CHAPTER 10 Surgical Management of the Difficult Adult Airway
Failure to maintain a patent airway may result in catastrophic events for the patient, including brain damage or death. In 1990, more than 85% of all respiratory event–related closed malpractice claims involved an anoxic brain injury or death.1 Difficulties with intubation and emergent airway issues remain the leading causes of serious intraoperative complications.2 As many as 30% of deaths attributable to anesthesia involve failure to manage the difficult airway.3 In any given patient, the risk of anoxic injury or death increases in direct proportion to the degree of difficulty in maintaining a patent airway.4 Any scientific or clinical description of the difficult airway must include clearly defined terminology. In this chapter, the difficult airway must be considered as involving three distinct but often clinically related scenarios.
The first of these is difficult tracheal intubation. This condition exists when multiple attempts are needed in the presence or absence of tracheal disease. An infinitely difficult intubation means the trachea cannot be intubated under direct vision, despite full paralysis, optimal head and neck positioning, cricoid pressure, forceful anterior elevation of the laryngoscope blade, and attempts by multiple operators with a variety of laryngoscope blades.4 The incidence of failed endotracheal intubation ranges from 5 to 35 in 10,000 patients.5,6
The second difficult airway scenario is the difficult laryngoscopy. Difficult laryngoscopy is described as not being able to visualize any portion of the larynx, vocal folds, or glottic aperture after multiple attempts at conventional laryngoscopy. Many investigators include grades III and IV or grade IV alone, according to the Cormack-Lehane original grading of the rigid laryngoscopic view.7 For studies of difficult laryngoscopy to be reliable and for the preceding laryngoscopic grading system to be helpful, the reported grades must describe the best view that was obtained, which in turn depends on the best performance of laryngoscopy.4 The technical components that optimize laryngoscopy include optimal position, complete muscle relaxation, firm forward and upward traction on the laryngoscope, and, if necessary, firm external laryngeal manipulation with cricoid pressure. External laryngeal pressure, for example, may reduce the incidence of grade III view from 9% to 1.3%.8 Theoretically, if the preceding technical components of laryngoscopy are used and the pitfalls avoided, all laryngoscopists (novice and expert) should have close to the same laryngoscopic view. Difficult laryngoscopy is synonymous with difficult intubation in most patients. A grade II or III laryngoscopic view requiring multiple attempts with different blades, or both, is relatively common, occurring in 100 to 1800 of 10,000 patients.9 The incidence of a higher grade III laryngoscopic view ranges from 100 to 400 of 10,000 patients.10,11
The third difficult airway scenario is difficult mask ventilation (DMV), which is a condition in which it is not possible to provide adequate face mask ventilation as a result of inadequate mask seal, or excessive resistance due to inadequate patency of airway.4 Langeron and colleagues compiled a list of predictors of difficult mask ventilation based on a survey of anesthesiologists asking them to rate difficulty of face mask ventilation.12 This difficulty was based on whether it was clinically relevant and could have led to potential problems if mask ventilation had to be maintained for a longer time. Six reasons for DMV were identified: (1) inability of the unassisted anesthesiologist to maintain oxygen saturation to greater than 92% using 100% oxygen and positive-pressure mask ventilation, (2) significant gas flow leak by the face mask, (3) necessity to increase the gas flow to greater than 15 L/min and to use the oxygen flush valve more than twice, (4) no perceptible gas movement, (5) necessity to perform a two-handed mask-ventilation technique, and (6) change of operator required.
The incidence of DMV is difficult to estimate in that there has not been a standard definition used universally by researchers, so each study needs to be carefully read to determine the definition used. Langeron and colleagues reported a 5% incidence of DMV with 1 in 1502 patients being impossible to ventilate with a face mask.13 A very significant finding from this study is that DMV conferred a fourfold increased risk for difficult intubation and 12-fold increase in the risk for an impossible intubation.
The incidence of the ultimately critical failed airway, the “can’t intubate, can’t ventilate” situation, can be estimated because it frequently results in anoxic brain injury or death, ranging from 0.01 to 2 of 10,000 patients.14,15 Although the cause of this dire apneic or obstructive state may be completely or partially related to the patient’s disease, there is often an identifiable iatrogenic component. The clinicians caring for the patient in this urgently life-threatening state must be prepared to act swiftly and decisively in order to sustain or recover oxygen saturation and ensure ventilation.
With these definitions in mind, it is important to realize that identification of the difficult airway before manipulation is crucial, and the key step in preparing for optimal patient care and a safe outcome.16 Selection of airway equipment or devices, techniques, and procedures all pivot on airway evaluation. The literature provides strong evidence that specific strategies and communication of these strategies with the airway team facilitate management of the difficult airway. The American Society of Anesthesiologists’ (ASA) algorithm on the management of the difficult airway is an example of such collaborative effort and should act as a foundation for locally developed institution-specific action strategies.17 Refer to the differences between 1993 and 2003 versions of the ASA algorithm (Box 10-1).
Box 10-1 Differences Between 1993 and 2003 ASA Management of the Difficult Airway Algorithms
From Hagberg CA, Benumof JL. The American Society of Anesthesiologists management of the difficult airway algorithm and explanation—analysis of the algorithm. In: Hagberg CA, ed. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:236-251.
Use of the LMA in the difficult airway situation has increased as equipment has evolved to facilitate intubation through the LMA. The success of this device led to the introduction of the intubating LMA (LMA Fastrach) into clinical practice.18,19 Intubation may be accomplished by passing the endotracheal tube (ETT) alone through the LMA (blind technique), or by intubation over a fiberoptic bronchoscope with or without an Aintree catheter placed over the bronchoscope. This technique provides visualization of the intubation and may be combined with videoendoscopy. The addition of the Aintree catheter facilitates intubation after removal of the LMA if this is required to proceed with surgery.
The Hollinger anterior commissure laryngoscope is the otolaryngologist’s most useful tool in difficult airway management, and should be considered when all other techniques have failed. This is particularly true for fixed laryngeal and subglottic lesions where a small cuffless ETT may be passed under direct visualization. The Hollinger scope will accommodate a 5.0 or smaller cuffed ETT, although the insufflation port may become lodged in the barrel of the scope. This should be determined before intubation. An Eschmann stylette may also be passed through the laryngoscope and the ETT passed over the stylette, allowing placement of a larger sized ETT (Figs. 10-1).
Awake Fiberoptic Nasotracheal Intubation
Historical Perspective
Flexible fiberoptic technology has provided the vehicle for developing and perfecting these intubation techniques. A flexible fiberoptic choledochoscope was first used for nasotracheal intubation on a patient with Stills’ disease as early as 1967.29 Five years later, nasotracheal intubation was successfully carried out in a patient with severe rheumatoid arthritis using a fiberoptic bronchoscope (FOB).30 Stiles and colleagues subsequently reported the first series of 100 fiberoptic endotracheal intubations.31 These intubations were performed both orally and nasally, and the authors suggested that with experience, fiberoptic intubation could be performed in less than 1 minute.
Davis and colleagues described the use of FOB for checking the ETT position in relation to carina.32 Use of FOB in assessing ETT position was shown to be as good as chest radiography in both adults and children.33,34 Many other uses of FOB technique were also described, especially in critically ill patients, to evaluate the upper and lower airway.35–38 Several authors cited advantages of the technique, including the ability to perform diagnostic and therapeutic procedures at the bedside without general anesthesia and without interruption of ongoing mechanical ventilation. Another advantage cited was the ability to perform the procedure orally, nasally, or through an in situ ETT or tracheostomy tube. One main disadvantage reported by many authors was the increased airway resistance particularly in pediatric patients because the FOB may occupy a significant portion of the lumen of the tube. FOB was not originally developed for the management of the difficult intubation.39 However, as technology was advanced and visualization improved, anesthesiologists soon appreciated the value of the FOB in the management of the difficult airway.40–43 Use of the FOB has been described for airway management in Ludwig’s angina,44 rheumatoid arthritis,45 trauma,46 unstable cervical spine,47 disruptive injuries of the soft tissue of the neck,48 acromegaly,49 and Pierre Robin syndrome,50 among other conditions. Several authors have reported successful use of the FOB in securing the airway in awake patients with increased risk of aspiration.51–54 As a result, the teaching of fiberoptic airway endoscopy is now an integral part of training for otolaryngology head and neck surgery (OHNS), anesthesiology, and emergency medicine.
Indications
When the airway is compromised or a difficult airway is anticipated, awake fiberoptic intubation (FOI) with or without sedation should be considered (Box 10-2). Awake fiberoptic intubation is an ideal procedure for patients with morbid obesity, supraglottic mass, or edema with the ability to visualize the glottic aperture, risk of aspiration, history of known DMV, patients with limited mandible excursion or trismus, and history of prior difficulty with transoral intubation using other techniques. Contraindications to awake fiberoptic intubation include fixed stenotic lesions at all levels that will not allow passage of an ETT without dilatation, active bleeding obscuring visualization, and acute obstructing supraglottitis, as well as in patients unable to cooperate during the examination. In general, many patients who might be denied general anesthesia or receive a tracheostomy can be safely intubated using the FOB. To intubate patients safely and quickly, certain preparatory steps should be taken.
Bronchoscopy Cart
Proper functioning equipment is essential to a successful outcome. A fully equipped videobronchoscopy cart should be available for use in the intensive care units, emergency department, critical care wards (e.g., otolaryngology patient floor) as well as in the operating room. All equipment and supplies for administration of anesthesia, resuscitation, and monitoring should be available and should be checked following a standard protocol before beginning a nonemergent fiberoptic intubation (Fig. 10-2). For awake intubations, the cart is placed on the left side at the head of the bed while a right-handed operator stands on the right side facing the patient. The light source should be checked for integrity and the necessary supplies are laid out on top of the fiberoptic cart, and suction is connected and at hand. The cart should contain ancillary equipment including nasopharyngeal airway and endotracheal tubes (standard and MLT long tubes), among other items.
Fiberoptic Intubation of the Conscious Patient
The advantages of awake fiberoptic intubation include maintenance of spontaneous ventilation and the ability to position the tip of the ETT precisely beyond the obstruction/compression. It is easiest in the awake near upright patient because the tongue does not fall back in the pharynx, and breathing tends to keep the airway open. In addition, with deep inspiration, the patient can assist the operator in locating the glottis when airway anatomy is distorted. Box 10-3 lists the factors that are crucial for successful completion of an awake fiberoptic intubation. These include training and preparation of the airway team, psychological and pharmacologic preparation of the patient, appropriate monitoring and delivery of oxygen during preparation of the patient and the procedure, expertise of the operator, and a well-functioning bronchoscopy cart.
Box 10-3 Keys to Successful Awake Fiberoptic Intubation
Modified from Wheeler M, Ovassapian A. Fiberoptic endoscopy–aided techniques. In: Hagberg CA, ed. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:399-438.
Preparing the Patient
Pharmacologic
There are three components to the pharmacologic preparation of the patient for awake fiberoptic intubation. These include premedication, limited sedation at the time of intubation, and application of topical anesthesia. The goals of premedication include sedation that augments the psychological preparation and provision of an anti-sialogogue to reduce secretions. Midazolam 1 to 3 mg intramuscularly or 10 to 20 mg by mouth provides adequate sedation in most patients. If the patient’s airway or physical condition is compromised, no sedative or opioid should be given before the patient’s arrival at a monitored setting where the airway team including an anesthesiologist is in attendance. An anti-sialogogue agent is essential for establishing good topical anesthesia and to optimize conditions for fiberoptic visualization of the glottis.55 Glycopyrrolate is considered the agent of choice in that it does not cross the blood-brain barrier and causes less tachycardia.
Conscious Sedation
When possible, conscious sedation is administered in immediate preparation for intubation. The goal of conscious sedation is to have a calm and cooperative patient who can follow verbal commands and maintain adequate oxygenation and ventilation. Depending on the indication for FOI, an opioid, a sedative, or a combination of both is used. A combination of fentanyl (1.5 µ/kg) and midazolam (30 µ/kg) has been used successfully for these procedures. Remifentanil infusion may also be used for sedation for fiberoptic intubation.56,57 This approach may not be indicated in the obese patient with obstructive sleep apnea, because it may precipitate an airway crisis. Use of sedation should be discussed with the anesthesiologist before initiating the procedure.
Topical Anesthesia
Instrumentation of the airway without using topical anesthesia is uncomfortable and distressing to an awake patient. Pharmacologic preparation should, therefore, include application of topical anesthesia. Adequate topical anesthesia eliminates pharyngeal, laryngeal, and tracheobronchial reflexes. Presence of excess secretions dilutes the local anesthetic solution, creates a barrier between the drug and the mucosa, and carries the local anesthetic away from the site of action.58,59 Using an effective antisialogogue is therefore necessary. Furthermore, the absorption rate and amount of topical anesthetic vary according to the site of application, the dose of the drug, and the general condition of the patient.60–62 The agents are absorbed more rapidly from alveoli than from the tracheobronchial tree. The rate and amount absorbed from the pharynx is even lower. The dosage for topical agents should be halved when applying them to the respiratory mucosa because the plasma levels rise more rapidly than do injections into the tissues.54
Nasotracheal Intubation of the Awake Patient
Technique
Intubating fiberoptically via the nasal route is easier than the oral approach because the scope is usually pointed straight at the vocal cords as it enters the oropharynx and is generally better tolerated by the patient.63 Our preferred method is to prepare the nose as described above while setting up the equipment and supplies. The ET tube is placed in warm saline to soften the tube. The cuff is tested by inflation, and to minimize resistance, when deflated the cuff is retracted back so that it is flush with the outer wall of the tube. The nasal passages are first dilated to appropriate diameter with increasingly larger nasal trumpets, which are generously lubricated with lidocaine gel and epinephrine solution (Fig. 10-3). The ET tube is then placed into the nostril, directed downward as it is advanced toward the nasopharynx. If the tube does not make the bend toward the oropharynx, it is pulled back and rotated 90 degrees to the right or left and then reintroduced.
The FOB, which is connected to a videoendoscopy unit, is then passed through the ETT and used to visualize the airway (Fig. 10-4). It is preferable to have the patient’s head elevated and if possible to be sitting near upright to minimize airway collapse; the operator faces the patient and video monitor (Fig. 10-5). The video-assisted FOI provides feedback to those assisting with the procedure and facilitates intubation. In 80% to 85% of patients, the epiglottis and vocal folds are seen with minimal or no manipulation of the tip of the scope. In patients who are not breathing spontaneously or are older, the tongue and pharyngeal tissue may fall back and block the exposure of the larynx. A few maneuvers, including extending the head, applying jaw thrust, or gently pulling the tongue forward, help visualize the cords. Once visualized, application of 3-mL aliquots of 2% lidocaine instilled through the scope should be performed at the level of the vocal folds before intubation and time should be allowed for effect. Patience is extremely helpful at this point and continuous communication with the patient, letting him or her know that all is proceeding as planned, helps minimize anxiety. It is also helpful to have multiple syringes with “slip-tip” preloaded with lidocaine, because repeated injections may be necessary until the patient no longer coughs in response to stimulation. The scope is then advanced through the vocal folds. Timing the entry of the scope with the breathing cycle is a useful trick. Once adequate visualization is achieved, the scope is then advanced into the cervical trachea and additional lidocaine in instilled. The scope is then advanced to a level just above the carina and the ET tube is passed over the scope into position. If there is resistance during this maneuver, it is helpful to rotate the ETT 90 degrees clockwise, changing the orientation of the bevel.64
Avoiding Failure in Fiberoptic Awake Intubation
Causes of Failure
Lack of training and expertise in the use of FOB is the single most common cause of failure in awake fiberoptic intubations. Other causes may be considered under the categories of team factors, patient factors, and equipment issues. These are outlined in Box 10-4.
Pearls to Ensure Successful Awake Fiberoptic Intubation
The training and expertise of the operator and the team is most critical (see Box 10-3 for a list of keys for successful awake intubation). There is no substitute for structured training during residency or fellowship.65,66 Preparing the team and keeping the communication lines open during the entire process helps ensure successful intubation. Maintaining a fully stocked, easily transportable, and functioning FOB cart at hand is highly desirable as well.
Advancing the ET tube into the trachea is not possible when the FOB is passed through the Murphy eye of the ET tube rather than the distal opening.67 Withdrawal of the FOB may be difficult or impossible without damaging the instrument. The scope and the tube should be withdrawn as a unit, the scope disengaged, and the procedure repeated. It is also necessary to use lubrication on the scope to facilitate movement of the tube into and out of the ET tube.68
Operative Surgical Airway Options
Historical Perspectives
The role of surgical airway as a lifesaving procedure has been appreciated for thousands of years.20 Egyptian tablets dating back to 3600 BCE depict surgical tracheostomy.21 Galen in the second century CE described a tracheostomy using a vertical incision as an emergency measure for airway obstruction.22 The first detailed description of tracheostomy was published in the sixteenth century by Vesalius, who proposed using the procedure to ventilate the lungs. The Spanish Inquisition, however, condemned his alleged resuscitation of a Spanish nobleman.23
The first recording of a successful tracheostomy in the United States was in 1852. The patient later died of airway stenosis, which remained a common complication of the procedure for decades to follow. A mortality rate of 50% was quoted for tracheostomy in an 1886 paper with many of these deaths attributed to a high incidence of stenosis.24 A landmark paper by Chevalier Jackson in 1909 described a safe surgical technique and principles that are still applicable.25 He described many factors that he considered to contribute to a lower mortality rate (3%) at the time. These included optimal securing of the airway before the procedure, the use of local anesthesia instead of sedation, using a specifically designed tube, and meticulous surgical technique and postoperative care. In the same paper, Jackson condemned the technique of high tracheostomy, which was the term used for cricothyrotomy at the time. In a later paper in 1921, Jackson described his series of 200 patients with postcricothyrotomy stenosis in patients referred to him. In addition to the obvious referral bias, the primary indication at the time (inflammatory lesions of the upper airway) might have accounted for high incidence of subglottic stenosis.26
After Jackson’s criticism, cricothyrotomy continued to be condemned for more than half a century. It was not until Brantigan and Grow published a series of 655 patients undergoing cricothyrotomy for chronic airway management.27 They described a procedure that was faster, simpler, and less likely to cause bleeding than tracheostomy. The stenosis rate was 0.01% and there were no major complication. Many subsequent studies support their conclusion that cricothyrotomy is a safe and effective surgical procedure and in fact preferable to tracheostomy for emergent airway control.28
Cricothyrotomy
Definition
Cricothyrotomy is the establishment of a surgical opening into the airway through the cricothyroid membrane (CTM) and placement of a tube for ventilation.20 It differs from tracheostomy in the anatomic location of entry into the airway.
Cricothyroid Membrane
The right and left cricothyroid arteries are branches of the right and left superior thyroid arteries. In most patients these vessels cross the superior aspect of the CTM to anastomose in the midline.69 Although at risk for injury during cricothyrotomy, the resulting bleeding is usually self-limiting and easily controlled with gauze packing.
Surgical Cricothyrotomy
Indications
The American Society of Anesthesiologists (ASA) Difficult Airway algorithm recommends a surgical airway as the final endpoint for the unsuccessful arm of the emergency pathway.70 Despite the introduction of numerous rescue devices for failed airway, the most common errors in the management of the difficult airway result from repeated unsuccessful attempts at intubation in these situations.71 Once the “can’t intubate, can’t ventilate” situation has been identified, immediate consideration should be given to a surgical airway. Failure to pursue this approach when unable to ventilate will most likely result in delay in achieving airway control, placing the patient at risk for subsequent hypoxic brain injury and/or death. Often the main obstacle is lack of experience or timely recognition of the need to perform this procedure. Adequate training in the laboratory, planning based on an algorithm and availability of equipment, as well as trained personnel, are the keys to successful performance of the procedure. Availability of necessary equipment, preferably packaged as a preassembled kit, prevents wasting crucial minutes gathering supplies in the emergency situation (Box 10-5).
Relative contraindications for cricothyrotomy include age younger than 10 years of age, severe neck trauma with inability to palpate the landmarks, and expanding neck hematoma. Preexisting laryngeal disease with subglottic extension (e.g., malignancy) is another relative contraindication. A planned urgent awake tracheostomy is preferred in this situation. Emergency or “slash” tracheostomy is a procedure that carries a very high risk of complications (up to 5 times that of elective procedure).72
Surgical Procedure
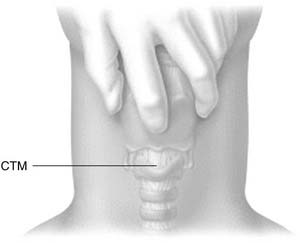
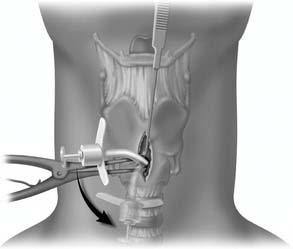
Identification and palpation of the sternal notch, thyroid cartilage (especially the superior thyroid notch), and cricoid cartilage (felt as an indentation below the thyroid cartilage) is the critical first step (Fig. 10-6). Stabilizing the upper airway with the nondominant hand of the operator during the rest of the procedure is the single most important factor determining successful outcome.73 This technique pins the larynx in the midline, facilitating dissection by maintaining anatomic positioning of the airway.
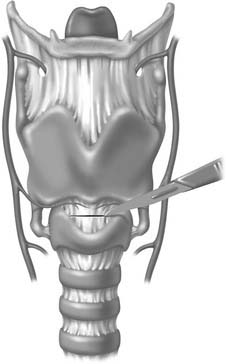
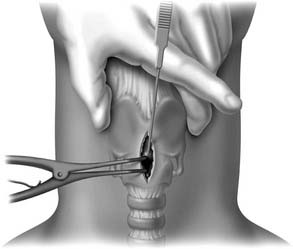
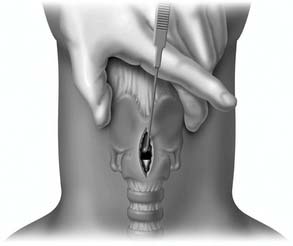
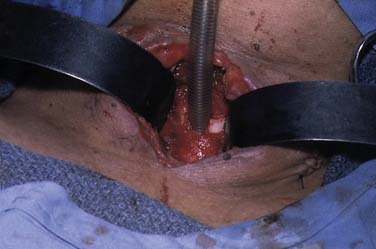
A midline vertical skin incision over the cricoid cartilage avoids the often engorged anterior jugular veins and minimizes the risk of injury to the carotid arteries and jugular veins (Fig. 10-7). The membrane is palpated through the incision (Fig. 10-8) and entered with a horizontal incision at the lower edge of the cricothyroid space (Fig. 10-9). This incision is then dilated with a hemostat or Kelly clamp placed in the airway and opened horizontally while lifting upward to expose the opening (Fig. 10-10). A cricoid hook may be placed at the inferior edge of the thyroid cartilage (Fig. 10-11) to facilitate elevating and opening the airway. A small (5.0) endotracheal tube or a tracheostomy tube is then placed and secured in position (Fig. 10-12). After stabilizing the patient, the cricothyrotomy should be converted to a formal tracheostomy preferably in the operating room if long-term intubation is anticipated. If early extubation is anticipated, the cricothyrotomy may be used until extubation. See reference 74 for basic technique of cricothyrotomy.
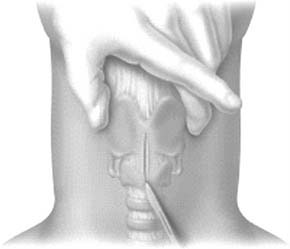
Figure 10-7. A vertical skin incision is made down to, but not through, the airway.
(From Walls RM, Murphy MF, Luten RC, et al. Manual of Emergency Airway Management. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004.)
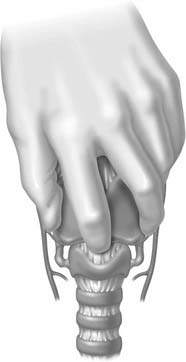
Figure 10-8. The index finger is used to directly palpate and relocate the cricothyroid membrane.
(From Walls RM, Murphy MF, Luten RC. Manual of Emergency Airway Management. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004.)
Rapid Five-Step Technique
A rapid four-step technique has been described and reported to be simple to learn and faster in obtaining a surgical airway.75 This technique has many advantages, including (1) the need for less equipment, (2) the need for only one operator without assistance, (3) the positioning is such that the operator stands at the head end of the patient.76 The authors prefer a slight modification and use a five-step rule.
The incidence of complications reported after cricothyrotomy varies from 6% to 39% depending on whether the procedure was done semielectively or as an emergent procedure. Reported complications include bleeding, trauma, or injury to the laryngeal framework and/or vocal folds (Fig. 10-13), mediastinal emphysema, pneumothorax, false passage with the tube placed in the mediastinum, esophageal laceration, and failure to secure the airway.
Percutaneous Cricothyrotomy
Primarily based on modified Seldinger technique, many commercially available kits and systems can help place a tracheal airway with limited surgical dissection beyond the skin incision. Although deemed simpler than surgical technique by many nonsurgeons, the procedure increases complexity and requires execution of more than five steps to secure an airway. Although no significant differences in performance times between open surgical cricothyrotomy and the percutaneous procedure have been noted in cadaver and dog models, as with the open cricothyrotomy, severe complications are more likely to occur in the emergent situation.77,78 One of the limitations of percutaneous cricothyrotomy, especially with the commercially available kits, is the relatively small lumen of the cannula.76
Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of difficult airway. Anesthesiology. 1989;71:769.
Brofeldt BT, Panacek EA, Richards JR. An easy cricothyrotomy approach: the rapid four-step technique. Acad Emerg Med. 1996;3(11):1060-1063.
Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: A closed claim analysis. Anesthesiology. 1990;72:828.
Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105-1111.
Edens ET, Sia RL. Flexible fiberoptic endoscopy in difficult intubations. Ann Otol Rhinol Laryngol. 1981;90(4 Pt 1):307-309.
Fasting S, Gisvold SE. Serious intraoperative problems—a five year review of 83,844 anesthetics. Can J Anaesth. 2002;49:545.
Gibbs MA, Walls RM. Surgical airway. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:678-696.
Hagberg CA, Benumof JL. The American Society of Anesthesiologist’s management of the difficult airway algorithm and explanation—analysis of the algorithm. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:236-251.
Holmes JF, Panacek EA, Sakles JC, et al. Comparison of 2 cricothyrotomy techniques: standard method versus rapid 4-step technique. Ann Emerg Med. 1998;32(4):442-446.
Hsiao J, Pacheco-Fowler V. Videos in clinical medicin. Cricothyroidotomy. N Engl J Med. 2008;358(22):e25.
Jackson C. High tracheostomy and other errors: The chief cause of chronic laryngeal stenosis. Surg Gynecol Obstet. 1921;32:392.
Klock PA, Benumof JL. Definition and incidence of the difficult airway. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:215-220.
Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92(5):1229-1236.
Ovassapian A, Yelich SJ, Dykes MH, et al. Fiberoptic nasotracheal intubation—incidence and causes of failure. Anesth Analg. 1983;62(7):692-695.
, 2003 Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98(5):1269-1277.
Rehm CG, Wanek SM, Gagnon EB, et al. Cricothyroidotomy for elective airway management in critically ill trauma patients with technically challenging neck anatomy. Crit Care. 2002;6(6):531-535.
Schwartz HC, Bauer RA, Davis NJ, et al. Ludwig’s angina: use of fiberoptic laryngoscopy to avoid tracheostomy. J Oral Surg. 1974;32(8):608-611.
Wilson ME, Spiegelhalter D, Robertson JA, et al. Predicting difficult intubation. Br J Anaesth. 1988;61(2):211-216.
1. Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: a closed claim analysis. Anesthesiology. 1990;72:828.
2. Fasting S, Gisvold SE. Serious intraoperative problems—a five year review of 83,844 anesthetics. Can J Anaesth. 2002;49:545.
3. Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of difficult airway. Anesthesiology. 1989;71:769.
4. Klock PA, Benumof JL. Definition and incidence of the difficult airway. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:215-220.
5. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434.
6. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42(5):487-490.
7. Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105-1111.
8. Wilson ME, Spiegelhalter D, Robertson JA, et al. Predicting difficult intubation. Br J Anaesth. 1988;61(2):211-216.
9. Finucane BT, Santora AH. Difficult Intubation, 2nd ed. St Louis: Mosby–Year Book; 1996.
10. Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth. 1994;41(5 Pt 1):372-383.
11. Williams KN, Carli F, Cormack RS. Unexpected, difficult laryngoscopy: a prospective survey in routine general surgery. Br J Anaesth. 1991;66(1):38-44.
12. Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92(5):1229-1236.
13. Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92(5):1229-1236.
14. Eichhorn JH. Documenting improved anesthesia outcome. J Clin Anesth. 1991;3(5):351-353.
15. Keenan RL, Boyan CP. Decreasing frequency of anesthetic cardiac arrests. J Clin Anesth. 1991;3(5):354-357.
16. Cass NM, James NR, Lines V. Difficult direct laryngoscopy complicating intubation for anaesthesia. BMJ. 1956;1(4965):488-489.
17. Hagberg CA, Benumof JL. The American Society of Anesthesiologist’s management of the difficult airway algorithm and explanation—analysis of the algorithm. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:236-251.
18. Benumof JL. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84(3):686-699.
19. Brain AI, Verghese C, Addy EV, et al. The intubating laryngeal mask. I: Development of a new device for intubation of the trachea. Br J Anaesth. 1997;79(6):699-703.
20. Gibbs MA, Walls RM. Surgical airway. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:678-696.
21. Mace SE. Blunt laryngotracheal trauma. Ann Emerg Med. 1986;15(7):836-842.
22. Boyle MF, Hatton D, Sheets C. Surgical cricothyrotomy performed by air ambulance flight nurses: a 5-year experience. J Emerg Med. 1993;11(1):41-45.
23. Morain WD. Cricothyroidostomy in head and neck surgery. Plast Reconstr Surg. 1980;65(4):424-428.
24. Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest. 1985;87(6):715-719.
25. Jackson C. Tracheotomy. Laryngoscope. 1909;18:285.
26. Jackson C. High tracheostomy and other errors: the chief cause of chronic laryngeal stenosis. Surg Gynecol Obstet. 1921;32:392.
27. Brantigan CO, Grow JBSr. Cricothyroidotomy: elective use in respiratory problems requiring tracheotomy. J Thorac Cardiovasc Surg. 1976;71(1):72-81.
28. Gillespie MB, Eisele DW. Outcomes of emergency surgical airway procedures in a hospital-wide setting. Laryngoscope. 1999;109(11):1766-1769.
29. Murphy P. A fibre-optic endoscope used for nasal intubation. Anaesthesia. 1967;22(3):489-491.
30. Conyers AB, Wallace DH, Mulder DS. Use of the fiber optic bronchoscope for nasotracheal intubation: case report. Can Anaesth Soc J. 1972;19(6):654-656.
31. Stiles CM, Stiles QR, Denson JS. A flexible fiber optic laryngoscope. JAMA. 1972;221(11):1246-1247.
32. Davis NJ. A new fiberoptic laryngoscope for nasal intubation. Anesth Analg. 1973;52(5):807-808.
33. Dietrich KA, Strauss RH, Cabalka AK, et al. Use of flexible fiberoptic endoscopy for determination of endotracheal tube position in the pediatric patient. Crit Care Med. 1988;16(9):884-887.
34. O’Brien D, Curran J, Conroy J, et al. Fibre-optic assessment of tracheal tube position. A comparison of tracheal tube position as estimated by fibre-optic bronchoscopy and by chest X-ray. Anaesthesia. 1985;40(1):73-76.
35. Fan LL, Flynn JW. Laryngoscopy in neonates and infants: experience with the flexible fiberoptic bronchoscope. Laryngoscope. 1981;91(3):451-456.
36. Fan LL, Sparks LM, Dulinski JP. Applications of an ultrathin flexible bronchoscope for neonatal and pediatric airway problems. Chest. 1986;89(5):673-676.
37. Lindholm CE, Ollman B, Snyder JV, et al. Cardiorespiratory effects of flexible fiberoptic bronchoscopy in critically ill patients. Chest. 1978;74(4):362-368.
38. Olopade CO, Prakash UB. Bronchoscopy in the critical-care unit. Mayo Clin Proc. 1989;64(10):1255-1263.
39. Ikeda S. Atlas of Flexible Bronchofiberoscopy. Baltimore: University Park; 1974.
40. Ovassapian A, Yelich SJ, Dykes MH, et al. Fiberoptic nasotracheal intubation—incidence and causes of failure. Anesth Analg. 1983;62(7):692-695.
41. Ovassapian A, Yelich SJ, Dykes MH, et al. Blood pressure and heart rate changes during awake fiberoptic nasotracheal intubation. Anesth Analg. 1983;62(10):951-954.
42. Ovassapian A, Dykes MH, Golmon ME. A training programme for fibreoptic nasotracheal intubation. Use of model and live patients. Anaesthesia. 1983;38(8):795-798.
43. Ovassapian A, Yelich SJ, Dykes MH, et al. Fiberoptic nasotracheal intubation—incidence and causes of failure. Anesth Analg. 1983;62(7):692-695.
44. Schwartz HC, Bauer RA, Davis NJ, et al. Ludwig’s angina: use of fiberoptic laryngoscopy to avoid tracheostomy. J Oral Surg. 1974;32(8):608-611.
45. Rogers SN, Benumof JL. New and easy techniques for fiberoptic endoscopy-aided tracheal intubation. Anesthesiology. 1983;59(6):569-572.
46. Mulder DS, Wallace DH, Woolhouse FM. The use of the fiberoptic bronchoscope to facilitate endotracheal intubation following head and neck trauma. J Trauma. 1975;15(8):638-640.
47. Sidhu VS, Whitehead EM, Ainsworth QP, et al. A technique of awake fibreoptic intubation. Experience in patients with cervical spine disease. Anaesthesia. 1993;48(10):910-913.
48. Davies JR. The fibreoptic laryngoscope in the management of cut throat injuries. Br J Anaesth. 1978;50(5):511-514.
49. Ovassapian A, Doka JC, Romsa DE. Acromegaly—use of fiberoptic laryngoscopy to avoid tracheostomy. Anesthesiology. 1981;54(5):429-430.
50. Scheller JG, Schulman SR. Fiber-optic bronchoscopic guidance for intubating a neonate with Pierre Robin syndrome. J Clin Anesth. 1991;3(1):45-47.
51. Edens ET, Sia RL. Flexible fiberoptic endoscopy in difficult intubations. Ann Otol Rhinol Laryngol. 1981;90(4 Pt 1):307-309.
52. Keenan MA, Stiles CM, Kaufman RL. Acquired laryngeal deviation associated with cervical spine disease in erosive polyarticular arthritis. Use of the fiberoptic bronchoscope in rheumatoid disease. Anesthesiology. 1983;58(5):441-449.
53. Nakayama M, Kataoka N, Usui Y, et al. Techniques of nasotracheal intubation with the fiberoptic bronchoscope. J Emerg Med. 1992;10(6):729-734.
54. Wheeler M, Ovassapian A. Fiberoptic endoscopy–aided techniques. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2006:399-438.
55. Bowes WAIII, Johnson JO. Pneumomediastinum after planned retrograde fiberoptic intubation. Anesth Analg. 1994;78(4):795-797.
56. Puchner W, Obwegeser J, Puhringer FK. Use of remifentanil for awake fiberoptic intubation in a morbidly obese patient with severe inflammation of the neck. Acta Anaesthesiol Scand. 2002;46(4):473-476.
57. Puchner W, Egger P, Puhringer F, et al. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand. 2002;46(4):350-354.
58. Reed AP, Han DG. Preparation of the patient for awake fiberoptic intubation. Anesth Clin North Am. 1991;9:69.
59. Ovassapian A, Wheeler M. Fiberoptic Endoscopy-Aided Techniques. Benumof’s Management: Principles and Practice, 1st ed. St Louis: Mosby; 1996.
60. Patterson JR, Blaschke TF, Hunt KK, et al. Lidocaine blood concentrations during fiberoptic bronchoscopy. Am Rev Respir Dis. 1975;112(1):53-57.
61. Bourke DL, Katz J, Tonneson A. Nebulized anesthesia for awake endotracheal intubation. Anesthesiology. 1985;63(6):690-692.
62. Ovassapian A, Yelich SJ, Dykes MH, et al. Fiberoptic nasotracheal intubation—incidence and causes of failure. Anesth Analg. 1983;62(7):692-695.
63. Ovassapian A, Yelich SJ, Dykes MH, et al. Blood pressure and heart rate changes during awake fiberoptic nasotracheal intubation. Anesth Analg. 1983;62(10):951-954.
64. Katsnelson T, Frost EA, Farcon E, et al. When the endotracheal tube will not pass over the flexible fiberoptic bronchoscope. Anesthesiology. 1992;76(1):151-152.
65. Ovassapian A, Dykes MH, Golmon ME. A training programme for fibreoptic nasotracheal intubation. Use of model and live patients. Anaesthesia. 1983;38(8):795-798.
66. Ovassapian A, Yelich SJ, Dykes MH, et al. Fiberoptic nasotracheal intubation—incidence and causes of failure. Anesth Analg. 1983;62(7):692-695.
67. Ovassapian A. Failure to withdraw flexible fiberoptic laryngoscope after nasotracheal intubation. Anesthesiology. 1985;63(1):124-125.
68. Siegel M, Coleprate P. Complication of fiberoptic bronchoscope. Anesthesiology. 1984;61(2):214-215.
69. Leibovici D, Fredman B, Gofrit ON, et al. Prehospital cricothyroidotomy by physicians. Am J Emerg Med. 1997;15(1):91-93.
70. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98(5):1269-1277.
71. Rehm CG, Wanek SM, Gagnon EB, et al. Cricothyroidotomy for elective airway management in critically ill trauma patients with technically challenging neck anatomy. Crit Care. 2002;6(6):531-535.
72. Goldenberg D, Ari EG, Golz A. Tracheotomy complications: a retrospective study of 1130 cases. Otolaryngol Head Neck Surg. 2000;123:495-500.
73. DiGiacomo C, Neshat KK, Angus LD, et al. Emergency cricothyrotomy. Mil Med. 2003;168:541-544.
74. Hsiao J, Pacheco-Fowler V. Videos in clinical medicine. Cricothyroidotomy. N Engl J Med. 2008;358(22):e25.
75. Holmes JF, Panacek EA, Sakles JC, et al. Comparison of 2 cricothyrotomy techniques: standard method versus rapid 4-step technique. Ann Emerg Med. 1998;32(4):442-446.
76. Gibbs MA, Walls R. Surgical airway. In: Hagberg CA, editor. Benumof’s Airway Management. 2nd ed. Philadelphia: Mosby Elsevier; 2009:678-696.
77. Bainton CR. Cricothyrotomy. Int Anesthesiol Clin. 1994;32(4):95-108.
78. Brofeldt BT, Panacek EA, Richards JR. An easy cricothyrotomy approach: the rapid four-step technique. Acad Emerg Med. 1996;3(11):1060-1063.