CHAPTER 61 Surgical Decompression for Foraminal Stenosis
ETIOLOGY OF FORAMINAL STENOSIS
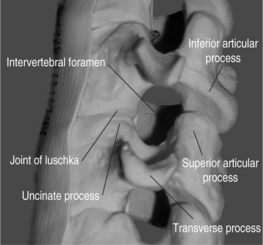
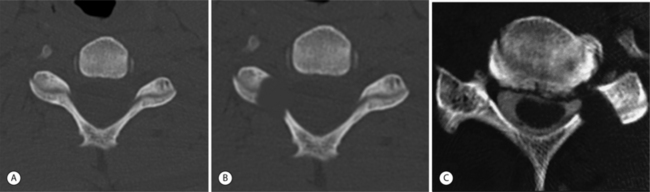
The cervical functional spinal unit consists of two vertebral bodies joined by an intervertebral disc anteriorly and two synovial facet joints posteriorly. The cranial surface of the vertebral body is typically concave from side-to-side and convex in the anteroposterior direction. The inferior surface is convex from side-to-side and concave in the anteroposterior direction. A projection on the lateral aspect of the superior surface of the inferior vertebral body is called the uncus. The uncus is intimately related to the convex lateral inferior surface of the cephalad vertebrae. This projection off the cephalad vertebral body is referred to as the enchanure or anvil. The ‘articulation’ between these two structures is referred to as the ‘uncovertebral joint’ or ‘joint of Luschka.’ Cervical nerve roots exit the spinal canal through the neural foramen in an anterolateral and inferior direction. The boundaries of the neural foramen are formed superiorly and inferiorly by the adjacent pedicles. The medial aspect of the facet joint and an adjacent portion of the articular column form the posterolateral border of the neural foramen. The anterolateral border is formed by the posterolateral portion of the uncus, the intervertebral disc, and the inferior portion of the superior adjacent vertebrae. Figure 61.1 shows a cervical spine model, viewed in an oblique projection, which illustrates the bony confines of the neural foramen.
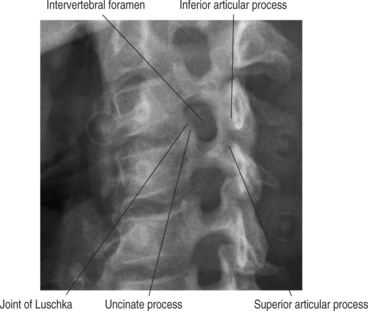
Neural foraminal narrowing can occur as a result of both static and dynamic processes. Abnormal biomechanics that result from biochemical changes in the intervertebral disc may lead to microinstability that contributes to dynamic neural foraminal narrowing. Changes in the bony architecture of the borders of the foramen lead to static narrowing of the neural foramen. Posteriorly protruding disc material, uncovertebral osteophytes, or thickened soft tissues within the foramen may result in extrinsic compression of the nerve root. An oblique radiograph of the cervical spine illustrates the anatomic borders of the foramen and how uncovertebral joint osteophytes can result in narrowing of the foramen. Both Figure 61.1 and Figure 61.2 demonstrate how osteophyte formation from the facet joint could result in narrowing in the anterior–posterior dimension. The height of the foramen may also be compromised as the intervertebral disc loses its biomechanical integrity and loses height. As mentioned earlier, the vertebral bodies move closer together, causing the adjacent pedicles to move closer, thereby narrowing the cephalad–caudad dimension of the neural foramen. Surgical treatment of neural foraminal stenosis must address the anatomic structures that are responsible for narrowing of the foramen and may include an arthrodesis to eliminate any dynamic foraminal narrowing.
CLINICAL PRESENTATION AND PATIENT EVALUATION
Foraminal stenosis typically results in cervical radiculopathy which refers to symptoms that occur in a specific dermatomal pattern in the upper extremity. Patients typically describe sharp pain and tingling or burning sensations in the involved area. Diminished sensation and motor loss corresponding to the involved nerve root may be present, as may diminished deep tendon reflexes. Patients typically have neck and arm pain that prevents them from finding a comfortable position. They may find comfort by placing their forearm on top of their head, the so-called ‘shoulder abduction sign.’1 Extension or lateral rotation of the head to the side of the pain (Spurling’s maneuver) may aggravate the symptoms. This finding may assist in the differentiation of radicular pain from other sources of shoulder pain and muscular neck pain. It is also paramount to remember that multiple sources of neck and upper extremity pain are extremely common and that neural compression may very well be occurring at two distinct locations.2,3 Adaptations to the initial radiculopathy symptoms may result in secondary pathologic changes in the shoulder, carpal tunnel, and ulnar nerve that may cloud the clinical picture and persist long after the cervical radiculopathy has resolved. In a series of 736 patients with cervical radiculopathy treated operatively, Henderson et al. reported the clinical signs and symptoms at presentation. They found that 99.4% had arm pain, 85.2% had sensory deficits, 79.7% had neck pain, 71.2% had diminished deep tendon reflexes, 68% had motor weakness, 52.5% had periscapular pain, 17.8% had anterior chest pain, and 9.7% had headaches. The neurologic deficits at presentation correlated with the offending disc level in approximately 80% of the patients.4
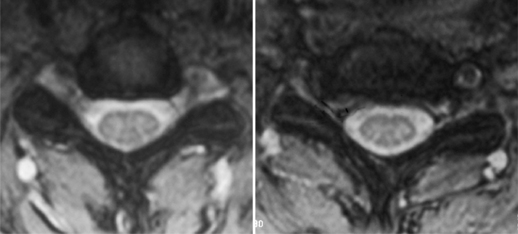
Additional studies that should be considered include bone scan, myelography combined with computed tomography (CT), electrodiagnostic testing, and magnetic resonance imaging (MRI). Consideration for bone scans should be given in patients with suspected malignancy, infection, or occult trauma realizing that bone scan may be normal in the setting of multiple myeloma. Myelography combined with computed tomography was the study of choice for the evaluation of suspected cervical stenosis until the widespread availability of MRI. Myelo/CT studies are still useful in those patients with contraindications to MRI and in potential revision situations. MRI has become the gold standard imaging modality for evaluation of patients with cervical radiculopathy and myelopathy. The advantages of MRI are that it provides complete visualization of the vertebrae, intervertebral discs, nerve roots, subarachnoid space, and spinal cord, including any intraspinal cord pathology. Multiplanar images are also routinely generated by this noninvasive, readily available imaging modality. It provides exquisite sensitivity in detecting subtle anatomic abnormalities. Axial images at the level of the neural foramen provide excellent contrast between the cerebrospinal fluid (CSF)-filled nerve root sleeve and the surrounding bony and soft tissue structures. Foraminal narrowing from disc herniation, uncovertebral osteophyte, facet hypertrophy, and in-folded ligamentum flavum can be visualized on these axial T2 images. An example of foraminal stenosis caused by uncovertebral osteophytes can be seen in Figure 61.3.
SURGICAL DECOMPRESSION OF FORAMINAL STENOSIS
Patients presenting with symptoms of cervical radiculopathy and imaging studies that coincide with the physical exam findings should be considered for active treatment. Medical rehabilitation and interventional spine treatment modalities including nonsteroidal antiinflammatories, mild narcotic analgesic medications, physical therapy, cervical epidural steroid injections, and selective nerve root blocks should be utilized initially for most patients. Absolute indications for surgical decompression of isolated foraminal stenosis are lacking although patients presenting radicular symptoms in combination with progressive myelopathy should be treated surgically. Strong indications for surgical intervention include profound muscle weakness and atrophy. Surgical decompression should also be considered if a patient has undergone a prolonged period of conservative management without resolution of their symptoms. A trial of 6–12 weeks of nonoperative modalities is considered adequate by most surgeons. The severity of the patient’s pain and the degree of muscle weakness play a role in the decision to abort conservative treatment and proceed with surgical intervention.
ANTERIOR CERVICAL DISCECTOMY AND FUSION
The anterior approach to the cervical spine allows for direct access to the compressive pathology responsible for foraminal stenosis. Bulging or herniated disc material and uncovertebral osteophytes lie anterior to the spinal cord and the nerve roots and can therefore be directly removed from an anterior approach. Figure 61.4 shows how these offending anterior structures are directly accessible for removal via an anterior approach. Robinson and Smith initially described anterior cervical discectomy and fusion in 1955.5 The anterior approach avoids direct manipulation of the neural elements. This original procedure relied on indirect decompression of the spinal cord and neural foramen by distraction of the disc space. Progression of spondylotic spur formation was prevented by stabilization of the segment and the authors believed that regression of these osteophytes occurred as a result of the arthrodesis. In 1958, Cloward published an alternative technique for anterior cervical discectomy and fusion in which direct decompression of the neural elements was performed.6 There are no comparative studies in the literature to support the clinical superiority of direct or indirect decompression of the neural elements in spondylotic radiculopathy. Individual surgeons must make a decision as to which philosophy they subscribe. The surgeon’s training as well as personal experience likely influence this decision.
Numerous modifications of the original Smith–Robinson and Cloward bone grafting techniques have been described. The Smith–Robinson technique involves an autogenous tricortical corticocancellous horseshoe graft placed into the evacuated disc space. The cortical component of the graft provides structural support that maintains the disc-space height while the cancellous portion facilitates graft incorporation. The original technique involved simply removing the cartilaginous endplate and incomplete decortication. More aggressive decortication, beyond simple cartilaginous endplate removal, has been demonstrated to improve fusion rates.7,8 Cloward’s autogenous graft was harvested from the iliac crest with an oversized hole saw. The oversized dowel graft was then impacted into an undersized hole at the level to be fused. The unicortical surface of this dowel graft was placed anteriorly, resting just posterior to the anterior surface of the vertebral bodies. Bailey and Badgley described a technique in which a ‘keystone-shaped’ corticocancellous graft was utilized.9 This trapezoidal-shaped graft is mortised into the prepared disc space. Excellent results with this technique were reported by Simmons in 1969.10
Contemporary choices for graft material include autograft, allograft, and synthetic cage devices. Good results have been reported with all of these graft choices when utilized for one-level discectomy and fusion without instrumentation, although the current literature does not support the superiority of any one single graft material or device.7,11–15 Higher rates of radiographic arthrodesis have been reported with the use of autogenous iliac crest grafts, especially with multiple-level discectomies.12,16 Higher radiographic fusion rates with autogenous grafts do not occur without consequences. Complications related to graft harvest include hematoma formation, infection, injury to the lateral femoral cutaneous nerve, iliac wing fractures, and, most importantly, persistent iliac crest pain have been reported.17,18 Silber et al. reported iliac crest graft site harvest complications in a series of patients undergoing one-level anterior cervical discectomy and fusion (ACDF). Twenty-six percent of patients reported chronic pain at the donor site and nearly half of these chronically used pain medications. They also report functional impairment in 13% of the patients studied.17
Allografts have been used in anterior cervical spine surgery since the 1950s. Cloward reported a 94% fusion rate in 44 patients who underwent ACDF with a dowel-shaped, frozen allogeneic iliac crest graft.6 Schneider utilized freeze-dried allogeneic iliac crest bone for ACDF with the Cloward technique. He reported an arthrodesis rate of 93% with one-level procedures and an 85% fusion rate for multiple-level discectomy and fusion.19 The use of allograft iliac crest grafts with the Smith–Robinson has also been reported in the literature. Rish reported a fusion rate of 80% in single-level and 69% in multiple-level patients undergoing a Smith–Robinson-type ACDF with freeze-dried allogeneic iliac crest.20 A 94% fusion rate was reported by Brown et al. in patients undergoing the same procedure with allogeneic, frozen iliac crest grafts.21 They also reported a high rate of graft collapse when allogeneic iliac crests are used. As a result of concerns over the compressive strength of allograft iliac crest grafts, some authors began utilizing fibular allograft for anterior cervical discectomy and fusion. Martin et al. reported on 289 patients who underwent ACDF with freeze-dried fibular allograft without instrumentation.11 Radiographic fusion was obtained in 90% of those undergoing one-level fusions. After two-level procedures, only 72% showed radiographic fusion at all levels. Although there was a trend towards higher rates of nonunion in those patients who were smokers, the difference was not statistically significant. The authors concluded that freeze-dried allogeneic fibula is an effective substrate for obtaining fusion following anterior cervical discectomy without instrumentation.
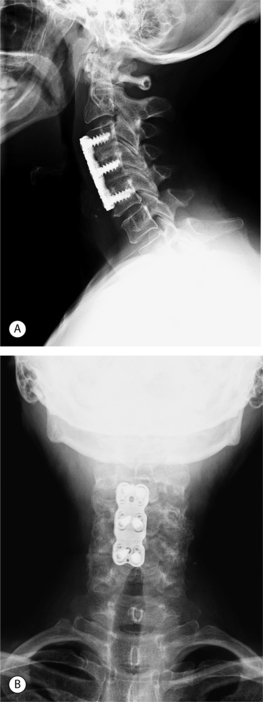
In an attempt to increase the likelihood of solid arthrodesis, many authors have advocated the addition of an anterior cervical plate, especially for multiple-level procedures. Radiographs of a two-level anterior discectomy and fusion with an anterior cervical plate are shown in Figure 61.5. Instrumentation increases the stability across the operative segment and decreases the motion between the graft and vertebral endplate, thereby increasing the chance of solid arthrodesis. The addition of anterior cervical instrumentation has been shown to decrease the rate of pseudoarthrosis in both single and multiple-level ACDFs. Nonunion rates ranging 11–63% have been reported depending on the number of levels at attempted fusion, the type of graft material used, and whether or not anterior instrumentation was utilized.12,22–25
Wang and colleagues reviewed the clinical and radiographic results of 80 patients treated with single-level ACDF.26 Pseudoarthrosis rates of 4.5% and 8.3% were reported for instrumented and noninstrumented, respectively. Patients stabilized with an anterior plate had significantly less graft collapse and kyphosis than those without a plate. The clinical outcomes based on Odom’s criteria were not significantly different between the two groups. In two separate reports, the same senior authors have also reported retrospectively on the use of anterior cervical plates in two- and three-level ACDFs.27,28 Autogenous tricortical iliac crest grafts were used in all patients in each study. In the review of two-level procedures, a statistically significant difference in pseudoarthrosis rate was found in the instrumented group (0%) compared to the noninstrumented group (7%).27 The use of a cervical plate did not alter clinical outcomes but those patients who developed a pseudoarthrosis had poorer clinical results. In the third report by this group, the results of three-level ACDF were reviewed.28 Forty of the fifty-nine patients were stabilized with an anterior cervical plate. Pseudoarthrosis developed in 18% (7/40) of the plated patients and 37% (7/19) of those treated without a plate. This difference was not statistically significant, perhaps because of the small number of patients, but the authors point out that the rate of nonunion in the noninstrumented group is nearly twice that of the instrumented group. Once again, the development of a pseudoarthrosis resulted in poorer clinical outcomes.
Other groups have analyzed the results of both one- and two-level ACDFs treated with and without plates.29,30 Caspar et al. retrospectively reviewed the charts of 356 patients who underwent one- and two-level ACDF with and without anterior instrumentation for degenerative disorders.30 Six percent of those undergoing noninstrumented fusions required reoperation for pseudoarthrosis compared to 0.7% in whom an anterior cervical plate was used. Two (out of 146) patients in whom a plate was used required a revision procedure secondary to hardware failure prior to fusion. The authors concluded that the use of anterior instrumentation for one- and two-level ACDFs results in a significant reduction in reoperation rates. In a retrospective manner, Kaiser et al. compared the radiographic results of a group of patients stabilized with anterior cervical plates following one- and two-level ACDF with a historical cohort of patients that were treated without plates.29 Cortical ring allograft struts were utilized in each of the groups. The fusion rates for one- and two-level ACDF with anterior instrumentation were 96% and 91%, respectively. Significantly lower fusion rates were found in those one- and two-level procedures (90% and 72%, respectively) that were not stabilized by an anterior plate.11 No infectious, neurologic, or graft-related complications were reported in the instrumented group. Statistical analysis revealed a higher graft complication rate in the noninstrumented group from the historical cohort. The authors conclude that the improved fusion rates and negligible complication rates justify the routine use of anterior cervical plating in the treatment of cervical spondylosis.
Surgical technique
Any remaining anterior osteophytes are removed with a rongeur or high-speed burr. This will allow the cervical plate to lie flat against the anterior aspect of the spine, thereby avoiding any possible issues with dysphagia from the instrumentation. Failure to completely remove this abnormal bone also results in the plate resting on the osteophytes with the screws obtaining some of their purchase in this soft bone. The distraction across the disc space is released and the distraction pins are removed. To provide additional compression of the graft, the neck is brought out of extension by placing a towel behind the occiput. The authors’ choice of plating systems is a slotted, dynamic plate that allows for both dynamic compression of the graft as the screws are tightened, and linear settling of the graft as the cranial screws slide through the slots in the plate. During the insertion of the plate care must be taken to assure that the cranial screws are entering the vertebral body at the graft–host bone junction. Failing to do so may result in damage to the superior adjacent segment as the operative level settles and the cranial edge of the plate approaches the disc space proximal to the fusion. This is more of a concern with multiple-level discectomies but should not be neglected with one-level fusions. An appropriate length of plate is placed flat against the anterior aspect of the spine. The drill holes are drilled for the inferior screws first with a drill bit with a stop at the appropriate length. The length of these screws is determined by the overall size of the patient and by preoperative radiographs. Alternatively, the depth of the vertebral body may be determined intraoperatively after the discectomy and decompression have been completed. Unicortical screws typically range 12–16 mm in length. The drill guide for the inferior screws is a ‘static’ guide, which places the screw in the center of the hole in the plate. The two caudal screws are placed but not tightened securely to the plate, thereby avoiding tipping the superior end of the plate anteriorly. The guide for the cranial screws is a ‘dynamic’ drill guide, which preferentially drills the guide hole eccentrically in the slot of the plate. The superior screws are placed but are also not secured tightly to the plate. Once all four screws have been placed, the inferior, or ‘static,’ screws are fully tightened. As the ‘dynamic’ screws at the top of the slots at the cranial end of the plate are tightened, a compressive force is place across the graft. Lastly, the screw-locking mechanism is engaged to prevent back-out of the screws. A lateral radiograph is obtained to confirm the position of the plate, the maintenance or recreation of the cervical lordosis, and the position of the screws. The self-retaining retractor is removed and sidewalls of the wound are inspected for any bleeding. The wound is irrigated with antibiotic saline and a small Jackson-Pratt drain placed directly over the plate and passed out the superior or lateral aspect of the wound. The platysma is closed with absorbable sutures followed by a running subcuticular closure of the skin. Because of the used of routine anterior cervical plating, all single-level discectomies are placed into a soft cervical collar that is worn for comfort only. Multilevel discectomies and corpectomies are placed into a semirigid cervical orthosis.
Posterior cervical foraminotomy
Concern exists over the large amount of muscle stripping and damage that must be done to perform a standard, open laminoforaminotomy. Higher rates of infection and postoperative neck pain have been reported in patients undergoing posterior procedures compared to anterior procedures.31 As a result of the concern over the large amount of muscle dissection, some have advocated a minimally invasive approach to posterior laminoforaminotomy. Fears of wrong-level surgery, creation of iatrogenic instability by excessive resection of the facet joint, and inadequate decompression also sway the surgeon away from performing laminoforaminotomies. Direct manipulation of the neural elements is also required to remove the offending pathologic structures that arise anterior to the nerve root. Theoretically, higher rates of neurologic complications should exist, although this has not been widely reported in the literature.
Clinical indications for posterior foraminotomy are similar to those outlined in the earlier portion of this chapter. Isolated radiculopathy in patients with imaging-confirmed neural compression that has failed to respond to conservative treatment modalities should be considered for operative treatment. Patients with any neck pain should be offered anterior decompression and fusion. Pathoanatomic indications for posterior cervical foraminotomy include posterolateral soft disc herniations, cervical lateral recess or foraminal stenosis from spondylosis, facet arthropathy with foraminal compression, continued radiculopathy following ACDF, and cervical disease in patients whom anterior approaches are contraindicated. As mentioned earlier, posterior cervical foraminotomy should also be considered when the cervical disease is located at the anatomic extremes, such as cervicothoracic junction or in the proximal cervical segments. Contraindications to foraminotomy include patients with vertebral body spondylotic bars, large central disc herniations, myelopathy, preexisting instability, and ossification of the posterior longitudinal ligament. Patients with significant kyphotic deformities should also not undergo posterior foraminotomies because of the nature of their neurologic compression. Posterior decompression in this setting, without correction of the deformity, may result in continued neurologic symptoms as the neural elements remained draped over the anterior compressive pathology. The indications and contraindications to posterior foraminotomy are summarized in Table 61.1.
Table 61.1 Indications and contraindications to posterior laminoforaminotomy
| Indications | Contraindications |
|---|---|
| Posterolateral soft disc herniations | Neck pain |
| Foraminal stenosis from uncovertebral joint osteophytes | Large central disc herniations |
| Facet arthropathy with foraminal compression | Myelopathy |
| Continued radiculopathy following ACDF | Preexisting instability |
| Disease at anatomic extremes (C2–3 or C7–T1) | Ossification of the PLL |
| Anterior approach contraindicated | Kyphotic sagittal alignment |
The effectiveness of the posterior cervical foraminotomy has been well documented in the literature over the past four decades.4,32–39 Scoville and associates reported 95% good or excellent results in patients who had lateral disc herniations.32 Rothman and Simeone reported 98% success rate in a group of patients that were followed for up to 8 years.33 Between 1963 and 1980, Henderson and Hennessy performed 846 posterior foraminotomy for cervical radiculopathy. They reported a 96% incidence of relief of significant arm pain and/or paresthesia and a 98% incidence of resolution of preoperative motor deficit.4 They also stated that there was no significant difference postoperatively regarding results or recurrence between the patients with suspected soft or hard disc protrusions and those with strictly spondylotic radiculopathy. Simeone and Dillin subsequently reported 96% good or excellent outcomes in their series of patients.34
Herkowitz, et al. prospectively compared anterior and posterior approaches for the treatment of cervical radiculopathy caused by soft disc herniations.35 Seventeen patients were randomized to undergo anterior fusion while sixteen patients had a posterior foraminotomy. Good to excellent results occurred in 85% of those undergoing an anterior discectomy and fusion and in 75% of patients treated with posterior foraminotomy. This difference was not statistically significant. Wirth et al. randomized 72 patients with acute radiculopathy from a unilateral cervical disc herniation to undergo posterior cervical foraminotomy, ACDF, or anterior cervical discectomy without fusion (ACD).40 All three procedures yielded excellent relief of symptoms in nearly all of the patients. Complications, operative time, length of hospital stay, and the use of analgesics in the immediate postoperative period were similar in all three groups. At 2-month follow-up, nearly all of the patients reported excellent pain relief and approximately 90% were back to work. Telephone interviews more than 2 years after the procedure showed a decrease in the percentage of patients in each group with complete pain relief although 79–92% were able to work. Return trips to the operating room for a disc operation at the same level were more common in the foraminotomy group while a higher incidence of adjacent segment disc problems requiring an operation occurred in the ACDF group. Proponents of motion-sparing procedures may argue that this is due to stress transfer to the adjacent segments when a fusion is performed.
In 1993, Zeidman and Ducker presented the results of posterior cervical foraminotomies performed in 172 patients with cervical radiculopathy.39 Some patients had multilevel disease and had multilevel foraminotomies; thus, a total of 243 foraminotomies were performed during 7-year period. Relief of radicular pain was obtained in 167 patients (97%). Recovery of motor deficit to baseline function was achieved in 36 of the 39 (93%) patients with preoperative motor weakness. Although the majority of patients who underwent single-level foraminotomy had improvement of their preoperative sensory abnormalities, the patients who had multilevel foraminotomies had minimal recovery of their sensory deficit. Also, no patient with depressed reflex had a recovery of this reflex after the operation.
More recently, Harrop et al. reported on the effectiveness of treating cervical radiculopathy via posterior approach.41 They found reduction of radicular pain from a mean score of 7.45 of 10 preoperatively to 0.2 postoperatively. Although preoperative neck pain was not as severe as the radicular pain, there was a reduction of preoperative neck pain from mean score of 2.55 of 10 to 0.5 postoperatively. Both of these differences were statistically significant.
Because of the concerns over the amount of muscle stripping needed to perform an open posterior foraminotomy, some have advocated the use of less invasive surgical techniques. The feasibility of a minimally invasive endoscopic approach (MED) to a posterior cervical laminoforaminotomy was evaluated in a cadaveric study.31 The authors compared the decompression provided by a minimally invasive approach with that of a standard open technique. The procedure time tended to be longer in the MED group and the laminotomy defect created with this minimally invasive technique larger. The average amount of the facet joint removed in the MED procedure was 37.5±12% versus 32.8±6.0% in the open group. This difference was statistically significant. Based on the biomechanical studies by Raynor and Zdeblick, this amount of facet removal does not lead to iatrogenic instability.42,43 Postprocedure CT myelography documented adequate neural decompression of the nerve roots at each level in both the open and MED groups. One dural laceration occurred during minimally invasive procedure but the authors felt that this was a ‘learning curve phenomenon.’ The authors concluded that the MED system provided adequate exposure and allowed for decompression equal to that of an open approach.
The clinical results of minimally invasive posterior foraminal decompression have been reported as well. Adamson reported results of 100 consecutive cases of posterior microendoscopic laminoforaminotomy used for the treatment of unilateral cervical radiculopathy secondary to disc herniation and/or spondylotic foraminal stenosis.44 Excellent or good results were obtained in 97 patients, with all returning to their preoperative occupation or level of functioning between 1 day and 4 weeks postoperatively. All patients were discharged from the hospital no later than postoperative day 1 with 90 of the patients being discharged the day of the procedure. Pain relief was excellent in a vast majority of patients, with 84 patients requiring no additional prescription medication after the first week. Fessler et al. reported their initial clinical experience with minimally invasive cervical microendoscopic foraminotomy (MEF) using the MED system.45 The MEF technique was performed on 25 patients with cervical radiculopathy from spondylotic foraminal stenosis or soft disc herniation. A group of 26 patients who underwent open cervical laminoforaminotomy for similar indications served as a comparison group. They reported that MEF group had lower overall operative times (115 versus 171 minutes), less blood loss (138 versus 246 mL per level), shorter postoperative hospital stay (20 versus 68 hours), and lower postoperative narcotic medication requirements (11 versus 40 equivalents) when compared to the patients who underwent open laminoforaminotomy. The first 12 patients in the MEF group were positioned in the prone position while the remaining 13 were placed in the seated position. Utilizing the seated position resulted in significantly shorter OR time, reduced blood loss, and shorter hospital stays. Outcomes were not significantly different between the two groups, with each achieving greater than 90% symptomatic improvement after the procedure.
Surgical techniques
Anatomic considerations
Posterior compression of the nerve root within the vertebral foramen can occur from the superior articular process, the ligamentum flavum, and the periradicular fibrous tissues.46 These compressive structures are easily removed via a posterior approach. In fact, one-quarter to one-half of the facet joint must be removed to ‘unroof’ the neural foramen during a posterior foraminotomy (Fig. 61.6). Anterior compressive structures such as uncovertebral osteophytes (unless very large) may be difficult to visualize and remove via the posterior approach. Nerve root decompression in this setting is provided by simply ‘unroofing’ the foramen. Soft disc herniations within the foramen may be visualized by gently retracting the nerve root cephalad to reveal the disc fragment. Compression of the neural structures medial to the foramen can simply not be addressed from the posterior approach, as this would require direct manipulation of the spinal cord itself. The foraminal decompression afforded by an anterior approach is often overestimated secondary to difficulty in lateral visualization. Pathology that exists far lateral within the foramen (for example, a soft disc herniation that has migrated laterally out of the interspace) can be easily overlooked unless careful scrutiny of the root anatomy is performed. This requires exposure of the vertebral artery and puts this structure at significant risk of injury. A thorough discussion of the advantages and limitations of both the anterior and posterior approaches was written by Raynor in 1983.47
Concerns over the stability of the cervical functional spinal unit after foraminotomy prompted biomechanical evaluation of the effects of resection of progressively larger portions of the cervical facets on the acute stability of the cervical spine. Progressive facet joint resection resulted in segmental hypermobility when 50% of both facet joints was removed. The authors of both studies recommended limiting resection of the facet joints to less than 50% to avoid iatrogenic instability after laminoforaminotomy.42,43 If more than 50% of the facet joint must be excised to perform an adequate decompression, serious consideration must be given to adding an arthrodesis to the procedure.
Microendoscopic foraminotomy
The patient positioning and set-up for this minimally invasive approach is the same as that used for the open technique. Monitoring for venous air embolism and for neurologic injury may also be performed as in the open procedure. Once the patient is positioned, a fluoroscope is brought into the operative field to localize the operative level with a spinal needle prior to a skin incision. A skin incision is made approximately 1 cm off midline at the level of the pathology. Under fluoroscopic guidance, a K-wire is inserted to the facet or lateral mass of the level of the pathology. It is imperative to dock the guide wire directly on the bone to avoid inadvertent dural penetration. A small incision is placed around the wire to allow the passage of a series of dilators through the soft tissues. These dilators must be docked directly onto the facet complex to minimize interference of visualization from the adjacent muscle tissue. Lateral fluoroscopic images should be obtained frequently to insure a proper working trajectory throughout this process. Finally, the tubular retractor is anchored to the operating room bed via a specific retractor arm. The working end of the retractor must be positioned at the junction of the lamina and lateral mass. Magnified visualization can be provided with an endoscope, an operative microscope, or with loupes. If loupe magnification is utilized, a retractor system with a built-in light source makes visualization much easier.
CASE STUDIES
Example 1
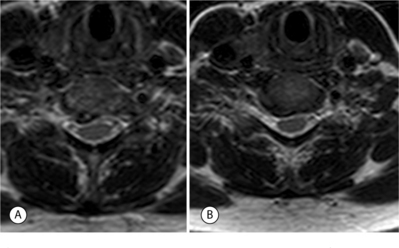
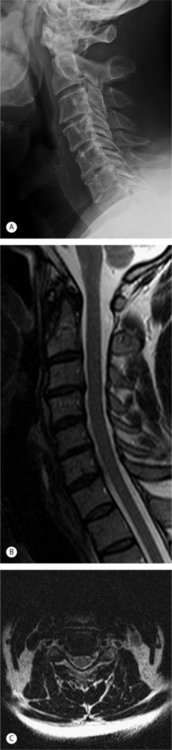
A 52-year-old man presented with a 2-month history of right arm pain and neck pain. He had been treated initially by his primary care physician with ibuprofen and physical therapy. Plain radiographs and an MRI were obtained; images of interest are presented in Figure 61.7. The axial MRI image shows right, unilateral foraminal stenosis from uncovertebral osteophytes. Mild deformation of the spinal cord is also present on the right as a result of a spondylotic bar from the inferior portion of the C6 vertebral body. The patient was sent for a series of cervical epidural steroid injections which gave him partial, temporary relief of his symptoms. After 5 months of symptoms, the patient wished to discuss surgical treatment. Because of his neck pain and the mild amount of spinal cord deformation, he was offered an ACDF with instrumentation as described in detail above. The procedure was performed without incident. In the recovery room, the patient reported that his arm pain was 90% improved compared to pre-op and that his neck pain was completely absent. He was immobilized in a soft cervical collar until his 2-week postoperative visit. At this visit, his arm pain had completely resolved. He had radiographic evidence of a solid arthrodesis at his 6-month postoperative visit. He is now 15 months out from surgery and has had no recurrence of arm pain or neck pain.
Example 2
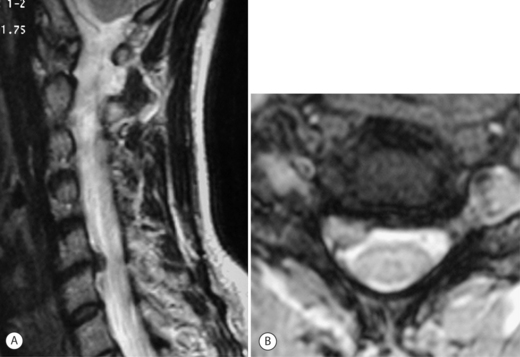
A 38-year-old female was seen in the emergency room complaining of severe, burning pain on the radial side of her right forearm and hand. She had no complaints of neck pain. Plain radiographs showed mild disc space narrowing at C5–6 and C6–7. An MRI (Fig. 61.8) showed a lateral soft disc herniation at C5–6 with no central spinal canal stenosis. She was initially treated with NSAIDs and physical therapy without any resolution of her symptoms. Selective nerve root blocks failed to lessen her C6 radiculopathy. Surgical intervention was discussed because of ongoing symptoms that interfered with her ability to work. Her lack of neck pain and the presence of a lateral soft disc herniation made her a perfect candidate for an open posterior laminoforaminotomy. The procedure was performed with the patient in the prone position. After ‘unroofing’ the foramen as described above, a large free fragment of disc was identified in the axilla of the C6 nerve root. A small, blunt nerve hook was passed under the C6 root and the fragment was removed with a small pituitary rongeur. The patient’s arm pain and paresthesias were completely absent when the patient was seen in the recovery room. She was discharged the following morning in a soft cervical collar. She returned to the office 2 weeks after surgery without her collar and with complaints of mild neck pain. Her neck pain resolved completely by her 6-week postoperative visit. She is now 9 months out from surgery and has no arm or neck pain.
CONCLUSIONS
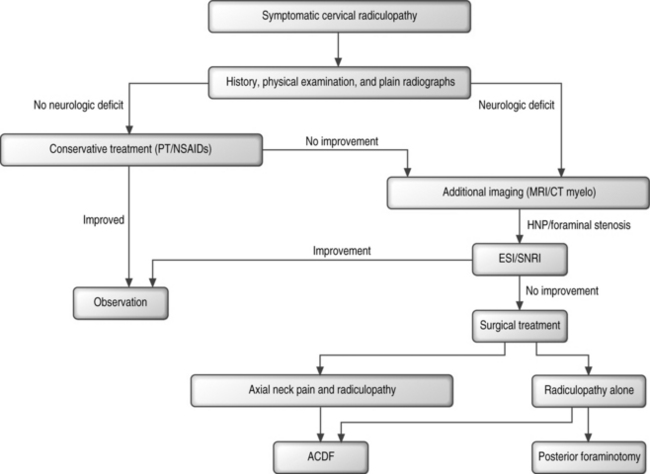
Successful surgical treatment of refractory radiculopathy from isolated cervical foraminal stenosis can be accomplished from both anterior and posterior surgical approaches. A simple algorithm is presented in Figure 61.9. After an appropriate trial of nonoperative management, usually lasting at least 6 weeks, patients will be considered for operative intervention. Any patient with neck pain is offered an anterior cervical discectomy and fusion with instrumentation. Patients with central spinal canal stenosis from either a soft disc herniation or spondylosis are not candidates for a posterior decompression and are also offered an anterior procedure. The available literature does not support the superiority of one graft type or plate. The authors use tricortical, patellar allograft to avoid the morbidity of harvesting iliac crest autograft and because of the theoretical advantages mentioned above. The authors’ choice of anterior cervical plate is a slotted, dynamic plate that allows limited, linear settling of the graft. Patients with only radicular complaints and isolated foraminal stenosis from either a lateral, soft disc herniation or spondylosis may be offered a posterior laminoforaminotomy. The authors have no experience with the microendoscopic technique described above. The authors perform the open procedure with the patient in the prone position with the head stabilized in Mayfield tongs.
1 Davidson RI, Dunn EJ, Metzmaker JN. The shoulder abduction test in the diagnosis of radicular pain in cervical extradural compressive monoradiculopathies. Spine. 1981;6(5):441-446.
2 Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2(7825):359-362.
3 Massey EW, Riley TL, Pleet AB. Coexistent carpal tunnel syndrome and cervical radiculopathy (double crush syndrome). South Med J. 1981;74:957-959.
4 Henderson CM, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13(5):504-512.
5 Robinson R, Smith G. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
6 Cloward R. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
7 Bohlman HH, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg [Am]. 1993;75(9):1298-1307.
8 Emery SE, et al. Robinson anterior cervical fusion comparison of the standard and modified techniques. Spine. 1994;19(6):660-663.
9 Bailey RW, Badgley CE. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg [Am]. 1960;42:565-594.
10 Simmons EH, Bhalla SK. Anterior cervical discectomy and fusion: a clinical and biomechanical study with eight-year follow-up. J Bone Joint Surg [Br]. 1969;51:225-237.
11 Martin GJJr, et al. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine. 1999;24(9):852-858. discussion 858–859
12 Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16(7):726-729.
13 Cauthen JC, Theis RP, Allen AT. Anterior cervical fusion: a comparison of cage, dowel and dowel-plate constructs. Spine J. 2003;3(2):106-117. discussion 117
14 van Limbeek J, et al. A systematic literature review to identify the best method for a single level anterior cervical interbody fusion. Eur Spine J. 2000;9(2):129-136.
15 Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9(5):398-403.
16 Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85(2):206-210.
17 Silber JS, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28(2):134-139.
18 Kurz LT, Garfin SR, Booth REJr. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14(12):1324-1331.
19 Schneider JR, Bright RW. Anterior cervical fusion using preserved bone allografts. Transplant Proc. 1976;8(2 Suppl 1):73-76.
20 Rish BL, McFadden JT, Penix JO. Anterior cervical fusion using homologous bone grafts: a comparative study. Surg Neurol. 1976;5(2):119-121.
21 Brown MD, Malinin TI, Davis PB. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop. 1976;119:231-236.
22 Boker DK, et al. Anterior cervical discectomy and vertebral interbody fusion with hydroxy-apatite ceramic. Preliminary results. Acta Neurochir (Wien). 1993;121(3–4):191-195.
23 Brown CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11(9):942-943.
24 Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine. 1997;22(22):2622-2624. discussion 2625
25 Mutoh N, et al. Pseudarthrosis and delayed union after anterior cervical fusion. Int Orthop. 1993;17(5):286-289.
26 Wang JC, et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12(6):467-471.
27 Wang JC, et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25(1):41-45.
28 Wang JC, et al. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine. 2001;26(6):643-646. discussion 646–647
29 Kaiser MG, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50(2):229-236. discussion 236–238
30 Caspar W, et al. Anterior cervical plate stabilization in one- and two-level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11(1):1-11.
31 Roh S, et al. Endoscopic foraminotomy using MED system in cadaveric specimens. Spine. 2000;25(2):260-264.
32 Scoville WB, Whitcomb BB. Lateral rupture of cervical intervertebral disks. Postgrad Med. 1966;39(2):174-180.
33 Rothman R, Simeone FA, editors. The spine, 2nd edn., Philadelphia: WB Saunders, 1982.
34 Dillin W, et al. Cervical radiculopathy. A review. Spine. 1986;11(10):988-991.
35 Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine. 1990;15(10):1026-1030.
36 Epstein JA, et al. Cervical spondylotic radiculopathy. The syndrome of foraminal constriction treated by foramenotomy and the removal of osteophytes. Clin Orthop. 1965;40:113-122.
37 Fager CA. Posterolateral approach to ruptured median and paramedian cervical disk. Surg Neurol. 1983;20(6):443-452.
38 Aldrich F. Posterolateral microdisectomy for cervical monoradiculopathy caused by posterolateral soft cervical disc sequestration. J Neurosurg. 1990;72(3):370-377.
39 Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 1993;33(3):356-362.
40 Wirth FP, et al. Cervical discectomy. A prospective analysis of three operative techniques. Surg Neurol. 2000;53(4):340-346. discussion 346–348
41 Harrop JS, et al. Cervicothoracic radiculopathy treated using posterior cervical foraminotomy/discectomy. J Neurosurg. 2003;98(2 Suppl):131-136.
42 Zdeblick TA, et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg [Am]. 1992;74(1):22-27.
43 Raynor RB, Pugh J, Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63(2):278-282.
44 Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95(1 Suppl):51-57.
45 Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(5 Suppl):37-45.
46 Tanaka N, et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine. 2000;25(3):286-291.
47 Raynor RB. Anterior or posterior approach to the cervical spine: an anatomical and radiographic evaluation and comparison. Neurosurgery. 1983;12(1):7-13.