CHAPTER 78 Surgical Management of Tremor
Spiegel and Wycis1–3 pioneered stereotactic surgery for the treatment of Parkinson’s disease (PD) by introducing coagulation of the globus pallidus—the stereotactic pallidotomy. Hassler4,5 subsequently introduced ablation of the ventral oral nucleus of the thalamus (consisting of the anterior [VOA] and posterior [VOP] subnuclei)—the terminus for pallidal afferents to the thalamus—to treat movement disorders. Microelectrode recordings later demonstrated that the area posterior to VOP—that is, the terminus of cerebellar afferents (the ventral intermediate [VIM] nucleus)—has rhythmic bursting activity close to the frequency of tremor.6 The VIM nucleus then became the lesioning target of choice for the treatment of all types of tremor.
DBS was first applied in the hypothalamus for the treatment of chronic pain by Pool and colleagues7 in the 1950s. Thereafter, DBS was applied primarily to the somatosensory thalamus and the paraventricular gray matter for the treatment of chronic pain.8,9 Surgeons noted, however, that the stimulation used to localize thalamic targets to be lesioned for pain relief also improved the symptoms of movement disorders.5,10 In addition, thalamic stimulation for the relief of chronic pain improved movement disorders associated with thalamic pain syndrome.11 In the 1980s and 1990s, stimulation was reported as an effective treatment for tremor12,13 and as a specific therapeutic modality for PD.14,15
The introduction of levodopa in the early 1970s led to a dramatic decrease in the number of stereotactic surgeries for movement disorders.16 Two decades later, side effects of drug treatment, including dyskinesias and fluctuations, had complicated the long-term care of PD patients and could become extremely disabling on their own.17,18 There was renewed interest in lesional surgical approaches to movement disorders after neurophysiologic studies in the realistic 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine (MPTP) model of PD demonstrated hyperactivity in the subthalamic nucleus (STN) and internal globus pallidus (GPi) that could be reversed by lesioning.19 This was demonstrated clinically in humans with advanced PD via a posteroventral GPi pallidotomy.20 Finally, the introduction of stimulation techniques led to a decreased role for ablative approaches in the treatment of movement disorders.13,21,22
Pathophysiology: Tremor Generation
Parkinson’s Tremor
In the case of Parkinson’s tremor, the most current hypothesis is that a thalamic oscillator is activated by hyperpolarization of the thalamocortical cells in ventral lateral (pars oralis)/VOP owing to increased inhibitory input from the pallidum in PD.19,23–27 When inhibited, these cells generate a somatic calcium spike that produces an action potential burst (low-threshold spike burst) followed by inhibition, leading to recurrent oscillations.23,28,29 However, such bursts are rarely found in recordings from the VIM nucleus and VOP of parkinsonian patients undergoing thalamotomy.30,31
Another proposed central generator is the GPi—specifically, neurons within the GPi that project to and inhibit VOP neurons. This hypothesis is inconsistent with higher rates of tremor-related activity in the cerebellar relay nucleus (VIM nucleus, 25%) compared with the pallidal relay in patients with PD (VOP, 21%).32,33 Furthermore, GPi activity at tremor frequency is rarely correlated with tremor in PD patients34,35 or in monkey models of parkinsonism.36 Based on these reports, the activity of cells in the thalamus and pallidum is not consistent with a pallidal generator for parkinsonian tremor.
Another proposed mechanism of parkinsonian tremor is peripheral feedback. This hypothesis proposes that tremor is the oscillation of unstable stretch reflex arcs (long-loop reflex arcs) that may traverse the motor cortex much as tendon tap reflexes traverse the spinal cord.37–39 The increased gain of these reflexes may cause parkinsonian rigidity and tremor, just as increased spinal reflexes cause spasticity and clonus.27 This hypothesis is supported by the finding that thalamic neuronal activity precedes tremor in thalamic neurons with sensory inputs but not in those without.33,40–42 Therefore, sensory cells participating in a reflex or feedback loop might cause tremor. Finally, a transfer function analysis has demonstrated a feedback loop in more than 90% of cells in the VIM nucleus and VOP.43
Essential Tremor
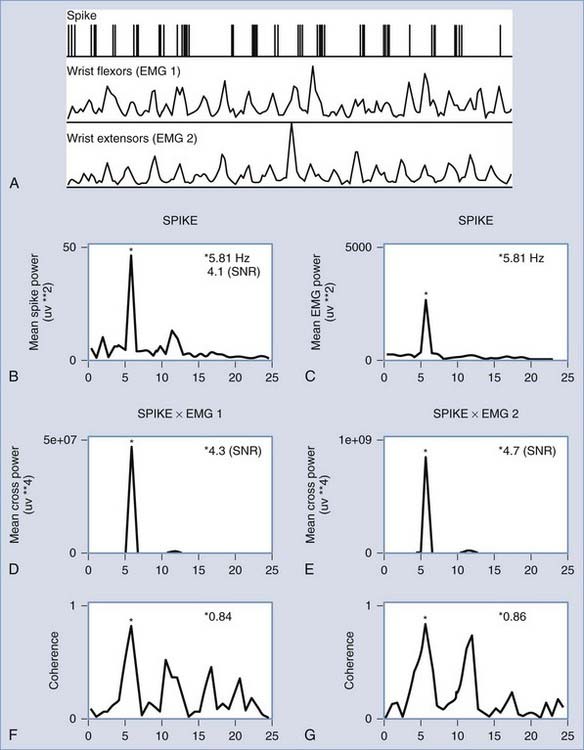
One particular animal model of tremor that reproduces many aspects of human essential tremor is the harmaline-induced tremor.44,45 Recordings in harmaline-treated cats indicate the importance of a cerebellobulbospinal pathway in the maintenance of this tremor.45 However, in humans with essential tremor there is clear thalamic activity that is directly correlated with tremor frequency46 (Fig. 78-1). Specifically, the VIM nucleus (which is the cerebellar relay nucleus) demonstrates the highest proportion of tremor-related neurons in intraoperative recording studies.47 These neurons become more active during voluntary movements, indicating that essential tremor may be facilitated by voluntary motor circuits that enable tremor-related thalamic activity. Additionally, many of these cells respond to proprioceptive input, meaning that sensory feedback from the periphery might also influence this central tremor circuitry.47
Surgical Approach
Targeting by Magnetic Resonance Imaging
Preoperative planning and target localization currently rely on MRI using one of the stereotactic frame-based systems. The MRI sequences used for electrode placement include T1, T2 fast spin echo, three-dimensional gradient echo, and axial inversion recovery images, which are particularly useful for determining target location relative to the midcommissural point of the anterior commissure (AC)–posterior commissure (PC) line or the PC itself.48–50 Target localization by MRI is not error free, and this can have serious clinical implications because of the small size of the target. The STN, for example, has an average dimension of 3 × 6 × 4 mm.51,52
Errors in electrode placement caused by systematic MRI errors have been measured against plain radiographs, which have less distortion. In a recent report on DBS placement accuracy using MRI target acquisition with the Leksell frame, the location of previous electrodes was examined during revision surgeries, and the error was measured using fluoroscopically derived images.53 The latter images of the old electrode position were taken before these electrodes were removed and compared with MRI-derived coordinates taken on the morning of the revision surgery. This study revealed that errors along the superior-inferior axis were generally larger than errors along the left-right and anterior-posterior axes, although all coordinate discrepancies were less than 1 mm. Larger errors were found in another postoperative review of 27 bilateral STN electrode placements.54 The postoperative MRI-derived coordinates of the tip of the electrode differed from the implantation coordinates, on average, by 0.48 mm, 0.69 mm, and 2.9 mm along the left-right, anterior-posterior, and superior-inferior axes, respectively.
Inhomogeneities within the primary gradient field can produce errors in MRI targeting.55 Artifacts can be induced by metal or magnetic susceptibility effects produced at the interface between materials (e.g., air and bone) that have different tendencies to alter the magnetic field in a region. Attempts to decrease errors in the MRI scan caused by these artifacts include software modifications and overlapping (fusion) of the three-dimensional MRI database with a computed tomography database that is not prone to these types of distortions.56 These databases can then be merged with atlas maps of anatomy to estimate the location of nuclei relative to the radiologic images. These atlas maps can be transformed to match either the AC-PC line in isolation or the AC-PC line along with other structures, such as the margins of the third ventricle or the internal capsule.57,58 Another approach is to estimate the target directly from the AC-PC line as determined radiologically. The target for the VIM nucleus used in our clinical practice is 3 mm anterior and 14 mm lateral to the PC and 2 mm above the AC-PC line. Alternatively, the target in the VIM nucleus can be estimated in the lateral plane midway between the internal capsule and the lateral edge of the T2-intense medial dorsal nucleus of the thalamus.59
Intraoperative Localization
Microelectrode recording as an adjunct to MRI-derived anatomic DBS placement plays an important role in improving the accuracy of targeting in stereotactic placement procedures.48,60–62 Useful features of microelectrode recording include differentiation of gray and white matter locations, differentiation of nuclei or subnuclei on the basis of intrinsic neuronal firing properties, localization of white matter tracts with particular responses to stimulation, and real-time correction for intraoperative shifts in implantation sites.49 The neuronal firing patterns detected in the various nuclei and white matter tracts for placement in the STN and VIM nucleus have been extensively reviewed.49,60–64 The microelectrodes typically consist of glass-insulated tungsten or a platinum-iridium alloy with an exposed tip measured in micrometers.48,60 During recording and stimulation, clinicians look for somatosensory-induced effects (e.g., receptive field–evoked responses, movements about joints producing signals, deep dermal receptive fields) as well as stimulation effects on the patient’s tremor or tone as an aid to localization.61–63 Microelectrodes are also used for stimulation during localization passes to map side effects, and some commercially available microelectrodes allow for macrostimulation at currents up to 2 mA. Although microelectrode recording might optimize the implant position, it does not necessarily help in choosing which of the DBS electrode elements should be used for chronic stimulation. In a study of 20 patients with PD undergoing DBS in the STN, there was no correlation between the mapped STN region and the most effective treatment electrode at 3- and 6-month follow-up.65 This may be related to the complexity of the fields produced by stimulation in the brain.66
In our institution, a map of physiologic results is made to the same scale as a set of transparent atlas maps from the sagittal sections of the Schaltenbrand and Bailey atlas.67 Parasagittal sections for different targets are as follows: 13.5-mm lateral atlas map for the VIM nucleus and 11 mm for the STN.67 The fusion and fitting of the imaging studies to the physiologic and atlas maps can also be accomplished through a number of commercially available surgical navigation systems. Microelectrodes for physiologic monitoring and recording are designed to isolate single action potentials60,68,69 and to withstand microstimulation, which degrades the electrode. Typically these characteristics are achieved by constructing electrodes from a platinum-iridium alloy or from tungsten, producing a tapered tip, and insulating with glass.60,69–73 The electrode impedance is usually greater than 500 kOhm,60,68,74 which is required to isolate single units.60
The assembled electrode is attached to a hydraulic or piezoelectric microdrive and mounted on the stereotactic frame. Some microdrive systems incorporate a coarse drive so that overlying structures can be traversed quickly. The tip is then retracted into a protective cylindrical housing while the whole assembly is advanced to a new depth.61 The microdrive can then be used from this new depth for detailed exploration of deeper structures. Another option is to use the microdrive throughout the trajectory, as in many commercial systems (Alpha Omega Co. USA, Alpharetta, GA). The signal from the microelectrode is amplified and filtered. Multiple neuronal discharges of varying sizes can be seen on a digitized trace and heard with the use of an audio monitor. In addition to recording, microstimulation of subcortical structures can be delivered. We employ biphasic, square-wave pulse trains of 0.1- to 0.3-msec pulses for up to 10 seconds at a frequency of 300 Hz.75 The current used in the stimulation determines the amount of local current spread.76
Semi-microelectode recordings can also be carried out using low-impedance microelectrodes with an impedance of less than 100 kOhm. The semi-microelectrode signal is often amplified against a concentric ring electrode that is mounted concentrically around the microelectrode.70,77,78 Recordings through a semi-microelectrode yield recordings of local field potentials (slow waves) or multiunit activity. Bipolar stimulation through a concentric ring electrode can also be used alone or in combination with recording through a semi-microelectrode.10,79,80
Localization of the Ventral Intermediate Nucleus
Sensory cells responding to sensory stimulation in small, well-defined, receptive fields are found in the ventral caudal (VC) nucleus, posterior to the VIM nucleus.63 There is a well-described mediolateral somatotopy within the VC nucleus, proceeding from representations of oral structures medially to the leg laterally.63,68 In the anterior VC nucleus, and further anteriorly in the VIM nucleus, neuronal firing is related to passive joint movement (deep sensory cells) or to active movement (voluntary cells).42,81 Some neurons respond both to active movement and to sensory stimulation such as joint movement (combined cells).33,42 The activity of deep sensory, combined, and voluntary cells is correlated with electromyographic activity during tremor,32,60,82 and they have a somatotopy parallel to that present in the VC nucleus. Stimulation in the VC nucleus evokes somatic sensations.75 Stimulation in the VIM nucleus may produce brief movements or alter ongoing tremor or dystonia.83
Thalamic semi-microelectrode recordings reveal patterns of neuronal activity parallel to those of microelectrode recordings.70,78,84 Macrostimulation through a low-impedance electrode (often <1000 kOhm) can reliably identify the VIM nucleus, by alterations in the movement disorder,10 and the capsule, by stimulation-evoked tetanic contraction of skeletal muscle at a low threshold.10,79 Stimulation of intralaminar nuclei, medial to the VC nucleus or the VIM nucleus, may evoke the recruiting response—long-latency, high-voltage, negative waves occurring over much of the cortex at the frequency of stimulation (usually <10 Hz).85,86
An analysis of the locations of tremor cells suggests that the optimal target for thalamic stimulation is 2 mm anterior to the VC nucleus and 3 mm above the AC-PC line.87 Alternatively, targets in thalamotomy and thalamic stimulation have been placed anterior to the site at which evoked potentials can be recorded in response to cutaneous stimulation of the fingers.88–90 Lesions have been made in the region where electrical stimulation produces effects on tremor and anterior to the region where electrical stimulation evokes sensations.10 Lesions have also been made in the region where cells respond to somatosensory stimulation of muscle, joint, and tendon and where electrical stimulation effects tremor.91
Localization of the Subthalamic Nucleus
Subthalamic exploration is also made from a coronal bur hole about 30 to 35 mm from the midline. Striatum and the VOA thalamus each have a characteristic pattern of neural activity that can be observed with microelectrode recording during mapping.62 As the electrode approaches the STN from an anterior direction, the dorsal striatal cells encountered are characterized by broad action potentials and a slow firing rate (approximately 1 Hz), often with long silent periods. When the thalamus is entered, the action potentials become narrower and often occur in bursts of the low-threshold spike type. Low-threshold spike bursts are preceded by a silent period of 20 to 100 msec and consist of an initial interspike interval of less than 6 msec, followed by interspike intervals of less than 16 msec.30,92 A relatively acellular gap of 1 to 6 mm is observed below the thalamus and above the STN, depending on the anterior-posterior location of the trajectory. The STN is characterized by multiple spike trains recorded from closely packed neurons, each with a mean rate of about 20 msec. Microstimulation evokes paresthesias posterior to the STN, muscle contractions lateral to the STN, and decreases in tremor or tone within STN.
Stimulation Settings
A multidisciplinary team is required for evaluation and clinical decision making in any patient with a movement disorder who is being considered for surgery.93 This clinical team should include a movement disorder neurologist who assists in the initial evaluation of the patient and later with the programming of stimulation parameters.
Outcome Studies
The efficacy of stimulation for the treatment of tremor has been demonstrated in randomized controlled trials94 and in large prospective uncontrolled studies, the latter with 6-year follow-up.95,96 The efficacy of stimulation for the treatment of PD was demonstrated by several prospective uncontrolled trials that demonstrated long-lasting improvements in parkinsonian symptoms, including tremor.13,21,97,98 The safety of this technique was established in these trials and by long-term follow-up of DBS for the treatment of chronic pain.99,100
Essential tremor is the usual indication for DBS therapy in patients with tremor, and the stereotactic target is the VIM nucleus—the cerebellar relay of the thalamus.33,101,102 At present, Parkinson’s tremor is more commonly treated by STN stimulation than by VIM nucleus stimulation.103 In its practice parameters for essential tremor, the American Academy of Neurology recommends the use of DBS primarily in the case of limb tremor; data for voice and head tremor show conflicting results.104
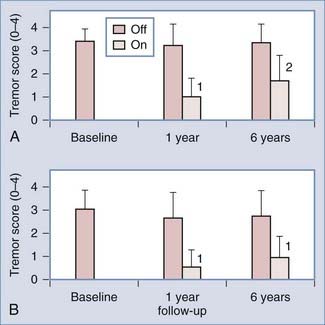
Long-term follow-up (5 to 6 years) of VIM-DBS was obtained from two studies consisting of 13 and 37 patients, respectively.96,105 The smaller study, which was conducted by Kumar and coworkers,105 included 5 patients with essential tremor, 2 of whom had become refractory to stimulation after 2 years of ongoing therapy, despite showing initial improvement. The other 8 patients had PD, and in 3 of these patients, their tremors improved sufficiently for the stimulators to be turned off. In the larger study, Sydow and colleagues96 demonstrated continued improvement in tremor rating in patients with essential tremor, even after 6 years of follow-up (Fig. 78-2). Other multicenter and single-center trials have demonstrated the efficacy and safety of VIM-DBS for treating essential tremor over shorter periods.95,106
Stimulation of the STN alone was demonstrated in a long-term series to be an effective treatment for Parkinson’s tremor, and it has the added benefit of treating the subsequent development of Parkinson’s bradykinesia and rigidity.107,108 Recent evidence indicates that stimulation of the subthalamic region might also be effective for treating essential tremor and torticollis. Specifically, STN stimulation has benefited patients with PD and torticollis, as well as a long history of essential tremor, in those with more than one diagnosis.108,109 Murata and coworkers110 placed stimulators in the posterior white matter of the subthalamic region in eight patients with severe essential tremor and obtained, on average, an 81% reduction in tremor scores. Lesions in the zona incerta were also considered effective for the treatment of tremor during the thalamotomy era.111
Optimal electrode positioning within the VIM nucleus has been investigated in an outcome study of 57 stimulators placed in 37 patients.112 In this group, postoperative Fahn-Tolosa-Marin tremor ratings were recorded and correlated with postoperative MRI DBS positioning within the VIM nucleus. The average optimal position determined by this study was 6.3 mm anterior to the PC, 12.3 mm lateral to the midline, or 10.0 mm lateral to the third ventricle, which placed the electrode at the anterior margin of the VIM nucleus. This is consistent with a postmortem examination of electrode location in a patient who had previously undergone effective DBS for essential tremor. Histologic analysis of the thalamus, including microrecording passes, demonstrated that the effective stimulation contacts were within the VIM nucleus according to both physiologic and anatomic criteria.113
Safety Issues: Complications
Stereotactic procedures have an obligatory risk of intracranial bleeding because the tip of the probe is never visualized as it passes through the brain. Early series of DBS indicated low rates of intracranial bleeding due to the procedure (three symptomatic bleeds in 117 patients, all without permanent deficits).21 A more recent patient study demonstrated symptomatic perioperative bleeds in 3 of 149 (2%) DBS implants.114 Other reported complications included hardware problems such as generator failure or lead breakage (6%; 9 of 149), stimulation-induced side effects (3%; 4 of 149), and behavioral changes (1%; 2 of 149).114 The infection rate is no higher than that for other surgical procedures, but if infection does occur, explantation of the device or generator may be necessary.115
Neurological complications specific to thalamotomy can be explained by lesion-induced disruption of a cerebellothalamocortical pathway or damage to thalamic and nearby structures. In a series of 60 patients with essential, parkinsonian, or cerebellar tremor, functional deficits in the immediate postoperative period were reported in 58% of patients.116 These transient deficits included weakness (34%), dysarthria (29%), ataxia (8%), dystonia (5%), and sensory deficits (3%). Cognitive deficits were also seen, including disorientation and somnolence, as well as speech and language deficits, including hypophonia. Functional deficits persisted in 23% of patients but were generally mild and did not increase disability.116
In a series of 34 patients operated on for parkinsonian tremor, there were persistent complications in 5 patients (14%): apraxia (1), dysarthria (2), dysphasia (1), and abulia (1).89 Transient complications in 61% included cognitive decline (5), central facial (10) or hand (7) weakness, and hand numbness (2). Half the transient deficits resolved by 1 week, and most deficits had resolved at the 3-month follow-up visit.
In the study by Wester and Hauglie-Hanssen,117 12 of 33 (36%) parkinsonian patients had mild complications “not yielding invalidity”: mild mental status changes (4), mild contralateral weakness (3), dysphasia (4), and dysarthria (1).117 Significant complications occurred in 6 of 33 (18%), including mental status changes (3), dysphasia (1), and dysarthria (2). Thus, significant or permanent side effects are often observed in patients with PD, as in those with essential and intention tremor. The best available evidence supports a rate of transient complications in approximately 60% of patients and persistent complications in the range of 15% to 20%.
Stimulation Mechanisms
Commercially available DBS leads are usually 40 cm long, with electrodes along the distal 1 cm.118,119 The diameter of the lead is about 1.3 mm, and the four stimulation electrodes are approximately 1.5 mm long, with spacings that range between 0.5 and 1.5 mm, depending on the model.119 Impedance is about 600 ohm. The adjustable system parameters (and their typical clinical ranges in PD) are the electrode voltage (1 to 3.5 V), pulse width (60 to 210 µsec), and stimulation frequency (130 to 185 Hz).120
Currently, there are only empirical schemes for the selection of optimal stimulus parameters for a given patient, owing to the variability of electrode placement, our lack of understanding of exactly which neural elements are being excited, and the range of clinical responses.120 There is reason to believe that this situation will improve as our understanding of stimulation physiology increases.121,122 For safety purposes, a charge-density limit has been derived for the range of allowed parameters in commercial devices, based on postmortem studies of tissue damage. The charge density is defined as the product of stimulation voltage and pulse width, divided by the impedance multiplied by the surface area of the contact. This implies a limit of 30 µC/cm2.120
The neural elements that are most excitable in response to externally applied electric fields are the myelinated axons and axon initial segments.76,122,123 In finite-element modeling studies of the DBS electrode in realistic axonal orientations derived from diffusion tensor MRI data, it was found that if typical clinical stimulation parameters are used, a large volume of tissue is excited during therapy.123 At 3V, the excitability effect spreads beyond the borders of the STN when it is modeled with realistic surrounding white matter pathways, and the pattern of excitation is consistent with the known stimulation side effects.123
Human brain tissue has variable electrical characteristics owing to its anatomic interleaving of gray matter nuclei and large fiber tracts. Therefore, the excitation volume is not a fixed cylinder around the stimulation electrode; it varies with electrode position.123 Chronaxy time measurements have also been useful for unraveling the direct excitatory effects of micro- and macrostimulation in the central nervous system,124 demonstrating the activation of neuronal somata as well as the predominantly excited axons.
The fact that axonal elements seem to be preferentially excited makes it conceivable that the extranuclear response to DBS is as important as the intrinsic response. This is certainly plausible in the case of a small nucleus like the STN, with its surrounding fiber tracts. DBS effects might also be location specific, depending on the excited cell types, as pointed out by Anderson and coworkers.125 These investigators demonstrated two groups of functionally inactivated thalamic neurons in the rat thalamic slice when stimulating in the ventral nuclei. The type I set exhibited rapid spike firing during stimulation but quickly returned to the baseline membrane potential. The type II set showed chronic depolarization without action potential generation. In both situations, the ongoing activity of these relay cells was dramatically altered. These authors hypothesized that tremor activity might be blocked in an analogous manner because of this stimulation effect on cell synapses.
McIntyre and colleagues126 recently reviewed this problem using the experimental and clinical evidence available for DBS therapeutic mechanisms. This group confirmed that a variety of hyperpolarization and depolarization effects on cell soma results from stimulation, which may lead to an overall suppression of firing from the cell soma because of inhibitory synaptic effects in the case of GPi stimulation.127 To a large extent, the efferent components of the target neurons are excited at the same time and are probably unaffected by these inhibitory synaptic effects. This points toward effective nuclear activation during stimulation just from the activation of efferent axons and could result in a “stimulation-induced modulation of pathological network activity.”126 Another model of PD cause and stimulation efficacy involves the observed pattern of abnormal 11- to 30-Hz oscillations recorded in the basal ganglia of Parkinson’s patients, as well as in experimental animals.128 These authors proposed that stimulating the STN at frequencies greater than 70 Hz results in disruption and suppression of this pathologic pallidal oscillation; there is evidence that it is also suppressed by levodopa.128
Conclusion
The success of DBS surgery for the treatment of movement disorders is multifactorial. First, it is based on recognized limitations in the medical treatment of movement disorders.18,116,129,130 Second, it is based on advances in our understanding of the anatomy and physiology of movement disorders such as essential tremor and PD. Third, it is based on the involvement of neurologists and neurosurgeons in outcome studies of DBS surgery, which include both large prospective trials using validated, blinded measurements and randomized trials. The long-term improvement and development of DBS will depend on our ability to understand its mechanism of action. Whether the stimulation is primarily affecting and suppressing local neuronal activity or whether it acts as an excitatory stimulus to passing axonal fiber pathways makes a large difference in choosing more effective implant locations and tailoring the electric field to provide better stimulation of the important neural elements.126 Additionally, because of the access to thalamic neurophysiology provided by these procedures, inroads are being made in our understanding of the pathophysiology of tremor,46,47 which will also aid therapeutic efforts.
Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403-406.
Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76-84.
Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73-78.
Garonzik IM, Hua SE, Ohara S, Lenz FA. Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord. 2002;17(Suppl 3):S135-S144.
Hariz MI. Complications of deep brain stimulation surgery. Mov Disord. 2002;17(Suppl 3):S162-S166.
Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117-127.
Hua SE, Lenz FA, Zirh TA, et al. Thalamic neuronal activity correlated with essential tremor. J Neurol Neurosurg Psychiatry. 1998;64:273-276.
Kelly PJ, Ahlskog JE, Goerss SJ, et al. Computer-assisted stereotactic ventralis lateralis thalamotomy with microelectrode recording control in patients with Parkinson’s disease. Mayo Clin Proc. 1987;62:655-664.
Kiss ZH, Anderson T, Hansen T, et al. Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur J Neurosci. 2003;18:728-732.
Koller WC, Lyons KE, Wilkinson SB, et al. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16:464-468.
Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934.
Krack P, Pollak P, Limousin P, et al. Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet. 1997;350:1675.
Lemstra AW, Verhagen ML, Lee JI, et al. Tremor-frequency (3-6 Hz) activity in the sensorimotor arm representation of the internal segment of the globus pallidus in patients with Parkinson’s disease. Neurosci Lett. 1999;267:129-132.
Lenz FA, Dostrovsky JO, Tasker RR, et al. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol. 1988;59:299-316.
Lenz FA, Kwan H, Dostrovsky JO, et al. Single unit analysis of the human ventral thalamic nuclear group: activity correlated with movement. Brain. 1990;113:1795-1821.
Lenz FA, Kwan HC, Martin RL, et al. Single neuron analysis of the human ventral thalamic nuclear group: tremor-related activity in functionally identified cells. Brain. 1994;117:531-543.
Lenz FA, Tasker RR, Kwan HC, et al. Cross-correlation analysis of thalamic neurons and EMG activity in parkinsonian tremor. Appl Neurophysiol. 1985;48:305-308.
McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239-1248.
McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40-50.
Merienne L, Mazars G. Treatment of various dyskinesias by intermittent thalamic stimulation. Neurochirurgie. 1982;28:201-206.
Okun MS, Tagliati M, Pourfar M, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centres. Arch Neurol. 2005;62:1250-1255.
Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
Siegfried J, Lippitz B. Chronic electrical stimulation of the VL-VPL complex and of the pallidum in the treatment of movement disorders: personal experience since 1982. Stereotact Funct Neurosurg. 1994;62:71-75.
Sydow O, Thobois S, Alesch F, Speelman JD. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry. 2003;74:1387-1391.
Zirh AT, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience. 1997;83:107-121.
1 Spiegel EA, Wycis HT. Ansotomy in paralysis agitans. Arch Neurol Psychiatry. 1954;71:598-614.
2 Spiegel EA, Wycis HT, Baird HW. Long-range effects of electropallidoansotomy in extrapyramidal and convulsive disorders. Neurology. 1958;8:734-740.
3 Cooper IS. Intracerebral injection of procaine into the globus pallidus in hyperkinetic disorders. Science. 1954;119:417-418.
4 Hassler R. The pathological and pathophysiological basis of tremor and parkinsonism. Proceedings of the Second International Congress of Neuropathology. 1955;2:29-40.
5 Hassler R. The influence of stimulations and coagulations in the human thalamus on the tremor at rest and its physiopathologic mechanism. Proceedings of the Second International Congress of Neuropathology. 1955;2:637-642.
6 Guiot G, Hardy J, Albe-Fessard DG. Delimitation precis des structures sous-corticales et identification de noyaux thalamiques chez l’homme par l’electrophyiologie stereotactic. Neurochirurgia. 1962;5:1-18.
7 Pool JL, Clark WK, Hudson P, Lombardo M. Hypothalamic-hypophysial dysfunction in man. Laboratory and clinical assessment. In: Guillemin R, Guillemin R, Carton CA, editors. Hypothalamic-Hypophysial Interrelationships. Springfield, IL: Thomas; 1956:114-124.
8 Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic and internal capsule stimulation for the control of central pain. Surg Neurol. 1975;4:91-92.
9 Mazars G, Merienne L, Ciolocca C. [Intermittent analgesic thalamic stimulation. Preliminary note]. Rev Neurol (Paris). 1973;128:273-279.
10 Tasker RR, Organ LW, Hawrylyshyn P. The thalamus and Midbrain in Man: A Physiologic Atlas Using Electrical Stimulation. Springfield, IL: Thomas; 1982.
11 Merienne L, Mazars G. Treatment of various dyskinesias by intermittent thalamic stimulation. Neurochirurgie. 1982;28:201-206.
12 Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403-406.
13 Siegfried J, Lippitz B. Chronic electrical stimulation of the VL-VPL complex and of the pallidum in the treatment of movement disorders: personal experience since 1982. Stereotact Funct Neurosurg. 1994;62:71-75.
14 Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76-84.
15 Siegfried J, Lippitz B. Bilateral chronic electrostimulation of the ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126-1130.
16 Levy R. A Short History of Stereotactic Neurosurgery. Park Ridge, IL: American Association of Neurological Surgeons; 1992.
17 Marsden CD, Fahn S. Problems in the dyskinesias. In: Marsden CD, Fahn S, editors. Movement Disorders II. London: Butterworths, 1987.
18 Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet. 1977;1:345-349.
19 Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
20 Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53-61.
21 Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203-214.
22 Clatterbuck R, Lee J-I, Lenz FA. Lesions versus stimulation for the neurosurgical treatment of movement disorders. Prog Neurol Surg. 2000;15:227-235.
23 Pare D, Curro Dossi R, Steriade M. Neuronal basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience. 1990;35:217-226.
24 Elble RJ, Koller W. Tremor. Baltimore: Johns Hopkins University Press; 1990.
25 Lamarre Y, Joffroy AJ. Experimental tremor in monkey: activity of thalamic and precentral cortical neurons in the absence of peripheral feedback. Adv Neurol. 1979;24:109-122.
26 Marsden CD. Origins of normal and pathological tremor. In: Findley LJ, Capildeo R, editors. Movement Disorders: Tremor. London: Macmillan Press Ltd.; 1984:37-85.
27 Stein RB, Lee RG. Tremor and clonus. In: Brooks VB, editor. Handbook of Physiology. Volume II: Motor Control Part II. Bethesda: American Physiological Society; 1981:325-343.
28 Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165-176.
29 Buzsaki G, Smith A, Berger S, et al. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36:1-14.
30 Zirh AT, Lenz FA, Reich SG, Dougherty PM. Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience. 1997;83:107-121.
31 Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549-564.
32 Hua S, Reich SG, Zirh AT, et al. The role of the thalamus and basal ganglia in parkinsonian tremor. Mov Disord. 1998;13(Suppl 3):40-42.
33 Lenz FA, Kwan HC, Martin RL, et al. Single neuron analysis of the human ventral thalamic nuclear group: tremor-related activity in functionally identified cells. Brain. 1994;117:531-543.
34 Lemstra AW, Verhagen ML, Lee JI, et al. Tremor-frequency (3-6 Hz) activity in the sensorimotor arm representation of the internal segment of the globus pallidus in patients with Parkinson’s disease. Neurosci Lett. 1999;267:129-132.
35 Hutchinson WD, Benko R, Dostrovsky JO, et al. Coherent relation of rest tremor to pallidal tremor cells in Parkinson’s disease patients. Mov Disord. 1998;13(Suppl 2):204.
36 Raz A, Fiengold A, Vaadia E, et al. Neuronal oscillations in the globus pallidus of tremulous MPTP-treated monkeys—are they synchronized? Mov Disord. 1998;13(Suppl 2):184.
37 Lenz FA, Tatton WG, Tasker RR. Electromyographic response to displacement of different forelimb joints in the squirrel monkey. J Neurosci. 1983;3:783-794.
38 Lenz FA, Tatton WG, Tasker RR. The effect of cortical lesions on the electromyographic response to joint displacement in the squirrel monkey forelimb. J Neurosci. 1983;3:795-805.
39 Desmedt JE. Cerebral motor control in man: long loop mechanisms. Progress in Clinical Neurophysiology. Vol. 4. Basel: Karger; 1978.
40 Vitek JL, Ashe J, DeLong MR, Alexander GE. Physiologic properties and somatotopic organization of the primate motor thalamus. J Neurophysiol. 1994;71:1498-1513.
41 Strick PL. Activity of ventrolateral thalamic neurons during arm movement. J Neurophysiol. 1976;39:1032-1044.
42 Lenz FA, Kwan H, Dostrovsky JO, et al. Single unit analysis of the human ventral thalamic nuclear group: activity correlated with movement. Brain. 1990;113:1795-1821.
43 Schnider SM, Kwong RH, Lenz FA, Kwan HC. Detection of feedback in the central nervous system using system identification techniques. Biol Cybern. 1989;60:203-212.
44 Lamarre Y. Animal models of physiological, essential, and parkinsonian-like tremors. In: Findley LJ, Capildeo R, editors. Movement disorders: Tremor. New York: Oxford University Press; 1984:183-194.
45 Weiss M. Rhythmic activity of spinal interneurons in harmaline-treated cats. J Neurol Sci. 1982;54:341-348.
46 Hua SE, Lenz FA, Zirh TA, et al. Thalamic neuronal activity correlated with essential tremor. J Neurol Neurosurg Psychiatry. 1998;64:273-276.
47 Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117-127.
48 Garonzik IM, Hua SE, Ohara S, Lenz FA. Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord. 2002;17(Suppl 3):S135-S144.
49 Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118-145.
50 Starr PA, Vitek JL, DeLong M, Bakay RA. Magnetic resonance imaging-based stereotactic localization of the globus pallidus and subthalamic nucleus. Neurosurgery. 1999;44:303-313.
51 Schaltenbrand G, Walker AE. Stereotaxy of the Human Brain. New York: Thieme-Stratton; 1982.
52 Benabid AL, Koudsie A, Benazzouz A, et al. Imaging of subthalamic nucleus and ventralis intermedius of the thalamus. Mov Disord. 2002;17(Suppl 3):S123-S129.
53 Simon SL, Douglas P, Baltuch GH, Jaggi JL. Error analysis of MRI and Leksell stereotactic frame target localization in deep brain stimulation surgery. Stereotact Funct Neurosurg. 2005;83:1-5.
54 Hamid NA, Mitchell RD, Mocroft P, et al. Targeting the subthalamic nucleus for deep brain stimulation: technical approach and fusion of pre- and postoperative MR images to define accuracy of lead placement. J Neurol Neurosurg Psychiatry. 2005;76:409-414.
55 Hardy PA, Barnett GH. Spatial distortion in magnetic resonance imaging: impact on stereotactic localization. In: Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:271-280.
56 Alexander E, Kooy HM, van Herk M, et al. Magnetic resonance image-directed stereotactic neurosurgery: use of image fusion with computerized tomography to enhance spatial accuracy. J Neurosurg. 1995;83:271-276.
57 Dostrovsky JO. The use of inexpensive personal computers for map generation and data analysis. In: Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:2031-2036.
58 Kelly PJ, Ahlskog JE, Goerss SJ, et al. Computer-assisted stereotactic ventralis lateralis thalamotomy with microelectrode recording control in patients with Parkinson’s disease. Mayo Clin Proc. 1987;62:655-664.
59 Hua SE, Garonzik IM, Lee J-I, Lenz FA. Thalamotomy for tremor. In: Winn RH, editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2004:2769-2784.
60 Lenz FA, Dostrovsky JO, Kwan HC. Techniques for microstimulation and recordings of single units and evoked potentials during stereotactic surgery. J Neurosurg. 1988;68:630-634.
61 Vitek JL, Bakay RA, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027-1043.
62 Hutchison WD, Allan RJ, Opitz H, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol. 1998;44:622-628.
63 Lenz FA, Dostrovsky JO, Tasker RR, et al. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol. 1988;59:299-316.
64 Garonzik IM, Ohara S, Hua SE, Lenz FA. Microelectrode techniques: single cell and field potential recordings in the thalamus and basal ganglia. In: Burchiel KJ, editor. Microelectrodes in movement disorder surgery. Stuttgart: Thieme, 2003.
65 Cintas P, Simonetta-Moreau M, Ory F, et al. Deep brain stimulation for Parkinson’s disease: correlation between intraoperative subthalamic nucleus neurophysiology and most effective contacts. Stereotact Funct Neurosurg. 2003;80:108-113.
66 McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40-50.
67 Schaltenbrand G, Bailey P. Introduction to Stereotaxis with an Atlas of the Human Brain. Stuttgart: Thieme; 1959.
68 Jasper HH, Bertrand G. Thalamic units involved in somatic sensation and voluntary and involuntary movements in man. In: Purpura DP, Yahr MD, editors. The Thalamus. New York: Columbia University Press; 1966:365-390.
69 Hubel DH. Tungsten microelectrode for recording from single units. Science. 1957;125:549-550.
70 Ohye C. Neural noise recording in functional neurosurgery. In: Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:941-948.
71 Umbach W, Ehrhardt KJ. Micro-electrode recording in the basal ganglia during stereotaxic operations. Confin Neurol. 1965;26:315-317.
72 Wolbarsht ML, MacNichol EF, Wagner HG. Glass insulated platinum microelectrode. Science. 1960;132:1309-1310.
73 Albe-Fessard DG. Electrophysiological methods for the identification of thalamic nuclei. Z Neurol. 1973;205:15-28.
74 Lozano AM, Hutchison W, Kiss Z, et al. Methods for microelectrode guided posteroventral pallidotomy. J Neurosurg. 1996;84:194-202.
75 Lenz FA, Seike M, Richardson RT, et al. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol. 1993;70:200-212.
76 Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417-440.
77 Taren J, Guiot G, Derome P, Trigo JC. Hazards of stereotaxic thalamectomy. Added safety factor in corroborating x-ray target localization with neurophysiological methods. J Neurosurg. 1968;29:173-182.
78 Burchiel KJ. Thalamotomy for movement disorders. In: Gildenberg PL, editor. Neurosurgery Clinics of North America. Philadelphia: WB Saunders; 1995:55-71.
79 Ojemann GA, Ward AA. Abnormal movement disorders. In: Youmans JR, editor. Neurological Surgery. Philadelphia: WB Saunders; 1982:3821-3857.
80 Laitinen LV, Hariz MI. Movement disorders. In: Youmans JR, editor. Neurological Surgery. Philadelphia: WB Saunders; 1996:3575-3609.
81 Raeva S. Localization in human thalamus of units triggered during verbal commands, voluntary movements, and tremor. Electroencephalogr Clin Neurophysiol. 1986;63:160-173.
82 Lenz FA, Tasker RR, Kwan HC, et al. Cross-correlation analysis of thalamic neurons and EMG activity in parkinsonian tremor. Appl Neurophysiol. 1985;48:305-308.
83 Lenz FA, Jaeger CJ, Seike MS, et al. Thalamic single neuron activity in patients with dystonia: dystonia-related activity and somatic sensory reorganization. J Neurophysiol. 1999;82:2372-2392.
84 Ohye C. Depth microelectrode studies. In: Schaltenbrand G, Walker AE, editors. Stereotaxy of the Human Brain. Anatomical, Physiological and Clinical Applications. Stuttgart: Georg Thieme; 1982:372-389.
85 Jones EG. The Thalamus. New York: Plenum; 1985.
86 Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841-851.
87 Lenz FA, Normand SL, Kwan HC, et al. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord. 1995;10:318-328.
88 Kelly PJ, Derome P, Guiot G. Thalamic spatial variability and the surgical results of lesions placed with neurophysiologic control. Surg Neurol. 1976;9:307-315.
89 Fox MW, Ahlskog EJ, Kelly PJ. Stereotactic ventrolateralis thalamotomy for medically refractory tremor in post-levodopa era Parkinson’s disease patients. J Neurosurg. 1991;75:723-730.
90 Guiot G, Derome P, Arfel G, Walter SG. Electrophysiological recordings in stereotaxic thalamotomy for parkinsonism. In: Krayenbuhl H, Maspes PE, Sweet WH, editors. Progress in Neurological Surgery. Basel: Karger; 1973:189-221.
91 Ohye CH, Narabayashi H. Physiological study of presumed ventralis intermedius neurons in the human thalamus. J Neurosurg. 1979;50:290-297.
92 Ramcharan EJ, Gnadt JW, Sherman SM. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci. 2000;17:55-62.
93 Okun MS, Tagliati M, Pourfar M, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centres. Arch Neurol. 2005;62:1250-1255.
94 Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461-468.
95 Koller WC, Lyons KE, Wilkinson SB, et al. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16:464-468.
96 Sydow O, Thobois S, Alesch F, Speelman JD. Multicentre European study of thalamic stimulation in essential tremor: a six-year follow up. J Neurol Neurosurg Psychiatry. 2003;74:1387-1391.
97 Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925-1934.
98 Kumar R, Lang AE, Rodriguez-Oroz MC, et al. Deep brain stimulation of the globus pallidus pars interna in advanced Parkinson’s disease. Neurology. 2000;55:S34-S39.
99 Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970-1984). J Neurosurg. 1986;64:543-553.
100 Bendok B, Levy RM. Brain stimulation for persistent pain management. In: Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1998:1539-1546.
101 Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117-127.
102 Deuschl G, Raethjen J, Baron R, et al. The pathophysiology of parkinsonian tremor: a review. J Neurol. 2000;247(Suppl 5):V33-V48.
103 Krack P, Pollak P, Limousin P, et al. Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet. 1997;350:1675.
104 Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008-2020.
105 Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601-1604.
106 Lee JYK, Kondziolka D. Thalamic deep brain stimulation for management of essential tremor. J Neurosurg. 2005;103:400-403.
107 Kleiner-Fisman G, Fisman DN, Sime E, et al. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489-495.
108 Stover NP, Okun MS, Evatt ML, et al. Stimulation of the subthalamic nucleus in a patient with Parkinson disease and essential tremor. Arch Neurol. 2005;62:141-143.
109 Chou KL, Hurtig HI, Jaggi JL, Baltuch GH. Bilateral subthalamic nucleus deep brain stimulation in a patient with cervical dystonia and essential tremor. Mov Disord. 2005;20:377-380.
110 Murata J, Kitagawa M, Uesugi H, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003;99:708-715.
111 Mohadjer M, Goerke H, Milios E, et al. Long-term results of stereotaxy in the treatment of essential tremor. Stereotact Funct Neurosurg. 1990;54:125-129.
112 Papavassiliou E, Rau G, Heath S, et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery. 2004;54:1120-1129.
113 Gross RE, Jones EG, Dostrovsky JO, et al. Histological analysis of the location of effective thalamic stimulation for tremor. Case report. J Neurosurg. 2004;100:547-552.
114 Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77:73-78.
115 Hariz MI. Complications of deep brain stimulation surgery. Mov Disord. 2002;17(Suppl 3):S162-S166.
116 Jankovic J, Cardoso F, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for parkinsonian, essential and other types of tremor. Neurosurgery. 1995;37:680-687.
117 Wester K, Hauglie-Hanssen E. Stereotaxic thalamotomy: experiences from the levodopa era. J Neurol Neurosurg Psychiatry. 1990;53:427-430.
118 Finelli DA, Rezai AR, Ruggieri PM, et al. MR imaging-related heating of deep brain stimulation electrodes: in vitro study. AJNR Am J Neuroradiol. 2002;23:1795-1802.
119 Alterman RL, Shils JL, Gudesblatt M, Tagliati M. Immediate and sustained relief of levodopa-induced dyskinesias after dorsal relocation of a deep brain stimulation lead. Case report. Neurosurg Focus. 2004;17:E6.
120 Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115:2431-2441.
121 Kiss ZH, Anderson T, Hansen T, et al. Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur J Neurosci. 2003;18:728-732.
122 McIntyre CC, Mori S, Sherman DL, et al. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589-595.
123 Durand DM. Electric field effects in hyperexcitable neural tissue: a review. Radiat Prot Dosimetry. 2003;106:325-331.
124 Kiss ZH, Wilkinson M, Krcek J, et al. Is the target for thalamic deep brain stimulation the same as for thalamotomy? Mov Disord. 2003;18:1169-1175.
125 Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004;559:301-313.
126 McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239-1248.
127 Dostrovsky JO, Levy R, Wu JP, et al. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570-574.
128 Brown JA. Motor cortex stimulation. In: Schmidt R, Willis WD, editors. Encyclopedia of pain. Berlin: Springer Verlag, 2004.
129 Marsden CD. Problems with long-term levodopa therapy for Parkinson’s disease. Clin Neuropharmacol. 1994;17(Suppl 2):S32-S44.
130 Watts RL, Koller WC. Movement Disorders. New York: McGraw Hill; 1998.
131 Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. Baltimore: Urban and Schwarzenberg; 1988:225-234.