27 Surgical Revascularization of the Posterior Circulation
Surgical anatomy and collateral circulation
Most patients have two patent vertebral arteries (VAs), and the left one is usually dominant. In some patients, one of the arteries ends in the PICA, or is occluded by atherosclerotic disease, which makes the inflow situation more tenuous. At the upper end, the posterior communicating arteries connect the ICAs with the PCAs; however, the size of these is variable. When there are two posterior communicating arteries (PCOM) that are 1 mm. or more in size, then the patient may safely tolerate basilar artery (BA)1 or bilateral VA occlusion, but in patients who have less than optimal collaterals (e.g., only one PCOM of ≥1 mm in size), the revascularization must be considered, prior to occlusion. Other variants may include bilateral (or unilateral) fetal PCOMs, fenestrations of the vertebrobasilar junction, persistent trigeminal arteries (with varying degrees of contribution to the upper BA), etc.
Endovascular alternatives
In patients with aneurysms, or ischemic disease, endovascular treatment assisted by endoluminal stents (stent with coiling, or angioplasty followed by a stent) may be an alternative.2–4 Based on the patient’s age and other risk factors, this should be discussed with the patient. Centers where the microsurgical treatment is performed should also have the appropriate endovascular expertise, and collaboration.
Skull base approaches
For operations involving bypass procedures, and for complex posterior circulation aneurysms in general, skull base approaches are essential. The correct approach to use for each case is determined by a careful study of the aneurysm anatomy, along with the landmarks on the skull, and the experience of the surgeon. The most common approaches used are the orbitozygomatic approach, the transpetrosal approach (retrolabyrinthine approach, or partial labyrynthectomy petrous apicectomy approach, and rarely, a total petrosectomy approach), a combined far-lateral retrosigmoid approach, the presigmoid approach (with the division and resuture of the sigmoid sinus in some patients), and the extreme lateral transtubercular approach.5 Some of the complex approaches take time to develop, and in elective cases, a staged approach is taken, with the approach being performed on one day, and the definitive operation on another day.
Bypass procedures
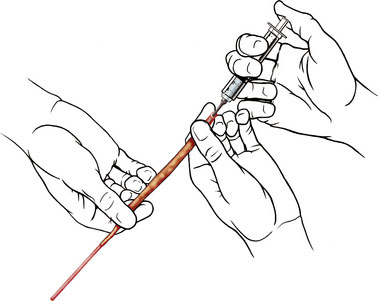
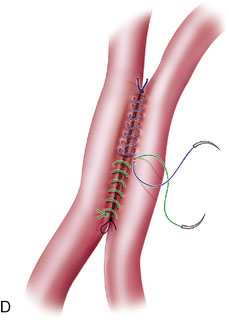
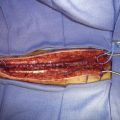
Two types of bypasses are used: in situ bypass procedures and bypass procedures using graft vessels for flow diversion. Bypasses using graft vessels such as the radial artery graft (RAG) and SV graft are generally high flow and can be either EC-IC (connecting extracranial vessel to intracranial vessel) or IC-IC (connecting two intracranial vessels). In general, the type of bypass performed depends upon the need (small vessel replacement needs an in situ or low-flow bypass, whereas large vessel replacement will require a high-flow bypass). The availability of donor and recipient vessels, and graft vessels, is also a factor. A bypass can only replace major vessels, and cannot solve perforator problems. In some patients with unruptured aneurysms, a distal (rather than proximal) occlusion of the aneurysm may be used in order to protect the flow through the perforating vessels. EC-IC bypasses include low-flow bypasses such as the superficial temporal to the superior cerebellar artery, and an occipital (OC) to PICA anastomosis (OC-PICA bypass), and high-flow bypasses that encompass RAGs or SV grafts. The choice between the RAG and the SVG is based on the size of the available donor vessel (diameter >0.23 cm for the RAG, and 0.3 cm for the SV), the passage of the Allen test for the RAG, and an adequate length available (at least 20 cm) for the SVG. We make this determination on the basis of a preoperative duplex examination. For RAGs, the use of the pressure distension technique has revolutionized the patency rate, by greatly reducing the incidence of postoperative vasospasm (Figure 27–1).6 High-flow bypasses are generally constructed from the ipsilateral VA, or the ECA, to end in the VA, or the PCA. In one case, the bypass was placed directly into the BA, under deep hypothermic circulatory arrest.
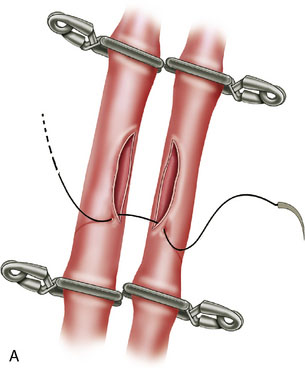
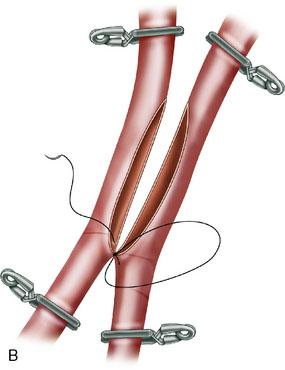
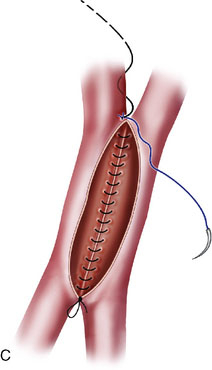
In situ bypasses are technically easier to perform than EC-IC bypasses. In situ bypasses include reimplantation of arteries, short interposition grafts, direct resuture of vessels, and side-to-side anastomosis. Reimplantation of vessels can be done in an end-to-side fashion after the resection of the aneurysm from which the vessel originates. Segmental resection with an interposition graft of similar caliber is performed when an aneurysm involves a segment of a vessel (e.g., fusiform aneurysm). Side-to-side anastomosis in posterior circulation is performed for flow replacement when clipping of aneurysm will result in total occlusion (or critical stenosis) of a small artery. Both the vessels of the anastomosis need to be approximately of the same caliber. Aneurysms involving PICA, AICA, or SCA can be addressed with PICA-PICA to AICA-PICA, or SCA-PICA, anastomosis (Table 27–1).7,8 Side-to-side anastomosis is a comparatively easier technique to perform for a small-vessel revascularization and generally stays patent (there were no known occlusions in our series) (Figure 27–2).7
Table 27–1 Bypass Procedures Performed for Various Conditions in Posterior Circulation.
| Treatment | Total number | |
|---|---|---|
| Aneurysms | ||
| Basilar tip aneurysms | M2 MCA to P2 PCA with microsurgical clipping (2), ECA to P2 PCA with distal basilar occlusion (1) | 3 |
| Midbasilar aneurysms | V3 VA to P2 PCA (SVG or RAG) with distal occlusion of basilar artery | 5 |
| Vertebral artery aneurysms | V3 VA to PCA with occlusion of vertebral artery | 4 |
| AICA aneurysms | AICA-PICA in situ side-to-side anastomosis | 1 |
| PICA aneurysms | OC-PICA anastomosis CCA to PICA RAG with occlusion of PICA (1), PICA to PICA (1) |
10 |
| Ischemia | ||
| Vertebral dissections | V3 VA to P2 PCA (SVG or RAG), with occlusion of vertebral artery | 3 |
| Tumors | ||
| Chordoma | V3 VA to P3 PCA (SVG or RAG) with gross total resection tumor along with encased portion of vertebral artery | 5 |
| Giant cell tumor (foramen magnum) | SVG interposition graft for vertebral artery with gross total resection of tumor | 4 |
| Meningioma (foramen magnum) | Vertebral artery resection and anastomosis, and gross total resection of tumor | 7 |
AICA, anterior inferior cerebellar artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery; VA, vertebral artery.
Case examples
Patient 1
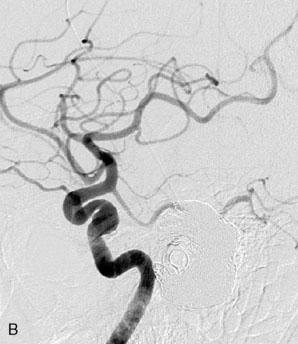
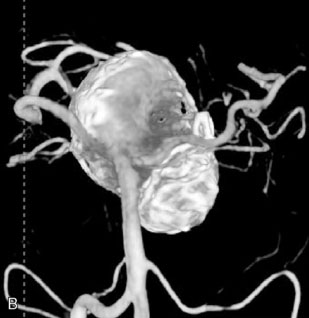
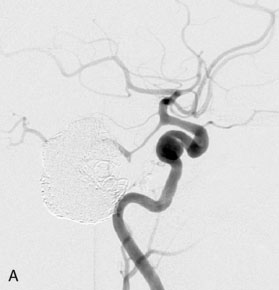
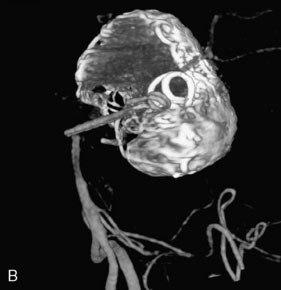
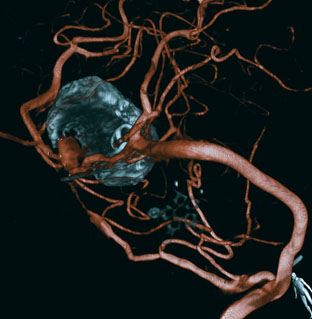
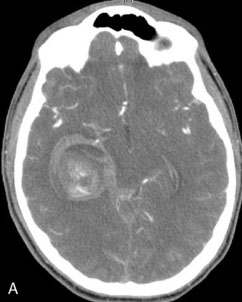
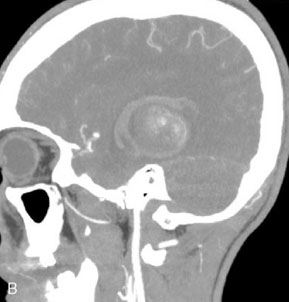
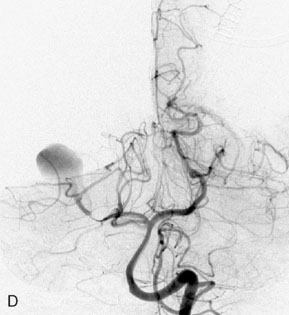
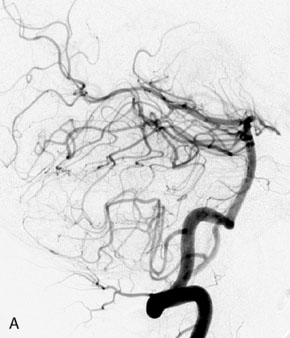
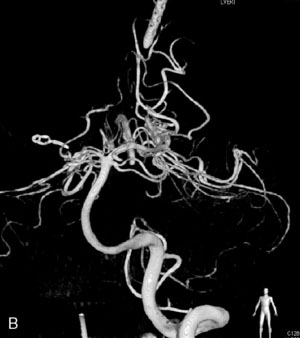
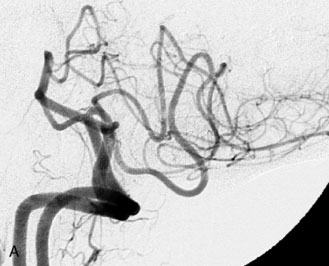
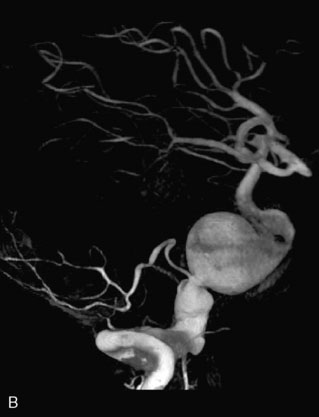
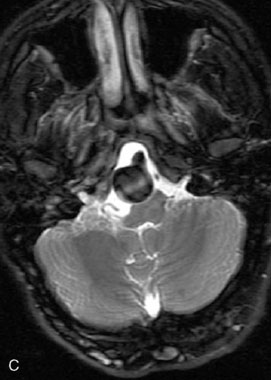
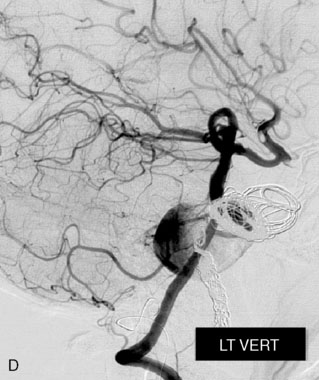
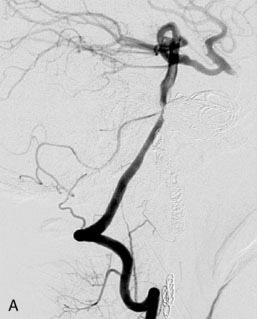
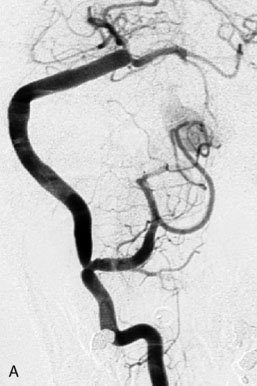
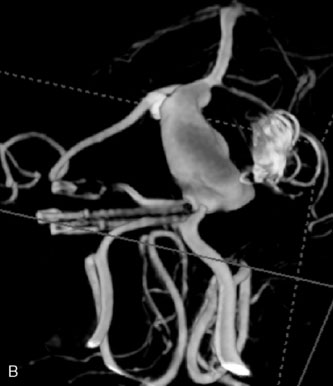
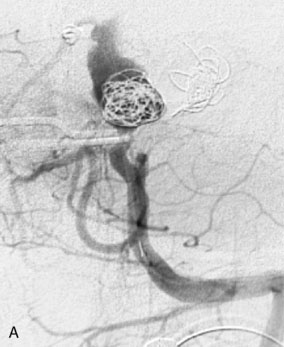
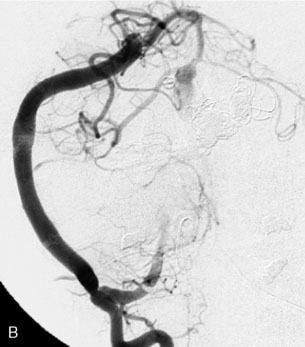
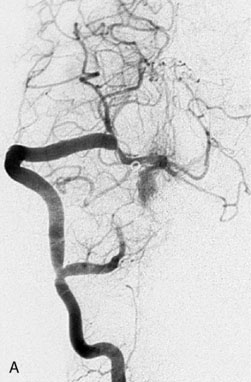
This 72-year-old woman presented with a subarachnoid hemorrhage (Hunt and Hess 4) secondary to a ruptured basilar tip aneurysm and was initially treated by endovascular coiling. She had various strokes during the hospitalization, and sustained permanent right third nerve palsy, but recovered to be independent for all daily living activities. Follow-up angiograms showed aneurysm recurrence due to coil compaction with the entire aneurysm mass measuring 21 × 19 × 25 mm, causing significant brainstem compression. She had four additional coiling procedures over the previous 5-year follow-up period (see Figures 27–1, 27–2A and 27–2B). Her examination indicated a right third nerve palsy, memory difficulties, and mild gait ataxia, and no other deficits. Her functional status was graded as modified Rankin score (mRS) 2.
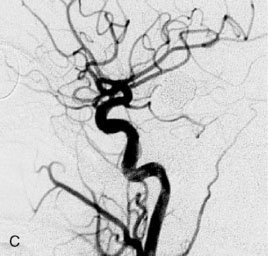
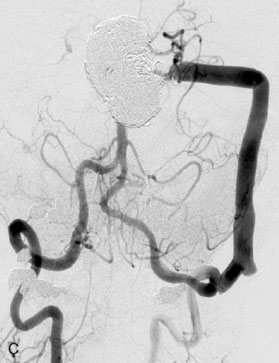
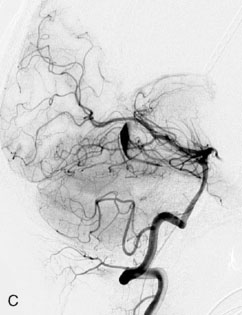
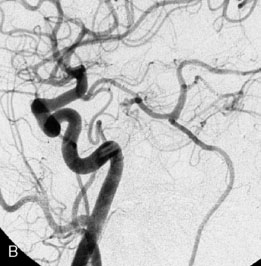
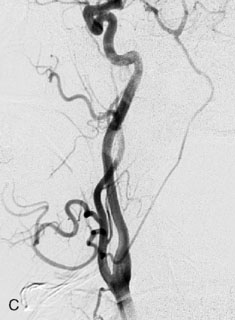
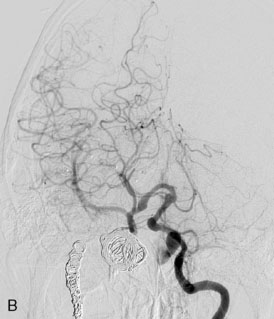
The patient suffered a transient neurological decline postoperatively, but recovered significantly with fluid administration. She recuperated in a rehabilitation facility, with recovery to her baseline at about 3 months. An angiogram immediately after the operation showed excellent flow through the bypass into the PCA, and occlusion of the aneurysm (Figure 27–3 and Figures 27–4A and 27–4B). Delayed angiography at 3 months showed sluggish flow through the bypass. Angiography at 18 months postoperatively demonstrated the complete occlusion of the bypass and the aneurysm, but good filling of the right PCA from the BA and through the right PCOM artery (Figures 27–4C and 27–4D). At this time, and at 4 years postoperatively, the patient was independent for activities of daily life. Her gait ataxia was worse than her preoperative baseline, with the patient needing a cane, and not driving. But she was mentally alert with a good memory, and able to go out to restaurants, exercise, and socialize (mRS 2).
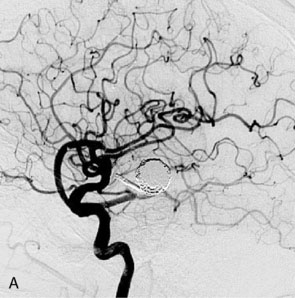
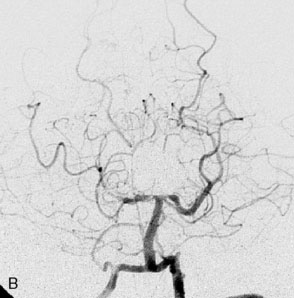
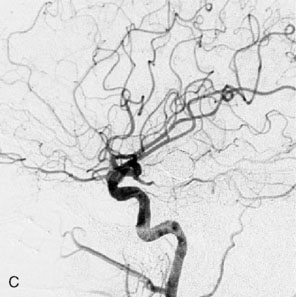
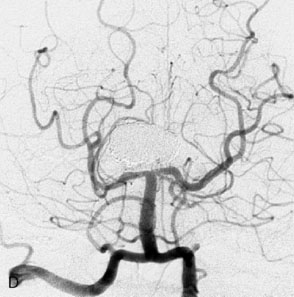
Figure 27–4 A, Immediate postoperative angiogram (right internal carotid angiogram, lateral view) showing the aneurysm completely occluded with the patent bypass and the PCOM. B, Immediate postoperative angiogram vertebral injection AP view. Note the filling of the right PCA. C, Angiograms 18 months postsurgery (RICA lateral view). Showing the patent PCOM and occluded bypass. D, Angiogram 18 months postsurgery (vertebral injection, AP view), showing the aneurysm completely occluded from the circulation. The right PCA is enlarged and filling better compared to immediately after surgery (see Figure 27-2D).
Patient 2
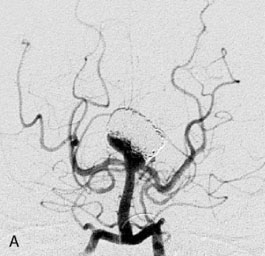
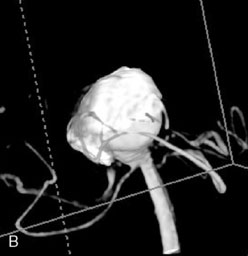
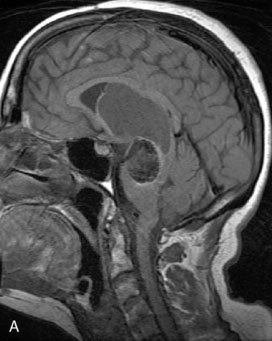
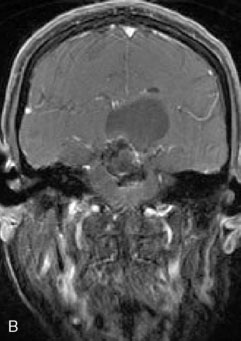
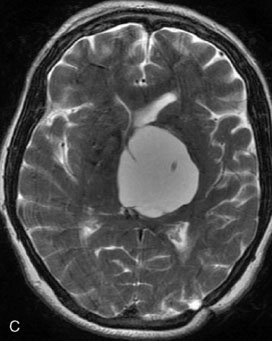
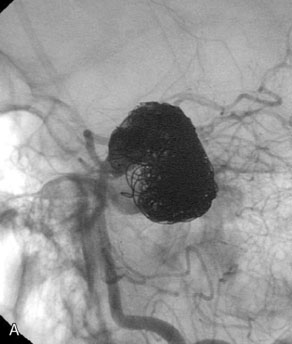
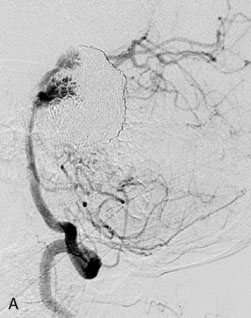
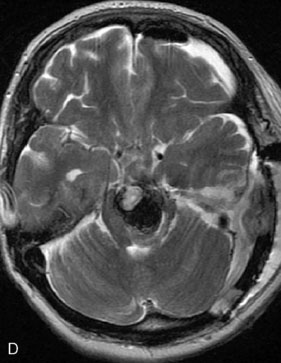
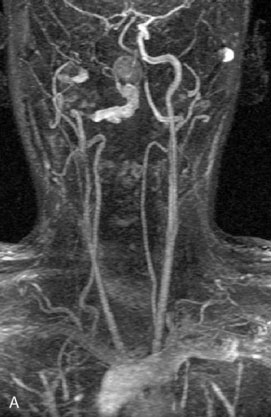
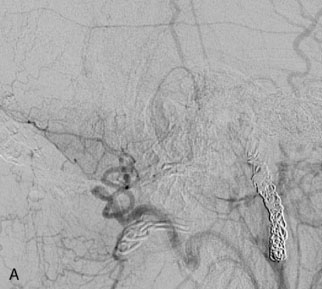
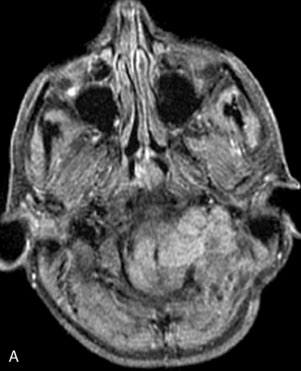
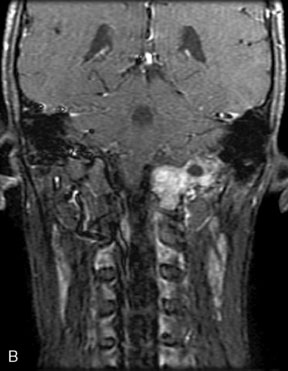
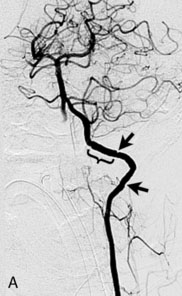
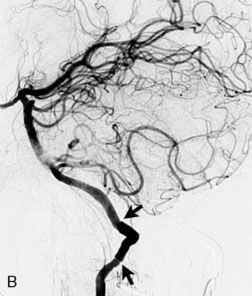
A 62-year-old woman presented with progressive deterioration in her daily abilities and difficulty swallowing over a period of 1 year. She had undergone four coiling procedures over a period of 4 years, for an unruptured basilar tip aneurysm measuring 27 × 22 × 21 mm (Figures 27–5 and 27–6). Her neurological condition had steadily declined. She was unable to swallow liquids, but could swallow solids. On examination, she was awake, was unable to speak, could follow commands intermittently, had right facial paralysis, and had severe right spastic hemiparesis as a result of which she was restricted to a wheelchair. An MRI scan showed a large cyst adjacent to the aneurysm, which was extending into the third and lateral ventricles (see Figure 27–5). The brain cyst had been previously treated using an Ommaya catheter, which had later been fenestrated when clogged. Cerebral angiography demonstrated continued filling of the aneurysm from the BA (see Figure 27–6A). The patient had a very small PCOM artery (<1 mm) on the left side and a large PCOM artery (≥1 mm) on the right (see Figure 27–6B).
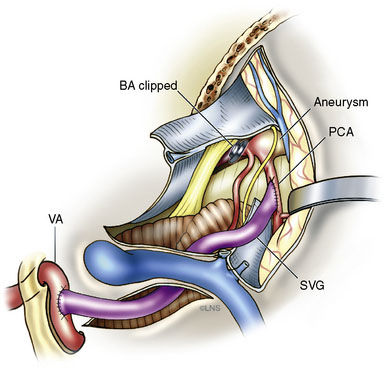
After the endoscopic fenestration of the cyst, the treatment of the aneurysm was done in two stages. The first stage was a transtemporal, transpetrosal approach to the posterior fossa with exposure of V2 and V3 segments of the VA and decompression of sigmoid sinus and facial nerve. This approach was the only one implemented due to the time taken for the procedure (about 6 hours). The second stage of the operation was performed 3 days afterward and involved the bypass procedure with a SV graft from the VA (V3 segment) to the right PCA (P2 segment), followed by occlusion of the upper BA (Figure 27–7). During this operation, the aneurysm could not be visualized due to the larger coil mass and the brainstem, which was draped around it. The BA was occluded just inferior to the SCA. A saphenous vein was chosen for the graft, since the radial arteries were not available to be used as a bypass conduit.
The patient recovered without any postoperative complications. An immediate postoperative angiogram and an MRI scan showed that the aneurysm remnant filling from the right PCOM artery and through the bypass (Figures 27-8 through 27-10). The graft was widely patent, and the BA terminated at the site of the clip. At 18 months follow-up, the patient had improved marginally from her condition at initial presentation. She was able to converse with a few words, remember and speak with family members, and walk a few steps with assistance with her hemiparesis improved. Angiograms at this time showed a patent graft and partially filling stable aneurysm remnant.
Patient 3
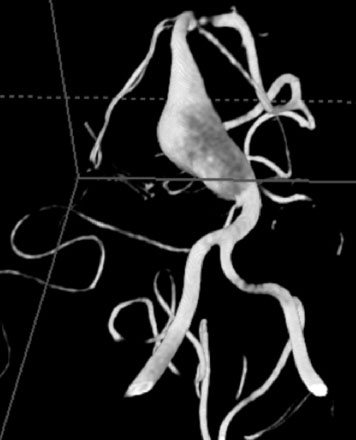
A 17-year-old boy presented with headaches for 3 days, and stabbing eye pain and vomiting for a day. On CT scan, he was found to have a giant aneurysm with hemorrhage measuring 5 × 4.9 × 4.0 (Figure 27–11A-D) centered around the right hippocampus.
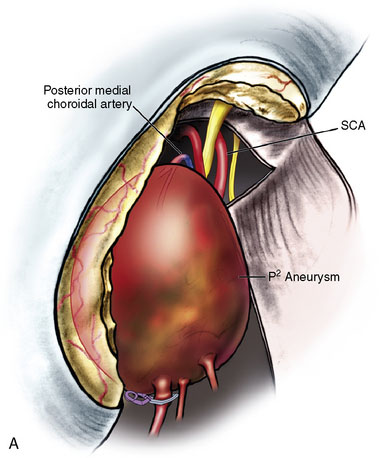
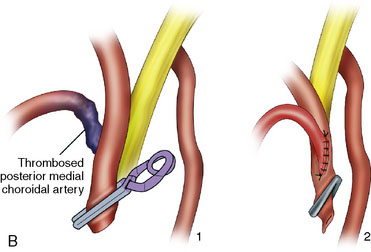
The angiogram showed a giant aneurysm arising from the P2 segment of the right PCA also associated with an additional fetal PCOM on the same side (see Figure 27–11A-D). A frontotemporal craniotomy with transsylvian approach was used to dissect the aneurysm, although it was found very difficult to identify proximal and distal vessels. Despite the ventriculostomy the brain was found to be rather full and the right temporal gyrus was resected to dissect the aneurysm. Following the puncturing and partial evacuation of blood clots, the aneurysm was clipped and resected completely with a segment of parent vessel (Figure 27–12). Side-to-side anastomosis of the remaining vessel ends was performed, which was later identified as the posterior lateral choroidal branch of the PCA (Figures 27–13 and 27–14).
Patient 4
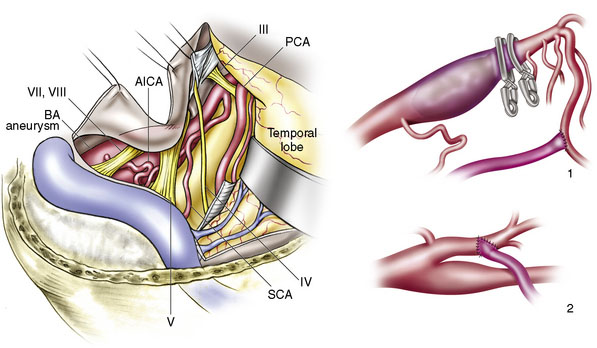
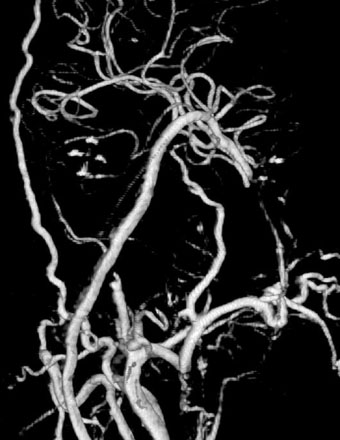
A 42-year-old man presented after an automobile accident, after suddenly passing out. The patient had spells of dizziness and problems with memory for 2 years. CTA showed a giant (27 × 13 mm) diffuse fusifom aneurysm of the BA, arising from below the AICA and ending 1 cm inferior to the origin of SUCA (Figure 27–15). Since the anatomy of the aneurysm was not favorable for endovascular coiling (even with stenting), revascularization was planned. Collateral circulation to the BA, distal to the aneurysm was poor. The patient had a fetal PCOM on the right side and no PCOM on the left side. Via a transpetrosal approach, a left-sided ECA-PCA bypass was performed with an RAG followed by the distal occlusion of the BA (Figures 27–16 and 27–17). The patient recovered well with no complications and was discharged home in 2 weeks. Follow-up angiograms showed a decrease in graft flow and good flow in the left PCA supplied by an enlarged PCOM.
Patient 5
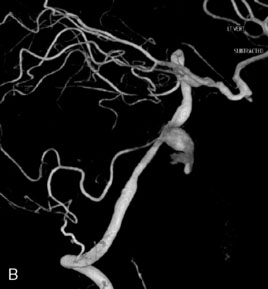
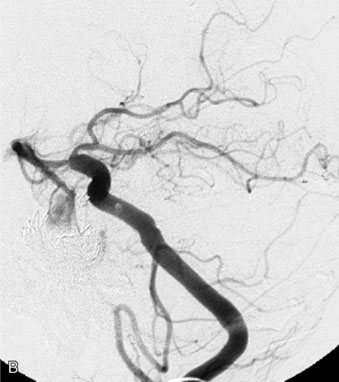
This 21-year-old man with a known history of collagen vascular disease more prominent on the right side of the body, presented for evaluation of a fusiform aneurysm of the VA (Figures 27–18 and 27–19). Nine years ago, he had a coil embolization of an ICA aneurysm, which resulted in occlusion of the right ICA. The aneurysm measured 1 × 2.2 cm extending from the V3 region to the origin of the PICA, without involving it. He tolerated the balloon occlusion test well, without symptoms. However, the right PICA was not filling with the left vertebral injections, indicating a need for its revascularization. An OC-PICA anastomosis was performed via a far lateral transtubercular approach with the clip occlusion of the right VA (Figure 27–20). Dissection of the occipital artery as well as performing the approach were tedious because the tissues were not elastic and exuberant. The patient recovered well postoperatively with no neurological deficits (Figures 27-21 through 27-23). He developed a CSF leak from the wound (which was re-explored and resutured) and developed hydrocephalus for which a shunt procedure was performed.
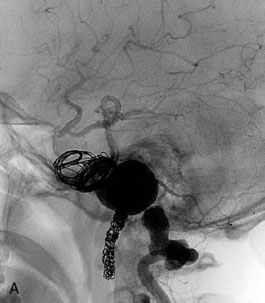
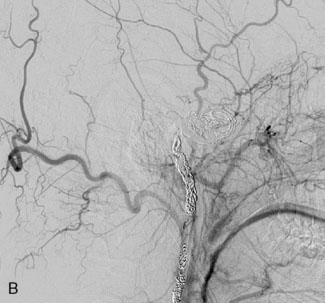
Figure 27–18 A, The aneurysm size and location. B, Previously occluded ICA circulation on the right side.
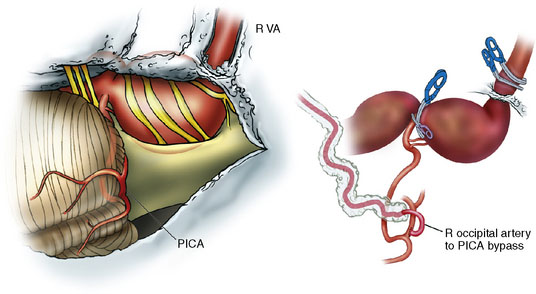
Figure 27–20 Illustration showing the clipping of vertebral with OC-PICA anastomosis.
(Courtesy of Laligam Sekhar.)
After 40 days, an anterior circulation revascularization was performed with an RAG from the ECA to the M2 segment of the MCA to treat the tenuous nature of blood supply to the right brain, which was dependent on the left ICA (see Figure 27–23). The left VA was chosen, since the right was abnormal, and the right MCA (M2) was found to have a normal caliber and wall thickness during surgery.
Patient 6
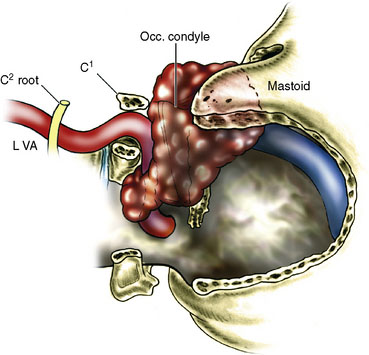
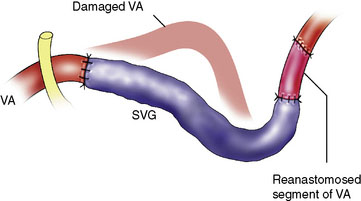
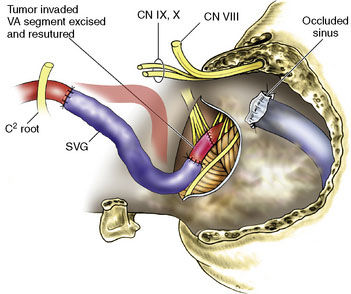
A 16-year-old boy had a recurrent giant cell tumor of the lower clivus, C1, occipital condyle, foramen magnum area on the left side, 4 years after initial resection (Figure 27–24). An extreme lateral complete transcondylar approach was performed for a total tumor resection. The tumor was filling the jugular bulb, occluding the sigmoid sinus and encasing the hypoglossal nerve. Gross total resection was pursued meticulously and all extensions of the tumor were removed (Figures 27–24A, 27–25A, and 27-5B). Extracranially, the nondominant left VA was encased by the tumor (Figure 27–26), supplied numerous branches to it, and was damaged during tumor removal. An SV graft was used to replace the affected V3 segment (Figure 27–27). Also, there was an additional area of tumor involvement found at the entrance point of the VA (V3-V4 junction). This segment was resected and the artery primarily resutured (Figure 27–28). He recovered well after surgery and follow-up angiogram confirmed the patency of the VA. Because of the unilateral condylar resection, occiput-C3 fusion was done followed by halo traction. Vocal cord medialization was done later to improve voice quality to compensate for cranial nerve X weakness. The patient remained tumor free 4 years after surgery.
Patient 7
A 10-year-old boy referred for the treatment of a recurrent giant fusiform basilar aneurysm previously coiled two times (Figure 27–29). A right far lateral and transpetrosal approach was used to perform SVG bypass from the V3 segment of the VA to the P2 segment of the PCA along with the proximal occlusion of both VAs for complete obliteration of the aneurysm (Figure 27–30). A postoperative MRI and angiograms showed presence of filling from the contralateral VA (see Figure 27–30). This residual filling was treated with further coil embolizations and the aneurysm was completely obliterated (Figures 27–31 and 27–32). This specific case illustrates the usefulness of endovascular therapy in obliterating minimal remnants, thereby augmenting the success of surgical therapy.
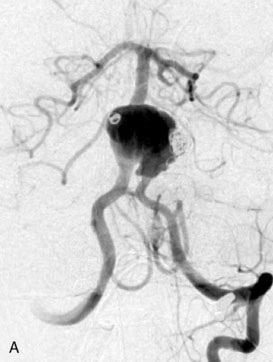
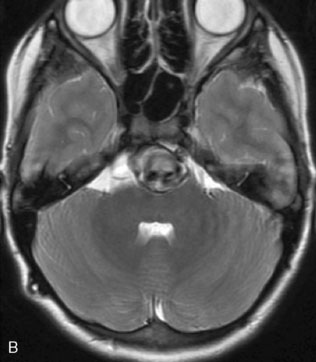
Figure 27–29 A, Fusiform aneurysm with coils inside from previous embolization. B, MRI showing the fusiform aneurysm of the BA.
Results and complications
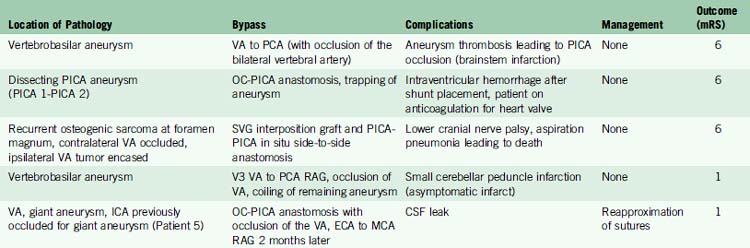
Tables 27-2 through 27-4 show the results of in situ and EC-IC posterior circulation bypasses in 42 patients (45 bypasses) performed from 1989 to December 2009. All of the bypasses (100%) were patent immediately postsurgery. Two patients had delayed graft occlusion, which was found on angiograms on follow-up visits. One of these patients had an RAG bypass from MCA to P2 PCA bypass for basilar tip aneurysm and the other had an OC-PICA anastomosis with occlusion of the VA (for a giant VA aneurysm dissection).
Table 27–4 Patency Rates of Bypasses in Posterior Circulation.
| Time Period Postbypass | Patency of Bypasses | Patency Rate |
|---|---|---|
| Immediate postoperative | All patent | 100% |
| Medium- to long-term follow-up (>6 weeks) | Two grafts spontaneously occluded—1 RAG (one MCA and P2 PCA delayed occlusion), 1 OC-PICA. Both patients asymptomatic with well-developed collateral flow | 95% |
There were three perioperative deaths and one major stroke (see following for details). The final outcome of the posterior circulation bypass patients was mRS 0-1 in 25 (60%), mRS 2 in nine (21%), mRS 3 in four (10%), mRS 4-5 in one (2%), and mRS 6 in three (7%) of the population (see Table 27–3).
Conclusion
The availability of flow diversion stents (if present studies show them to be safe and effective) will make them the treatment of choice for some complex posterior circulation aneurysms in the future.2,9 However, various types of bypasses will continue to remain effective options for the treatment of some of these aneurysms. Microsurgeons and endovascular surgeons need to be aware of these options, and work collaboratively in order to achieve the best outcomes for their patients.
1 Steinberg G.K., Drake C.G., Peerless S.J. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161-173.
2 Fiorella D., et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6-16. discussion 16–17
3 Thorell W.E., et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035-1040. discussion 1035–40
4 Wanke I., Gizewski E., Forsting M. Horizontal stent placement plus coiling in a broad-based basilar-tip aneurysm: an alternative to the Y-stent technique. Neuroradiology. 2006;48:817-820.
5 Babu R.P., Sekhar L.N., Wright D.C. Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg. 1994;81:49-59.
6 Sekhar L.N., et al. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646-658. discussion 658–9
7 Ramanathan D., Hegazy A., Mukherjee S., et al. Intracranial in-situ side-to-side microvascular anastomosis: principles, operative technique and applications. World Neurosurgery. 2010. In press
8 Quinones-Hinojosa A., Lawton M.T. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2008;62(Suppl 3):1442-1449.
9 Biondi A., et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61:460-468. discussion 468–9