Chapter 83 Surgical Management of Cerebral Arteriovenous Malformations
History
AVM neurosurgery is a relatively recent innovation. Even though there have long been reports about the surgical management of this kind of lesion, only from 1960 onward has the veritable surgery become a reality. In the 1960s, endovascular treatments started to be performed as a way to assist neurosurgeons in the resection of such lesions. Since the first description of embolization by Luessenhop and Spence in 1960,1 endovascular procedures have been improved, enabling a dramatic change in the direction of AVM treatments. In that same decade, with the appearance of the operating microscope, surgery took a 180-degree turn in everything regarding neurosurgery, including neurovascular surgery.1 Permanent laboratory practice in microsurgery, better and continued training in neuroanatomy, and constant development of surgical instruments for microsurgery, together with the supraspecialization in vascular neurosurgery, have led neurosurgeons to acquire more practice in the resection of such lesions, with a consequent decrease in morbidity and mortality. Eventually, radiosurgery came up and started to become popular as an adjuvant treatment—or as the only treatment for some cerebral AVMs—in the 1980s.
Classification
As AVMs became known, particularly their characteristics and the difficulties they posed in each case, they started to be classified according to different factors. Such classifications sought and still seek to help neurosurgeons decide whether to treat a cerebral AVM and what therapeutic procedures would be most appropriate for each case.2
In 1977, Luessenhop and Gennarelli proposed a classification based on the arterial pedicles that supplied AVMs.1,17 This classification did not take into account the draining veins or whether AVMs were located in eloquent areas. In 1984, Luessenhop, with Rosa’s collaboration, published a grading scale based on AVM size.4,18 Even though it was an easily applicable classification, it was not practical for surgery because it did not consider the venous drainage or whether it was in an eloquent area. Sugita also classified AVMs located at the sylvian fissure, taking into account whether they were directly in the fissure, lateral, medial, or deep seated.3 In 1986, Shi and Chen published a classification that took into account size, location, depth, the feeding arteries, and the venous drainage.4,18 It was a complex classification, one difficult to remember due to the various combinations therein (since it had seven grades), and the determination of eloquent or noneloquent areas was imprecise. But in that same year Spetzler and Martin’s classification was published, which took into account size, location, and venous drainage.4 This classification turned out to be the most practical one, and its diffusion and acceptance worldwide were fast and unanimous. As time went by, and with the application of this classification, it became evident that AVMs in grade III presented different difficulties in treatment and prognosis. This was motivated by the variability of the lesions included in this grade: for example, it included a small AVM with deep venous drainage located in an eloquent area, such as the brain stem, as well as a 6-cm-wide cortical lesion with superficial venous drainage located in a noneloquent area. This range led several authors to propose modifications to this grade of the classification. The most accepted ones are grades IIIA and IIIB, regarding location.3–6
Even though modifications are still being proposed, the Spetzler-Martin classification is the most commonly used at present, and treatment criteria are mostly based on it. Regardless, with the existing research possibilities on brain function, it is becoming increasingly difficult to speak of eloquent and noneloquent areas. In one way or another, practically the whole brain is “eloquent.”
Based on my experience, as well as on the work that has been carried out in Uruguay for year, and the concepts given by Borovich in the 1980s,6 I would add to the Spetzler-Martin classification the arterial territories involved in AVMs. They are grouped according to whether one, two, or three vascular territories are involved. Most AVMs are included in the groups for one or two vascular territories, which correspond, to a great extent, to grades I to III in the Spetzler-Martin classification.6
Treatment
In a publication about the management of AVMs in 1979, almost 20 years after the beginning of the AVM era, Drake proposed five options neurosurgeons could use when dealing with such a lesion7:
• Expectant behavior (which certainly refers to aggressive treatments, not to treatment symptoms, e.g., epilepsy)
After complete surgical resection of an AVM, with no evidence of remaining lesions in the angiographic control, the patient can be deemed cured. Although this happens in the vast majority of the cases, AVM relapses have been reported.8 It is my opinion that in such cases the angiographic subtraction control study failed to reveal small pathologic pedicles, from which the lesion subsequently reproduced.
Better knowledge of the natural history of AVMs, their flow, the pressure at the nidus, and AVM functionality, as well as that of the surrounding brain, has permitted substantial improvement in the prognosis of such lesions in the last two decades. This has been achieved through the use of dynamic studies, such as supraselective digital angiographies, digital subtraction angiography combined with color scaling, diffusion and perfusion magnetic resonance imaging with flow study, magnetic resonance imaging tractography, magnetoencephalography, and positron emission tomography. All these techniques allow better knowledge of the malformation, its behavior, and the characteristics of the surrounding brain, thus enabling the surgical team to plan the best treatment or combination of treatments for each case.9–16
Arteriovenous Malformations
Vascular malformations of the brain (excluding aneurysms) constitute a heterogeneous group of lesions that includes AVMs, pial malformations, cavernous angiomas, venous malformations, dural fistulae, and telangiectasias. Therefore, AVMs widely dominate the spectrum of lesions.17
Cerebral AVMs are lesions of likely congenital origin or predisposition constituted by a conglomerate of abnormal vessels (both arterial and venous) of variable size and number. There is no intermediate capillary network; there is no parenchyma among vessels. Usually, AVMs are surrounded by a thin layer of nonfunctioning brain tissue (reactional gliosis).6,17
Independent of their classification, AVMs can be divided into one of two categories, superficial (also called “of the convexity,” “cortical,” or “corticosubcortical”) or deep, when planning for their treatment.6,18 Given the variability of these lesions, in both their anatomy and their physiology, there is no universal consensus about how to treat them, even those AVMs that a priori could be similar. Thus, there have long been attempts to establish standards and guidelines for the treatment of AVMs. The problem lies in rapid changes to the proposed guidelines arising from development in diagnostic techniques and knowledge about AVMs and their treatments.
Regardless, the guidelines are recommendations formulated by groups of professionals dedicated to a particular pathology and must be taken as a base for the treatment of AVMs. They are flexible and can be modified according to the results of the different possible treatments and to the appearance of new therapeutics.19,20
Through its chapter on vascular neurosurgery, the Latin American Federation of Neurosurgical Societies in 2003 formulated the first recommendations for the management of AVMs.17 In 2009, I led a group of neurosurgeons dedicated to vascular surgery who published an updated version of those recommendations under the (translated) title “Recommendations for the Management of Brain Arteriovenous Malformations.”17 This publication aims to offer the specialist a guide for managing such lesions, but by no means is strict compliance necessary.17,19
In the last decade, several authors have published guides with the same objective (e.g., Vazquez and Larrea in 2000,21 Ogilvy et al. in 2001,19 and Starke et al. in 200922). These guides do not substantially differ in their recommendations, proposing surgery for grades I and II, combination of treatment for grade III, and conservative treatment for grades IV and V. Starke et al. conclude that surgery is the ideal and curative treatment. They advocate surgery, endovascular surgery, radiosurgery, or combination therapies depending on various factors: angioarchitecture of the AVM, topography, experience of the surgical team, and so on.19–22
AVMs are lesions whose incidence ranges between 1.4% and 4.3% of the population.23,24 The percentage of symptomatic AVMs is unknown, but it has been proved that they are “dynamic” lesions.23,24 In other words, a cerebral AVM diagnosed but not treated can show in a high percentage of the cases, under a periodic follow-up, changes in its anatomy, size, and symptomatology.23,24 Hence, the conservative (do nothing) treatment should not be considered in low-grade malformations or in high-grade AVMs that have shown important clinical aggressiveness, such as repeated bleeding and epilepsy that is difficult to control.
When a treatment for an AVM is proposed—which should be a curative one—it should have a lower morbidity and mortality than those accorded by the natural history of that particular malformation. The treatment or treatments to be carried out in each case issue from the discussion of a multidisciplinary group formed by neurosurgeons, neurointerventionists, neurophysiologists, neurointensivists, neuroanesthesiologists, and neurologists. With all the necessary studies that enable understanding of an AVM (computed tomography, magnetic resonance with functional components, digital angiography, etc.), the team can then decide whether to treat the lesion. If treatment is chosen, one or a combination of the previously mentioned options should be chosen. Every time the team decides to treat an AVM, a curative treatment should be sought, which means the elimination of the lesion. If the treatment was meant to be curative but a remnant of the lesion remained, the treatment is considered a failure. Frequently, palliative treatments are considered, for instance, when dealing with grade V AVMs with recurrent bleeding. In such cases, we know that the possibility of curing the patient is low, but a palliative treatment may significantly reduce the possibility of future bleeding.25–28
When and How to Treat a Cerebral AVM
Cerebral AVMs manifest clinically through bleeding in 50% of the cases, followed by epilepsy. When an AVM has bled, it must be treated. When we face epilepsy refractory to the medical treatment, it must also be treated. When we face an AVM with no bleeding or significant clinical manifestations but with studies that show it presents intranidal aneurysms, stenosis of the afferent vessels, or significant venous stasis, it also must be treated. If AVMs are small and deep seated, they also must be treated, since it is accepted that they have a greater chance of developing complications. Regardless, studies have not been able to demonstrate—based on levels of evidence—a clear correlation between the characteristics of the lesion and its chances of bleeding.26,27,29,30
Following the analysis of malformations according to the Spetzler-Martin grading scale, the following guidelines could be established.17
Grade I and II AVMs
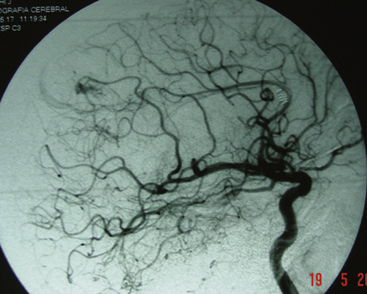
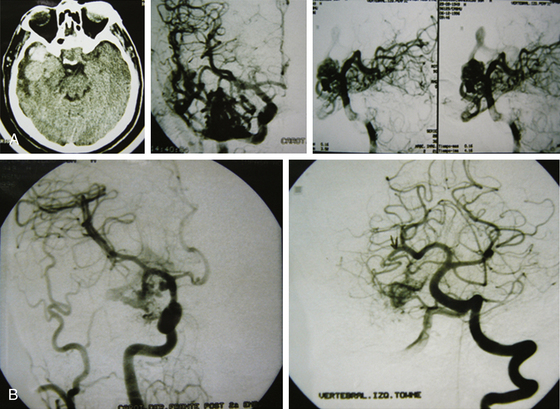
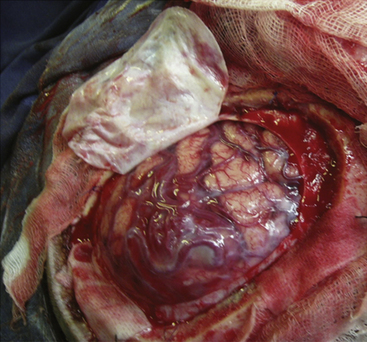
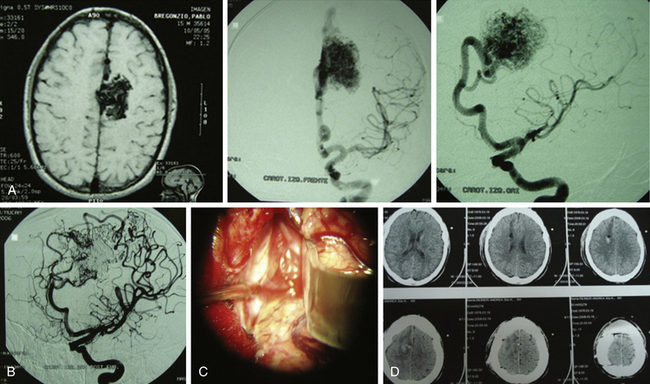
The endovascular option would be, for this group, in the third place. Even though they should not represent any difficulty for the endovascular surgeon, the angiographic disappearance of the lesions does not ensure they have been cured. Small, nonvisible pedicles may exist that may make the nidus reappear.2,5,6–19,21–23,25,31–37 Even though previous papers mentioned total and definitive occlusion with histoacryl glue,38 recent publications about treatment with Onyx reinforce that grade I AVMs can be cured with endovascular treatment (Fig. 83-1).39,40
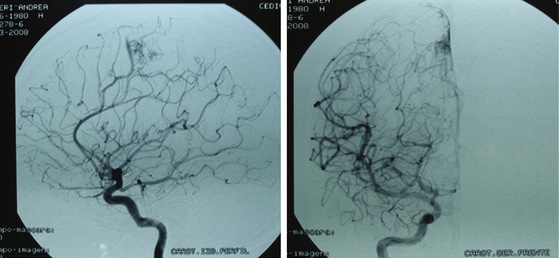
Radiosurgery is not indicated as a stand-alone option in this group of lesions. It can be considered a second-line treatment after endovascular therapy has been carried out, but it has been proved (as in AVMs of higher volume) that the area to be radiated does not include the whole nidus and that areas treated by endovascular means may not be included, thus leading to lesion reproduction in the long term.13,17,75 Even though the proposal to always treat grade I and II AVMs may be controversial, the specialized bibliography supports my strong recommendation for surgery (Fig. 83-2).2,5,6–19,21–23,25,29,31–37
Grade I and II AVMs have with surgery (alone or combined) a cure rate close to 100%.17,31,32,36,37,41,42
Grade III AVMs
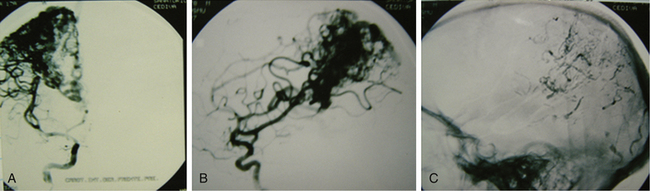
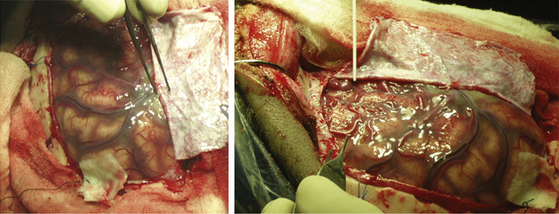
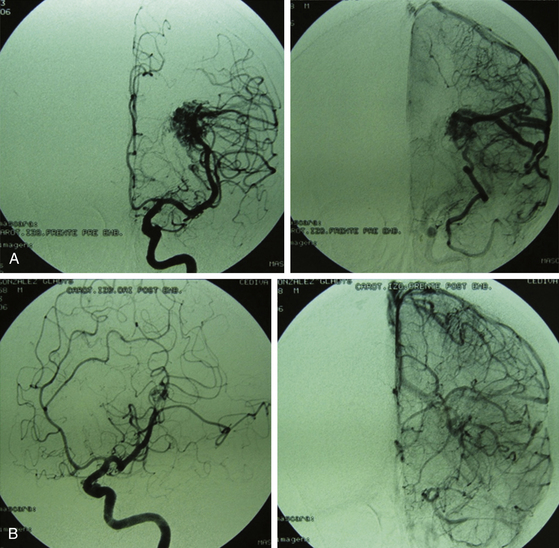
Grade III AVMs must always be treated. They can become highly problematic due to the extensive number of variants that can appear within this group. AVMs that have a large nidus, are superficial (corticosubcortical), and involve one or two vascular territories must always be treated by means of combined techniques. Endovascular therapy is essential in these cases (in either one or two sessions) and is aimed at progressively occluding the afferent vessels (through which inversion of the flow is achieved) but not causing a sudden redistribution of it, which could be harmful. Endovascular therapy with Onyx offers excellent results in this group of AVMs as a treatment prior to surgery.43,44 A prudent amount of time should elapse between this treatment and surgery (no less than 4 or 5 days). After the surgery, and by means of a control angiogram, the complete elimination of the nidus must be verified. If small remnants remain, the treatment can be complemented with radiosurgery.
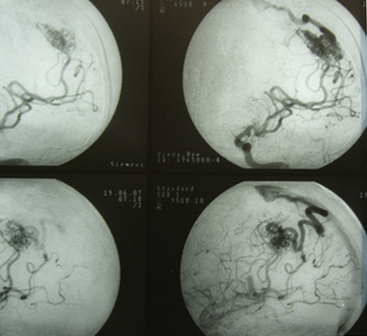
Most lesions within this grade, when treated, have a cure rate close to 100% but a morbidity rate close to 25%.27,31,37 As with grades I and II, the preceding proposal may result in controversy, but the specialized bibliography recommends such treatment (Figs. 83-3 and 83-4).17,21–23,25,31–37,45
Grade IV AVMs
Grade IV AVMs that have not bled and do not have angiographic signs suggestive of complications (e.g., intranidal aneurysms) should be controlled with clinical and imagenologic evaluation.19,22,33,34 The morbidity and mortality rates of the treatment are higher than those supplied by spontaneous course. If there was hemorrhage, these AVMs must be treated, since it is well known that if a lesion of such characteristics has bled and the patient survived, the AVMs will bleed again. In addition, it is highly likely that after hemorrhage the patient will present with neurologic sequelae, which diminishes the rate of sequelae occurrence due to treatment.
When these cerebral vascular malformations are treated (using a combination of options), the cure rate is close to 100%, but the possibility of sequelae rises to almost 50%.17,21,22,32–34
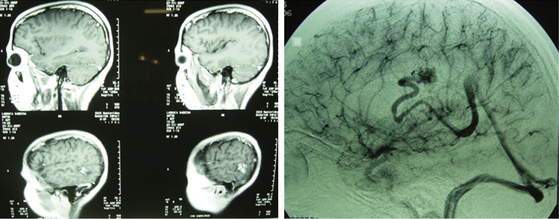
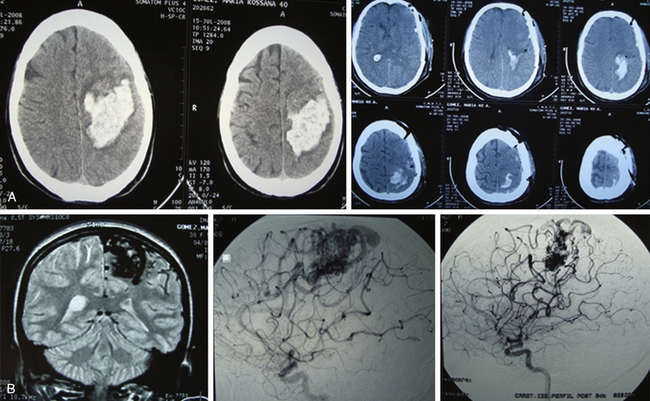
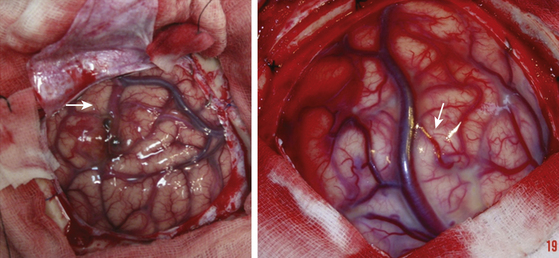
Sometimes a palliative treatment will be proposed: endovascular surgery in association with radiosurgery or as a stand-alone option. With such treatment, the possibility of bleeding is reduced but the patient is not necessarily cured (Fig. 83-5).
Grade V AVMs
In principle, grade V AVMs are not treated.17,19,37 Risks are high; cure is possible, but at the cost of sequelae. If they are aggressive lesions, in the sense of presenting recurrent bleeding, treatments must be tried. In my opinion, those treatments will always be palliative and will be dominated by endovascular surgery and the possibility of being completed with radiosurgery. Direct surgery should not be an option in malformations of this grade. Regardless, a grade V malformation can be operated on, but always with the prior endovascular therapy as an adjuvant. Surgery will always be difficult. The sequelae rate is high. In my opinion, surgery of grade V AVMs must be reserved exclusively for those cases in which there is recurrent bleeding and the patient, already with sequelae, has a life risk in case of new hemorrhage.17,19,33,35,45–48 Some authors propose radiosurgery as the first step of treatment and then endovascular therapy. In all cases, it will be a palliative treatment (Fig. 83-6).17,21,22,37,46–48
2. Endovascular therapy + radiosurgery
4. Endovascular therapy + surgery
A recent publication49 studied the safety of surgery in the treatment of AVMs. The conclusion was that in grades I and II the surgical risk (morbidity) is under 1%, while in grade III to V AVMs it varies according to the involvement of eloquent areas; surgical morbidity and mortality range from 17% to 21%. The comments of Batjer and Hernesniemi on this study support surgery as the best option in the treatment of the AVMs.49
Preparation for Surgery
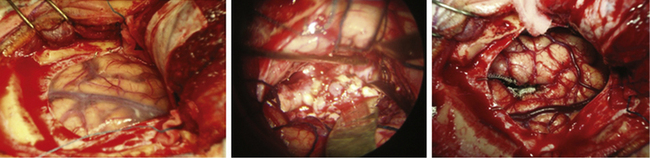
Unlike in other neurosurgical interventions, the preparation for AVM surgery requires the fulfillment of several indispensable steps that allow the surgical act to be performed with fewer risks. First, the surgical intervention of an AVM should always be scheduled. A patient with an AVM complicated by a hemorrhage may require an emergency operation—a case that supports such action. But the indicated action in these cases is to proceed exclusively to the hemorrhagic area evacuation and not seek the malformation in this urgent surgical act. A swollen brain, a distorted anatomy, vessels compressed by the hematoma, venous stasis, and associated ischemic phenomena due to several of these factors conspire in such conditions against the surgical attempt on the AVM, rendering it a surgical failure, not to mention affecting the vital and functional prognosis of the patient. The exception can lay in small malformations found in the wall of the hematoma. In such cases, the resection can be performed. Although a hemorrhagic lesion was evacuated and nothing was found on searching in its “walls,” the pathologist may later report that a small AVM was found among the clots (Fig. 83-7).
Second, when a malformation is going to be operated on, the patient must be prepared for surgery. This implies complete studies and, if necessary, the performance of endovascular techniques. When occlusions of afferent pedicles and part of the malformation nidus have been carried out by endovascular means, surgery must be performed no fewer than 4 or 5 days after the treatment was carried out.50,51 Apart from this requirement, the timing for the surgery depends on the characteristics of the AVM, its flow, its size, the afferents and efferents, and the grade of occlusion achieved. Most importantly, it depends on the patient’s clinical condition after the procedure. An asymptomatic patient can be operated on prematurely, but those who start to present a neurologic deficit and whose imaging studies reveal an ischemic lesion must be differed until clinical stabilization is achieved. Therefore, the surgical team, taking into consideration these factors, should determine the best moment for surgery in each case. The existence of an AVM provokes an alteration in the brain’s blood flow. The occlusion determines a new redistribution of the brain’s blood flow, regardless of the percentage occluded by endovascular means. Such modification, which has been progressive with the endovascular therapy, experiences an abrupt change with surgery. Thus, allowing time for the changes in blood flow to be induced by the therapy before surgery takes place reduces the possibility of associated morbidity.51
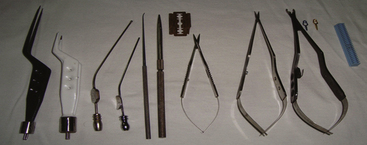
Operating Room
• Microscalpel or arachnoid knife.
• Two aspirators, one that is 5 or 6 French and another that is 8 French, for use in case of bleeding.
• Microsurgery scissors. In the case of a superficial AVM, straight 12- or 15-cm microscissors are enough. When dealing with a deep-seated AVM or subcortical components, 18-cm bayonet scissors may be necessary.
• Bipolar forceps for coagulation. For cortical lesions, short forceps no longer than 15 cm should suffice. As with microscissors, in deep-seated lesions, longer bayonet forceps (18 cm) are needed.
• Microdissector, an accessory device that can be necessary to separate or dissect an arachnoid.
• Hemoclips and aneurysm or AVM clips, both transitory and definitive, together with their corresponding application forceps.
Of these, the essential “tools” that surgeons use in most malformation dissections are the aspirator and the bipolar coagulator (Fig. 83-8).
Surgical Technique
Superficial Malformations
In superficial (corticosubcortical) AVMs, a large flap must be performed centered on the malformation. Even in lesions only a few centimeters wide, the best option is for the flap to include not only the lesion but also the surrounding area, giving the surgeon a view of the normal surrounding brain and thus allowing identification of the sulcus, the different circumvolutions, and the eloquent areas. This also enables the surgeon to assess the normal and not-so-normal vessels that supply and leave the malformation. Thus, the surgeon ensures control of the afferent arterial vessels and of veins, as well as the capacity to identify the arterialized veins. Once structures have been visualized and recognized, the anatomy is compared with the imaging, which must be carefully studied before and during the surgical act (Fig. 83-9).
In addition to the previously described care that must be used at the opening of the dura mater, if the sinus and the interhemispheric fissure have to be exposed, the dural opening must be done slowly and cautiously. In a brain with no vascular lesions, like the ones dealt with in this chapter, there are usually thick veins and adhesions in the vicinity of the sinus. When there is an AVM, such situations become more frequent; hence, the dissection and separation of veins and dural adhesions must be performed carefully.
Venous bleeding in the vicinity of the midline is not rare in such patients. Thus, it is recommended in these cases that the small dural vessels be coagulated and cut against the arachnoid and not against the dura. In case of tearing or bleeding of large-caliber vessels, they must not be coagulated, but a hemostasis must be performed by means of hemostatic materials and later compression with cottons. Trying to perform hemostasis with coagulation of large-caliber venous vessels is a technical mistake and will not solve the problem; on the contrary, the bleeding will increase and will lead the surgery to failure (Fig. 83-10).
Afterward, and always with the corresponding magnification by means of an operating microscope, the area to be operated on is delimited. Arteries are studied as the surgeon tries to identify whether they are vessels heading to the malformation or en passage vessels; the process is repeated with veins. Usually, the veins that exit the nidus have an abnormal coloration, looking more like an artery, hence their designation as “arterialized veins” (Fig. 83-11).
An essential step is to identify the plane between the nidus and the surrounding tissue. Because of the chronic ischemia that determines the malformation on the neighboring parenchyma, there is always at least a small layer of nonfunctioning tissue (gliotic or cicatricial) that surrounds the lesion. This tissue is the margin within which the surgeon has to work without provoking lesions for compression and/or coagulation. In each step, it is important to seek the pedicles and coagulate them. The thickest can be coagulated or clipped. The use of clips is reserved for thick vessels, where coagulation or placement of hemoclips is controversial regarding their efficiency. If they are used, they must always be small or miniature clips. The placement of transitory clips is reserved for those vessels that the surgeon wants to preserve, because it is uncertain whether they are en passage arteries, go to the malformation, or supply the nidus. However, clipping distal to the malformation is preferred to enable the surgeon to manage it better, identify the branches that come to the nidus, and eliminate them. The new clips for AVMs are useful at this point in the surgery.
Occasionally, there is a zone of major bleeding that cannot be controlled with these maneuvers. This usually indicates that remnants of the malformation were not removed. In such cases, and by means of higher magnification, the zone must be explored. This small, remaining nest must be surrounded and then resected in the same way as with the main nidus.6,29,37,52–56
Deep-Seated Malformations
Whatever the therapeutic maneuver, it entails life risk and especially a considerable morbidity risk. Therefore, in those lesions that did not bleed, abstention is probably the best choice. However, it is well known that deep-seated lesions have higher possibilities of bleeding than those located in other areas of the brain. Morbidity of these lesions has diminished significantly in recent years,18,23,24,37 basically because of advances in neuroanesthesia, the practice of neurosurgeons in microsurgery of sulcus and cisterns, and the strict knowledge of gyri (Fig. 83-12).
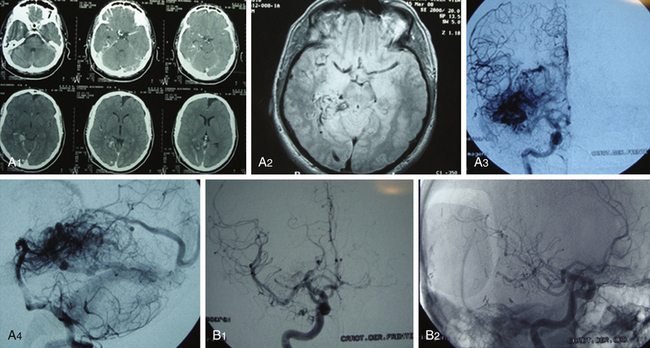
FIGURE 83-12 A, Deep grade III AVM preembolization. B, The same case after embolization and surgery.
This subgroup of malformations poses an important challenge to neurosurgeons, and every case raises a dilemma about how to reach the final and curative solution. Prior to describing surgery of deep-seated AVMs, I must mention the usual topographies of this group of lesions. At the supratentorial level, they are localized in the region of the basal ganglia and the insula, periventricular and intraventricular. At an infratentorial level, it is possible to find them along the brain stem, in the IV ventricle, or deep at the level of the vermis or cerebellar hemispheres.
Solomon and Stein57 divide these malformations into three groups: the basal ganglia ones, especially the thalamocaudate; those of the brain stem; and those neighboring the tentorium. Among the first group, he includes those neighboring the atrium of the ventricle, and among the last group are those placed at the quadrigeminal cistern, mesencephalon, and cerebellar vermis.
Surgery
For external lesions of the basal ganglia, it is necessary to seek some sulcus that, due to its proximity, leads the surgeon to the lesion. It is always necessary to avoid injuring the parenchyma, so correct dissection of a cistern or sulcus permits the surgeon to make enough room to reach the malformation, with minimal or no brain compression. In this case, the head must be placed laterally toward the side opposite the lesion, always avoiding compressions of the neck. It is fixed with a cranial headrest (Fig. 83-13).
In deep-seated lesions of the basal ganglia, centered on the thalamus or the so-called thalamocaudate, an interhemispheric transcallous approach might be the most indicated. In these cases, the patient can be placed in a half-sitting position, with the head fixed with a cranial fixation headrest. The same position can be adopted for the intraventricular or subependymal lesions. This position has my preference. However, the patient could also be placed in lateral decubitus.
In malformations of the cerebellum, my preference is the half-sitting position with a cranial fixation headrest. This position requires correct monitoring by means of thoracic Doppler to search for embolisms and act in consequence. Other authors prefer to position the patient in ventral decubitus. Though this position is safer as concerns avoiding embolisms, there is a higher accumulation of blood in the bed as the surgeon goes forward through the different planes. Regardless, well-trained surgeons should never find difficulties in this position. I consider the ideal position to be the one to which the surgeon is accustomed and for which it is most comfortable for that surgeon to operate (Fig. 83-14).
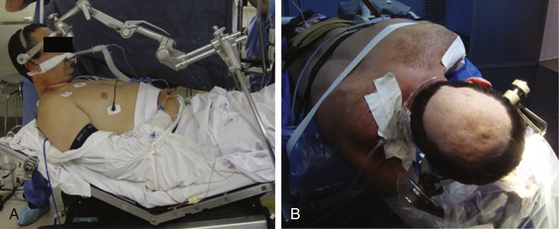
FIGURE 83-14 Half-sitting position (A) and park bench position (B), both for an interhemispheric approach.
Unlike what has been explained regarding superficial lesions (where wide flaps are my preference), in these cases and with corresponding assistance by means of the aforementioned equipment, small lesion–centered flaps can be performed.
Eventually, the walls of the sulcus and/or of the cistern (through which the approach was performed) are covered with hemostatic materials to avoid bleeding of these walls, usually rich in vessels and congestive since it presents near a malformation and due to the neurosurgeon’s work.3,18,24,52,53,55–62
Endovascular Surgery
For the treatment of an AVM, endovascular surgery, endovascular therapy, or neurointerventionism, a well-trained specialist in the management of such lesions is required. Before the treatment, correct planning of the surgery must be carried out by means of a throughout analysis of the imaging, widely dominated by the digital angiography of brain vessels. At present, it is also fundamental to have functional magnetic resonance, which permits the surgeon to delimit the areas of work.9,11,12,14
This method seeks the greatest occlusion of the nidus, as well as of its afferents and of the origin of the draining vein, without causing damage to the patient. Many of those who work in the area of endovascular surgery ponder the possibility of reaching complete endovascular occlusion of an AVM with this method. The permanent and complete occlusion of the nidus is posed.63,64 I think that sometimes it is possible to achieve the complete radiologic occlusion, but this does not mean the complete disappearance of the AVM. In those cases in which complete closure of the lesion is ensured—and that later undergoes surgery—there may be small vessels that did not refill in the endovascular procedure and that eventually would make the lesion reappear if surgery was not performed. In the cases of apparent complete endovascular occlusion with no surgery, it occasionally happens that in successive angiographic controls, which can be performed after 3, 6, or 9 months—or even later—pathologic vessels and draining veins reappear.44 This is controversial, because endovascular surgeons consider complete and definitive occlusion to be achievable, especially in small AVMs and when Onyx is used.39,40,43,44,64–66 In recent publications, Weber et al.39,66 demonstrated that Onyx penetrates small vessels, achieving occlusion. Even as these authors stated that complete angiographic occlusion can be achieved, they remarked that some cases of recanalization have been found in long-term controls. However, in dural fistulae, complete occlusion was reported in another study, and at 5-year follow-up no recanalizations were found.67
Weber et al.39 referred to the benefit of treatment with Onyx, achieving high occlusion rates, especially in cortical AVMs. Van Rooij et al.,40 in a 5-year treatment series, referred to complete occlusion with Onyx in small AVMs. The authors recommended that several years of use of this method pass before the endovascular therapists’ opinion is accepted as true. Up to now, there have been no papers with enough evidence to sustain that position.
In large lesions, neuromonitoring is fundamental, as well as the arterial occlusion tests. When the interventionist knows that navigating up to the origin of the nidus will not be possible, a test to assess the possible occlusion of proximal arterial branches or the stem, itself an important artery, is necessary. By means of administration of amobarbital sodium, Wada’s test can be performed in arteries distant from the nidus, as well as in the distal vessels against the nidus. This is a highly valued test to determine the tolerance of a certain vessel to occlusion.6,65
The particles and histoacrylic glue were long the materials of choice for closure of the vessels of a malformation. Later, surgeons began to use some coils in some cases. Finally, the appearance of Onyx has been of great value in the treatment of such lesions. This material is administered in a liquid form that hardens when it comes into contact with blood, with scant risk of migration. At present, histoacrylic glue (n-butyl cyanoacrylate) and particularly Onyx are the materials of choice for the endovascular treatment of AVMs.39,40,43,44,65,66,68–71
The treatment must be always performed under general anesthesia. This allows the endovascular surgeon to work more comfortably and, if complications occur, enables the anesthesiologist to act rapidly, correcting parameters, improving oxygenation, controlling the capnography, provoking arterial hypertension if necessary, enabling delivery of volume and inotropes, and so on.
Never to proceed to surgery immediately after embolization; rather, allow time for the flow redistribution to be determined by the endovascular therapy. In this period, the patient can also recover from neurologic deficits that may have appeared due to the treatment. Even though endovascular therapies conducted by a well-trained surgeon usually exhibit low morbidity, if morbidity takes place, the surgeon can let several days or weeks to elapse before advising surgery. On the opposite end of the spectrum, there is the risk of adding morbidity to the surgery (Figs. 83-15 and 83-16).72,73
Radiosurgery
Radiosurgery has a widely spread use in neurosurgery, and the AVM is one of the pathologies to which it is frequently applied. In this application, the nidal thrombosis is sought. This is achieved through progressive hyperplasia of the tunica intima up to occlusion of the vessels. Although it is usually well tolerated, positive results are never immediate.74,75 On average, 2 years must elapse until complete sclerosis; thus, the AVM occlusion can be verified. Therefore, during the degenerative process that leads to closure of the nidus, the risk of bleeding persists. This is why radiosurgical therapy has some precautions. Such therapy is clearly indicated for some cases, whereas for other cases other options should be discussed; it is mostly indicated for AVMs that did not bleed and that have a volume below 8 cc. If we take the AVM as the integral dose (dose/volume relationship, expressed in millijoules) it would be 170 mJ. This is comparable with the 8-cc volume.17 If the patient presented with bleeding due to an AVM, it is best to wait no less than 6 months before proceeding to treatment with radiation.
Recently published works refer to the benefits of radiosurgery as the only treatment for some AVMs but also refer to its value as an adjuvant to the surgery. It has been proposed that a malformation subjected to stereotactic radiosurgery can have better surgical management and fewer risks of intraoperative blood loss. However, it is also accepted that AVMs that underwent stereotactic radiosurgery but 2 years later have not yet disappeared should be operated on if are accessible to the surgeon. In all cases, when surgery has been performed after the radiant treatment, morbidity has been low.74
In 2008, a scale for radiosurgery and AVMs was proposed, with the aim of predicting the prognosis after the radiant treatment.75 It needs to be used for some years before its value can be assessed.76–89
Awad J.A., Magdinec M., Schubert A. Intracranial hypertension after resection of brain arteriovenous malformations: predisposing factors and management strategy. Stroke. 1994;25:611-620.
Brown R. Simple risk predictions for arteriovenous malformations hemorrhage. Neurosurgery. 2000;46:1024.
Brown R., Flemming K., Meyer F., et al. Natural history, evaluation and management of intracranial vascular malformations. Mayo Clin Proc. 2005;80:269-281.
Cood P., Mitha A., Ogilvy C. A recurrent brain malformation in an adult. J. Neurosurg. 2008;109(3):486-491.
Cover K., Lagerwaard F., Van den Berg R. Color intensity projection of digitally subtracted angiography for the visualization of brain arteriovenous malformations. Neurosurgery. 2007;60(3):511-515.
Davidson A., Morgan M. How safe is AVM surgery? A prospective observational study of surgery as first line treatment for brain arteriovenous malformations. Neurosurgery. 2010;66:498-505.
Drake C. Brain arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979;26:145-208.
Guglielmi G. Analysis of the hemodynamic characteristics of brain arteriovenous malformations using electrical models: baseline setting, surgical extirpation, endovascular embolization and surgical bypass. Neurosurgery. 2008;63(1):1-11.
Hashimoto N., Nosaki N., Takogi Y. Surgery of arteriovenous malformations. Neurosurgery. 2007;61:375-389.
Hernesniemi A., Dashti R., Juvela S., et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63(5):823-831.
Heros R.C. Spetzler-Martin grade IV and V arteriovenous malformations. J Neursurg. 2003;98:1-2.
Hoh B.L., Chapman P.H., Loeffler J.S., et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizures incidence and seizures outcomes. Neurosurgery. 2002;51:303-309.
Kim L., Albuquerque F., Spetzler R. Postembolization neurological deficits in brain AVMs. Neurosurgery. 2006;59:53-59.
Laakso A., Dashti R., Seppänen J., et al. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery. 2008;63(2):244-255.
Lawton M.T. Spetzler-Martin grade III AVMs: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-749.
Maruyama K., Shin M., Tago M., et al. Radiosurgery to reduce the risk hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60(3):453-459.
Morgan M., Rochford A. Surgical risk associated with the management of grade I and II brain arteriovenous malformations. Neurosurgery. 2007;61:417-424.
Ogilvy C.L., Awad I., Brown R., et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
Sanchez-Mejía R., McDermott M. Radiosurgery Facilitates Resection of Brain arteriovenous malformations and reduces surgical morbidity. Neurosurgery. 2009;64(2):231-240.
Sinclair J., Kelly M., Steinberg G. Surgical management of posterior fossa AVMs. Neurosurgery. 2006;58:189-201.
Spagnuolo E. Malformaciones arteriovenosas supratentoriales corticales. In: Pedroza A., Quintana L., Perilla T. Tratado de Neurocirugía Vascular Latinoamericana. Bogotá: FLANC; 2008:412-423. Chap 29
Spagnuolo E., Lemme-Plaghos L., Revilla F., et al. Recomendaciones para el manejo de las malformaciones arteriovenosas cerebrales. Neurocirugía-Rev. Española de Neurociencias. 2009;20:5-14.
Starke R., Komotor R., Hwang B. Treatment guidelines for arteriovenous malformations microsurgery. BJNS. 2009;23:376-385.
Weber W., Kis B., Siekmann R. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-254.
Weber W., Kis B., Siekman R., et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx. Technical aspects. AJNR. 2007;28:371-377.
1. Luessenhop A.J., Spence W.T. Artificial embolization of brain arteries: report of use in a case of arteriovenous malformation. JAMA. 1960;172:1153-1155.
2. Lawton M.T. Spetzler-Martin grade III AVMs: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-749.
3. Lawton M.T., Lu D., Young W. Sylvian fissure arteriovenous malformations: an application of the Sugita classification to 28 surgical patients. Neurosurgery. 2007;61(1):29-38.
4. Spetzler R.F., Martin N.A., Carter L.P., et al. Surgical management of large AVMs by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
5. Fischer W.S. III: therapy of AVMs: a decision analysis. Clin Neurosurg. 1995;42:294-312.
6. Spagnuolo E. Malformaciones arteriovenosas supratentoriales corticales. In: Pedroza A., Quintana L., Perilla T. Tratado de Neurocirugía Vascular Latinoamericana. Bogotá: FLANC; 2008:412-423. Chap 29
7. Drake C. Brain arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979;26:145-208.
8. Cood P., Mitha A., Ogilvy C. A recurrent brain malformation in an adult. J. Neurosurg. 2008;109(3):486-491.
9. Kikuta K., Takagi Y., Nozaki K., et al. Early experience with 3-T magnetic resonance tractography in the surgery of brain arteriovenous malformations in and around the visual pathway. Neurosurgery. 2006;58(2):331-337.
10. Mäkelä J., Forss N., Jääskeläinen J., et al. Magnetoencephalography in neurosurgery. Neurosurgery. 2006;59(3):493-511.
11. Cover K., Lagerwaard F., Van den Berg R. Color intensity projection of digitally subtracted angiography for the visualization of brain arteriovenous malformations. Neurosurgery. 2007;60(3):511-515.
12. Kondziolka D., Lunsford L.D., Kanal E., et al. Stereotactic magnetic resonance angiography for targeting in arteriovenous malformations radiosurgery. Neurosurgery. 1994;35:585-591.
13. Colombo F., Cavedon C., Francescon P., et al. Three-dimensional angiography for radiosurgical treatment planning for arteriovenous malformations. J Neurosurg. 2003;98:536-543.
14. Pouratian N., Bookheimer S.Y., Rex D.E., et al. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002;97:21-32.
15. Lehericy S., Biondi A., Sorour N., et al. Arteriovenous brain malformations: is functional MR imaging reliable for studying language reorganization in patients? Initial observations. Radiology. 2002;223:672-682.
16. Latchaw R.E., Hu X., Ugurbil K., et al. Functional magnetic resonance imaging as a management tool for brain arteriovenous malformations. Neurosurgery. 1995;37:619-626.
17. Spagnuolo E., Lemme-Plaghos L., Revilla F., et al. Recomendaciones para el manejo de las malformaciones arteriovenosas cerebrales. Neurocirugía-Rev. Española de Neurociencias. 2009;20:5-14.
18. Spagnuolo E. Malformaciones arteriovenosas profundas. In: Pedroza A., Quintana L., Perilla T. Tratado de Neurocirugía Vascular Latinoamericana. Bogotá: FLANC; 2008:425-436. Chap: 30
19. Ogilvy C.L., Awad I., Brown R., et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of Stroke Council, American Stroke Association. Stroke. 2001;32:1458-1471.
20. Brown R., Flemming K., Meyer F., et al. Natural history, evaluation and management of intracranial vascular malformations. Mayo Clin Proc. 2005;80:269-281.
21. Vazquez F, Larrea, J: Guía de tratamiento de las malformaciones arterivenosas. Guía práctica para la realización del tratamiento endovascular. In: GENI: Grupo español de neurorradiología intervencionista. geni@senr.org. 1–22, 2000.
22. Starke R., Komotor R., Hwang B. Treatment guidelines for arteriovenous malformations microsurgery. BJNS. 2009;23:376-385.
23. Brown R. Simple risk predictions for arteriovenous malformations hemorrhage. Neurosurgery. 2000;46:1024.
24. Spagnuolo E., Johnston E., Calvo A. Cirugía guiada por estereotaxia de malformaciones vasculares pequeñas y profundas. Arch Neurological Institute Montevideo. 1999;2:19-26.
25. Pawlikowska L., Poon K., Achrol A., et al. Apolipoprotein E2 is associated with new hemorrhage risk in brain arteriovenous malformations. Neurosurgery. 2006;58(5):838-843.
26. Stüer C., Ikeda T., Stoffel M., et al. Norepinephrine and brain blood flow regulation in patients with arteriovenous malformations. Neurosurgery. 2008;62(6):1254-1261.
27. Guglielmi G. Analysis of the hemodynamic characteristics of brain arteriovenous malformations using electrical models: baseline setting, surgical extirpation, endovascular embolization and surgical bypass. Neurosurgery. 2008;63(1):1-11.
28. Laakso A., Dashti R., Seppänen J., et al. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery. 2008;63(2):244-255.
29. Hernesniemi A., Dashti R., Juvela S., et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63(5):823-831.
30. Hoh B.L., Chapman P.H., Loeffler J.S., et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizures incidence and seizures outcomes. Neurosurgery. 2002;51:303-309.
31. Roda J.M. Tratamiento de la patología vascular del Sistema Nervioso Central. Neurocirugía. 2004;15:400-402.
32. Weerskkody R., Trivedi R., Santurios T., et al. Arteriovenous malformations. BJNS. 2009;23:494-498.
33. Stapf C., Mast H., Sciacca R., et al. The New York Islands Arteriovenous Malformations Study. Design, study, progress and initial results. Stroke. 2003;34:229-233.
34. Halim A., Johnston C., Singh V. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformations of the brain within a defined population. Stroke. 2004;35:1697-1709.
35. Viñuela F. Neurorradiología intervencionista en malformaciones arteriovenosas cerebrales y durales. In: Mercader J., Viñuela F. Neurorradiología diagnóstica y terapéutica. Barcelona: Masson; 2004:745-753.
36. Choi J., Mast H., Siacca R. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformations. Stroke. 2006;37:1243-1247.
37. De Oliveira E., Tedeschi H., Raso J. Comprehensive management of AVMs. Neurological Research. 1998;20:673-683.
38. Debrun G., Aletich V., Ausman J., et al. Embolization of the nidus of brain arteriovenous malformations with butyl cyanoacrylate. Neurosurgery. 1997;40:112-121.
39. Weber W., Kis B., Siekmann R., et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx. Technical aspects. AJNR. 2007;28:371-377.
40. Van Rooij W., Sluzewski M., Bente G. Brain AVM embolization with Onyx. AJNR. 2007;28:172-178.
41. Andrade-Souza Y., Ramani M., Tsao M. Embolization before radiosurgery reduces the obliteration rate of AVMs. Neurosurgery. 2007;60:453-459.
42. Morgan M., Rochford A. Surgical risk associated with the management of grade I and II brain arteriovenous malformations. Neurosurgery. 2007;61:417-424.
43. Natarajan Sabareesh K., Ghodke Basavaraj B., Gavin W., et al. Multimodality treatment of brain arteriovenous malformations with microsurgery after embolization with Onyx: single-center experience and technical nuances. Neurosurgery. 2008;62(6):1213-1226.
44. Sabareesh K., Donald B., Sekhar L., et al. Histopathological changes in brain arteriovenous malformations after embolization using Onyx. or n-butyl cyanoacrylate. J Neurosurg. 2009;111(1):105-113.
45. Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95:633-637.
46. Chang S.D., Marcellus M.L., Marks M.P., et al. Multimodality treatment of giant intracranial AVMs. Neurosurgery. 2003;53:1-13.
47. Han J.P., Ponce F.A., Spetzler R.F. Intention to treat analysis of Spetzler-Martin grade IV and V AVMs: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
48. Heros R.C. Spetzler-Martin grade IV and V arteriovenous malformations. J Neursurg. 2003;98:1-2.
49. Davidson A., Morgan M. How safe is AVM surgery? A prospective observational study of surgery as first line treatment for brain arteriovenous malformations. Neurosurgery. 2010;66:498-505.
50. Picard L., Da Costa E., Anxionnat R., et al. Acute spontaneous hemorrhage after embolization of brain arteriovenous malformation with n-butyl cyanoacrylate. J Neuroradiol. 2001;3:147-165.
51. Peerless S.J., Friedman W.A., Sypert G.W., et al. Successful treatment of the normal perfusion pressure breakthrough syndrome. Neurosurgery. 1982;11:625-630.
52. Hashimoto N., Nosaki N., Takogi Y. Surgery of arteriovenous malformations. Neurosurgery. 2007;61:375-389.
53. Yasargil M.G. `Microneurosurgery, vol. III B: AVM of the Brain. Stuttgart: Georg Thieme Verlag; 1998. 25-53
54. Sugita K. Microneurosurgical Atlas. Berlin: Springer-Verlag; 1985. pp. 136-177
55. Hashimoto N. Microsurgery for brain AVMs: dissection technique and its theoretical implications. Neurosurgery. 2001;48:1278-1281.
56. Awad J.A., Magdinec M., Schubert A. Intracranial hypertension after resection of brain arteriovenous malformations: predisposing factors and management strategy. Stroke. 1994;25:611-620.
57. Solomon R., Stein B. Management of deep supratentorial and brain stem arteriovenous malformations. In: Barrow D., editor. Intracranial Vascular Malformations. Park Ridge, IL: AANS Publications; 1991:125-140. Chap 9
58. Solomon R., Stein B. Surgical management of arteriovenous malformations that follow the tentorial ring. Neurosurgery. 1986;18:708-715.
59. Sisti M., Solomon R., Stein B. Stereotactic craniotomy in the resection of small AVM. J Neurosurg. 1979;75:40-44.
60. Sinclair J., Kelly M., Steinberg G. Surgical management of posterior fossa AVMs. Neurosurgery. 2006;58:189-201.
61. Pik J.H.T., Morgan M.K. Microsurgery for small AVMs of the brain: results in 110 consecutive patients. Neurosurgery. 2000;47:571-577.
62. Gross B., Duckworth E., Getch C., et al. Challenging traditional beliefs: microsurgery for arteriovenous malformations of the basal ganglia and thalamus. Neurosurgery. 2008;63(3):393-411.
63. Debrun G., Viñuela F., Fox A., et al. Embolization of brain arteriovenous malformations with bucrylate: experience in 46 cases. J Neurosurg. 1982;56:615-627.
64. Andreou A., Ioannidis I., Laloo S., et al. Endovascular treatment of intracranial microarteriovenous malformations. J Neurosurg. 2008;109(6):1091-1097.
65. Hartman A., Pile-Spellman J., Stapf C., et al. Risks of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;7:1816-1820.
66. Weber W., Kis B., Siekmann R. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-254.
67. Carlson A., Taylor C., Yanos H. Treatment of dural arteriovenous fistula using Onyx. Arterial embolization as the primary modality. Short term results. J Neurosurg. 2007;107:1120-1125.
68. Kerber C.W., Wong W. Liquid acrylic adhesive agents in interventional neuroradiology. Neurosurg Clin N Am. 2000;11:85-99.
69. Levrier O., Mekkaoui C., Rolland P.H., et al. Efficacy and low vascular toxicity of embolization with radical versus anionic polymerisation of n-butyl-2-cyanoacrylate (nBCA): an experimental study in the swine. J Neuroradiol. 2003;2:95-102.
70. Meisel H.J., Mansmann U., Alvarez H., et al. Effect of partial targeted n-butyl-cyanoacrylate embolization in brain AVM. Acta Neurochir (Wien). 2002;9:879-887.
71. Jafar J.J., Davis A.J., Berenstein A., et al. The effect of embolization with n-butyl cyanoacrylate prior to surgical resection of brain arteriovenous malformations. J Neurosurg. 1993;78:60-69.
72. Kim L., Albuquerque F., Spetzler R. Postembolization neurological deficits in brain AVMs. Neurosurgery. 2006;59:53-59.
73. Ledesma C., Hoh B., Carter B., et al. Complications of brain arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58(4):602-611.
74. Sanchez-Mejía R., McDermott M. Radiosurgery facilitates resection of brain arteriovenous malformations and reduces surgical morbidity. Neurosurgery. 2009;64(2):231-240.
75. Pollock B., Flickinger J. A proposed radiosurgery based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
76. Leksell L. The stereotaxic method and RS of the brain. Acta Chir Scand. 1951;102:316-319.
77. Russel S., Woo H., Joseffer S., et al. Role of frameless stereotaxy in the surgical treatment of brain AVMs: technique and outcome in a controlled study of 44 consecutive patients. Neurosurgery. 2002;51:1101-1331.
78. Betti O., Munari C., Roster R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311-321.
79. Flickinger J., Kondziolka D., Maitz A., et al. An analysis of the dose response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002;63:347-354.
80. Friedman W., Bova F., Bollampally S., et al. Analysis of factors predictive of success or complication in arteriovenous malformative radiosurgery. Neurosurgery. 2003;52:296-307.
81. Pollock B., Gorman D., Coffey R. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery. 2003;52:1291-1296.
82. Inoue H., Ohye C. Hemorrhage risks and obliteration rates of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2002;97:474-476.
83. Nataf F., Schlienger M., Bayram M., et al. Microsurgery or radiosurgery for brain arteriovenous malformations? A study of two paired series. Neurosurgery. 2007;61(1):39-50.
84. Jahan R., Solberg T., Viñuela F., et al. An arteriovenous malformation model for stereotactic radiosurgery research. Neurosurgery. 2007;61(1):152-159.
85. Lišcák R., Vladyka V., Šimonová G., et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60(6):1005-1016.
86. Maruyama K., Shin M., Tago M., et al. Radiosurgery to reduce the risk hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60(3):453-459.
87. Reyns N., Blond S., Gauvrit J. Role of radiosurgery in the management of brain arteriovenous malformations in the pediatric age group: data from a 100-patient series. Neurosurgery. 2007;60(2):268-276.
88. Tu Jian S., Marcus A., Morgan M., et al. Responses of arteriovenous malformations to radiosurgery: ultrastructural changes. Neurosurgery. 2006;58(4):749-758.
89. Celix J., Douglas J., Haynor D., et al. Thrombosis and hemorrhage in the acute period following gamma knife surgery for arteriovenous malformation. J Neurosurg. 2009;111(1):124-131.