CHAPTER 71 Surgical Management of Upper Airway Stenosis
Pathophysiology
Adult laryngotracheal stenosis has numerous etiologies (Box 71-1). The pathophysiology of the specific cause plays a role in the timing of surgical management as well as the surgical procedure chosen.
Box 71-1 Causes of Adult Laryngeal and Upper Tracheal Stenosis
The most common causes of laryngotracheal stenosis are external trauma to the neck and prolonged endotracheal intubation. Both may result in acute stenosis and chronic stenosis; which occur through different pathophysiologic processes. Anatomically, the larynx and trachea can be regarded as a semirigid tubular structure. When the laryngotracheal complex is injured by external trauma, disruption of the cartilaginous framework, hematoma in the laryngeal spaces, and mucosal disruption usually result. Resorption of the hematoma can cause cartilage loss and extensive deposition of collagen. Subsequent scar contracture leads to stenosis and loss of mobility. The location, mechanism, and severity of the laryngeal injury caused by external trauma vary.1
In contrast, the injury from endotracheal intubation is usually initiated by ischemic necrosis of the mucosa from pressure of the cuff of the endotracheal tube or the endotracheal tube itself.2 Mucosal ulceration in the presence of bacterial infection can lead to perichondritis and chondritis with subsequent cartilage resorption. Healing occurs by secondary intention with subsequent submucosal fibrosis and scar contraction. Injuries from endotracheal intubation occur primarily in the posterior glottis as a result of pressure exerted by the wall of the tube and the pressure of the cuff on the tube’s tip. The latter injury has been reduced significantly with low-pressure, high-volume cuffs. Other factors, including tube size and composition, duration of intubation, and laryngeal movement, contribute to the development of laryngotracheal stenosis. With the widespread acceptance and use of endotracheal intubation to provide ventilatory and airway support, efforts have been directed to modifying endotracheal tube design.3
Patient disease has also shown to be correlated with the development of subglottic and tracheal stenosis. Diabetes mellitus, congestive heart failure, and a history of stroke have been shown to be associated with a higher incidence of severe acute laryngeal injury from intubation. In some cases, this condition prompts early tracheotomy.4 Laryngopharyngeal reflux in the setting of gastroesophageal disease has also been demonstrated to be a causative agent and potentiator of laryngotracheal stenosis. Treatment with H2 blockers and proton pump inhibitors in the perioperative and acute injury phases has been demonstrated to prevent scar formation and subsequent contracture.5
Classification of Stenosis
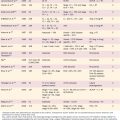
Multiple staging systems exist for airway stenosis; these are based on function or location of the stenotic section. As shown in Table 71-1, the most prevalent is the Cotton-Myers classification; however, the McCaffrey system and others also add to the diagnostic and therapeutic plan in providing not only size of stenosis but location and inclusion of multiple subsites, which are associated with a poorer prognosis following surgery.
Table 71-1 Classification and Staging Systems for Airway Stenosis
| Myers-Cotton Classification: circumferential stenosis limited to subglottic region | |
| Grade I | Obstruction of 0%-50% of the lumen |
| Grade II | Obstruction of 51%-70% of the lumen |
| Grade III | Obstruction of 71%-99% of the lumen |
| Grade IV | No detectable lumen; obstruction of 100% of the lumen |
| McCaffrey Classification: laryngotracheal stenosis based on the subsites and length of the stenosis | |
| Stage I | Subglottic or tracheal lesions < 1 cm long |
| Stage II | Subglottic lesions > 1 cm long |
| Stage III | Subglottic/tracheal lesions not involving the glottis |
| Stage IV | Glottic lesions |
| Lano Classificaiton: based on number of subsites involved | |
| Stage I | One subsite involved |
| Stage II | Two subsites involved |
| Stage III | Three subsites involved |
Surgical Principles
Goals and Assessment
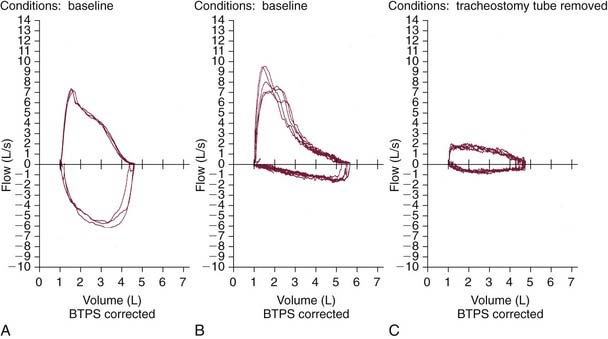
Several factors should be considered, including the location, dimensions, and quality (soft vs. fibrous) of stenosis, associated vocal fold motion impairment, and extent of functional impairment. Initial evaluation should involve a thorough history that includes details of the degree of subjective impairment (perceived by the patient) along with objective measures (e.g., observing the patient walking 200 feet, climbing a flight of stairs, pulmonary function testing).6 Pulmonary function testing is helpful in delineating the site of obstruction and severity of airway compromise (Fig. 71-1).
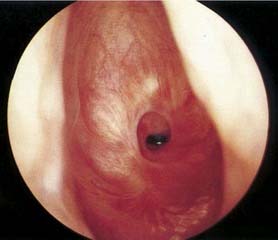
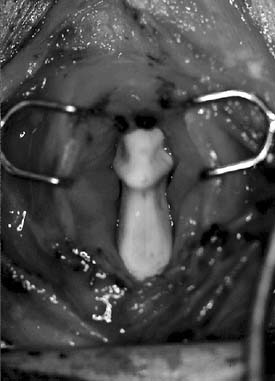
Physical examination, including an indirect laryngeal examination followed by direct laryngoscopy and bronchoscopy, is essential (Fig. 71-2). High-resolution computed tomography of the larynx and trachea may be useful in assessing the level and extent of the stenosis. Three-dimensional computed tomography with volume rendering or virtual endoscopy has been employed to evaluate the extent or severity of the stenosis.7,8
An important consideration is whether the site of stenosis requires reestablishment of structural support, usually by repositioning of existing cartilage or, more commonly, through the use of cartilage or bone grafts.9 Even with grafts, the preservation of existing mucosa and the judicious use of antibiotics, stents, skin or mucosal grafts are all adjunctive treatments intended to minimize formation of granulation tissue, and subsequent deposition of collagen.
Timing of Repair
A chronic laryngotracheal stenosis repair is generally elective, once the appropriate workup is completed. The onset of symptoms may be insidious, depending on the extent of scar formation and contracture, and in intubated patients, several attempts at extubation usually fail, requiring tracheotomy.13 Such patients subsequently cannot be decannulated or, after a brief decannulation, experience gradually progressive upper airway symptoms. Irreversible stenosis is usually diagnosed at this time, and repair can be performed electively after thorough evaluation. A key consideration is that patients with inflammatory or autoimmune diseases require stabilization of their underlying disease processes before surgical management of the stenosis.
Endoscopic versus Open Repair
The last few decades have seen the growing popularity of the carbon dioxide (CO2) laser, which offers the advantages of delayed formation and maturation of collagen in wounds, thus allowing reepithelialization before scar formation as well as minimal deep tissue injury. The laser has enabled precise control of the areas removed and hemostasis with the aim of preserving existing mucosa that may be utilized for the repair. Limitations of the CO2 laser include the risk of an airway fire, and the danger of the laser plume for the surgeon and hospital staff if they are working with infectious lesions.10 In addition, it is often difficult to control thermal injury, which may result in perioperative edema and postoperative scarring of the vocal folds.11 “Due to these risks,” some authors advocate initial endoscopic management of chronic laryngotracheal stenosis, reserving open repair for patients whose stenosis does not resolve with endoscopic management.12 The advantage of this approach is that an open surgical repair may be avoided in some instances; however, several endoscopic procedures may be required for long-term success. Current recommendations include treatment of mild stenosis (Cotton-Myer grades I and II) with endoscopic techniques. Failure of initial endoscopic technique is associated with previous surgery, circumferential scarring, loss of cartilaginous support, exposure of cartilage leading to chondritis, posterior inlet scarring with subsequent arytenoid fixation, postoperative bacterial infection, and initial poor choice of candidates, such as patients with combined laryngeal and tracheal stenosis or vertical scar length greater than 1 to 1.5 cm. With prudent choice of candidates and careful postoperative care and monitoring, success of decannulation at 1 year after endoscopic surgery for stenosis has risen from 60% to 65% to 80% to 85%.
Microsurgical Débridement
Much like the impact of advanced instrumentation for endoscopic techniques in laryngeal surgery, the success of powered instrumentation in many arthroscopic procedures has led to the popularity of microsurgical debriders in airway surgery. This technique combines the use of flexible forceps and a microsurgical debrider with videoendoscopic control.14 The debrider has a powered rotating blade and suction so that soft tissue lesions are aspirated into the tip of the blade and cleanly cut. Use of this instrumentation in endoscopic sinus surgery provided for more efficient and precise tissue removal and reduced injury to normal mucosa. Development of a laryngeal microsurgical shaver in the 1990s allowed for removal of bulky, exophytic laryngeal papilloma in patients with recurrent respiratory papillomatosis, obstructing carcinoma, and Reinke’s edema.15–17 In comparison with the carbon dioxide laser, the laryngeal microsurgical debrider eliminates the risk of airway fire because there is no laser plume and therefore a lower infectious risk for the operating room staff. Anecdotal data show that use of this instrument in patients with recurrent respiratory papillomatosis, which was previously treated with the laser, have less postoperative pain and a quicker return to a usable speaking voice. A decrease in operative time has also been observed, thus reducing both the patient’s exposure to a general anesthetic and overall operating room costs.18
We use the microdebrider for many laryngeal lesions (Box 71-2) by maneuvering the rigid telescope with the laryngeal shaver, thus promoting camera steadiness and good visualization of the blade tip relative to the lesion as it is removed. Subglottic and tracheal stenotic lesions are typically removed with a surgical microdebrider (4-mm) subglottic blade to excise the fibrous scar. Softer, bulky lesions, such as laryngeal and tracheal papilloma, are rapidly removed with the 4.0-mm round window (skimmer) blade. Papillomas and other lesions involving the true vocal folds are removed with the 3.5-mm round window (skimmer) laryngeal blade (Figs. 71-3 and 71-4).
Local Application of Mitomycin C
Topical mitomycin C may be useful for the treatment and prevention of subsequent re-stenosis and scar formation in the larynx and trachea. Mitomycin C is an antineoplastic antibiotic that acts as an alkylating agent by inhibiting DNA and protein synthesis. It can inhibit cell division, protein synthesis, and fibroblast proliferation.19
The use of topical mitomycin C remains controversial, with one study demonstrating that after 15 months of mitomycin application, all patients had clinical improvement of symptoms without evidence of recurrence.19 Another study performed on the pediatric airway found no statistical difference between a single topical dose of mitomycin and isotonic sodium chloride after laryngotracheal reconstruction.20 Animal studies have shown a statistically significant reduction in the rate of restenosis after surgical lysis with the use of mitomycin C.21
Surgical Planning for Repair
Each of these graft materials may be limited by size, lack of pliability, and variable resorption due to lack of blood supply. The rib or costal cartilage graft is most commonly used because of its size, strength, and relative ease of harvesting. Vascularized hyoid and thyroid cartilage grafts have shown considerable success22,23 but are limited by the amount of cartilage available. Composite auricular and nasal septal cartilage has the advantage of providing simultaneous epithelial repair, but it generally lacks adequate size and rigidity for cases in which large grafts are required. Vascularized perichondrial grafts and periosteal grafts have been evaluated for their chondrogenic or osteogenic capabilities and show potential for providing a pliable, vascularized graft that generates viable rigid bone or cartilage. In a rabbit model, vascularized perichondrium was shown to form significantly more cartilage when placed in a subglottic defect, and it tolerated a 2-hour ischemia time24; it has also been successfully maneuvered into tube form for reconstruction of a segmental tracheal defect.25 The sternocleidomastoid myoperiosteal flap has been successfully used for subglottic and tracheal reconstruction, with a low incidence of bony overgrowth on long-term follow-up.26
Use of Skin and Mucosal Grafts
The second consideration of the successful repair of acute and chronic laryngotracheal stenosis is the establishment of a completely epithelialized lumen of reasonably normal size and shape. In acute injuries, the ideal method is primary closure of mucosal lacerations after minimal débridement of nonviable tissue. In chronic stenosis, scar tissue should be excised, with preservation of as much overlying mucosa as possible. Small mucosal defects can be left to remucosalize, resulting in minimal detrimental effects.27 The controversy arises in the use of epithelial grafts to resurface large defects in acute and chronic stenosis. Wounds that heal by secondary intention form granulation tissue and deposit collagen, which may contract until later covered by epithelium.27 When the denuded area is secondarily infected—particularly after tracheotomy—epithelialization can be prolonged, leading to excessive granulation tissue and scar formation.28
Epidermal grafts have been advocated to interrupt this process and to result in healing similar to that of primary intention with minimal collagen deposition. When a stable cartilaginous framework is present, the tendency for the epidermal graft to contract is less, because the laryngeal skeleton acts as an external splint.29 The techniques of skin grafting as an important adjunct in laryngotracheal reconstruction have been well described.30
Stent Placement
Internal laryngeal and subglottic stenting is widely used with acceptable results; however, questions about the indications for stenting, the optimal type of stent, and the duration of stenting are largely unanswered. Internal stenting with soft or firm materials increases the risk of local infection, mucosal ulceration, and granulation tissue, which is directly related to the duration of stenting. Capillary blood flow ceases with a pressure of 20 to 40 mm Hg in mucosa closely adherent to cartilage, possibly initiating ischemic mucosal injury.31 Therefore, several writers advocate avoiding internal stenting (unless absolutely necessary) and minimizing the duration of stenting. However, internal stenting is commonly used for the following specific indications: (1) to provide support for cartilage and bone grafts or to splint displaced cartilage fragments in the desired position, (2) to allow approximation and immobilization of epidermal grafts to a recipient site, (3) to separate opposing raw surfaces during healing, and (4) to maintain lumen in a reconstructed area that lacks adequate cartilaginous support and requires scar formation.
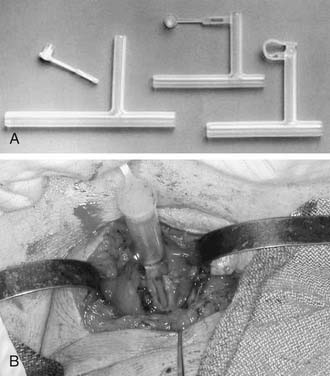
The optimal stent has not been determined, and a variety of options exist. A tantalum32 or umbrella silicone33 keel is useful for anterior glottic webs, but for more severe laryngotracheal stenosis, various stents are commonly used, including the Montgomery laryngeal stent (Fig. 71-5), the Aboulker stent, the “Swiss roll” siliconized rubber stent, and the finger cot stent; each has proponents who describe good results.34,35 In general, a soft stent should be used to minimize pressure on mucosal surfaces when cartilaginous support is satisfactory and when the indication for stenting is to splint an epidermal graft or to separate opposing raw mucosal surfaces. A finger cot with a povidone-iodine–soaked sponge is an excellent soft stent; an alternative is a Montgomery laryngeal stent, which is firm but conforms to endolaryngeal contours. A firm stent is needed when the cartilaginous framework or graft requires splinting or when inadequate cartilage is present, thereby requiring that luminal support be achieved through collagen deposition and scar contraction. A solid stent is preferable to a hollow stent to minimize aspiration. However, a soft, hollow stent such as the Montgomery T-tube allows phonation. The T-tube is commonly used for subglottic and upper tracheal stenosis; appropriate patient selection and education are essential, because this is a single-lumen tube with a greater risk of airway obstruction.36 An Aboulker stent can be used for subglottic or combined subglottic and laryngeal stenosis. The advantage of this stent is that it is used with a double-cannula metal tracheostomy tube, and is secured to the tube, thus preventing removal. Modifications of this technique that allow tracheotomy changes have been described.37
There is no uniformly accepted optimal duration of stent placement. Most authorities recommend 6 to 8 weeks, but the duration varies from 2 weeks to several months.38 Usually 1 to 3 weeks is sufficient if only mucosal healing or epidermal graft take is required. If the laryngeal skeleton is adequate but requires splinting to maintain the appropriate position, 6 to 8 weeks is the generally accepted time frame.
However, if the cartilaginous framework is deficient and mature scar is important for structural strength, prolonged stenting is indicated to allow scar contraction around an inert, firm object. Successful prolonged stenting has been reported for intervals of up to 14 months. In this situation, the problem arises in premature stent removal, usually before sufficient scar contraction has occurred.38 The presence of a paratubal air envelope, which manifests as linear air densities between the outside wall of the stent and the inside wall of the larynx and trachea, is reported to be a reliable indicator of laryngotracheal structural integrity and functional stability and is a useful measure to determine optimal timing for stent removal.39
Surgical Management of Chronic Stenosis
Supraglottic Stenosis
Chronic hypopharyngeal stenosis usually results from unrecognized acute blunt injury to the hyoid and thyrohyoid membrane. The direction of force is posterior to superior and results in the following discrete injuries: (1) the epiglottis can be adherent to the posterior or lateral hypopharyngeal walls, (2) the hyoid bone can be fractured and displaced posteriorly with the epiglottis, thereby producing laryngeal inlet stenosis, (3) a horizontal web of the posterior hypopharyngeal wall may form at the level of the superior aspect of the epiglottis, or (4) postcricoid stenosis may result in stenosis of the esophageal introitus.40
Adhesions of the epiglottis to the hypopharyngeal walls may be managed by division of the adhesion along its long axis, submucosal excision of scar tissue, and primary mucosal closure. When a horizontal web or shelf is present, a vertical incision is made through the web, and scar tissue is excised. Mucosal flaps are undermined to allow closure of the incision in a horizontal line. If necessary, superiorly based mucosal advancement flaps can be used. If the hypopharyngeal stenosis is extensive, the division of bands and the excision of scar tissue may result in an extensive denuded area over the constrictor muscle, and skin grafting should be considered. Hollow silicone esophageal stents have also been used to assist in the healing of a skin graft.40 If resection of scar tissue results in an extensive full-thickness pharyngeal defect, a radial forearm revascularized flap may be used to provide thin, pliable, soft tissue, which may, however, result in an adynamic hypopharyngeal segment.
Laryngeal inlet stenosis results from posterior displacement of the hyoid and epiglottic cartilage and can be accompanied by a fracture through the thyroid notch. This injury is approached through a laryngofissure approach with division of the thyrohyoid membrane and elevation of the mucoperichondrium on the laryngeal surface to allow for excision of this inverted wedge of the base of the epiglottic cartilage, perichondrium, fascia, and scar. With suturing of the resultant flaps to the free edge of the anterior epiglottic perichondrium, the thyrotomy is closed, and stenting is not required.40
Glottic Stenosis
Anterior Glottic Stenosis
Anterior glottic stenosis may be caused by external trauma resulting in a thyroid cartilage fracture and mucosal disruption or from circumferential scarring after denuding of mucosa by an endotracheal tube. A thin web that extends more than 3 to 4 mm posteriorly along the vocal fold can produce hoarseness, whereas a thicker web or a web that extends farther posteriorly can cause significant airway obstruction.41 Hoarseness or airway compromise is an indication for surgical intervention. Excision of the anterior glottic scar recreates the conditions that caused the original stenosis. Successful surgical repair requires physical separation of the opposing edges until remucosalization is complete; this may be done endoscopically when the web does not extend below the inferior edge of the true vocal fold and the posterior commissure is normal.42 Microlaryngeal instruments allow for better development and preservation of mucosal flaps than are possible with the laser. Excess scar tissue is removed, and a keel is placed and secured externally with wires or with a heavy suture placed through the thyrohyoid membrane. Alternatively, a mini-cricothyrotomy may be performed to place the keel with endoscopic visualization. Differing keel designs and compositions have been described. In principle, the keel should be composed of an inert material of sufficient length to extend from the cricothyroid membrane to at least 2 to 3 mm above the anterior commissure; if the keel extends superiorly over the petiole of the epiglottis, the anterior edge of the keel should make a 120-degree angle (i.e., the angle of the epiglottis and the anterior tracheal wall) to minimize granulation tissue formation at the petiole of the epiglottis. Of note, the posterior wing of the keel should lie at the vocal processes and should not touch the posterior commissure.41,42 The keel is usually removed after 2 to 4 weeks with any granulation tissue adherent to it.
If the anterior subglottic stenosis extends more than 5 mm subglottically, an external laryngofissure approach should be utilized. Resection of scar tissue should not be overzealous, and mucosa should be preserved whenever possible. When mucosa is deficient, Isshiki and others52 have described obtaining superior voice results by resurfacing the vocal fold with labial mucosa instead of split-thickness skin grafts and short-term stenting. Successful results have been achieved with the Montgomery umbrella silicone keel used for 2 to 3 weeks or with the McNaught tantalum keel.41
Posterior Glottic Stenosis
The extent of injury is variable. Type III and type IV posterior glottic stenoses are difficult to manage successfully, particularly when they are associated with subglottic stenosis.3,43 The major therapeutic challenges are to reestablish a satisfactory airway and to preserve the voice, which is often normal.
Endoscopic repair of posterior glottic stenosis is successful in carefully selected cases. Patients with an interarytenoid web and posterior sinus tract have been managed with a simple division of the web. Many patients experience restenosis, particularly those who have had a tracheotomy.44 A soft, sponge-filled finger cot stent has been advocated in this situation to separate the raw opposing surfaces; it can be placed endoscopically and remain for 2 weeks.45
Posterior commissure stenosis can be managed endoscopically with a CO2 laser or a laryngofissure approach. Creation of a posterior micro-trapdoor flap is successful when a posterior sinus or a 3- to 4-mm posterior interarytenoid scar is present.44 This technique fails when there is arytenoid fixation or a scar that is more than 1 cm in height. When posterior interarytenoid mucosa is insufficient to create a trapdoor flap (>3-4 mm), interarytenoid scar tissue is resected, with the mucosa preserved through a hockey-stick incision.
A synthetic implantation fabric keel may be placed endoscopically and left in place for 4 to 6 weeks to cause sufficient widening of the interarytenoid mucosa to allow for a micro-trapdoor flap. However, Dedo and Sooy,44 who first described this procedure, reported that failures occurred in all of their patients who had arytenoid fixation. Several other writers recommend open repair through a laryngofissure approach.45,46 The surgeon effects such a repair by developing a superiorly based mucosal flap, excising scar tissue and fibrosed interarytenoid muscle, and repositioning the mucosal flap to provide immediate epithelial coverage. Internal stenting is not required when both arytenoids are mobile. Care is taken to not enter the cricoarytenoid joints if both arytenoids are mobile. Successful surgical repair is an elusive goal.
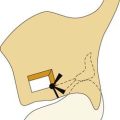
Posterior glottic stenosis with arytenoid fixation has been traditionally managed through an external approach. If, after development of a mucosal flap and resection of scar, one arytenoid is freely mobile, resection of the fixed arytenoid is unnecessary. When bilateral arytenoid fixation exists, removal of the less mobile arytenoid is necessary to achieve a satisfactory airway. Denuded mucosal surfaces are covered with mucosal flaps, skin, or mucosal grafts. Stenting for 2 to 3 weeks with a conforming silicone laryngeal stent or with a soft finger cot stent is required. A posterior cricoid split with rib graft may also be considered (Fig. 71-6).
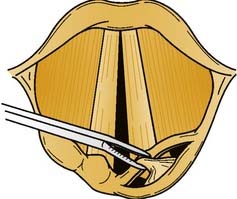
In 1983, Ossoff and associates47 first described complete arytenoidectomy via an endoscopic approach. Endoscopic laser arytenoidectomy is also useful for managing type IV posterior commissure stenosis when subglottic involvement is limited, because this procedure avoids the complications of an external approach and allows for the immediate assessment of airway size (Fig. 71-7). Endoscopic laser arytenoidectomy requires the removal of the entire arytenoid except for the muscular process. Arytenoid perichondritis is a potential complication, and the administration of perioperative antibiotics and antireflux measures, as always, are recommended. The degree of vocal fold lateralization obtained is variable, and the use of a lateralization suture or a finger cot stent may improve the final result by controlling the extent of lateralization.
The external approaches for an arytenoidectomy are through a laryngofissure or a Woodman arytenoidectomy. The latter approach exposes the arytenoid cartilage through a posterolateral extralaryngeal approach and the entire arytenoid cartilage is resected, except for the vocal process. A submucosal suture is placed through the vocal process and anchored around the inferior thyroid cornu to lateralize the vocal fold (6 mm in men, 5 mm in women).48 Other writers believe that tethering the vocal process around the inferior thyroid cornu may reposition the vocal fold inferior to the opposite fold; they recommend suturing to the thyroid lamina at the vocal fold level5 or around the superior cornu (success rate, 60%-80%).43 If the procedure is unsuccessful, completion arytenoidectomy through a laryngofissure approach with suture lateralization of the vocal fold can be performed.49
Exploration of the fixed cricoarytenoid joints with lysis of adhesions without arytenoidectomy has also been advocated. Fibrous bands on the medial aspect of the cricoarytenoid joints are divided, and the arytenoids are stented in full abduction for 2 to 3 weeks; stent removal and aggressive speech therapy follow. Return of vocal cord mobility has been described in 90% of patients managed in this way.50
Complete Glottic Stenosis
Complete glottic stenosis usually results from unrecognized severe extralaryngeal trauma, in which injury is rarely isolated to the glottis and associated subglottic injury is common. Endoscopic management is rarely indicated because of the severity and nature of the injury, and only a few instances have been reported in the literature. The mainstay of management involves an open laryngofissure approach. The stenosis should be divided at the midline and excessive scar tissue resected; the mucosa is preserved and mucosal flaps are developed from the aryepiglottic folds for coverage. If extensive areas are devoid of mucosa, epithelial grafts of buccal mucosa, grafts of nasal septal mucosa, or split-thickness skin grafts may be used. Large split-thickness skin grafts may cause excessive crusting. The grafts are sutured in place and stented with a form-fitting laryngeal stent for 4 to 8 weeks.51 When the stent is removed, an umbrella keel is placed into the anterior commissure for 2 weeks.
An alternative approach is reconstruction with an epiglottic flap. This approach is indicated in the following situations: severe glottic stenosis, a more than 50% reduction in the anteroposterior dimension of the glottis, glottic-subglottic stenosis, and glottic-supraglottic stenosis with an intact epiglottis.52,53 The repair is approached through a midline thyrotomy, and the thick scar is transected in the midline. Submucosal excision of scar tissue can be attempted, and the mucosa can be repositioned and secured with chromic suture. The base of the epiglottis is identified, and the median thyroepiglottic ligament is transected. The epiglottis is pulled inferiorly to reach the anterior cricoid arch, and the epiglottic flap is sutured to the outer anterior edges of the thyroid cartilage laterally and to the cricoid cartilage inferiorly. This procedure results in an epithelialized, widened anterior commissure. Several cartilage-splitting incisions may be used to allow the petiole to be folded onto itself to make a sharper anterior commissure.36
Subglottic Stenosis
Endoscopic management of subglottic stenosis requires careful patient selection. The predictive factors for failure were described earlier.43,54,55 CO2 laser excision followed by repair with a micro-trapdoor flap is moderately reliable for managing circumferential subglottic stenosis; it requires laser incision of the superior surface of the scar and the submucosal excision of scar tissue. An inferiorly based rectangular mucosal flap is created by two lateral knife incisions. The preserved flap does not completely cover the lasered area, but it does divide the raw concave area into two flat lateral raw areas, which heal rapidly. One study reported a 90% success rate in all patients undergoing the procedure, in whom scars that were less than 10 mm in height.43 A large flap that can potentially obstruct the airway through a ball-valve effect should not be created. Other writers44 have confirmed the efficacy of this procedure in adults and emphasize that it is useful when the stenosis is less than 10 mm in height, but a tracheotomy should be performed if the flap is longer than 4 mm. The procedure is uniformly unsuccessful in children, however.55 Failure also occurs if associated posterior glottic stenosis or arytenoid fixation is present. Shapshay and coworkers proposed an alternative method, consisting of radial incisions followed by bronchoscopic dilation.56 Their series of patients was small, however, and follow-up observation was only 1 year; thus, success was not assumed.
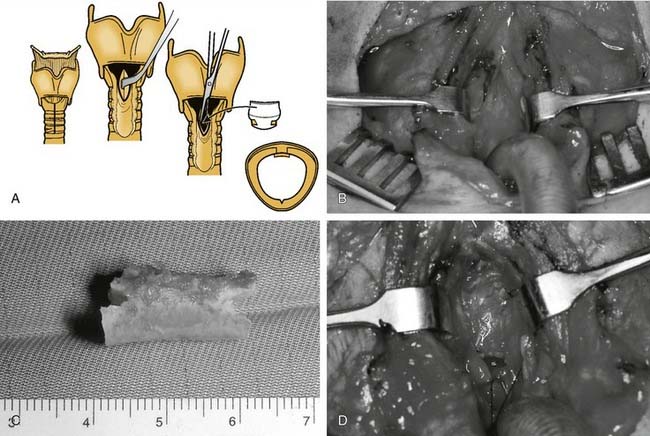
Subglottic scar is resected to cricoid perichondrium and is relined with the buccal mucosa or a split-thickness skin graft. The graft is preferably sewn around a stent that is fixed in place. When cartilaginous support or luminal augmentation is also required, various autogenous cartilage grafts are available. The hyoid-sternohyoid muscle interposition graft has been extensively used, with a success rate of approximately 70% in adults.57 This technique can be applied to isolated subglottic stenosis or subglottic stenosis in combination with anterior glottic or tracheal stenosis.
Various free autogenous cartilage grafts have been used for luminal augmentation. These grafts have variable amounts of resorption and require prolonged stenting to allow resorbed graft to be replaced by firm, mature scar tissue. Costal cartilage grafts have the advantage of providing large grafts that are easily sculpted. Specific details of graft tailoring have been well described.55 Composite nasal septal cartilage grafts have been used successfully in patients with stenosis of the larynx and upper trachea.15 Stenosis of up to 3 cm in height can be repaired, and the respiratory epithelium is a theoretic advantage, because it provides immediate resurfacing with a match of epithelium from the stenotic area. In children, a stent is not used, and the graft is not used so as to prevent injury to nasal growth centers. Various other autogenous grafts, including auricular cartilage, clavicular bone, and free hyoid bone, have been used but they have not received uniform acceptance.
Grillo and others58,59 refined this procedure to incorporate a posterior membranous tracheal wall flap to resurface the bared cricoid cartilage. In a series of 80 patients with subglottic and upper tracheal stenosis, these researchers reported failure in only 2 out of 77 patients with at least 6 months follow-up; one perioperative death occurred. Of 77 patients, 66 had no restriction of daily activities due to dyspnea, and tracheotomy was rarely required.
Cervical Tracheal Stenosis
When surgical repair of tracheal stenosis is considered (Box 71-3), regardless of the cause, it is essential to determine the location, length, composition, extent of airway stenosis, and neurologic integrity of the larynx through indirect laryngeal examination, bronchoscopy, and radiography. In most instances, cervical tracheal stenosis can be categorized as cicatricial membranous stenosis, anterior wall collapse, or complete stenosis. Airway management should be considered preoperatively and discussed with the anesthesiologist, as for all of these procedures. If a tracheotomy is not present or is not planned, it is safest to have the patient breathe spontaneously until the airway is secured. If a Montgomery T-tube is used as part of the reconstruction, the patient should breathe spontaneously after the T-tube is inserted (Fig. 71-8). If the airway is 5 mm or smaller at the time of reconstruction, dilation is performed initially with use of general anesthesia; microsurgical debridement resection may also be performed. If the lumen is larger than 6 mm in diameter, intubation is carried out above the lesion, and dissection is performed carefully to prevent airway obstruction. If a postoperative tracheotomy is planned, it should be performed with use of local anesthesia at the outset of the reconstruction procedure.
Cicatricial Membranous Stenosis
Granular or fibrous stenosis of the cervical trachea with intact cartilage can initially be managed endoscopically. CO2 laser excision of granular stenosis is useful because tracheotomy can be avoided, and the granular stenosis can be resected accurately with minimal bleeding. If the stenosis is circumferential, partial resections should be staged 2 to 4 weeks apart to prevent the formation of a circumferential denuded area of trachea in which restenosis can occur. Fibrous stenosis can be excised with a micro-trapdoor technique.44
Anterior Wall Collapse
Collapse of the anterior laryngeal wall can result from anterior blunt neck trauma, but it more commonly results from tracheotomy. Montgomery categorizes these injuries as suprastomal, stomal, or infrastomal.32 In this type of stenosis, a well-mucosalized posterolateral wall and residual cartilage in the area of the stenosis should be present.36
A suprastomal and stomal stenosis can be approached and managed as described for cicatricial membranous stenosis. However, restoration of the anterior wall is essential. The surgeon can accomplished this restoration by (1) stenting the lumen open with a T-tube or a firm stent of a tracheostomy tube, such as the Aboulker stent, and repairing the anterior wall with a cartilage graft or (2) mobilizing the sternohyoid muscles and securing them together over the defect. In both instances, prolonged stenting is required.36
Wedge resection of an anterior tracheal wall stenosis can be considered if (1) the stenosis is limited to two or three rings, with significant loss of cartilage that would preclude stenting, (2) the posterior wall mucosa is intact, or (3) the stenosis is stomal or suprastomal. The procedure involves adequate exposure of the involved trachea, including at least one ring above and one ring below the stenosis. Subperichondrial dissection is carried out carefully around the involved trachea to prevent injury to the recurrent laryngeal nerves. The stenosis is resected, with care taken to preserve posterior tracheal wall mucosa. An oroendotracheal tube is passed through the anastomosis, and the cartilaginous trachea is reanastomosed with submucosal sutures tied extraluminally. The patient is extubated when awake and alert, and granulation tissue is excised endoscopically as early as possible.36
Complete Tracheal Stenosis
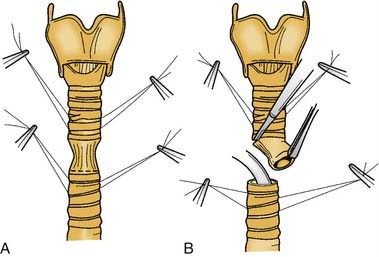
Segmental resection and primary anastomosis provide optimal results for complete tracheal stenosis. The average length of the adult trachea is 11 cm (range, 10-13 cm), and there are 14 to 20 tracheal rings. With hyperextension of the neck, 50% of the trachea is cervical. Approximately 50% of the trachea (5-7 cm) can be safely resected and anastomosed primarily with appropriate mobilization techniques. Other factors also should be considered in the determination of how much trachea can be resected. Older patients with calcification between tracheal rings may have less tracheal elasticity; a patient with a short, thick neck may have a deep trachea, with the cricoid at the level of the sternal notch. Tracheal mobilization is limited in a patient with decreased ability to extend the neck. The procedure is essentially the same as a wedge resection, except that dissection requires excision of the posterior membranous wall. The anterior cricoid arch and the posterior cricoid lamina are resected to just below the cricothyroid joints. An oroendotracheal tube is passed distal to the anastomotic site, and the anastomosis is performed with submucosal sutures placed around a trachea ring and tied extraluminally. Adequate mobilization of the larynx and trachea is required to relieve tension from the anastomosis. The neck is placed in extreme flexion, and a stay stitch is placed from chin to sternum.36
Segmental resection with primary anastomosis shows promise as the most efficacious procedure for managing near complete or complete tracheal stenosis (Fig. 71-9). In patients in whom stenosis is limited to the trachea and subglottis and the glottis is normal, success rates in several large series are 95% to 97%.59 When the stenosis also involves the glottis, resection with end-to-end anastomosis has been performed simultaneously with laryngofissure and decannulation repair of the glottic stenosis, which is achieved in approximately 95% of patients. In these instances, a Montgomery T-tube and prolonged stenting were uniformly used.
Anand VK, Alemar G, Warren ET. Surgical considerations in tracheal stenosis. Laryngoscope. 1992;102:237-243.
Cotton RT. Management of subglottic stenosis. Otolaryngol Clin North Am. 2000;33:111-130.
Hartnick CJ, Hartley BE, Lacy PD, et al. Topical mitomycin application after laryngotracheal reconstruction: a randomized, double-blind, placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 2001;127:1260.
Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116:1553-1557.
Koshkareva Y, Gaughan JP, Soliman AM. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol. 2007;116:206-210.
Kurrus JA, Gray SD, Elstad MR. Use of silicone stents in the management of subglottic stenosis. Laryngoscope. 1997;107:1553-1558.
Nouraei SA, Nouraei SM, Upile T, et al. A proposed system for documenting the functional outcome of adult laryngotracheal stenosis. Clin Otolaryngol. 2007;32:407-4099.
Nouraei SA, Ma E, Patel A, et al. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol. 2007;32:411-412.
Perepelitsyn I, Shapshay SM. Endoscopic treatment of laryngeal and tracheal stenosis—has mitomycin C improved the outcome? Otolaryngol Head Neck Surg. 2004;131:16-20.
Weymuller EA. Laryngeal injury from prolonged endotracheal intubation. Laryngoscope. 1988;98:1.
Zalzal GH. Use of stents in laryngotracheal reconstruction in children: indications, technical considerations, and complications. Laryngoscope. 1988;98:849.
1. Stell PM, Maran AG, Stanley RE, et al. Chronic laryngeal stenosis. Ann Otol Rhinol Laryngol. 1985;94:108.
2. Weymuller EA. Laryngeal injury from prolonged endotracheal intubation. Laryngoscope. 1988;98:1.
3. Santos PM, Afrassiabi A, Weymuller EA. Prospective studies evaluating the standard endotracheal tube and a prototype endotracheal tube. Ann Otol Rhinol Laryngol. 1989;98:935.
4. Volpi D, Lin PT, Kuriloff DB, et al. Risk factors for intubation injury of the larynx. Ann Otol Rhinol Laryngol. 1987;96:684.
5. Jindal JR, Milbrath MM, Shaker R, et al. Gastroesophageal reflux disease as a likely cause of “idiopathic” subglottic stenosis. Ann Otol Rhinol Laryngol. 1994;103:186.
6. Davidson J, Gullane P, Havas T, et al. Functional assessment after laryngotracheoplasty. Otolaryngol Head Neck Surg. 1987;97:294.
7. Goldenberg D, Shreiber R, Golz, et al. Volume rendering in the preoperative assessment and localization of subglottic stenosis. Presented at the AAO-HNS Foundation 104th Annual Meeting, San Diego, CA. September 2002.
8. Hoppe H, Walder B, Sonnenschein M, et al. Multidetector CT virtual bronchoscopy to grade tracheobronchial stenosis. AJR Am J Roentgenol. 2002;178:1195.
9. Choi SS, Zalzal GH. Pitfalls in laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1999;125(Jun):650.
10. Pasquale K, Wiatrak B, Woolley A, et al. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113:139.
11. Flint P. Powered surgical instruments for laryngeal surgery. Otolaryngol Head Neck Surg. 2000;122:263.
12. Dedo HH, Sooy CD. Endoscopic laser repair of posterior glottic, subglottic and tracheal stenosis by division or microtrapdoor flap. Laryngoscope. 1984;94:445.
13. Simpson GT, Strong MS, Healy GB, et al. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol. 1982;91:384.
14. Mayer HM, Brock M. Percutaneous endoscopic lumbar discectomy (PELD). Neurosurg Rev. 1993;16:115.
15. McNaught RC. Surgical correction of the anterior web of the larynx. Laryngoscope. 1950;60:264.
16. Simoni P, Peters GE, Magnuson JS, et al. Use of the endoscopic microdebrider in the management of airway obstruction from laryngotracheal carcinoma. Ann Otol Rhinol Laryngol. 2003;112:11.
17. Sant’Anna GD, Mauri M. Use of the microdebrider for Reinke’s edema surgery. Laryngoscope. 2000;110:2114.
18. Patel N, Rowe M, Tunkel D. Treatment of recurrent respiratory papillomatosis in children with the microdebrider. Ann Otol Rhinol Laryngol. 2003;112:7.
19. Rahbar R, Valdez TA, Shapshay SM. Preliminary results of intraoperative mitomycin-C in the treatment and prevention of glottic and subglottic stenosis. J Voice. 2000;14:282.
20. Hartnick CJ, Hartley BE, Lacy PD, et al. Topical mitomycin application after laryngotracheal reconstruction: a randomized, double-blind, placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 2001;127:1260.
21. Spector JE, Werkhaven JA, Spector NC, et al. Prevention of anterior glottic restenosis in a canine model with topical mitomycin-C. Ann Otol Rhinol Laryngol. 2001;110:1007.
22. Burstein FD, Canalis RC, Ward PH. Composite hyoid-sternohyoid interposition grafts revisited: UCLA experience 1974-1984. Laryngoscope. 1986;96:516.
23. Fry TL, Fischer ND, Pillsbury HC. Tracheal reconstruction with pedicled thyroid cartilage. Laryngoscope. 1985;95:60.
24. Hartig GK, Esclamado RM, Telian SA. Chondrogenesis by free and vascularized rabbit auricular perichondrium. Ann Otol Rhinol Laryngol. 1994;103:901.
25. Naficy S, Esclamado RM, Clevens R. Reconstruction of the rabbit trachea with vascularized auricular perichondrium. Ann Otol Rhinol Laryngol. 1996;105:356.
26. Friedman M, Mayer AD. Laryngotracheal reconstruction in adults with the sternocleidomastoid myoperiosteal flap. Ann Otol Rhinol Laryngol. 1992;101:897.
27. Olson NR. Wound healing by primary intention in the larynx. Otolaryngol Clin North Am. 1979;12:735.
28. Sasaki CT, Horiuchi M, Koss N. Tracheotomy related subglottic stenosis: bacteriologic pathogenesis. Laryngoscope. 1979;86:857.
29. Thomas GK, Stevens MH. Stenting in experimental laryngeal injuries. Arch Otolaryngol Head Neck Surg. 1975;101:217.
30. Olson NR. Skin grafting of the larynx. Otolaryngol Head Neck Surg. 1991;104:503.
31. Nordin U, Lindholm CE, Wolgast M. Blood flow in rabbit tracheal mucosa and the influence of tracheal intubation. Acta Anesthesiol Scand. 1977;21:84.
32. Montgomery WW, editor. Surgery of the Upper Respiratory System, Vol 2. Philadelphia: Lea & Febiger. 1989.
33. Montgomery WW, Gamble JE. Anterior glottic stenosis. Arch Otolaryngol Head Neck Surg. 1970;92:560.
34. Evans JN. Laryngotracheoplasty. Otolaryngol Clin North Am. 1977;10:119.
35. Harris HH, Tobin HA. Acute injuries of the larynx and trachea in 49 patients. Laryngoscope. 1970;80:1376.
36. Cummings CW, et al. Tracheal stenosis. In: Cummings CW, et al, editors. Atlas of Laryngeal Surgery. St Louis: Mosby, 1984.
37. Mohr RM. A modification of the Aboulker stent for reduction of granulation tissue that allows tracheotomy changes. Laryngoscope. 1992;102:350.
38. Schuller DE. Long term stenting for laryngotracheal stenosis. Ann Otol Rhinol Laryngol. 1980;89:515.
39. Gallivan GJ. Laryngotracheal paratubal air envelope: a useful sign in voice and airway reconstructive surgery. J Voice. 1994;8:168.
40. Gates GA, Tucker JA. Sliding flap tracheoplasty. Ann Otol Rhinol Laryngol. 1989;98:926.
41. Dedo HH. Endoscopic Teflon keel for anterior glottic web. Ann Otol Rhinol Laryngol. 1979;88:467.
42. Parker DA, Das Gupta AR. An endoscopic Silastic keel for anterior glottic webs. J Laryngol Otol. 1987;101:1055.
43. Zalzal GH. Use of stents in laryngotracheal reconstruction in children: indications, technical considerations, and complications. Laryngoscope. 1988;98:849.
44. Dedo HH, Sooy CD. Endoscopic laser repair of posterior glottic, subglottic and tracheal stenosis by division or microtrapdoor flap. Laryngoscope. 1984;94:445.
45. Bogdasarian RS, Olson NR. Posterior glottic laryngeal stenosis. Otolaryngol Head Neck Surg. 1980;88:765.
46. Montgomery WW. Posterior and complete glottic stenosis. Arch Otolaryngol Head Neck Surg. 1973;98:170.
47. Ossoff RH, Karlan MS, Sisson GA. Endoscopic laser arytenoidectomy. Lasers Surg Med. 1983;2:293.
48. Sessions D, Ogura JH, Henneman H. Surgical management of bilateral vocal cord paralysis. Laryngoscope. 1976;86:559.
49. Remsen K, Lawson W, Patel N, et al. Laser lateralization for bilateral vocal cord abductor paralysis. Otolaryngol Head Neck Surg. 1985;93:645.
50. Goodwin WJJr, Isaacson G, Kirchner JC, et al. Vocal cord mobilization by posterior laryngoplasty. Laryngoscope. 1988;98:846.
51. Isshiki N, Taira T, Nose K, et al. Surgical treatment of laryngeal web with mucosa graft. Ann Otol Rhinol Laryngol. 1991;100:95.
52. Delaere PR, Ostyn F, Segers A, et al. Epiglottoplasty for reconstruction of posttraumatic laryngeal stenosis. Ann Otol Rhinol Laryngol. 1991;100:447.
53. Kennedy TL. Epiglottic reconstruction of laryngeal stenosis secondary to cricothyroidotomy. Laryngoscope. 1980;90:1130.
54. Simpson GT, Strong MS, Healy GB, et al. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol. 1982;91:384.
55. Beste DJ, Toohill RJ. Microtrapdoor flap repair of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol. 1991;100:420.
56. Shapshay SM, Beamis JFJr, Hybels RL, et al. Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol. 1987;96:661.
57. Zalzal GH, Cotton RT. A new way of carving cartilage grafts to avoid prolapse into the tracheal lumen when used in subglottic reconstruction. Laryngoscope. 1986;96:1039.
58. Imhoff AB, Ledermann T. Arthroscopic subacromial decompression with and without the holmium: YAG-laser: a comparative study. Arthroscopy. 1995;5:549.
59. Grillo HC, Wright CD, Vlahakes GJ, et al. Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardio Surg. 2002;123:145-152.
60. Hall RR. The healing of tissues incised by carbon dioxide laser. Br J Surg. 1971;58:222.