Surgical Infectious Disease
Despite improvements in antimicrobial therapy, surgical technique, and postoperative intensive care, infection continues to be a significant source of mortality and morbidity for pediatric patients. Widespread antibiotic use has brought with it the complication of resistant organisms, and the selection of the appropriate antibiotic has become increasingly complex as newer antibiotics are continually developed.1,2 In addition, infections with uncommon organisms are becoming more frequent with diminished host resistance from immunosuppressive states such as immaturity, cancer, systemic diseases, and medications after transplant procedures. Surgical infections generally require some operative intervention, such as drainage of an abscess or removal of necrotic tissue, and seldom respond to antibiotics alone.
Two broad classes of infectious disease processes affect a surgical practice: those infectious conditions brought to the pediatric surgeon for treatment and cure, and those that arise in the postoperative period as a complication of an operation.3
Components of Infection
The virulence of any microorganism depends on its ability to cause damage to the host. Exotoxins, such as streptococcal hyaluronidase, are digestive enzymes released locally that allow the spread of infection by breaking down host extracellular matrix proteins. Endotoxins, such as lipopolysaccharides, are components of gram-negative cell walls that are released only after bacterial cell death. Once systemically absorbed, endotoxins trigger a severe and rapid systemic inflammatory response by releasing various endogenous mediators such as cytokines, bradykinin, and prostaglandins.4 Surgical infections are often polymicrobial, involving various interactions among the microorganisms.
The size of the inoculum is the second component of an infection. The number of colonies of microorganisms per gram of tissue is a key determinant. Predictably, any decrease in host resistance decreases the absolute number of colonies necessary to cause clinical disease. In general, if the bacterial population in a wound exceeds 100,000 organisms per gram of tissue, invasive infection is present.5
For any inoculum, the presence of suitable nutrients for the organism is essential and comprises the third component of any clinical infection. Accumulation of necrotic tissue, hematoma, and foreign matter is an excellent nutrient medium for continued organism growth and spread. Of special importance to the surgeon is the concept of necrotic tissue and infection.6 This tissue often needs to be debrided to restore the host–bacterial balance and lead to effective wound healing.7 Neutrophils, macrophages and cytokines accumulate in necrotic tissue initiating an inflammatory secondary response.8
Defense Against Infection
Anatomic Barriers
Intact skin and mucous membranes provide an effective surface barrier to infection.9 These tissues are not merely a mechanical obstacle. The physiologic aspects of skin and mucous membranes provide additional protection. In the skin, the constant turnover of keratinocytes, temperature of the skin, and acid secretion from sebaceous glands inhibits bacterial cell growth. The mucosal surfaces also have developed advanced defense mechanisms to prevent and combat microbial invasion. Specialized epithelial layers provide resistance to infection. In addition, mechanisms such as the mucociliary transport system in the respiratory tract and normal colonic flora in the gastrointestinal tract prevent invasion of organisms. Anything that affects the normal function of these anatomic barriers increases the host’s susceptibility to infection. A skin injury or a burn provides open access to the soft tissues, and antibiotic use disrupts normal colonic flora.10 Such breakdowns in surface barriers are dealt with by the second line of defenses, the immune system.
Immune Response
The immune system involves complex pathways and many specialized effector responses. The first line of defense is the more primitive and nonspecific innate system, which consists primarily of phagocytic cells and the complement system. The neutrophil is able to rapidly migrate to the source of the infection and engulf and destroy the infecting organisms by phagocytosis. Cytokines, low molecular weight proteins including tumor necrosis factor (TNF), and many interleukins attract and activate neutrophils, and play a significant role in mediating the inflammatory response. In addition, the complement system, when activated, initiates a sequential cascade that also enhances phagocytosis and leads to lysis of pathogens. Neonates, particularly premature infants, have an immature immune system and are helped by the protective agents in human breast milk.11,12 The more specialized, adaptive immune system involves a highly specific response to antigens as well as the eventual production of a variety of humoral mediators.13
Humoral and Cell-Mediated Immunity
Specific, adaptive immunity has two major components. The humoral mechanism (B-cell system) is based on bursa cell lymphocytes and plasma cells. The cellular mechanism (T-cell system) consists of the thymic-dependent lymphocytes.14 The adaptive immune system is an antigen-specific system that is regulated by the lymphocytes. A myriad of receptors on the T-cells that are matched to particular individual antigens create these specific responses. Furthermore, antibody production from B-cells enhances the antigen-specific interaction.
Immunodeficiencies
Susceptibility to infection is increased when one of the components of the host defense mechanism is absent, reduced in numbers, or curtailed in function. Some of these derangements may be congenital, although the majority are acquired as a direct result of medications, radiation, endocrine disease, surgical ablation, tumors, or bacterial toxins. Immunodeficiencies from any cause significantly increase the risk of infection both in hospitalized and postoperative patients. Mycotic infections are an increasing problem in immunocompromised pediatric patients.15
Systemic diseases lead to diminished host resistance. For example, in diabetes mellitus, leukocytes often fail to respond normally to chemotaxis. Therefore, more severe, recurrent, and unusual infections often occur in diabetic patients.16 In addition, malignancy and other conditions that impair hematopoiesis lead to alterations in phagocytosis, resulting in an increased predilection for infection. Human immunodeficiency virus (HIV) infection in children is another major source of immunodeficiency. Vertical transmission from mother to child is the dominant mode of HIV acquisition among infants and children. Finally, poor nutritional status has adverse effects on immune function owing to a wide variety of negative influences on specific defense mechanisms, including decreased production of antibodies and phagocytic function.17
In patients with a primary immune defect, susceptibility to a specific infection is based on whether the defect is humoral, cellular, or a combination. Primary immunodeficiencies are rare but important because prompt recognition can lead to life-saving treatment or significant improvement in the quality of life.18 B-cell deficiencies are associated with sepsis from encapsulated bacteria, especially pneumococcus, Haemophilus influenzae, and meningococcus. Often a fulminating course rapidly ends in death, despite timely therapeutic measures. Although congenital agammaglobulinemia or dysgammaglobulinemia has been widely recognized, other causes of humoral defects include radiation, corticosteroid and antimetabolite therapy, sepsis, splenectomy, and starvation. Chronic granulomatous disease is caused by a deficiency in the respiratory burst action of phagocytes that leads to severe and recurrent bacterial and fungal infections in early childhood. Children with chronic granulomatous disease are prone to develop hepatic abscesses as well as suppurative adenitis of a single node or multiple nodes, both of which may require surgical drainage or excision.19
Antibiotics
The pharmacokinetics and monitoring of drug dosages in infants and children is also important when treating them with antibiotics. The efficacy and safety of many drugs have not been established in the pediatric patient, especially in the newborn.20 Dosages based on pediatric pharmacokinetic data offer the most rational approach. Dosage requirements constantly change as a function of age and body weight. Furthermore, the volume of distribution and half-life of many medicines are often increased in neonates and children compared with adults for a variety of reasons.21,22 Knowledge of a drug’s pharmacokinetic profile allows manipulation of the dose to achieve and maintain a given plasma concentration.
Newborns usually have extremely skewed drug-distribution patterns. The entire body mass can be considered as if it were a single compartment for the purposes of dose calculations. For the majority of drugs, dose adjustments can be based on plasma drug concentration. Administering a loading dose is advisable when rapid onset of drug action is required. For many drugs, loading doses (milligrams per kilogram) are generally greater in neonates and young infants than in older children or adults.22 However, prolonged elimination of drugs in the neonate requires lower maintenance doses, given at longer intervals, to prevent toxicity. Monitoring serum drug concentrations is useful if the desired effect is not attained or if adverse reactions occur.
The neonate undergoing extracorporeal membrane oxygenation (ECMO) presents a special challenge to drug delivery and elimination. Because the ECMO circuit may bind or inactivate drugs and make them unavailable to the patient, dosing requires careful attention to drug response and serum levels. The pharmacokinetics under these conditions generally include a larger volume of distribution and prolonged elimination, with a return to baseline after decannulation.23
Prevention of Infections
The most effective way to deal with surgical infectious complications is to prevent their occurrence. The clinician must recognize the variables that increase the risk of infection and attempt to decrease or eliminate them. A summary of the category 1 recommendations published by the Hospital Infection Control Practices Advisory Committee (HICPAC) of the Centers for Disease Control and Prevention is listed in Box 9-1.
Patient Characteristics
In adults, comorbidities often increase the risk of a surgical site infection (SSI). However, these chronic diseases are infrequently encountered in children. A prospective multicenter study of wound infections in the pediatric population found that postoperative wound infections were more likely related to factors at the operation rather than to patient characteristics.24 In this study of more than 800 children, the only factors associated with increased SSI were contamination at the time of operation and the duration of the procedure. Other investigators have similarly found that local factors at the time of operation, such as degree of contamination, tissue perfusion, and operative technique, play a more important role in initiation of an SSI than the general condition of the patient.25
Surgical Preparation
Preoperative preparation of the operative site and the sterility of the surgical team are very important in reducing the risk of postoperative infection. Hand scrubbing remains the most important proactive mechanism to reduce infection by reducing the number of microorganisms present on the skin during the operation. In the USA, the conventional method for scrubbing consists of a five-minute first scrub followed by subsequent two- or three-minute scrubs for subsequent cases with either 5% povidone-iodine or 4% chlorhexidine gluconate. These scrubbing protocols can achieve a 95% decrease in skin flora.26,27 Newer alcohol-based antiseptic cleaners with shorter applications, usually 30 seconds, have been shown to be as effective as or even more effective than hand washing in decreasing bacterial contamination.28–30 In addition, these solutions increase compliance and are less drying to the surgeon’s skin.
Normothermia has also been suggested as a means to decrease the incidence of wound infections. Infants and children are at particular risk for experiencing hypothermia during surgery due to an increased area-to-body weight ratio leading to greater heat loss.31 Intraoperative hypothermia can potentially lead to serious complications, including coagulopathy, SSIs, and cardiac complications. A prospective randomized trial of 200 adult patients undergoing colorectal surgery showed that intraoperative hypothermia caused delayed wound healing and a greater incidence of infections.32 A number of techniques are available to warm infants and children during surgery, including warming intravenous fluids or using forced-air warming systems. In addition, supplemental oxygen given during the perioperative period in adults has been shown to decrease the rate of wound infection by as much as 40–50%.33,34 Finally, adequate control of glucose levels perioperatively has also been demonstrated to decrease morbidity and mortality in both adult and pediatric surgical patients, particularly in those patients undergoing cardiac surgery.35,36
Antibiotic Prophylaxis
Operative procedures can be classified into one of four types, as outlined in Table 9-1. In adults, several well-designed prospective trials have documented a decreased incidence of infection for all types of operative procedures with established antibiotic recommendations.37 Important points for preoperative antibiotic prophylaxis include using agents that cover the most probable intraoperative contaminants for the operation, optimal timing for the initial dose of antibiotic so that bactericidal concentrations are reached at the time of incision, and maintaining the contribution levels throughout the operation.38 Timing of the perioperative antibiotic coverage is crucial. The first dose is generally given 30 minutes to one hour before the start of the operation. In operations that take more than the half-life of the administered drug, a second dose of prophylactic antibiotics is indicated to re-achieve adequate serum levels.39
TABLE 9-1
| Class | Definition |
| Clean | An uninfected operative wound in which no inflammation is encountered and the respiratory, alimentary, genital, or infected urinary tract is not entered. In addition, clean wounds are closed primarily and, if necessary, drained with closed drainage |
| Clean–contaminated | An operative wound in which the respiratory, alimentary, genital, or urinary tract is entered under controlled conditions and without unusual contamination |
| Contaminated | Open, fresh, accidental wounds. This includes operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract and incisions in which acute, nonpurulent inflammation is encountered |
| Dirty | Old traumatic wounds with retained devitalized tissue and those that involve existing clinical infection or perforated viscera |
Prophylaxis accounts for nearly 75% of antibiotic use on pediatric surgical services. As such, prophylaxis is the major cause of the inappropriate use of antimicrobials in children. In one study of children younger than 6 years of age undergoing surgical procedures, prophylactic antibiotics were administered inappropriately to 42% of children receiving preoperative antibiotics.40 A more recent study demonstrated that 82% of patients received prophylactic antibiotics when indicated and that 40% of patients received antibiotics when there was no indication.41 In pediatric surgery, it is clear that antibiotic coverage is required during clean-contaminated, contaminated, or dirty cases. Antibiotic prophylaxis in a clean case in the pediatric population is now at the discretion of the operating surgeon.
Bowel Preparation
The efficacy of bowel preparation before an elective colon operation is well documented.42,43 Bowel preparation includes mechanical irrigation and flushing of the colon to remove stool, oral antibiotics against colonic aerobes and anaerobes, and preoperative intravenous antibiotics that cover both common skin and colonic flora.44 The preparation can be started on an outpatient basis the day before the operation, and the parenteral drugs are added to the regimen just before the procedure. Recently, there has been debate in the adult literature regarding the necessity of mechanical bowel preparation. In infants and children, protocols for bowel preparation have largely been extrapolated from the adult colorectal literature. It appears that the majority of pediatric surgeons use bowel preparations for elective colorectal surgery.45 Recently, others have proposed that omitting mechanical bowel preparation carries no increased risk of infectious or anastomotic complications.46 If bowel preparation is used in the pediatric population, care must be taken to avoid dehydration.
Types of Infection
Postoperative Surgical Site Infection
Despite meticulous technique and perioperative antibiotics, infectious complications still occur. Postoperative wound infections can be divided into superficial or deep.47 Early diagnosis and prompt intervention help to avoid morbidity and occasional mortality. Erythema, fever, leukocytosis, tenderness, crepitus, and suppuration are diagnostic signs but are not always present. When confronted with one or more of these signs, clinical judgment is important. Treatment may include oral or intravenous antibiotics, simple incision and drainage, or extensive surgical debridement.
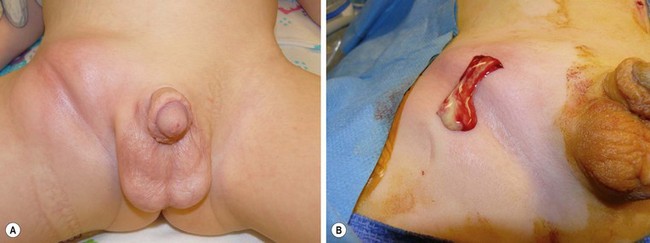
An abscess is a localized collection of pus in a cavity formed by an expanding infectious process (Fig. 9-1A) Pus is a combination of leukocytes, necrotic material, bacteria, and extracellular fluid. The usual cause is the staphylococcal species in combination with one or more organisms. The treatment is incision and drainage (Fig. 9-1B), followed by antibiotic therapy if associated with localized cellulitis or an immunocompromised patient. Drainage must be complete, or the abscess will reform. A phlegmon is an area of diffuse inflammation with little pus and some necrotic tissue. A phlegmon can often be treated with antibiotics, although it can progress to an abscess.
Nosocomial Infection
Nosocomial infections are defined as those infections that are hospital acquired.48 As such, they are a potential threat to all hospitalized patients and increase morbidity and mortality significantly. Their incidence appears to be increasing as surgical care becomes more advanced and patients survive longer. Recent focus on patient safety has made prevention of nosocomial infections increasingly important. One report describing 676 operative procedures in 608 pediatric patients showed a nosocomial infection rate of 6.2%.48 The infectious complications included septicemia, pulmonary, urinary tract, abdominal, and diarrhea. The highest overall occurrence of infection was in the infant group. The most common isolates were Staphylococcus epidermidis from septic patients and gram-negative enteric bacteria from organ and wound infections. Infection was associated with impaired nutrition, multiple disease processes, and multiple operations. In addition, ECMO use has been shown to correlate with an increased incidence of nosocomial infection as does length of the preoperative stay and exposure to invasive medical devices.49–51
Pneumonia can be a lethal nosocomial infection, with mortality ranging from 20–70% and accounting for 10–15% of all pediatric hospital-acquired infections.52 The mortality rate is dependent on the causative organism. The risk factors for nosocomial pneumonia in the pediatric population include serious underlying illness, immunosuppression, and length of time on a ventilator.53 Measures to prevent ventilator-associated pneumonia in children include elevating the head of the bed, daily assessment of readiness for extubation, and age-appropriate mouth care.54
Clostridium difficile is a well-recognized cause of infectious diarrhea that develops after antibiotic therapy in many patients, although it likely only accounts for 20% of antibiotic-associated diarrhea. It is a very common cause of nosocomial infection, and its incidence is increasing in frequency with associated increasing mortality.55,56 The best method of prevention is the judicious and appropriate use of antibiotics.
Catheter Infections
Central venous catheters (CVCs) are essential for managing critically ill patients. The use of CVCs in infants and children has increased as prolonged vascular access has become increasingly necessary to provide parenteral nutrition, chemotherapy, antimicrobial therapy, and hemodynamic monitoring. However, catheter-related infections are common, despite considerable effort to reduce their occurrence, and are associated with increased hospital costs and length of stay. Infection is manifested as erythema at the site of insertion, tachycardia, and/or leukocytosis. Rates of infection are influenced by patient-related factors, by type and severity of illness, and by catheter-related parameters (catheter type, purpose, and conditions under which it was placed).57 Coagulase-negative staphylococci, followed by enterococci, were the most frequently isolated causes of hospital-acquired bloodstream infections in a report from the National Nosocomial Infections Surveillance System.58 A number of factors are associated with the development of catheter-related infections, including the sterility of the insertion technique, type of solution being administered through the line, care of the catheter once inserted, proximity of the catheter to another wound, and the presence of another infection elsewhere. Updated guidelines for the prevention of intravascular catheter-related infections were published in 2011.59 For catheters that will remain for a long time, tunneling the catheter has been shown to significantly reduce the risk of catheter-related infection.60,61
Absolute sterile techniques should be maintained in all instances of line insertion whenever possible. Emergency situations may necessitate less-than-sterile technique. The use of maximal sterile barriers, including sterile gown and gloves and a large sterile sheet, has been shown in adults to greatly reduce the risk of catheter-related infection.62 Studies suggest that chlorhexidine significantly reduces the incidence of microbial colonization compared with povidone-iodine, and 0.5% chlorhexidine preparations with alcohol is now recommend for skin antisepsis.63 The safety and efficacy of chlorhexidine is unknown in infants < 2 months.
The skin and catheter hub are the most common sources of colonization and infection. Thus, various methods have been used to combat these risks. Silver ions have broad antimicrobial activity, and the use of silver-impregnated cuffs have been tried as a preventive measure.64,65 In addition, antimicrobial and antiseptic catheters and cuffs may decrease the incidence of catheter-related infections.66,67 Catheters have been coated with chlorhexidine/silver sulfadiazine as well as minocycline/rifampin along with other agents. The use of these coated catheters has been approved by the ultrasound Food and Drug Administration for use in patients weighing more than 3 kg. It is likely that the efficacy for reducing infection decreases after being in place for longer than three weeks because of a decrease in the antimicrobial activity.67 These impregnated catheters and sponge dressings can be used if the infection rate is not decreasing with other measures. Of note, no studies in adults have demonstrated a benefit for systemic antibiotic prophylaxis after insertion of a CVC. Studies in high-risk neonates and children have demonstrated conflicting results. However, concern exists for the emergence of resistance with the routine use of antimicrobial prophylaxis.68,69
Other Infections Requiring Surgical Care and Treatment
Necrotizing Soft Tissue Infection
Necrotizing fasciitis is a rapidly progressing infection of the fascial tissues and overlying skin. Although it can occur as a postoperative complication or as a primary infection, necrotizing fasciitis is more likely in immunocompromised patients.70 However, in the pediatric population, necrotizing fasciitis often affects previously healthy children and infants.71 Because the diagnosis is often not obvious, the clinician must look for clinical clues such as edema beyond the area of erythema, crepitus, skin vesicles, or cellulitis refractory to intravenous antibiotics. Skin necrosis is generally a late sign and is indicative of thrombosis of vessels in the subcutaneous tissue. Necrotizing fasciitis often occurs in the truncal region in children as opposed to adults where infection in the extremities is most common (Figs 9-2 and 9-3).72 Although infections with a single organism often occur in adults with necrotizing fasciitis, polymicrobial infections predominate in children.73 Prompt surgical intervention, including wide excision of all necrotic and infected tissue, along with the institution of antibiotics including penicillin, is mandatory to avoid progression and mortality. Necrotizing fasciitis can also occur as a complication of chickenpox.74 In neonates, necrotizing fasciitis can occur as a secondary infection of omphalitis, balanitis, and fetal monitoring.75
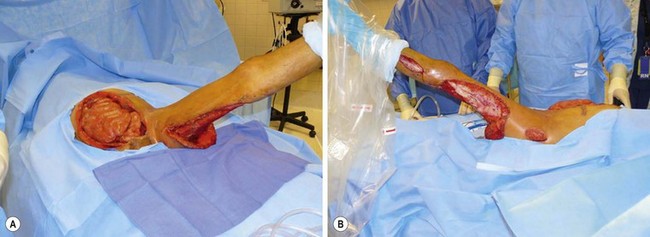
FIGURE 9-2 This 15-year-old was ill for two weeks with perforated appendicitis and presented in shock. After a midline incision for exploration for a rigid abdomen, his appendix was removed. The peritoneal cavity was extensively and copiously irrigated and the abdominal incision was left open. He returned to the operating room two days later for evaluation and was found to have necrotizing fasciitis of the rectus abdominis muscles bilaterally. Eventually, despite aggressive surgical debridement, this process spread to the retroperitoneum and down the left inguinal canal through a patent processus vaginalis. One week postoperatively, he was found to have edema and erythema of the left leg that prompted exploration. The necrotizing fasciitis had progressed down all compartments of the left thigh and the lateral compartment of the left lower leg. In the upper thigh, the semimembranosus and semitendinosus muscles had to be excised due to necrotic musculature. These photographs were taken on his ninth postoperative day. (A) The abdomen is seen to be open and the medial aspect of the left thigh is visualized. (B) The incisions in the left buttock area, the left lateral thigh, and the left lateral lower leg are seen.
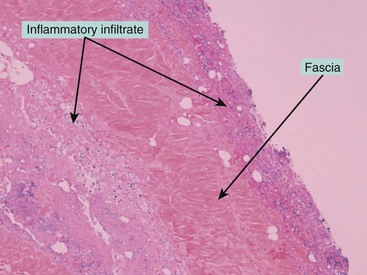
FIGURE 9-3 This photomicrograph depicts the histologic findings of necrotizing fasciitis in the patient shown in Figure 9-2. Note the inflammatory infiltrate on both sides of the fascia. The fascial cultures grew Escherichia coli.
Sepsis
Sepsis, by contemporary definition, defines the systemic derangements that are caused by the infectious organisms and their byproducts as opposed to those derangements that are caused by the host-systemic inflammatory response. In 1992, the Society of Critical Care Medicine published the results of a consensus conference to define accurately the terms regarding sepsis and the inflammatory response to injury and infection.76 These definitions were updated in the 2001 Consensus Conference.77 Although there has been a significant decrease in the mortality rate among children with sepsis, severe sepsis remains one of the leading causes of death in the pediatric population. In 2002, a group of experts gathered to focus specifically on pediatric sepsis.78 Systemic inflammatory response syndrome (SIRS) has previously been defined in adults as the nonspecific inflammatory process after a variety of insults with sepsis specifically occurring from infection.79 The main pediatric modifications include the inclusion of temperature or leukocyte abnormalities in addition to tachycardia and tachypnea because these last two indices are common in many pediatric disease processes. Another difference is that hypotension is not necessary for the diagnosis of septic shock, but cardiovascular dysfunction must be present. SIRS may progress to multi-organ dysfunction and death. Gram-negative organisms possess a lipopolysaccharide moiety on the cell wall that has been shown to incite most, if not all, of the toxic effects of end-organ failure.
Neonatal sepsis is defined as a generalized bacterial infection accompanied by a positive blood culture within the first month of life.80 Neonatal sepsis occurring during the first week of life is caused primarily by maternal organisms transferred during delivery. Maternal contamination can be transmitted through the placenta to the newborn via the birth canal or by direct contamination of the amniotic fluid. The mortality of this early onset sepsis approaches 50%. Late-onset neonatal sepsis is primarily nosocomial and is most often secondary to indwelling catheters or bacterial translocation from the gut. In the surgical neonate, three factors promote bacterial translocation and sepsis: (1) intestinal bacterial colonization and overgrowth; (2) compromised host defenses; and (3) disruption of the mucosal epithelial barrier.81 The mortality of late-onset sepsis approaches 20%. The clinician must be alert for the subtle signs and symptoms of neonatal sepsis, which include lethargy, irritability, temperature instability, and a change in respiratory or feeding pattern. Neonates may not demonstrate leukocytosis. Empirical triple antibiotic coverage may be started, pending the results of blood and other cultures.
Peritonitis
Peritonitis is defined as inflammation of the peritoneum.82 It is divided into primary, secondary, and tertiary. Spontaneous primary peritonitis is a bacterial infection without enteric perforation. Primary peritonitis is usually caused by a single organism. An infant with primary peritonitis usually does not exhibit signs of peritonitis but may have poor feeding, lethargy, distention, vomiting, and mild to severe abdominal tenderness. Definitive treatment may require only a course of broad-spectrum antibiotics. Secondary peritonitis is associated with gastrointestinal tract disruption. This can be caused directly by intestinal perforation, bowel wall necrosis, trauma, or postoperatively as a result of iatrogenic injury or an anastomotic leak. In addition, secondary peritonitis also may result from an indwelling dialysis catheter or ventriculoperitoneal shunt.83 These infections are generally polymicrobial. Treatment of secondary peritonitis is a combination of operative intervention, removal of any prosthetic device, and antibiotics. Tertiary peritonitis, also called recurrent peritonitis, is characterized by organ dysfunction and systemic inflammation in association with recurrent infection. The mortality rate is very high, and management is difficult.84 Treatment consists of broad-spectrum antibiotics because the infection often includes nosocomial organisms and multidrug-resistant bacteria.
References
1. Brueggemann, AB. Antibiotic resistance mechanisms among pediatric respiratory and enteric pathogens: A current update. Pediatr Infect Dis J. 2006; 25:969–973.
2. Liu, HH. Antibiotics and infectious diseases. Prim Care. 1990; 17:745–774.
3. Kosloske, AM. Surgical infections in children. Curr Opin Pediatr. 1994; 6:353–359.
4. DeLa Cadena, RA, Majluf-Cruz, A, Stadnicki, A, et al. Activation of the contact and fibrinolytic systems after intravenous administration of endotoxin to normal human volunteers: Correlation with the cytokine profile. Immunopharmacol. 1996; 33:231–237.
5. Robson, MC, Stenberg, BD, Heggers, JP. Wound healing alterations caused by infection. Clinics in plastic surgery. 1990; 17:485–492.
6. Baxter, CR. Immunologic reactions in chronic wounds. Am J Surg. 1994; 167:12S–14S.
7. Bowler, PG. Wound pathophysiology, infection and therapeutic options. Ann Med. 2002; 34:419–427.
8. Harris, BH, Gelfand, JA. The immune response to trauma. Semin Pediatr Surg. 1995; 4:77–82.
9. Forslind, B, Lindberg, M, Roomans, GM, et al. Aspects on the physiology of human skin: Studies using particle probe analysis. Microscopy research and technique. 1997; 38:373–386.
10. Godet, AS, Williams, RD. Postoperative clostridium difficile gastroenteritis. J Urol. 1993; 149:142–144.
11. Newburg, DS, Walker, WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007; 61:2–8.
12. Newburg, DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009; 87:26–34.
13. Fleisher, TA. Back to basics: Primary immune deficiencies: Windows into the immune system. Pediatr Rev. 2006; 27:363–372.
14. Fleisher, TA, Bleesing, JJ. Immune function. Pediatr Clin North Am. 2000; 47:1197–2109.
15. Hilfiker, ML, Azizkhan, RG. Mycotic infections in pediatric surgical patients. Semin Pediatr Surg. 1995; 4:239–244.
16. Menne, EN, Sonabend, RY, Mason, EO, et al. Staphylococcus aureus infections in pediatric patients with diabetes mellitus. J Infect. 2012; 65:135–141.
17. Scrimshaw, NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003; 133:316S–321S.
18. Puck, JM. Primary immunodeficiency diseases. JAMA. 1997; 278:1835–1841.
19. Berescher, E. Infectious complications of dysfunction or deficiency of polymorphonuclear and mononuclear phagocytes. In Long S, Pickering LK, Prober CG, eds.: Principles and Practice of Pediatric Infectious Diseases, 2nd ed, New York: Churchill Livingstone, 2003.
20. Musoke, RN. Rational use of antibiotics in neonatal infections. East Afr Med J. 1997; 74:147–150.
21. Hall, P, Kaye, CM, McIntosh, N, et al. Intravenous metronidazole in the newborn. Arch Dis Child. 1983; 58:529–531.
22. Routledge, PA. Pharmacokinetics in children. J Antimicrob Chemother. 1994; 34(Suppl. A):19–24.
23. Buck, ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: Implications for drug therapy of neonates. Clin Pharmacokinet. 2003; 42:403–417.
24. Horwitz, JR, Chwals, WJ, Doski, JJ, et al. Pediatric wound infections: A prospective multicenter study. Ann Surg. 1998; 227:553–558.
25. Bhattacharyya, N, Kosloske, AM. Postoperative wound infection in pediatric surgical patients: A study of 676 infants and children. J Pediatr Surg. 1990; 25:125–129.
26. Pereira, LJ, Lee, GM, Wade, KJ. The effect of surgical handwashing routines on the microbial counts of operating room nurses. Am J Infect Control. 1990; 18:354–364.
27. Wheelock, SM, Lookinland, S. Effect of surgical hand scrub time on subsequent bacterial growth. AORN J. 1997; 65:1087–1094.
28. Tanner, J, Swarbrook, S, Stuart, J. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2008.
29. Parienti, JJ, Thibon, P, Heller, R, et al. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates: A randomized equivalence study. JAMA. 2002; 288:722–727.
30. Girou, E, Loyeau, S, Legrand, P, et al. Efficacy of handrubbing with alcohol based solution versus standard handwashing with antiseptic soap: Randomised clinical trial. BMJ. 2002; 325:360–362.
31. Serour, F, Weissenberg, M, Boaz, M, et al. Intravenous fluids warming by mattress is simple and efficient during pediatric surgery. Acta Anaesthesiol Scand. 2002; 46:80–84.
32. Kurz, A, Sessler, DI, Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. 1996; 334:1209–1215.
33. Greif, R, Akça, O, Horn, EP, et al. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000; 342:161–167.
34. Belda, FJ, Aguilera, L, García de la Asunción, J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: A randomized controlled trial. JAMA. 2005; 294:2035–2042.
35. Krinsley, J. Perioperative glucose control. Curr Opin Anaesthesiol. 2006; 19:111–116.
36. Yates, AR, Dyke, PC, Taeed, R, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006; 7:351–355.
37. Nichols, RL. Surgical antibiotic prophylaxis. Med Clin North Am. 1995; 79:509–522.
38. Mangram, AJ, Horan, TC, Pearson, ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999; 27:97–132.
39. Nichols, RL. Preventing surgical site infections. Clin Med Res. 2004; 2:115–118.
40. Kesler, RW, Guhlow, LJ, Saulsbury, FT. Prophylactic antibiotics in pediatric surgery. Pediatrics. 1982; 69:1–3.
41. Rangel, SJ, Fung, M, Graham, DA, et al. Recent trends in the use of antibiotic prophylaxis in pediatric surgery. J Pediatr Surg. 2011; 46:366–371.
42. Bartlett, JG, Condon, RE, Gorbach, SL, et al. Veterans Administration cooperative study on bowel preparation for elective colorectal operations: Impact of oral antibiotic regimen on colonic flora, wound irrigation cultures and bacteriology of septic complications. Ann Surg. 1978; 188:249–254.
43. Debo Adeyemi, S, Tai da Rocha-Afodu, J. Clinical studies of 4 methods of bowel preparation in colorectal surgery. Eur Surg Res. 1986; 18:331–336.
44. Le, TH, Timmcke, AE, Gathright, JB, et al. Outpatient bowel preparation for elective colon resection. South Med J. 1997; 90:526–530.
45. Breckler, FD, Fuchs, JR, Rescorla, FJ. Survey of pediatric surgeons on current practices of bowel preparation for elective colorectal surgery in children. Am J Surg. 2007; 193:315–318.
46. Leys, CM, Austin, MT, Pietsch, JB, et al. Elective intestinal operations in infants and children without mechanical bowel preparation: A pilot study. J Pediatr Surg. 2005; 40:978–982.
47. Upperman, JS, Sheridan, RL, Marshall, J. Pediatric surgical site and soft tissue infections. Pediatr Crit Care Med. 2005; 6:S36–S41.
48. Allen, U, Ford-Jones, EL. Nosocomial infections in the pediatric patient: An update. Am J Infect Control. 1990; 18:176–193.
49. Coffin, SE, Bell, LM, Manning, M, et al. Nosocomial infections in neonates receiving extracorporeal membrane oxygenation. Infect Control Hosp Epidemiol. 1997; 18:93–96.
50. Bizzarro, MJ, Conrad, SA, Kaufman, DA, et al. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011; 12:277–281.
51. Yogaraj, JS, Elward, AM, Fraser, VJ. Rate, risk factors, and outcomes of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2002; 110:481–485.
52. Stein, F, Trevino, R. Nosocomial infections in the pediatric intensive care unit. Pediatr Clin North Am. 1994; 41:1245–1257.
53. Jarvis, WR. The epidemiology of colonization. Infect Control Hosp Epidemiol. 1996; 17:47–52.
54. Sandora, TJ. Prevention of healthcare-associated infections in children: New strategies and success stories. Curr Opin Infect Dis. 2010; 23:300–305.
55. Benson, L, Song, X, Campos, J, et al. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007; 28:1233–1235.
56. Nylund, CM, Goudie, A, Garza, JM, et al. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adoles Med. 2011; 165:451–457.
57. O’Grady, NP, Alexander, M, Dellinger, EP, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention. Pediatrics. 2002; 110:e51.
58. System ArftN. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004; 32:470–485.
59. O’Grady, NP, Alexander, M, Burns, LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011; 52:1087–1099.
60. Randolph, AG, Cook, DJ, Gonzales, CA, et al. Tunneling short-term central venous catheters to prevent catheter-related infection: A meta-analysis of randomized, controlled trials. Crit Care Med. 1998; 26:1452–1457.
61. Timsit, JF, Sebille, V, Farkas, JC, et al. Effect of subcutaneous tunneling on internal jugular catheter-related sepsis in critically ill patients: A prospective randomized multicenter study. JAMA. 1996; 276:1416–1420.
62. Raad, II, Hohn, DC, Gilbreath, BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994; 15:231–238.
63. Maki, DG, Ringer, M, Alvarado, CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. The Lancet. 1991; 338:339–343.
64. Dahlberg, PJ, Agger, WA, Singer, JR, et al. Subclavian hemodialysis catheter infections: A prospective, randomized trial of an attachable silver-impregnated cuff for prevention of catheter-related infections. Infect Control Hosp Epidemiol. 1995; 16:506–511.
65. Groeger, JS, Lucas, AB, Coit, D, et al. A prospective, randomized evaluation of the effect of silver impregnated subcutaneous cuffs for preventing tunneled chronic venous access catheter infections in cancer patients. Ann Surg. 1993; 218:206–210.
66. Raad, I, Darouiche, R, Dupuis, J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997; 127:267–274.
67. Veenstra, DL, Saint, S, Saha, S, et al. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: A meta-analysis. JAMA. 1999; 281:261–267.
68. Kacica, MA, Horgan, MJ, Ochoa, L, et al. Prevention of gram-positive sepsis in neonates weighing less than 1500 grams. J Pediatr. 1994; 125:253–258.
69. Spafford, PS, Sinkin, RA, Cox, C, et al. Prevention of central venous catheter-related coagulase-negative staphylococcal sepsis in neonates. J Pediatr. 1994; 125:259–263.
70. Farrell, LD, Karl, SR, Davis, PK, et al. Postoperative necrotizing fasciitis in children. Pediatrics. 1988; 82:874–879.
71. Bingol-Kologlu, M, Yildiz, RV, Alper, B, et al. Necrotizing fasciitis in children: Diagnostic and therapeutic aspects. J Pediatr Surg. 2007; 42:1892–1897.
72. Murphy, JJ, Granger, R, Blair, GK, et al. Necrotizing fasciitis in childhood. J Pediatr Surg. 1995; 30:1131–1134.
73. Moss, RL, Musemeche, CA, Kosloske, AM. Necrotizing fasciitis in children: Prompt recognition and aggressive therapy improve survival. J Pediatr Surg. 1996; 31:1142–1146.
74. Waldhausen, JH, Holterman, MJ, Sawin, RS. Surgical implications of necrotizing fasciitis in children with chickenpox. J Pediatr Surg. 1996; 31:1138–1141.
75. Hsieh, W-S, Yang, P-H, Chao, H-C, et al. Neonatal necrotizing fasciitis: A report of three cases and review of the literature. Pediatrics. 1999; 103:e53.
76. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992; 20:864–874.
77. Levy, MM, Fink, MP, Marshall, JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003; 29:530–538.
78. Goldstein, B, Giroir, B, Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6:2–8.
79. Bone, RC, Sprung, CL, Sibbald, WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992; 20:724–726.
80. Wolach, B. Neonatal sepsis: Pathogenesis and supportive therapy. Semin Perinatol. 1997; 21:28–38.
81. Jackson, RJ, Smith, SD, Wadowsky, RM, et al. The effect of E coli virulence on bacterial translocation and systemic sepsis in the neonatal rabbit model. J Pediatr Surg. 1991; 26:483–485.
82. Heemken, R, Gandawidjaja, L, Hau, T. Peritonitis: Pathophysiology and local defense mechanisms. Hepato-gastroenterology. 1997; 44:927–936.
83. Levy, M, Balfe, JW, Geary, D, et al. Exit-site infection during continuous and cycling peritoneal dialysis in children. Perit Dial Int. 1990; 10:31–35.
84. Nathens, AB, Rotstein, OD, Marshall, JC. Tertiary peritonitis: Clinical features of a complex nosocomial infection. World J Surg. 1998; 22:158–163.