CHAPTER 37 Surgical Peritonitis and Other Diseases of the Peritoneum, Mesentery, Omentum, and Diaphragm
Secondary peritonitis is often referred to as surgical peritonitis because the many and varied disease processes which present with peritonitis frequently require procedural intervention for treatment. Primary or spontaneous bacterial peritonitis is discussed in Chapter 91.
ANATOMY AND PHYSIOLOGY
GROSS ANATOMY
The peritoneum is divided into parietal and visceral components. The parietal peritoneum covers the anterior, lateral, and posterior abdominal walls; the inferior surface of the diaphragm; and the pelvis. A large portion of the surface of the intraperitoneal organs (stomach, jejunum, ileum, transverse colon, liver, and spleen) is covered by visceral peritoneum, whereas only the anterior aspect of the retroperitoneal organs (duodenum, left and right colon, pancreas, kidneys, and adrenals) is covered by visceral peritoneum. The intraperitoneal organs are suspended by thickened bands of peritoneum, or abdominal ligaments. The nine ligaments and two mesenteries identified by Meyers and colleagues are the coronary, gastrohepatic, hepatoduodenal, falciform, gastrocolic, duodenocolic, gastrosplenic, splenorenal, and phrenicocolic ligaments, the transverse mesocolon, and the small bowel mesentery.1 These ligamentous structures, which are apparent at laparotomy, as well as on computed tomography (CT), subdivide the abdomen into interconnected compartments. Familiarity with the anatomy can be used to predict the route of spread of disease; for example, the gastrohepatic and gastrocolic ligaments allow a gastric tumor to spread to the liver and colon. The spread of infection within the peritoneal cavity is governed by the site of infection, the sites of fibrinous and fibrous adhesions, intraperitoneal pressure gradients, and the position of the patient. After leakage of visceral contents, dependent recesses (e.g., paracolic gutters, pelvis, lesser sac, and subhepatic and subphrenic spaces) tend to become sites of abscess formation. For instance, patients with perforated peptic ulcer disease may present with right lower quadrant pain secondary to the dependent nature of the right lower quadrant and the right paracolic gutter. A common practice before modern imaging and percutaneous drainage methods was to place the patient in a semirecumbent position (Fowler’s position) to encourage pooling of contaminated fluids within the pelvis, in order to palpate the resultant abscess and drain it through the rectum.
MICROSCOPIC ANATOMY
The peritoneum can regenerate after injury or surgery. In animal models of abdominal wall hernias repaired with composite mesh grafts a functional neoperitoneum covers the graft in 7 to 14 days.2
BLOOD SUPPLY AND INNERVATION
The visceral peritoneum is supplied by the splanchnic blood vessels, and the parietal peritoneum by intercostal, subcostal, lumbar, and iliac vessels. The visceral peritoneum is supplied by nonsomatic nerves, whereas the parietal peritoneum is supplied by somatic nerves. Therefore, visceral pain is poorly localized, diffuse, and vague (see Chapter 10). Visceral pain is caused by stretching, distention, torsion, and twisting. The visceral peritoneum does not produce pain when it is cut or burned. When visceral pain fibers of midgut structures are stimulated, a vague periumbilical discomfort results because the visceral pain fibers enter the spinal cord at the same level as the T10 dermatome somatic fibers (see Chapters 10 and 11). This sensation is, therefore, experienced as discomfort in the dermatomal distribution. Likewise, visceral stimulation from foregut structures produces epigastric (T8 distribution) discomfort, and visceral stimulation in the hindgut produces suprapubic (T12) discomfort. Parietal (somatic) pain fibers are activated by such stimuli as cutting, burning, and inflammation. This type of pain is sharply localized. A good example of this process is appendicitis. Early in the disease process the patient experiences periumbilical discomfort secondary to distention of the appendiceal lumen, and this progresses to localized right lower quadrant pain and tenderness as the inflammation becomes transmural and stimulates the parietal peritoneum.
PHYSIOLOGY
Particles, solutes, and fluids are absorbed from the peritoneal cavity by two different routes. Substances smaller than 2 kd may be absorbed through peritoneal mesothelial venous pores and are directed to the portal circulation.3 Particles larger than 3 kd are absorbed through peritoneal mesothelial lymphatics, entering the lymphatic thoracic duct and from there the systemic circulation.4 This last route of absorption plays an important role in controlling abdominal infections because it has a huge capacity for absorption. The anatomic structure of these large channels between the peritoneal cavity and the diaphragmatic vessels and the negative pressure of the thorax during inspiration make this mechanism extremely effective in the removal of bacteria and cells. The large surface area and semipermeability of the peritoneal membrane can be exploited therapeutically in peritoneal dialysis of patients with kidney failure and in rewarming hypothermic patients with peritoneal lavage.
SURGICAL PERITONITIS
CAUSES AND PATHOGENESIS
Bacteria reach the peritoneal cavity by a variety of pathologic processes: transmural inflammation with luminal obstruction (see Chapter 119), perforation of the gastrointestinal (GI) tract, and ischemia (see Chapter 114). The initial inoculum of bacteria is determined by the normal flora in the involved portion of the GI tract (see Chapter 102).
Flora
Although the flora of the gut, especially of the large bowel, is diverse and extensive, the numbers of types of organisms rapidly decrease after leakage of gut contents into the peritoneal cavity.5 Aerobes such as Escherichia coli and enterococci and anaerobes such as Bacteroides fragilis and Clostridium organisms predominate. A recent study of infections associated with ruptured colonic diverticulitis reported anaerobes only in 15% of cases, aerobic bacteria only in 11%, and mixed aerobic and anaerobic flora in 74%; cultures from peritoneal abscesses detected anaerobic bacteria in 18%, aerobes alone in 5%, and mixed aerobic and anaerobic flora in 77%.6 In addition to bacteria, the presence of fungi in intra-abdominal infection has recently been more frequently recognized and may have clinical significance. For instance, a positive fungal culture is quite common in perforated peptic ulcer disease and may adversely affect outcome.7
On the basis of an animal model of monomicrobial and polymicrobial peritonitis with various combinations of bacteria, it is apparent that (a) E. coli is the organism most often responsible for death from this form of iatrogenic peritonitis, at least in part because of its ability to cause bacteremia, and (b) that combinations of anaerobes and facultative organisms lead to abscess formation.8 As stated, 77% of bacterial cultures from peritoneal abscesses are polymicrobial.6 Other adjuvant substances, such as devitalized tissue, mucus, bile, hemoglobin, and barium, can act synergistically with microorganisms to increase mortality in surgical peritonitis through their ability to interfere with phagocytosis and killing of bacteria. These considerations form the basis for the treatment of surgical peritonitis, which is described later.
The peritoneal cavity possesses several lines of defense against bacterial infection (Table 37-1). Peritonitis results when these are overwhelmed.
| Removal Mechanisms |
ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule.
Peritoneal Clearance of Bacteria
Once bacteria enter the peritoneal cavity, clearance of the offending microorganisms begins immediately. Within 6 minutes of intraperitoneal inoculation of bacteria in dogs, bacteria can be cultured in thoracic lymph, indicating passage of organisms through the diaphragm. Twelve minutes later, bacteremia may be evident. This clearance mechanism is probably important in survival because blockade of the thoracic duct in an animal model of peritonitis decreases bacteremia episodes4 but increases mortality and induces liver necrosis. This appears to be directly related to the amount of endotoxin to which the liver is exposed.9 Decades before it was known that the diaphragm was the predominant site of clearance of bacteria, Fowler, in 1900, proposed his head-up, pelvis-down position for prevention of absorption of toxins from infected peritoneal cavities. In the preantibiotic era, documentation of the delayed clearance of bacteria from experiments in infected dogs in the head-down position confirms the wisdom of this positioning for patients with peritonitis.
Killing Mechanisms
In addition to mechanisms of bacterial clearance through the diaphragm, intraperitoneal defense mechanisms include cellular and humoral responses (see Chapter 2). Macrophages and neutrophils are attracted to the peritoneal cavity, and in this setting, microvilli of the mesothelial cells play a significant role in leukocyte migration into the peritoneal cavity by providing the needed substrates for their adhesion, namely intercellular adhesion molecule-1 (ICAM-1, or CD 54), and vascular cell adhesion molecule-1 (VCAM-1, or CD 106).10
The degree of cellular recruitment may be a key factor in a patient’s survival because a prolonged peritoneal inflammatory response has been observed to be adversely correlated with survival in an animal model of peritonitis.11 Humoral antibacterial agents, such as complement factors, fibronectin, and globulins, are released into the peritoneal cavity. These opsonins coat bacteria and render them recognizable as foreign; then they are entrapped and killed by phagocytes.12
Sequestration Mechanisms
Sequestration mechanisms include fibrin trapping of bacteria, fibrinous adhesions, and omental loculation of foci of infection (see Table 37-1).13 It has been known since 1950 that bacteria are more readily destroyed on a surface than in a liquid medium. The microscopic and macroscopic networks of surfaces provided by fibrin and the omentum assist phagocytes in locating, trapping, ingesting, and killing bacteria. The volume of peritoneal fluid in which infection develops has a remarkable effect on mortality; 20% of rats inoculated with E. coli diluted in 1 mL of saline die, whereas 75% of rats inoculated with the same number of viable bacteria but diluted in 30 mL of saline die.14 This phenomenon explains in part the risk of development of spontaneous bacterial peritonitis in relation to the ascitic fluid total protein concentration.15 The more voluminous the ascitic fluid, the lower the concentration of proteins and opsonins, the less efficient the trapping of bacteria, and the higher the risk of an uncontrolled infection (see Chapter 91). Patients undergoing chronic ambulatory peritoneal dialysis may be vulnerable to peritonitis because of dilution of opsonins by dialysis fluids.
Bacterial contamination in the peritoneal cavity and the subsequent response of immune cells such as neutrophils and macrophages lead to an inflammatory response including the release of cytokines. The systemic inflammatory response syndrome (SIRS) is marked by fever, a hyperdynamic cardiovascular response, muscle protein breakdown,16 and respiratory failure. If the underlying cause is treated by surgical intervention, antibiotics, or the body’s own defense mechanisms, these processes can be thwarted or reversed. However, if the process goes unchecked, multisystem organ failure and death will result. In addition, even if the underlying cause is treated, the inflammatory response can lead to multisystem organ failure and death if the treatment is delayed or the inflammatory response is particularly vigorous.
Patients with severe peritonitis may have a higher mortality from a shift from type 1 to type 2 T-helper cells leading to greater immunosuppression.17 When treating peritonitis or operating within the abdomen, the clinician’s goal is to minimize or eliminate inflammation. For instance, laparoscopic operations may induce less of a systemic inflammatory response than their open counterparts.18,19 In addition, laparoscopy differs from laparotomy in regard to peritoneal macrophage response,20 less cortisol release,21 and less reduction in natural killer (NK) cell subsets.22 Laparoscopic operations may well confer an immunologic advantage over conventional open operations.23 The additional benefits of smaller incisions, less tissue trauma, decreased postoperative pain, and shorter recovery are driving a trend to laparoscopic operations over open operations even in acute settings.
HISTORY AND PHYSICAL EXAMINATION
Clinical history and careful physical examination are the key factors in making a timely diagnosis of surgical peritonitis. In general, the sooner the diagnosis is made, the better the prognosis. Abdominal pain is the hallmark of peritonitis. The exact details of the onset of pain can be helpful in drawing attention to the affected organ (see Chapter 10). The pain’s character, location, area of radiation, change over time, and provocative and palliative factors are key pieces of information in assisting with the diagnosis. Peritoneal inflammation is typically associated with ileus, and therefore nausea and vomiting are common symptoms.
The ability of the clinician to elicit an accurate history of abdominal pain and peritoneal signs is limited in patients with neurologic and immunologic compromise. The pain of peritonitis can be reduced or even absent in older adult patients. Infants and children may be incapable of furnishing any history or cooperating with the physical examination. Notoriously difficult patients to assess for secondary peritonitis include emergency room patients under the influence of alcohol or illicit drugs, trauma patients with central nervous system or spinal cord injuries, and sedated and ventilated intensive care unit (ICU) patients. Analgesics typically will not relieve the pain of peritonitis on examination but may relieve some discomfort as related to the history of present illness. In fact, it has been shown that early provision of analgesia to patients with undifferentiated abdominal pain does not affect diagnostic accuracy.24 Diabetic patients have deficits in neurologic and immune function. Patients receiving immunosuppressive and anti-inflammatory drugs, such as glucocorticoids and chemotherapeutic drugs, may have blunted perception of pain and minimal signs of peritoneal irritation. Patients with cirrhosis and ascites may show no pain during episodes of spontaneous bacterial peritonitis unless the parietal peritoneum becomes involved with the inflammatory process (see Chapter 91).
On examination, the patient with surgical peritonitis is usually immobile because any movement acutely worsens the pain. Fever of 100° F or higher is typical, as is tachycardia, which may be in part secondary to pain. Hypotension is usually a late finding accompanying sepsis. Fever is a basic endogenous mechanism to help fight infection. In fact, the increase in body temperature that is usually found during bacterial infections, including peritonitis, seems to be essential for optimal host defense against bacteria.25 The absence of percussible hepatic dullness suggests the presence of free air in the peritoneal cavity. Exquisite tenderness to percussion should lead to very gentle palpation. Overly vigorous palpation of a very tender abdomen may cause patients such pain that they are subsequently unable to cooperate for the remainder of the examination.
Peritoneal signs signify inflammation of the parietal peritoneum secondary to an intra-abdominal process. Peritoneal signs consist of rebound tenderness, involuntary guarding and extreme tenderness on palpation. Peritonitis can be diffuse, such as that associated with perforated ulcer, or localized, such as that of diverticulitis confined to the left lower quadrant. Significant septic processes may be confined to the pelvis by overlying bowel and omentum with a resulting absence of peritoneal signs in the anterior abdominal wall. Therefore, careful rectal and pelvic exams are essential in order to detect pelvic peritonitis. The presence of iliopsoas and obturator signs (described in Chapter 116) can be helpful in detecting retroperitoneal or pelvic inflammation and abscesses.
LABORATORY TESTS AND IMAGING
Free air may be detected on upright chest radiograph or on upright or decubitus abdominal films, but this finding may be only 60% sensitive in detecting gut perforation.26 The absence of free air should not delay surgical intervention in an otherwise appropriate clinical setting. Ultrasonography can be helpful in demonstrating abscesses, bile duct dilatation, and large fluid collections. CT scan of the abdomen and pelvis, generally with both oral (occasionally rectal) and intravenous contrast, is increasingly preferred as the most sensitive and specific imaging modality for acute abdominal pain. Multidetector CT scanners are capable of imaging the entire abdomen and pelvis in a single breath-hold. The axial images are of extremely high resolution and can be reconstructed in coronal, sagittal, and three dimensional sets of images.27 CT is much more sensitive than plain films for the detection of free air, and with multidetector CT it is possible to visualize the actual site of perforation.28 Although CT images are increasingly accurate and the images compelling, they should not delay surgical consultation, resuscitation, and operation in a patient with suspected peritonitis.
DIAGNOSIS
The diagnosis of surgical peritonitis is suspected on the basis of history, physical examination, and laboratory and imaging tests and is confirmed at laparotomy or laparoscopy when purulent fibrinous peritonitis is found. In those patients whose history and physical examinations are unreliable, CT and peritoneal lavage are extremely valuable in confirming the diagnosis of surgical peritonitis. CT is less invasive but peritoneal lavage can be performed quickly in hemodynamically unstable patients. Peritoneal lavage is performed by inserting a catheter under sterile conditions into the peritoneal cavity and infusing 1 L of normal saline. If the effluent contains more than 500 white blood cells (WBCs) per cubic millimeter, an amylase or bilirubin level greater than the corresponding serum value, or bacteria on Gram stain, there is approximately a 90% likelihood of surgical peritonitis.29 Laparotomy is usually indicated in this setting. Finally, diagnostic laparoscopy is extremely accurate in making the diagnosis of surgical peritonitis and many of the underlying diseases can be dealt with laparoscopically, avoiding the need for laparotomy.30
TREATMENT
The following two principles in the management of surgical peritonitis cannot be overemphasized. First, not all patients with peritonitis require surgery. For example, a patient with localized left lower quadrant peritonitis secondary to diverticulitis can be managed with bowel rest and intravenous antibiotics alone. Another patient with the same clinical presentation and findings of a diverticular abscess on CT scan can be successfully treated with antibiotics and percutaneous drainage (see Chapter 26). Second, the absence of peritonitis does not exclude the possibility of surgical emergency. The classic example of this clinical situation is early acute mesenteric ischemia with abdominal pain out of proportion to findings on physical examination findings. Likewise, a complete mechanical small bowel obstruction without peritoneal signs, an indication of perforation or vascular compromise, still requires operation.
For most cases of secondary peritonitis fluid resuscitation and antibiotic therapy followed by urgent laparotomy or laparoscopy are the mainstays of treatment. Fluid resuscitation is guided by frequent monitoring of physiologic parameters in an ICU, including blood pressure (by arterial line if shock is present), heart rate, central venous pressure or pulmonary capillary wedge pressure, and urine output. Hematocrit, WBC, electrolytes, glucose, creatinine, and blood gases should also be monitored. Hypovolemia, hypotension, metabolic acidosis, hypoxia, and hemoconcentration from loss of plasma into the peritoneal cavity are expected. Glucocorticoids have been shown not to provide benefit in the setting of septic shock.31
Antibiotics
Antibiotic therapy is required before, during, and after surgical intervention. The type of bacteria causing secondary peritonitis depends in part on the normal flora of the part of the GI tract that is the source of sepsis and in part on the clinical setting. In community-acquired peritonitis, susceptible gram-negative bacilli, strict anaerobic bacteria, and enterococci are typically found. In general, antibiotics directed against the most likely pathogens should be chosen. For instance, colonic processes require coverage for gram-negative aerobes and anaerobes. In animal models, antibiotics directed against gram-negative enteric aerobic organisms minimize mortality, and drugs effective against anaerobes prevent abscess formation.32 It has been shown in experimental models of peritonitis that there is synergism between aerobic and anaerobic bacteria.33 The coverage of all potential organisms is not necessary.34 The flora of surgical peritonitis simplifies with time, even before initiation of antibiotics. Killing certain key species may change the microenvironment sufficiently to prevent growth and allow killing of other flora. If a Candida species is cultured from the peritoneal cavity in a patient with secondary peritonitis, this organism should be treated if the patient is in septic shock or is immunocompromised despite being hemodynamically stable. Hemodynamically stable immunocompetent patients with secondary peritonitis do not need treatment for Candida.35
A variety of antibiotic regimens have been proposed using the following classes of antibiotics alone or in combination: second-generation cephalosporins, third-generation cephalosporins, broad-spectrum beta-lactams, fluoroquinolones and metronidazole, and aminoglycosides with clindamycin or metronidazole. Many controlled trials of antibiotic regimens show equivalency. For example, it has been shown that monotherapy with a broad-spectrum beta-lactam is as effective as combination therapy with a beta-lactam and an aminoglycoside.36
Data-supported guidelines regarding optimal treatment have been hampered by suboptimal study design and nonuniform efficacy criteria in the controlled trials that have been performed. A recent Cochrane review of 40 randomized trials involving 16 different regimens showed no difference in mortality.37 The specific antibiotics chosen should take into account other considerations such as the avoidance of toxicities, the sensitivity profile of cultured organisms, the ease and route of administration, and cost. The availability of broad-spectrum antibiotics, including beta-lactams, fluoroquinolones, and third- and fourth-generation cephalosporins, makes it unnecessary to use aminoglycosides with their potential nephrotoxicity in patients with compromised renal function.36
The failure to clear secondary peritonitis after an appropriate course of antibiotic therapy or the recurrence of peritonitis is termed tertiary peritonitis. Nosocomial infections occurring in patients after long periods of hospitalization may include infections with multiresistant Pseudomonas, Enterobacter, Enterococcus, Staphylococcus, and Candida species. The development of multiple organ dysfunction syndrome (MODS) after an initial operation should prompt an aggressive search for inadequate source control and abscesses, involving repeat CT scans, percutaneous or operative drainage, and culture of persistent fluid collections, in addition to antimicrobial therapy.38
Surgical Intervention
Antibiotics help treat or prevent fatal bacteremia but do not cure most patients with surgical peritonitis unless operative intervention is also undertaken. Neither free leakage of gut contents nor large abscesses can be sterilized by antibiotics alone in the absence of drainage. Surgical intervention should occur as soon as possible after the patient is stabilized and resuscitated and antibiotics have been given. Laparotomy remains the gold standard for definitive diagnosis and mainstay of therapy in surgical peritonitis. However, a recent review confirms the success of an increasing number of laparoscopic procedures for some forms of peritonitis.30 With either laparoscopic or conventional open operations, the aims of surgical treatment include source control, peritoneal decontamination, and prevention of recurrent infection.
Repeat laparotomy and laparostomy, in which the abdomen is left open, are useful tools when control of the source of infection is not possible at the initial operation.38 Surgical re-exploration may be undertaken for the following reasons: (1) tenuous control of the source of infection, (2) reassessment of bowel viability, (3) inadequate or poor drainage, (4) hemodynamic instability, (5) infected pancreatic necrosis or diffuse fecal peritonitis at the initial operation, (6) reassessment of a tenuous anastomosis, and (7) the development of intra-abdominal hypertension (abdominal compartment syndrome). This syndrome is described in more detail in Chapter 10. An abdominal compartment syndrome results when the closure of the abdomen at either the level of the fascia or skin causes intra-abdominal pressure to rise to a degree that impairs respiratory, hepatic, and renal function.39
Preoperative and postoperative fluid and nutritional support are crucial to prompt wound healing and survival. Peritonitis has been compared with a 50% total body surface area burn, and even a calorie intake of 3000 to 4000 kcal per day may not achieve a positive nitrogen balance. Inability to achieve positive nitrogen balance may, however, be secondary to accelerated proteolysis11 and negative nitrogen balance associated with pathologic proteolysis will not be treated by any amount of caloric intake. This proteolysis may only be thwarted with treatment of the septic process and recovery of the patient. The enteral route of nutrition is preferred over parenteral. Placement of a feeding jejunostomy tube at the initial operation is prudent in these critically ill patients.
PROGNOSIS
Despite the modern approach to the diagnosis and treatment of surgical peritonitis, mortality remains high in certain subgroups of patients, especially older adult patients and patients who suffer multiple organ failure before the development of peritonitis.40 In general, peritonitis-related mortality may be as low as 14%,41 with appendicitis and perforated duodenal ulcer at the low end of the spectrum (10%) and postoperative (tertiary) peritonitis at the high end (as high as 50%).40
PERITONITIS OF OTHER CAUSES (see Table 37-2)
PRIMARY PERITONITIS
Spontaneous bacterial peritonitis (SBP), or peritonitis without a known surgical source, is the most common cause of primary peritonitis. This occurs predominantly in patients with cirrhosis and ascites and is discussed in Chapter 91. Primary peritonitis may also occur in patients with ascites due to nephrotic syndrome.42 Primary peritonitis in the absence of cirrhosis or nephrosis is much less common and usually occurs in children. Primary peritonitis is treated without surgical intervention, using antibiotics directed against the offending organism.
Table 37-2 Causes of Nonsurgical Peritonitis
PERITONITIS WITH CONTINUOUS AMBULATORY PERITONEAL DIALYSIS
Continuous ambulatory peritoneal dialysis (CAPD) is a common treatment of end-stage kidney disease.43 Bacterial peritonitis develops in this setting about 1.4 times per patient-year of treatment.44 The most common isolates in patients treated with CAPD are Staphylococcus epidermidis and other skin flora.45 Other pathogens, such as fungi or Mycobacterium tuberculosis, are less frequent. The most probable explanation for this high incidence of infection is inadvertent contamination of the indwelling catheter. Even with better patient education regarding sterile technique, peritonitis in this group of patients is a major source of morbidity and the largest single cause of patient failure on CAPD.46 New technical maneuvers47 or special management of insertion site48 may decrease the incidence of infections in these patients.
Abdominal pain and tenderness are found in about 75% of patients, but fever is found in only about one third.49 A consistent feature is cloudy effluent, noted in 98%.50 The diagnosis is suspected on the basis of signs and symptoms and is confirmed by a fluid WBC count greater than 100 neutrophils/mm3 or the presence of organisms on Gram stain. Treatment should be started immediately without waiting for the culture results, similar to the empiric treatment of patients with cirrhosis and neutrocytic ascites.50 Initial treatment of suspected CAPD peritonitis should cover the most frequently isolated bacteria. Vancomycin and second- or third-generation cephalosporins are good options. The intraperitoneal route of administration is probably the most effective.50 The sensitivity of the organism isolated determines the subsequent antibiotic choice. Most of these patients are successfully treated on an outpatient basis without stopping dialysis. Prompt treatment ensures survival; however, recurrent infection is common and may lead to catheter removal or scarring of the peritoneum and poor dialysis exchange. Addition of heparin to the dialysis bag in cases of peritonitis may decrease the formation of fibrin and thereby the incidence of postinfection adhesions. However, these infections often require removal of the catheter if they do not respond to antibiotic treatment. Repeated infections lead to sclerosing encapsulating peritonitis (abdominal cocoon syndrome) and loss of surface area for effective dialysis.
TUBERCULOUS PERITONITIS
The number of patients with tuberculous peritonitis has increased in recent years, due in part to the development of this disease in patients with acquired immunodeficiency syndrome (AIDS), with a high rate of multiresistant strains of M. tuberculosis.51
Noncirrhotic patients with this form of peritonitis usually have ascites with a high protein content, low glucose concentration, and a low serum-to-ascites albumin gradient of less than 1.1 g/dL.52 Patients almost always have an elevated ascitic fluid WBC count and a lymphocytic predominance. The algorithm in evaluation of patients with high-lymphocyte-count ascites includes cytologic evaluation of the fluid and consideration of laparoscopy.52 Patients with lymphocytic ascites and fever usually have tuberculosis, whereas afebrile patients usually have malignancy-related ascites. Cancer is the cause of lymphocytic ascites about 10 times more frequently than is tuberculosis (see Chapter 91). If peritoneal carcinomatosis is present, the cytologic findings are positive more than 90% of the time, and the laparoscopy can be avoided.53 If the cytology is negative, laparoscopy is performed and is nearly 100% sensitive in detecting tuberculous peritonitis. Tuberculous peritonitis may also appear in a miliary form or as a pelvic mass with high serum levels of CA125, making the diagnosis difficult to distinguish from metastatic ovarian cancer.54 Adenosine deaminase levels are typically elevated in the ascitic fluid in tuberculous ascites, and this finding can help differentiate tuberculous peritonitis from other causes of peritonitis and ascites.
A six-month treatment course consisting of isoniazid, rifampin, and pyrazinamide for the first eight weeks, followed by isoniazid and rifampin for the next four months, is considered adequate.55 More antituberculous drugs may be necessary, depending on local susceptibility testing. More than half of patients with tuberculous peritonitis in the United States have underlying cirrhosis, usually alcohol related,56 whereas in Third World countries, peritoneal tuberculosis usually occurs in the absence of cirrhosis. The presence of cirrhosis affects the results of ascitic fluid tests, including reducing the sensitivity of adenosine deaminase to only 30% (see Chapter 91).56 Furthermore, ascites in tuberculous peritonitis may diminish or disappear with diuretics, but fever usually persists, as does a high ascitic fluid leukocyte count. Antituberculous therapy must be supervised carefully by public health personnel, as well as physicians. Erratic treatment leads to emergence of resistant strains.
PERITONITIS ASSOCIATED WITH ACQUIRED IMMUNODEFICIENCY SYNDROME (see Chapter 33)
Patients with AIDS may develop peritonitis from many different pathogens: bacteria (monomicrobial or polymicrobial); viruses (cytomegalovirus, herpes, and others) and fungal organisms (Histoplasma, Cryptococcus, and Coccidioides); parasites (Pneumocystis jiroveci, Trypanosoma cruzi); and mycobacteria (M. tuberculosis and Mycobacterium avium-intracellulare). Also, neoplastic lesions, such as Kaposi’s sarcoma and non-Hodgkin’s lymphoma, may metastasize to the peritoneum. Like other forms of peritonitis, the common features of presentation are abdominal pain; anorexia; fever; and ascites, which typically has a high protein content. The diagnosis of a rare form of peritonitis with one of these organisms sometimes leads to a diagnosis of AIDS57 in a human immunodeficiency virus (HIV)-positive patient. The treatment of these opportunistic infections involving the peritoneum is generally pharmacologic (e.g., antibiotics, amphotericin B, ganciclovir) unless bowel involvement has led to gut perforation, which may occur with cytomegalovirus, for instance. Also, laparotomy may be indicated for obstructive symptoms, as with lymphoma. Bowel resection is required in this instance. With advances in highly active antiretroviral therapy (HAART) there has been a three-fold reduction in the mortality rate from AIDS from 1995 to 2002,58 a decline in the prevalence of opportunistic GI disease,59 and a major decrease in the number of operations for AIDS-related surgical illness.58
CHLAMYDIA PERITONITIS
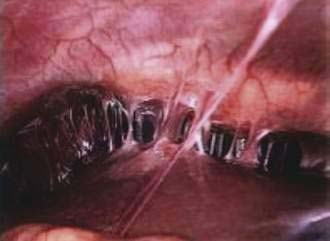
Fitz-Hugh–Curtis syndrome, or perihepatitis (Fig. 37-1), was formerly most commonly associated with Neisseria gonorrhoeae. However, in recent years Chlamydia is increasingly implicated in perihepatitis.60 Chlamydia perihepatitis occurs only in women, owing to seeding of bacteria into the peritoneal cavity from the fallopian tubes. Symptoms presenting in these patients include inflammatory ascites, pain in the right upper abdominal quadrant, fever, and a hepatic friction rub. If there is enough ascitic fluid to be clinically detectable, it has an elevated white cell count with a predominance of neutrophils and a high protein content, even in excess of 9 g/dL.60 Laparoscopy is very helpful in confirming the diagnosis, revealing “violin strings” and “bridal veil” adhesions from the abdominal wall to the liver. Doxycycline is usually curative. Also, these adhesions may be an incidental finding during laparoscopy or laparotomy for another reason. In this situation, no treatment is required.
FUNGAL AND PARASITIC PERITONITIS
Fungal peritonitis can be due to gut perforation, especially perforation of the upper gastrointestinal tract. It can also be a complication of acquired immunodeficiency (see Chapter 33). Fungal peritonitis may be limited to the pelvis in cases of gynecologic dissemination; this may be treated with fluconazole.61 The most common isolate is Candida spp., probably because routine blood culture media can detect Candida. Although infrequent, fungal peritonitis has been described in patients undergoing chronic ambulatory peritoneal dialysis.62
Although rare in the United States, peritoneal histoplasmosis, coccidioidomycosis, and cryptococcal infection are increasing in frequency in the setting of acquired immunodeficiency. Schistosomiasis, pinworms, ascariasis, strongyloidiasis, and amebiasis also may involve the peritoneal cavity (see Chapters 109 and 110).
STARCH PERITONITIS
Years ago, approximately 1 of 1000 patients who underwent laparotomy developed fever and migratory abdominal pain two to three weeks postoperatively due to contamination of the peritoneum by glove powder starch. This is much less frequent today63 probably because starch has been replaced by other more inert substances. Glove powder is known to be a source of formation of abdominal granulomas.64 Glove powder granulomas also may mimic peritoneal carcinomatosis. These lesions should be biopsied and sent for frozen section if the etiology is in question and if the results could change the operative procedure.65 Starch peritonitis is a difficult diagnosis to make, and a high index of suspicion is required. Treatment is nonoperative, and glucocorticoids may be of benefit.66
RARE CAUSES OF PERITONITIS
Connective tissue diseases lead to peritonitis as a manifestation of serositis in approximately 5% of patients with lupus and approximately 10% of patients with polyarteritis and scleroderma.67 Treatment of the underlying disease usually controls the serositis (see Chapter 35).
Familial Mediterranean fever is an autosomal recessive hereditary disease that affects the peritoneum, as well as other serous membranes. It is more frequently found in patients of Ashkenazi Jewish, Armenian, and Arabic ancestry. It is an aseptic form of recurrent peritonitis; no infectious agent has been observed to be related to this disease. Patients usually present with sporadic episodes of abdominal pain and fever, and synovitis and pleuritis may also be present. Treatment with colchicine appears to prevent attacks and can prevent fatal renal amyloidosis (see Chapter 34).68
INTRA-ABDOMINAL ADHESIONS
The aftermath of secondary peritonitis and the surgery to correct it is the variable formation of intra-abdominal adhesions, abnormal fibrous bands between peritoneal surfaces that are usually separate. Adhesions may be congenital, but the vast majority is acquired as result of peritoneal injury. Intraperitoneal foreign bodies such as suture material, clips, and mesh also contribute to adhesion formation. Intra-abdominal adhesions can be a considerable source of morbidity and mortality. They are the most common cause of small bowel obstruction (Chapter 119). Adhesions are a leading cause of secondary infertility in women, accounting for 15% to 20% of cases. Pelvic adhesions may be a source of chronic lower abdominal and pelvic pain. Adhesions may preclude peritoneal dialysis or intraperitoneal chemotherapy should they be necessary. Extensive adhesions may preclude laparoscopic procedures and have been shown to increase blood loss, operative time, and risk of enterotomy in reoperative surgery. These patients are then at increased risk for postoperative complications and prolonged hospital stay. The socioeconomic cost of adhesive disease is considerable.69
Formidable effort has been devoted to the prevention of adhesion formation. Tissue damage, hemorrhage, and inflammation in the peritoneal cavity lead to fibrin deposition on the peritoneal surfaces allowing adjacent surfaces to adhere in this sticky matrix. Various strategies for prevention of adhesion formation include reduction of peritoneal injury, inhibition of the inflammatory response, prevention of fibrin formation, promotion of fibrinolysis, prevention of collagen deposition, and barrier separation of the peritoneal surfaces. Although various experimental strategies in animal experiments have reduced the number and severity of adhesions, few of these have translated into clinical practice. The preponderance of evidence in human as well as animal studies shows decreased adhesions at the incision sites and at the operative site in laparoscopic surgery compared with open surgery.70 Seprafilm, a hyaluronic-carboxymethylcellulose membrane, has been shown in human trials to reduce intra-abdominal adhesions after general surgical procedures but there has been no demonstrable reduction in bowel obstruction. The risk of abscesses and anastomotic leaks was increased.71
PERITONEAL TUMORS
TUMORS METASTATIC TO THE PERITONEUM
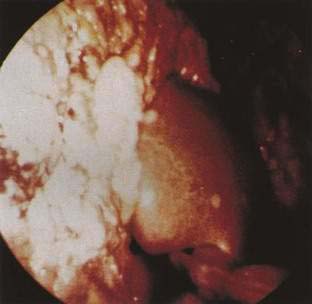
Metastatic cancer is by far the most common peritoneal tumor (Fig. 37-2). Although it is frequently assumed that tumors cause ascites only when malignant cells line the peritoneal cavity (i.e., peritoneal carcinomatosis), extraperitoneal tumors, including massive liver metastases, hepatocellular carcinoma with or without cirrhosis, malignant lymph node obstruction as in lymphoma, and Budd-Chiari syndrome with or without inferior vena cava obstruction, are associated with ascites.53 Ascitic fluid characteristics often allow their distinction,53 which is important because each may require different treatment (see Chapter 91 for details of pathogenesis and ascitic fluid analysis).
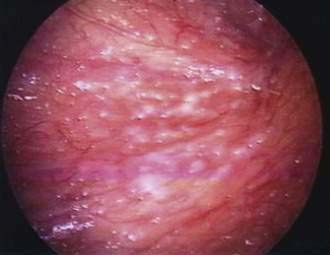
Figure 37-2. Intraoperative photograph of peritoneal carcinomatosis.
(From Free Picture Gallery, 2001. www.laparoscopyhospital.com/gallery6.htm.)
Tumors that preferentially metastasize to the peritoneum include adenocarcinomas of the ovary, stomach, colon, breast, pancreas, and lung, as well as lymphoma and other sarcomas (see Fig. 37-2).
Clinical Features
Patients with malignancy-related ascites of recent onset usually tolerate its presence poorly, probably because of less compliance of the abdominal wall compared with patients with cirrhosis who have chronic ascites. As the malignancy progresses, the fluid component tends to be replaced by solid tumor, leading to bowel obstruction. Some common myths about peritoneal carcinomatosis are that the cytology is insensitive and that the fluid is frequently bloody (see Chapter 91).
Treatment
Paracentesis
Therapeutic paracentesis for symptomatic palliation is the mainstay of treatment for the majority of patients with peritoneal carcinomatosis. The recommendation to use diuretics for treatment was based largely on supposition rather than hard data. A study of ascitic fluid volume and blood volume in patients with peritoneal carcinomatosis who lost weight taking large doses of diuretics demonstrated that the weight was lost at the expense of blood volume, not ascitic fluid volume. The characteristics of the ascitic fluid may help direct diuretic use. In general, ascites with a high serum-ascites albumin gradient (≥1.1g/dL) responds to diuretics.72 Therefore, in cancer patients, diuretics should be reserved for those with edema or some specific indication other than peritoneal carcinomatosis.
Surgery and Intraperitoneal Chemotherapy
Because the usual response to routine therapy is poor, new treatments have been suggested, such as peritonectomy combined with hyperthermic antiblastic perfusion.73 Recent results show that cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion can be performed with success.74 In some instances this approach allows better survival for patients with extensive carcinomatosis who were no longer responsive to traditional therapies. The rationale for application of intraperitoneal chemotherapy is that its use would allow larger local concentrations of drugs delivered to tumor cells, and the increased temperature makes the chemotherapeutic agents more effective. Mitomycin C is a commonly used chemotherapeutic drug for this purpose. Hyperthermic intraperitoneal chemoperfusion may be most useful when complete tumor cytoreduction is possible or in cases with positive cytology or gross perforation.75 Other treatment options that are under investigation include gene therapy76 and the use of angiogenesis inhibitors to reduce the ability of the peritoneal tumor to spread.77 Also, systemic “antidotes” to certain chemotherapy drugs (e.g., leucovorin or methotrexate) could be administered to reduce toxicity further.
Treatment of Ovarian Cancer
The results of treatment for ovarian cancer are the most encouraging. Seventy-five percent of patients with epithelial ovarian cancer present with advanced (stage III/IV) disease. Cytoreductive surgery and chemotherapy (cisplatinum and paclitaxel) have led to long-term survival. The degree of cytoreduction that can be achieved surgically correlates with improved five-year survival (52% for microscopic residual versus 29% for macroscopic <1 cm residual).78 Experimental approaches are emerging with bevucizumab, an inhibitor of vascular endothelial growth factor (VEGF) receptors, to control ascites formation and tumor growth.79
Prognosis
Prognosis is very poor in general for patients with peritoneal involvement with metastatic cancer.80 In one large study, only 70% of patients survived one month, 25% survived 3 months, 12% survived 6 months, and 4% survived longer than one year after diagnosis.80 Their course involves recurrent and progressive bowel obstruction, malnutrition, and wasting before death.
PSEUDOMYXOMA PERITONEI
Pseudomyxoma peritonei represents a rare (≈2 in 10,000 laparotomies) and special case in metastatic peritoneal tumors.81 Seventy-five percent of these patients are women between 45 and 75 years of age. This tumor causes gelatinous implants on the peritoneum. The sites of origin of the tumor are ovary and appendix. Its degree of malignant potential is variable; about 50% of patients live 5 years.81 Lymphatic or extraperitoneal spread of the tumor is rare. Presenting symptoms and signs include painless abdominal distention and an ovarian mass; mucin may accumulate intraperitoneally many years after resection of an ovarian mass.81 Definitive diagnosis is made when the jelly-like material is encountered at laparotomy or laparoscopy. However, there are often characteristic findings on CT that can suggest the diagnosis preoperatively.82 Cytoreductive surgery with intraperitoneal hyperthermic perfusion is effective current treatment for pseudomyxoma peritonei and has acceptable morbidity and mortality rates.83 The largest series of patients reports an operative mortality of 2%, morbidity of 40%, and a median survival of 13 years.84 Unfortunately, recurrence usually causes bowel obstruction, malnutrition, and death. The optimal treatment for mucinous cystadenocarcinoma of the appendix in the presence of pseudomyxoma peritonei is right hemicolectomy with aggressive tumor debulking.85
MESOTHELIOMA
Sixty-five percent to 70% of mesotheliomas arise in the pleura, and 25% in the peritoneum.86 Most peritoneal mesotheliomas are malignant, associated with asbestos exposure, and detected 35 to 40 years after initial exposure. The families of asbestos workers are also at risk. Diagnosis is usually made at laparotomy or laparoscopy, but occasionally diagnostic malignant mesothelial cells are found on ascitic fluid analysis. Serum osteopontin levels may help distinguish pleural mesothelioma from asbestosis without mesothelioma.87 Distinction of mesothelioma from peritoneal carcinomatosis of unknown primary may be difficult, even at autopsy. Classic treatments for localized peritoneal mesothelioma include cytoreductive surgery, hyperthermic intraoperative or postoperative intraperitoneal chemotherapy, and immunotherapy.88 Mesothelioma is a nearly uniformly lethal neoplasm with a median survival of only six months.
PELVIC LIPOMATOSIS
Fat deposits normally found in the perirectal and perivesical spaces may develop nonmalignant overgrowth and are recognized as a distinct clinicopathologic entity, pelvic lipomatosis. It occurs predominantly in African American men (male-to-female ratio 18 : 1) between 20 and 60 years of age89 and may cause hypertension; proliferative cystitis, urinary tract obstruction, and occasionally gastrointestinal symptoms. The abnormal proliferation of fat is accompanied by varying degrees of fibrous reaction. Transrectal ultrasonography and CT are important in diagnosis, particularly in differentiating pelvic lipomatosis from liposarcoma. The disease does not progress in most patients; however, in some, urinary obstruction requires diversion.
DISEASES OF THE MESENTERY AND OMENTUM
Diseases of the mesentery and omentum (in decreasing order of frequency) include hemorrhage, tumors, inflammatory and fibrotic conditions, and infarction. Abscesses are covered in Chapter 26.
HEMORRHAGE
Mesenteric and retroperitoneal bleeding and their complications are usually due to trauma or anticoagulants. In rare cases, aneurysms of the splanchnic arteries may rupture, leading to intraperitoneal hemorrhage. Traumatic hematomas may or may not require surgical intervention, depending on the site of the lesion and whether the trauma was blunt or penetrating.90 Intraperitoneal bleeding may be a consequence of a previous surgical procedure, such as cytoreductive surgery for gynecologic cancer.91 A special case of spontaneous hemoperitoneum is found in patients with cirrhosis and hepatocellular carcinoma.
Symptoms usually are pain and those from mass effects of the hematoma such as symptoms of intestinal obstruction. Diagnosis depends on a high index of suspicion and ultrasonography or CT, which demonstrates the collection of blood. An ultrasound-guided fine-needle aspiration may help in confirming the diagnosis. Treatment consists of discontinuation of anticoagulants (in those being so treated) and reversal of anticoagulation. In others treatment is dictated by the local or systemic symptoms of hemorrhage. In certain cases angiographic embolization may help treat intraperitoneal hemorrhage.92
TUMORS
Mesenteric Cysts
Mesenteric cysts are probably the most uncommon among these rare tumors.93 A review of the English-language literature revealed only 139 such lesions as of 1986.93 They occur in children and adults. Symptoms include pain in 58% and abdominal distention in 50%. Some cases may present with fever and chills, and others are asymptomatic, discovered incidentally and misdiagnosed before laparotomy.94 These are typically large (13 cm), fluid-filled (≈2000 mL) lesions and, despite their size, are malignant in only 3% of cases and cause death in only 2% of cases.93 They are usually cured by complete excision. If a small mesenteric cyst is found incidentally at laparotomy, it does not need to be resected. The treatment of choice for a complication (i.e., cyst rupture or hemorrhage) is excision, and this has been performed laparoscopically.95
Solid Tumors
Solid tumors appear to be next in decreasing order of frequency. Among mesenteric tumors, two thirds are benign, including fibromas, xanthogranulomas, lipomas, leiomyomas, capillary and cavernous hemangiomas, neurofibromas, and mesenchymomas. The malignant tumors include hemangiopericytomas, fibrosarcomas, liposarcomas, leiomyosarcomas, and malignant mesenchymomas. Solid tumors of the omentum are remarkably similar in histologic type and prevalence of malignancy.96 Typical of mesenteric and omental tumors, symptoms and signs include pain and distention with large lesions. Treatment is surgical resection. Prognosis is generally fair: about 18% of patients die of the tumor, overall, and the rate of five-year survival for patients with malignant tumors is only 21%.97 Needle biopsy may be attempted with these tumors, although laparoscopy or laparotomy may be required for diagnosis as well as treatment.
Multifocal Leiomyomas (Leiomyomatosis Peritonealis Disseminata)
Multifocal leiomyomatous tumors are even less common, can be malignant, and can mimic peritoneal carcinomatosis. They may appear together with other leiomyomatous lesions98 or endometriosis.99 These lesions consist of small, rubbery nodules and appear to be hormone sensitive, developing sometimes during pregnancy or estrogen therapy and regressing with hormone withdrawal. These tumors can cause abdominal pain or GI bleeding
Castleman’s Disease
Castleman’s disease, giant lymph node hyperplasia, is rare. There is considerable heterogeneity in the disease but it is classified in unicentric and multicentric forms.100 Castleman’s disease is associated with a variety of other autoimmune diseases. The central lymph nodes of the mesentery and mediastinum are more frequently involved in the unicentric form. In the unicentric form of the disease surgical removal of the mass is successful and prognosis is good. The multicentric form is treated with systemic therapies with variable success. The prognosis is considerably worse, with patients at risk for conversion to frank lymphoma.101
INFLAMMATORY AND FIBROTIC CONDITIONS
This subset of diseases of the mesentery and retroperitoneum is the most confusing, in part because of their rarity and because of overlapping clinical and histologic features. At least a dozen terms are used to describe the three basic diseases: retractile mesenteritis, mesenteric panniculitis, and retroperitoneal fibrosis. To add to the confusion, some cases have been reported with different names. These diseases could easily represent different aspects of the same spectrum of inflammation and scarring of these structures. Retractile mesenteritis was the name used in the first description of these diseases. This entity represents the fibrotic end of the spectrum and has been known as sclerosing mesenteritis, multifocal subperitoneal sclerosis, fibromatosis, and desmoid tumor.102 The inflammatory end of the spectrum has been called mesenteric panniculitis, mesenteric lipodystrophy, lipogranuloma of the mesentery, liposclerotic mesenteritis, mesenteric Weber-Christian disease, and systemic nodular panniculitis.103 There have been attempts to subclassify this disease into diffuse, single, and multiple forms and to suggest an association with lymphoma.104 Overlapping names such as sclerosing lipogranuloma, the well-documented progression and conversion of mesenteric panniculitis to retractile mesenteritis over a 12-year period, and the concurrence of sclerosing mesenteritis and retroperitoneal fibrosis indicate that these are simply stages of one basic underlying process.
Although mesenteric panniculitis and retractile mesenteritis are usually manifested by abdominal pain, symptoms of gut obstruction, and a mass lesion,104 cases associated with prolonged high-grade fever and autoimmune hemolytic anemia without abdominal symptoms have been described.105 Retractile mesenteritis and mesenteric panniculitis are always idiopathic, but retroperitoneal fibrosis has a cause approximately 30% of the time, including drugs, malignancy, trauma, or inflammation.106 Most of the reported cases have been drug induced (methysergide, ergotamine). The process of fibrosis may lead to ureteral or vascular obstruction.
Histologically, retractile mesenteritis and mesenteric panniculitis can have inflammation with lymphocytes and neutrophils, fat necrosis, fibrosis, and calcification.102 In contrast, only mesenteric panniculitis has multinucleate giant cells, cholesterol clefts, lipid-laden macrophages, and lymphangiectasia.102 Retroperitoneal fibrosis consists of dense connective tissue, with or without inflammation.
Diagnosis and Treatment
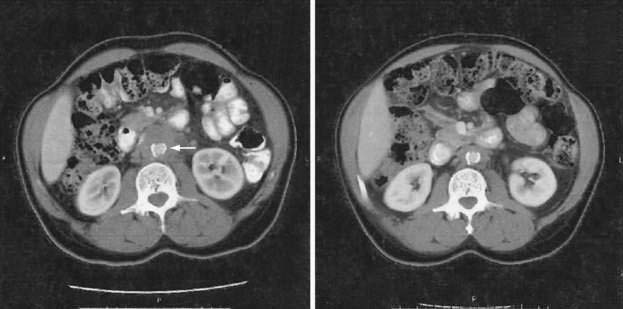
These diseases have usually been diagnosed at laparotomy or autopsy in the past; however, noninvasive techniques such as CT scan (Fig. 37-3) or MRI may assist in preoperative diagnosis.107,108 Radiologic findings suggestive of mesenteric panniculitis have been found in 0.6% of patients in a large series of abdominal CT scans. There was a female predominance and an association with malignancy in 34 of 49 patients with radiologic features of mesenteric panniculitis.108 Retroperitoneal fibrosis is more common in men and typically causes the ureters to deviate medially on radiographic evaluation. Treatment may be necessary in patients with retractile mesenteritis if it obstructs the intestine. Treatment is usually surgical, but administration of progesterone has been reported to down-regulate fibrogenesis.109 The prognosis of patients with retroperitoneal fibrosis seems to be better than in the past. Successful treatment of this entity with immunosuppressives, such as azathioprine with steroids, has been reported.110 In other cases, ureterolysis may be required.
INFARCTION OF THE OMENTUM
Infarction of the omentum occurs when a portion of the omentum twists around a narrow vascular pedicle.111 If a diagnosis by imaging techniques (such as CT scan or MRI) is achieved preoperatively,112 laparoscopic resection of the necrotic mass is curative.113 However, the diagnosis is difficult and often delayed.
DISEASES OF THE DIAPHRAGM
HERNIAS AND EVENTRATION
Diaphragmatic hernias consist of herniation of an abdominal organ through the diaphragm into the thorax and are discussed in detail in Chapter 24.
TUMORS
Diaphragmatic tumors are usually of connective tissue origin and may be benign or malignant or may consist of simple cysts.114,115 They are detected by screening chest films or in evaluation of pleuritic chest pain.
HICCUPS (see Chapter 12)
Hiccups are quick inhalations that follow abrupt rhythmic involuntary contractions of the diaphragm and closure of the glottis. When they last only a few minutes, they are considered a form of physiologic myoclonus.116 For hiccups of longer duration, home remedies include breath holding, sudden fright, rebreathing from a paper bag, eating dry granulated sugar, and drinking cold liquids. Intractable hiccups can last weeks or even longer, can be familial, and are usually due to diaphragmatic irritation, gastric distention, thoracic or central nervous system irritation or tumors, hyponatremia, or other metabolic derangements.
Treatment includes pharmacologic agents, noninvasive phrenic nerve stimulation, or rarely phrenic nerve crushing. Drugs that have been reported to be successful include chlorpromazine, metoclopramide, quinidine, phenytoin, valproic acid, baclofen, sertraline, gabapentin, and nifedipine. The implantation of breathing pacemakers that control the diaphragmatic excursions may be an interesting approach for treatment of chronic hiccups.116 Postoperative hiccups after abdominal surgery may be due to subphrenic abscess or other sources of diaphragmatic irritation such as acute gastric dilatation, and this should be considered before assuming a more benign cause.
LAPAROSCOPY IN THE EVALUATION OF PERITONEAL DISEASES
GENERAL CONSIDERATIONS
Diagnostic laparoscopy, as first described by Kelling in 1901, is a safe and effective means of evaluating the abdominal cavity. It allows direct visualization of the liver surface, peritoneal lining, and mesentery for directed biopsies. (See Figs. 37-2 and 37-4 for illustrations of peritoneal carcinomatosis and lymphoma.) Ascitic fluid can be collected easily. Although less invasive imaging techniques such as CT have reduced its necessity, laparoscopy continues to have a role in the evaluation of liver and peritoneal diseases. Although generally well tolerated, possible complications include prolonged abdominal pain, vasovagal reaction, viscus perforation, bleeding (either from biopsy sites or within abdominal wall), splenic laceration, ascites fluid leakage, and postlaparoscopy fever.117 It has been suggested that abdominal insufflation during laparoscopy could increase bacterial translocation, making the practice of laparoscopy dangerous in certain clinical settings, such as septic peritonitis.118 These observations, however, are not uniformly accepted.119 The adverse hemodynamic consequences of abdominal insufflation can be overcome in the vast majority of patients with aggressive resuscitation and careful anesthetic management. Despite these concerns, laparoscopy is becoming a common technique used in patients requiring operation for diseases causing peritonitis. A laparoscopic approach has been effective in treating perforated gastroduodenal ulcer120 and has been advocated as the treatment of choice for patients with appendicitis.121 Laparoscopic cholecystectomy is safe and effective treatment of acute cholecystitis,122 and laparoscopic colectomy can be performed for acute diverticulitis.123 Evidence-based guidelines for the application of laparoscopic operation in surgical peritonitis have been developed.30
EVALUATION OF ASCITES OF UNKNOWN ORIGIN (see Chapter 91)
Clinical presentation, conventional laboratory examinations, and ascitic fluid analysis identify the cause of ascites in the majority of patients; however, conventional paracentesis occasionally fails to make a diagnosis. In these instances diagnostic laparoscopy affords direct and sensitive technique for obtaining specimens for histology and culture. In the United States, occult cirrhosis and peritoneal malignancy account for the majority of cases.117 In studies from Asian countries, peritoneal malignancy is also the most common cause of unexplained ascites, but tuberculous peritonitis accounts for an increasing number of cases.124 In patients with HIV, peritoneal involvement may result from a variety of opportunistic infections and neoplasms (see earlier section and Chapter 33). Non-Hodgkin’s lymphoma (Fig. 37-4) accounts for the majority of these peritoneal lesions revealed by laparoscopy, but M. tuberculosis, M. avium-intracellulare, and P. jiroveci are often revealed.125
STAGING LAPAROSCOPY
Laparoscopy has found increasing utility in the staging of malignant solid tumors of the gastrointestinal tract. Diagnostic laparoscopy coupled with laparoscopic ultrasound, peritoneal fluid cytology, and biopsy allow for improved selection of patients that will benefit from larger, definitive operations for curative intent. In hepatocellular carcinoma, the use of diagnostic laparoscopy and laparoscopic ultrasonography demonstrates that 25% to 33% of patients with potentially resectable disease are found to be unresectable and can be spared unnecessary laparotomy.126 Staging laparoscopy and laparoscopic ultrasonography are commonly used to select patients under consideration for hepatic resection of metastatic colorectal cancer. In an extensive laparoscopic staging procedure for pancreatic cancer, Conlon and associates identified 36% of patients with metastatic disease after an initial negative CT.127 The overall accuracy of staging laparoscopy in this series of patients for selection of patients for pancreatic resection was 98%. In a large review of 420 patients with upper gastrointestinal malignancy, laparoscopic staging prevented unnecessary laparotomy in 5% of esophageal cancers and 20% of patients with a tumor of the gastroesophageal junction.128 The finding of metastatic disease on staging laparoscopy in esophageal and gastric cancers will not necessarily obviate the need for palliative operations.
Bridda A, Mencarelli R, Frego M. Peritoneal mesothelioma: A review. Med Gen Med. 2007;9:32. (Ref 88.)
D’Angelica M, Spiros P, Hiotis HJK, et al. Laparoscopic staging for liver, biliary, pancreas, and gastric cancer. Curr Prob Surg. 2007;44:228-69. (Ref 126.)
Dunn DL, Barke RA, Knight NB, et al. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985;49:257-64. (Ref 13.)
Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions in laparoscopic surgery? Surg Endosc. 2004;18:898-906. (Ref 70.)
Higgins PM, Aber GM. Idiopathic retroperitoneal fibrosis: An update. Dig Dis. 1990;8:206-22. (Ref 100.)
Leschka S, Alkadhi H, Wildermuth S, Marincek B. Multi-detector computed tomography of acute abdomen. Eur Radiol. 2005;15:2435-47. (Ref 27.)
Lorber B, Swenson RM. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975;55:1349-54. (Ref 5.)
Meyers MA, Oliphant M, Berne AS, Feldberg MAM. The peritoneal ligaments and mesenteries: Pathways of intra-abdominal spread of disease. Radiology. 1987;163:593-604. (Ref 1.)
Novitsky YW, Litwin DEM, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-19. (Ref 23.)
Ordonez CA, Puyana JC. Management of peritonitis in critically ill patients. Surg Clin North Am. 2006;86:1323-49. (Ref 38.)
Runyon BA, Morrissey R, Hoefs JC, Wyle F. Opsonic activity of human ascitic fluid: A potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985;5:634-7. (Ref 12.)
Saltzman DJ, Williams RA, Gelfand DV, Wilson SE. The surgeon and AIDS: Twenty years later. Arch Surg. 2005;140:961-7. (Ref 58.)
Sauerland S, Agresta F, Bergamaschi R, et al. Laparoscopy for abdominal emergencies: Evidence based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2006;20:14-29. (Ref 30.)
Wong PF, Gilliam AD, Kumar S, et al. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. Cochrane Database Syst Rev. (Issue 2):2005. (Ref 37.)
Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2006;14(2):484-92. (Ref 84.)
1. Meyers MA, Oliphant M, Berne AS, Feldberg MAM. The peritoneal ligaments and mesenteries: Pathways of intra-abdominal spread of disease. Radiology. 1987;163:593-604.
2. Bellon JM, Garcia-Carranza A, Jurado F, et al. Peritoneal regeneration after implant of a composite prosthesis in the abdominal wall. World J Surg. 2001;25:147.
3. Kraft AR, Tomplins RK, Jesseph JE. Peritoneal electrolyte absorption: Analysis of portal, systemic venous, and lymphatic transport. Surgery. 1968;64:148.
4. Aydin M, Guler O, Yigit MF, et al. The effect on survival of thoracic duct ligation in experimental peritonitis. Hepatogastroenterology. 1999;46:308.
5. Lorber B, Swenson RM. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975;55:1349-54.
6. Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol. 2000;49:827.
7. Shan YS, Hsu HP, Hsieh YH, et al. Significance of intraoperative peritoneal culture of fungus in perforated peptic ulcer. Br J Surg. 2003;90:1215.
8. Onderdonk AB, Bartlett JG, Louie T, et al. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976;13:22.
9. Guler O, Ugras S, Aydin M, et al. The effect of lymphatic blockage on the amount of endotoxin in portal circulation, nitric oxide synthesis, and the liver in dogs with peritonitis. Surg Today. 1999;29:735.
10. Liang Y, Sasaki K. Expression of adhesion molecules relevant to leukocyte migration on the microvilli of liver peritoneal mesothelial cells. Anat Rec. 2000;258:39.
11. Martineau L, Shek PN. Peritoneal cytokine concentrations and survival outcome in an experimental bacterial infusion model of peritonitis. Crit Care Med. 2000;28:788.
12. Runyon BA, Morrissey R, Hoefs JC, Wyle F. Opsonic activity of human ascitic fluid: A potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985;5:634-7.
13. Dunn DL, Barke RA, Knight NB, et al. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985;49:257-64.
14. Dunn DL, Barke RA, Ahrenholz DH, et al. The adjuvant effect of peritoneal fluid in experimental peritonitis: Mechanism and implications. Ann Surg. 1984;199:37.
15. Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343.
16. Hasselgren PO. Pathways of muscle protein breakdown in injury and sepsis. Curr Opin Clin Nutr Metab Care. 1999; March;2:155.
17. Russ MA, Reith HB. The severity of infection induces a shift in the Type 1/Type 2 T-helper cell balance in patients with or without peritonitis. Surg Infect (Larchmt). 2003;4:247.
18. Nguyen NT, Goldman CD, Ho HS, et al. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194:557. discussion 566
19. Grande M, Tucci GF, Adorisio O, et al. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313.
20. Romeo C, Impellizzer P, Antonuccio P, et al. Peritoneal macrophage activity after laparoscopy or laparotomy. J Pediatr Surg. 2003; Jan:97.
21. Luo K, Li JS, Li LT, et al. Operative stress response and energy metabolism after laparoscopic cholecystectomy compared to open surgery. World J Gastroenterol. 2003;9:847.
22. Walker CB, Bruce DM, Heys SD, et al. Minimal modulation of lymphocyte and natural killer cell subsets following minimal access surgery. Am J Surg. 1999;177:48.
23. Novitsky YW, Litwin DEM, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-19.
24. Thomas SH, Silen W, Cheema F, et al. Effects of morphine analgesia on diagnostic accuracy in emergency department patients with abdominal pain: A prospective, randomized trial. J Am Coll Surg. 2003;196:18.
25. Jiang Q, Cross AS, Singh IS, et al. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265.
26. Lee PWR, Costen PDM, Wilson DH, Halsall AK. Pneumoperitoneum in perforated duodenal ulcer disease: A further look. Br J Clin Pract. 1977;31:108.
27. Leschka S, Alkadhi H, Wildermuth S, Marincek B. Multi-detector computed tomography of acute abdomen. Eur Radiol. 2005;15:2435-47.
28. Ghekiere O, Lesnik A, Millet I, et al. Direct visualization of perforation sites in patients with a non-traumatic free pneumoperitoneum: Added diagnostic value of thin transverse slices and coronal and sagittal reformations for multi-detector CT. Eur Radiol. 2007;17:2302.
29. Lobbato V, Cioroiu M, LaRaja RD, et al. Peritoneal lavage as an aid to diagnosis of peritonitis in debilitated and elderly patients. Am Surg. 1985;51:508.
30. Sauerland S, Agresta F, Bergamaschi R, et al. Laparoscopy for abdominal emergencies: Evidence based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2006;20:14-29.
31. Bone RC, Fisher CJ, Clemmer TP, et al. A controlled trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653.
32. Bartlett JG, Louie TJ, Gorbach SL, Onderdonk AB. Therapeutic efficacy of 29 antibiotic regimens in experimental intraabdominal sepsis. Rev Infect Dis. 1981;3:535.
33. Chalfine A, Carlet J. Antibiotic treatment of peritonitis. J Chir (Paris). 1999;136:15.
34. Solomkin JS, Meakins JL, Allo MD, et al. Antibiotic trials in intra-abdominal infections: A critical evaluation of study design and outcome reporting. Ann Surg. 1984;200:29.
35. Blot SI, Vanderwoude KH, De Waele JJ. Candida peritonitis. Curr Opin Crit Care. 2007;13:195-9.
36. Dupont H, Carbon C, Carlet J. Monotherapy with a broad-spectrum beta-lactam is as effective as its combination with an aminoglycoside in treatment of severe generalized peritonitis: A multicenter randomized controlled trial. Antimicrob Agents Chemother. 2000;44:2028.
37. Wong PF, Gilliam AD, Kumar S, et al. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. Cochrane Database Syst Rev. (Issue 2):2005.
38. Ordonez CA, Puyana JC. Management of peritonitis in critically ill patients. Surg Clin North Am. 2006;86:1323-49.
39. Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care. 2005;11:156.
40. Bohnen J, Boulanger M, Meakins JL, McLean APH. Prognosis in generalized peritonitis. Arch Surg. 1983;118:285.
41. Seiler CA, Brugger L, Forssmann U, et al. Conservative surgical treatment of diffuse peritonitis. Surgery. 2000;127:178.
42. Chuang TF, Kao SC, Tsai CJ, et al. Spontaneous bacterial peritonitis as the presenting feature in an adult with nephrotic syndrome. Nephrol Dial Transplant. 1999;14:181.
43. Nolph KD, Lindblad AS, Novak JW. Continuous ambulatory peritoneal dialysis. N Engl J Med. 1988;318:1595.
44. Rubin J, Rogers WA, Taylor HM, et al. Peritonitis during continuous ambulatory peritoneal dialysis. Ann Intern Med. 1980;92:7.
45. Golden GT, Stevenson TR, Ritchie WP. Primary peritonitis in adults. South Med J. 1975;68:413.
46. Saklayen MG. CAPD peritonitis. Incidence, pathogens, diagnosis, and management. Med Clin North Am. 1990;74:997.
47. Kagawa K, Park S, Tokioka K, et al. Reduction of peritonitis with the rectus abdominis muscle flap in a CAPD patient. Pediatr Nephrol. 2000;14:114.
48. Montenegro J, Saracho R, Aguirre R. Exit-site care with ciprofloxacin otologic solution prevents polyurethane catheter infection in peritoneal dialysis patients. Perit Dial Int. 2000;20:209.
49. Paterson PK, Matzke G, Keane WF. Current concepts in the management of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Rev Infect Dis. 1987;9:604.
50. Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669.
51. Hopewell PC. Impact of human immunodeficiency virus infection on the epidemiology, clinical features, management, and control of tuberculosis. Clin Infect Dis. 1992;15:540.
52. Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330:337.
53. Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104.
54. Geisler JP, Crook DE, Geisler HE, et al. The great imitator: Miliary peritoneal tuberculosis mimicking stage III ovarian carcinoma. Eur J Gynaecol Oncol. 2000;21:115.
55. Combs DL, O’Brien RJ, Geiter LJ. USPHS tuberculosis short-course chemotherapy trial 21: Effectiveness, toxicity, and acceptability: The report of final results. Ann Intern Med. 1990;112:397.
56. Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408.
57. Libbrecht E, Brissart N, Roger M, Fur A. Pneumococcal pelvioperitonitis revealing HIV seropositivity. Presse Med. 2000;29:246.
58. Saltzman DJ, Williams RA, Gelfand DV, Wilson SE. The surgeon and AIDS: Twenty years later. Arch Surg. 2005;140:961-7.
59. Monkemuller KE, Call SA, Lazenby AJ, Wilcox CM. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol. 2000;95:457.
60. Lopez-Zeno JA, Keith LG, Berger GS. The Fitz-Hugh–Curtis syndrome revisited. J Reprod Med. 1985;30:567.
61. Mikamo H, Sato Y, Hayasaki Y, Tamaya T. Current status and fluconazole treatment of pelvic fungal gynecological infections. Chemotherapy. 2000;46:209.
62. Warady BA, Bashir M, Donaldson LA. Fungal peritonitis in children receiving peritoneal dialysis: A report of the NAPRTCS. Kidney Int. 2000;58:384.
63. Malinger G, Ginath S, Zeidel L, et al. Starch peritonitis outbreak after introduction of a new brand of starch powdered latex gloves. Acta Obstet Gynecol Scand. 2000;79:610.
64. Ellis H. The hazards of surgical glove dusting powders. Surg Gynecol Obstet. 1990;171:521.
65. Giercksky KE, Quist H, Giercksky TC, et al. Multiple glove powder granulomas masquerading as peritoneal carcinomatosis. J Am Coll Surg. 1994;179:299.
66. Sternlieb JJ, McInlrath DC, Van Heerden JA, Harrison EGJr. Starch peritonitis and its prevention. Arch Surg. 1977; Apr;112:458.
67. Matolo NM, Albo D. Gastrointestinal complications of collagen vascular diseases. Am J Surg. 1971;122:678.
68. Zemer D, Pras M, Sohar E, et al. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314:1001.
69. Attard JP, MacLean AR. Adhesive small bowel obstruction: epidemiology, biology and prevention. Can J Surg. 2007;50:291.
70. Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions in laparoscopic surgery? Surg Endosc. 2004;18:898-906.
71. Zeng Q, Zhengping Y, You J, Zhang Q. Efficacy and safety of Seprafilm for preventing postoperative abdominal adhesion: systematic review and meta-analysis. World J Surg. 2007;31:2125.
72. Pockros PJ, Esrason KT, Nguyen C, et al. Mobilization of malignant ascites with diuretics is dependent on ascitic fluid characteristics. Gastroenterology. 1992;103:1302.
73. Cavaliere F, Di Filippo F, Botti C, et al. Peritonectomy and hyperthermic antiblastic perfusion in the treatment of peritoneal carcinomatosis. Eur J Surg Oncol. 2000;26:486.
74. Ahmad SA, Kim J, Sussman JJ, et al. Reduced morbidity following cytoreductive surgery and intraperitoneal chemoperfusion. Ann Surg Oncol. 2004;11:387.
75. Ceelen WP, Hesse V, Dehemptinne B, Pattyn P. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg. 2000;87:1006.
76. Sumantran VN, Lee DS, Baker VV, et al. A bcl-x(S) adenovirus demonstrates therapeutic efficacy in an ascites model of human breast cancer. J Soc. Gynecol Invest. 2000;7:184.
77. Yoshikawa T, Yanoma S, Tsuburaya A, et al. Angiogenesis inhibitor, TNP-470, suppresses growth of peritoneal disseminating foci. Hepatogastroenterology. 2000;47:298.
78. Chobanian N, Dietrich CS. Ovarian cancer. Surg Clin North Am. 2008;88:285.
79. Xu L, Yoneda J, Herrera C, et al. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol. 2000;16:445.
80. Yamada S, Takeda T, Matsumoto K. Prognostic analysis of malignant pleural and peritoneal effusions. Cancer. 1983;51:136.
81. Mann WJ, Wagner J, Chumas J, et al. The management of pseudomyxoma peritonei. Cancer. 1990;66:1636.
82. Zissin R, Gayer G, Fishman A, et al. Synchronous mucinous tumors of the ovary and appendix associated with pseudomyxoma peritonei: CT findings. Abdom Imaging. 2000;25:311-16.
83. Deraco M, Baratti D, Inglese MG, et al. Peritoneotomy and intraperitoneal hyperthermic perfusion (IPHP): A strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11:393.
84. Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Onc. 2006;14:484-92.
85. Lo NS, Sarr MG. Mucinous cystadenocarcinoma of the appendix. The controversy persists: A review. Hepatogastroenterology. 2003;50:432.
86. McDonald AD, McDonald JC. Malignant mesothelioma in North America. Cancer. 1980;46:1650.
87. Pass HI, Lonardo F, Harbut M, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564.
88. Bridda A, Mencarelli R, Frego M. Peritoneal mesothelioma: A review. Med Gen Med. 2007;9:32.
89. Heyns CF. Pelvic lipomatosis: A review of its diagnosis and management. J Urol. 1991;146:267.
90. Feliciano DV. Management of traumatic retroperitoneal hematoma. Ann Surg. 1990;211:109.
91. Campagnutta E, Giorda G, De Piero G, et al. Different patterns of postoperative bleeding following cytoreductive surgery for gynecological cancer. Eur J Gynaecol Oncol. 2000;21:91.
92. Velmahos GC, Chahwan S, Falabella A, et al. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J Surg. 2000;24:539.
93. Kurtz RJ, Heimann TM, Beck AR, et al. Mesenteric and retroperitoneal cysts. Ann Surg. 1986;203:109.
94. Yasoshima T, Mukaiya M, Hirata K, et al. A chylous cyst of the mesentery: Report of a case. Surg Today. 2000;30:185.
95. Dequanter D, Lefebvure JC, Belva P, et al. Mesenteric cysts. A case treated by laparoscopy and a review of the literature. Surg Endosc. 2002;16:1493. Epub July 29, 2002
96. Stout AP, Hendry J, Pardie FJ. Primary solid tumors of the greater omentum. Cancer. 1963;16:231.
97. Schwartz RW, Reames M, McGrath PC, et al. Primary solid neoplasms of the greater omentum. Surgery. 1991;109:543.
98. Horiuchi K, Yabe H, Mukai M, et al. Multiple smooth muscle tumors arising in deep soft tissue of lower limbs with uterine leiomyomas. Am J Surg Pathol. 1998;22:897.
99. Herrero J, Kamali P, Kirschbaum M. Leiomyomatosis peritonealis disseminata associated with endometriosis: A case report and literature review. Eur J Obstet Gynecol Reprod Biol. 1998;76:189.
100. Dpenzieri A, Gertz MA. Treatment of Castleman’s disease. Curr Treat Options Oncol. 2005;6:255.
101. Waterston A, Bower M. Fifty years of multicentric Castleman’s disease. Acta Oncol. 2004;43:698.
102. Reske M, Nimiki H. Sclerosing mesenteritis: Report of two cases. Am J Clin Pathol. 1975;64:661.
103. Kipfer RE, Moertel CG, Dahlin DC. Mesenteric lipodystrophy. Ann Intern Med. 1984;80:582.
104. Parra-Davila E, McKenney MG, Sleeman D, et al. Mesenteric panniculitis: Case report and literature review. Am Surg. 1998;64:768.
105. Papadaki HA, Kouroumalis EA, Stefanaki K, et al. Retractile mesenteritis presenting as fever of unknown origin and autoimmune haemolytic anaemia. Digestion. 2000;61:145.
106. Higgins PM, Aber GM. Idiopathic retroperitoneal fibrosis: An update. Dig Dis. 1990;8:206-22.
107. Kronthal AJ, Kang YS, Fishman EK, et al. MR imaging in sclerosing mesenteritis. Am J Radiol. 1991;156:517.
108. Daskalogiannaki M, Voloudaki A, Prassopoulos P, et al. CT evaluation of mesenteric panniculitis: Prevalence and associated diseases. AJR Am J Roentgenol. 2000;174:427.
109. Mazure R, Fernandez J, Marty P, et al. Successful treatment of retractile mesenteritis with oral progesterone. Gastroenterology. 1998;114:1317.
110. Netzer P, Binek J, Hammer B. Diffuse abdominal pain, nausea and vomiting due to retroperitoneal fibrosis: A rare but often missed diagnosis. Eur J Gastroenterol Hepatol. 1997;9:1005.
111. Poujade O, Ghiles E, Senasli A. Primary torsion of the greater omentum: case report—Review of literature. Surg Laparosc Endosc Percutan Tech. 2007;17:54.
112. Stella DL, Schelleman TG. Segmental infarction of the omentum secondary to torsion: Ultrasound and computed tomography diagnosis. Australas Radiol. 2000;44:212.
113. Gassner PE, Cox MR, Cregan PC. Torsion of the omentum: Diagnosis and resection at laparoscopy. Aust N Z J Surg. 1999;69:466.
114. Anderson L, Forrest J. Tumors of the diaphragm. AJR Am J Roentgenol. 1973;119:259.
115. Greenberg M, Maden V, Ataii E, et al. Intradiaphragmatic cyst: A diagnostic challenge. JAMA. 1974;230:1176.
116. Dobelle WH. Use of breathing pacemakers to suppress intractable hiccups of up to thirteen years duration. ASAIO J. 1999;45:524.
117. Vargas C, Jeffers LJ, Bernstein D, et al. Diagnostic laparoscopy: A 5-year experience in a hepatology training program. Am J Gastroenterol. 1995;90:1258.
118. Evasovich MR, Clark TC, Horattas MC, et al. Does pneumoperitoneum during laparoscopy increase bacterial translocation? Surg Endosc. 1996;10:1176.
119. Tug T, Ozbas S, Tekeli A, et al. Does pneumoperitoneum cause bacterial translocation? J Laparoendosc Adv Surg Tech A. 1998;8:401.
120. Seelig MH, Seelig SK, Behr C, Schonleben K. Comparison between open and laparoscopic technique in the management of perforated gastroduodenal ulcers. J Clin Gastroenterol. 2003;37:226.
121. Palesty JA, Wang XJ, Rutland RC, et al. Fifty-five consecutive laparoscopic appendectomy procedures without conversion. JSLS. 2004;8:141.
122. Asoglu O, Ozmen V, Karanlik H, et al. Does the complication rate increase in laparoscopic cholecystectomy for acute cholecystitis? J Laparoendosc Adv Surg Tech A. 2004;14:81.
123. Fine AP. Laparoscopic surgery for inflammatory complications of acute sigmoid diverticulitis. J Soc Laparoendosc Surg. 2001;5:233.
124. Chu C, Lin S, Peng S, et al. The role of laparoscopy in the evaluation of ascites of unknown origin. Gastrointest Endosc. 1994;40:285.
125. Jeffers LJ, Alzate I, Agulfer H, et al. Laparoscopic and histologic findings in patients with the human immunodeficiency virus. Gastrointest Endosc. 1994;40:160.
126. D’Angelica M, Spiros P, Hiotis HJK, et al. Laparoscopic staging for liver, biliary, pancreas, and gastric cancer. Curr Prob Surg. 2007;44:228-69.
127. Conlon KC, Dougherty E, Klimstra DS, et al. The value of minimal access surgery in the staging of patients with potentially resectable perpancreatic cancer. Ann Surg. 1996;223:134.
128. vanDijkum EJ, deWit LT, vanDelden OM. Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg. 1999;189:459.