Chapter 9 Surgical Management of Low-Grade Gliomas
The term “low-grade glioma” refers to a series of primary brain tumors characterized by benign histology (low proliferation, low neoangiogenesis phenomena) and aggressive behavior related to the slowly progressive tendency to invade the normal brain parenchyma.1–4 These neoplasms are classified as grade II (out of IV) by the World Health Organization classification of brain tumors and include the following entities: grade II astrocytoma (further divided in fibrillary and protoplasmic), grade II oligoastrocytoma, and grade II oligodendroglioma.5 Pilocytic astrocytomas, or grade I astrocytomas, are occasionally referred to as low-grade gliomas but due to their peculiar behavior, require separate considerations. In this chapter, low-grade gliomas refer only to WHO grade II tumors.
Low-grade gliomas are slow growing tumors, typically affecting younger individuals (median age 35), and mainly males (male/female ratio 1.5) who clinically present with seizures (often partial seizures).6 Headache, personality changes, and focal neurologic deficits represent the other most common symptoms. The neurologic symptoms include motor/sensory deficits, dysphasia/aphasia, disinhibition, apathy, and visuospatial disturbances according to tumor location and size.1,7,8 Interestingly, some authors report the tendency of low-grade gliomas to occur in eloquent areas or in their proximity.9
The optimal treatment for low-grade gliomas has yet to be determined. Watchful observation, needle biopsy, and open biopsy, as well as surgical resection have all been advocated by different authors.2,10–16 No evidence of class I or II exists regarding the optimal management of these patients, even if the more modern tendency is to obtain at least some type of tissue diagnosis.17,18 The rationale behind the observational or “wait-and-see” policy was the occasionally indolent or very slowly progressive behavior of these tumors.14,16 On the other hand, following the modern oncologic concepts, some authors proposed performing a biopsy to obtain a histopathologic confirmation of the nature of the neoplasm before deciding on further management. Surgical resection of low-grade gliomas is still matter of debate, although recent studies are increasingly supporting its role.10,13,17,18–22 Surgery can in fact achieve multiple aims: more reliable histologic diagnosis with eventual molecular profile (e.g., 1p/19q loss and MGMT status), symptom relief; beneficial effect on seizure control, and lower rate of recurrence and malignant transformation.13,18,20 Nevertheless, surgery carries unavoidable (albeit low) risks that can potentially and permanently affect the patient’s quality of life.
Given this general information on low-grade glioma behavior and the possibility of treatment, it is clear that a modern surgical approach to these tumors has the goal of maximal resection of the mass and minimizing postoperative morbidity to preserve the patient’s functional integrity.13,18–20,23 Since the natural history of the tumor can be relatively long (with or without surgery), the conservation of simple and complex neurologic functions of patients is mandatory. To achieve the goal of a satisfactory tumor resection associated with full preservation of the patient’s abilities, a series of neuropsychological, neurophysiologic, neuroradiologic, and intraoperative investigations must be performed. In this chapter, we will describe the rationale, indications, and modality for performing a safe and rewarding surgical removal of low-grade gliomas.
Rationale and Indications
The major aims of surgical treatment are1 obtaining adequate specimens and representative tissue to reach a correct histologic and molecular diagnosis;2 achieving cytoreduction to decrease rate of recurrence and malignant transformation, possibly prolonging survival;3 improving patient neurologic symptoms; and4 obtaining better seizure control. These goals can be reached by tailoring the surgical approach on location, modality of growth, and biological behavior of the tumor, as well and patient characteristics.
Histologic and Molecular Diagnosis
It is well known that astrocytomas represent a challenge for the neuropathologist, mainly in terms of grading the tumor. The size or number of needle biopsy specimens does not always permit all tests eventually required for immunohistochemical or molecular analysis. In addition, the biopsy site can significantly change the final results because gliomas are typically very heterogeneous with areas of different malignity. Recently, proton MR spectroscopy or MR perfusion has been used to partially overcome the latter problem, providing information on the presence of choline peaks (index of membrane production and malignancy) or areas of increased angiogenesis that can guide the surgeon in identifying the best location for performing the biopsy.24–26 In any case, the risk of underestimating, or more rarely overestimating, the grade is a distinct possibility for needle and even open biopsies eventually resulting in significant changes in the choice of the most appropriate treatment.
Molecular markers have become a standard in determining the type of low-grade glioma. In fact, chromosome 1p/19q loss of heterozygosity plays a very important role in the distinction between oligodendrogliomas or astrocytomas. This molecular marker is relevant not only in the histotype definition but also in therapeutic implications.18,27,28 In fact, 1p/19q loss as well as MGMT methylation (another important marker) facilitate predicting the response to certain chemotherapeutic agents. More recently, unexpected mutations affecting the isocitrate dehydrogenase (IDH1) gene at codon 132 have been found in 77% of grade II gliomas, and it was found associated with 1p19q deletions and MGMT methylated status, and with a better outcome.29 Obviously, inadequate or incorrect sampling of the tumor can dramatically impair the possibility of a molecular analysis.
Size, Location, and Growth
Most of low-grade gliomas are localized close or within the so-called eloquent areas, such as the areas of the brain that control motor, language, or visuospatial functions. In a recent series, as well as in the experience of our group, 82.6% of tumors were located within eloquent motor or language areas (27.3% of cases within the SMA, 25.0% in the insula, 18.9% in language centers, 6.0% in the primary somatosensory area, 4.5% in the primary motor area).9,30,31 As for the modality of growth, these tumors are characterized by a prevalent diffusive pattern of growth.9,32 Groups of tumor cells or single tumor cells diffuse away from the main tumor mass along vessels or short and long white matter tracts. These features are responsible for the typical aspect of low-grade gliomas seen in MR images, which is characterized by a morphology strictly resembling that of white matter tracts along which the tumor grows and diffuses. In addition, despite their occasional apparently indolent behavior, low-grade gliomas are characterized by a continuous growth, with periods of faster and lower rates of growth during the entire time of the natural history of the tumor.32 Most of the lesions judged as stable actually did show various degrees of growth; minor changes in the diameter (e.g., 1 to 2 mm) reflect a significant cellular growth in terms of volume.32 For the sake of simplicity, the rate of growth of a tumor can be quantified by measuring the maximal diameter onto FLAIR MR images. Repetitive measurement on representative sections demonstrated that the tumor continuously grows and that the mean increase of the tumor diameter is around 4 mm/year. Furthermore, an increase in tumor diameter larger than 8 mm/year, even in the absence of contrast enhancement or modification of T2 or FLAIR images, is associated with a high tendency toward malignant transformation and aggressive biological behavior. These data stress the point that serial measurements of tumor volumes are an important tool to determine the biological behavior of the tumor. At the same time, it is clear that tumor volume is an important prognostic factor, able to determine per se the biological behavior of the tumor overtime. In fact, larger tumor volumes are more frequently associated with a higher risk of malignant transformation and shorter patient survival.18 Tumor volume is associated with the risk of developing neurologic symptoms, increase in the risk of seizures, and probability of impacting in the social and professional life of patients.
Neurologic Symptoms
The majority of patients who are diagnosed with low-grade gliomas usually come to medical attention because of sudden occurrence of seizures.7,18 These patients are generally intact at the gross neurologic examination, but they frequently present more subtle symptoms affecting complex neurologic functions (memory, language, character, visuospatial orientation, etc.) that require a specific testing by a neuropsychologist.31,33,34 As will be detailed below, this type of testing is mandatory when considering surgery for this type of lesion because it allows tailoring the intraoperative testing to the patient and permits finely assessing the impact of surgery on the patients’ superior neurologic functions.35
Seizures
As mentioned above, surgery for gliomas aims to maximally remove the tumor mass and at the same time to preserve the patient’s functional integrity. This policy applies to the resection of any glioma but more specifically to those located close or within eloquent areas. The concept of eloquence refers not only to areas involved in motor, language, or visuospatial functions, but also, more widely, to any area affecting the well being of the individual (e.g., memory, socioaffective behavior, specific tasks performance, etc.). In all these cases, extensive resection and maximal functional integrity can still be achieved through the intraoperative use of brain mapping techniques.11,18,19,30,36–38
Intraoperative Mapping
Neuropsychological Evaluation
Neuropsychological evaluation comprises a large number of tests to assess various neurologic functions such as cognitive, emotional, intelligence, and basic language functions. Such a broad-spectrum evaluation provides information on how the tumor has impacted on the social, emotional, and cognitive life of the patient. It is important that the testing be the most extensive possible because the tumor that grows along fiber tracts may alter connectivity between separate areas of the brain, resulting in impairment of functions that may not be documented in the case of a neuropsychological examination limited to testing of functions strictly related to the area of the brain in which the tumor has grown.13,30,31,38 When this extensive testing is administered, changes can be documented in more than 90% of patients.13,30,31 These data represent the baseline with which the effect of surgical and future treatment should be compared. Additionally, when the tumor involves language or visuospatial areas or pathways, a more extensive specific evaluation should be added.
The neuropsychological assessment also allows one to build up a series of tests composed of various items that will be used intraoperatively for the evaluation and mapping of various functions, among which memory, language in its various components, and visuospatial orientation are some of the most important. For language evaluation, a battery of preoperative tests evaluates verbal language production and comprehension, together with repetition.30,39–41 Hemispheric language dominance is evaluated through the Edinburgh Inventory Questionnaire and fMRI. Most tests generally used have been standardized on the normal population. In addition, various tests can be adjusted according to the nationality of the patient. It is important to include in the battery both qualitative and quantitative tests, and normative data must be available for the quantitative procedure. It is also important that a speech therapist and a (neuro)psychologist manage patient assessments.
Preoperative language evaluation is also used to prepare a series of tests that will be used intraoperatively for assessing language during surgery. Among these tests, object naming is probably the most important. In the case of a tumor located in the dominant or parietal areas, number recognition and reading, as well as calculations or writing should be added to preoperative testing and considered for intraoperative evaluation.9,42,43 When the patient is bilingual or speaks more than two languages, it is important to include evaluation of these languages in the preoperative testing.32,44–48 In any case, bi- or multi-lingual assessment is generally recommended also intraoperatively.44 Visuospatial functions are usually evaluated for tumor located in the parietal lobe, generally on the right side.13 Unilateral spatial neglect is a complex and disabling syndrome that typically results from right hemisphere damage, and it is characterized by impaired awareness of the contralesional left half of space, objects, and mental images. In this case, the patient is presented with various tests such as the line bisection test or star cancellation test to evaluate spatial awareness.
Neuroradiologic Evaluation
The neuroradiologic examination consists of basic exams, such as morphologic T1, T2, and FLAIR images, as well as postcontrast T1 images. These images together with volumetric sequences provide information on the site and location of the tumor, and allows to determine its relationship with various structures, such as major vessels, and to measure tumor volume, and when performed at different time points to establish the speed of growth. Further MR studies include MR spectroscopy, which allows designing a map of areas within the tumor in which tumor metabolism is more or less pronounced (multipixel MR spectroscopy map).25,26 This is of great assistance in tissue sampling at the time of surgery for histologic and molecular purposes. Perfusion MR studies are useful for designing perfusion maps,49,50 which provide additional and complementary information of the biological behavior of the tumor and help in the tissue collection for histologic and molecular purposes at the time of surgery.24 Metabolic information may be also obtained by performing SPECT or PET, and these data may be incorporated into the navigation system for surgical guidance as well.51,52
The neuroradiologic investigations include functional studies, such as fMRI, and anatomic studies such as DTI-FT. The former provides functional information on the location of cortical sites, which activates in response to motor or various language tasks. Motor fMRI is generally used to design a map of the cortical motor sites and to establish their relationship with the tumor.53 fMRI for language provides a map of the cortical sites that activate during language tasks, such as denomination (object naming), famous face naming, verb generation, and verbal fluency.48,54 All these data form a complex map of how the various components of language are organized at the cortical level and allow establishment of spatial relationship between these cortical areas and the tumor mass. It is usually recommended that language fMRI be performed with the same tests that are used for language evaluation to increase its reliability.
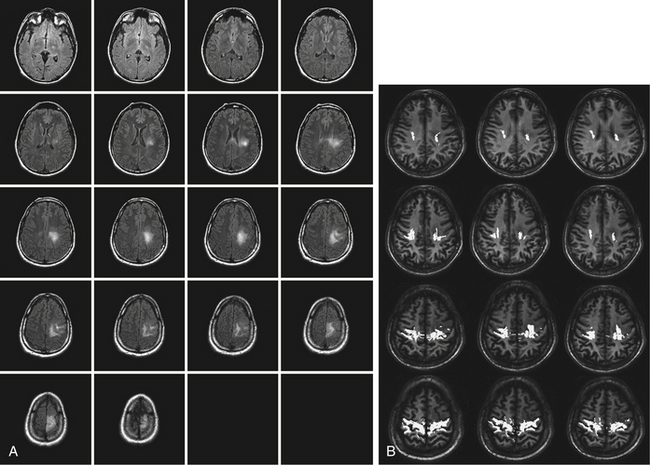
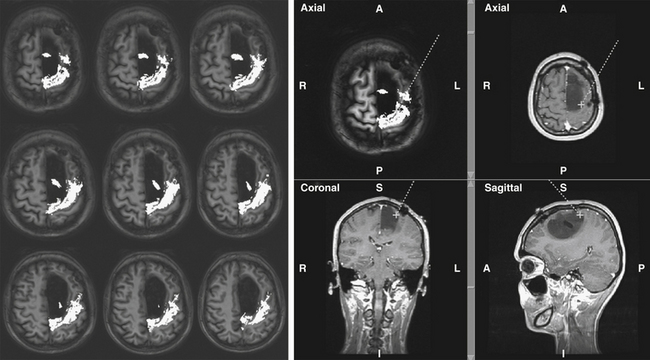
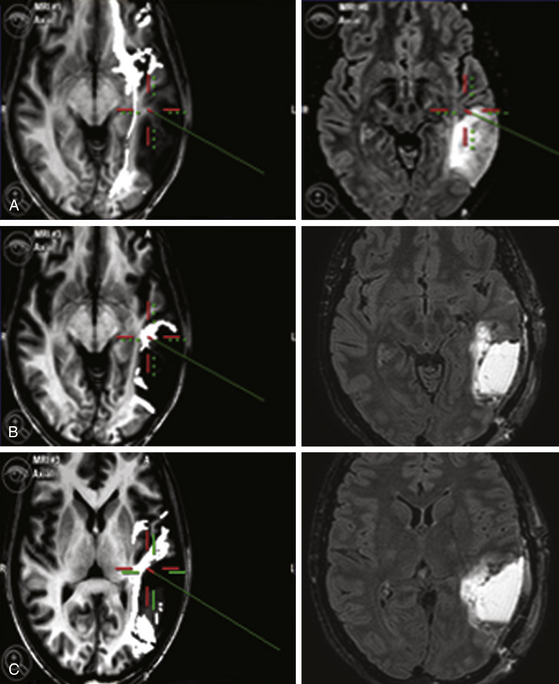
DTI-FT techniques allow depicting the connectivity around and inside a tumor, by reconstructing and visualizing the fiber tracts, which run around or inside the tumor mass55 (Fig. 9-1). DTI-FT provides anatomic information on the location of motor tracts, mainly the corticospinal tract (CST), and various language tracts, involved either in the phonologic or semantic components of language.56–59 For a better visualization of tracts in low-grade gliomas, an FA (fraction of anisotropy) of 0.1 should be used, and additional regions of interest (ROIs) for a particular tract such as the anterior part of the superior longitudinalis or the SMA portion of the CST can be added.56,60,61 The basic DTI-FT map includes the CST for the motor part, and the superior longitudinalis (SLF), which includes the fasciculus arcuatus, and the inferior fronto-occipital (IFO) tract for the language part.38,39,56 The SLF is the basic tract involved in the phonologic component of language; the IFO is the basic tract involved in the semantic component of language. Additional tracts that can be reconstructed are the uncinatus (UNC) and the inferior longitudinalis (ILF) tracts, which provide information on the semantic and phonologic component of language in the frontal and temporal lobe, or the subcallosum fasciculus, involved in the phonologic component of language, sited in the lateral border of the lateral ventricle.56,60,62 Preoperative neuroimaging produces an impressive amount of information concerning the anatomic and functional boundaries of the lesion to be resected. Together with the volumetric morphologic images, the DTI-FT images are usually loaded into the neuronavigation system and help in the perioperative period in performing the resection. However, the imaging gives information based on probabilistic measurements, and although they may have a relatively high sensitivity or specificity, they still carry a certain amount of mistake, which cannot, at least nowadays, be considered as sufficient for performing a safe and effective resection.
Anesthesiologic Evaluation
Besides the standard anesthesiologic work-up, the patient should be examined for his or her ability to experience intraoperative awake monitoring when needed. Preparation and selection of patients by anesthesiologists with expertise in awake surgery is recommended.63,64 In our institution, the only absolute contraindications to awake surgery are the lack of cooperation, age older than 70 years, obesity, and difficult airway or airway affected by severe cardiovascular or respiratory diseases. In addition, common contraindications to any general anesthesia regimen, communication difficulties (moderate to severe aphasia), psychological imbalance (extreme anxiety), prone position, and inability to lie still for many hours are also included.
• Lesions in the nondominant hemisphere, away from eloquent areas and without relationship with areas of activation according to fMRI: motor monitoring (optional)
• Lesions in the nondominant hemisphere, in central or precentral area or in relationship with the CST (e.g., insular, temporomesial tumors) and small central lesions in the dominant hemisphere: motor mapping and monitoring
• Lesions in the nondominant hemisphere, in postcentral region: motor mapping and monitoring, visuospatial mapping
• Lesions in the dominant hemisphere: motor mapping and monitoring, language mapping more or less visuospatial mapping for parietal lesions
Intraoperative Protocol
Anesthesia
Total intravenous anesthesia with propofol and remifentanil is used in our institution for performing these procedures. Newer drugs, such as dexmedetomidine, are emerging as effective and safe in producing sedation without inducing respiratory depression and without affecting electrophysiologic monitoring. In patients requiring only motor mapping, the patient is intubated through the nose and a light surgical anesthesia is maintained throughout the procedure. No muscle relaxants are employed during surgery to allow neurophysiologic assessment. When language or the visuospatial functions have to be tested during surgery, the patient can be maintained either awake during the entire surgery, or awakened for the phase of the surgery during which the mapping is performed.18,30,36,39,44,56,63–65 In our institution, patients receive a laryngeal mask that is maintained until after the craniotomy and dural opening. At this point, the patient is awakened, while adequate analgesia is maintained to allow function monitoring. Time for awakening varies between 20 to 50 minutes, depending on the ability of the patient to metabolize the anesthetics. The anesthesiologist should be able to keep the patient awake for the entire time of subcortical mapping, which may be required particularly during long-lasting operations to alternate rest periods with those awake and responsive periods. Fatigue is observed in most of the patients, and its appearance correlates with duration of mapping, and the test difficulties (extensive language and visuospatial mapping).25,44 Five percent of patients require suspension of mapping for a period longer than 20 minutes. The occurrence of seizures is the most important complication during the awake time of surgery, and can be controlled either by cold saline irrigation or by the infusion of a small bolus (1 ml) of propofol. Partial seizures occurred in our series in 4% of patients during surgery, and were related to mapping. Generalized seizures occurred in two patients at the end of the craniotomy. These two patients required reintubation. Vomiting is a rare complication, and can be controlled by the administration of antiemetics at the beginning of the mapping phase.
Neurophysiology
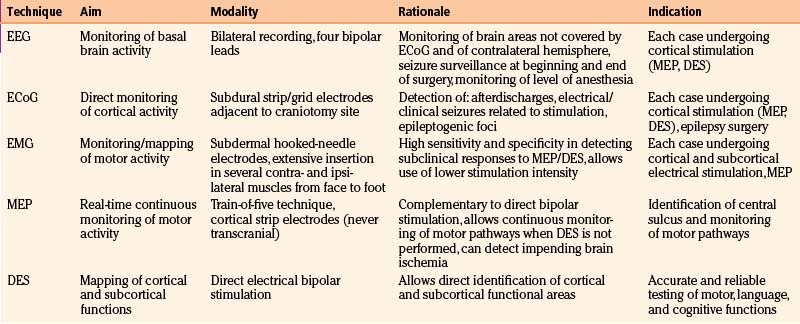
The major components of the neurophysiologic protocol are monitoring (EEG, ECoG, EMG, MEP) and mapping (DES) procedures11,31,60,66–68 (Table 9-1).
TABLE 9-1 Summary of Clinical Experience for 503 Patients with Low-Grade Gliomas Treated at University of Milan
MEP recording allows continuous monitoring of motor function. The “train of five technique,” which was introduced for surgery in anesthetized patients, has been described as sensitive in detecting imminent lesions of the motor cortex and the pyramidal pathways.69 For this purpose, a strip containing four to eight electrodes is placed over the precentral gyrus. A single stimulus or a double pulse stimulus (individual pulse width 0.3–0.5 millisecond, anodal constant current stimulation, interstimulus interval 4 milliseconds, stimulation intensity close to motor threshold) is usually delivered. MEP recording is usually alternated with direct cortical and subcortical motor mapping. MEP monitoring is very useful because it provides real-time information on the integrity of the motor pathways during the resection of large parts of the tumor not closely related to the functional structures. In addition, MEP provides warnings of impending brain ischemia, due to critical vessel interruption, mostly in deep temporal or insular regions.52
For language mapping, the initial test used is counting. The current is usually applied to the premotor cortex related to the face, and the test is aimed at determining whether the current stops the patient from counting. This has to be repeated several times and counting stopped at least three times in order to be reliable.41 If not, the current intensity is increased until these results are produced. When the current is established, DES is applied to the entire exposed surface of the brain, and the occurrence of afterdischarges checked in the ECoG. The stimulus duration is between 1 to 4 seconds. Only the current that is not inducing afterdischarges in the entire stimulated cortex is used for mapping. In case of afterdischarges, the current intensity is decreased by at least 0.5 mA.
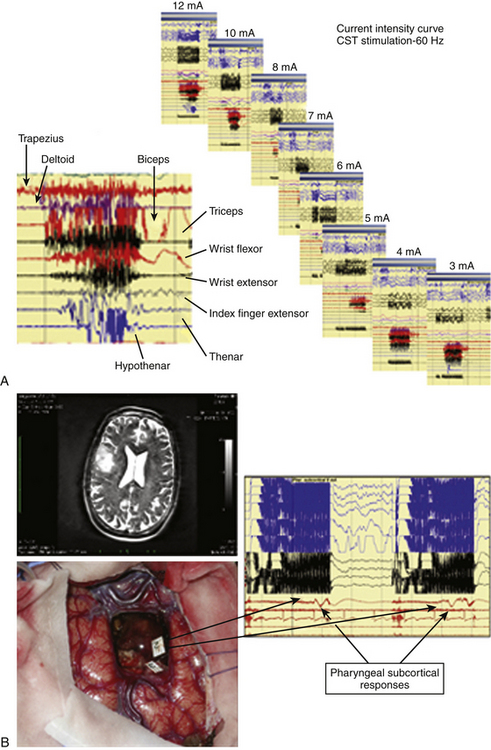
For subcortical mapping, either the same current used for cortical mapping or a current raised to 2 mA is applied, and the stimulus is continuously alternated with the resection. When a response was induced at a subcortical level, performing an intensity–response curve is recommended to assess maintenance of the response either at very low current-intensity levels. This can help in estimating the distance between the point of stimulation and the functional tract (Fig. 9-2). Also, during subcortical mapping, ECoG is continuously monitored to look for the occurrence of afterdischarges and seizures, in order to verify reliability of responses.
MEP monitoring is typically used at the beginning of the procedure, and helps in identifying the location of the motor strip. During resection, MEP recording is alternated with subcortical motor mapping and provides additional information on the integrity of motor pathways.70
Results of Mapping or Monitoring Procedures
Motor Mapping
Occasionally, in patients under general anesthesia and receiving a large amount of antiepileptic medications, it might be difficult to evoke cortical motor responses, even after the current intensity has been increased until that which might induce the appearance of afterdischarges. In these patients, the use of monopolar stimulation can be useful for identifying the location of the motor cortex and to plan the site of incision, allowing continuing resection. During subcortical stimulation, motor responses appeared as focal (few muscles) when the tract is stimulated in close vicinity to the surface, while they appeared on multiple muscle groups with deep stimulation (Figs. 9-3 and 9-4). For resection of tumors located in the premotor cortex, the placement of electrodes in the ipsilateral muscles allows detection of responses coming from these segments during resection. In addition, when resection is approaching the deep portion of the tumor, subcortical stimulation permits detection of small motor responses without overt muscle activity, which indicate that the resection is getting close to motor pathways. When these warning responses are identified, resection should proceed carefully in this region until more pronounced motor responses are identified, usually when the tip probe is touching and stimulating the motor pathways. This can be confirmed by performing a current intensity curve.
The simultaneous use of CUSA and DES at the subcortical level in proximity to the corticospinal tract may result in the abolition of previously evident motor responses. This abolition is generally fully reversible after turning the CUSA off. An analogous pattern of inhibition of motor responses can also be evident when the DES is applied cortically and CUSA is used subcortically when close to motor pathways. This interference with motor mapping may be interpreted as a transitory inhibition of axonal conduction. This should be kept in mind by the surgeon when using both tools during resection.67
Motor Monitoring
For continuous motor monitoring with MEP, a strip electrode is placed over M1, delivering monopolar pulses to elicit motor-evoked potentials (MEPs) in a few target muscles. MEPs are monitored throughout the surgery, except when the surgeon needs direct subcortical mapping. MEP monitoring is very useful because it provides on-line information of the motor pathway integrity during resection of a large part of the tumor not closely located to functional structures. MEP provides warnings of impending brain ischemia due to critical vessel interruption, mostly in deep temporal or insular regions.70
Language and Visuospatial Monitoring
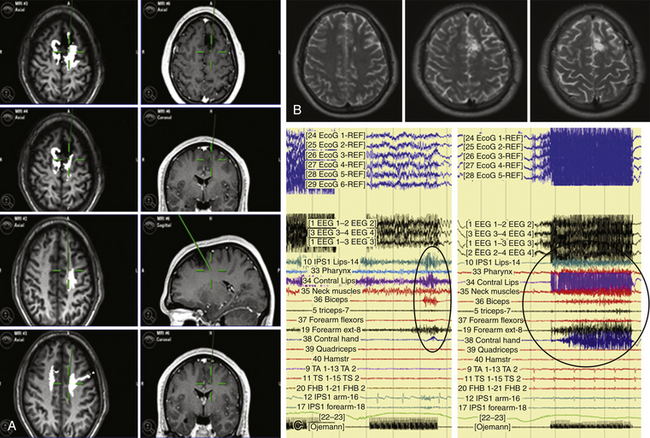
Various types of errors are possible during test administration. During the administration of each test, the ECoG and EEG must be checked for afterdischarges or electrical seizures. Only errors in the absence of ECoG disturbances are reliable. A site can be defined as essential for language when it produces language disturbances at least three times during various nonconsecutive stimulations. Cortical language sites coding for object naming, verb generation, face naming, word or sentence comprehension, numbers, or colors can be identified in several regions in the frontal, temporal, or parietal lobes, which differ according to patient gender and other characteristics.18,30 For subcortical language mapping, the patient is asked to perform an object-naming and a verb-generation task during which the surgeon can continue to perform resection, which is alternated with stimulation. When a language disturbance is produced, the site is then carefully tested for the occurrence of semantic or phonemic paraphasia. Each tract can be recognized at a subcortical level by the appearance of semantic (inferior fronto-occipital tract, uncinatus), or phonemic (superior longitudinalis, inferior longitudinalis) paraphasia associated with typical language disturbances, such as speech arrest in proximity to the subcallosum (Figs. 9-4 and 9-5).
EEG and ECoG Monitoring
EEG and ECoG recordings should be kept during the entire duration of the procedure because they permit monitoring for the occurrence of afterdischarges, electrical seizures, and even clinical seizures. The occurrence of afterdischarges is quite common during these procedures, and the main objective of monitoring is to recognize those that occur in response to stimulation, in order to maintain testing reliability. Groups of ECoG spikes or electrical seizures occur in up to 30% to 40% of cases, and may be related to stimulation. In any case, when they appear irrigating the cortex and surgical cavity with cold saline is recommended, as in most instances this results in control and reversal of the situation. Clinical seizures occur in 4% of cases, and most of them are focal. In these cases, the EEG is useful to look for diffusion of the seizure, either in the same or the contralateral hemisphere. In selected cases, ECoG can be used to detect the generation of spikes in specific areas of the cortex, either near or distant from the tumor mass, that are responsible for sustained electrical activity. ECoG is also used to titrate and monitor the level of anesthesia, particularly in sleeping patients. A continuous trace recording is usually recommended in this setting to ensure optimal response to cortical and subcortical stimulation.
Results of Intraoperative Imaging
Both morphologic volumetric T1, T2, or FLAIR images, along with motor and language fMRI and DTI-FT images, are usually loaded into the neuronavigation system. Neuronavigation helps during surgery to localize the tumor, and to define the relationship between the tumor and the surrounding functional and anatomic structures, both at cortical and subcortical levels. To estimate clinical navigation accuracy, the target registration error localizing a separate fiducial (not used for registration) is usually performed at the beginning of surgery. The target registration error should be less than 2 mm. The main limitation in using a neuronavigation system, particularly for large tumors, is the occurrence of brain shift, which occurs already at the beginning of surgery when the dura is opened, and increases with the progress of tumor removal.53,56,71–73 To reduce the problem of brain shift during resection, repeated landmark checks are performed during surgery to ensure overall ongoing clinical navigation accuracy. Using a craniotomy limited to the minimum necessary to expose the tumor area and a small portion of the surrounding brain, minimizes brain shift. For frontal tumors located in proximity of the CST, resection is started from the posterior border where the CST is located and, after its identification, the tract is followed inside the tumor mass. Afterward, the remaining anterior part of the tumor is removed. Similarly, in the case of parietal tumors, resection is started from the anterior border following the same principle.
When preoperative fMRI is correlated with intraoperative findings, motor fMRI usually matches with data obtained via DES, although the extent of the functional activations is larger than the area defined with intraoperative mapping, and results are strictly dependent on the type of task used for testing.19,74,75 In any case, motor fMRI can be safely used for planning surgery. For language correlation, the results are variable and different according to series. Naming and verb generation tasks are most widely used for language fMRI studies. Language fMRI data obtained with naming or verb generation tasks are imperfectly correlated with intraoperative brain mapping results (sensitivity 59% and specificity 97% when the two fMRI are combined).48,54,76 fMRI shows greater activation than observed with direct cortical mapping, which on the contrary, demonstrates only essential language sites. In our experience, sensitivity can be increased up to 72% by using the same figures in fMRI naming tasks as employed during surgery. Nevertheless, false negatives have been documented in up to 8% of patients, even when using the same naming tasks. Therefore, language fMRI cannot be used to make critical decisions in the absence of direct brain mapping. Language fMRI is useful to establish language laterality and can effectively replace the Wada test.
In low-grade gliomas, preoperative DTI-FT shows that the tracts were mostly infiltrated and interrupted or dislocated by the tumor mass. In addition, a large portion of the tracts were documented inside the tumor mass.61 As for the correspondence with DES and their clinical use, we have to remember that DTI-FT is providing anatomic information, whereas subcortical mapping provides functional data.56,57,61,62 This is of relatively less importance for CST, but of particular relevance for language tracts, in which the anatomic distribution of the tract as depicted by DTI is greater than the functional distribution obtained with mapping. Therefore, large portions of tracts as depicted by DTI-FT can be removed because they are not pertinent to the function tested at that time.
Additional problems may derive from the FA used for tract reconstruction, which can vary inside the same tumor according to its grade of heterogeneity. In cases of rolandic tumors, DTI reconstructs the CST mainly inside the tumor mass (98% of cases). In the majority of bulky tumors, the tract is displaced anteriorly (22%), or more frequently posteriorly (78%), and highly infiltrated by the tumor mass. Less frequently, and in case of highly infiltrating and diffuse low-grade gliomas, the tract is depicted inside the tumor mass and as highly infiltrated. In the first tumor group, subcortical DES locates the tract in the same position where it is depicted by DTI-FT (Figs. 9-3 and 9-4). Some discrepancies are observed only in the superior portion of the tract, close to the cortical surface, where DTI-FT fails to reconstruct fibers, and instead DES locates motor responses. Even the placement of additional ROIs does not improve the fiber reconstruction. More problematic are cases of highly diffuse low-grade gliomas, where DTI-FT usually reconstructs the tract as highly infiltrated and inside the tumor mass.
When a portion of the tumor is removed, and the CST partially decompressed, the 60-Hz stimulation starts again to identify motor responses, usually in the same location where DTI-FT reconstructs the deeper portion of the CST. As for the SLF, the anatomic distribution of this tract is usually larger than the functional distribution when language subcortical mapping is performed. This is particularly the case for frontal and temporal tumors. As for the IFO tract, its anatomic distribution is small and usually corresponds to the functional one depicted by subcortical mapping (Figs. 9-4 and 9-5). Some problems may occur for F3 low-grade gliomas in which DTI-FT fails in reconstructing the more superior part of the tract at the inferior border of the tumor, when the tumor infiltration in this area is quite extensive.
The anatomic distribution of the UNC tract is small and usually corresponds to the functional one depicted by subcortical mapping. The reconstruction of this tract in F3 tumors requires placement of an additional ROI at this level. In F3 low-grade gliomas, the tract is usually inside the tumor mass, and the depicted fibers are typically identified as functional by subcortical mapping.
In temporal LGG tumors, the tract is still described as inside the tumor mass, but the fibers are extensively infiltrated and interrupted, and not functional. Our experience on a large number of patients showed that the combined use of DTI-FT and DES is a feasible approach that can be effectively and safely applied in routine clinical activity.56,61 When available and loaded into the neuronavigation system, DTI-FT can help in reducing time spent in surgery by helping the surgeon locate where in the tract to start subcortical stimulation and thus proceed with a careful resection. This may result in a smaller number of stimulations needed to safely locate a tract, fewer seizures, and less patient fatigue.
Intraoperative MR has been more widely used for surgical treatment of low-grade gliomas20,71,77 by using both low (0.2 or 0.5) or high1.5 magnetic fields. The advantage of using intraoperative MR images is to have a precise judgment of surgical removal while the patient is still in the operating room. In addition, by performing repeated images during surgery, it is possible to update morphologic images and transfer them into the neuronavigation system to overcome the problem of brain shift. Progression of surgery can be followed and the occurrence of intraoperative complications monitored. In at least 20% of cases of low-grade gliomas, remnants of the tumor can be visualized in the field and further removed.
The major limitation of the intraoperative MR system is cost of the machine and instruments. Lower magnetic fields may permit the use of nonmagnetic surgical instruments, and thus lower cost of machine and installation. Various lower-field machines are available, such as the 0.2 Polestar or the 0.5 GE. The 0.5 GE prototype allows on-time intraoperative images during surgery,20 but is limited by the restricted surgical room and by the need to use nonmagnetic surgical tools. In addition, low magnetic fields do not permit fMRI or DTI-FT studies. The intraoperative high-field magnetic resonance (MR) system provides high-quality images and offers various modalities beyond standard anatomic imaging, such as MR spectroscopy, diffusion tensor imaging, and functional MR imaging, providing not only data on the extent of resection and localization of tumor remnants but also on metabolic changes, tumor invasion, and localization of functional eloquent cortical and deep-seated brain areas. Various systems have been developed and used. In most of them, the patient is located in a bed and moved into the magnet for MR images. Recently 3T MR systems have been put in place, or are under construction, including in our institution (University of Milan).
Ultrasound is another imaging option used for intraoperative visualization of low-grade gliomas. Advances in ultrasound technology have made the image quality of the ultrasound comparable to intraoperative MR.78 Recent studies and the experience of our center showed that the integration of intraoperative ultrasound with neuronavigation represents an efficient and inexpensive tool for intraoperative imaging and surgical guidance. Brain shift detected with intraoperative ultrasound could be used to update preoperative image data such as fMRI and DTI-FT in order to increase the value of this information through the operation. However, intraoperative MR systems are superior to ultrasound methods in revealing tumor remnants.
The Concept of Subpial Resection
Surgical removal is usually performed via tailored craniotomy exposing the tumor area and limited surrounding cortex. In the case of temporal or frontal tumors in the dominant hemisphere, the craniotomy should expose the face premotor cortex to allow testing at the beginning of resection to establish in the awakened patient the current intensity to be used. In other cases, the placement of a subdural strip permits reaching the motor cortex and performing MEP monitoring. When the relationship between the tumor and the functional areas is established and the point of entry identified, resection begins using a transcortical subpial approach. This allows removal of nonfunctional tumor tissue until functional borders are identified and preservation of arteries and veins. Resection cavities are eventually connected to one another, maintaining the vasculature skeleton. The safety of this microsurgical strategy is indicated by the patient morbidity profile.13,18,31,37,56,61
Functional Results of Surgery
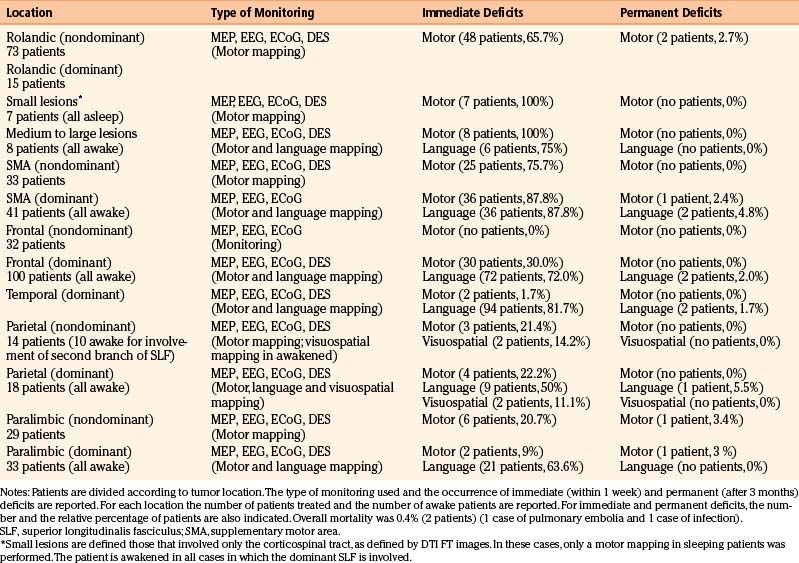
Resection margins are usually kept 5 mm apart from essential cortical sites, and are usually coincident with subcortical sites (Tables 9-2 and 9-3). When this is achieved, motor or language deficits develop in the immediate postoperative period in 72.8% and 65.4% of cases, respectively. When no subcortical sites are identified, this risk is very low (3%–5%).12,30,38,39,56,77,79,80 In our experience, most of the deficits were transient and disappeared within 1 month from surgery. Overall, in the group of patients in which a subcortical functional site was identified during the resection, the likelihood of developing a permanent deficit was less of 4%, independent of histology and location. This percentage reached 7% in patients with a pre-existing motor or language deficit.
TABLE 9-3 Functional Borders Encountered during Resection According to Tumor Location
| Location | Functional Borders |
|---|---|
| Rolandic or SMA nondominant | Posteriorly: CST and leg component of SMA fibers |
| Rolandic or SMA dominant | Posteriorly: CST and leg component of SMA fibers |
| Laterally: SLF | |
| Inferiorly: IFO, subcallosum | |
| Precentral nondominant | Posteriorly: CST (if reachable) |
| Precentral dominant | Posteriorly: SLF |
| Laterally and inferiorly: SLF, IFO, UNC | |
| Parietal nondominant | Anteriorly: CST |
| Laterally: second branch SLF | |
| Parietal dominant | Anteriorly: CST |
| Laterally: SLF | |
| Inferiorly: IFO, visual pathways | |
| Temporal dominant | Medial: UNC, IFO, ILF |
| Posteriorly: SLF | |
| Insular dominant | Anteriorly: IFO, SLF, UNC |
| Medially: IFO, CST | |
| Posteriorly: CST, SLF |
CST, corticospinal tract; IFO, inferior longitudinalis fasciculus; SLF, superior longitudinalis fasciculus; SMA, supplementary motor area; subcallosum, subcallosum fasciculus; UNC, uncinatus fasciculus.
In contrast, when no subcortical sites were found at the time of surgery, the likelihood of inducing a permanent deficit was even lower (2%). These results further reinforce the concept that when a subcortical site is found, the surgeon is very close to the subcortical pathway. Therefore, when a subcortical response is reliably detected, the resection must be stopped and then continued in adjacent structures because there is a great potential for damage to functional structures.21,30,77,80 If no subcortical structures are found, the resection can be continued because the probability of injury to essential structures is low. These data indicate subcortical stimulation as a reliable tool for guiding surgical resection, as well as for predicting the likelihood of developing a deficit postoperatively.
The low incidence of postoperative deficits in patients in whom no subcortical tracts were identified is usually due to vascular damage and the development of ischemic areas. MEP monitoring can help in preventing motor deficits due to vascular injury.52 Long-term postoperative neuropsychological evaluation found that 79.5% of patients had long-term postoperative normal language, 18.6% showed mild disturbances but still compatible with normal daily life, and only 2.3% showed long-term impairment. Similar figures were observed for the resection of low-grade gliomas close to motor areas or pathways. These functional results were totally different from those obtained when subcortical stimulation was not applied. Analysis of patients with high- or low-grade gliomas operated on in our institution before the use of direct electrical stimulation showed 23% with permanent language or motor deficits, in accordance with results reported in other series.12,30,81
Oncologic Results of Surgery
Surgery performed with the aid of brain mapping techniques permits attainment of several oncologic objectives. It permits collection of a large amount of material, which helps the pathologist the histologic and molecular diagnosis. It increases the number of cases submitted to surgical treatment: in accordance with previous reports in the literature, this percentage in our series moved from 11% of cases when mapping was not available, to 81% when mapping was applied, with a significant decrease in the number of cases that were submitted to biopsy only.12,18,30,81 Moreover, it reduces the percentage of postoperative permanent deficits, which fell from 33% to 2.3% for language or motor functions. Another important effect is the decline in the incidence of seizures, particularly in low-grade glioma patients with a long epileptic history and affected by insular tumors.
Seizure control is more likely to be achieved after gross total resection than after subtotal resection/biopsy alone. In fact when total or subtotal resection is achieved, in more than 80% of cases a positive impact on seizures is documented, with reduction in the number of antiepileptic drugs (AEDs) administered. In addition, suppression of AEDs is possible in 30% of cases.40 Lastly, and most important, is the impact that these techniques have on the extent of resection. The use of brain mapping techniques increased the percentage of patients in which a total and subtotal resection was achieved. In our series of low-grade gliomas, the percentage of total and subtotal resections was 11% in the period in which no mapping was available, and 69.8% in the time in which brain mapping techniques were applied. These figures are in accordance with the results of other groups.
A large number of class III and II studies suggests that more extensive resection at the time of initial diagnosis may be a favorable prognostic factor for this type of tumors.12,17–22,31,38,77,82 The evaluation of resection extent is usually performed on postoperative FLAIR volumetric images with the aid of semiautomatic segmentation software.21,71 The ability to achieve a complete resection (no abnormalities seen on postoperative FLAIR images) or subtotal resection (a postoperative volume on volumetric postoperative FLAIR images of less than 10 ml) is influenced by both preoperative tumor volume and tumor involvement of eloquent tissue, particularly at the subcortical level.18 Preoperative tumor volume is a significant predictor of patient survival and progression-free survival per se, as well as the involvement of subcortical tracts. Extent of resection as well as pre- and post-operative tumor volume strongly influence progression-free survival and time to malignant transformation. In addition, extent of resection has also an influence of patient survival. Total resection (no abnormalities in postoperative FLAIR volumetric images) is seen in 37.5% of patients in our series, and can usually be reached in small or well-demarcated tumors. In addition, no tumor recurrence is found in these patients at 5 years follow-up. Because tumor size is inversely related to patient outcome, delaying surgical intervention may increase the risk of malignant transformation. Moreover, all efforts made to increase the extent of resection are warranted.18
Strategy for Large Diffuse or Recurrent Tumors: The Concept of Brain Plasticity
Low-grade gliomas present as a variable type of tumors ranging from discrete and apparently well defined lesion, to either diffuse or less discrete lesion. The therapeutic strategies for the more defined type of tumors are those we previously described. Large diffuse tumors still represent a challenge. Most of them are histologically diffuse astrocytoma, and contain functional subcortical tracts. In these cases, a total or subtotal resection as initial strategy is quite difficult to be achieved. Although partial removal may still be beneficial,18 particularly in cases where a mass effect is present, the majority of these patients underwent stereotactic biopsy only, usually guided by spectroscopy MR images, followed by adjuvant treatments. A recent strategy to increase the rate of resection in these tumors is represented by the use of up-front preoperative chemotherapy. TMZ administered up front for a period of up to 6 months in a limited group of patients resulted in a decrease in tumor cell invasion, and reduced tumor cell infiltration along large fiber tracts, which help in reaching a larger proportion of tumor removal (ref. 80, Soffietti et al., 2010 in press). Alternatively, chemotherapy may be used as adjuvant treatment after partial removal, and in these cases it may further decrease postoperative tumor volume.27,28,83 In addition, in the case of large tumors, a two-time surgical strategy may be chosen, particularly in cases involving language areas or pathways. In these instances, the initial surgery is continued as long as patient collaboration and responsiveness are maintained, and then is resumed from 1 week to several months later. In our institution, the patient is subjected to a second surgery 4 to 6 months later, which permits patient recovery from the initial surgery, and brain plasticity to occur.40
Cerebral plasticity could be defined as the continuous processing allowing short-, middle-, and long-term remodeling of the neurono-synaptic organization.13 Plasticity may occur in the preoperative period in low-grade gliomas and in this case, is the results of the progressive functional brain reshaping induced by these slow growing lesions.13,84,85 Brain plasticity also occurs in the postoperative period. This has been shown by submitting patients that have recovered from postoperative deficit status, to functional neuroimaging studies some months after surgery and when a recovery has occurred, demonstrating the activation of different areas of the brain, close or remote to those were involved in the preoperative period. Plasticity may occur at a cortical level or (less frequently) at a subcortical level, where it can be explained by the recruitment or unmasking of parallel and redundant subcortical circuits.38 The occurrence of such phenomenon of compensation is of particular relevance because it allows extending surgical indications. It allows extending the initial surgery until functional boundaries are encountered, which allows the patient to recover in the postoperative period due to activation of redundant functional areas when the essential are preserved at the cortical or subcortical levels. Second, the functional reshaping induced by the initial surgery can be used to perform a second surgery with the aim of removing areas of the brain initially essential for function, and that due to the functional reshaping induced by the initial surgery or to the continuous slow growth of the tumor, have lost their essential nature in terms of function. This functional reshaping phenomenon can be observed up to a period of 6 months after the initial surgery, and allows performing a more radical second surgery with an increase in oncologic benefit for the patient.
Despite aggressive and early treatment, low-grade gliomas recur. As already discussed, the rate of recurrence is influenced by the preoperative tumor volume and to a lesser extent by the extent of surgical removal.10,18,23,38 The duration of the longest-lasting symptom, tumor size, and presence of preoperative contrast enhancement are associated with tumor recurrence at last follow-up. A diagnosis of FA does not have a statistical association with tumor recurrence.86 A tumor recurrence may still retain the morphologic feature of low-grade gliomas, or may show signs of tumor progression, such as contrast enhancement. The appearance of contrast enhancement is usually associated with a large preoperative volume, and with the presence of limited or focal enhancement in the preoperative MR images. Generally, when total or subtotal removals were achieved at the time of initial surgery, the recurrent tumor has a greater chance of recurring as a low-grade one. When only a partial removal was obtained, the potential for recurrence toward a higher grade is much greater. When a tumor recurs, various therapeutic options are available: surgery, chemotherapy, radiotherapy, or a wait-and-see policy.13,83 Surgery usually is intermingled with the other therapeutic modalities, and is the treatment of choice when a subtotal or even a total removal can be predicted, such as for discrete lesions. When this is feasible, the prognosis of the patient is still favorable. Brain mapping techniques can still be applicable in cases of recurrent tumors, even after radiotherapy. Alternatively, surgery may be used to decrease tumor volume to enhance the effect of chemo- or radio-therapy. Generally, a patient with low-grade gliomas may undergo several surgeries during the entire time of the disease, and surgery is used for various objectives and strictly associated with the other therapeutic modalities. Up to 30% of patients in our series underwent four surgeries, and 12% had up to five operations. We observed a decrease in the extent of resection with the increase in the number of surgeries, but this was not associated with an increase in the occurrence of transient and permanent postoperative deficits.
Conclusive Remarks
Another critical technical issue is the relationship between stimulating current intensity and distance from the functional site, in particular when subcortical mapping is performed. In the literature, there are no available works studying the penetration distance of subcortical bipolar stimulation in the white matter, while the range of bipolar stimulation on the cortex has been observed to be approximately 2 to 10 mm.11,33,66 When a response was induced at a subcortical level, we always performed an intensity–response curve, in order to assess the maintenance of the response at very low current intensity levels. This can help in estimating the distance between the point of stimulation and the functional tract. In addition, we commonly observed that as we approximated the end of the resection, a lower current intensity was needed to induce a response. Functional structures probably regain their normal excitability threshold once the mass effect exerted by the tumor is relieved. Anesthesiologic factors may also play a role (e.g., progressive clearance of anesthetic drugs). In order to maintain mapping reliability and to avoid false-positive findings that could lead to premature interruption of the resection, verifying and eventually decreasing the working current once a large part of the tumor has been removed are recommended. Nevertheless, further studies are needed to clarify this point.
Globally considered, surgery accomplished according to functional and anatomic boundaries allows maximal resection of the tumor and maximal preservation of patient functional integrity. This can be reached at the time of the initial surgery, depending on the functional organization of the brain, or may require additional surgeries, eventually intermingled with adjuvant treatments. The use of brain mapping techniques extends surgical indications with greater oncologic impact.
Bello L., Castellano A., Fava E., et al. Intraoperative use of DTI FT and subcortical mapping for surgical resection of gliomas: technical considerations. Neurosurg Focus. 2010;28:E6.
Bello L., Fava E., Carrabba G., et al. Present day’s standards in microsurgery of low-grade gliomas. Adv Tech Stand Neurosurg. 2010;35:113-157.
Bello L., Gallucci M., Fava M., et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67-80. discussion 80-82
Bello L., Gambini A., Castellano A., et al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. Jan. 2008;1(39):369-382. Epub August 29, 2007
Berger M.S., Deliganis A.V., Dobbins J., et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
Bertani G., Fava E., Casaceli G., et al. Intraoperative mapping and monitoring of brain functions for the resection of low-grade gliomas: technical considerations. Neurosurg Focus. 2009;27:E4.
Capelle L., Duffau H., Lopes M., et al. Recurrence and malignant degeneration after resection of adult hemispheric low grade gliomas. J Neurosurg. 2010;112:10-17.
Claus E.B., Horlacher A., Hsu L., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898-914.
Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476-486.
Duffau H., Capelle L., Sichez N., et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125:199-214.
Duffau L., Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622-2626.
Hoang-Xuan K., Capelle L., Kujas M., et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133-3138.
Kaloshi G., Benouaich-Amiel A., Diakite F., et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;22(68):1831-1836.
Keles G.E., Chang E.F., Lamborn K.R., et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34-40.
Keles G.E., Lamborn K.R., Berger M.S. Lowgrade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735-745.
Keles G.E., Lundin D.A., Lamborn K.R., et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369-375.
Klein M., Heimans J.J. The measurement of cognitive functioning in low-grade glioma patients after radiotherapy. J Clin Oncol. 2004;22:966-967.
Lote K., Egeland T., Hager B., et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
Mandonnet E., Jbabdi S., Taillandier L., et al. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro-oncology. 2007;9:63-69.
Mokhtari K., Poirier J., Sahel M., et al. WHO grade 2 gliomas in adults: a study of prognostic factors with special emphasis on the role of surgery. J Neurooncol. 2002;4:S17-S69.
Pignatti F., van den Bent M., Curran D., et al. European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group: Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076-2084.
Ricard D., Kaloshi G., Amiel-Benouaich A., et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61:484-490.
Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-764. discussion 264-266. Review
Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
1. Bello L., Acerbi F., Giussani C., et al. Intraoperative language localization in multilingual patients with gliomas. Neurosurgery. 2006;59:115-125.
2. Bello L., Gallucci M., Fava M., et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67-80. discussion 80–82
3. Bello L., Gambini A., Castellano A., et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39:369-382. Epub August 29, 2007
4. Bello L., Fava E., Casaceli G., et al. Intraoperative mapping for tumor resection. Neuroimaging Clin North Am. 2009;19:597-614.
5. Bello L., Fava E., Carrabba G., et al. Present day’s standards in microsurgery of low-grade gliomas. Adv Tech Stand Neurosurg. 2010;35:113-157.
6. Bello L., Castellano A., Fava E., et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for surgical resection of gliomas: technical considerations. Neurosurg Focus. 2010;28:1-14.
7. Bertani G., Fava E., Casaceli G., et al. Intraoperative mapping and monitoring of brain functions for the resection of low-grade gliomas: technical considerations. Neurosurg Focus. 2009;27:E4.
8. Berger M.S., Ojemann G.A., Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurg Clin North Am. 1990;1:65-70.
9. Berger M.S., Deliganis A.V., Dobbins J., et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
10. Berger M.S. Functional mapping-guided resection of low-grade gliomas. Clin Neurosurg. 1995;42:437-452.
11. Berger M.S., Rostomily R.C. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34:85-101.
12. Berman J.I., Berger M.S., Mukherjee P., Henry R.G. Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004;101:66-72.
13. Bogomolny D.L., Petrovich N.M., Hou B.L., et al. Functional MRI in the brain tumor patient. Top Magn Reson Imaging. 2004;15:325-335.
14. Capelle L., Duffau H., Lopes M., et al. WHO grade 2 gliomas in adults: a study of prognostic factors with special emphasis on the role of surgery. J Neurooncol. 2002;4:S17-S69.
15. Carrabba G., Fava E., Giussani C., et al. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: impact on postoperative morbidity and extent of resection. J Neurosurg Sci. 2007;51:45-51.
16. Carrabba G., Fava E., Mandonnet E., et al. Transient axonal inhibition induced by CUSA during brain mapping: a case report with motor EMG evidence. Neurosurgery. 2008;63:178-179.
17. Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77-94.
18. Cavaliere R., Lopes M.B., Schiff D. Low-grade gliomas: an update on pathology and therapy. LancetNeurol. 2005;4:760-770.
19. Cha S., Tihan T., Crawford F., et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2005;26:266-273.
20. Chaichana K.L., McGirt M.J., Laterra J. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J of Neurosurg. 2010;112:10-17.
21. Claus E.B., Horlacher A., Hsu L., et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227-1233.
22. Clark C.A., Barrick T.R., Murphy M.M., Bell B.A. White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? NeuroImage. 2003;20:1601-1608.
23. Danks R.A., Rogers M., Aglio L.S., Gugino L.D., Black P.M. Patient tolerance of craniotomy performed with the patient under local anesthesia and monitored conscious sedation. Neurosurgery. 1998;42:28-36.
24. Danks R.A., Aglio L.S., Gugino L.D., Black P.M. Craniotomy under local anesthesia and monitored conscious sedation for the resection of tumors involving eloquent cortex. J Neurooncol. 2000;49:131-139.
25. Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898-914.
26. De Witte O., Levivier M., Violon P., et al. Prognostic value positron emission tomography with [18F]fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery. 1996;39:470-476.
27. Duffau H., Capelle L., Sichez J., et al. Intraoperative direct electrical stimulations of the central nervous system: The Salpêtrière experience with 60 patients. Acta Neurochir (Wien). 1999;141:1157-1167.
28. Duffau H., Denvil D., Lopes M., et al. Intraoperative mapping of the cortical areas involved in multiplication and subtraction: an electrostimulation study in a patient with a left parietal glioma. J Neurol Neurosurg Psychiatry. 2002;73:733-738.
29. Duffau H., Capelle L., Sichez N., et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomofunctional study. Brain. 2002;125:199-214.
30. Duffau H., Capelle L., Denvil D., et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74:901-907.
31. Duffau L., Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622-2626.
32. Duffau H., Khalil I., Gatignol P. Surgical removal of corpus callosum infiltrated by low-grade glioma: functional outcome and oncological considerations. J Neurosurg. 2004;100:431-437.
33. Duffau H., Lopes M., Arthuis F., et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without 1985-96 and with 1996-2003 functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76:845-851.
34. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476-486.
35. Duffau H., Gatignol P., Mandonnet E., et al. New insights into the anatomofunctional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797-810.
36. Duffau H., Taillandier L., Capelle L. Radical surgery after chemotherapy: a new therapeutic strategy to envision in grade II glioma. J Neurooncol. 2006;80:171-176.
37. Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity—a review. J Neurooncol. 2006;79:77-115.
38. Ebel H., Ebel M., Schillinger G. Surgery of intrinsic cerebral neoplasms in eloquent areas under local anesthesia. Minim Invasive Neurosurg. 2000;43:192-196.
39. Galanaud D., Chinot O., Nicoli F., et al. Use of proton magnetic resonance spectroscopy of the brain to differentiate gliomatosis cerebri from low-grade glioma. J Neurosurg. 98, 2003. 269-276
40. Gasparini F.M., Cohen L., Lopes M., et al. A clinical study of the number processing system: decimal size effects on reading numbers in patients with left parieto-occipital gliomas. Rev Neurol (Paris). 2005;161:427-435.
41. Gerganov V.M., Samii A., Akbariam A. Reliability of intraoperative high resolution 2D ultrasound as an alternative to high field strength MR imaging for tumor resection control: a prospective comparative study. J Neurosurg. 2009;111:512-514.
42. Giussani C., Roux F.E., Lubrano V., et al. Review of language organisation in bilingual patients: what can we learn from direct brain mapping? Acta Neurochir (Wien). 2007;149:1109-1116. Nov; discussion 1116. Epub 2007 Aug 23. Review
43. Goldstein B., Obrzut J.E., John C. The impact of frontal and non-frontal brain tumor lesions on Wisconsin Card Sorting Test performance. Brain Cogn. 2004;54:110-116.
44. Gossl C., Fahrmeir L., Putz B. Fiber tracking from DTI using linear state space models: detectability of the pyramidal tract. Neuroimage. 2002;16:378-388.
45. Guillevin R., Menuel C., Duffau H., et al. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J Neurooncol. 2008;87:181-187.
46. Hoang-Xuan K., Capelle L., Kujas M., et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133-3138.
47. Holodny A.I., Schulder M., Liu W.C. The effect of brain tumors on BOLD functional MR Imaging activation in the adjacent motor cortex: Implications for image-guided neurosurgery. Am J Neuroradiol. 2000;21:1415-1422.
48. Johannesen T.B., Langmark F., Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854-862.
49. Kaloshi G., Benouaich-Amiel A., Diakite F., et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;22(68):1831-1836.
50. Keles G.E., Lamborn K.R., Berger M.S. Lowgrade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735-745.
51. Keles G.E., Lamborn K.R., Berger M.S. Coregistration accuracy and detection of brain shift using intraoperative sononavigation during resection of hemispheric tumors. Neurosurgery. 2003;53:556-564.
52. Keles G.E., Lundin D.A., Lamborn K.R., et al. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369-375.
53. Keles G.E., Chang E.F., Lamborn K.R., et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34-40.
54. Kleihues P., Louis D.N., Scheithauer B.W., et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-229.
55. Klein M., Heimans J.J. The measurement of cognitive functioning in low-grade glioma patients after radiotherapy. J Clin Oncol. 2004;22:966-967.
56. Kuznetsov Y.E., Caramanos Z., Antel S.B., et al. Proton magnetic resonance spectroscopic imaging can predict length of survival in patients with supratentorial gliomas. Neurosurgery. 2003;53:565-576.
57. Laws E.R., Shaffrey M.E., Morris A., Anderson F.A.Jr. Surgical management of intracranial gliomas—does radical resection improve outcome? Acta Neurochir Suppl. 2003;85:47-53.
58. Lehericy S., Duffau H., Cornu P., et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589-598.
59. Leighton C., Fisher B., Bauman G., et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294-1301.
60. Lote K., Egeland T., Hager B., et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
61. Lucas T.H., McKhann G.M., Ojemann G.A. Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J Neurosurg. 2004;101:449-457.
62. Mandonnet E., Jbabdi S., Taillandier L., et al. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro-oncology. 2007;9:63-69.
63. Meyer P.T., Sturz L., Schreckenberger M., et al. Preoperative mapping of cortical language areas in adult brain tumor patients using PET and individual non-normalised SPM analyses. Eur J Nucl Med Mol Imaging. 2003;30:951-960.
64. Minn H. PET and SPECT in low-grade glioma. Eur J Radiol. 2005;56:171-178.
65. Neuloh G., Schramm J. Motor evoked potential monitoring for the surgery of brain tumours and vascular malformations. Adv Tech Stand Neurosurg. 2004;29:171-228.
66. Nikas D.C., Bello L., Zamani A.A., Black P.M. Neurosurgical considerations in supratentorial low-grade gliomas: experience with 175 patients. Neurosurg Focus. 1998;15(4):e4.
67. Nimsky C., Ganslandt O., Fahlbusch R. Functional neuronavigation and intraoperative MRI. Adv Tech Stand Neurosurg. 2004;29:229-263.
68. Nimsky C., Ganslandt O., Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 58(4) (Suppl 2), 2006. ONS-292–303; discussion ONS-303-4
69. Ojemann G., Ojemann J., Lettich E., Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316-326.
70. Petrovich N., Holodny A.I., Tabar V., et al. Discordance between functional magnetic resonance imaging during silent speech tasks and intraoperative speech arrest. J Neurosurg. 2005;103:267-274.
71. Pignatti F., van den Bent M., Curran D., et al. European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group: Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076-2084.
72. Reinges M.H., Nguyen H.H., Krings T. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien). 2004;146:369-377.
73. Reijneveld J.C., Sitskoorn M.M., Klein M. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001;56:618-623.
74. Ricard D., Kaloshi G., Amiel-Benouaich A., et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61:484-490.
75. Rostomily R.C., Keles G.E., Berger M.S. Radical surgery in the management of low-grade and high-grade gliomas. Baillieres Clin Neurol. 1996;5:345-369.
76. Roux F.E., Tremoulet M. Organization of language areas in bilingual patients: A cortical stimulation study. J Neurosurg. 2002;97:857-864.
77. Roux F.E., Boulanouar K., Lotterie J.A. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335-1345.
78. Roux F.E., Boetto S., Sacko O., et al. Writing, calculating, and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome. J Neurosurg. 2003;99:716-727.
79. Rutten G.J., Ramsey N.F., van Rijen P.C. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann Neurol. 2002;51:350-360.
80. Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-764. discussion 264-266. Review
81. Sanson M., Marie Y., Paris S., et al. Isocitrate Deydrogenase 1 codon 132 mutations i san important prognostic biomarker in gliomas. J Clin Oncol. 2009;1:4150-4154.
82. Sarang A., Dinsmore J. Anesthesia for awake craniotomy—-evolution of a technique that facilitates awake neurological testing. Br J Anaesth. 2003;90:161-165.
83. Smith J.S., Chang E.F., Lamborn K.R., et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
84. Stupp R., Janzer R.C., Hegi M.E. Prognostic factors for low-grade gliomas. Semin Oncol. 2003;30:23-28.
85. Szelenyi A., Camper B., Beck J., et al. Transcranial and direct cortical stimulation for evoked potential monitoring in intracerebral aneurysm surgery. Neurophysiol Clin. 2007;27:391-398.
86. van Veelen M.L., Avezaat C.J., Kros J.M., et al. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64:581-587.