Chapter 7 Surgical Principles
Histologic Diagnosis
Percutaneous radiograph-directed FNAC has gained great popularity over the past 25 years.1 Ultrasonography or CT scanning is routinely used to obtain hepatic, renal, pancreatic, and retroperitoneal biopsies. Percutaneous biopsy techniques are particularly useful to confirm the presence of metastases in a patient with a prior malignant tumor. Adrenal FNAC should be avoided if a pheochromocytoma has not been excluded. If potentially curable metastases (e.g., limited hepatic metastases from colorectal cancer) are present, this diagnostic test is unwarranted and potentially dangerous because it rarely results in tumor seeding. If unresectable cancer is present at exploratory laparotomy, a confirmatory biopsy should be obtained.
Incisional biopsy excises more of a tumor mass than needle biopsy. A full-thickness biopsy using a scalpel or punch biopsy tool of a larger skin tumor is a commonly performed incisional biopsy. A full-thickness sampling of the thickest portion of the lesion should be removed to allow accurate tumor staging. Sometimes an incisional biopsy of a sarcoma is performed to provide the diagnosis before proceeding with definitive treatment (e.g., amputation, preoperative chemotherapy or radiation), but core-needle biopsies are another option. An incisional biopsy of an extremity tumor is preferably obtained through a longitudinal rather than a transverse incision because the longitudinal incision can be more readily incorporated with a wide local excision.2 Intraoperative incisional biopsies during thoracotomy and laparotomy are rarely indicated; tumor spillage is more likely than with FNAC or core-needle biopsies.
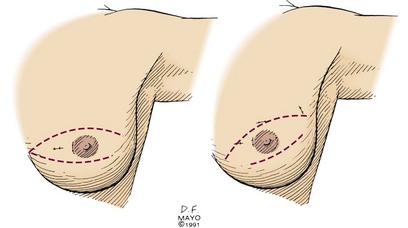
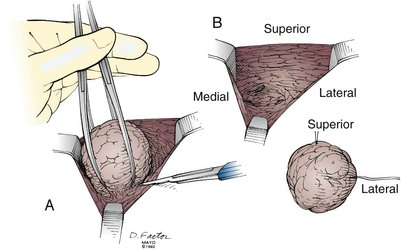
Excisional biopsy of a breast mass illustrates the key principles of a diagnostic surgical biopsy. This procedure can be used for small, palpable lesions amenable to easy complete excision. A diagnostic excisional breast biopsy is now rarely performed. Almost all breast cancers are diagnosed with FNAC or core biopsy preoperatively. If possible, the skin incision should be circumareolar or situated within the elliptical incision that would be used for a mastectomy (Fig. 7-1). If the breast mass is believed to be malignant, it should be excised with a 1-cm margin of normal tissue, centering the suspicious lesion in the specimen (Fig. 7-2A). The biopsy specimen should be oriented to permit the pathologist to specify which of the resection margins, if any, are histologically involved by tumor. Specimen orientation can be denoted with two sutures (see Fig. 7-2B) or by painting the specimen with multiple colored stains. If frozen-section evaluation of the resection margins is available, re-excisions can be performed immediately when necessary. When frozen-section evaluation is unavailable, separate, individually labeled margins may be sent in addition to the primary tumor specimen. Selective re-excision can be performed at a later date if the main specimen has been appropriately orientated with sutures, clips, multicolored inks, or separate margins. Surgical clips left at the base of the biopsy cavity facilitate accurate partial breast or boost-field radiation therapy after breast-conservation surgery. Small titanium clips provide accurate localization for the radiation oncologist with minimal interference on future images, including MRI.
Staging
Clinical and pathologic stages of disease for most cancers have been standardized in the American Joint Committee for Cancer (AJCC) TNM system.3 In this nomenclature, T refers to the primary tumor, N indicates the status of regional lymph nodes, and M denotes the presence or absence of metastatic disease. For most cancers, the size (e.g., in lung, liver, or breast cancers) or the degree of invasion (e.g., in melanoma or stomach or colorectal cancers) of the primary tumor correlates with the probability of metastases.
Role of Laparoscopy
The introduction of laparoscopy in general surgical practice has led to more precise staging of many intra-abdominal malignancies, particularly gastric,4 pancreatic,5 and hepatobiliary cancers.6,7 Thoracoscopy allows inspection and biopsy of the pleural cavity to assess for intrathoracic tumor spread. When used for diagnosis, laparoscopy allows visualization of peritoneal surfaces; histologic evaluation of peritoneal, omental, or hepatic tumor masses; biopsy of lymph nodes; and collection of ascites or peritoneal washings for cytologic examination. Laparoscopic ultrasonography further improves the staging of pancreatic and hepatobiliary malignancies.8–10 If the laparoscopic findings confirm metastatic malignancy, the attendant morbidity from tumor resection by traditional or minimally invasive techniques can be avoided. Laparoscopic techniques can be used for cancer therapy to palliate patients with advanced malignancy and curatively resect others.11
Staging of Pancreatic Malignancy
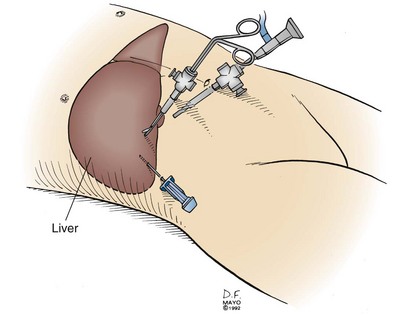
Laparoscopy is frequently used to stage pancreatic and periampullary carcinomas because hepatic or peritoneal metastases undetectable by radiographic means occur in up to 30% of patients with tumors believed to be resectable preoperatively.5,12,13 A complete laparoscopic inspection of the abdominal cavity is undertaken using a 5- or 10-mm port at the umbilicus (Fig. 7-3). Additional subcostal cannulae allow for retraction of the liver, omentum, and loops of intestine. Inspection of the upper abdomen, looking for small hepatic metastases or drop metastases on the parietal and visceral peritoneum or the greater omentum, is readily accomplished. Any suspicious lesions should be sampled using a biopsy forceps or core-needle biopsy tool. The remainder of the abdomen can be examined, including the peritoneal surfaces, for seeding and dependent areas for ascites. Evidence of direct spread of a pancreatic carcinoma into the transverse mesocolon and small bowel mesentery should be assessed.
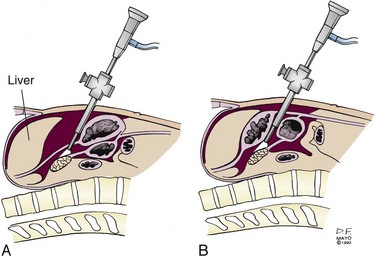
With the patient in the reverse Trendelenburg position, limited visualization of the anterior surface of the pancreas may be obtained with a supragastric approach after division of the lesser omentum (Fig. 7-4A) or an infragastric approach, entering the lesser sac through the gastrocolic omentum (see Fig. 7-4B). Biopsy of peritoneal implants within the lesser sac should be obtained. Laparoscopic ultrasonography provides a more accurate assessment of visceral vascular involvement and deep hepatic metastases,10 although endoscopic ultrasonography (EUS) provides highly accurate staging in these sites.
Perioperative Care of the Oncology Patient
The extent and type of operative management must take into account the patient’s preexisting and malignancy-induced comorbidities. Cancer cachexia resulting from anorexia is common and results in significant lean tissue loss and immunodeficiency. Because malnutrition significantly increases the risk for perioperative morbidity, the surgeon must carefully assess the degree of preoperative malnutrition. Although retrospective studies evaluating preoperative nutritional support show a reduction in postoperative complications for patients with severe malnutrition,14 a meta-analysis did not support routine preoperative nutrition for oncologic patients.15 Total parenteral nutrition should be reserved for patients unable to tolerate enteral nutrition, those unable to take sufficient calories by oral or enteric routes during therapy, and those felt to be unsuitable for operative or combined-modality therapy until their nutritional status improves. Enteral rather than parenteral nutrition should be used whenever possible. Postoperative nutritional support should always be considered, especially in patients with upper gastrointestinal malignancies such as esophageal, gastric, and pancreatic cancers. A feeding jejunostomy catheter placed intraoperatively facilitates delivery of postoperative nutrition.
Patients with malignancy are often anemic. Blood transfusions result in immunosuppression, including depression of specific cellular immunity, and nonspecific immune responses, including natural killer cell activity and macrophage phagocytosis. Some retrospective studies have shown higher cancer recurrence rates in cancer patients receiving blood transfusions, but controlled trials have not demonstrated a poorer disease-specific survival rate specifically related to perioperative transfusions.16,17 Blood transfusion must be administered as appropriate in oncologic patients.
Radiation Therapy and Wound Healing
Tissues exposed to radiation therapy develop acute inflammatory changes in proportion to the total dose. Higher-dose fractions cause more significant changes. Acute radiation injury is manifested by vasodilation (erythema) and tissue edema. Following moderate-dose preoperative radiation (45 to 50 Gy in 1.8- to 2.0-Gy fractions), a 3- to 6-week preoperative recovery period is generally allowed for partial resolution of acute radiation changes.18 Late radiation changes include atrophy and fibrosis, which result from decreased tissue vascularity.
Wound healing is impaired in irradiated tissue by several factors, including diminished blood supply, impaired collagen formation, and the increased risk of infection resulting in part from decreased leukocyte function. After high-dose irradiation, slow and nonhealing wounds are commonplace. In this situation, nonirradiated tissues, such as vascularized myocutaneous flaps, need to be transferred into the radiation field to allow proper wound healing.19 This is preferably done at the time of tumor resection rather than after a nonhealing postoperative wound develops. If partial resection of an irradiated hollow organ (e.g., bowel, bile duct, trachea) is necessary, one side of the anastomosis should be nonirradiated tissue whenever possible. This precaution provides a better blood supply for healing of the anastomosis. This policy will reduce the incidence of early postoperative leak and fistula formation and late anastomotic strictures.
Surgical Treatment of Breast Cancer
Most women with breast cancer can now choose breast conservation. Data from multiple mature, controlled trials have demonstrated no significant difference in disease control or survival between patients who elect breast-conservation therapy and those who choose mastectomy.20,21,22 Mastectomy is a suitable option if the woman chooses this form of treatment. Mastectomy is preferable for the management of multicentric disease and most large primary tumors (neoadjuvant chemotherapy may allow breast conservation in patients with a sufficient response) and in patients unable to receive postoperative radiation therapy. The majority of patients undergoing mastectomy can have immediate breast reconstruction, if desired. Consultation with a plastic surgeon should be obtained preoperatively to allow the patient to assess the immediate reconstruction options. The timing of breast reconstruction depends partly on patient preference. In addition, if chest wall irradiation is indicated by the breast cancer stage, the immediate reconstruction results are often cosmetically affected.
Early Breast Cancer
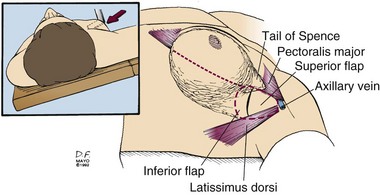
Sentinel lymph node biopsy or axillary lymph node dissection is best performed through a separate incision. The axillary incision should be placed between the axillary folds and not cross the lateral border of the pectoralis major muscle (Fig. 7-5). Axillary staging aids patient management in determining prognosis and systemic adjuvant treatment. Patients with positive axillary nodes are almost routinely advised to receive systemic treatment, usually combination chemotherapy. Postoperative therapy commences after sufficient wound healing has occurred, usually within 4 weeks.
Sentinel Lymph Node Biopsy
A sentinel lymph node biopsy identifies and excises the first draining regional node(s). First popularized in the management of cutaneous malignant melanoma, in 1994 Giuliano and colleagues23 reported their adaptation of the sentinel node technique for patients with breast cancer. Using isosulfan blue to isolate the sentinel lymph node, they identified a sentinel node in 93% of patients with high accuracy for detecting axillary metastases. Given their success with sentinel node biopsy to identify nodal metastases, Giuliano and colleagues23,24 stopped performing axillary lymphadenectomy for patients with a negative sentinel node biopsy.
To successfully predict nodal metastasis requires an orderly spread of tumor cells in the lymphatics, not a random distribution. The initial draining node or sentinel lymph node is the site of first metastasis. After several confirmatory reports, sentinel lymph node biopsy became standard practice for breast cancer patients who had no clinical evidence of axillary metastasis.25,26,27 The sentinel lymph nodes can be localized with a dye (usually isosulfan blue or methylene blue), a radiolabeled colloid (usually sulfur colloid or human serum albumin), or with both dye and colloid. Through a small axillary incision (2 to 3 cm), the blue-stained lymphatics are frequently located at the lateral border of the pectoralis major muscle and followed to the blue-stained sentinel node. Alternately, a hand-held gamma probe allows localization of the radioactive lymph nodes. All nodes stained with dye, those with high radioactivity counts, and those with both staining and high counts are excised and evaluated histologically. Any hard lymph node should also be removed and examined by the pathologist because a tumor-replaced node has no lymphatic flow. Tumor-involved nodes found on frozen-section evaluation allow immediate completion of the axillary dissection. The prognostic importance of minimal tumor involvement of a sentinel node, such as that detected only by immunohistochemical staining (i.e., N0[i+]) and micrometastases (i.e., a tumor deposits less than 2 mm in diameter; i.e., N1mi), remains controversial. Recommendations for complete axillary lymph node dissection and adjuvant treatment based on these limited metastases are still under development.
Multimodality Therapy for Pancreatic Adenocarcinoma
Preoperative staging of pancreatic carcinoma should determine if a curative operation is possible. High-quality, thin-cut, spiral CT scanning with oral and intravenous contrast media delineates the primary pancreatic mass, local vascular invasion, and liver metastasis in most patients. Endoscopic ultrasonography (EUS) and laparoscopy with or without ultrasonography provide more sensitive evaluation of limited metastatic disease.8–13 With EUS, a high-frequency transducer within the gastric and duodenal lumen is positioned near the pancreas. This technique can detect small pancreatic masses (<1 cm), identify enlarged regional lymph nodes (and allow FNA biopsy of both), and better define the presence and extent of visceral vascular involvement. Laparoscopy for staging pancreatic carcinoma is the best means to detect peritoneal or liver metastases not detected with preoperative imaging.
Only 10% to 20% of patients with carcinoma of the pancreas can be completely resected (R0 resection). Palliation of symptoms to improve quality of life becomes the therapeutic goal for most pancreatic cancer patients. Obstructive jaundice can be palliated surgically or nonoperatively. Several prospective, randomized studies showed nonoperative biliary stenting to be as effective in the relief of jaundice as surgical biliary bypass.28,29 Pain from posterior tumor invasion is effectively treated with a combination of oral analgesics and a celiac plexus block. Operative (laparoscopic or open) gastrojejunostomy or expanding metal endoluminal stents are both reasonable options for the management of duodenal obstruction caused by tumor invasion. Stenting is generally less durable than surgical bypass, requiring multiple procedures when patient survival is lengthy.
The operative results and long-term survival rates after pancreaticoduodenectomy have improved most significantly in medical centers with larger patient experiences. Operative management includes a full abdominal exploration, focusing on inspection of the liver, peritoneum, and omentum for distant metastases. The regional lymph nodes are also palpated to assess for tumor involvement. Periaortic, porta hepatis, small bowel mesentery, or celiac lymph node metastases indicate tumor spread beyond the limits of standard resection. Japanese surgeons have advocated radical lymphadenectomy to improve patient outcome,30 but randomized trials of extended lymph node dissection have not resulted in improved patient survival rates.31,32
Local tumor factors that usually prevent curative resection include retroperitoneal extension to involve the inferior vena cava or aorta or direct involvement or encasement of the superior mesenteric artery or celiac axis. Several centers have shown that en bloc resection of the involved superior mesenteric or portal vein results in survivals comparable to those of standard pancreaticoduodenectomy.33,34 After excluding unresectable tumors, the surgeon can proceed with pancreaticoduodenectomy. The classic Whipple procedure, which resects the gastric antrum, or the pylorus-preserving modification provides similar results.
Because approximately 50% of patients have locoregional recurrence after curative resection, adjuvant irradiation could have beneficial effects on patient survival rates.35 The Gastrointestinal Tumor Study Group36 first reported encouraging results from a prospective, randomized trial in 1985. The efficacy of adjuvant postoperative irradiation and chemotherapy for adenocarcinoma of the head of the pancreas was evaluated. Forty-three patients were randomized to receive adjuvant therapy with irradiation and 5-fluorouracil (5-FU) or no adjuvant therapy. The median survival time for the 21 patients who received adjuvant therapy was 20 months, and 3 (14%) survived more than 5 years. Among the 22 patients who did not receive adjuvant therapy, the median survival time was 11 months, and only 1 patient (4.5%) survived 5 years.37 Similar results were noted in a collaborative study from Johns Hopkins Hospital and the Mayo Clinic38 favoring adjuvant postoperative chemoradiation therapy over surgery alone. A European Study Group for Pancreatic Cancer (ESPAC-1)39 trial showed benefit for adjuvant chemotherapy, with a deleterious impact of concurrent chemotherapy and irradiation. Major flaws in the ESPAC-1 trial are discussed in Chapter 46, Pancreatic Cancer, in this textbook.
For locally advanced, unresectable pancreatic carcinomas, the use of external beam irradiation (EBRT) plus chemotherapy has been reported to result in a doubling of median survival compared with surgical bypass or biliary stenting. The 2-year survival range increases from 0% to 5% up to 10% to 20% with palliative chemoirradiation. Five-year survivors are rare, and nonprogression of the primary tumor is uncommon. The addition of intra-operative irradiation therapy (IORT) to EBRT with or without 5-FU improves local control, as shown by physicians at the Mayo Clinic and Massachusetts General Hospital.40,41 This benefit did not translate into an improved patient survival, because of liver and peritoneal metastatic progression. If the full course of EBRT, with or without 5-FU, is delivered preoperatively, this sequence allows restaging 2 to 3 months after treatment initiation. The 2-year survival rate appeared to be improved with this sequence of treatment followed by IORT.42 Improvement presumably resulted from altered patient selection, because the incidence of liver plus peritoneal failure was not different. Until better systemic therapy is developed, the improved local control of unresectable pancreatic adenocarcinoma observed with IORT will not translate into improved survival for patients with locally advanced disease.
Therapy for Adenocarcinoma of the Rectum
Despite significant evolution in the multimodality therapy of rectal adenocarcinoma over the past decades, surgical resection continues to be the primary curative modality in these patients. The goal of surgery is to resect all known malignant tissue from the pelvis, thereby optimizing survival and minimizing local failure, while preserving normal bowel, bladder, and sexual function whenever possible. Several patient- and tumor-related factors influence not only the choice of the operation but also the coordination of surgical and nonsurgical treatment modalities. Preoperative clinical tumor staging should assess the level of the tumor from the anal verge, the extent of circumferential involvement, the depth of tumor invasion (T category), and the presence of locoregional adenopathy (N category) and of metastatic disease (M category).3 Important patient-related factors may include body habitus, preoperative bowel and sphincter function, and the presence of conditions that may contraindicate the creation of a colostomy, such as blindness, severe arthritis, or mental incapacity.
Accurate preoperative staging of rectal carcinoma is of central importance for selection of surgical strategy and for determining the need for preoperative irradiation and/or chemotherapy. As discussed below, the majority of patients with T3-4 or node-positive disease currently undergo preoperative therapy. The exact location of the tumor is measured in reference to the anal verge or to the top of the anal sphincter complex (i.e., anorectal ring) by digital rectal examination and proctosigmoidoscopy. The operative surgeon should ideally perform this evaluation before the patient receives preoperative treatment. Distant metastatic disease can be detected by CT scanning with 75% to 87% sensitivity. Although nodal staging by CT scanning carries a lower sensitivity (45% to 73%), visible perirectal adenopathy should be suspected as malignant.43,44 At present, T and N stages are most commonly assessed by transrectal EUS. A radial scanning echoendoscope is inserted transrectally for inspection of each layer of the rectal wall and the perirectal structures. Fine-needle aspiration of suspicious perirectal nodes or extramural lesions can be performed. The reported stage-specific sensitivities and specificities of EUS are T1 (88% and 98%), T2 (81% and 96%), T3 (96% and 91%), and T4 (95% and 98%); corresponding sensitivity and specificity for nodal staging are 73% (95% CI, 71% to 76%) and 76% (95% CI, 74% to 78%), respectively.45,46 EUS is operator-dependent and is of limited value when severe luminal stenosis is present or when EUS is performed in the postirradiation setting because inflammatory changes of the soft tissues reduce the accuracy of EUS.47
In addition to T and N staging, there has been increasing emphasis on assessing the circumferential resection margin (CRM) or the degree of tumor infiltration of the mesorectal fascia.48 Preoperative recognition of a CRM where malignant infiltration is present within 1 to 2 mm of the mesorectal fascia allows surgical planning for a resection plane that will result in a negative CRM.49 In the multicenter Magnetic Resonance Imaging and Rectal Cancer European Equivalence Study (MERCURY),50 high-resolution MRI accurately predicted the involvement of the mesorectal margin to within 1 mm and extratumoral extension with a specificity of 92%. Using surgical resection specimens as the gold standard, 94% of the patients did have a negative CRM.50,51 For patients with locally advanced primary cancer or locally recurrent rectal tumor, pelvic MRI best assesses for tumor involvement or fixation to adjacent pelvic organs, helping the surgeon plan en bloc resection of involved organs for a microscopically negative margin.49,52 The need for urologic, orthopedic, and plastic surgical assistance can be anticipated preoperatively with MRI staging.
Surgical resection of curative intent removes the primary tumor, draining lymphatic tissue, and any involved pelvic structures in an en bloc fashion. Standard resection includes sphincter-preserving procedures such as low anterior resection (LAR) and proctectomy with coloanal anastomosis, or abdominoperoneal resection (APR) with a permanent colostomy. The choice of the procedure is mainly dictated by the distance between the tumor and the anal verge because adequate proximal and distal margins must be achieved. Previous studies have shown that tumor deposits in nodal tissue are rarely seen more than 2 to 4 cm distal to the tumor. A distal margin of 2 cm resulted in no difference in overall survival or local failure rates when compared with a greater distal margin.53,54 Patients are candidates for sphincter preservation if the tumor is located at least 2 cm above the anorectal ring. All operations involve (1) proximal ligation of the inferior mesenteric and superior hemorrhoidal vessels and (2) full mobilization of the rectum either to a level well below the gross tumor or to the level of the levator ani muscles. The adequacy of the distal margin is then assessed to determine the feasibility of sphincter preservation. If sphincter preservation is possible, the rectum is transected, usually with a cutting linear stapler. An end-to-end colorectal anastomosis is then created using either a hand-sewn or stapling technique. When the line of rectal transaction is at the level of the anal sphincter, the rectal division may be accomplished transanally. A hand-sewn coloanal anastomosis using interrupted sutures placed transanally is then performed. Although intersphincteric dissection followed by hand-sewn anastomosis has been reported, its role remains highly limited.55 When sphincter preservation is not possible, an APR is performed. Recently, a “cylindrical” approach to APR has been advocated, with an extended perineal dissection including the origin of the levator ani muscle from the lateral pelvic sidewall. This en bloc resection of the levator muscles results in a more cylindrical specimen and had been associated with less CRM positivity and intraoperative specimen perforation.56
Sphincter-preserving procedures carry the risk of anastomotic leakage and altered bowel function. In an analysis of 5187 patients undergoing LAR in five randomized control trials, the symptomatic anastomotic leakage rate was 9.7%.57 Leakage was associated with reduced overall survival rates. A diverting stoma lessened the symptoms of the leakage. Construction of a diverting stoma is prudent with a low rectal anastomosis or in patients who received neoadjuvant chemoradiation or will have adjuvant chemoradiation, or both.58,59 Sphincter-preservation procedures are also accompanied by altered bowel function, most commonly stool frequency, urgency, and incontinence. Reconstructive techniques, including the colonic J pouch, transverse coloplasty, or side-to-end anastomosis, have been developed. A recent Cochrane review showed that functional outcomes were better with the colonic J pouch than with a straight rectal anastomosis in the short term (18 months). There was insufficient evidence for any long-term benefit.60 These reconstructive options may not be feasible in patients with a bulky mesentery or narrow pelvis. Therefore, sphincter preservation must be considered in the context of (1) adequate oncologic margin, (2) risk of anastomotic leakage, and (3) altered bowel function.
In addition to distal and proximal margins, CRM involvement is a key determinant of outcome after standard rectal resection.61 For tumors confined within the mesorectum, an involved CRM is less likely with total mesorectal excision (TME) compared with a less radical resection.62 Popularized by Heald and associates,62 TME involves sharp dissection of the avascular plane between the visceral covering of the mesorectum and the parietal fascia of the pelvis. The surgical planes between the integral visceral mesentery of the hindgut and the surrounding tissues provide a unique opportunity for defining a surgically achievable “tumor package,” and, serendipitously, the field of spread of rectal cancer is commonly limited within this package, or mesorectum.63,64 TME encompasses virtually every tumor satellite except those in patients in whom the tumor is widely disseminated. The excised specimen includes the entire posterior, distal, and lateral mesorectum to the plane of the inferior hypogastric nerve plexus, which is carefully preserved whenever possible. Anteriorly, the specimen includes the intact Denonvilliers fascia and the peritoneal reflection. The characteristic smooth, bilobed, encapsulated appearance posteriorly and distally reflects the contours of the pelvic floor and the midline anococcygeal raphe. In contrast to the local recurrence rates of 20% to 45% reported with traditional resections, rates between 4% and 7% and 5-year disease-free survival rates approaching 80% have been reported with TME alone.63,64
Several less-invasive surgical approaches for patients with rectal cancer have gained attention in recent years. Transanal local excision, either conventionally or via transanal endoscopic microsurgery, may be an option in select patients with well-differentiated T1 tumors located within 8 cm of the anal verge, less than 3 cm in diameter, occupying less than 30% of the luminal circumference, and without suspicious perirectal adenopathy.65 Excised specimens should be full-thickness, nonpiecemeal specimens with an adequate margin of resection for pathologic examination. Patients treated in this fashion enjoy minimal morbidity but face increased risks for local failure and, perhaps, decreased overall survival rates.66–68 Although laparoscopic-assisted operations for rectal cancer are technically feasible, long-term data demonstrating oncologic equivalence to open procedures are not available. Particular concerns of the laparoscopic approach include inadvertent spillage of luminal contents, handling of the tumor specimen, implantation of tumor cells in the pelvis, and the potential for port-site recurrence. In the short term, patients have reduced analgesia requirements, ambulate more readily, and appear to have less paralytic ileus. The United Kingdom Medical Research Council trial of conventional versus laparoscopic-assisted surgery in colorectal cancer (UK MRC CLASSIC trial) included a subgroup of 381 rectal cancer patients who were randomized to open versus laparoscopic procedures. After accounting for a 34% conversion rate, 189 and 87 patients were treated in each arm, respectively. No statistically significant difference was seen in the rates of positive proximal, distal, or circumferential margins, 3-year local recurrence, or overall survival. However, in the subgroup of patients who underwent laparoscopic versus open LAR, the CRM was positive in 12% versus 6% of the open LAR patients (p = .19).69 Results from more adequately powered trials with longer follow-up are needed. Finally, robotic surgery for rectal cancer has emerged, but its oncologic outcomes remain unestablished.70
Despite surgical resection of rectal cancer with curative intent, approximately 40% of the patients develop disease relapse, usually during the first 3 postoperative years. Local pelvic recurrences can be highly morbid, causing pain, bowel obstruction, hemorrhage, and malignant fistulization. The risk of relapse correlates with the T and N stages of the tumor, as well as completeness of surgical excision71 (Table 7-1). Local failure is also more common with a positive CRM.48,72 Consequently, adjuvant therapy has evolved with the goals of preventing local tumor recurrence, eliminating distant metastases, and increasing disease-free and overall survival rates.73 Both preoperative and postoperative regimens of irradiation or chemoirradiation have been developed. Although early trials mainly involved postoperative irradiation, the potential for tumor downstaging, less tissue hypoxia, and less exposure to small bowel in the radiation field has shifted the focus toward preoperative treatment. Two meta-analyses showed that when compared with surgery alone, the addition of preoperative irradiation to surgery decreased the local failure rate (odds ratio [OR] 0.49; 95% CI 0.38 to 0.62; p < .001)74 and that the yearly local failure rate was 46% lower with preoperative irradiation (p < .001) and 37% lower with postoperative irradiation (p = .002).75 In the era of TME surgery, the German Rectal Cancer Study Group trial demonstrated the added benefit of preoperative combination chemoirradiation: the local recurrence rate was 6% with preoperative treatments versus 13% with postoperative treatments (p = .006), although no difference was seen in overall survival rates.76 Although a second trial of similar design, NSABP R-03, did not meet its accrual goal, a superior 5-year disease-free survival rate was observed in the preoperative versus postoperative arm (65% vs. 53%; p = .011). A 5-year local recurrence rate of 10.7% was reported for both treatment arms.77 Although preoperative combination chemoirradiation has been widely adopted in the United States for nearly all T3-4 and node-positive rectal cancer patients, its use is more selective in Europe. A recent trial from the Medical Research Council (MRC CR07) compared short-course preoperative irradiation with TME surgery and selective postoperative chemoirradiation and demonstrated that preoperative treatment still led to a 6% lower 3-year local recurrence rate, although no difference was seen in overall survival rates.78 Finally, based on pooled data from five phase III North American rectal adjuvant trials, selected patients with intermediate-risk rectal cancers (T1-2N1 and T3N0) might not need radiation therapy as a component of adjuvant treatment.71 Ongoing trials continue to investigate the optimal regimen that provides oncologic benefit but spares patients potential adverse effects.
Management of Retroperitoneal Soft Tissue Sarcoma
Soft tissue sarcomas constitute less than 1% of all malignant tumors in the United States. Approximately 15% of sarcomas arise in the retroperitoneum.79 Retroperitoneal sarcomas provide a challenge for the surgical oncologist. Most retroperitoneal sarcoma patients eventually die of their disease, despite never developing disease outside the abdomen. The locally invasive growth pattern, the lack of anatomic boundaries in the retroperitoneum, and the large size of most sarcomas at presentation make complete R0 resection difficult. The rate of complete gross tumor resection (R0 and R1), which was approximately 50% in major centers,79 has increased to 67% to 75%.80,81 The local recurrence rate at 5 years after complete surgical resection ranges from 40% to 50%.80–82,83 Among patients who are disease free at 5 years, 40% will experience a recurrence by 10 years after their operation.84 Uncontrollable local recurrence is the most common cause of death from retroperitoneal sarcoma. Retrospective analysis of more than 1000 retroperitoneal sarcoma patients found primary tumor size; tumor fixation to nerve, vessels, or bone; regional lymph node involvement (rare); presence of metastatic disease; and tumor grade predictive of survival.85
The surgical treatment of retroperitoneal sarcomas consists of en bloc resection of the tumor plus adjacent organs invaded by or adherent to the sarcoma. Recent reports recommend removal of any adjacent organs regardless of apparent tumor involvement.86,87 This is feasible for some sarcomas but not for others.88 This policy has not been shown to give better results in a controlled study.85 Most retroperitoneal sarcomas involve left-sided or right-sided retroperitoneal structures, with few arising in the midline. Because of their large dimensions, en bloc resection often requires ipsilateral nephrectomy and colectomy, partial small bowel resection, distal pancreatectomy, and splenectomy. Less frequently, a partial gastrectomy, pancreaticoduodenectomy, major hepatectomy, or vascular resection may be indicated. Any adherence of the sarcoma to an adjacent structure must be assumed to be malignant in nature. These structures should be excised en bloc whenever feasible. If extensive venous collaterals are present from vena caval occlusion, the inferior vena cava may be resected without reconstruction; a prosthetic graft reconstruction is indicated in other patients. An aortic resection and prosthetic reconstruction should be considered for a sarcoma encasing the aorta when the sarcoma can be completely excised. When a retroperitoneal sarcoma has an extensive volume of necrotic tissue, care must be used during mobilization to avoid tumor rupture.
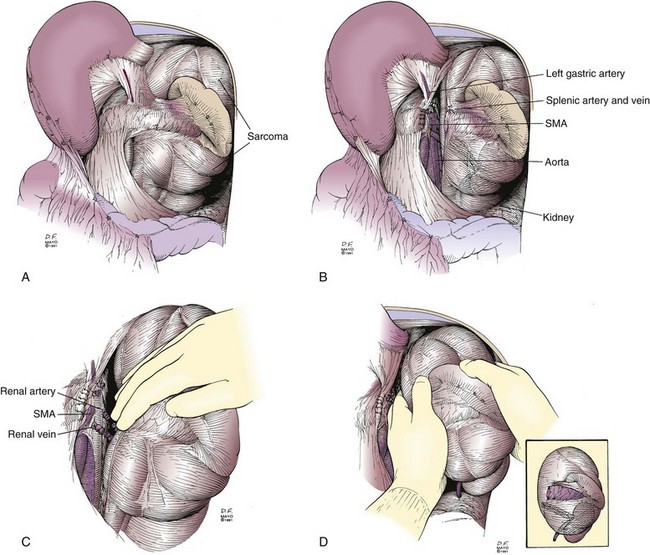
Resection of a large retroperitoneal sarcoma en bloc is presented in Figures 7-6 and 7-7. This patient presented with pain and an abdominal mass. A preoperative CT scan image appears in Figure 7-6. The sarcoma filled the left upper quadrant, displacing the spleen and distal pancreas anteriorly. On abdominal exploration through a midline incision, the tumor was found to be limited to the left retroperitoneum. The stomach was not involved by tumor. After opening the lesser sac, the stomach was reflected cephalad. The splenic flexure of the colon was also uninvolved. It was retracted caudad, providing excellent exposure of the distal pancreas, spleen, left kidney, and anterior surface of the sarcoma (see Fig. 7-7A). The neck of the pancreas was divided, allowing ligation of the splenic artery and vein (see Fig. 7-7B). To minimize intraoperative bleeding, the vascular supply along the medial aspect of the sarcoma was ligated before the sarcoma was mobilized and en bloc resection was performed. The left renal artery and vein were then exposed and ligated (see Fig. 7-7C). The resected retroperitoneal sarcoma with the distal pancreas, spleen, and left kidney are shown in Figure 7-7D.
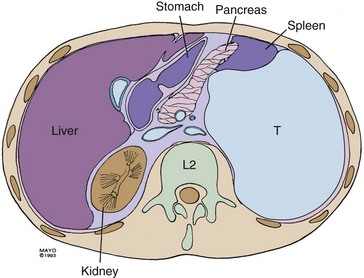
Figure 7-6 Appearance of left upper quadrant mass on CT scan. T, tumor.
Courtesy the Mayo Foundation.
Because local tumor recurrence is the most common pattern of treatment failure, radiation therapy should theoretically improve results. Some retrospective series have suggested an improvement in the local control and survival of retroperitoneal sarcoma patients with adjuvant irradiation.89,90 The large-field radiotherapy needed to treat the resection bed following retroperitoneal sarcoma excision can cause significant complications. IORT plus EBRT has been considered a better alternative to full-dose EBRT because of less toxicity to normal tissues. A prospective, randomized clinical trial performed at the National Cancer Institute compared IORT plus postoperative low-dose EBRT with postoperative high-dose EBRT alone. This study showed no statistically significant difference in survival rates between patient groups, but the pattern of disease failure differed between the two groups. Patients receiving IORT plus EBRT had significantly better local disease control within irradiation fields and a significantly lower prevalence of small bowel toxicity.91 A trial sponsored by the American College of Surgeons Oncology Group to study whether adjuvant EBRT improved local control and survival rates compared with resection alone for retroperitoneal sarcoma patients closed prematurely because of poor patient accrual. Currently, there is no proven benefit for the use of either adjuvant radiation therapy or chemotherapy for retroperitoneal sarcomas.
3 Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual, ed 7. New York: Springer-Verlag; 2010.
4 D’Ugo DM, Pende V, Persiani R, et al. Laparoscopic staging of gastric cancer. An overview. J Am Coll Surg. 2003;196:965.
7 D’Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy. Prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183.
19 Mathes SJ, Alexander J. Radiation injury. Surg Oncol Clin North Am. 1996;5:809.
21 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2003;347:1233.
25 Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer. A multicenter validation study. N Engl J Med. 1998;339:941.
27 Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546.
28 Shepherd AH, Royle G, Ross APR, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct. A randomized trial. Br J Surg. 1988;75:1166.
34 Siriwardana HPP, Siriwardena AK. Systemic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br. J Surg. 2006;93:662-673.
37 Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006.
38 Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma. The Johns Hopkins Hospital—Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981.
39 Neoptolemas JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200.
46 Puli SR, Reddy JB, Bechtold ML, et al. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers. A meta-analysis and systematic review. Ann Surg Oncol. 2009;16(5):1255.
50 Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1):132.
56 West NP, Finan PJ, Anderin C, et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26(21):3517.
62 Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer. A Scandinavian consensus. Br J Surg. 1995;82(10):1297.
65 Network NCC. Rectal cancer: the NCCN oncology practice guidelines. Rockledge, Pa: NCCN; 2010.
69 Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma. 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061.
71 Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer. A pooled analysis. J Clin Oncol. 2004;22(10):1785.
76 Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731.
83 Stoeckle E, Coindre J-M, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma. A multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359.
84 Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832.
91 Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized clinical trial. Arch Surg. 1993;128:402.
1 Gazelle GS, Haaga JR. Guided percutaneous biopsy of intraabdominal lesions. Am J Radiol. 1989;153:929.
2 Karakousis CP. Principles of surgical resection for soft tissue sarcomas of the extremities. Surg Oncol Clin North Am. 1993;2:547.
3 Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual, ed 7. New York: Springer-Verlag; 2010.
4 D’Ugo DM, Pende V, Persiani R, et al. Laparoscopic staging of gastric cancer. An overview. J Am Coll Surg. 2003;196:965.
5 Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409.
6 Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2000;235:392.
7 D’Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy. Prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183.
8 Schirmer B. Laparoscopic ultrasonography. Enhancing minimally invasive surgery. Ann Surg. 1994;220:709.
9 John TG, Greig JD, Carter DC, Garden OJ. Carcinoma of the pancreatic head and periampullary region. Tumor staging with laparoscopy and laparoscopic ultrasonography. Ann Surg. 1995;221:156.
10 John TG, Greig JD, Corsbie JL, et al. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg. 1994;220:711.
11 Bogen GL, Mancino AT, Scott-Conner CEH. Laparoscopy for staging and palliation of gastrointestinal malignancy. Surg Clin North Am. 1996;76:557.
12 Conlon KC, Dougherty E, Klimstra DS, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134.
13 Pisters PWT, Lee JE, Vauthey JN, et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325.
14 Daly JM, Massar E, Giacco G, et al. Parenteral nutrition in esophageal cancer patients. Ann Surg. 1982;196:203.
15 Detsky AS, Baker JP, ORourke K, Goel V. Perioperative parenteral nutrition. A meta-analysis. Ann Intern Med. 1987;107:195.
16 Busch ORC, Hop WCJ, van Papendrecht MAW, et al. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372.
17 Houbiers JGA, Brand A, van de Watering LMG, et al. Randomized controlled trial comparing transfusion of leukocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344:573.
18 Bernstein EF, Sullivan FJ, Mitchell JB, et al. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993;20:435.
19 Mathes SJ, Alexander J. Radiation injury. Surg Oncol Clin North Am. 1996;5:809.
20 van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy. European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143.
21 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2003;347:1233.
22 Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy. The National Cancer Institute randomized trial. Cancer. 2003;98:697.
23 Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391.
24 Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394.
25 Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer. A multicenter validation study. N Engl J Med. 1998;339:941.
26 Derossis AM, Fey J, Yeung H, et al. A trend analysis of the relative value of blue dye and isotope localization in 2,000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg. 2001;193:473.
27 Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546.
28 Shepherd AH, Royle G, Ross APR, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct. A randomized trial. Br J Surg. 1988;75:1166.
29 Andersen JR, Sorensen SM, Kruse A, et al. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132.
30 Nagakawa T, Nagamori M, Futakami F, et al. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996;77:640.
31 Yeo CJ, Cameron JL, Lillimoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Part 2. Randomized controlled trial evaluating survival, morbidity and mortality. Ann Surg. 2002;236:355.
32 Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618.
33 Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection. Margin status and survival duration. J Gastrointest Surg. 2004;8:935-950.
34 Siriwardana HPP, Siriwardena AK. Systemic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br. J Surg. 2006;93:662-673.
35 Tepper J, Nardi G, Suit H. Carcinoma of the pancreas. Review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1977;37:1519.
36 Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;97:28.
37 Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006.
38 Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma. The Johns Hopkins Hospital—Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981.
39 Neoptolemas JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200.
40 Shipley WU, Wood WC, Tepper JE, et al. Intraoperative irradiation for patients with unresectable pancreatic carcinoma. Ann Surg. 1984;200:289.
41 Gunderson LL, Nagorney DM, Martenson JA, et al. External beam plus intraoperative irradiation for gastrointestinal cancers. World J Surg. 1995;19:191.
42 Garton GR, Gunderson LL, Nagorney DM, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153.
43 Hundt W, Braunschweig R, Reiser M. Evaluation of spiral CT in staging of colon and rectum carcinoma. Eur Radiol. 1999;9(1):78.
44 Thoeni RF. Colorectal cancer. Radiologic staging. Radiol Clin North Am. 1997;35(2):457.
45 Puli SR, Bechtold ML, Reddy JB, et al. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254.
46 Puli SR, Reddy JB, Bechtold ML, et al. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers. A meta-analysis and systematic review. Ann Surg Oncol. 2009;16(5):1255.
47 Maor Y, Nadler M, Barshack I, et al. Endoscopic ultrasound staging of rectal cancer. Diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21(2):454.
48 Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89(3):327.
49 Shihab OC, Heald RJ, Rullier E, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009;10(12):1207.
50 Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1):132.
51 Wieder HA, Rosenberg R, Lordick F, et al. Rectal cancer. MR imaging before neoadjuvant chemotherapy and radiation therapy for prediction of tumor-free circumferential resection margins and long-term survival. Radiology. 2007;243(3):744.
52 Schaefer O, Langer M. Detection of recurrent rectal cancer with CT, MRI and PET/CT. Eur Radiol. 2007;17(8):2044.
53 Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198(2):159.
54 Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes’ B and C rectal carcinoma. A report of the NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. Ann Surg. 1986;204(4):480.
55 Tilney HS, Tekkis PP. Extending the horizons of restorative rectal surgery. Intersphincteric resection for low rectal cancer. Colorectal Dis. 2008;10(1):3. discussion 15-16
56 West NP, Finan PJ, Anderin C, et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26(21):3517.
57 den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96(9):1066.
58 Matthiessen P, Hallbook O, Rutegard J, et al. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer. A randomized multicenter trial. Ann Surg. 2007;246(2):207.
59 Chude GG, Rayate NV, Patris V, et al. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer. Should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology. 2008;55(86-87):1562.
60 Brown CJ, Fenech DS, McLeod RS. Reconstructive techniques after rectal resection for rectal cancer, Cochrane Database Syst Rev 2:CD006040, 2008.
61 Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303.
62 Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer. A Scandinavian consensus. Br J Surg. 1995;82(10):1297.
63 MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457.
64 Heald RJ. Rectal cancer. The surgical options. Eur J Cancer. 1995;31A(7-8):1189.
65 Network NCC. Rectal cancer: the NCCN oncology practice guidelines. Rockledge, Pa: NCCN; 2010.
66 De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol. 2009;35(12):1280.
67 Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52(4):577.
68 Bach SP, Hill J, Monson JR, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96(3):280.
69 Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma. 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061.
70 Wexner SD, Bergamaschi R, Lacy A, et al. The current status of robotic pelvic surgery, Results of a multinational interdisciplinary consensus conference. Surg Endosc. 2009;23(2):438.
71 Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer. A pooled analysis. J Clin Oncol. 2004;22(10):1785.
72 Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer. A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821.
73 O’Connell MJ, Gunderson LL. Adjuvant therapy for adenocarcinoma of the rectum. World J Surg. 1992;16:510.
74 Camma C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. JAMA. 2000;284(8):1008.
75 Adjuvant radiotherapy for rectal cancer. A systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358(9290):1291.
76 Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731.
77 Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum. NSABP R-03. J Clin Oncol. 2009;27(31):5124.
78 Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016). A multicentre, randomised trial. Lancet. 2009;373(9666):811.
79 Dalton RR, Donohue JH, Mucha JJr, et al. Management of retroperitoneal sarcomas. Surgery. 1989;106:725.
80 Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma. Analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355.
81 Hassan I, Park SZ, Donohue JH, et al. Operative management of primary retroperitoneal sarcomas. A reappraisal of an institutional experience. Ann Surg. 2004;239:244.
82 Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138:248.
83 Stoeckle E, Coindre J-M, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma. A multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359.
84 Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832.
85 Russell WO, Cohen J, Enzinger F, et al. A clinical and pathological staging system for soft tissue sarcomas. Cancer. 1977;40:1562.
86 Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24.
87 Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas. A multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31.
88 Pisters PW. Resection of some—-but not all—clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6.
89 Tepper JR, Suit HD, Wood WC, et al. Radiation therapy of retroperitoneal soft-tissue sarcomas. J Radiat Oncol Biol Phys. 1984;10:825.
90 van Doorn RC, Gallee MPW, Hart AAM, et al. Resectable retroperitoneal soft tissue sarcomas. The effect of extent of resection and postoperative radiation therapy on local tumor control. Cancer. 1994;73:637.
91 Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized clinical trial. Arch Surg. 1993;128:402.