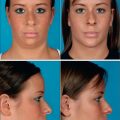
Chapter 22 Surgical Management of Migraine Headaches
Summary
Introduction
Migraine headaches (MH) are a significant cause of morbidity, affecting over 28 million Americans, with a lifetime prevalence estimated to be between 11% and 32% across several countries.1–6 MH are ranked as #19 among all diseases worldwide causing disability.7–9 The cost of medical treatment of MH in the USA has been estimated at 14 billion US dollars.10 There is an additional burden of 13 billion US dollars for loss of work. There are two subtypes of migraine: without aura, and with aura. Auras develop over 5-20 minutes, but last for less than 60 minutes, and are followed by a migraine. One out of three patients with MH experience aura.
MH are usually frontotemporal, typically unilateral, and are characterized by recurrent attacks of pulsating, intense pain associated with nausea and photophobia. The diagnostic criteria of migraines are shown in Table 22.1. Traditional, nonsurgical treatment of migraines can be nonpharmacologic or pharmacologic. Nonpharmacologic treatment of migraines consists of avoidance of triggers, usually caffeine, alcohol, or tobacco. Pharmacologic treatment can be further subdivided into acute analgesic, acute abortive, and prophylactic treatment. Acute analgesic treatment consists of pain control, acetaminophen, non-ste-roidal anti-inflammatory drugs (NSAIDS), analgesics, benzodiazepines, opioids, and barbituates. First-line acute abortive treatment is triptans, although IV antiemetics and ergotamine can be used as well. Prophy-lactic treatment consists of beta blockers, tricyclic antidepressants, and valproic acid.11
(Adapted from International Headache Society. The International Classification of Headache Disorders. 2nd edn. Cephalalgia 2004; 24(Suppl 1):9-160).
Though tension headaches can be confused with migraine without aura, it is possible to distinguish them from migraine, because tension headaches are not associated with nausea and are not affected by activity. Another group of headaches to be separated from MH are cluster headaches. Cluster headaches are marked by severe pain and affect the orbital, supraorbital, or temporal regions exclusively.12 They are 15-180 minutes in duration, and are associated with unilateral autonomic disturbances, such as conjunctival injection, lacrimation, nasal congestion, rhinorrhea, forehead and facial sweating, miosis, ptosis, and eyelid edema. During an attack, the headaches occur once every other day up to eight times a day. In contrast to MH, patients with cluster headaches are typically restless and agitated.
Nonpharmacologic treatment of cluster headache consists of avoidance of ethanol, histamine, nitroglycerine, or tobacco. Abortive treatment consists of 100% oxygen, triptans, cafergot, and dihydroergotamine. Prophylactic treatment consists of verapamil, lithium, methysergide, ergotamine tartrate, and prednisone taper.13
Pathophysiology
Pathophysiology of MH
Though migraine pathophysiology has not been completely elucidated, there are several plausible and experimentally substantiated theories. Importantly, these explanations incorporate both central and peripheral nervous-system activity, and these factors interact and lead to MH. There are four mechanisms in the pathogenesis of MH.15 First, patients with MH have some experimental evidence of interictal cortical derangements, specifically hyperexcitability of cortical neurons. Second, periaqueductal gray matter (PAG), which is an antinociceptive modulator, becomes progressively dysfunctional in MH. Burstein14 provided evidence that the sensitization of nociceptors causes an increase in neuronal discharges, which causes an increase in sensitivity to all stimuli. In other words, the central nervous system is ramped up, compared to normal, in response to both normal and painful stimuli. Third, auras are caused by cortical spreading depression, and the cortical spreading depression itself might cause irritation of the trigeminal nerve nucleus caudus.15 Finally, trigeminal nerve irritation causes release of substance P, calcitonin gene-related peptide, and neurokinin A in the cell bodies of the trigeminal nerve.15–17 The substances then travel along the nerve and produce a localized meningitis, and dilation of large vessels and dura mater innervated by the trigeminal nerve.1,2,15,18,19 Although this dilation is thought to cause MH, in our view it is the consequence of inflammation.
What causes the initial trigeminal nerve irritation is not exactly known. The anatomic relationship between trigeminal nerve branches and head and neck musculature provides the basis of the trigger site hypothesis of trigeminal nerve irritation in our opinion. Triggers sites are where nerves are irritated either by traversing the muscle or due to contact with it. This concept is borne out by anatomic studies. The trunk of the supratrochlear nerve, and branches of the supraorbital, which are both branches of the ophthalmic division of the trigeminal nerve, pierce the corrugator and depressor supercilii. The fact that many patients with MH have evidence of corrugator hypertrophy provides support for this hypothesis, which is further reinforced by the beneficial effects attained from injection with botulinum toxin A. Branches of the ZMTBTN pierce the temporalis muscle. The greater occipital nerve pierces the semispinalis capitus muscle.20,21 In some ways, this is analogous to piriformis syndrome, in which contraction of the piriformis muscle results in sciatic nerve irritation.22,23
Like MH, the pathophysiology of cluster headaches is not well understood. What is known is that pain in cluster headaches is mediated by the first division of the trigeminal nerve, while autonomic activation is mediated by cranial parasympathetic outflow from the facial nerve. There is no brainstem activation in cluster headaches, which distinguishes it pathophysiologically from MH.24
Surgical Treatment
Rationale for surgical treatment
In 1931, Walter Dandy removed the inferior cervical and first thoracic sympathetic ganglions in two patients, hypothesizing that migraine pain had a clinical profile consistent with nerve involvement.25 In 1946, Gardner reported on the resection of the greater superficial petrosal nerve in 26 patients. Though he reported some success in migraine patients, he noted complications including nasal dryness, decreased tear production, and corneal ulcerations.26 Slightly more than two decades later, Murillo described temporal neurovascular bundle resection in 34 patients for treatment of migraine headaches.27 Shortly after, Murphy reported greater occipital neurectomy for occipital migraine.28 Murillo and Murphy both had shortcomings in the length of follow-up of their studies, and there was a sequela of numbness resulting from these procedures. These radical surgeries with morbid sequelae led to unacceptable surgical outcomes, but the idea that surgery has potential in the treatment of headaches has persisted.
The understanding that the glabellar muscle group (GMG, including the corrugator, depressor supercilii, and procerus) itself may contribute to the pathophysiology, means that resection of these muscles makes intuitive sense. Not only do these patients benefit functionally from the surgery, they also observe some aesthetic improvement. Furthermore, the involvement of the ZMTBTN, which is sometimes resected in facial rejuvenation procedures,2 makes transection of that nerve justifiable for the treatment of headaches, since temporalis muscle resection may result in a depression and may also be of some, although minor, functional consequence.
Preoperative History and Considerations
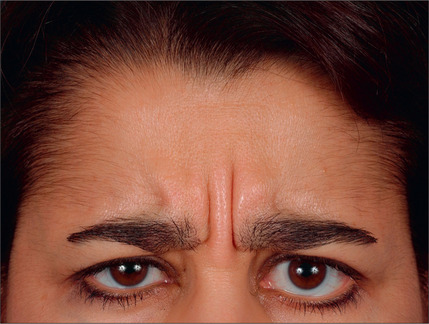
With the type of headache in mind, the ideal candidate for surgery must be identified. Studies have traditionally evaluated patients by the following criteria: proper diagnosis of headache (by headache questionnaire and evaluation by a physician trained in evaluating the headache patient), corrugator supercilii hypertrophy (Fig. 22.1), absence of medical or neurological conditions likely to cause headache, and the absence of unacceptable surgical risk. Pregnant and nursing women are also typically excluded from surgical consideration.
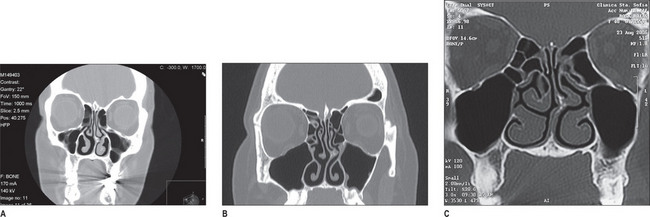
The constellation of symptoms leads the examiner to suspect the potential trigger sites,1–4 which is further validated by a nasal exam. The nasal exam includes direct and indirect endoscopic examination – which must be performed to detect septal deviation, turbinate hypertrophy, and concha bullosa – and findings are confirmed using a CT scan (Fig. 22.2).
Operative Approach for Migraine Headaches
Trigger sites
Currently, there are four common trigger sites: frontal triggers, where glabellar muscles irritate the supratrochlear and supraorbital nerves and cause frontal headaches; temporal triggers, where contraction of the temporalis muscle causes inflammation of the ZTBTN and causes temporal headaches; occipital triggers, where the semispinatus capitus stimulates the occipital nerve and causes occipital headaches; and septonasal triggers, where intranasal structures compress the trigeminal end branches and cause paranasal and retrobulbar headaches.20,21,29 There are several less common trigger sites, most of which are at intersections of nerves and arteries, such as the superficial temporal artery and the auriculotemporal nerve, or the greater occipital nerve and occipital arteries. The constellation of the symptoms that aid in detection of the trigger sites have been outlined in Table 22.2.
Table 22.2 The constellation of symptoms that aid in the diagnosis of migraine headache trigger sites
| Frontal headache | Occipital headache |
| Frontal pain | Upper neck/occipital pain |
| Late afternoon | Stress |
| Stress | Heavy-exercise related |
| Strong frowning muscles | Muscle tightness |
| Eyebrow/eyelid ptosis | Trigger point tenderness |
| Tenderness | Response to Botox |
| Temporal headache | Rhinogenic headaches |
| Temporal pain | Pain behind the eyes |
| Morning | Early am |
| Stress | Weather-related |
| Clenching/grinding | Allergy-related |
| Trigger point tenderness | Hormone-related |
| TMJ pain | Rhinorrhea |
| Cyclic |
The role of botulinum toxin A
Botulinum toxin A (Botox, Allergan, Irvine, California) blocks the release of acetylcholine at the neuromuscular junction; a deadly neurotoxin produced by Clostridium botulinum, its clinical utility for in MH has only been recently discovered.30,31. Clostridium botulinum actually produces seven subtypes of the neurotoxin-labeled A, B, C, D, E, F, and G, each of which has its own cellular substrate and target cleavage site.
It was first introduced over three decades ago as a treatment for strabismus,32–35 and since has been used in many neuromuscular conditions, including oromandibular dystonia, laryngeal dystonia, cervical dystonia, writer’s cramp, and hemifacial spasm.35
At the time of this writing, the use of botulinum toxin A for clinical treatment of migraines is investigational and still controversial.36–38 We use botulinum toxin A to confirm suspected trigger sites.
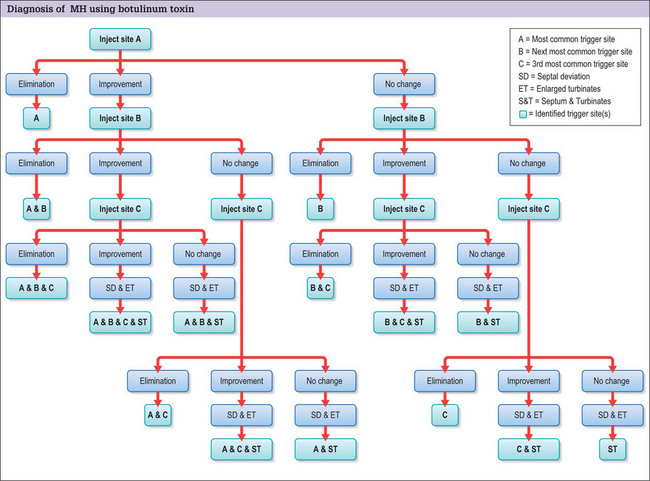
The trigger points are injected systematically, starting with the most common and severe trigger site based on the patient’s reported symptoms and physical examination. This is most often the corrugator supercilii muscle. First, 12.5 U of botulinum toxin A are injected with a long 30-gauge needle into the glabellar muscle sites. Trigger sites are injected 1 month apart, up to a maximum of 3. Patients then keep a headache diary, and refrain from taking prophylactic migraine medication. Those patients with trigger sites that respond, either completely or partially (defined as a 50% reduction of headache intensity or frequency from baseline for 4 weeks), to botulinum toxin A are considered for surgical intervention2 (Fig. 22.3). Nerve blocks could be used similarly.
The primary complication of botulinum toxin A injected into the temporal area is atrophy of the temporal muscles.39 This disuse atrophy is temporary, and patients should be counseled appropriately. Eyelid ptosis is the second most common complication. Some patients have developed antibodies to botulinum toxin A, rendering it relatively ineffective. This has been estimated to occur in over 7% of treated patients.35,40 The use of non-A botulinum toxins for patients resistant to type A is currently being investigated.35 It should be noted that while the lethal dose is estimated to be very high in humans (the LD50 parenteral dose is 3000 U), the maximum recommended dose is 300-400 U per 3-month period, which minimizes the risk of resistance.35
Frontal triggers
Frontal triggers include any of the glabellar muscles: corrugator supercilii, depressor supercilii, and procerus. The goal of surgery is to prevent further muscular irritation of the supraorbital and supratrochlear nerves, and complete resection is necessary for a favorable outcome.41 The approach can be either transpalpebral42,43 or endoscopic. The endoscopic approach can be valuable because a corrugator resection, temporal release, and ZMTBTN resection can all be performed through this approach.
Transpalpebral approach
Potential pitfall: Irregular resection of the corrugator supercilii muscle can cause contour deformities and recurrence of MH; fat grafting the corrugator site minimizes this risk.
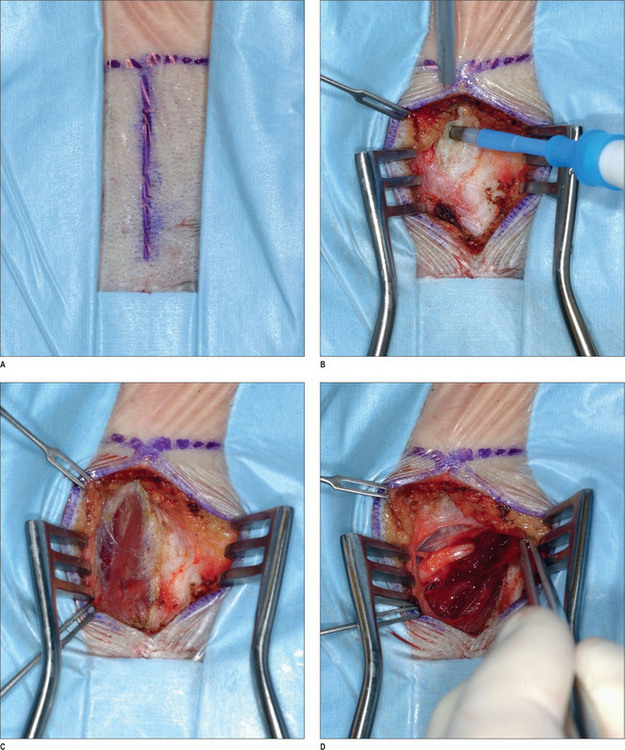
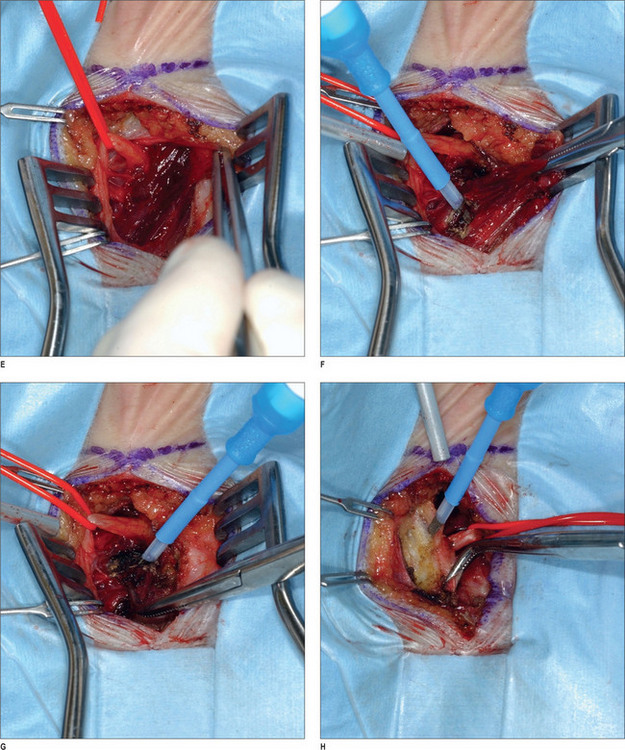
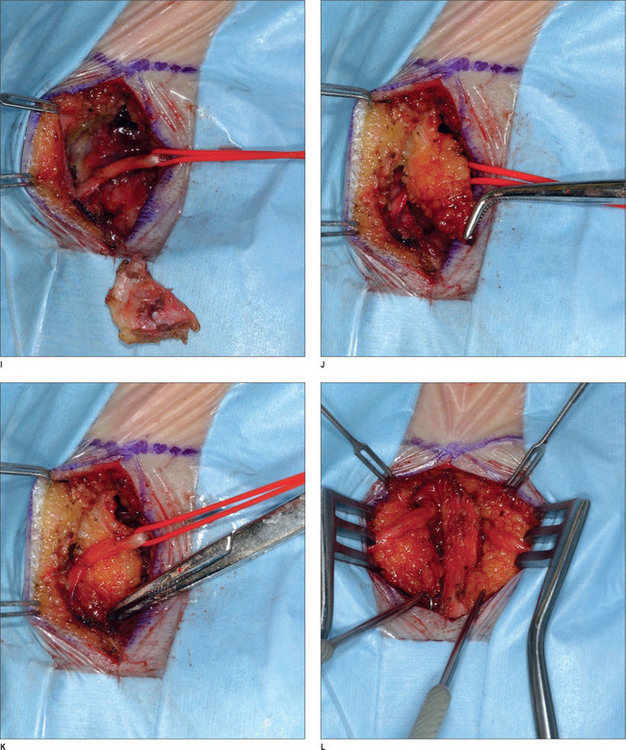
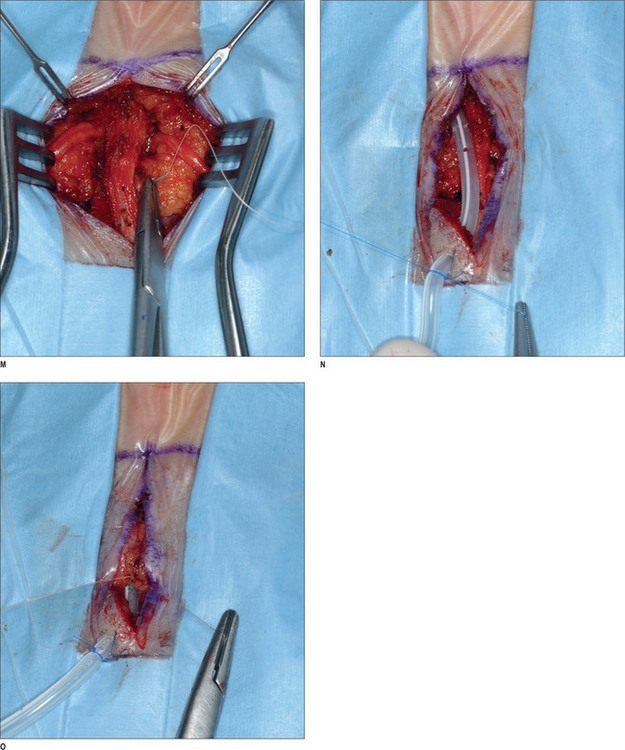
Under intravenous sedation, the patient is prepped and draped. The upper tarsal crease is marked on each eyelid, with an incision length of approximately 1 inch. Local anesthesia (0.5% lidocaine with 1 : 100 000 epinephrine) is infiltrated into the eyelid. A skin incision is made with a 10 c blade, and is extended through the orbicularis muscle. The plane between the orbicularis muscle and the septum is identified, and dissection continues cephalad, in that plane, with baby Metzenbaum scissors. The depressor supercilii muscle comes into view first. The muscle is lighter in color than the corrugator supercilii and it is less friable. The muscle is then removed as thoroughly as possible. The corrugator supercilii muscle is identified by its position over the supraorbital rim, as well as its darker color compared with the orbicularis oculi and depressor supercilii muscles. The corrugator muscle is undermined. A small communicating vein is often seen between the suprorbital and supratrochlear vessels. The corrugator supercilii muscle is removed in a lateral and medial fashion, using electrocautery, while the supraorbital and supratrochlear nerves are preserved. Then, attention is turned to the medial aspect of the upper eyelid. Fat is harvested from the medial compartment and grafted to the site of resected muscle. The fat graft serves three purposes: it minimizes contour deformity resulting from muscle resection, it protects the exposed nerve branches, and it also minimizes the current function. The graft is sutured with 6-0 polygalactin (Vicryl), and the skin incision is sutured with 6-0 plain catgut.1,43
Endoscopic approach
After proper preparation of the face under sedation, the incision sites are marked. There are five total incisions: one midline incision and two on either temple, all placed within the hair-bearing skin. Xylocaine containing 1 : 100 000 epinephrine is injected in the non-hair-bearing skin and xylocaine containing 1 : 200 000 epinephrine is injected in the hair-bearing skin. The dissection is performed in the subperiosteal plane to the supraorbital rim and lateral orbital rim, and zygomatic and malar arches. The procedure for the ZMTBTN is described below, and requires exposure of the zygomatic and malar arches. For corrugator resection, attention is concentrated in the glabellar area. The supraorbital nerves and corrugator muscle are exposed, and the periosteum is then released laterally leaving the central portion intact over the mid-glabellar area to prevent too much resection in the medial eyebrows. The corrugator muscle is removed, and fat harvested from the temporal region is grafted to the corrugator site. Fascial sutures of 3-0 polydioxanone are placed laterally for resuspension. A suction drain is placed in the incision and is fixed with 5-0 plain catgut. Incisions are repaired with 5-0 polygalactin (Vicryl) and 5-0 plain catgut1 (Fig. 22.4) (see Chapter 7, Forehead rejuvenation).
Complications
Alopecia can occur at port sites. Temporal or scalp paresthesias or anesthesia can result from this approach, as well as inadequate resection of muscle. Eyebrow elevation can be affected (resulting in either too much or too little elevation uni- or bi-laterally), and dimpling can occur on animation.44 The temporal branch of the facial nerve is vulnerable to injury during the dissection, which can result in frontalis paralysis. These complications are rare.
Temporal triggers
Temporal headaches are triggered primarily by contraction the temporal muscle around the ZMTBTN.
After appropriate preparation of the head and face, five 1.5 cm incisions are marked – one midline incision, and two on either temple, 7 cm and 10 cm from the midline. The forehead, temple, and malar regions are then injected with 1% lidocaine with 1 : 100 000 epinephrine; the scalp is injected with 0.5% lidocaine with 1 : 200 000 epinephrine. After the incisions are made with a 15 scalpel, they are deepened with spreading effects of baby Metzenbaum scissors to the deep temporal fascia. The dissection continues using a periosteal elevator until the endoscopic access device (EAD) (Applied Medical Technology, Cleveland, Ohio) is inserted to allow for the endoscope to be introduced. The periosteal elevator then raises periosteum posteriorly and cephalically. Once dissection is completed for the right side, the same is done on the left side. A subperiosteal dissection is then carried under endoscopic visualization to the supraorbital rim, lateral orbital rim, zygomatic, and malar arches. The dissection is continued immediately superficial to the deep temporal fascia and the ZMTBTN is exposed. Grasping forceps are used to hold it while it is avulsed. It is important that as much length of the nerve as possible is transected to prevent re-coaptation, and we usually remove approximately 3 cm. Any bleeding vessels are coagulated and the proximal nerve end is allowed to retract into temporalis muscle to reduce the risk of neuroma formation. The periosteum and arcus marginalis are released in the lateral orbital and supraorbital regions for transposing and fixing the soft tissues.1 The endoscopic devices are removed, and a single hook is placed on either side of the caudal portion of the incision. A 3-0 PDS suture is used to fix the superficial and intermediate temporal fascia to the deep temporal fascia laterally. A #10 TLS drain is placed, and the skin incisions are repaired with 5-0 poliglecaprone 25 (Monocryl) and 5-0 plain catgut interrupted stitches.
Occipital triggers
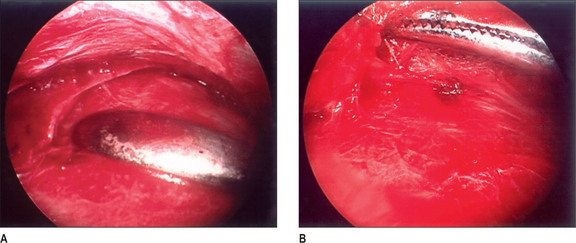
A 2 × 2-cm subcutaneously flap based caudally is elevated and passed under the nerve and is sutured to the midline raphe. A drain is placed, and the wound is closed with 5-0 polygalactin (Vicryl) and 5-0 plain catgut. This procedure is then repeated on the other side (Fig. 22.5).
Operative Approach for Cluster Headaches
Early surgery for cluster headaches consisted of partial or complete transection of the trigeminal nerve.45 This turned out to be a procedure with many complications, including dysesthesias, corneal numbness, and masseter weakness. Simply transecting the ZMTBTN is a safer alternative.
Conclusion
The decision-making and treatment algorithm is shown in Figure 22.2. A patient who presents for surgical treatment for headaches should have a thorough headache evaluation. Once the headaches are identified as either MH or cluster headaches, careful documentation of current and past medications is made. For MH, trigger sites are then identified by systematically injecting 12.5 U of botulinum toxin A in frontal, temporal, and occipital trigger sites. For frontal trigger sites, corrugator resection is performed. For temporal trigger sites, the zygomaticotemporal branch of the trigeminal nerve is resected. For occipital trigger sites, we resect the semispinalis capitus muscle medial to the greater occipital nerve. Septal and turbinate surgery is performed for patients who have evidence of septal deviation and enlarged turbinates, and whose clinical profile suggests septal and turbinate triggers.
For patients with cluster headaches, partial or complete transection of the trigeminal nerve can be performed.
1. Guyuron B., Tucker T., Davis J. Surgical treatment of migraine headaches. Plast Reconstr Surg. 2002;109(7):2183-2189.
2. Guyuron B., Kriegler J.S., Davis J., Amini S.B. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115(1):1-9.
3. Henry P., Auray J.P., Gaudin A.F., et al. Prevalence and clinical characteristics of migraine in France. Neurology. 2002;59(2):232-237.
4. Patel N.V., Bigal M.E., Kolodner K.B., Leotta C., Lafata J.E., Lipton R.B. Prevalence and impact of migraine and probable migraine in a health plan. Neurology. 2004;63(8):1432-1438.
5. Adeney K.L., Flores J.L., Perez J.C., Sanchez S.E., Williams M.A. Prevalence and correlates of migraine among women attending a prenatal care clinic in Lima, Peru. Cephalalgia. 2006;26(9):1089-1096.
6. Mattsson P., Svardsudd K., Lundberg P.O., Westerberg C.E. The prevalence of migraine in women aged 40-74 years: a population-based study. Cephalalgia. 2000;20(10):893-899.
7. Leonardi M., Mathers C. Summary of methods and data sources, global burden of disease. Geneva: World Health Organization, 2000.
8. Leonardi M., Steiner T.J., Scher A.T., Lipton R.B. The global burden of migraine: measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF). J Headache Pain. 2005;6(6):429-440.
9. WHO. Reducing risks, promoting healthy life. Geneva: World Health Organization, 2002.
10. Hu X.H., Markson L.E., Lipton R.B., Stewart W.F., Berger M.L. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999;159(8):813-818.
11. Lim C. Headache, migraine. In Ferri F., editor: Ferri’s clinical advisor 2007: instant diagnosis and treatment, 9th edn, Philadelphia: Mosby Elsevier, 2007.
12. International Headache Society. The International Classification of Headache Disorders. Cephalalgia. 2004;24(Suppl 1):9-160. 2nd edn
13. Lim C. Headache, cluster. In Ferri F., editor: Ferri’s clinical advisor 2007: instant diagnosis and treatment, 9th ed, Philadelphia: Mosby Elsevier, 2007.
14. Burstein R., Cutrer M.F., Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703-1709.
15. Welch K.M. Contemporary concepts of migraine pathogenesis. Neurology. 2003;61(8 Suppl 4):S2-S8.
16. Goetz C. Textbook of clinical neurology, 3rd edn. Philadelphia: Saunders, 2007.
17. Moskowitz M.A. The neurobiology of vascular head pain. Ann Neurol. 1984;16(2):157-168.
18. Novak V.J., Makek M. Pathogenesis and surgical treatment of migraine and neurovascular headaches with rhinogenic trigger. Head Neck. 1992;14(6):467-472.
19. Feindel W., Penfield W., Mc N.F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555-563.
20. Mosser S.W., Guyuron B., Janis J.E., Rohrich R.J. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113(2):693-697. discussion 698-700
21. Dash K.S., Janis J.E., Guyuron B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115(6):1752-1758. discussion 1759-1760
22. Benzon H.T., Katz J.A., Benzon H.A., Iqbal M.S. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98(6):1442-1448.
23. Kuncewicz E., Gajewska E., Sobieska M., Samborski W. Piriformis muscle syndrome. Ann Acad Med Stetin. 2006;52(3):99-101. discussion 101
24. Leone M., Proietti Cecchini A., Mea E., et al. Functional neuroimaging and headache pathophysiology: new findings and new prospects. Neurol Sci. 2007;28(Suppl 2):S108-S113.
25. Dandy W.E. Treatment of hemicrania (migraine) by removal of the inferior cervical and first thoracic sympathetic ganglion. Johns Hopkins University Bulletin. 1931;48:357-361.
26. Gardner W.J., Stowell A., Dutlinger R. Resection of the greater superficial petrosal nerve in the treatment of unilateral headache. J Neurol. 1947;4:105-114.
27. Murillo C.A. Resection of the temporal neurovascular bundle for control of migraine headache. Headache. 1968;8(3):112-117.
28. Murphy J.P. Occipital neurectomy in the treatment of headache. Results in 30 cases. Md State Med J. 1969;18(6):62-66.
29. Guyuron B., Tucker T., Kriegler J. Botulinum toxin A and migraine surgery. Plast Reconstr Surg. 2003;112(5 Suppl):171S-173S. discussion 174S-176S
30. Evers S. Status on the use of botulinum toxin for headache disorders. Curr Opin Neurol. 2006;19(3):310-315.
31. Argoff C.E. The use of botulinum toxins for chronic pain and headaches. Curr Treat Options Neurol. 2003;5(6):483-492.
32. Scott A.B., Rosenbaum A., Collins C.C. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12(12):924-927.
33. Scott A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J Pediatr Ophthalmol Strabismus. 1980;17(1):21-25.
34. Scott A.B. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981;79:734-770.
35. Brin M.F. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997;6:S146-S168.
36. Smuts J.A., Schultz D., Barnard A. Mechanism of action of botulinum toxin type A in migraine prevention: a pilot study. Headache. 2004;44(8):801-805.
37. Welch K.M. Botulinum toxin type A for the treatment of headache: con. Headache. 2004;44(8):831-833.
38. Blumenfeld A. Botulinum toxin type A for the treatment of headache: pro. Headache. 2004;44(8):825-830.
39. Guyuron B., Rose K., Kriegler J.S., Tucker T. Hourglass deformity after botulinum toxin type A injection. Headache. 2004;44(3):262-264.
40. Greene P., Fahn S., Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9(2):213-217.
41. Guyuron B., Varghai A., Michelow B.J., Thomas T., Davis J. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106(2):429-434. discussion 435-437
42. Guyuron B., Michelow B.J., Thomas T. Corrugator supercilii muscle resection through blepharoplasty incision. Plast Reconstr Surg. 1995;95(4):691-696.
43. Guyuron B., Knize D.M. Corrugator supercilii resection through blepharoplasty incision. Plast Reconstr Surg. 2001;107(2):604-607.
44. Guyuron B. Endoscopic forehead rejuvenation: limitations, flaws, and rewards [reply]. Plast Reconstr Surg. 2007;119:1116-1119.
45. Maxwell R.E. Surgical control of chronic migrainous neuralgia by trigeminal ganglio-rhizolysis. J Neurosurg. 1982;57(4):459-466.