Chapter 80 Surgical Management of Cranial Dural Arteriovenous Fistulas
DAVFs account for approximately 5% to 20% of all intracranial vascular malformations.1,2 They can occur at any age, but the mean age at presentation in most studies lies between the sixth and seventh decades of life.2,3 The term “dural arteriovenous malformation” has been applied by some authors to all types of DAVFs, both pediatric and adult. However, malformation implies a congenital origin. We prefer to use the term DAVF because, at least in adults, there is good evidence that these lesions are acquired rather than congenital.4–6 The rare exception is in the pediatric age group in whom congenital malformations of dural venous sinuses are associated with high-flow arteriovenous fistulas.7
Pathogenesis
The etiologic factors and mechanisms involved in the pathogenesis of DAVFs are poorly understood. DAVFs are associated with several conditions including head injury, previous craniotomy, and dural venous sinus thrombosis, suggesting that they are acquired rather than congenital lesions.4,7 According to the most popular theory, DAVFs are formed as a consequence of thrombosis and subsequent recanalization of dural venous sinuses.8,9 Venous hypertension is thought to play an important role in this process. Indeed animal studies have shown that venous hypertension, even in the absence of sinus thrombosis, can elicit the formation of DAVFs.6 Whether sinus thrombosis is in fact the initial event in the genesis of DAVFs is controversial.8 Only a small number of patients with sinus thrombosis go on to develop DAVFs, and not all DAVFs are associated with sinus thrombosis.4,10
What follows sinus thrombosis and venous hypertension is also controversial. Two hypotheses have been proposed. The first suggests that DAVFs arise from opening up of preexisting microscopic vascular channels within the dura mater. These preexisting channels are thought to open up or enlarge as a result of venous hypertension secondary to sinus thrombosis.7 The second hypothesis suggests that DAVFs result from the formation of new vascular channels in the dura, a process stimulated and regulated by angiogenic factors. To support this, surgical DAVF specimens have been shown to contain basic fibroblastic growth factor and vascular endothelial growth factor, which were absent in control specimens.11,12 Angiogenic factors may originate either directly as part of the inflammatory process that occurs during organization and recanalization of a thrombosed sinus or indirectly as a result of cerebral ischemia secondary to venous hypertension.13 If the angiogenic theory is true, antiangiogenic agents may provide an adjuvant therapy for patients with untreatable DAVFs.8
Many cranial DAVFs ultimately undergo spontaneous resolution. The exact mechanism for this is unknown, but it is thought to result from progressive thrombosis of the involved dural sinus. This is paradoxical in that the cause of the abnormality is also thought to be the curing process. In some cases, however, spontaneous resolution has occurred despite sinus patency.14
Classification
Ideally, a classification system should predict the clinical behavior of a lesion and aid in therapeutic decision making. Several classification schemes have been devised for DAVFs.15–19 The most commonly used are those of Borden and colleagues16 (Borden classification) and Cognard and colleagues17 (Cognard classification) (Table 80-1 and Fig. 80-1). Both are based on the pattern of venous drainage of the lesion, the factor that best predicts the clinical presentation and natural history of DAVFs. The Cognard classification, which is a modification of the classification of Djindjian and colleagues,18 divides cranial DAVFs into five types, based on the presence or absence of CVR, sinusal drainage, and direction of flow in the involved dural sinus.17
Table 80-1 Venous Drainage Pattern of Dural Arteriovenous Fistulas According to Borden and Cognard Classification Schemes
| Borden Classification | Cognard Classification |
|---|---|
| Type I: Drainage into dural venous sinus or meningeal vein only | Type I: Drainage into dural venous sinus only, with normal antegrade flow Type IIa: Drainage into dural venous sinus only, with retrograde flow |
| Type II: Drainage into dural venous sinus or meningeal vein + CVR | Type IIb: Drainage into dural venous sinus (antegrade flow) + CVR Type IIa + b: Drainage into dural venous sinus (retrograde flow) + CVR |
| Type III: CVR only | Type III: CVR only without venous ectasia Type IV: CVR only with venous ectasia Type V: Drainage into spinal perimedullary veins |
CVR, cortical venous reflux. This has also been referred to as cortical venous drainage,3 retrograde leptomeningeal venous drainage,20 and drainage into subarachnoid veins.16
Data from Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179; Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
The Borden classification16 is a more simplified scheme with three types based on the presence or absence of CVR and sinusal drainage, without taking into account the direction of flow in the venous sinus. In a study by the University of Toronto Brain Vascular Malformation Study Group, Davies and colleagues19 validated the classification systems of Borden and of Cognard with respect to clinical presentation. Aggressive presentation (i.e., intracranial hemorrhage [ICH], focal neurologic deficit, or death) occurred in 2% of the Borden classification type I, 39% of type II, and 79% of type III cranial DAVFs. Similar correlation was found between the Cognard classification and clinical presentation (Table 80-2).19 Subsequent studies by our group have demonstrated that the pattern of venous drainage and in particular the presence or absence of CVR also correlate with the natural history of DAVFs after the initial presentation.3,20
Table 80-2 Relationship between Aggressive Clinical Presentation and Classification of 102 Cranial DAVFs
| Classification and Type | % of DAVFs with Aggressive Clinical Presentation (i.e., ICH or NHND) |
|---|---|
| Borden type I (n = 55) | 2 |
| Cognard type I (n = 40) | 0 |
| Congard type IIa (n = 15) | 7 |
| Borden type II (n = 18) | 39 |
| Cognard type IIb (n = 8) | 38 |
| Cognard type IIa + b (n = 10) | 40 |
| Borden type III (n = 29) | 79 |
| Cognard type III (n = 13) | 69 |
| Cognard type IV (n = 12) | 83 |
| Cognard type V (n = 4) | 100 |
DAVFs, dural arteriovenous fistulas; ICH, intracranial hemorrhage; NHND, nonhemorrhagic neurologic deficit.
From Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
DAVFs were previously classified according to their anatomic location.21 Anterior cranial fossa and tentorial lesions were associated with a higher risk of aggressive clinical behavior than lesions in other locations.1,19 However, it was later shown that the poor prognosis of lesions in these “dangerous” locations was purely a function of their pattern of venous drainage.19,22 It is the presence of CVR, not the anatomic location per se, that leads to aggressive clinical behavior.1,19 Hemodynamically, DAVFs may be classified into high-flow or low-flow fistulas. The classification of Barrow and colleagues23 is described later in the section on carotid cavernous fistulas. The classification of pediatric DAVFs is not discussed in this chapter.
Clinical Presentation
Other than pulsatile tinnitus, the symptoms of DAVFs are related to venous hypertension.7,24 This may lead to venous congestion, cerebral edema, cerebral infarction, and ICH. The clinical features of cranial DAVFs are shown in Table 80-3. ICH, nonhemorrhagic neurologic deficit (NHND), and death are considered aggressive.1,19 The most significant risk factor for aggressive clinical presentation is the presence of CVR.1,17,19 Therefore, type II and III lesions of the Borden classification are frequently associated with aggressive clinical behavior, whereas type I lesions are rarely so (see Table 80-2). ICH associated with DAVFs typically results from rupture of an arterialized cortical vein and is usually intraparenchymal but may also occur into the subarachnoid, subdural, and intraventricular spaces (Fig. 80-2). NHND is caused by cerebral ischemia secondary to venous congestion. This category does not include ophthalmoplegia secondary to cranial nerve dysfunction. The neurologic deficit may be focal or global.
Table 80-3 Clinical Manifestations of Dural Arteriovenous Fistulas
| Intracranial hemorrhage |
| Focal neurologic deficit (e.g., motor weakness, aphasia, cerebellar signs, progressive myelopathy) |
| Global neurologic deficit (e.g., dementia) |
| Pulsatile tinnitus, objective bruit |
| Proptosis, conjunctival injection, chemosis |
| Ophthalmoplegia (secondary to extraoccular muscle swelling, or compression of cranial nerves III, IV, VI) |
| Visual loss (secondary to orbital congestion and increased intraocular pressure, retinal hemorrhages, or optic neuropathy) |
| Glaucoma |
| Papilledema (secondary to hydrocephalus or pseudotumor cerebri caused by impaired venous drainage) |
| Facial pain (secondary to compression of the first and second divisions of trigeminal nerve in lateral wall of cavernous sinus) |
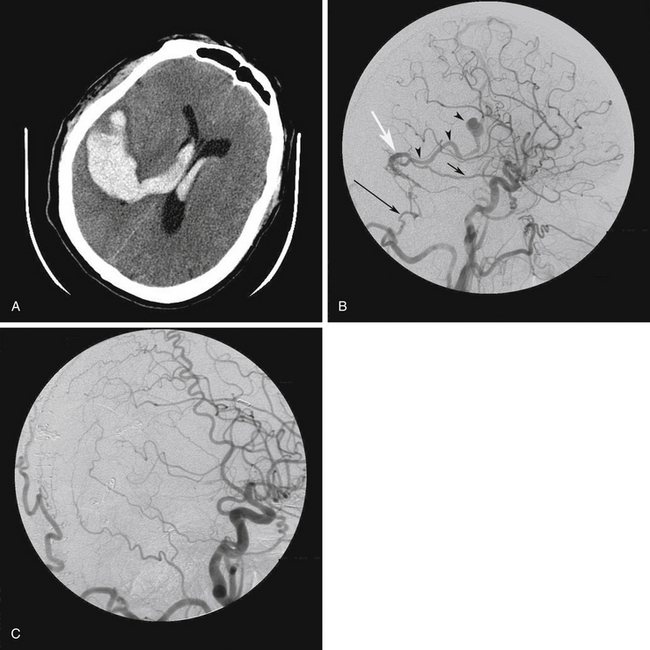
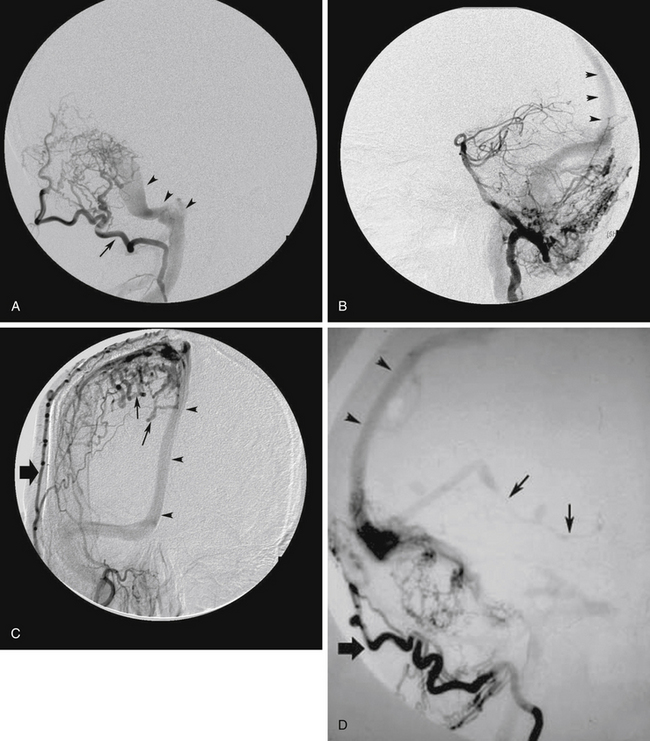
FIGURE 80-2 A, Axial computed tomography scan of a 56-year-old man who presented with sudden headache followed by loss of consciousness shows intracerebral and intraventricular hemorrhage. B, Right common carotid artery angiogram, lateral projection, shows a transverse sinus dural arteriovenous fistula (DAVF), fed by branches of the right middle meningeal artery (arrow) and the right occipital artery (large arrow). Venous drainage was by reflux into a cortical vein (white arrow) leading to an ectasia (arrowheads). There was no sinusal drainage, making this a Borden type III/Cognard type IV DAVF. In this patient, selective angiography was not possible because of severe atherosclerosis and tortuosity of the carotid arteries. C, Postoperative angiogram after right occipital craniotomy exposing the transverse sinus. The dura was opened and reflected inferiorly based on its attachment to the transverse sinus. The DAVF was treated by coagulation and division of this vein close to the transverse sinus, at the site shown by the white arrow in Figure 80-3B.
Benign symptoms include pulsatile tinnitus and orbital symptoms. Pulsatile tinnitus is produced by turbulent flow in the diseased dural sinus.4 Objective bruit is heard in 40% of patients with tinnitus. Ophthalmologic symptoms and signs are most commonly seen with cavernous sinus DAVFs, but they can also occur with lesions in other locations, if the venous drainage involves the cavernous sinus and ophthalmic veins.25 The symptoms are caused by venous congestion.4,26 The ophthalmologic symptoms may be progressive and disabling and may even lead to blindness and therefore may not be considered benign by the patient. In infants, heart failure and craniomegaly may occur.
Radiographic Evaluation
Computed Tomography and Magnetic Resonance
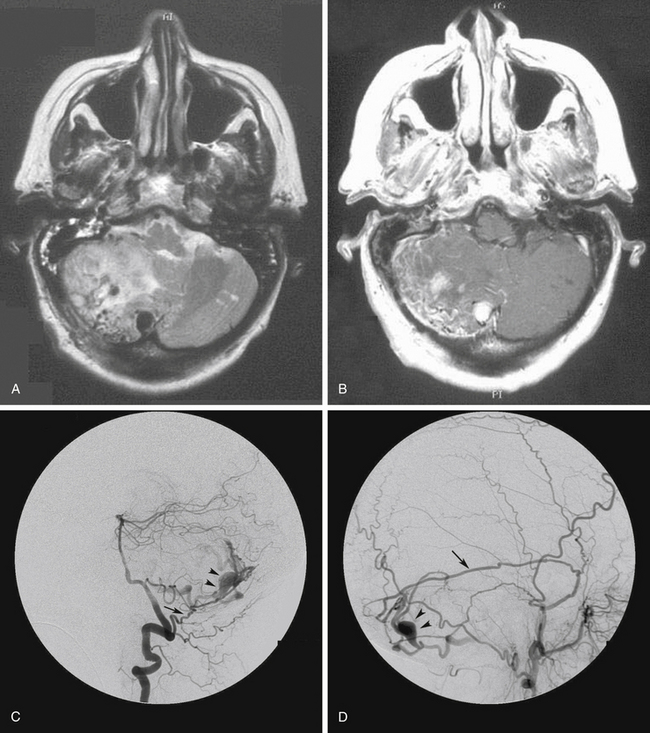
In cases of DAVF without CVR, computed tomography and magnetic resonance imaging (MRI) of the brain parenchyma are typically normal. However, MRI and magnetic resonance angiography may show the stenosis or occlusion of the dural sinuses.7 Hydrocephalus may be seen in any DAVF that causes venous hypertension in the superior sagittal sinus. In cases of DAVF with CVR, computed tomography and MRI of the brain may show ICH, engorged pial vessels, and diffuse white matter edema secondary to venous congestion. The ICH is usually intraparenchymal but may also have subdural, subarachnoid, or intraventricular components (see Fig. 80-2). The pattern of hemorrhage is not specific to DAVFs, and a high index of suspicion is required. Dilated pial vessels and white matter edema are more likely to be seen on MRI than on computed tomography.7 On T2-weighted MRI, dilated pial vessels are seen as flow voids on the surface of the brain, and diffuse white matter edema is seen as hyperintensity in the cerebral or cerebellar hemispheres, brain stem, or spinal cord (Fig. 80-3). Recent reports have demonstrated improvements in the ability of magnetic resonance angiography to diagnose DAVFs and to detect CVR.27 However, these results have been reported in small selected series and require further study. At present, the diagnosis of DAVFs cannot be excluded with negative computed tomography, MRI, or magnetic resonance angiography. If clinically suspected, catheter angiography is required to confirm or exclude the presence of a DAVF.
Intra-arterial Catheter Angiography
This is the gold standard method for diagnosis and evaluation of DAVFs. The characteristic angiographic feature of DAVFs is premature visualization of intracranial veins or venous sinuses during the arterial phase (see Fig. 80-3C; Fig. 80-4A).28 This is caused by shunting of arterial blood into the venous system through the fistula. To obtain the necessary information regarding the arterial supply and venous drainage of the DAVF and the venous drainage of the brain, imaging must start in the ultraearly arterial phase and be carried well into the late venous phase. Selective angiography with magnification and subtraction techniques is essential. The study should include injections of both internal carotid arteries (ICAs), both external carotid arteries (ECAs), and both vertebral arteries. This is because a single DAVF may have multiple feeding arteries (see Figs. 80-3 and 80-4) and also because 8% of patients have multiple DAVFs.29 Detailed knowledge of dural arterial anatomy is essential in angiographic evaluation of DAVFs. Meningeal arteries that are invisible or difficult to see on normal angiograms may be dilated and clearly visible when supplying a DAVF. For example, the tentorial branch of the meningohypophyseal trunk of the ICA (the artery of Bernasconi and Cassinari) or the meningeal branch of the posterior cerebral artery (the artery of Davidoff and Schecter) may be dilated and easily seen on the angiograms (see Fig. 80-3C).
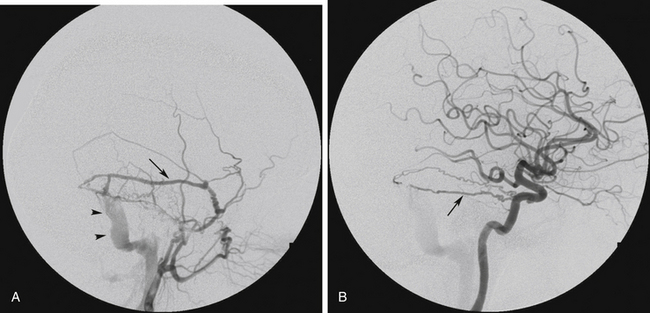
FIGURE 80-4 Angiograms of a 52-year-old woman who presented with pulsatile tinnitus and audible bruit behind the right ear. A, Right external carotid artery angiogram, lateral projection shows premature appearance of the sigmoid sinus (arrowheads) in the ultraearly arterial phase of the angiogram. A transverse sinus dural arteriovenous fistula (DAVF) is shown, fed by the posterior branch of the right middle meningeal artery (arrow) and draining antegradely into the transverse/sigmoid sinus (arrowheads) without any cortical venous reflux (CVR) (Borden type I, Cognard type I). The same DAVF is shown in Figure 80-1A being fed also by the right occipital artery. B, Right internal carotid artery angiogram, lateral projection, revealed a hypertrophied artery of Bernasconi and Cassinari, also supplying the DAVF (arrow). As there was no CVR, the patient was managed with observation alone.
The nidus of a DAVF is the site of arteriovenous shunting and refers to that part of the dura where there is convergence of all feeding arteries and the origin of venous draining channels. The best views of the nidus are often obtained during the ultraearly arterial phase of the angiogram and by injections of distant arterial feeders.28 Images obtained when injecting the main arterial feeders, particularly in the later arterial phase or in the venous phase, are often obscured by engorged feeding arteries and draining veins.
Assessment of the venous drainage pattern of DAVFs is extremely important as this factor determines the natural history of the lesion and aids in selecting the most appropriate management strategy. The presence or absence of CVR, venous sinus occlusion, direction of flow in the venous sinuses, and the venous drainage pattern of the brain must be determined. The exact site of CVR must be determined to allow treatment planning.7 At angiography, a delayed circulation time is compatible with venous congestion.30 Focal areas of delayed venous drainage in the brain correspond to the site of CVR.7 In some cases, tortuous, dilated pial veins may be seen that develop as a result of venous hypertension (see Fig. 80-3F). Willinsky and colleagues30 have described this finding as the pseudophlebitic pattern, which is a sign of venous congestion of the brain and may be associated with an aggressive natural history.7
Natural History
The natural history of a disease refers to the disease course after presentation if left untreated. Knowledge of the natural history is essential in patient management. The results and complications of available treatment strategies must be compared with the outcome of the natural history of the disease. Recently, our group reported the results of the largest prospective natural history studies of patients with cranial DAVFs.3,20 The results, summarized in the following, showed that the presence of CVR is the most significant predictive factor for an aggressive natural history.3,20
Dural Arteriovenous Fistulas with Cortical Venous Reflux
Of 236 cranial DAVFs, 119 had CVR (Borden classification type II or III and Cognard classification type IIb, IIa + b, III, or IV). Of these, 96 patients successfully underwent curative treatment, and three patients were lost to follow-up. Van Dijk and colleagues20 followed the remaining 20 patients with persistent CVR (14 patients who refused treatment and 6 who had partial treatment only) for a mean follow-up period of 4.3 years (86.9 patient-years). In these 20 patients, the annual risks of ICH and NHND (disregarding aggressive events at presentation) were found to be 8.1% and 6.9%, respectively, adding up to an annual event rate (i.e., ICH and NHND) of 15%. The annual mortality rate was 10.4%. These results demonstrate that DAVFs with CVR have an aggressive natural history.
Dural Arteriovenous Fistulas without Cortical Venous Reflux
Of the 236 cranial DAVFs, 117 had no CVR (Borden type I, Cognard type I or IIa). Five patients were lost to follow-up. The remaining 112 patients were followed up clinically for a median of 27.9 months (range, 1 month to 17.5 years), amounting to 348 patient-years. Of these, 68 underwent observation alone, and 44 underwent palliative treatment for symptomatic control (43 endovascular, 1 surgery). Palliative treatment, never aimed at cure, was performed if the patient had intolerable symptoms or if there was persistent high intraocular pressure or decreasing visual acuity. Using this conservative management strategy, 98% of patients had a benign and well-tolerated clinical course, without any ICH or NHND.3 Long-term angiographic follow-up in 50 patients showed that DAVFs without CVR have a 2% to 3% risk of developing CVR.3
Indications for Treatment
Decisions regarding treatment of DAVFs should be guided by their natural history. Therefore, DAVFs with CVR should be treated to eliminate the risk of hemorrhage and neurologic deficit. The aim of treatment in these cases is elimination of CVR or complete cure if possible. Lesions without CVR do not require treatment unless they are associated with intolerable symptoms such as intolerable tinnitus and ophthalmologic symptoms including visual deterioration and pain. For these lesions, the aim of treatment is not cure, but palliative symptom control.
Management Options
Observation
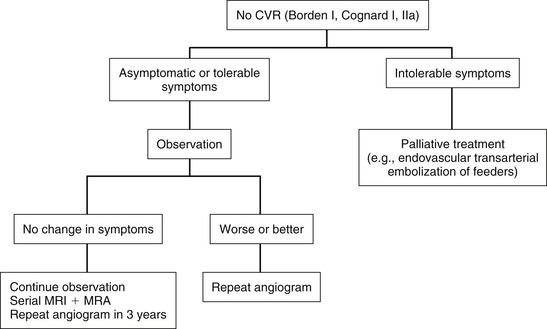
DAVFs without CVR usually behave in a benign manner and are rarely associated with hemorrhage or neurologic deficit. Therefore, observation is the most appropriate management option for these patients if they are asymptomatic or are tolerating their symptoms.4,7 As they have a 2% to 3% chance of developing CVR, all patients should be followed up clinically and radiographically. Any change in symptoms (worsening or improvement) may be a warning signal for development of CVR and should prompt repeat cerebral angiography.3,7 In patients with a stable clinical condition, serial MRI and magnetic resonance angiography and a conventional angiogram after 3 years is advised.7 Observation is not a valid treatment option for DAVFs with CVR.
Compression Therapy
Intermittent manual carotid compression by the patient has been used by Halbach and colleagues,8,31 to treat DAVFs of the cavernous sinus in patients with no evidence of carotid atherosclerosis. The patient is instructed to compress the carotid-jugular area ipsilateral to the DAVF, with the contralateral hand for as long as 30 minutes per session. The compression should be terminated if any weakness develops.8 Halbach and colleagues8,31 reported a cure rate of 27% with this technique after 4 to 6 weeks. However, this treatment has been the subject of debate because the 27% cure rate in the short term may reflect the natural history of the disease.7
Transarterial Embolization
Superselective embolization of the dural feeding vessels to a DAVF can be an effective treatment in some cases.8 The ideal goal is to cure the DAVF by occluding the fistula itself. To achieve this from the arterial side, a microcatheter must be navigated into the distal part of a feeding artery and wedged, so that cyanoacrylate glue can be pushed through the nidus and into the most proximal venous outlet. This is essentially a venous treatment performed through the arterial side. Liquid adhesive agents (e.g., n-butyl cyanoacrylate), the rate of polymerization of which can be controlled, are the best agents to permanently occlude a fistula.7 Successful occlusion of the fistula using this technique has been reported in a small number of patients.7,32
In most cases, transarterial embolization involves occlusion of the feeding arteries without occlusion of the fistula. This may decrease flow through the fistula but is rarely curative because most DAVFs have multiple small feeding arteries that are not amenable to transarterial embolization. Furthermore, in most cases, the lesion will continue to recruit feeding arteries from other sources, leading to recurrence.8,32 Transarterial embolization is commonly used as an adjunct before surgical treatment or transvenous embolization of DAVFs. It is also used as palliative treatment for benign DAVFs with intolerable symptoms. Preoperative embolization of large feeding arteries, particularly in the external carotid territory, carries a relatively low risk and helps to reduce intraoperative blood loss. Particles (e.g., polyvinyl alcohol) may be used for this purpose.7
Endovascular therapy is not without risk. Knowledge of the important anastomoses between the external carotid, internal carotid, and vertebrobasilar systems is crucial for safe embolization.33 Embolic agents can inadvertently travel through these anastomoses and lead to occlusion of important branches. Embolization of feeding arteries that arise directly from the ICA or vertebral artery may lead to reflux of embolic material into the parent artery and result in stroke. In the case of benign DAVFs, it is important to ensure that the embolic material does not flow past the fistula site and obstruct venous outflow. If this occurs, venous drainage may be diverted to cortical veins, thereby converting a benign fistula to an aggressive one.
Transvenous Embolization
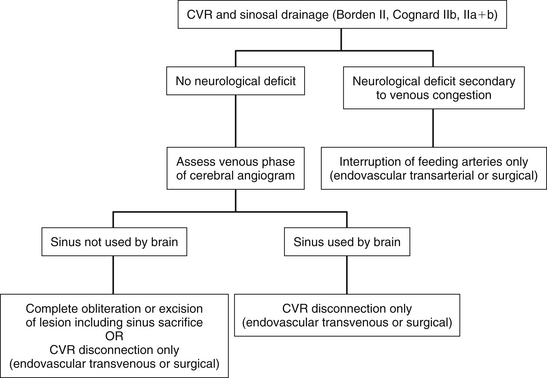
Transvenous embolization is increasingly used in the management of cranial DAVFs.34,35 This involves a retrograde approach through the veins with deposition of materials such as coils into the venous compartment at the fistula site. Usually the transfemoral route is employed. In the majority of cases, transvenous embolization involves sacrificing the involved dural venous sinus. This can only be performed if detailed study of the venous phase of the cerebral angiogram has shown that the involved dural sinus is not being used by the brain and that alternate pathways for venous drainage of the brain have developed.7 In some cases it may be possible to use a retrograde transvenous approach to disconnect CVR without sacrificing the dural sinus. However, in most cases this is not possible because of the difficulty in access to the refluxing cortical veins and the tortuosity of these veins.
In some cases of transverse/sigmoid sinus DAVFs, the lesion may be draining into a venous pouch, parallel to and separate from the transverse sinus. The University of California-San Francisco interventional neuroradiology group have reported 10 such cases and named this entity the parallel venous channel. In their series, transvenous embolization was used to occlude the parallel venous channel and cure the fistula in all 10 cases while preserving the transverse/sigmoid sinus.36 Identification of this separate venous channel is important as it may allow curative treatment of the fistula without sacrificing the transverse sinus. However, this type of channel has been identified in a small number of cases only.
The mainstay of transvenous embolization is occlusion of the affected venous sinus. Neurologic complications after embolization occur if outflow to normally draining veins is obstructed, resulting in venous hypertension and venous infarction. After embolization of cavernous sinus DAVFs, paradoxical worsening of ophthalmologic symptoms may develop secondary to excessive thrombosis and venous hypertension involving the ophthalmic veins. Other complications include guidewire injury to the wall of dural sinuses resulting in subarachnoid hemorrhage and injury to cranial nerves.34
Surgery
Surgery to Obtain Venous Access
In some cases, the usual venous access routes may not be available for transfemoral transvenous embolization. This can occur due to thrombosis or steno-occlusive disease of the dural venous sinuses. In these cases, access may be gained to the fistula by direct surgical exposure and catheterization of the involved sinus. Direct packing of the sinus can then be performed using coils or other thrombogenic materials such as Gelfoam and silk sutures.37
Surgical Excision
Traditionally, the goal of surgery for aggressive DAVFs has been complete excision of the lesion. This involves interruption of the arterial feeders, extensive coagulation, and excision of the pathologic dural leaflet, and disconnection of arterialized leptomeningeal veins (i.e., CVR). In some cases, the involved dural venous sinus is also excised if it is not participating in the venous drainage of normal brain. We use preoperative transarterial embolization of the arterial feeders whenever possible to reduce intraoperative blood loss. Prophylactic intravenous antibiotics are given at induction. Intraoperative mannitol and cerebrospinal fluid drainage using a lumbar spinal drain may be used when necessary to reduce the need for brain retraction. Under general endotracheal anesthesia, the scalp incision and craniotomy are fashioned in the appropriate location. Frameless or frame-based stereotaxy may be helpful in localizing the lesion, particularly if enlarged cortical veins or a venous varix can be visualized on computed tomography or MRI. Once the pathologic region of dura has been exposed, the involved dura and feeding arteries are extensively coagulated. If the involved venous sinus can be sacrificed, the dura is incised on both sides of, and parallel to, the sinus. Feeding arteries are coagulated or occluded using hemostatic clips as they are encountered. The involved segment of the venous sinus can then be ligated proximally and distally and excised together with the pathologic dura. All arterialized leptomeningeal veins are coagulated or clipped at their entrance into the sinus and divided. Intraoperative angiography is used to ensure complete obliteration of the fistula.
If the sinus is being used for normal venous drainage of the brain, it should be skeletonized and left in situ.38 Skeletonization of the sinus involves interruption of the dural arterial supply to the fistula, and excision of dural segments on both sides of the sinus, while preserving the venous sinus and the nonarterialized cortical veins. In cases of transverse/sigmoid or superior sagittal sinus DAVFs, the tentorium or the falx adjacent to the sinus should also be coagulated and cut to ensure complete interruption of arterial supply to the fistula.
The extensive procedures used for total excision of DAVFs may be associated with significant complications. Access may be difficult when the lesion is in a deep location (e.g., tentorial and posterior fossa lesions) and may require extensive skull base procedures. Significant hemorrhage may occur at any stage of the procedure. Torrential hemorrhage may occur from engorged vessels when incising the scalp and from engorged osseous and dural vessels when performing the craniotomy.39 Bleeding from bone should be controlled using bone wax. Bleeding from the enlarged dural arteries feeding the fistula should be controlled using extensive coagulation or hemostatic clips. Preoperative transarterial embolization of the feeding vessels is extremely helpful in reducing intraoperative hemorrhage.
Disconnection of Cortical Venous Reflux Alone
The goal of treatment for aggressive DAVFs is to eliminate the risk of ICH and neurologic deficit. Because CVR is the predisposing factor for aggressive natural history, it has been suggested that a procedure limited to disconnection of arterialized leptomeningeal veins (CVR) is sufficient in the treatment of aggressive lesions and that complete excision of the involved dural leaflet and the venous sinus is not necessary.7,40–42 Disconnection of CVR is a simpler procedure with less morbidity and changes the natural history of the lesion from aggressive to benign. There are now several reports showing that this technique is effective, and we now use it in preference to complete excision in most cases.43
Stereotactic Radiosurgery
There is a limited number of reports on the radiosurgical treatment of intracranial DAVFs.44–49 Pan and colleagues47 reported complete angiographic resolution of the nidus in 47% of 19 transverse/sigmoid sinus DAVFs, after a median follow-up of 19 months. Pollock and colleagues45,46 have used a combination of stereotactic radiosurgery and transarterial embolization. Although radiosurgery is effective in obliterating some DAVFs, its main disadvantage is the long interval between treatment and the expected therapeutic effects. In patients with CVR, this delay is not acceptable because of the high risk of ICH and neurologic deficit (15% per year) while waiting for obliteration of the lesion.7,20,42 In patients without CVR, curative treatment is not required because of the benign natural history of these lesions. Most of these patients can be managed by observation alone or by palliative transarterial embolization. The risk of radiosurgery in these patients may not be acceptable, especially in view of the limited data available on the efficacy of this treatment.3,7 Therefore, the role of radiosurgery in the management of intracranial DAVFs is currently unclear.
Comprehensive Management Strategy
Dural Arteriovenous Fistulas without Cortical Venous Reflux (Benign Fistulas: Borden Type I, Cognard Types I and IIa)
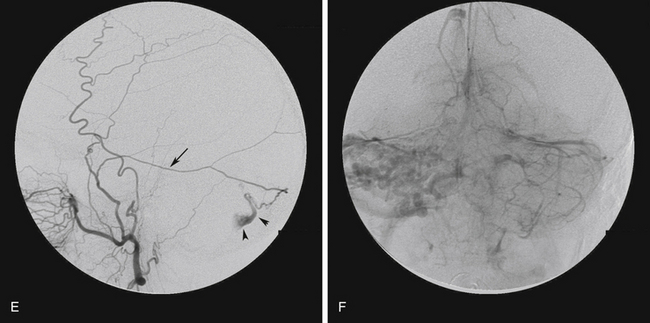
For patients with DAVFs without CVR who are asymptomatic or have tolerable symptoms, observation is the most appropriate treatment option.4,7 If the patient has intolerable symptoms, such as tinnitus or ophthalmologic symptoms, treatment may be indicated. In these cases, curative treatment is not necessary as the patient is not at risk of ICH or neurologic deficit. Palliative treatment in the form of transarterial embolization of the feeding arteries is usually effective in symptom control and is associated with fewer complications compared with curative transvenous embolization or surgery. All patients should be followed up clinically and radiographically, and any change in symptoms (deterioration or improvement) should prompt repeat conventional angiography to look for development of CVR (Fig. 80-5).
Dural Arteriovenous Fistulas with Cortical Venous Reflux (Aggressive Fistulas: Borden Types II and III, Cognard Types IIb through V)
These lesions must be treated aggressively because they are associated with a 15% annual risk of hemorrhage and neurologic deficit.20 Treatment should be performed soon after the diagnosis has been made.4 There is evidence that rebleeding may occur early after an initial hemorrhagic presentation. In one series, 35% of patients had a rebleed within 2 weeks after the first hemorrhage.50 Complete obliteration of the fistula is ideal but not critical because there is increasing evidence that disconnection of CVR alone results in long-term protection against hemorrhage and neurologic deficit.4,40–43,51
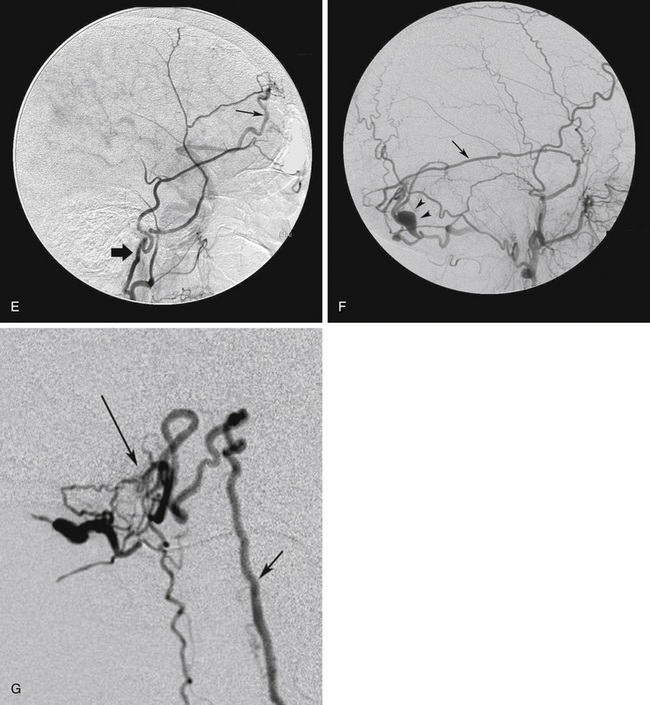
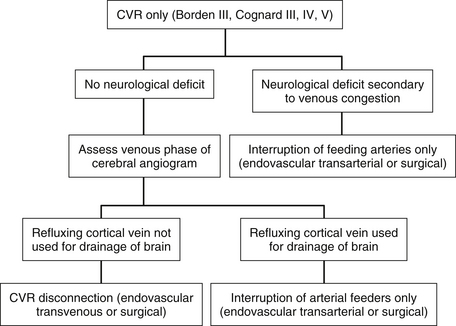
For lesions with CVR only without any sinusal drainage (Borden type III and Cognard types III, IV, and V), CVR disconnection alone is all that is required. Transvenous embolization is rarely successful in these cases because the adjacent venous sinus cannot be used for access to the refluxing cortical vein. Most of these cases therefore require surgical disconnection of CVR. Before disconnection, it must be decided whether it is safe to interrupt the veins involved in CVR. This is particularly important when the vein to be disconnected is a major cortical draining vein, such as the vein of Labbé. The venous phase of the cerebral angiograms must be studied in detail to ensure that normal brain is not draining into the concerned veins and that other venous routes are being used by the brain (Fig. 80-6).
The most difficult situation arises in patients with preoperative neurologic deficit. In these patients, an assumption that the sinus can be sacrificed, based solely on angiographic findings may be incorrect. In other words, the patient’s neurologic deficit may be a consequence of the retrograde flow in the sinus and the resulting venous hypertension, which may improve if normal flow in the sinus can be reestablished. Occlusion of the sinus or the refluxing cortical veins in these cases would eliminate the chance of recovery of neurologic function and may lead to venous infarction. In these patients, the safest option may be to limit the procedure to the arterial side of the lesion. This can be achieved by extensive transarterial embolization of the feeding arteries or by surgical skeletonization of the sinus. All cortical veins and the venous sinuses should be left intact. Fortunately, this situation arises rarely (Fig. 80-7).
Anatomic Considerations for Dural Arteriovenous Fistulas in Specific Locations
In itself, anatomic location of a DAVF has no direct correlation with aggressive clinical events, although this has been suggested in the past.3,19 DAVFs in any location can develop CVR (Table 80-4).1,19 However, DAVFs in particular locations such as the anterior cranial fossa and the tentorium are more often associated with CVR and are therefore more likely to behave aggressively than are lesions in other locations. This reflects the pattern of regional venous anatomy in these locations. For example, anterior cranial fossa DAVFs cannot drain into any venous sinuses and can only drain into cortical veins and are therefore always associated with CVR.19 Table 80-4 shows the anatomic location of intracranial DAVFs. They are named according to the dural sinus with which they are associated.52 This section deals with the anatomy and surgical management of DAVFs in specific locations.
Table 80-4 Anatomic Location of 236 DAVFs Managed by University of Toronto Brain Vascular Malformation Study Group
| Anatomic Location | No. (% of all DAVFs) | No. with CVR (% of DAVFs in This Location) |
|---|---|---|
| Transverse sigmoid | 100 (42.4) | 38 (38) |
| Cavernous sinus | 79 (33.5) | 29 (36.7) |
| Deep venous/tentorial incisural | 20 (8.5) | 20 (100) |
| Superior sagittal sinus and convexity | 12 (5.1) | 12 (100) |
| Foramen magnum | 11 (4.6) | 9 (81.8) |
| Anterior cranial fossa | 9 (3.8) | 9 (100) |
| Temporal fossa | 4 (1.7) | 1 (25) |
| Superior petrosal sinus | 1 (0.4) | 1 (100) |
CVR, cortical venous reflux; DAVFs, dural arteriovenous fistulas.
Transverse/Sigmoid Sinus Dural Arteriovenous Fistulas
Transverse/sigmoid sinus DAVFs are the most common intracranial DAVFs, accounting for 4% to 60% of cases (see Table 80-4).28,36,43 Their arterial supply is derived mainly from branches of the ECA, namely, the transmastoid branches of the occipital artery, the posterior auricular artery, the posterior branch of the middle meningeal artery, and the neuromeningeal division of the ascending pharyngeal artery. They may also receive arterial supply from the posterior meningeal branch of the vertebral artery, the tentorial branch of the meningohypophyseal trunk of the ICA, and the petrous bone.8,28 Venous drainage may be through the ipsilateral transverse/sigmoid sinuses or through the contralateral side if the ipsilateral sinus is occluded. Occasionally, these lesions drain into the diploic veins, which may lead to significant hemorrhage during craniotomy. CVR may occur through temporal, occipital, and cerebellar veins.4,7
If endovascular therapy fails, surgery should be performed in cases with CVR. Preoperative transarterial embolization is very helpful in reducing intraoperative bleeding and should be used whenever possible. The patient is placed in the park-bench position with the side of the lesion up. It is important to have access to the region between the inion and the mastoid. A lazy “S”-shaped incision is made two fingerbreadths behind the ear. The occipital and posterior auricular arteries, which are usually enlarged, should be doubly ligated and divided. In some cases, this interrupts the main arterial supply to the DAVF. The nuchal muscles are incised using monopolar cautery, leaving a small cuff of fascia at the superior nuchal line. The muscles are then scraped off the bone using subperiosteal dissection. A craniotomy is performed to expose the transverse sinus and the dura superior and inferior to it. Great care must be taken to prevent a dural laceration while performing the craniotomy as this may lead to torrential hemorrhage from enlarged dural feeding arteries. The cutting bit of a high-speed drill may be used for performing the craniotomy. The bone is gradually removed along the margin of the bone plate until the dura can be seen through a thin layer of bone. The dura is gently separated from the bone flap, and the bone flap is elevated using a periosteal elevator.39 Significant hemorrhage from the dura when elevating the bone flap may be controlled by placing a large piece of Gelfoam or Surgicel over the dura and applying pressure with a wet sponge for a few minutes. Dural feeders can be coagulated using bipolar cautery or occluded using hemostatic clips.
If the goal of treatment is complete excision of the fistula and the sinus can be sacrificed, then after performing the craniotomy as described previously, the mastoid and posterolateral portion of the petrous bone are removed using a diamond burr on a high-speed drill to expose the dura lateral and anterior to the sigmoid sinus.38,39 Knowledge of the anatomy of this region or assistance from an otolaryngologist is important to avoid damage to hearing or to the facial nerve during this stage of the procedure.38 At this point, bleeding is mainly from arterial feeders contained within the petrous bone, which should be controlled using bone wax. The dura is incised above and below the transverse sinus and parallel to its long axis. Feeding arteries are coagulated or clipped as they are encountered. The transverse sinus is then occluded medially using two curved hemostats placed approximately 2 cm apart. The sinus is cut between the two hemostats, the ends are closed using 3-0 silk transfixion sutures, and the hemostats are removed. The dural incisions above and below the sinus are then continued laterally. Gentle retraction on the occipital lobe and the cerebellum exposes the tentorium, which should also be cut in a medial to lateral direction to interrupt any tentorial feeding arteries. All arterialized leptomeningeal veins are also coagulated and divided. The dural and tentorial incisions are extended laterally, distal to the junction of the transverse and sigmoid sinuses, which is usually the site of the fistula. The skeletonized sinus is resected distal to this site. The sigmoid sinus is then evaluated for patency. If there is a significant vein of Labbé, the sigmoid sinus should be left patent to drain it. However, if the vein of Labbé is arterialized and is not used for venous drainage of the brain, it can be clipped and divided, and the sigmoid sinus is then packed with Surgicel or Gelfoam and ligated. Duraplasty using fascia lata, pericranium, or synthetic dura is then performed to close the dural defect. The mastoid air cells are completely obliterated using bone wax, and the bone flap is replaced. The nuchal muscles and fascia are closed in multiple layers to prevent cerebrospinal fluid leak. The scalp is closed in the usual manner. Intraoperative angiography is used to ensure complete excision of the nidus.
Cavernous Sinus Dural Arteriovenous Fistulas
These are the second most common intracranial DAVFs, accounting for a third of all lesions in our series (see Table 80-4).8,28,43 They are supplied by branches from the ICAs, ECAs, or both. Venous drainage is via the cavernous sinus, with subsequent anterior drainage into the superior and inferior ophthalmic veins, posterior drainage into the superior and inferior petrosal sinuses (IPSs), or cortical venous drainage into the sphenoparietal sinus, sylvian, uncal, and anterior pontomesencephalic veins.7,28 Barrow and colleagues23 classified carotid cavernous fistulas into four categories based on the type of feeding arteries detected on angiography. Type A fistulas are direct fistulas between the ICA and the cavernous sinus. Types B, C, and D are DAVFs involving the cavernous sinus. Type B are fed by meningeal branches of the ICA, type C by meningeal branches of ECA, and type D by meningeal branches of both ICA and ECA. Type A fistulas are high-flow fistulas and usually result from a traumatic tear in the cavernous ICA or spontaneously from a rupture of an intracavernous ICA aneurysm. These high-flow fistulas rarely resolve spontaneously and require treatment to prevent progressive visual loss, intolerable bruit, or pain.19 They are almost always treated by endovascular embolization and are not dealt with in this chapter. Most spontaneous carotid cavernous fistulas are of the low-flow types B, C, or D and are referred to as cavernous sinus DAVFs. They are usually idiopathic and occur most commonly in middle-aged women. They may present with proptosis, chemosis, diplopia, visual loss, pulsatile bruit, retro-orbital pain, and facial pain.35 Cavernous sinus DAVFs may present with ophthalmologic symptoms on the contralateral side. Unlike type A fistulas, a significant proportion of these low-flow fistulas undergo spontaneous resolution.
Cavernous sinus DAVFs can also be classified according to the pattern of venous drainage based on the Borden or Cognard classification. As with other DAVFs, it is the presence or absence of CVR that determines the natural history of these lesions. CVR occurs in 19% to 37% of patients with cavernous sinus DAVFs (see Table 80-4).35,53 CVR is more common in patients with bilateral orbital signs but can also occur in those with unilateral signs. In a study of 118 patients with cavernous sinus DAVFs, 43% of patients with bilateral orbital signs and 9% of those with unilateral orbital signs had CVR.53 Cavernous sinus DAVFs without CVR usually have a benign clinical course. Many of these lesions undergo spontaneous involution, with symptom resolution over weeks or months.28 The majority of patients can be managed conservatively but must be followed up closely by a neuro-ophthalmologist. Although these lesions have been referred to as benign, in some cases, spontaneous venous thrombosis and venous hypertension may result in increased intraocular pressure and progressive visual loss.7 Medical therapy including topical agents, oral corticosteroids, and systemic anticoagulation may be used to prevent blindness. However, if medical therapy fails to control intraocular pressure and deteriorating vision, prompt endovascular treatment is indicated.7 Transarterial embolization using particles may be tried first to slow down the flow through the fistula. If this fails, transvenous embolization is required.
If CVR exists, these DAVFs must be treated with the same aggressiveness as DAVFs in other locations. This is usually done using transvenous embolization. Before this, transarterial embolization may be performed, particularly if there is significant supply through ECA branches. For transvenous embolization, access to the cavernous sinus is usually obtained by the femoral route through the internal jugular vein and the IPS. This venous approach may be difficult or impossible in cases in which the IPS is partially or completely thrombosed. However, with improved materials and experience, some surgeons have successfully navigated an angiographic catheter, even through a thrombosed IPS.54 If access through the IPS is not possible or has failed, other routes may be employed. These may involve endovascular and/or surgical techniques. The approach depends on the direction of venous drainage of the fistula and may use the superior ophthalmic vein (SOV), the facial and angular veins, the pterygoid plexus, the superior petrosal sinus, the refluxing cortical veins, or direct surgical puncture of the cavernous sinus.34 The goal of transvenous embolization is to occlude the venous side of the lesion. This is usually achieved by placing coils into the cavernous sinus. Obliteration of the venous side of the lesion can result in the total disappearance of the fistula.28 However, in some cases of successful treatment, thrombosis may extend into the orbital veins, and this may lead to venous hypertension and paradoxical visual deterioration.7 This may require treatment with oral corticosteroids, antiplatelet therapy, or anticoagulation.
If endovascular therapy fails, surgery is indicated in cases with persistent CVR or when vision is at risk. The role of surgery in the treatment of cavernous sinus DAVFs is mainly in providing venous access to the cavernous sinus in cases in which access through the femoral route is not possible. Several options are available, including cannulation of the SOV, cannulation of refluxing intracranial veins such as the sylvian veins, or direct exposure of the cavernous sinus.34
The SOV approach is often performed by a team consisting of an ophthalmologist, a neurosurgeon, and a neuroradiologist.55 The procedure is performed under general anesthesia and under fluoroscopic guidance. A sheath is placed in a common femoral artery to allow intraoperative angiography. The skin incision is made in the nasal part of the superior sulcus of the upper eyelid, under magnification provided by an operating microscope.55 The incision is carried down through the orbicularis oculi muscle. The orbital septum is then opened, exposing the retroseptal orbital fat. The SOV is then identified and cleaned of the surrounding orbital fat to expose a segment of vein 5 to 20 mm in length. Two ligatures (2-0 silk suture) are placed around the vein approximately 1 cm apart. A sheath is then inserted into the vein between the ligatures, and thrombogenic material (such as platinum coils) is injected into the cavernous sinus under fluoroscopy. At the end of the procedure, an intraoperative angiogram is performed through the femoral sheath to ensure complete closure of the fistula. The SOV sheath is then removed, the vein is suture ligated, and the lid incision is closed. Alternatives to surgical exposure of the SOV have been reported and include direct percutaneous puncture of the SOV under angiographic guidance, direct percutaneous puncture of the facial vein under sonographic guidance, and transvenous transfemoral access to the facial vein.34,35 In cases in which the facial vein is catheterized, the catheter is advanced into the angular vein, then into the SOV, and finally into the cavernous sinus.
In rare instances, a cavernous sinus DAVF may only drain by retrograde flow into cortical veins, and there may be no access through the IPSs, the ophthalmic veins, or the pterygoid plexus. In such cases, access to the cavernous sinus may be achieved via the refluxing cortical vein (e.g., a refluxing sylvian vein). This has been described using the percutaneous transfemoral technique and also by surgical exposure of the cortical vein.34
Direct microsurgery of cavernous sinus DAVFs has also been described,9,56–58 but with advances in endovascular embolization, these techniques are rarely required. These procedures require surgical experience and detailed knowledge of the anatomy of the cavernous sinus. The cavernous sinus is approached through triangles that are formed by convergence and divergence of the cranial nerves in the region of the cavernous sinus and the middle fossa.25 The selection of approach is determined by the pattern of venous drainage of the fistula, and the degree of familiarity of the surgeon. The two main approaches used are described below.
The anteroinferior extradural approach to the cavernous sinus is suitable for anteriorly draining fistulas and is performed through a standard pterional craniotomy. After administration of intravenous mannitol, the dura is reflected away from the anterior and anteromedial walls of the middle fossa to expose the lateral wall of the cavernous sinus. The posterior aspect of the orbital roof is then drilled all the way to the lateral aspect of the anterior clinoid process. The superior orbital fissure region is exposed, the periorbita is opened, and the cranial nerves are identified. The superior or inferior orbital vein is then identified. An intraoperative Doppler probe may be used to localize the high arterial flow in these veins.58 An atraumatic intravenous cannula is then inserted into the vein, and a guidewire is directed posteriorly into the cavernous sinus. A catheter is then introduced over the guidewire into the cavernous sinus and used to introduce thrombogenic material such as coils to obliterate the fistula. An intraoperative angiogram is performed to confirm adequate obliteration of the sinus. It must be noted that it may be difficult to identify the ophthalmic veins in a low-flow fistula.9
The posterosuperior approach is appropriate for posteriorly draining fistulas. It is performed through a pterional craniotomy. After opening the dura, the sylvian fissure is split widely to allow gentle retraction of the frontal and temporal lobes. The oculomotor nerve is then identified as a landmark. Underneath the oculomotor nerve, the superior petrosal sinus is identified and followed proximally to its origin from the cavernous sinus. Thrombogenic material is injected through the superior petrosal sinus into the cavernous sinus.9 If the superior petrosal sinus cannot be identified, the cavernous sinus can be packed through Parkinson’s triangle, bounded by the lower margin of the trochlear nerve, the upper margin of the ophthalmic division of trigeminal nerve, and a line connecting the point of entry of trochlear nerve into the dura to the point of entry of trigeminal nerve into Meckel’s cave.25 An intraoperative angiogram is performed to confirm complete occlusion of the fistula. The dura is closed as usual, the bone flap is repositioned, and the temporalis muscle, galea, and skin are closed in layers. Combined and more extensive approaches to the cavernous sinus, with opening and packing of all triangles, have been described but are rarely necessary.56
Anterior Cranial Fossa Dural Arteriovenous Fistulas
The arterial supply to the anterior cranial fossa dura is derived mainly from the ICA through the ophthalmic artery, which gives off the anterior and posterior ethmoidal arteries within the orbit. The ethmoidal arteries leave the orbit to enter the ethmoidal air cells. The smaller of the two, the posterior ethmoidal artery, supplies the dura in the region of planum sphenoidale. The anterior ethmoidal artery gives origin to the falcine artery and supplies the dura of the medial and inferior portions of the anterior cranial fossa. As well as supply from the ethmoidal arteries, DAVFs in this region may also recruit dural arterial supply via the anterior division of the middle meningeal arteries from both dural convexities and transcranial arterial supply via engorged scalp arteries.28 The venous drainage of anterior cranial fossa DAVFs is always via cortical venous drainage through the frontal and olfactory veins.4,7 The cortical veins may often be variceal. This constant presence of CVR explains the high incidence of aggressive clinical behavior for lesions in this location. They must therefore be treated aggressively to eliminate CVR. Anterior fossa DAVFs are rarely cured by endovascular means. Transarterial embolization is usually limited to ECA branches. Embolization of ophthalmic artery branches is usually avoided as it carries the risk of central retinal artery occlusion and visual compromise.59 Transvenous embolization has been described in case reports60 but is not possible for most lesions in this location due to lack of venous access. Surgery is associated with high cure rates and low complication rates and is the treatment of choice for most anterior fossa DAVFs.59 This is performed through a low unilateral frontal craniotomy in the supine position with minimal retraction of the frontal lobes. A unilateral craniotomy allows disconnection of leptomeningeal veins on both sides via the transfalcine route. Alternatively, a bifrontal craniotomy may be performed. The next step is to disconnect the arterialized leptomeningeal veins, which should be coagulated and divided. Excision of the dura involved with the fistula is not necessary.59
Superior Sagittal Sinus (Convexity) Dural Arteriovenous Fistulas
The arterial supply to these lesions is from the middle meningeal arteries. The venous drainage may be into the superior sagittal sinus, into cortical veins, or both. If CVR exists, the lesion must be treated to prevent future hemorrhage or neurologic deficit. Few cases of successful curative endovascular treatment have been reported.8,61 Surgical treatment is therefore required in most cases and involves disconnection of CVR. The lesion is usually approached through a unilateral craniotomy on the side of the fistula. Preoperative transarterial embolization is used to reduce the amount of hemorrhage from scalp and dura.
In some cases without CVR, there may be a high-flow fistula that shunts into a patent superior sagittal sinus. These lesions may be associated with papilledema.28 These cases may be managed with extensive transarterial embolization or surgical skeletonization of the superior sagittal sinus to reduce the flow through the fistula. Other options include the use of lumboperitoneal shunting or optic nerve sheath decompression for treatment of papilledema.28
Deep-Seated and Posterior Fossa Dural Arteriovenous Fistulas
This group consists of lesions associated with the deep veins (tentorial incisura), superior petrosal sinus, IPS, or the marginal sinus (foramen magnum). As with DAVFs in other locations, deep-seated and posterior fossa lesions without CVR can be observed or treated palliatively by transarterial embolization of the arterial feeders. Lesions with CVR should be treated aggressively until all CVR is disconnected. This may be achieved by endovascular or surgical means.42,43,52 Extensive resection of the nidus, which was performed in the past, often requires complex skull base approaches, carries significant risk of morbidity, and is unnecessary. If endovascular treatment fails to eliminate CVR, surgical disconnection can be performed with relatively little risk to the patient.42,43
Deep Venous (Tentorial Incisura) Dural Arteriovenous Fistulas
These lesions are located at the tentorial incisura and involve the deep venous system. They may extend along the straight sinus to involve the falx. Their arterial supply may be bilateral and is derived from the tentorial dural branches of the ECA, ICA, and vertebral and posterior cerebral arteries. Angiography may reveal hypertrophy of embryonic dural arteries, including the artery of Bernasconi and Cassinari (tentorial branch of the meningohypophyseal trunk of the ICA) and the artery of Davidoff and Schecter (dural branch of the posterior cerebral artery).28 Venous drainage is almost always leptomeningeal and involves the basal vein of Rosenthal, the lateral mesencephalic veins, and the vein of Galen.7,8 Fistulas at this location may have infra- or supra-tentorial cortical venous drainage or drainage into spinal medullary veins.42 The presence of CVR explains the high incidence of ICH and neurologic deficit with these lesions. Drainage into the spinal medullary veins may be associated with progressive quadriparesis.62 Surgical exposure of these lesions may be performed using a posterior parasagittal craniotomy and interhemispheric approach with the trajectory posterior to the splenium of the corpus callosum.63 Surgical treatment consists of CVR disconnection.
Superior Petrosal Sinus Dural Arteriovenous Fistulas
These lesions are often included in the same group as the deep venous DAVFs and together are referred to as tentorial DAVFs. Other authors have grouped them separately because they are located at the petrous ridge and involve the superior petrosal sinus.8,52 The arterial supply is from dural branches of the ECA, ICA, and the vertebrobasilar system. Most cases are associated with thrombosis of the superior petrosal sinus on either side of the fistula site, with venous drainage diverted into cortical veins.8,52 ICH is the most common mode of presentation. In a series of 18 patients reported by Ng and colleagues,52 17 (94%) had CVR and 9 (50%) presented with ICH. Ocular symptoms may occur if there is venous reflux into the cavernous sinus and the SOV. Trigeminal neuralgia may occur secondary to irritation of the trigeminal ganglion in Meckel’s cave by dilated tortuous feeding arteries or compression of the trigeminal nerve at the root entry zone by a dilated petrosal vein. Surgical excision of this type of DAVF may require an extensive skull base approach consisting of subtemporal craniotomy, posterior petrosectomy, and suboccipital craniotomy.63 These extensive procedures are usually unnecessary, and treatment should focus on disconnection of CVR only.
Inferior Petrosal Sinus Dural Arteriovenous Fistulas
The main arterial supply to these lesions is usually from the dural and muscular branches of the vertebral arteries and dural branches from the ascending pharyngeal, middle meningeal, and occipital branches of the ECA. Venous drainage may be retrograde up the IPS and into the cavernous sinus. This may result in presentation with ocular symptoms similar to those of cavernous sinus DAVFs. Alternatively, venous drainage may flow into the ipsilateral jugular bulb, resulting in pulsatile tinnitus.8 Retrograde flow up the ipsilateral sigmoid and transverse sinuses and into the contralateral transverse/sigmoid sinuses or into the straight and superior sagittal sinuses may also occur.63 Surgical exposure may be performed using the far lateral suboccipital approach.63 Treatment consists of CVR disconnection.
Marginal Sinus (Foramen Magnum Region) Dural Arteriovenous Fistulas
These lesions are rare. The marginal sinus encircles the foramen magnum and communicates with the basal venous plexus of the clivus anteriorly and with the occipital sinus posteriorly. It normally drains to the sigmoid sinus or jugular bulb through a series of small sinuses.64 Fistulas in this area have often been grouped together with posterior fossa DAVFs or referred to as foramen magnum region DAVFs. Their arterial supply is usually from the muscular branches of the vertebral artery, the transmastoid perforators of the occipital artery, the neuromeningeal division of the ascending pharyngeal artery, and the posterior clival dural branches of the meningohypophyseal trunk of the ICA.64 Venous drainage may be into the sigmoid sinus/jugular vein, in which case the patient may complain of pulsatile tinnitus, or into the IPS, which may lead to venous engorgement in the ipsilateral cavernous sinus and ocular symptoms.28,64 CVR may lead to ICH, and reflux into the spinal medullary veins may result in progressive myelopathy and quadriparesis.65 These lesions are surgically approached through a posterior paramedian craniocervical exposure, with the patient in the semiprone position. The venous plexus surrounding the vertebral artery may be arterialized and require meticulous hemostasis during exposure. Suboccipital craniotomy and laminectomy at the appropriate levels are then performed. The dura is extensively coagulated and opened. Refluxing leptomeningeal veins communicate with arterialized tortuous posterior and/or anterior medullary veins. The refluxing leptomeningeal veins are coagulated or clipped and divided, whereas the anterior and posterior medullary veins should be left intact.
Outcome and Complications of Treatment
Between 1984 and 2001, 236 cranial DAVFs have been managed by the University of Toronto Brain Vascular Malformation Study Group. Of these, 117 did not have CVR (Borden type I and Cognard types I and IIa) and were managed by observation alone or by palliative treatment. Of these patients, 98% had a benign clinical course.3 The other 119 patients had DAVFs with CVR (Borden types II and III, and Cognard type IIb, IIa + b, III, or IV). Three patients were lost to follow-up after initial assessment, and 14 patients declined treatment. The remaining 102 patients were treated.43 Forty-seven patients (46.1%) were treated by disconnection of CVR, 49 (48%) had complete obliteration of the fistula, and 6 (5.9%) were partially treated. The six partially treated patients had persistent CVR but refused or were physically unable to undergo further treatment. Therefore, complete obliteration of the fistula or CVR disconnection was achieved in 94.1% of patients. This was achieved by endovascular treatment alone in 26 patients (25.5%), by surgery alone in 25 patients (24.5%), and by a combination of endovascular and surgical treatments in 45 patients (44%). Multiple treatment sessions were required in some patients, resulting in 120 endovascular and 74 surgical procedures. The complications of treatment were all transient and are outlined in Table 80-5. No deaths or permanent neurologic deficits occurred in this series. The mean follow-up period was 3.7 years (741 patient-years). Control angiography was performed in 93 patients (91.2%). CVR disconnection was equally effective in lowering the risk of aggressive events as total obliteration of the fistula.43
Table 80-5 Complications of Treatment in 102 Patients with Cranial Dural Arteriovenous Fistulas with Cortical Venous Reflux
| Complication | Surgery (74 Procedures) | Endovascular Embolization (120 Procedures) |
|---|---|---|
| Significant blood loss | 8 | 0 |
| Memory dysfunction | 1 | 1 |
| Seizures | 1 | 0 |
| Cranial nerve deficit | 1 | 0 |
| Hemorrhage | 1 | 0 |
| Meningitis | 1 | 0 |
| Embolic transient ischemic attack | 0 | 1 |
| Groin hematoma | 0 | 1 |
From Van Dijk JM, Ter Brugge KG, Willinsky RA, Wallace MC. Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg. 2004;101:31-35.
Al-Shahi R., Bhattacharya J.J., Currie D.G., et al. Prospective, populationbased detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
Aminoff M.J. Vascular anomalies in the intracranial dura mater. Brain. 1973;96:601-612.
Awad I.A., Little J.R., Akarawi W.P., et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;2:839-850.
Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
Davies M.A., TerBrugge K., Willinsky R., et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
Djindjian R., Merland J.J., Theron J. Superselective Arteriography of the External Carotid Artery. New York: Springer-Verlag; 1977.
Hamada Y., Goto K., Inoue T., et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452-458.
Herman J.M., Spetzler R.F., Bederson J.B., et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539-545.
Lalwani A.K., Dowd C.F., Halbach V.V. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11-15.
Lasjaunias P., Chiu M., ter Brugge K., et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724-730.
Lawton M.T., Jacobowitz R., Spetzler R.F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267-274.
Luciani A., Houdart E., Mounayer C., et al. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001;22:992-996.
Malek A.M., Halbach V.V., Higashida R.T., et al. Treatment of dural arteriovenous malformations and fistulas. Neurosurg Clin North Am. 2000;11:147-166.
Malik G.M., Pearce J.E., Ausman J.I., et al. Dural arteriovenous malformations and intracranial hemorrhage. Neurosurgery. 1984;15:332-339.
Mullan S. Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979;50:131-144.
Rhoton A.L.Jr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002;51:S375-S410.
Sarma D., ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol. 2003;46:206-220.
Satomi J., van Dijk J.M., Terbrugge K.G., et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
Terada T., Higashida R.T., Halbach V.V., et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
Terada T., Tsuura M., Komai N., et al. The role of angiogenic factor bFGF in the development of dural AVFs. Acta Neurochir (Wien). 1996;138:877-883.
Uranishi R., Nakase H., Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781-786.
van Dijk J.M., terBrugge K.G., Willinsky R.A., et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
van Dijk J.M., Willinsky R.A. Venous congestive encephalopathy related to cranial dural arteriovenous fistulas. Neuroimaging Clin North Am. 2003;13:55-72.
1. Awad I.A., Little J.R., Akarawi W.P., et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
2. Al-Shahi R., Bhattacharya J.J., Currie D.G., et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
3. Satomi J., van Dijk J.M., Terbrugge K.G., et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
4. Sarma D., ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol. 2003;46:206-220.
5. Herman J.M., Spetzler R.F., Bederson J.B., et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539-545.
6. Terada T., Higashida R.T., Halbach V.V., et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
7. van Dijk J.M., Willinsky R.A. Venous congestive encephalopathy related to cranial dural arteriovenous fistulas. Neuroimaging Clin North Am. 2003;13:55-72.
8. Malek A.M., Halbach V.V., Higashida R.T., et al. Treatment of dural arteriovenous malformations and fistulas. Neurosurg Clin North Am. 2000;11:147-166.
9. Mullan S. Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979;50:131-144.
10. Hamada Y., Goto K., Inoue T., et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452-458.
11. Terada T., Tsuura M., Komai N., et al. The role of angiogenic factor bFGF in the development of dural AVFs. Acta Neurochir (Wien). 1996;138:877-883.
12. Uranishi R., Nakase H., Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781-786.
13. Lawton M.T., Jacobowitz R., Spetzler R.F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267-274.
14. Luciani A., Houdart E., Mounayer C., et al. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001;22:992-996.
15. Lalwani A.K., Dowd C.F., Halbach V.V. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/ sigmoid sinus. J Neurosurg. 1993;79:11-15.
16. Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
17. Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
18. Djindjian R., Merland J.J., Theron J. Superselective Arteriography of the External Carotid Artery. New York: Springer-Verlag; 1977.
19. Davies M.A., TerBrugge K., Willinsky R., et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
20. van Dijk J.M., terBrugge K.G., Willinsky R.A., et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
21. Aminoff M.J. Vascular anomalies in the intracranial dura mater. Brain. 1973;96:601-612.
22. Malik G.M., Pearce J.E., Ausman J.I., et al. Dural arteriovenous malformations and intracranial hemorrhage. Neurosurgery. 1984;15:332-339.
23. Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
24. Lasjaunias P., Chiu M., ter Brugge K., et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724-730.
25. Rhoton A.L.Jr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002;51:S375-S410.
26. Stiebel-Kalish H., Setton A., Nimii Y., et al. Cavernous sinus dural arteriovenous malformations: patterns of venous drainage are related to clinical signs and symptoms. Ophthalmology. 2002;109:1685-1691.
27. Noguchi K., Melhem E.R., Kanazawa T., et al. Intracranial dural arteriovenous fistulas: evaluation with combined 3D time-of-flight MR angiography and MR digital subtraction angiography. AJR Am J Roentgenol. 2004;182:183-190.
28. Awad I.A. Dural arteriovenous malformations. In: Carter L.P., Spetzler R.F., Hamilton M.G. Neurovascular Surgery. New York: McGraw-Hill; 1995:905-932.
29. van Dijk J.M., TerBrugge K.G., Willinsky R.A., et al. Multiplicity of dural arteriovenous fistulas. J Neurosurg. 2002;96:76-78.
30. Willinsky R., Goyal M., terBrugge K., et al. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol. 1999;20:1031-1036.
31. Halbach V.V., Higashida R.T., Hieshima G.B., et al. Dural fistulas involving the cavernous sinus: results of treatment in 30 patients. Radiology. 1987;163:437-442.
32. Nelson P.K., Russell S.M., Woo H.H., et al. Use of a wedged microcatheter for curative transarterial embolization of complex intracranial dural arteriovenous fistulas: indications, endovascular technique, and outcome in 21 patients. J Neurosurg. 2003;98:498-506.
33. Lasjaunias P., Berenstein A., ter Brugge K. Surgical Neuroangiography: Clinical Vascular Anatomy and Variations. Vol 1, 2nd ed. Berlin: Springer-Verlag; 2001.
34. Klisch J., Huppertz H.J., Spetzger U., et al. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003;53:836-857.
35. Meyers P.M., Halbach V.V., Dowd C.F., et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85-92.
36. Caragine L.P., Halbach V.V., Dowd C.F., et al. Parallel venous channel as the recipient pouch in transverse/sigmoid sinus dural fistulae. Neurosurgery. 2003;53:1261-1267.
37. Houdart E., Saint-Maurice J.P., Chapot R., et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg. 2002;97:280-286.
38. Ojemann R.G., Heros R.C., Crowell R.M. Dural arteriovenous malformations. In Surgical Management of Cerebrovascular Disease, 2nd ed., Baltimore: Williams & Wilkins; 1988:415-425.
39. Sundt T.M.Jr., Piepgras D.G. The surgical approach to arteriovenous malformations of the lateral and sigmoid dural sinuses. J Neurosurg. 1983;59:32-39.
40. Collice M., D’Aliberti G., Arena O., et al. Surgical treatment of intracranial dural arteriovenous fistulae: role of venous drainage. Neurosurgery. 2000;47:56-67.
41. Thompson B.G., Doppman J.L., Oldfield E.H. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg. 1994;80:617-623.
42. Tomak P.R., Cloft H.J., Kaga A., et al. Evolution of the management of tentorial dural arteriovenous malformations. Neurosurgery. 2003;52:750-762.
43. Van Dijk J.M., Ter Brugge K.G., Willinsky R.A. Wallace MC: Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg. 2004;101:31-35.
44. Shin M., Kurita H., Tago M., et al. Stereotactic radiosurgery for tentorial dural arteriovenous fistulae draining into the vein of Galen: report of two cases. Neurosurgery. 2000;46:730-734.
45. Friedman J.A., Pollock B.E., Nichols D.A., et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulas of the transverse and sigmoid sinuses. J Neurosurg. 2001;94:886-891.
46. Pollock B.E., Nichols D.A., Garrity J.A., et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45:459-467.
47. Pan D.H., Chung W.Y., Guo W.Y., et al. Stereotactic radiosurgery for the treatment of dural arteriovenous fistulas involving the transverse sigmoid sinus. J Neurosurg. 2002;96:823-829.
48. O’Leary S., Hodgson T.J., Coley S.C., et al. Intracranial dural arteriovenous malformations: results of stereotactic radiosurgery in 17 patients. Clin Oncol (R Coll Radiol). 2002;14:97-102.
49. Guo W.Y., Pan D.H., Wu H.M., et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998;19:1081-1087.
50. Duffau H., Lopes M., Janosevic V., et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78-84.
51. Collice M., D’Aliberti G., Talamonti G., et al. Surgical interruption of leptomeningeal drainage as treatment for intracranial dural arteriovenous fistulas without dural sinus drainage. J Neurosurg. 1996;84:810-817.
52. Ng P.P., Halbach V.V., Quinn R., et al. Endovascular treatment for dural arteriovenous fistulae of the superior petrosal sinus. Neurosurgery. 2003;53:25-33.
53. Stiebel-Kalish H., Setton A., Berenstein A., et al. Bilateral orbital signs predict cortical venous drainage in cavernous sinus dural AVMs. Neurology. 2002;58:1521-1524.
54. Benndorf G., Bender A., Lehmann R., et al. Transvenous occlusion of dural cavernous sinus fistulas through the thrombosed inferior petrosal sinus: report of four cases and review of the literature. Surg Neurol. 2000;54:42-54.
55. Miller N.R., Monsein L.H., Debrun G.M., et al. Treatment of carotid cavernous sinus fistulas using a superior ophthalmic vein approach. J Neurosurg. 1995;83:838-842.
56. Day J.D., Fukushima T. Direct microsurgery of dural arteriovenous malformation type carotid-cavernous sinus fistulas: Indications, technique, and results. Neurosurgery. 1997;41:1119-1126.
57. Tu Y.K., Liu H.M., Hu S.C. Direct surgery of carotid cavernous fistulae and dural arteriovenous malformations of the cavernous sinus. Neurosurgery. 1997;41:798-806.
58. Krisht A.F., Burson T. Combined pretemporal and endovascular approach to the cavernous sinus for the treatment of carotid cavernous dural fistulae: technical case report. Neurosurgery. 1999;44:415-418.
59. Lawton M.T., Chun J., Wilson C.B., Halbach V.V. Ethmoidal dural arteriovenous fistulae: an assessment of surgical and endovascular management. Neurosurgery. 1999;45:805-811.
60. Defreyne L., Vanlangenhove P., Vandekerckhove T., et al. Transvenous embolization of a dural arteriovenous fistula of the anterior cranial fossa: preliminary results. AJNR Am J Neuroradiol. 2000;21:761-765.
61. Fukai J., Terada T., Kuwata T., et al. Transarterial intravenous coil embolization of dural arteriovenous fistula involving the superior sagittal sinus. Surg Neurol. 2001;55:353-358.
62. Ricolfi F., Manelfe C., Meder J.F., et al. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology. 1999;41:803-812.
63. Lewis A.I., Rosenblatt S.S., Tew J.M.Jr. Surgical management of deep-seated dural arteriovenous malformations. J Neurosurg. 1997;87:198-206.
64. McDougall C.G., Halbach V.V., Dowd C.F., et al. Dural arteriovenous fistulas of the marginal sinus. AJNR Am J Neuroradiol. 1997;18:1565-1572.
65. Pierot L., Chiras J., Meder J.F., et al. Dural arteriovenous fistulas of the posterior fossa draining into subarachnoid veins. AJNR Am J Neuroradiol. 1992;13:315-323.