Chapter 32 Surgical Management of Parasagittal and Convexity Meningiomas
Epidemiology and Significance
Meningiomas are the most common primary brain and central nervous system tumor (incidence rate, 6.29 per 100,000 persons). Data from the 2004–2006 Central Brain Tumor Registry reveal that these tumors account for approximately 34% of all primary brain tumors. Meningiomas occur in females 2.25 times more frequently than males (incidence: 8.44 per 100,000 females vs. 3.76 per 100,000 males).2 An increased incidence has been reported among Africans and black Americans.3,4 The incidence rate increases with older age and peaks in persons over 85 years of age (incidence over age of 85 years of age: 36.9 cases per 100,000 persons).2 Meningiomas occur most frequently on the convexity (19% to 34%) and parasagittal locations (18% to 25%), followed by the sphenoid wing and middle cranial fossa (17% to 25%), anterior skull base (10%), posterior fossa (9% to 15%), cerebellar convexity (5%) and clivus (<1%).5–7 To optimize patient management and surgical outcome, understanding appropriate surgical objectives and technique based on the location and biology of these neoplasms is critical.
Etiology
Sporadic
The etiology of the majority of meningiomas is unknown. Although head injury, viral infection, and cell phone use have been implicated in the development of intracranial meningiomas,8,9 the data are inconclusive and conflicting for each of these potential etiologies. The strongest support for an etiologic role in the development and progression of sporadic meningiomas is hormonal. Specifically, studies have shown a potential progesterone influence in the development of meningiomas based on the propensity of these tumors to occur in females and the presence of progesterone receptors in the majority of meningiomas.10–13 While few definitive etiologic correlations have been made for meningiomas, there are certain iatrogenic, environmental exposure and genetic causes that have been linked to their development. These tumorigenic etiologies have significant implications for the management of a meningioma patient who presents for treatment.
Radiation Induced
Meningioma development is associated with irradiation exposure. This association was established by two large cohorts of individuals exposed to radiation that subsequently developed tumors, including meningiomas. Studies published on a group of nearly 11,000 Israeli children treated with low-dose (approximately 1.5 Gy) radiation for tinea capitis revealed a 9.5 times risk for development of meningioma compared to matched controls and untreated siblings.14,15 Similarly, the incidence of meningioma formation among 68 survivors of the Hiroshima atomic bomb within a 2-km radius of the hypocenter identified a 2.9 times risk for the development of meningioma with higher risk for patients within a 1-km radius (6.7 times risk).16 Other iatrogenic forms of ionizing radiation exposure have also been linked to the subsequent development of intracranial meningiomas, including higher-dose, full-mouth dental radiographs and radiotherapy provided for treatment of other cancers.17–20 Generally, radiation-induced meningiomas have a dose dependent latency to development of 10 to over 30 years and have a higher propensity to recur after resection.21
Radiation-induced meningiomas tend to be a higher pathologic grade when compared to their sporadic counterparts, but even WHO grade I radiation–induced meningiomas exhibit more aggressive clinical behavior and have an increased proclivity to recur when compared to their sporadic counterparts.19,22,23 Patients with radiation-induced meningiomas also have an increased incidence of multiple tumors, which must be accounted for when planning placement of the incison.17,24,25 Further, atrophic changes in the scalp that commonly accompany cranial irradiation can require modification of standard multilayer scalp closure. Because radiation exposure can result in other delayed side effects, patients with radiation-induced meningiomas require close medical follow-up for other sequelae, including pituitary dysfunction, visual disturbances (optic atrophy), radiation necrosis, and development of other neoplasms (sarcoma, glioma).26
Neurofibromatosis Type 2 (NF2)
NF2 is an autosomal dominant heritable tumor predisposition syndrome that leads to the development of central and peripheral nervous system tumors (meningiomas, schwannomas, ependymomas), ophthalmologic findings (cataracts, epiretinal membranes, retinal hamartomas) and cutaneous findings (skin plaques, subcutaneous tumors).27 Intracranial meningiomas are identified in approximately half of NF2 patients and are a significant source of morbidity and mortality. NF2-associated intracranial meningiomas are frequently multiple and develop at a younger age than compared to sporadic cases of meningiomas.28–31 Up to 20% of children presenting with a meningioma will have NF2, necessitating full clinical screening and longitudinal follow-up.28,32 The presence of intracranial meningiomas is associated with a 2.5-fold rise in relative risk of mortality in patients with NF2.33 Meningiomas associated with NF2 frequently have increased proliferative activity and a greater rate of atypical and anaplastic grades than do sporadic meningiomas.34,35 Because of the frequent multiplicity of lesions, we perform resection of tumors based on the development of symptoms rather than radiographic tumor growth.
Multiple Meningiomas
Examination of a large series reporting on the presence of multiple meningiomas found that 1% to 10.5% of patients may present with multiple tumors.34 Identification of patients with multiple meningiomas has increased significantly with the advent of improved imaging techniques. Both sporadic and familial forms of multiple meningiomas have been described in the literature, independent of NF2 or history of radiation exposure.37–40 Familial forms of the disease follow an autosomal dominant inheritance pattern and are caused by a mutation independent of the NF2 gene.41,42 Tumors in patients with multiple sporadic meningiomas appear to originate from a single clone (suggesting metastatic spread), whereas others may develop these tumors independently, based on cytogenetic differences among tumors.43–45 Patients with sporadic or familial forms of multiple meningiomas present dilemmas in management similar to patients with NF2, resection is often reserved until the development of symptoms rather radiographic tumor growth.
Classification
Meningiomas originate from arachnoid cap cells, which are distributed along the entire neuroaxis and reflect the wide spectrum of tumor localization. The preoperative classification of meningiomas is based on location of the tumor, primary dural attachment and relationship to neurovascular structures. In the Cavendish Lecture presented in 1922, Harvey Cushing coined the term “meningioma” and classified these tumors based on their site of origin.1 Later, in 1938, Cushing and Eisenhardt used preoperative anatomic classification of meningiomas as a method to correlate clinical findings, help determine surgical approach and aide in developing a prognosis.46 To this day, preoperative anatomic localization remains of critical importance, because it determines surgical positioning, placement of incision and risks involved with resection of the tumor.
Convexity Meningiomas
Convexity meningiomas refer to tumors of the supratentorial space that have their sole attachment to the dura covering the convexity of the cerebral hemispheres. The original classification of convexity meningiomas by Cushing included temporal, frontal, paracentral, parietal and occipital locations.1 Several studies examining the distribution of convexity tumors reveal that the frontal region is the most common site of origin (over 50%), with the posterior locations least frequent (7% to 11%), and the remainder equally distributed between the temporal and paracentral locations.47,48 Because of improvements in the ability to localize tumors and their relationship to functional cortices based on anatomic and functional imaging studies, it is possible to preoperatively classify convexity tumors, anticipate clinical symptoms and the specific risks associated with their management.
Parasagittal Meningiomas
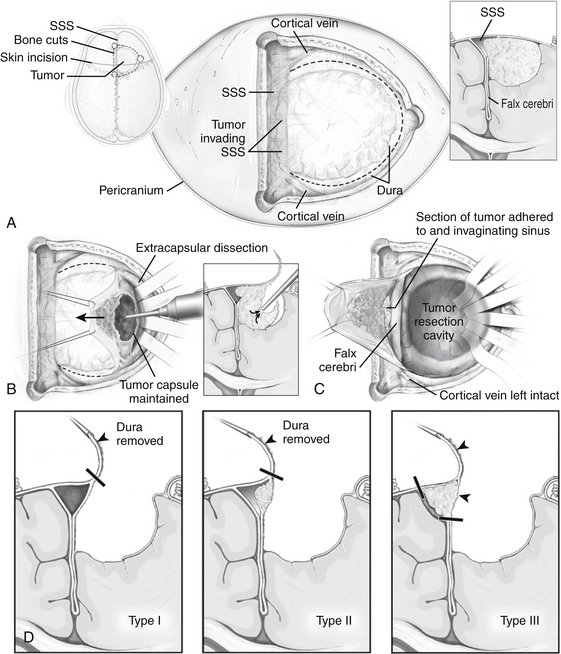
Parasagittal meningiomas have an attachment to the dura forming the outer layer of the SSS and occupy the parasagittal angle displacing brain from this location (Fig. 32-1). Olivecrona first classified parasagittal meningiomas based on their anatomic location along the superior sagittal sinus (SSS). He divided the SSS into thirds anatomically (anterior, middle, posterior) because of the potential consequences of sinus occlusion during complete removal of meningiomas in each area.49 The most common location for parasagittal meningiomas to arise is along the middle third of the SSS (from coronal suture to bregma). Tumors in this location account for 37% to 70% of parasagittal tumors. Fifteen to 42% of parasagittal meningiomas are located along the anterior third of the SSS (from the glabella to the coronal suture) and 9% to 16% of parasagittal tumors are located along the posterior third of the SSS (between the bregma and torcula).50,51
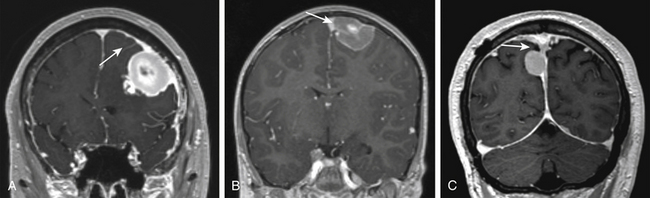
Differentiating parasagittal meningiomas from convexity and falcine meningiomas has significant implications for preoperative evaluation and surgical planning. In the instance where tumors of the convexity approach the SSS, they are distinguished from parasagittal tumors by the presence of brain tissue at the parasagittal angle and lack of attachment to the meninges that create the outer wall of the superior sagittal sinus itself. A similar distinction is made of falcine meningiomas, which are delineated from parasagittal tumors because their sole dural attachment is the falx cerebri. Parasagittal meningiomas vary in the amount of attachment to convexity dura and the falx cerebri, but they all have an attachment to the dura forming the outer layer of the SSS and occupy the parasagittal angle displacing brain tissue from this location (Fig. 32-1).
Clinical Presentation
Meningiomas are discovered incidentally or as a result of the development of related signs and/or symptoms. The increasing use of computed tomography (CT) and magnetic resonance (MR)-imaging has led to an increase in the number of incidentally discovered meningiomas over the last two decades. Currently, incidentally discovered meningiomas represent 10% to 20% of all meningiomas that are brought to clinical attention. Overall, clinical symptoms from meningiomas develop as a result of raised intracranial pressure, disruption of cortical electrophysiology or direct mass effect on adjacent neural structures.
Convexity Meningiomas
Similar to other mass-occupying lesion in the central nervous system, convexity meningiomas can present with a variety of signs and symptoms based on their anatomic location. Symptomatic convexity meningiomas most commonly are associated with headache (39% to 48%), seizures (20% to 34%), and/or hemiparesis (10% to 21%).47,48,52 Other specific symptomatology, such as dysphasia, sensory changes, and visual disturbance occur less frequently but develop when tumors are located specifically over the respective eloquent cortices.
Parasagittal Meningiomas
Signs and symptoms associated with parasagittal meningiomas depend on their location along the SSS. Tumors of the anterior third of the SSS often cause headache and personality changes. Tumors of the middle third of the SSS often are associated with Jacksonian seizures, headache and progressive hemiparesis. Tumors of the posterior third of the SSS often cause headache, seizures and gradual hemianopsia.50,51 Like convexity tumors, the most common symptoms associated with parasagittal meningiomas at the time of presentation are seizures (46% to 51%), headache (42% to 54%), and/or motor weakness (39% to 49%).
Indications for Surgery
Incidental Meningiomas
Because they exhibit no growth or slow linear growth, the majority of asymptomatic, incidental meningiomas may be observed without surgical intervention.53,54 Several studies reporting on the long-term natural history of these tumors also reveal that a smaller subset of tumors may demonstrate exponential growth or sigmoidal growth.55,56 Variability in growth rates and patterns necessitate close surveillance until these properties can be established.57 We perform follow-up MR-imaging 3 months after initial diagnosis for incidentally discovered meningiomas. If the tumor demonstrates no growth or slow linear growth, we will monitor the tumor at 6 to 12 month intervals. Alternatively, we monitor tumors exhibiting exponential growth at 3 to 6 month intervals. Specific imaging and patient characteristics associated with more rapid meningioma growth rates have been identified. The likelihood of tumor growth is higher in young patients and tumors larger than 3 cm in size.53,58,59 Tumors that lack calcification and are hyperintense on T2-weighted MR-imaging are also more likely to display an aggressive growth pattern.55,57,60 For each clinical situation, the decision to recommend surgery should be evaluated on an individual basis, incorporating patient comorbidities, age, observed growth rate, and image-based predictive factors for growth.
Parasagittal Meningiomas
In addition to the indications presented for convexity tumors, timing of parasagittal meningioma resection is influenced by the extent of SSS involvement. Because acute surgical obstruction of the patent SSS can cause significant cerebral edema and venous infarction, a tumor causing partial obstruction may be followed closely to observe for the development of complete occlusion, allowing that the intracranial component of the tumor does not greatly increase in size, cause symptoms, or encroach on large adjacent superficial anastomosing veins. Alternatively, an asymptomatic meningioma without evidence of invasion into the SSS may require intervention at the earliest sign of growth so that a complete resection can be performed without entering the SSS.
Preoperative Evaluation
Superior Sagittal Sinus Involvement
With advancements in imaging and microsurgical techniques, reconstruction and bypass of the SSS have been used in an effort to enhance resection parasagittal meningiomas. Several large series have been published on the complete removal of tumor with reconstruction or bypass of the SSS.61 Despite performing more extensive resections (Simpson grade I, Table 32-1) with sinus reconstruction or bypass, which increased perioperative morbidity and mortality, the authors found that local recurrence remains a significant problem.62 Subsequently, based on the potential morbidity with these cases, better understanding of the natural history of meningiomas and efficacy of adjuvant radiosurgery63–65 in the management of residual and/or recurrent disease, many surgeons have adopted a more conservative approach to management of SSS invasion by meningioma.62,63,66
Table 32-1 Simpson Classification of Extent of Resection for Intracranial Meningiomas and Recurrence Rate
| Grade | Extent of Resection | Recurrence Rate |
|---|---|---|
| I | Gross total resection of tumor, dural attachments and abnormal bone | 9% |
| II | Gross total resection of tumor, coagulation of dural attachments | 19% |
| III | Gross total resection of tumor without resection or coagulation of dural attachment or its extradural extensions | 29% |
| IV | Partial resection of tumor | 44% |
| V | Simple decompression |
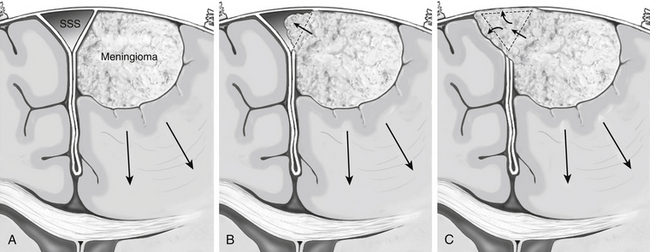
Derived from a surgical approach that does not include resection and repair/reconstruction of a patent or partially occluded SSS, a simple classification system can be used that permits stratification of parasagittal meningiomas based on preoperative imaging (Fig. 32-2). This classification scheme divides parasagittal tumors into three types that have direct surgical implications. Type I tumors are defined as tumors that attach to the external layer of dura forming the SSS. Type II tumors are identified as tumors that visibly invade the SSS and narrow its lumen, but do not cause complete obstruction. Grade III tumors are defined as tumors that invade the SSS and cause its complete obstruction. Each of the three types has surgical implications as defined below.
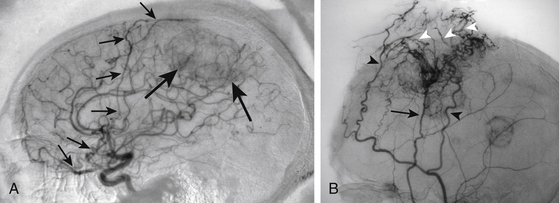
The evaluation of SSS patency, invasion and the development of collateral venous pathways are performed with contrast-enhanced MR-venography (CE-MRV). The sensitivity and specificity for these findings on CE-MRV is similar to that of digital subtraction angiography (DSA), with a reduction in risk and patient discomfort.67,68 DSA is still performed if tumor embolization is considered or if arterial supply to the tumor needs to be better defined. In the setting of SSS obstruction, CE-MRV, and DSA may reveal significantly dilated scalp veins and diploic veins in addition to engorged cortical venous collaterals. These supplementary collateral venous pathways play a critical role in venous drainage, especially in the setting of parasagittal meningiomas causing occlusion of the middle or posterior third of the superior sagittal sinus. When possible, surgical approaches should avoid transgression of these structures with tailored scalp incisions and bone flaps.62,69 Although convexity and parasagittal meningiomas typically receive their primary blood supply from dilated meningeal arteries, the surgeon should be aware that some tumors may develop additional blood supply through the internal carotid artery (pial parasitization) or dilated scalp arteries (Fig. 32-3).
Bony Changes
Preoperative evaluation of bone involvement is useful in assessing the need for bone removal and cranioplasty. Because MR imaging alone significantly underestimates bony changes associated with meningiomas, we routinely perform a cranial CT for evaluation of the extent of bony involvement of all parasagittal and convexity meningiomas.70 Bony changes associated with meningiomas can be caused by hyperostotic changes or direct bone invasion by tumor. Hyperostotic changes are generally considered a benign form of inductive ossification caused by tumoral increases in alkaline phosphatase production.71 In managing hyperostotic changes without bony invasion by tumor, we use the high-speed drill to remove the bulk of hyperostotic bone, at times just leaving the outer cortical table of bone intact. For tumors that present with significant bone destruction and replacement by tumor, removal of the invaded bone is necessary for complete tumor resection.
Tumor and Brain Characteristics
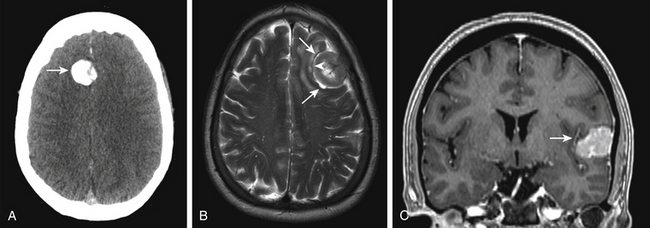
The “gold standard” for evaluation of meningioma size, location, and impact on adjacent brain structures is MR-imaging performed with and without contrast. Meningiomas typically appear as isointense masses on pre-contrast T1-weighted imaging and enhance homogenously after the administration of contrast. Postcontrast MR imaging often reveals a trailing linear enhancing structure along its dural attachment referred to as the “dural tail.”72 The dural tail is not specific to the diagnosis of meningioma, but its management continues to be a point of controversy.72,73 Although evidence suggests that most of the dural tail is an imaging correlate of dilated meningeal vessels and dural congestion,74 many still advocate extensive resection of the dural tail believing that it can contain tumor. Generally, we resect at least a 1-cm margin of dura from its interface with the tumor.
T2-weighted MR-image sequences typically show more variability in tumoral intensity (50% isointense, 40% hyperintense, and 10% hypointense),75 but may help predict clinical behavior of meningiomas. Identification of a T2-weighted hyperintense arachnoid cleft between tumor and brain is often indicative of a distinct anatomic plane (Fig. 32-4). Absence of an arachnoid cleft in combination with significant peritumoral edema may be indicative of pial vessel parasitization and/or brain invasion. Addressing brain invasion is a key aspect in the operative management of parasagittal and convexity meningiomas. Management balances preoperative expectations, age of the patient, and eloquence of the involved cortices. If extrapial resection is not possible in an area of eloquence, a small amount of tumor may be left attached to the cortical surface and observed postoperatively.76 For tumors near eloquent areas with variable representation, such as Broca’s and Wernicke’s area, fMR imaging or awake surgery with mapping is valuable in elucidating their anatomic relationship and determining risks of resection.
Surgical Treatment
Surgical advances
In 1743, Lorenz Heister,77 performed the first documented attempt of surgical treatment of a meningioma. During the operation, he applied a caustic of lime to the tumor of a 34-year-old Prussian soldier in an attempt to destroy the tumor tissue. Infection ensued shortly thereafter, leading to the death of the patient. In 1835, the first meningioma was successfully resected by Zanobi Pecchioli. The parietal convexity tumor, which had eroded through the calvarium, was excavated with a triangular craniectomy, and “a fine linen soaked in almond oil” was placed over the exposed brain. The open wound healed by granulation tissue formed over the course of 4 months. While wound healing occurred, the patient utilized a “skullcap of boiled leather with padded lining” to protect the brain.78
The development of monopolar cautery, in 1926, and then the widespread use of bipolar cautery, in the late 1950s, significantly improved meningioma resection results by reducing the significant blood loss that often occurred before their development.79,80 The use of the operating microscope, beginning in the 1960s, enhanced the safe and complete resection of many meningiomas through improved visualization of the tumor–tissue plane, tumor associated vessels and other tumor-nervous system relationships. Recently, intraoperative image-guided surgical navigation systems have made it possible to more accurately localize these tumors before incision and craniotomy. This has enhanced the precision of incision placement and has led to minimization of the craniotomy size necessary for complete resection.
Preoperative Care
For patients who present with seizure, antiepileptic medications are optimized to prevent complications that may result from intra- and peri-operative seizures including venous hypertension and brain swelling. For tumors with surrounding edema, preoperative steroids (dexamethasone) can be started 4 to 7 days before surgery to allow reduce peritumoral vasogenic edema and swelling. Concurrent administration of proton pump inhibitors for gastrointestinal ulcer prophylaxis is recommended.81 Because most parasagittal and convexity tumors have a broad attachment to convexity dura, we prefer not to give diuretics such as Mannitol (osmotic diuretic) or Furosemide (loop diuretic). We typically reserve the use of these agents in the setting of a large parasagittal tumor with a primary falcine attachment to minimize the need for brain retraction. For deep venous thrombosis prophylaxis, all patients have graduated compression stockings and/or an intermittent pneumatic compression device applied before induction. According to guidelines, preoperative antibiotics (for appropriate gram-positive coverage) are administered within 60 minutes of incision.82
Embolization
Since embolization for preoperative management of meningiomas was introduced in 1971,83 reports have indicated that it can be used to reduce intraoperative blood loss, which can potentially reduce surgical complications and improve overall outcome.84–86 Despite these theoretical advantages, the surgeon must balance the use of embolization against its known complications (6%), including ischemic stroke, hemorrhage, and acute cerebral edema.87,88 While there is little benefit in performing preoperative embolization for small tumors,89 the risks of angiography and embolization can be considered for treatment of larger tumors. In our experience, we have rarely found it necessary to embolize convexity or parasagittal meningiomas of any size, because the source of tumor bleeding is readily apparent at the working surface and can be controlled with meticulous hemostasis.
Parasagittal Meningiomas
Positioning
Positioning for resection of parasagittal meningiomas is determined by the anteroposterior location of the tumor along the SSS. For tumors located along the anterior third of the SSS, a supine position with the head and neck in a neutral or gently flexed position will permit direct access to the tumor. For tumors located along the middle third of the SSS, a supine semisitting position with slight head and neck flexion will often allow direct access to the tumor. Another positioning option for tumors in this location includes placing the patient lateral with their head elevated so that the scalp over the tumor is at the highest point in the field.62 Finally, for parasagittal tumors located along the posterior third of the SSS, a prone position with the head and neck in neutral or slightly extended position can provide direct tumor access. Another positioning option for tumors in this location includes placing the patient three quarters prone with the tumor below the midline, allowing for the brain to fall away with minimal retraction.76,90
Incision
After patient positioning, frameless stereotaxic navigation is registered to patient. The use of frameless navigation facilitates the identification of the surface presentation of the underlying tumor with reference to the overlying scalp. Surgical navigation can be used to assist in determining the precise placement, shape and size of incision, as well as facilitate the planning and size of the craniotomy after incision and calvarial exposure.91 During the course of resection (provided there is little shift of brain parenchyma), frameless neuronavigation is also helpful in affirming local relationships to major vascular structures (dural venous sinuses, superficial anastamotic veins and feeding arteries) and eloquent cortices. After the skin incision is marked, the incision site and surrounding region is sterilely prepared and draped. The skin of the incision site is then infiltrated with a local anesthetic with epinephrine (1:200,000).
Generally, parasagittal meningiomas of the anterior third of SSS are exposed via a bicoronal incision. Meningiomas of the middle and posterior third of the SSS can be exposed through a bicoronal, S-shaped or a U-shaped incision. Bicoronal or S-shaped incisions are centered over the tumor mass in the anteroposterior plane and should permit exposure of the uninvolved side of the SSS. Similarly, U-shaped incisions for parasagittal tumors should extend at least 2 cm past the midline to provide exposure to the uninvolved side of the SSS. U-shaped incisions should have a wide base that is 1.5 times the length of the pedunculated flap to ensure adequate perfusion to the distal portions. During incision, it is important to attempt to maintain large anastamotic collaterals that may have developed within the scalp and diploe to prevent venous congestion, brain swelling, and/or infarction.69
Resection
The major obstacles to a safe resection pertain to the management of the superior sagittal sinus and bridging superficial cortical vessels entering this structure. After craniotomy, intraoperative ultrasound or the neuronavigation can supplement the surgeon’s observation and tactile tracing of the underlying tumor and adjacent neurovascular structures. A C-shaped dural opening, with its base towards the SSS is planned and small durotomy is made with the #15 blade over the ipsilateral brain convexity 1 cm from the tumor margin (Fig. 32-5). As the durotomy is extended along the “C” in both directions towards the SSS, great care is taken to examine cortical veins that may cross to bridge with the SSS. Because of the potential importance of these veins for critical drainage we prefer to alter the course of the durotomy than cause damage to these vessels.
Once the durotomy is completed, bipolar cautery applied to the center of the dural leaflet causes eversion of the cut margins and allows visualization of the intradural compartment. Initial separation of the tumor from the underlying cortex along arachnoid planes proceeds and the dura is reflected progressively over the contralateral hemisphere causing the tumor to evert. Cottonoid patties are placed in the tumor–brain arachnoid cleft. As this commences, we perform central debulking of the tumor to avoid avulsion or disruption of venous elements on the falcine side of the tumor and detachment of the convexity dura mater. Central debulking of the tumor is carried out with the use of the ultrasonic aspirator. For fibrous and heavily calcified tumors, the loop cautery device can sometimes be more effective than the ultrasonic aspirator. Care is taken never to breach the capsule of the tumor while internal debulking is performed. Alternating between extracapsular dissection and intratumoral debulking permits tumor dissection and removal, while minimizing brain tissue manipulation. The primary dural attachment at the convexity and/or falx is resected to within a few millimeters from the SSS, leaving a small portion attached to the SSS.
SSS involvement
Type I tumors can be peeled from their attachment to the dura overlying the SSS (Fig. 32-5). After this is accomplished, the external layer of dura is cauterized to achieve a Simpson grade II resection. If preoperative assessment indicates tumor is present within the lumen of the SSS (type II), we perform a Simpson grade III resection. Tumor is debulked at its insertion through the wall of the superior sagittal sinus, but cannot completely be removed or peeled without entering the lumen of the SSS. Instead, this residual tumor is cauterized and the tumor residual within the lumen of the SSS left in situ. Type III tumors allow for ligation and resection of the SSS. Because immediately adjacent veins that enter the SSS just anterior or posterior to the obstruction represent important collaterals, it is imperative that these vessels not be compromised in an attempt for greater resection. In order to ligate the SSS, the dura over the contralateral hemisphere is opened approximately 1 cm from the SSS along the length of the planned SSS resection. At the anterior and posterior margins of the resection, 2-0 silk suture is passed through the falx under direct visualization, encircling the SSS. Once the SSS is ligated in this fashion, it may be transected and removed, achieving a Simpson grade I resection.
Closure
For all tumors, the pericranium is harvested at the time of exposure, kept moistened during resection and available for closure. Because shrinkage of dura is inevitable from management of meningeal feeding vessels, achieving a primary watertight dural closure is often not feasible. In cases with small convexity defects, portions of the pericranial graft may be placed as interposition grafts to gain a better seal. The pericranial graft can also be used to replace the entirety of large defects created by Simpson grade I resections (type III tumors) or resection of a large primary attachment to the convexity dura at the parasagittal angle (type I and II tumors). Several small, nonbraided, nonresorbable interrupted suture are used to tack the graft in position and augmented with a running suture. The dural closure is then completed with a running suture. The suture line is then augmented with a thin layer of fibrin sealant. Two to three central dural tack-up sutures are placed through the dura and bone flap at closure.
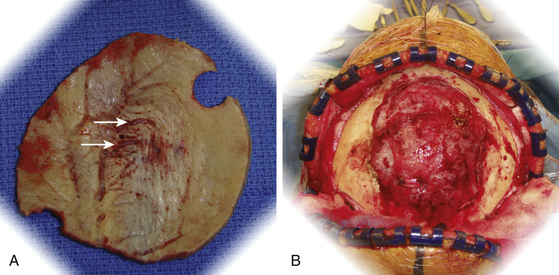
The bone flap is examined for evidence of bony changes (Fig. 32-6). Because direct tumor invasion can be identified with preoperative CT imaging, with co-register CT and MR-imaging in neuronavigation system to identify the extent of tumor involvement of bone before the craniotomy flap is elevated, as described previously. This allows accurate resection of the portion of the bone flap that is involved, instead of relying solely on visual inspection. Either titanium mesh or methylmethacrylate can be utilized to bridge the defect that is created in the craniotomy flap. If a craniectomy is needed because of extensive invasion of the bone by tumor, a cranioplasty is performed. A custom-designed cranioplasty for these larger defects can be included in the preoperative planning. Hyperostotic regions, without direct invasion by tumor, are removed with a high-speed cutting burr until the appropriate contour and thickness are achieved. The bone flap is then returned to its native position and aligned without gap in portions that are covered with thinned or nontrichogenic scalp. Titanium burr-hole cover plates and miniplates are held in place with titanium screws. Subsequently, the galea is reapproximated with inverted absorbable suture and the scalp reapproximated with staples.
Convexity Meningiomas
Positioning
During positioning, the head placed above the level of the heart and situated to present the tumor-bearing convexity parallel to the ceiling. This may require positioning the patient in a park-bench or prone position, especially for parietal and occipital tumors, depending on how mobile the neck and torso are in accommodating rotation. To simplify positioning, avoid the risk of venous air embolism,92 and ideally place the tumor at the highest point of the operative field so that brain retraction can be minimized, we generally avoid the sitting position for operation of convexity tumors.
Exposure
Once the pericranium is dissected and the calvarium is exposed, we use neuronavigation and anatomic landmarks to outline the planned craniotomy and mark any area of tumor-invaded bone. Depending on the size of the tumor and age of the patient, anywhere from one to three burr holes may be placed circumferentially around the underlying tumor. More burr holes are placed in elderly patients because of the adherence of dura to the calvarium that increases with age. A right-angle dural elevator or the Penfield-3 is used to separate the underlying dura along the craniotomy flap. The craniotome is used to connect the outside margin of the burr holes. Preferably under direct visualization, the craniotomy flap is separated from the underlying dura. Tumor-invaded bone is detached sharply from the underlying intracranial mass. Bleeding from exposed tumor and dural tears are aggressively cauterized with the use of bipolar cautery once the craniotomy flap is completely elevated. Bleeding from arachnoid granulations and venous lakes near the superior sagittal sinus are stopped with the application of woven oxidized cellulose polymer and Gelfoam. The bone flap is set aside for tumor resection. Removal of suspected areas of bony invasion and reshaping of hyperostotic bone is done at the time of closure. After the bone is removed, circumferential tack-up sutures are placed.
Resection
Large tumors are centrally debulked for several reasons. First, this allows retraction on the tumor capsule into the tumor itself. Second, as tumors enlarge, they often begin to parasitize blood supply through the development of pial collateral feeding vessels. Careful identification, cautery, and sharp transection of these vessels allow minimal disruption of the underlying cortex. Lastly, debulking allows extracapsular differentiation between feeding vessels and vessels of passage, critical in removal of tumors near the Sylvian fissure (Fig. 32-4). If central debulking is planned, we generally acquire tissue for histologic evaluation at this time because subsequent cautery effect on the capsule causes significant histopathologic artifacts. Central debulking of the tumor is carried out with the use of the ultrasonic aspirator. For fibrous and heavily calcified tumors, the loop cautery device can be more effective than the ultrasonic aspirator. While proceeding during internal debulking of the tumor, it is useful to have hemostatic agents, such as thrombin-soaked Gelfoam available to place in the tumor cavity. With gentle application of pressure on top of thrombin-soaked Gelfoam, most intratumoral bleeding will stop. It is important to avoid breaching the capsule as internal debulking proceeds, making alternating internal debulking and subsequent extracapsular dissection an ideal way to proceed without significant lapses in time. Proceeding in this fashion, the tumor is gradually excavated from its bed.
Postoperative Care and Complications
Immediately following placement of the dressing, patients are awoken from anesthesia and their neurologic status assessed. It is imperative that during awakening, pain and hypoxia be managed appropriately to prevent excessive agitation.93 Coughing and valsalva maneuvers should be avoided during emergence and extubation. Once recovered from anesthesia, all patients are kept under neurologic observation with bedside examinations performed by nursing staff every 1 to 2 hours overnight. The head of bed is kept elevated at 30 degrees. Initiation of new antiepileptic drug therapy for postoperative seizure prophylaxis is not recommended.94 Postoperatively, initiation of steroids is avoided and preoperative dosing is stopped (if patient on steroids less than 7 days before surgery) or systematically tapered over days. Strict assessment of fluid intake and outputs are recorded and euvolemia maintained.
For uncomplicated convexity surgeries that less than 3 hours, we routinely remove the Foley catheter before emergence and encourage increased activity as tolerated after the immediate postanesthetic recovery period. For patients undergoing longer operations or who have preoperative motor deficits, activity level is monitored and advanced as tolerated. Perioperative antibiotic prophylaxis is discontinued within 24 hours of surgery. Deep venous thrombosis prophylaxis with low-dose, unfractionated subcutaneous heparin or low-molecular-weight heparin is added to graduated compression stockings and/or an intermittent pneumatic compressive device on the first postoperative day and continued until patient discharge.95
Despite standard implementation of extensive preoperative evaluations, medical morbidity still occurs in 2% to 5% of all convexity meningioma operations. While perioperative mortality from surgery of convexity meningiomas is very low, the morbidity remains at approximately 10%. These are equally divided between neurosurgical complications, including development of new neurological deficits (approximately 2%), wound infection (3%), cerebrospinal fluid leak (1%), postoperative hematoma (1%), seizure (1%), and delayed hydropcephalus (1%) and medical complications, including nonwound infections (1%), cardiac events (1%), deep venous thrombosis (3%), and pulmonary embolus (1%).47,48
Both medical and surgical complications are increased in patients undergoing resection of parasagittal meningiomas compared to convexity tumors. This is likely due to the increased complexity of surgical resection and management of the critical venous structures. Perioperative mortality, although significantly reduced in the microsurgical era, still occurs in 2% to 3% of cases.51,61 Operations involving the SSS have the additional risk of air embolism (1%) and significant blood loss.61 Permanent neurologic deficit and worsening of functional status (approximate 10%) occurs as a result of postoperative brain swelling and/or venous infarction, likely from acute occlusion of important collateral vessels.51,61 Postoperative hematomas (3%) and wound infections (5%) are also more frequent in patients undergoing resection of parasagittal tumors and may be related to the increased time often required to remove meningiomas in this location compared to convexity tumors.
Outcomes and Follow-Up
The extent of surgical resection is the most important factor in the prevention of recurrence.96,97 Simpson reviewed the recurrence rate among 265 patients who underwent operation for meningiomas and noted a proportional increase in recurrence that was associated with the amount of residual tumor remaining after surgery97 (Table 32-1). Because convexity tumors are free of involvement of the major venous sinuses and are amenable to resection of involved dura, the 5-year recurrence rate of benign meningiomas in this location is 0% to 3%.47,96 In contrast, the 5-year recurrence rate for parasagittal meningiomas is 2% to 18%.50,51,61,96
In addition to extent of surgical resection, the World Health Organization (WHO) histopathologic grading of meningiomas is the other major predictive factor of recurrence.35,98 Eighty to 90% of meningiomas are benign tumors (WHO grade I), whose gross total resection results in recurrence rates of 7% to 25%.99,100 Although some histologic variants of benign (WHO grade I) meningioma (secretory) are associated with increased findings of edema on preoperative imaging, no differential growth or recurrence rate has been identified between histologic subgroups of benign meningiomas.101 Atypical meningiomas (WHO grade II) are associated with recurrence rates of 29% to 52% while anaplastic meningiomas (WHO grade III) recur in 50% to 94% of cases.100 The WHO no longer classifies brain invasion as a feature of atypical (WHO grade II) meningiomas, although its clinical presence is an indicator of higher chance for recurrence.
Al-Mefty O. Meningiomas. New York: Raven Press; 1991.
Al-Mefty O., Kersh J.E., Routh A., Smith R.R. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73(4):502-512.
Asthagiri A.R., Parry D.M., Butman J.A., et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974-1986.
Bratzler D.W., Houck P.M. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
Claus E.B., Bondy M.L., Schildkraut J.M., et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088-1094.
Cushing H., Eisenhardt L. Meningiomas: their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield, IL: Charles C. Thomas; 1938.
Geerts W.H., Bergqvist D., Pineo G.F., et al. Prevention of venous thromboembolism: american College of Chest Physicians evidence-based clinical practice guidelines 8th ed. Chest, 2008;6 suppl. 6:133
Hancq S., Baleriaux D., Brotchi J. Surgical treatment of parasagittal meningiomas. Semin Neurosurg. 2003;14(3):203-210.
Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Neurosurgery. 1998;43(3):405-413.
Kuijlen J.M.A., Teernstra O.P.M., Kessels A.G.H., et al. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta-analysis. Seizure. 1996;5(4):291-298.
Larson J.J., Tew J.M.Jr., Simon M., Menon A.G. Evidence for clonal spread in the development of multiple meningiomas. J Neurosurg. 1995;83(4):705-709.
Manelfe C., Lasjaunias P., Ruscalleda J. Preoperative embolization of intracranial meningiomas. Am J Neuroradiol. 1986;7(5):963-972.
Mirimanoff R.O., Dosoretz D.E., Linggood R.M. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18-24.
Morokoff A.P., Zauberman J., Black P.M. Surgery for convexity meningiomas. Neurosurgery. 2008;63(3):427-433. discussion 433-434
Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62-71.
Perry A., Louis D.N., Cheithauer B.W., et al. Meningiomas. In: Louis D., Ohgaki H., Wiestler O.D., Cavenee W.K. WHO Classification of Tumours of the Central Nervous System. 3rd ed. Geneva: International Agency for Research on Cancer; 2007:164-172.
Ron E., Modan B., Boice J.D.Jr., et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033-1039.
Sanai N., Sughrue M.E., Shangari G., et al. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010;112(5):913-919.
Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22-39.
Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105(4):514-525.
Umansky F., Shoshan Y., Rosenthal G., et al. Radiation-induced meningioma. Neurosurg Focus. 24(5), 2008.
Wen P.Y., Schiff D., Kesari S., et al. Medical management of patients with brain tumors. J Neuro-Oncol. 2006;80(3):313-332.
Wilkins R. Parasagittal meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:329-343.
Yano S., Kuratsu J.I. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105(4):538-543.
1. Cushing H. The meningioma (dural endotheliomas): their source and favoured seats of origin. Brain. 1922;45:282-316.
2. Central Brain Tumor Registry of the United States. 2009-2010 CBTRUS Statistical Report: primary Brain and Central Nervous System Tumors Diagnosed in Eighteen States in 2002-2006. CBTRUS, 2009 www.cbtrus.org Accessed August 17, 2010
3. Bahemuka M. Worldwide incidence of primary nervous system neoplasms. Geographical, racial and sex differences, 1960-1977. Brain. 1988;111(3):737-755.
4. Preston-Martin S. Descriptive epidemiology of primary tumors of the brain, cranial nerves and cranial meninges in Los Angeles County. Neuroepidemiology. 1989;8(6):283-295.
5. Al-Mefty O. Meningiomas. New York: Raven Press; 1991.
6. Buetow M.P., Buetow P.C., Smirniotopoulos J.G. Typical, atypical, and misleading features in meningioma. 1991;11(6):1087-1106.
7. Kaye A.H., Laws E.R. Brain Tumors: An Encyclopedic Approach. Edinburgh and New York: Churchill Livingstone; 1995.
8. Khurana V.G., Teo C., Kundi M. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. 2009;72(3):205-214.
9. Claus E.B., Bondy M.L., Schildkraut J.M. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088-1094.
10. Hsu D.W., Efird J.T., Hedley-Whyte E.T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113-120.
11. Blaauw G., Blankenstein M.A., Lamberts S.W.J. Sex steroid receptors in human meningiomas. Acta Neurochir. 1986;79(1):42-47.
12. Maxwell M., Galanopoulos T., Neville-Golden J., Antoniades H.N. Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg. 1993;78(3):456-462.
13. Perrot-Applanat M., Groyer-Picard Th M., Kujas M. Immunocytochemical study of progesterone receptor in human meningioma. Acta Neurochir. 1992;115(1-2):20-30.
14. Modan B., Baidatz D., Mart H. Radiation induced head and neck tumours. Lancet. 1974;1(7852):277-279.
15. Ron E., Modan B., Boice J.D.Jr., et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033-1039.
16. Shintani T., Hayakawa N., Hoshi M., et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Radiat Res (Tokyo). 1999;40(1):49-57.
17. Sadetzki S., Flint-Richter P., Ben-Tal T., Nass D. Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg. 2002;97(5):1078-1082.
18. Walter A.W., Hancock M.L., Pui C.H., et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998;16(12):3761-3767.
19. Harrison M.J., Wolfe D.E., Lau T.S. Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg. 1991;75(4):564-574.
20. Longstreth W.T.Jr., Phillips L.E., Drangsholt M., et al. Dental x-rays and the risk of intracranial meningioma: a population-based case–control study. Cancer. 2004;100(5):1026-1034.
21. Kondziolka D., Kano H., Kanaan H., et al. Stereotactic radiosurgery for radiation-induced meningiomas. Neurosurgery. 2009;64(3):463-469.
22. Umansky F., Shoshan Y., Rosenthal G. Radiation-induced meningioma. Neurosurg Focus. 24(5), 2008.
23. Musa B.S., Pople I.K., Cummins B.H. Intracranial meningiomas following irradiation—a growing problem? Br J Neurosurg. 1995;9(5):629-637.
24. Rubinstein A.B., Shalit M.N., Cohen M.L. Radiation-induced cerebral meningioma: a recognizable entity. J Neurosurg. 1984;61(5):966-971.
25. Soffer D., Pittaluga S., Feiner M., Beller A.J. Intracranial meningiomas following low-dose irradiation to the head. J Neurosurg. 1983;59(6):1048-1053.
26. Al-Mefty O., Kersh J.E., Routh A., Smith R.R. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73(4):502-512.
27. Asthagiri A.R., Parry D.M., Butman J.A., et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974-1986.
28. Evans D.G.R., Birch J.M., Ramsden R.T. Paediatric presentation of type 2 neurofibromatosis. Arch Dis Child. 1999;81(6):496-499.
29. Mautner V.F., Lindenau M., Baser M.E., et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996;38(5):880-886.
30. Nunes F., MacCollin M. Neurofibromatosis 2 in the pediatric population. J Child Neurol. 2003;18(10):718-724.
31. Parry D.M., Eldridge R., Kaiser-Kupfer M.I. Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994;52(4):450-461.
32. Evans D.G.R., Watson C., King A. Multiple meningiomas: differential involvement of the NF2 gene in children and adults. J Med Genet. 2005;42(1):45-48.
33. Baser M.E., Friedman J.M., Aeschliman D., et al. Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet. 2002;71(4):715-723.
34. Antinheimo J., Haapasalo H., Haltia M., et al. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J Neurosurgery. 1997;87(4):610-614.
35. Perry A., Giannini C., Raghavan R., et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: A clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994-1003.
36. Akalan N., Ozgen R. Multiple meningiomas. In: Pamir M., Black P., Fahlbusch R. Meningiomas: A Comprehensive Text. Philadelphia: Saunders; 2010:601-609.
37. Antinheimo J., Sankila R., Carpén O. Population-based analysis of sporadic and type 2 neurofibromatosis- associated meningiomas and schwannomas. Neurology. 2000;54(1):71-76.
38. Butti G., Assietti R., Casalone R., Paoletti P. Multiple meningiomas: A clinical, surgical, and cytogenetic analysis. Surg Neurol. 1989;31(4):255-260.
39. McDowell J.R. Familial meningioma. Neurology. 1990;40(2):312-314.
40. Turgut M., Palaoğlu S., Ozcan E.O. Multiple meningiomas of the central nervous system without the stigmata of neurofibromatosis. Clinical and therapeutic study. Neurosurg Rev. 1997;20(2):117-123.
41. Pulst S.M., Rouleau G.A., Marineau C. Familial meningioma is not allelic to neurofibromatosis 2. Neurology. 1993;43(10):2096-2098.
42. Shen Y., Nunes F., Stemmer-Rachamimov A., et al. Genomic profiling distinguishes familial multiple and sporadic multiple meningiomas. BMC Med Genomics. 2, 2009.
43. Larson J.J., Tew J.M.Jr., Simon M., Menon A.G. Evidence for clonal spread in the development of multiple meningiomas. J Neurosurg. 1995;83(4):705-709.
44. Stangl A.P., Wellenreuther R., Lenartz D., et al. Clonality of multiple meningiomas. J Neurosurg. 1997;86(5):853-858.
45. Zhu J.J., Maruyama T., Jacoby L.B., et al. Clonal analysis of a case of multiple meningiomas using multiple molecular genetic approaches: pathology case report. Neurosurgery. 1999;45(2):409-416.
46. Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield, IL: Charles C. Thomas; 1938.
47. Morokoff A.P., Zauberman J., Black P.M. Surgery for convexity meningiomas. Neurosurgery. 2008;63(3):427-433. discussion 433-434
48. Sanai N., Sughrue M.E., Shangari G. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010;112(5):913-919.
49. Olivecrona H. Die Parasagittalen Meningeome. Leipzig: Georg Thieme; 1934.
50. Wilkins R. Parasagittal meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:329-343.
51. DiMeco F., Li K.W., Casali C., et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery. 2004;55(6):1263-1272.
52. Fahlbusch R., Hofmann B. Surgery of Convexity Meningiomas. In: Pamir M., Black P., Fahlbusch R. Meningiomas: A Comprehensive Text. Philadelphia: Saunders; 2010:339-348.
53. Yoneoka Y., Fujii Y., Tanaka R. Growth of incidental meningiomas. Acta Neurochir. 2000;142(5):507-511.
54. Yano S., Kuratsu J.I. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105(4):538-543.
55. Nakasu S., Fukami T., Nakajima M. Growth pattern changes of meningiomas: long-term analysis. Neurosurgery. 2005;56(5):946-954.
56. Nakasu S., Nakasu Y., Fukami T. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neuro-Oncol. 2010:1-8.
57. Hashiba T., Moto N.H., Izumoto S., et al. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas: clinical article. J Neurosurg. 2009;110(4):675-684.
58. Herscovici Z., Rappaport Z., Sulkes J. Natural history of conservatively treated meningiomas. Neurology. 2004;63(6):1133-1134.
59. Niiro M., Yatsushiro K., Nakamura K. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68(1):25-28.
60. Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62-71.
61. Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105(4):514-525.
62. Hancq S., Baleriaux D., Brotchi J. Surgical treatment of parasagittal meningiomas. Semin Neurosurg. 2003;14(3):203-210.
63. Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Neurosurgery. 1998;43(3):405-413.
64. Kondziolka D., Lunsford L.D., Coffey R.J., Flickinger J.C. Stereotactic radiosurgery of meningiomas. J Neurosurg. 1991;74(4):552-559.
65. Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53(4):815-822.
66. Caroli E., Orlando E.R., Mastronardi L., Ferrante L. Meningiomas infiltrating the superior sagittal sinus: surgical considerations of 328 cases. Neurosurg Rev. 2006;29(3):236-241.
67. Bozzao A., Finocchi V., Romano A., et al. Role of contrast-enhanced MR venography in the preoperative evaluation of parasagittal meningiomas. Eur Radiol. 2005;15(9):1790-1796.
68. Brotchi J., Patay Z., Baleriaux D. Surgery of the superior sagittal sinus and neighboring veins. In: Hakuba A., editor. Surgery of the Intracranial Venous System. Tokyo: Springer-Verlag; 1996:207-219.
69. Waga S., Handa H. Scalp veins as collateral pathway with parasagittal meningiomas occluding the superior sagittal sinus. Neuroradiology. 1976;11(4):199-204.
70. Kizana E., Lee R., Young N. A review of the radiological features of intracranial meningiomas. Australas Radiol. 1996;40(4):454-462.
71. Heick A., Mosdal C., Jorgensen K., Klinken L. Localized cranial hyperostosis of meningiomas: a result of neoplastic enzymatic activity? Acta Neurol Scand. 1993;87(3):243-247.
72. Goldsher D., Litt A.W., Pinto R.S. Dural “tail” associated with meningiomas on Gd-DTPA-enhanced MR images: characteristics, differential diagnostic value, and possible implications for treatment. Radiology. 1990;176(2):447-450.
73. Guermazi A., Lafitte F., Miaux Y. The dural tail sign—beyond meningioma. Clin Radiol. 2005;60(2):171-188.
74. Kawahara Y., Niiro M., Yokoyama S., Kuratsu J. Dural congestion accompanying meningioma invasion into vessels: the dural tail sign. Neuroradiology. 2001;43(6):462-465.
75. Raksin P.B. Imaging of meningiomas. Semin Neurosurg. 2003;14(3):193-201.
76. Hofmann B., Fahlbusch R.. Surgical management of convexity, parasagittal, and falx meningiomas, Schmidek H.H., Roberts D.W., editors. Operative Neurosurgical Techniques, 3rd ed., Vol. 1. Philadelphia: Saunders Elsevier, 2006;726.
77. Al-Rodhan N.R.F., Laws E.R.Jr. Meningioma: a historical study of the tumor and its surgical management. Neurosurgery. 1990;26(5):832-846.
78. Giuffre R. Successful radical removal of an intracranial meningioma in 1835 by Professor Pecchioli of Siena. J Neurosurg. 1984;60(1):47-51.
79. Voorhees J.R., Cohen-Gadol A.A., Laws E.R., Spencer D.D. Battling blood loss in neurosurgery: harvey Cushing’s embrace of electrosurgery. J Neurosurg. 2005;102(4):745-752.
80. Bulsara K.R., Sukhla S., Nimjee S.M. History of bipolar coagulation. Neurosurg Rev. 2006;29(2):93-96. discussion 96
81. Wen P.Y., Schiff D., Kesari S. Medical management of patients with brain tumors. J Neuro-Oncol. 2006;80(3):313-332.
82. Bratzler D.W., Houck P.M. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
83. Manelfe C., Guiraud B., David J. Embolisation par Catheterisme des Meningiomes Intracraniens. Rev Neurol (Paris). 1973;128(5):339-351.
84. Manelfe C., Lasjaunias P., Ruscalleda J. Preoperative embolization of intracranial meningiomas. Am J Neuroradiol. 1986;7(5):963-972.
85. Richter H.P., Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery. 1983;13(3):261-268.
86. Macpherson P. The value of pre-operative embolisation of meningioma estimated subjectively and objectively. Neuroradiology. 1991;33(4):334-337.
87. Carli D.F.M., Sluzewski M., Beute G.N., Van Rooij W.J. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. Am J Neuroradiol. 2010;31(1):152-154.
88. Bendszus M., Monoranu C.M., Schuütz A. Neurologic complications after particle embolization of intracranial meningiomas. Am J Neuroradiol. 2005;26(6):1413-1419.
89. Lilov M., Juszkat R., Petkov A., Todorov I. Preoperative endovascular embolization of intracranial meningiomas. Rentgenologiya Radiologiya. 2009;48(3-4):179-185.
90. Shevach I., Cohen M., Rappaport Z.H., Tew J.M.Jr. Patient positioning for the operative approach to midline intracerebral lesions: technical note. Neurosurgery. 1992;31(1):154-155.
91. Barnett G.H., Steiner C.P., Weisenberger J. Intracranial meningioma resection using frameless stereotaxy. J Image Guided Surg. 1995;1(1):46-52.
92. Porter J.M., Pidgeon C., Cunningham A.J. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82(1):117-128.
93. Bruder N.J. Awakening management after neurosurgery for intracranial tumours. Curr Opin Anaesthesiol. 2002;15(5):477-482.
94. Kuijlen J.M.A., Teernstra O.P.M., Kessels A.G.H. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta-analysis. Seizure. 1996;5(4):291-298.
95. Geerts W.H., Bergqvist D., Pineo G.F., et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines 8th ed. Chest. 133(6 Suppl. 6), 2008.
96. Mirimanoff R.O., Dosoretz D.E., Linggood R.M. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18-24.
97. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22-39.
98. Perry A., Giannini C., Raghavan R., et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994-1003.
99. Perry A., Stafford S.L., Scheithauer B.W. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455-1465.
100. Lamszus K. Meningioma pathology, genetics K., biology J. Neuropathol Exp Neurol. 2004;63(4):275-286.
101. Perry A., Louis D.N., Cheithauer B.W. Meningiomas. In: Louis D., Ohgaki H., Wiestler O.D., Cavenee W.K. WHO Classification of Tumours of the Central Nervous System. 3rd ed. Geneva: International Agency for Research on Cancer; 2007:164-172.
102. Nakamura M., Roser F., Michel J. Volumetric analysis of the growth rate of incompletely resected intracranial meningiomas. Zentralbl Neurochir. 2005;66(1):17-23.