CHAPTER 108 Surgical Procedures for the Control of Chronic Pain
The results of surgical therapy for chronic benign pain syndromes are poor.1,2 Surgical experience with central deafferentation (e.g., cordotomy)3–9 suggested that patients with neoplastic pain achieved satisfactory results more frequently than those with benign pain. Apparently, the longer life expectancy of patients with benign pain allowed neural plasticity to overcome surgical interruption.10 This was borne out in studies of cordotomy or dorsal rhizotomy.11–17 A distinct characteristic of refractory benign pain is that it appears to follow lesions of the nervous system. This is in contrast to malignant pain, which is thought to be nociceptive.
Mechanisms of Chronic Pain Production
The immediate effect of injury is to activate receptors to cause firing in specific nerve fiber types. These include large myelinated Aβ fibers, small myelinated Aδ fibers, and small unmyelinated C fibers. The interaction of these afferents, through the substantia gelatinosa, forms the basis for the gate control theory of pain. The cell bodies of primary nociceptive neurons are located in the dorsal root ganglion, with afferent synapses in layer I or V. Layer I cells are nociceptor specific and somewhat less discriminatory. Layer V cells respond to many inputs, mainly repetitive nociceptive stimuli and nociceptive input. The primary nociceptive neurons synapse on rostrally projecting second-order neurons in the dorsal horn, the theoretic target of the “gate.”18 After postinjury discharge, the next normal event would be for the fibers to return to rest. In the face of persisting injury, however, repeated firings are provoked; some receptors become more sensitive to subsequent stimulation and can, in fact, fire without further stimulation. This sensitization can arise from direct change in the structure of the nociceptor13,19,20 or as a response to substances released in its milieu.21–24 As damaged tissue heals with scar formation, granulation tissue containing nerve sprouts and capillaries invades the area. This further changes the local environment and the properties of the nerve endings. Such changes have relevance in the healing of surgical wounds such as those from laminectomy or spinal fusion; during healing, there may be significant pain problems generated by the wound itself.
The physiologic responses to nerve damage are complex and variable. Devor broadly classifies these into three groups.25 The first, sensitization of the nociceptor endings, is characterized by a reduced activation threshold for activation; in such a state, non-noxious stimuli may become capable of producing pain. Second, the nociceptive fibers themselves become a source of pain when they are activated at abnormal locations along their course; this is the phenomenon of ectopic electrogenesis. Third, pain could result from abnormal central processing of afferent impulses. In the setting of chronic spinal pain syndromes, the first two possibilities are most pertinent, with true central pain syndromes being uncommon.
Chemical21,22,24 and mechanical26–28 stimuli can invoke or modify repetitive discharge in the damaged nerve. Epinephrine and norepinephrine can both activate afferent fiber endings in neuromas; these responses are thought to be mediated by α-adrenergic receptors.23,29,30 Experimental observation indicates that sympathetic system activity can produce abnormal sensation through neural transmitter release that stimulates afferent nerve sprouts possessing ectopic adrenergic chemosensitivity.30 The abnormal sensations produced by these mechanisms may explain causalgia and other sympathetic dystrophies,31,32 along with the potential benefit of sympathectomy for such disorders.
The phenomenon of ectopic electrogenesis, which occurs in neuromas, can also develop in axons that have become demyelinated but remain in continuity. This issue relates directly to chronic low back and leg pain, in which a neuroma would not be expected to form, but in which demyelination of nerve roots is a known complication. Such demyelinated roots may exhibit either hyposensitivity or hypersensitivity. Spontaneous discharge has been shown to occur at sites of peripheral demyelination.32 These discharge patterns are similar to those found in neuromas.25 Hence nerves with regions of demyelination can demonstrate ectopic electrogenesis, which transfers nociceptor-like information into the central nervous system. Rhythmic firing, a characteristic of cell behavior not elicited until a certain threshold level of generation current is reached,33 can be provoked in demyelinated regions by mechanical stimuli. This threshold characteristic is important because many injured nerves appear to be poised near the rhythmic firing threshold. Hence brief or weakened stimuli can set off prolonged discharge that may persist beyond removal of the stimulus.25 In experimental preparations, tetanic stimulation produces this so-called after-discharge, which is followed by a period of prolonged electrical silence.34 It is evident that this could have implications in pain relief from spinal cord stimulation.
The dorsal root ganglion demonstrates mechanical sensitivity in its normal state and has such a high level of baseline excitability that some discharge occurs spontaneously35; after discharge occurs, stimulation is common. This baseline excitability is heightened after peripheral nerve injury. In this instance, the dorsal root ganglion contributes ectopic barrages above and beyond those generated by the region of peripheral injury.36,37 The state of excitability of the dorsal root ganglion is thus of clinical importance in root compromise.32 In the chronically injured root, deafferentation in the form of ganglionectomy would, theoretically, remove this focus of irritability.
Damage to a peripheral nerve causes changes central to the lesion that may not be reversed by treating the original injury.10 As noted earlier,25,35 these central changes include heightened sensitivity of the dorsal root ganglion to mechanical distortion and to neurotransmitters. Axons central to a nerve lesion also diminish their conduction velocity. Cells in the dorsal root ganglion may degenerate, with consequent degeneration of central axons. This leads to substantial loss of afferent fibers and produces deafferentation, which is another mechanism of pain. Additionally, the central terminals of C fibers change in response to peripheral nerve injury. The result is a failure of feedback mechanisms that produce prolonged depolarization and inhibition. Peripheral nerve section is thus followed by a reduction in inhibition of afferent fibers.37 The cord “responds” to diminished input (deafferentation) by diminishing inhibitions to the remaining input.38 The spinal cord itself thus becomes a location for continuing provocation of pain through mechanisms of chronic afferent barrage accompanied by reduced inhibition. Not surprisingly, many central ablative procedures such as cordotomy have been proposed as treatments for chronic pain syndromes. However, the role of these more central procedures in the treatment of chronic spinal pain syndromes is limited.
Finally, the individual motion segment is richly innervated and thus capable of generating postinjury pain in the absence of frank neural compression.39–44 Many of the procedures discussed next are intended for the treatment of continued extremity pain caused by persistent neurogenic dysfunction; such procedures are not, in general, successful in dealing with disorders of the motion segment per se. Thus entities such as posttraumatic lumbar strain, postdecompressive segmental instability, and persistent discogenic pain are not well served by deafferentation procedures. Additionally, reversible sources of neural compression producing continued sciatica such as disc herniation, lateral recess, or central or foraminal stenosis must be meticulously excluded before the consideration of any of these procedures. Indeed, the most effective way to deal with the “failed back” (failed back surgery syndrome, FBSS) is to avoid creating it by judicious and appropriately indicated initial treatment and surgery. Given the historical and current rates of spine surgery,45 in the settings of favorable natural history of many degenerative syndromes,46 it is unlikely that the incidence of FBSS will decrease.
Modulatory therapies that are germane to the concept of inhibition are nerve and cord stimulation and epidural implants. Destructive therapies are essentially deafferentation procedures: rhizotomy and ganglionectomy. More central ablative procedures have no place in the current treatment of failed lumbar surgery syndromes. For example, the dorsal root entry zone (DREZ) lesion, produced by electrocoagulation, has been reported to yield a success rate of 54% to 82% in brachial plexus avulsion47–50 and 50% in neurogenic pain from spinal cord injury; however, in benign pain syndromes or arachnoiditis, dismal results have been reported.51–54 Cordotomy55 has been extensively studied in cases of neoplastic pain and can be of major benefit in this instance.3,5,7,9 The procedure, in which the anterolateral pathways of the spinal cord are divided, thus interrupting pain and temperature transmission, has also been investigated for cases of lower cord or cauda equina injury. Porter and colleagues8 reported a 62% rate of significant pain relief in follow-up ranging from 8 to 20 years. White and Sweet cited a 60% rate of pain relief in patients with cord injuries and in four of seven with cauda equina damage. The complications of this procedure are significant, however: urinary incontinence, sexual dysfunction, and leg weakness. Additionally, genitourinary dysfunction rates of 8% to 92% have been reported.5,6,8,9
Deafferentation Procedures
Little attention in the recent literature has been focused on the use of rhizotomy as a treatment for chronic backache and sciatica. It has, however, been widely investigated in other areas. As noted previously, results in tumor patients are generally superior to those achieved in chronic benign pain patients.12,56–60 In a comprehensive review, Barrash and Leavens61 analyzed dorsal rhizotomy for relief of tumor pain. Promising results of rhizotomy were noted in cases of central neoplasms, as well as neoplasms involving the breast, colon, head and neck, lung, and rectal and urogenital systems. The problem of trigeminal neuralgia has been widely addressed as well. Van Loveren and colleagues62 reviewed their experience of 1000 patients with trigeminal neuralgia, comparing the techniques of percutaneous stereotactic rhizotomy and posterior fossa exploration. Of the 700 treated by percutaneous stereotactic rhizotomy, excellent or good results regarding pain relief were achieved in 125 patients treated with microsurgical vascular decompression or partial sensory rhizotomy. These favorable results were corroborated by the report of Bederson and Wilson63 in 252 patients. Additionally, glycerol rhizotomy for trigeminal neuralgia has been investigated.64–66 In general, good or excellent results are reported in 70% to 72% of the cases using this technique. Selective dorsal rhizotomy for spasticity in children with cerebral palsy, although controversial, has also been recommended. Cahan and colleagues67 and Kundi and colleagues68 emphasized the safety of the procedure, citing preservation of cortical somatosensory evoked responses. Good results have also been reported by others.11,17,69–71 Intraspinal rhizotomy has been reported to diminish spasticity in patients with myelomeningocele.72 Sacral rhizotomy in the treatment of hypertonic neurogenic bladder has also been investigated,31,73–75 as has control of spasticity resulting from posttraumatic paraplegia.76 Percutaneous radiofrequency rhizotomy resulted in improvement of spasticity in 24 of 25 patients in the series of Kadson and Lathi.77 In Turnbull’s series, percutaneous rhizotomy improved lower extremity spasm in paraparetic patients who were not hospitalized.78
Rhizotomy and Ganglionectomy
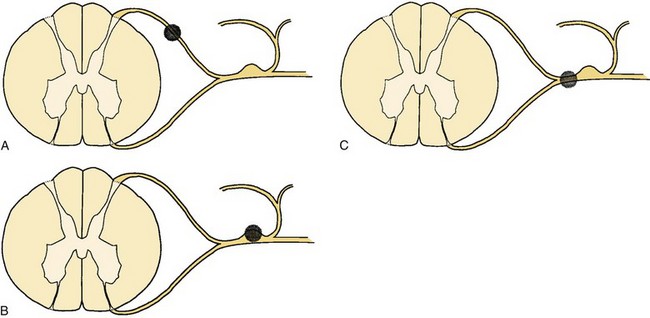
Sectioning of the spinal nerves or excision of dorsal root ganglia can be accomplished at multiple levels. Rhizotomy may be performed by intradural section of the dorsal root, extradural section of the dorsal root, or extradural section of the mixed root (Fig. 108–1). Additionally, the median branch of the posterior primary ramus may be interrupted, although this is usually by a percutaneous technique such as radiofrequency ablation (Fig. 108–2).
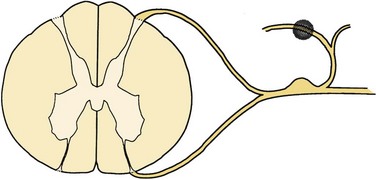
FIGURE 108–2 Facet rhizotomy. The shaded area represents the surgical lesion created.
(From Wetzel FT: Chronic benign cervical pain syndromes: Surgical considerations. Spine 17:S367-S374, 1992.)
Sensory rhizotomy for the relief of chronic pain was first carried out by Abbe in 1888 but had been nearly abandoned by 1925 because of the relatively high failure rate and the subsequent interest in cordotomy.11–79 Rhizotomy may be performed at this level to include selective sensory fibers, or it may take the form of a complete rhizotomy. Characteristically, both ablative procedures are performed proximal to the ganglion.14
The goal of rhizotomy or ganglionectomy is denervation of the area in which pain is felt. It has frequently been assumed that root section should remove pain that is peripheral and circumscribed because the afferent territory of a few adjacent nerves presumably completely delineates the pain for that region.14,80 Long-term results of rhizotomy fail to support this, however. In addition, results of selective sensory rhizotomy may be compromised because of the presence of denervation hypersensitivity, intersegmental cross-linking,16 and overlapping dermatomes81–84 and the presence of afferent unmyelinated axons in the ventral roots.85,86 Intraoperative stimulation of these roots has been shown to provoke pain. If these ventral afferents comprise a significant portion of the ventral root, dorsal sensory rhizotomy may be providing insufficient deafferentation to interrupt pertinent sensory pathways.
One of the central problems in planning surgery for persistent limb pain is the precise delineation of the involved roots. Many authors have attempted to select patients on the basis of their response to individual nerve root sheath blockade, as guided by electrophysiologic evidence of chronic radiculopathy and neurologic examination. Onofrio and Campa16 reported their results in 286 patients who underwent rhizotomy. Fifty-eight patients underwent lumbar rhizotomies. Only 6 of the 45 undergoing S1 rhizotomies were believed to have long-term pain relief. Three of 13 patients who underwent lumbar rhizotomies had clinically successful results. These results were obtained despite consideration of dermatomal overlap and the use of selective nerve root blockade to plan the surgery. Loeser15 reported similar results with a 14% success rate at 10 years. Arachnoiditis, in the setting of failed disc surgery, seemed to be correlated with poor results, and preoperative nerve root blocks provided little diagnostic or prognostic information. Loeser offered several reasons for these results including incomplete root sectioning, inadequate numbers of roots divided, and a higher threshold of fibers in adjacent nerves, which may begin to produce chronic pain syndromes after the effects of local anesthetics from root blocks have worn off. He also speculated that central alterations may be important. Additionally, the utility of “diagnostic” nerve root blocks must be questioned. The selective root sheath injection appears to be nonspecific in not only a dermatomal sense but in a central and peripheral sense as well: Several authors have reported on the ability of distal blocks to produce temporary relief.87–89 Jain90 believed that selective extradural sensory rhizotomy was not successful in the setting of arachnoiditis. Other authors have reported similarly discouraging results.13,88,91
In a compendium of results from multiple sources,11 Dubuisson noted a 74% rate of immediate success following rhizotomy at L4, L5, and S1, which dropped to 33% 3 months after surgery.14–16,59,90,91 These results are corroborated by the reports of others.13,88,91 White, in reviewing a series of sensory rhizotomies for 10 patients with failed lumbar surgery, noted 80% good to excellent results; however, follow-up was variable, ranging from 4 months to 11 years, and no temporal specifics were reported. He did, however, agree that there was little pain relief when arachnoiditis was present.57 Wetzel and colleagues92 reported poor outcomes (14% success) in patients undergoing selective sensory rhizotomy at a mean follow-up of 2 years. All patients had undergone previous unsuccessful lumbar surgery.
Thus it is difficult to recommend rhizotomy for the treatment of chronic benign lower extremity pain. Seemingly, the most reliable indication for rhizotomy is pain caused by deafferentation itself. Tasker and colleagues31 reviewed 168 patients. The pain was divided into two groups: spontaneous and hyperpathic. The latter implies pain production induced by normally non-noxious stimulation within adjacent areas of increased somatosensory thresholds. Overall, the pain in this group was nearly always causalgic or dysesthetic in quality and was associated with sensory loss. This was dramatically ameliorated by intravenous sodium thiopental, but not by morphine, and was usually relieved by proximal local anesthetic blockade. Various deafferentation procedures including rhizotomy, neurectomy, cordectomy, and cordotomy were reviewed, and each of these ablative procedures failed to relieve most patients of deafferentation pain. Hyperpathia, which occurred in incompletely deafferented areas, however, was partially relieved by surgical completion of the deafferentation, although the authors noted that pain may persist at the periphery of the sensory loss.
Sectioning of the dorsal root ganglion has been shown to provide the best results in terms of pain relief when performed for benign truncal neuralgias.93 The results of ganglionectomy (see Fig. 108–1) at the caudal lumbar roots in cases of failed lumbar surgery are as disappointing as those reported for rhizotomy.15,16,90,93,94 At this time, meaningful differentiation between rhizotomy and ganglionectomy as distinct therapeutic tools in this setting is impossible.
Technique
The patient is placed in a prone position under general anesthesia, and hemilaminectomy and partial facetectomy are used to expose the involved root. The root sheath is clearly identified and opened longitudinally for 8 to 10 mm proximal to the dorsal root ganglia. The dural septum, which separates dorsal and ventral roots, is identified, and a small nerve hook is passed between root filaments. Osgood and colleagues93 noted that several distinct root fascicles are usually present. With electrocautery at a low setting, electrical stimulation is used to distinguish between motor and sensory fibers. As Bertrand has noted, however, caution must be used in relying on this test exclusively because chronically damaged roots may exhibit a higher threshold for motor excitation response than normal roots.91 Thus a wake-up test may be required. When appropriate sensory fibers are identified, they can be sectioned with electrocautery or a microsurgical blade.
Facet Rhizotomy
Facet denervation is not nerve root surgery in the same sense that open rhizotomy is; rather, the theory behind this procedure involves destruction of the median branch of the posterior primary afferent nerve that supplies the facet joint (see Fig. 108–2). The median branch of the posterior primary ramus descends through a notch at the base of the transverse process and is covered by a ligament at the anteroinferior border of the facet joint at this level. This ligament is a continuation of the intertransverse membrane, and it is here that several small twigs are given off to the facet joint. These twigs then enter the facet joint capsule.44 Each posterior primary ramus supplies at least two facet joints, and each facet joint receives innervation from at least two spinal levels. Clinical features of facet joint syndrome have been described by several investigators. Mooney and Roberson are generally credited with one of the earliest descriptions.42 Subsequent authors have attempted to improve the sensitivity and specificity of diagnosis and investigate diagnostic maneuvers, specifically response to facet blockade as selection criteria for facet rhizotomy95 or even fusion.96 Saal, in reviewing current diagnostic techniques, noted that the capacity of diagnostic facet blocks to correlate specifically with findings of the history and physical examination is limited.97 Although a variety of signs and symptoms have been described as being associated with facet mediated pain, these signs or symptoms are not specific enough to delineate a patient population suffering from facet mediated pain per se.98,99 Several studies have noted that the prevalence of facet pain among patients with chronic back pain is relatively low when measured by facet injection; however, the anesthetic response to a single uncontrolled block is as high as 50%, an unacceptably high false-positive rate.100 It is generally felt that the “gold standard” by which facet joint blockade is judged is the appropriateness of the pain relief response based on duration of effect appropriate to the agent used for blockade and sustained relief by subsequent facet rhizotomy. This has been demonstrated in the cervical spine. In a study by Lord and colleagues,101 patients experiencing neck pain from whiplash, who responded to facet blocks, were randomized into active and sham groups for radiofrequency lesioning. The median duration of pain relief in the active group was significantly greater that in the sham group. No patients who failed to respond to blockade were included in the study.
Rees is generally credited with performing the first facet rhizotomies. These were done percutaneously with a knife and reportedly resulted in immediate relief of symptoms in 998 of the 1000 patients who had facet pain in concert with the “intervertebral disc syndrome.” Shealy102 performed the procedure in North America but had an unusually high frequency of wound hematomas. This led to the adoption of a radiofrequency probe. Success rates as high as 90% were initially reported in previously nonoperated patients. That pain relief was achieved by the interruption of afferent impulses of facet joint has been suggested by the anecdotal reports of many authors who have noted immediate relief of pain in patients undergoing lumbar fusion.
Candidates for facet rhizotomy are those patients with back pain caused by facet dysfunction who have failed to respond to conservative therapy.103 The key diagnostic maneuver is thought to be the facet block. This involves percutaneous insertion of a needle into the joint, under fluoroscopic guidance, followed by joint injection with lidocaine (Xylocaine) combined with steroids or contrast agents.42,104,105 Patients in whom this procedure yields temporary relief may be candidates for facet rhizotomy.103,104
In the series of Shealy,106 a satisfactory clinical result was noted in 79% of patients who had not had previous surgery. In patients who had undergone previous laminectomy, the success rate fell to 41%, and in those who had undergone previous fusion, success was only 27%. Of the 82 patients McCulloch followed from 6 to 20 months after facet rhizotomy, only 50% had satisfactory results.107 Interestingly, three patients in this group required repeat surgery. Schaerer reported on 71 patients who underwent lumbar facet rhizotomy.108 There were five distinct subgroups in his review: (1) lumbar facet disease without disc involvement (discography negative), (2) lumbar facet involvement with disc involvement (discography positive), (3) lumbar facet disease with discopathy and root signs, (4) facet signs with osteoarthritis, and (5) postlaminectomy pain. At a mean follow-up of 13.7 months, patients were evaluated using a pain profile. Thirty-five of 71 patients had satisfactory results. The highest percentage of success was in the author’s first group—those who had a “pure” facet syndrome (7 of 15 patients). No attempt was made to determine statistically significant differences in outcome between these groups. Florez and colleagues109 reported a series of 30 patients, achieving satisfactory results in 76%. Twenty-six of the patients were followed for 3 to 9 months. The best results were noted in patients without previous operations and those with shorter duration of symptoms. Oudenhoven110 reported 377 patients with “pseudoradicular” pain in whom a lumbar facet syndrome was diagnosed by facet blocks. At a mean follow-up of 26 months, 83% were judged to be clinical successes. The author noted that a unilateral facet rhizotomy did not control pain and reported that 22% of patients who were judged to be clinical successes noted some return of symptoms at 18 to 24 months postoperatively. None of the authors reported any significant complications with the procedure. Lord and colleagues111 reported on 19 patients who underwent cervical percutaneous neurotomy for neck pain of at least 3 months’ duration following motor vehicle accidents. They found that results varied by level. Of the 10 patients who underwent C2-C3 rhizotomy, 3 obtained greater than 6 months of pain relief and 1 was pain free at the 4-month follow-up; the remaining 6 had return of symptoms over 3 weeks. Of the 10 who underwent more caudal neurotomies, seven obtained “clinically useful” pain relief. The authors noted that C2-C3 results may have been compromised by technical failure including the relatively large diameter of the nerves and their variable course.
One may be tempted to recommend this procedure after diagnosis of facet syndrome with facet arthrography and blockade. The current literature, however, does not substantiate the rates of clinical success (83%) reported earlier.110 North and colleagues95 retrospectively reviewed prognostic factors for facet rhizotomy and found that only 42% of those selected for rhizotomy by response to facet blockade had pain relief at 2 years post procedure. Seventeen percent of those who experienced relief from blockade, but did not undergo rhizotomy, were improved. As noted previously, one randomized prospective study of rhizotomy for cervical pain noted a longer duration of relief in the active lesion group.101 The reported complication rates are low,112 and the apparent risk to the patient is minimal. There remain no convincing studies in the literature, however, suggesting that pain relief from the procedure is permanent.
Technique
The technique for this procedure is well described.103,107 It is recommended that it be performed in the operating room or radiology department. Local anesthetic is adequate; the patient should be in the supine position for cervical rhizotomy or the prone position for thoracic and lumbar rhizotomy. Image intensification is required. Fourteen-gauge needles are placed unilaterally in the region of the appropriate facet(s) and nerves. A 5-mm bare-tipped probe is then positioned in the area of the facet and the 14-gauge needle is partially withdrawn, leaving only the probe in the space between the superior facet and the transverse process immediately adjacent to the superior facet. The depth of the probe is controlled by lateral image intensification. A stimulation frequency of 100 Hz and from 0.1 to approximately 3 V is used to localize the tip away from the anterior ramus, as noted by the absence of paresthesia in the ipsilateral extremity. Once the depth is appropriate, stimulation adjacent to the posterior primary ramus reproduces a pain pattern familiar to the patient and the lesioning is then performed. A temperature-controlled lesion is produced by setting the controls at 25 V and 100 mA for approximately 60 seconds, at 80°C. During the final 20 seconds, the amperage is slowly increased to the point where the milliamperage starts to diminish and voltage rises. This takes the temperature to approximately 90°C. After this, the probe is withdrawn, the wound dressed, and the patient mobilized.
Sympathectomy
Sympathetic dystrophy represents a constellation of disorders of sympathetic nerve functions that accentuate or perpetuate chronic pain. Historically, Lankford113 divided sympathetic dystrophy into two types, causalgia and dystrophy, based on the type of injury. More recently, Stanton-Hicks and colleagues114 presented a revised taxonomic classification for reflex sympathetic dystrophy (RSD), regrouping the subtypes into a single entity, complex regional pain syndrome (CRPS). The diagnosis of CRPS requires regional pain and sensory changes following the index injury, coupled with skin color changes, temperature changes, abnormal sudomotor response, and edema. CRPS Type I occurs without a specific nervous lesion and corresponds to sympathetic or traumatic dystrophy; Type II refers to a discrete nervous lesion, equivalent to the former definition of causalgia. Obviously, many features of chronic spinal pain syndrome may fall into these various categories.
The anatomic constancy of the sympathetic trunk, with respect to the outer annulus, has been well demonstrated by Bogduk and colleagues.41 Pain fibers traveling lateral to the vertebral column in the sympathetic trunk may be prone to irritation owing to injury to the motion segment. Likewise, tears of the annulus fibrosus have been thought to be capable of producing a cold, painful limb on the ipsilateral side,39 and Hodgson described a pattern of intractable lower extremity pain, associated with diminished temperature, in patients with failed lumbar surgery.115
Sympathectomy has been investigated in the treatment of limb distress of other causes, most notably vascular disease. Norman and House reported the results of lumbar sympathectomy for peripheral vascular disease in 153 patients.116 Five years postoperatively, 67% of those who experienced claudication and 54% of those who experienced rest pain had avoided further surgery. Repelaer van Driel and colleagues117 reported favorable results from sympathectomy in 66 patients who had suffered from lower limb ischemia. Jones also noted a beneficial effect of digital sympathectomy in treating ischemia of the hand in systemic disease.118
In the setting of persistent neurogenic dysfunction, however, the results of sympathectomy have been far less predictable. A central problem remains in establishing the diagnosis. The sine qua non for diagnosis remains response to sympathetic blockade.40,87,119,120 Thermography has been suggested to be another diagnostic technique for detecting skin surface temperature changes associated with autonomic dysfunction.88,121,122 Even with the use of both modalities, however, doubts have been raised regarding the validity of patient selection in chronic lumbar pain syndromes.88
Mockus and colleagues123 reported on 34 patients who underwent lumbar sympathectomy. In 13, the precipitating incident was lumbar disc surgery. In this series, only one patient failed to obtain satisfactory relief. Wetzel and colleagues reported acceptable pain relief in only 4 of 17 patients at the 2-year follow-up. In this group, the patients’ response to block was not as significant in predicting response to surgery as was their initial thermographic diagnosis.88 Overall, including the results of even upper thoracic ganglionectomy, less than two thirds of patients report satisfactory pain relief at 2 years with less than one third reporting satisfactory pain relief at 5 years.112 Thus at the present time, lumbar sympathectomy cannot be recommended with any conviction in the setting of chronic benign lumbar pain syndromes.
Stimulation Therapy
Epidural and Intraspinal Implants
Selective nerve root blockade or epidural blockade has been successfully used in many intraoperative and postoperative situations.56,124–126 Likewise, implanted epidural narcotic reservoirs have been used in the treatment of intractable spinal and limb pain in a variety of neoplastic conditions.127–132 Downing and colleagues131 reported excellent relief of refractory pain in 23 cancer patients. In the series of Sjogren and colleagues133 29 of 48 patients were able to be stabilized on epidural opioid treatment, and of these, 21 were judged to be clinical successes. A tendency toward better relief of non-neurogenic pain was noted. In a large series reported by Liew and Hui,134 good to excellent pain relief was obtained in 85% of patients. In patients who survived more than 3 months, the mean daily morphine requirement increased progressively from 3.5 to 19.5 mg per day, consistent with both drug tolerance and disease progression.
Overall, the results of intraspinal narcotics, usually morphine, are less consistent in benign pain than in pain from cancer. In an early report of 43 patients, 32 of whom had continuous delivery systems, Auld and colleagues135 noted good to excellent pain relief in 65% of patients at greater than 2-year follow-up. Neither serious side effects nor evidence of addiction were observed. The same authors reported comparable results using a smaller system consisting of an epidural catheter and subcutaneous reservoir.136 Prager recently summarized the current literature, noting comparable outcomes reported in most studies to date with approximately 80% of cancer patients reporting greater than 50% pain relief with somewhat lower rates being reported for benign pain patients.137
The implantation of an indwelling narcotic reservoir is a promising technique for the management of benign refractory benign spinal pain syndromes; however, several notes of caution must be sounded. As previously noted, neuroplasticity in the setting of chronic benign pain tends to diminish the results of ablative therapies over time. In an earlier report, increasing narcotic requirements were seen in cancer patients.134 Paice and colleagues138 made a similar observation in chronic benign pain patients. In a multicenter study of 429 cases provided by 35 physicians, the temporal profile of drug use differed between cancer and noncancer patients: Cancer patients had a higher initial dose, which increased quickly and then reached a plateau. Benign pain patients had a gradual, virtually linear increase in dose. Bearing this in mind, the following guidelines appear to be reasonable: An appropriate patient is one who is chronically disabled, not a surgical candidate, not appropriate for behavioral operant therapy, and psychologically sound. Whether a trial of electrical stimulation (see later) should precede consideration of an intraspinal implant is a matter of debate; additionally, it appears to be the case that certain types of pain (e.g., nociceptive predominant) may be relatively stimulator resistant and are treated more effectively by intrathecal therapy.
For both intrathecal infusion and spinal cord stimulation, a psychologic assessment should be undertaken as a component of patient selection.139 This analysis is best performed before initiating a trialing procedure. Certain mood and behavioral abnormalities are considered a contraindication for implantable device therapy (Box 108–1). It is important to recognize that the prevalence of depression, anxiety, and other mood disorders within a population of chronic pain patients is quite high and that these psychologic abnormalities do not represent a contraindication for implantable pain technology.140 The methodology for “psychologic clearance” is not standardized. Some commonly used testing measures include the Minnesota Multiphasic Personality Inventory, the Beck Depression Inventory, the Sickness Impact Profile, and the Oswestry Disability Index. Certain measures have failed to demonstrate significant prognostic value.141 It is pertinent to note that psychologic screening is not 100% effective in eliminating patients with significant psychopathology from trialing and implantation.142,143 Additionally, it may also be appropriate to maintain psychologic interventions into the chronic phase of therapy because a synergistic effect on pain reduction has been reported with a combination of behavioral and interventional therapy.144
Although several agents are commonly used for chronic intrathecal delivery, only three medications currently have U.S. Food and Drug Administration (FDA) approval for long-term intrathecal use: baclofen, morphine, and ziconotide. Baclofen, a GABA-B agonist,145,146 has FDA approval for spasticity of both cerebral and spinal origin.147,148 Although baclofen has shown some analgesic properties in animal studies, its utility in chronic pain is probably limited to musculoskeletal pain associated with spasticity and dystonias associated with CRPS.149–152 Morphine, considered by many clinicians to be the first-line agent for intrathecal pain management, has been FDA approved for clinical use for more than 2 decades. Other opiates such as hydromorphone, fentanyl, and sufentanil have been used for chronic intrathecal delivery, but these agents lack formal FDA approval. Intraspinal opiates are thought to exert their therapeutic effect presynaptically by inhibiting calcium ion influx and postsynaptically by increasing potassium outflow.153,154 The newest medication for intrathecal pain control is ziconotide, a synthetic form of ω-conotoxin MVIIa (Prialt, Élan Pharmaceuticals, San Diego, Calif.). This compound was originally isolated from the venom of the cone snail, Conus magus. This drug was formerly known as SNX-111 and is a 25 amino acid peptide that blocks a neural specific calcium channel on small myelinated and unmyelinated nociceptive afferents that are primarily localized in the superficial Rexed laminae (I and II).155 The analgesic effect of ziconotide is produced when this channel blockade results in diminishing neurotransmitter release from these primary nociceptive afferents. Thus the transmission of the pain signal never crosses the synaptic cleft from the primary afferents to the second order neurons. Other commonly used agents for chronic intraspinal infusion include bupivacaine and clonidine. Intrathecal bupivacaine exerts its therapeutic action by directly inhibiting neuronal voltage-gated sodium channels and thus hindering nerve transmission. In general, the degree of blockade is related to the diameter, myelination, and conduction velocity of nerve fibers. Because the axons of pain transmitting neurons tend to be thinner and poorly myelinated, bupivacaine can diffuse more readily into them than into thicker and more heavily myelinated nerve axons.156 The mechanism of action for intrathecal clonidine, an α2-adrenoceptor agonist, is not fully elucidated and may include interactions with NMDA receptors.157
Treatment algorithms for use of these agents, both in monotherapy and combination therapy, are far from standardized. It is important to recognize that although combination therapy may have the therapeutic advantage of synergy between multiple agents, it also adds complexity with regards to stability, compatibility, and dosing adjustments. No combination therapy has FDA approval. One level of evidence-based decision making for intrathecal pain therapy is consensus statements of experienced clinicians. In January 2007, a consensus of experts convened to review the current medical literature and formulate updated guidelines for intrathecal pain therapy. The 2007 groups recommended three first-line agents: morphine, hydromorphone, and ziconotide. Second-line agents include fentanyl monotherapy, as well as morphine or hydromorphone in combination with ziconotide, morphine, or hydromorphone in combination with bupivacaine, and morphine or hydromorphone in combination with clonidine. The complete algorithm from this consensus panel is outlined in Figure 108–3.158
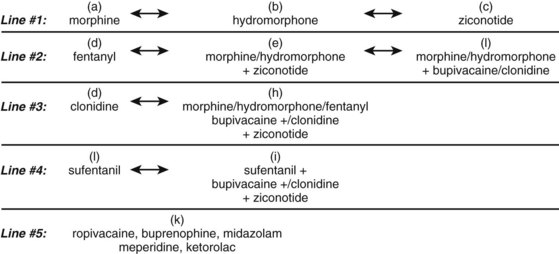
FIGURE 108–3 2007 Polyanalgesic Consensus conference algorithm for intrathecal pain therapy.
(Adapted from Deer T, Krames ES, Hassenbusch SJ, et al: Polyanalgesic consensus conference 2007: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation 2007;10:300-328.)
Intrathecal delivery of medications can result in several potential adverse events including sedation, cognitive impairment, nausea, vomiting, pruritus, urinary retention, constipation, hormonal dysfunction, and edema.159 The majority of these problems can be managed with adjunctive care and/or adjustments of intrathecal delivery. However, the development of an inflammatory mass at the tip of the intraspinal catheter may require surgical intervention. These masses are called granulomas and were first described by North more than 2 decades ago.160 The precise pathophysiology of these masses is not well understood. The relative contributions of drug dose, drug concentration, flow rate, continuous versus bolus dosing, catheter position, and cerebrospinal (CSF) dynamics remains somewhat unknown. Although several medications have been associated with granuloma development, morphine and increasingly hydromorphone are implicated in the majority of cases. There is consensus among experienced providers that high drug concentrations, especially for morphine, combined with low flow rate increase the risk of granuloma development. There is an increasing recognition of these masses with widespread use of MRI imaging, which makes prevalence reporting uncertain. The clinical presentation for granulomas ranges from asymptomatic to full-blown progressive myelopathy. Patients should be routinely queried for new-onset neurologic symptoms and undergo motor, sensory, and reflex examinations on a regular basis. This investigation is especially important in individuals who have demonstrated loss on pain control or new-onset pain complaints. Computed tomography myelography or magnetic resonance imaging with gadolinium contrast of the catheter tip region is necessary to confirm the diagnosis.161 In asymptomatic and nonprogressive patients, weaning of intrathecal medications and initiation of saline infusion can produce spontaneous disintegration of the mass. In patients with progressive or severe neurologic compromise, urgent surgical decompression and excision are recommended.162
Spinal Cord Stimulation
With the introduction of the gate control theory began a new era of thinking regarding chronic pain.18 This theory held that low-threshold, primary, afferent fibers might be electrically activated peripherally, resulting in central suppression of nociceptive influences. Several authors163,164 applied this idea to the treatment of pain in the distribution of a peripheral nerve, with encouraging results. Subsequently, Shealy and colleagues165,166 suggested stimulation of the dorsal columns of the spinal cord to control chronic, intractable lower extremity pain. This seemed physiologically correct because the dorsal column fibers represent direct extensions of large-diameter primary afferent fibers running centrally. Thus their stimulation should allow pain control over a wide region of the body.81,167,168 Although the key concept revolved around stimulation of the dorsal columns, Larson and colleagues169 demonstrated an effect from ventral electrode placements as well. Thus active pathways are difficult to determine, and the physiologic basis of pain relief from spinal cord stimulation remains an active area of research. Clinically the neurogenic component of pain is more effectively treated than the nociceptive component; sympathetically mediated pain is more effectively treated than somatic pain. This suggests that the “gate” may be closed in the spinothalamic tract by activation of large-diameter afferents. Activation of supraspinal loops may provide ascending and descending inhibition. Additionally, direct stimulation or activation of descending pathways in the cord may block ascending impulses.170 Additionally, the mechanism by which spinal cord stimulation achieves the desired effect may not be limited to electrical depolarization of certain fiber populations. The work of Basbaum and Fields171 has characterized a descending modulatory pathway; this lends credence to theories recommending the electrical and pharmacologic stimulation of descending pathways for the treatment of chronic pain. In this descending pathway, fibers from the midbrain and hypothalamus synapse in the dorsal pons and rostroventral medulla. Efferent, inhibitory impulses travel via the dorsolateral funiculus to synapses in the dorsal horn. These projections may functionally inhibit the target cells that give rise to the spinothalamic tract. The release of neuromediators in the dorsal horn has been documented in animal models. Linderoth and colleagues,172,173 in a cat model, noted increased levels of substance P–like immunoreactivity in microdialysate from the dorsal horn after spinal cord stimulation. Release of serotonin was also noted, but no effects on extracellular levels of amino acids were detected. The authors speculate that serotonin release may be relevant for pain on the basis of the existence of descending neurons in the dorsolateral funiculus that store both substance P and serotonin and have a putative role in pain inhibition. Recent studies have also implicated gamma-amino butyric acid (GABA), demonstrating GABAergic activity following spinal cord stimulation (SCS).172–174 Current thoughts on the neurochemistry of SCS have been elegantly summarized by Oakely and Prager.170 SCS decreases dorsal horn excitability, releases dorsal horn GABA, substance P, serotonin, glycine, and adenosine and reduces the release of glutamate, aspartate, and excitatory amino acids.
Initially, it was assumed that most of the effects of stimulation were attributed directly to stimulation of the dorsal columns. It has become clear, however, that application of an electrical field to the dorsal epidural space may activate a larger number of neural structures. It is likely that paresthesias are elicited from intraspinal neural structures both inside and outside the cord. Barolat and colleagues175 noted paresthesias ipsilateral to stimulating electrodes perceived as tingling sensations, reflecting stimulation of large myelinated fibers, involving the dorsal columns, dorsal roots, dorsal root entry zone, and dorsal horn. Meyerson and colleagues176 noted evoked activity in the dorsal and ventral spinal cord at the level of the dorsal horn. Thus as proposed by Oakley and Prager,170 the older term “dorsal column stimulation” should be abandoned in favor of the term “spinal cord stimulation.”
Clinically, the results of stimulation appear to have improved over time. Krainick and colleagues177 reported a 38% success rate in 726 patients, which subsequently declined to 22.5% in long-term follow-up. These figures, however, represent the results for various etiologies. Husson and colleagues124 reported good preliminary results in 20 patients with radicular pain from arachnoiditis. They based their selection criteria on a pain relief level of at least 50% after a month of transcutaneous stimulation.124 Wester, in a series of 35 patients, 30 of whom were selected for implants, noted that 15 months postoperatively 43.5% used the stimulator regularly. He found an increase in the amount of pain relief among patients with failed lumbar surgery syndromes and arachnoiditis.125 Young, in a series of 51 patients who underwent stimulator implantation, reported much less satisfactory results.56 Twenty-five of the patients had undergone previous lumbar surgery, and in all patients pain had been present for at least 24 months. Thirty-seven patients underwent open laminectomy for implantation, 11 had electrodes placed percutaneously, and three, after an initial trial of percutaneous implantation, required open laminectomy. The author noted no major complications but stated that minor complications (e.g., paresthesia not in the desired location, infection, CSF fluid leak, lead migration, and breakage) required an additional 33 procedures. At a mean follow-up of 38 months, his results were disappointing. In the immediate postoperative period, 47% had significant relief, but no functional improvement (e.g., return to work) was noted in any patient. This raised the question of whether or not a spinal cord stimulator is an effective treatment for chronic benign pain.
Overall, with increased technical support, clinical experience, and the use of multichannel programmable devices, results have improved. Pain relief ranging from 0% to 85% has been reported.52,106,130,175,177–203 All these studies, however, suffer from the same flaws: retrospective study design, variable definitions of criteria for success, and outcome comparisons independent of pain etiology. As the techniques and selection criteria have improved, an overall trend of increased maintenance of pain relief over time has been noted. Regarding efficacy in benign pain syndromes, the work of North and colleagues196 appears to be illustrative. Successful outcomes, defined as 50% or greater pain relief, have been realized in 50% to 53% of patients with follow-up as long as 20 years.
Work by North and colleagues178 bears special mention. In that prospective randomized study, spinal cord stimulation was compared with reoperation (laminectomy) for “failed back surgery syndrome.” Fifty-one patients were randomized into either stimulation or reoperation groups, with crossover permitted. Failure of stimulation was defined as crossover into the surgical group from the stimulator group, and failure of reoperation was defined as crossover into the stimulator group. Results for the first 27 patients reaching the 6-month crossover point showed a statistically significant (P = 0.018) advantage of spinal cord stimulation over reoperation. Additionally, in a group of patients followed outside the study who opted for reoperation, 42% crossed over to spinal cord stimulation at 6-month follow-up. Kemler and colleagues204 conducted a randomized prospective study to evaluate SCS in CRPS. Thirty-six patients were assigned to the SCS plus physical therapy group, while 18 received therapy alone. Of the 36, 24 had a successful trial and underwent implantation. At 6-month follow-up, patients in the SCS plus physical therapy group had significantly lower visual analog pain scale (VAS) scores, although there were no functional differences between the groups.
Turner and colleagues205 performed a meta-analysis of SCS for the Failed Back Surgery Syndrome (FBSS). In the 39 studies that met inclusion criteria, mean follow-up was 16 months, satisfactory pain relief (>50%) was reported by 59%, and complications occurred in 42%. All studies were case controlled. On the basis of these data, the authors concluded that no firm conclusions could be reached regarding efficacy or the effect of SCS on patient work status, disability, or medication use. It should be recalled that although this study did reflect the bulk of the available literature, it did not include either of the randomized prospective studies reviewed earlier.
The selection criteria for patients who may be candidates for spinal cord stimulation are of paramount importance. Krainick and colleagues177 performed an initial trial with an electrode inserted into the arachnoid space using a small cannula. Stimulation was performed above the segmental level of pain for 30 minutes. In 73 patients, 28 obtained more than 50% pain relief. The value of a trial stimulation was also addressed by Neilson and colleagues.168 In 96 of 221 patients who underwent a percutaneous trial, the stimulation was found to provide insufficient pain relief. Surprisingly, 28 of these 96 underwent permanent spinal cord stimulator implantation and, not surprisingly, failed to obtain any relief from the procedure.
Use of spinal cord stimulation to treat benign lumbar pain syndromes remains controversial. The most rigorous set of guidelines espoused for patient selection and technique are those of Krainick167: use of multiple electrodes, open epidural placement, localization of electrodes above the pain segments, absence of secondary gain, and localized rather than diffuse pain. These guidelines represent the ideal candidates for spinal cord stimulation. With the use of multiple electrodes to treat more complex pain syndromes, the open laminotomy or laminectomy approach is becoming more common, although percutaneous insertion may still be used as well (Fig. 108–4).194,206
Technique
For most procedures, the patient is taken to the operating room and positioned prone. If the procedure is to be done percutaneously, local anesthesia with intravenous sedation is used. If an open laminotomy is preferred, the epidural space is directly visualized and the lead inserted. It is recommended that, for chronic lumbar pain syndromes, leads be inserted in the T12-L1 interlaminar space and traverse at least three levels cranially. This must be done under fluoroscopic control. The intradural pouch, well described by Burton, also requires an open laminotomy.180
Summary
Pearls
Pitfalls
Key Points
1 Dubuisson D. Root surgery. In: Wall PD, Melzack R, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:784-789.
2 Loeser JD. Dorsal rhizotomy for the relief of chronic pain. J Neurosurg. 1972;36:745-750.
3 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: A physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
4 Wetzel FT, LaRocca SH, Adinolfi M. The treatment of chronic extremity pain in failed lumbar surgery. the role of lumbar sympathectomy. Spine. 1992;17:1462-1468.
5 Wetzel FT, Phillips FM, Aprill CN, et al. Extradural sensory rhizotomy in the management of chronic lumbar radiculopathy: A minimum 2-year follow-up study. Spine. 1997;22:2283. discussion 2291-2292
6 North RB, Kidd DH, Campbell JN, Long DM. Dorsal root ganglionectomy for failed back surgery syndrome: A 5-year follow-up study. J Neurosurg. 1991;74:236-242.
7 North RB, Kidd DH, Zahurak M, Piantadosi S. Specificity of diagnostic nerve blocks: A prospective, randomized study of sciatica due to lumbosacral spine disease. Pain. 1996;65:77-85.
8 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
9 Lord SM, Barnsley L, Wallis BJ, et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721-1726.
10 Prager JP. Neuraxial medication delivery: The development and maturity of a concept for treating chronic pain of spinal origin. Spine. 2002;27:2593. discussion 2606
11 Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: A retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
12 North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine: Case report and laboratory experience. Neurosurgery. 1991;29:778-784.
13 Deer T, Krames ES, Hassenbusch S, et al. Management of intrathecal catheter-tip inflammatory masses: An updated 2007 consensus statement from an expert panel. Neuromodulation. 2008;11:77-91.
14 Oakley JC, Prager JP. Spinal cord stimulation: Mechanisms of action. Spine. 2002;27:2574-2583.
15 North RB, Kidd DH, Lee MS, Piantodosi S. A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: Initial results. Stereotact Funct Neurosurg. 1994;62:267-272.
16 Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: A systematic literature synthesis. Neurosurgery. 1995;37:1088. discussion 1095-1096
1 Tasker RR, Dostrovsky JO. Deafferentation and central pain. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:154-186.
2 Tasker RR, DeCarvalho GT, Dolan EJ. Intractable pain of spinal cord origin: Clinical features and implications for surgery. J Neurosurg. 1992 Sep;77:373-378.
3 Cowie RA, Hitchcock ER. The late results of antero-lateral cordotomy for pain relief. Acta Neurochir (Wien). 1982;64:39-50.
4 Tasker RR. Percutaneous cordotomy: The lateral high cervical technique. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques: Indications, Methods and Results. New York: Grune & Stratton; 1988:1191-1205.
5 Frankel SA, Prokop JD. Value of cordotomy for the relief of pain. N Engl J Med. 1961;264:971-974.
6 French LA. High cervical tractotomy: Technique and results. Clin Neurosurg. 1974;21:239-245.
7 O’Connell JE. Anterolateral chordotomy for intractable pain in carcinoma of the rectum. Proc R Soc Med. 1969;62:1223-1225.
8 Porter RW, Hohmann GW, Bors E, French JD. Cordotomy for pain following cauda equina injury. Arch Surg. 1966;92:765-770.
9 Raskind R. Analytical review of open cordotomy. Int Surg. 1969;51:226-231.
10 Noordenbos W, Wall PD. Implications of the failure of nerve resection and graft to cure chronic pain produced by nerve lesions. J Neurol Neurosurg Psychiatry. 1981;44:1068-1073.
11 Abbe R. Intradural section of the spinal nerves for neuralgia. Bos Med Surg J. 1989;135:329-335.
12 Arens LJ, Peacock WJ, Peter J. Selective posterior rhizotomy: A long-term follow-up study. Childs Nerv Syst. 1989 Jun;5:148-152.
13 Bernard TNJr, Broussard TS, Dwyer AP, LaRocca SH. Extradural sensory rhizotomy in the management of chronic lumbar spondylosis with radiculopathy. Ortho Trans. 1987;11:23.
14 Dubuisson D. Root surgery. In: Wall PD, Melzack R, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:784-789.
15 Loeser JD. Dorsal rhizotomy for the relief of chronic pain. J Neurosurg. 1972;36:745-750.
16 Onofrio BM, Campa HK. Evaluation of rhizotomy. review of 12 years’ experience. J Neurosurg. 1972;36:751-755.
17 Oppenheim WL. Selective posterior rhizotomy for spastic cerebral palsy. A review. Clin Orthop Relat Res, 253. 1990 Apr:20-29. 253
18 Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150(699):971-979.
19 Beck PW, Handwerker HO, Zimmermann M. Nervous outflow from the cat’s foot during noxious radiant heat stimulation. Brain Res. 1974;67:373-386.
20 Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025-1043.
21 Fock S, Mense S. Excitatory effects of 5-hydroxytryptamine, histamine and potassium ions on muscular group IV afferent units: A comparison with bradykinin. Brain Res. 1976;105:459-469.
22 Richardson BP, Engel G. The pharmacology and function of 5HT3 receptors. Trends Neurosci. 1986;9:424.
23 Korenman EM, Devor M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp Neurol. 1981 Apr;72(1):63-81.
24 Levine JD, Gooding J, Donatoni P, et al. The role of the polymorphonuclear leukocyte in hyperalgesia. J Neurosci. 1985 Nov;5(11):3025-3029.
25 Devor M. Nerve pathophysiology and mechanisms of pain in causalgia. J Auton Nerv Syst. 1983;7:371-384.
26 Handwerker HO, Anton F, Reeh PW: Discharge patterns of afferent cutaneous nerve fibers from the rat’s tail during prolonged noxious mechanical stimulation. Exp Brain Res 65:493-504.
27 Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation–a paradox. Hum Neurobiol. 1984;3:53-58.
28 Reeh PW, Bayer J, Kocher L, Handwerker HO. Sensitization of nociceptive cutaneous nerve fibers from the rat’s tail by noxious mechanical stimulation. Exp Brain Res. 1987;65:505-512.
29 Scadding JW. Development of ongoing activity, mechanosensitivity, and adrenaline sensitivity in severed peripheral nerve axons. Exp Neurol. 1981;73:345-364.
30 Wall PD, Gutnick M. Ongoing activity in peripheral nerves: The physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974;43:580-593.
31 Tasker RR, Organ LW, Hawrylyshyn P. Deafferentation and causalgia. In: Bonica JJ, editor. Pain. New York: Raven Press; 1980:305-329.
32 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: A physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
33 Calvin WH. Generation of spike trains in CNS neurons. Brain Res. 1975;84:1-22.
34 Lisney SJ, Devor M. Afterdischarge and interactions among fibers in damaged peripheral nerve in the rat. Brain Res. 1987;415:122-136.
35 Kirk EJ. Impulses in dorsal spinal nerve rootlets in cats and rabbits arising from dorsal root ganglia isolated from the periphery. J Comp Neurol. 1974;155:165-175.
36 Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321-339.
37 Wall PD, Devor M. The effect of peripheral nerve injury on dorsal root potentials and on transmission of afferent signals into the spinal cord. Brain Res. 1981;209:95-111.
38 Wall PD. Introduction. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:13.
39 O’Brien JP. Mechanisms of spinal pain. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:244.
40 Walsh JA, Glynn CJ, Cousins MJ, Basedow RW. Blood flow, sympathetic activity and pain relief following lumbar sympathetic blockade or surgical sympathectomy. Anaesth Intensive Care. 1985;13:18-24.
41 Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(Pt 1):39-56.
42 Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res, 115. 1976:149-156. 115
43 Pallie W. The intersegmental anastomoses of posterior spinal rootlets and their significance. J Neurosurg. 1959;16:188-196.
44 Pedersen HE, Blunck CF, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerve (sinu-vertebral nerves); with an experimental study of their functions. J Bone Joint Surg Am. 1956;38-A:377-391.
45 Wynn Parry CB. The failed back. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:341-354.
46 Hakelius A. Prognosis in sciatica. A clinical follow-up of surgical and non-surgical treatment. Acta Orthop Scand Suppl. 1970;129:1-76.
47 Dieckmann G, Veras G. Plexus avulsion pain (neurogenic pain): High frequency coagulation of the dorsal root entry in patient with deafferentation pain. acta neurosurg. 1984;33(Suppl):445-450.
48 Friedman AH, Bullitt E. Dorsal root entry zone lesions in the treatment of pain following brachial plexus avulsion, spinal cord injury and herpes zoster. Appl Neurophysiol. 1988;51:164-169.
49 Friedman AH, Nashold BSJr, Bronec PR. Dorsal root entry zone lesions for the treatment of brachial plexus avulsion injuries: A follow-up study. Neurosurgery. 1988;22:369-373.
50 Thomas DG, Sheehy JP. Dorsal root entry zone lesions (nashold’s procedure) for pain relief following brachial plexus avulsion. J Neurol Neurosurg Psychiatry. 1983;46:924-928.
51 Wiegand H, Winkelmuller W. Treatment of deafferentation pain by high-frequency intervention on the dorsal root entry zone. Dtsch Med Wochenschr. 1985;110:216-220.
52 Nashold BSJr, Ostdahl RH. Dorsal root entry zone lesions for pain relief. J Neurosurg. 1979;51:59-69.
53 Powers SK, Adams JE, Edwards MS, et al. Pain relief from dorsal root entry zone lesions made with argon and carbon dioxide microsurgical lasers. J Neurosurg. 1984;61:841-847.
54 Powers SK, Barbaro NM, Levy RM. Pain control with laser-produced dorsal root entry zone lesions. Appl Neurophysiol. 1988;51:243-254.
55 Spiller WG, Martin E. The treatment of persistent pain of organic origin in the lower part of he body by division of the anterolateral column of the spinal cord. JAMA. 1912;58:1489-1490.
56 Young RF. Evaluation of dorsal column stimulation in the treatment of chronic pain. Neurosurgery. 1978;3:373-379.
57 White JC. Posterior rhizotomy: A possible substitute for cordotomy in otherwise intractable neuralgias of the trunk and extremities of nonmalignant origin. Clin Neurosurg. 1965;13:20-41.
58 Scoville WB. Extradural spinal sensory rhizotomy. J Neurosurg. 1966;25:94-95.
59 Strait TA, Hunter SE. Intraspinal extradural sensory rhizotomy in patients with failure of lumbar disc surgery. J Neurosurg. 1981;54:193-196.
60 Toczek SK, McCullough DC, Gargour GW, et al. Selective sacral rootlet rhizotomy for hypertonic neurogenic bladder. J Neurosurg. 1975;42:567-574.
61 Barrash JM, Leavens ME. Dorsal rhizotomy for the relief of intractable pain of malignant tumor origin. J Neurosurg. 1973;38:755-757.
62 van Loveren H, Tew JMJr, Keller JT, Nurre MA. A 10-year experience in the treatment of trigeminal neuralgia. comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg. 1982;57:757-764.
63 Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359-367.
64 Arias MJ. Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia. A prospective study of 100 cases. J Neurosurg. 1986;65:32-36.
65 Beck DW, Olson JJ, Urig EJ. Percutaneous retrogasserian glycerol rhizotomy for treatment of trigeminal neuralgia. J Neurosurg. 1986;65:28-31.
66 Bennett MH, Lunsford LD. Percutaneous retrogasserian glycerol rhizotomy for tic douloureux: Part 2. results nd implications of trigeminal evoked potential studies. Neurosurgery. 1984;14:431-435.
67 Cahan LD, Kundi MS, McPherson D, et al: Electrophysiologic studies in selective dorsal rhizotomy for spasticity in children with cerebral palsy. Appl Neurophysiol 50:459-462.
68 Kundi M, Cahan L, Starr A. Somatosensory evoked potentials in cerebral palsy after partial dorsal root rhizotomy. Arch Neurol. 1989;46:524-527.
69 Fasano VA, Broggi G, Zeme S. Intraoperative electrical stimulation for functional posterior rhizotomy. Scand J Rehabil Med Suppl. 1988;17:149-154.
70 Neville BG. Selective dorsal rhizotomy for spastic cerebral palsy. Dev Med Child Neurol. 1988;30:395-398.
71 Storrs BB, Nishida T. Use of the ‘H’ reflex recovery curve in selective posterior rhizotomy. Pediatr Neurosci. 1988;14:120-123.
72 McLaughlin TP, Banta JV, Gahm NH, Raycroft JF. Intraspinal rhizotomy and distal cordectomy in patients with myelomeningocele. J Bone Joint Surg Am. 1986;68:88-94.
73 Mulcahy JJ, Young AB. Percutaneous radiofrequency sacral rhizotomy in the treatment of the hyperreflexic bladder. J Urol. 1978;120:557-558.
74 Rockswold GL, Chou SN, Bradley WE. Re-evaluation of differential sacral rhizotomy for neurological bladder disease. J Neurosurg. 1978;48:773-778.
75 Rockswold GL, Bradley WE, Chou SN. Differential sacral rhizotomy in the treatment of neurogenic bladder dysfunction. preliminary report of six cases. J Neurosurg. 1973;38:748-754.
76 Laitinen LV, Nilsson S, Fugl-Meyer AR. Selective posterior rhizotomy for treatment of spasticity. J Neurosurg. 1983;58:895-899.
77 Kasdon DL, Lathi ES. A prospective study of radiofrequency rhizotomy in the treatment of posttraumatic spasticity. Neurosurgery. 1984;15:526-529.
78 Turnbull IM. Percutaneous lumbar rhizotomy for spasms in paraplegia. Paraplegia. 1983;21:131-136.
79 Wilkin RH. Neurosurgical classics. New York: Johnson Reprint Corp; 1968.
80 Davis L, Pollock LJ. The peripheral pathway for painful sensation. Arch Neurol Psychiatry. 1930;24:883-898.
81 Head H. On disturbances of sensation with especial reference to the pain of nerve disease. Brain. 1930;16:133.
82 Dykes RW, Terzis JK. Spinal nerve distributions in the upper limb: The organization of the dermatome and afferent myotome. Philos Trans R Soc Lond B Biol Sci. 1981;293:509-554.
83 Foerster O. The dermatomes in man. Brain. 1933;56:1-39.
84 Sherrington CS. Experiments in the examination of the peripheral distribution of the fibers of the posterior roots of some spinal nerves. Philos Trans R Soc Lond B Biol Sci. 1898;190:45-186.
85 Coggeshall RE. Afferent fibers in the ventral root. Neurosurgery. 1979;4:443-448.
86 Sykes MT, Coggeshall RE. Unmyelinated fibers in the human L4 and L5 ventral roots. Brain Res. 1973;63:490-495.
87 Kilber RF, Nathan PW. Relief of pain and paraesthesiae by nerve block distal to a lesion. J Neurol Neurosurg Psychiatry. 1960;23:91-98.
88 Wetzel FT, LaRocca SH, Adinolfi M. The treatment of chronic extremity pain in failed lumbar surgery. the role of lumbar sympathectomy. Spine. 1992;17:1462-1468.
89 Xavier AV, McDanal J, Kissin I. Relief of sciatic radicular pain by sciatic nerve block. Anesth Analg. 1988;67:1177-1180.
90 Jain KK. Nerve root scarring and arachnoiditis as a complication of lumbar intervertebral disc surgery–surgical treatment. Neurochirurgia (Stuttg). 1974;17:185-192.
91 Bertrand G. The “battered” root problem. Orthop Clin North Am. 1975;6:305-310.
92 Wetzel FT, Phillips FM, Aprill CN, et al. Extradural sensory rhizotomy in the management of chronic lumbar radiculopathy: A minimum 2-year follow-up study. Spine. 1997;22:2283. discussion 2291-2292
93 Osgood CP, Dujovny M, Faille R, Abassy M. Microsurgical ganglionectomy for chronic pain syndromes. technical note. J Neurosurg. 1976;45:113-115.
94 North RB, Kidd DH, Campbell JN, Long DM. Dorsal root ganglionectomy for failed back surgery syndrome: A 5-year follow-up study. J Neurosurg. 1991;74:236-242.
95 North RB, Kidd DH, Zahurak M, Piantadosi S. Specificity of diagnostic nerve blocks: A prospective, randomized study of sciatica due to lumbosacral spine disease. Pain. 1996;65:77-85.
96 Esses SI, Moro JK. The value of facet joint blocks in patient selection for lumbar fusion. Spine. 1993;18:185-190.
97 Saal JS. General principles of diagnostic testing as related to painful lumbar spine disorders: A critical appraisal of current diagnostic techniques. Spine. 2002;27(22):2538. discussion 2546
98 Revel M, Poiraudeau S, Auleley GR, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. proposed criteria to identify patients with painful facet joints. Spine. 1998;23:1972.
99 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: Identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73:824-828.
100 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195-200.
101 Lord SM, Barnsley L, Wallis BJ, et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721-1726.
102 Shealy CN. Percutaneous radiofrequency denervation of spinal facets. treatment for chronic back pain and sciatica. J Neurosurg. 1975;43:448-451.
103 Sluijter ME. Percutaneous facet denervation and partial posterior rhizotomy. Acta Anaesthesiol Belg. 1981;32:63-79.
104 Moran R, O’Connell D, Walsh MG. The diagnostic value of facet joint injections. Spine. 1988;13:1407-1410.
105 Destouet JM, Gilula LA, Murphy WA, Monsees B. Lumbar facet joint injection: Indication, technique, clinical correlation, and preliminary results. Radiology. 1982;145:321-325.
106 Shealy CN. Dorsal column stimulation: Optimization of application. Surg Neurol. 1975;4:142-145.
107 McCulloch JA. Percutaneous radiofrequency lumbar rhizolysis (rhizotomy). Appl Neurophysiol. 1976-1977;39:87-96.
108 Schaerer JP. Radiofrequency facet rhizotomy in the treatment of chronic neck and low back pain. Int Surg. 1978;63:53-59.
109 Florez G, Eiras J, Ucar S. Percutaneous rhizotomy of the articular nerve of luschka for low back and sciatic pain. Acta Neurochir (Wien), Suppl 24. 1977:67-71.
110 Oudenhoven RC. The role of laminectomy, facet rhizotomy, and epidural steroids. Spine. 1979;4:145-147.
111 Lord SM, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: A caution. Neurosurgery. 1995;36:732-739.
112 Whitworth LA, Feler CA. Application of spinal ablative techniques for the treatment of benign chronic painful conditions: History, methods, and outcomes. Spine. 2002;27:2607. discussion 2613
113 Lankford LL. Reflex sympathetic dystrophy. In Rehabilitation of the hand, 2nd ed, St. Louis: Mosby; 1989:509-532.
114 Stanton-Hicks M, Janig W, Hassenbusch S, et al. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain. 1995;63:127-133.
115 Hodgson AR. Mechanism of spinal pain. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:245.
116 Norman PE, House AK. The early use of operative lumbar sympathectomy in peripheral vascular disease. J Cardiovasc Surg (Torino). 1988;29:717-722.
117 Repelaer van Driel OJ, van Bockel JH, van Schilfgaarde R. Lumbar sympathectomy for severe lower limb ischaemia: Results and analysis of factors influencing the outcome. J Cardiovasc Surg (Torino). 1988;29:310-314.
118 Jones NF. Ischemia of the hand in systemic disease. the potential role of microsurgical revascularization and digital sympathectomy. Clin Plast Surg. 1989;16:547-556.
119 Wang JK, Johnson KA, Ilstrup DM. Sympathetic blocks for reflex sympathetic dystrophy. Pain. 1985;23:13-17.
120 Amadio PC. Pain dysfunction syndromes. J Bone Joint Surg Am. 1988;70:944-949.
121 Perelman RB, Adler D, Humphreys M. Reflex sympathetic dystrophy: Electronic thermography as an aid in diagnosis. Orthop Rev. 1987;16:561-566.
122 Pochaczevsky R. Thermography in posttraumatic pain. Am J Sports Med. 1987;15:243-250.
123 Mockus MB, Rutherford RB, Rosales C, Pearce WH. Sympathectomy for causalgia. patient selection and long-term results. Arch Surg. 1987;122:668-672.
124 Husson JL, Meadeb J, Eudier F, et al. Treatment of chronic pain of the musculoskeletal system by epidural stimulation. J Chir (Paris). 1988;125:522-524.
125 Wester K. Dorsal column stimulation in pain treatment. Acta Neurol Scand. 1987;75:151-155.
126 Meglio M, Cioni B, Prezioso A, Talamonti G. Spinal cord stimulation (SCS) in the treatment of postherpetic pain. Acta Neurochir Suppl (Wien). 1989;46:65-66.
127 Coombs DW, Saunders RL, Gaylor M, Pageau MG. Epidural narcotic infusion reservoir: Implantation technique and efficacy. Anesthesiology. 1982;56:469-473.
128 Onofrio BM, Yaksh TL, Arnold PG. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin Proc. 1981;56:516-520.
129 St Marie B. Administration of intraspinal analgesia in the home care setting. J Intraven Nurs. 1989;12:164-168.
130 De La Porte C, Van de Kelft E. Spinal cord stimulation in failed back surgery syndrome. Pain. 1993;52:55-61.
131 Downing JE, Busch EH, Stedman PM. Epidural morphine delivered by a percutaneous epidural catheter for outpatient treatment of cancer pain. Anesth Analg. 1988;67:1159-1161.
132 Greenberg HS, Taren J, Ensminger WD, Doan K. Benefit from and tolerance to continuous intrathecal infusion of morphine for intractable cancer pain. J Neurosurg. 1982;57:360-364.
133 Sjogren P, Banning AM, Henriksen H. High-dose epidural opioid treatment of malignant pain. Ugeskr Laeger. 1989;151:25-28.
134 Liew E, Hui YL. A preliminary study of long-term epidural morphine for cancer pain via a subcutaneously implanted reservoir. Ma Zui Xue Za Zhi. 1989;27:5-12.
135 Auld AW, Murdoch DM, O’Laughlin KA. Intraspinal narcotic analgesia. pain management in the failed laminectomy syndrome. Spine. 1987;12:953-954.
136 Auld AW, Maki-Jokela A, Murdoch DM. Intraspinal narcotic analgesia in the treatment of chronic pain. Spine. 1985;10:777-781.
137 Prager JP. Neuraxial medication delivery: The development and maturity of a concept for treating chronic pain of spinal origin. Spine. 2002;27(22):2593.
138 Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: A retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
139 Doleys DM. Psychological factors in spinal cord stimulation therapy: Brief review and discussion. Neurosurg Focus. 2006;21:E1.
140 Carlsson AM. Personality characteristics of patients with chronic pain in comparison with normal controls and depressed patients. Pain. 1986;25:373-382.
141 North RB, Kidd DH, Wimberly RL, Edwin D. Prognostic value of psychological testing in patients undergoing spinal cord stimulation: A prospective study. Neurosurgery. 1996;39:301.
142 Ferrante FM, Rana MV, Ferrante MA. Conversion disorder mimicking dejerine-roussy syndrome (thalamic stroke) after spinal cord stimulation. Reg Anesth Pain Med. 2004;29:164-167.
143 Parisod E, Murray RF, Cousins MJ. Conversion disorder after implant of a spinal cord stimulator in a patient with a complex regional pain syndrome. Anesth Analg. 2003;96:201.
144 Molloy AR, Nicholas MK, Asghari A, et al. Does a combination of intensive cognitive-behavioral pain management and a spinal implantable device confer any advantage? A preliminary examination. Pain Pract. 2006;6:96-103.
145 Orsnes G, Crone C, Krarup C, et al. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin Neurophysiol. 2000;111:1372-1379.
146 Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290:149-152.
147 Ivanhoe CB, Tilton AH, Francisco GE. Intrathecal baclofen therapy for spastic hypertonia. Phys Med Rehabil Clin N Am. 2001;12:923.
148 Saulino M, Jacobs BW. The pharmacological management of spasticity. J Neurosci Nurs. 2006;38:456-459.
149 Slonimski M, Abram SE, Zuniga RE. Intrathecal baclofen in pain management. Reg Anesth Pain Med. 2004;29:269-276.
150 Dykstra DD, Mendez A, Chappuis D, et al. Treatment of cervical dystonia and focal hand dystonia by high cervical continuously infused intrathecal baclofen: A report of 2 cases. Arch Phys Med Rehabil. 2005;86:830-833.
151 Ward AB, Kadies M. The management of pain in spasticity. Disabil Rehabil. 2002;24:443-453.
152 van Rijn MA, Munts AG, Marinus J, et al. Intrathecal baclofen for dystonia of complex regional pain syndrome. Pain. 2009;143:41-47.
153 Pirec V, Laurito CE, Lu Y, Yeomans DC. The combined effects of N-type calcium channel blockers and morphine on A delta versus C fiber mediated nociception. Anesth Analg. 2001;92:239-243.
154 Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357-1358.
155 Kristipati R, Nadasdi L, Tarczy-Hornoch K, et al. Characterization of the binding of omega-conopeptides to different classes of non-L-type neuronal calcium channels. Mol Cell Neurosci. 1994;5:219-228.
156 Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiol Clin North America. 2000;18:217.
157 Roh DH, Kim HW, Yoon SY, et al. Intrathecal clonidine suppresses phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesth Analg. 2008;107:693-700.
158 Deer T, Krames ES, Hassenbusch SJ, et al. Polyanalgesic consensus conference 2007: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation. 2007;10:300-328.
159 Ruan X. Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician. 2007 Mar;10:357-366.
160 North RB, Cutchis PN, Epstein JA, Long DM. Spinal cord compression complicating subarachnoid infusion of morphine: Case report and laboratory experience. Neurosurgery. 1991;29:778-784.
161 Deer T, Krames ES, Hassenbusch S, et al. Management of intrathecal catheter-tip inflammatory masses: An updated 2007 consensus statement from an expert panel. Neuromodulation. 2008;11:77-91.
162 McMillan MR. Intrathecal morphine and inflammatory masses. Anesthesiology. 2004;101:255.
163 Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109.
164 Sweet WH, Wepsic JG. Treatment of chronic pain by stimulation of fibers of primary afferent neuron. Trans Am Neurol Assoc. 1968;93:103-107.
165 Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg. 1970;32:560-564.
166 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967;46:489-491.
167 Krainick JU. Spinal cord stimulation. In: Melzack R, Wall PD, editors. Textbook of Pain. 2nd ed. New York: Churchill Livingston; 1989:924.
168 Nielson KD, Adams JE, Hosobuchi Y. Experience with dorsal column stimulation for relief of chronic intractable pain: 1968-1973. Surg Neurol. 1975 Jul;4(1):148-152.
169 Larson SJ, Sances A, Cusick JF, et al. A comparison between anterior and posterior spinal implant systems. Surg Neurol. 1975 Jul;4(1):180-186.
170 Oakley JC, Prager JP. Spinal cord stimulation: Mechanisms of action. Spine. 2002 Nov 15;27(22):2574-2583.
171 Basbaum AI, Fields HL. Endogenous pain control mechanisms: Review and hypothesis. Ann Neurol. 1978 Nov;4(5):451-462.
172 Linderoth B, Fedorcsak I, Meyerson BA. Peripheral vasodilatation after spinal cord stimulation: Animal studies of putative effector mechanisms. Neurosurgery. 1991 Feb;28(2):187-195.
173 Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992 Aug;31(2):289. 96; discussion 296-297
174 Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996 Aug;39(2):367. 74; discussion 374-375
175 Barolat G, Massaro F, He J, et al. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78:233-239.
176 Meyerson BA, Cui JG, Yakhnitsa V, et al. Modulation of spinal pain mechanisms by spinal cord stimulation and the potential role of adjuvant pharmacotherapy. Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):129-140.
177 Krainick JU, Thoden U, Riechert T. Spinal cord stimulation in post-amputation pain. Surg Neurol. 1975;4:167-170.
178 North RB, Kidd DH, Lee MS, Piantodosi S: A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: Initial results. Stereotact Funct Neurosurg 62:267-272.
179 Blume H, Richardson R, Rojas C. Epidural nerve stimulation of the lower spinal cord and cauda equina for the relief of intractable pain in failed low back surgery. Appl Neurophysiol. 1982;45:456-460.
180 Burton C. Instrumentation for dorsal column stimulator implantation. Surg Neurol. 1974;2:39-40.
181 Clark K. Electrical stimulation of the nervous system for control of pain: University of texas southwestern medical school experience. Surg Neurol. 1975;4:164-166.
182 de la Porte C, Siegfried J. Lumbosacral spinal fibrosis (spinal arachnoiditis). its diagnosis and treatment by spinal cord stimulation. Spine. 1983;8:593-603.
183 Devulder J, De Colvenaer L, Rolly G, et al. Spinal cord stimulation in chronic pain therapy. Clin J Pain. 1990;6:51-56.
184 Hoppenstein R. Electrical stimulation of the ventral and dorsal columns of the spinal cord for relief of chronic intractable pain: Preliminary report. Surg Neurol. 1975;4:187-194.
185 Kumar K, Nath R, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: A 10-year experience. J Neurosurg. 1991;75:402-407.
186 Kumar K, Wyant GM, Ekong CEU. Epidural spinal cord stimulation for relief of chronic pain. Pain Clinic. 1986;1:91-99.
187 Law JD. Targeting a spinal stimulator to treat the “failed back surgery syndrome”. Appl Neurophysiol. 1987;50:437-438.
188 Leclercq TA, Russo E. Epidural stimulation for pain control (author’s transl). Neurochirurgie. 1981;27:125-128.
189 LeDoux MS, Langford KH. Spinal cord stimulation for the failed back syndrome. Spine. 1993;18:191-194.
190 LeRoy PL. Stimulation of the spinal neuraxis by biocompatible electrical current in the human. Appl Neurophysiol. 1981;44(4):187-193.
191 Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain. A 9-year experience. J Neurosurg. 1989;70:519-524.
192 Meilman PW, Leibrock LG, Leong FT. Outcome of implanted spinal cord stimulation in the treatment of chronic pain: Arachnoiditis versus single nerve root injury and mononeuropathy. brief clinical note. Clin J Pain. 1989;5:189-193.
193 Mittal B, Thomas DG, Walton P, Calder I. Dorsal column stimulation (DCS) in chronic pain: Report of 31 cases. Ann R Coll Surg Engl. 1987;69:104-109.
194 North RB, Ewend MG, Lawton MT, Piantadosi S. Spinal cord stimulation for chronic, intractable pain: Superiority of “multi-channel” devices. Pain. 1991;44:119-130.
195 North RB, Ewend MG, Lawton MT, et al. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692-699.
196 North RB, Kidd DH, Zahurak M, et al. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery. 1993;32:384.
197 Pineda A. Dorsal column stimulation and its prospects. Surg Neurol. 1975;4:157-163.
198 Richardson RR, Siqueira EB, Cerullo LJ. Spinal epidural neurostimulation for treatment of acute and chronic intractable pain: Initial and long term results. Neurosurgery. 1979;5:344-348.
199 Shelden CH, Paul F, Jacques DB, Pudenz RH. Electrical stimulation of the nervous system. Surg Neurol. 1975;4:127-132.
200 Siegfried J, Lazorthes Y. Long-term follow-up of dorsal cord stimulation for chronic pain syndrome after multiple lumbar operations. Appl Neurophysiol. 1982;45:201-204.
201 Simpson BA. Spinal cord stimulation in 60 cases of intractable pain. J Neurol Neurosurg Psychiatry. 1991;54:196-199.
202 Vogel HP, Heppner B, Humbs N, Schramm J, Wagner C. Long-term effects of spinal cord stimulation in chronic pain syndromes. J Neurol. 1986;233:16-18.
203 Waisbrod H, Gerbershagen HU. Spinal cord stimulation in patients with a battered root syndrome. Arch Orthop Trauma Surg. 1985;104:62-64.
204 Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618-624.
205 Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: A systematic literature synthesis. Neurosurgery. 1995;37:1088. discussion 1095-1096
206 Law JD. A new method for targeting a spinal stimulator: Quantitatively paired comparisons. Appl Neurophysiol. 1987;50:436.