CHAPTER 34 Surgical Management of Rheumatoid Arthritis
Rheumatoid arthritis is a chronic, progressive, systemic disease with widespread involvement of connective tissues and primarily synovial joints. Women are affected two to three times more often than men with most cases manifesting in the 4th or 5th decades, although patients may be affected at any age. More recent improvements in medical management have correlated with a decrease in hospitalizations for severe rheumatoid disease; however, one study found that although admission for treatment of several medical complications of rheumatoid arthritis decreased significantly from 1983-2001, the rate of admission for cervical spine surgery in this population remained unchanged.1 The cervical spine is involved in 25% to 90% of patients with rheumatoid arthritis, making it the most commonly affected site after the metatarsophalangeal and metacarpophalangeal joints.2 In contrast to rheumatoid arthritis of the thoracic and lumbar spine, cervical spine involvement carries significant risks of spinal cord and medullary and vertebral artery compromise and sudden death.3,4
Numerous patients with cervical instability may be asymptomatic or may have symptoms indistinguishable from symptoms in patients without instability. Neva and colleagues5 reviewed 154 patients with rheumatoid arthritis who were on a waiting list for various orthopaedic procedures at a Finnish hospital. Overall, 44% of patients had radiographic evidence of cervical subluxation or prior cervical fusion. When excluding patients with previous fusions, these investigators noted that 69% of patients with radiographic evidence of subluxations reported neck pain compared with 65% of patients without subluxation. Similarly, rates of occipital, temporal, retro-orbital, and upper extremity radicular pain were not significantly different in patients with or without cervical instability. Many patients with significant cervical involvement may be overlooked by the treating physician because of the lack of clear neurologic deficits on physical examination. The physician must be cognizant of the natural history, pathophysiology, clinical presentation, radiologic findings, and treatment options to avoid the grave consequences of rheumatoid disease of the cervical spine.
Historical Perspective
There is some evidence that rheumatoid arthritis was described in the 17th and 18th centuries.6,7 More concrete evidence points to a period around 1800, according to a description by Landré-Beauvais, a French medical student, in his doctoral thesis.8 The description of rheumatoid arthritis of the cervical spine occurred much later, with a report in 1890 by Garrod.9
Interest increased in the 1950s and 1960s with reports describing cervical instability.10–13 Articles on the surgical treatment of cervical instability in patients with rheumatoid arthritis followed in the 1960s and 1970s.14–18 For the most part, C1-2 fusion with Gallie-type19 wiring and graft techniques were used early on. Brooks and Jenkins,20 Wertheim and Bohlman,21 and Clark and colleagues22 subsequently described modified wiring techniques. Other types of C1-2 fixation emerged later with the use of the Halifax interlaminar clamp,23 the Magerl transarticular screw technique,24–28 and posterior C1 lateral mass–C2 pedicle screw fixation.29
Foerster30 originally described occipitocervical procedures in 1927. Kahn and Yglesias31 were the first to add iliac crest bone graft to occipitocervical fusion in 1935. Hamblen16 later described the combined use of wires and iliac crest graft for occipitocervical fusion in four patients with rheumatoid arthritis and cervical instability in 1967. In a 1976 report, Brattström and Granholm14 modified the wiring technique for occipitocervical fusion by adding methyl methacrylate. Modifications of occipitocervical fusion techniques continued in the 1980s with the emergence of the looped rod with iliac crest bone graft, as described by Ransford 32 and Flint 33 and their colleagues and later occipitocervical plating, as described by Grob34,35 and Smith36 and their colleagues. Current use of plate or rod fixation extends to the subaxial spine, especially with fixation of the lateral masses described by Magerl and Roy-Camille.25–27
Pathophysiology
The inflammatory process in the cervical spine mirrors the inflammatory process in other sites of the body and consists predominantly of T lymphocytes, macrophages, and plasma cells in a hypervascular synoviocyte pannus.37,38 The inflammatory reaction has a predilection for synovial joints, and the multiple synovial joints39 of the cervical spine (facets, uncovertebral, atlanto-occipital, atlantodens) are particularly affected. In contrast to the thoracic and lumbar spine, the cervical spine relies heavily on ligaments and joint congruency for stability and so is at greater risk for instability. With significant inflammation, pannus formation and erosion of the joint and capsular structures can occur. Progressive instability can result, manifesting as atlantoaxial (C1-2) instability, subaxial subluxation, cranial settling (also known as basilar invagination or atlantoaxial impaction), or a combination thereof. Rheumatoid discitis may also occur at the discovertebral junctions with noninfectious erosion of the endplates.3
Atlantoaxial instability is the most common instability pattern in the rheumatoid cervical spine and may be the result of the development of periodontoid pannus and progressive inflammatory destruction of the upper cervical spine.39–41 Pannus formation localizes around the synovial joint formed between the transverse ligament, the posterior arch of the atlas, and the base of the odontoid. The soft tissue may compress the spinal cord and often distends and erodes the surrounding periodontoid-ligamentous structures (alar, apical, and transverse ligaments), the odontoid process (dens), and the lateral articular masses between C1 and C2.42 Erosion of the dens may lead to the development of occult, atraumatic odontoid fractures.43 Subsequent static or dynamic instability or subluxation occurs in 50% to 70% of patients.18,44 Most often, the subluxation is anterior (70%), but lateral, posterior, and rotational subluxations can also occur.45,46 Anterior subluxation of 0 to 3 mm is normal in adults, subluxation of 3 to 6 mm suggests instability with disruption of the transverse ligament, and subluxation of 9 mm or more suggests disruption of the entire periodontoid-ligamentous and capsular structures with gross instability and is a clear indication for surgery.18,47,48
The combination of periodontoid pannus buildup and instability may cause spinal cord and root impingement leading to myeloradiculopathy and sudden death. Delamarter and Bohlman49 conducted postmortem studies suggesting that paralysis can be due to mechanical neural compression, vascular impairment of the neural structures, or both. Henderson and colleagues50 reported that diffuse axonal injury with or without frank necrosis may irreversibly affect the spinal cord owing to mechanical damage. These findings may partly explain the smaller diameter and cross-sectional area of the spinal cord found in patients with severe disease.51 Some evidence suggests that instability promotes worsening periodontoid pannus formation and that stabilization reverses the buildup.40
Posterior atlantoaxial subluxation is rare and should raise the possibility of an anterior arch defect of the atlas or erosion or fracture of the odontoid.48 Patients usually present with myelopathy and posterior kinking of the cord without radiographic compression.52 Lateral subluxation is defined as more than 2 mm of lateral displacement of the C1-2 lateral masses and occurs in 21% of atlantoaxial subluxations.48 Lateral subluxations occur more commonly in patients with spinal cord compression than patients without compression and are often accompanied by rotational subluxation.53
Subaxial subluxation is the second most common instability pattern and results from destruction of the facet joints, interspinous ligaments, and discovertebral joints.44,54 These pathologic changes can lead to longitudinal collapse, bony erosion, soft tissue hypertrophy, and sagittal plane instability along multiple spinal segments, causing a “stepladder”-type pattern of deformity. Concurrent spondylodiscitis may occur with pain, neural compression, and instability.
Natural History
It is difficult to study the natural history of rheumatoid arthritis because patients are usually treated at some stage of the disease and it would be unethical to do otherwise. From studies on rheumatoid arthritis, it seems consistent that the inflammatory processes in the cervical spine begin early after the onset of rheumatoid arthritis and progress along with peripheral involvement. Atlantoaxial instability may be detected within 2 to 10 years of disease onset in most patients,55 and there is a strong correlation between cervical spine subluxation and peripheral erosions of the hands and feet.56 Approximately 10% of patients with cervical spine involvement eventually require surgery.57
The natural history of rheumatoid arthritis of the spine without surgical intervention, especially in patients with myelopathy, seems to be progressive disability and risk of sudden death. In one study of 21 patients treated medically, 76% showed deterioration at an average of 6 years of follow-up. All patients in the study became bedridden within 3 years of developing myelopathy, and all died within 7 years with one third dying suddenly for unknown reasons.58 Risk factors for progression include mutilating articular disease, history of high-dose corticosteroid use, high seropositivity, rheumatoid subcutaneous nodules, vasculitis, and male gender. Other potential but unproven risk factors for cervical involvement include high C-reactive protein level and HLA-Dw2 or HLA-B27 positivity.59,60 As the disease progresses, pain, neurologic deficits, and sudden death are the primary risks.
Clinical Presentation
Neck pain is the most common symptom in patients with cervical spine involvement. Peripheral erosive changes similarly occur along the apophyseal joints and surrounding soft tissues and may be a source of pain. Cervical instability may cause secondary impingement of the posterior rami of the lesser and greater occipital nerves, which may lead to occipital headaches. Pain in the suboccipital region generally suggests atlantoaxial pathology or cranial settling. Middle or lower cervical pain should suggest subaxial subluxation. These are not hard and fast rules because pain localization in rheumatoid cervical disease may be poor. Subaxial involvement may also lead to painful neck deformity with loss of sagittal plane supporting structures, which may later become fixed deformities owing to postinflammatory ankylosis. Patients may complain of a “clunking” sensation in the neck with neck motion. This sensation is most common in patients with C1-2 instability, owing to spontaneous reduction of the subluxation with neck extension (known as the Sharp-Purser test).61
Progressive instability leads to decreased effective canal diameter and brainstem compression. Myelopathy and vertebrobasilar dysfunction may result because of mechanical and ischemic damage to the white and gray matter evidenced on histologic specimens by long tract demyelinization, lateral column necrosis, and focal gliosis.49 According to Bell,62 C1-2 instability may lead to a phenomenon termed cruciate paralysis, characterized by upper motor neuron dysfunction of the upper extremities with paresis and paralysis and normal lower extremities similar to a mild central cord syndrome after trauma. Pathologically, there is selective injury to the decussating corticospinal tracts of the upper extremities at the level of the cervicomedullary junction with sparing of the uncrossed lower extremity pyramidal and extrapyramidal tracts.
Additional neurovascular changes may occur because of vertebral artery occlusion with decreased flow into the posterior inferior cerebellar artery and cephalad brainstem circulation. Without adequate collateral blood flow, these patients may develop Wallenberg syndrome or lateral medullary infarction. These clinical syndromes are characterized by ipsilateral cranial nerve palsies (cranial nerves V, IX, X, and XI), cerebellar ataxia, Horner syndrome (ptosis, miosis, anhidrosis, and enophthalmos), facial pain, and contralateral loss of pain and temperature sensation.63 Rarely, patients may develop quadriplegia, quadriplegia with facial muscle paralysis (locked-in syndrome),64 and sudden death.38,58
In contrast to pain symptoms, neurologic signs are less straightforward. Myelopathy is progressive but may not be evident by loss of fine motor control, gait imbalance, or global numbness of the hands, but rather by the slow onset of deteriorating independence and becoming wheelchair bound. In many instances, hand deformities mask motor deficits in the upper extremities, and the patients’ deteriorating ambulatory status may be attributed to large joint involvement rather than myelopathy. Patients must be assessed keeping their global disease in mind. Progression of myelopathy, especially early disease, may be misinterpreted as progression of peripheral disease. A high index of suspicion is necessary in these patients to detect early myelopathy because patients do poorly without early surgical intervention.38,58
The Ranawat grading system may provide some useful clinical information in assessing patients with neurologic deficits (Table 34–1).65 This system classifies pain as none (grade 0), mild (1), moderate (2), or severe (3). Neurologic function falls into three classes: Class I has no neurologic deficit; class II has subjective weakness, dysesthesia, and hyperreflexia; and class III has objective weakness and long tract signs. This last class is subdivided further into class IIIA, in which patients are ambulatory, and class IIIB, in which patients have quadriparesis and are unable to walk. Casey and colleagues66 reported that only 25% of 55 Ranawat class IIIB patients who underwent surgical decompression and stabilization had a favorable outcome. The mortality rate was 13% after 30 days and 60% by 4 years.
| Pain Assessment | |
| Grade 0 | None |
| Grade 1 | Mild, intermittent, requiring only aspirin analgesia |
| Grade 2 | Moderate; cervical collar needed |
| Grade 3 | Severe; pain not relieved by either aspirin or collar |
| Neural Assessment | |
| Class I | No neural deficit |
| Class II | Subjective weakness with hyperreflexia and dysesthesias |
| Class IIIA | Objective findings of paresis and long tract signs, but walking possible |
| Class IIIB | Quadriparesis with resultant inability to walk or feed oneself |
Adapted from Ranawat CS, O’Leary P, Pellici P, et al: Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am 61:1003-1010, 1979.
Radiologic Evaluation
Historically, the AADI was used to screen patients who required surgical stabilization for atlantoaxial instability based on an AADI greater than 9 mm, with AADI 0 to 6 mm suggesting instability and AADI 0 to 3 mm being normal.48 AADI is limited by the occasional difficulty of obtaining accurate measurements in some patients because of erosive changes that distort the normal anatomic landmarks and because it has not been correlated clinically with the mass effect of retrodental pannus that may compress the spinal cord. Boden and colleagues67 showed that PADI may be more reliable in predicting neurologic outcome because it is a more accurate indicator of the space available for the cord. Patients with a PADI of at least 14 mm were more likely to have neurologic recovery after surgical stabilization, whereas patients with a PADI less than 10 mm had no neurologic recovery. These observations can also be explained by the anatomy of the spinal cord at the C1 level because the dura requires an average space of 1 mm on the anterior and posterior sides, the cerebrospinal fluid requires 2 mm, and the cord requires 10 mm for a total space of 14 mm.68
The open-mouth view is useful to identify lateral subluxation, defined as more than 2 mm of lateral displacement of C1-2 lateral masses, which occurs more commonly in patients with spinal cord compression than in patients without compression.48,53 Erosive changes of the dens and C1-2 articulations may also be visualized. Taniguchi and colleagues69 defined the atlantodental lateral shift (ADLS) as a means of assessing lateral atlantoaxial instability. Using dynamic open-mouth odontoid views (taken in maximal left and right lateral bending), ADLS is calculated by dividing the distance from the center of the dens to the medial edge of the C1 lateral mass (in the direction of lateral bending) by the distance between the medial borders of the bilateral C1 lateral masses and is expressed as a percentage. With this method, these investigators noted that in patients with rheumatoid arthritis, the ADLS averaged 14.8% compared with 6.1% in control patients. Among the subgroup of patients with rheumatoid arthritis and increased AADI, the ADLS averaged 20.6% versus 12.7% in patients without anterior atlantoaxial instability.
Posterior subluxation, although rare, may be seen on lateral radiographs of the cervical spine and should be suspected in patients with an absent or fractured odontoid process.52 The AADI decreases with worsening cranial settling as the arch of the atlas approaches the wider base of the odontoid process and is a potential source of mistaken radiographic improvement.
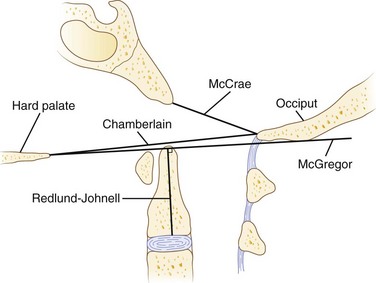
Cranial settling carries the greatest risk of neurologic deficits and sudden death, especially when superimposed on atlantoaxial instability. As a consequence, several measurement techniques have been used with varying sensitivity and specificity, based on lateral plain radiographs of the upper cervical spine (Fig. 34–1).70 Most of these techniques assess the relationship between the tip of the odontoid, the hard palate, and the base of the skull. Traditionally, McGregor line (drawn from the posterosuperior tip of the hard palate to the caudad base of the occiput) has been widely used for its simplicity. Cranial settling occurs when the tip of the dens is more than 4.5 mm above this line.
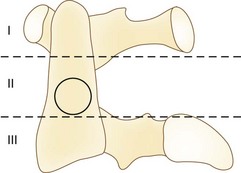
Erosive changes in the odontoid anatomy may make it difficult to analyze many of the measurement techniques, so Redlund-Johnell and Pettersson71 described a technique that measured the vertical line from the midpoint of the caudad margin of C2 to McGregor line. Cranial settling occurs when the distance is less than 34 mm in men or less than 29 mm in women. Ranawat and colleagues65 described a similar technique in which the distance along the odontoid was measured from the C2 pedicle to the transverse axis of the ring of C1. This technique avoided problems with changes in odontoid anatomy and identifying the bony landmarks at the base of the skull and hard palate. Cranial settling was positive if the distance was less than 15 mm in men or less than 13 mm in women. Clark and colleagues22 defined the “station of the atlas” (Fig. 34–2), which indicates the position of the anterior ring of C1 to parts of the body of the axis divided into thirds. Normally, the atlas is adjacent to the upper third (station I) of the axis. Riew and colleagues70 noted that none of the current radiographic measurements alone had a sensitivity, specificity, or negative or positive predictive value greater than 90% and recommended the combination of the Clark station, Redlund-Johnell criteria, and Ranawat criteria with sensitivity and negative predictive values of 94% and 91%.
Subaxial subluxation is the second most common instability pattern and occurs secondary to inflammatory destruction of the apophyseal and discovertebral joints and supporting soft tissues. White and Panjabi72 defined radiographic cervical instability as 3.5 mm or more of vertebral translation and greater than 11 degrees of angular changes between adjacent motion segments, although their study is more representative of acute conditions. The space available for the cord should also be considered in these patients because critical stenosis may be present with or without instability. Boden and colleagues67 correlated anatomic measurements with neurologic findings and reported that a sagittal diameter of less than 14 mm was considered critically stenotic in the rheumatoid subaxial spine. This is in contrast to 13 mm in cervical spondylotic patients who do not have pannus in the canal.
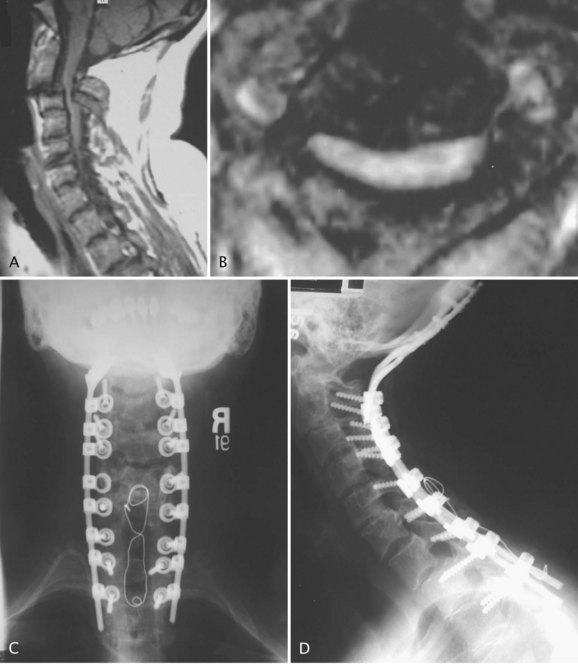
Computed tomography (CT), especially with intrathecal contrast medium, is a valuable addition to plain radiographs in delineating the bony anatomy and identifying cord compression and cranial settling. The degree of medullary compression on CT scans has been shown to correlate with myelopathy.40 Static or dynamic magnetic resonance imaging (MRI) is the best option, however, to assess the soft tissues and to look for the presence of periodontoid pannus (Fig. 34–3) and spinal cord or brainstem compression.73–75 MRI is also useful for postoperative evaluation of the periodontoid pannus, which has been shown to decrease after stabilization.40 Myelopathic signs correlate with MRI findings75 of a cervicomedullary angle of less than 135 degrees (normal 135 to 175 degrees). This angle measures the intersection of vertical lines drawn along the anterior surface of the brainstem and the cord on sagittal MRI.
Nonsurgical Treatment
Although life expectancy is lower in patients with rheumatoid arthritis, many are living longer with their disease because of more aggressive medical therapy that has slowed disease progression with fewer side effects.59 Only a few of these patients eventually need surgical stabilization. Patients with early cervical disease and intermittent pain without radiographic instability or myelopathy can be managed with a soft cervical orthosis or trials of physical therapy. Medication options include nonsteroidal anti-inflammatory drugs (NSAIDs); oral steroids (prednisone); and disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, gold, sulfasalazine, hydroxychloroquine, penicillamine, and azathioprine.
In recent years, biologic agents such as tumor necrosis factor (TNF)-α and interleukin-1 antagonists have become increasingly available. The addition of antiresorptive osteopo-rosis therapy may improve vertebral bone strength in patients with rheumatoid arthritis.76 Patients should be followed closely for neurologic or radiographic changes. Cervical collars provide support, warmth, some pain relief, and a feeling of stability; however, rigid collars are often poorly tolerated, especially in patients with temporomandibular disease, dental problems, and skin sensitivity, and may have further detrimental effects by blocking spontaneous reduction of anterior atlantoaxial subluxation in extension.
Surgical Management
Indications
Patients with any one or more of the instability patterns described earlier (atlantoaxial instability, cranial settling, subaxial subluxation) associated with or without pain, myelopathy, or neurologic deficits should be considered for early surgery. As noted previously, radiographic indicators for surgery include AADI of 9 mm or more; PADI of less than 14 mm; mobile subaxial subluxation greater than 3.5 mm; cord compression or space available for the cord of less than 14 mm; and cranial settling measured most accurately by the combination of Clark station, Redlund-Johnell criteria, and Ranawat criteria.70 CT myelography and MRI showing cord compression or a cervicomedullary angle of less than 135 degrees are suggestive of impending neurologic deficits and should be weighed heavily as an indication for surgery within the context of the remaining clinical picture. Patients with headaches in the distribution of the greater or lesser occipital nerve are likely to have atlantoaxial instability and may need C1-2 arthrodesis for pain relief.
Timing of surgical intervention is crucial. In a retrospective review of 110 patients who underwent cervical spine fusion for rheumatoid arthritis, only 3 of 55 patients (5.5%) with early C1-2 fusion for isolated atlantoaxial instability developed subaxial subluxation requiring surgery an average of 9 years later. This review contrasted with a 36% incidence of recurrent cervical instability at a mean of 2.6 years in patients who initially underwent occiput-C3 fusion for atlantoaxial instability combined with cranial settling. The investigators recommended early surgery before the development of cranial settling to decrease the risks of recurrent instability.39 In a series of 28 patients, Schmitt-Sody and colleagues77 reported that 7 of 10 patients who were Ranawat class II improved to class I after surgery, whereas only 1 of 11 class III patients improved (class IIIA to class II), and 2 patients deteriorated (class IIIA to class IIIB) postoperatively. Similarly, these authors recommended early surgical stabilization before the development of neurologic symptoms.
Preoperative Assessment
The goals of surgery should be relief of pain, spinal alignment, decompression to relieve neurologic deficits where necessary, and stabilization of the involved motion segments. Thorough preoperative assessment is a prerequisite to good outcome in these often fragile patients. The quality of the bone stock, the presence of irreducible subluxations, and the medical condition of the patients should guide the preoperative decision-making process, including the potential need for awake fiberoptic nasal or endotracheal intubation.78
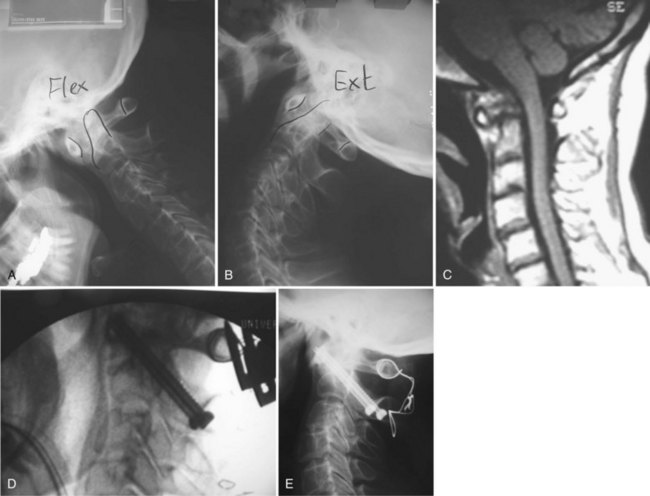
Patients with severe basilar invagination should be considered for preoperative skeletal traction using a halo ring. The ring can be incorporated into a halo vest after surgery. Constant preoperative traction can improve alignment, neurologic symptoms, and pain. Traction may be required for 3 to 7 days or longer,52,79,80 and some authors have used a halo wheelchair to allow the patient to be more mobile and avoid secondary problems such as decubitus ulcers and infection from prolonged bed rest.52,79,80 Gentle traction along the midline longitudinal axis with approximately 7 to 12 lb while avoiding hyperflexion or hyperextension is recommended. Patients are monitored with frequent neurologic examinations, and plain radiographs should be obtained to avoid overdistraction during the process. The authors do not routinely use preoperative traction in atlantoaxial instability or subaxial subluxation. Many of these instabilities that moved little on preoperative flexion-extension views are better reduced when the patient is under general anesthesia positioned with the head holder.
When in the operating room, obtaining baseline neurophysiologic data including somatosensory evoked potentials and motor evoked potentials before positioning a myelopathic patient may help prevent cord injury during or after positioning of the head and neck. Finally, the surgical approach should be determined by the underlying pathology and its location, in part analogous to the approach for compressive lesions in patients with cervical spondylosis.81,82
Perioperative Management of Rheumatoid Medication
Because of the risk of increased bleeding time and potentially increased intraoperative blood loss, NSAIDs should be discontinued 3 to 5 half-lives before surgery.83 In addition, NSAIDs have been previously shown to inhibit bone healing and so should be held as long as possible postoperatively so as not to impair formation of a spinal fusion.
Corticosteroids may produce immunosuppression, which can adversely affect wound and bone healing. Sudden stoppage of long-term corticosteroid therapy may lead to adrenal insufficiency, necessitating the continued use of corticosteroids perioperatively and potentially requiring the use of stress dosing. Generally, perioperative management of corticosteroids depends on the long-term dosage and the potential degree of surgical stress. Most spinal procedures for these patients would be considered highly stressful, and patients on long-term moderate-dose to high-dose regimens (>20 mg/day of prednisone) should receive stress dose steroids on the day of surgery with rapid tapering over 1 to 2 days to their usual long-term dosage.83,84
Continued use of methotrexate in the perioperative period has not been shown to increase infection rates.85 It may affect bone healing, however86; this has not been studied in a spinal fusion model. Although withholding methotrexate may increase the likelihood of a flare of rheumatoid symptoms, the authors routinely discontinue its use postoperatively and recommend that patients remain off of methotrexate for 6 to 8 weeks if possible.
Few studies exist to direct perioperative management of the newer biologic agents such as TNF-α and interleukin-1 antagonists. Because of their strong immunoregulatory effects, these agents may predispose patients for opportunistic infections.83,84 In a retrospective review, Giles and colleagues87 reported that 10 of 91 (11%) patients with rheumatoid arthritis who underwent an orthopaedic surgical procedure developed early, major postoperative infection (septic arthritis, osteomyelitis, deep wound infection). Of the 10 patients with infection, 7 (70%) were receiving TNF-α inhibitor therapy during the perioperative period. There are no data on the effects of these agents on bone fusion. Current recommendations are to discontinue biologic agents preoperatively (at the end of the dosing cycle), and restart 10 to 14 days postoperatively.
Operative Procedures
Atlantoaxial (C1-2) Instability
Posterior fusion is considered the standard procedure using sublaminar or interspinous wires, hook-claw constructs, or transarticular screws. Gallie19 and Brooks20 wiring techniques require the presence of the posterior arch of C1, supplemental bone graft, and external immobilization. Claw-type constructs such as the Halifax clamp are rarely used today because of biomechanical limitations and better options. Transarticular screw fixation is popular because of its multidirectional rigidity, but it requires intraoperative fluoroscopy and preoperative axial imaging to visualize the vertebral artery anatomy. Direct screw fixation of the C1 lateral masses and C2 pedicles, pars, or lamina has been described more recently.29,88,89
Wire Techniques
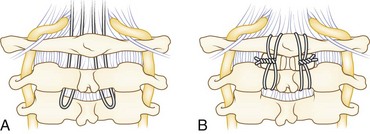
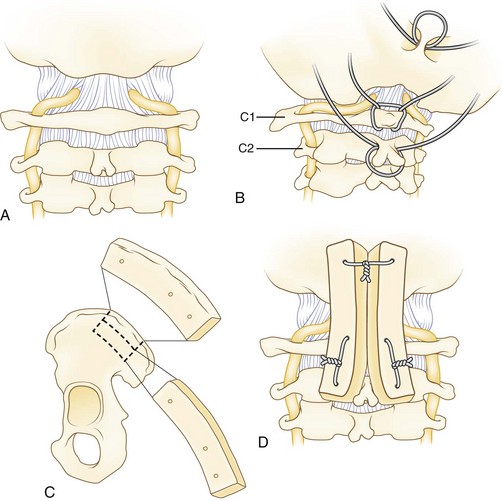
The Gallie wiring technique plus bone graft was described in 1939 for fracture fixation in the cervical spine.19 Sublaminar wire fixation is obtained on the C1 ring and around the C2 spinous process (Fig. 34–4). The authors recommend a modification of the Gallie technique. The patient’s head is placed in a tong-type holder for rigid positioning. Intraoperative imaging should be used to determine the final position of the head and neck and to confirm adequate reduction of C1. The chin should be tucked to open the space between the occiput and C1 to facilitate passage of sublaminar wire. An occiput-to-C2 fusion may be safer with or without a C1 laminectomy in cases of an inadequate posterior space available for safe wire passage of an unreducible C1 ring.
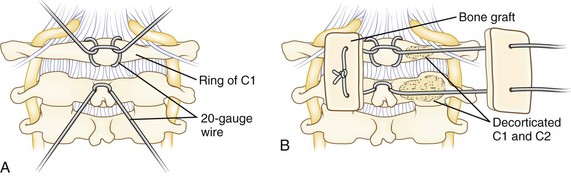
A Brooks-type modification of the Gallie technique uses bilateral sublaminar wires beneath C1 and C2 (Fig. 34–5). Positioning and exposure are as described for the Gallie technique. A laminotomy may be done between C2 and C3 to facilitate wire passage, a 20-gauge wire is looped, and the looped end is threaded beneath the lamina of C2 and C1 on either side of the midline. Near full–thickness corticocancellous iliac crest bone grafts are harvested and placed on the lamina of C1 and C2. The grafts should be contoured to fit snugly to reduce the risk of graft slippage. Alternatively, a full bone block may be used with a space in the caudad end to fit around the C2 spinous process. Decortication is unnecessary and risks weakening the lamina. The wires are tightened down in a longitudinal fashion over the bone block on either side. This fixation may provide more rotational stability over the Gallie technique because of fixation on both sides of the midline. A halo vest may be used at the discretion of the surgeon based on the need for maintaining reduction and the adequacy of intraoperative fixation.
Transarticular Screw Fixation
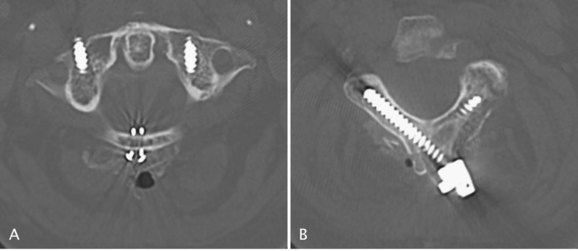
The technique of transarticular screw fixation provides the most rigid fixation for atlantoaxial fusion and can be used in conjunction with wire techniques to provide three-point fixation (Figs. 34-6 and 34-7).25 It rigidly fixes the C1 and C2 facets, minimizing the need for postoperative external immobilization, which is desired in these patients with fragile skin. Careful preoperative assessment with a CT scan is necessary to define the vertebral artery anatomy adequately. It also ensures that the C2 isthmus is wide enough to accept at least a 3.5-mm screw and that the lateral mass of C1 is reduced and aligned with the superior facet of C2. Other considerations that may preclude the use of this technique include significant cranial settling with collapsed lateral masses, irreducible subluxations, substantial osteoporosis and osteopenia, comminuted fractures of the atlas and axis vertebrae, and an anomalous vertebral artery.
Posterior C1-2 Intra-articular Screw Fixation
A variation is the use of a C1-2 intra-articular interference screw, as described by Tokuhashi and colleagues.90 In this technique, posterior dissection exposes the C1-2 joints bilaterally. The joint capsule is dissected off the bone and retracted superiorly with the greater occipital nerve. When placing a screw on one side, atlantoaxial subluxation is reduced and maintained by application of a Halifax interlaminar clamp to the contralateral side. A 1-mm Kirschner wire is inserted directly into the C1-2 joint, and a cannulated tap is used to prepare the site for insertion of the cannulated 5.6-mm or 6.5-mm titanium interference screw. These screws are typically 8 mm or 10 mm long. The procedure is performed on the opposite side. Finally, corticocancellous bone graft is fashioned to fit the intralaminar space and is secured beneath bilateral Halifax clamps. Patients are placed in a cervical collar postoperatively.
In a more recent study, Tokuhashi and colleagues91 used this technique in 22 patients with rheumatoid arthritis. All patients reported significant improvement in pain, and all patients who were Ranawat class IIIA or above improved neurologically by 2-year follow-up. Atlantoaxial reduction was maintained, and bony fusion was noted in all patients, although four patients eventually had changes in alignment of the subaxial cervical spine (none of which required surgical intervention).
Posterior C1-2 Screw-Rod Constructs
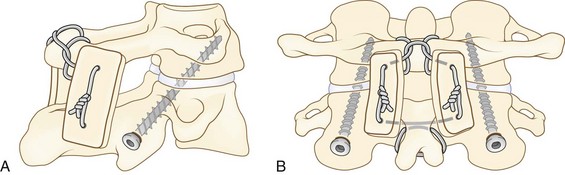
Several methods of posterior C1-2 rigid instrumentation have been described more recently. Harms and Melcher29 reported a technique involving placement of 3.5-mm polyaxial screws directly into the lateral mass of C1 and into the pars interarticularis or pedicle of C2 bilaterally (Fig. 34–8). Vertical rods are attached to these screws followed by bone grafting to obtain arthrodesis. This method allows for direct manipulation and reduction of C1 on C2 as needed. The advantages of this technique include the theoretical decreased risk to the vertebral artery compared with C1-2 transarticular screws. Bleeding from the venous lakes covering the C1 lateral masses can easily be encountered and is a disadvantage of this technique. This bleeding is usually minimized by meticulous subperiosteal dissection and can typically be controlled by absorbable gelatin sponge (Gelfoam). Either technique requires an intimate knowledge of the anatomy by the operating surgeon aided by good fluoroscopic technique.
The entry point for the C2 pedicle screw is in the upper and medial quadrants of the isthmus surface. The direction of the pilot hole is approximately 20 to 30 degrees in a convergent and cephalad direction. The medial border of the C2 pars interarticularis can initially be palpated with a small elevator to help with accurate placement of C2 screws. A typical length for C2 pedicle screws would be 22 to 28 mm, which would again be confirmed with fluoroscopy. In their study, Harms and Melcher29 did not wire in grafts for their fusion technique; however, this is an option to augment the stability of the screw-rod construct and to help insure a successful arthrodesis (see Fig. 34–7).
An alternative to the C2 pedicle or pars screw is placement of an intralaminar C2 screw (Fig. 34–9). As described by Wright,88 this technique provides excellent fixation of C2 with virtually no risk of injury to neurovascular structures. Exposure is as described earlier for the placement of C1 lateral mass and C2 pedicle screws. Placement of the C1 lateral mass screws is done by the previously described technique. For placement of the C2 intralaminar screws, a high-speed bur is used to open a small cortical window at the cranial end of the junction of the C2 spinous process and lamina. A hand drill is used to drill the intercortical space of the contralateral lamina to a depth of approximately 30 mm, while maintaining alignment of the drill with the exposed dorsal aspect of the lamina.
A more recent biomechanical study examined the relative stability of several C1-2 screw-rod constructs in an odontoid fracture model.92 Using cadaveric specimens, C1 lateral mass screws were placed and connected to C2 intralaminar screws, C2 pars screws, or C2 pedicle screws. Insertional torque for C2 pedicle screws and intralaminar screws was similar, and both of these were significantly higher than pars screws. Pullout strength was greatest for pedicle screws. In intact models, intralaminar screws provided superior resistance to axial rotation compared with pars screws and similar resistance to pedicle screws. After experimentally induced odontoid fracture, pars and pedicle screws were superior in resisting lateral bending. Although similar to the intralaminar technique, pedicle screws overall provided the greatest stability of C1-2 in all planes, particularly after experimental odontoid fracture. Lapiswala and colleagues93 noted similar results with C2 intralaminar screws providing less resistance to lateral bending than either C2 pedicle screws or C2-C1 transarticular screws. In their study, all posterior constructs, when supplemented with posterior cable fixation, provided similar stiffness in flexion-extension and axial rotation.
Occipitocervical Fusion
The technique of choice to treat patients with cranial settling or fixed atlantoaxial subluxations causing posterior cord impingement from the ring of C1 is occipitocervical fusion.52,80,94 In the latter case, an occiput-to-C2 fusion with a C1 laminectomy is preferred. Patient positioning is the same as with the wire techniques described earlier. The base of the occiput from the external occipital protuberance is exposed with caudad extension to the level of the subaxial spine to be fused.
Wiring and Graft Technique
The wiring and graft technique uses corticocancellous bone blocks wired to the occiput and laminae (Fig. 34–10).52,80,94 The prominent bony protuberance (inion) that is present approximately 2 cm below the external occipital protuberance is the site of wire fixation (approximately 5 to 7 cm from the base of the skull). A 2-mm diamond bur is used to make unicortical holes to form a tunnel with a bridge of superficial cortical bone on each side of the midline. The holes are connected with a towel clamp between the inner and outer tables of the skull. A 20-gauge wire is passed through the tunnel and looped back under and out again to wrap around the bony bridge. A second wire is passed beneath the C1 lamina (if it has not been removed) and looped onto itself to cinch down on the lamina. A wire is passed transversely through the base of the C2 spinous process and looped around and passed back through to distribute stress. Other interspinous or sublaminar wires can be used in more caudad segments as needed. Long, thick corticocancellous iliac crest grafts are harvested, typically 9 to 10 cm in length and 1.5 cm wide.
For occiput-to-C2 fusions, a single block of graft may be used with the caudad end fashioned to fit around the C2 spinous process. A 1.5-mm drill bit is used to place holes through the graft. The wires are threaded through the graft with the cancellous side facing the exposed skull and laminae. The concavity of the central part of the iliac crest facilitates excellent contact between the occiput and the laminae. In severely osteopenic patients, a wire mesh may be placed over the cortical side of the graft to prevent the wires from cutting through the graft.52 The wires are tightened down cautiously to prevent breakage or cutting through the graft. Cancellous bone chips may be added to the construct. A drain is typically used, and the wound is closed in layers. Without adjuvant fixation, most patients are managed in a halo vest postoperatively, but a two-poster brace or other cervical orthosis may be used based on the stability of the construct.
Occipitocervical Plating
Occipitocervical plating provides more rigid fixation compared with wiring techniques,34,36,95,96 even in patients with significant osteoporosis. Early instrumentation consisted of acetabular reconstruction plates,36 but current improvements are more versatile and incorporate plate-rod hybrid constructs (Fig. 34–11). It may be technically challenging to produce the correct amount of contour required at the base of the skull. In longer fusions, it is recommended that fixation is achieved in C2 and in the lower vertebrae before fixation into the skull because the more distal fixation points require more precise anatomic locations. C2 fixation can be done with pedicle screws, transarticular screws, or intralaminar screws (described earlier). Because of their inferior pullout strength, C2 pars screws should not be used unless the fusion is to be carried down into the subaxial cervical spine.
Fixation to the skull requires knowledge of the venous sinus anatomy to avoid bleeding complications. Screws should be placed distal to the transverse sinus, which lies at the level of the external occipital protuberance. The thickness of the skull decreases laterally away from the midline and closer to the foramen magnum.97 Unicortical fixation is adequate in the thicker areas of the skull, but bicortical fixation can be performed safely. Two to three screws should be used on each side of the midline. A 2-mm diamond bur is used to minimize the chance of a spinal fluid leak, which is common when using more aggressive carbide burs. The holes are tapped, and 3.5- to 4.5-mm screws 6 to 12 mm in length are placed. In case of a spinal fluid leak, bone wax may be sufficient to stop the leak; otherwise, placing the screw usually tamponades the leak. There does not seem to be any adverse effect from breaching the inner table. The head and neck are immobilized in a two-poster brace or a halo vest in patients with osteoporotic bone and questionable fixation. Patients are typically changed to a soft collar for comfort after 8 weeks.
Contoured Loop and Rod Technique
The contoured loop and rod technique was first proposed by Ransford and colleagues in 198632 and is used with segmental wiring to provide occipitocervical fixation.32,98–100 The exposure is similar to that described earlier for plate fixation. Wires are placed in the occiput and beneath the lamina of C1 and the subaxial vertebrae. Earlier constructs relied on a horseshoe-shaped loop of  threaded stainless steel rods. The horizontal limb should be approximately 3.5 to 4 cm wide, and the length of the occipital portion of the loop should be 2.5 to 3 cm. More recent constructs use a custom-contoured threaded titanium loop with titanium-threaded cables, which allows for postoperative MRI.
threaded stainless steel rods. The horizontal limb should be approximately 3.5 to 4 cm wide, and the length of the occipital portion of the loop should be 2.5 to 3 cm. More recent constructs use a custom-contoured threaded titanium loop with titanium-threaded cables, which allows for postoperative MRI.
Resection of the Odontoid
Mild to moderate cervicomedullary compression can be treated with posterior C1 laminectomy and occipitocervical fusion as described earlier. The pannus resorbs with stabilization.40 Anterior decompression may be needed, however, to treat an irreducible anterior extradural compression of the cervicomedullary junction by pannus or a severely migrated odontoid.101,102 The transoral approach is associated with an increased risk of infection with mouth flora; the high retropharyngeal approach is a good alternative in many patients.103 Supplemental posterior fusion is required unless there is a solid prior posterior fusion mass. In case of a prior posterior fusion, resection of the odontoid without grafting suffices. In cases without prior posterior fusion, anterior strut grafting with iliac crest or fibula is required between C2 or C3 and the clivus, followed by posterior occipitocervical fusion. Posterior stabilization may be staged depending on the medical status of the patient. Thorough preoperative assessment is necessary and includes swallowing and respiratory function considerations, dental assessment, and the presence of a mobile temporomandibular joint to allow about 2.5 to 3 cm of opening.
Subaxial Fusion
Wiring Techniques
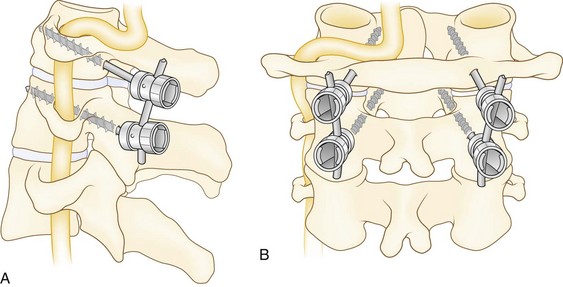
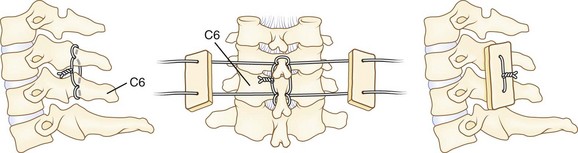
Posterior wiring and autogenous bone grafting may provide stable fixation at a high fusion rate. The authors use the triple wire technique described by McAfee and Bohlman (Fig. 34–12).103 The first 20-gauge wire is woven in a figure-of-eight fashion through holes drilled into the base of the spinous processes of the involved vertebrae. This is the midline tethering wire. The wire is typically looped onto itself after passing through the spinous process to help distribute stress; 22-gauge wires can then pass individually through the same holes to loop around the spinous processes at the ends of the segments to be fused. The ends of these wires pass through drill holes in the rectangular corticocancellous iliac crest autografts. The wires are tightened down on the graft to force the graft against the laminae. Decortication is unnecessary. The wound is closed in layers over a drain. A two-poster cervical brace is used for 6 to 8 weeks to allow solid fusion.
Lateral Mass Plating and Screw and Rod Constructs
Lateral mass plating and screw and rod constructs typically provide excellent fixation of the subaxial spine. Several modifications exist in the way the screws are angled in the lateral mass.25–28 According to An and colleagues,26 15 degrees of superior angulation and 30 degrees of lateral angulation best avoids violating the facet joints and the nerve roots. All the techniques avoid the vertebral artery, which lies anterior to the lateral mass. The starting point is 1 mm medial to the center of the lateral mass. Current instrumentations come with standard drill kits. The screws average 14 to 18 mm, depending on the size of the patient. The C7 lateral mass is thin and may require pedicle screw placement or supplemental posterior wiring between the C6 and C7 spinous processes as described earlier. Fluoroscopy is helpful in placing C7 pedicle screws if the shoulders are not in the way, but a C7 laminotomy provides direct palpation of the medial wall of the pedicle for safer screw placement. A 25- to 30-degree medial angulation is recommended.26 Pedicle screw fixation of all involved levels of the cervical vertebrae has been described frequently in other patient populations but has limitations of pedicle size and risks to the vertebral artery.104
Complications
The progressive nature of rheumatoid disease of the spine demands long-term follow-up to assess for the development of new symptoms in previously asymptomatic segments (see Fig. 34–12). In a study of 51 patients undergoing cervical fusion for instability secondary to rheumatoid arthritis, Clarke and colleagues105 reported that at average 8.3-year follow-up, 39% of patients undergoing C1-2 fixation developed subaxial subluxation. Slightly more than half of these patients required extension of fusion for symptomatic or unstable subaxial subluxation. This subluxation occurred most commonly at C3-4 (62% of patients). Of the patients who underwent long posterior fusions (C1 to C6 through T1) for combined atlantoaxial and subaxial instability, none required secondary procedures. Including all involved unstable levels in the initial fusion procedure may reduce the risk of subsequent adjacent level subluxation. Age, presence of atlantoaxial instability, and perioperative complications have been shown to be independent predictors of long-term mortality after cervical surgery in this population.106
Thoracolumbar Disease
In contrast to the cervical spine, rheumatoid disease of the thoracic and lumbar spine leads to severe neurologic symptoms less frequently and has received less attention. Cases of thoracic myelopathy, lumbar radiculopathy, and cauda equina syndrome have been reported, however, and seem to be due to bony destruction leading to instability and subluxation with or without the presence of pannus or nodules.107–109 Radiographic findings include poorly defined margins of the vertebral endplates and a relative lack of osteophytes.110,111 Involvement of the facet joints may lead to spondylolisthesis and instability on flexion-extension films. Heywood and Meyers110 postulated that the pathologic process in the lumbar spine begins with synovitis of the facet joints causing functional incompetence, allowing anteroposterior and lateral translation. This synovitis is followed by discitis, which may start as an enthesopathy at the junction of the endplate and disc, resulting in loss of disc height and contributing further to instability. In the thoracic spine, the disease may also spread directly into the spine via the costovertebral joints. Nerve root impingement may be due to spinal instability and direct impingement from rheumatoid pannus.110
To correlate radiographic findings with clinical symptoms, Kawaguchi and colleagues111 studied 106 patients with rheumatoid arthritis and found that 40% of patients complained of back pain, 18% had leg pain and 12% exhibited symptoms of neurogenic claudication. They reported abnormal radiographic findings in 57% of patients, with disc space narrowing the most frequent finding, in 37% of patients; coronal plane deformity in 28%; spondylolisthesis in 23%; and endplate or facet erosion in 20%. Finally, Kawaguchi and colleagues111 noted a high percentage of osteoporosis, particularly in patients on steroid therapy. Using MRI, Sakai and colleagues112 reported a 45.2% incidence of lumbar involvement in rheumatoid arthritis and described two distinct patterns of disc destruction: narrowing, in which the disc space progressively collapsed, and ballooning, in which changes in the endplates give the illusion of increasing disc height. Additionally, they found that the degree of lumbar involvement related to the severity of peripheral joint involvement.
The literature regarding surgical treatment of lumbar involvement is sparse. One more recent study retrospectively compared outcomes of 19 patients with rheumatoid arthritis who underwent posterior lumbar decompression and fusion with a matched control group of patients without rheumatoid arthritis.113 Given the small number of patients in the study, there were no significant differences in complications, fusion, or patient satisfaction between the two groups. Two patients with rheumatoid arthritis showed evidence of loosening of pedicle screws on postoperative radiographs, however, and one patient underwent noninstrumented fusion owing to severe osteoporosis noted intraoperatively. None of the control patients had any hardware complications. In addition, there was a trend toward more wound complications in the rheumatoid arthritis group.
Summary
Most patients with rheumatoid arthritis of the cervical spine can be managed nonoperatively. These patients should be monitored closely, however, because the progression of disease may be silent, and the clinical findings are nonspecific. Screening flexion and extension lateral radiographs should be obtained periodically before neurologic symptoms develop and before any planned surgical procedure that may require general anesthesia, intubation, or manipulation of the cervical spine. The benefits of early detection and surgical intervention in patients with progressive cervical instability cannot be overemphasized, and the current trend is to perform prophylactic fusion before the development of cranial settling or frank neurologic symptoms.39,51,80 Otherwise, mortality and morbidity significantly increase as patients become less functional and develop Ranawat class III criteria.51,58,65,79,103 Although surgery in patients with quadriparesis may provide some neurologic and functional recovery, the results are not as predictable as with early surgery.
Pearls
Pitfalls
Key Points
1 Boden SD, Dodge LD, Bohlman HH, et al. Rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am. 1993;75:1282-1297.
2 Bundschuh C, Modic MT, Kearney F, et al. Rheumatoid arthritis of the cervical spine: Surface-coil MR imaging. AJNR Am J Neuroradiol. 1988;9:565-571.
3 Larsson E-M, Holtås S, Zygmunt S. Pre- and postoperative MR imaging of the craniocervical junction in rheumatoid arthritis. AJNR Am J Neuroradiol. 1989;152:561-566.
4 Ranawat CS, O’Leary P, Pellici P, et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003-1010.
5 Riew KD, Hilibrand A, Palumbo MA, et al. Diagnosing basilar invagination in the rheumatoid patient. J Bone Joint Surg Am. 2001;83:194-200.
6 Harms J, Melcher R. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976). 2001;26:2467-2471.
1 Ward MW. Decreases in rates of hospitalization for manifestations of severe rheumatoid arthritis, 1983-2001. Arthritis Rheum. 2004;50:1122-1131.
2 Bland JH. Rheumatoid arthritis of the cervical spine. J Rheumatol. 1974;3:319-341.
3 Heywood AWB, Meyers OL. Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surg Br. 1986;68:362-368.
4 Kawaguchi Y, Matsuno H, Kanamori M, et al. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech. 2003;16:38-43.
5 Neva MH, Hakkinen A, Makinen H, et al. High prevalence of asymptomatic cervical spine subluxation in patients with rheumatoid arthritis waiting for orthopaedic surgery. Ann Rheum Dis. 2006;65:884-888.
6 Hansen SE. The recognition of rheumatoid arthritis in the eighteenth century: The contribution of Linné and Bosissier de la Croix de Sauvages. Scand J Rheumatol. 1993;22:178-182.
7 Short CL. The antiquity of rheumatoid arthritis. Arthritis Rheum. 1974;17:193-205.
8 Snorrason E. Landré-Beauvais and his Goutte Asthénique Primitive. Acta Med Scand. 1952;142(Suppl 266):115-118.
9 Garrod AE. A Treatise on Rheumatism and Rheumatoid Arthritis. London: Griffin; 1890.
10 Lourie H, Stewart WA. Spontaneous atlantoaxial dislocation: A complication of rheumatoid disease. N Engl J Med. 1961;265:677-681.
11 Pratt TLC. Spontaneous dislocation of the atlanto-axial articulation occurring in ankylosing spondylitis and rheumatoid arthritis. J Fac Radiol. 1959;10:40-43.
12 Werne S. Spontaneous dislocation of the atlas (as a complication of rheumatoid arthritis). Acta Rheumatol Scand. 1957;3:101-107.
13 Wilson PDJr, Dangelmajer RC. The problem of atlanto-axial dislocation in rheumatoid arthritis. In Proceedings of the American Orthopaedic Association. J Bone Joint Surg Am. 1963;45:1780.
14 Brattström H, Granholm L. Atlanto-axial fusion in rheumatoid arthritis: A new method of fixation with wire and bone cement. Acta Orthop Scand. 1976;47:619-628.
15 Ferlic DC, Clayton ML, Leidholt JD, et al. Surgical treatment of the symptomatic unstable cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1975;57:349-354.
16 Hamblen DL. Occipito-cervical fusion: Indications, technique and results. J Bone Joint Surg Br. 1967;49:33-45.
17 McGraw RW, Rusch RM. Atlanto-axial arthrodesis. J Bone Joint Surg Br. 1973;55:482-489.
18 Rana NA, Hancock DO, Taylor AR, et al. Atlanto-axial subluxation in rheumatoid arthritis. J Bone Joint Surg Br. 1973;55:458-470.
19 Gallie WE. Fractures and dislocations of the cervical spine. Am J Surg. 1939;46:495-499.
20 Brooks AL, Jenkins EB. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg Am. 1978;60:279-284.
21 Wertheim SB, Bohlman HH. Occipitocervical fusion: Indications, technique, and long-term results in thirteen patients. J Bone Joint Surg Am. 1987;69:833-836.
22 Clark CR, Goetz DD, Menezes AH. Arthrodesis of the cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1989;71:381-392.
23 Cybulski GR, Stone JL, Crowell RM, et al. Use of Halifax interlaminar clamps for posterior C1-C2 arthrodesis. Neurosurgery. 1988;22:429-431.
24 Grob D, Jeanneret B, Aebi M, et al. Atlanto-axial fusion with transarticular screw fixation. J Bone Joint Surg Br. 1991;73:972-976.
25 Magerl F, Seeman PS. Stable posterior fusion of the atlas and axis by transarticular screw fixation. In: Kehr P, Weidner A, editors. Cervical Spine. Berlin: Springer-Verlag; 1986:322-327.
26 An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine (Phila Pa 1976). 1991;16(10 Suppl):S548-S551.
27 Heller JG, Carlson GD, Abitbol JJ, et al. Anatomic comparison of the Roy-Camille and Magerl techniques for screw placement in the lower cervical spine. Spine (Phila Pa 1976). 1991;16(10 Suppl):S552-S557.
28 Anderson PA, Henley MB, Grady MS, et al. Posterior cervical arthrodesis with AO reconstruction plates and bone graft. Spine (Phila Pa 1976). 1991;16(3 Suppl):S72-S79.
29 Harms J, Melcher R. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976). 2001;26:2467-2471.
30 Foerster O. Die Leitungsbahnen des Schmerzgefühls. Berlin: Urban und Schwarzenburg; 1927. p 266
31 Kahn EA, Yglesias L. Progressive atlanto-axial dislocation. JAMA. 1935;105:348-352.
32 Ransford AO, Crockard HA, Pozo JL, et al. Craniocervical instability treated by contoured loop fixation. J Bone Joint Surg Br. 1986;68:173-177.
33 Flint GA, Hockley AD, McMillan JJ, et al. A new method of occipitocervical fusion using internal fixation. Neurosurgery. 1987;21:947-950.
34 Grob D, Dvorak J, Panjabi M, et al. The role of plate and screw fixation in occipitocervical fusion in rheumatoid arthritis. Spine (Phila Pa 1976). 1994;19:2545-2551.
35 Grob D, Dvorak J, Panjabi M, et al. Posterior occipitocervical fusion: A preliminary report of a new technique. Spine (Phila Pa 1976). 1991;16(Suppl 3):S17-S24.
36 Smith MD, Anderson P, Grady MS. Occipitocervical arthrodesis using contoured plate fixation: An early report on a versatile fixation technique. Spine (Phila Pa 1976). 1993;18:1984-1990.
37 Kontinnen Y, Santavirta S, Bergroth V, et al. Inflammatory involvement of cervical spine ligaments in rheumatoid arthritis. Acta Orthop Scand. 1986;57:587.
38 Mikulowski P, Wollheim FA, Rotmil P, et al. Sudden death in rheumatoid arthritis with atlanto-axial dislocation. Acta Med Scand. 1975;198:445-451.
39 Agarwal AK, Peppelman WC, Kraus DR, et al. Recurrence of cervical spine instability in rheumatoid arthritis following previous fusion: Can disease progression be prevented by early surgery? J Rheumatol. 1992;19:1364-1370.
40 Larsson E-M, Holtås S, Zygmunt S. Pre- and postoperative MR imaging of the craniocervical junction in rheumatoid arthritis. AJR Am J Roentgenol. 1989;152:561-566.
41 Toolanen G, Larsson SE, Fagerlund M. Medullary compression in rheumatoid atlanto-axial subluxation evaluated by computerized tomography. Spine (Phila Pa 1976). 1986;11:191-194.
42 Chen TY, Lin KL, Ho HH. Morphologic characteristics of atlantoaxial complex in rheumatoid arthritis and surgical consideration among Chinese. Spine (Phila Pa 1976). 2004;29:1000-1004.
43 Lewandrowski KU, Park PP, Baron JM, et al. Atraumatic odontoid fractures in patients with rheumatoid arthritis. Spine J. 2008;6:529-533.
44 Smith PH, Benn RT, Sharp J. Natural history of rheumatoid cervical luxations. Ann Rheum Dis. 1972;31:431-439.
45 Bogduk N, Major GA, Carter J. Lateral subluxation of the atlas in rheumatoid arthritis: A case report and postmortem study. Ann Rheum Dis. 1984;43:341-346.
46 Rana NA. Natural history of atlanto-axial subluxation in rheumatoid arthritis. Spine (Phila Pa 1976). 1989;14:1054-1056.
47 Papadopoulos SM, Dickman CA, Sonntag VK. Atlantoaxial stabilization in rheumatoid arthritis. J Neurosurg. 1991;74:1-7.
48 Weissman BN, Aliabadi P, Weinfeld MS, et al. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 1982;144:745-751.
49 Delamarter RB, Bohlman HH. Postmortem osseous and neuropathologic analysis of the rheumatoid cervical spine. Spine (Phila Pa 1976). 1994;19:2267-2274.
50 Henderson FC, Geddes JF, Crockard HA. Neuropathology of the brainstem and spinal cord in end-stage rheumatoid arthritis: Implications for treatment. Ann Rheum Dis. 1993;52:629-637.
51 Casey ATH, Crockard HA, Bland JM, et al. Surgery on the rheumatoid cervical spine for the non-ambulant myelopathic patient—too much, too late? Lancet. 1996;347:1004-1007.
52 Lipson SJ. Cervical myelopathy and posterior atlanto-axial subluxation in patients with rheumatoid arthritis. J Bone Joint Surg Am. 1985;67:593-597.
53 Burry HC, Tweed JM, Robinson RG, et al. Lateral subluxation of the atlanto-axial joint in rheumatoid arthritis. Ann Rheum Dis. 1978;37:525-528.
54 Kudo H, Iwano K. Surgical treatment of subaxial cervical myelopathy in rheumatoid arthritis. J Bone Joint Surg Br. 1991;73:474-480.
55 Winfield J, Cooke D, Brook AS, et al. A prospective study of the radiological changes in the cervical spine in early rheumatoid disease. Ann Rheum Dis. 1981;40:109-114.
56 Winfield J, Young A, Williams P, et al. Prospective study of the radiological changes in hands, feet, and cervical spine in adult rheumatoid disease. Ann Rheum Dis. 1983;42:613-618.
57 Pellicci PM, Ranawat CS, Tsairis P, et al. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am. 1981;63:342-350.
58 Sunahara N, Matsunaga S, Mori T, et al. Clinical course of conservatively managed rheumatoid arthritis patients with myelopathy. Spine (Phila Pa 1976). 1997;22:2603-2608.
59 Paimela L, Laasonen L, Kankaanpää E, et al. Progression of cervical spine changes with early rheumatoid arthritis. J Rheumatol. 1997;24:1280-1284.
60 Young A, Corbett M, Winfield J, et al. A prognostic index for erosive changes in the hands, feet, and cervical spines in early rheumatoid arthritis. Br J Rheumatol. 1988;27:94-101.
61 Sharp J, Purser DW. Spontaneous atlantoaxial dislocation in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis. 1961;20:47-50.
62 Bell HS. Paralysis of both arms from injury of the upper portion of the pyramidal decussation: “Cruciate paralysis.”. J Neurosurg. 1970;33:376-380.
63 Gurley JP, Bell GR. The surgical management of patients with rheumatoid cervical spine disease. Rheum Dis Clin North Am. 1997;23:317-332.
64 Rana NA, Hancock DO, Taylor AR, et al. Upward translocation of the dens in rheumatoid arthritis. J Bone Joint Surg Br. 1973;55:471-477.
65 Ranawat CS, O’Leary P, Pellicci P, et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003-1010.
66 Casey AT, Crockard HA, Bland JM, et al. Predictors of outcome in the quadriparetic nonambulatory myelopathic patient with rheumatoid arthritis: A prospective study of 55 surgically treated Ranawat class IIIb patients. J Neurosurg. 1996;85:574-581.
67 Boden SD, Dodge LD, Bohlman HH, et al. Rheumatoid arthritis of the cervical spine: A long term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75:1282-1297.
68 Koehler PR, Haughton VM, Daniels DL, et al. MR measurement of the normal and pathologic brainstem diameters. AJNR Am J Neuroradiol. 1985;6:425-427.
69 Taniguchi D, Tokunaga D, Hase H, et al. Evaluation of lateral instability of the atlanto-axial joint in rheumatoid arthritis using dynamic open-mouth view radiographs. Clin Rheumatol. 2008;27:851-857.
70 Riew KD, Hilibrand AS, Palumbo MA, et al. Diagnosing basilar invagination in the rheumatoid patient: The reliability of radiographic criteria. J Bone Joint Surg Am. 2001;83:194-200.
71 Redlund-Johnell I, Pettersson H. Radiographic measurements of the craniovertebral region: Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiol Diagn. 1984;25:23-28.
72 White AA, Panjabi MM. Clinical Biomechanics of the Spine, ed 2. Philadelphia: JB Lippincott; 1990.
73 Dvorak J, Grob D, Baumgartner H, et al. Functional evaluation of the spinal cord by magnetic resonance imaging in patients with rheumatoid arthritis and instability of upper cervical spine. Spine (Phila Pa 1976). 1989;14:1057-1064.
74 Bell GR, Stearns KL. Flexion-extension MRI of the upper rheumatoid cervical spine. Orthopedics. 1991;14:969-974.
75 Bundschuh C, Modic MT, Kearney F, et al. Rheumatoid arthritis of the cervical spine: Surface-coil MR imaging. AJR Am J Roentgenol. 1988;151:181-187.
76 Mawatari T, Miura H, Hamai S, et al. Vertebral strength changes in rheumatoid arthritis patients treated with alendronate, as assessed by finite element analysis of clinical computed tomography scans: A prospective randomized clinical trial. Arthritis Rheum. 2008;58:3340-3349.
77 Schmitt-Sody M, Kirchhoff C, Buhmann S, et al. Timing of cervical spine stabilisation and outcome in patients with rheumatoid arthritis. Int Orthop. 2008;32:511-516.
78 Wattenmaker I, Concepcion M, Hibberd P, et al. Upper-airway obstruction and perioperative management of the airway in patients managed with posterior operations on the cervical spine for rheumatoid arthritis. J Bone Joint Surg Am. 1994;76:360-365.
79 van Asselt KM, Lems WF, Bongartz EB, et al. Outcome of cervical spine surgery in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:448-452.
80 Peppelman WC, Krauss DR, Donaldson WFIII, et al. Cervical spine surgery in rheumatoid arthritis: Improvement of neurologic deficit after cervical spine fusion. Spine (Phila Pa 1976). 1993;18:2375-2379.
81 Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80:941-951.
82 Chin KR, Ozuna R. Options in the surgical treatment of cervical spondylotic myelopathy. Curr Opin Orthop. 2000;11:151-157.
83 Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14:544-551.
84 Scanzello CR, Figgie MP, Nestor BJ, et al. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSS J. 2006;2:141-147.
85 Grennan DM, Gray J, Loudon J, et al. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60:214-217.
86 Gerster JC, Bossy R, Dudler J. Bone non-union after osteotomy in patients treated with methotrexate. J Rheumatol. 1999;26:2695-2697.
87 Giles JT, Bartlett SJ, Gelber AC, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55:333-337.
88 Wright NM. Posterior C2 fixation using bilateral, crossing C2 laminar screws: Case series and technical note. J Spinal Disord Tech. 2004;17:158-162.
89 Payer M, Luzi M, Tessitore E. Posterior atlanto-axial fixation with polyaxial C1 lateral mass screws and C2 pars screws. Acta Neurochir. 2009;151:223-229.
90 Tokuhashi Y, Matsuzaki H, Shirasaki Y, et al. C1-C2 intra-articular screw fixation for atlantoaxial posterior stabilization. Spine (Phila Pa 1976). 2000;25:337-341.
91 Tokuhashi Y, Ajiro Y, Oshima M, et al. C1-C2 Intra-articular screw fixation for atlantoaxial subluxation due to rheumatoid arthritis. Orthopedics. 2009;32:1.
92 Dmitriev AE, Lehman RA, Helgeson MD, et al. Acute and long term stability of atlantoaxial fixation methods: A biomechanical comparison of pars, pedicle, and intralaminar fixation in an intact and odontoid fracture model. Spine (Phila Pa 1976). 2009;34:365-370.
93 Lapiswala SB, Anderson PA, Oza A, et al. Biomechanical comparison of four C1 to C2 rigid fixative techniques: Anterior transarticular, posterior transarticular, C1 to C2 pedicle, and C1 to C2 intralaminar screws. Neurosurgery. 2006;58:516-521.
94 McAfee PC, Cassidy JR, Davis RF, et al. Fusion of the occiput to the upper cervical spine: A review of 37 cases. Spine (Phila Pa 1976). 1991;16(Suppl 10):S490-S494.
95 Huckell CB, Buchowski JM, Richardson WJ, et al. Functional outcome of plate fusions for disorders of the occipitocervical junction. Clin Orthop. 1999;359:136-145.
96 Sasso RC, Jeanneret B, Fischer K, et al. Occipitocervical fusion with posterior plate and screw instrumentation: A long-term follow-up study. Spine (Phila Pa 1976). 1994;19:2364-2368.
97 Roberts DA, Doherty BJ, Heggeness MH. Quantitative anatomy of the occiput and the biomechanics of occipital screw fixation. Spine (Phila Pa 1976). 1998;23:1100-1107.
98 Fehlings MG, Errico T, Cooper P, et al. Occipitocervical fusion with a five-millimeter malleable rod and segmental fixation. Neurosurgery. 1993;32:198-207.
99 Matsunaga S, Ijiri K, Koga H. Results of a longer than 10-year follow-up of patients with rheumatoid arthritis treated by occipitocervical fusion. Spine (Phila Pa 1976). 2000;25:1749-1753.
100 Moskovich R, Crockard HA, Shott S, et al. Occipitocervical stabilization for myelopathy in patients with rheumatoid arthritis: Implications of not bone-grafting. J Bone Joint Surg Am. 2000;82:349-365.
101 Kerschbaumer F, Kandziora F, Klein C, et al. Transoral decompression, anterior plate fixation, and posterior wire fusion for irreducible atlantoaxial kyphosis in rheumatoid arthritis. Spine (Phila Pa 1976). 2000;25:2708-2715.
102 Crockard HA, Calder I, Ransford AO. One-stage transoral decompression and posterior fixation in rheumatoid atlanto-axial subluxation. J Bone Joint Surg Br. 1990;72:682-685.
103 McAfee PC, Bohlman HH. One-stage anterior cervical decompression and posterior stabilization with circumferential arthrodesis: A study of twenty-four patients who had a traumatic or a neoplastic lesion. J Bone Joint Surg Am. 1989;71:78-88.
104 Kast E, Mohr K, Richter HP, et al. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J. 2006;15:327-334.
105 Clarke MJ, Cohen-Gadol AA, Ebersold MJ, et al. Long term incidence of subaxial cervical spine instability following cervical arthrodesis surgery in patients with rheumatoid arthritis. Surg Neurol. 2006;66:136-140.
106 Ronkainen A, Niskanen M, Auvinen A, et al. Cervical spine surgery in patients with rheumatoid arthritis: Longterm mortality and its determinants. J Rheumatol. 2006;33:517-522.
107 Nakamura C, Kawaguchi Y, Ishihara H, et al. Upper thoracic myelopathy caused by vertebral collapse and subluxation in rheumatoid arthritis: Report of two cases. J Orthop Sci. 2004;9:629-634.
108 Hirohashi N, Sakai T, Sairyo K, et al. Lumbar radiculopathy caused by extradural rheumatoid nodules: Case report. J Neurosurg Spine. 2007;7:352-356.
109 Kawaji H, Miyamoto M, Gembun Y, et al. A case report of rapidly progressing cauda equine symptoms due to rheumatoid arthritis. J Nippon Med Sch. 2005;72:290-294.
110 Heywood AWB, Meyers OL. Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surg Br. 1986;68:362-368.
111 Kawaguchi Y, Matsuno H, Masahiko K, et al. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech. 2003;16:38-43.
112 Sakai T, Sairyo K, Hamada D, et al. Radiological features of lumbar spinal lesions in patients with rheumatoid arthritis with special reference to the changes around intervertebral discs. Spine J. 2008;8:605-611.
113 Crawford CH3rd, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes in patients with rheumatoid arthritis. Eur Spine J. 2008;17:822-825.