7 Surgical Anatomy of EC-IC Bypass Procedures
Introduction
The number of cerebral revascularization procedures performed by neurosurgeons has declined after the Cooperative Study of Extracranial-Intracranial Arterial Anastomosis1 failed to demonstrate that this procedure reduced the risk of stroke in patients with cerebral ischemia. However, cerebral revascularization is now well recognized as an important element in the treatment of complex intracranial aneurysms, cranial base tumors, and certain kinds of ischemic diseases.
Results
Arterial Relationships
The diameters of vessels measured, which are frequently used for cerebral revascularization procedures, are shown in Tables 7–1 and 7–2.
Table 7–1 Diameter of Vessels for Cerebral Revascularization in Anterior Circulation (MM).
| ICA |
| Cervical segment: 8.57 ± 1.34 Petrous segment: 5.42 ± 0.68 Supraclinoid segment: 3.95 ± 0.56 |
| ECA |
| Cervical portion: 5.75 ± 0.94 |
| STA |
| At the level of zygoma: 1.93 ± 0.48 |
| MCA |
| M2 (largest branch near the central sulcus): 1.76 ± 0.36 M4 (largest branch in the area) Frontal branch: 1.19 ± 0.32 Temporal branch: 1.22 ± 0.23 Parietal branch: 1.36 ± 0.24 |
| ACA |
| A2: 2.35 ± 0.60 A3: 1.98 ± 0.35 A4: 1.94 ± 0.40 A5: 1.55 ± 0.12 |
Table 7–2 Diameter of Vessels for Cerebral Revascularization in Posterior Circulation (MM).
| PCA |
| P2A: 2.13 ± 0.38 P2P: 1.73 ± 0.33 P3: 1.67 ± 0.16 |
| SCA |
| Anterior pontomesencephalic segment: 1.67 ± 0.16 Lateral pontomesencephalic segment Single trunk: 1.51 ± 0.12 Rostral trunk: 1.25 ± 0.17 Caudal trunk: 1.15 ± 0.30 |
| AICA |
| Anterior pontomesencephalic segment: 1.34 ± 0.28 Cortical segment: 1.07 ± 0.29 |
| PICA |
| Anterior medullary segment: 1.84 ± 0.45 Tonsillomedullary segment (caudal loop): 1.68 ± 0.38 |
| OA |
| At the digastric groove: 2.05 ± 0.48 At the level of the superior nuchal line: 2.01 ± 0.45 |
Internal carotid artery
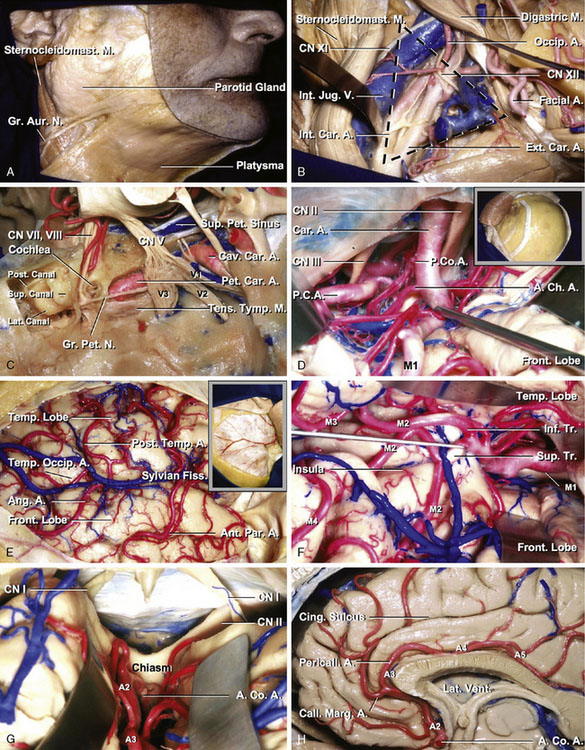
The common carotid artery divides into the internal (ICA) and external carotid artery (ECA) at the level of C2-C3 below the mandibular angle. The cervical, petrous, and supraclinoid ICAs are the sites of cerebral revascularization procedures. The average diameters of the cervical, petrous, and supraclinoid ICAs were 8.57, 5.42, and 3.95 mm, respectively (Table 7–1). The cervical portion of the ICA is covered by the skin, superficial cervical fascia, platysma, deep fascia, anterior margin of the sternocleidomastoid muscle, and internal jugular vein. Dissecting these anatomic structures exposes the carotid triangle, which is formed by the posterior belly of the digastric, omohyoid, and sternocleidomastoid muscles. In the carotid triangle, the ICA begins at the bifurcation of the common carotid, opposite the upper border of the thyroid cartilage, and runs perpendicularly upward, in front of the transverse processes of the upper three cervical vertebrae, to the carotid canal in the petrous portion of the temporal bone (Figures 7–1A and 7–1B).
The petrous portion of the ICA courses within the carotid canal and ends where the artery enters the cavernous sinus. The carotid artery, at the point where it enters the carotid canal, is surrounded by a strong layer of connective tissue that makes it difficult to mobilize the artery. The roof of the carotid canal opens below the trigeminal ganglion near the distal end of the carotid canal. The greater petrosal nerve runs beneath the dura of the middle fossa, immediately superior and anterolateral to the horizontal segment of the petrous carotid. The cochlea lies below the floor of the middle fossa, just posterosuperior to the lateral genu of the petrous carotid artery. The Eustachian tube and the tensor tympani muscle are located parallel to and along the anterior margin of the horizontal segment, where they are separated from the artery by a thin layer of bone (Figure 7–1C).
The supraclinoid segment of the ICA begins where the artery emerges from the dura mater and enters the cranial cavity by passing along the medial side of the anterior clinoid process and below the optic nerve. It reaches the lateral side of the optic chiasm and bifurcates below the anterior perforated substance at the medial end of the sylvian fissure to give rise to the anterior cerebral artery (ACA) and middle cerebral artery (MCA). The supraclinoid segment of the ICA gives rise to three branches: the ophthalmic, posterior communicating, and anterior choroidal arteries. In addition, this segment gives off perforating branches including the superior hypophyseal artery (Figure 7–1D).
External carotid artery
The external carotid artery (ECA) begins at the bifurcation of the common carotid in front of the ICA and ascends backward to the space behind the neck of the mandible, where it divides into the superficial temporal and maxillary arteries. The average diameter of the ECA was 5.75 mm (Table 7–1). The ECA is crossed by the hypoglossal nerve, common facial and superior thyroid veins, and the digastric and stylohyoid muscles. The superior thyroid artery arises from the external carotid artery just below the level of the greater cornu of the hyoid bone and ends in the thyroid gland. The STA, the smaller of the two terminal branches of the ECA, appears, from its direction, to be the continuation of that vessel. The STA plays an important role as a donor vessel in cerebral revascularization. The STA begins in the substance of the parotid gland, behind the neck of the mandible, and crosses over the posterior root of the zygomatic process of the temporal bone. The average diameter of the STA at the level of the zygoma was 1.93 mm (Table 7–1). The STA divides into two branches, frontal and parietal. The frontal branch (anterior temporal) runs tortuously upward and forward to the forehead, supplying the muscles, integument, and pericranium in this region, and anastomosing with the supraorbital and frontal arteries. The parietal branch (posterior temporal), larger than the frontal, curves upward and backward on the side of the head, lying superficial to the temporal fascia and anastomosing with its fellow of the opposite side and with the posterior auricular and occipital arteries (Figure 7–1B).
Middle cerebral artery
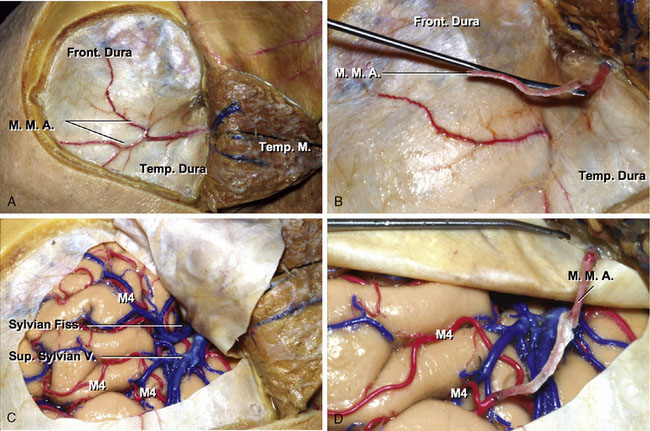
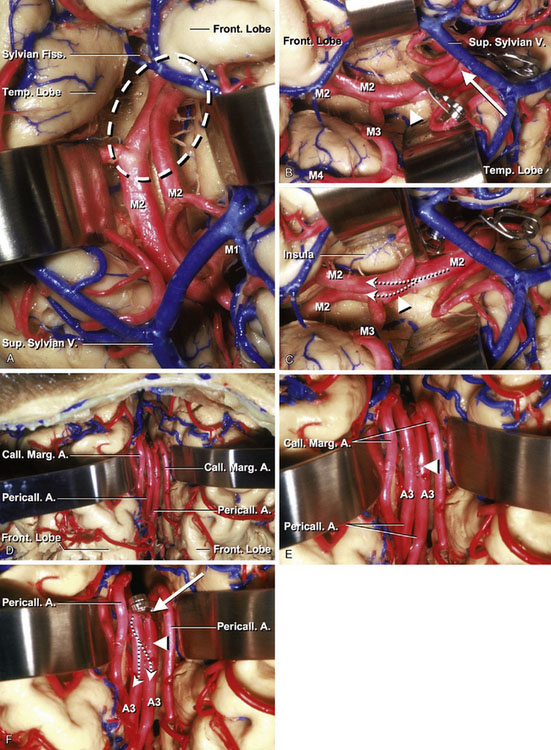
M2 and M4 are the sites of cerebral revascularization procedures. The M2 segment includes the trunk that lie on and supply the insula. This segment begins at the genu where the middle cerebral artery (MCA) trunk passes over the limen insulae and terminates at the circular sulcus of the insula. The greatest branching of the MCA occurs distal to the genu as these trunks cross the anterior part of the insula. The M2 branches are used for a bypass procedure, especially for a high-flow bypass. Important factors in selecting an artery for the procedure are its diameter, the length of artery available on the cortical surface, and perforating arteries to the basal ganglia. The average diameter of the largest branch near the central sulcus of the insula was 1.76 mm (Table 7–1).
The M4 is composed of the branches to the lateral convexity. They begin at the surface of the sylvian fissure and extend over the cortical surface of the cerebral hemisphere. The largest cortical artery is the temporo-occipital artery. Nearly two-thirds are 1.5 mm or more in diameter, and 90% are 1 mm or more in diameter. The smallest cortical artery is the orbitofrontal artery; approximately one-quarter are 1 mm or more in diameter. The average diameters of the largest M4 in the parietal, temporal, and frontal area were 1.36, 1.22, and 1.19 mm, respectively (Table 7–1). The central sulcal artery is the largest branch to the frontal lobe, and the angular artery is the largest branch to the parietal lobe. The temporo-occipital and the posterior temporal arteries are the largest branches to the temporal lobe. The minimum length of a cortical artery needed to complete a bypass is 4 mm. The angular, posterior parietal, and temporo-occipital arteries have the longest segments on the cortical surface, and the orbitofrontal and temporopolar arteries have the shortest cortical segment (Figures 7–1E and 7–1F).
Anterior cerebral artery
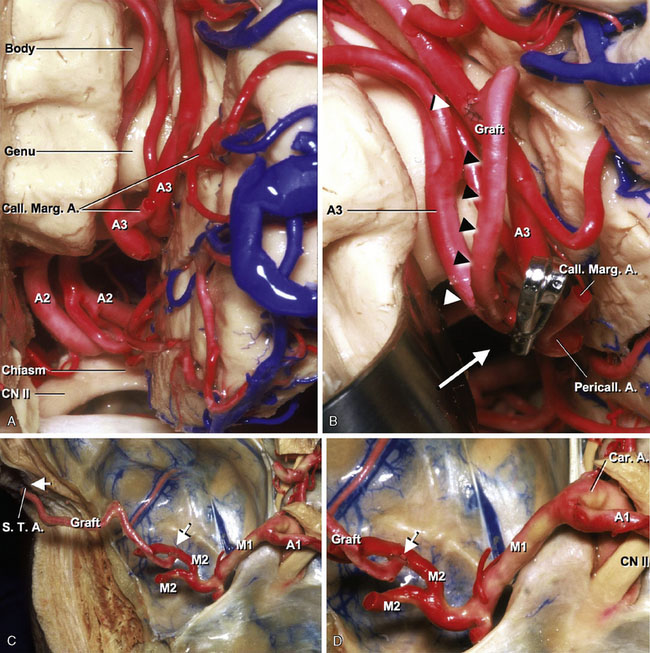
The anterior cerebral artery (ACA) is divided at the anterior communicating artery (AComA) into two parts, proximal (A1) and distal (A2–5). The A3 segment of the ACA is the dominant site of cerebral revascularization procedures, including side-to-side anastomosis and short arterial and venous interposition grafting. The average diameters of A2, A3, A4, and A5 were 2.35, 1.98, 1.94, and 1.55 mm, respectively (Table 7–1). The pericallosal artery is the portion of the ACA distal to the AComA and is constantly present. The pericallosal artery ascends in front of the lamina terminalis to pass into the interhemispheric fissure (A2). Above the lamina terminalis, the artery makes a smooth curve around the genu of the corpus callosum (A3) and then courses backward above the corpus callosum in the pericallosal cistern (A4 and A5).
The callosomarginal artery is defined as the artery that courses in or near the cingulate sulcus and gives rise to two or more major cortical branches.2 When the callosomarginal artery is well formed, it lies in the cingulate sulcus above the cingulate gyrus and follows a course roughly parallel to that of the pericallosal artery. Its origin varies from just distal to the AComA to the level of the genu of the corpus callosum. Its frequent origin was from the A3 segment, followed by the A2 segment and AComA. The size of the pericallosal artery distal to the callosomarginal origin varies inversely with the size of the callosomarginal artery (Figures 7–1G and 7–1H).
Posterior cerebral artery
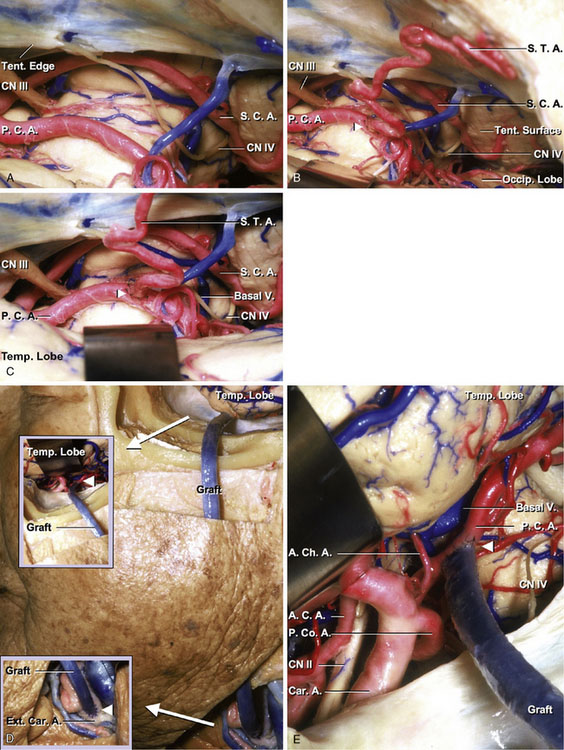
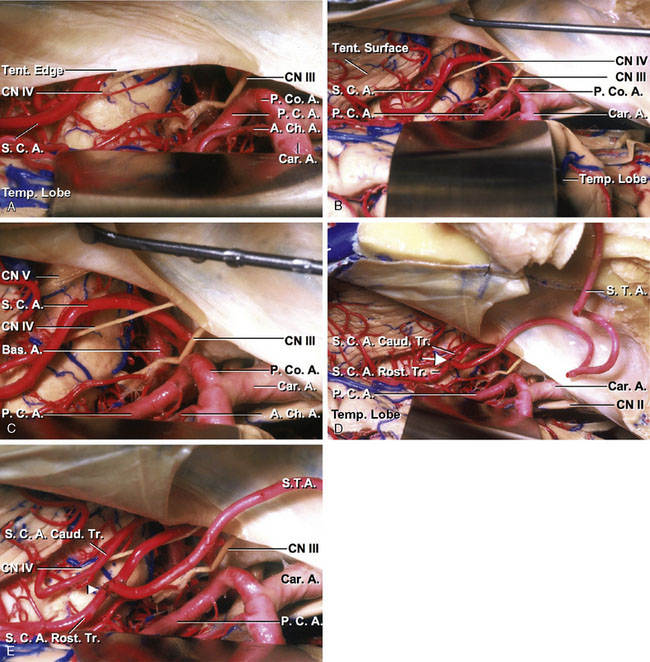
The posterior cerebral artery (PCA) arises at the basilar bifurcation, is joined by the posterior communicating artery (PComA) at the lateral margin of the interpeduncular cistern, encircles the brainstem passing through the crural and ambient cisterns to reach the quadrigeminal cistern, and is distributed to the posterior part of the hemisphere. The PCA supplies not only the posterior part of the cerebral hemispheres, but also sends critical branches to the thalamus, midbrain, and other deep structures, including the choroid plexus and walls of the lateral and third ventricles. Each segment of the PCA is classified according to our already proposed system.3 The P1 is the segment proximal to the PComA. The P2 segment extends from the PComA to the point at which the PCA enters the quadrigeminal cistern. The P2 segment is subdivided into equal anterior (P2A) and posterior (P2P) halves. The P2A begins at the PComA and courses between the cerebral peduncle and uncus that forms the medial and lateral walls of the crural cistern, and inferior to the optic tract and basal vein that crosses the roof of the cistern, to enter the proximal portion of the ambient cistern. The P2P begins at the posterior edge of the cerebral peduncle at the junction of the crural and ambient cisterns. It courses between the lateral midbrain and the parahippocampal and dentate gyri, which form the medial and lateral walls of the ambient cistern, below the optic tract, basal vein, and geniculate bodies and the inferolateral part of the pulvinar in the roof of the cistern, and superomedial to the trochlear nerve and tentorial edge. The P3 segment begins at the posterior midbrain, courses within the quadrigeminal cistern, and ends at the anterior limit of the calcarine fissure. The P4 segment is the distal branch of the P3 segment. The average diameters of P2A, P2P, and P3 were 2.13, 1.73, and 1.67 mm, respectively (Table 7–2). The PCA gives rise to three types of branches:1 central perforating branches to the diencephalon and midbrain,2 ventricular branches to the choroid plexus and walls of the lateral and third ventricles and adjacent structures,3 and cerebral branches to the cerebral cortex and splenium of the corpus callosum. The central branches include the direct and circumflex perforating arteries, including the thalamoperforating, peduncular perforating, and thalamogeniculate arteries. The ventricular branches are the lateral and medial posterior choroidal arteries. The cerebral branches include the inferior temporal group of branches, which are divided into hippocampal and the anterior, middle, posterior, and common temporal branches, plus the parieto-occipital, calcarine, and splenial branches. The long and short circumflex, thalamoperforating, and medial posterior choroidal arteries arise predominantly from P1, and the other PCA branches most frequently arise from P2 or P3. The hippocampal, anterior temporal, peduncular perforating, and medial posterior choroidal arteries most frequently arise from P2A. The middle temporal, posterior temporal, common temporal, and lateral posterior choroidal arteries most frequently arise from P2P. The thalamogeniculate arteries arise only slightly more frequently from P2P than from P2A. The calcarine and parieto-occipital arteries most frequently arise from P3 (Figure 7–2A).
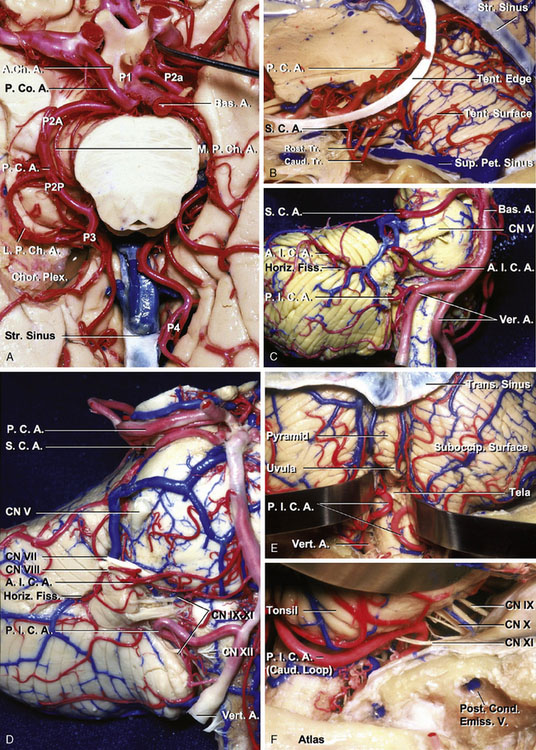
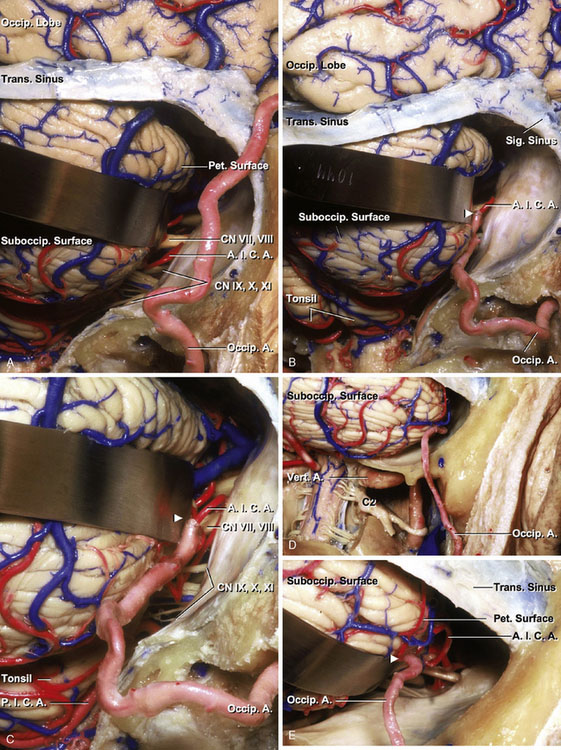
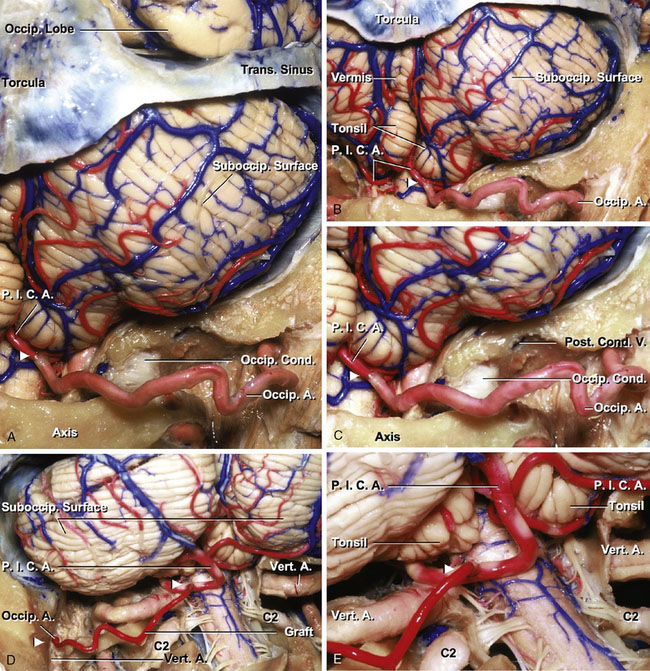
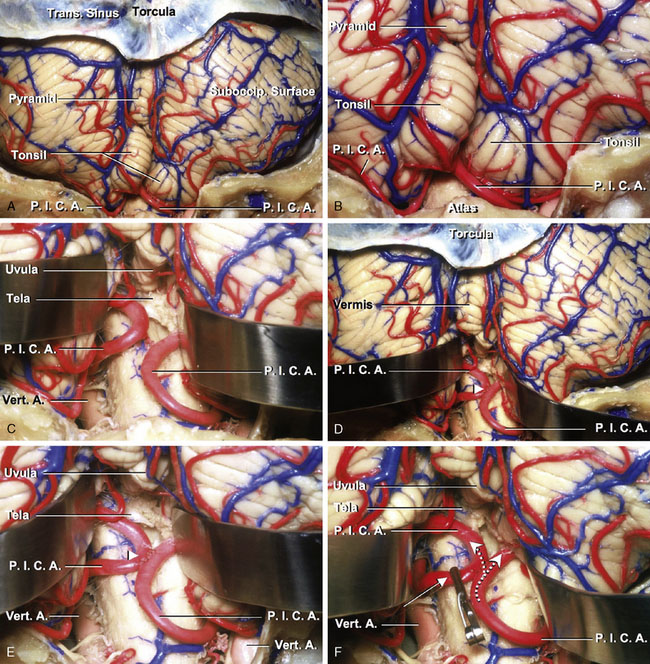
Figure 7–2 Arterial relationships. A and B. Inferior and anterosuperior views, PCA. The PCA arises at the basilar bifurcation, is joined by the PComA at the lateral margin of the interpeduncular cistern, encircles the brainstem passing through the crural and ambient cisterns to reach the quadrigeminal cistern above the edge of the cerebellar tentorium, and is distributed to the posterior part of the hemisphere. The PCA is divided into P1 to P4. The PCA gives rise to three types of branches:1 central perforating branches to the diencephalon and midbrain,2 ventricular branches to the choroid plexus and walls of the lateral and third ventricles and adjacent structures,3 and cerebral branches to the cerebral cortex and splenium of the corpus callosum. C and D. Anterolateral and anterior views of the brainstem and the cerebellum, SCA, AICA, and PICA. The SCA arises in front of the midbrain, usually from the basilar artery near the apex, and passes below the oculomotor nerve. Its proximal portion courses medial to the free edge of the tentorium cerebelli around the brainstem near the pontomesencephalic junction, and its distal part passes below the tentorium, making it the most rostral of the infratentorial arteries. All of the SCAs that arise as a single vessel bifurcate into two major trunks, one rostral and one caudal, most commonly near the point of maximal caudal descent of the artery on the lateral side of the brainstem (B). The AICA originates from the basilar artery, usually as a single trunk, and encircles the pons near the abducent, facial, and vestibulocochlear nerves. After coursing near and sending branches to the nerves entering the acoustic meatus and to the choroid plexus protruding from the foramen Luschka, it passes around the flocculus on the middle cerebellar peduncle to supply the cerebellopontine fissure and the petrosal surface. The PICA arises from the vertebral artery near the inferior olive and passes posteriorly around the medulla. E and F. Posterior and posterolateral views of the brainstem and the cerebellum and PICA. After passing the lateral aspect of the medulla, the PICA courses around the cerebellar tonsil and enters the cerebellomedullary fissure and passes posterior to the lower half of the roof of the fourth ventricle. On exiting the cerebellomedullary fissure, its branches are distributed to the vermis and hemisphere of the suboccipital surface. In the tonsillomedullary segment of the PICA, the loop passing near the lower part of the tonsil, referred to as the caudal loop, forms a caudally convex loop that coincides with the caudal pole of the tonsil, but it may also course superior or inferior to the caudal pole of the tonsil without forming a loop. The telovelotonsillar segment commonly forms a loop with a convex rostral curve, called the cranial loop. The apex of the cranial loop usually overlies the central part of the inferior medullary velum. A., artery; A.Ch.A., anterior choroidal artery; A.I.C.A., anterior inferior cerebellar artery; Bas., basilar; Caud., caudal; Chor. Plex., choroid plexus; CN, cranial nerve; Cond., condylar; Emiss., emissary; Fiss., fissure; Horiz., horizontal; L.P.Ch.A., lateral posterior choroidal artery; M.P.Ch.A., medial posterior choroidal artery; P.C.A., posterior cerebral artery; P.Co.A., posterior communicating artery; Pet., petrosal; P.I.C.A., posterior inferior cerebellar artery; Post., posterior; Rost., rostral; S.C.A., superior cerebellar artery; Str., straight; Suboccip., suboccipital; Sup., superior; Tent., tentorial; Tr., trunk; Trans., transverse; V., vein; Vert., vertebral.
Superior cerebellar artery
The superior cerebellar artery (SCA) arises in front of the midbrain, usually from the basilar artery near the apex, and passes below the oculomotor nerve. Its proximal portion courses medial to the free edge of the tentorium cerebelli around the brainstem near the pontomesencephalic junction, and its distal part passes below the tentorium, making it the most rostral of the infratentorial arteries. All of the SCAs that arise as a single vessel bifurcate into two major trunks, one rostral and one caudal, most commonly near the point of maximal caudal descent of the artery on the lateral side of the brainstem. The SCA is divided into four segments: anterior pontomesencephalic, lateral pontomesencephalic, cerebellomesencephalic, and cortical. The anterior pontomesencephalic segment begins at the origin of the SCA and extends below the oculomotor nerve to the anterolateral margin of the brainstem. The average diameter of the vessel was 1.67 mm in the segment (Table 7–2). The lateral pontomesencephalic segment begins at the anterolateral margin of the brainstem and frequently dips caudally onto the lateral side of the pons. This segment terminates at the anterior margin of the cerebellomesencephalic fissure. The average diameters of rostral and caudal trunks in this segment were 1.25 and 1.15 mm, respectively. If it is a single trunk, the average diameter was 1.51 mm in the segment (Table 7–2). The cerebellomesencephalic segment courses within the cerebellomesencephalic fissure, giving off branches that penetrate fissure’s opposing walls. The cortical segment includes the branches distal to the cerebellomesencephalic fissure that pass under the tentorial edge and are distributed to the tentorial surface (Figures 7–2B through 7–2D).
Anterior inferior cerebellar artery
The anterior inferior cerebellar artery (AICA) originates from the basilar artery, usually as a single trunk, and encircles the pons near the abducent, facial, and vestibulocochlear nerves. After coursing near and sending branches to the nerves entering the acoustic meatus and to the choroid plexus protruding from the foramen Luschka, it passes around the flocculus on the middle cerebellar peduncle to supply the cerebellopontine fissure and the petrosal surface. It commonly bifurcates near the facial-vestibulocochlear nerve complex to form a rostral and a caudal trunk. The AICA is divided into four segments: anterior pontine, lateral pontine, flocculonodular, and cortical. Each segment may include more than one trunk, depending on the level of bifurcation of the artery. The average diameters of the anterior pontine and cortical segments were 1.34 and 1.07 mm, respectively (Table 7–2). The most common pattern is for the AICA to supply the majority of the petrosal surface, but overlap of the SCA onto the upper part of the petrosal surface and the PICA onto the lateral part of the suboccipital surface is not uncommon, depending on the size of the AICA (Figures 7–2C and 7–2D).
Posterior inferior cerebellar artery
The PICA has the most complex, tortuous, and variable course and area of supply of the cerebellar arteries. The PICA arises from the vertebral artery near the inferior olive and passes posteriorly around the medulla. After passing the lateral aspect of the medulla, it courses around the cerebellar tonsil and enters the cerebellomedullary fissure and passes posterior to the lower half of the roof of the fourth ventricle. On exiting the cerebellomedullary fissure, its branches are distributed to the vermis and hemisphere of the suboccipital surface. Most PICAs bifurcate into a medial and a lateral trunk. The medial trunk supplies the vermis and adjacent part of the hemisphere, and the lateral trunk supplies the cortical surface of the tonsil and the hemisphere. The PICA is divided into five segments: anterior medullary, lateral medullary, tonsillomedullary, telovelotonsillar, and cortical. The anterior medullary segment begins at the origin of the PICA anterior to the medulla and extends backward to the inferior olivary prominence, passing near the hypoglossal rootlets. The lateral medullary segment begins where the artery passes the most prominent point of the inferior olive and ends at the level of the origin of the glossopharyngeal, vagus, and accessory rootlets. The tonsillomedullary segment begins where the PICA passes posterior to the glossopharyngeal, vagus, and accessory nerves and extends medially across the posterior aspect of the medulla near the caudal half of the tonsil. It ends where the artery ascends to the midlevel of the medial surface of the tonsil. This segment commonly passes medially between the lower margin of the tonsil and the medulla before turning rostrally along the medial surface of the tonsil. The loop passing near the lower part of the tonsil, referred to as the caudal loop, forms a caudally convex loop that coincides with the caudal pole of the tonsil, but it may also course superior or inferior to the caudal pole of the tonsil without forming a loop. The average diameter of the caudal loop was 1.68 mm, which is one of the largest distal vessels in the cerebellar arteries (Table 7–2). The telovelotonsillar segment begins at the mid-portion of the PICA’s ascent along the medial surface of the tonsil toward the roof of the fourth ventricle and ends where it exits the fissures between the vermis, tonsil, and hemisphere to reach the suboccipital surface. This segment commonly forms a loop with a convex rostral curve, called the cranial loop. The apex of the cranial loop usually overlies the central part of the inferior medullary velum, but its location varies from the superior to the inferior margin and from the medial to the lateral extent of the inferior medullary velum. The cortical segment begins where the trunks and branches leave the groove between the vermis medially and the tonsil and the hemisphere laterally, and includes the terminal cortical branches (Figures 7–2C through 7–2F).
Occipital artery
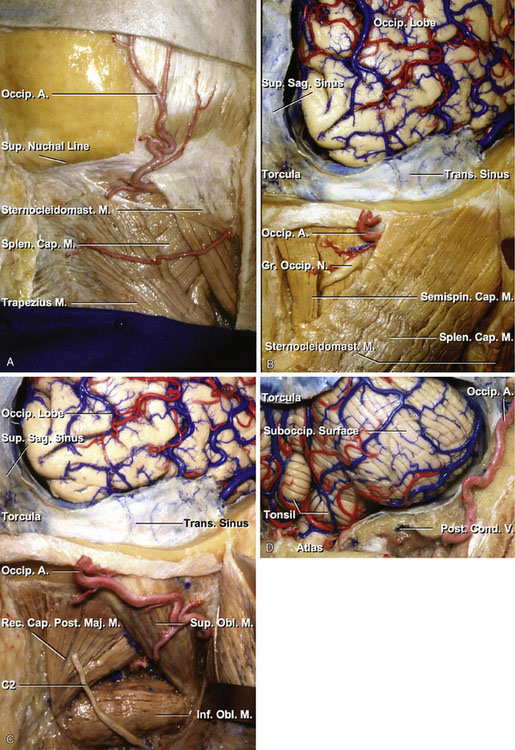
The occipital artery (OA) arises from the posterior part of the external carotid, opposite the external maxillary, near the lower margin of the posterior belly of the digastric muscle, and ends in the posterior part of the scalp. At its origin, it is covered by the posterior belly of the digastric and the stylohyoid muscles, and the hypoglossal nerve winds around it from behind forward; higher up, it crosses the internal carotid artery, the internal jugular vein, and the vagus and accessory nerves. It ascends to the interval between the transverse process of the atlas and the mastoid process of the temporal bone, and passes horizontally backward, grooving the surface of the mastoid bone, being covered by the sternocleidomastoid, splenius capitis, longissimus capitis, and digastric muscles, and resting upon the rectus capitis lateralis, the superior oblique, and semispinalis capitis muscles. The average diameter of the OA was 2.05 mm in the exit of the digastric groove (Table 7–2). It then changes its course and runs vertically upward, pierces the fascia connecting the cranial attachment of the trapezius with the sternocleidomastoid muscles, and ascends in a tortuous course in the superficial fascia of the scalp, where it divides into numerous branches, which reach as high as the vertex of the skull and anastomose with the posterior auricular and superficial temporal arteries. The average diameter of the OA was 2.01 mm at the level of the superior nuchal line (see Table 7–2). In three specimens (four sides), length of the OA from the exit of the digastric groove to the level of the superior nuchal line was measured. An average distance between them was 81.9 mm. Its terminal portion is accompanied by the greater occipital nerve (Figures 7–3A through 7–3D).
Graft
Artery graft, radial artery
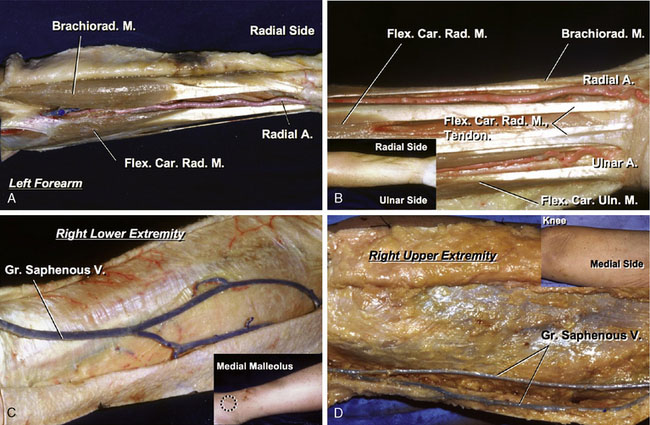
Before the dissection of the radial artery, an Allen test4 is necessary to check the collateral circulation to its territory. The radial artery is exposed at the wrist where it is located beneath the skin. The artery is traced proximally in the forearm, between the brachioradialis and the pronator teres muscles, up to the bifurcation of the brachial artery. Branches joining the artery are either ligated or bipolar-cauterized. Heparinized saline solution is used to wash out the blood inside the vessel and to check for leaks. The harvested radial artery graft is placed in heparinized saline (Figures 7–4A and 7–4B).
Vein graft, great saphenous vein
The vein is harvested from the leg or the thigh, depending on the caliber of the graft required, with care to avoid vascular injury and potential thrombosis. The incision is begun 1 cm anterior and 1 cm rostral to the medial malleolus and the vein is located in the subcutaneous tissue. The vein is traced superiorly toward the medial side of the knee for a distance of 15 to 20 cm, as needed. Branches joining the vein are either ligated or occluded with small clips. Before the saphenous vein is dissected, it is marked superficially with a skin marker to avoid later kinking. After complete dissection of the saphenous vein, it is ligated proximally and distally, sectioned, and removed. Heparinized saline solution is used to wash out the blood inside the vessel and to check for leaks. The harvested saphenous graft is placed in heparinized saline (Figures 7–4C and 7–4D).
Procedures for Cerebral Revascularization
STA-MCA anastomosis
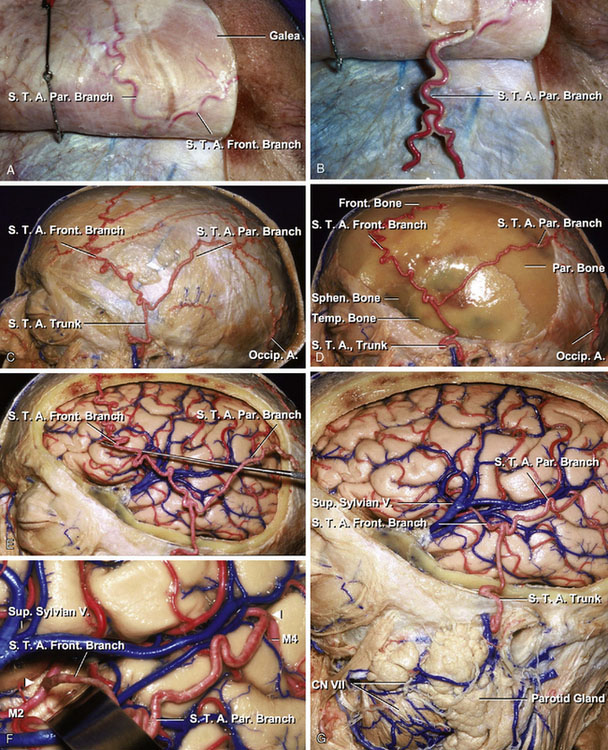
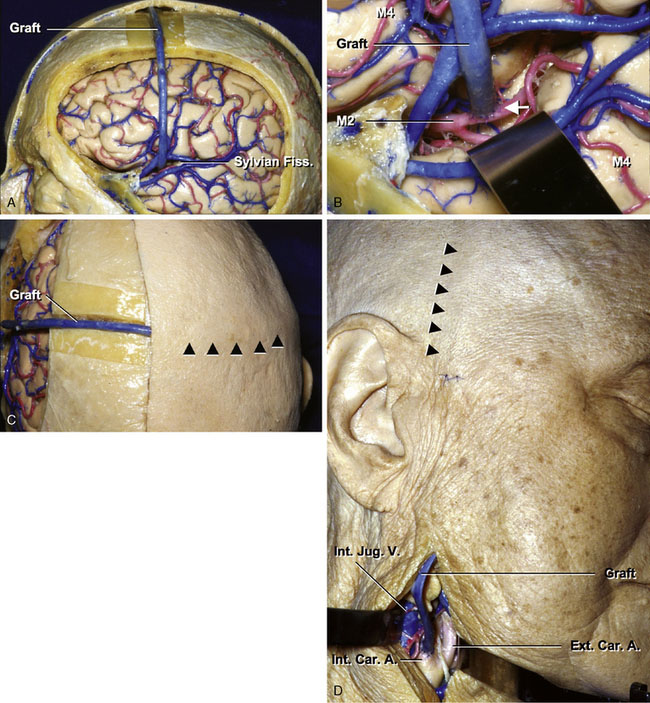
An STA to MCA anastomosis is the most common operative procedure performed. The patient is positioned supine, with the head turned so that the side of bypass is easily accessible. The STA courses in the subcutaneous tissue above the galea (Figure 7–5A). The STA is outlined either by palpation of the artery or with a portable Doppler device. The severed end of the STA is stripped of its fascial layer, and the vessel is exposed over 7 to 8 mm (Figure 7–5B). Once dissection of the STA is completed, the temporal muscle and fascia are incised at the site of the craniotomy (Figures 7–5C and 7–5D). Removing the bone flap and dura mater exposes cerebral cortex, where the anastomosis is performed. The frontal branch of the STA courses above the frontotemporal region, whereas the parietal branch of the STA passes above the parietotemporal region (Figure 7–5E). The recipient artery, which is over 1 mm in size and have a segment at least 6 to 8 mm for microanastomosis, is chosen according to its supplying area. One or two small branches from the recipient vessel may need to be coagulated and sacrificed. A colored background material is then placed beneath the recipient artery. The tip of the STA is then cut, making the fish-mouthed shape suitable for the anastomosis site. The recipient vessel is prepared by placing the temporary clips across the vessel and performing the small arteriotomy. Placing two stay-sutures, anastomosis of the STA to the recipient vessel is performed using interrupted 10-0 nylon sutures (Figures 7–5F and 7–5G).
MMA-MCA anastomosis
The MMA is anastomosed to the MCA in this procedure. After a frontotemporal craniotomy, the MMA is dissected from between the dural leaves. The anastomosis is accomplished with interrupted 10-0 nylon sutures (Figures 7–6A through 7–6D).
Side-to-side anastomosis (MCA and ACA)
This procedure is mainly used for cerebral revascularization for MCA branches and distal ACA (A3), combined with occlusion of their proximal branch vessels. Arteriotomies, 4 mm in length, were made on both donor and recipient arteries. Most difficult procedure here is to make a tight suture on a back wall. After two stay-sutures were placed in the distal and caudal edges of the arteriotomies, the back wall was sutured using a continuous running fashion from the intravascular side and then the anterior wall closed with interrupted sutures (10-0 nylon). Then, occlusion of the proximal vessel is completed. The distal part of the artery beyond the occlusion site is supplied by the adjacent artery (Figures 7–7A through 7–7F).
Short arterial interposition grafting anastomosis
The STA or occipital artery is used for an arterial graft. A section of STA graft approximately 4 cm in length is dissected from a skin flap and then both severed ends of the STA are stripped of their fascial layer. The graft is anastomosed between bilateral A3s with interrupted sutures (10-0 nylon). Then the right pericallosal and callosomarginal junction is occluded. The distal part of the right pericallosal artery is supplied by the left pericallosal artery (Figures 7–8A and 7–8B).
Short venous interposition grafting anastomosis
In this technique, a short vein graft was used to connect the donor and recipient arteries. Cerebral revascularization was performed using a shot saphenous vein graft or a superior temporal vein graft, 5 to 10 cm in length, extending from the STA trunk anterior to the ear to M2 or M4. An end-to-side anastomosis of the vein to the recipient artery was carried out using 10-0 nylon interrupted sutures. Following completion of the anastomosis at the distal end, an end-to-end anastomosis of the vein to the STA trunk immediately above the zygoma was carried out in using 8-0 nylon interrupted sutures (Figures 7–8C and 7–8D).
Cervical ECA/ICA–M2 anastomosis
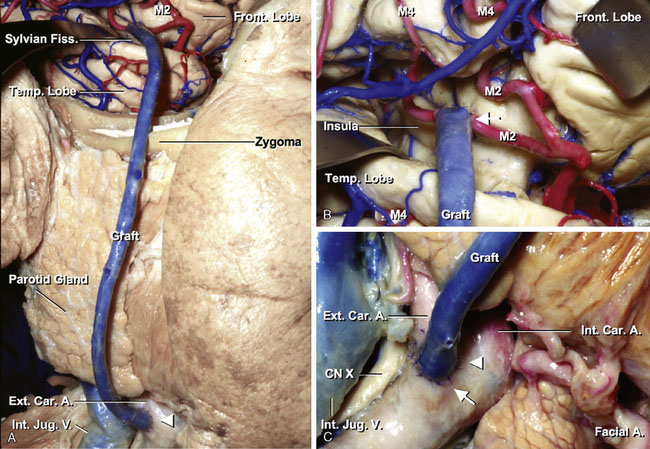
Either a saphenous vein graft or a radial artery graft is used for this bypass procedure. The common carotid artery, ICA, and ECA are exposed in the carotid triangle making a cervical incision along the anterior aspect of the sternocleidomastoid muscle. The zygomatic arch is drilled to fashion a conduit for the graft when it is placed in a preauricular position. After the craniotomy, wide dissection of the sylvian fissure is performed. The MCA trunk is identified and the secondary branches are dissected at their origin. The secondary vessel, which is thick and with the least number of side branches, is chosen and mobilized for the anastomosis. The diameter of the vessels in the insular area is normally larger than 1 mm. Care should be taken to avoid damaging the lenticulostriate perforators from the M2 branches. The main trunk of the MCA is occluded distal to the lenticulostriate perforators, and its branches are also occluded. The distal anastomosis between M2 and the graft is then performed in an end-to-side fashion with interrupted 10-0 nylon sutures. After completion of the distal anastomosis, the cervical ECA is occluded proximally and distally. The graft is pulled down through the subcutaneous tunnel. An arteriotomy, approximately 6 to 8 mm, is made on the ECA. The graft is anastomosed to the artery in an end-to-side fashion, using 7-0 nylon sutures (Figures 7–9A through 7–9C).
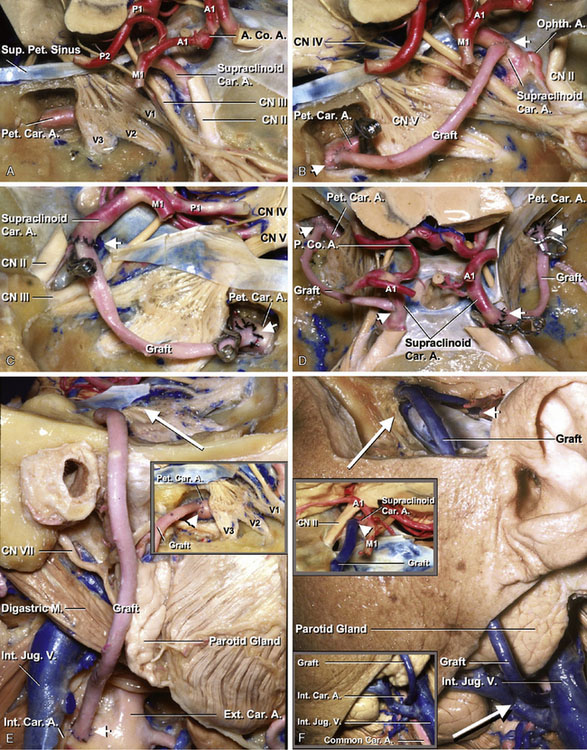
Petrous ICA–supraclinoid ICA anastomosis
After the pterional craniotomy, the sylvian fissure is opened widely. The sphenoid ridge, anterior clinoid, and proximal optic canal are drilled away. The distal dural ring is cut to provide room to place a clip on the ICA proximal to the ophthalmic artery. Sufficient ICA is then available between the ophthalmic and posterior communicating arteries for anastomosing a saphenous vein graft. Exposure of the petrous segment of the ICA is performed extradurally under the middle fossa dura. The dura is elevated along the course of the middle meningeal artery to the foramen spinosum. The middle meningeal artery is divided, and exposure is continued medially and anteriorly to the foramen ovale. A small area of the bone posterior to the third branch of the trigeminal nerve and medial to the foramen spinosum is drilled away to expose approximately a 1-cm length of the petrous ICA. The Eustachian tube and the tensor tympani muscle will be identified with attempted preservation. The saphenous vein is anastomosed to the petrous segment of the ICA in an end-to-side or end-to-end fashion between temporary clips. Interrupted sutures are made using 8-0 nylon. The other end of the saphenous vein graft is then anastomosed to the ICA in an end-to-side or end-to-end fashion between the ophthalmic and posterior communicating arteries, using 8-0 or 10-0 nylon depending on the wall thickness of the ICA and vein graft. The anastomosis on this site is often difficult, because sclerotic change of the arterial wall is frequently observed in the supraclinoid segment of the ICA (Figures 7–10A through 7–10D).
Cervical ICA–petrous ICA anastomosis
The cervical segment of the ICA in the carotid triangle and the horizontal segment of the petrous ICA are exposed as previously described. A radial artery graft is passed from the cervical area through beneath the subcutaneous tissue on the zygomatic arch toward the petrous ICA. The proximal end of the radial artery graft is anastomosed in an end-to-side or end-to-end fashion to the cervical ICA with interrupted sutures (7-0 nylon). The distal end of the radial artery graft is then anastomosed in an end-to-side fashion to the petrous ICA with interrupted sutures (8-0 or 6-0 nylon) (Figure 7–10E).
Cervical ICA–supraclinoid ICA anastomosis
The supraclinoid segment of the ICA can be used as the recipient vessel when the MCA is not suitable for anastomosis. The cervical and supraclinoid segments of the ICAs are exposed as previously described. The proximal end of the saphenous vein graft is anastomosed in an end-to-side or end-to-end fashion to the cervical ICA with interrupted sutures (7-0 nylon). The distal end of the saphenous vein graft is then anastomosed in an end-to-side fashion to the supraclinoid ICA with interrupted sutures (8-0 or 6-0 nylon). Frequently the intima of the ICA in the supraclinoid portion is atherosclerotic (Figure 7–10F).
Bonnet bypass
In the absence of an available ipsilateral donor artery in a patient requiring cerebral revascularization, an anastomosis between the contralateral STA or ICA/ECA and the branch of the MCA (M2 or M4) should be performed using the saphenous vein or radial artery graft. After a left frontotemporal craniotomy, the saphenous vein graft is anastomosed to the contralateral ICA. Then, the graft is placed over the skull through the subcutaneous tunnel and anastomosed to left M2 (Figures 7–11A through 7–11D).
STA–PCA anastomosis
The anastomosis is performed via subtemporal route. After positioning the temple region flat on the table, the STA is outlined either by palpation of the artery or with a portable Doppler device. Once dissection of the STA is completed, the temporal muscle and fascia are incised at the site of the craniotomy for the subtemporal approach. After the dura has been opened, the temporal lobe is elevated until the tentorial edge is identified. Care should be taken not to damage the vein of Labbé or posterior temporal veins as they enter the transverse sinus. The PCA is dissected free from the arachnoid as it courses around the cerebral peduncle. It is not necessary to expose the P1 segment of the PCA. The portion of the vessel that is isolated for temporary occlusion is the P2A segment of the PCA. A portion of the vessel about 1.5 cm in length, which is free of perforating vessels, is selected for the anastomosis. Care should be taken to avoid damaging these vessels that pass near the anastomotic site, including the long and short circumflex, thalamoperforating, medial and lateral posterior choroidal, hippocampal, anterior temporal, middle temporal, posterior temporal, common temporal, and peduncular perforating arteries. It may be difficult to see the PCA in the center of the operative field when the artery courses the upper part of the cistern around the brainstem. However, excess retraction of the temporal lobe should be avoided to protect the temporal lobe and bridging veins. After the PCA has been exposed, the severed end of the STA is stripped of its fascial layer, and the vessel is exposed over 7 to 8 mm. The tip of the STA is then cut making the shape suitable for the anastomotic site. The recipient vessel is prepared by placing the temporary clips across the vessel and performing the small arteriotomy. Placing two stay-sutures, anastomosis of the STA to the recipient vessel is performed using interrupted 10-0 nylon sutures (Figures 7–12A through 7–12C).
ECA–PCA anastomosis
Either a saphenous vein graft or a radial artery graft is used for this bypass procedure. The common carotid artery, ICA, and ECA are exposed in the carotid triangle, making a cervical incision along the anterior aspect of the sternocleidomastoid muscle. The zygomatic arch is drilled to fashion a conduit for the graft when it is placed in a preauricular position. After the craniotomy for the subtemporal approach, dissection of the P2 segment of the PCA is performed through the subtemporal route. The P2 segment of the PCA, which is thick and with the least number of side branches, is chosen and mobilized for the anastomosis. Care should be taken to avoid damaging the perforators. The distal anastomosis between P2 and the graft is then performed in an end-to-side fashion with interrupted 8-0 nylon sutures. After completion of the distal anastomosis, the cervical ECA is occluded proximally and distally. The graft is pulled down through the subcutaneous tunnel. An arteriotomy, approximately 6 to 8 mm, is made on the ECA. The graft is anastomosed to the artery in an end-to-side fashion, using 6-0 nylon sutures (Figures 7–12D and 7–12E).
STA–SCA anastomosis
The STA-SCA anastomosis is performed in the same operative view described in the STA-PCA anastomosis. After completion of dissecting the STA, the tentorial edge is identified through the subtemporal space. The edge of the tentorium is elevated and sectioned to provide more exposure. The flap of the tentorium is retracted and reflected, and is anchored to a more lateral aspect of the tentorium. Care is taken not to damage the fourth nerve, which courses beneath the tentorial edge. The SCA is then identified in its lateral pontomesencephalic segment. The artery usually has no perforating branches as it travels from the lateral portion of the midbrain to the superior portion of the cerebellum. If there are branches to the brainstem, a portion of the SCA beyond the area within the brainstem branch is selected for the anastomosis. As the SCA frequently divides into rostral and caudal branches in this segment, the larger of the two branches is used for the anastomosis. After the SCA has been exposed, the severed end of the STA is stripped of its fascial layer, and the vessel is exposed over 7 to 8 mm. The tip of the STA is then cut making the shape suitable for the anastomotic site. The recipient vessel is prepared by placing the temporary clips across the vessel and performing the small arteriotomy to equal the length prepared on the SCA. Placing two stay-sutures, anastomosis of the STA to the recipient vessel is performed using interrupted 10-0 nylon sutures (Figures 7–13A through 7–13E).
OA–AICA anastomosis
The course of the OA was outlined the scalp with a portable Doppler device. The OA can be dissected from its point of penetration through the occipital muscle to its most distal extent. It is important to dissect sufficient length of OA to be able to reach the cerebellar arteries; therefore, the dissection may be extended to the level of the mastoid. This dissection is generally difficult because the OA is more tortuous and deeper than the STA. The OA is gently retracted off the field, and a lateral suboccipital craniotomy is completed from the foramen magnum to the transverse sinus and from the edge of the mastoid to the midline. Generally, removal of the arch of C1 is not necessary. After opening the dura mater, the suboccipital surface of the cerebellum can be seen. With careful retraction of the cerebellum laterally, the AICA in the petrous surface is visualized (Figure 7–14A). To perform the anastomosis to the proximal portion of the AICA near the basilar artery would require continuous compression of the cerebellum with compromise of the collateral branches between AICA and PICA or AICA and SCA. Therefore, the flocculonodular (Figures 7–14B and 7–14C) or cortical segment (Figures 7–14D and 7–14E) of the AICA distal to the facial-vestibulocochlear nerve complex is selected for the anastomosis. The vessel is freed of the petrous surface of the cerebellum and is isolated. After the AICA has been exposed, the served end of the OA is stripped of its fascial layer, and the vessel is exposed over 7 to 8 mm. The tip of the OA is then cut making the shape suitable for the anastomotic site. The recipient vessel is prepared by placing the temporary clips across the vessel and performing the small arteriotomy to equal the length prepared on the AICA. Placing two stay-sutures, anastomosis of the OA to the recipient vessel is performed using 10-0 nylon interrupted sutures. Generally, the anastomosis procedure for the AICA is more difficult than that for the PICA because of depth of the operative field and smaller diameter of the recipient vessel.
OA–PICA anastomosis
After completion of dissecting the OA, a suboccipital craniotomy is performed described in the OA-AICA anastomosis. The arch of C1 is removed, if needed. After the dura is opened, exposure of the caudal loop of the PICA is obtained. Then, the vessel is freed from the arachnoid. After the PICA has been exposed, the severed end of the OA is stripped of its fascial layer, and the vessel is exposed over 7 to 8 mm. The tip of the OA is then cut making the shape suitable for the anastomotic site. The recipient vessel is prepared by placing the temporary clips across the vessel and performing the small arteriotomy to equal the length prepared on the PICA. Placing two stay-sutures, anastomosis of the OA to the recipient vessel is performed using interrupted 10-0 nylon sutures (Figures 7–15A through 7–15C).
Short arterial interposition grafting anastomosis (OA–PICA)
The STA or OA is used for an arterial graft. OA–AICA, OA–PICA, or vertebral artery to PICA anastomoses can be performed using an interposition graft. A section of STA or OA graft of approximately 8 cm in length is dissected from a skin flap and then both severed ends of the donor vessel are stripped of their fascial layer. The graft is anastomosed between the OA and the caudal loop of the PICA with interrupted 10-0 nylon sutures (Figures 7–15D and 7–15E).
Side-to-side anastomosis (PICA)
This procedure is chiefly used for cerebral revascularization for the distal portion of the PICA after occlusion of its proximal portion. The proximity and parallel courses of the tonsillomedullary and the telovelotonsillar segments of the PICAs permit their side-to-side anastomosis. Arteriotomies, 4 mm in length, were made on both donor and recipient arteries. The most difficult procedure here is to make a tight suture on a back wall. After two stay-sutures are placed, the back wall is sutured using a continuous running fashion from the intravascular side and then the anterior wall closed with interrupted sutures (10-0 nylon). The distal part of the artery beyond the occlusion site is supplied by the adjacent artery (Figures 7–16A through 7–16F).
Conclusion
Cerebral revascularization is an important procedure in the treatment of complex intracranial aneurysms, cranial base tumors, and certain ischemic diseases.5,6 In this study, we examined microsurgical procedures for cerebral revascularization in the anterior and posterior circulation and showed each procedure using cadaveric specimen. It will help not only surgeons, but also neurologists, neuroradiologists, and other physicians to further understand the mechanism of cerebral revascularization.
1 The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313(19):1191-1200.
2 Perlmutter D., Rhoton A.L.Jr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49(2):204-228.
3 Zeal A.A., Rhoton A.L.Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978;48(4):534-559.
4 Allen E.V. Thromboangitis obliterans: Methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am J Med Sci. 1929;178:237-243.
5 Kawashima M., Rhoton A.L.Jr, Tanriover N., et al. Microsurgical anatomy of cerebral revascularization, Part I: Anterior circulation. J Neurosurg. 2005;102(1):116-131.
6 Kawashima M., Rhoton A.L.Jr, Tanriover N., et al. Microsurgical anatomy of cerebral revascularization, Part II: Posterior circulation. J Neurosurg. 2005;102(1):132-147.