CHAPTER 64 Surgical Management of Lumbar Spinal Stenosis
Indications for Surgery
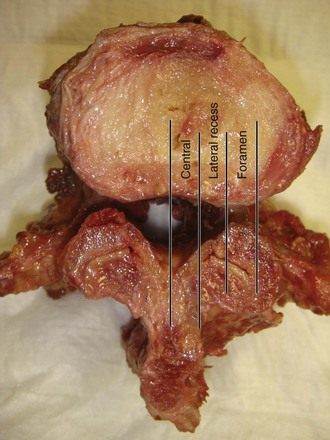
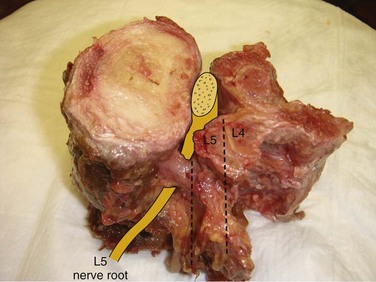
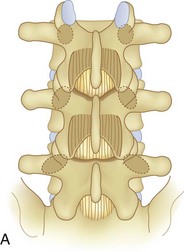
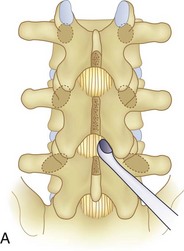
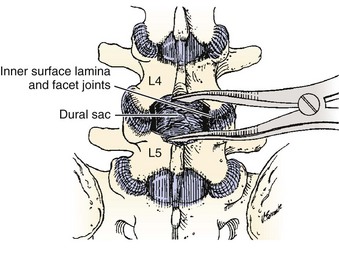
Spinal stenosis is the most common reason for lumbar spine surgery in adults older than the age of 65 years.1 Lumbar stenosis occurs secondary to spondylotic changes at the facet joints, instability, or a congenitally small canal. Pathologic changes affect the central vertebral canal, lateral recess, and neural foramina (Fig. 64–1). Stenosis at the lateral recess from spondylotic changes is typically from the superior articular process of the caudal vertebra compressing the traversing nerve root, leading to neurogenic claudication (i.e., stenosis at the L4-5 level leading to compression of the L5 nerve root by the superior articular process of L5) (Fig. 64–2). The natural course of spinal stenosis is that a substantial proportion of patients do not automatically deteriorate and will remain unchanged or even improved by nonoperative treatment.2 Ultimately, patient desire combined with failure of conservative treatment with physical therapy, activity modification, medication, and steroid injections drives the decision for operative treatment. Proper patient selection is critical to achieving a good outcome with spinal stenosis surgery. The ideal patient has symptoms of neurogenic claudication, which includes pain, numbness, and paresthesias in the posterolateral legs and thighs associated with prolonged walking or with activities causing back extension such as walking up stairs. Neurogenic claudication may also manifest as cramping and exhaustion of the lower extremities. Activities such as sitting, leaning forward on a walker or shopping cart, and riding a bicycle typically alleviate the pain. Lastly, lower extremity symptoms secondary to vascular claudication must be ruled out.
Deen and colleagues3 found that the most common cause of early failure after lumbar laminectomy was the absence of classical symptoms of neurogenic claudication coupled with the absence of stenosis on preoperative imaging studies. A predominance of low back pain over radicular pain is also associated with worse surgical outcomes.4,5 In addition, a selective nerve root block is a good prognostic indicator of surgical outcome because patients who obtain greater than 50% relief of leg pain for at least 1 week after an injection tend to have greater than 50% relief of leg pain after surgery.6 Patients who do not respond to a selective nerve root injection and have pain for more than 1 year will generally have a poor surgical outcome.6 Surgical decompression can be done on an elective basis unless the patient has a rapidly progressing neurologic deficit and bowel/bladder dysfunction, both of which are rare presentations of spinal stenosis. Before surgical intervention we routinely obtain standing anteroposterior (AP), lateral, and flexion/extension radiographs, as well as magnetic resonance imaging (MRI) or computed tomography (CT) myelogram. It is important to recognize that instability is a dynamic process and may not necessarily be apparent on static supine MRI. Instability must be carefully assessed on flexion/extension radiographs in all patients. Any degree of instability is assessed, and all stenotic levels are identified. Although several surgical techniques have been described to treat lumbar stenosis, there is no clear evidence of the most effective technique.7 Surgical options include decompressive laminectomy with or without fusion, laminotomy, minimally invasive decompression, or placement of an interspinous device.
Laminectomy
In the absence of instability, laminectomy remains the gold standard for treating central, lateral recess, and foraminal stenosis. After induction of anesthesia, the patient is placed in the kneeling position on an Andrews frame (Orthopedic Systems, Union City, Calif.). Use of the Andrews frame has been shown to decrease vena caval and central venous pressures, leading to a reduction in blood loss when compared with a Cloward surgical saddle.8,9 In addition, flexion of the hips reduces normal lumbar lordosis and widens the interlaminar space, making it easier to access the spinal canal. Use of a Wilson frame is also able to reduce lordosis from the lumbar spine during the decompression, although not to the same degree as the Andrews frame. It is important to note that placing a patient in this position can underestimate the true degree of stenosis when compared with the lordotic position obtained with a Jackson table. If a fusion is planned in addition to a laminectomy, we use a Jackson table in order to assist fusion in a lordotic position. If a Wilson frame is used in the fully cranked position for the laminectomy, it is important to uncrank the frame before the final fusion so as to prevent fusing in a nonlordotic position.
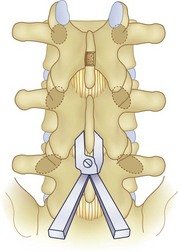
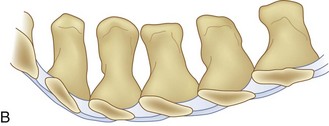
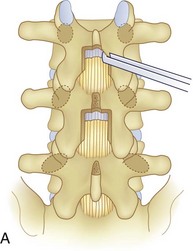
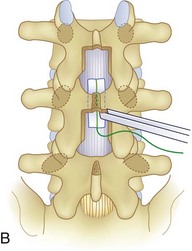
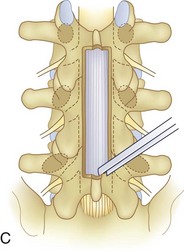
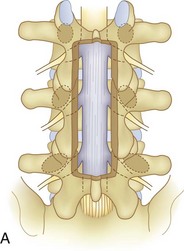
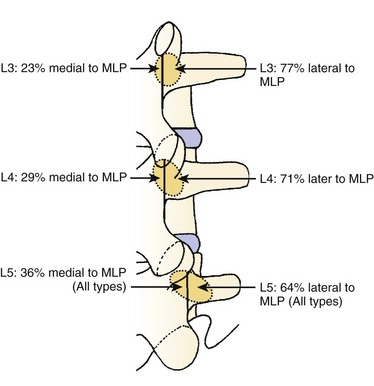
We believe that flexion of the knees with the use of pillows under the anterior calf is also important because it has the potential to reduce tension on the sciatic nerve. We routinely use 2.5× or 3.5× loupe magnification and an operating headlight. The relationship of the iliac crests to the lower lumbar levels on preoperative radiographs should be carefully examined to help guide the incision. A standard midline skin incision is made over the desired levels. The incision should be long enough to allow for exposure of the pedicles of the cephalad and caudal levels to be decompressed. For example, for an L3 to L5 pedicle-to-pedicle decompression, there should be enough exposure so that the inferior aspect of the L3 pedicle and the superior aspect of the L5 pedicle are easily palpated at the end of the decompression. Dissection is carried down to the thoracolumbar fascia with electrocautery. The fascia should be clearly exposed approximately 1 cm off of midline with a Cobb elevator to ensure a distinct layer for fascial closure at the conclusion of the case. The spinous processes are easily palpated and can be used to fine-tune the fascial incision for exposure of the appropriate levels. Electrocautery is then used to dissect just lateral to the spinous process, taking care to preserve the supra and interspinous ligaments. At this point a Kocher clamp is placed on the cephalad aspect of one of the spinous processes so that the clamp is in line with the pedicle of interest. Alternatively, a Woodson elevator can be placed in the intralaminar space to mark the appropriate surgical level. An intraoperative radiograph is then taken. It includes the Kocher clamp or Woodson elevator and the sacrum in order to confirm the appropriate level. This step is critical because 71% of wrong-level spine surgery occurs in the lumbar spine.10 Once the correct levels are identified, subperiosteal dissection of the paraspinal muscles is carried laterally with a combination of a Cobb elevator and electrocautery exposing the spinous process and lamina. During the dissection, the midlateral pars must be clearly identified so that it can be later used as a guideline for bony resection (Fig. 64–3). One should remember that the facet joints are superficial to the level of the lamina and midlateral pars and that as the dissection is carried out laterally, care should be taken to preserve the capsule of the facet joint. It has been demonstrated in cadaveric models that excision of the capsule and cartilage of the facets results in increased motion in both sagittal and axial planes, potentially leading to clinical instability.11 For an isolated laminectomy, dissection should be carried out just to the lateral aspect of the facet capsule. Hemostasis with bipolar electrocautery is critical for visualization, particularly around the facet joint secondary to bleeding from the parafacetal arteries medial and lateral to the facets.12 If a posterolateral fusion is planned, the dissection needs to extend laterally to the transverse process taking care to maintain the integrity of the intertransverse membrane between each level. The cephalad and caudal limits of the laminectomy are delineated with a Leksell rongeur. This includes the inferior half of the spinous process at the top of the decompression and the superior half of the spinous process of the inferior level to be decompressed. This is typically adequate to be able to palpate the pedicles of the cephalad and caudal levels to be decompressed. A Horsley bone cutter is used to remove the remaining spinous processes up until the spinous process/lamina junction (Fig. 64–4). The rongeur is once again used to thin the lamina. Any bleeding from cancellous bone should be controlled with bone wax. The decompression should begin centrally because the central zone is the last region to become stenotic. Because of the relatively shorter length, the oblique orientation, and larger attachment areas of the ligamentum flavum, the laminas of the lower spine have a much greater proportion of their anterior surface covered by the deep layer of the ligamentum flavum (Fig. 64–5).13 A curette is used to dissect the underlying ligamentum flavum from the inferior aspect of the lamina (Fig. 64–6). A Kerrison punch is then used to remove bone from the inferior aspect of the lamina using the ligamentum flavum as a protective layer. The decompression is carried cephalad until the ligamentum flavum ends and epidural fat or dura is encountered. In our experience this is approximately at the inferior aspect of the pedicle of the lamina being removed. In an L3 to L5 decompression, we recommend beginning at the inferior aspect of L4 and, once the dura is identified, moving on to removing the inferior aspect of L3 (Fig. 64–7A). This is followed by placement of a cottonoid between the dura and remaining laminar bridge of L4 and removing the residual L4 lamina and superior laminar edge of L5 (Fig. 64–7B). Adhesions between the dura and the flavum or lamina can be released with the use of a Woodson elevator or a Penfield No. 3 dissector. A central trough is thus safely created with complete removal of the lamina and flavum (Fig. 64–7C). The next step involves decompression of the lateral recesses. It is critical to adequately decompress the lateral recesses because it is well known that the most common technical error for early failure after lumbar laminectomy is inadequate neural decompression.3 A high-speed bur may be used to thin the lamina down and delineate the bony resection with extreme caution, particularly in the cephalad aspect of the lamina, where the cortical bone is thin and not protected by the ligamentum flavum. Lateral recess decompression should be performed with a Kerrison punch with care taken to preserve at least 50% of the facet joint (Fig. 64–8). Care must also be taken to preserve approximately 1 cm of the pars interarticularis in order to prevent an iatrogenic pars fracture. The location of the medial aspect of the pedicle to the midlateral pars (MLP) varies with each lumbar level. Su and colleagues14 recently described the percentage of pedicle medial and lateral to the MLP and found that with more cephalad levels, there is less pars when the decompression is carried out to the medial aspect of the pedicle (Fig. 64–9). Particular care should be taken at levels cephalad to L4 so as to not take too much of the lamina, leaving a narrow pars susceptible to a pars fracture. As such, with a decompression from L2 to S1, the laminectomy should appear to be trapezoidal with a narrower laminectomy trough in the more cephalad levels and a wider trough in the more caudal levels. Depending on the surgeon’s experience, the medial aspect of the inferior facet can be removed using an osteotome, exposing the underlying superior facet to be removed with a Kerrison punch. Cadaveric studies have shown that medial facetectomy does not affect lumbar spinal stability and, conversely, total facetectomy, even created unilaterally, makes the lumbar spine unstable.15 In addition, postlaminectomy CT scans of patients with resection of more than one half of the bone immediately above the flare of the inferior articular process at the level of the laminectomy resulted in a facet fracture.16 Undercutting the superior facet using a Kerrison punch assists bone removal while preserving the facet joint and the pars interarticularis. Because of the angle of approach, this maneuver should be performed from the opposite side of the operating table to ensure complete visualization of the dura during the decompression. Decompression should progress laterally until the medial wall of the pedicle is easily palpated with a Woodson or angled dural elevator. In the cranial caudal dimension, the pedicles of the superior and inferior segments should be palpable. Decompression of the lateral recess often leads to bleeding from the epidural venous plexus. Hemostasis is obtained with the use of bipolar electrocautery and hemostatic agents such as an absorbable gelatin sponge (Gelfoam) and cross-linked gelatin granules and topical human thrombin (Floseal). The last stage of the decompression involves foraminotomies at each level. Each nerve root should be traced as it passes underneath the pedicle and into the foramen. Any bone or soft tissue is removed with a Kerrison punch placed dorsal and parallel to the root as it exits the foramen. In the presence of exit-zone stenosis, bony spurs from the dorsal and lateral aspect of the superior facet should be resected. As in decompression of the lateral recesses, this should also be done from the contralateral side to prevent iatrogenic durotomy of the nerve root sleeve. A Woodson elevator or Murphy probe is used to assess the decompression by passing it into the foramen dorsal to the nerve root; a 4-mm ball tip probe should pass without difficulty.17 The nerve root can also be assessed for mobility by gently retracting the dural sac and nerve root medially into the canal with a Penfield No. 4 dissector; if adequately decompressed, the nerve should demonstrate 1 cm of medial displacement.17 At the end of the decompression, any sharp spikes of bone should be smoothed with a curette or a Kerrison punch to prevent it from lacerating the dura. Lastly, each level should be examined for an extruded herniated disc fragment, which should then be removed in a routine manner. We do not recommend a discectomy for well-contained discs. Before closure, hemostasis is once again obtained and the wound is irrigated. We routinely place a No. 15 French fully fluted Blake drain in the deep wound. The fascia is closed with interrupted figure-of-eight No. 1 absorbable sutures. The subcutaneous tissue is closed with buried 2-0 absorbable sutures, and the skin is closed with a nonlocked running 3-0 nylon suture or 3-0 absorbable subcuticular suture.
Outcomes of Laminectomy
Turner attempted a meta-analysis in 1992 on the basis of 74 studies with only 3 studies being prospective and found that on average, 64% of patients treated surgically with a laminectomy for lumbar spinal stenosis were reported to have good to excellent outcomes. However, there was wide variation across studies in the percentage with good outcomes ranging from 26% to 100% with none of the studies being randomized trials.18 One of the studies examined the surgical outcome of 140 patients and found that at 4 years after surgical decompression, average leg pain improvement was 82% and average back pain improvement was 71%.19
Subsequent prospective observational cohort studies attempted to delineate the efficacy of laminectomy for stenosis.4,5,20,21 Katz and colleagues21 prospectively followed 194 patients who had a decompressive laminectomy and found that at 6-month follow-up, 78% of patients were satisfied with the outcome. Patients bothered predominantly by back pain preoperatively and those with greater medical comorbidity and functional disability were significantly less satisfied with the results of surgery.21 In a separate analysis of 7- to 10-year follow-up of the same cohort, 75% were satisfied with the results of surgery and 23% had undergone reoperation.20 Atlas and colleagues4 assessed the surgical versus nonsurgical outcome of 148 patients with lumbar stenosis. At 1 year, 55% of surgically versus 28% of nonsurgically treated patients reported definite improvement in their symptoms (P = 0.003); the maximal benefit of surgery was observed at 3 months postoperatively.4 Although few nonsurgically treated patients experienced a worsening of their condition, there was little improvement in functional status.4 Four-year follow-up of the same cohort of patients showed that 70% of the surgically treated and 52% of the nonsurgically treated patients reported that their symptoms were better (P = 0.05).5 The initial improvement seen in the surgically treated patients modestly decreased over the subsequent 4 years.5
A 2002 review by Benoist2 and a 2005 Cochrane review by Gibson and Waddell7 found that there is limited Level I evidence regarding the efficacy of spinal stenosis surgery when compared with nonoperative treatment with no scientific evidence to support surgical treatment. Both reviews identified one randomized study that examined a small cohort of patients for 10 years following randomization to operative or nonoperative treatment.22 After 4 years, excellent or fair results were found in half of the patients selected for conservative treatment and in 80% of the patients selected for surgery.22 Patients with an unsatisfactory result from conservative treatment were offered delayed surgery, which led to similar results to that of the initial group.22
Subsequently, there have been two randomized prospective studies that evaluated the efficacy of surgical versus nonoperative treatment for lumbar stenosis.23,24 Malmivaara and colleagues23 reported on a study from Finland that randomized 94 patients to operative or nonoperative treatment and were evaluated with functional outcome scores. Although patients in both groups improved over the 2-year follow-up period, those undergoing surgery reported greater improvement regarding leg pain, back pain, and overall disability. The relative benefit of initial surgical treatment diminished over time, but outcomes of surgery remained favorable at 2 years.23 The lack of well-designed randomized control trials that evaluated the efficacy of surgical treatment of conditions associated with low back and leg pain such as lumbar stenosis led to the design of the multicenter Spine Patient Outcomes Research Trial (SPORT) in the United States.25 In 2008 the 2-year outcomes of SPORT evaluating laminectomy versus conservative treatment for lumbar stenosis were reported.24 All patients who had a history of at least 3 months of lumbar stenosis without spondylolisthesis were surgical candidates. A total of 289 patients were enrolled in the surgical cohort, and 365 patients were enrolled in the observational cohort. At 2-year follow-up, 43% of those who were randomly assigned to receive nonsurgical care underwent surgery. Despite the high incidence of crossover, the intention-to-treat analysis showed a significant treatment effect favoring surgery on the SF-36 scale for bodily pain; however, there was no significant difference in scores on physical function or on the Oswestry Disability Index (ODI). The as-treated analysis showed a significant advantage for surgery compared with nonoperative treatment at 3 months for all primary outcomes including the SF-36 and ODI. These changes remained significant for all time points. At 2-year follow-up, on the basis of the as-treated analysis, 63% of patients treated surgically rated themselves as having major improvement with their condition versus 29% in the group treated nonoperatively.24 Evidence from SPORT is the best Level I data for the efficacy of surgical decompression for lumbar stenosis without instability. Longer follow-up of this cohort of patients will elucidate the long-term benefits of surgical decompression.
Arthrodesis After Laminectomy
Some authors have recommended that an arthrodesis be performed when stenosis is associated with instability or in the setting of a spondylotic spine associated with complaints of low back pain.26 Grob and colleagues27 prospectively evaluated the results of decompression with and without arthrodesis in 45 patients with stenosis without instability. Instability was defined as greater than 5 mm of motion in the saggital plane between segments or greater than 5 mm of lateral offset in the coronal plane.27 All patients had significant clinical improvement compared with preoperative values at an average of 28-month follow-up with no significant differences regardless of whether or not a fusion was performed.27 The results indicate that in the absence of instability, decompression with care taken to not destabilize the spine does not require an arthrodesis.27 However, other authors in prospective observational studies have reported that in patients with stenosis (the majority of which did not have instability or scoliosis), decompression and arthrodesis versus laminectomy alone led to greater relief of back pain at 2-year follow-up.28 The most likely explanation is that in patients with stenosis without instability, spondylotic changes contribute to a component of low back pain. We currently do not fuse patients who have stenosis secondary to spondylosis unless there is a significant component of low back pain associated with it.
Herkowitz and colleagues29 reported on the results of 50 patients who had spinal stenosis associated with degenerative spondylolisthesis to determine if noninstrumented arthrodesis provided better results than decompressive laminectomy alone. At a mean of 3-year follow-up, patients with an arthrodesis had significantly more relief of pain in the back and lower limbs.29 In regards to use of instrumentation for fusion, Fischgrund and colleagues30 conducted a prospective study in 76 patients with spondylolisthesis and stenosis randomized to decompression and fusion with and without instrumentation. Although use of instrumentation increased the percentage of patients with a successful arthrodesis (82% vs. 45%), clinical outcomes at 2-year follow-up were similar between the two groups with approximately 80% excellent or good relief of leg and back pain.30 However, longer-term follow-up (average 7 years, 8 months) of the cohort of patients who had an uninstrumented fusion in the study by Herkowitz and colleagues29 and Fischgrund and colleagues30 found that clinical outcome was excellent to good in 86% of patients with a solid arthrodesis versus 56% of patients with a pseudarthrosis (P = 0.01).31 Benefits of a successful arthrodesis over pseudarthrosis were demonstrated, contradicting previous reports with shorter-term follow-up that indicated no differences in clinical outcome between the two groups.31 Although use of instrumentation increases rates of fusion30 and success of uninstrumented arthrodesis leads to better clinical outcomes,31 caution must be taken when implying that use of instrumentation leads to better clinical outcomes.
It is our current recommendation that arthrodesis with pedicular fixation be performed in the setting of a decompressive laminectomy if there is associated instability at the involved motion segments (>5 mm), degenerative scoliosis (curve progression or >30 degrees), revision decompression at the same level, or resection of greater than 50% of the facet joints.12
Surgical costs for patients with stenosis in the SPORT study were estimated by Tosteson and colleagues32 using the 2004 Medicare payment rate. The reported cost per Quality Adjusted Life Years (QALY) gained with surgical treatment of spinal stenosis was $77,600, and the reported cost per QALY gained with surgical treatment of stenosis with degenerative spondylolisthesis was $115,600.32 The majority of degenerative spondylolisthesis cases were treated with fusion, which carries a higher upfront surgical cost.32 With longer-term follow-up, the value of the surgical treatment of degenerative spondylolisthesis may improve, assuming that the observed improvements in quality of measures are lasting and that the need for additional treatment and revision surgery is minimal.32
Laminotomy
In patients with primarily lateral recess stenosis, laminotomy is an alternative to laminectomy. Laminotomy involves performing the decompression through a microdiscectomy-like approach targeting the stenotic levels either unilaterally or bilaterally. Advocates argue that by leaving the midline structures intact, there is less chance of iatrogenic instability and back pain.33 Other surgeons think little additional stability is gained by performing multiple laminotomies compared with a facet-sparing laminectomy because the majority of stability is provided by the intervertebral disc and the facet joint–capsule complex.33 Thomas and colleagues34 evaluated postoperative radiographs and MRIs in patients who underwent laminectomy or laminotomy. They found that a laminotomy can adequately decompress lumbar canal stenosis and that laminectomy and laminotomy have the same degree of postoperative listhesis.34 Aryanpur and colleagues35 described the results of decompressive laminotomies and foraminotomies in 32 patients with focal lateral recess stenosis. At last follow-up, 90% of the patients treated reported an excellent outcome with no significant postoperative morbidity or mortality, concluding that in a subgroup of patients with lumbar stenosis, multilevel laminotomies may be an acceptable alternative to laminectomy.35
McCulloch described the results of a “less invasive” one- or two-level decompression and uninstrumented fusion in 21 patients with degenerative spondylolisthesis and stenosis.36 The decompression is performed through bilateral fascial incisions with preservation of the midline structures and with the aid of a microscope.36 This technique begins with excision of the lower half of the anterior portion of the cephalad lamina proximal to the origin of the ligamentum flavum. The insertion of the ligamentum flavum is then removed by excising the superior edge of the trailing (caudal) lamina. After removing the medial edge of the facet joint so that it is flush with the medial border of the pedicle, the stenosing ligamentum flavum is removed from the top down or from the bottom up (Fig. 64–10). Twenty of 21 patients had relief of their claudication and the overall fusion rate was 86%.36 Costa and colleagues recently reported on the clinical and radiographic results of unilateral laminotomy and bilateral microdecompression of 374 patients with stenosis and found that 88% had a clinical benefit at a mean follow-up for 30 months.37
Bilateral laminotomy, also described as the “port-hole technique,” was evaluated by Kleeman and colleagues38 in 54 patients with lumbar stenosis with and without degenerative spondylolisthesis. The patients were evaluated specifically to determine if bilateral laminotomy without fusion would alleviate the symptoms of neurogenic claudication without causing further spondylolisthesis. Eighty-eight percent of the patients had good or excellent results, and 87% with degenerative spondylolisthesis showed no change in the amount of slip.38 Fu recently reported on a prospective study of 152 patients who underwent either laminectomy or a window laminotomy, which preserved the midline structures.39 Patients with spondylolisthesis (>3 mm of translation on flexion extension radiographs or >10 degrees of angulation) were excluded. Functional outcomes were evaluated at approximately 40 months postoperatively. Eighty-nine percent of the patients in the laminotomy group had a good to excellent results, whereas 63% of the patients in the laminectomy group had a good result, indicating that a laminoforaminotomy can lead to good long-term results with few complications. It is our recommendation that in one- or two-level lateral recess stenosis without significant central stenosis, a less invasive unilateral or bilateral laminoforaminotomy is a reasonable option.
Fenestration
The use of “fenestration” to treat stenosis has been described in multiple reports but with notable variability in surgical technique relating to the nomenclature.35,40–42 The concept, similar to a laminoforaminotomy, is to preserve the midline structures and minimize soft tissue and bony resection while addressing sites of neurologic compression. Young and colleagues42 described decompression in 32 patients with a bilateral subarticular fenestration technique. This involved removing the medial third of each facet joint with a drill and then undercutting the remaining two thirds of the joint to allow a generous fenestration in the ligamentum flavum and adjacent laminae. Sixty-one percent of the patients obtained complete relief at final follow-up.42 Mackay and colleagues40 reported on the results of a unilateral fenestration technique that involved entering the canal through the ligamentum flavum and expanding the hole with Kerrison bone punches to open the lateral recess, preserving the facet joint and lamina as much as possible. Forty-two patients were treated only at the clinically symptomatic levels of stenosis with excellent or good outcomes at a mean follow-up time of 32 months in 60% of the patients.40 It should be noted that despite the nomenclature of “fenestration,” the techniques described by Young42 and Mackay40 are similar to the laminotomy and foraminotomy procedures for lateral recess stenosis previously discussed.34–36,38,39
Shenouda and Gill41 described a unique fenestration technique that involves decompression through a 5-mm drill hole in the pars interarticularis immediately below the superior facet. The hole exposes the inferior aspect of the pedicle and the root in the nerve root canal. Through an operating microscope and using a 2-mm diamond drill bit, the inferior aspect of the superior pedicle is drilled away and the dorsal aspect of nerve root is then decompressed by undercutting the lamina and hypertrophied facet (Fig. 64–11). The authors note that unlike other previously described techniques of “fenestration,” their method addresses foraminal in addition to lateral recess stenosis. Although there were no clinical or radiographic outcomes reported, the authors suggest that their technique preserves spinal stability and provides early mobility, subsequently shortening hospital stay.41
Laminoplasty
Distraction laminoplasty is a technique for lumbar decompression of the central and lateral recess stenosis that allows minimal bony resection. This technique involves mechanical distraction of the stenotic interspace to assist spinal canal access with minimal bony resection, and it allows for visualization of the spinal canal during decompression while minimizing removal of the posterior bony elements.43 The procedure begins as a standard laminectomy by removing the inferior one half of the spinous process and lamina of the cephalad vertebra and the superior edge of the caudad vertebra.43 Distraction is then applied across the spinous processes of the segments, effectively opening up the interlaminar working space by mobilizing the cephalad lamina proximally and the caudad lamina distally (Fig. 64–12). The motion occurs through the facet joints and disc, and the increase in interlaminar space (typically 1 cm) allows for increased visualization. After removal of the ligamentum flavum, the lateral recesses are then decompressed by removing 10% to 20% of the facet joint in a tapered fashion.43 The undersurface of the cephalad lamina is then thinned from inside out to 30% to 50% of its thickness.43 Caution is required in patients with significant osteoporosis in whom vigorous distraction may result in fracture of the spinous process or laminar edge.43 Although the technique has been well described, there is a lack of clinical outcomes data to show the efficacy of distraction laminoplasty when compared with a standard laminectomy. In the setting of an open decompression, we have found that use of a laminar spreader between the spinous processes tenses the dura, making neural decompression easier. In our experience, it also decreases the chance of dural violation.
Tsuji and colleagues44 developed the technique of expansive lumbar laminoplasty to alleviate the problems of conventional laminectomy in the treatment of spinal stenosis. The technique is analogous to laminoplasty in the cervical spine and involves opening one side of the lamina and using the contralateral side as a hinge. Bone grafts from excised spinous processes were placed in the opened laminae and were fixed with braided wire or nylon sutures.45 The initial reports of this procedure in a small group of manual laborers with an average follow-up of 3 years yielded satisfactory results.46 However, Kawaguchi45 reported the long-term outcomes (average follow-up of 5.5 years) in 54 patients undergoing lumbar laminoplasty with the specific purpose of investigating postoperative problems with the procedure. At final follow-up, the Japanese Orthopaedic Association score became worse for seven patients, six patients had lesions develop at the level adjacent to the laminoplasty, and five patients had spondylolisthesis develop. Interlaminar fusion was observed in 22 patients (41%). The authors concluded that the best indications for the lumbar laminoplasty procedure were young and active patients with isolated central spinal stenosis.45 Because of the inability of this operation to fully address lateral recess stenosis and the high interlaminar fusion rate, we do not currently use this procedure for lumbar stenosis.
Microendoscopic Decompressive Laminotomy
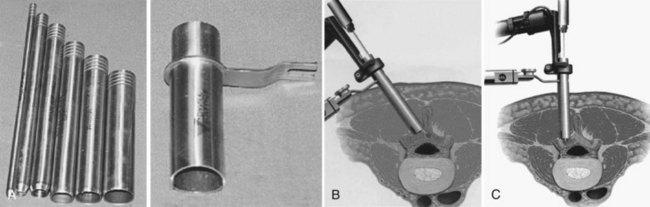
Many authors have advocated the concept of a minimally invasive decompression, which is based on decompression of the neural elements using a smaller skin incision with preservation of soft tissue and bony anatomy. Although procedures such as laminotomy, fenestration, and laminoplasty are all considered less invasive than a standard laminectomy, the term “minimally invasive” has been tied by surgeons, patients, and the industry to microendoscopic decompression. The technology surrounding endoscopic visualization has made tremendous strides in the past decade, and as such, successful results from performing a microendoscopic discectomy through a percutaneously placed tube have occurred.47 Since the visualization required for a discectomy is similar to that of a decompression for stenosis, microendoscopic decompressive laminotomy (MEDL) as an even less invasive approach than a microscopic laminotomy was developed. MEDL was investigated in cadaveric studies that used postdecompression CT scans to validate that equivalent bony decompressions were achieved either endoscopically or open.48 Asgarzadie and Khoo47 have recently reported on the rationale, indications, and surgical techniques for bilateral decompression through a unilateral MEDL approach using the METRx system (Medtronic, Sofamor-Danek, Memphis, Tenn.). After localization of the level with fluoroscopy, a series of dilators are passed over a Steinman pin centered over the spinolaminar junction. Typically, the incision is 2.5 cm for use of an 18-mm working channel (Fig. 64–13).47 The contralateral lamina is first decompressed by medial angulation of the retractor tube and drilling the anterior aspect of the lamina to the contralateral lateral recess and foramen with a high-speed bur.47 Specialized endoscopic Kerrison rongeurs are used to perform the laminotomy and decompression.47 After the contralateral side is decompressed, the ipsilateral side is then addressed.47 The authors presented 4-year outcome data of 48 patients who underwent MEDL. All patients had central and/or lateral recess stenosis. Eighty percent of patients had an increase in walking endurance, and 88% of patients reported an improvement in symptoms. Notably, the overall rate of dural violations was 4% as compared with a previous report from 5 years prior49 by the same authors of 16%.47 The authors attribute the reduction of dural violations to decompressing the contralateral side before decompression of the ipsilateral side. For procedures in which a cerebrospinal fluid leak was encountered, direct repair was difficult secondary to the small surgical working field. The authors use fibrin glue or fat and muscle grafts to tamponade the leak rather than repairing it directly. Should a large dural tear occur, it may require enlarging the incision or converting to a nonendoscopic technique so that direct repair of the dura can be performed. There is a learning curve for all new techniques, and it is essential to become familiar with the anatomy and endoscopic system by performing the procedure in cadaveric specimens before performing the surgery in a clinical setting. A recent randomized clinical trial comparing tubular versus conventional open microdiscectomy for lumbar disc herniations in 328 patients found that at 1 year, tubular discectomy resulted in less favorable results for patient self-reported leg pain and back pain.50 Although these data imply that similar outcomes may be found, caution needs to be taken when extrapolating these data to outcomes of tubular versus conventional laminoforaminotomy for lumbar degenerative stenosis.
Interspinous Devices
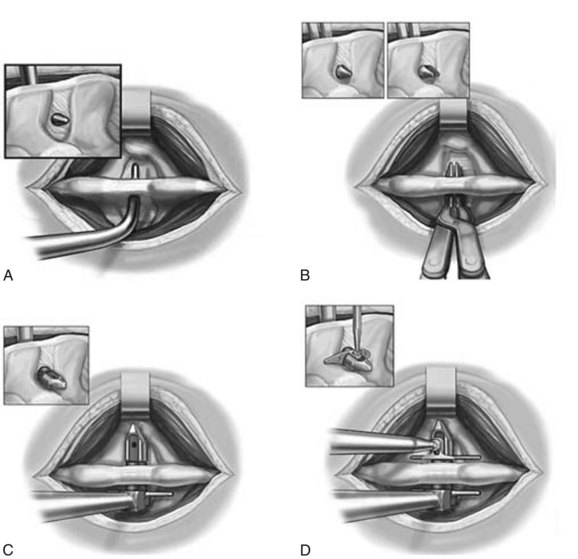
The use of an interspinous spacer to distract the interspinous space and block extension is an old concept that was abandoned secondary to device displacement necessitating removal.51,52 In the past 5 years, there has been a surge of interspinous process (ISP) devices introduced for indirect decompression of the spinal canal. Distraction of the interspinous processes leads to flexion and stretching of the infolding ligamentum flavum and indirect decompression of the neural foramina. The X-STOP device (St. Francis Medical Technologies, Alameda, Calif.) was the first interspinous device approved by the U.S. Food and Drug Administration (FDA). It is also the most well-studied interspinous implant. The implant is composed of an oblong central core that is stabilized by two lateral wings (Fig. 64–14). It is inserted as two components that are fixed to each other to straddle the interspinous region (Fig. 64–15). The device is composed of titanium so that it is compatible with postoperative MRI.
Richards quantified the spinal canal and neural foramina dimensions of cadaveric lumbar spines using MRI before and after placement of the X-STOP implant.53 In extension, the implant significantly increased the subarticular diameter by 50%, the canal diameter by 10%, and the foraminal area by 25%.53 Clinically, positional MRIs in patients before and 6 months after implantation of the X-STOP device demonstrated that, in extension, the left and right exit foramens increased by 34.2% and 25.4%, respectively. Similarly, there was an increase in dural sac area by 20% in standing and 16.3% in neutral.54
Three prospective studies with separate cohorts of patients treated with the X-STOP device have been conducted.55–57 It should be noted that the authors have a financial interest through the device manufacturer. Zucherman and colleagues reported on 1-55 and 2-year58 results of a prospective, randomized trial of 200 patients at nine U.S. centers randomized to the X-STOP or nonoperative treatment. Eligible patients had neurogenic claudication secondary to lumbar stenosis at one or two levels completely relieved during flexion. Patients also had to be able to sit for 50 minutes without pain, walk 50 feet or more, and have completed at least 6 months of nonoperative therapy. Radiographic exclusion criteria were a scoliotic Cobb angle greater than 25 degrees and spondylolisthesis greater than grade 1 at the affected level.55 At 1 year, 59% of the patients in the X-STOP group and 12% of the nonoperative patients were significantly improved.55 Two-year follow-up of the same cohort of patients demonstrated that benefits from the surgery at 1 year were maintained.58 A separate analysis of the same cohort of patients demonstrated an improvement in quality of life compared with nonoperative treatment.59
Kondrashov reported on the long-term (4-year) results of 18 patients (33% had a grade I spondylolisthesis) who received the X-STOP device as part of an FDA investigational device exemption (IDE) trial.60 It should be emphasized that the results of this study are not the 4-year outcome results of the same cohort of patients from the FDA randomized control trial.55,58 Using an arbitrary 15-point improvement from baseline in the ODI score as a success criterion, 14 out of 18 patients (78%) had successful outcomes.60 Caution should be used in interpreting these conclusions because the 200-patient randomized control trial used the severity and physical function domains of the Zurich Claudication Questionnaire (ZCQ) and not the ODI as a primary outcome measure.55,58 Longer-term follow-up of the larger randomized control trial will provide valuable information into whether or not results after X-STOP placement are sustained after 2 years.
Siddiqui and colleagues56 reported on another prospective study on the X-STOP device. One-year results in 40 patients who received the X-STOP device were reported. Of the patients who had completed all of the questionnaires at follow-up, 54% reported clinically significant improvement in their symptoms and 71% expressed satisfaction with the procedure.56 Notably, 29% of the patients required an epidural 12 months after surgery for recurrence of their symptoms of neurogenic claudication.56 These short-term results, although promising, are not as good as the results reported in the Zucherman FDA trial at 1-year follow-up.55
Although the cohort of patients for the prospective studies on the X-STOP device included some patients with less than a grade I spondylolisthesis,55 Anderson and colleagues57 reported on a prospective study of only patients with a grade I spondylolisthesis (average of 14% slippage) and stenosis. Seventy-five patients were randomized to either X-STOP placement or nonoperative treatment. Functional and radiographic outcomes at 2 years demonstrated significant improvement in ZCQ and SF-36 scores in the X-STOP patients, but not in the nonoperative control patients at all intervals. Overall clinical success occurred in 63% of X-STOP patients and only 13% of controls. Radiographic measurements of spondylolisthesis and kyphotic angulation were unchanged at follow-up.57 Despite these promising outcomes, Verhoof and colleagues57a recently reported on the results of 12 patients after X-STOP placement, all of whom had a spondylolisthesis. The study included patients with less than a 30% slip. Most of the patients had a grade I spondylolisthesis, although 25% of the patients had greater than a grade I slip; the average preoperative slip was 20%. Immediately postoperatively, 67% of patients had relief of symptoms. However, at mean follow-up of 30 months, 7 of 12 (58% of) patients had recurrence of pain, neurogenic claudication, and worsening of neurologic symptoms, of whom all but one had an original grade I slip. The X-STOP was removed, and a decompression and posterolateral fusion with instrumentation was performed.
The X-STOP implant technique relies on being able to dock onto the spinous processes; as such, patients with severe osteoporosis were excluded from clinical trials.55 Idler and colleagues61 recently reported on a technique for augmenting the spinous process with polymethylmethacrylate (PMMA) in osteoporotic patients in order to allow for X-STOP device placement without fracturing the spinous process. After injecting osteoporotic cadaveric spinous processes with PMMA, a two-level X-STOP was placed. The mean failure load of the PMMA treated group was significantly higher than the control group (2386 N versus 1250 N).61
Complications
Although spondylotic spinal stenosis is typically a condition of older patients with greater comorbidities, several studies have demonstrated that decompression and/or fusion in elderly patients is safe with an acceptable rate of morbidity when compared with younger patients.62–64 Benz and colleagues62 found that preoperative medical comorbidities did not predict early postoperative complications in patients older than 70 years of age who underwent a spinal decompression. The early mortality rate was 1.4%, and serious complications potentially affecting quality of life occurred in 12% of patients with the total complication rate being 40%.62 Ragab and colleagues63 examined an even older population, ages 70 to 101 years, and found that the overall morbidity rate was 20%. They concluded that advanced age did not increase the morbidity associated with this operation because the results reported in this study are comparable with those from other studies of a younger population.63
In a study comparing complications in patients ages 65 to 80 years old who underwent elective spinal decompression with a similar group of randomly selected patients who underwent total hip arthroplasty, both groups had a similar number of life-threatening complications (approximately 20%).64 However, it should be noted that there were twice as many minor complications in the decompression group.64 Most recently, Glassman and colleagues65 studied the 2-year outcome of patients 65 years and older compared with patients younger than 65 years old who underwent a single-level posterolateral arthrodesis with iliac crest bone graft for single-level degenerative disc disease. At 2-year follow-up, older patients had a similar improvement in leg pain and ODI scores. However, there were significantly more postoperative serious adverse events in the older patient group (38% vs. 17%) including significantly more cardiac events, respiratory events, and infections.65
The vascular complications of posterior lumbar surgery include deep venous thrombosis (DVT), pulmonary embolism, postoperative hematoma, and catastrophic vascular events. In a systematic review of the literature, Glotzbecker and colleagues66 found that DVT risk ranged from 0.3% to 31% depending on patient population and method of diagnosis. The overall rate of DVT was 2.2%, and the PE rate was 0.3%.66 In the setting of elective posterior lumbar decompression with or without fusion, the DVT risk either based on venography or clinical diagnosis in patients receiving no prophylaxis is approximately 5%.66 Some studies suggest that mechanical prophylaxis for thromboembolism may not be sufficiently protective for patients undergoing combined anterior/posterior spine surgery.67 However, there is currently insufficient evidence to support the use of pharmacologic prophylaxis or DVT screening in patients undergoing routine elective lumbar decompression.66 As such, in a recent survey of spine surgeons, there was wide variability in the surgeon’s estimation of DVT rates, as well as the choice and timing of thromboembolic prophylaxis.68 We currently feel that the low risk of DVT and PE combined with the risk of a postoperative epidural hematoma development does not support the use of pharmacologic DVT prophylaxis with lumbar decompression surgery. We routinely use sequential compression stockings and early ambulation in patients undergoing elective posterior spinal surgery. Although the probability of a major vascular injury is infrequent (<.02%), it is important to recognize it in the early postoperative period because the best prognosis for recovery of acute vascular interruption occurs with immediate treatment within 24 to 48 hours.69 Patients with preexisting vascular disease are predisposed to injury. Unexplained hypotension is highly suggestive of a vascular catastrophe, and is an indication for either laparotomy or arteriography.69
Dural tears are a well-known complication of lumbar stenosis surgery, with rates in the literature ranging from 4%70 to 16%.71 Revision surgery is twice as likely as primary surgery to result in this complication72 with use of the Kerrison punch being the most commonly used instrument when a dural tear was created.71 Primary watertight closure of the dural tear has been shown to result in good results without having long-term deleterious effects or to increase the risk of postoperative infection, neural damage, or arachnoiditis.73 In a large series of patients Khan and colleagues72 found that after primary closure, 1.8% of all patients needed a secondary procedure for a persistent spinal fluid leak. Persistent spinal fluid leaks and pseudomeningoceles after the primary procedure should be addressed promptly with a secondary procedure with direct repair or a fascia lata graft, which leads to good results.70,72 It is our protocol to perform a closure of all dural tears at the time of durotomy. Primary repair is performed with 6-0 Gortex suture followed by placement of a spinal sealant system (DuraSeal and/or Duragen). All repairs are tested with a Valsalva maneuver. Closed-suction wound drainage does not seem to aggravate the leak and can be used safely in the presence of a dural repair.73 Patients are placed on bed rest for 24 to 48 hours, given ceftriaxone for meningitis prophylaxis, and slowly allowed to ambulate. Meningitis is most likely to occur if the dura is broached because this structure provides an effective barrier to the passage of organisms.74
Deyo and colleagues,75 using a statewide hospital discharge registry in Washington, reported on the morbidity and mortality of a large series of patients who underwent surgery on the lumbar spine and reported a 0.4% postoperative infection rate. It should be noted that this was based on a discharge registry and not a patient cohort. Infection rates in other large series for lumbar spine surgery have ranged from 2%76 to 4.4%.77 Fang and colleagues77 found that the majority of infections occurred during the early postoperative period (<3 months). Age older than 60 years, smoking, diabetes, previous surgical infection, increased body mass index, and alcohol abuse were statistically significant preoperative risk factors.77 Others have reported that infection was more common in patients undergoing fusion with instrumentation.76 It is critical to diagnose an infection early in the postoperative period. Other than physical examination findings, use of C-reactive protein (CRP) has been shown to be useful. Mok and colleagues,78 in a study of 149 patients, 13% of whom had a postoperative infection, examined the trend of CRP and erythrocyte sedimentation rate (ESR). After a peak at 2.7 days postoperatively, CRP showed an exponential decrease with a half-life of 2.6 days in patients who did not have an infection.78 A second rise or failure to decrease as expected had a sensitivity, specificity, positive predictive value, and negative predictive value of 82%, 48%, 41%, and 86% for infectious complications, respectively, indicating that CRP is more applicable, predictable, and responsive in the early postoperative period compared with ESR.78 Once diagnosed, the infection should be aggressively managed with operative débridement and irrigation including the deep subfascial layer. Hardware and viable bone graft can be preserved. The choice of one versus multiple débridements can be made on the basis of the appearance of the wound, patient factors, and nutritional status.79 This approach has led to good clinical results76 with similar functional outcome scores at a mean follow-up of 62 months when compared with a cohort of patients in whom this complication did not occur.80 Meningitis is a rare complication of spinal surgery with an incidence of 0.18% of 2180 spinal operations with a good outcome with early diagnosis and prompt management.74 In that series, patients presented with fever, headache, photophobia, and neck stiffness within 6 to 14 days after surgery.74 Good outcomes can be expected with prompt diagnosis and treatment.74 We routinely give perioperative antibiotics for 24 hours after surgery. A recent study has indicated that up to 2 days of antibiotics does not elevate the infection rate after spinal surgery using instrumentation, and long-term administration of antibiotics prolongs the duration of hospital stay, inhibits normalization of body temperature, and elevates CRP levels.81
Despite adequate decompression, substantial back and leg symptoms develop in up to 10% to 15% of patients who have undergone an adequate lumbar decompression.82 Substantial osseous regrowth after decompression may be the reason symptoms recur and can decrease patient satisfaction.83,84 In a study by Postacchini and Cinotti,83 40 patients were treated for lumbar stenosis with an average follow-up time of 8.6 years after surgery. On the basis of AP radiographs, the amount of bone regrowth at the laminectomy site was assessed.83 Only 12% of patients showed no regrowth of the previously resected posterior vertebral arch, whereas 40% of the patients demonstrated more than 40% regrowth of the lamina.83 The clinical results were better in those who had mild regrowth when compared with those with marked regrowth (84% vs. 40% satisfactory results). This study showed that in certain patients, bone regrowth could lead to recurrent stenosis.83 In a similar analysis, Chen and colleagues84 found that at 4.5 years after laminectomy, 44% of patients had moderate or marked regrowth of the lamina with spinal instability accelerating bone regrowth. Patients with moderate and marked bone regrowth had poorer clinical outcomes than those with no significant and mild bone regrowth.84
In the setting of an arthrodesis, Harrop and colleagues,85 in a systematic review, reported that adjacent-level degeneration in 34% of patients with symptomatic disease was found in 14% of patients. Ghiselli and colleagues86 reported on 215 patients who underwent posterior lumbar arthrodesis with follow-up of 6.7 years. On the basis of radiographs, 27% of the patients had evidence of degeneration at the adjacent levels and elected to have an additional decompression or arthrodesis.86 However, there was no significant correlation between the preoperative arthritic grade and the need for additional surgery.86 On the basis of these data, the rate of symptomatic degeneration at an adjacent segment warranting either decompression or arthrodesis was predicted to be 17% at 5 years and 36% at 10 years.86
Pearls and Pitfalls
Key Points
1 Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine. 1990;15:1142-1147.
2 Boden SD, Martin C, Rudolph R, et al. Increase of motion between lumbar vertebrae after excision of the capsule and cartilage of the facets. A cadaver study. J Bone Joint Surg Am. 1994;76:1847-1853.
3 Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis: Four-year outcomes from the Maine Lumbar Spine Study. Spine. 2000;25:556-562.
4 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794-810.
5 Grob DT, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am. 1995;77:1036-1041.
6 Katz JN, Lipson SJ, Lew RA, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine. 1997;22:1123-1131.
7 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
8 Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
9 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
10 Glassman SD, Polly DW, Bono CM, et al. Outcome of lumbar arthrodesis in patients sixty-five years of age or older. J Bone Joint Surg Am. 2009;91:783-790.
11 Deen HGJr, Zimmerman RS, Lyons MK, et al. Analysis of early failures after lumbar decompressive laminectomy for spinal stenosis. Mayo Clin Proc. 1995;70:33-36.
12 Wang JC, Bohlman HH, Riew KD. Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg Am. 1998;80:1728-1732.
1 Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441-1445. discussion 6-7
2 Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine. 2002;69:450-457.
3 Deen HGJr, Zimmerman RS, Lyons MK, et al. Analysis of early failures after lumbar decompressive laminectomy for spinal stenosis. Mayo Clin Proc. 1995;70:33-36.
4 Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996;21:1787-1794. discussion 94-5
5 Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis: Four-year outcomes from the Maine Lumbar Spine Study. Spine. 2000;25:556-562.
6 Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17:S176S183.
7 Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine. 2005;30:2312-2320.
8 DiStefano VJ, Klein KS, Nixon JE, et al. Intra-operative analysis of the effects of position and body habitus on surgery of the low back. A preliminary report. Clin Orthop Relat Res. 1974;Mar-Apr(99):51-56.
9 McNulty SE, Weiss J, Azad SS, et al. The effect of the prone position on venous pressure and blood loss during lumbar laminectomy. J Clin Anesth. 1992;1(4):220-225.
10 Mody MG, Nourbakhsh A, Stahl DL, et al. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33:194-198.
11 Boden SD, Martin C, Rudolph R, et al. Increase of motion between lumbar vertebrae after excision of the capsule and cartilage of the facets. A cadaver study. J Bone Joint Surg Am. 1994;76:1847-1853.
12 Yuan PS, Booth REJr, Albert TJ. Nonsurgical and surgical management of lumbar spinal stenosis. Instr Course Lect. 2005;54:303-312.
13 Olszewski AD, Yaszemski MJ, White AA3rd. The anatomy of the human lumbar ligamentum flavum. New observations and their surgical importance. Spine. 1996;21:2307-2312.
14 Su BW, Kim PD, Cha TD, et al. An anatomical study of the mid-lateral pars relative to the pedicle footprint in the lower lumbar spine. Spine (Phila Pa 1976). 2009;34:1355-1362.
15 Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine. 1990;15:1142-1147.
16 Rosen C, Rothman S, Zigler J, et al. Lumbar facet fracture as a possible source of pain after lumbar laminectomy. Spine. 1991;16:S234-S238.
17 Truummees E, Herkowitz H. Lumbar Spinal Stenosis: Treatment Options. AAOS Instructional Course Lectures. 2003:107-115.
18 Turner JA, Ersek M, Herron L, et al. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1-8.
19 Herron LD, Mangelsdorf C. Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord. 1991;4:26-33.
20 Katz JN, Lipson SJ, Chang LC, et al. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine. 1996;21:92-98.
21 Katz JN, Lipson SJ, Brick GW, et al. Clinical correlates of patient satisfaction after laminectomy for degenerative lumbar spinal stenosis. Spine. 1995;20:1155-1160.
22 Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine. 2000;25:1424-1435. discussion 35-6
23 Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1-8.
24 Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794-810.
25 Birkmeyer NJ, Weinstein JN, Tosteson AN, et al. Design of the Spine Patient outcomes Research Trial (SPORT). Spine. 2002;27:1361-1372.
26 Nasca RJ. Rationale for spinal fusion in lumbar spinal stenosis. Spine. 1989;14:451-454.
27 Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am. 1995;77:1036-1041.
28 Katz JN, Lipson SJ, Lew RA, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine. 1997;22:1123-1131.
29 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
30 Fischgrund JS, Mackay M, Herkowitz HN, et al. 997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
31 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733. discussion 33-34
32 Tosteson AN, Lurie JD, Tosteson TD, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: Cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845-853.
33 Benz RJ, Garfin SR. Current techniques of decompression of the lumbar spine. Clin Orthop Relat Res. 2001:75-81.
34 Thomas NW, Rea GL, Pikul BK, et al. Quantitative outcome and radiographic comparisons between laminectomy and laminotomy in the treatment of acquired lumbar stenosis. Neurosurgery. 1997;41:567-574. discussion 74-5
35 Aryanpur J, Ducker T. Multilevel lumbar laminotomies: an alternative to laminectomy in the treatment of lumbar stenosis. Neurosurgery. 1990;26:429-432. discussion 33
36 McCulloch JA. Microdecompression and uninstrumented single-level fusion for spinal canal stenosis with degenerative spondylolisthesis. Spine. 1998;23:2243-2252.
37 Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7:579-586.
38 Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: the “Port-Hole” technique. Spine. 2000;25:865-870.
39 Fu YS, Zeng BF, Xu JG. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine. 2008;33:514-518.
40 Mackay DC, Wheelwright EF. Unilateral fenestration in the treatment of lumbar spinal stenosis. Br J Neurosurg. 1998;12:556-558.
41 Shenouda EF, Gill SS. Laminal fenestration for the treatment of lumbar nerve root foraminal stenosis. Br J Neurosurg. 2002;16:494-496. discussion 7
42 Young S, Veerapen R, O’Laoire SA. Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: Preliminary report. Neurosurgery. 1988;23:628-633.
43 O’Leary PF, McCance SE. Distraction laminoplasty for decompression of lumbar spinal stenosis. Clin Orthop Relat Res. 2001;Mar(384):26-34.
44 Tsuji H, Itoh T, Sekido H, et al. Expansive laminoplasty for lumbar spinal stenosis. Int Orthop. 1990;14:309-314.
45 Kawaguchi Y, Kanamori M, Ishihara H, et al. Clinical and radiographic results of expansive lumbar laminoplasty in patients with spinal stenosis. J Bone Joint Surg Am. 2004;86-A:1698-1703.
46 Matsui H, Tsuji H, Sekido H, et al. Results of expansive laminoplasty for lumbar spinal stenosis in active manual workers. Spine. 1992;17:S37-S40.
47 Asgarzadie F, Khoo LT. Minimally invasive operative management for lumbar spinal stenosis: overview of early and long-term outcomes. Orthop Clin North Am. 2007;38:387-399. abstract vi-vii
48 Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine. 2002;27:432-438.
49 Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51:S146-S154.
50 Arts MP, Brand R, van den Akker ME, et al. Tubular diskectomy vs conventional microdiskectomy for sciatica: A randomized controlled trial. JAMA. 2009;302:149-158.
51 Whitesides TEJr. The effect of an interspinous implant on intervertebral disc pressures. Spine. 2003;28:1906-1907. author reply 7-8
52 Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20:255-261.
53 Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine. 2005;30:744-749.
54 Siddiqui M, Nicol M, Karadimas E, et al. The positional magnetic resonance imaging changes in the lumbar spine following insertion of a novel interspinous process distraction device. Spine. 2005;30:2677-2682.
55 Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22-31.
56 Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345-1348.
57 Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: Application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463-471.
57a Verhoof OJ, Bron JL, Wapstra FH, van Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188-192.
58 Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: Two-year follow-up results. Spine. 2005;30:1351-1358.
59 Hsu KY, Zucherman JF, Hartjen CA, et al. Quality of life of lumbar stenosis-treated patients in whom the X STOP interspinous device was implanted. J Neurosurg Spine. 2006;5:500-507.
60 Kondrashov DG, Hannibal M, Hsu KY, et al. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: A 4-year follow-up study. J Spinal Disord Tech. 2006;19:323-327.
61 Idler C, Zucherman JF, Yerby S, et al. A novel technique of intra-spinous process injection of PMMA to augment the strength of an inter-spinous process device such as the X STOP. Spine. 2008;33:452-456.
62 Benz RJ, Ibrahim ZG, Afshar P, et al. Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res. 2001:116-121.
63 Ragab AA, Fye MA, Bohlman HH. Surgery of the lumbar spine for spinal stenosis in 118 patients 70 years of age or older. Spine. 2003;28:348-353.
64 Reindl R, Steffen T, Cohen L, et al. Elective lumbar spinal decompression in the elderly: Is it a high-risk operation? Can J Surg. 2003;46:43-46.
65 Glassman SD, Polly DW, Bono CM, et al. Outcome of lumbar arthrodesis in patients sixty-five years of age or older. J Bone Joint Surg Am. 2009;91:783-790.
66 Glotzbecker MP, Bono CM, Wood KB, et al. Thromboembolic disease in spinal surgery: A systematic review. Spine. 2009;34:291-303.
67 Dearborn JT, Hu SS, Tribus CB, et al. Thromboembolic complications after major thoracolumbar spine surgery. Spine. 1999;24:1471-1476.
68 Glotzbecker MP, Bono CM, Harris MB, et al. Surgeon practices regarding postoperative thromboembolic prophylaxis after high-risk spinal surgery. Spine. 2008;33:2915-2921.
69 Smith DW, Lawrence BD. Vascular complications of lumbar decompression laminectomy and foraminotomy. A unique case and review of the literature. Spine. 1991;16:387-390.
70 Eismont FJ, Wiesel SW, Rothman RH. Treatment of dural tears associated with spinal surgery. J Bone Joint Surg Am. 1981;63:1132-1136.
71 Sin AH, Caldito G, Smith D, et al. Predictive factors for dural tear and cerebrospinal fluid leakage in patients undergoing lumbar surgery. J Neurosurg Spine. 2006;5:224-227.
72 Khan MH, Rihn J, Steele G, et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: A review of 3,183 consecutive degenerative lumbar cases. Spine. 2006;31:2609-2613.
73 Wang JC, Bohlman HH, Riew KD. Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg Am. 1998;80:1728-1732.
74 Twyman RS, Robertson P, Thomas MG. Meningitis complicating spinal surgery. Spine. 1996;21:763-765.
75 Deyo RA, Cherkin DC, Loeser JD, et al. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis, and procedure. J Bone Joint Surg Am. 1992;74:536-543.
76 Weinstein MA, McCabe JP, Cammisa FPJr. Postoperative spinal wound infection: A review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422-426.
77 Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine. 2005;30:1460-1465.
78 Mok JM, Pekmezci M, Piper SL, et al. Use of C-reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine. 2008;33:415-421.
79 Beiner JM, Grauer J, Kwon BK, et al. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15:E14.
80 Mok JM, Guillaume TJ, Talu U, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: A matched cohort analysis. Spine. 2009;34:578-583.
81 Ohtori S, Inoue G, Koshi T, et al. Long-term intravenous administration of antibiotics for lumbar spinal surgery prolongs the duration of hospital stay and time to normalize body temperature after surgery. Spine. 2008;33:2935-2937.
82 Diwan AD, Parvartaneni H, Cammisa F. Failed degenerative lumbar spine surgery. Orthop Clin North Am. 2003;34:309-324.
83 Postacchini F, Cinotti G. Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Joint Surg Br. 1992;74:862-869.
84 Chen Q, Baba H, Kamitani K, et al. Postoperative bone re-growth in lumbar spinal stenosis. A multivariate analysis of 48 patients. Spine. 1995;19:2144-2149.
85 Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine. 2008;33:1701-1707.
86 Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497-1503.