Chapter 76 Surgical Management of Terminal Basilar and Posterior Cerebral Artery Aneurysms
Background
In the early 1940s, following surgery for a posterior fossa aneurysm that ultimately took the life of the patient, Walter Dandy stated, “I know of no successful outcome from operative attack upon an aneurysm of the posterior cranial fossa, but for those upon the vertebral and posterior inferior cerebellar arteries, which afford good exposure, cures will certainly come in time.”1
The first description of a basilar aneurysm dates back to 1779, when Morgagni presented a case of an aneurysm involving the basilar artery and both posterior cerebral arteries (PCAs).2 Little progress was made concerning the identification and treatment of posterior circulation aneurysms until the mid-20th century. In 1954, Herbert Olivecrona was the first surgeon to describe clipping of a basilar artery aneurysm through a subtemporal approach.3 It was during this time that Charles Drake emerged as a pioneer in the surgical management of basilar tip aneurysms. In 1959, he presented his experience with four patients presenting with a subarachnoid hemorrhage (SAH) from basilar bifurcation aneurysms at the American Academy of Neurological Surgeons in Pebble Beach, CA.4 Although two of his patients succumbed to complications due to their initial hemorrhage, his case description laid the groundwork for standards of BA aneurysm treatment. Review of the literature over 2 decades prior to the publication of Drake’s paper had identified 38 cases of aneurysms involving the vertebrobasilar (VB) circulation. Following Drake’s publication, a plethora of literature were published concerning the treatment and outcome of basilar aneurysms. Kenneth Jamieson reviewed his operative experience of 19 patients with VB aneurysms.5 Only four of those patients made sufficient recovery to return to work. As Drake’s experience with basilar aneurysms grew, his results also improved in parallel.
Part of his success was also attributable to advances in microinstruments and microsurgical technique, including the use of the surgical microscope. Ultimately, these factors culminated in improved surgical outcome for Drake’s patients harboring BA aneurysms. In a 1968 report, Drake described his experience after treating 17 basilar artery aneurysms with only one fatality.6 One year later, Drake presented a review of 43 operative cases involving the VB circulation with 70% of patients attaining “satisfactory outcome.”7 As part of his legacy, Drake published his 25 years of experience treating 1767 aneurysms of the VB system 2 years prior to his death in 1998.8 The vast majority of these patients (1286) harbored aneurysms at the basilar bifurcation or PCAs. Drake reported excellent outcomes in 70% to 80% of his patients who initially presented with a good-grade SAH and those who harbored nongiant BA aneurysms. Drake’s 1996 chapter continues to be the standard that other series involving basilar artery aneurysms are judged against.
Neuroanesthesia
Improvements in the field of neuroanesthesia have allowed neurosurgeons to more safely attack aneurysm in the BA region. Our patients undergo a thorough preoperative evaluation by the anesthesiology team that includes review of the patients’ relevant medical history, as well as their general surgical risk factors. Most patients receive anxiolytic medications (e.g., benzodiazepines) prior to transport into the operating room. The patient may also receive light opioid sedation before insertion of a radial arterial line (many patients also receive a central venous catheter). After placement of standard monitors (pulse oximeters, electrocardiogram leads, noninvasive blood pressure cuff, and temperature probe), induction of anesthesia is performed. An intravenous anesthetic agent such as propofol or thiopental is typically administered, along with opioids and intravenous lidocaine, to blunt the hemodynamic response to intubation.
We do not routinely perform electroencephalography or brain stem auditory evoked potentials (BAEPs) when treating BA aneurysms. An exception is made when we have treated “giants” or complex BA aneurysms using complete circulatory arrest. This technique has been shown to be a useful adjunct in the treatment of complex intracranial aneurysms, particularly when prolonged cerebral hypoperfusion is needed.9,10 In these situations, we use electroencephalography, spontaneous somatosensory evoked potentials (SSEPs), and BAEPs, in conjunction with deep hypothermic (18°C) circulatory arrest. The suppression of electroencephalogram activity by barbiturates is used to titrate an effective dose for cerebral protection. The SSEPs are an indicator of intact sensory pathway conduction and persist despite burst suppression. A BAEP is a useful tool, especially if manipulation of brain stem structures is anticipated during the procedure.
Surgical Strategies for BA Aneurysms
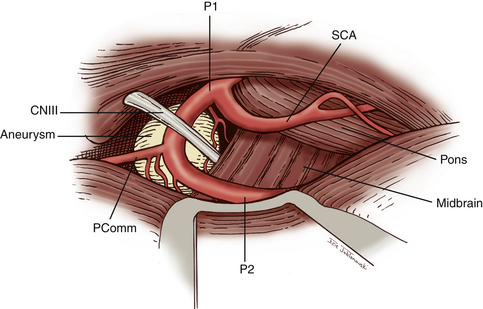
Surgical treatment of BA aneurysms is extremely challenging due to the complex anatomy in and around the interpeduncular cistern. Surgeons are forced to navigate through deep and narrow channels that make visualization of the anatomy in this region particularly difficult. The basilar tip and PCAs are located in the confined spaces of the interpeduncular cistern. They are enclosed by the posterior clinoids and clivus anteriorly, mesiotemporal lobes laterally, cerebral peduncles posteriorly, and mammillary bodies superiorly. The BA is approximately 15 mm posterior to the internal carotid artery (ICA).11 The termination of the basilar artery gives rise to bilateral PCAs. The superior cerebellar arteries (SCAs) arise immediately proximal to the basilar bifurcation. The oculomotor nerve exits between the PCAs and the SCAs and is therefore vulnerable to injury during surgery. The segment of the PCA from its origin from the BA to the ostium of the posterior communicating artery (Pcomm) is referred to as the P1 segment of the PCA. The PCA distal to the Pcomm is also known as the P2 segment. The P2 segment extents from the Pcomm to the posterior edge of the midbrain. Flow to the PCA territory may be predominantly from the Pcomm in cases of a fetal Pcomm that occurs in 15% to 40% of the population. Anterior thalamoperforators typically arise from the Pcomm. These vessels supply a portion of the cerebral peduncles, posterior thalamus, subthalamic nucleus, optic chiasm, tuber cinereum, and mammillary bodies. The posterior thalamoperforators usually arise from the BA or the proximal P1 segments. These vessels supply the thalamus, hypothalamus, posterior limb of the internal capsule, and subthalamic nucleus. There may be considerable variation in the configuration and areas supplied by the anterior and posterior thalamoperforators. Due to the extent of the vascular territories supplied by these vessels, compromise of any of these perforators can lead to devastating results.
Approaches for BA Aneurysms
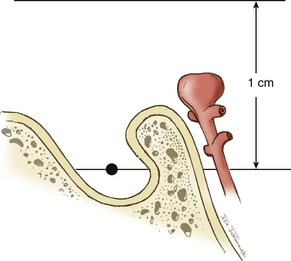
The approach to BA aneurysms largely relies on the relationship of the basilar bifurcation to the sella (Fig. 76-1). Multiple surgical approaches may be used to treat aneurysms in the BA region. These craniotomy routes include subtemporal, orbitozygomatic, and pterional craniotomies. These approaches describe an increasingly anterior trajectory to approaching basilar bifurcation aneurysms. Aneurysms arising at or below the middle depth of the sella are best approached via a subtemporal craniotomy, often combined with additional skull base techniques such as removal of the petrous apex (Kawase approach).12 Aneurysms associated with a high bifurcation (more than 1 cm above the posterior clinoids) can be treated via an orbitozygomatic craniotomy that allows for better superior visualization due to more aggressive bone removal. Aneurysms that arise above the sella and up to 1 cm above the clinoids are accessible via a trans-sylvian pterional craniotomy. We have employed all three techniques in treating BA aneurysms at our institution. However, we favor the use of a pterional trans-sylvian craniotomy, in combination with some elements of the subtemporal craniotomy (a half-and-half approach), when targeting lesions in this location.
Subtemporal Approach
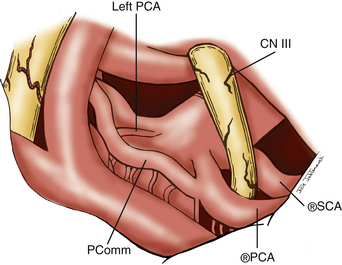
The subtemporal approach was popularized by Peerless and Drake, who were pioneers in successfully treating aneurysms in the basilar bifurcation region.13,14 This approach targets the aneurysm from a lateral trajectory as the temporal lobe is elevated. We utilize this approach for aneurysms originating from below the middle depth of the sella turcica and for posteriorly projecting aneurysms. Advantages to the subtemporal approach are many. Lateral trajectory facilitates visualization and dissection of posterior perforators, especially for posteriorly projecting aneurysms. Preservation of these perforators is perhaps the most crucial objective of these procedures. Proximal control is also easy to obtain with this approach. As the surgeon is working along the axis of the aneurysm neck, aneurysm clips can be placed with optimal visualization of the aneurysm neck as well as the thalamoperforators. Lastly, division of the tentorium and even removal of the petrous apex through this approach allows for exposure of the upper third of the clivus for access to low-lying bifurcations. The subtemporal craniotomy does have some disadvantages: there is poor visualization of the contralateral P1; cranial nerve III (CN III) is centered in the field, which often leads to postoperative oculomotor nerve palsies; and excessive retraction may lead to temporal lobe injury.
Positioning and Scalp Incision
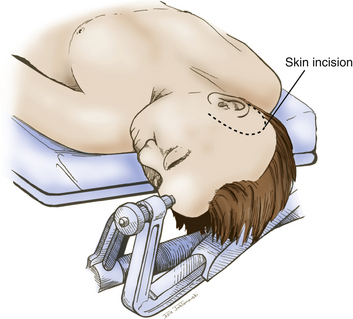
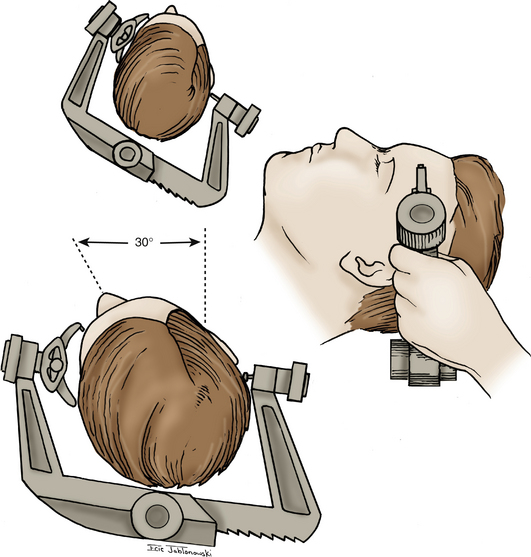
The patient is positioned supine with a shoulder roll placed on the right side. The head is fixed in a radiolucent Mayfield headrest. One pin is positioned over the forehead, and two pins are positioned over the occiput (Fig. 76-2). The head is rotated until the midline plane parallels the floor. The head is angled approximately 15 to 20 degrees until the floor of the middle cranial fossa is parallel to the line of sight. This maneuver also allows the temporal lobe to fall away from the middle cranial fossa, minimizing retraction during the procedure.
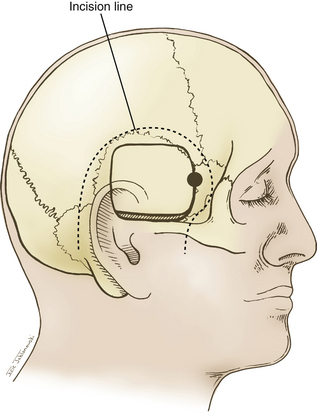
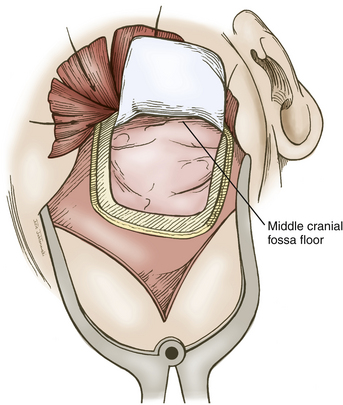
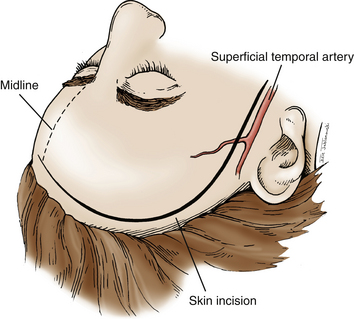
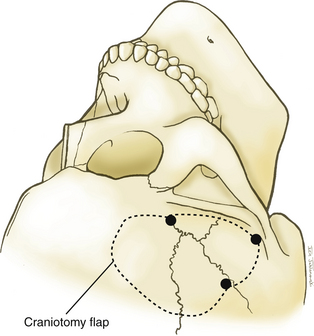
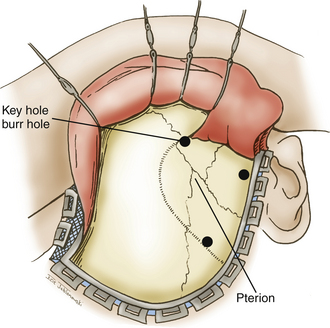
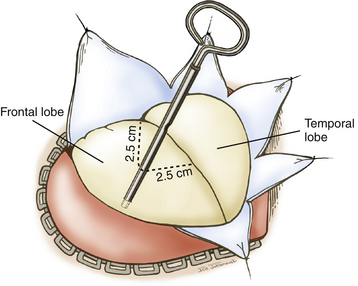
A question mark–shaped incision is made over the right temporal region (Fig. 76-3). The incision originates 1 cm anterior to the tragus and extends superiorly approximately 4 cm. The incision is then curved anteriorly to a point 1 cm behind the hairline. The line then follows the path of the superior temporal line, terminating just superior and posterior to the mastoid. The scalp flap is retracted anteriorly and secured with fishhooks connected to a Leyla bar. The temporalis muscle is divided and retracted anteriorly. An approximately 4 × 4 cm bone flap is made, based over the zygomatic root, using a high-speed pneumatic drill. Our craniotomies are slightly larger for patients with a SAH. We drill the temporal bone inferiorly and anteriorly until it is flush with the floor of the middle fossa to visualize the middle fossa floor, as well as the temporal tip. The dura is incised in a square based at the floor of the middle fossa (Fig. 76-4). The dura is retraced and secured to the overlying tissue using a 3-0 Vicryl suture. A ventricular catheter or lumbar drain, if present, is opened, allowing for cerebrospinal fluid (CSF) drainage and brain relaxation. If sufficient brain relaxation is not achieved with CSF diversion, diuresis or hyperventilation subpial resection of the inferior temporal gyrus may be performed. The Budde Halo retraction system (Integra) is used to elevate the temporal gyrus, exposing the tentorial incisura. Incising the tentorium may be necessary for low-lying aneurysms. The surgical microscope is now brought into the field. It may be helpful at this point to place a 3-0 Vicryl suture into the free edge of the tentorium, anchoring it to the floor of the middle cranial fossa. This maneuver provides 3 to 4 mm of additional exposure in the surgical field.
Subarachnoid Dissection and Clip Application
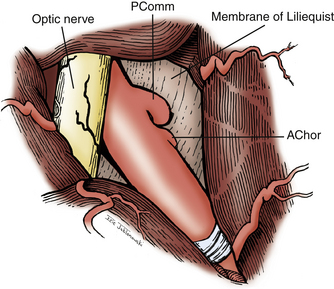
Attention is now directed to dissecting through the subarachnoid space. The arachnoid between the third CN and the uncus is excised. The dissection is extended forward below the course of CN III. This arachnoid continues anteriorly, forming a band that runs medially across the interpeduncular fossa, known as the membrane of Liliequist. This membrane is sharply divided across the anterior aspect of the pons to allow for clot removal in the interpeduncular fossa if necessary. The SCA is identified and followed around the curve of the cerebral peduncle to the basilar artery. Arachnoid surrounding the basilar artery is cleared circumferentially in preparation for temporary clip placement. The inferior aspect of the ipsilateral P1 is identified. The P1 is followed medially across the basilar artery until the contralateral P1 is also identified. CN III can be seen passing between the SCAs and the PCAs. The dissection is now concentrated to the posterior aspect of the aneurysm complex. Perforators arising from the posterior aspect of the basilar artery often adhere to the neck and dome of the aneurysm. It is crucial to identify these vessels and dissect them off of the aneurysm (Fig. 76-5). The great advantage of the subtemporal approach arises from these important perforators potentially being easier to identify through a lateral approach. At this time, if temporary clip occlusion is needed, burst suppression is initiated by our anesthesiologists and the blood pressure is pharmacologically elevated. Temporary clipping of the basilar artery allows for more aggressive dissection of the aneurysm neck, perforators, and P1 arteries. Once the neck is well visualized, an aneurysm clip compatible with magnetic resonance imaging is placed across the neck of the aneurysm. A standard straight clip is used for anteriorly or posteriorly projecting aneurysms. However, most frequently, aneurysms in this location project superiorly. In these instances, a fenestrated clip is utilized. The aperture of the clip is used to enclose the P1 and any perforators, while the clip blades trap the neck of the aneurysm.
Orbitozygomatic Approach
The orbitozygomatic approach is similar to a pterional craniotomy with the additional removal of the superior and lateral portions of the orbit. The origins of the orbitozygomatic approach can be traced back to the description of the supraorbital craniotomy by Jane et al.15 This approach subsequently evolved to its contemporary form through additional modifications by several authors.16–18 The orbitozygomatic approach provides several advantages over the traditional trans-sylvian approach due to the increased bone removal at the skull base. The orbitozygomatic approach provides a better operative trajectory, provides a surgical field that is shallower than both the subtemporal and the trans-sylvian approaches, and requires less brain retraction. Due to the additional bone work, the orbitozygomatic approach may pose additional risks. These risks include frontalis nerve injury, extraocular muscle injury, and pulsatile enophthalmos. The orbitozygomatic approach offers extensive exposure of the anterior and middle cranial fossae by the removal of the superior and lateral portions of the orbit. As discussed previously, this exposure leads to a more anterior trajectory and a more upward view of the BA regions compared to the standard pterional approach. We utilize this approach for high-bifurcating aneurysms that are located 1 cm or more above the posterior clinoids. The orbitozygomatic craniotomy allows the surgeon’s line of sight to pass from the globe and angle superiorly. We used a modified one-piece orbitozygomatic approach similar to that described by Lemole et al.19
Patient positioning is similar to that used for standard pterional craniotomies. Our scalp flap is a slight modification to the pterional flap. The scalp incision is extended across the midline to the contralateral midpupillary line adjacent to the hairline (Fig. 76-6). The temporalis muscle and fascia are dissected and retracted anteriorly, with the scalp flap connected to fishhooks and a Leyla bar. The orbitozygomatic modification poses little risk to injury of the frontalis branch of the facial nerve, since the malar eminence and the zygomatic root are not exposed. A standard pterional bone flap is made with a high-speed pneumatic drill (Fig. 76-7). The craniotomy stops at the orbital rim lateral to the supraorbital notch. An isolated area of bone remains between the supraorbital rim and the bur hole at the anatomic keyhole. The orbitotomy cuts must be made from within the orbit, since the intracranial compartment has not been entered. Two additional cuts are made to the pterional craniotomy with a reciprocating saw for a modified one-piece orbitozygomatic approach. The first cut extends from the orbital rim lateral to the supraorbital notch and connects to the craniotomy superiorly. The second cut extends from the frontozygomatic suture over the orbital rim toward the bur hole at the pterion. Twisting and countertraction with a Penfield dissector may be necessary to extend fractures along the osteotomy lines to connect the cuts.
The intradural exposure of the modified orbitozygomatic approach is the same as in a standard pterional approach. The advantage with this approach is that the basilar region is viewed more easily due to the extra space created by the supraorbital osteotomy. The fulcrum of the trajectory is lower when the orbital roof is removed. This provides the surgeon with a higher view above the posterior clinoids.
Extended Lateral (Half-and-Half) Approach
We employ a half-and-half approach for the vast majority of our basilar tip aneurysms. This approach represents a hybrid, combining the advantages of the trans-sylvian and subtemporal approaches while minimizing the limitations associated with each approach. Variations of the extended lateral approach have been previously described in the literature.20
The half-and-half approach has many similarities to the standard pterional craniotomy pioneered by Yasargil.21,22 In addition to the pterional craniotomy, an extensive subtemporal craniectomy is performed, providing exposure of the anterior temporal tip and the floor of the middle cranial fossa. The additional bone removal allows the surgeon to mobilize the temporal lobe superiorly and laterally, providing the surgeon with a wider corridor for approaching BA aneurysms.
Positioning and Scalp Incision
The patient is placed supine on the operating room table, with the head placed in rigid pin fixation. We use a radiolucent headrest that allows unobstructed views during intraoperative angiography. A single pin is placed over the left frontal region, and two pins are placed in the right mastoid and occipital region. The head is rotated approximately 30 degrees to the left with slight neck extension, placing the malar eminence at the highest point of the operating field (Fig. 76-8). The Budde Halo retraction system is secured to the headrest in preparation for retraction once the craniotomy has been performed. The microscope and surgical chair are also draped and ready for use prior to skin incision. The incision begins 1 cm anterior to the tragus at the root of the zygomatic arch. The incision curves forward gently past the midline to where the hairline intersects with the contralateral midpupillary line. The scalp flap is elevated anteriorly with blunt and sharp dissection, leaving the fascia of the temporalis muscle intact. The skin flap is retracted using fishhooks connected to a Leyla bar. The subgaleal fat pad is identified in the pterional region. The temporalis muscle and fascia are divided posterior to the fat pad and perpendicular to the zygomatic root to avoid injury to the frontalis branch of the facial nerve. The muscle is also divided along the superior temporal line, leaving a fascial cuff to facilitate reapproximation of the muscle later in the procedure. The posterior limb of the muscle is retracted posteriorly and is secured using a 2-0 Vicryl suture. The anterior limb of the dissected muscle is retracted anteriorly with the scalp flap and secured using the fishhook–Leyla construct (Fig. 76-9).
Craniotomy
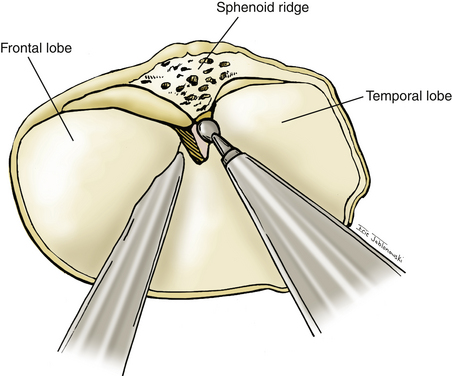
A high-speed pneumatic drill is used to perform the craniotomy. Bur holes are placed at the posterior extent of the superior temporal line, the keyhole, and the root of the zygoma. A craniotome is used to connect the bur holes, making a circular bone flap with its superior edge extending to the level of the ipsilateral midpupillary line. The posterior edge extends to the edge of the scalp flap. The inferior edge is marked by the root of the zygoma, and the anterior border is marked by the sphenoid ridge. A round cutting bur is used to perform an extensive subtemporal craniectomy that allows us to visualize the anterior temporal tip. The same drill attachment is used to generously drill the lateral sphenoid wing flat to the level of the superior orbital fissure (Fig. 76-10). Once the bone work is complete, hemostasis is achieved using bone wax and oxidized cellulose.
Subarachnoid Dissection and Clip Application
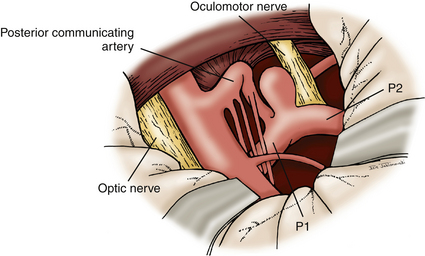
The dura is opened in a curvilinear fashion with an anteriorly based flap and secured anteriorly using a 3-0 Vicryl suture. The dural opening extends from the anterior extent of the exposed frontal fossa, curves posteriorly around the proximal origin of the sylvian fissure, and terminates posteroinferiorly in the middle cranial fossa. If further relaxation is needed and a ventriculostomy is not in place, one is inserted at Paine’s point, allowing CSF drainage from the lateral ventricles.23 This technique typically provides the relaxation needed for proper brain mobilization (Fig. 76-11). The surgical microscope is brought into the field at this time. The operating table is lowered, and the operating chair is positioned for optimal comfort. The sylvian fissure is opened in a distal to proximal fashion, identifying the M2 branch of the middle cerebral artery. The dissection is continued medially until the anterior temporal branch is identified. A subfrontal brain retractor is used to elevate the frontal lobe, stretching the arachnoid of the deep sylvian fissure. The dissection is carried proximally and medially in the sylvian fissure until the optic nerve and supraclinoid ICA are visualized. Microdissectors and microscissors are used to dissect and clearly visualize the Pcomm, anterior choroidal artery, CN III, and ICA terminus (Fig. 76-12). The opticocarotid and prechiasmatic cisterns are opened sharply to free the frontal lobe further. Attention is then directed to elevating the temporal lobe posteriorly and laterally out of the middle cranial fossa. The bridging veins connecting the temporal lobe to the floor of the middle cranial fossa are coagulated and cut, avoiding potential bleeding during the critical clipping period. CN III is disconnected from its connection to the uncus. Retractors are used to elevate the frontal lobe medially and the temporal lobe laterally, providing a wide working corridor. Further arachnoid dissection is performed medial to the carotid, in the retrocarotid space along the Pcomm, and in the supracarotid region. The membrane of Liliequist is opened to provide clear visualization of the interpeduncular cistern. The Pcomm is followed to its junction with the PCA (P1–P2 junction). The SCA is identified and followed to its junction with the basilar trunk. The basilar artery is dissected free of its arachnoid adhesions in preparation for temporary clipping. The P1 segment is then identified above CN III and followed medially to the BA (Fig. 76-13). Dissection of the arachnoid around the BA also brings the contralateral P1 into view.
Once these vessels are identified, attention is given to the posterior perforators that arise from the proximal P1 segments and the BA. We typically ask our anesthesiologist to initiate burst suppression at this time. A temporary clip is placed across the basilar trunk lateral to CN III. From this point, the dissection can be more aggressive as the proximal and distal neck of the aneurysm is identified. Gentle traction with the microsuction can be placed on the fundus of the aneurysm to displace it anteriorly. This maneuver allows better visualization of the posterior thalamoperforators that need to be dissected off of the aneurysm and displaced posteriorly. Utilizing the half-and-half approach, dissection of the thalamoperforators occurs as it would during a subtemporal approach.
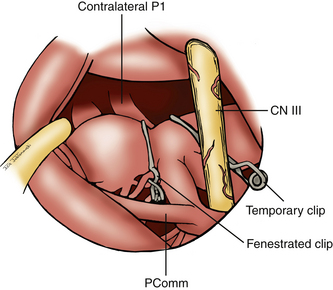
Several techniques can be used to clip aneurysm of the BA region (Fig. 76-14). It is typically necessary to use multiple clips to secure the neck of these aneurysms. Smaller aneurysm can typically be clipped using standard straight clips placed in tandem. Using the suction tip, the aneurysm is retracted anteriorly. The posterior blade of the clip is visualized first as it passes across the neck above the contralateral P1. Once the posterior blade is in position, the anterior blade is closed to trap the distal neck of the aneurysm. Once the initial clip is applied, it may be necessary to place a second clip above the initial one to completely secure the aneurysm. We routinely use intraoperative indocyanine green videography and intraoperative angiography following clipping to evaluate perforators, larger vessels, and any residual aneurysm. Alternatively, for larger aneurysms with a wide neck, we initially use a fenestrated clip. The clip is often placed with its fenestration enclosing the ipsilateral P1 and the proximal neck. The blades are passed across the aneurysm to secure the distal neck. A second standard straight clip is then used to clip the remaining aneurysm, pushing the blades until they are adjacent to the fenestration of the first clip.
PCA Aneurysms
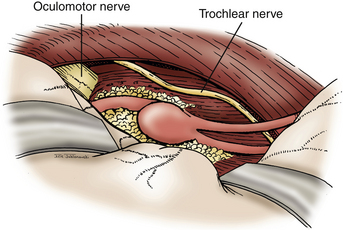
For proximal PCA aneurysms, a standard pterional or a half-and-half approach provides sufficient exposure for clip ligation (Fig. 76-15). As with basilar tip aneurysms, careful attention must be given to dissecting perforators from the aneurysm neck and dome prior to clip placement. For anterior temporal artery aneurysms, we typically employ a subtemporal approach. These aneurysms may be hidden under the hippocampus, which may require partial resection of that gyrus (Fig. 76-16). It is important to visualize the PCA proximally from the P1 and follow it laterally to avoid disorientation, which commonly occurs in this region. The trochlear nerve should be identified along the tentorial hiatus and moved laterally to avoid injury during the procedure. Large aneurysms in this location can be treated with parent vessel occlusion distal to the origin of the choroidal arteries. Thalamoperforators and thalamogeniculate arteries typically have originated prior to the origin of the anterior temporal artery. In addition, the PCA territory has a rich collateral supply, allowing occlusion of the distal P2 with only minor risk for ischemic injury. Aneurysm of the more distal PCA segment (P3) cannot be treated by the approaches described here. These lesions may be treated by an interhemispheric occipital approach.
Endovascular Therapy for Management of BA Aneurysms
The introduction of GDCs in 1990 for treatment of saccular aneurysms introduced a new chapter in the field of cerebrovascular surgery.24 The GDC technology provided a completely new approach to treating intracranial aneurysms. No longer was it necessary to treat every aneurysm by a major craniotomy procedure, particularly challenging ones in the posterior circulation. The use of GDCs for aneurysms did not gain wide acceptance until the milestone publication of the International Subarachnoid Aneurysm Trial (ISAT).25 That study reported an absolute risk reduction of 6.9% for dependence or death when comparing patients who underwent endovascular therapy (23.7%) versus craniotomy (30.6%) for their aneurysms. Although the risk of rebleeding was higher in the endovascular group (4.2%) at 1 year and 7 years (seven patients) compared to the surgical group (3.6%) after 1 year and 7 years (two patients), the mortality rate after 7 years continued to be greater for the surgical group.26 This data from ISAT helped mainstream the use of endovascular therapy for treatment of saccular aneurysms. The International Study of Unruptured Intracranial Aneurysms in 2003 further supported endovascular therapy as a treatment modality for cerebral aneurysms.27 The investigators concluded that for any posterior circulation aneurysm 12 mm or larger, poor outcome was significantly greater in the surgical group compared to the endovascular group. Successful endovascular therapy for treatment of BA aneurysms has been well documented in the literature.28–30 The procedural morbidity and mortality rates range from 3% to 9%. This has made coil embolization of these aneurysms the first line of treatment at many institutions. However, with reported recurrence rates as high as 17.5%, coiling of these aneurysms may not be the definitive treatment in every instance. In addition, with recurrence rates greater than 50% for large and giant BA aneurysms, endovascular therapy is not suitable for these larger lesions.
Wide-neck aneurysms of the BA have posed another challenge for neurointerventionalists. Initially, these aneurysms were difficult to embolize due to their poor dome-to-neck ratio (less than 2:1). In an effort to address some of these limitations, innovation led to new technology. The U.S. Food and Drug Administration approved the use of the Neuroform (Smart Therapeutics) and Enterprise (Cordis Endovascular) vascular construction devices (VCDs) under their Humanitarian Device Exemption in 2002 and 2007, respectively. These devices have enabled interventionalists to treat aneurysms that were not previously amenable to coil embolization. There have been limitations with the use of these devices as well. The use of these VCDs is limited in patients with SAHs. The Neuroform and Enterprise VCDs require the use of dual antiplatelet therapy for the prevention of in-stent stenosis. The use of antiplatelet medications has been shown to have unacceptable complication rates associated with new hemorrhages when used in patients with ruptured aneurysms.31
Baumgartner W.A., Silverberg G.D., Ream A.K., et al. Reappraisal of cardiopulmonary bypass with deep hypothermia and circulatory arrest for complex neurosurgical operations. Surgery. 1983;94:242-249.
Bendok B.R., Getch C.C., Parkinson R., et al. Extended lateral transsylvian approach for basilar bifurcation aneurysms. Neurosurgery. 2004;55:174-178.
Drake C.G. Bleeding aneurysms of the basilar artery: Direct surgical management in four cases. J Neurosurg. 1961;23:230.
Drake C.G. Further experience with surgical treatment of aneurysms of the basilar artery. J Neurosug. 1968;29:372.
Drake C.G. The surgical treatment of vertebral–basilar aneurysms. Clin Neurosurg. 1969;16:114-169.
Drake C.G., Peerless S.J., Hernesniemi J.A.. Surgery of Vertebrobasilar Aneurysms: London, Ontario Experience on 1767 Patients, Vienna, Springer-Verlag, 1996:300-329
Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach; a new surgical technique. Surg Neurol. 1986;26:271-276.
Jamieson K.G. Aneurysms of the vertebrobasilar system: surgical intervention in 19 cases. J Neurosurg. 1964;21:781.
Kawase T., Toya S., Shiobara R., Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
Lemole G.M.Jr., Henn J.S., Zabramski J.M., et al. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99:924-930.
Molyneux A.J., Kerr R.S., Yu L.M., et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group: International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
Paine J.T., Batjer H.H., Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
Peerless S.J., Drake C.G. Management of aneurysms of the posterior circulation. In: Youmans J.R., editor. Neurological Surgery: A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems. Philadelphia: WB Saunders; 1990:1764-1806.
Peluso J.P., van Rooij W.J., Sluzewski M., Beute G.N. Coiling of basilar tip aneurysms: results in 154 consecutive patients with emphasis on recurrent hemorrhage and retreatment during mid and long term follow-up. J Neurology, Neurosurg, and Psych. 2008;79:706-711.
Samson D.S., Hodosh R.M., Clark W.K. Microsurgical evaluation of the pterional approach to aneurysms of the distal basilar circulation. Neurosurgery. 1978;3:135-141.
Spetzler R.F., Hadley M.N., Rigamonti D., et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868-879.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. International Study of Unruptured Intracranial Aneurysms Investigators: Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
Yasargil M. Operative anatomy. In: Yasargil M., editor. Microneurosurgery. Stuttgart: George Thieme Verlag; 1984:5.
Yasargil M.G., Antic J., Laciga R., et al. Microsurgical pterional approach to aneurysm of the basilar bifurcation. Surg Neurol. 1976;6:83-91.
Zabramski J.M., Kiris T., Sankhla S.K., et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
1. Schwartz H.G. Arterial aneurysms of the posterior fossa. J Neurosurg. 1948;5:312.
2. Morgagni J.B. De sedibus et causis morborum per antomen indagatis libri quinque. Switzerland: Embrun; 1779. lib.1, epist. 4,9
3. Hook O., Norlen G., Guzman J. Saccular aneurysms of the vertebral–basilar arterial system. A report of 28 cases. Acta Neurol Scand. 1963;39:271-304.
4. Drake C.G. Bleeding aneurysms of the basilar artery: direct surgical management in four cases. J Neurosurg. 1961;23:230.
5. Jamieson K.G. Aneurysms of the vertebrobasilar system: surgical intervention in 19 cases. J Neurosurg. 1964;21:781.
6. Drake C.G. Further experience with surgical treatment of aneurysms of the basilar artery. J Neurosug. 1968;29:372.
7. Drake C.G. The surgical treatment of vertebral–basilar aneurysms. Clin Neurosurg. 1969;16:114-169.
8. Drake C.G., Peerless S.J., Hernesniemi J.A.. Surgery of Vertebrobasilar Aneurysms: London, Ontario Experience on 1767 Patients, Vienna, Springer-Verlag, 1996:300-329
9. Baumgartner W.A., Silverberg G.D., Ream A.K., et al. Reappraisal of cardiopulmonary bypass with deep hypothermia and circulatory arrest for complex neurosurgical operations. Surgery. 1983;94:242-249.
10. Spetzler R.F., Hadley M.N., Rigamonti D., et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868-879.
11. Samson D.S., Hodosh R.M., Clark W.K. Microsurgical evaluation of the pterional approach to aneurysms of the distal basilar circulation. Neurosurgery. 1978;3:135-141.
12. Kawase T., Toya S., Shiobara R., Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
13. Peerless S.J., Drake C.G. Management of aneurysms of the posterior circulation. In: Youmans J.R., editor. Neurological Surgery: A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems. Philadelphia: WB Saunders; 1990:1764-1806.
14. Drake C.G. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96-144.
15. Jane J.A., Park T.S., Pobereskin L.H., et al. The supraorbital approach: technical note. Neurosurgery. 1982;11:537-542.
16. Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach; a new surgical technique. Surg Neurol. 1986;26:271-276.
17. Pellerin P., Lesoin F., Dhellemmes P., et al. Usefulness of the orbitofrontomalar approach associated with bone reconstruction for frontotemporosphenoid meningiomas. Neurosurgery. 1984;15:715-718.
18. Zabramski J.M., Kiris T., Sankhla S.K., et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
19. Lemole G.M.Jr., Henn J.S., Zabramski J.M., Spetzler R.F. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99:924-930.
20. Bendok B.R., Getch C.C., Parkinson R., et al. Extended lateral transsylvian approach for basilar bifurcation aneurysms. Neurosurgery. 2004;55:174-178.
21. Yasargil M. Operative anatomy. In: Yasargil M., editor. Microneurosurgery. Stuttgart: George Thieme Verlag, 1984. p 5
22. Yasargil M.G., Antic J., Laciga R., et al. Microsurgical pterional approach to aneurysm of the basilar bifurcation. Surg Neurol. 1976;6:83-91.
23. Paine J.T., Batjer H.H., Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
24. Guglielmi G., Vinuela F., Dion J., Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:1-14.
25. Molyneux A.J., Kerr R.S., Yu L.M., et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2002;366(9488):809-817.
26. Molyneux A.J., Kerr R.S., Yu L.M., Clarke M., et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809-817.
27. Wiebers D.O., Whisnant J.P., Huston J.III, et al. International Study of Unruptured Intracranial Aneurysms Investigators: Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
28. Klein G.E., Szolar D.H., Leber K.A., et al. Basilar tip aneurysm: endovascular treatment with Guglielmi detachable coils—midterm results. Radiology. 1997;205:191-196.
29. Eskridge J.M., Song J.K. The participants: endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81-86.
30. Peluso J.P., van Rooij W.J., Sluzewski M., Beute G.N. Coiling of basilar tip aneurysms: results in 154 consecutive patients with emphasis on recurrent hemorrhage and retreatment during mid and long term follow-up. J Neurology, Neurosurg, and Psych. 2008;79:706-711.
31. Tumialan L.M., Zhang Y.J., Cawley C.M., et al. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: a cautionary report. J Neurosurg. 2008;108:1119-1121.