Chapter 42 Surgical Management of Posterior Fossa Meningiomas
Posterior fossa meningiomas are uncommon lesions that are most often slow-growing neoplasms manifesting in clinically indolent fashion. Based on clinical and radiographic information, the differential diagnosis is relatively straightforward; however, the range of management options can be considerable, including observation, surgery, and radiation in various forms and combinations. Although chemotherapeutic agents have been and continue to be investigated, this form of therapy is generally considered only for intractable, atypical, and malignant tumors and is not part of the general armamentarium in the majority of patients. Although the surgical management of meningiomas located in the posterior fossa may be challenging, patient-based outcomes studies suggest that long-term results can be encouraging.1
Posterior fossa meningiomas can be found anywhere in the posterior fossa, and individualized management recommendations depend primarily on size, growth rate, clinical presentation, and location (i.e., surgical accessibility). Tumors are primarily classified by their anatomic origin along the suboccipital surface of the cerebellum, lateral (i.e., lateral to the internal auditory meatus) petrous bone, cerebellopontine angle (CPA), petroclival region and clivus, jugular foramen, foramen magnum, pineal region, fourth ventricle, or tentorium (Table 42-1). Surgical accessibility varies based on location, with the most straightforward originating on the suboccipital surface of the cerebellum and lateral petrous bone; the more difficult originating on the tentorial surface, in the fourth ventricle, and at the CPA; and the most difficult originating at the petroclival junction. Each location is associated with different clinical implications regarding presentation and treatment strategy. Contemporary radiation therapy and radiosurgical decisions also depend on tumor location and the proximity of the tumor to critical neural elements. This chapter reviews the topic of posterior fossa meningioma from diagnosis though management and follow-up, in terms of both general and specific approaches relative to the various anatomic sites.
General Clinical Presentation and Diagnosis
The clinical presentation of posterior fossa meningiomas varies according to the location and size of the tumor, but because meningiomas are mostly slow-growing tumors, size plays less of a role in presentation and more of a role in choice of treatment strategy. Anatomically, the presenting clinical symptoms most often are related to involvement of cranial nerves and to a lesser degree the cerebellum, brain stem (i.e., long tracts), and fourth ventricle (i.e., hydrocephalus). Headaches are a common but nonspecific symptom. More specific clinical features based on location are discussed in the individual sections (see Table 42-1).
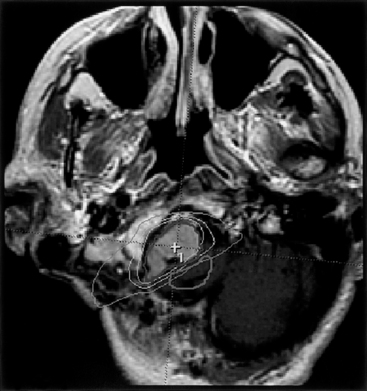
The diagnosis of meningioma is generally made radiographically, and most tumors share certain imaging characteristics. Plain radiographs, although no longer commonly utilized, often demonstrate bony hyperostosis, bony erosion, calcification, and occasionally, evidence of enlarged vascular channels. Transaxial computed tomography (CT) most often shows a hyperdense tumor signal (75% of cases) that intensely and homogeneously enhances after contrast administration. Cystic regions within the tumor can be seen but are uncommon, and peritumoral low-density suggestive of edema is noted in up to 60% of tumors.2 Calcification can also be noted on CT, but hemorrhage is distinctly uncommon. Three-dimensional computed tomography angiography (3D CTA) is useful in patients with skull base meningiomas. 3D CTA provides images that clearly depict the anatomic relationship between the meningioma and the bony structures of the skull base, as well as the relationship between the tumor and the neighboring vessels.3 Magnetic resonance imaging (MRI) most often reveals an isointense or hyperintense lesion on T1-weighted imaging, which is heterogeneous on T2-weighted imaging. These tumors almost always intensely enhance after gadolinium administration. The dural tail sign is one of the most revealing characteristics of meningioma, even though it is not pathognomonic (Fig. 42-1). A high-intensity signal noted on T2-weighted imaging is typical of peritumoral edema.2 While unproven in a large series of patients, diffusion-weighted MR imaging has been noted to correlate with the histopathologic features of certain meningiomas before resection.4 Cerebral angiography commonly (but not invariably) reveals an intense vascular supply, primarily from the dural arteries; a prolonged stain in the late venous phase of the angiogram is also common. Given the efficacy of magnetic resonance angiography (MRA), magnetic resonance venography (MRV), and 3D CTA for diagnosis and surgical planning, conventional angiography is used less frequently today. Nevertheless, it is still required for preoperative embolization.
Cerebral Angiography and Embolization
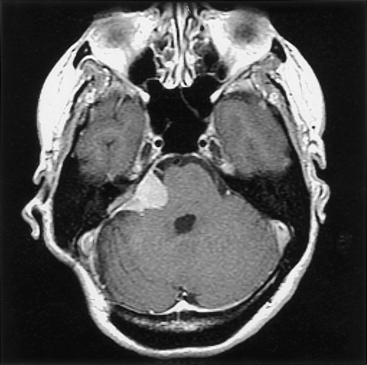
Given the limited space in the posterior fossa, the surgical removal of a highly vascular lesion can be fraught with difficulty (e.g., persistent bleeding obscures the operative field, requiring greater patience on the part of the surgeon to avoid increased morbidity), and although ideally the surgeon will select an approach that allows eradication of the tumor’s vascular supply early in the operation, this is not always possible or sufficient. In these instances, preoperative arterial embolization can be helpful, especially for selected tumors in the posterior fossa5 (Fig. 42-2). The primary reservations regarding embolization generally include risk of stroke, retroperitoneal hematoma, and secondary tumor necrosis and swelling (possibly leading to clinical deterioration). In addition, because the vascular supply is commonly made up of internal and external vessels, external carotid embolization may shift vascular supply to more-difficult-to-control internal carotid branches. Nevertheless, our experiences and those of others suggest that embolization can be helpful.6,7 Most authors conclude that to be most helpful, embolization must achieve complete or near-complete eradication of the vascular supply to the tumor: partial interruption of the blood supply may leave the surgeon with a significant intraoperative bleeding challenge.
An additional issue relates to the timing of the surgical procedure relative to embolization. After an investigation of 50 patients, Chun and colleagues observed that more favorable results of embolization were noted with delays of longer than 24 hours, although the optimal delay was not specified.8 It has become the practice with all posterior fossa tumors at Henry Ford Hospital to delay the surgical procedure 48 to 72 hours from the time of embolization; because of the potential risk (albeit rare) of clinical deterioration from tumor swelling, the patient is admitted to the hospital until surgery.
General Treatment Considerations
The general management considerations surrounding meningiomas located in the posterior fossa run parallel to those for treating these lesions located in other regions of the brain. Because meningiomas are mostly slow-growing neoplasms, urgent and emergent management decisions are seldom required. Recent reports on residual disease after surgical resection of petroclival tumors indicate that the average growth rate is 0.37 cm/yr; this is associated with a median time to progression of 36 months, a median progression-free survival of 66 months, and a 5-year progression-free survival rate of 60%.9 Older age, menopause, and previous radiation therapy can be associated with slower growth. In addition, we found that meningiomas with evidence of calcification on CT scan have longer doubling times. Despite this indolent nature, the neoplasms seem to grow relentlessly, often by en-plaque growth along tissue planes, along the dura, and through the basal cranial foramina, thereby making accurate growth assessment by CT or MRI a challenge. For practical purposes, four management options are available: (1) observation alone, (2) surgical resection, (3) radiation therapy or radiosurgery, and (4) some combination of surgery and radiation.
Surgery
Realistically, most surgeons focus primarily on the removal of the significant bulk (i.e., Simpson grade III) of the tumor and “decompression” of neurologic structures, including the brain stem and cranial nerves.10 Although it is known that recurrence often follows when the tumor is not entirely removed, the morbidity associated with attempts at total resection is clearly higher. However, in the modern era, and in the hands of experienced surgeons, the extent of resection has dramatically improved and has been associated with a marked decrease in morbidity.11 This decrease in morbidity largely relates to the use of skull base surgical approaches that rely on bone removal as opposed to brain retraction to create tumor exposure.
General concepts are important for the successful removal of most meningiomas; these include adequate bony exposure, early eradication of vascular supply, debulking of tumor mass, and maintenance of the arachnoid plane. A sufficient bony exposure greatly facilitates surgical manipulation and allows the surgeon an opportunity to move from one section of the tumor to another. Although it is preferable to internally debulk most tumors so that the tumor capsule can be more easily separated from adjacent normal structures, for many vascular lesions, an approach that provides access to and early elimination of the vascular supply is desirable (Fig. 42-3). Once the vascular supply is eliminated, a far less challenging resection may be possible.
Caused partially by the location of the cells of origin of meningiomas, these tumors are commonly situated next to cerebral venous structures, the preservation of which is often critical. Generally, acute occlusion of major draining veins or sinuses as a result of surgical approach and/or dissection is likely to lead to cerebral edema and significant neurologic deficit, and it should be avoided. Despite this, instances of resection of the tumor-infiltrated walls of a patent sagittal sinus with successful repair, complete tumor resection, and maintenance of sinus patency have been reported.12 In addition, division of the sigmoid and transverse sinuses during petrosal and pineal approaches, respectively, have been reported.13,14 In principle, however, it is safest to avoid division of major veins and sinuses. It is quite reasonable to consider radical subtotal resections, leaving patent venous structures intact and following the patient to monitor growth of the residual tumor with consideration of delayed radiation.
Radiation
Conventional Radiation Therapy
Meningiomas are best managed with total excision, if that is achievable with acceptable morbidity. However, about one third of meningiomas are not fully resectable because of tumor location, size, and proximity to adjacent tissue and vascular structures. The posterior fossa is also a common site of residual postoperative disease.15 Because subtotal resection is associated with a higher chance of clinically significant recurrence than is total resection, postoperative radiotherapy has been common practice and is associated with improved local tumor control and low morbidity.16,17 The goals of radiotherapy are to prevent tumor progression, prolong the interval to recurrence, and improve survival whether administered as adjuvant or primary therapy.
As primary therapy, radiation therapy has been shown to be efficacious for tumor control and long-term relapse-free survival.18–21 The standard recommendation for external beam radiotherapy is a dose of 5400 to 6000 cGy in fractionated doses of 180 to 200 cGy to the entire tumor plus a 2-cm margin. Histologically benign meningiomas have most often been treated with a lower dose of about 5400 cGy, and malignant meningiomas have been treated with 6000 cGy. To these dosing standards, the recent addition of intensity modulated radiation therapy (IMRT) has significantly improved the delivery process. IMRT allows multiple and highly conformal radiation beams to be delivered to the tumor volume while significantly decreasing the dose to immediately adjacent tissue (e.g., the optic tracts, acoustic and facial nerves, and brain stem). Three-dimensional radiography, coupled with CT- or MRI-assisted computerized treatment planning, and precise tumor localization and patient immobilization techniques have all served to improve progression-free survival as compared with the techniques used previously.22
Radiotherapy has been used primarily as an adjuvant treatment after subtotal resection, for infiltrating tumor into the adjacent brain parenchyma or pathologic finding of atypia, and after multiple recurrences. Although postoperative radiotherapy has never been evaluated in a randomized trial, there have been large retrospective analyses, and the results of these strongly support its role following subtotal resection.17,18 Recurrence rates of 60% after subtotal resection versus 32% after subtotal resection plus radiotherapy at mean follow-up of 78 months, and 5-year freedom from recurrence of 59% after subtotal resection alone versus 77% after postoperative radiation therapy, have been noted.16 These studies also found that median time to recurrence was doubled by radiotherapy (i.e., 66 vs. 125 months), and this analysis was notable in that many of the irradiated patients had more adverse factors such as surgically unfavorable sites (e.g., posterior fossa) than did patients treated with surgery alone.
In another report, findings for skull base meningiomas (including those in the posterior fossa) did not differ significantly in terms of progression-free survival from similar findings for other, more favorable sites.15 An interesting observation suggests that the progression-free survival rate at 5 years for those patients treated before 1980 was 77% versus 98% for those treated after 1980, when CT and MRI became available for tumor localization and treatment planning.22 The Royal Marsden Hospital experience also supported the role for postoperative radiotherapy, particularly following subtotal resection.21 All patients were treated to a total dose of 5000 to 5500 cGy over 6 to 6.5 weeks. Interestingly, there was no significant difference in disease-free survival of minimum postoperative residual versus bulky residual; with actuarial disease-free survivals at 5, 10, and 15 years, patients with minimum residual tumor had survival rates of 78%, 67%, and 56%, respectively, whereas patients with bulky residual tumor had survival rates of 81%, 68%, and 61%, respectively.
As salvage therapy for patients with recurrent or progressive disease, radiotherapy may be used either alone or as an adjuvant to surgical resection. In these cases, local control with the radiotherapy appears equal or superior to that seen with resection alone.17,23,24 With a total dose of 4500 to 5500 cGy using older megavoltage radiation, the crude salvage rate was 50% as compared with 37% for reoperation alone.23 More recent reports showed 10-year tumor control rates in the range of 80% with adjuvant radiotherapy following reoperation, as opposed to 10% to 30% for surgery alone.24 Although the local control rate following salvage radiotherapy compares favorably with rates seen with immediate postoperative radiotherapy, these data should not be interpreted as sole justification for withholding radiation in the early postoperative period. Given the natural history of many meningiomas, progression, although common, is often extremely slow, and the decision as to when to radiate (i.e., immediate postoperative period vs. time of recurrence) is a matter for the treating physician and patient. In our practice, radiation is generally postponed to the time of demonstrable radiographic or clinical progression.
• Group I (low risk) includes gross total resection (Simpson grade I-III) and subtotal resection with World Health Organization (WHO) grade I. These patients will be closely observed.
• Group II (intermediate risk) includes gross total resection with WHO grade II and recurrent WHO grade I meningioma, irrespective of resection extent. These patients will undergo radiotherapy (54 Gy/30 Fr) by 3D CTA or IMRT.
• Group III (high risk) includes newly diagnosed or recurrent WHO grade III of any resection extent, recurrent WHO grade II of any resection extent, and newly diagnosed WHO grade II subtotal resection. These patients will be treated with radiotherapy (60 Gy/30 Fr) by IMRT.
Stereotactic Radiation Therapy and Radiosurgery
Despite the favorable results obtained with conventional fractionated radiotherapy for subtotally resected, unresectable, and “inoperable” meningiomas, the use of radiosurgery has evolved as both adjuvant and primary therapy. Controversy still exists, however, regarding the use and efficacy of radiosurgery. The arguments against the routine use of radiosurgery are largely based on the lack of long-term radiosurgical data and on the fear of potential delayed radiation injury to the brain tissue. However, considering the encapsulation of most benign meningiomas, the relatively low risk of infiltration into the adjacent brain tissue, the steep dose gradient characteristics, and the low reported morbidity (albeit after relatively short-term follow-up), the use of radiosurgery has become firmly established and is readily applied to meningiomas in the posterior fossa. Another significant concern has been the likelihood that secondary tumors will develop in the wake of radiosurgery, although few reports have appeared in the literature to date.25 Fractionated radiosurgery has also become readily available (Fig. 42-4), largely as a result of noninvasive patient immobilization methods, including “head mask” frame (i.e., noninvasive) and frameless image-guidance systems.
A recent report evaluating fractionated stereotactic radiotherapy showed survival rates of 97% for 5 years and 96% for 10 years after treatment, with local failure seen in only 3 out of 189 patients with WHO grade I meningiomas (22.5% located in the posterior fossa).26 These researchers noted tumor shrinkage (i.e., more than 50% reduction) in 14% of patients and resolution of preexisting cranial nerve symptoms in 28% of patients. Clinically significant treatment-related toxicity was seen in 1.6% of the patients.26 Outcomes data for radiosurgery are available for intracranial meningiomas generally, as well as for those located in the posterior fossa. Engenhart et al. reported an experience of radiosurgery in 17 patients with histologically proven benign meningiomas. Using a linear accelerator radiosurgery system, a maximum single dose of 10 to 50 Gy was used, with mean dose of 29 Gy prescribed to the 80% isodose line matching the tumor volume.27 The dose was chosen based on tumor volume, tumor location, and radiosensitivity of the adjacent brain tissue. Freedom from tumor progression was noted in 80% of patients, and late complications included transient neurologic deficits with perifocal brain edema and one case of visual loss.
Relating to intracranial meningiomas in general, Kondziolka et al. reported 2-year radiosurgical tumor control rates of 96% in 50 patients.28,29 The radiosurgery dose was 10 to 25 Gy (mean 17 Gy) to the tumor margin using a gamma knife unit; the doses were chosen based on tumor volume, location, previous radiotherapy dose, and adjacent critical normal tissue (e.g., optic chiasm). Tumors greater than 35 mm in diameter and located within 5 mm from the optic chiasm were excluded for radiosurgery. Three patients experienced delayed neurologic complications, thought to be consistent with radiation injury at 3 to 12 months following radiosurgery.28–30 In another report from the Mayo Clinic on radiosurgical results for meningioma (in which 77% of 206 tumors were located at the cranial base), the median tumor margin dose was 16 Gy and survival rates at 5 and 7 years were 94% and 92%, respectively.31 The overall tumor control rate was 89% at 5 years, with 56% of tumors decreasing in size. Taken together, the available data relating intracranial meningiomas (with the endpoint being freedom from progression after radiosurgery) indicate that tumor control appears to be in the range of 90%, with tumor shrinkage noted in approximately one third of radiosurgical patients.
Meningiomas in the posterior fossa often require radiation as either adjuvant or primary therapy. The radiosurgical experiences for acoustic and other schwannomas, as well as those for various benign and malignant tumors, have provided an important database regarding the responses of the cranial nerves and brain stem. For practical purposes, most cranial nerves are tolerant to radiosurgical doses up to 16 Gy, whereas the optic nerves, chiasm, and tracts seem more sensitive; for these, lower doses up to 10 Gy seem reasonable.32 The radiosurgical response may vary depending on the length of nerve irradiated; the microenvironment of the nerve (e.g., ischemia), the degree of compression by the tumor, prior surgery and radiotherapy effects, retreatment and treatment intervals, and additional host factors33,34 (Fig. 42-5). Motor morbidity rates appear to be less than 5%. However, the assessment of sensory nerve function poses more difficult problems, depending on the tumor type and location. For example, whereas the radiosurgical impact on cochlear nerve function may be less significant when dealing with meningiomas, radiosurgical dosing for acoustic neuroma has undergone a gradual decrease, possibly as a result of the intimate relation of tumor to nerve. One recent report on 55 patients with skull base meningiomas (including three of the petrous apex, four of the tentorium, two of the clivus, five of the CPA, and three of the jugular foramen), with average follow-up of 48.4 months, noted tumor stabilization in 69%, shrinkage in 29%, and enlargement in 2%.35 Mean tumor volume was 7.33 cm3, and doses ranged from 12 to 25 Gy. All complications were transient, including seven trigeminal pareses and three patients with diplopia. Radiosurgical data on 62 patients with petroclival meningiomas treated with tumor margin doses of 11 to 20 Gy documented tumor shrinkage in 23%, stabilization in 68%, and growth in 8%.36 Median follow-up was 37 months.
For tumor residuals intimately attached to the dural sinuses, similar principles apply. Based on the current literature, it is reasonable to use radiosurgery for a tumor located adjacent to or inside a dural sinus. There does not appear to be increased risk of weakening of the walls of the sinus, venous infarction, or other vascular-related complications, and it seems unlikely that radiosurgery will lead to progressive sinus occlusion. Larger experience and longer-term follow-up are needed to confirm these findings.
Combined Surgery and Radiation
Ultimately, it seems logical to consider a combination of surgical resection and postoperative radiation treatment for the management of many meningioma patients. Many published reports have supported a planned, staged, microsurgery followed by stereotactic radiosurgery for selected patients with meningioma when indicated.37,38 In these situations, microsurgical cytoreduction of a portion of the tumor can be achieved with minimal morbidity, as well as creation of adequate margins of space between the residual tumor surface and the differentially radiosensitive surrounding structures.
The optimal timing of the two stages remains a matter for debate. Delay of 12 to 18 months between stages allows for maximal recovery of any partial cranial neuropathy that occurs as a result of microsurgery before exposing the cranial nerves to an additional pathologic stress with radiation. However, delay between stages increases the likelihood of interval tumor growth and leads to loss of safety space achieved with surgery, because the previously compressed and displaced normal surrounding tissue returns to its natural position. If a delay between stages is contemplated, regular neuroimaging at 3-month intervals is recommended to detect and react to any unexpected circumstances.39 This allows the surgeon to accelerate the radiation treatment to respond to the changing situation.
Perioperative Issues
Preoperative Edema and Postoperative Swelling
The postoperative problems secondary to edema are best dealt with during the operation by ensuring sufficient bony exposure, thereby limiting the extent of cerebellar retraction, which can aggravate the edema. A difficult tumor dissection may distract the surgeon, and when tumor removal requires ever-greater retraction, it is often best to resect the portion of the commonly contused cerebellum underlying the retractor rather than leave it alone, because this area can further swell and create significant, if not fatal, postoperative problems. Steroids are continued into the postoperative period and osmotic diuretics (i.e., mannitol and/or hypertonic saline solutions) may also be useful. Standard skull base approaches specifically for petroclival tumors now substitute bony removal for cerebellar retraction as the primary means to achieve the necessary exposure for safe tumor resection.
Hyperostosis
Although still somewhat controversial, the hypertrophic bony changes often noted adjacent to meningiomas are known in most instances to be secondary to tumor invasion. Previous theories have suggested that these bony changes were related to vascular disturbances, “irritation” of the bone, osteoblastic stimulation by tumor, and direct tumor-derived bone formation.40 Our experience, as well as the plentiful evidence in the literature, demonstrates that in many cases these changes are secondary to tumor invasion. The practical issue then relates to the potential for tumor recurrence, and it is well known that the best chance of cure follows radical tumor resection including the involved dura and bone (i.e., Simpson grade I).
Venous Obstruction and Infarction
Although more obvious with supratentorial tumors, venous infarction can complicate surgery in the posterior fossa. Adequate exposure and internal debulking (when possible) of the tumor are the best means of avoiding this complication. After debulking, the tumor falls away or can be more easily separated from the adjacent vein. The effects of disrupting the venous sinuses (deep and superficial draining veins) are not completely predictable. The veins near the roof of the fourth ventricle (i.e., around the cerebellar vermis and tonsils, petrosal veins, and veins of the cerebellomedullary fissure) can usually be sacrificed without major consequences. However, sacrificing the Galen vein, torcula, dominant lateral, or sigmoid sinuses is considered high risk.13,14 Resection of a meningioma involving unilateral transverse sinus is feasible when the contralateral traverse sinus is open and close to the size of involved sinus. If a significant size discrepancy exists, prior to division of the involved transverse sinus, temporary sinus occlusion and pressure measurement should be considered.14 When present, significant changes in manometric pressures during temporary occlusion and acute but temporary brain swelling indicate high risk.
Specific Locations in the Posterior Fossa
Occipital and Lateral Petrous Surface of the Posterior Fossa
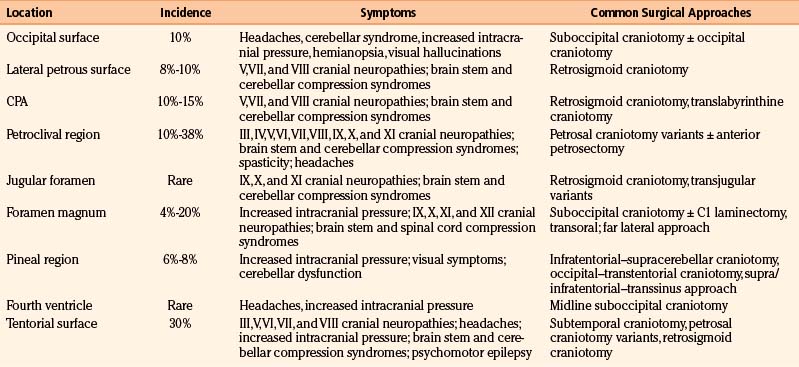
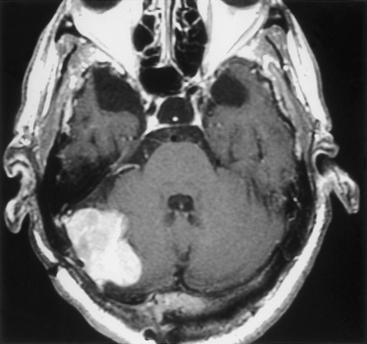
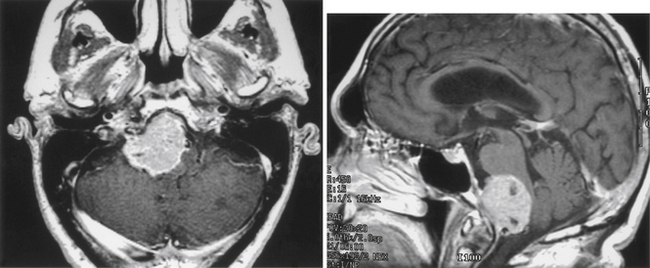
Tumors located along the midline and lateral aspect of the occipital surface and lateral petrous bone (i.e., lateral to the internal auditory meatus) are, surgically, the least problematic (Fig. 42-6). Meningiomas in this region often present with symptoms of cerebellar dysfunction and headache, but they may also present as an incidental finding during workup for unrelated central nervous system symptoms. Occasionally, these tumors present with signs of elevated intracranial pressure. A suboccipital craniectomy/craniotomy is generally sufficient, and standard surgical techniques are employed to remove the tumor. The main surgical challenge is not to inadvertently open a patent dural sinus, although a simple suture or graft generally suffices as closure. The surgeon must realize that collateral venous drainage around an occluded sinus may be critical and should be protected. For those lesions located on the petrous bone lateral to the CPA, careful attention to the nerves coursing into the internal auditory meatus is required, and electrophysiologic monitoring of both the facial and the acoustic nerves is recommended. Although these lateral petrous tumors may be seen on MRI (T2-weighted images are usually best for this purpose) to be adjacent but lateral to the facial and acoustic nerve complex, the ability to stimulate the facial nerve, especially because it is located behind the tumor (i.e., in the surgeon’s view), can be reassuring. It is important after the resection is complete to assess whether the adjacent cerebellum has been too heavily retracted or contused. In either case, resection of the injured brain may be prudent to avoid the postoperative risks associated with swelling.
Tentorial Surface
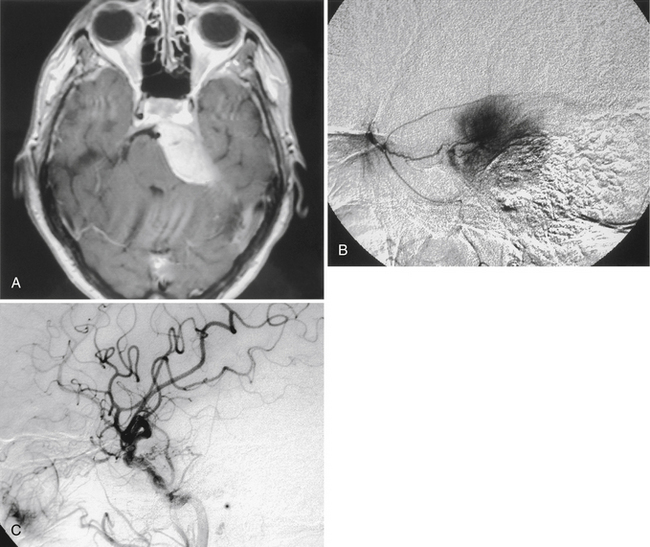
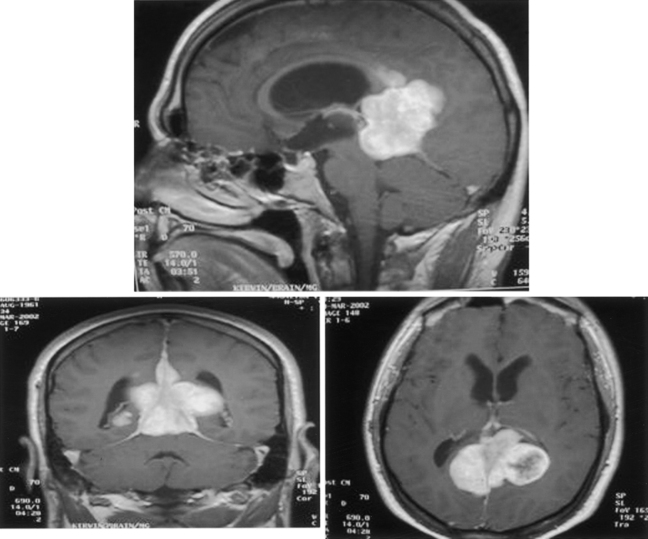
The clinical presentation of patients with tentorial meningioma is generally nonspecific. When the tumor originates in the anterior region of the tentorium, compression of the trigeminal and abducens nerves may lead to tic doloreaux and oculomotor paresis, but trochlear nerve signs are uncommon. Tumors originating from the posterior region of the tentorium rarely present with other than headache and, perhaps, mild cerebellar signs. Occasionally, as a result of occlusion of the dural sinuses, a meningioma may present with signs of elevated intracranial pressure and visual loss (Fig. 42-7).
An additional but rare clinical presentation involves that of trigeminal neuralgia and hemifacial spasm secondary to a tumor that arises on the posterior portion of the tentorium but not involving the CPA.41 In this case, the presumed cause of the neurologic symptoms and signs was rotation of the brain stem secondary to the large size of the tumor such that the cranial nerves were brought in contact with an ectatic vertebral artery. All symptoms and signs resolved after tumor removal.
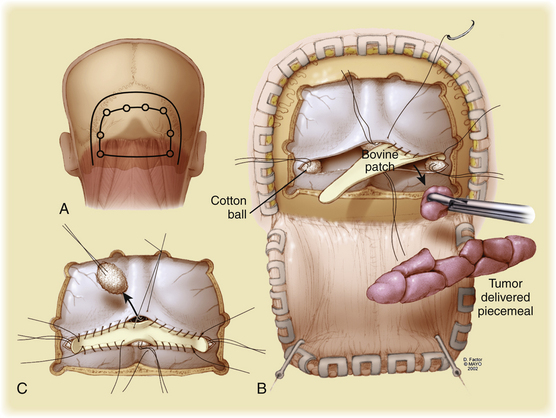
Although not a unique feature of tentorial meningiomas, an interesting surgical challenge concerns the handling of the dural venous sinuses. Generally, resection of these sinuses is not recommended if there is any indication of patency on CTA, angiography, or MRV or by direct intraoperative observation. When the sinus is completely occluded, it can be resected, but attention to the collateral venous drainage is critical because disruption of significant collateral drainage can be fatal.42 Collignon et al. recently reported a case of occlusion of the torcula and bilateral transverse sinuses with hemangiopericytoma43 (Fig. 42-8). The patient presented with visual blurring and bilateral papilledema, which was managed by bilateral occipital and suboccipital craniotomy. The tumor was noted to be filling the sinuses without extension beyond the walls of the sinuses. Total resection was achieved, and control of venous bleeding was accomplished with cotton balls, after which the sinus was repaired with bovine pericardial graft. Radiation therapy was used adjunctively, and 4 months after surgery the sinus remained patent, the patient was well, and papilledema had resolved. Although this unique case and others demonstrates the feasibility of sinus resection, it should not be undertaken lightly.
Cerebellopontine Angle
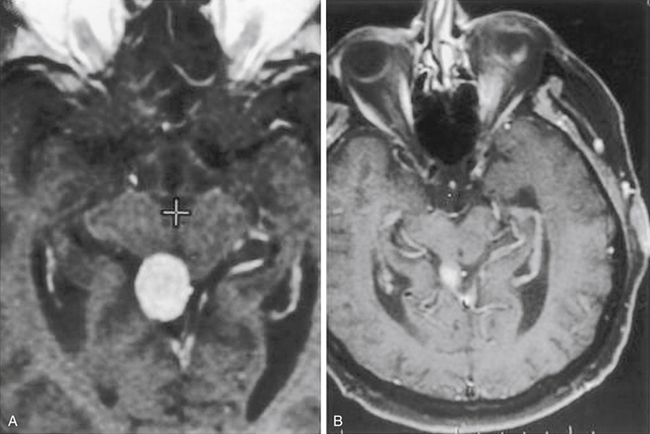
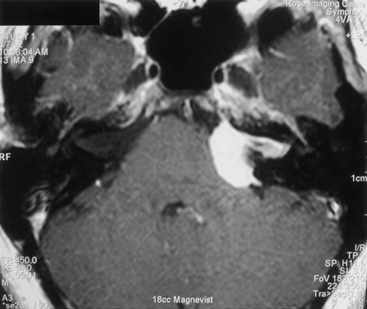
The CPA is the most common location for posterior fossa meningiomas, which are thought to originate from arachnoid cap cells in this region. The primary differential diagnostic consideration is acoustic neuroma, which arises from Schwann cells on the vestibular division of the statoacoustic nerve. Patients generally present in their fourth and fifth decades of life.44 The clinical presentation, as for meningiomas as a whole, depends on the site of origin and direction of growth. Tumors growing superior to the internal auditory meatus commonly present with trigeminal symptoms (notably numbness and/or neuralgia), whereas those growing alongside the meatus may present with auditory and facial nerve dysfunction, the latter being distinctly uncommon with vestibular schwannoma. Those tumors growing inferior to the meatus may present with dysfunction of the lower cranial nerves (i.e., IX through XII). Symptoms and signs secondary to brain stem and cerebellar compression, because of the slow growth of the typical meningioma, are a late occurrence and often are not apparent even with very large tumors. CPA meningiomas cannot be distinguished from other tumor types based on clinical presentation alone, even though hearing loss and vestibular dysfunction are far less common with meningioma than with acoustic schwannoma.
MRI is the study of choice, although various auditory tests (e.g., pure-tone audiometry, speech discrimination, acoustic reflex, and brain stem auditory evoked responses) are commonly performed in neuro-otology practice, where many patients presenting with hearing loss are first evaluated. MRI findings (i.e., extra-axial CPA mass) can include an enlarged CPA cistern, gray–white distortion of the adjacent cerebellum, and an indication of contrast enhancement extending along the skull base beyond the main tumor mass (i.e., dural tail) (Fig. 42-9). Calcification and cystic change are more common and less common, respectively, with meningiomas, whereas expansion of the porus acousticus is uncommon for the meningiomas. Although a typical acoustic schwannoma has the appearance of an ice cream cone, with sharply demarcated and rounded borders along the CPA portion with tumor extension filling the fundus of the internal auditory canal (IAC), meningiomas most often fill the CPA cistern with lateral extensions, irregular borders, and distinctly less notable growth into the IAC.
Because the epicenter of the tumor is the IAC, a standard lateral suboccipital approach generally provides sufficient exposure for complete tumor removal.45 The patient is placed in a “park bench” position with the head slightly flexed and tilted toward the floor, after which the nose is rotated toward the floor. Mannitol and spinal drainage may facilitate the exposure and help to maintain this exposure, especially with larger tumors and longer dissections. The facial and acoustic nerves are routinely monitored. Given that the cranial nerves do not generally adhere to the tumor capsule, hearing preservation and even improvement should be reasonable expectations.
Other surgical approaches can be considered, but these have limitations. The translabyrinthine approach may be suitable when hearing is absent and may allow for early devascularization and identification of the facial and statoacoustic nerves, but the bony drilling is time consuming. The standard petrosal approach is generally too extensive for most lesions limited to the CPA, and the subtemporal approach does not provide complete exposure to the CPA for most of these tumors.
Petroclival Region
Anatomically, the petroclival region extends along the midline from the level of the posterior clinoid process to the jugular foramen and laterally to the CPA, incorporating cranial nerves III through XI and the brain stem. In a recent publication, Cho and Al-Mefty described hearing loss, facial and trigeminal pareses, gait disturbance, dysarthria, spasticity, and headache as the predominant clinical findings in patients with petroclival meningiomas prior to surgery.46
Pioneered by Malis, transpetrous approaches provide exposure above and behind the petrous bone into the middle and posterior fossa, respectively.47 Subsequent modifications of this approach provided additional exposure by incorporating various extents of petrous bone resection (i.e., retrolabyrinthine), which in keeping with standard skull base techniques, markedly decreases the need for temporal lobe, cerebellar, and brain stem retraction, thereby minimizing morbidity and allowing for total to near-total tumor resection.48 This approach follows a route anterior to the sigmoid sinus and shortens the distance from the operator to the tumor. The approach also increases the risk to hearing loss by inadvertent drilling into the posterior semicircular canal; however, even this approach cannot easily visualize the most anteromedial portion of tumors extending along the midline clivus.
The anterior transpetrous approach is well suited for smaller lesions centered medial and superior to the internal auditory meatus and is based on the original description of the extended middle fossa approach.46,49,50 In 1992, Spetzler et al. reported the results of various extents of petrous bone resection (i.e., retrolabyrinthine, translabyrinthine, and transcochlear) for 46 lesions including 18 petroclival meningiomas.13 A recent report describes the addition of an anterior transpetrous bony removal (i.e., anteromedial to the cochlea, posteromedial to the petrous carotid, and inferior and medial to the gasserian ganglion) to a standard petrosal approach.46 The authors report that in five of seven tumors, total resection was accomplished with neither mortality nor decrease in performance status, and only one patient lost hearing.
In the combined approach, the patient is placed in a supine position with a bolster under the shoulder and head turned about 30 to 45 degrees to the contralateral side.46 The supra- and infratentorial craniotomy utilizes four bur holes placed above and below the transverse–sigmoid junction, plus one hole immediately above the zygoma, another at the superior temporal line, and one hole in the posterior fossa. The plate can frequently be removed as one piece. Next, a mastoidectomy with retrolabyrinthine bony removal is performed. The middle fossa dura is elevated, and the middle meningeal artery and greater superficial petrosal nerve are divided. The horizontal portion of the carotid is then exposed, and after identification and elevation of the third division of the trigeminal nerve, the anteromedial petrous apex (i.e., anteromedial to the cochlea) is removed, exposing the upper clivus. The dura is then opened above and below the superior petrosal sinus (SPS). The SPS is divided after the surgeon has carefully examined the MRV to ensure that no significant temporal bridging vein will be interrupted.51 This maneuver exposes the middle and inferior clival regions. The tumor can now be appreciated, and piecemeal removal follows with initial attention to the vascular supply. By working back and forth between the inferior and the superior extremes of this exposure, the surgeon often can radically remove the tumor and safely work around vascular and nervous elements. The initial phases of the dissection may be facilitated by image guidance, especially regarding the anterior transpetrous resection. The approach provides excellent exposure for lesions centered on the midline and extending ipsilaterally, as well as for extensions across the midline. The most anteromedial portions of the tumor may occasionally be better visualized through the retrosigmoid portion of the exposure toward the end of the dissection (see Fig. 42-3). If hearing is to be preserved, this combined approach is generally required (especially for larger tumors); however, when hearing is not an issue, the translabyrinthine and transcochlear bony variations add considerably to the exposure.
Jugular Foramen
The jugular foramen region is a relatively uncommon origin for meningiomas.
Symptoms and signs reflect the local anatomy with involvement of cranial nerves IX to XI (and occasionally V and VIII), and larger tumors may be associated with brain stem compression.52 Because of the nature of the preoperative neurologic deficits, otolaryngology evaluation should be performed to establish a preoperative baseline, but postoperative testing is also required and is more critical. Although most often temporary, postoperative swallowing deficits can be debilitating and require gastrostomy and tracheostomy.
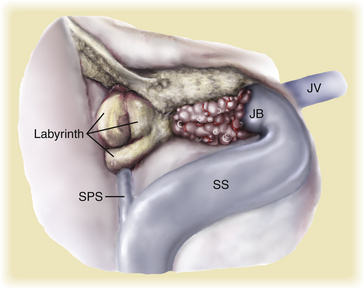
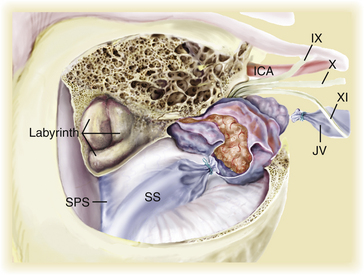
Monitoring of cranial nerves VII, VIII, IX, XI, and XII should be performed because these nerves may be hidden (especially IX, X, and XI) by the tumor capsule. Arnautovic et al. describe a suprajugular, retrojugular, and transjugular approach for tumors in this region, with the patency and dominance of the jugular bulb dictating the choice.53 The infralabyrinthine suprajugular approach requires a mastoidectomy, followed by skeletonization of the superior petrosal and sigmoid sinuses and jugular bulb, after which the tumor is dissected from the cranial nerves (Fig. 42-10). In the retrojugular approach, a lateral suboccipital craniotomy and mastoidectomy with skeletonization of the sigmoid sinus and jugular bulb are followed by removal of a portion of the occipital condyle and jugular tubercle. A durotomy posterior to the sigmoid sinus provides the exposure for tumor removal. In the transjugular variation, a mastoidectomy that exposes the sigmoid sinus, jugular bulb, and jugular vein and suboccipital craniotomy is performed. The posterior belly of the digastric muscle and the stylohyoid muscle are divided, after which the styloid process is removed. The sigmoid sinus and jugular vein are ligated above and below the tumor, respectively, and the tumor is dissected from the cranial nerves and carotid artery (Fig. 42-11). In this series of eight patients (nine surgeries), worsened or new deficits in three patients were reported, and some degree of recovery or stabilization was noted in all eight patients.52
Foramen Magnum
Meningiomas at the foramen magnum arise from the dura of the craniocervical junction; they account for 4.2% to 20% of posterior fossa meningiomas, depending on the referral status of the reporting institution47 (Fig. 42-12). These tumors tend to go undiagnosed or misdiagnosed for long periods because of the variety of presenting symptoms. Tumors can present with cerebellar signs, evidence of increased intracranial pressure, lower cranial nerve deficits, and brain stem or spinal cord signs mimicking cervical myelopathy with radicular signs and dysesthesias. In addition, symptoms of suboccipital pain may be seen.
Surgical approaches have included the standard posterior midline suboccipital approach with C1 laminectomy, the transoral approach, and the transcondylar approach.54–56 For true ventral lesions, the posterior midline approach is insufficient for anything better than a Simpson grade IV removal, and it can be associated with significant morbidity because the spinal cord and lower brain stem will be interposed between the surgeon and the pathology. However, for lesions arising more laterally and dorsal to the dentate ligaments and displacing the spinal cord and brain stem medially, standard midline approaches may well be sufficient, although removal of the most medial extents of the tumor can be difficult in the final phases of the operation when the brain stem and spinal cord expand into the operative field. Theoretically reasonable from an anatomic viewpoint, the transoral approach provides limited exposure, carries the serious risk of postoperative cerebrospinal fluid leak through the oropharyngeal mucosa, and is best left to surgeons experienced with transoral techniques.55 The transcondylar approach has been detailed by Sen and others56–58 and is most suitable for practically all meningiomas in this location.
Preoperative considerations include the precise origin of the tumor, the relationship of the vertebral artery to the tumor, the relative sizes (i.e., contribution to stability) of the occipital–C1 joint, the vascular anatomy, the patency of the vertebral arteries (i.e., noted with CTA, MRA, and MRV), and the status of lower cranial nerve function (especially swallowing). Patients are placed in a park-bench position and are monitored for somatosensory and possibly auditory brain stem function, along with cranial nerves X through XII. The transcondylar approach for ventrally situated tumors generally requires transposition of the vertebral artery, which must be carefully rotated such that thrombosis will not occur during the commonly lengthy procedure. The microvascular Doppler can be helpful in locating the vertebral artery in the early stages of the operation. To create the greatest extent of exposure, especially for tumors with extension across the midline, one half to two thirds of the condyle may be removed. Only rarely does this require surgical stabilization. The final element in the initial exposure involves the extent of venous engorgement in the so-called suboccipital cavernous sinus, as detailed by Caruso et al., which can complicate the dissection.59 From this point, careful attention to the cranial nerves traversing the tumor capsule is required while the tumor is debulked and dissected. In a review of 18 patients with ventral foramen magnum tumors followed for a mean of 40 months after first-time surgery, Arnautovic et al. described 75% with gross total resection (Simpson I-II), 12.5% with near-total resection (Simpson III), and 12.5% with subtotal excision (Simpson IV).53 Ninth and tenth cranial nerve deficits were the most common consequence, and there was no 30-day operative mortality.
Pineal Region
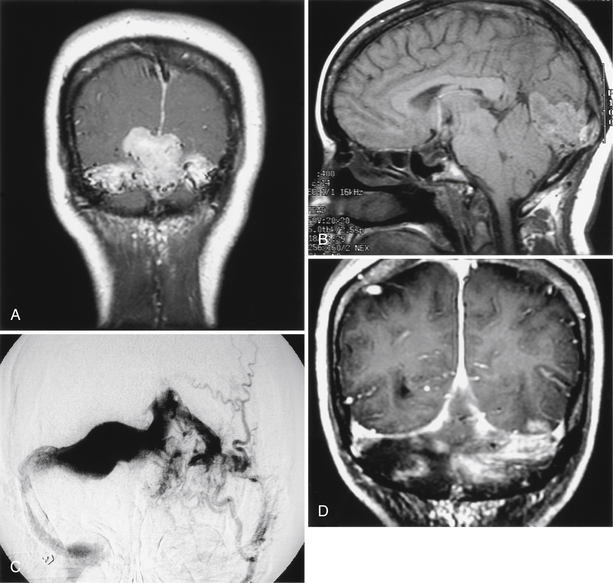
Meningiomas in the pineal region are a rare entity constituting approximately 6% to 8% of neoplasms.60 Most meningiomas arise from the tentorial edge or from the junction of tentorium and falx, although some instances of lesions with no dural attachment have been reported, in which case the tumor is believed to arise from the telachoroidea in the roof of the third ventricle.61,62 Presenting symptoms are quite variable, partly reflecting the local anatomy. Common presenting symptoms are related to increased intracranial pressure (i.e., secondary to either tumor size or deep venous occlusion) and/or the visual system.63 As with many meningiomas in various locations, headache is a common but nonspecific finding. Involvement of the tectal region may manifest as various degrees of upgaze paresis, pupillary, and convergence abnormalities culminating occasionally in a complete Parinaud syndrome. Symptoms and signs of cerebellar dysfunction may also occur but are uncommon. When the tumor compresses the cerebral aqueduct, hydrocephalus may develop.
The imaging study of choice is contrast-enhanced MRI with MRA and MRV, although with larger tumors cerebral angiography may be more useful for arterial and venous delineation (i.e., internal cerebral veins, basal Rosenthal vein, Galen vein, sinuses, and superficial venous system). Preoperative embolization, although rare, may also be considered (Fig. 42-13). Feeding vessels are commonly the medial and lateral posterior choroidal arteries, as well as the tentorial arteries. Given the usual operative choices (i.e., infratentorial–supracerebellar and occipital–transtentorial approaches), a clear understanding of the arterial and venous anatomy may be important because early arterial occlusion during the surgical procedure is not always possible.
In the past, surgical treatment of pineal region meningiomas was associated with significant morbidity and mortality; however, as with meningiomas in all other locations, recent subspecialization and improvements in surgical techniques have markedly reduced these morbidities.64 The main surgical approaches currently used are infratentorial–supracerebellar and occipital–transtentorial, with a supra/infratentorial–transsinus approach recently reported.14 When significant obstructive hydrocephalus is detected preoperatively, insertion of a ventriculoperitoneal shunt may be considered; however, endoscopically guided third ventriculostomy is becoming the procedure of choice.
With the occipital–transtentorial approach, it is preferable to approach the tumor from the nondominant side, but tumor shape and vascular anatomy primarily determine this orientation. The patient may be sitting or semisitting, but we prefer a three quarter–prone position with the operated side down, which diminishes the degree of occipital lobe retraction.65 The occipital lobe is gently retracted laterally, with attention paid to the bridging veins because their injury can lead to hemianopsia. After a paramedian tentorial incision, the tumor should come into view. The advantage of this approach is surgeon comfort (i.e., for those unfamiliar with the sitting position); disadvantages include limited space, potential for retraction injury to occipital lobe, off-midline anatomic orientation, and venous anatomy oriented between the surgeon and the tumor.
The advantages of the infratentorial–supracerebellar approach are the midline orientation, excellent tumor visibility below the venous system, and minimal cerebellar retraction. The main disadvantages of this approach, done with the patient in the sitting position, are limited exposure of tumors extending above the deep venous complex and fatigue in the surgeon’s arm and hand, although with appropriate flexion of the patient’s head and back such fatigue can be minimized.66 As for any operation with the patient in a sitting position, air embolism is a possibility.63
For large tumors in the pineal region, a combined supra/infratentorial–transsinus approach has been described.14 A craniotomy in three pieces is made over the torcula and transverse sinuses, with subsequent section of transverse sinus and tentorium. This approach provides a wide surgical corridor and large exposure with access to the tumor extending into the third ventricle, minimal retraction of cerebellum and occipital lobes, and a comfortable position for the surgeon.14
Fourth Ventricle
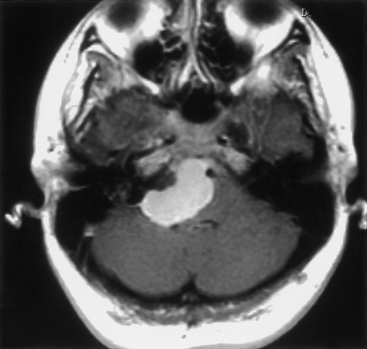
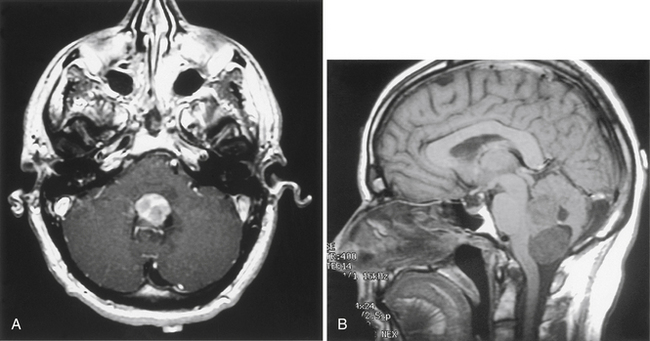
Meningiomas located in the fourth ventricle are rare and usually present with signs of obstructive hydrocephalus; rarely, they present with cranial neuropathies secondary to compression of cranial nerves and nuclei in the floor of the ventricle (Fig. 42-14). The surgical approach generally requires a midline suboccipital craniotomy with vermian split and early devascularization of the tumor. Care to limit iatrogenic compression of the structures in the floor and not to intrude through the floor of the ventricle is paramount. With larger tumors, it is generally recommended to monitor somatosensory and brain stem auditory evoked responses along with lower cranial nerves (i.e., VI to XII).
Akagami R., Napolitano M., Sekhar L.N. Patient-evaluated outcome after surgery for basal meningiomas. Neurosurgery. 2002;50:941-949.
Ausman J.I., Malik G.M., Dujovny M. Three-quarter prone approach to the pineal–tentorial region. Surg Neurol. 1988;29:298-306.
Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
Bendszus M., Rao G., Burger R., et al. Is there a benefit to preoperative meningioma embolization. Neurosurgery. 2000;47:1306-1312.
Bonnal J., Brotchi J. Reconstruction of the superior sagittal sinus in parasagittal meningiomas. In: Schmidek H.H., editor. Meningiomas and their Surgical Management. Philadelphia: WB Saunders; 1991:221-229.
Carella R.J., Ransohoff J., Newall J. Role of radiation therapy in the management of meningioma. Neurosurgery. 1982;10:332-339.
Cho C.W., Al-Mefty O. Combined petrosal approach to petroclival meningiomas. Neurosurgery. 2002;51:708-716.
Chun J.Y., McDermott M.W., Lamborn K.R., et al. Delayed surgical resection reduces intraoperative blood loss for embolized meningiomas. Neurosurgery. 2002;50:1231-1237.
Elster A.D., Challa V.R., Gilbert G.H., et al. Meningiomas: MR and histopathologic features. Radiology. 1989;170:857-862.
Forbes A.R., Goldberg I.D. Radiation therapy in the treatment of meningioma: The Joint Center for Radiation Therapy experience. J Clin Oncol. 1984;2:1139-1143.
House W.F., Hitselberger W.E., Horn K.L. The middle fossa transpetrous approach to the anterior–superior cerebellopontine angle. Am J Otol. 1986;7:1-4.
Kinjo T. Al-Mefty O, Imad K: Grade zero removal of supratentorial convexity meningiomas: clinical study. Neurosurgery. 1993;33:394-399.
Kondziolka D., Lunsford L.D. Radiosurgery of meningioma. Neurosurg Clin N Am. 1992;3:219-230.
Kondziolka D., Lundsford L.D., Flickinger J.C.. Stereotactic radiosurgery of meningiomas, Lundsford L.D., Kondziolka D., Flickinger J.D., editors, Gamma Knife Brain Surgery, Basel, Karger, 1998;Vol.14:104-113.
Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53(4):815-822.
Mirabell R., Linggood R.M., de la Monte S., et al. The role of radiotherapy in the treatment of subtotally resected meningiomas. J Neurooncol. 1992;13:157.
Rock J.P., Monsell E.M., Schmidek H.H. Meningiomas of the cerebellopontine angle. In: Schmidek H.H., editor. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders; 1991:417-425. Harcourt Brace Jovanovich
Sekhar L.N., Janetta P.J. Cerebellopontine angle meningiomas. J Neurosurg. 1984;60:500.
Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
Simpson D. The recurrence of intracranial meningioma after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22.
Spetzler R.F., Daspit P., Pappas C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: Experience with 46 cases. J Neurosurg. 1992;76:588-599.
Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurg. 2001;49:1029-1038.
Tishler R.B., Loeffler J.S., Lundsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
Yasargil M.G., Mortara R.W., Curcic M. Meningiomas of basal posterior cranial fossa. Adv Tech Stand Neurosurg. 1980;7:1-15.
Ziyal I.M., Sekhar L.N., Salas E., Olan W.J. Combined supra/infratentorial–transsinus approach to large pineal region tumors. J Neurosurg. 1998;88:1050-1057.
1. Akagami R., Napolitano M., Sekhar L.N. Patient-evaluated outcome after surgery for basal meningiomas. Neurosurgery. 2002;50:941-949.
2. Elster A.D., Challa V.R., Gilbert G.H., et al. Meningiomas: MR and histopathologic features. Radiology. 1989;170:857-862.
3. Tsuchiya K., Hachiya J., Mizutani Y., et al. Three-dimensional Helical CT angiography of skull base meningiomas. AJNR. 1996;17:933-936.
4. Filippi C.G., Edgar M.A., Ulug A.M., et al. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR. 2001;22:65-72.
5. Bendszus M., Rao G., Burger R., et al. Is there a benefit to preoperative meningioma embolization. Neurosurgery. 2000;47:1306-1312.
6. Dean B., Flom R.A., Wallace R.C., et al. Efficacy of endovascular treatment of meningiomas: Evaluation of matched samples. AJNR Am J Neuroradiol. 1993;15:1675-1680.
7. Macpherson P. The value of pre-operative embolization of meningioma estimated subjectively and objectively. Neuroradiology. 1991;33:334-337.
8. Chun J.Y., McDermott M.W., Lamborn K.R., Wilson C.B., Higashida R., Berger M.S. Delayed surgical resection reduces intraoperative blood loss for embolized meningiomas. Neurosurgery. 2002;50:1231-1237.
9. Jung H., Yoo H., Paek S.H., Choi K.S. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567-575.
10. Simpson D. The recurrence of intracranial meningioma after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22.
11. Kinjo T. Al-Mefty O, Imad K: Grade zero removal of supratentorial convexity meningiomas: clinical study. Neurosurgery. 1993;33:394-399.
12. Bonnal J., Brotchi J. Reconstruction of the superior sagittal sinus in parasagittal meningiomas. In: Schmidek H.H., editor. Meningiomas and their Surgical Management. Philadelphia: WB Saunders; 1991:221-229.
13. Spetzler R.F., Daspit P., Pappas C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588-599.
14. Ziyal I.M., Sekhar L.N., Salas E., Olan W.J. Combined supra/infratentorial–transsinus approach to large pineal region tumors. J Neurosurg. 1998;88:1050-1057.
15. Mirimanoff R.A., Dorseretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
16. Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
17. Taylor B.W., Marcus R.B., Friedman W.A., et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
18. Forbes A.R., Goldberg I.D. Radiation therapy in the treatment of meningioma: The Joint Center for Radiation Therapy experience. J Clin Oncol. 1984;2:1139-1143.
19. Carella R.J., Ransohoff J., Newall J. Role of radiation therapy in the management of meningioma. Neurosurgery. 1982;10:332-339.
20. Condra K., Buatti J., Mendenhall W.M., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427-436.
21. Glaholm J., Bloom H.J.G., Crow J.H. The role of radiotherapy in the management of intracranial meningiomas: The Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18:755.
22. Goldsmith B.J., Wara W.M., Wilson C.B., et al. Postoperative irradiation for subtotally resected meningioma. J Neurosurg. 1994;80:195-201.
23. Wara W.M., Sheline G.E., Newman H., et al. Radiation therapy of meningiomas. Am J Roentgenol Rad Ther Nucl Med. 1975;123:453.
24. Mirabell R., Linggood R.M., de la Monte S., et al. The role of radiotherapy in the treatment of subtotally resected meningiomas. J Neurooncol. 1992;13:157.
25. Loeffler J.S., Niemierko A., Chapman P.H. Second tumors after radiosurgery: tip of the iceberg or a bump on the road? Neurosurgery. 2003;52:1436-1442.
26. Debus J., Wuendrich M., Pirzkall A., et al. High efficacy of fractionated stereotactic radiotherapy of large base of skull meningiomas: long-term results. J Clin Oncol. 2001;19:3547-3553.
27. Engenhart R., Kimming B.N., Hover K.H., et al. Stereotactic single high dose radiation therapy of benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 1991;19:1021-1026.
28. Kondziolka D., Lunsford L.D. Radiosurgery of meningioma. Neurosurg Clin N Am. 1992;3:219-230.
29. Kondziolka D., Lundsford L.D., Flickinger J.C.. Stereotactic radiosurgery of meningiomas, Lundsford L.D., Kondziolka D., Flickinger J.D., editors, Gamma Knife Brain Surgery, Basel, Karger, 1998;Vol 14:104-113.
30. Flickinger J.C., Lundsfors L.D., Kondziolka D. Dose prescription and dose volume effects in radiosurgery. Neurosurg Clin N Am. 1992;3:51-59.
31. Stafford S.L., Pollock B.E., Foote R.L., et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurg. 2001;49:1029-1038.
32. Tishler R.B., Loeffler J.S., Lundsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
33. Ryu S., Gorty S., Kazee A.M., et al. “Full dose” re-irradiation of human cervical spinal cord. Am J Clin Oncol. 2000;23:29-31.
34. Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53(4):815-822.
35. Chang S.D., Adler J.R. Treatment of cranial base meningiomas with linear accelerator radiosurgery: a clinical study. Neurosurgery. 1997;41:1019-1027.
36. Subach B.R., Lunsford L.D., Kondziolka D., et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-445.
37. Zachenhofer I., Wolfsberger S., Aichholzer M., Bertalanffy A., et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58(1):28-36. discussion 28–36
38. Couldwell W.T., Cole C.D., Al-Mefty O. Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg. 2007;106(1):30-35.
39. Linskey M.E., Davis S.A., Ratanatharathorn V. Relative roles of microsurgery and stereotactic radiosurgery for the treatment of patients with cranial meningiomas: a single-surgeon 4-year integrated experience with both modalities. J Neurosurg. 2005;102:59-70.
40. Pieper D.R., Al-Mefty O., Hanada Y., Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-747.
41. Ogasawara H., Oki S., Kohno H., et al. Tentorial meningioma and painful tic convulsive. J Neurosurg. 1995;82:895-897.
42. Sindou M. Meningiomas invading the sagittal and transverse sinuses, resection and venous reconstruction. J Clin Neurosci. 2001;8(suppl 1):8-11.
43. Collignon F.P., Cohen-Gadol A.A., Piepgras D.G. Hemangiopericytoma of the confluence of the sinuses and the transverse sinus. J Neurosurg. 2003;99:1085-1088.
44. Sekhar L.N., Janetta P.J. Cerebellopontine angle meningiomas. J Neurosurg. 1984;60:500.
45. Rock J.P., Monsell E.M., Schmidek H.H. Meningiomas of the cerebellopontine angle. In: Schmidek H.H., editor. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders; 1991:417-425. Harcourt Brace Jovanovich
46. Cho C.W., Al-Mefty O. Combined petrosal approach to petroclival meningiomas. Neurosurgery. 2002;51:708-716.
47. Malis L.I.. Surgical resection of tumors of the skull base, Wilkin R.H., Rengachary S.S., editors, Neurosurgery, New York, McGraw-Hill, 1985;Vol 1:1011-1021.
48. Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510-517.
49. House W.F., Hitselberger W.E., Horn K.L. The middle fossa transpetrous approach to the anterior–superior cerebellopontine angle. Am J Otol. 1986;7:1-4.
50. Kawase T., Shiobara R., Toya S. Anterior transpetrosal–transtentorial approach for sphenoclival meningiomas: surgical methods and results in 10 patients. Neurosurgery. 1991;28:869-976.
51. Sakata K., Al-Mefty O., Yamamoto I. Venous consideration in the petrosal approach: microsurgical anatomy of the temporal bridging vein. Neurosurgery. 2000;47:153-161.
52. Arnautovic K.I., Al-Mefty O. Primary meningiomas of the jugular fossa. J Neurosurg. 2002;97:12-20.
53. Arnautovic K.I., Al-Mefty O., Husain M.. Ventral foramen magnum meningiomas, J Neurosurg (Spine 1), 2000;92:71-80
54. Yasargil M.G., Mortara R.W., Curcic M. Meningiomas of basal posterior cranial fossa. Adv Tech Stand Neurosurg. 1980;7:1-15.
55. Miller E., Crockard H.A. Transoral transclival removal of anteriorly placed meningiomas at the foramen magnum. Neurosurgery. 1987;20:966-968.
56. Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
57. Babu R.P., Sekhar L.N., Wright D.C. Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg. 1994;81:49-59.
58. George B., Lot G. Anterolateral and posteriolateral approaches to the foramen magnum: technical description and experience from 97 cases. Skull Base Surg. 1995;5:9-19.
59. Caruso R.D., Rosenblaum A.E., Chang J.K., et al. Craniocervical junction venous anatomy on enhanced MRI images: the suboccipital cavernous sinus. AJNR Am J Neuroradiol. 1999;20:1127-1131.
60. Asari S., Maeshiro T., Tomita S. Meningiomas arising from the falcotentorial junction: clinical features, neuroimaging studies, and surgical treatment. J Neurosurg. 1995;82:726-738.
61. Madari A.A., Crochard H.A., Stevens J.M. Pineal region meningioma without dural attachment. Br J Neurosurg. 1996;10:305-307.
62. Roda J.M., Perez-Higueras A., Oliver B. Pineal region meningiomas without dural attachment. Surg Neurol. 1982;17:147-151.
63. Konovalov A.N., Spallone A., Pitzkhelauri D.I. Meningioma of the pineal region: a surgical series of 10 cases. J Neurosurg. 1996;85:586-590.
64. Bruce J.N., Stein B.M. Surgical management of pineal region tumors. Acta Neurochir (Wien). 1995;134:130-135.
65. Ausman J.I., Malik G.M., Dujovny M. Three-quarter prone approach to the pineal–tentorial region. Surg Neurol. 1988;29:298-306.
66. Bloomfield S.M., Sonntag V.K.H., Spetzler R.F. Pineal region lesions. BNI. 1985;Q1:10-23.