Chapter 60 Surgical Management of Spinal Dysraphism
The term “spinal dysraphism” describes many different forms of congenital malformations of the neural tube.∗ Table 60-1 classifies dysraphic malformations according to accepted theories of embryogenesis and conveniently divides most of them into primary and secondary neurulation lesions, plus an additional class of preneurulation malformations whose basic error of embryogenesis probably occurred before primary neurulation. The surgical repair of these malformations varies as widely as their morbid anatomy. The “surgical” classification in Table 60-2 therefore has less to do with embryogenesis, structural characteristics, neurology, or region of involvement within the neuraxis, than with whether the lesion is “open” or “closed” in its external boundary, a factor that strongly influences the timing and urgency of surgery. A transitional class incorporates lesions that may have “limited” exposure to the outside, although their main features are mostly enclosed. Each class consists of malformations that have radically dissimilar features. The difference in surgical techniques necessitates individual description under the appropriate lesion heading.
Table 60-1 Classification of Spinal Dysraphic Malformations According to Theories of Embryogenesis
| Primary Neurulation Malformations |
| Open neural tube defect, terminal and segmental |
| Spinal cord lipomas (dorsal and transitional) |
| Limited dorsal myeloschisis (LDM) |
| Dermal sinus tract (cyst) |
| Secondary Neurulation Malformation |
| Caudal agenesis (caudal cell mass abnormalities) |
| Thickened or fatty filum |
| Spinal cord lipomas (terminal, chaotic?) |
| Terminal myelocystocele |
| Retained medullary cord |
| Malformation of Gastrulation |
| Split cord malformations, types I and II |
Table 60-2 Classification of Spinal Dysraphic Malformations According to Surgical Significance
| Open Dysraphism |
| Open neural tube defect with terminal neural placode |
| Open neural tube defect with segmental neural placode |
| Closed Dysraphism |
| Spinal cord lipomas |
| Dorsal |
| Transitional |
| Chaotic |
| Terminal |
| Thickened filum |
| Split cord malformations, types I and II |
| Caudal agenesis and associated caudal spinal cord malformations |
| Terminal myelocystocele |
| Retained medullary cord |
| Transitional Forms of Dysraphism |
| Limited dorsal myeloschisis |
| Dermal sinus tract (cyst) |
Open Spinal Dysraphism
Embryology and Morbid Anatomy
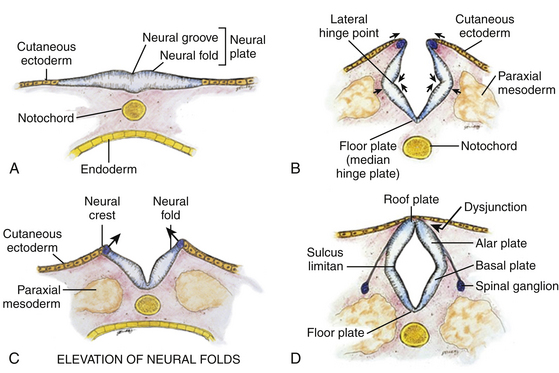
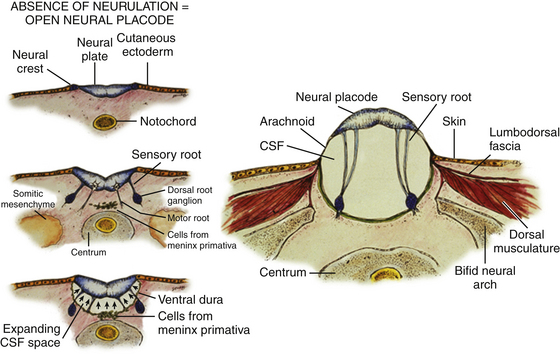
Normal development of the spinal cord begins around postovulatory day (POD) 22 to 23, when the neural groove deepens and the neural folds meet in the dorsal midline to form the primary neural tube (eFigs. 60-1 and 60-2).∗ The dorsal midline fusion of the neural folds proceeds in a “zipper-fashion” both caudally and rostrally beginning near the sixth cervical somite. This phase of development, called primary neurulation, ends with the formation of the lower lumbar cord segments opposite somites 30/31 around POD 28. As the primitive streak shortens to almost nothing with elongation of the primary neural tube, the caudal cell mass, a cluster of pluripotent primitive stem cells appearing around POD 27 to 28 (O’Rahilly and Muller’s stage 12),2 begins forming (among other caudal embryonic tissues) a solid cord of future neural cells called the medullary cord.2 This connects with the primary neural tube, and then undergoes central vacuolization (cavitation) to form a secondary neural canal. This second phase of development, called secondary neurulation, culminates in the conus from somites 30/31 downward. A final process of degeneration involving extensive apoptosis of the coccygeal segments of the medullary cord occurs resulting in the filum terminalis.3–5
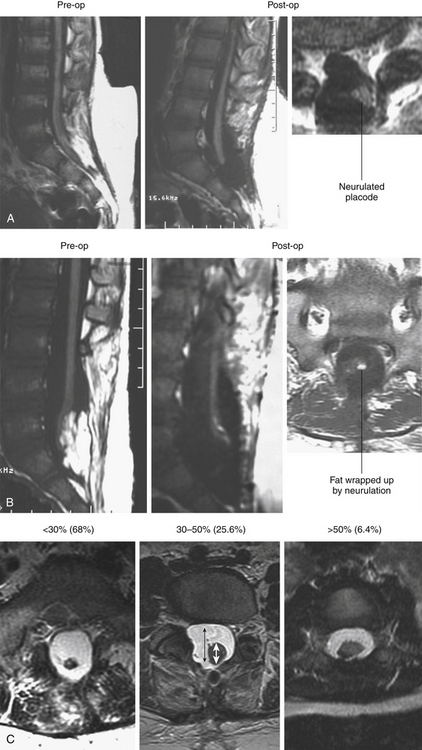
Most ONTDs contain a terminal neural placode with no recognizable neural tube caudal to the flattened neural plate.6 It appears that in most cases, the complete failure of dorsal folding and fusion of the primary neural plate inhibits secondary neurulation so that no conus is formed and the placode ends abruptly. In some cases, remains of an abnormal secondary medullary cord can be found in the form of a filum-like band attached to the lower margin of the neural placode. The dorsal surface of the terminal placode corresponds to what would have been the ependymal lining of the cord if neurulation had taken place, and its ventral surface corresponds to the outer surface of the “would-be” neural tube. The sensory and motor roots from the neural placode therefore project from its ventral surface only, the more lateral sensory roots issuing from the alar plates and the medial motor roots from the basal plates. The cutaneous ectoderm from each side of the embryo normally destined to fuse in the midline is kept widely apart by the unneurulated placode. The dorsal surface of the placode is therefore either “naked,” or covered by an epithelial membrane of variable thickness grown in from the surrounding pia-arachnoid layer (eFig. 60-3A). The placode also effectively prevents dorsomedial migration of the mesenchyme, and thus the completion of the posterior neural arch (hence the term spina bifida), dorsal paraspinous muscles, and lumbodorsal fascia.
Because the meninges develop adjacent to the basal surface of the neuroepithelium, only the ventral (basal) surface of the unneurulated placode receives meningeal investment.7,8 As CSF accumulates between the ventral surface of the placode and underlying leptomeninges, the flat placode is subjected to increasing dorsally directed forces and, lacking dorsal integumentary and myofascial support, is ultimately pushed out dorsally to ride on the dome of the distended cyst (eFig. 60-3B). The remaining dorsal wall of the sac on each side of the placode is composed of leptomeninges that were also ballooned out by the CSF and stretched between the lateral edge of the placode and the margin of abnormal skin. Intact dura lines the ventral portion of the sac, being also prevented from dorsal midline fusion and instead fuses with the margin of the unclosed skin, dorsal musculature, fascia, and periosteum of the incomplete neural arch on both sides of the myelomeningocele sac.8
Preparation for Surgery
The goals of surgical management of ONTD are (1) preservation of functional neural tissues, (2) reconstruction of the dural tube, (3) securing sound myofascial and skin closure, and (4) minimizing the chances of future retethering of the cord. Most open lesions are closed within 24 hours after birth. If the child is initially unstable, closure may be safely delayed for up to 72 hours without an increase in complications. Performing surgery after that time carries a substantial risk of meningitis, wound abscess,9,10 and neurologic deterioration.
Surgical Technique
Opening the Sac
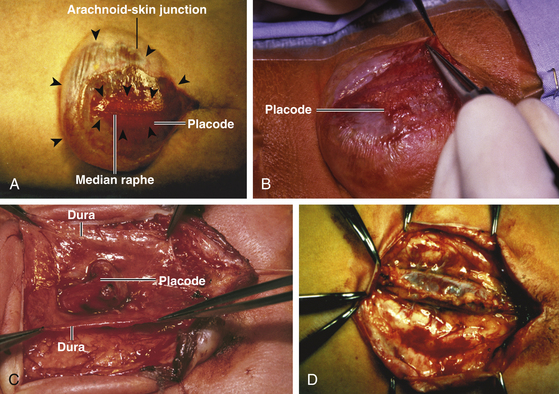
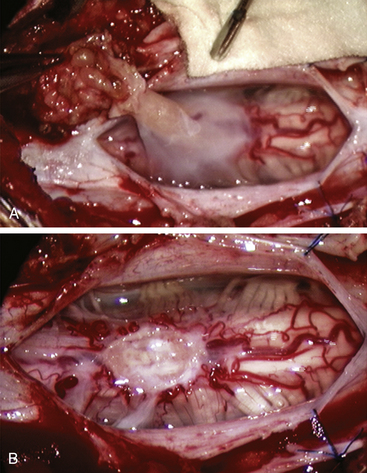
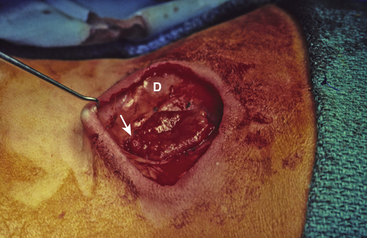
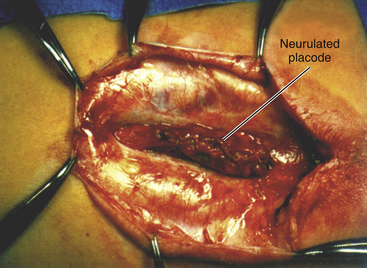
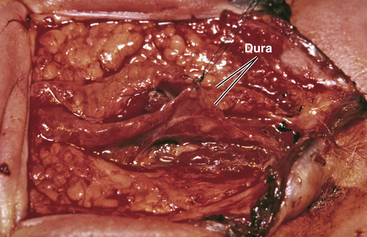
Magnification is used from the very beginning. The sac is entered through the diaphanous leptomeningeal membrane halfway between the margin of healthy skin and the edge of the placode (eFig. 60-4). Neural tissue of the placode is recognizable by its pink, felty surface, transverse wrinkles, and a straight, longitudinal median raphe (Fig. 60-1A) and since the epithelialized membrane itself is relatively avascular, any substantial bleeding from the cut edges signifies breaching of the neural tissue (Fig. 60-1B). Bleeding is controlled with a pair of ultrafine irrigating bipolar cautery forceps. After the initial gush of CSF and collapse of the cyst, the edge of the neural placode is gently flipped up to identify the ventral nerve roots. Several crossing blood vessels may have to be coagulated to free the placode margins. The pearly epithelium must be meticulously trimmed circumferentially from the placode to avoid later occurrence of inclusion dermoid cyst. At the caudal extreme of a terminal placode, the epithelial membrane may remain thin, or one may encounter a band-like thickening probably representing remnant of the medullary cord that must be divided to free the tip. At the rostral extreme of the placode, careful incision of the epithelium–neural tissue junction on both sides exposes the delicate bevel-shaped transition between the neurulated cylindrical spinal cord and the un-neurulated, flat placode (eFig. 60-5). At the apex of the bevel, the central canal of the normal cord can be seen unfurling into the median raphe of the placode, from which CSF sometimes slowly oozes.
Handling the Neural Placode
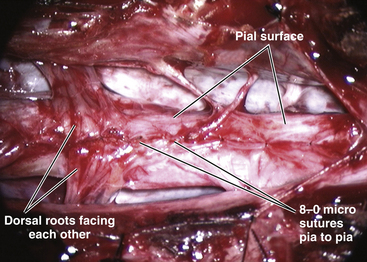
The neural placode is always handled gently with jeweler’s micro-forceps. If the placode is pliable and thin enough, it is rolled on itself and sewn up with 8-0 nylon sutures through the delicate pial edges (eFig. 60-6). There is no evidence that this improves neurologic function, but it reduces the “sticky” surface from a flat plate to a seam and theoretically lessens the chances of later tethering. If the placode is too thick or too stiff, it should not be forced to roll up for fear of strangulation. Also, tugging too hard on the proximal spinal cord will cause ascension of the neurologic level. In addition, the caudal end of the placode must be checked for the presence of a neurulated cord in the rare case of a segmental placode (see below).
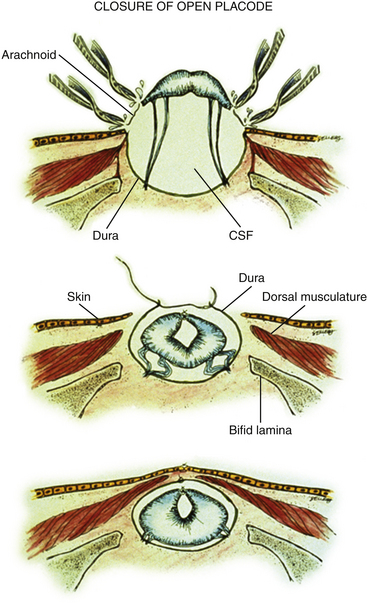
Dural Closure
The margins of the dural flaps are created by sharply incising the ventral dura from the lumbosacral fascia and periosteum (Fig. 60-1C and eFig 60-7; see also eFig. 60-4A and B), and a new dural tube is reconstructed in the midline (Fig. 60-1D). One aims to obtain as capacious a dural sac as possible commensurate with the size of the placode, the theory being if the placode passively flops freely within a large CSF space, it is less likely to adhere to the dorsal dura. This is almost always achievable with the patient’s own dura, even if some of the periosteum overlying the bifid neural arches have to be mobilized with the dura proper to enlarge the sac. In the rare event of insufficient dura, bovine pericardium can be used as a graft because its texture is compatible with newborn dura and because it seldom has suture-hole leakage of CSF. At the end of closure, the suture line is tested with a Valsalva maneuver held at a pressure of 30 to 35 cm of H2O for 10 seconds.
Skin and Myofascial Closure
The true size of the skin defect is only apparent after completely excising the epithelialized membrane back to full thickness skin. Defects up to 5 or 6 cm diameter can usually be closed primarily after the subcutaneous layer is mobilized a short distance centrifugally, just enough to reduce the tension on the skin edges. The subdermal layer is closed with interrupted absorbable sutures, and the skin with fine nylon sutures. A number of surgical techniques have been developed to minimize suture line tension in large defects. Lateral relaxing incisions with bipedicle flap closure in the midline have been effective,11 but the relaxing incisions themselves then require skin grafting at the same time or at a later date. Complex multiple rotation skin flaps have also been tried, but this necessitates extensive skin undermining and still does not altogether eliminate all tension spots. For impossibly large defects, I favor using composite skin–muscle (myocutaneous) flaps. There are three advantages to this technique. Cadaver vaso-latex studies show that a rich vascular anastomosis exists in the skin overlying the gluteus maximus and the latissimus dorsi muscles on each side and across the midline; the muscles themselves are supplied by the gluteal and thoracodorsal arteries, respectively. As long as these arterial pedicles are preserved, blood supply to the lumbosacral skin is well maintained when the four muscles are apposed in the midline, even if the short paraspinous arterial perforators deep to the muscle are taken.12 Secondly, because there is no undermining of the subcutaneous tissues, the skin tension is mostly absorbed by the muscle closure. Finally, this method results in a triple-layered (muscle, subcutaneous tissue, and skin) closure with extra insurance against CSF leak. The flaps survival rate even for enormous defects is greater than 92% with this technique, and the blood loss and extra anesthetic time are quite acceptable.12
Postoperative Management
Early Complications (First Postoperative Week)
The operative mortality for children undergoing repair of an ONTD should be close to 0.13–15 The most common cause of postoperative death is related to hindbrain dysfunction (73%),10,16 but this seldom occurs acutely in the first week of life. Most of the immediate complications pertain to the wound itself.
Wound Dehiscence
A study of the nutritional status of newborn infants who have had myelomeningocele surgery using body weight, nitrogen balance, serum protein, and total lymphocyte count as parameters, showed that these neonates undergo an initial period of severe catabolic changes that do not readjust themselves for as long as 1 month after surgery. This nonspecific catabolic response is caused by rises in circulating levels of ACTH, cortisol, thyroxin, growth hormone, and antidiuretic hormone, stimulated by the extreme stress of surgery, general anesthesia, and blood transfusion.8 During this period, the resistance to infection is lowered, and all anabolic processes, including wound healing, are temporarily slowed. This metabolically unstable time also coincides with feeding difficulties caused by hydrocephalus, postoperative ileus, neurogenic dysphagia (due to brainstem compression), and prematurity. It is no surprise that wound dehiscence is the single most common complication during the first postoperative week.8
Sloughing of only the epidermis in small areas requires only simple dressing changes because the wound eventually epithelializes over the underlying dermal and subdermal layers. Skin grafting is unnecessary. If the skin necrosis is full thickness but there is healthy muscle underneath, the wound edges should be carefully debrided back to bleeding skin. It may then be dressed for second intention healing from below, but this will take some time and delay CSF shunting. A faster way would be partial-thickness skin grafting, which should take well over a well vascularized bed. If full thickness necrosis exposes the dural tube, some measure of immediate coverage must be instituted to prevent desiccation and meningitis. This usually means a more radical and extensive flap rotation or even pediculated full thickness skin flap grafting.17
Finally, parenteral or enteral hyperalimentation should be set up to ensure adequate nutrition.
Wound Infection
Considering how badly the exposed neural placode is contaminated during and shortly after birth, it is surprising how rarely wound (extradural) infections occur after closure of an ONTD. The wound infection rate is about 1.5% to 2.5%, which is only moderately higher than clean neurosurgical procedures.8 However, if one counts the intradural infections, the infection rate rises to 7% to 10% even for early closure.15,18
Systemic signs of sepsis due to gram-negative meningitis are usually present 1 to 3 days after closure. In neonates, these early signs tend to be nonspecific, such as poor feeding, lethargy, or an ashen complexion. It is more common to see hypothermia than pyrexia, and the systemic white blood cell count often drops below 4000/mm.3 If the dural sac is well invested with a myocutaneous coverage, an intradural abscess may eventually form without any external signs. It is important to obtain CSF for culture from a ventricular tap if there is clinical suspicion of sepsis, for the long-term prognosis of gram-negative ventriculitis in the newborn depends almost solely on the promptness of diagnosis and treatment.
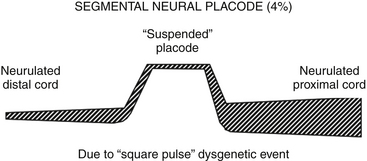
ONTD with Segmental Placode
The term segmental placode describes a portion of open neural plate bounded both rostrally and caudally by perfectly neurulated spinal cord (eFig. 60-8). It is found in approximately 4% of all open neural tube defects. The exact mechanism of its genesis is unknown, but somehow it must involve a “square-pulse” type teratogenic insult to the process of primary neurulation; that is, normal neurulation resumes post facto to an isolated failure of neural plate fusion, both in space and time. Or, it could be a manifestation of “collision site” failure from two adjacent neurulation sites proceeding in opposite directions, although no proof of the multisite closure hypothesis yet exists.19 The most common site for the segmental placode seems to be midthoracic to thoracolumbar. It is unclear why the teratogenic insult in these cases, unlike in terminal placodes, does not disrupt secondary neurulation, and allows for normal closure of the posterior neuropore and formation of the conus.
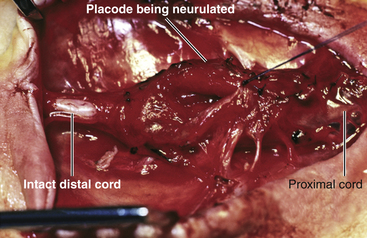
It is important to recognize the placode as segmental before surgical closure because the surgeon should be mentally prepared to handle the distal end of the placode delicately. One reliable clinical clue is the preservation of distal lower extremity movements while the open defect is located high up in the thoracic region. A preoperative MR should be obtained, not only to visualize the distal spinal cord beyond the placode, but to spot other associated paradysraphic malformations such as a split cord malformation (SCM),20,21 a lipoma,22 or a thickened filum. It is even possible the segmental placode represents a hemimyelomeningocele in that the other hemicord of the SCM is fully neurulated and stays uninvolved in the open defect itself.23,24
The technique of closure of the segmental placode is the same as for the terminal one. Every bit of neural tissue must be preserved during trimming of the extraneural membrane, and every effort should be made to reconstruct the tube (eFig. 60-9). The critical decision is whether to deal with the other associated malformation at the same time or at a later date. I recommend the latter since one wants to inflict as little stress to the newborn infant as possible and the immediate goals of infection prevention and neural conservation have been met by the mere closure of the open sac. The definitive procedure of “complete” untethering usually involves more extensive bone and soft tissue dissection, and should be left till 2 to 3 months later when the infant can better withstand a longer anesthesia and larger blood loss, when hydrocephalus is no longer an issue, and after thorough neuroimaging studies have been obtained.
ONTD and Kyphectomy
The apex of the kyphus is resected through the intervertebral discs with the monopolar cautery. The extent of resection must take into account the feasibility of apposing the remaining ends of the spine to fill the gap. The two ends of the stump are then cleared of cartilaginous endplates, and brought together using two parallel wires forced through the bony part of the vertebral bodies with sharp cutting needles. A certain amount of downward pressure must be exerted on the bodies during the apposition and twisting of the wires. During twisting of the wires, the fusion surfaces and the wire loops are subjected to tremendous distracting and persistent dorsal-pointing stresses. The wires should never pass through any cartilaginous part of the body or the intervertebral disc. The infant is immediately immobilized in a fitted thermoplastic body brace for a minimum of 3 months and up to 2 years. Nonunion is a serious problem because discarding one or two crumbled and defunct vertebral bodies essentially means widening the gap even more and an even greater stress for the new construct.8,25
Spinal Cord Lipomas
We advocate strongly for total resection of spinal cord lipomas and radical reconstruction of the neural placode over partial resection because aggressive surgery, contrary to traditional view, is safe and gives far better long-term progression-free survival.26 The rationale for total lipoma resection is based on three hypotheses: (1) the high rate of symptomatic recurrence after partial resection is due to retethering; (2) retethering is promoted by three factors: a tight content-container relationship between spinal cord and dural sac, a large “sticky” raw surface of residual fat, and incomplete detachment of the terminal neural placode from residual lipoma; and (3) total resection can eliminate the factors conducive to retethering and thus reduces the probability of symptomatic recurrence.
The object of surgery is therefore to create conditions that will minimize retethering. The first condition relates to the fact that the normal spinal cord exhibits intradural motions to gravity and postural changes on ultrasonography and dynamic imaging.27,28 Reducing the content-container ratio and amplifying the degree of freedom of the cord within the dural sac must lessen resticking by limiting sustained contact between cord and dura, this sustained contact being intuitively a necessary condition preceding the formation of fibrous adhesions. To do this, the cord bulk must be drastically reduced. For large rambling “virgin” lipomas, this means resection of all or most of the fat down to the thin, supple neural placode. For redo lipomas, the hard, grasping cicatrix must also be removed. The aim is to render the thinnest, most pliant neural placode possible that can be atraumatically neurulated without distortion or strangulation to form a slender, round tube. The raw, sticky lipoma bed is simultaneously concealed within the tube and the sac is enlarged by a capacious dural graft. Finally, total resection also enhances the chances of terminal untethering.
Anatomy and Classification
In the literature, the nomenclature of spinal cord lipoma is imprecise and inconsistent. Here, we are defining the types of lipomas as follows, based loosely on Chapman’s original classification.29
Dorsal Lipoma
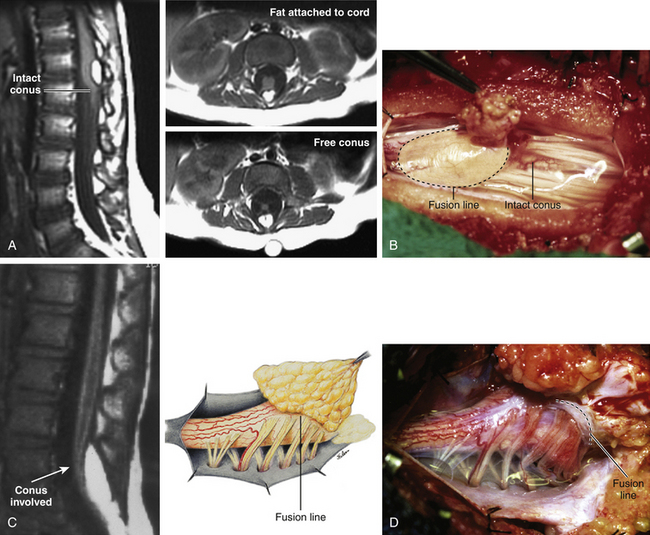
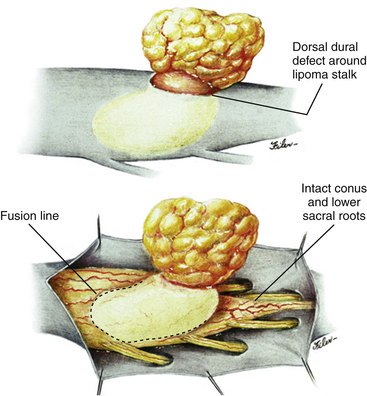
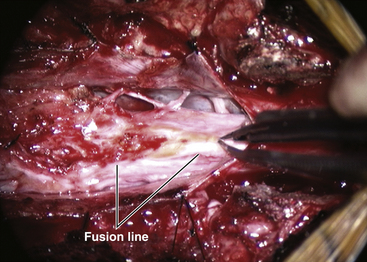
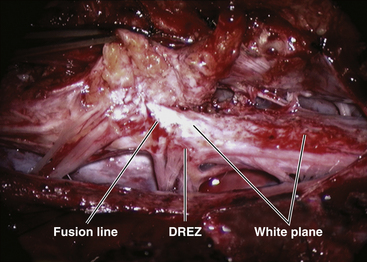
The lipoma–cord interface is entirely on the dorsal surface of the lumbar spinal cord, sparing the distal conus (Fig. 60-2A). The junctional demarcation between lipoma, cord, and pia, the fusion line, can always be traced neatly along a roughly oval track, separating fat from the dorsal root entry zone (DREZ) and dorsal nerve roots laterally (Fig. 60-2B and eFig 60-10). The lipoma therefore never contains nerve roots. The lipomatous stalk runs through an equally discrete dorsal dural defect to blend with extradural fat. The uninvolved conus often ends in a thickened filum terminale.
Transitional Lipoma
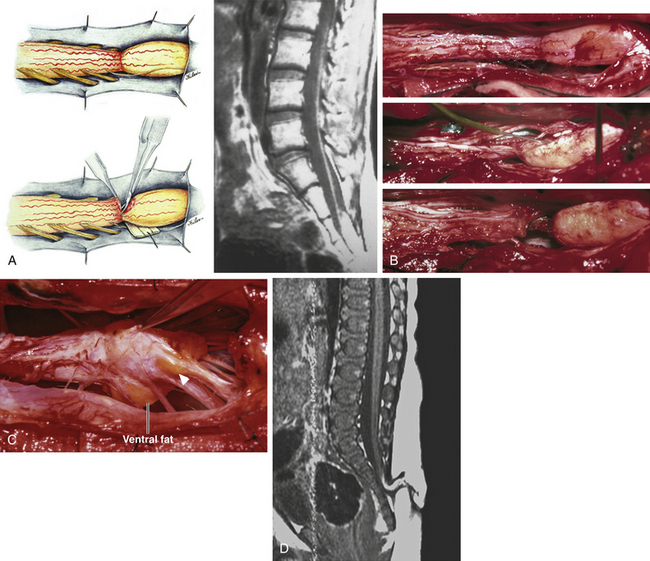
The rostral portion of this type is identical to that of a dorsal lipoma, with a discrete fusion line and easily identifiable DREZ and dorsal roots. Unlike the dorsal type, however, which always spares the conus, the transitional lipoma then plunges caudally to involve the conus as the plane of the fusion line cuts ventrally and obliquely towards the tip of the conus likened to making a slanting, beveled cut on a stick (Fig. 60-2C). The lipoma–cord interface thus created may be undulating and tilted so that the neural placode is rotated to one side or even spun into a parasagittal edge-on orientation, but the neural tissue is always ventral to it and the DREZ and the nerve roots are predictably localizable lateral and ventral to the fusion line and therefore do not course through the fat (Fig. 60-2D). There may or may not be a discrete filum. The dorsal dural defect extends to the caudal end of the thecal sac and may be much larger on the biased side.
Terminal Lipoma
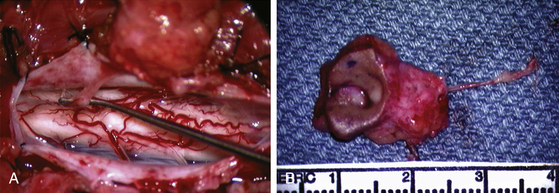
Unlike the dorsal and transitional types, terminal lipomas insert into the caudal extremity of the conus without blending with the spinal cord or its root entry zones. All the sacral roots unmistakably leave the conus rostral to the lipoma, and in most cases the conus itself looks normal. The dural sac and the dorsal myofascial coverings are intact. The lipoma either replaces the filum entirely, or is separated from the conus tip by a short, thickened filum (Fig. 60-3A and B).
Chaotic Lipoma
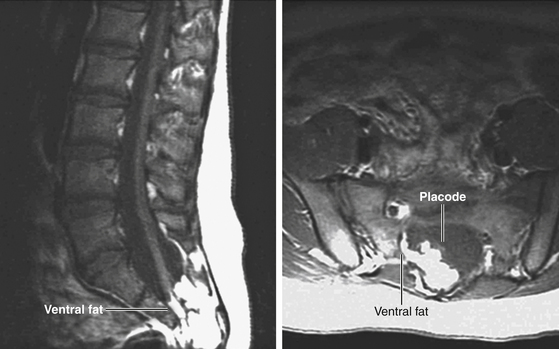
This previously undescribed type is so named because it does not “follow the rules” of either the dorsal, transitional, or terminal lipoma. It begins dorsally in an orderly fashion as in a dorsal or transitional lipoma, but its caudal portion is ventral to the neural placode and does engulf neural tissue and nerve roots (eFig. 60-11). The fusion line may be distinct rostrally but quickly becomes blurred distally, and the location of the DREZ and nerve roots is less predictable. The moniker “chaotic” depicts the sometimes confusing blend of the ventral fat and neural placode, and the often impossible task of separating fat from neural tissue at surgery (Fig. 60-3C). Chaotic lipomas are uncommon but are characteristically seen with sacral agenesis.
The literature30,31 describes one other lipoma type, the lipomyelomeningocele, in which part of the distal conus extends into the extraspinal compartment, dragging with it a small collar of dural sac (Fig. 60-3D). The basic structure is either that of a transitional or a dorsal lipoma. Accordingly, we choose to classify this type as either a transitional or dorsal lipoma with a descriptive qualifier of “extraspinal extension.”
Surgically Relevant Embryology
Embryogenesis of Dorsal and Transitional Lipomas
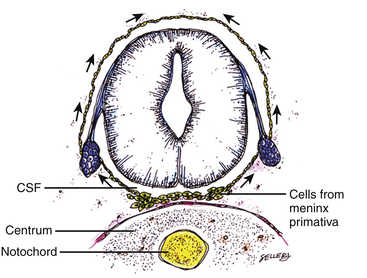
In the embryo, a progressive disparity exists between the spinal cord and vertebral column as a result of the faster growth rate of the latter.32–35 The caudal end of the cord ascends gradually from opposite the coccyx in the 30-mm human embryo to the L1–L2 level at birth.34–37 Proper ascent of the cord requires a well-formed neural tube and a smooth pia-arachnoid covering. If during early development a dorsal defect exists in the dura (duraschisis) and neural tube (myeloschisis), mesodermal elements from the surrounding mesenchyme will enter the dural sac and form attachment with the sliding neural tube in the form of a fibro-fatty stalk, resulting in its entrapment. This theory features a fundamental defect in neural tube closure during primary neurulation (secondary neurulation does not involve dorsal neural fold closure), and thus applies only to the dorsal and transitional lipomas (see below). It is compatible with the observation that these two types of lipomas are always associated with neural arch defects.
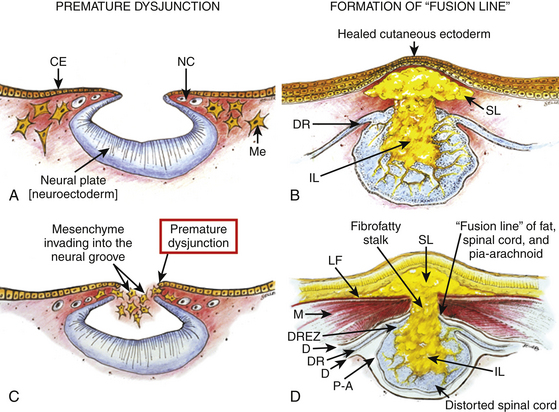
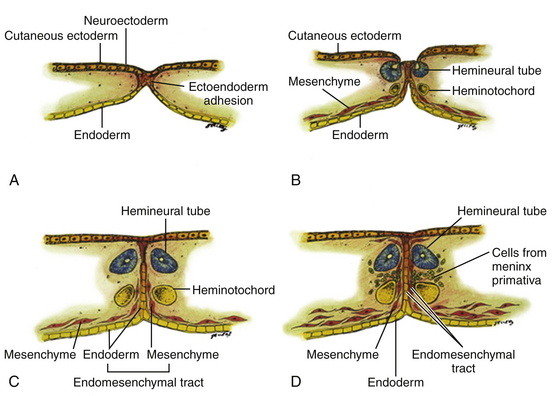
The embryologic error leading to the mesodermal invasion of the neural tube probably lies in premature disjunction between the cutaneous and neural ectoderms38–40; that is, the separation of one from the other occurs before the converging neural folds fuse with each other. This allows the paraxial mesenchyme to roll over the still gaping neural folds and enter the central canal. Once contact between mesenchyme and ependymal neuroectoderm is made, further closure of the neural tube is permanently prevented and a segmental dorsal myeloschisis is created (eFig. 60-12A and B). Alternatively, the fault may lie in a delay in neural folds fusion secondary to an insufficiency of the paraxial mesoderm in impelling their dorsal convergence,41–47 so that ectodermal disjunction again precedes neural folds fusion. Lastly, faulty fusion of the neural folds due to metabolic disturbance of the cell membrane-bound glycosaminoglycans, which are vital to cell–cell recognition and adhesion,48–51 could likewise reverse the temporal relationship between disjunction and neural folds fusion.
Experimental studies show that the pluripotent mesenchyme forms derivatives according to the inductive properties of the adjacent neuroectoderm (eFig. 60-12C).6,9 The ependymal side of the neural tube induces mesenchyme to form fat, muscles, collagen, and occasionally bone and cartilage, whereas the outer surface of the neural tube induces the formation of meninges.52 However, no dura can now form over the dorsal opened portion of the neural tube, and the dural defect neatly surrounds the evolving lipomatous stalk, which tethers the neural tube to the subcutaneous adiposity. In like manner, deficiencies in the overlying myofascial layers (from myotomal mesoderm) and neural arches (from scleromesoderm) also neatly surround the lipomatous stalk (eFig. 60-12D).
Within the neural tube, the intramedullary fat and muscles fuse with the developing alar and basal plates. Since the dorsal root ganglions develop from neural crest cells at the outer aspect of the neural fold lateral to the site of failed fusion, the dorsal nerve roots grow outward ventrolateral to, but never traverse, the lipomatous stalk. The DREZ must correspondingly lie very near, but always lateral to, the exact junctional boundary between lipoma and spinal cord. This boundary, called fusion line, is of tremendous surgical significance22,53,54 (eFig. 60-12D). Meanwhile, the cutaneous ectoderm, long detached from the neuroectoderm, heals over in the dorsal midline to form wholesome skin over the subcutaneous lipoma.
The genesis of a dorsal lipoma perfectly exemplifies mistimed disjunction during primary neurulation. Its fibro-fatty stalk always involves cord segments above the conus, which mainly forms from secondary neurulation. Furthermore, failure of primary neural tube closure appears to be segmental, normal closure takes place “business-as-usual” immediately following the abnormal event. This “square pulse” nature is illustrated by the fact that the sharp fusion line between fat, spinal cord, and pia-arachnoid can be neatly traced circumferentially around the lipomatous stalk53–55 (see Fig. 60-2B and eFig. 60-10). Dorsal lipomas therefore result from a segmental closure abnormality involving only primary neurulation. They are found in less than 15% of spinal cord lipomas in our series.22,26
In transitional lipoma, the myeloschisis involves much more than an isolated segment of the primary neural tube. Even though its rostral part resembles the dorsal lipoma, the involvement of the whole of the caudal spinal cord means that not only primary but also secondary neurulation have been profoundly disturbed by the mesodermal invasion. This is supported by the observations that in many transitional lipomas the filum is incorporated into the distal fat, and within the lipomas are often spaces resembling the vacuoles within the secondary neural tube. Also, while the rostral part of the transitional lipoma is always dorsal and aptly reflects premature disjunction of primary neurulation, the distal part sometimes involves both the dorsal and ventral aspects of the conus, a situation compatible with misguided mesenchymal inclusion during the much less orderly events of secondary neurulation. Intramedullary mesenchyme may migrate within the neural tube after invasion and travel caudally across the boundary from the primary to the secondary neural canal, since the two neural canals are in continuity.56 In fact, the hypothesis that the rostral part of the transitional lipoma arises from aberrant primary neurulation (involving only the dorsal cord) and the caudal lipoma arises from abnormal condensation of the secondary neural cord (affecting more ventral aspects of the conus) furnishes at least one explanation for the dorsoventral obliquity of the lipoma–cord interface.
Embryogenesis of Chaotic Lipomas
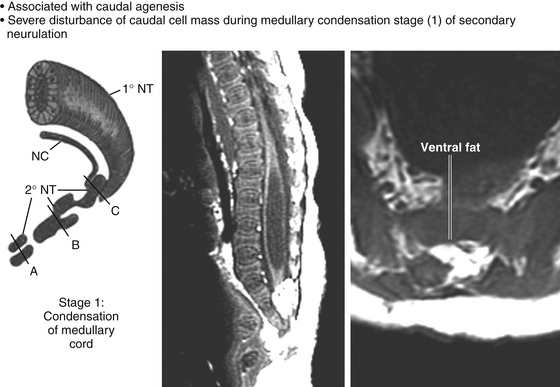
This degree of anatomic unpredictability in chaotic lipoma and its strong association with caudal agenesis (82% in our series22) suggest that the embryogenetic error occurs during the early stage of secondary neurulation as part of the general failure of the caudal cell mass (eFig. 60-13).57,58 Secondary neurulation comprises three distinct stages: (1) condensation of neural material from the caudal cell mass to form the solid medullary cord, (2) intrachordal cavitation of the medullary cord56,58,59 and its integration with the primary neural tube, and (3) partial degeneration of the cavitary medullary cord through massive apoptosis to result in the thin filum terminale.34,56 It is possible that formation of the chaotic lipoma involves the entanglement of lipogenic mesenchymal stem cells with cells from the caudal cell mass during aberrant condensation of the medullary cord, forming an inseparable mixture of neural tissue and fat, with nerve roots projecting out haphazardly.22
Embryogenesis of Terminal Lipoma
Terminal lipomas result from abnormal secondary rather than primary neurulation, as evidenced by the unexcepted rule that the lumbar and upper sacral cord segments, products of primary neurulation, are never affected in a terminal lipoma. Furthermore, dorsal myeloschisis and duraschisis, both hallmarks of failed (primary) neural fold fusion, are never seen. Lastly, the terminal lipoma either replaces or forms part of a thickened filum, which temporally places the pathogenetic process at secondary neurulation. The fact that the distal conus in terminal lipomas always remains fat-free argues against an abnormal condensation phase during early secondary neurulation. On the other hand, the terminal lipoma often contains (disorganized) spinal cord elements and ependymal tubules,60,61 which suggests rather an incomplete or ineffective degeneration phase at late secondary neurulation.
Intraoperative Electrophysicologic Monitoring
Intraoperative monitoring has become sine qua non in lipoma surgery.22,62,63 Electromyography (EMG) is routinely used to accurately identify the motor roots and to detect functional spinal cord within ambiguous tissues. The muscles commonly employed are sartorius (L1), rectus femoris (L2, L3), anterior tibialis (L4, L5), extensor hallucis longus (L5), gastrocnemius (S1), and abductor hallucis (S2). Half-inch long, 25- to 27-gauge–needle recording electrodes and input gains of 50 to 80 microvolts are selected to enable maximum capturing of far-field evoked action potentials of the indexed muscle without undue artifacts. Smaller needle electrodes are inserted directly through the anal verge to record activities of the external anal sphincter (S2-S4). All stimulations and recordings in our cases are done with the Cadwell Cascade Intraoperative Monitoring System (Cadwell Laboratories, Kennewick, WA) using the Cascade Software Version 2.0.
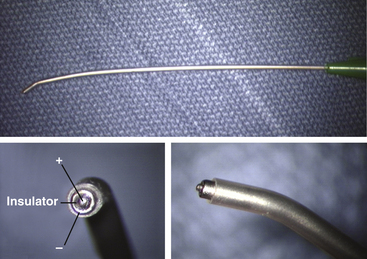
For nerve root and direct spinal cord stimulation, we use a concentric coaxial bipolar microprobe (Kartush Concentric Bipolar, Medtronic Xomed, www.xomed.com) (Fig. 60-4) capable of generating extremely focused and confined current spread at its 1.75-mm tip, and is thus best suited in precise localization of small functioning neuron-axonal units. Larger double-pronged bipolar electrodes, or worse, monopolar electrode, which in essence converts the spinal cord into a giant volume conductor, are undesirable because they cause unwanted recruitment of adjacent depolarizable tissues. Stimulating currents from 0.5 to 3.0 milliamperes are used depending on target impedance. The stimulation frequency is usually set at 10 per second. This allows spontaneous random firing due to nerve irritation from surgical manipulation to be distinguishable from the rhythmic evoked contractions.
Surgical Technique of Total/Near-Total Resection
Step 2. Detachment of Lipoma from Dura
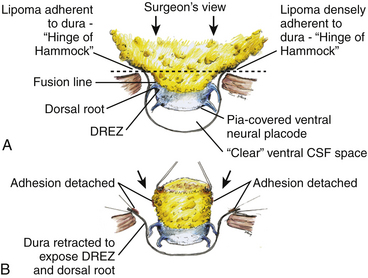
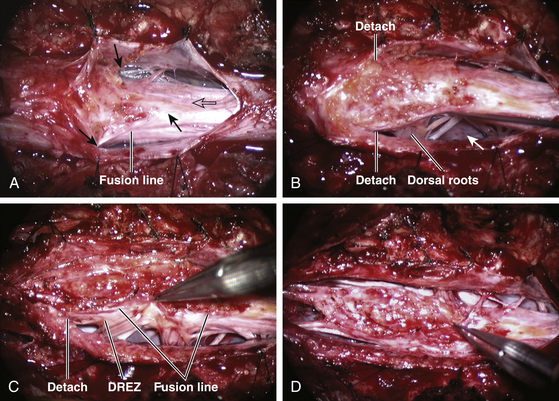
The dura rostral to the lipoma is opened in the midline. For dorsal lipoma, the dural incision is carried circumferentially around the discrete lipoma stalk and then down the middle again to expose the conus (eFig. 60-10). For transitional lipoma, the dura is cut close to the lipoma edge on each side as far caudal as possible, though the two side incisions seldom could meet distally. The dural edges are then tautly and widely retracted with sutures. This is a crucial maneuver because full lateral exposure of the intradural span, made possible by the generous bone removal, reveals the “crotch” where the far lateral fringe of the lipoma attaches to the inner surface of the dura (see Fig. 60-6A).
Next, the fusion line is identified where pia, spinal cord, and lipoma join in a continuous furrowed border that travels from rostral to caudal outlining the entire attachment of the lipoma stalk to the cord. In a dorsal lipoma, the fusion line forms a neat complete oval or circle from side to side, usually upon a leveled horizontal plane, often bilaterally symmetrical, and always sparing the conus below (Fig. 60-2B and eFig 60-10). In a transitional lipoma, the rostral fusion line starts distinctly enough but then edges ventrally towards the tip of the conus and tends to wander laterally and asymmetrically, often becomes sheltered by the overhanging fat, and never meets its mate from the other side at the caudal end (see Fig. 60-2C and D).
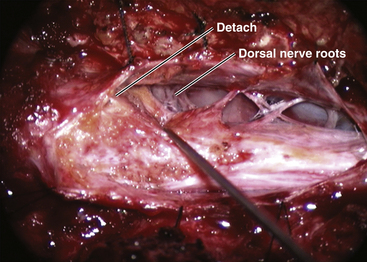
True to the events of embryogenesis, the DREZ and dorsal roots are always lateral to the fusion line, and at the rostral end of the fatty stalk of both lipoma types, this orderly arrangement can be depended upon on both sides, thus presenting a convenient place to start the dissection. In most transitional lipomas, however, the more caudal nerve roots are quickly covered from view by the overflowing fat which tends to fuse with the dura at a far lateral point (Fig. 60-5A). Hence the term “crotch dissection,” which depicts the key step of grasping the overhanging fat and pulling it medially under tension against the tagged dural edge, then sharply separating the fat-dura attachment with dissecting scissors (Fig. 60-5B and eFig. 60-14). It is absolutely requisite to lean the round curve of the scissors firmly against the inner lining of the dura while cutting this attachment to avoid blindly injuring the nerve roots, which project from the cord slightly medial to the “crotch” and lie just deep to the fat. The hidden roots should spring into view wherever the detached fat is pulled back, and can be gently coaxed away from the dura by blunt dissection toward the exit foramina (Fig. 60-6B). At the same time, the free CSF space ventral to the dorsal roots, and the fat-free, pia-covered ventral surface of the neural placode, hitherto hidden by the overhang, now “pop” into view (Fig. 60-6C).
It is clear from this description that in each successive axial slice, all lipomas big or small, dorsal or transitional, are roughly divided by a transverse line joining the points of the far lateral fat-dura attachment on each side, where the lipoma–cord assembly is in effect suspended like a hammock against two lateral hinges over an uncluttered ventral CSF pool. Dorsal to this transverse line is the visible but disorderly, massive, and unrevealing fat and ventral to this line is the orderly fusion line, DREZ, dorsal nerve roots, neural placode, and ventral CSF space but all initially rendered invisible to the surgeon by the overhanging fat (Fig. 60-5A). The purpose of the “crotch dissection” is therefore to release this suspension so that the hammock of neural placode and nerve roots can be folded inward enough to be identified and preserved during the next phase of lipoma resection (Fig. 60-5B).
This laborious but indispensable step of “crotch dissection” is carried all the way caudally (Fig. 60-6C) until all the “useful” nerve roots are identified and the entire neural placode, with a profusion of lipoma still attached, is completely unsuspended from the dura and has literally “fallen” to the basin of the dural trough (Fig. 60-6D).
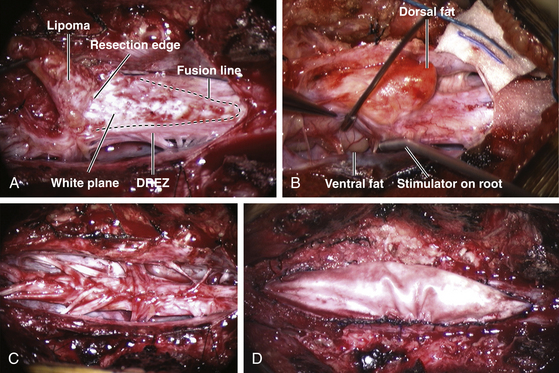
Step 3. Lipoma Resection
Resection of the lipoma begins at the rostral end where the anatomic relationships between fat, DREZ, and nerve roots are clearly decipherable (Fig. 60-7 and eFig. 60-15). Sharp dissection with microscissors is used to locate a thin but distinct silvery white plane between fat and cord at the demi-lune of the rostral fusion line (Fig. 60-8A). It takes some determination, for the initiate, to cut into this traditionally forbidden place, seemingly straight into the spinal cord right at the fusion line, but with experience, this white plane can be found in every case. We strongly discourage using the CO2 laser because it chars the surface (no more white plane!) and negates the tactile feedback through the microscissors on which the surgeon depends to differentiate between cutting through the grittiness of fibrous fat and the formless softness of spinal cord. Bleeding on the white plane can be handled with the ultra-fine irrigating bipolar cautery (0.2-mm tips) and a very low current setting. The cold irrigation mitigates against sticking but more importantly it dissipates heat rapidly from the cord. Minimal cauterization is used on the DREZ to avoid postoperative dysesthesia.
As long as all the activities are rendered medial to the fusion line, thus also medial to the DREZ and nerve roots, dissection along the white plane can be conducted safely all the way to the end with no damage to the cord or nerve roots (eFigs. 60-16 and 60-17). In a dorsal lipoma, this is a simple feat because the white plane is basically horizontal and flat, the two banks are symmetrical, and the caudal end well defined rostral to the conus so that a completely circumscribed attack on the fat is possible from multiple angles (eFig. 60-18). In a large transitional lipoma, navigating the white plane is more difficult because it always slopes ventrally, often undulates, and one side may be tilted so steeply that the corresponding DREZ and nerve roots are shifted ventrally and the placode so rotated that its ventral surface now faces the side. Such a white plane is almost turned vertically “on edge,” its orientation confusing unless one remembers the transverse line concept dividing one “clean” ventral hemisphere from the “messy” dorsal one.
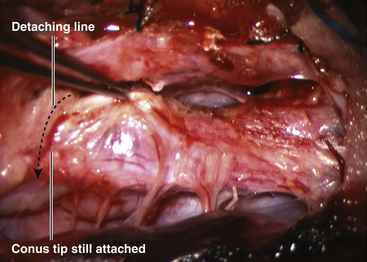
The white plane sometimes seems never-ending in large transitional lipomas and the caudal thecal sac is thronged with fat admixed with suspicious strands. This is when systematic stimulation and identification of the ventral nerve roots become invaluable in localizing the termination of the functional spinal cord. As soon as two to three pairs of sphincter-activating sacral roots are identified, any tissue distal to the last pair can be considered nonessential and be cleanly cut across to consummate the final liberation of the placode (eFig. 60-19). A good chunk of the now isolated distal fatty stump should be excised to prevent reconnection with the terminal placode.
In chaotic lipomas, electrophysiologic determination of the functional extent of the neural placode may be the only way to achieve final untethering; the caudal fat-cord-fibrous jumble can only be sorted out functionally and not anatomically by direct stimulation of the placode and the projecting nerve roots. The handling of the white plane on the dorsal side of a chaotic lipoma is the same as with the other lipoma types, but the billows of fat on the ventral side of the placode should be left alone and its smooth pial surface left unviolated (Fig. 60-8B and eFig. 60-20). It is always the dorsal and never the ventral part (unless iatrogenically invaded) of the lipoma that actually tethers the spinal cord.22
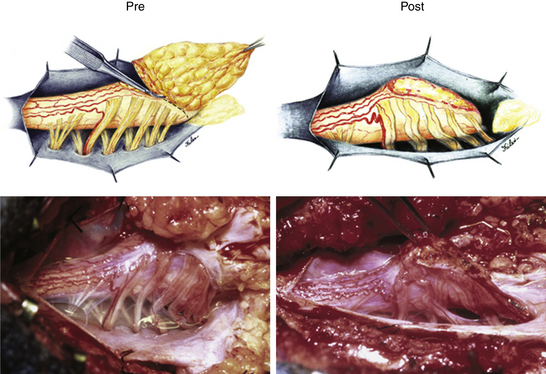
Step 4. Neurulation of the Neural Placode
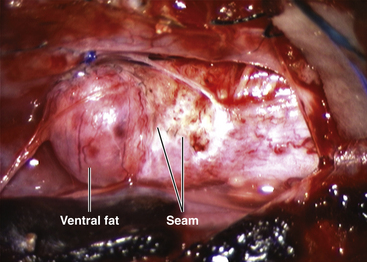
Total resection of the dorsal fat and thorough unhinging of the placode convert a bulky, transfixed lipoma–cord complex into a free-floating, thin, supple, purely neural plate (eFig. 60-17), eminently suitable for pia-to-pia, midline, dorsal closure with interrupted 8-0 nylon sutures without strain or strangulation to the neural tissue (eFig. 60-21). It is helpful to leave a narrow cuff of pia along the cut edges of the white plane to accommodate the sutures, which are tied with inverted knots. Neurulation thus transforms a broad, wafery, sticky sheet into a trim, sturdy, pia-covered tube bearing a single seam, evocative of the natural neurulation process (Fig. 60-8C).
Step 5. Expansile Graft Duraplasty
The argument for a graft dural closure comes from the belief that if the neurulated placode could slosh about in ample CSF within a capacious sac, the likelihood of reattachment to the dura would be diminished. Thus we prefer a slightly full-bodied yet texturally compatible (to infant dura) material such as bovine pericardium that can maintain its shape, to a filmy-soft graft such as autologous fascia lata that may swell and ebb with respiration and body movements and thus collapse on the cord. The bovine graft is carefully measured and shaped to prevent inward folds. Running prolene sutures are used to achieve watertight closure, confirmed with Valsalva maneuvers (Fig. 60-8D).
Technical Points
Our data show that total or near-total resection of spinal cord lipomas can be done in more than 90% of cases22 (Fig. 60-9A). In most instances, the small amount of residual fat is adherent to the DREZ and had been invaginated out of mischief during neurulation (Fig. 60-9B). In the 8% of patients with unusually large amount of residual fat, the fat belongs to the ventral component of a chaotic lipoma and has been intentionally left pia-covered and therefore harmless.
To assess the “looseness” or degree of freedom of the reconstructed placode within the newly formed dural sac, we created the cord–sac ratio, defined as the ratio of the diameter of the cord to the diameter of the sac on the postoperative axial MRI at the bulkiest portion of the reconstructed segment. The ratios are grouped into low, less than 30%, in the loosest sacs; medium, between 30% and 50%, in moderately loose sacs; and high, greater than 50%, in the tightest sacs (Fig. 60-9C). In the total group of 238 patients, 162 (68%) had low cord–sac ratios, 61 (25.6%) had medium ratios, and only 15 (6.3%) had high ratios.22
The size of the lipoma is not an important determinant of the completeness of resection. Much more relevant is the configuration of the lipoma–cord interface, which contains the “white plane.” The white plane is a filmy netting of relatively compact collagen fibers. It may be extremely contorted and asymmetrical in some transitional lesions, and there is no better way than boldly cutting into the tongue of the rostral lipoma to find the glistening fibers beneath the first few globules of yellow fat. Once the plane is located, one can follow it with sharp dissection by feeling the grittiness through the microscissors and by spotting the glistening white stripes between yellow fat and pink spinal cord. With large dorsolateral transitional lipomas, the placode may be twisted 90 degrees and the lipoma–cord interface can look almost vertical. Access to the DREZ of the ventral (down) side can be awkward unless the “hammock” is first unhinged, and the placode is swung back to a more horizontal position. On the more involved side, festoons of overhanging fat may obscure the emergent dorsal roots to give the false impression that they course through and not underneath the lipoma. In fact, this overhang can be readily teased and lifted off the “knee-turn” of the dorsal rootlets to allow these to be traced under the verandah of fat into the true DREZ, at which site the white plane can once again be picked up. In general, a sinuous and severely rotated white plane makes it more likely to leave behind residual fat.
Redo lesions are associated with a higher rate of residual fat and a higher cord–sac ratio. When the fat layer is infiltrated by heavy cicatrix from previous surgery, the cementing hold to the surrounding dura is much more tenacious and harder to detach, and the bright yellow of “virgin” fat is lost to a gray dense concretion much harder to distinguish from the white plane. The dissection often stops short of the white plane for fear of cutting too deep, and the result is a stiffer slab of residual scar-studded fat that not only augments the bulk of the placode, but also makes it awkward to fold at neurulation. The presence of this unyielding fat-scar at the DREZ often leads to “gouging,” which may well be the cause of postoperative dysesthetic pain in redo patients.26
Incomplete terminal untethering of the placode predictably ends in recurrence of symptoms.64–66 We ascribe two explanations for the surgeon’s hesitation to commit the final disconnecting cut. With very large transitional lipomas, the distal neural placode is buried in fat, and unless visualization is improved by substantial removal of fat, safe untethering cannot be done. Also, nerve twigs are sometimes seen issuing in pairs from the distal placode, making it seem impossible to complete the detachment without sacrificing functional cord. This assumption is spurious, for as long as two or three anal sphincter-activating roots, presumably S2 to S4, are identified and preserved, there should be no loss of function if the terminal cut is made just caudal to these roots. The small nerve twigs within the discarded stump that did not stimulate are probably coccygeal roots and therefore vestigial in human and have no essential function.22,26,29
Thus, thorough lipoma and scar resection and terminal untethering impart the optimal bulk, texture, and maneuverability to the neural placode for tensionless neurulation. A low postoperative cord–sac ratio has been shown to be the single most important factor in securing a long progression-free survival (PFS) in lipoma surgery.26
Finally, our experience unequivocally shows that chaotic lipomas are the most treacherous lesions. They can be recognized in the preoperative MRI by the presence of ventral fat medial to the ventral nerve roots and by their association with sacral agenesis (eFig. 60-13). Compared to other lipoma types, chaotic lipomas are more likely to show conspicuous residual fat and high cord–sac ratio on the postoperative MRI.22,26 The strategy for chaotic lesion is knowing just when to stop excavating deeper after the dorsal portion of the lipoma has been removed to enable neurulation. If the ventral fat is judged not amenable to total excision and neurulation, then its pial surface that had hitherto laid freely against the adjacent ventral dura should be left unsullied to avoid creating new adhesions (eFig. 60-20).
Complications
Our combined neurologic-urologic deterioration rate following total/near-total resection is 4.2%, which compares favorably with rates in the literature ranging from 0.6% to 10%30,31,66–80 and averaging 3% to 7%, associated with partial resection using more conservative techniques. Only 1.7% of patients had new weakness and approximately 4% had neuropathic pain, which, we suspect, is due to close encounters of the DREZ and dorsal roots with heat from the electrocautery. Most minor bleeding on the cord can be stopped with gentle tamponade and Gelfoam, and if electrocoagulation has to be applied, only the ultrafine microtipped irrigating bipolar cautery is used with very low current intensity.
The CSF leak rate (0.8%) and wound complication (1.3%) with total resection are much lower than almost all of the published series (of partial resection), which record CSF leak rates from 2% to 47%,31,66,69,70,74–76,78,80 and wound dehiscence and infection rates of 2% to 26%.31,66,70,73,74,76,78,80 Good results are owed to the following technical stipulations: (1) Enough bony exposure must be done caudally so that a cuff of healthy dura past the lowest extent of the lipoma can be made available for graft anastomosis. The graft should never be sewn to the web of fat at the remaining lipoma stump. (2) Absolute water-tight closure of the graft with Prolene must be achieved, and challenged by Valsalva maneuvers. (3) Synthetic or organic tissue glues are used if there is even a suggestion of a leak. (4) In large sacral lesions, there are often gaping muscle and fascial defects that cannot be primarily approximated. In these cases, paramedian relaxing incisions can be used on the flanking lumbodorsal fascia to facilitate sliding midline closure of the myofascial edges. (5) The large subcutaneous lipoma is never removed at the time of intraspinal surgery. The creation of this immense dead space will encourage collection of CSF which may turn into an enlarging pseudomeningocele and ultimately threaten skin flap viability.
Results of Total Resection
When the outcome of total lipoma resection is analyzed against preoperative symptoms, the best result is seen with pain. Most of the sharp, dysesthetic leg and perineal pain will significantly diminish within 3 months, but not necessarily low back pain that is likely mechanical in origin.81,82 Children also become more active and playful, and virtually never have chronic back complaints. Sensorimotor deficits also respond favorably with surgery. Although less than 20% of patients have actual normalization of motor function, the majority will substantially improve.22,26 As with other forms of tethered cord, the milder and more recent deficits have better chance for good recovery. Bladder dysfunction responds favorably in about 20% to 30% of patients.22 The subtype of neuropathic bladder with the best prognosis is the small capacity, spastic bladder with uninhibited detrusor contraction. Atonic bladders seldom improve with surgery, and intermittent catheterization usually needs to be continued indefinitely. The response of detrusor-sphincter dyssynergia to surgery is unpredictable. It is mandatory that cystometry and voiding cystourethrogram be repeated 3 to 6 months after surgery to determine what other urologic procedures such as bladder augmentation or ureteral conduits may be necessary to prevent reflux and frequent infections. Surgery has been known to arrest the rapid worsening of existing scoliosis in tethered cord. However, severe scoliosis still requires surgical realignment and fixation with instrumentation and fusion.
The immediate benefits of total lipoma resection are due to the abrupt cessation of spinal cord tethering and are thus comparable to those from partial resection.22 However, the long-term benefits of total resection become very obvious if progression-free survival (PFS) of the two techniques are calculated for periods of 15 to 16 years. The total resection PFS for all lipoma types and clinical subgroups is 84% versus 34% for partial resection.26 When the select group of young children without symptoms or prior lipoma surgery is considered, the PFSs for total versus partial resection are 98.9% and 43%, respectively.26 Considering conservative management of lipoma without surgery carries a PFS of only 60%,83 these robust statistics argue strongly for total resection, not only for symptomatic patients but also as prophylactic treatment for all comers.26
Terminal Lipoma
There is no controversy surrounding the technique of resecting terminal lipomas, and the surgical result is essentially that of filum surgery. The terminal lipoma inserts directly into the caudal extremity of the conus. The junction between lipoma and conus is usually readily identifiable by the yellow color of the fat, the sparse vasculature compared to the conus, and the lack of nerve roots exit. Roots may become adherent to the ventral surface of the lipoma. It is important to identify and preserve “hitch hiker” roots arising from the conus but become adherent to the pia over the lipoma (see Fig. 60-3B). The conus–lipoma junction is then cut across transversely.
Split Cord Malformations
Embryogenesis, Morbid Anatomy, and Classification
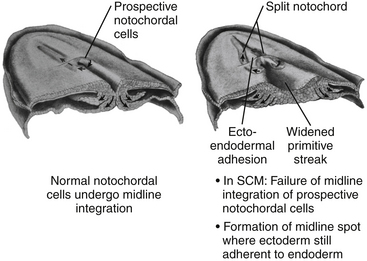
All double spinal cord malformations probably arise from a common embryogenetic error during gastrulation: a failure of prospective notochordal cells to achieve midline integration following their ingress through Hensen’s node, allowing the simultaneously lengthening ectoderm and endoderm to form an adhesion across this central fenestration in the notochord20,24,32,84 (eFig. 60-22). The subsequent incorporation of pluripotent mesenchyme into this adhesion constitutes the endomesenchymal tract, which not only permanently bisects the notochord but also forces each overlying hemineural plate to neurulate against its own heminotochord in a severely compromised manner. The basic malformation, therefore, consists of two heminotochords and two hemineural plates separated by a midline tract containing ectoderm, mesenchyme, and endoderm (eFig. 60-23). Further evolution of this basic form into the full-grown malformation depends on four factors: (1) the ability of the heminotochords and hemicords to achieve midline healing, (2) the interaction between each heminotochord and hemineural plate during neurulation, (3) persistence of the endomesenchymal tract, and (4) the developmental fates of the three germ elements.
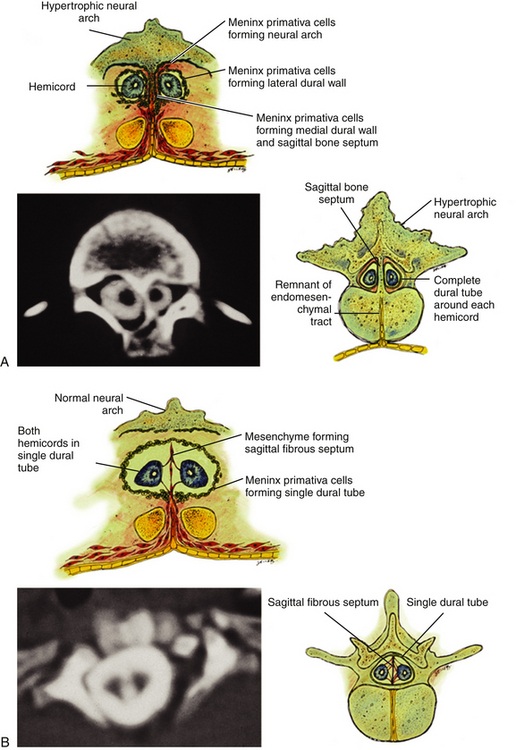
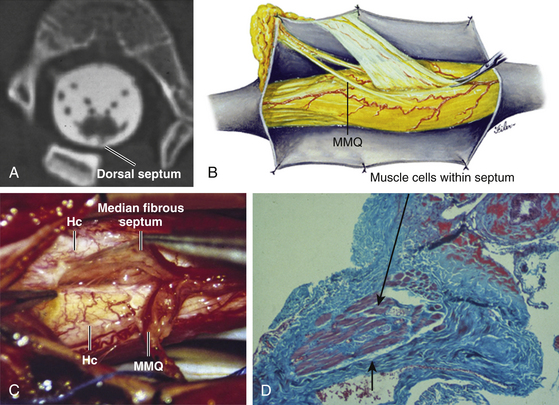
Classification of split cord malformations (SCM) into the well-known types I and II lesions depends on activities of specialized mesodermal cells destined to form dura and neural arch. These meninx primitive cells are normally found in the region between the notochord and neural tube around postovulation days 27 to 29 (eFigs. 60-2 and 60-23D), slightly later than the formation of the endomesenchymal tract. If they become incorporated into the endomesenchymal tract, a median dural layer forms next to the medial aspect of the hemicord and joins the dura that normally grows around the lateral aspect of the hemicord to complete a separate dural tube for each hemicord. Additionally, in accordance with their sclerogenic function, the meninx primitiva cells within the endomesenchymal tract facing away from the hemicords also form a midline bone spur between the two median dural walls, continuous with the bone of the developing vertebral centrum. This configuration, designated type I SCM, therefore consists of two hemicords each contained within its own dural tube, separated by a dura-sheathed rigid osseocartilaginous median septum (Fig. 60-1A). Inasmuch as the endomesenchymal tract frequently reaches the neural arches, the median bone spur bisects the spinal canal into two separate compartments. The spinal cord is transfixed solidly to the spinal canal by the bony and dural septum. The sclerogenic effect of the meninx primitiva cells when these admix with cells of the developing neural arches accounts for the often massively hypertrophic fusion of several adjacent laminae at the level of a type I SCM (Fig. 60-10A, lower right).
In contrast, the endomesenchymal tract in a type II SCM does not recruit meninx primitiva cells. A thin fibrous septum, texturally different from dura, will form from the “ordinary” mesenchyme in the space between the hemicords. Here also, no arachnoid, bone, or cartilage will form. Both hemicords will lie within a single arachnoid and dural tube inside a noncompartmentalized spinal canal, separated by a fibrous rather than a rigid osseocartilaginous median septum (Fig. 60-10B). However, this fibrous septum is always adherent to the medial aspect of the hemicords, and by virtue of its firm peripheral attachment to the ventral and/or dorsal dural wall, it is as real a tethering lesion as the bone spur of a type I SCM (Fig. 60-10B, lower right).
Operative Technique
Type I SCM
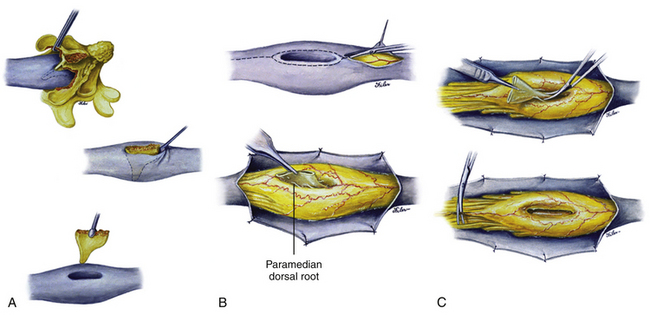
The extent of the laminectomy should include at least one level rostral and one caudal to the septum-bearing laminae. The hypertrophic laminae are rongeured away piecemeal around the attachment of the septum until only a small island of lamina is left attached to the dorsal end of the septum (Fig. 60-11A, upper). This affords a circumferential view of the bone spur still within its dural sleeve so that its dural attachment can be safely dissected off the bone deep within the cleft. However, once the dorsal support of the septum (by the laminae) is eliminated, the septum is no longer held rigidly at both ends and could be pushed from side to side depending on how solidly it is anchored ventrally. Excessive lateral movement of the septum thus may injure the subjacent hemicords and must be avoided. A Woodson dental elevator with a thin, angulated, sharp edge is well suited to peel off the dura with minimal lateral “wedging” motions (Fig. 60-11A, middle).
For most type I septa, their dorsal attachment is broad but their ventral junction with the vertebral body is usually flimsy; they could thus be easily avulsed from the dural cleft (Fig. 60-11A, lower). If the ventral attachment happens to be stout and bony, the septum may be carefully chipped away using a small rongeur. Complete extradural removal of the bone spur at this stage greatly facilitates later resection of the dural sleeve. Also, most type I septa are either purely bony or partly cartilaginous. Purely cartilaginous septa are rare. Embedded in the septum is a fairly constant central artery which can bleed briskly on avulsion. A deft plunge with some bone wax on a cotton patty deep into the cleft should handle the bleeding.
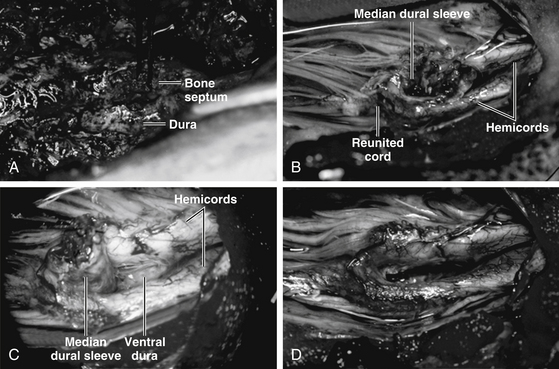
The dura is opened on both sides of the now-empty dural cleft to isolate the median dural sleeve (Fig. 60-11B, upper). The medial aspect of each hemicord is often tightly adherent to the dural sleeve by fibrous bands that must be cut. Paramedian dorsal nerve roots, when present in a type I lesion, typically stretch from the dorsomedial aspect of the hemicords to end blindly within the median dural sleeve (Fig. 60-11B, lower). These are nonfunctional and must be cut prior to resection of the dural sleeve. The dural sleeve is always wedged against the caudal reunion site of the hemicords, and the split cord rostral to the septum is therefore a safe, relatively spacious area to begin resection of the dural sleeve. Proceeding caudally from the rostral edge of the sleeve where the hemicords are least adherent, the surgeon cauterizes the ventral attachment of the sleeve to seal the central vessels and then cuts it flush with the ventral dural wall (Fig. 60-11C, upper). Not having the enclosed stump of the bone spur greatly simplifies the low resection of the sleeve. The most hazardous part of this undertaking is at the caudal end where the reunited “crotch” of the hemicords hugs tightly against the caudal end of the sleeve. The pressure on the crotch is readily felt here, and upward migration of the cord is often seen right after the sleeve resection.
Complete resection of the dural sleeve exposes the ventral extradural space in the sagittal midline (Fig. 60-11C, lower). Any remaining bony stump must now be trimmed down until it is no longer in contact with the ventral surface of the hemicords (Fig. 60-12). Closure of the anterior dural defect is unnecessary because of the abundant adhesions of the ventral dura to the posterior longitudinal ligament which would naturally prevent CSF leakage. Anterior dural closure is actually undesirable because the anterior suture line can potentially tether the hemicords.84 Posterior dural closure ultimately converts the double dural tubes into a single sac.
Frequently, a fibroneurovascular stalk containing paramedian dorsal nerve roots, fibrous bands, and large blood vessels (myelomeningocele manqué) tautly tethers the hemicords to the dorsal dura. These bands always penetrate the dura at a level more caudal than their origin from the hemicords, and they often form an exuberant tuft of fibroadipose tissue which, unlike ordinary loose extradural fat, clings tenaciously to the outer surface of the dura, and marks the underlying myelomengocoele manqué. These bands must be cut flush with the hemicords to complete the untethering process.
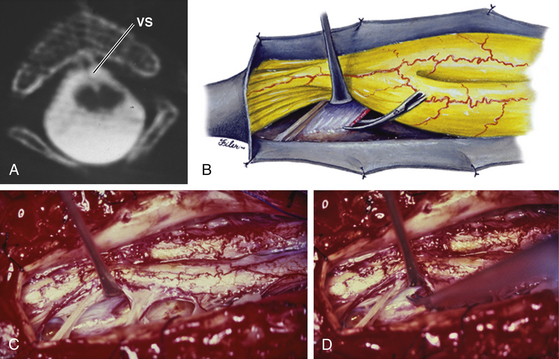
Type II SCM
In all cases of type II SCM, some form of fibrous (mesenchymal) septum or band is found within the midline cleft. Three patterns of such nonrigid median septa are encountered in type II lesions: (1) The least common is a complete fibrous septum stretching between the ventral and dorsal surfaces of the dural sac. Except for it being nonosseous, the complete fibrous septum transfixes the hemicords to the surrounding dura in the same manner as does the type I bony septum. (2) Slightly more common is the purely ventral fibrous septum. Its intimate adherence to the ventromedial aspects of the hemicords in effect anchors the cord ventrally where the septum arises from the ventral dura. (3) By far the most prevalent kind is the purely dorsal septum that attaches the dorsomedial aspects of the hemicords to the dorsal dura. A tuft of fibrovascular tissue in the extradural space is sometimes found connected to the septum through a small defect in the dorsal dura (Fig. 60-13B).
Hypertrophic and fused laminae, common in type I lesions, are seldom found in type II SCMs. In fact, the neural arches of type II lesions are often attenuated or even bifid. Laminectomy for these SCMs is technically easy and safe. A midline dural opening immediately exposes a dorsal or complete septum (Fig. 60-13) but a purely ventral septum has to be sought for either between the hemicords or by gently rotating the hemicords to one side (Fig. 60-14). Like the type I bony septa, all type II fibrous septa are found near the caudal end of the split. However, the length of the split in type II SCMs is, by comparison, much shorter than that of the type I SCM, and, because all fibrous septa are thin, the hemicords in type II SCM are apposed much closer together, with very little “free” part. Intracleft exploration is not recommended. Fortunately, the excessive width of the spinal canal should allow the hemicords to be rolled gently to one side for ventral inspection.
The point of attachment between hemicords and septum is invariably rostral to the point of attachment between dura and septum (Figs. 60-13 and 60-14). This is true of all three kinds of fibrous septa, giving testament to the fact that the fibrous septum was dragged upward by rostral movement of the cord after formation of the primordium of the septum. This upward dragging converts the incomplete (ventral or dorsal) septum into an oblique backward-pointing sheet and the complete septum into a V-shaped sheet with the apex pointing rostrally. Blood vessels often skirt the edge of the septum (Fig. 60-14C) or are loosely incorporated within the centromedian fibroneural bands. Very rarely, a true arteriovenous malformation is found within the median cleft. Unless these large vessels are contributing to the tethering of the hemicords, they should be left alone. Paramedian dorsal nerve roots and myelomeningocele manqués are commonly found in type II SCMs (Fig. 60-13), coursing dorsally from the dorsomedial aspect of the hemicords. The puny nerve roots usually end blindly in the septum but the more robust myelomeningocele manqués often penetrate the dorsal dura caudal to their hemicord origin (Fig. 60-13). These bands contribute to the tethering and must be cut. Untethering is completed by simply cauterizing the central vessels and excising the median fibrous septum. The ventral septum and bands come through a small defect in the ventral dura, presumably where the original endomesenchymal tract arose, but this small defect never leaks CSF and does not need to be repaired.
Special Circumstances
Composite SCMs and Multiple SCMs
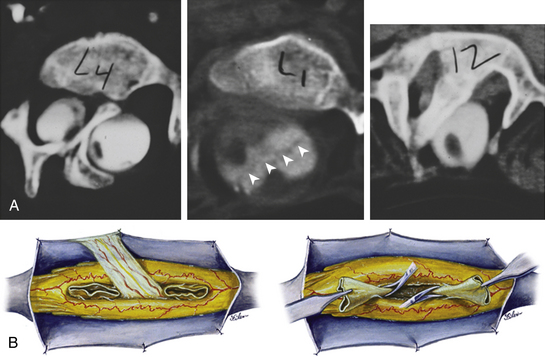
A composite SCM consists of two or more SCMs of differing types occurring in tandem, with no normal cord in between. The most common constituents of a composite SCM are a type I–type II–type I combination (Fig. 60-15A). Each individual component is typical of its kind: the type I lesion has an extradural bone spur and a median dural sleeve, and the type II lesion has a median fibrous septum within a single dural tube. The three median elements are continuous, suggesting that the entire lesion results from a single (but very large) endomesenchymal tract in which meninx primitiva precursor cells have been included at both ends to cause the type I lesion, but not in the middle where the median septum remains fibrous. The total length of the septa can sometimes span as many as seven vertebral levels. The split cord is coextensive with the septa so that the rostral and caudal reunion sites of the hemicords hug tightly the rostral margin of the rostral bone spur and the caudal margin of the caudal bone spur, respectively.
The dural opening for composite SCMs begins rostral to the rostral dural sleeve, skirts around it, returns to the midline over the type II lesion, and finally skirts around the second dural sleeve (Fig. 60-15B, upper). Resection of the middle type II fibrous septum should then be done to gain a “free” area within the midportion of the median cleft. This will provide working room to begin resection of the type I dural sleeves above and below: for the rostral dural sleeve, resection goes from caudal to rostral, whereas resection goes from rostral to caudal for the caudal dural sleeve. This way, the manipulation of the dural sleeve is always directed toward and never away from the tight reunion site of the hemicords at either end (Fig. 60-15B, lower). Release of the cord is accomplished with total excision of all three septa (eFig. 60-24).
If two or more SCMs occur in the same patient but are separated by an interval of normal spinal cord, they are true multiple SCMs. These are rare because they result from multiple endomesenchymal tracts, that is, from multiple embryologic errors in the same neural tube. The individual SCMs may be all type I or type II, or of mixed types.23 Their surgical treatment is as described.
Associated Dermal Sinus Tract and Dermoid Cyst
A dermal sinus tract is formed when the original connection between the endomesenchymal tract and the cutaneous ectoderm is retained (eFig. 60-25A). Because of this embryologic relationship, the deep end of the sinus tract is always in continuity with the mesenchymal median septum, but because the septum is either extra- or intrathecal depending on the SCM type, the clinical significance of the retained dermal sinus tract also varies with the SCM type.
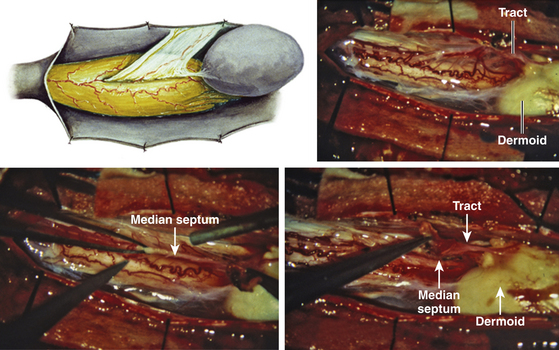
For a type I lesion, the dermal sinus tract can be traced all the way from the skin pit through defects in the myofascial layers and neural arches to the bone spur (eFig. 60-25B). Thus, the tract is always extradural and usually does not contribute to the tethering. It is removed together with the bone spur (eFig. 60-25A). Even if the tract may occasionally encyst to form a dermoid cyst, it lies outside the dural sac and seldom becomes large enough to cause compression of the hemicords.
In a type II SCM, however, the dermal sinus tract is of necessity intradural where it retains connection with the median fibrous septum. In its intradural course, the sinus tract is often densely adherent to the hemicords, thereby exerting a separate tethering effect (eFig. 60-26). In addition, over 50% of dermal sinus tracts in a type II SCM will develop a dermoid cyst within the dura, often large enough to cause cord compression. The entire intradural sinus tract and cyst must be excised to prevent recurrence. The cyst is first collapsed by intracapsular evacuation of its cheesy content and the cyst wall is then carefully peeled off the pial surface of the cord. Its deep end is removed with resection of the fibrous median septum (Fig. 60-16).
Associated Myelomeningocele and Hemimyelocele
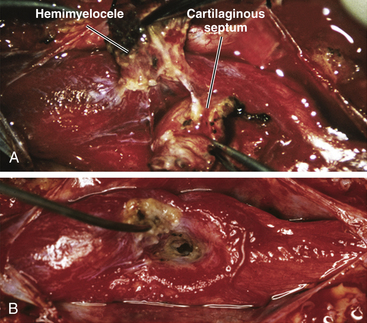
Approximately 25% to 35% of SCMs have an associated ONTD.20,22,23 Depending on whether one or both hemicords are involved in the dysraphic sac, the lesion may contain a hemimyelocele or a full-blown myelomeningocele, respectively.
In most myelomeningoceles, the open neural placode is terminal and thus is caudal to the SCM. Less commonly, the open placode is segmental and may therefore be rostral to the SCM. Because the open dysraphic sac is usually treated at birth, by the time the SCM is diagnosed by later neuroimaging studies and explored via a second surgical procedure, the original neural placode would have already developed dense adhesions to the dorsal dura. Because the placode is always adjacent to the split cord and may well be contributing to the tethering, it is always freed from the dura when the SCM is being treated. Depending on whether the placode is rostral or caudal to the SCM, it is carefully detached from the dorsal dura by sharp dissection either before or after the median septum is excised.
Most hemimyeloceles are segmental (eFig. 60-27). The unaffected hemicord is usually hidden from view by the median septum during the original sac closure, when the hemiplacode is mistaken to be the whole lesion. During the definitive procedure for the SCM, the entire dural sac (double or single) must be exposed to give access to both hemicords (Fig. 60-17). This often requires cutting into dense scar tissue. Removing the adjacent normal laminae helps to define the full width of the dura rostral to the scar. After identifying and resecting the median septum, the hemiplacode is detached from the dorsal dura with sharp dissection.
Associated Neurenteric Cyst and Other Intestinal Anomalies
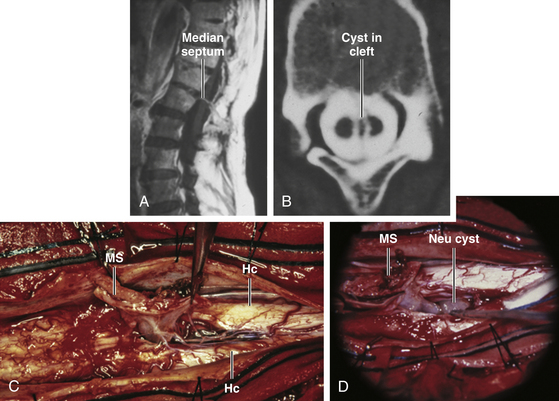
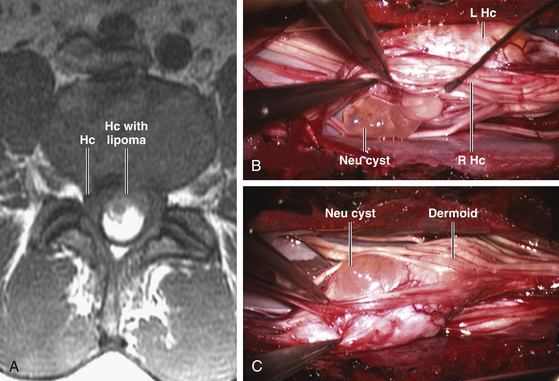
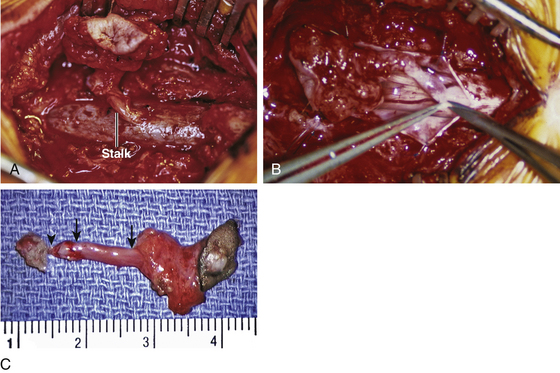
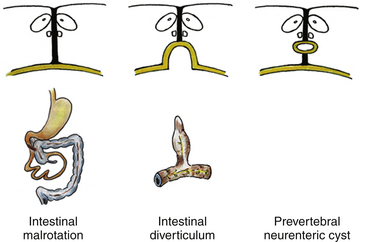
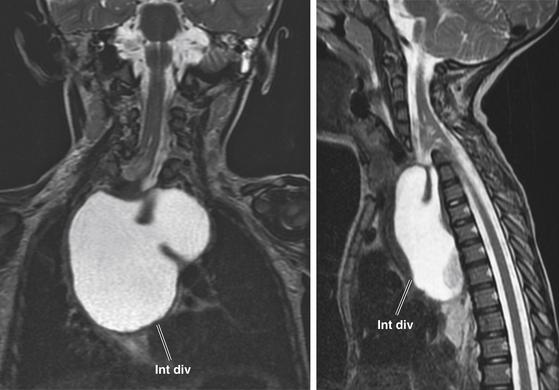
Since the ventral endomesenchymal tract is continuous with and partially composed of yolk sac endoderm, it is no surprise that enterogenous remnants and other intestinal anomalies coexist with SCMs. Neurenteric (enterogenous) cysts are rare (presumably because extrinsic induction of endodermal progenitor cells is fastidious) but have been found along the entire endomesenchymal tract, from subcutaneous layers to the prevertebral space in front of the split cord84 (eFig. 60-28). These cysts can cause compression when found in between the hemicords (Fig. 60-18) or as intramedullary masses within the hemicords (Fig. 60-19). They must be resected in toto to avoid recurrence.
Intestinal malrotation results when the ventral endomesenchymal tract remains as a tethering band in the proximal intestine (Ladd’s band) that prevents normal clockwise rotation of the primitive fore-gut (eFig. 60-29). If the portion of the endomesenchymal tract continuous with the yolk sac differentiates into mature bowel epithelium, a patent intestinal diverticulum linking small bowel with the prevertebral tissue may occur as an impressive mass lesion in the thoracic cavity (eFig. 60-30). This will require a combined thoracotomy and spinal exposure for complete resection.
Complications and Precautions
Neurologic Injury
Worsening of neurologic function occurs in less than 3% of patients following surgery for SCM. The surgical morbidity is higher with a type I SCM due to hemicord injury during removal of the bone spur. This is especially true when the bony septum is oblique and the delicate minor hemicord is tucked under the overhanging septum. The risk of hemicord injury is minimized by the following precautions:
1. Accurate preoperative depiction of the peculiar angulation of the bony septum and the relationship of the minor hemicord to the bony overhang.
2. Wide laminectomy at the site of the septum to improve exposure of both dural sacs. Without wide exposure, the oblique bone spur may be mistaken for the lateral wall of the spinal canal.
3. Extremely careful piecemeal resection of the dorsal end of the septum. As more of this end of the septum is bitten away, more of the minor dural tube is exposed.
Postoperative Bladder Management
Transient detrusor weakness and urinary retention is not uncommon after the resection of a type I SCM. It can last from a few days to several months and tends to be more common in adults than in children. Permanent worsening in bladder function occurs in about 3% of patients.20,23,24 A bladder catheter is left in the first two postoperative days when the patient is confined to complete bed rest. The catheter is removed on the third postoperative day as the patient is encouraged to ambulate and use bathroom facilities. If there is obvious difficulty in micturition or if a large postvoid residual urine volume is obtained repeatedly, the patient is given a cholinergic agent such as bethanechol chloride to strengthen detrusor contraction, or an α-sympathetic blocker such as prazosin hydrochloride to encourage relaxation of the internal urethral sphincter. If the postvoid residual urine volume remains high on medication, an intermittent catheterization program is started in the hospital and is continued on an outpatient basis. After 4 to 6 weeks, a cystometrogram is obtained to evaluate bladder function and to determine whether therapy can be discontinued.
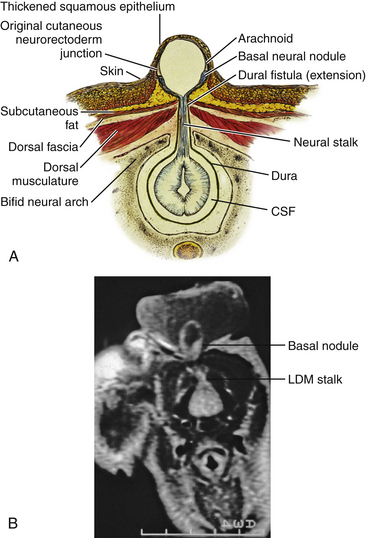
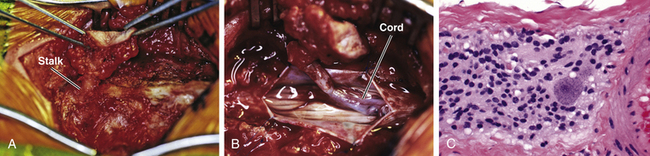
Limited Dorsal Myeloschisis
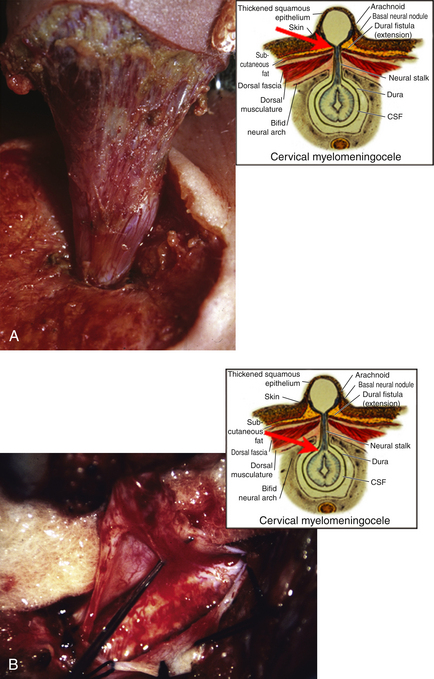
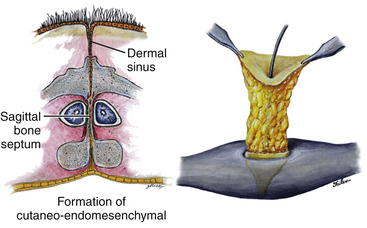
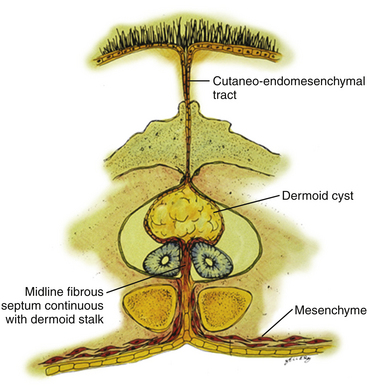
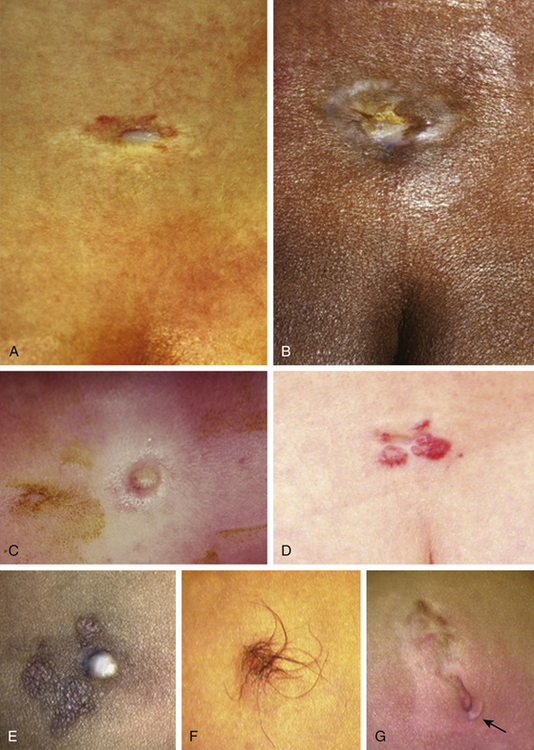
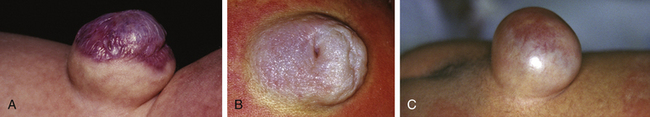
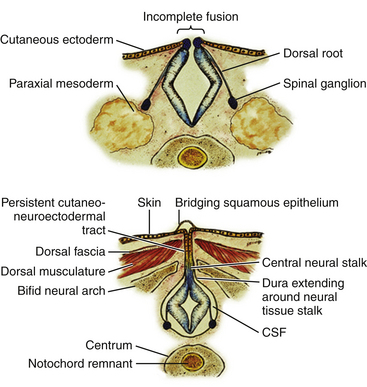
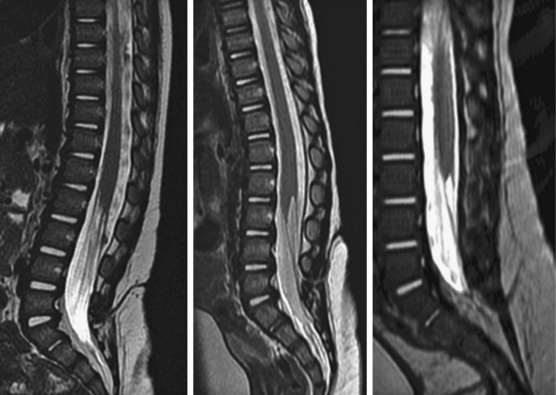
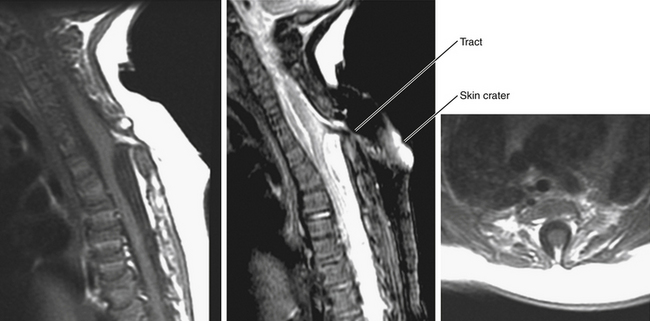
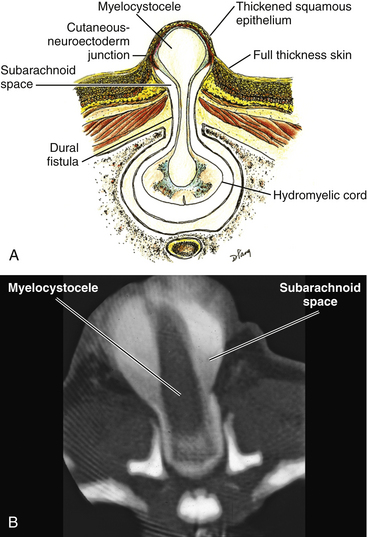
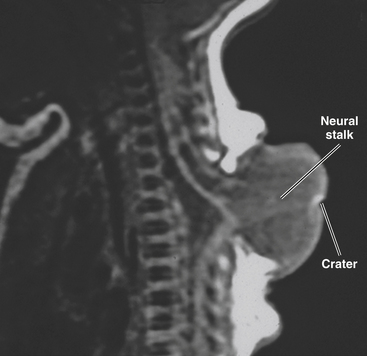
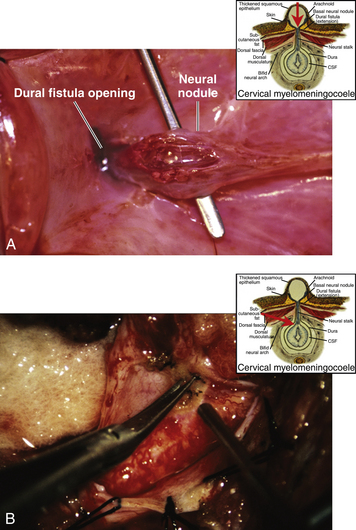
This form of spinal dysraphism is called transitional because it is neither open, with a leaking sac, nor is it truly closed, with its obvious cutaneous signature and a potentially patent tract. The basic structure of a limited dorsal myeloschisis (LDM) consists of a fibroneurovascular stalk containing neurons, glia, and nerves emanating from the dorsal surface of the spinal cord and penetrating through a narrow dorsal dural opening to reach the surface of the skin. The presence of an LDM is always indicated on the overlying skin by one of two main types of cutaneous manifestations: (1) the flat or nonsaccular type, consisting of a prominent dimple or crater made of pearly, squamous epithelium without an underlying dermis, often surrounded by elevated skin edges, hemangioma, or hair tufts (eFig. 60-31), and (2) the saccular type consisting of either a fluid-filled, fluctuant mass with a skin-covered base capped by a thick, purplish, raw-looking squamous epithelial membrane (eFig. 60-32A), a skin sac with an unobtrusive epithelial crater at the dome (eFig. 60-32B), or a sac with a thin epithelial cap that is not full-thickness skin (eFig. 60-32C). LDMs are rare lesions found in all areas of the spine, but more commonly in the lumbar and thoracolumbar regions.20,24 In the cervical region, LDMs are almost always of the sac type, particularly with the purplish lichenized squamous epithelial cap21,85 forming an imposing protrusion in the back of the newborn’s neck.
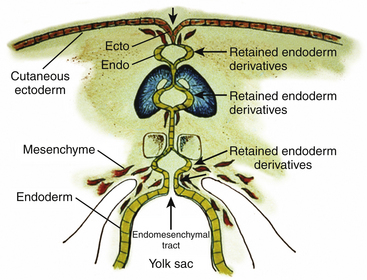
Embryogenesis
Embryogenetically, LDM is a defect of primary neurulation and thus never involves the conus. It probably differs from the open myelomeningocele only in the degree of the incompleteness of neurulation. In LDM, most of neurulation has occurred except for the final fusion of the opposed neural folds (hence dorsal myeloschisis). The basic configuration of the neural tube has taken shape but for a thin slip in the dorsal midline (hence limited). Here, disjunction between cutaneous ectoderm and neuroectoderm never truly happens, but the midline gap between the converging cutaneous ectoderm and dorsal scleromyotomes from opposite sides of the embryo remains very narrow (eFig. 60-33A). Further development of the full-thickness, dorsal myofascial tissues (except for a narrow strip in the midline) progressively sets the integument farther away from the neural tube, which ultimately retains its primarily intraspinal location. A dorsal median stalk of central nervous system tissue, however, remains as the original link between the nearly closed neural tube and the still slightly gaping cutaneous ectoderm (eFig. 60-33B). Underneath, normal meninges develop around the neural tube and extend around the midline neural stalk as a sleeve that projects to the surface (eFigs. 60-34 and 60-35). Superficially, the small dimple or pearly thin membrane belies the small unclosed gap of the cutaneous ectoderm. In the flat or nonsaccular LDM, thick squamous epithelium grows across and bridges this gap, even though this membrane lacks a full-thickness epidermis and dermis.21 In the saccular LDM, the formation of the requisite fibroneural stalk linking cutaneous with neuroectoderm is the same as in the flat type, but in the sac type, CSF squeezes into the dorsal dural fistula around the neural stalk and ultimately distends the thinner, less well-supported squamous epithelial portion of the gap into a fluid-filled and mostly skin-covered sac (eFig. 60-36A). The crater, dimple, and otherwise “un-skin-like” cap of the dome always belie the original dorsal myeloschisis.
The internal structure of the saccular LDM dorsal to the fibroneural stalk can be of three forms. If there is an associated hydromyelia in the part of the cord bearing the dorsal myeloschisis, CSF may distend the center of the distal neural stalk dorsal to the myofascia into a large myelocystocele within the meningocele sac21,86 (eFig. 60-36). If there is no hydromyelia, the neural stalk remains solid and narrow and fans out on the dome of the sac (eFig. 60-37). Or the CSF pressure within the sac may compress the neural stalk ventrally into a series of basal neural nodules lining the bottom of the sac (eFig. 60-38). In all 3 variants, the spinal cord underneath the sac is tethered to the myofascial tissue by way of the fibroneural stalk and its meningeal investment. Neurologic deficits develop because of this tethering effect and vary according to the location of the LDM.
Surgical Technique
LDMs are frequently associated with other tethering lesions that should be simultaneously treated. For example, a thickened filum is invariably found in thoracolumbar and lumbar LDMs and in some cervical LDMs; about 50% of cervical LDMs have coexisting Chiari II malformation21,85 and hydrocephalus; and SCMs have also been reported.
The operation for the crater-type LDM is usually uncomplicated (Fig. 60-20). The superficial crater or pit is excised with an elliptical cuff of skin; the fibroneural stalk is followed through to the laminar defect and ultimately to the dorsal dura after laminectomy. The fibroneural stalk itself may be thin or thick, but the principle is always to resect it flush with the dorsal surface of the cord and leave as inconspicuous a scar as possible (Fig. 60-21 and eFigs. 60-39 and 60-40).
The operation for saccular LDMs is the same whether the internal structure of the sac contains a myelocystocele, a basal neural nodule, or a slender, simple fibroneural band. The skin incision incorporates an elliptical split around the base of the sac exposing at least one set of intact laminae both above and below the dural-neural stalk. The often thick subcutaneous tissues are carefully dissected to reveal the funnel of dura at the base of the sac where the discrete stalk penetrates the myofascial layer (Fig. 60-22A and eFig. 60-41A). Laminectomy is done to display the normal dural tube rostral and caudal to this dural fistula, which is thus seen in-continuity with the dorsal dural outpouching. The dura is opened in the midline to show the fibroneural stalk and the almost always discrete attachment of the stalk with the spinal cord (Fig. 60-22B). The stalk is simply cut flush with the surface of the cord (eFig. 60-41B), and the dura is closed primarily. The superficial sessile sac, now detached, can be resected en masse.
Chapman P.H. Congenital intraspinal lipomas. Anatomic considerations and surgical treatment. Child’s Brain. 1982;9:37-47.
Chapman P.H., Davis K.R. Surgical treatment of spinal lipomas in childhood. Pediatr Neurosurg. 1993;19:267-275.
Dias M., Pang D. Human neural embryogenesis: a description of neural morphogenesis and a review of embryonic mechanisms. In: Pang D., editor. Disorders of the Pediatric Spine. New York: Raven Press, 1994.
Kulkarni H.V., Pierre-Kahn A., Zerah M. Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery. 2004;54:868-875.
La Marca F., Grant J.A., Tomita T., McLone D.G. Spinal lipomas in children: outcome of 270 procedures. Pediatr Neurosurg. 1997;26:8-16.
McLone D.G., Naidich T.P. Spinal dysraphism: experimental and clinical. In: Holtzman R.N., Stein B.M. The Tethered Spinal Cord. New York: Thieme-Stratton, 1985.
McLone D.G., Suwa J., Collins J.A., et al. Neurulation: Biochemical and morphological studies on primary and secondary neural tube defects. Concepts Pediatr. Neurosurg. 1983;4:15-29.
Muller F., O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at Stage 12. Anat Embryol (Berl). 1974;176:413-430.
O’Rahilly R., Meyer D.B. The timing and sequence of events in the development of the human vertebral column during the embryonic period proper. Anat Embryol (Berl). 1973;157:167-176.
O’Rahilly R., Müller F. Developmental Stages in Human Embryos, Including a Revision of Streeter’s Horizons and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institution of Washington; 1987.
Pang D.. Electrophysiological monitoring for tethered cord surgery, Yamada S., editor. Tethered cord syndrome, 2nd ed, New York and Stuttgart: Thieme, 2010.
Pang D. Split cord malformations, theories and practice. In: Batjer H., Loftus C. Textbook of Neurological Surgery. Philadelphia: Lippincott, Williams & Wilkins; 2002:916-945.
Pang D. Ventral tethering in split cord malformation. Neurosurg Focus. 2001;0(1):e6.
Pang D. Surgical complications of open spinal dysraphism. Neurosurg Clin North Am. 1995:6243-6257.
Pang D. Cervical myelomeningoceles. Neurosurgery. 1993;33(3):363-373.
Pang D. Split cord malformation. Part II: the clinical syndrome. Neurosurgery. 1992;31:481-500.
Pang D., Dias M., Ahdab-Barmada M.. Split cord malformation. Part I: A unified theory of embryogenesis for double spinal cord malformation. Neurosurgery, 1992;31:451-480
Pang D., Zovickian J.G., Oviedo A. Long term outcome of total and near total resection of spinal cord lipomas and radical reconstruction of neural placode. Part II: Outcome analysis and preoperative profiling. Neurosurgery. 2010;66(2):253-273.
Pang D., Zovickian J.G., Ovieda A. Long-term outcome of total and near total resection of spinal cord lipomas and radical reconstruction of the neural placode. Part I: surgical technique. Neurosurgery. 2009;65:511-529.
Pierre-Kahn A., Lacombe J., Pichon J., et al. Intraspinal lipomas with spina bifida: prognosis and treatment in 73 cases. J Neurosurg. 1986;65:756-761.
Pierre-Kahn A., Zerah M., Renier D., et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997;13:298-334.
Saitsu H., Yamada S., Uwabe C., et al. Development of the posterior neural tube in human embryos. Anat Embryol (Berl). 2004;209:107-117.
Schoenwolf G.C. Histological and ultrastructural observations of tail bud formation in the chick embryo. Anat Rec. 1979;193:131-148.
Schoenwolf G.C., Nichols D.H. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 1984;169:361-376.
Steinbok P. Dysraphic lesions of the cervical spinal cord. Neurosurg Clin North Am. 1995;6:367-376.
Numbered references appear on Expert Consult.
eFIGURE 60-17 Completed resection of lipoma, leaving thin, supple, purely neural placode free from all adhesions.
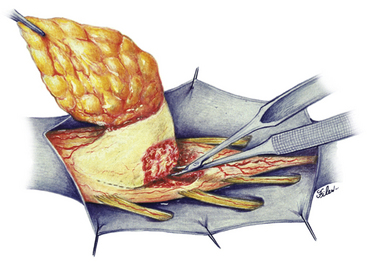
eFIGURE 60-18 Resection of dorsal lipoma can be done with a completely circumscribed perspective from all sides of the fusion line.
1. Warkany J. Morphogenesis of spina bifida. In: McLaurin R.L., editor. Myelomeningocele. New York: Grune and Stratton; 1977:31.
2. O’Rahilly R., Müller F. Developmental Stages in Human Embryos, Including a Revision of Streeter’s Horizons and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institution of Washington; 1987.
3. Saitsu H., Yamada S., Uwabe C., et al. Development of the posterior neural tube in human embryos. Anat Embryol (Berl). 2004;209:107-117.
4. Saitsu H., Yamada S., Uwabe C., et al. Aberrant differentiation of the axially condensed tail bud mesenchyme in human embryos with lumbosacral myeloschisis. Anat Rec. 2007;290:251-258.
5. Sapunar D., Vilović K., England M., Saraga-Babić M. Morphological diversity of dying cells during regression of the human tail. Ann Anat. 2001;183:217-222.
6. Detwiler S.R., Hotzer H. The inductive and formative influence of the spinal cord upon the vertebral column. Bull Hosp Jt Dis Orthop Inst. 1954;15:114-123.
7. Sensenig E.C. The early development of the meninges of the spinal cord in human embryos. Contrib Embryol. 1949;33:21-41.
8. Pang D. Surgical complications of open spinal dysraphism. Neurosurg Clin North Am. 1995;6:243-257.
9. Kallen B. Early embryogenesis of central nervous system with special reference to closure defects. Dev Med Child Neurol. 1968;19(suppl):44-53.
10. McLone D.G., Dias M. Complications of myelomeningocele closure. Pediatr Neurosurg. 1991-1992;17:267.
11. Habal M.B., Vried J.K. Tension-free closure of large meningomyelocele defects. Surg Neurol. 1977;8:177-180.
12. Ramirez O.M., Ramasastry S.S., Granick M.S., et al. A new surgical approach to closure of large lumbosacral meningomyelocele defects. Plast Reconstr Surg. 1987;80:799-807.
13. Ames M.D., Shut L. Results of treatment of 171 consecutive myelomeningoceles 1963 to 1968. Pediatrics. 1972;50:466.
14. McLone D.G. Treatment of myelomeningocele: arguments against selection. Clin Neurosurg. 1986;33:359.
15. Reigel D.H., McLone D.G. Myelomeningocele: operative treatment and results. In: Marlin A.E., editor. Concepts in Pediatric Neurosurgery. Basel: S. Karger; 1988:41.
16. McLone D.G. Technique for closure of myelomeningocele. Childs Brain. 1980;6:65.
17. Ramasastry S.S., Cohen M. Soft tissue closure and plastic surgical aspects of large open myelomeningoceles. Neurosurg Clin North Am. 1995;6:279-291.
18. McLone D.G., Naidich T.P. Myelomeningocele: outcome and late complications. In: McLaurin R.L., Schut L., Venes J.L. Pediatric Neurosurgery. 2nd ed. Philadelphia: WB Saunders; 1989:53.
19. Pang D. Commentary to the paper double neural tube defect: a case report and discussions on neural tube development by V Ravindran. Childs Nerv Syst. 2010;26(5):703.
20. Pang D. Split cord malformation. Part II: the clinical syndrome. Neurosurgery. 1992;31:481-500.
21. Pang D. Cervical myelomeningoceles. Neurosurgery. 1993;33(3):363-373.
22. Pang D., Zovickian J.G., Ovieda A. Long-term outcome of total and near total resection of spinal cord lipomas and radical reconstruction of the neural placode. Part I: surgical technique. Neurosurgery. 2009;65:511-529.
23. Pang D. Split cord malformations: theories and practice. In: Batjer H., Loftus C. Textbook of Neurological Surgery. Philadelphia: Lippincott, Williams & Wilkins; 2002:916-945.
24. Pang D., Dias M., Ahdab-Barmada M.. Split cord malformation. Part I: a unified theory of embryogenesis for double spinal cord malformation. Neurosurgery, 1992;31:451-480
25. Pang D. Surgical management of spinal dysraphism. In: Fessler R., Sekhar L. Atlas of Neurosurgical Techniques. New York: Thieme Medical and Scientific Publishers; 2006:729-758.
26. Pang D., Zovickian J.G., Oviedo A. Long-term outcome of total and near total resection of spinal cord lipomas and radical reconstruction of neural placode. Part II: outcome analysis and preoperative profiling. Neurosurgery. 2010;66(2):253-273.
27. Brunelle F., Sebag G., Baraton J., et al. Lumbar spinal cord motion measurement with phase-contrast MR imaging in normal children and in children with spinal lipomas. Pediatr Radiol. 1996;26(4):265-270.
28. Dick E.A., deBruhn R. Ultrasound of the spinal cord in children: its role. Eur Radiol. 2003;13(3):552-562.
29. Chapman P.H. Congenital intraspinal lipomas. Anatomic considerations and surgical treatment. Childs Brain. 1982;9:37-47.
30. Arai H., Sato K., Wachi A. Surgical management in 81 patients with congenital intraspinal lipoma. Childs Nerv Syst. 1992;8:171.
31. Pierre-Kahn A., Zerah M., Renier D., et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997;13:298-334.
32. Dias M., Pang D. Human neural embryogenesis: a description of neural morphogenesis and a review of embryonic mechanisms. In: Pang D., editor. Disorders of the Pediatric Spine. New York: Raven Press, 1994.
33. Hamilton H.L., Boyd J.D., Mossman H.M. Human Embryology, 4th ed. Baltimore: Williams & Wilkins; 1972. 437–525
34. Kunitomo K. The development and reduction of the tail and of the caudal end of the spinal cord. Contrib Embryol Carnegie Inst. 1918;8:163-198.
35. Streeter G.L. Factors involved in the formation of the filum terminalis. Am J Anat. 1919;25:1-12.
36. Barson A.J. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106:489-497.
37. Jone P.H., Love J.G. Arch Surg. 1956;73:556-566.
38. Caldarelli M., McLone D.G., Colins J.A., et al. Vitamin A induced neural tube defects in a mouse. Concepts Pediatr Neurosurg. 1985;6:161-171.
39. Caldarelli M., McLone D.G., Collins J.A., et al. Vitamin A induced neural tube defects in a mouse. Concepts Pediatr Neurosurg. 1985;6:161-171.
40. McLone D.G., Suwa J., Collins J.A., et al. Neurulation: biochemical and morphological studies on primary and secondary neural tube defects. Concepts Pediatr Neurosurg. 1983;4:15-29.
41. Marin-Padilla M.. Clinical and experimental rachischisis, Vinken P.S., Bruyn G.W., editors, Handbook of Clinical Neurology, Amsterdam, North-Holland, 1978;vol. 32:159-191.
42. Marin-Padilla M. Mesodermal altercations induced by hypervitaminosis A. J Embryol Exp Morph. 1966;15:261-269.
43. Marin-Padilla M. Morphogenesis of anencephaly and related malformations. Curr Top Pathol. 1970;51:145-174.
44. Marin-Padilla M. Morphogenesis of experimentally induced Arnold-Chiari malformation. J Neurol Sci. 1981;50:29-55.
45. Marin-Padilla M. Morphogenesis of experimentally induced encephalocele (cranioschisis occulta). J Neurol Sci. 1980;46:83-99.
46. Marin-Padilla M. Notochordal-basochondrocranium relationships: abnormalities in experimentally induced axial skeletal (dysraphic) disorders. J Embryol Exp Morph. 1979;53:15-38.
47. Marin-Padilla M. The tethered cord syndrome: developmental considerations. In: Holtzmann R.N.N., Stein B.M. The Tethered Spinal Cord. New York: Thieme; 1985:3-13.
48. McLone D.G., Knepper P.A. Role of complex carbohydrates and neurulation. Pediatr Neurosci. 1986;1:2-9.
49. Morris-Kay G.M., Crutch B. Culture of rat embryos with B-D-xyloside: evidence of a role for proteoglycans in neurulation. J Anat. 1982;134:491-506.
50. O’Shea K.S., Kaufmann M.H. Phospholipace C-induced neural tube defects in the mouse embryo. Experientia. 1980;36:1217-1219.
51. Toole B.P. Glycosaminoglycans in morphogenesis. In: Hay E., editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1981:229-294.
52. McLone D.G., Naidich T.P. Spinal dysraphism: experimental and clinical. In: Holtzman R.N., Stein B.M. The Tethered Spinal Cord. New York: Thieme-Stratton, 1985.
53. Pang D. Spinal cord lipomas. In: Pang D., editor. Disorders of the Pediatric Spine. New York: Raven Press; 1995:175-201.
54. Pang D. Spinal cord lipoma. In: Batjer H., Loftus C. Textbook of Neurological Surgery: Principles and Practice. Philadelphia: Lippincott, Williams & Wilkins, 2002.
55. Pang D. Tethered cord syndrome. In: Hoffman H.J., editor. Advances in Pediatric Neurosurgery. Philadelphia: Hanley and Belfus; 1986:45-79.
56. Schoenwolf G.C., Nichols D.H. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 1984;169:361-376.
57. Muller F., O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at Stage 12. Anat Embryol (Berl). 1974;176:413-430.
58. O’Rahilly R., Meyer D.B. The timing and sequence of events in the development of the human vertebral column during the embryonic period proper. Anat Embryol (Berl). 1973;157:167-176.
59. Schoenwolf G.C. Histological and ultrastructural observations of tail bud formation in the chick embryo. Anat Rec. 1979;193:131-148.
60. Talwalker V.C., Datsur D.K. Meningoceles and meningomyeloceles (ectopic spinal cord). Clinicopathological basis of a new classification. J Neurol Neurosurg Psychiatry. 1970;33:251-262.
61. Talwalker V.C., Datsur D.K. Ectopic spinal cord myelomeningocele with tethering: a clinicopathological entity. Dev Med Child Neurol. 1974;16(suppl 32):159-160.
62. Pang D.. Electrophysiological monitoring for tethered cord surgery, Yamada S., editor. Tethered Cord Syndrome, 2nd ed, New York: Thieme, 2010.
63. Pang D. Use of an anal sphincter pressure monitor during operations on the sacral spinal cord and nerve roots. Neurosurgery. 1983;13:562-568.
64. Chapman P.H., Davis K.R. Surgical treatment of spinal lipomas in childhood. Pediatr Neurosurg. 1993;19:267-275.
65. Pierre-Kahn A., Lacombe J., Pichon J., et al. Intraspinal lipomas with spina bifida: prognosis and treatment in 73 cases. J Neurosurg. 1986;65:756-761.
66. Xenos C., Sgouros S., Walsh R., Hockley A., et al. Spinal lipomas in children. Pediatr Neurosurg. 2000;32:295-307.
67. McGuire E.J. The innervation and function of the lower urinary tract. J Neurosurg. 1986;65:278-285.
68. Atala H., Sato K., Wachi A. Bladder functional changes resulting from lipomyelomeningocele repair. J Urol. 1992;148:592-595.
69. Cochrane D.D., Finley C., Kestle J., et al. The patterns of late deterioration in patients with transitional lipomyelomeningocele. Eur J Pediatr Surg. 2000;10(suppl 1):13-17.
70. Hoffman H.J., Taecholarn C., Hendrick E.B., et al. Management of lipomyelomeningoceles. J Neurosurg. 1985;62:1-8.
71. James H.E., Canty T.G. Human tails and associated spinal anomalies. Clin Pediatr (Phila). 1995;34:286-288.
72. James C.C.M., Lassman L.P. Diastematomyelia and the tight filum terminale. J Neurol Sci. 1970;10:193-196.
73. James C.C.M., Williams J., Brock W., et al. Radical removal of lipomas of the conus and cauda equina with laser microsurgery. Neurosurgery. 1984;13:340-345.
74. Kanev P.M., Lemire R.J., Loeser J.B., Berger M.S. Management and Long-term follow-up review of children with lipomyelomeningocele, 1952-1987. J Neurosurg. 1990;74:48-52.
75. Koyanagi I., Iwasaki Y., Hida K., et al. Surgical treatment supposed natural history of the tethered cord with occult spinal dysraphism. Childs Nerv Syst. 1997;13:268-274.
76. La Marca F., Grant J.A., Tomita T., McLone D.G. Spinal lipomas in children: outcome of 270 procedures. Pediatr Neurosurg. 1997;26:8-16.
77. McGuire E.J., Woodside J.R., Borden T.A., Weiss R.M. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205-209.
78. McLone D.G., Naidich T.P. Laser resection of fifty spinal lipomas. Neurosurgery. 1986;18:611-615.
79. Sathi S., Madsen J.R., Bauer S., Scott R.M. Effect of surgical repair on neurologic function in infants with lipomeningocele. Pediatr Neurosurg. 1993;19:256-259.
80. Sutton L.N. Lipomyelomeningocele. Neurosurg Clin North Am. 1995;6:325-338.
81. Hoffman H.J., Hendrick E.B., Humphreys R.P. The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain. 1976;2:145-155.
82. Pang D., Wilberger J.E. Tethered cord syndrome in adults. J Neurosurg. 1982;57:32-47.
83. Kulkarni H.V., Pierre-Kahn A., Zerah M. Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery. 2004;54:868-875.
84. Pang D. Ventral tethering in split cord malformation. Neurosurg Focus. 2001;10:e6.
85. Steinbok P., Cochrane D.D. The nature of congenital posterior cervical or cervicothoracic midline cutaneous mass lesions. Report of eight cases. J Neurosurg. 1991;75:206-212.
86. Steinbok P. Dysraphic lesions of the cervical spinal cord. Neurosurg Clin North Am. 1995;6:367-376.