Chapter 13 Surgical Management of Brain Stem Tumors in Adults
Brain stem tumors account for 1.5% to 2.5% of all intracranial tumors in adults while comprising 10% to 20% of all pediatric tumors.1–4 These tumors are less common in adults and, therefore, more clinical studies have been conducted in children with brain stem tumors, specifically brain stem gliomas, since they are more common in this patient population.3–10 Adults with brain stem gliomas have a median survival of 5 to 7 years which is longer than that of children.2–5 Historically, brain stem tumors were treated as a homogenous group of lesions with radiotherapy, which usually proceeded without histopathologic confirmation. Surgery for these tumors was rare and typically limited to biopsy, extirpation of cystic lesions, and the placement of shunts for obstructive hydrocephalus. However, Pool documented one of the first surgical resections of a brain stem tumor located in the area of the cerebral aqueduct in 1968.11 This reported case led to the advent of further investigation and development of surgical techniques to treat lesions located in the brain stem.
Imaging and Classification
Prior to the advent of MRI, CT was utilized to assess the pathology of brain stem lesions and biopsy was often needed to guide management. MRI has become the primary diagnostic modality for patients with brain stem tumors because it has advanced the diagnosis and categorization of these lesions by providing superior anatomic detail of the brain stem and posterior fossa.12–16 T1- and T2-weighted MR imaging have provided the ability to differentiate the tissue characteristics of tumors in many cases. In adults, the differential diagnosis for brain stem lesions may be broad and may include such pathologic entities like metastatic tumors, demyelinating processes, infectious processes, granulomas, cavernous malformations, hemangioblastomas, or hematomas that should be differentiated from gliomas.
Based upon MRI and CT data, classification schemes have been developed for grouping brain stem tumors according to growth patterns and the feasibility of surgical resection (Table 13-1).13,14,17–21 The earliest classification schemes were based on CT images and surgical observation.17,19,21 Later schemes relied on MR imaging, in which better neuroanatomic details were made available.13,14,18 The most recently developed classification system relied on both CT and MRI.20 All of these schemes classify the tumor into either a focal or diffuse growth pattern, while the more complex classifications further subdivide the tumors into location within the brain stem, presence or absence of an exophytic component, and the presence of hydrocephalus or hemorrhage. The more complex schemes were developed in an attempt to predict tumor behavior and guide operative versus nonoperative management. In general, tumors with a focal growth pattern have been considered amenable to surgical extirpation in contrast to those with a diffuse growth pattern.
| Author | Method Used to Create System | Classification System |
|---|---|---|
| Epstein21 | CT | Intrinsic |
| Diffuse | ||
| Focal | ||
| Cervicomedullary | ||
| Exophytic | ||
| Anterolateral into cerebellopontine angle | ||
| Posterolateral and into brachium pontis | ||
| Disseminated | ||
| Positive cytology | ||
| Positive myelography | ||
| Epstein and McCleary19 | CT, MRI, and surgical observation | Diffuse |
| Focal | ||
| Cervicomedullary | ||
| Stroink et al.17 | CT | Group I—dorsal exophytic glioma |
| Group IIa—intrinsic brainstem tumors | ||
| Hypodense, no enhancement | ||
| Group IIb—intrinsic brainstem tumors | ||
| Hyperdense, contrast enhancing exophytic | ||
| Group III—focal cystic tumor with contrast enhancement | ||
| Group IV—focal intrinsic isodense contrast enhancement | ||
| Barkovich et al.14 | MRI | Location (midbrain, pons, medulla) |
| Focality (diffuse or focal) | ||
| Direction and extent of tumor growth | ||
| Degree of brainstem enlargement | ||
| Exophytic growth | ||
| Hemorrhage or necrosis | ||
| Evidence of hydrocephalus | ||
| Albright18 | MRI | Focal (midbrain, pons, medulla) |
| Diffuse | ||
| Fischbein et al.13 | MRI | MidbrainDiffuse |
| Focal | ||
| Tectal | ||
| Pons | ||
| Diffuse | ||
| Focal | ||
| Medulla | ||
| Diffuse | ||
| Focal | ||
| Dorsal exophytic | ||
| Choux et al.20 | CT and MRI | Type I—diffuse |
| Type II—intrinsic, focal | ||
| Type III—exophytic, focal | ||
| Type IV—cervicomedullary |
Focal Tumors
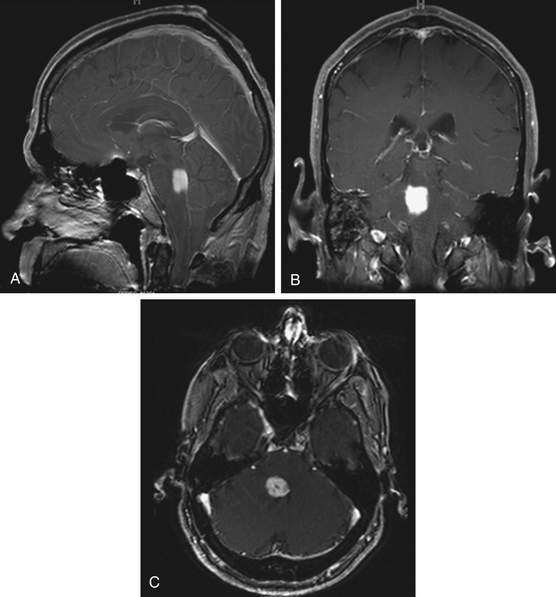
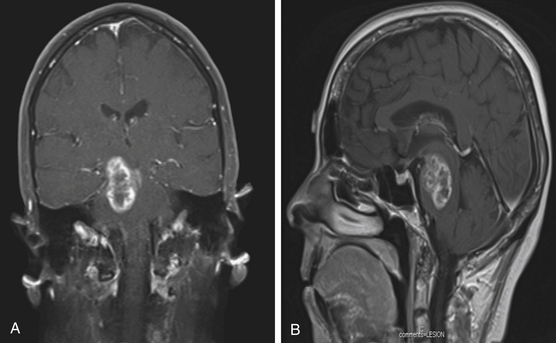
Focal tumors are intrinsic to the brain stem and may be cystic or solid (Fig. 13-1). They are typically not associated with edema and contrast enhancement may be variable. A majority of these tumors are low-grade gliomas, but some malignant tumors, such as a World Health Organization (WHO) grade-IV astrocytoma, may imitate a focal one, known as a pseudofocal tumor. In pseudofocal tumors, MRI and CT imaging demonstrate focal enhancement after the administration of contrast with normal signal characteristics from the peritumoral area that imitates the focal lesion.
Furthermore, the majority of cervicomedullary gliomas are low-grade, noninfiltrative tumors, and their growth is usually confined rostrally by the white matter of the corticospinal tract and medial lemniscus.22
Exophytic Tumors
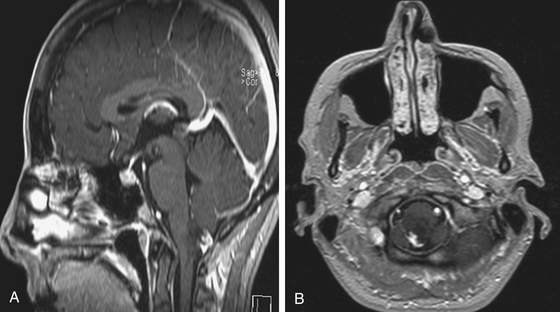
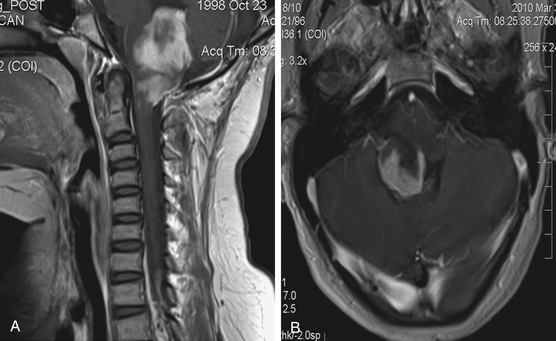
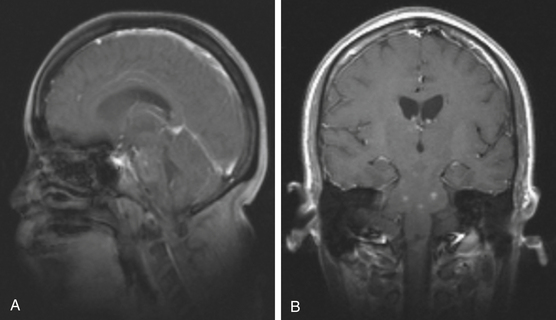
Focal brain stem tumors with an exophytically growing component are usually low-grade and well circumscribed (Fig. 13-2). They are typically dorsally exophytic tumors growing into the fourth ventricle or cervicomedullary tumors with exophytic growth into the cisterna magna and fourth ventricle (Fig. 13-3). It should be mentioned that, in addition to focal tumors, some diffuse tumors may cause bulging into the fourth ventricle, cerebellopontine angle, and prepontine and other cisterns.
Diffuse Tumors
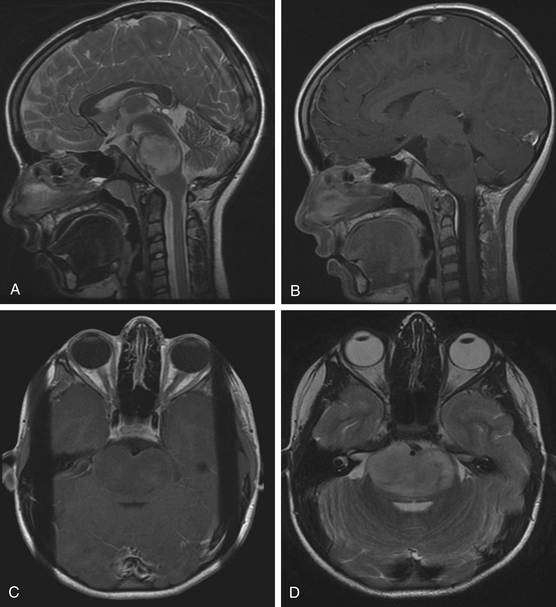
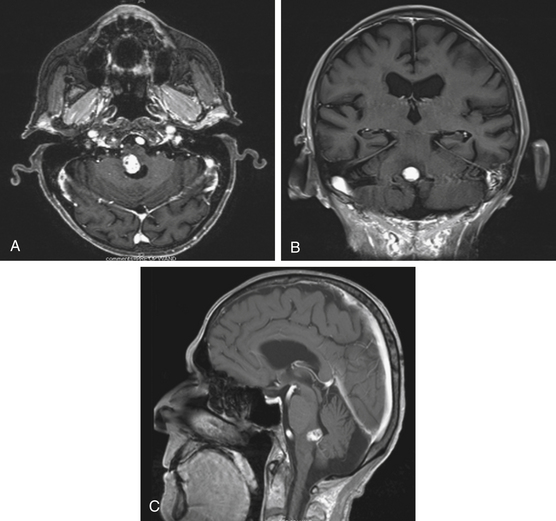
Diffuse brain stem tumors appear hypo- to iso-intense and hyper-intense on T1-weighted and T2-weighted MR images, respectively (Fig. 13-4). There is variable enhancement with contrast administration, and contrast enhancement in these tumors may be indicative of malignant degeneration. Moreover, the tumor boundaries are not able to be delineated on MRI, and the brain stem is typically enlarged and deformed.
Additional Imaging Modalities
Imaging of the central nervous system has evolved with the continual development of more sophisticated techniques. Positron emission tomography (PET) has been used in an attempt to differentiate low-grade from high-grade brain stem gliomas, particularly in the pediatric population.23–26 Although the MR imaging characteristics of gliomas may be highly diagnostic in the pediatric population allowing for the potential use of PET imaging to attempt to determine the degree of malignancy, the heterogeneity of lesions in the adult population may not allow for the procurement of useful data until studies are conducted correlating preoperative PET imaging with histologic diagnosis. One study utilized PET imaging in two adults with dorsal midbrain lesions and obstructive hydrocephalus.27 The lesions were hyperintense on T2-weighted images and partial contrast enhancement was observed in one patient. The patients underwent preoperative PET studies followed by an endoscopic third ventriculostomy combined with endoscopic biopsy sampling of the lesions. Glial proliferation, which contained the partial enhancement with contrast, and a possible low-grade glioma were diagnosed in the two patients, respectively. Results of the PET imaging in both patients suggested that the lesions had nontumorous characteristics and portended a good prognosis. With larger-scale studies, PET imaging may someday prove to be more informative and predictive of the biological behavior of dorsal midbrain lesions than a biopsy.
Magnetic resonance spectroscopy (MRS) is a noninvasive imaging modality used for tissue characterization and complements data obtained from MRI. The concentrations of creatine, phosphocreatine, choline, N-acetylaspartate, lactate, and lipids are analyzed in an effort to differentiate various pathologic processes. It has been used to distinguish between normal and abnormal tissues and grading of brain tumors. Inflammation, infectious processes, and tumors may potentially be distinguished with MRS.28 Studies have been conducted with MRS to investigate brain stem lesions with histologic correlation in some cases.28–31 MRS can provide additional information on brain stem lesions, but further studies are needed with histologic confirmation given the broad differential diagnosis of brain stem lesions in adults.
Diffusion tensor imaging (DTI) is an imaging modality that demonstrates white matter tracts. The relationship of sensory and motor tracts to brain stem tumors has been investigated in pediatric patients.32,33 With further studies and evolution of this technique for use in the brain stem, DTI may play a role in assisting the treatment planning for brain stem lesions in adults.
Clinical Manifestations
The clinical presentation of brain stem tumors varies and has been correlated with the growth pattern, location, and degree of malignancy. Focal tumors tend to have a slow progression to neurologic signs in contrast to diffuse malignant tumors, which have a fast progression to neurologic signs. The manifestations include headaches, nausea, vomiting, diplopia, weakness, ataxia, numbness, cranial neuropathies, and/or vertigo. Pontine and cervicomedullary tumors typically present with cranial neuropathies and long tract signs. Midbrain tumors can present with obstructive hydrocephalus, oculomotor deficit, hemiparesis, and ataxia, while dorsally exophytic tumors typically present with signs of obstructive hydrocephalus.
Stereotactic Biopsy
Since the late 1970s, stereotactic biopsies of brain stem lesions have been performed and account for 5% to 12% of all brain biopsies.34–56 This procedure was not used widely in the pediatric population because it was reported that characteristic MRI features were sufficient to diagnose diffuse brain stem gliomas without the need for a biopsy.14,57–59 Biopsies of brain stem lesions are not as common as other brain biopsies because of the risk involved in obtaining tissue. However, studies have shown that stereotactic brain stem biopsies can be routinely performed in a safe and effective manner.34,40,42–46,51–56 In some published series, the reported rate of complications has ranged between 2.5% and 7.7%.54,56,60
In adults with contrast-enhancing brain stem lesions, other pathologic processes must be considered in the differential diagnosis because studies have demonstrated preoperative radiographic diagnoses to be incorrect in 10% to 25% of cases in patients over 20 years of age presenting with a contrast-enhancing lesion in the brain stem.60,61 In addition to malignant glioma, the differential diagnosis may include infectious processes, such as abscess, tuberculomas, and toxoplasmosis, demyelinating disease, sarcoidosis, progressive multifocal leukoencephalopathy, metastasis, lymphoma, and vascular processes, such as vasculitis and infarction (Figs. 13-5, 13-6, and 13-7).2 This heterogeneity of brain stem lesions in adults makes it difficult to make a diagnosis on the basis of imaging alone. Therefore, image-guided stereotactic biopsies are indicated in many adult brain stem lesions that enhance with contrast and generally in cases in which the diagnosis of the lesion is in doubt to determine the histology of the abnormal process.
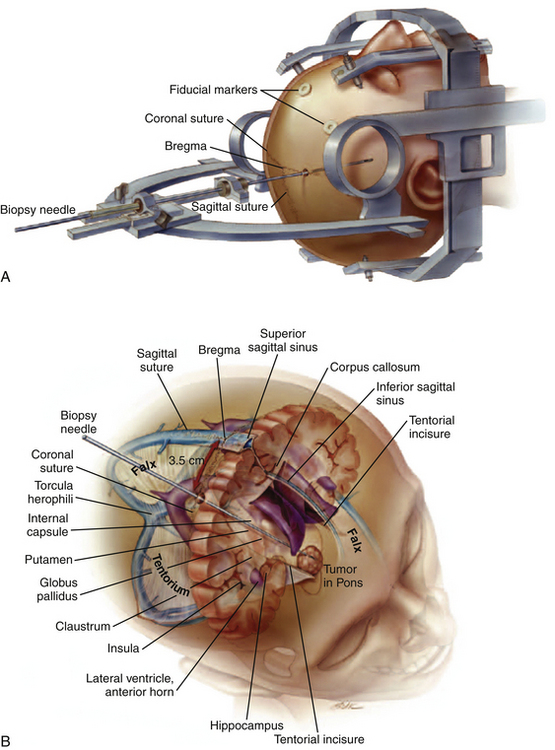
Stereotactic biopsies of the brain stem are performed with image guidance utilizing either CT or MRI. Approaches used include the transfrontal, transtentorial, and transcerebellar routes depending on the location of the lesion within the brain stem. The transtentorial approach, which is really not used, places vital vasculature and cranial nerves at risk and may cause pain with tentorial puncture.62,63 In addition, this route crosses the pia two additional times above and below the tentorium. The ipsilateral transfrontal route frequently requires traversing the lateral ventricle and is limited to midline regions of the pons and medulla by the tentorial incisura.41,51,64,65 Moreover, the suboccipital transcerebellar approach has been used for lesions in the lower midbrain, pons, middle cerebellar peduncle, and rostral medulla.34,46,47,54,66,67 Due to patient positioning, intubation and general anesthesia is generally required although the use of local anesthesia has been reported.54,67 With this approach, a potential drawback is the discomfort associated with muscle dissection prior to placement of the twist-drill hole.
An alternative contralateral, transfrontal, extraventricular approach using a Leksell stereotactic frame system has been described for reaching lesions in the lateral pons and middle cerebellar peduncle (Fig. 13-8).53 The needle’s trajectory crosses only one pial surface and avoids the ventricle and tentorium. In a reported series of six patients, diagnostic samples were obtained in all patients and there was no surgical morbidity.53
Indications for Surgery
In general, patients with a clinical presentation and imaging consistent with a diffuse glioma will not benefit from surgical intervention, although experimental studies are being conducted for the local intracranial delivery of therapeutic agents to these lesions.1,68–72 Focal brain stem tumors are considered amenable to surgical resection, which is often the primary treatment of choice. Dorsal midbrain tumors, such as tectal gliomas known to be indolent and stable clinically and radiographically for many years, may be initially managed in a nonoperative manner with serial imaging, and surgery is performed in cases of tumor progression on MRI.73 Obstructive hydrocephalus, if present, may be treated with an endoscopic third ventriculostomy and the tumor can be followed with serial MR imaging.
Intraoperative Neurophysiologic Monitoring and Mapping
The complex neuroanatomic organization of the brain stem presents a formidable challenge to neurosurgeons when operating on lesions in this complex, small structure. Within the brain stem, cranial nerve nuclei and ascending and descending pathways are contained here. Tumors can distort the normal anatomy of the brain stem, such as anatomic landmarks on the floor of the fourth ventricle like the facial colliculus and striae medullares, and make surgery even more challenging. The introduction of intraoperative neurophysiologic monitoring and mapping has allowed for safer surgery and assisted in formulating the operative plan while minimizing risk to critical brain stem structures.
Functional mapping of the fourth ventricular floor has allowed the identification of brain stem cranial nerve motor nuclei and their relationship to the tumor.74–76 The responses from muscles innervated by cranial nerves VII, IX, X, and XII can be recorded during surgery as the neurosurgeon stimulates the floor of the fourth ventricle with a hand-held monopolar probe. To prevent the occurrence of damage to the floor of the fourth ventricle during stimulation, the tip of the probe is round and approximately 0.75 mm in size. Muscle action potentials, through the utilization of EMG, can be recorded from the orbicularis oculi and oris muscles (CN VII), posterior pharyngeal wall (CN IX, X), and tongue muscles (CN XII). Confirmation should be obtained that the muscle relaxant does not interfere with EMG recordings. Moreover, the extent of brain stem compression, pathology, and distance to the cranial nerve motor nuclei play important roles in determining the threshold intensity, which can be as low as 0.2 mA for a hematoma, while brain stem tumors usually require higher threshold intensities up to 2.0 mA. After a muscle response is recorded, utilization of the threshold intensity allows for localization of the nuclei by moving the stimulation probe every 1 mm. This information allows for a safe site for an incision and attempts to minimize injury during tumor removal.74–76 If muscle responses are not detected, technical problems with the stimulator and/or recording system may be present, or the cranial nerve motor nuclei may be located ventral to the pathology. If the nuclei are located ventral to the brain stem pathology, then repeated stimulation would be required through the lesion for detection of the nuclei.
Moreover, the corticospinal tracts can be mapped at the level of the cerebral peduncles.77 A Kartush handheld stimulator (Medtronic Xomed, Inc.) is used to focally stimulate the midbrain with EMG monitoring of specific muscles. This intraoperative modality allows the surgeon to determine the location of the aforementioned pathways in relation to the tumor and safely make an incision in the midbrain. Localization of the corticospinal tracts assists the surgeon in an endeavor to protect them during the dissection around and mobilization of the lesion.
Surgical Approaches
The subtemporal approach provides access to lesions of the lateral midbrain. An orbitozygomatic approach provides additional access to the rostral pons, interpeduncular region, and pontomesencephalic junction in addition to the lateral midbrain.78–80 These approaches may be combined with an anterior petrosectomy for lesions located more inferior in the ventral pons.
Surgical Techniques
Intrinsic tumors necessitate an incision in the surface of the brain stem and require a thorough understanding of the anatomy. Although many tumors can be accessed where it is closest to the surface, it must bear in mind that this may not be the optimal route in some cases. The incision is usually small and less than 1 cm, which typically provides enough space for the resection of large tumors. Tumors in the vicinity of the corticospinal tracts in the cerebral peduncles can be mapped for a safe entry point. For tumors approached through the fourth ventricular floor, mapping of the floor is extremely important for placement of the incision to avoid cranial nerve nuclei, such as VII, IX, X, and XII. In addition, there are areas of the brain stem that can be entered relatively safely. Dorsal pontine tumors approached through the fourth ventricular floor can be entered through the median sulcus above the facial colliculus, suprafacial, infrafacial, and area acoustica. Tectal mesencephalic tumors can be accessed through the supracollicular, infracollicular, and lateral mesencephalic sulcus, while longitudinal myelotomies can be performed in the posterior median fissure below the obex, posterior intermediate sulcus, and posterior lateral sulcus for medullary and cervicomedullary tumors.81
For focal tumors with an exophytic component, this protruding aspect of the tumor provides an avenue for surgical access to the tumor. Moreover, tumors that penetrate the subarachnoid space may contact or encase vital arteries, and those that penetrate the ventral brain stem may contact the vertebral or basilar arteries and their branches; therefore, these tumors must be approached with extreme caution. Tumors arising from the subependymal aspect of the pons or medulla with no or minimal brain stem invasion and protruding into the fourth ventricle comprise a subgroup of dorsally exophytic tumors that are benign and can be resected with success.22,82,83
Abernathey C.D., Camacho A., Kelly P.J. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195-200.
Amundson E.W., McGirt M.J., Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg. 2005;102:565-570.
Barkovich A.J., Krischer J., Kun L.E., et al. Brain stem gliomas: A classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73-83.
1. Frazier J.L., Lee J., Thomale U.W., et al. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009;3:259-269.
2. Laigle-Donadey F., Doz F., Delattre J.Y. Brainstem gliomas in children and adults. Curr Opin Oncol. 2008;20:662-667.
3. Guillamo J.S., Monjour A., Taillandier L., et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528-2539.
4. Kesari S., Kim R.S., Markos V., et al. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175-183.
5. Salmaggi A., Fariselli L., Milanesi I., et al. Natural history and management of brainstem gliomas in adults. A retrospective Italian study. J Neurol. 2008;255:171-177.
6. Landolfi J.C., Thaler H.T., DeAngelis L.M. Adult brainstem gliomas. Neurology. 1998;51:1136-1139.
7. Grigsby P.W., Garcia D.M., Simpson J.R., et al. Prognostic factors and results of therapy for adult thalamic and brainstem tumors. Cancer. 1989;63:2124-2129.
8. Selvapandian S., Rajshekhar V., Chandy M.J. Brainstem glioma: comparative study of clinico-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wien). 1999;141:721-726. discussion 726-727
9. Guiney M.J., Smith J.G., Hughes P., et al. Contemporary management of adult and pediatric brain stem gliomas. Int J Radiat Oncol Biol Phys. 1993;25:235-241.
10. Shrieve D.C., Wara W.M., Edwards M.S., et al. Hyperfractionated radiation therapy for gliomas of the brainstem in children and in adults. Int J Radiat Oncol Biol Phys. 1992;24:599-610.
11. Pool J.L. Gliomas in the region of the brain stem. J Neurosurg. 1968;29:164-167.
12. Zimmerman R.A. Neuroimaging of primary brainstem gliomas: Diagnosis and course. Pediatr Neurosurg. 1996;25:45-53.
13. Fischbein N.J., Prados M.D., Wara W., et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9-23.
14. Barkovich A.J., Krischer J., Kun L.E., et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73-83.
15. Lee B.C., Kneeland J.B., Walker R.W., et al. MR imaging of brainstem tumors. AJNR Am J Neuroradiol. 1985;6:159-163.
16. Packer R.J., Zimmerman R.A., Luerssen T.G., et al. Brainstem gliomas of childhood: magnetic resonance imaging. Neurology. 1985;35:397-401.
17. Stroink A.R., Hoffman H.J., Hendrick E.B., et al. Transependymal benign dorsally exophytic brain stem gliomas in childhood: diagnosis and treatment recommendations. Neurosurgery. 1987;20:439-444.
18. Albright A.L. Brain stem gliomas. In: Youmans J., editor. Neurological Surgery. 4th ed. Philadelphia: WB Saunders Co; 1996:2603-2611.
19. Epstein F., McCleary E.L. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11-15.
20. Choux M., Lena G., Do L. Brainstem tumors. In: Choux M., Di Rocco C., Hockley A. Pediatric Neurosurgery. New York: Churchill Livingstone; 2000:471-491.
21. Epstein F. A staging system for brain stem gliomas. Cancer. 1985;56:1804-1806.
22. Jallo G.I., Kothbauer K.F., Epstein F.J.. Surgical management of cervicomedullary and dorsally exophytic brain stem tumors, Spetzler R.F., editor, Operative Techniques in Neurosurgery. Brain Stem Surgery, Philadelphia, WB Saunders, 2000;Vol 3:131-136.
23. De Witte O., Lefranc F., Levivier M., et al. FDG-PET as a prognostic factor in high-grade astrocytoma. J Neurooncol. 2000;49:157-163.
24. Delbeke D., Meyerowitz C., Lapidus R.L., et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995;195:47-52.
25. Kwon J.W., Kim I.O., Cheon J.E., et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol. 2006;36:959-964.
26. Padma M.V., Said S., Jacobs M., et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64:227-237.
27. Yamaguchi S., Terasaka S., Kobayashi H., et al. Indolent dorsal midbrain tumor: new findings based on positron emission tomography. J Neurosurg Pediatr. 2009;3:270-275.
28. Rand S.D., Prost R., Haughton V., et al. Accuracy of single-voxel proton MR spectroscopy in distinguishing neoplastic from nonneoplastic brain lesions. AJNR Am J Neuroradiol. 1997;18:1695-1704.
29. Smith J.K., Londono A., Castillo M., et al. Proton magnetic resonance spectroscopy of brain-stem lesions. Neuroradiology. 2002;44:825-829.
30. Porto L., Hattingen E., Pilatus U., et al. Proton magnetic resonance spectroscopy in childhood brainstem lesions. Childs Nerv Syst. 2007;23:305-314.
31. Curless R.G., Bowen B.C., Pattany P.M., et al. Magnetic resonance spectroscopy in childhood brainstem tumors. Pediatr Neurol. 2002;26:374-378.
32. Helton K.J., Phillips N.S., Khan R.B., et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am J Neuroradiol. 2006;27:786-793.
33. Helton K.J., Weeks J.K., Phillips N.S., et al. Diffusion tensor imaging of brainstem tumors: axonal degeneration of motor and sensory tracts. J Neurosurg Pediatr. 2008;1:270-276.
34. Abernathey C.D., Camacho A., Kelly P.J. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195-200.
35. Apuzzo M.L., Chandrasoma P.T., Cohen D., et al. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-937.
36. Beatty R.M., Zervas N.T. Stereotactic aspiration of a brain stem hematoma. Neurosurgery. 1983;13:204-207.
37. Bosch D.A., Beute G.N. Successful stereotaxic evacuation of an acute pontomedullary hematoma. Case report. J Neurosurg. 1985;62:153-156.
38. Coffey R.J., Lunsford L.D. Diagnosis and treatment of brainstem mass lesions by CT-guided stereotactic surgery. Appl Neurophysiol. 1985;48:467-471.
39. Frank F., Fabrizi A.P., Frank-Ricci R., et al. Stereotactic biopsy and treatment of brain stem lesions: combined study of 33 cases (Bologna-Marseille). Acta Neurochir Suppl (Wien). 1988;42:177-181.
40. Giunta F., Grasso G., Marini G., et al. Brain stem expanding lesions: stereotactic diagnosis and therapeutical approach. Acta Neurochir Suppl (Wien). 1989;46:86-89.
41. Hood T.W., Gebarski S.S., McKeever P.E., et al. Stereotaxic biopsy of intrinsic lesions of the brain stem. J Neurosurg. 1986;65:172-176.
42. Kondziolka D., Lunsford L.D. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg Neurol. 1995;43:558-562.
43. Kratimenos G.P., Nouby R.M., Bradford R., et al. Image directed stereotactic surgery for brain stem lesions. Acta Neurochir (Wien). 1992;116:164-170.
44. Lobato R.D., Rivas J.J. Stereotactic suboccipital transcerebellar biopsy of pontine lesions. J Neurosurg. 1989;71:466-467.
45. Massager N., David P., Goldman S., et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93:951-957.
46. Mathisen J.R., Giunta F., Marini G., et al. Transcerebellar biopsy in the posterior fossa: 12 years experience. Surg Neurol. 1987;28:100-104.
47. Neal J.H., Van Norman A.S. Transcerebellar biopsy of posterior fossa lesions using the Leksell gamma model stereotactic frame. Neurosurgery. 1993;32:473-474. discussion 474-475
48. Nicolato A., Gerosa M., Piovan E., et al. Computerized tomography and magnetic resonance guided stereotactic brain biopsy in nonimmunocompromised and AIDS patients. Surg Neurol. 1997;48:267-276. discussion 276-267
49. Ostertag C.B., Mennel H.D., Kiessling M. Stereotactic biopsy of brain tumors. Surg Neurol. 1980;14:275-283.
50. Sawin P.D., Hitchon P.W., Follett K.A., et al. Computed imaging-assisted stereotactic brain biopsy: a risk analysis of 225 consecutive cases. Surg Neurol. 1998;49:640-649.
51. Steck J., Friedman W.A. Stereotactic biopsy of brainstem mass lesions. Surg Neurol. 1995;43:563-567. discussion 567-568
52. Valdes-Gorcia J., Espinoza-Diaz D.M., Paredes-Diaz E. Stereotactic biopsy of brain stem and posterior fossa lesions in children. Acta Neurochir (Wien). 1998;140:899-903.
53. Amundson E.W., McGirt M.J., Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg. 2005;102:565-570.
54. Sanai N., Wachhorst S.P., Gupta N.M., et al. Transcerebellar stereotactic biopsy for lesions of the brainstem and peduncles under local anesthesia. Neurosurgery. 2008;63:460-466. discussion 466-468
55. Roujeau T., Machado G., Garnett M.R., et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107:1-4.
56. Goncalves-Ferreira A.J., Herculano-Carvalho M., Pimentel J. Stereotactic biopsies of focal brainstem lesions. Surg Neurol. 2003;60:311-320. discussion 320
57. Albright A.L. Diffuse brainstem tumors: when is a biopsy necessary? Pediatr Neurosurg. 1996;24:252-255.
58. Albright A.L., Packer R.J., Zimmerman R., et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery. 1993;33:1026-1029. discussion 1029-1030
59. Epstein F. Intrinsic brain stem tumors of childhood. Surgical indications. Prog Exp Tumor Res. 1987;30:160-169.
60. Rajshekhar V., Chandy M.J. Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976-981.
61. Boviatsis E.J., Kouyialis A.T., Stranjalis G., et al. CT-guided stereotactic biopsies of brain stem lesions: Personal experience and literature review. Neurol Sci. 2003;24:97-102.
62. Apuzzo M.L., Sabshin J.K. Computed tomographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery. 1983;12:277-285.
63. Coffey R.J., Lunsford L.D. Stereotactic surgery for mass lesions of the midbrain and pons. Neurosurgery. 1985;17:12-18.
64. Blond S., Lejeune J.P., Dupard T., et al. The stereotactic approach to brain stem lesions: A follow-up of 29 cases. Acta Neurochir Suppl (Wien). 1991;52:75-77.
65. Kondziolka D., Lunsford L.D. Stereotactic biopsy for intrinsic lesions of the medulla through the long-axis of the brainstem: technical considerations. Acta Neurochir (Wien). 1994;129:89-91.
66. Guthrie B.L., Steinberg G.K., Adler J.R. Posterior fossa stereotaxic biopsy using the Brown-Roberts-Wells stereotaxic system. Technical note. J Neurosurg. 1989;70:649-652.
67. Spiegelmann R., Friedman W.A. Stereotactic suboccipital transcerebellar biopsy under local anesthesia using the Cosman-Roberts-Wells frame. Technical note. J Neurosurg. 1991;75:486-488.
68. Jallo G.I., Volkov A., Wong C., et al. A novel brainstem tumor model: functional and histopathological characterization. Childs Nerv Syst. 2006;22:1519-1525.
69. Lee J., Jallo G.I., Guarnieri M., et al. A novel brainstem tumor model: guide screw technology with functional, radiological, and histopathological characterization. Neurosurg Focus. 2005;18:E11.
70. Sandberg D.I., Edgar M.A., Souweidane M.M. Convection-enhanced delivery into the rat brainstem. J Neurosurg. 2002;96:885-891.
71. Lonser R.R., Walbridge S., Garmestani K., et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002;97:905-913.
72. Murad G.J., Walbridge S., Morrison P.F., et al. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg. 2007;106:351-356.
73. Yeh D.D., Warnick R.E., Ernst R.J. Management strategy for adult patients with dorsal midbrain gliomas. Neurosurgery. 2002;50:735-738. discussion 738-740
74. Morota N., Deletis V. The importance of brainstem mapping in brainstem surgical anatomy before the fourth ventricle and implication for intraoperative neurophysiological mapping. Acta Neurochir (Wien). 2006;148:499-509. discussion 509
75. Morota N., Deletis V., Epstein F.J., et al. Brain stem mapping: neurophysiological localization of motor nuclei on the floor of the fourth ventricle. Neurosurgery. 1995;37:922-929. discussion 929-930
76. Strauss C., Romstock J., Nimsky C., et al. Intraoperative identification of motor areas of the rhomboid fossa using direct stimulation. J Neurosurg. 1993;79:393-399.
77. Quinones-Hinojosa A., Lyon R., Du R., et al. Intraoperative motor mapping of the cerebral peduncle during resection of a midbrain cavernous malformation: technical case report. Neurosurgery. 2005;56:E439. discussion E439
78. Fujitsu K., Kuwabara T. Zygomatic approach for lesions in the interpeduncular cistern. J Neurosurg. 1985;62:340-343.
79. Hakuba A., Liu S., Nishimura S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271-276.
80. Zabramski J.M., Kiris T., Sankhla S.K., et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
81. Bricolo A.. Surgical management of intrinsic brain stem gliomas, Spetzler R.F., editor, Operative Techniques in Neurosurgery. Brain Stem Surgery,2000;Vol 3:137-154.
82. Hoffman H.J. Dorsally exophytic brain stem tumors and midbrain tumors. Pediatr Neurosurg. 1996;24:256-262.
83. Pollack I.F., Hoffman H.J., Humphreys R.P., et al. The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993;78:859-863.