Chapter 72 Surgical Treatment of Paraclinoid Aneurysms
The portion of the proximal intradural internal carotid artery (ICA) adjacent to the anterior clinoid process (ACP) is called the paraclinoid segment. Aneurysms arising from the ICA between the roof of the cavernous sinus and the origin of the posterior communicating artery (PComA) are defined as paraclinoid aneurysms.1–5 These aneurysms are of considerable surgical interest due to their particular anatomic features and technical difficulties. Some of these aneurysms were considered in the past as unclippable or associated with very bad results when surgically approached.6 Fortunately, with the progressive refinement of microsurgical techniques their management has changed from the very conservative surgery to direct neck clipping, with results surpassing those of the endovascular therapy in terms of total neck obliteration and long-term recanalization (Table 72-1).4,5,7 The classification of these aneurysms according to the origin of their necks and projection of the aneurysms is particularly important to select the optimum microsurgical approach.1,8–12
Anatomic Aspects
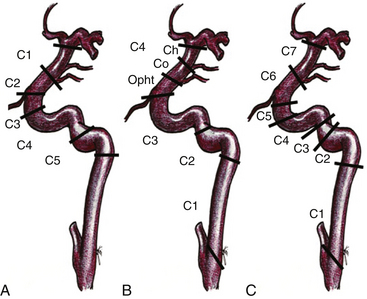
In 1938, Fisher12a published an anatomic nomenclature for the ICA based on the angiographic course of the artery, describing five segments designated C1 through C5. However, these segments were numbered opposite to the direction of blood flow and the extracranial ICA was excluded (Fig. 72-1A). Recently, other classifications have been published that include the extracranial and intracranial segments, and the carotid segments have been numbered according to the direction of the blood flow. Therefore, the paraclinoid segment of the ICA comprises the C2 and C3 segments of the original Fisher classification (1938), the distal C3 and Proximal C4 segment of Gibo et al. (1981),13 and the C5 and C6 segment of the Bouthillier classification (1996)14 (Fig. 72-1). Because of the close topographical vicinity of these aneurysms to osseous, fibrous, nervous and vascular structures of the skull base they may present with clinical symptoms due to compression of the optic nerve or other surrounding structures instead of the classic subarachnoid hemorrhage frequently seen in aneurysms in other locations.3,15 The anatomic structures of the paraclinoid area not only produce a limited space for expanding vascular lesions, but also for the neurosurgeon during operation, thus, sufficient proximal control and minimal manipulation of the vascular and nerve structures around are of utmost importance for the postoperative outcome.1,2,4,5,7,16–18
Classification
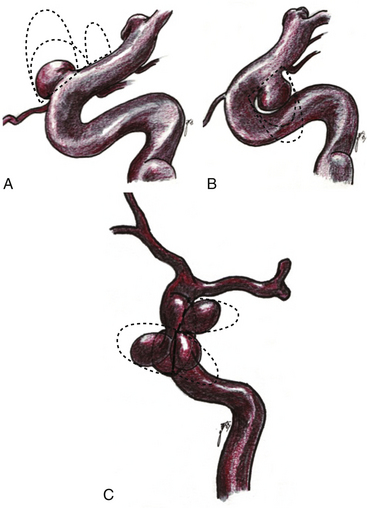
Given the variability in projection, size and origin of these aneurysms, various authors in the past have classified them. Most of the classifications are based on the site of origin of the neck, the projection of the dome and its relationship with branches arising from the ICA.2,7–10,12,17 The vast majority of saccular aneurysms arise within the angle formed by the parent artery and a significant arterial branch. Therefore, aneurysms related to these arteries are called accordingly, for example, ophthalmic and superior hypophyseal artery aneurysms.2,8 In addition, aneurysms unrelated to branches occur only rarely in this segment (distal ophthalmic aneurysms).12 Ventral paraclinoid carotid aneurysms seem to belong to the same category as infraophthalmic aneurysms, which originate from the ventral surface of the ICA and in which the proximal aspect of the neck is located approximately at the level of the ophthalmic artery and the distal aspect of the neck is located proximal to the posterior communicating artery19 (Fig. 72-2). These aneurysms also project straight or slightly medial and downward. Carotid cave aneurysms are another distinct type of aneurysm located at non-branching sites of this segment. This type of aneurysm arises from the medial wall of the proximal intradural ICA, and grows within a small dural recess, with the apex of the sac directed toward the cavernous sinus10 (Fig. 72-3). All these denominations have contributed to create a rather complex view of these aneurysms. Another confusing characteristic is that they are not always related to a branching artery and may point in any direction, as laterally, medially, ventrally, or dorsally (Fig. 72-2).
In order to simplify the classification of these aneurysms, we prefer to name them according to their site of origin in relation to the circumference of the ICA and some branching artery (if any), because this is also relevant at the time of clipping. Thus, the paraclinoid aneurysms can be classified as follows: dorsal type aneurysms, ventral type aneurysms, carotid cave aneurysms, and global type aneurysms (Figs. 72-3 to 72-7).
Dorsal Type Aneurysms
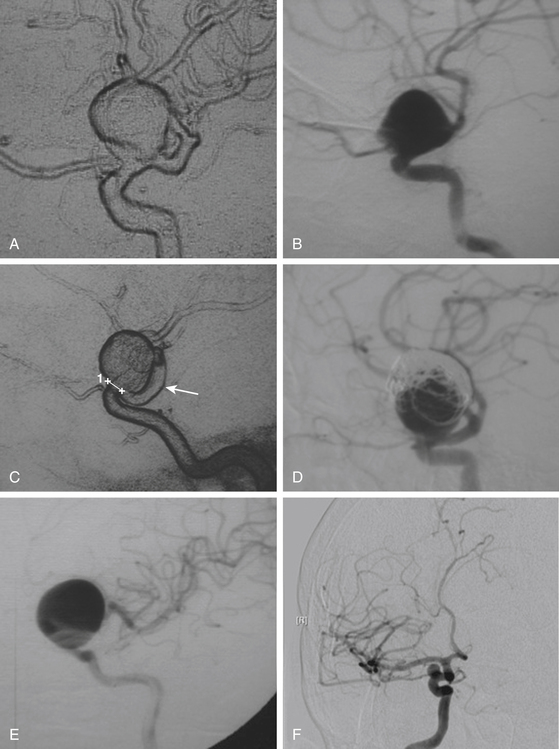
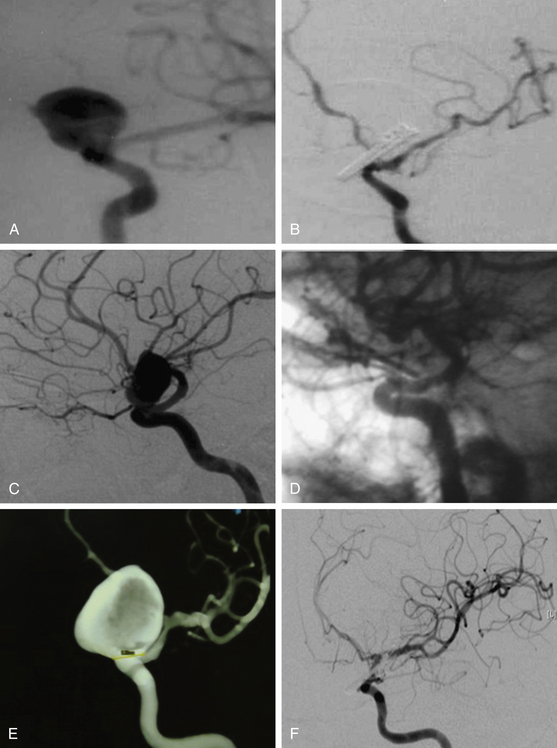
These include the proximal dorsal type aneurysms that correspond to the carotid ophthalmic aneurysms. They arise from the ophthalmic segment of the ICA in close relationship with the ophthalmic artery.7,9,11,15,18 On the lateral view of an angiogram the neck of the aneurysm is located just distal to the origin of this artery (Figs. 72-2A, 72-4, and 72-8). They grow upwards and cause compression of the optic nerve. As they have a tendency to grow, many of these lesions are detected because of visual deficits. The second main type is the distal dorsal type aneurysm (also known as dorsal wall aneurysms). This aneurysm grows upward at the dorsal surface of the ICA (Fig. 72-5). They are located distal to the ophthalmic artery and seem not to origin from any branch of the ICA.9,12 Whether they arise at bifurcations of vestigial arteries or because hemodynamic stress at the curvature of the carotid siphon is unknown.20 The dorsal surface of the ICA is also a common site of blood blister–like aneurysms. These are dangerous small lesions with fragile walls consisting of normal adventitia or fibrin nets. Primary treatment in the acute stage is challenging due to the substantial risk of intraoperative bleeding, resulting in the formation of a large defect in the ICA. To treat these aneurysms, sometimes it is necessary to use especially designed encircling clips or some wrapping procedure, otherwise, a bypass procedure with trapping of the aneurysm can be used as an alternative.
Ventral Type Aneurysms
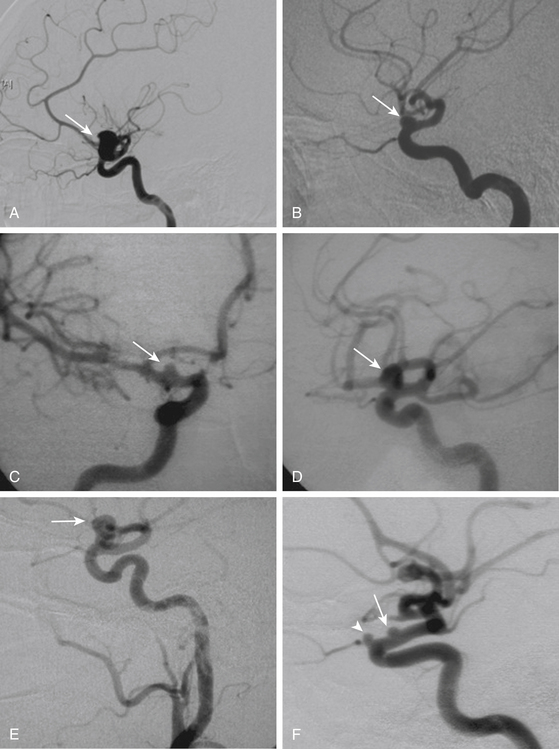
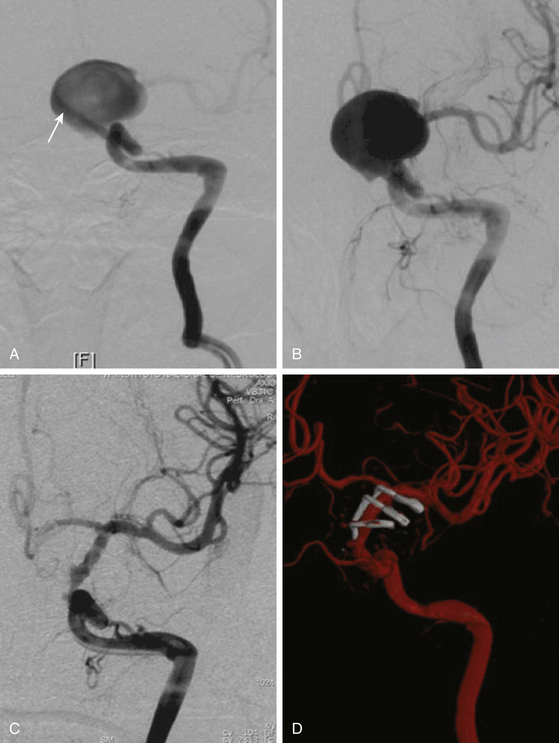
These aneurysms grow at the ventral or ventromedial surface of the ICA (Figs. 72-2B, 72-6, and 72-9). They are located opposite to the origin of the ophthalmic artery and in close relationship with the superior hypophyseal artery. As they increase in size, they are directed downward and medially. When large or giant, they produce an upward displacement of the ICA; however, visual disturbance is not as frequent as in dorsal type aneurysms.1,2,5,8,19
Carotid Cave Aneurysms
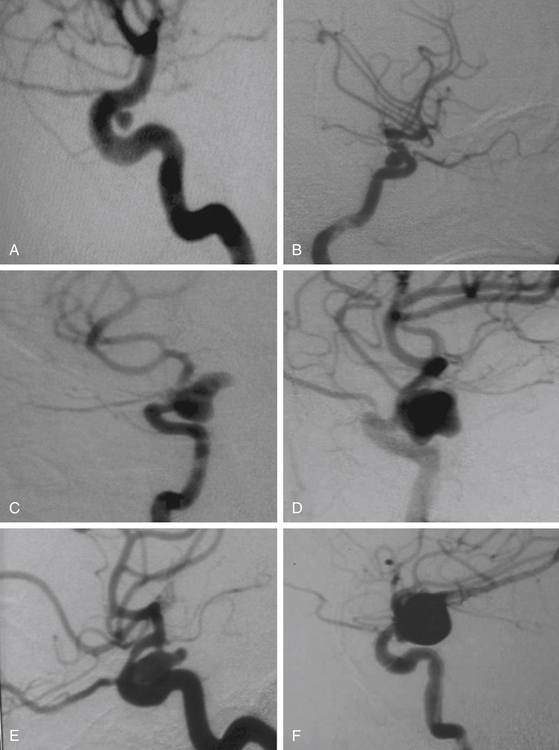
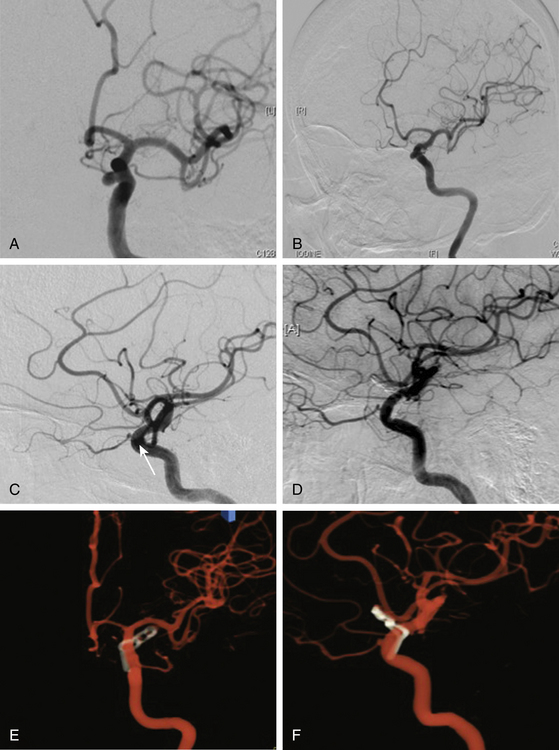
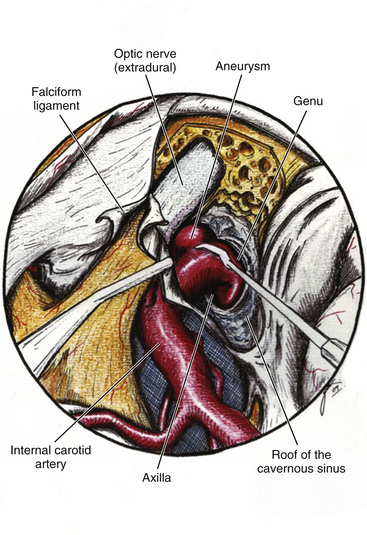
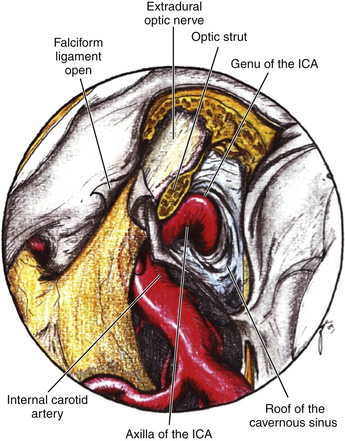
This is a special type of aneurysm originating between the proximal and distal carotid rings. They grow ventromedially proximal to the ophthalmic artery, and are visible mainly on the anterior or oblique angiographic views. On the lateral view, they remain hidden by the ICA (Figs. 72-3 and 72-10). Carotid cave aneurysms are transitional in type between paraclinoid intradural and cavernous sinus aneurysms. They may grow out of the cave into the intradural subarachnoid space. During surgery, they project ventrally at the level of the carotid genu10 (Fig. 72-11). In this sense, the surgical genu is located more proximal than the angiographic genu, which roughly corresponds to the location of the dural ring.
Global Type Aneurysms
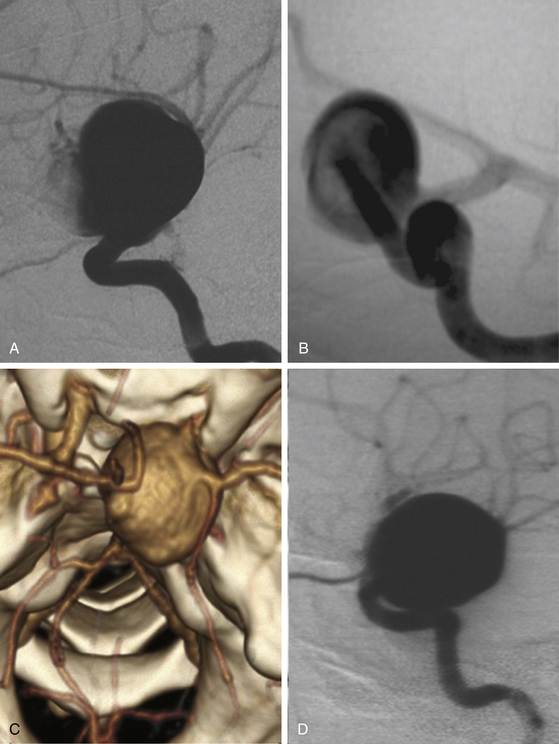
These aneurysms involve the entire circumference of the ICA; they are large or giant in size, and during angiography or surgery the origins of the neck are not as easy to identify as ventral or dorsal types even when the origin was surely at any of these points. Most cases are associated with degeneration of the carotid wall. The importance of this type of aneurysm is that the treatment is based on deconstructive techniques (parent artery obliteration and bypass surgery). This type of aneurysm should not be diagnosed only because of size and shape (Fig. 72-7).
It should be emphasized that during the growth process of a paraclinoid aneurysm, the dome could occupy anatomic spaces at the medial or lateral side of the ICA (Fig. 72-2). We do not believe that the medial or lateral sides of the ICA are origin sites of such aneurysms. When we carefully analyze the angiographic videos and films or the intraoperative recordings (except for global type aneurysms), the origin can be traced to the dorsal or ventromedial surface of the ICA.
Preoperative Planning
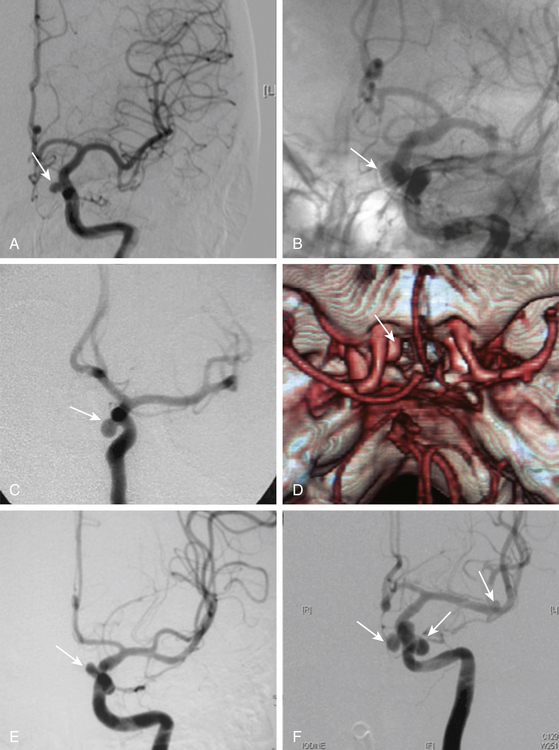
A very careful preoperative plan should be obtained for every case, taking into account the size and position of neck and dome of the aneurysm and its relationship with surrounding structures, especially the optic nerve. In addition, the length of the ICA should be assessed as well as the position of the ophthalmic and posterior communicating arteries. The summary of all this information will provide important hints for selecting the craniotomy and the technique for drilling the ACP (extra or intradural), and to prepare the availability of proper instruments to be used in the operation including a complete set of ring (fenestrated) clips for ventral or carotid cave aneurysms. To prevent premature intraoperative rupture or prolonged ICA occlusion, the preoperative angiography should include a four-vessel angiography with a balloon or manual compressive test occlusion in the awake patient. This will provide information about the position of the aneurysm and the collateral circulation at the circle of Willis, especially from one side to the other through the anterior communicating artery and from the posterior to the anterior circulation. The results of this preoperative test allow for reliable estimation of the tolerance of temporary or even permanent ICA occlusion or to be prepared for a bypass procedure before attacking a complex aneurysm in case of poor collateral circulation2,3,21 (Fig. 72-12).
Proximal Control of the ICA
Techniques to achieve proximal control of the ICA are briefly described in the following:
Proximal control at the neck. This is the simplest and safest method to obtain vascular control before beginning the craniotomy.2 Exposure of the cervical ICA typically requires 15 minutes of operative time and secures the ICA early in the procedure, which is very important in the event of premature rupture of the aneurysm.
Exposure of the petrous ICA in the middle fossa.22,23 In this technique, the middle fossa is approached extradurally after the craniotomy is completed. The components of Glasscock’s triangle are identified (arcuate eminence, foramen spinosum, and foramen ovale). The course of the greater superficial petrosal nerve is identified and the nerve is sectioned to avoid traction injury to the geniculate ganglion and facial nerve. The middle fossa floor is drilled along the course of the canal of the petrosal nerve. The bone covering the petrous carotid canal is usually thin, and sometimes a membranous layer covers the carotid. Unroofing the petrous bone over the ICA should not proceed caudally beyond the point where the ICA turns vertically if injury to the cochlea is to be avoided. Once identified, the petrous carotid should be dissected from its adherence to the petrous canal. Temporary occlusion of the ICA at this point can be obtained by packing the carotid petrous segment with surgical patties (Fukushima’s technique), by the introduction of a Fogarty catheter (Spetzler’s technique), or by a standard clipping technique after 360-degree dissection of the carotid artery from the petrous wall. The main disadvantage of this technique is that the neurosurgeon should be very familiar with the anatomy of the floor of the middle fossa based on laboratory work and previous surgical experience.
Intracranial control of the ICA after opening the distal dural ring. This technique is feasible during clipping of small- or medium-size aneurysms; however, it is more difficult when dealing with large or giant aneurysms with a short intradural ICA.4 It has the disadvantage of obtaining proximal control after the neurosurgeon faces the aneurysm; therefore, if rupture occurs early, rapid proximal control is difficult.
Endovascular ICA occlusion via femoral artery catheter with the option to perform an intraoperative angiography. It has the advantage to perform a suction-decompression technique of large or giant aneurysms before clipping.24 Another related endovascular technique is the Dallas maneuver. A deflated, double-lumen balloon catheter is placed in the appropriate ICA, 2 cm above the common carotid bifurcation. Proximal control is achieved by inflating the balloon. Temporary clipping just proximal to the origin of the posterior communicating artery then gains distal control. Retrograde suction decompression through the catheter collapses the aneurysm, which is then permanently clipped.25 However, with these techniques, it has been reported traumatic dissection and thromboembolism from the end of the catheter that caused patient injury. The technique to get proximal control of the ICA, however, should be selected according to experience of the neurosurgeon and local facilities.
Patient Positioning and Craniotomy
The patient is positioned supine with the head fixed with a three-point skeletal fixation device such as a Mayfield-Kees or Sugita. The head is directed 20 degrees vertex down and rotated about 30 degrees to the opposite side of the approach. The exact position of the head will allow a visual axis along the sphenoid ridge to the ACP and the parasellar area.
A standard pterional craniotomy is performed for small- or medium-size aneurysms. Craniotomy should provide enough space to complete the ACP drilling and unroofing the optic nerve, to expose the ICA and the aneurysm, and to proceed to clip the aneurysm (Fig. 72-13). In cases of large or giant aneurysms, the extent of the craniotomy should be large enough to expose the aneurysm with minimal brain retraction, and to get the space for free hand movements to ensure a comfortable clipping without limitations.
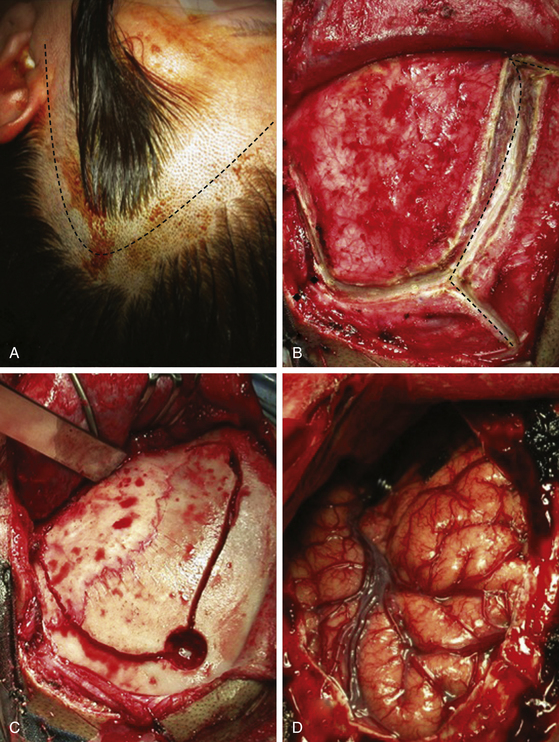
A skin incision is made 1 cm in front of the tragus, at the level of the zygomatic arch, and carried behind the hairline after curving over the superior temporal line at the midpupilar level. The skin flap is reflected rostrally. Particular attention is paid to dissection of the superficial and deep fascia of the temporalis muscle to prevent injury to the facial nerve and maintain the arterial vascularization to the muscle. The muscular flap in also reflected rostrally and fixed with sutures or hooks. A frontotemporal craniotomy is performed with one or more burr holes depending of the adherence of the dura to the bone. We start the first burr hole at the most caudal part of the craniotomy, at the level of the superior temporal line (Fig. 72-13). The bone flap is retired and bone hemostasis is completed with bone wax. If the proximal control will be obtained at the petrous carotid level, the craniotomy should be extended at the temporal side until the middle fossa floor level and the dura dissected from the orbital roof, the sphenoid ridge, and the middle fossa. Otherwise, the dura is only dissected around the sphenoid ridge down to the ACP. The sphenoid ridge is drilled with a diamond-tip drill until the ACP. If the aneurysm is of ventral type, the ACP could be resected extradurally.4,26
Next we proceed to dissect the middle fossa separating the dura from the media fossa floor. At first it is identified as the foramen spinosum following the course of the middle meningeal artery that is usually visible at the dura surface. The foramen is expanded with a diamond tip drill to facilitate the coagulation and cutting of the artery. The foramen ovale is then identified rostral and medial to the foramen spinosum. Afterward, the dura is dissected from the arcuate eminence in a caudal to rostral fashion to avoid stretching of the petrosal nerve. Once the greater superficial petrosal nerve is identified, it is separated from the adherent dural layer and followed until it reaches the lateral border of V3. The petrosal nerve is sectioned at its mid-portion avoiding any stretching that can be transmitted to the geniculate ganglion and facial nerve. The canal of the nerve is followed with a small diamond-tip drill until the petrous carotid is exposed. Sometimes, a thin layer of membranous tissue covers the petrous carotid, but in other cases it is located 2 to 3 mm from the surface of the middle fossa. During drilling you may find a soft tissue structure laterally, corresponding to the tensor tympani muscle. At this point you should direct the drilling medially, because lateral to the tensor tympani muscle is located the Eustachian tube. After exposure of the petrous carotid, the artery is dissected from the wall of the petrous canal to get space for the placement of surgical patties, Fogarty catheter, or temporary clip. If necessary, drilling can be extended medially to obtain more space through Kawase’s triangle without harm to any structure.
Sylvian Fissure and Basal Cisterns
The dura is opened in a semi lunar fashion and reflected rostrally. The sylvian fissure is widely open to obtain a larger exposure with minimal brain retraction. Any unnecessary cutting of bridging veins should be avoided because of the risk of venous infarctions. The arachnoid space is open to include the sylvian fissure, carotid and chiasmatic cisterns, and the Lilliequist membrane. This will expose the ACP, optic nerves and chiasm, lamina terminalis, and the ICA until the bifurcation (Fig. 72-14). In case of a recent bleeding, the lamina terminalis is open to release the maximal amount of cerebrospinal fluid (CSF) and to prevent hydrocephalus. In case of a contralateral paraclinoid aneurysm, the arachnoid tissue is further opened around the opposite optic nerve to expose the contralateral paraclinoid ICA.
Anterior Clinoid Process
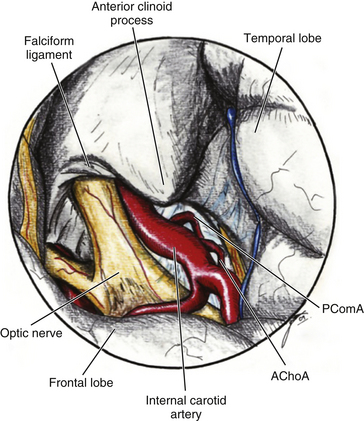
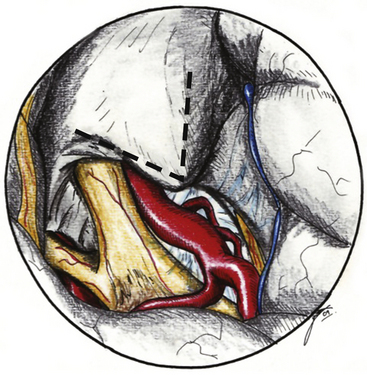
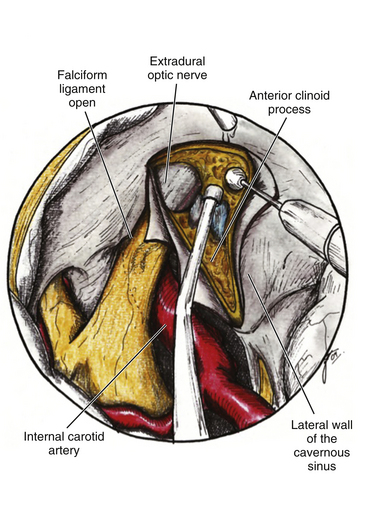
The ACP should be removed in any case of paraclinoid aneurysm approached ipsilaterally independently of their size. This will provide enough space and vision of the neck and dome even in small aneurysms. Clipping one aneurysm with partial exposure has been one of the main causes of poor surgical results due to intraoperative rupture or neck remnant. As mentioned previously, the ACP can be resected in an extradural or intradural fashion.4,26 If an extradural resection could not be completed due to a large clinoid process or a dorsal type aneurysm in which the dome is close to the ACP or may have eroded it, a combined extradural-intradural technique is selected. In general, diamond drills are preferred to avoid damage to surrounding structures. In the case of intradural resection of the ACP, an incision is performed following the long axis of the ACP and curving the dura incision over the falciform ligament (Fig. 72-15). The ACP is drilled until the tip is reached and resected with the aid of a small rongeur or dissector. As an alternative, a bone ultrasonic aspirator can be used; unfortunately, this device is expensive and not available everywhere.27 The resection of the ACP should be very careful in cases of dorsal type aneurysms because of the risk of rupture. At this level of the operation the proximal control should be fully obtained. In addition, it is important to extend the level of bone resection to include the optic strut, which connects the ACP to the lateral wall of the sphenoid sinus and comprises the lateral and ventral border of the optic foramen (Fig. 72-16). Microsurgical resection of the optic strut is of paramount importance in visualizing the origin of the ophthalmic artery. The origin of the ophthalmic artery often demarcates the junction of the proximal aneurysm neck with normal ICA.
Distal Dural Ring and Optic Nerve
Opening the dura of the optic nerve from the falciform fold along the entire length of the optic canal longitudinally allows slight but significant mobilization of the nerve and dissection away from the aneurysm. Intermittent retraction of the optic nerve is preferred over single, protracted retraction. The origin of the ophthalmic artery is carefully dissected away from the aneurysm. Opening the distal dural ring anchoring the ICA reveals the proximal portion of the neck and the proximal ICA (Fig. 72-17). This opening is extended toward the third cranial nerve, and any resulting venous bleeding is easily controlled with packing with small pieces of Surgicel or injection of fibrin glue.28 Stepwise dissection of the aneurysm from the surrounding structures follows. Papaverine or nimodipine is routinely applied to the ICA and its branches early in the surgical procedure and is repeated as necessary to avoid mechanical vasospasm.
Dissection and Clipping of the Aneurysm
Small- and Medium-Size Aneurysms
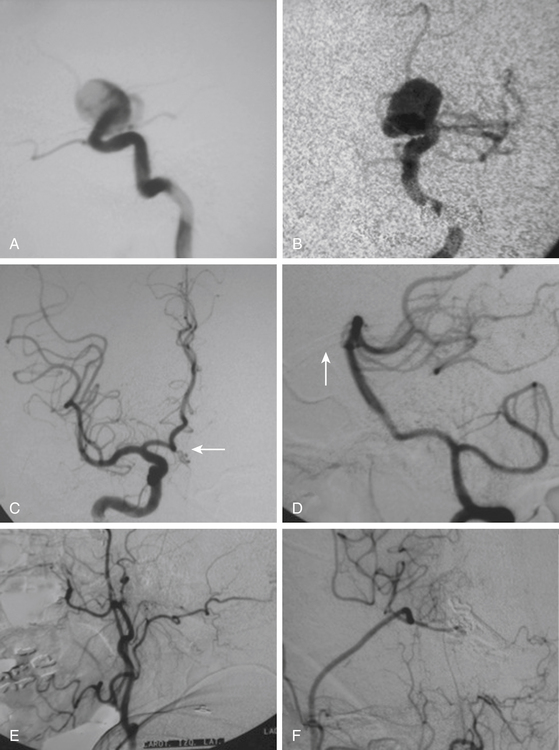
After the above–mentioned steps are completed and the ICA and aneurysm are brought into view, a space is created between the origin of the posterior communicating artery and the neck of the aneurysm for later placement of a distal temporary clip. The course of the anterior choroidal artery is also confirmed as well as all the branching arteries in the vicinity of the aneurysm. In small- and medium-size aneurysms, the wall can be thin and the blood flow could be seen through the wall of the aneurysm, warning the neurosurgeon to avoid aggressive movements over the dome of the aneurysm. On the other side, aneurysms with a thick or atherosclerotic wall could be gently compressed, as the aneurysm occasionally must be lifted to establish a plane between it and the ICA. At this stage, the aneurysm collapses and is carefully separated from the ICA. The neck of the aneurysm is dissected and permanently clipped. More than one clip may be necessary to exclude the aneurysm and to prevent circulation pressure from opening or pulsating the clip blades. Bipolar coagulation of the aneurismal sac does not have an effect in some patients because either the aneurysm wall or neck is thick with prominent atherosclerosis. After clipping the aneurysm, the blood flow is reestablished. Some additional aid, such as intraoperative angiography or microscope-based indocyanine green (ICG) video angiography could be used to confirm total obliteration of the aneurysm sac. Most paraclinoid aneurysms have a broad neck; therefore, neck occlusion will be difficult. The necessary number of clips to secure complex aneurysms should be used (Figs. 72–8B, D, and F;72-9D;and 72-10E).
Giant Aneurysms
Treating giant or large paraclinoid aneurysms is more difficult than other anterior circulation aneurysms and demands particular operative technique. The risk of treatment is higher and is associated with greater operative hazards. Patients with giant aneurysms tend to be older, and have a higher risk for complications associated with a general anesthesia or systemic medical conditions. Giant aneurysms have also a higher rate of wall calcifications, atherosclerotic plaque, and intraluminal thrombus. All these aspects complicate direct clip reconstruction. Various techniques for conservative or indirect treatment have been advocated, including carotid occlusion, arterial extracranial-intracranial bypass, and subsequent ligation of the ICA with heparinization3,21,22 (Fig. 72-12). However, most neurosurgeons agree that the best treatment for giant or large paraclinoid aneurysms is direct clipping. The goals should be to eliminate the risk of primary or recurrent subarachnoid hemorrhage, prevent further visual loss by decompressing the optic apparatus, and secure the hemodynamics by maintaining the patency of the ICA and its branches.
Perhaps the greatest technical difficulties in giant aneurysms are gaining proximal control and relieving the tension within the aneurysm to allow dissection and successful clipping. In this sense, Yasargil described the technique of trapping these aneurysms followed by puncture or excision of the dome, suction of the contents, deflation and coagulation of the sac, and finally clipping. Proximal vascular control and the suction decompression technique now comprise the best surgical adjunct to facilitate dissection and clipping of giant paraclinoid aneurysms. When this method is used, however, the duration of temporary occlusion of the ICA is an important issue. It is usually not possible to complete dissection and clipping of the aneurysm within a few minutes. The occlusion time ranges from 10 to 60 minutes, with a mean of 30 minutes. Accordingly, a balloon occlusion test (BOT) should be performed routinely to select the best treatment. For patients at high risk from temporary arterial occlusion, the use of the retrograde suction decompression technique should be considered more carefully. Although the BOT is not perfect in predicting the tolerable occlusion time in each case, this test can select patients who cannot withstand even a short period of temporary occlusion. Such patients usually develop motor disturbance, aphasia, or loss of consciousness within 1 minute after test occlusion. For patients who are intolerant of the test occlusion, a combination of high-flow graft and proximal ICA occlusion (trapping technique) is used, or direct clipping using the suction decompression technique after performing a high flow bypass graft in the same operative session22,23,29(Fig. 72-12).
Clip Selection
Selection of the clip shape and size will depend on aneurysm size and direction. In this sense, it is important to take into consideration the main types of paraclinoid aneurysms described previously. Dorsal type aneurysms are in close relationship with the optic nerve and the anterior clinoid process and clinical presentation is very often with visual deficit more than with SAH.15 In this case the resection of the anterior clinoid process and unroofing of the optic nerve should be more extensive because of the need of mobilization, especially in large aneurysms. Evacuation of the aneurismal sac permits immediate decompression of the optic pathway, and it may be the most effective method for the recovery of visual dysfunction caused by the aneurysm. Sometimes, however, despite a careful dissection of the sac away from the optic nerve, postoperative deterioration of visual acuity occurs. Nowadays, this remains as a significant problem related to the direct surgical clipping of large ophthalmic aneurysms. This kind of aneurysm is best clipped using straight or slightly curved clips because the course of the ICA remains under the sac of the aneurysm (Fig. 72-8). If necessary, according to the neurosurgeon’s preference, clipping could be performed under temporary occlusion of the ICA to reduce the intraluminal pressure of a broad neck aneurysm. In giant aneurysms, proximal balloon occlusion and suction will facilitate clip positioning and will avoid intraoperative rupture.24,25 If endovascular aid is not available, a proximal and distal temporary occlusion followed by direct puncture and suction of the dome will facilitate the clipping.
On the other side, ventral type aneurysms are located behind the circumference of the ICA. They grow downward and the optic nerve is not displaced in the same extent as with the dorsal type aneurysms. Therefore, the use of standard clips across the neck will produce stretching and kinking of the ICA or will left a remnant sac. For this type of aneurysm a combination of ring (fenestrated) clips should be used2,3,8,16,18 (Fig. 72-9).
The carotid cave aneurysms are directed ventromedially between the proximal and distal dural rings at the level of the carotid genu. As this type of aneurysm projects ventral to the ICA circumference, they are best clipped using ring clips (Fig. 72-10). Kobayashi et al. have described a specially designed clip for these aneurysms taking into consideration the curve of the ICA when located on the left or right side.10 Again, use of standard (nonfenestrated) clips runs the risk of incomplete clipping.
Multiple Aneurysms
Paraclinoid region is a common site of location for mirror aneurysms. Optimally, all aneurysms should be clipped simultaneously. The frequency of multiple aneurysms also justifies an aggressive surgical approach. For patients with mirror aneurysms, the contralateral approach has been described without having visual obstruction caused by the ACP as when the aneurysm is approached through the same side.30 In these cases, a wide opening of the arachnoid cisterns around the chiasmatic area will permit a proper visualization of the aneurysm as well as the ophthalmic and superior hypophyseal arteries. Therefore, clipping small- or medium-size contralateral aneurysm could be performed with a high grade of efficiency.31 In case of large or giant contralateral aneurysms, approaching the aneurysm from the side or origin in a second operation is recommended. On the other hand, in case of multiple nonmirror aneurysms, the possibility to clip them will depend on aneurysm location. For aneurysms located on the same side, including those at the anterior communicating artery or contralateral ICA bifurcation, they can be clipped starting from the deepest to the most superficial one in order to avoid visual limitation by the head of the clip.
Assessment of Blood Flow at Parent Artery
After the aneurysm is clipped, the patency of the ICA should be confirmed by visual inspection of the artery. In case of arteries with a thick-wall or broad-neck aneurysm, the external inspection is not enough to confirm the patency of the ICA; using the intraoperative Doppler should then be mandatory. This device provides qualitative or quantitative information about blood flow. Other alternatives are the intraoperative angiography, or recently, use of intraoperative fluorescence-based angiography through the surgical microscope.
Results
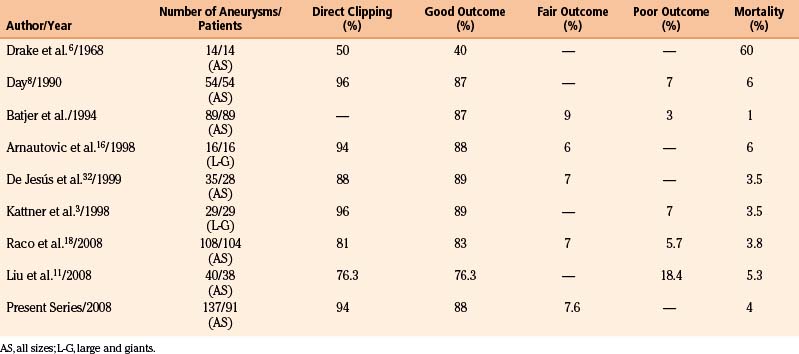
The series of direct surgical treatment of paraclinoid aneurysms (among them giant and large ones) reported more than 2 decades ago had relatively high mortality rates, ranging from 20% to 60%.6 Nonetheless, these authors should be credited for being the first to directly attack these difficult lesions. The postoperative mortality and morbidity decreased markedly over time, and the rate of direct surgical approaches to these lesions increased, as did the rate of successful clipping of these giant and large aneurysms. In the recently reported series of giant and large paraclinoid aneurysms, the mortality rates of direct surgery are lowered to less than 10% in the majority of series4,5,7,8,11,18,25 (Table 72-1). Furthermore, in his series, Batjer et al.2 reported 59% good, 23% fair, and 14% poor outcomes, as well as permanent ICA occlusions in 14%; Day,8 which also included small paraclinoid aneurysms, reported a 96% rate of successful clipping, with 87% good and 7% poor outcomes, and a 6% mortality rate. Overall, the direct surgery of giant aneurysms has recently increased, as has the rate of successful clipping. Furthermore, good outcomes range from 59% to 92%, and the number of patients with poor and fair outcomes is decreasing. Al-Mefty et al. reported a series of 16 large and giant aneurysms, with 88% good, 6% fair, and 6% mortality. In our personal experience in two reference centers, we have operated 992 aneurysms between 1992 and 2008. Of these, 137 (13.8%) were located at the paraclinoid region. Nine (6.5%) of them were giants. Of 91 patients, 30 (27%) had multiple aneurysms. Our mortality rate was 4.3% (4 patients). The overall complications rate was 20% with a reduction to less than 8% after 3 months.
Complications
Visual Outcome
Paraclinoid aneurysms may compress the optic apparatus and present clinically with visual deterioration. Also, the proximity of these aneurysms to the optic nerve accounts for the risk of visual deficit after surgical clipping. The risk of this complication seems to occur in 5% to 10% of patients regardless of the treatment strategy, and is more common when treating large or giant aneurysms.15 The risk to develop a new visual deficit is also related with the type of aneurysm. Proximal dorsal type aneurysms (true ophthalmic aneurysms) (Figs. 72-2A and 27-4) arise in close proximity to the ophthalmic artery, in contact with the undersurface of the optic nerve. When the aneurysm grows, direct compression of the optic nerve results, causing a progressive visual deficit. On the other hand, distal dorsal type aneurysms (Figs. 72-2A and 72-5) grow distal to the origin of the ophthalmic artery and do not produce a direct contact with the optic nerve until they become large or giant, situation that occurs less frequent when compared with the ophthalmic aneurysms. Ventral type aneurysms do not have a direct contact with the optic apparatus, and visual deficit may appear after upward displacement of the ICA in case of giant aneurysms (Fig. 72-9). After surgery, the main sources of optic nerve damage are (1) heat lesion caused by the drilling during the extradural unroofing of the nerve; (2) excessive mobilization of the nerve during clipping; (3) direct compression of the nerve caused by the clips, especially if large or multiple clips were used to treat a dorsal type aneurysm; and (4) sudden decompression of the nerve after clipping causing vascular damage.
Summary
Paraclinoid aneurysms have traditionally been considered difficult. The principal obstacles to safe clipping have been the initial poor view of the parent artery to get vascular control and the lack of exposition of the aneurysm dome. When one aneurysm is operated under these conditions, rupture during dissection or while clipping produces a very stressful scenario to the neurosurgeon. The life of the patient is put in danger or there exists the risk of an extensive and disabling infarction produced by the closure of the parent artery under desperate conditions. Past efforts by respectable neurosurgeons led to the present techniques and tools aimed at safe clipping of these lesions. The other important factor is that a wide variety of aneurysms and dome projections occur in only 1.5 to 2 cm of the ICA length, making the region a complex one. However, it is important to make the surgical approach a systematic one. The approach should include a good surgical planning evaluating the collateral blood flow conditions to anticipate the need for an indirect revascularization procedure if necessary. The type of the aneurysm should be identified because this will influence extension of the surgical approach and the kind of clips to be used. The surgical technique should include considerations about proximal control of the ICA, extent of the craniotomy, the need for an additional skull base approach (e.g., orbitozygomatic approach), dural opening, arachnoid cistern dissection, ACP resection technique, and aneurysm clipping. The availability of surgical tools should be checked (high-speed drill, set of clips, intraoperative Doppler, etc.). Even when this seems to be a very basic recommendation, having in mind all these aspects in a systematic way will permit the neurosurgeon to proceed to clip an aneurysm in this area with the highest rate of success.
Barami K., Hernandez V.S., Diaz F.G., Guthikonda M. Paraclinoid carotid aneurysms: surgical management, complications, and outcome based on a new classification scheme. Skull Base. 2003;13(1):31-41.
Batjer H.H., Kopitnik T.A., Giller C.A., Samson D.S. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650-658.
Batjer H.H., Samson D.S. Retrograde suction decompression of giant paraclinoidal aneurysms: technical note. J Neurosurg. 1990;73:305-306.
Bouthillier A., van Loveren H.R., Keller J.T. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38(3):425-433.
Bulsara K.R., Patel T., Fukushima T. Cerebral bypass surgery for skull base lesions: technical notes incorporating lessons learned over two decades. Neurosurg Focus. 2008;24(2):E11.
Day A. Aneurysms of the ophthalmic segment: a clinical and anatomical analysis. J Neurosurg. 1990;29:24-31.
De Jesús O., Sekhar L.N., Riedel C.J. Clinoid and paraclinoid aneurysms: surgical anatomy, operative techniques, and outcome. Surg Neurol. 1999;51:477-488.
Dolenc V. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667-672.
Drake C.G., Vanderlinded R., Amacher A. Carotid-ophthalmic aneurysm. J Neurosurg. 1968;29:24.
Fries G., Perneczky A., van Lindert E., Bahadori-Mortasawi F. Contralateral and ipsilateral microsurgical approaches to caroti-ophthalmic aneurysms. Neurosurgery. 1997;12:333-342.
Gibo H., Lenkey C., Rhoton A.L.Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560-574.
Hadeishi H., Suzuki A., Yasui N., Satou Y. Anterior clinoidectomy and opening of the internal auditory canal using an ultrasonic bone curette. Neurosurgery. 2003;52:867-871.
Kattner K.A., Bailes J., Fukushima T. Direct surgical management of large bulbous and giant aneurysms involving the paraclinoid segment of the internal carotid artery: report of 29 cases. Surg Neurol. 1998;49:471-480.
Khan N., Yoshimura S., Roth P., et al. Conventional microsurgical treatment of paraclinoid aneurysms: state of the art with the use of the selective extradural anterior clinoidectomy SEAC. Acta Neurochir Suppl. 2005;94:23-29.
Kobayashi S., Kyoshima K., Gibo H., et al. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989;70:216-221.
Krayenbühl N., Hafez A., Hernesniemi J.A., Krisht A.F. Taming the cavernous sinus: technique of hemostasis using fibrin glue. Neurosurgery. 61(Suppl 3), 2007. E-52
Mastronardi L., Sameshima T., Ducati A., et al. Extradural middle fossa approach. Proposal of a learning method: the “rule of two fans.” Technical note. Skull Base. 2006;16(3):181-184.
Nakagawa F.F., Kobayashi S., Toshiki T., Sugita K. Aneurysms protruding from the dorsal wall of the internal carotid artery. J Neurosurg. 1986;65:303-308.
Nonaka T., Haraguchi K., Baba T., et al. Clinical manifestations and surgical results for paraclinoid cerebral aneurysms presenting with visual symptoms. Surg Neurol. 2007;67(6):612-619.
Nutik S. Subclinoid aneurysms. J Neurosurg. 2003;98:731-736.
Yasargil M.G., Gasser J.C., Hodosh R.M., Rankin T.V. Carotid-ophthalmic aneurysm: direct microsurgical approach. Surg Neurol. 1977;8:155-165.
1. Barami K., Hernandez V.S., Diaz F.G., Guthikonda M. Paraclinoid carotid aneurysms: surgical management, complications, and outcome based on a new classification scheme. Skull Base. 2003;13(1):31-41.
2. Batjer H.H., Kopitnik T.A., Giller C.A., Samson D.S. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650-658.
3. Kattner K.A., Bailes J., Fukushima T. Direct surgical management of large bulbous and giant aneurysms involving the paraclinoid segment of the internal carotid artery: report of 29 cases. Surg Neurol. 1998;49:471-480.
4. Khan N., Yoshimura S., Roth P., et al. Conventional microsurgical treatment of paraclinoid aneurysms: state of the art with the use of the selective extradural anterior clinoidectomy SEAC. Acta Neurochir Suppl. 2005;94:23-29.
5. Xu B.N., Sun Z-H., Jiang J.L., et al. Surgical management of large and giant intracavernous and paraclinoid aneurysms. Chin Med J (Engl). 2008;121(12):1061-1064.
6. Drake C.G., Vanderlinded R., Amacher A. Carotid-ophthalmic aneurysm. J Neurosurg. 1968;29:24.
7. Yasargil M.G., Gasser J.C., Hodosh R.M., Rankin T.V. Carotid-ophthalmic aneurysm: direct microsurgical approach. Surg Neurol. 1977;8:155-165.
8. Day A. Aneurysms of the ophthalmic segment: a clinical and anatomical analysis. J Neurosurg. 1990;29:24-31.
9. Kinouchi H., Mizoi K., Nagamine Y., et al. Anterior paraclinoid aneurysms. J Neurosurg. 2002;96:1000-1005.
10. Kobayashi S., Kyoshima K., Gibo H., et al. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989;70:216-221.
11. Liu Y., You C., He M., Cai B.W. Microneurosurgical management of the clinoid and paraclinoid aneurysms. Neurol Res. 2008;30(6):552-556.
12. Nakagawa F.F., Kobayashi S., Toshiki T., Sugita K. Aneurysms protruding from the dorsal wall of the internal carotid artery. J Neurosurg. 1986;65:303-308.
12a. Fisher E. Die Labeabweichungen der vorderen Hirnarterie im Gefalblid. Zentralbl Neurochir. 1938;3:300-313.
13. Gibo H., Lenkey C., Rhoton A.L.Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560-574.
14. Bouthillier A., van Loveren H.R., Keller J.T. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38(3):425-433.
15. Nonaka T., Haraguchi K., Baba T., et al. Clinical manifestations and surgical results for paraclinoid cerebral aneurysms presenting with visual symptoms. Surg Neurol. 2007;67(6):612-619.
16. Arnautovic K.I., Al-Mefty A., Angtuaco E. A combined microsurgical skull-base and endovascular approach to paraclinoid aneurysms. Surg Neurol. 1998;50:504-520.
17. Dolenc V.A. Combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667-672.
18. Raco A., Frati A., Santoro A., et al. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008;108:1200-1210.
19. Nutik S. Subclinoid aneurysms. J Neurosurg. 2003;98:731-736.
20. Jou L.D., Lee D.H., Morsi H., Mawad M.E. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 2008;9:1761-1767.
21. Murakami K., Shimizu H., Matsumoto Y., Tominaga T. Acute ischemic complications after therapeutic parent artery occlusion with revascularization for complex internal carotid artery aneurysms. Surg Neurol. 2009;71:434-471.
22. Bulsara K.R., Patel T., Fukushima T. Cerebral bypass surgery for skull base lesions: technical notes incorporating lessons learned over two decades. Neurosurg Focus. 2008;24(2):E11.
23. Mastronardi L., Sameshima T., Ducati A., et al. Extradural middle fossa approach. Proposal of a learning method: the “rule of two fans.” Technical note. Skull Base. 2006;16(3):181-184.
24. Thorell W., Rasmussen P., Perl J., et al. Balloon-assisted microvascular clipping of paraclinoid aneurysms. Technical note. J Neurosurg. 2004;100(4):713-716.
25. Batjer H.H., Samson D.S. Retrograde suction decompression of giant paraclinoidal aneurysms: technical note. J Neurosurg. 1990;73:305-306.
26. Noguchi A., Balasingam V., Shiokawa Y., et al. Extradural anterior clinoidectomy. Technical note. J Neurosurg. 2005;102:945-950.
27. Hadeishi H., Suzuki A., Yasui N., Satou Y. Anterior clinoidectomy and opening of the internal auditory canal using an ultrasonic bone curette. Neurosurgery. 2003;52:867-871.
28. Krayenbühl N., Hafez A., Hernesniemi J.A., Krisht A.F. Taming the cavernous sinus: technique of hemostasis using fibrin glue. Neurosurgery. 61(Suppl 3), 2007. E-52
29. Lui J.K., Fukushima T., Sameshima T., Al-Mefty O., Couldwell W.T. Increasing exposure of the petrous internal carotid artery for revascularization using the transzygomatic extended middle fossa approach: a cadaveric morphometric study. Neurosurgery. 2006;59(Suppl 2):ONS309-ONS318.
30. Clatterbuck R.E., Tamargo R. Contralateral approaches to multiple cerebral aneurysms. Neurosurgery. 2005;57(Suppl 1):160-163.
31. Fries G., Perneczky A., van Lindert E., Bahadori-Mortasawi F. Contralateral and ipsilateral microsurgical approaches to carotid-ophthalmic aneurysms. Neurosurgery. 1997;12:333-342.
32. De Jesús O., Sekhar L.N., Riedel C.J. Clinoid and paraclinoid aneurysms: surgical anatomy, operative techniques, and outcome. Surg Neurol. 1999;51:477-488.