 1986;8:130-145.
3 Ballantine H, Ojemann R, Drew J. Syringomyelia. In: Kraybuhl H, Maspes PE, Sweet WH, editors. Progress in Neurological Surgery, Vol 4. New York: S. Karger; 1971:220-244.
4 Foster JB, Hudgson P. Historical introduction. London, Philadelphia: Saunders; 1973.
5 Hoffman HJ, Neill J, Crone KR, et al. Hydrosyringomyelia and its management in childhood. Neurosurgery. 1987;21:347-351.
6 Barnett HJM. The epilogue. In: Barnett HJM, Foster JB, Hudgson P, editors. Syringomyelia. Major problems in neurology. Philadelphia: Saunders; 1973:302-313.
7 Milhorat TH, Capocelli ALJr., et al. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802-812.
8 Milhorat TH, Johnson RW, Milhorat RH, et al. Clinicopathological correlations in syringomyelia using axial magnetic resonance imaging. Neurosurgery. 1995;37:206-213.
9 Milhorat TH, Johnson WD, Miller JI. Syrinx shunt to posterior fossa cisterns (syringocisternostomy) for bypassing obstructions of upper cervical theca. J Neurosurg. 1992;77:871-874.
10 Milhorat TH, Johnson WD, Miller JI, et al. Surgical treatment of syringomyelia based on magnetic resonance imaging criteria. Neurosurgery. 1992;31:231-244. discussion 244-235
11 Milhorat TH, Miller JI, Johnson WD, et al. Anatomical basis of syringomyelia occurring with hindbrain lesions. Neurosurgery. 1993;32:748-754. discussion 754
12 Milhorat TH, Nobandegani F, Miller JI, Rao C. Noncommunicating syringomyelia following occlusion of central canal in rats. Experimental model and histological findings. J Neurosurg. 1993;78:274-279.
13 Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
14 Tamaki K, Lubin A. Pathogenesis of syringomyelia: case illustrating the process of cavity formation from embryonic cell rests. Arch Neurol Psychiatry. 1938;40:748.
15 Poser C. The relationship between syringomyelia and neoplasm. Springfield, IL: Charles C. Thomas; 1956.
16 MacKay R, Favill J. Syringomyelia and intramedullary tumor of the spinal cord. Arch Neurol Psychiatry. 1935;33:1255.
17 Gardner WJ. Anatomic anomalies common to myelomeningocele of infancy and syringomyelia of adulthood suggest a common origin. Cleve Clin Q. 1959;26:118-133.
18 Gardner WJ. Diastematomyelia and the Klippel-Feil Syndrome. Relationship to Hydrocephalus, Syringomyelia, Meningocele, Meningomyelocele, and Iniencephalus. Cleve Clin Q. 1964;31:19-44.
19 Gardner WJ. Hydrodynamic Mechanism of Syringomyelia: Its Relationship to Myelocele. J Neurol Neurosurg Psychiatry. 1965;28:247-259.
20 Gardner WJ. Myelocele: rupture of the neural tube? Clin Neurosurg. 1968;15:57-79.
21 Gardner WJ, Angel J. The mechanism of syringomyelia and its surgical correction. Clin Neurosurg. 1958;6:131-140.
22 Gardner WJ, McMurray FG. “Non-communicating” syringomyelia: a non-existent entity. Surg Neurol. 1976;6:251-256.
23 Griffiths ER, McCormick CC. Post-traumatic syringomyelia (cystic myelopathy). Paraplegia. 1981;19:81-88.
24 McLaurin R, Bailey OT, Schurr PH, Ingraham FD. Myelomalacia and multiple cavitations of spinal cord secondary to adhesive arachnoiditis; an experimental study. AMA Arch Pathol. 1954;57:138-146.
25 Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet. 1972;2:799-801.
26 West R, Williams B. Radiographic studies of the ventricles in syringomyelia. Neuroradiology. 1980;20:5.
27 Milhorat TH, Kotzen RM, Anzil AP. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg. 1994;80:716-722.
28 Williams B. On the pathogenesis of syringomyelia: a review. J R Soc Med. 1980;73:798-806.
29 Gardner WJ, Goodall RJ. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7:199-206.
30 Gardner WJ, Abdullah AF, Mc CL. The varying expressions of embryonal atresia of the fourth ventricle in adults: Arnold-Chiari malformation, Dandy-Walker syndrome, arachnoid cyst of the cerebellum, and syringomyelia. J Neurosurg. 1957;14:591-605.
31 Appleby A, Bradley W, Foster J, et al. Syringomyelia due to chronic arachnoiditis at the foramen magnum. J Neurol Sci. 3, 1969.
32 Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990;53:106-113.
33 Liber A, Lisa J. Rosenthal fibers in non-neoplastic syringomyelia; a note on the pathogenesis of syringomyelia. J Nerv Ment Dis. 1937;86:549.
34 Nelson J. Intramedullary cavitation resulting from adhesive spinal arachnoiditis. AMA Arch Neurol Psychiatry. 1943;50:1.
35 Williams B. The distending force in the production of “communicating syringomyelia.”. Lancet. 1969;2:189-193.
36 Chang HS, Nakagawa H. Theoretical analysis of the pathophysiology of syringomyelia associated with adhesive arachnoiditis. J Neurol Neurosurg Psychiatry. 2004;75:754-757.
37 Barnett HJM. Syringomyelia associated with spinal arachnoiditis. In: Barnett HJM, Foster JB, Hudgson P, editors. Syringomyelia. Major problems in neurology. Philadelphia: Saunders; 1973:220-244.
38 Koyanagi I, Iwasaki Y, Hida K, Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005;63:350-355. discussion 355-356
39 Barnett HJM, Rewcastle N. Syringomyelia and tumors of the nervous system. In: Barnett HJM, Foster J, Hudgson P, editors. Syringomyelia, Major problems in neurology. London, Philadelphia: Saunders; 1973:261-301.
40 Feigin I, Ogata J, Budzilovich G. Syringomyelia: the role of edema in its pathogenesis. J Neuropathol Exp Neurol. 1971;30:216-232.
41 Russell D. Capillary hemangiomas of the spinal cord associated with syringomyelia. J Pathol Bacteriol. 1932;35:103.
42 Ferry DJ, Hardman JM, Earle KM. Syringomyelia and intramedullary neoplasms. Med Ann Dist Columbia. 1969;38:363-365.
43 Peerless SJ, Durward QJ. Management of syringomyelia: a pathophysiological approach. Clin Neurosurg. 1983;30:531-576.
44 Slooff J, Kernohan J, MacCarty C. Primary Intramedullary Tumors of the Spinal Cord and Filum Terminale. Philadelphia: WB Saunders Co.; 1964.
45 Conway LW. Hydrodynamic studies in syringomyelia. J Neurosurg. 1967;27:501-514.
46 Putnam T. Syringomyelia-diagnosis and treatment. Med Clin North Am. 1936;19:1571.
47 Wechsler I. Syringomyelia (including spinal gliosis). In: A Textbook of Clinical Neurology. Philadelphia: WB Saunders Co; 1927:159-164.
48 Wilson S. Syringomyelia: Syringobulbia. In: Bruce AN, editor. Neurology, Vol 2. Baltimore: Williams and Wilkins; 1955:1187-1202.
49 Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 1994;35:865-873. discussion 873
50 Hida K, Iwasaki Y, Imamura H, Abe H. Posttraumatic syringomyelia: its characteristic magnetic resonance imaging findings and surgical management. Neurosurgery. 1994;35:886-891. discussion 891
51 Rossier AB, Foo D, Shillito J, Dyro FM. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108(Pt 2):439-461.
52 Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61-67.
53 Cushing H. Haematomyelia from gunshot wounds of the spine: a report of two cases, with recovery following symptoms of hemilesion of the cord. Am J Med Sci. 1891;115:654.
54 Fairholm D, Turnbull I. Microangiographic study of experimental spinal injuries in dogs and rabbits. Surg Forum. 1970;21:453-455.
55 Holmes G. The Goulstonian lectures on spinal injuries of warfare: Part I The pathology of acute spinal injury. Br Med J. 1915;2:769.
56 Kao CC, Chang LW, Bloodworth JM. The mechanism of spinal cord cavitation following spinal cord transection. Part 3: Delayed grafting with and without spinal cord retransection. J Neurosurg. 1977;46:757-766.
57 Kao CC, Chang LW, Bloodworth JMJr. The mechanism of spinal cord cavitation following spinal cord transection. Part 2. Electron microscopic observations. J Neurosurg. 1977;46:745-756.
58 Mair WG, Druckman R. The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs; a report of four cases. Brain. 1953;76:70-91.
59 McVeigh J. Experimental cord crushes: with special reference to the mechanical factors involved and subsequent changes in the areas of the cord affected. Arch Surg. 1923;7:573.
60 Wolman L. The Disturbance of Circulation in Traumatic Paraplegia in Acute and Late Stages: a Pathological Study. Paraplegia. 1965;2:213-226.
61 Woodard JS, Freeman LW. Ischemia of the spinal cord; an experimental study. J Neurosurg. 1956;13:63-72.
62 Barbaro NM, Wilson CB, Gutin PH, Edwards MS. Surgical treatment of syringomyelia. Favorable results with syringoperitoneal shunting. J Neurosurg. 1984;61:531-538.
63 Hughes J. Spinal cord dysfunction: Assessment, in Oxford medical publications. In: Illis LS, editor. Spinal cord dysfunction: Assessment, in Oxford medical publications. Oxford [England]; New York: Oxford University Press; 1988:34-40.
64 Stoodley MA, Jones NR, Brown CJ. Evidence for rapid fluid flow from the subarachnoid space into the spinal cord central canal in the rat. Brain Res. 1996;707:155-164.
65 Williams B. Simultaneous cerebral and spinal fluid pressure recordings. 2. Cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochir (Wien). 1981;59:123-142.
66 Williams B. Simultaneous cerebral and spinal fluid pressure recordings. I. Technique, physiology, and normal results. Acta Neurochir (Wien). 1981;58:167-185.
67 Asano M, Fujiwara K, Yonenobu K, Hiroshima K. Post-traumatic syringomyelia. Spine (Phila Pa 1976). 1996;21:1446-1453.
68 Dyste GN, Menezes AH, VanGilder JC. Symptomatic Chiari malformations. An analysis of presentation, management, and long-term outcome. J Neurosurg. 1989;71:159-168.
69 Hall P, Lindseth R, Campbell R, et al. Myelodysplasia and developmental scoliosis: a manifestation of syringomyelia. Spine (Phila Pa 1976). 1976:48.
70 Schlesinger EB, Antunes JL, Michelsen WJ, Louis KM. Hydromyelia: clinical presentation and comparison of modalities of treatment. Neurosurgery. 1981;9:356-365.
71 Zager EL, Ojemann RG, Poletti CE. Acute presentations of syringomyelia. Report of three cases. J Neurosurg. 1990;72:133-138.
72 Boman K, Iivanainen M. Prognosis of syringomyelia. Acta Neurol Scand. 1967;43:61-68.
73 Seki T, Fehlings MG. Mechanistic insights into posttraumatic syringomyelia based on a novel in vivo animal model. Laboratory investigation. J Neurosurg Spine. 2008;8:365-375.
74 Honan WP, Williams B. Sensory loss in syringomyelia: not necessarily dissociated. J R Soc Med. 1993;86:519-520.
75 Pojunas K, Williams AL, Daniels DL, Haughton VM. Syringomyelia and hydromyelia: magnetic resonance evaluation. Radiology. 1984;153:679-683.
76 Sindou M, Chavez-Machuca J, Hashish H. Cranio-cervical decompression for Chiari type I-malformation, adding extreme lateral foramen magnum opening and expansile duroplasty with arachnoid preservation. Technique and long-term functional results in 44 consecutive adult cases—comparison with literature data. Acta Neurochir (Wien). 2002;144:1005-1019.
77 Steinmetz A, Aschoff A, Kunze S. The iatrogenic tethering of the cord. Acta Neurochir (Wien). 1993;123:219.
78 Roser F, Ebner FH, Liebsch M, et al. A new concept in the electrophysiological evaluation of syringomyelia. J Neurosurg Spine. 2008;8:517-523.
79 Roser F, Ebner FH, Sixt C, et al. Defining the line between hydromyelia and syringomyelia. A differentiation is possible based on electrophysiological and magnetic resonance imaging studies. Acta Neurochir (Wien). 2009.
80 Nakamura M, Ishii K, Watanabe K, et al. Clinical significance and prognosis of idiopathic syringomyelia. J Spinal Disord Tech. 2009;22:372-375.
81 Abbe R, Coley W. Syringo-myelia, operation-exploration of cord-withdraw of fluid-exhibition of patient. J Nerv Ment Dis. 1892;19:512.
82 Quencer RM, Morse BM, Green BA, et al. Intraoperative spinal sonography: adjunct to metrizamide CT in the assessment and surgical decompression of posttraumatic spinal cord cysts. AJR Am J Roentgenol. 1984;142:593-601.
83 Freeman LW. Ascending spinal paralysis; case presentation. J Neurosurg. 1959;16:120-122.
84 Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
85 Sgouros S, Williams B. Management and outcome of posttraumatic syringomyelia. J Neurosurg. 1996;85:197-205.
86 Edgar R, Quail P. Progressive post-traumatic cystic and non-cystic myelopathy. Br J Neurosurg. 1994;8:7-22.
87 Elsberg CA. Surgical diseases of the spinal cord, membranes, and nerve roots; symptoms, diagnosis, and treatment, First edition. [New York]: P. B. Hoeher; 1941.
88 Frazier C. Shall syringomyelia be added to the lesions appropriate for surgical intervention? JAMA. 1930;95:1911.
89 Aschoff A, Donauer E, Huwel N. Evaluation of syrinx-surgery: a critical comment on requirements for reliable follow up studies. Acta Neurochir (Wien). 1993;123:224.
90 Aschoff A, Kunze S. 100 Years syrinx-surgery—a review. Acta Neurochir (Wien). 1993;123:157-159.
91 Falci S, Charlotte I, Lammertse D. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. J Neurosurg Spine. 2009;11:445-460.
92 Padovani R, Cavallo M, Gaist G. Surgical treatment of syringomyelia: favorable results with syringosubarachnoid shunting. Surg Neurol. 1989;32:173-180.
93 Suzuki M, Davis C, Symon L, Gentili F. Syringoperitoneal shunt for treatment of cord cavitation. J Neurol Neurosurg Psychiatry. 1985;48:620-627.
94 Gardner WJ, Angel J. The cause of syringomyelia and its surgical treatment. Cleve Clin Q. 1958;25:4-8.
95 Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg. 1988;68:726-730.
96 Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382-388.
97 da Silva JA, Holanda MM. Basilar impression, Chiari malformation and syringomyelia: a retrospective study of 53 surgically treated patients. Arq Neuropsiquiatr. 2003;61:368-375.
98 Guyotat J, Bret P, Jouanneau E, et al. Syringomyelia associated with type I Chiari malformation. A 21-year retrospective study on 75 cases treated by foramen magnum decompression with a special emphasis on the value of tonsils resection. Acta Neurochir (Wien). 1998;140:745-754.
99 Bejjani GK, Cockerham KP, Rothfus WE, et al. Treatment of failed Adult Chiari Malformation decompression with CSF drainage: observations in six patients. Acta Neurochir (Wien). 2003;145:107-116. discussion 116
100 Logue V, Edwards MR. Syringomyelia and its surgical treatment—an analysis of 75 patients. J Neurol Neurosurg Psychiatry. 1981;44:273-284.
101 Rascher K, Donauer E. Experimental models of syringomyelia—personal observations and a brief look at earlier reports. Acta Neurochir (Wien). 1993;123:166-169.
102 Aghakhani N, Parker F, David P, et al. Long-term follow-up of Chiari-related syringomyelia in adults: analysis of 157 surgically treated cases. Neurosurgery. 2009;64:308-315. discussion 315
103 Sahuquillo J, Rubio E, Poca MA, et al. Posterior fossa reconstruction: a surgical technique for the treatment of Chiari I malformation and Chiari I/syringomyelia complex—preliminary results and magnetic resonance imaging quantitative assessment of hindbrain migration. Neurosurgery. 1994;35:874-884. discussion 884-875
104 Sakamoto H, Nishikawa M, Hakuba A, et al. Expansive suboccipital cranioplasty for the treatment of syringomyelia associated with Chiari malformation. Acta Neurochir (Wien). 1999;141:949-960. discussion 960-941
105 Nishizawa S, Yokoyama T, Yokota N, et al. Incidentally identified syringomyelia associated with Chiari I malformations: is early interventional surgery necessary? Neurosurgery. 2001;49:637-640. discussion 640-631
106 Tator CH, Meguro K, Rowed DW. Favorable results with syringosubarachnoid shunts for treatment of syringomyelia. J Neurosurg. 1982;56:517-523.
107 Hida K, Iwasaki Y, Koyanagi I, Abe H. Pediatric syringomyelia with chiari malformation: its clinical characteristics and surgical outcomes. Surg Neurol. 1999;51:383-390. discussion 390-381
108 Schaller B, Mindermann T, Gratzl O. Treatment of syringomyelia after posttraumatic paraparesis or tetraparesis. J Spinal Disord. 1999;12:485-488.
108a Adelstein LJ. Surgical treatment of syringomyelia. Am J Surg. 1938;40:384-395.
109 Pitts FW, Groff RA. Syringomyelia: Current Status of Surgical Therapy. Surgery. 1964;56:806-809.
110 Love JG, Olafson RA. Syringomyelia: a look at surgical therapy. J Neurosurg. 1966;24:714-718.
111 Anderson N, Willoughby W, Wrightson P. The natural history and the influence of surgical treatment in syringomyelia. Acta Neurol Scand. 1985;71:472.
112 Lee TT, Alameda GJ, Camilo E, Green BA. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine (Phila Pa 1976). 2001;26:S119-S127.
113 Paul KS, Lye RH, Strang FA, Dutton J. Arnold-Chiari malformation. Review of 71 cases. J Neurosurg. 1983;58:183-187.
114 Pillay PK, Awad IA, Little JR, Hahn JF. Symptomatic Chiari malformation in adults: a new classification based on magnetic resonance imaging with clinical and prognostic significance. Neurosurgery. 1991;28:639-645.
115 Menezes AH. Chiari I malformations and hydromyelia—complications. Pediatr Neurosurg. 1991;17:146-154.
116 Hida K, Iwasaki Y, Koyanagi I, et al. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery. 1995;37:673-678. discussion 678-679
117 Blagodatsky MD, Larionov SN, Alexandrov YA, Velm AI. Surgical treatment of Chiari I malformation with or without syringomyelia. Acta Neurochir (Wien). 1999;141:963-968.
118 Depreitere B, Van Calenbergh F, van Loon J, et al. Posterior fossa decompression in syringomyelia associated with a Chiari malformation: a retrospective analysis of 22 patients. Clin Neurol Neurosurg. 2000;102:91-96.
119 Goel A, Desai K. Surgery for syringomyelia: an analysis based on 163 surgical cases. Acta Neurochir (Wien). 2000;142:293-301. discussion 301-292
120 Asgari S, Engelhorn T, Bschor M, et al. Surgical prognosis in hindbrain related syringomyelia. Acta Neurol Scand. 2003;107:12-21.
121 Dones J, De Jesus O, Colen CB, et al. Clinical outcomes in patients with Chiari I malformation: a review of 27 cases. Surg Neurol. 2003;60:142-147. discussion 147-148
122 Navarro R, Olavarria G, Seshadri R, et al. Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst. 2004;20:349-356.
123 Arruda JA, Costa CM, Tella OIJr. Results of the treatment of syringomyelia associated with Chiari malformation: analysis of 60 cases. Arq Neuropsiquiatr. 2004;62:237-244.