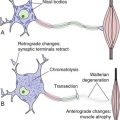
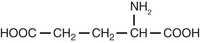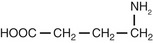8 Synaptic Transmission between Neurons
In contrast to the way in which information travels within individual neurons as electrical signals, information is usually transmitted between neurons through the release of neurotransmitters at specialized junctions called synapses. And in contrast to unvarying, always depolarizing action potentials, a wide variety of slow graded potentials may be produced at the synapses on an individual neuron—some depolarizing, some hyperpolarizing, some milliseconds in duration, others seconds, minutes, or even hours.
There Are Five Steps in Conventional Chemical Synaptic Transmission
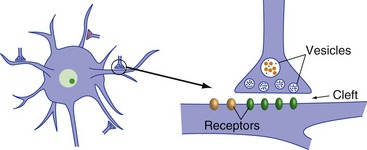
The fundamental elements of a chemical synapse (Fig. 8-1) are a presynaptic ending from which neurotransmitter is released, a synaptic cleft across which it diffuses, and a postsynaptic element containing receptor molecules to which the neurotransmitter binds. Although the presynaptic ending is usually an axon terminal and the postsynaptic ending usually a dendrite, any part of a neuron can be presynaptic to any part of another neuron. The essential processes at chemical synapses are presynaptic synthesis, packaging, and release of neurotransmitter; binding to postsynaptic receptors; and termination of neurotransmitter action.
Presynaptic Endings Release Neurotransmitters into the Synaptic Cleft
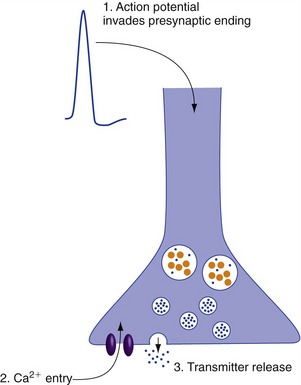
Neurotransmitter release is a secretory process triggered by an increase in presynaptic Ca2+ concentration. The membranes of presynaptic terminals contain voltage-gated Ca2+ channels that open when an action potential spreads into the terminal (Fig. 8-2). Ca2+ influx causes one or more vesicles to fuse with the presynaptic membrane and dump its neurotransmitter content into the synaptic cleft. Because small vesicles are close to the synaptic cleft, they are the first to release their contents. Because the large vesicles are farther away, release of their contents requires more Ca2+ entry (hence, more presynaptic action potentials) and more time.
Synaptic Transmission Can Be Rapid and Point-to-Point, or Slow and Often Diffuse
Rapid Synaptic Transmission Involves Transmitter-Gated Ion Channels
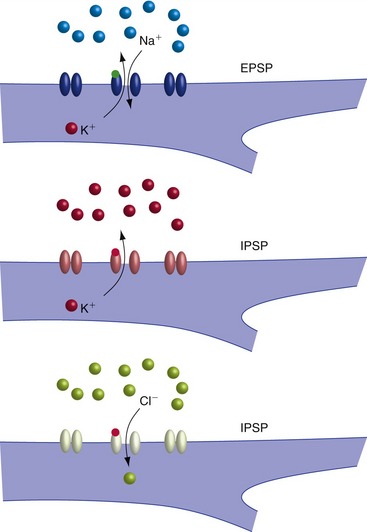
Fast EPSPs and IPSPs are produced by the binding of neurotransmitter to a receptor that is itself a ligand-gated ion channel (Fig. 8-3); because of the direct coupling to ion flow, these are also referred to as ionotropic receptors. The permeability change induced by the binding of transmitter determines the postsynaptic response. Some receptors become permeable to both Na+ and K+, causing depolarization (EPSP). Others become permeable to K+ or Cl−, causing hyperpolarization (IPSP).
Slow Synaptic Transmission Usually Involves Postsynaptic Receptors Linked to G Proteins
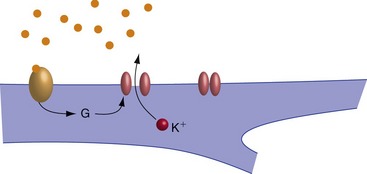
Slow EPSPs and IPSPs are produced by a multistep process involving changes in the postsynaptic concentration of a second messenger. They typically begin with binding of neurotransmitter to a receptor linked to an adjacent guanine nucleotide-binding protein (G protein). The G protein then dissociates and one of its subunits triggers subsequent steps. In the simplest case, the G protein subunit is itself the second messenger and binds to a ligand-gated ion channel (Fig. 8-4). In most cases, however, the G protein subunit increases or decreases the activity of an enzyme, which in turn causes changes in the concentration of something else. Because of the intermediate metabolic steps, the receptors involved in slow EPSPs and IPSPs are also referred to as metabotropic receptors.
Synaptic Strength Can Be Facilitated or Depressed
Most Neurotransmitters Are Small Amine Molecules, Amino Acids, or Neuropeptides
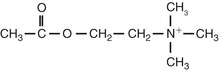
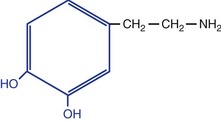
Nearly all neurotransmitters are either small molecules (amines or amino acids) or neuropeptides. There are dozens of known or suspected neuropeptide transmitters, but a much smaller number of important small-molecule transmitters, each with a more or less distinctive role (Tables 8-1 and 8-2). Acetylcholine mediates rapid, point-to-point, excitatory transmission in the PNS. Glutamate and γ-aminobutyric acid (GABA) mediate rapid excitatory and inhibitory transmission, respectively, in the CNS. Amines and neuropeptides almost without exception mediate slow, second-messenger effects in the CNS and PNS.
| Amines |
| Acetylcholine |
| Monoamines |
| Serotonin |
| Catecholamines |
| Dopamine |
| Norepinephrine |
| Amino acids |
| Glutamate |
| GABA (γ-aminobutyric acid) |
Table 8-2 Structures, locations, and actions of the principal small-molecule transmitters
| Transmitter | Principal Neurons Using It | Major Action* |
|---|---|---|
| Acetylcholine |
* Major but not all actions. For example, there are metabotropic glutamate and GABA receptors.
† Important exception: the NMDA receptor (THB6 Figure 8-20, p. 193) has additional voltage-gated properties.
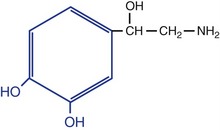
‡ The blue part is the catechol group for which catecholamines are named
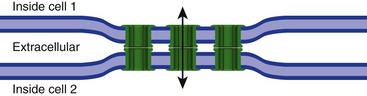
Gap Junctions Mediate Direct Current Flow from One Neuron to Another
A minority of synaptic connections eschews neurotransmitters altogether, and instead are gap junctions at which current can flow directly from one neuron into another (Fig. 8-5). These electrical synapses are useful for groups of neurons that need to fire synchronously and for networks of neurons designed to spread information laterally with little computation, but otherwise are fairly rare in the mammalian CNS.