Chapter 81 Surgical Management of Cavernous Malformations of the Nervous System
Cavernous malformations (CMs) have long been recognized as one of the major clinicopathologic categories of vascular malformations of the nervous system.1–3 Because no abnormal vascularity is seen on angiography, CMs have been included in the descriptions of cryptic or occult vascular malformations, a term that has been used to describe any vascular malformation that cannot be seen on angiography.4–9 The term cavernous angioma was used by Russell and Rubinstein3 in their excellent description of the pathology of these lesions. CMs have also been called cavernous hemangiomas or cavernomas, but the term cavernous malformation has become more widely accepted, explicitly distinguishing these lesions from true vascular neoplasms as suggested by the term angioma.
Pathologic Features
CMs can occur throughout the brain or spinal cord parenchyma, as well as on cranial and spinal nerves.10,11 The lesions, which may be multiple, can range in size from a few millimeters to several centimeters. Often, an associated venous malformation of the brain may be present; rarely, similar lesions may be found in other parts of the body.3,12
How a CM develops is unknown. In some patients, the lesions are clearly acquired, appearing in areas of brain that were normal on prior MRI studies.9 These include patients with familial lesions and those in whom the CM has developed in an area of previously irradiated brain tissue or even developed sporadically.13–17 Wilson9 has proposed a possible pathogenesis for the development of acquired lesions.
The lesion is well defined and usually has a lobulated appearance. There is often a characteristic gross appearance that has been likened to a mulberry, characterized by a dark red or purple color. Inside the lesion is a honeycomb of thin-walled vascular spaces.2 Small hemorrhages adjacent to or within the lesion may occur, but large hemorrhages are rare. A variable number of small blood vessels enter the lesion. Gliotic tissue surrounds the mass, and it is usually stained yellow. In some patients, the lesion may gradually enlarge as a result of small hemorrhages, progressive hyalinization, thickening of the vascular walls, or gradual thrombosis.11,18
Radiologic Features
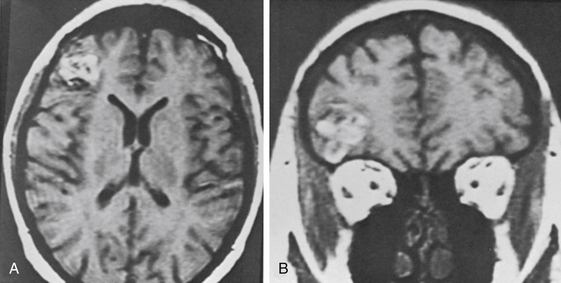
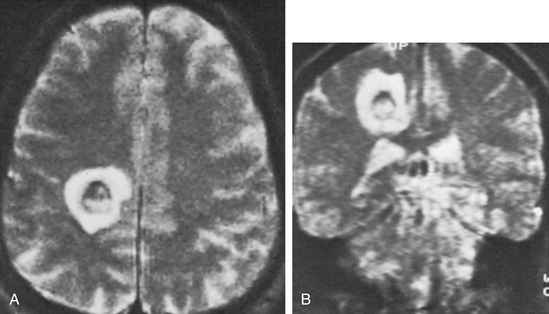
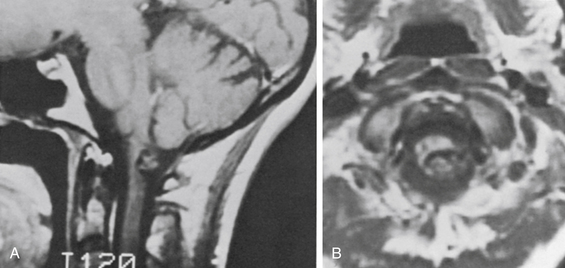
MRI accurately establishes the diagnosis in most cases and often is the only study needed. The criteria for MRI diagnosis is a well-circumscribed lesion with a combination of a reticulated or mottled core of mixed signal intensity and a prominent surrounding rim of decreased signal intensity (Fig. 81-1).19 Hemorrhages of different ages may be seen within or around the lesion, and an associated venous anomaly may be seen. On T2-weighted images, an increased signal may be present in the adjacent brain as a result of edema. Usually little or no enhancement follows the administration of gadolinium. Although appearance on MRI has a high correlation with the diagnosis of CM, occasionally an occult arteriovenous malformation or a tumor can have a similar appearance.9,20,21 Significant enhancement suggests the possibility of a tumor. Advances in MRI techniques have resulted in the use of susceptibility-weighted imaging as the gold standard for diagnosis of CMs. Head-to-head comparisons of susceptibility weighted imaging (SWI) against T2 gradient echo and other T2-weighted sequences have shown a significantly higher sensitivity for relatively small lesions that would have classically been considered occult.22 This is particularly relevant in familial cases where multiple CMs are present and for locating otherwise-cryptogenic seizure foci in these patients.
The angiographic results are almost always normal because the lesion has small blood vessels with low flow and no hypertrophied feeding arteries or early draining veins. Rarely, an avascular mass or capillary blush can be identified.19,23 Since the development of MRI, angiography is rarely indicated. Occasionally, angiography is needed to obtain information regarding the vascular anatomy to help plan a surgical approach. If the MRI scan suggests an association with another vascular malformation, or if the diagnosis is in doubt, angiography should be done.
Familial Occurrence and Genetics
CMs are known to be present in both sporadic and familial forms.17,24,25 The familial form of the disease affects up to 30% to 50% of patients harboring a CM19,24 and seems most common among Hispanic Americans.17 Studies have shown that familial CMs are more prevalent than previously believed, and there is a greater incidence of multiple lesions (Fig. 81-2).17 Rigamonti et al.25 estimated a 73% familial incidence of multiple CMs of the brain, compared with a 10% to 15% incidence in the sporadic form. Further reports have indicated that familial CMs can be found in diverse ethnic groups, including the Japanese and French.26,27 Familial and sporadic forms of the disease appear to be similar clinically28; however, as many as 75% of patients who present with multiple CMs likely harbor the hereditary form of the disease.26
An autosomal dominant pattern of inheritance with variable penetrance was first described in a 122-member Hispanic lineage, of whom 5 harbored symptomatic lesions.24 This autosomal pattern of inheritance was confirmed by a subsequent description of 6 unrelated Hispanic families.17 Subsequent study of other families utilizing MRI to detect silent lesions revealed a more complete expression than previously suspected.26 The location of the responsible gene was mapped to the long arm of chromosome 7, to a locus named cerebral cavernous malformation 1 (CCM1).29,30 CCM1 locus homogeneity was identified in an analysis of 14 Hispanic families with CMs31 but did not extend to kindreds of different ethnicity. Investigation of 20 non-Hispanic Caucasian families revealed linkage to two additional loci: CCM2 at chromosome 7p and CCM3 at chromosome 3q.32 The clinical, radiographic, and pathologic characteristics of the disease in kindreds mapping to these other loci does not appear to differ from those seen in kindreds mapping to the CCM1 locus. Linkage to one of these three loci (i.e., 7q, 7p, or 3q) accounted for inheritance in all 20 Caucasian kindreds studied. CCM1 was considered to be the locus involved in only 40% of non-Hispanic kindreds, with the remaining lineages linked to CCM2 (20%) or CCM3 (40%).32 Subsequent analysis of further kindreds seems to confirm that about 40% of familial CMs are attributable to CCM1 mutations.33 The discovery of genetic heterogeneity in familial CMs has important implications both for the ability to provide reliable genetic testing in presymptomatic diagnosis and for understanding the pathogenesis of the disease. It has been suggested that there may be locus-specific differences in the penetrance of the disease, although such differences may also be attributable to the particular mutation involved.32
Given that the clinical phenotypes of mutations at all three loci are essentially identical, the presumption is that the involved proteins likely converge on a conserved pathway that is required for the regulation of endothelial development. This has been borne out in a series of detailed molecular studies aimed at identifying the molecular pathogenesis of the disease. The responsible gene associated with CCM1, at the chromosome 7q locus, has been identified as human Krev interaction trapped 1 (KRIT1). Mutations in KRIT1 were first identified in a study of 57 French kindreds with CMs; 12 different mutations were discovered.34 Further KRIT1 mutations have been described in families of different ethnicities.35,36 Although the exact function of KRIT1 remains unknown, its interaction with Krev/rap 1a (a ras family guanosine triphosphatase) and with integrin cytoplasmic domain-associated protein-1 alpha and evidence that KRIT1 is a microtubule-associated protein suggest a role in endothelial cell matrix interactions that in turn could play a role in abnormal vascular development.37 Gene products associated with CCM2 have been recently reported as proteins with potential involvement in integrin signaling encoded by the gene MGC4607 (malcavernin, osmosensing scaffold for MEKK3).38,39 Subsequent molecular studies have identified this gene as a key regulator of the Rho family of guanosine triphosphatases involved in endothelial proliferation and migration through effects on microtubule stability and expression of cell adhesion molecules.40 The most recent breakthrough was the identification of the gene product of the CCM3 locus, a protein known as programmed cell death 10 (PDC10).41,42 While the exact mechanism has not been worked out, preliminary studies have shown that the CCM3 gene functions in the same Rho signaling pathway as its family members.43 In a striking example of how the study of human disease has resulted in the advancement of our understanding of cellular biology, investigations into the CCM proteins have uncovered a signaling pathway that is fundamental to vascular biology.
Based on the identified genetic abnormalities, it could be proposed that familial CMs result from inherited mutations and that sporadic CMs result from either a germline mutation in an individual or a somatic mutation in a single cell. There are reports both for de novo germline mutations44 and for somatic cell mutations in KRIT1 leading to sporadic CMs.45 Support for the notion of somatic mutations may be found in the development of CMs following radiation therapy, where cerebral lesions have been described within the irradiated field and were not present prior to treatment.14 The possibility of radiation-induced mutagenesis resulting in CM formation is one explanation for this observation.
Clinical Presentation
In 1976, Voigt and Yasargil46 analyzed 163 cases reported up to 1974. They found that these lesions occurred in every age group and that the gender incidence was equal. In 1988, Simard et al.47 reviewed 126 cases published since 1960 and added 12 of their own. The male-to-female ratio was 0.9:1, and the ages ranged from neonate to 75 years. In 1991, two publications were based on the analysis of consecutive MRI scans performed over several years.48,49 In the report by Robinson et al.48 of 66 patients, the male-to-female ratio was 1.2:1, and the ages ranged from 4 months to 84 years (mean 34.6 years). In the report of 32 patients by Curling et al.,49 the male-to-female ratio was 1.1:1, the ages ranged from 16 to 72 years (mean 37.6 years), and multiple lesions were present in 6 patients (19%). Scott et al.50 reported a series of 19 children ranging in age from 7 months to 17 years. In the MGH series of 116 patients, the ages ranged from 4 to 69 years (mean 35.5 years), with a male-to-female ratio of 1:1.2. Multiple lesions were present in 12 patients (10.3%) in this series, and associated venous malformations were present in 9 patients (7.8%).
Most CMs that come to attention are supratentorial in location46; the distribution of CMs within the central nervous system seems to reflect the volume of tissue, without specific predilection for any particular location. The locations of the 121 operated CMs in our series of 116 patients are listed in Table 81-1.
| Location | CMs | Percentage of Total CMs |
|---|---|---|
| Cerebrum | 84 | 69.4 |
| Frontal | 36 | |
| Parietal | 16 | |
| Temporal | 28 | |
| Occipital | 4 | |
| Brain stem | 17 | 14.0 |
| Mesencephalon | 2 | |
| Pontomesencephalon | 4 | |
| Pons | 8 | |
| Pontomedullary | 2 | |
| Medulla | 1 | |
| Cerebellum | 8 | 6.6 |
| Cranial nerves | 4 | 3.3 |
| Spinal cord | 8 | 6.6 |
| Cervicomedullary | 2 | |
| Cervical | 3 | |
| Thoracic | 2 | |
| Lumbar | 1 | |
| Total CMs | 121 | 99.9 |
The four general categories of clinical presentation are seizures, headache, neurologic deficit, and asymptomatic presentation.46–49 Seizure is the most common presenting symptom, affecting 35% to 55% of patients.25,47–49,51,52 In many patients, more than one symptom is present. Within each of the symptomatic categories, some patients have had a hemorrhage into the adjacent brain parenchyma. The hemorrhages are usually small but on rare occasion can be large, with the patient having rapid deterioration. In some patients, the CM gradually enlarges, and the lesion can act as a mass that causes a progressive neurologic deficit. The clinical symptoms arising from the 121 CMs in the MGH surgical series are presented in Table 81-2; among the intracranial lesions, seizures were the presenting symptom in 45%, followed by neurologic deficits (38%) and headaches (17%).
Natural History
In 1985, Wilkins8 reviewed the natural history of vascular malformations. He concluded that not enough information was present in the literature to describe the natural history of cavernous angiomas. In 1991, two reports of CMs diagnosed in large consecutive series of MRI scans gave some information about the short-term natural history,48,49 and data from familial cases have contributed to our knowledge.17
Most hemorrhages are noncatastrophic, but there are occasional exceptions. Zimmerman et al.53 reported one patient who died from rehemorrhage of a tectal CM. There are consequences, however, even of repeated small hemorrhages.
Progressive deterioration with successive hemorrhages has been described,6 and Robinson et al.54 found a strong association between hemorrhage and neurologic disability in patients with CMs. Knowledge regarding the long-term risk of hemorrhage is of importance in management decisions, especially for CMs presenting incidentally or with minimal symptoms.
Overall, available estimates of the risks of initial hemorrhage from CMs have indicated low hemorrhage rates. In a retrospective study, Curling et al.49 reported a 0.25% per person-year and 0.1% per lesion-year hemorrhage rate among 32 patients with 76 lesions. Robinson et al.48 followed 57 symptomatic patients with 66 lesions for a mean of 26 months and observed only 1 hemorrhage in 143 lesion-years, resulting in a 0.7% per lesion-year hemorrhage rate. Porter et al.52 reported a 1.6% per person-year hemorrhage rate among 110 patients followed for a mean of 46 months. For 68 prospectively followed patients, Moriarity et al. reported an overall 3.1% per person-year hemorrhage rate.55 The risk of bleeding may be higher in deep or brain stem CMs.52 In their cohort of 110 prospectively followed patients, Porter et al.52 found a 10-fold higher hemorrhage rate among infratentorial lesions at 3.8% per year compared with supratentorial lesions at 0.4% per year. This may reflect the eloquence of the surrounding tissue, with even small brain stem hemorrhages being more likely than lesions to be clinically manifest in the cerebral hemispheres.
The risk of hemorrhage in familial CMs appears to be similar to that in nonfamilial CMs, when considering the increased frequency of lesion multiplicity. In their follow-up of six families with familial CMs, Zabramski et al.17 found a 1.1% per lesion-year (6.5% per person-year) rate of bleeding over a follow-up period of 26 months. In 40 patients with familial CMs, Labauge et al. reported a 2.5% per lesion-year hemorrhage risk.56
Several reports suggest a higher risk of bleeding after a first hemorrhage. Kondziolka et al.57 followed 122 patients for 34 months and noted a low 0.6% per person-year hemorrhage rate among those without history of prior hemorrhage but a higher 4.5% per person-year rate among those with a previous hemorrhage. Kim et al. also reported a slightly higher recurrent hemorrhage rate of 3.8% per person-year compared with 2.3% per person-year for first hemorrhage.58 For brain stem lesions, Kupersmith et al. reported a bleeding rate of 2.5% per person-year, with a rebleeding rate of 5.1% per person-year.59 Tung et al.6 reported recurrent hemorrhage occurring in 7 patients whose diagnosis of CM was confirmed at surgery. The median interval from the initial hemorrhage to the recurrent hemorrhage was 12 months, with only 2 months until a second rebleed. Aiba et al.60 also found a much higher incidence of hemorrhage in those with prior bleeds at 22.9% per lesion-year versus 0.4% per lesion-year in those without prior bleeds. There is also evidence for temporal clustering of hemorrhages, with rates of rehemorrhage initially as high as 2% per month in a selected population but decreasing to less than 1% per month after 2 to 3 years.61
Information regarding the long-term risk of seizure development is scarce. Kondziolka et al.57 reported that 4 of 94 patients without seizures developed seizures over the mean 34-month follow-up. Curling et al.49 estimated the risk of seizure development to be 1.5% per person-year based on 32 patients. In Zabramski et al.’s17 group of patients with familial CMs followed over a mean period of 2.2 years, 1 of 6 asymptomatic individuals developed seizures. The rate of new seizures in the 68 patients reported by Moriarity et al. was 2.4% per person-year.55
The natural history of familial CMs has been addressed in several reports. Zabramski et al.17 reported six families with familial CMs; 31 patients among these families harbored CMs, 21 of whom were followed clinically and with serial imaging. A total of 128 CMs were identified radiographically in these patients. During the mean follow-up of 2.2 years, 5 lesions were found to change in size, 13 lesions showed changes in signal characteristics, and 17 new lesions were identified in 6 patients. Given the dynamic nature of the CMs, the report’s authors recommended serial MRI at 12-month intervals for symptomatic individuals, in addition to screening of family members. This approach can clarify the risk of morbidity in these patients and the need for close radiographic and clinical follow-up while providing data regarding the natural history. Labauge et al. noted 23 new lesions in 11 patients (27.5%) during follow-up of 40 patients with familial CM harboring 232 CMs over a mean follow-up of 3.2 years.56 Nine lesions (3.9%) changed in size, and signal change was observed in 14 lesions (6%) over the same follow-up period.
De novo lesions have been described in nonfamilial cases of CMs as well. The primary risk factor identified has been radiation therapy, with reports of de novo development of CMs in the spinal cord62 and the brain14,15 years following irradiation. However, cases without an identifiable risk factor have also been documented.63,64
Little information is available about asymptomatic patients and their risks for developing symptoms. In the report by Robinson et al.,48 four of nine asymptomatic patients developed symptoms related to the CM over a relatively short follow-up period of 6 months to 2 years (mean 18 months).
Management Considerations
Surgery
The current, well-established indications for surgical resection of CMs are recurrent hemorrhage, progressive neurologic deterioration, and intractable epilepsy, unless the location is associated with an unacceptably high surgical risk.48,49,65 When the surgical risk is high, observation or radiosurgery should be considered. Because the risk of surgery is low for lesions in many locations, there are groups of patients (e.g., those with CMs of the cerebrum or cerebellum with a single overt hemorrhage, those with the onset of a seizure disorder, and those who are worried about the presence of the lesion) in whom surgery should be considered.66 In children, Scott et al.50 have a “policy to recommend surgery for patients with cavernous angiomas if the lesion is safely accessible, is currently symptomatic either by mass effect and/or hemorrhage or seizure, or shows evidence of having bled in the past.”
In a special category is the young woman who wants to become pregnant (Fig. 81-3). Robinson et al.48 noted that two of their six patients with acute hemorrhage were in the first trimester of pregnancy. They suggested that in women contemplating pregnancy, one of the indications for surgical excision was an accessible lesion. Other authors have also commented on the possible role of hormonal influences as a contributing factor.16,60 Aiba et al.60 reported that women predominated in the group of patients presenting with hemorrhage and that young women had a higher rate of subsequent hemorrhage.
In some patients, splitting a cortical fissure may be possible rather than performing a full corticectomy to approach the lesion. For lesions in critical areas, cortical mapping and stimulation may be used. When removal of a CM in the deep portions of the cerebral hemispheres is indicated, the lesion is localized with stereotactic techniques and intraoperative ultrasound.6,50,67 A comparison of microsurgical resection with or without neuronavigation demonstrated that the size of resection was significantly smaller when stereotaxy was used. While this study did not show a difference in outcome, it clearly demonstrated that neuronavigation can result in a safe, better-defined resection.68 This is particularly important for deeply seated lesions in the basal ganglia, thalamus, or brain stem. For brain stem lesions, special monitoring with evoked potential responses may be helpful, and arrangements for temporary cardiac pacing may be prudent.
Radiosurgery
Kondziolka et al.69 reported on gamma knife use in the treatment of 47 patients with surgically inaccessible lesions with at least one prior hemorrhage. Over the mean follow-up of 3.6 years, they found a significant decline in the hemorrhage rate, from 32% per lesion-year pretreatment to 1.1% per lesion-year at 2 years after treatment. There was a high incidence of radiation-induced complications after treatment, with mean center doses of 32 Gy (range 20-40 Gy): 12 of the 42 patients (27%) were affected, although only 2 patients (4%) were reported to suffer permanent deficits. In a subsequent report from the same institution, 82 patients were analyzed. Again, a reduction in hemorrhage risk was reported from 33.9% to 12.3% during the first 2 years post-treatment and by 0.76% per patient-year thereafter.70 Overall incidence of radiosurgical morbidity was 13.4%. Karlsson et al.71 also reported experience with gamma knife, treating 22 patients with symptomatic CMs using maximum doses of 11 to 60 Gy (mean and median 33 Gy) and minimum doses of 9 to 35 Gy (mean and median 18 Gy). Over the mean follow-up of 6.5 years, they noted a decreasing trend in hemorrhages 4 years after treatment. However, they also found a high rate of morbidity, with 6 patients (27%) suffering radiation-related complications; in 5 of them (23%), these led to permanent deficits. Pollock et al. also noted a high incidence of radiation-related complications, occurring in 41% of their 17 patients with deep lesions, although hemorrhage rates did decline from 40.1% pretreatment to 2.9% more than 2 years after treatment.72 Another series of gamma knife radiosurgery noted a reduced frequency of seizures following treatment, with 18 of 28 patients (64%) who had a chief complaint of seizure experiencing this benefit.73
More recent studies have shown similar results. Lunsford et al. recently published their results of a retrospective cohort of 103 patients who demonstrated propensity to bleed (more than two bleeds) and had CMs in locations deemed too risky for microsurgical resection. Their analysis again demonstrated a reduction in hemorrhage risk from 32.5% to 4.6%. Most of the risk of hemorrhage was in the first 2 years after surgery (10.8%), because the rate of hemorrhage after this initial latency period dropped to 1.06%.74 The studies’ authors commented that this latency period likely reflects the time period over which the endothelium undergoes progressive hyalinization and luminal obliteration, reminiscent of the mechanism of action on arteriovenous malformations. One report of histopathlogic analysis of a CM that was microsurgically resected 1 year after receiving 40 Gy of radiation supports this hypothesis.75 Despite the use of a slightly lower mean dose (30.2 Gy, range 21.7-40 Gy) and confining the target volume to the hemosiderin ring as defined by high-resolution T2 images, the incidence of postradiation T2 signal change surrounding the CM was 18.4%. New neurologic deficit was noted in 13.5% of patients, though the report’s authors commented that these deficits were all transient, with only 1 patient demonstrating persistent deficits.74 Similar results were found by the same group looking at a cohort of 68 patients with hemorrhagic brain stem CMs that were also deemed surgically inaccessible.76 Moreover, the authors bring up the point that previous studies demonstrating much higher and more permanent adverse radiation effects likely reflected treatment of CMs with associated developmental venous anomaly (DVAs), which are at intrinsically high risk for venous congestion and ischemic events if treated with stereotactic radiosurgery. As such, the authors advocate for better patient selection rather than abandonment of stereotactic radiosurgery for CMs in surgically high-risk areas.
Stereotactic charged-particle radiosurgery, both helium-ion radiosurgery and proton beam therapy, has also been used to treat cavernous angiomas. Fabrikant et al.77 noted that the clinical results after helium-ion radiosurgery for cavernous angiomas were not as good as those for arteriovenous malformations. Radiosurgery using a linear accelerator has also been reported but with small numbers of patients treated and a lack of long-term data.78 Chang et al.79 summarized the results of 57 patients treated using helium ion (47 patients) or linear accelerator (10 patients). All patients harbored CMs that had bled previously and were treated with mean doses of 18 GyE. Hemorrhage rates decreased 3 years after treatment to 1.6% per patient-year. In this study, 5 patients (9%) suffered radiation-related edema or necrosis, resulting in permanent deficits in 2 patients (4%). Analysis of Kjellberg’s experience at the Harvard Cyclotron Laboratory using proton beam therapy revealed a decline in hemorrhage rates from 17.3% per lesion-year before treatment to 4.5% per lesion-year after a latency period of 2 years.80 Among the 98 lesions treated with a median center dose of 18 Gy, 26 (26.5%) were associated with radiation-related complications, 16 of which were permanent and 3 of which resulted in mortality.
Because the long-term natural history of CMs is not well defined, current evidence favors expectant management, given the high rates of complications with radiosurgery. The risk of radiation-related complications appears to be significantly higher than that found for arteriovenous malformations of similar size and location72; although the basis for this is unknown, a role for the potential radiosensitizing properties of the hemosiderin ring around CMs has been proposed.81 The decline in hemorrhage rates observed in most studies cannot definitively be ascribed to the treatment, because it may reflect the poorly characterized natural history of bleeding in these lesions.
Temporal clustering of hemorrhages, with a spontaneous 2.4-fold decline in hemorrhage rates after 2 years, has been observed and may affect the interpretation of hemorrhage risk reduction in radiosurgical series.61 This modality is presently considered only rarely for deep, inaccessible lesions associated with repeated hemorrhage and progressive neurologic deficit.
CM of the Cerebrum
Management
Patients Presenting with Seizures
The treatment of patients with CMs presenting with seizures continues to evolve. With the good results of surgical removal, the indications for operation have expanded beyond conditions in which medical control of seizures is difficult and in which the diagnosis is in question (more common before the advent of MRI). Surgery is recommended in most patients who have had a parenchymal hemorrhage and in many patients who have not had a hemorrhage when the surgical risk is low. These patients are usually in their 20s to 40s but are sometimes younger, and they are often concerned about the presence of the lesion. Although the seizures can often be controlled with medication, the removal of the CM and the adjacent gliotic yellow-stained tissue may reduce the long-term frequency and severity of seizures and allow the patients to discontinue their medications.2,48,49 Robinson et al.48 reported that all 18 patients who did not have surgery continued to require medical control of their seizures. In the surgically treated group, 50% had no more seizures, and the others had a reduction in seizure frequency.
Rengachary and Kalyan-Raman2 described a possible basis for recommending surgery. They suggest that slow lysis of red cells sequestrated in the cavernous spaces allows red cell pigment to diffuse out of the lesion into the adjacent tissue. This pigment seems to induce gliosis, which may contribute to the development of a seizure focus. The authors quote experimental studies that suggest chemical compounds containing iron play important roles in inducing a seizure focus.
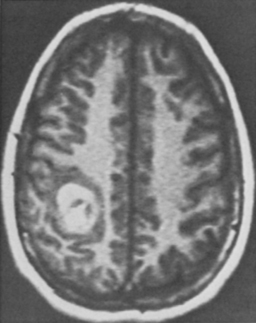
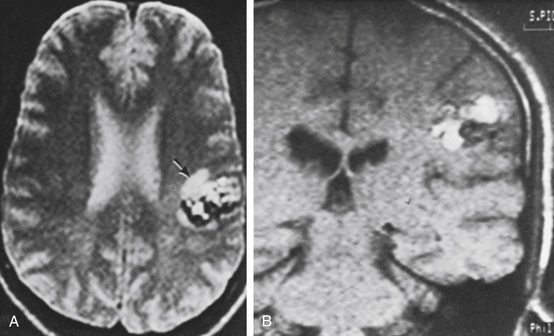
In the MGH series, a seizure was the primary presenting symptom in 51 of 84 patients with lesions of the cerebrum (61%) (Table 81-2). Some of these patients had other symptoms (usually headache), and slightly more than half had an associated parenchymal hematoma. Patients with CMs presenting with seizure are shown in Figs. 81-1 (temporal), 81-3 and 81-4 (frontal), 81-5 (parietal), and 81-6 (motor–sensory cortex).
Patients Presenting with Headache
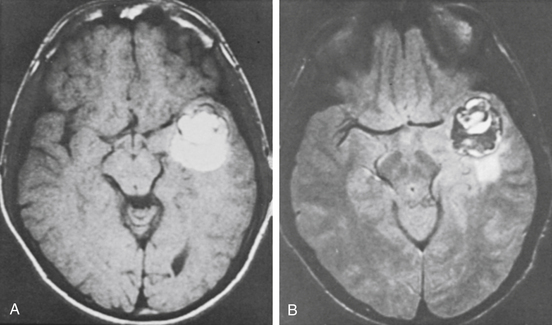
If the headache is a new symptom or is recurrent, it can often be related to the CM. In these patients, MRI often shows a recent hemorrhage. In our series, 16 of 18 patients with headache (89%) had a parenchymal hemorrhage (Table 81-2). Surgery is indicated in most of these patients to avoid future neurologic disability from recurrent hemorrhage (except in patients with unacceptably high surgical risk because of a critical anatomic location). An example is illustrated in Fig. 81-7.
Patients Presenting with Neurologic Deficit
In most patients with a progressive or acute neurologic deficit, there is an associated hematoma, and surgical removal of the lesion is indicated to prevent further neurologic damage and to help restore neurologic function. Some of these patients also have had headaches. In the patient whose deficit is improving, observation can be the initial strategy used. If the surgical risks are judged to be relatively low, however, the lesion should be electively removed to prevent the effects of rehemorrhage. These patients are generally younger than 60 years and are at risk for repeated hemorrhages and neurologic deficits.6 Involvement of the speech or motor–sensory cortex should not preclude the consideration of surgery, but a final decision should include an assessment of the surgical accessibility of the lesion.
In our series, most patients (13 of 15, or 87%) presenting with a neurologic deficit had an associated acute hemorrhage. The indications for surgery were progressive neurologic deficit, sudden severe neurologic deficit, recurrent episodes of neurologic deficits lasting days to weeks, and history of a hemorrhage followed by recovery. An example is illustrated in Fig. 81-8.
Results
Several reports documented good results in the surgical treatment of CMs of the cerebral hemispheres.9,46,50,51,66,82–87 With accessible lesions, surgical morbidity is minimal and mortality is almost zero. Acciarri et al.82 found a 90% incidence of good outcomes in 55 patients with hemispheric lesions, with 0% mortality. Smaller series have found similarly favorable results, with good results in 100% of patients.51,85,86 Chadduck et al.88 reviewed patients with intraventricular CMs. Although they found good outcomes, particularly in patients with lateral ventricular lesions, 2 of 15 surgically treated patients (13%) died, and 1 patient was rendered comatose. Acciarri et al.82 reported two deaths among 4 patients with intraventricular lesions.
The treatment of CMs of the basal ganglia and thalamus has been controversial, and these lesions (along with brain stem lesions) may be considered dangerous to excise. Some practitioners suggest that surgery be considered when there are recurrent episodes of hemorrhage or progressive worsening neurologic deficit.50,89 Lorenzana et al.90 reported on a patient with a CM that involved the anterior third of the lentiform nucleus and a large part of white matter anterior to the nucleus. The patient was cured by use of CT-assisted stereotactic craniotomy with an approach through the second frontal sulcus. In other case reports, the results of treatment of CMs in the thalamus were discouraging.86,91 Bertalanffy et al.92 found permanent neurologic complications in 6 of 12 patients (50%) after resection of deep-seated lesions within the basal ganglia, thalamus, and insula. Steinberg et al. reported worsening in 16 of a group of 56 patients (29%) with deep or brain stem lesions, of which 15 were located in the basal ganglia or thalamus; long-term outcome, however, was improved condition in 52%, unchanged in 43%, and worse in 5%.93
The effects of resection on seizure control are pertinent given the frequency of seizures among supratentorial CMs. Lesionectomy alone has resulted in favorable results.82,94,95 Despite the implication of iron products as potential seizure foci, there is considerable controversy as to whether removal of the surrounding hemosiderin ring should be a primary goal during microsurgical resection. Early studies did not show a correlation between removal of the hemosiderin-stained brain and improved outcome.96,97 However, more recent literature seems to suggest the contrary: at least three recent studies have demonstrated a significantly higher proportion of patients classified as Engel class I (free from disabling seizures) after complete removal of the hemosiderin ring versus partial or no removal.98–100 Length of seizure history and total number of seizures, however, do not seem to affect surgical outcome negatively.95,97,101 Nevertheless, seizure control has obvious implications for long-term quality of life. Retrospective studies reveal that 75% to 100% of patients with fewer than five seizures or shorter than a 12-month history of seizures are seizure free after lesionectomy, compared with 50% to 62.5% of patients with more than five seizures or a seizure history longer than 1 year.95,101 A newer study looked at 53 patients with epilepsy related to supratentorial CMs and demonstrated that complete microsurgical resection, along with the hemosiderin rim, resulted in 84.9% of patients being classified as Engel class I. This study also demonstrates a significant improvement in seizure control rate in patients with complete resection of the hemosiderin rim versus in those in whom the hemosiderin rim was left (77.8% vs. 65.7%, p < 0.037).100
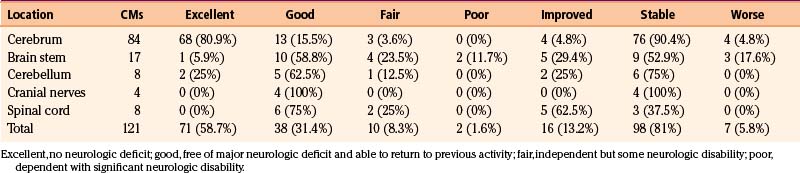
In the MGH series of 84 patients with CMs of the cerebrum outside the basal ganglia and thalamus, operative mortality was zero, and all patients had complete removal of the lesion. Postoperatively, 81 of the 84 patients (96%) had an excellent or good outcome neurologically (i.e., were able to return to their previous level of activity) and, in most cases, had normal results on neurologic examination. Three patients (4%) were classified as fair because of residual disability. Neurologic outcome and comparison of presurgical and postsurgical neurologic status are shown in Table 81-3. Of the 52 patients presenting with seizures, 29 underwent surgery primarily for seizure control; the remainder underwent surgery to eliminate risk of further hemorrhage rather than primary treatment of the seizure disorder. Of the 52 patients, 50 (96%) were seizure free on or off anticonvulsants after surgery. Of the 29 patients treated primarily for epilepsy, 26 (90%) experienced improvement in seizure control after surgery.
CM of the Brain Stem
Management
Based on their experiences with 8 patients (4 of whom had surgery), Falbusch et al.102 recommended that CMs of the brain stem that are associated with recurrent episodes of hemorrhage, MRI-confirmed diagnosis, negative angiographic results, and progressive neurologic disability should be removed. Patients with recovery or stabilization of their neurologic deficit, or those who are asymptomatic, should be observed. The report by Zimmerman et al.53 summarized 24 patients (16 of whom had surgery). They recommended that neurologically intact patients with CMs that did not touch the pial surface should be observed. When the lesion was associated with repeated hemorrhages or with progressive neurologic deficit and was close to the pial surface, surgery was performed. They also suggested that any symptomatic lesion of the brain stem located superficially be considered for surgery if eloquent tissue could be spared. Based on the poor overall natural history of symptomatic CMs in a brain stem location, resection is advocated for lesions abutting a pial surface or surrounded by only a thin rim of tissue.103–107 The optimal timing of surgery following hemorrhage is less well defined. Operation in the subacute phase following hemorrhage (several days to a few weeks) may be preferable.107–109 This allows time to stabilize the patient’s condition while conferring the benefit of earlier reduction of mass effect by evacuation of hematoma. Furthermore, the presence of a subacute hematoma may create a better surgical plane than would be present after delayed surgery following complete clot resorption, or with acute surgery with a firm clot.93 Mathiesen et al. found better outcomes among their patients with brain stem cavernomas if surgery was performed within 1 month of the hemorrhagic ictus.108 On the other hand, Samii et al. reported that there was no difference in long-term outcomes based on the timing of surgery in 36 patients.106
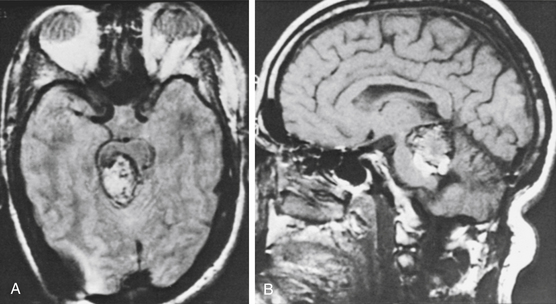
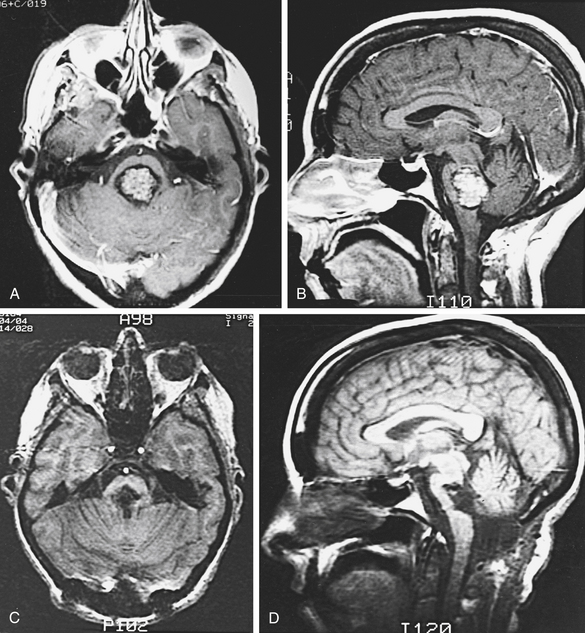
In the past, some CMs of the brain stem were treated with radiosurgery when recurrent hemorrhages and increased neurologic deficit were present. The patient whose MRI scan is illustrated in Fig. 81-9 was treated for this reason several years ago. The lesion did not become smaller, and recurrent hemorrhage with worsening symptoms occurred. The patient declined surgical management.
Careful study of the MRI scan aids in planning of the precise corridor of exposure and resection. Neuronavigation can be of considerable help, especially for lesions that are covered by a thin rim of tissue and that are not directly visible on the surface.109 Electrophysiologic monitoring (e.g., somatosensory evoked potentials, brain stem auditory evoked potentials, and cranial nerve V, IIV, and XII function monitoring) can be utilized in an effort to minimize complications.93,109 While there is benefit to resecting the hemosiderin ring in supratentorial lesions with regard to seizure control, this is clearly not a concern in the brain stem. Moreover, high-resolution diffusion tensor imaging and tractography have shown that while the CM itself neatly displaces white matter tracts, the hemosiderin ring is often intimately involved with them. As such, resection of the hemosiderin ring should be avoided in cases of infratentorial CMs.68
Pontomedullary CMs can be approached through a midline suboccipital craniotomy and exposure of the fourth ventricle. Lesions in the pontomesencephalic region are more difficult to manage but may be treated by a subtemporal or combined subtemporal–suboccipital approach. Falbusch et al.102 used a supracerebellar infratentorial approach for midline pontomesencephalic lesions.
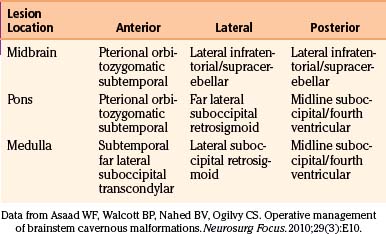
In our patients, the approach to brain stem CMs is dictated by the relative rostral–caudal, as well as anterior–posterior, localization of the lesion (Table 81-4). Upper brain stem CMs were removed by a subtemporal approach (Fig. 81-10); in one patient, a laterally placed brain stem lesion was approached through the cerebellopontine angle between the fifth and seventh cranial nerves. In patients with lesions in the pons or medulla, a midline exposure through the floor of the fourth ventricle was used (Fig. 81-11).
Results
Zimmerman et al.53 reported that among 16 patients who underwent surgery, some had transient postoperative worsening, but the outcome in all except for 1 patient was the same or improved. Isamat and Louesa110 had 6 patients with CMs in the pons or medulla that were in contact with the floor of the fourth ventricle and were removed through the floor of the ventricle. All patients returned to their previous activities and had improvement in their neurologic deficits. Three other patients in their study are being followed because the lesion was completely surrounded by normal brain stem tissue.
Fritschi et al.111 reviewed 93 cases of brain stem CMs treated operatively from their own experience and from the literature up to 1992. They found good outcomes in 84% of cases. The authors compared the surgical results with 30 patients treated conservatively without surgery. Although most nonoperative patients recovered completely or with minimal deficits (67%), 20% died, and 7% suffered poor outcomes. Porter et al. reported improved outcomes in 87% of 84 patients who underwent operation for brain stem CMs; 10% worsened postoperatively, and 4% died.103 A 12% incidence of severe or permanent morbidity was reported. Despite successful resection of brain stem lesions, CMs within this location clearly carry a higher risk of operative morbidity and mortality. Rates of transient complications within the literature range from 25% to 70%,53,92,104,108,112 rates of permanent complications are up to 25%,92,103,104,112,113 and mortality rates are up to 6%.53,92,103,111,114
In the MGH series of 17 patients with CMs of the brain stem, 5 improved, but some residual neurologic deficits that had been present before surgery were usually persistent (see Table 81-3). Eight patients (47%) experienced transient postoperative neurologic deficits, all of which resolved to baseline within 1 month. Three patients (17.6%) experienced permanent postoperative deficits. One of these patients harbored a mesencephalic CM and initially presented with hemorrhage, in coma; clot evacuation and partial lesion resection was undertaken. He made a gradual recovery but had a small rehemorrhage 2 years later. He underwent reoperation, which resulted in poor outcome. Another patient presented with a progressive quadriparesis (Fig. 81-11), and at operation, the large lesion was calcified and difficult to remove. Complete resection was achieved, but the patient had further neurologic deficits postoperatively, leaving her bedridden. The third patient had a pontomedullary CM and presented with progressive diplopia and ataxia. Postoperatively, the patient’s condition was worse, with difficulty swallowing and hoarseness that improved but with residual effect.
Abla et al. recently published the largest series of brain stem CMs, in which they examine their experience with treating 300 patients between 1989 and 2005. Their data underscore the difficulty of operative management of these lesions, because the rate of postoperative neurologic deficit was approximately 51%, with 35% of patients having permanent deficits. Perioperative complications, most commonly from tracheostomy or feeding tube placement and cerebrospinal fluid leak, were noted in 28% of the cohort. Rehemorrhage was observed in 6.9% of patients, and the overall annual rate of rehemorrhage was 2.0%,115 which represents a significant decrease compared to rates as high as 34%116 that are quoted in the literature. The study spans a period during which dramatic improvements in microsurgical technique, MRI resolution, and intraoperative neuronavigation have been made. As such, it is not surprising that patients operated on at an earlier point in the study were more likely to have a poor outcome. However, this study reiterates the perils of operating in the brain stem and highlights the need for appropriate patient selection and serious consideration of the relative risks and benefits of surgery for brain stem CMs.
CM of the Cerebellum
Results
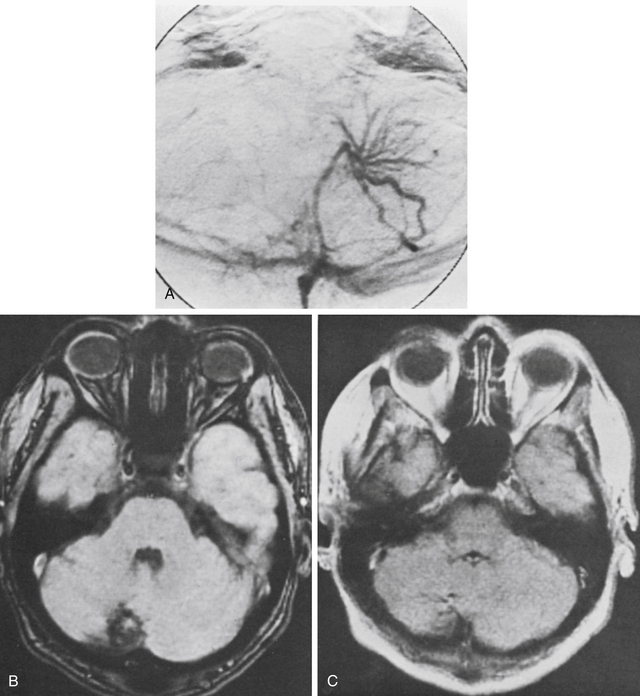
There were eight patients with CMs of the cerebellum in the MGH series: all made a good recovery and could be classified as having excellent or good outcomes (see Table 81-3) except for one patient classified as a fair outcome secondary to persistence of a preoperative deficit. One patient had an associated venous anomaly (Fig. 81-12). At operation, a typical CM was resected, and the large venous angioma was carefully preserved. The patient made an uneventful recovery. Another patient with a cerebellar CM and associated venous anomaly developed a venous infarction 1 month postoperatively, despite efforts to leave the venous anomaly intact. This complication required reoperation for cerebellar decompression, but the patient ultimately regained her preoperative neurologic status.
CM of the Cranial Nerves
Management
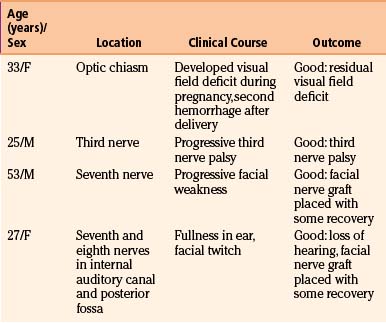
CMs have been reported to involve cranial nerves in the following locations: optic nerves and chiasm, third nerve, seventh nerve in the temporal bone, and seventh and eighth nerves in the internal auditory canal (Table 81-5).117–121 The presenting symptoms relate to the nerve involved with the lesion. The MRI scan usually suggests the diagnosis, but in some patients, the pathology may not be evident until surgery. Surgery does not usually restore function in the involved nerve, although some improvement in deficit can be observed.121 Surgery is indicated to prevent hemorrhage into adjacent neural structures or, as in a patient with a facial nerve lesion, to remove the malformation and place a sural nerve graft, which may restore some function. Gross total resection of the lesion is required to prevent progression of the cranial nerve dysfunction.
Results
Improvement in function has been documented with removal of cranial nerve CMs.122 Others, however, report a low potential for restoration of function118,119 and note that complete resection is difficult without incurring permanent nerve injury.119,120
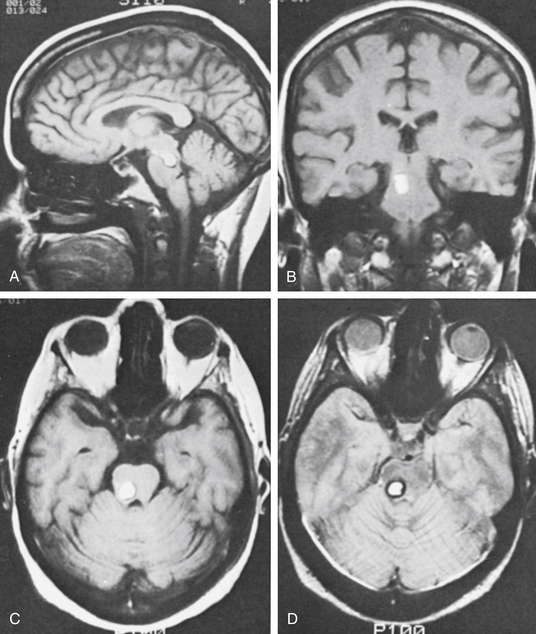
In the MGH series of four patients (see Table 81-4), one patient had a CM in the optic chiasm. A field deficit developed during pregnancy as a result of hemorrhage and then improved, only to worsen after a second hemorrhage a few months after delivery. The field deficit improved somewhat after removal of the lesion. Malik et al.118 reported the partial removal of a CM from the underside of the optic nerve and chiasm in one patient. Vision did not improve, but no further symptoms occurred. Shibuya et al.122 reported visual improvement after resection of a chiasmal CM. Deshmukh et al. reported visual improvement in all four of their patients with chiasmal CMs.121 A retrospective review of the literature on optic pathway CMs showed rates of vision preservation were highest in patients who underwent complete resection.123 As such, resection is generally favored for preservation of vision and prevention of rehemorrhage.
One patient in the MGH series had a lesion involving the seventh and eighth nerves in the internal auditory canal. Involvement in the internal auditory canal has been reported in four patients, including the one in the MGH series.119,124 The eighth nerve could not be saved in any patient, but reasonable facial nerve function remained in three patients. When the facial nerve cannot be saved, a nerve graft is placed at operation. One patient in the MGH series had a malformation involving the seventh nerve in the temporal bone. The malformation and nerve were removed, and a sural nerve graft was placed. The last patient in the series had a lesion of the third cranial nerve120 that was extensively involved and was resected to remove the malformation. The case reported by Scott125 was treated in the same way.
CM of the Spinal Cord
Clinical Presentation
Reviewing spinal cord CMs reported within the literature through 1991, Ogilvy et al.11 described 36 patients with 37 spinal lesions, including 6 patients from the MGH series. The series included 25 (69%) women and 11 (31%) men. The locations of the lesions along the spinal axis were cervical medullary junction in 3 (8%), cervical in 12 (32%), thoracic in 20 (54%), lumbar spinal cord in 1 (3%), and conus medullaris in 1 (3%). The review found 2 patients had a history of familial CM, 4 had a CM at some other site in the central nervous system, and 1 had a second spinal lesion.
1. Thirteen patients presented with episodes of neurologic deterioration with variable degrees of neurologic recovery between episodes. The episodes lasted hours to days. The interval between events was often months to years.
2. Twelve patients had a clinical course characterized by slowly progressive neurologic deterioration. The duration of the progression was usually several years. Two patients had discrete episodes of gradual worsening separated by many years.
3. Eight patients had an acute onset of symptoms followed by neurologic worsening over several days.
4. Three patients had the acute onset of mild symptoms followed by weeks or months of deterioration of neurologic function.
Review of the brain imaging studies of 17 patients in a recent series of spinal CMs demonstrated a higher incidence of multiple neuraxis CMs, with 8 (47%) such patients harboring multiple CMs126; patients with spinal CMs may warrant complete neuraxis imaging to evaluate for additional lesions.
Management
The diagnosis of a CM of the spinal cord is established by the characteristic MRI appearance (Fig. 81-13). From the history obtained from symptomatic patients, it is evident that in some patients there can be intervals of several years between episodes of recurrent neurologic problems that presumably result from hemorrhage. In these intervals, a neurologic deficit is stable or the patient is symptom free. Also, in recurrent hemorrhages, the neurologic deficit often increases and does not always fully recover. Rehemorrhage rates as high as 66% per year have been reported, favoring resection of symptomatic lesions.127 An alternative strategy has been proposed by Kharkar et al., who studied 14 symptomatic patients at their institution with a mean follow-up of 80 months. In this group, 10 patients were managed conservatively and did not demonstrate any new hemorrhage or neurologic deficit. They found that 2 of the patients showed significant improvement in neurologic status, while the rest remained stable. In addition, 4 patients were treated surgically; 2 of these remained the same, 1 improved, and 1 deteriorated significantly.128 While this is a relatively small study, the authors’ conclusion was that observation of patients, even those with symptomatic intramedullary spinal cord malformations, is not unreasonable. Consensus on this issue is not available, and treatment must be tailored to the individual clinical scenario.
Results
Several small case series have shown favorable results with complete resection of intramedullary CMs.129,130 Transient neurologic complications in the early postoperative period can be prominent, however.130,131 Spetzger et al.130 noted such complications in 5 of 9 patients (55%) but found that over long-term follow-up, 8 of 9 patients (88%) were normal or improved compared with preoperative status. Operative intervention for spinal cord CMs generally results in long-term improvement or stabilization of function.11,127,129–133 Cantore et al.134 found a correlation between surgical outcome and severity and length of preoperative symptoms in 6 patients, and Canavero et al.135 found that preoperative status was the most important factor affecting outcome from surgery. Mitha et al. published the largest series to date of surgical outcomes in patients with spinal CMs. At 5-year follow-up, 10% of their cohort of 80 patients were worse, 68% were the same, and 23% were improved compared to their preoperative status. Moreover, they noted an interesting correlation between outcome and anterior–posterior length of the lesion, but not with craniocaudal or transverse length. They surmise that this likely relates to the vast majority of the lesions operated on being posteriorly located. As lesions increased in anterior–posterior size, they extended more anteriorly, placing them in proximity with motor tracts.136 With more frequent availability of MRI, a higher proportion of patients are being diagnosed with spinal CMs prior to neurologic deterioration, when the only symptom present is pain. Surgery for spinal CMs that are causing significant pain appears to be effective at reducing pain at 1 month and 1 year.137
Conclusions
1. Patients who are asymptomatic are observed.
2. Patients with acute severe or progressive neurologic deficits undergo surgical resection.
3. Patients presenting with medically uncontrolled seizures undergo surgical resection; patients with new onset seizures may undergo resection, but some are observed, depending on the factors discussed in this chapter.
4. Patients with a single hemorrhage in the cerebrum, cerebellum, or spinal cord usually undergo surgical resection. When the hemorrhage is in the brain stem, thalamus, or basal ganglia, the patients are observed.
5. Patients with a recurrent hemorrhage usually undergo resection, but there are exceptions when the lesion is in a deep area with high surgical risk.
Abla A.A., Lekovic G.P., Turner J., et al. Advances in the Treatment and Outcome of Brain Stem Cavernous Malformation Surgery: a Case Series of 300 Surgically Treated Patients. Neurosurgery. 2010 Nov 25. [Epub ahead of print]
Amin-Hanjani S., Ogilvy C.S., Candia G.J., et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery. 1998;42:1229-1238.
Asaad W.F., Walcott B.P., Nahed B.V., Ogilvy C.S. Operative management of brainstem cavernous malformations. Neurosurg Focus. 2010 Sep;29(3):E10.
Bertalanffy H., Benes L., Miyazawa T., et al. Cerebral cavernomas in the adult: review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1-53.
Deshmukh V.R., Albuquerque F.C., Zabramski J.M., et al. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. 2003;53:352-357.
De Souza J.M., Dominges R.C., Cruz L.C.Jr., et al. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with T2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008 Jan;29(1):154-158.
Fabrikant J.I., Levy R.P., Steinberg G.K., et al. Charged-particle radiosurgery for intracranial vascular malformations. Neurosurg Clin N Am. 1992;3:99-139.
Hammen T., Romstöck J., Dörfler A., et al. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: a study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007 Apr;16(3):248-253. Epub 2007 Feb 2
Kharkar S., Shuck J., Conway J., et al. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007 May;60(5):865-872. discussion 865–872
Kondziolka D., Lunsford L.D., Flickinger J.C., et al. Reduction of hemorrhage risk after stereotactic radisourgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
Kupersmith M.J., Kalish H., Epstein F., et al. Natural history of brain stem cavernous malformations. Neurosurgery. 2001;48:47-54.
Little J.R., Awad I.A., Jones S.C., et al. Vascular pressures and cortical blood flow in cavernous angioma of the brain. J Neurosurg. 1990;73:555-559.
Louvi A., Chen L., Two A.M., et al. Loss of cerebral cavernous malformation 3 (CCM3) in neuroglia leads to CCM and vascular pathology. Proc Natl Acad Sci USA. 2011 Mar 1;108(9):3737-3742.
Lunsford L.D., Khan A.A., Niranjan A., et al. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg. 2010 Jul;113(1):23-29.
Mitha A.P., Turner J.D., Abla A.A., et al. Outcomes following resection of intramedullary spinal cord cavernous malformations: a 25-year experience. J Neurosurg Spine. 2011 May;14(5):605-611. Epub 2011 Feb 25
Moriarity J.L., Wetzel M., Clatterbuck R.E., et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. 1999;44:1166-1171.
Ogilvy C.S., Heros R.C., Ojemann R.G., et al. Angiographically occult arteriovenous malformations. J Neurosurg. 1988;69:350-355.
Ojemann R.G., Heros R.G., Crowell R.M. Surgical Management of Cerebrovascular Disease. Baltimore: Williams & Wilkins; 1988. 401-403
Rigamonti D., Hadley M.N., Drayer B.P., et al. Cerebral cavernous malformations: incidence and familial occurrence. N Engl J Med. 1988;19:343-347.
Roda J.M., Alvarez F., Isla A., et al. Thalamic cavernous malformations. J Neurosurg. 1990;72:642-649.
Samii M., Eghbal R., Carvalho G.A., et al. Surgical management of brain stem cavernomas. J Neurosurg. 2001;95:825-832.
Sandalcioglu I.E., Wiedemayer H., Gasser T., et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253-256.
Stavrou I., Baumgartner C., Frischer J.M., et al. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008 Nov;63(5):888-896. discussion 897
Whitehead K.J., Chan A.C., Navankasattusas S., et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009 Feb;15(2):177-184.
Zimmerman R.S., Spetzler R.F., Lee K.S., et al. Cavernous malformations of the brain stem. J Neurosurg. 1991;75:32-39.
1. McCormick W.F. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg. 1966;24:807-816.
2. Rengachary S.S., Kalyan-Raman U.P.. Other cranial intradural angiomas, Wilkins R.H., Rengachary S.S., editors, Neurosurgery, New York, McGraw-Hill, 1985 :1465-1472 vol. 2
3. Russell D.S., Rubinstein L.J. Pathology of Tumors of the Nervous System. London: Edward Arnold; 1959. 79-83
4. Ogilvy C.S., Heros R.C., Ojemann R.G., et al. Angiographically occult arteriovenous malformations. J Neurosurg. 1988;69:350-355.
5. Ojemann R.G., Heros R.G., Crowell R.M. Surgical Management of Cerebrovascular Disease. Baltimore: Williams & Wilkins; 1988. 401-403
6. Tung H., Giannotoa S.L., Chandrasoma P.T., et al. Recurrent intraparenchymal hemorrhage from angiographically occult vascular malformations. J Neurosurg. 1990;73:174-180.
7. Wakai S., Ueda Y., Inoh S., et al. Angiographically occult angiomas: a report of 13 cases with analysis of the cases documented in the literature. Neurosurgery. 1985;17:549-556.
8. Wilkins R.H. Natural history of intracranial vascular malformations: a review. Neurosurgery. 1985;16:421-430.
9. Wilson C.B. Cryptic vascular malformations. Clin Neurosurg. 1992;38:49-84.
10. Maraire J.N., Awad I.A. Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery. 1995;37:591-605.
11. Ogilvy C.S., Louis D.N., Ojemann R.G. Intramedullary cavernous angiomas of the spinal cord: clinical presentation, pathologic features and surgical management. Neurosurgery. 1992;31:219-230.
12. Rigamonti D., Spetzler R.F. The association of venous and cavernous malformations: report of four cases and discussion of the pathophysiological, diagnostic, and therapeutic implications. Acta Neurochir (Wien). 1988;92:100-105.
13. Detwiler P.W., Porter R.W., Zabramski J.M., et al. De novo formation of a central nervous system cavernous malformation: implications for predicting risk of hemorrhage: case report and review of the literature. J Neurosurg. 1997;87:629-632.
14. Larson J.J., Ball W.S., Bove K.E., et al. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg. 1998;88:51-56.
15. Pozatti E., Giangaspero F., Marliani F., et al. Occult cerebrovascular malformations after irradiation. Neurosurgery. 1996;39:677-682.
16. Pozzati E., Acciarri N., Tognetti F., et al. Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery. 1996;38:662-669.
17. Zabramski J.M., Wascher T.M., Spetzler R.F., et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 80, 1994. 422–432
18. Pozzati E., Giuliani G., Nuzzo G., et al. The growth of cerebral cavernous angiomas. Neurosurgery. 1989;25:91-97.
19. Rigamonti D., Drayer B.P., Johnson P.C., et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67:518-524.
20. Muras I., Confronti R., Scuotto A., et al. Cerebral cavernous angioma: diagnostic considerations. J Neuroradiol. 1993;20:34-41.
21. Roda J.M., Carceller F., Perez-Higueras A., et al. Encapsulated intracerebral hematoma: a defined entity. J Neurosurg. 1993;78:829-833.
22. De Souza J.M., Dominges R.C., Cruz L.C.Jr, et al. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with T2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008 Jan;29(1):154-158.
23. Servo A., Porras M., Raininko R. Diagnosis of cavernous haemangiomas by computed tomography and angiography. Acta Neurochir (Wien). 1984;71:273-282.
24. Hayman L.A., Evans R.A., Ferrell R.E., et al. Familial cavernous angiomas: natural history and genetic study over a 5-year period. Am J Med Genet. 1982;11:147-160.
25. Rigamonti D., Hadley M.N., Drayer B.P., et al. Cerebral cavernous malformations: incidence and familial occurrence. N Engl J Med. 1988;19:343-347.
26. Labauge P., Laberge S., Brunereau L., et al. Hereditary cerebral cavernous angiomas: clinical and genetic features in 57 French families. Societe Francaise de Neurochirurgie. Lancet. 1998;352:1891-1897.
27. Sunada I., Nakabayashi H., Tsuchida K., et al. A case of familial cerebral cavernous angioma and review of Japanese cases. No Shinkei Geka. 2001;29:359-365.
28. Siegel A.M. Familial cavernous angioma: an unknown, known disease. Acta Neurol Scand. 1998;98:369-371.
29. Gil-Nagel A., Dubovsky J., Wilcox K.J., et al. Familial cerebral cavernous angioma: a gene localized to a 15-cm interval on chromosome 7q. Ann Neurol. 1996;39:807-810.
30. Gunel M., Awad I.A., Anson J., et al. Mapping of a gene causing cerebral cavernous malformations to 7q11.2-q21. Proc Natl Acad Sci U S A. 1995;92:6620-6624.
31. Gunel M., Awad I.A., Finberg K., et al. A founder mutation as a cause of cerebral cavernous malformation in Hispanic Americans. N Engl J Med. 1996;334:946-951.
32. Craig H.D., Gunel M., Cepeda O., et al. Multilocus linkage identifies two new loci for a Mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2-27. Hum Mol Genet. 1998;7:1851-1858.
33. Davenport W.J., Siegel A.M., Dichgans J., et al. CCM1 gene mutations in families segregating cerebral cavernous malformations. Neurology. 2001;56:540-543.
34. Laberge-le Couteulx S., Jung H.H., Labauge P., et al. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet. 1999;23:189-193.
35. Zhang J., Clatterbuck R.E., Rigamonti D., et al. Mutations in KRIT1 in familial cerebral cavernous malformations. Neurosurgery. 2000;46:1272-1277.
36. Marini V., Ferrera L., Dorcaratto A., et al. Identification of a novel KRIT1 mutation in an Italian family with cerebral cavernous malformations by the protein truncation test. J Neurol Sci. 2003;212:75-78.
37. Gunel M., Laurans M.S.H., Shin D., et al. KRIT1, a gene mutated in cerebral cavernous malformations, encodes a microtubule-associated protein. PNAS. 2002;99:10677-10682.
38. Liquori C.L., Berg M.J., Siegel A.M., et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am J Hum Genet. 2003;73:1459-1464.
39. Whitehead K.J., Chan A.C., Navankasattusas S., et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009 Feb;15(2):177-184.
40. Stockton R.A., Shenkar R., Awad I.A., et al. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010 Apr 12;207(4):881-896.
41. Bergametti F., Denier C., Labauge P., et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005 Jan;76(1):42-51.
42. Guclu B., Ozturk A.K., Pricola K.L., et al. Mutations in apoptosis-related gene, PDC10, cause cerebral cavernous malformation 3. Neurosurgery. 2005 Nov;57(5):1008-1013.
43. Louvi A., Chen L., Two A.M., et al. Loss of cerebral cavernous malformation 3 (CCM3) in neuroglia leads to CCM and vascular pathology. Proc Natl Acad Sci USA. 2011 Mar 1;108(9):3737-3742.
44. Lucas M., Costa A.F., Montori M., et al. Germline mutations in the CCM1 gene, encoding KRIT1, cause cerebral cavernous malformations. Ann Neurol. 2001;49:529-532.
45. Kehrer-Sawatzki H., Wilda M., Braun V.M., et al. Mutation and expression analysis of the KRIT1 gene associated with cerebral cavernous malformations (CCM1). Acta Neuropathol. 2002;104:231-240.
46. Voigt K., Yasargil M.G. Cerebral cavernous haemangiomas or cavernomas: incidence, pathology, localization, diagnosis, clinical features and treatment: review of the literature and report of an unusual case. Neurochirurgia (Stuttg). 1976;19:59-68.
47. Simard J.M., Garcia-Bengochea F., Ballinger W.E.Jr, et al. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery. 1986;18:162-172.
48. Robinson J.R.Jr, Awad I.A., Little J.R. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-719.
49. Curling O.D.Jr, Kelly D.L., Elster A.D., et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
50. Scott R.M., Barnes P., Kupsky W., et al. Cavernous angiomas of the central nervous system in children. J Neurosurg. 1992;76:38-46.
51. Giombini S., Morello G. Cavernous angiomas of the brain: account of 14 personal cases and review of the literature. Acta Neurochir (Wien). 1978;40:61-82.
52. Porter P.J., Willinsky R.A., Harper W., et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with and without hemorrhage. J Neurosurg. 1997;87:190-197.
53. Zimmerman R.S., Spetzler R.F., Lee K.S., et al. Cavernous malformations of the brain stem. J Neurosurg. 1991;75:32-39.
54. Robinson Sr J.R., Awad I.A., Magdinee M., et al. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993;32:730-736.
55. Moriarity J.L., Wetzel M., Clatterbuck R.E., et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. 1999;44:1166-1171.
56. Labauge P., Brunereau L., Levy C., et al. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology. 2000;42:327-332.
57. Kondziolka D., Lunsford L.D., Kestle J.R.W. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
58. Kim D.S., Park Y.G., Choi J.U., et al. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48:9-17.
59. Kupersmith M.J., Kalish H., Epstein F., et al. Natural history of brain stem cavernous malformations. Neurosurgery. 2001;48:47-54.
60. Aiba T., Tanaka R., Koike T., et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56-59.
61. Barker F.G., Amin-Hanjani S., Butler W.E., et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-25.
62. Narayan P., Barrow D.L. Intramedullary spinal cavernous malformation following spinal irradiation: case report and review of the literature. J Neurosurg. 2003;98(Suppl 1):68-72.
63. Massa-Micon B., Luparello V., Bergui M., et al. De novo cavernoma case report and review of the literature. Surg Neurol. 2000;53:484-487.
64. Ludemann W., Ellerkamp V., Stan A.C., et al. De novo development of a cavernous malformation of the brain: significance of factors with paracrine and endocrine activity: case report. Neurosurgery. 2002;50:646-649.
65. Golfinos J.G., Wascher T.M., Zabramskik J.M., et al. The management of unruptured intracranial vascular malformation. Barrow Neurol Inst Q. 1992;8:2-11.
66. Amin-Hanjani S., Ogilvy C.S., Ojemann R.G., et al. Risks of surgical management for cavernous malformations of the nervous system. Neurosurgery. 1998;42:1220-1227.
67. Esposito V., Oppido P.A., Delfini R., et al. A simple method for stereotactic microsurgical excision of small deep-seated cavernous angiomas. Neurosurgery. 1994;34:515-519.
68. Winkler D., Lindner D., Strauss G., et al. Surgery of cavernous malformations with and without navigational support-—a comparative study. Minim Invasive Neurosurg. 2006 Feb;49(1):15-19.
69. Kondziolka D., Lunsford L.D., Flickinger J.C., et al. Reduction of hemorrhage risk after stereotactic radisourgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
70. Hasegawa T., McInerney Kondziolka D, et al. Long-term results after stereotactic radisourgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197.
71. Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
72. Pollock B.E., Garces Y.I., Stafford S.L., et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
73. Zhang N., Pan L., Wang B.J., et al. Gamma knife radiosurgery for cavernous hemangiomas. J Neurosurg. 2000;93(Suppl 3):74-77.
74. Lunsford L.D., Khan A.A., Niranjan A., et al. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg. 2010 Jul;113(1):23-29.
75. Nyáry I., Major O., Hanzély Z., et al. Histopathological findings in a surgically resected thalamic cavernous hemangioma 1 year after 40-Gy irradiation. J Neurosurg. 2005;102(Suppl):56-58.
76. Monaco E.A., Khan A.A., Niranjan A., et al. Stereotactic radiosurgery for the treatment of symptomatic brainstem cavernous malformations. Neurosurg Focus. 2010 Sep;29(3):E11.
77. Fabrikant J.I., Levy R.P., Steinberg G.K., et al. Charged-particle radiosurgery for intracranial vascular malformations. Neurosurg Clin N Am. 1992;3:99-139.
78. Alexander E.III, Loeffler S.S. Radiosurgery using a modified linear accelerator. Neurosurg Clin N Am. 1992;3:167-190.
79. Chang S.D., Levy R.P., Adler J.R., et al. Stereotactic radiosurgery of angiographically occult vascular malformations: 14-year experience. Neurosurgery. 1998;43:213-220.
80. Amin-Hanjani S., Ogilvy C.S., Candia G.J., et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery. 1998;42:1229-1238.
81. St George E.J., Perks J., Plowman P.N. Stereotactic radiosurgery XIV: the role of the haemosiderin “ring” in the development of adverse reactions following radiosurgery for intracranial cavernous malformations: a sustainable hypothesis. Br J Neurosurg. 2002;16:385-391.
82. Acciarri N., Padovani R., Giulioni M., et al. Intracranial and orbital cavernous angiomas: a review of 74 surgical cases. Br J Neurosurg. 1993;7:529-539.
83. Little J.R., Awad I.A., Jones S.C., et al. Vascular pressures and cortical blood flow in cavernous angioma of the brain. J Neurosurg. 1990;73:555-559.
84. Schneider R.C., Liss L. Cavernous hemangiomas of the cerebral hemispheres. J Neurosurg. 1958;15:391-399.
85. Tagle P., Huete I., Mendez J., et al. Intracranial cavernous angioma: presentation and management. J Neurosurg. 1986;64:720-723.
86. Vaquero J., Leunda G., Martinez R., et al. Cavernomas of the brain. Neurosurgery. 1983;12:208-210.
87. Yamasaki T., Hande H., Yamashita J., et al. Intracranial and orbital cavernous angiomas. J Neurosurg. 1986;64:197-208.
88. Chadduck W.M., Binet E.F., Farrell F.W.Jr, et al. Intraventricular cavernous hemangioma: report of three cases and review of the literature. Neurosurgery. 1985;16:189-197.
89. Pozzati E. Thalamic cavernous malformations. Surg Neurol. 2000;53:30-39.
90. Lorenzana L., Cabezudo J.M., Porras L.F., et al. Focal dystonia secondary to cavernous angioma of the basal ganglia: case report and review of the literature. Neurosurgery. 1992;31:1108-1112.
91. Roda J.M., Alvarez F., Isla A., et al. Thalamic cavernous malformations. J Neurosurg. 1990;72:642-649.
92. Bertalanffy H., Gilsbach J.M., Eggert H.R., et al. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir (Wien). 1991;108:91-99.
93. Steinberg G.K., Chang S.D., Gewirtz R.J., et al. Microsurgical resection of brain stem, thalamic and basal ganglia angiographically occult vascular malformation. Neurosurgery. 2000;46:260-271.
94. Bertalanffy H., Kuhn G., Scheremet R., et al. Indications for surgery and prognosis in patients with cerebral cavernous angiomas. Neurol Med Chir (Tokyo). 1992;32:659-666.
95. Cohen D.S., Zubay G.P., Goodman R.R. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83:237-242.
96. Casazza M., Broggi G., Franzini A., et al. Supratentorial cavernous angiomas and epileptic seizures: preoperative course and postoperative outcome. Neurosurgery. 1996;39:26-34.
97. Zevgaridis D., van Velthoven V., Ebeling U., et al. Seizure control following surgery in supratentorial cavernous malformations: a retrospective study in 77 patients. Acta Neurochir (Wien). 1996;138:672-677.
98. Hammen T., Romstöck J., Dörfler A., et al. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: a study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007 Apr;16(3):248-253. Epub 2007 Feb 2
99. Baumann C.R., Schuknecht B., Lo Russo G., et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006 Mar;47(3):563-566.
100. Stavrou I., Baumgartner C., Frischer J.M., et al. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008 Nov;63(5):888-896. discussion 897
101. Cappabianca P., Alfieri A., Maiuri F., et al. Supratentorial cavernous malformations and epilepsy: seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg Psychiatry. 1997;99:179-183.
102. Falbusch R., Strauss C., Huk W., et al. Surgical removal of pontomesencephalic cavernous hemangioma. Neurosurgery. 1990;26:449-457.
103. Porter R.W., Detwiler P.W., Spetzler R.F., et al. Cavernous malformations of the brain stem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
104. Bertalanffy H., Benes L., Miyazawa T., et al. Cerebral cavernomas in the adult: review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1-53.
105. Ziyal I.M., Sekhar L.N., Salas E., et al. Surgical management of cavernous malformations of the brain stem. Br J Neurosurg. 1999;13:366-375.
106. Samii M., Eghbal R., Carvalho G.A., et al. Surgical management of brain stem cavernomas. J Neurosurg. 2001;95:825-832.
107. Wang C., Liu A., Zhang J., et al. Surgical management of brain stem cavernous malformations: report of 137 cases. Surg Neurol. 2003;59:444-454.
108. Mathiesen T., Edner G., Kihlstrom L. Deep and brain stem cavernomas: a consecutive 8-year series. J Neurosurg. 2003;99:31-37.
109. Sandalcioglu I.E., Wiedemayer H., Secer S., et al. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. 2002;72:351-355.
110. Isamat F., Louesa G. Cavernous angioma of the brain stem. Neurosurg Clin N Am. 1993;4:507-518.
111. Fritschi J.A., Reulen H.J., Spetzler R.F., et al. Cavernous malformations of the brain stem. Acta Neurochir (Wien). 1994;130:35-46.
112. Weil S.M., Tew J.M. Surgical management of brain stem vascular malformations. Acta Neurochir (Wien). 1990;105:14-23.
113. Scott R.M. Brain stem cavernous angiomas in children. Pediatr Neurosurg. 1990;16:281-286.
114. LeDoux M., Aronin P.A., Odrezin G.T. Surgically treated cavernous angiomas of the brain stem: report of two cases and review of the literature. Surg Neurol. 1991;35:395-399.
115. Abla A.A., Lekovic G.P., Turner J., et al. Advances in the Treatment and Outcome of Brain Stem Cavernous Malformation Surgery: a Case Series of 300 Surgically Treated Patients. Neurosurgery. 2011;68(2):403-414. discussion 414–415
116. Ferroli P., Sinisi M., Franzini A., et al. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery. 2005;56:1203-1204.
117. Corboy J.R., Galetta S.L. Familial cavernous angiomas manifesting with an acute chiasmal syndrome. Am J Ophthalmol. 1989;108:245-250.
118. Malik S., Cohen B.H., Robinson J., et al. Progressive vision loss: a rare manifestation of familial cavernous angiomas. Arch Neurol. 1992;49:170-173.
119. Matias-Guiu X., Alejo M., Sole T., et al. Cavernous angiomas of the cranial nerves. J Neurosurg. 1990;73:620-622.
120. Ogilvy C.S., Pakzaban P., Lee J.M. Oculomotor nerve cavernous angioma in a patient with Robert’s syndrome. Surg Neurol. 1993;33:39-47.
121. Deshmukh V.R., Albuquerque F.C., Zabramski J.M., et al. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. 2003;53:352-357.
122. Shibuya M., Baskaya M.K., Saito K., et al. Cavernous malformations of the optic chiasm. Acta Neurochir (Wien). 1995;136:29-36.
123. Liu J.K., Lu Y., Raslan A.M., et al. Cavernous malformations of the optic pathway and hypothalamus: analysis of 65 cases in the literature. Neurosurg Focus. 2010 Sep;29(3):E17.
124. Sundaresan N., Ellen T., Civic I. Hemangiomas of the internal auditory canal. Surg Neurol. 1976;6:119-121.
125. Scott R.M. Third nerve palsy in a 14-year-old boy due to cavernous hemangioma of the third nerve. In: Raimond A.J., editor. Concepts in Pediatric Neurosurgery. New York: Karger; 1983:100-107.
126. Vishteh A.G., Zabramski J.M., Spetzler R.F. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuraxis cavernous malformations. Neurosurgery. 1999;45:30-32.
127. Sandalcioglu I.E., Wiedemayer H., Gasser T., et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253-256.
128. Kharkar S., Shuck J., Conway J., et al. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007 May;60(5):865-872. discussion 865–872
129. Gordon C.R., Crockard H.A., Symon L. Surgical management of spinal cord cavernoma. Br J Neurosurg. 1995;9:459-464.
130. Spetzger U., Gilsbach J.M., Bertalanffy H. Cavernous angiomas of the spinal cord clinical presentation, surgical strategy, and postoperative results. Acta Neurochir (Wien). 1995;134:200-206.
131. Anson J.A., Spetzler R.F. Surgical resection of intramedullary spinal cord cavernous malformations. J Neurosurg. 1993;78:446-451.
132. Cosgrove G.R., Bertrand G., Fontaine S., et al. Cavernous angiomas of the spinal cord. J Neurosurg. 1988;68:31-36.
133. McCormick P.C., Michelson W.J., Post K.D., et al. Cavernous malformations of the spinal cord. Neurosurgery. 1988;23:459-463.
134. Cantore G., Delfini R., Cervoni L., et al. Intramedullary cavernous angiomas of the spinal cord: report of six cases. Surg Neurol. 1995;43:448-452.
135. Canavero S., Pagni C.A., Duca S., et al. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41:381-388.
136. Mitha A.P., Turner J.D., Abla A.A., et al. Outcomes following resection of intramedullary spinal cord cavernous malformations: a 25-year experience. J Neurosurg Spine. 2011 May;14(5):605-611. Epub 2011 Feb 25
137. Deutsch H. Pain outcomes after surgery in patients with intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010 Sep;29(3):E15.
138. Asaad W.F., Walcott B.P., Nahed B.V., Ogilvy C.S. Operative management of brainstem cavernous malformations. Neurosurg Focus. 2010 Sep;29(3):E10.