Chapter 40 Surgical Management of Petroclival Meningiomas
Posterior fossa meningiomas comprise 10% of intracranial meningiomas. Clival and petroclival meningiomas comprise only 3% to 10% of the posterior fossa meningiomas. Petroclival meningiomas arise from the petroclival junction, above the jugular tubercle, medial to the trigeminal nerve, and anteromedial to the facial–vestibulocochlear nerve complex.1–5 Petroclival meningiomas arise in the area surrounding the spheno-occipital synchondrosis. Tumors can involve the anterior portion of the tentorial incisura, Meckel’s cave, middle cranial fossa floor, and parasellar region and cavernous sinuses (sphenopetroclival meningioma). Petroclival meningiomas can displace the brain stem and encase the surrounding critical neurovascular structures. Meningiomas arising posterolateral to the internal auditory canal (IAC) are defined as cerebellopontine angle meningiomas. The position of cranial nerves VII and VIII is the critical landmark to differentiate petroclival meningiomas from cerebellopontine angle meningiomas. Petroclival meningiomas originate anterior to the IAC and displace cranial nerves VII and VIII posteriorly. In contrast, cerebellopontine angle meningiomas originate posterior to the IAC and displace cranial nerves VII and VIII anteriorly. Foramen magnum meningiomas arise inferior to the jugular tubercles.1,2,4–7
History
In 1874, “a 50-year-old woman developed paralysis of the arms, followed by the legs, then of respiration…and died after a few months. The autopsy revealed a nut-sized tumor of the inferior basilar gutter.” This case, presented by Hallopeau,8 may represent the first report of a petroclival meningioma in the literature. In the past, petroclival meningiomas were described only in postmortem studies.9,10 In 1922, Cushing11 acknowledged the difficulties in treating meningiomas of the clival region. He concluded his Cavendish lecture by saying, “The difficulties are admittedly great, sometimes insurmountable, and though the disappointments still are many, another generation of neurologic surgeons will unquestionably see them largely overcome.” The petroclival clival region is an anatomically complex area. It has an irregular bony topography and contains complex collection of blood vessels, cranial nerves, and related brain stem. Despite Cushing’s optimism, petroclival meningiomas remain the most demanding and formidable meningiomas to treat.
In 1927, Olivecrona12 started his surgical exploration of clival meningiomas. He deemed them inoperable after an unacceptable experience with six patients. Early surgical experience with petroclival meningiomas was depressing. The reported surgical mortality exceeded 50%.10,13,14 With the introduction of microsurgical techniques, Yasargil et al.5 reported more promising results in 1980, with a mortality rate of 10%. With improved three-dimensional understanding of microsurgical anatomy and advancement and refinement of skull base techniques and approaches, management of petroclival meningiomas has been accomplished with much lower morbidity and mortality rates according to the recent literature.
Natural History
The incidence of petroclival meningiomas is low compared to that of other skull base meningiomas. Petroclival meningiomas are known to be slowly growing. However, the literature on the growth rates and natural history of petroclival meningiomas is insufficient. Jääskeläinen et al.15 reported the growth rates of intracranial meningiomas among tumors with the same histologic grade. In 2003, Van Havenbergh et al.16 reported the results of a long-term clinical and radiologic follow-up study of 21 patients with petroclival meningiomas treated conservatively. The follow-up period was from 48 to 120 months (mean 82 months, median 85 months). During follow-up, radiologic tumor growth was documented in 76% of the patients, and clinical deterioration occurred in 63% of these patients. The mean growth rates were 1.16 mm/yr in diameter and 1.10 cm3/yr in volume. Rapid growth spurts were documented in small and medium-sized tumors. Small to medium-sized tumors tended to grow more than larger tumors. Therefore, active treatment was recommended for symptomatic patients with small or medium-sized tumors. The authors recommended surgical resection for younger and healthy patients and focused beam radiation therapy (stereotactic radiosurgery or radiotherapy) for elderly patients or unhealthy patients who cannot undergo surgery. The authors have the same recommendations for large growing asymptomatic tumors.
Recurrence Rate
It is difficult to compare the recurrence and regrowth rates in the published series due to the variation in the follow-up duration and the definition of the degree of resection. Recurrence rate depends on location, cavernous sinus involvement, brain stem infiltration, grade of resection, and histopathologic result.17 Mathiesen et al.18 provide comprehensive long-term results of resected skull base meningiomas. Recurrence rate after 5 years for Simpson grade I resection was 3.5%, for grade II resection was 4%, for grade III resection was 25%, and for grade IV to V resection 36% to 45%. After 15 years, recurrence rate for Simpson grade I resection was 7% to 10%, for grade II resection was 11% to 15%, for grade III resection was 37% to 43%, and for grade IV to V resection was 63% to 100%. Recurrence rate after 25 years for Simpson grade I resection was 13% to 16%, for grade II resection was 15% to 20%, and for grade III resection was 39% to 76%.
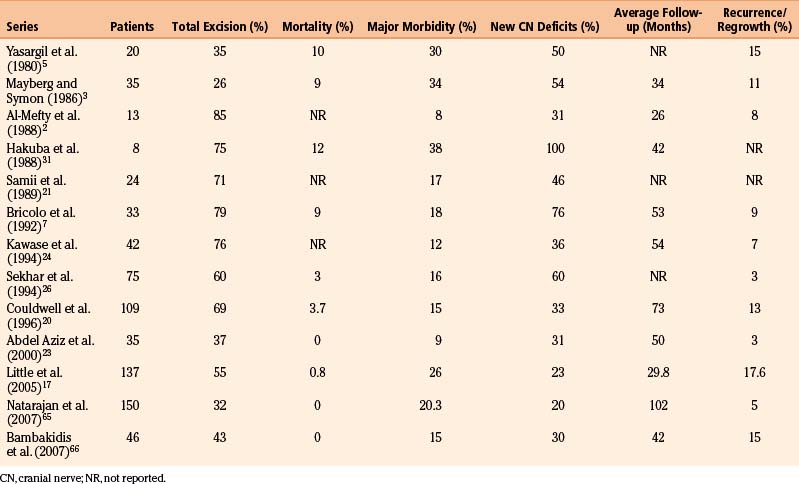
Recurrence rates for petroclival meningiomas in the published literature ranged from 0% to 42%2,3,5,7,17,19–24,26,30,31,65,66 (Table 40-1). Jung et al. followed 38 patients after subtotal resection of petroclival meningiomas for a mean period of 47.5 months. Radiographic evidence of tumor progression and recurrence occurred in 16 patients (42%). Among the previously mentioned factors, there were correlations among growth rate, old age, and occurrence of menopause.19
Clinical Picture
Clinical findings in patients with petroclival meningiomas can be related to four major etiologies: (1) involvement of cranial nerves, (2) cerebellar compression, (3) brain stem compression, and (4) increased intracranial pressure.2,5,14 Presenting symptoms in petroclival meningiomas are nearly the same in all series reported in the literature. Cranial nerves affected, in order of greatest incidence, are V, VIII, VI, VII, IX, and X. Cranial nerves are affected by compression, stretching, incorporation into the tumor, or less frequently, invasion by the tumor. Other common neurologic findings include gait ataxia and motor and sensory deficits due to cerebellar and brain stem compression. Increased intracranial pressure, dementia, and change in visual acuity were frequently associated with hydrocephalus, secondary to aqueduct compression.
Based on the location of the tumor’s dural attachment, Ichimura et al.22 created four subtypes of petroclival meningiomas: the upper clivus type, the cavernous sinus type, the tentorial type, and the petrous apex type. Trigeminal neuropathy was the most commonly found symptom (45%); there was no significant difference in the frequency of trigeminal neuropathy among the four types. The upper clivus type comprises tumors medial to the trigeminal nerve with the primary presentation of ataxia. The cavernous sinus type comprises tumors originating from the posterior cavernous sinus medial to the trigeminal nerve and extends into the posterior fossa, with the characteristic symptom being abducens nerve palsy. The tentorial type originates from the tentorium and the petroclival junction; it displaces the trigeminal nerve inferomedially. The petrous apex type of tumor attaches to the petrous apex lateral to the trigeminal nerve and pushes it superomedially. Trigeminal neuralgia was common in patients with the petrous apex and tentorial types.
Neuroradiologic Evaluation
Thorough evaluation of preoperative neuroimaging studies is of utmost importance in the management of petroclival meningiomas. Multimodality imaging methods are utilized to assess the tumor’s size, consistency, vascularity, location “zone” and extension of dural attachment, tumor–brain stem interface, degree of brain stem displacement encasement, displacement of the vertebrobasilar arterial system, and tumor extension into the cavernous sinus.23
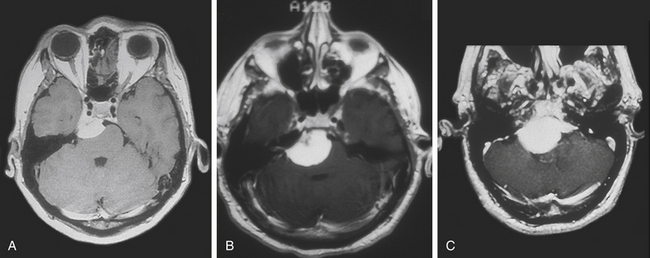
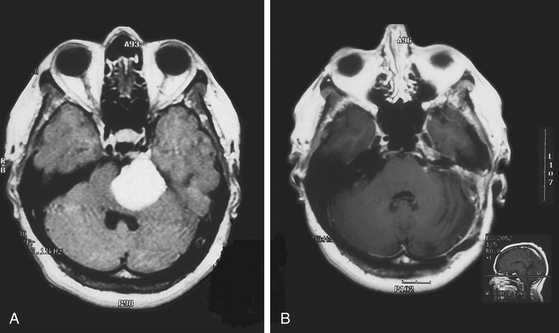
Magnetic resonance imaging (MRI) T1-weighted images with and without contrast delineates the tumor and its relationships to the surrounding structures. MRI T2-weighted images are useful for assessment of the arachnoid cleavage plane, brain stem edema, and infiltration. Flow voids on MRI T2-weighted images can reveal the location of major vertebrobasilar vessels. The absence of a definite arachnoid cleavage plane between the tumor and the brain stem can make resection extremely challenging. This is one of the main obstacles preventing safe total resection of petroclival meningiomas. The lack of a definite arachnoid cleavage plane can be associated with brain stem pial infiltration. This can be predicted from preoperative MRI studies (Fig. 40-1) and has been associated with postoperative morbidity.4,24–27 Understanding the venous anatomy can reduce related complications during surgical approaches for petroclival meningiomas. Magnetic resonance venogram can illustrate the variations of the torcula and the relative sizes and patency of the transverse and sigmoid sinuses on either side. In addition, the vein of Labbé (posterior temporal venous drainage) and its drainage area are often visualized. This information may be useful if a sigmoid sinus needs to be divided (lateral to the temporal venous drainage) during surgery.
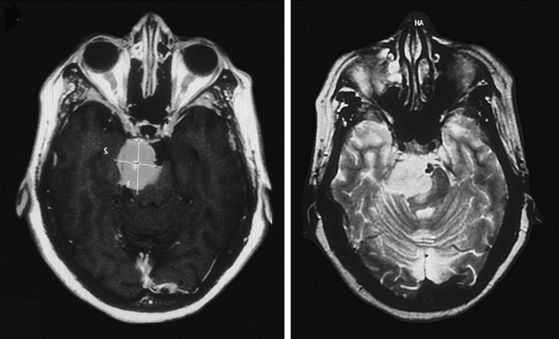
FIGURE 40-1 T1- and T2-weighted MRI images revealing brain stem infiltration by a petroclival meningioma.
(From Abdel Aziz KM, Sanan A, van Loveren HR, et al. Petroclival meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery. 2000;47(1):139-152.)
Cerebral angiography should be considered during the preoperative evaluation of petroclival meningiomas. Not only does it show the tumor’s blood supply, it also can indirectly demonstrate the tumor causing mass effect on the vertebrobasilar system and its branches. Petroclival meningiomas are supplied by the meningohypophysial trunk of the internal carotid artery, the posterior branch of the middle meningeal artery, the meningeal branch of the vertebral artery, the clivus artery from the carotid siphon, the petrosal branches of the meningeal arteries, and the ascending pharyngeal branches of the external carotid artery.28 Angiography can also be used for preoperative tumor embolization to reduce surgical blood loss.
Anesthetic Considerations
Brain relaxation can be provided by the judicious use of mannitol (0.25 to 1.0 mg/kg) and furosemide (10 to 20 mg), hypertonic saline (NaCl 3%), and a lumbar drain. It is preferred to keep the patient normocapnic with maintained euvolemia. The ability to maintain and manipulate blood pressure in response to changes in monitoring parameters and surgical needs is crucial. Muscle relaxation is not utilized because of cranial nerve and motor tract monitoring. Most anesthesiologists use a narcotic-based technique, a remifentanil or sufentanil infusion for analgesia, and a low-solubility inhalational agent such as sevoflurane or an intravenous agent such as propofol for hypnosis. The hypnotic agents can be titrated based on bispectral index monitoring. This is especially useful when propofol is being used for long cases, because the context-sensitive half-life of propofol increases with the duration of use. Nitrous is not utilized with such a regimen. A phenylephrine drip is used to titrate the blood pressure to desired levels. Burst suppression can also be provided in conjunction with electroencephalogram monitoring.
Intraoperative Neurophysiologic Monitoring
The following modalities are generally used:
• Somatosensory evoked potentials (SSEPs). SSEPs are recorded by electric stimulation of peripheral afferent nerves and recorded with the assistance of scalp electrodes. The sensory system is monitored from the peripheral nerves in upper and lower extremities via plexi, tracts, fasciculi, thalami, and the sensory cortex. The median nerve at the wrist is the most common stimulation site for upper extremity monitoring, and the posterior tibial nerve, just posterior to the medial malleolus, is most commonly used for lower extremity monitoring.
• Motor evoked potentials (MEPs). The motor system is monitored by transcranial stimulation of the motor cortex bilaterally and recording electromyogram activity in the muscles. Muscle relaxants cannot be used during MEP monitoring. MEPs can be difficult to detect at baseline, especially in older patients, and are very sensitive to the depth of anesthesia.
• Brain stem auditory evoked potentials (BSAEPs). BSAEPs record cortical responses to auditory stimuli. This allows monitoring of the function of the entire auditory pathway, including the acoustic nerve, brain stem, and cerebral cortex. Positive deflections are termed waves I to VII. Waves I, III, and V are the waves most consistently seen in healthy subjects (obligate waves). Wave V is the most reliably seen wave. A shift in latency of 1 msec or a drop in amplitude of 50% could be significant and should be reported to the neurosurgeon. Auditory evoked potential monitoring is crucial during dissection and retraction around cranial nerve VIII.
• Electroencephalography. This is generally used to monitor burst suppression. It may also detect ischemia with unilateral slowing.
Goals of Surgical Management
Neurosurgeons must weigh the increased morbidity produced by aggressive surgery against the natural history of residual tumor, particularly in older patients. The optimal therapeutic strategy for each patient involves various considerations. These include prognostic factors, applicability of other options such as radiosurgery, and consequences of the outcome on the patient’s quality of life. Evaluating the surgical results of petroclival meningioma removal requires special considerations. Grading schemes used for convexity meningiomas cannot be used to evaluate the excision of petroclival meningiomas. It is extremely difficult to achieve total excision of a petroclival meningioma, including excision of its dural attachment, its dural tail, and the involved bone. Therefore, it is impractical to apply the Simpson grading system to assess the degree of resection of petroclival meningiomas.29
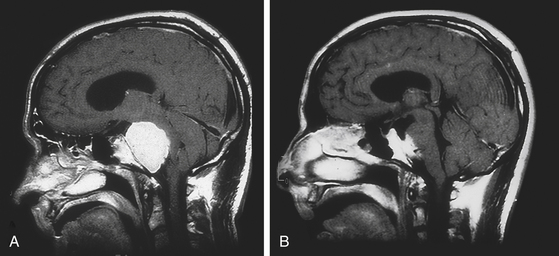
Untreated petroclival meningiomas produce morbidity from the continuous brain stem compression (Fig. 40-2). Therefore, our primary goal is brain stem decompression to restore clinical function with either total or subtotal excision. We have developed a grading scale that evaluates the extent of resection and the degree of brain stem reexpansion. The extent of resection is considered maximal if the tumor is grossly removed. Brain stem reexpansion is maximal if the brain stem regains its normal contour and position (Table 40-2).
Table 40-2 Grading Scale for Excision of Petroclival Meningiomas
| Grade I | Total resection with coagulation of the dural attachment |
| Grade II | Total resection without coagulation of the dural attachment |
| Grade II | <10% residual tumor |
| Grade IV | 10%-50% residual tumor |
| A: Complete brain stem expansion | |
| B: >75% brain stem reexpansion | |
| C: <75% brain stem reexpansion | |
| Grade V | >50% residual tumor |
| A: Complete brain stem expansion | |
| B: >75% brain stem reexpansion | |
| C: <75% brain stem reexpansion |
Petroclival meningiomas grow very slowly, and they may have a tendency to invade the brain stem, cranial nerves, and the basilar artery and its perforators. For tumors with neurovascular invasion, we prefer performing an excision of the tumor that leaves the parts infiltrating the neurovascular structures undisturbed, rather than attempting total excision of the tumor, which may leave the patient with a major neurologic deficit. Devascularization of the residual capsule of a petroclival meningioma may result in limited growth for a long period.3,30
Surgical Approaches
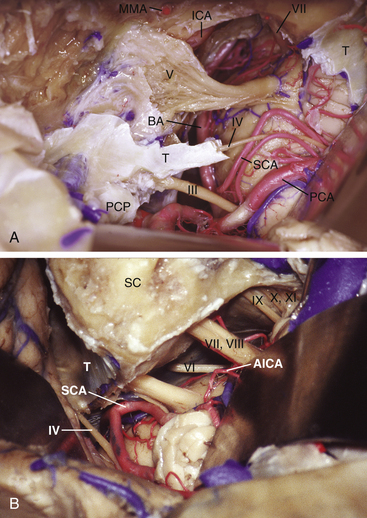
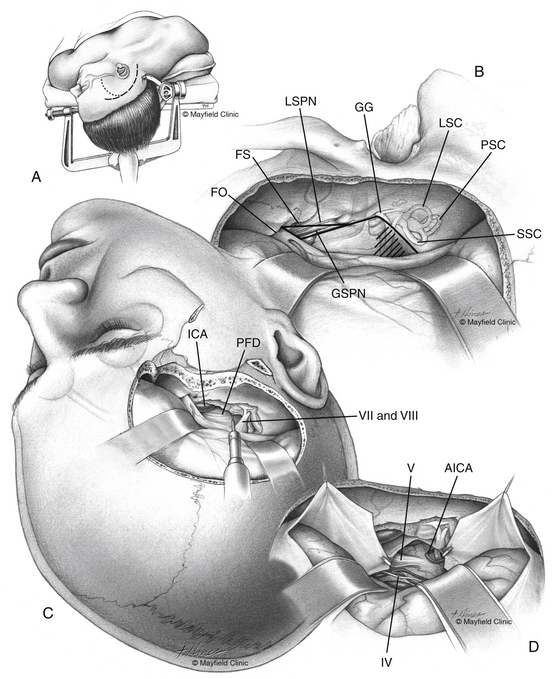
Petroclival meningiomas have been mainly exposed through the petrous portion of the temporal bone. Petrosal approaches have a distinctive advantage for exposure of the petroclival region compared to the other conventional approaches.1,2,24,31–37 The variety of names for the petrosal approaches fall into one of two categories: anterior or posterior petrosectomy.33 The two approaches can be combined (i.e., combined petrosal approach), or they can be used as part of a more elaborate surgical exposure. We prefer to collaborate with the neuro-otologists to perform the temporal bone drilling. However, some neurosurgeons prefer to perform the whole approach in either one or two stages without a neuro-otologist. On the basis of surgical experience and anatomic dissections, we have designed an algorithm to predict the extent of resection possible via transpetrosal approaches.23 The extent of exposure achieved via the anterior petrosal approach is from cranial nerve III down to the IAC (Fig. 40-3A). The extent of exposure achieved via the posterior petrosal (retrolabyrinthine) approach was from cranial nerve IV to the upper border of the jugular tubercle (Fig. 40-3B). Some pioneers achieved successful results through the lateral suboccipital “retrosigmoid” approach.38–40
Clivus and Petroclival Zones
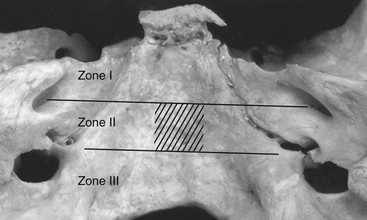
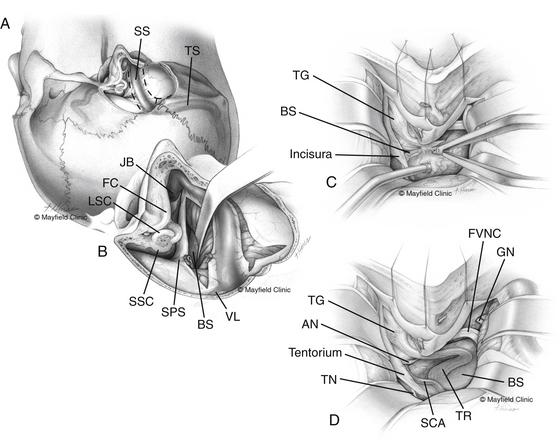
Based on the extent of surgical exposure achieved by surgical approaches, the clivus and petroclival region is divided into three zones (I, II, and III)23 (Fig. 40-4). Petroclival meningiomas involving multiple zones require combined approaches.
Zone I (upper zone) extends from the dorsum sellae to the upper border of the IAC. This involves the retrosellar region and the region medial to the trigeminal impression (Meckel’s cave) down to the IAC. Zone I can be exposed utilizing the Kawase approach,32 also known as the anterior petrosal approach.23,33 Zygomatic osteotomy can be added to the anterior petrosectomy to enhance the angle of exposure.41 If the tumor only involves the retrosellar region of zone I, exposure can be via a trans-sylvian transcavernous approach, which involves mobilization of the oculomotor nerve and drilling of the posterior clinoid process and the dorsum sellae.42 With endoscopic assistance, zone I of the petroclival region can be exposed via a subtemporal keyhole approach.43
Zone II (middle zone) extends from the IAC to the upper border of the jugular tubercle, representing the exposure provided via the posterior petrosal approach. The posterior petrosal approach achieves exposure from the trigeminal impression (in zone I) to the jugular tubercle. Tumors involving both zone I and zone II require a combined petrosal approach.44 This is a combination of anterior and posterior petrosal approaches (Fig. 40-5).
Horgan et al. described the “transcrusal” approach, which adds drilling of the posterior and superior semicircular canals, while preserving hearing, to enhance the exposure of the petroclival region.45
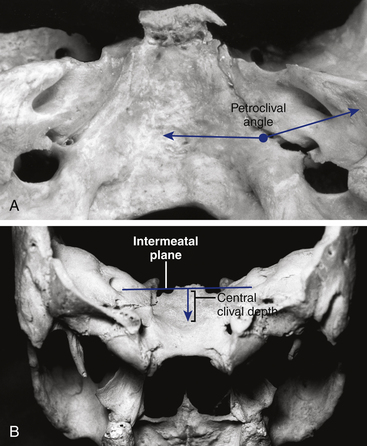
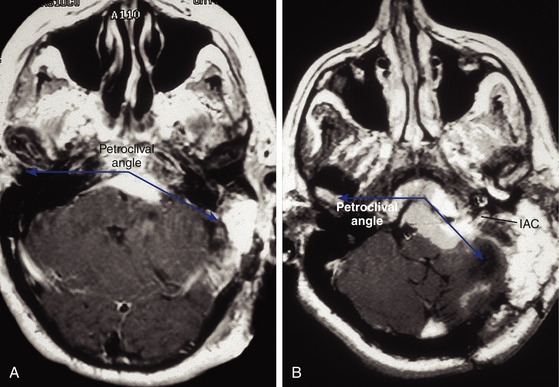
The angle between the petrous bone and the clivus (the petroclival angle) at the level of the IAC varies between patients.23 There is no direct access to the small irregular volume of space bounded by the midline medially, the intermeatal plane superiorly, and the jugular tubercle inferiorly (Fig. 40-6). This is a relatively difficult-to-access or an inaccessible space called the “central clival depression.”23 The less obtuse the petroclival angle, the more difficult the exposure of the central clival depression (Figs. 40-7 and 40-8).
With posterior petrosectomy (retrolabyrinthine), our line of sight could not reach portions of tumor attached to the central clival region between the IAC and the upper border of the jugular tubercle. Exposure of the central clival depression necessitates the posterior petrosal translabyrinthine/transcochlear approach with posterior mobilization of the facial nerve. Resection of the labyrinth results in hearing loss, and posterior mobilization of the facial nerve to drill the cochlea results in a facial palsy, with limited expectation of facial recovery. To achieve posterior mobilization of the facial nerve, the nerve is unroofed from the IAC back to the stylomastoid foramen. The chorda tympani and the greater superficial petrosal nerve (GSPN) are cut, and the facial nerve is mobilized from the geniculate ganglion posteriorly. As there are few anastomotic arteries between the pregeniculate and the postgeniculate portions of the facial nerve, manipulation around the geniculate ganglion leads to ischemic injury of the facial nerve.46–50 Posterior mobilization of the facial nerve results in complete postoperative nerve paralysis in 100% of patients. Facial nerve paralysis improves in about one third of patients to House-Brackmann grade III within 1 year.51 In 1994, Cass et al.52 reported the use of total petrosectomy (the translabyrinthine/transcochlear approach) for large petroclival meningiomas. Despite the sacrifice of hearing and a significant rate of facial nerve paralysis, only 55% of lesions were totally excised.
Anterior Petrosal Approach
The anterior petrosal approach32 (Fig. 40-9) allows exposure of the middle fossa floor, the petrous bone apex, and zone I of the petroclival region. The anterior petrosal approach includes a subtemporal or frontotemporal craniotomy and anterior petrosectomy. The anterior petrosal approach can be combined with the posterior petrosal approach to expand the exposure down to the jugular tubercle (zone II). Patient positioning is the most crucial step of all operative procedures. For the subtemporal/anterior petrosal approach, we prefer placement of a lumbar drain prior to positioning. The patient is placed in a supine position with the head fixed in a three-point skull fixation device and rotated 90 degrees (the sagittal suture is parallel to the floor). The ipsilateral shoulder is elevated with a gelatin roll to prevent kinking and occlusion of the jugular vein and stretching of the brachial plexus. The head is tilted 15 degrees downward; this places the zygoma in the highest point of the surgical field, and the gravity effect minimizes the extent of temporal lobe retraction during surgery. The patient’s upper back is elevated 25-30 degrees to facilitate venous drainage. The arms are securely padded to avoid pressure on the ulnar and radial nerves. Slight flexion of the knees prevents joint tension from hyperextension. Thromboembolic deterrent hose and compression stockings are placed around the legs to reduce venous stasis.
If the petroclival meningioma extends anteriorly beyond zone I into the interpeduncular fossa and the cavernous sinus, a frontotemporal craniotomy is designed. The previously mentioned skin flap is extended anteriorly, and a mycutaneous flap is reflected anteroinferiorly. The sphenoid wing is totally exposed and completely drilled; our craniotomy extends anteriorly to allow more exposure. The sphenoid wing is completely drilled (flush to the roof and lateral wall of the orbit). Extradural partial anterior clinoidectomy can be added. This allows exposure of the frontotemporal junction of the basal dura and facilitates extradural dissection and mobilization of the lateral wall of the cavernous sinus using the Hakuba technique53 (directed from anterolateral to posteromedial). The dura is elevated from the middle fossa floor, and petrous bone via a posterior to anterior approach; elevation starts at the arcuate eminence and proceeds anteriorly. The middle meningeal artery is controlled with bipolar cautery and sectioned, and the foramen spinosum is packed with mixed bone wax–Oxycel. The GSPN is identified and kept intact; we always leave a thin dural layer covering the nerve. Geniculate ganglion location is confirmed with facial nerve monitoring. The GSPN is preserved, and dissection follows the GSPN from posterior to anterior until it courses under the third division of the trigeminal nerve (V3). More anteriorly, the mandibular division (V2) is identified at the foramen rotundum. Occasionally, the petrous segment of the internal carotid artery is visualized through a bony dehiscence in the petrous apex. Dissection continues medially to the petrous ridge indenting the superior petrosal sinus. Separation of the dura propria continues until the connective tissue sheath over V2, V3, and the Gasserian ganglion is visible (Meckel’s cave). Kawase’s quadrilateral is drilled under the microscope starting medially at the petrous ridge to identify the IAC. The arcuate eminence forms a 120-degree angle to the GSPN (or the internal carotid artery), and the IAC bisects this angle. Another, less commonly used, method to identify the IAC is to follow the geniculate ganglion to the labyrinthine segment of the facial nerve marking the IAC. This is associated with a high incidence of facial nerve injury. Drilling of the IAC continues to the bone crest dividing the facial nerve and the superior vestibular nerve (Bill’s bar). The bone overlying the cochlea is drilled until the cochlea appears as a blue line. After identification of the dura covering the IAC posteriorly, the Kawase’s quadrilateral is drilled to the GSPN (preserved) laterally, the petrous segment of the internal carotid artery anterolaterally, V3 anteriorly, the superior petrosal sinus medially, and the posterior fossa dura and inferior petrosal sinus inferiorly. the inferior temporal lobe dura is open above and parallel to the superior petrosal sinus. The dura is reflected inferiorly; the superior petrosal sinus is secured with titanium hemoclips and is split. The tentorium is cut medially toward the tentorial incisura posterior to the dural entry of the trochlear nerve. The posterior fossa dura is further split inferiorly. This allows panoramic exposure from the oculomotor nerve superiorly, to the vestibulocochlear nerve inferiorly, to the nerve inferiorly (see Fig. 40-3A).
After completion of surgical resection, watertight dural closure is demanding. The IAC bony opening is plugged with a small piece of fat or muscle. The dura is approximated utilizing a synthetic dural graft and is sprayed with fibrin glue. If there is a big filling defect, it can be judiciously obliterated with pieces of fat graft to prevent postoperative fluid collection and cerebrospinal fluid (CSF) leak. Bone flaps are connected and fixed with titanium plates and secures. Bony defects are filled with bone cement for cosmetic reconstruction and prevention of CSF leak. We prefer to keep the lumbar drain in place for 48 hours after obtaining an immediate postoperative computed tomography scan.
Posterior Petrosal Approach
The posterior petrosal approach (Fig. 40-10) consists of a temporal craniotomy combined with a presigmoid craniectomy and a small lateral retrosigmoid craniectomy. Depending on the preoperative hearing status, either retrolabyrinthine or translabyrinthine bony temporal bone drilling is added. The addition of a retrosigmoid craniotomy, sectioning of the superior petrosal sinus and tentorium, and a relaxing incision in the dura above the lateral transverse sinus frees the sigmoid sinus and allows mobilization of the sigmoid sinus posteriorly to expand the presigmoid space. These steps are crucial to improving the presigmoid exposure of posterior fossa contents.
Retrolabyrinthine mastoidectomy is performed by a neuro-otologist. The spine of Henle is a landmark to the antrum. The mastoid bone is drilled, and the middle fossa and meatal bone plates are dissected until the antrum is identified. The floor of the antrum is the cortical bone of the lateral semicircular canal. After drilling the bone over the sinodural angle, the sigmoid sinus, superior petrosal sinus, and posterior semicircular canal are exposed. Following the lateral semicircular canal until it bisects the posterior semicircular canal is another technique to expose the posterior semicircular canal. The posterior semicircular canal is followed superiorly to expose the superior semicircular canal. The air cells of the mastoid tip are removed to expose the digastric ridge, which is a landmark to the stylomastoid foramen and the beginning of the fallopian canal. Preservation of the cortical bone of the fallopian canal avoids injury to the facial nerve. The combination of a temporal and suboccipital craniotomy with petrosectomy allows mobilization of the sigmoid sinus so that the surgeon can operate presigmoidally through the petrosectomy and retrosigmoidally through the suboccipital craniotomy.
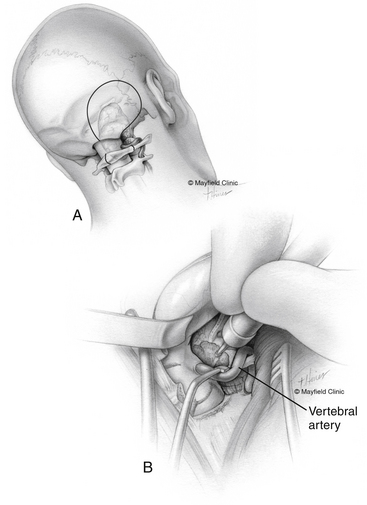
The dural opening is made as follows: (1) the Dura is incised along the undersurface of the temporal lobe parallel to the superior petrosal sinus, taking care to preserve the vein of Labbé, which generally enters the transverse sinus within 1 cm of the transverse–sigmoid junction. (2) The posterior fossa dura in the presigmoid space is incised longitudinally between the superior petrosal sinus and the jugular bulb. (3) With gentle traction on the temporal lobe and cerebellum, the superior petrosal sinus is sectioned between two titanium hemoclips. (4) A relaxing dural incision is made along the upper border of the transverse sinus. (5) The tentorium is sectioned into the incisura at a point posterior to entrance of the trochlear nerve; the vein of Labbé on the surface of the temporal lobe is protected during the final dural cut. This wide dural opening allows panoramic posterior fossa exposure between the trigeminal nerve (cranial nerve V) and the upper border of the jugular tubercle (see Fig. 40-3B). If additional exposure caudal to the jugular tubercle (zone III) is required, a retrosigmoid craniectomy is expanded with partial condylar resection and C1 laminectomy to expose the foramen magnum (transcondylar approach) (Fig. 40-11; see also Fig. 40-8).
Lateral Suboccipital Approach
For petroclival meningiomas involving zones I, II, and III, the lower lateral suboccipital (retrosigmoid) approach is a viable alternative.54,55 This provides exposure of the posterior surface of the petrous bone, the anterolateral brain stem, and the craniocervical junction. Intradural drilling of the petrous apex allows exposure of the tumor portion extending to the middle cranial fossa through Meckel’s cave.56
The lateral oblique position is preferred for the lateral suboccipital approach. However, some surgeons prefer the supine position for lateral suboccipital approaches. This is applicable in slim patients with flexible necks. The lateral oblique position avoids excessive neck rotation and facilitates exposure of the posterior fossa and the upper cervical spine. The head is secured with a three-point skull fixation while the patient is in the lateral position. An axillary roll is placed under the axilla to prevent brachial plexus compression. The lower extremities are flexed and separated with a pillow. Pressure points are padded; this includes the head of the fibula with the overlying peroneal nerve. The body is secured to the table with adhesive tape and safety belts. The patient’s upper back is elevated 15 degrees to allow venous drainage. The head is maintained in a neutral position. The neck is flexed and tilted slightly downward so that three fingerbreadths can be easily placed between the chin and the manubrium. After the skin incision and subperiosteal muscle separation, craniotomy or craniectomy is designed. A keyhole bur hole is placed inferomedial to the asterion to avoid injury of the transverse sigmoid junction. The craniotomy flap does not extend beyond three to four fingerbreadths lateral to the external auditory canal. Mastoid bone is drilled to expose the medial edge of the sigmoid sinus and the inferior edge of the transverse sinus. We always prefer to open the foramen magnum. For a tumor that extends to zone III, C1 laminectomy and drilling of the posteromedial third of the occipital condyle and lateral mass of C1 allow lateral extension of the dural opening and better exposure to tumor portion anterior to the brain stem (see Fig. 40-11). The V3 segment of the vertebral artery is easily identified at the groove of the C1 lamina. This step can be achieved by following the periosteum covering the C1 lamina laterally; this usually keeps the neurosurgeon on top of the prevertebral venous plexus. If venous bleeding is encountered, it is controlled with Oxycel and bipolar cautery. The dural incision starts at C1, extends superiorly through the foramen magnum, and extends superolaterally to the top of craniotomy edge. After tumor resection, the dura is closed watertight. An autologous pericranial graft or a synthetic graft is usually required during closure, and the suture line is sprayed with fibrin glue. A craniotomy flap is placed using titanium plates and screws, and bony defects are filled with bone cement to minimize postoperative headache.
Radiosurgery
Stereotactic radiosurgery can be applied as a primary treatment modality for small petroclival meningiomas of less than 3 cm in diameter.57 It can be utilized as an adjuvant treatment to prevent tumor regrowth and recurrence after maximal safe surgical resection. Subach et al.58 retrospectively reviewed a heterogeneous group of 62 patients with a petroclival meningioma treated by gamma knife radiosurgery. During a median follow-up period of 37 months, tumor control was obtained in 92% of cases. Neurologic status improved in 21%, remained stable in 66%, and declined in 13%. Park et al.59 reported on 12 patients who underwent stereotactic radiosurgery treatment with a median follow-up period of 52 months. These patients refused surgical treatment, suffered multiple medical problems, had a tumor that was asymptomatic and incidentally diagnosed, or in elderly patients, had a tumor that was small. The authors reported no clinical deterioration except in one patient, and two patients showed radiologic evidence of tumor progression during the follow-up period. The two patients with tumor progression underwent surgical treatment. Flannery et al.60 reported the largest series in the literature of 168 patients with petroclival meningiomas treated with stereotactic radiosurgery as a primary or an adjuvant treatment with a median follow-up period of 72 months. The median tumor volume was 6.1 cm3, and the median radiation dose to the tumor margin was 13 Gy. During the follow-up period, status improved in 44 patients (26%) who showed neurological improvement, 98 patients (58%) remained stable, and 26 patients (15%) showed neurologic deterioration. Tumor volume decreased in 78 patients (46%), remained stable in 74 patients (44%), and increased in 16 patients (10%). Major risk factors for tumor progression were tumor volume of 8 cm3 and male sex.
In 2007, Couldwell et al.61 report 13 patients with benign (World Health Organization grade I) skull base meningiomas who demonstrated tumor progression after stereotactic radiosurgery treatment. Radiosurgery was the primary or an adjuvant treatment. Tumor progression occurred within 6 months to 8 years after treatment in 10 patients and was between 8 and 14 years in 3 patients. With these recent data, it is strongly recommended to follow patients for longer durations after stereotactic radiosurgery treatment.
Outcomes
Various authors have reported gross total resection rates of 20% to 85%. Mortality varies from 0% to 10%, cranial nerve deficits range from 29% to 76%, and major morbidity is reportedly 8% to 45%.2,3,5,7,17,23,24,26,30,31,62–66 The published series on petroclival meningiomas is summarized in Table 40-1.
Conclusions
As stated by Cushing in 1922, “There is today nothing in the whole realm of surgery more gratifying than the successful removal of a meningioma with a subsequent perfect functional recovery.”11 Despite the major attempts to perform total resection of petroclival meningiomas, there are microscopic nests of cells on the involved basal dura; dural attachment cannot be completely excised in the majority of cases. Ultimately, for meningiomas attached to the dura at the central clival depression in zone II, the surgical morbidity associated with total removal becomes prohibitive, and the chance of total removal, even with excellent exposure, remains poor.
Abdel Aziz K.M., Sanan A., van Loveren H.R., et al. Petroclival meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery. 2000;47(1):139-152.
Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510-517.
Bambakidis N.C., Kakarla U.K., Kim L.J., et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurg. 2007;61(ONS suppl 2):202-211.
Couldwell W.T., Weiss M.H. Surgical approaches to petroclival meningiomas. I: upper and midclival approaches. Contemp Neurosurg. 1994;16:1-6.
Flannery T.J., Kano H., Lunsford L.D., et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. September 4, 2009.
Kawase T., Shiobara R., Toya S. Anterior transpetrosal–transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869-876.
Kawase T., Shiobara R., Toya S. Middle fossa transpetrosal–transtentorial approaches for petroclival meningiomas: selective pyramid resection and radicality. Acta Neurochir (Wien). 1994;129:113-120.
Little K.M., Friedman A.H., Sampson J.H., et al. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56(3):546-559.
Natarajan S.K., Sekhar L.N., Schessel D., Morita A. Petroclival meningiomas: multimodality treatment and outcomes at long term follow-up. Neurosurg. 2007;60:965-981.
Samii M., Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien). 1992;118:27-32.
1. Al-Mefty O., Smith R.R. Clival and petroclival meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:517-537.
2. Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510-517.
3. Mayberg M.R., Symon L.D. Meningiomas of the clivus and apical petrous bone: report of 35 cases. J Neurosurg. 1986;65:160-167.
4. Sekhar L.N., Jannetta P.J., Burkhart L.E., Janosky J.E. Meningiomas involving the clivus: six years experience with 41 patients. Neurosurgery. 1990;27:764-781.
5. Yasargil M.G., Mortara R.W., Curcic M.. Meningioma of basal posterior cranial fossa, Krayenbühl H., editor, Advances and Technical Standards in Neurosurgery, Vienna, Springer-Verlag, 1980;vol. 7:1-115.
6. Samii M., Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien). 1992;118:27-32.
7. Bricolo A.P., Turazzi S., Talacchi A., Cristofori L. Microsurgical removal of petroclival meningiomas: a report of 33 patients. Neurosurgery. 1992;31:813-828.
8. Hallopeau H. Note sur deux faits de tumeurs du mésocéphale. Gaz Med Paris. 1874;3:111-112.
9. Castellano F., Ruggiero G. Meningioma of the posterior fossa. Acta Radiol Suppl. 1953;104:1-157.
10. Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield: Charles C Thomas; 1938.
11. Cushing H: The meningioma (dural endothelioma): their source and favoured seats of origin–Cavendish Lecture. June 13, 1922.
12. Olivecrona H. The surgical treatment of intracranial tumors. In: Olivecrona H., Tonnis W. Handbuch der Neurochirurgie. Berlin: Springer-Verlag; 1967:1-301.
13. Campbell E., Whitfield R.D. Posterior fossa meningiomas. J Neurosurg. 1948;5:131-153.
14. Hakuba A., Nishimura S., Tanaka K. Clivus meningioma: six cases of total removal. Neurol Med Chir (Tokyo). 1977;17:63-77.
15. Jääskeläinen J., Haltia M., Laasonen E. The growth rate of intracranial meningiomas and its relation to histology: an analysis of 43 patients. Surg Neurol. 1985;24:165-172.
16. Van Havenbergh T., Carvalho G., Tatagiba M. Natural history of petroclival meningiomas. Neurosurgery. 2003;52(1):55-62.
17. Little K.M., Friedman A.H., Sampson J.H. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56(3):546-559.
18. Mathiesen T., Lindquist C., Kihlström L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-9.
19. Jung H.W., Yoo H., Paek S.H., Choi K.S. Long term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567-575.
20. Couldwell W.T., Fukushima T., Giannotta S.L., Weis M.H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20-28.
21. Samii M., Ammirati M., Mahran A. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24:12-17.
22. Ichimura S., Kawase T., Onozuka K., Ohira T. Four subtypes of petroclival meningiomas: differences in symptoms and operative findings using the anterior transpetrosal approach. Acta Neurochir (Wein). 2008;150:637-645.
23. Abdel Aziz K.M., Sanan A., van Loveren H.R., et al. Petroclival Meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery. 2000;47(1):139-152.
24. Kawase T., Shiobara R., Toya S. Middle fossa transpetrosal–transtentorial approaches for petroclival meningiomas: selective pyramid resection and radicality. Acta Neurochir (Wien). 1994;129:113-120.
25. Samii M., Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien). 1992;118:27-32.
26. Sekhar L., Swamy N.K.S., Jaiswal V., et al. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860-868.
27. Couldwell W.T., Weiss M.H. Surgical approaches to petroclival meningiomas. I: upper and midclival approaches. Contemp Neurosurg. 1994;16:1-6.
28. Bricolo A., Turazzi S.. Petroclival meningiomas, Schmidek and Sweet’s Operative Technique and Approaches, 5th ed. Saunders, 2005, Chapter 69
29. Simpson D. The recurrence of intracranial meningioma after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
30. Samii M., Ammirati M., Mahran A. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24:12-17.
31. Hakuba A., Nishimura S., Jang B.J. A combined retroauricular and preauricular transpetrosal–transtentorial approach to clivus meningiomas. Surg Neurol. 1988;30:108-116.
32. Kawase T., Shiobara R., Toya S. Anterior transpetrosal–transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869-876.
33. Miller C.G., van Loveren H.R., Keller J.T., et al. Transpetrosal approach: surgical anatomy and technique. Neurosurgery. 1993;33:461-469.
34. Sekhar L.N., Swamy N.K., Jaiswal V., et al. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994;81:860-868.
35. Sekhar L.N., Wright D.C., Richardson R., Monacci W. Petroclival and foramen magnum meningiomas: surgical approaches and pitfalls. J Neurooncol. 1996;29:249-259.
36. Spetzler R.F., Daspit C.P., Pappas C.T.E. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588-599.
37. van Loveren H.R., Abdel Aziz K.M.. Tauber M: Skull base exposures: posterior and lateral, Kaye A.H., Black P.M.c.L., editors, Operative Neurosurgery, Edinburgh, Churchill Livingstone, 2000;vol. 2:1385-1398.
38. Samii M., Tatagiba M., Carvalho G.A. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. Journal of Neurosurgery. Feb 2000;92(2):235-241. 235-241
39. Ammirati M., Samii M. Presigmoid sinus approach to petroclival meningiomas. Skull base surgery. 1992 July;2(3):124-128.
40. Samii M., Tatagiba M. Suprameatal extension of the retrosigmoid approach: microsurgical anatomy. Neurosurgery. March 1999;44(3):560.
41. Zhao J., Liu J.K. Transzygomatic extended middle fossa approach for upper petroclival skull base lesions. Neurosurg Focus. 2008;25(6):E5.
42. Figueiredo E.G., Zabramski J.M., Deshmukh P., et al. Anatomical and quantitative description of the transcavernous approach to interpeduncular and prepontine cisterns. J Neurosurg. 2006;104:957-964.
43. Taniguchi M., Perneczky A. Surgical anatomy and technique subtemporal keyhole approach to the suprasellar and petroclival region: microanatomic considerations and clinical application. Neurosurgery. 1997;41(3):592-601.
44. Zhu W., Mao Y., Zhou L.-F. Combined subtemporal and retrosigmoid keyhole approach for extensive petroclival meningioma surgery: report of experience with 7 cases. Minim Invas Neurosurg. 2007;50:106-110.
45. Horgan M.A., Delashaw J.B., Schwartz M.S., et al. Transcrusal approach to the petroclival region with hearing preservation. Technical note and illustrative cases. J Neurosurg. 2001;94:660-666.
46. Balkany T., Fradis M., Jafek B.W., Rucker N.C. Intrinsic vasculature of the labyrinthine segment of the facial nerve: implications for site of lesion in Bell’s palsy. Otolaryngol Head Neck Surg. 1991;104:20-23.
47. Blunt M.J. The blood supply of the facial nerve. J Anat. 1954;88:520-526.
48. Guerrier Y. Surgical anatomy, particularly vascular supply of the facial nerve. In: Fisch U., editor. Facial Nerve Surgery. Birmingham: Aesculapius; 1977:13-23.
49. Larouere M.J., Lundy L.B. Anatomy and physiology of the facial nerve. In: Jackler R.K., Brackman D.E. Neurootology. St. Louis: Mosby; 1994:1274-1276.
50. May M. Anatomy of the facial nerve for the clinician. In: May M., editor. The Facial Nerve. New York: Thieme; 1986:34-62.
51. Selesnick S.H., Abraham M.T., Carew J.F. Rerouting of the intratemporal facial nerve: an analysis of the literature. Am J Otol. 1996;17:793-805.
52. Cass P.C., Sekhar L.N., Pomeranz S. Excision of petroclival tumors by a total petrosectomy approach. Am J Otol. 1994;15:474-484.
53. Hakuba A., Tanaka K., Suzuki T., Nishimura S. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71(5 Pt 1):699-704.
54. Samii M., Tatagiba M., Carvalho G.A. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. Journal of Neurosurgery. Feb 2000;92(2):235-241. 235-241
55. Ammirati M., Samii M. Presigmoid sinus approach to petroclival meningiomas. Skull base surgery. 2(3), 1992 July. 124–112
56. Samii M., Tatagiba M. Suprameatal extension of the retrosigmoid approach: microsurgical anatomy. Neurosurgery. March 1999;44(3):560.
57. Roche P.H., Pellet W., Fuentes S. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir. 2003;145:883-888.
58. Subach B.R., Lunsford L.D., Kondziolka D. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurg. 1998;42:437-445.
59. Park C.K., Jung H.W., Kim J.E. The selection of optimal therapeutic strategy for petroclival meningiomas. Surgical Neurol. 2006;66:160-166.
60. Flannery T.J., Kano H., Lunsford L.D., et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. September 4, 2009.
61. Couldwell W.T., Cole C.D., Al-Mefty O. Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg. 2007;106:30-35.
62. Jung H.W., Yoo H., Paek S.H., Choi K.S. Long term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567-575.
63. Couldwell W.T., Fukushima T., Giannotta S.L., Weis M.H. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg. 1996;84:20-28.
64. Samii M., Ammirati M., Mahran A. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24:12-17.
65. Natarajan S.K., Sekhar L.N., Schessel D., Morita A. Petroclival meningiomas: multimodality treatment and outcomes at long term follow-up. Neurosurg. 2007;60:965-981.
66. Bambakidis N.C., Kakarla U.K., Kim L.J., et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurg. 2007;61(ONS suppl 2):202-211.