Chapter 6 Surgery of Ventilation and Mucosal Disease
Bilateral myringotomy with placement of ventilation tubes is the most common surgical procedure performed in the United States. An estimated 1.05 million tympanostomy tube procedures are performed annually in the United States.1 In addition, otitis media is the most common diagnosis of patients who make office visits to physicians in the United States—the diagnosis increased from about 10 million visits in 1975 to 25 million in 1990.2 The annual visit rate for children younger than 2 years statistically increased by 224% during one study period.3 Otitis media with effusion (OME) incurs approximately $5 billion annually in direct and indirect costs.1 Because of the magnitude of the disease and its impact on society, and conflicting reports over the most appropriate and cost-effective management of the problem,1,3–5 consensus on the treatment of OME has been difficult to achieve. Attempts have been made to devise an algorithm for the management of OME in young children,6 and these guidelines have been recently reviewed and updated (see later).7,8 This chapter reviews the terminology, epidemiology, pathophysiology, and medical and surgical treatment of OME.
TERMINOLOGY
Acute Otitis Media without Effusion
Acute otitis media (AOM) without effusion is characterized by an inflamed middle ear mucosa and tympanic membrane in the absence of an effusion; this can be seen in the early stages of AOM or during its resolution. The tympanic membrane appears dull, erythematous, hypervascular, and inflamed; normal landmarks are often lost. In infants and children, AOM without effusion is usually caused by the same organisms that are isolated from acute OME.9 Treatment principles are the same and are discussed later.
Acute Otitis Media with Effusion
Acute OME occurs most frequently in infants. Redness with or without bulging of the tympanic membrane, fever, irritability, and pain are the hallmark signs and symptoms. An older child with acute OME has a red tympanic membrane and middle ear effusion, but may not have pain or fever. The middle ear effusion is generally purulent. Casselbrant and associates10 reported a cumulative incidence of acute OME of 43% in a study of 198 newborns followed monthly until the age of 2 years. In the Greater Boston Otitis Media Study Group, infants had an average of 1.2 and 1.1 episodes per year, with 46% of children having had 3 or more episodes by the age of 3 years.11
Otitis Media with Effusion
Otitis media with effusion simply refers to fluid in the middle ear without signs or symptoms of ear infection. Asymptomatic OME can be classified as acute (<3 weeks), subacute (3 weeks to 3 months), or chronic (>3 months).12 Acute and chronic refer to the temporal course of the disease, not to severity. Synonyms of OME include secretory otitis media, nonsuppurative otitis media, or serous otitis media; the most commonly used term is OME.
Chronic Suppurative Otitis Media
Chronic suppurative otitis media (CSOM) is a stage of ear disease in which there is chronic infection of the middle ear and mastoid, and in which a central perforation of the tympanic membrane (or a patent tympanostomy tube) and discharge (otorrhea) are present.13 To meet the requirement for “chronic,” the otorrhea should be present for 6 weeks or longer. The infection involves the mastoid and the middle ear, and usually drains through a central perforation. Chronic otorrhea through a nonintact tympanic membrane (perforation or ventilation tube) may or may not be accompanied by cholesteatoma. Cholesteatoma may or may not result in CSOM. CSOM should not be confused with chronic OME; in the latter, no perforation is present, and the fluid is not purulent.
EPIDEMIOLOGY
Teele and colleagues11,14,15 found that 13% of children in their study groups had at least one episode of AOM by age 3 months; that percentage increased to 67% by 12 months. By age 3 years, 46% of children had three or more episodes of AOM. The highest incidence of AOM was found in children 6 to 11 months old. Most children with multiple recurrences of otitis media have their first episode before age 12 months.
An episode of AOM is a significant risk factor for the development of OME. Many investigators have documented persistent middle ear effusion after a single episode of AOM.14–17 Middle ear effusion has been shown to persist after an episode of AOM for 1 month in 40% of children, 2 months in 20%, and 3 or more months in 10%.15
Risk Factors
Risk factors for OME include male gender, recent upper respiratory infection, allergic rhinitis, first-degree relative with allergy, bottle feeding, cigarette smoke in the house, increased number of siblings in the house, and, probably the most important, daycare.18 Children in a public daycare facility have a fivefold increase in otitis media at age 2 years compared with children in home care.19 Whites and Hispanics are more susceptible than African Americans; Native Americans and Inuit are at even greater risk.
Microbiology
Bluestone and coworkers20 obtained aspirates of middle ear effusions by tympanocentesis in infants and children with AOM or OME. Of aspirates from ears with AOM, 35% grew Streptococcus pneumoniae, 23% grew Haemophilus influenzae, and 14% grew Moraxella catarrhalis.20 S. pneumoniae remains the most common bacterium causing AOM.21–23 Introduction of the pneumococcal vaccine may significantly reduce the incidence of pneumococcal disease, including otitis media.24–26
Asymptomatic middle ear effusion (OME) had been previously thought to be sterile. Newer, more sensitive cultures and the introduction of polymerase chain reaction (PCR) testing have shown bacteria and bacterial DNA in asymptomatic middle ear effusions.27 These investigators found that 77% of middle ear effusions had evidence of the three major organisms by PCR (with or without being culture positive), whereas only 28% were culture positive. The most common bacteria were H. influenzae (54.5%), M. catarrhalis (46.4%), and S. pneumoniae (29.9%).27 By comparison, in an earlier study of ears with OME, 30% of aspirates did not grow bacteria, 45% grew “other” strains, 15% had H. influenzae, 10% had M. catarrhalis, and 7% grew S. pneumoniae.20 Other bacteria include Staphylococcus aureus and gram-negative enteric bacilli. In infants younger than 6 weeks, gram-negative bacilli cause about 20% of AOM episodes.21
The bacteriology of CSOM with or without cholesteatoma is different. Most frequently isolated bacteria include Pseudomonas aeruginosa (most common), S. aureus, Corynebacterium, Klebsiella pneumoniae, and anaerobes.23 With better culture techniques, anaerobes have been increasingly isolated from chronic suppurating ears; these organisms include Bacteroides spp., Peptococcus spp., Peptostreptococcus spp., and Propionibacterium acnes.23
PATHOPHYSIOLOGY
Acute Otitis Media
AOM is principally a sequela of a viral upper respiratory infection. The upper respiratory infection impairs ciliary motility and breaks down mucosal barriers that prevent bacterial adherence and growth. Poor clearance of secretions results in stasis and allows bacteria to infect the host. Pathogenic bacteria that appear in the nasopharynx after an upper respiratory infection are the same as the bacteria cultured from middle ear effusions (S. pneumoniae and H. influenzae).28 The adenoid seems to be the source of infecting bacteria in middle ear disease; Pillsbury and associates29 showed higher bacterial colony counts in the adenoids of children with recurrent otitis media than in children undergoing adenoidectomy for adenoid hypertrophy without otitis media. During an upper respiratory infection, sneezing, blowing the nose, and swallowing in the presence of nasal obstruction may create a pressure differential between the nasopharynx and middle ear, forcing bacteria through the Eustachian tube into the middle ear.
Chronic Otitis Media with Effusion
Two theories have been proposed to account for the persistence of middle ear effusion in the absence of acute infection. As shown by the Boston Collaborative Group, persistent effusion is a natural sequela of acute middle ear infection.15 Effusion persists for 1 month in 40% of children after an episode of AOM, 2 months in 20%, and 3 or more months in 10%.15 Because pathogenic bacteria and bacterial DNA have been recovered from “nonacutely infected” fluid in the middle ear,21,27,30–31 it seems that Eustachian tube obstruction and retained secretions in these cases are the result of the acute infection, rather than the cause.
Eustachian tube dysfunction may be a primary disorder that causes acute and chronic OME. Primary Eustachian tube dysfunction results in underaeration and poor ventilation of the middle ear space; this leads to negative pressure in the middle ear with resultant transudation of fluid. Negative middle ear pressure also causes hypoxia and hypercapnia of the middle ear mucosa, resulting in goblet cell hyperplasia and hypersecretion.32,33 The result is a sterile fluid that becomes secondarily infected. The fluid resolves only after adequate ventilation is restored, either by return of Eustachian tube function or by placement of alternative ventilation, such as a ventilation tube.
According to Gates,34 the available evidence lends support to the theory that the secretory changes in the middle ear that exist in cases of chronic OME are the histologic sequelae of chronic infection, rather than a separate pathologic disorder. Most cases of chronic OME begin as acute infection of the middle ear; postinflammatory alterations in the mucosa of the middle ear and Eustachian tube lead to persistent effusion. Obstruction of the Eustachian tube is secondary to the infection and not the cause of it. Eustachian tube obstruction prevents clearance of secretions, impedes ventilation and drainage, and perpetuates the inflammatory process.
Allergic rhinitis has been recognized as a risk factor in the development of chronic OME. The actual prevalence of “allergic” chronic OME has been reported in the literature with a very broad range of 10% to 90%.32
Although it is beyond the scope of this chapter to provide extensive details of the type I hypersensitivity response, we provide a short summary. Atopic disease is initially characterized by antigen exposure and specific sensitization. Subsequent re-exposure results in mast cell degranulation and the release of numerous inflammatory mediators, including histamine, cysteinyl leukotrienes, and cytokines, and the infiltration of eosinophils, mast cells, basophils, and other inflammatory cells.35 These cells further the release of histamine and cysteinyl leukotrienes, resulting in increased mucosal blood flow, vascular permeability, and mucus production, and continued recruitment of inflammatory cells.
As in the nose, continued allergic inflammation in the middle ear is associated with a marked increase in the number of mucosal mast cells and cell bound IgE.36 In addition to degranulation, mast cells may also pre-sent antigen to B lymphocytes, dendritic cells, and monocytes, resulting in the further release of proinflammatory36 cytokines, including tumor necrosis factor, interleukin (IL)-1, and IL-6.36 IL-4 is released by activated mast cells and circulating basophils, and naïve T cells. IL-4 eventually mediates an isotypic switch from a predominantly TH1 lymphocytic profile of naïve T cells to the proinflammatory TH2 cells that are the hallmark of atopic disease. The further release of IL-5, IL-9, and IL-13 by TH2 lymphocytes results in the recruitment and activation of more basophils, mast cells, and eosinophils, insuring the development of a chronic allergic milieu.33
Nose
The nose is the target organ most commonly involved in the allergic reaction, and numerous publications have supported the concept that allergic inflammation resulting in nasal congestion may result in significant Eustachian tube obstruction.37–40 The sensitized mucosa of the nose, adenoid bed, or nasopharynx exposed to allergens releases cytokines, resulting in increased mucus secretion, edema, and inflammation around the torus tubarius. The resulting Eustachian tube obstruction has a negative effect on middle ear ventilation, resulting in the production of chronic OME.
Middle Ear Space
Although it is intuitively tempting to consider the middle ear the most likely target organ of an allergic reaction resulting in the production of chronic OME, there is likely little antigen exposure to the middle ear mucosa. The Eustachian tube is normally closed, unless one is swallowing or yawning, which would make it much less likely than the nose to allow physical contact with inhaled aeroallergens. Supporting this limited role, Bernstein39 suggested, based on a study determining the local production of IgE in middle ear mucosal biopsy specimens of atopic children undergoing pressure equalization tube insertion, that the middle ear mucosa likely serves as the target organ of an allergic reaction less than 10% of the time. Other authors disagree, however. Eustachian tube dysfunction has been reported from transtympanic challenge on antigen in sensitized animals.41,42 Ebert and colleagues43 showed a similar finding with intratympanic histamine mast cells found to be present in the middle ear effusions and biopsy specimens of children with chronic OME, suggesting local inflammation and reaction.44–47
Eustachian Tube
Several mechanisms for Eustachian tube dysfunction have been proposed. First, congestion of the nasal mucosa may produce a retrograde spread of edema and Eustachian tube dysfunction. Second, poor mucociliary function, either innate or resulting from allergic or other inflammatory etiologies, may lead to retention of secretions resulting in obstruction of the Eustachian tube.48,49 Third, inhalation of aeroallergens with subsequent direct allergic inflammation within the Eustachian tube may produce venous engorgement and hypersecretion of mucus, with the subsequent obstruction affecting gas exchange in the middle ear space.50 The resulting negative pressure allows a transudation of fluids into the middle ear space by the interruption of cellular tight junctions.39,50 Later, persistent obstruction of the Eustachian tube with mucus results in chronic middle ear inflammation, with resulting mucosal metaplasia and increased glandular activities of goblet cells.39,50
Increasingly, physicians who treat allergic patients embrace the concept of one airway, whereby the inflammatory response in one portion of the airway may also lead to inflammatory changes in other portions. The classic example is the recognition of the intimate relationship existing between the upper and lower airways. In a study of atopic subjects, inhaled allergen challenge isolated to the nose produced inflammatory changes in the upper and lower airways. These changes included increased adhesion molecules, increased bronchial hyperreactivity, and eosinophil infiltration.51 Although it is important that we continue research to define the immunology involved in the subset of patients with chronic OME who have allergy as an underlying etiology, we should keep in mind that allergy does affect the common airway, and that lessening significant allergic inflammation in the entire anatomic area would be beneficial.
TREATMENT AND PATIENT SELECTION
Acute Otitis Media and Recurrent Acute Otitis Media
For a single episode of AOM, antimicrobial therapy targets the most common offending pathogens: S. pneumoniae, H. influenzae, and M. catarrhalis. We recommend a 10-day course of amoxicillin as first-line empirical therapy. Clinical practice guidelines from the Agency for Healthcare Research and Quality (AHRQ) argue for observation without antibiotic therapy in selected patients with AOM (Table 6-1).7 Studies have shown an increase in the β-lactamase-producing organisms, H. influenzae and M. catarrhalis.19,52 β-Lactamase renders the organism that produces it resistant to penicillin (and ampicillin). Persistent or recurrent AOM may be secondary to a β-lactamase-producing organism and requires a broader spectrum antibiotic53; good choices in this setting include cefuroxime, erythromycin-sulfisoxazole, trimethoprim-sulfamethoxazole, amoxicillin-clavulanate, and cefaclor. Antipyretics (but not aspirin) are also indicated for children with AOM.
TABLE 6-1 Criteria for Initial Antibacterial Agent Treatment or Observation in Children with Acute Otitis Media
| Age | Certain Diagnosis | Uncertain Diagnosis |
|---|---|---|
| <6 mo | Antibacterial therapy | Antibacterial therapy |
| 6 mo–2 yr | Antibacterial therapy | Antibacterial therapy if severe illness; observation option if nonsevere illness |
| ≥2 yr | Antibacterial therapy if severe illness; observation option if nonsevere illness | Observation option |
From American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media: Diagnosis and management of acute otitis media. Pediatrics 113:1451-1465, 2004.
A child (or adult) with an infectious complication of otitis media requires more aggressive therapy, including intravenous antibiotics and possible surgical intervention. This subject is beyond the scope of this chapter and is discussed in Chapter 19.
Placement of tympanostomy tubes is effective treatment in the prevention of recurrent otitis media. Many authorities accept four episodes of AOM in 6 months as a criterion for tympanostomy tube placement. Gebhart54 was the first to show a reduction in the number of new episodes of AOM after insertion of tympanostomy tubes.
The role of adenoidectomy in the treatment of recurrent AOM is controversial. Although Paradise and coworkers55 found a significant reduction (28% and 35%) in the incidence of AOM in the first and second years after adenoidectomy, a formal study examining the role of adenoidectomy in the treatment of recurrent AOM has not been done. Results of studies of chronic OME and adenoidectomy may or may not be applicable for patients with recurrent AOM. For patients with recurrent AOM and persistent effusion, adenoidectomy is an appropriate surgical treatment (see the following section on chronic OME).
Chronic Otitis Media with Effusion
As mentioned, 10% of children with AOM have persistent middle ear effusion 3 or more months after resolution of the acute infection.15 Most children clear their effusion within 1 to 2 months; these patients need no further therapy. The few patients who retain fluid in the middle ear longer than 3 months are at risk for other sequelae, including hearing loss, language delay, vertigo or imbalance, tympanic membrane changes (including atelectasis or retraction pockets or both), further middle ear pathology (including ossicular problems and adhesive otitis), and discomfort with nighttime wakefulness and irritability.
Numerous treatment strategies have been proposed for chronic OME: antimicrobial therapy, antihistamines/decongestants, corticosteroids, tympanostomy tubes with or without adenoidectomy, and mastoidectomy. Updated clinical practice guidelines from the AHRQ do not recommend antibiotics, antihistamines, decongestants, or corticosteroids for the treatment of chronic OME.8 These modalities and allergic strategies are now reviewed.
Antimicrobial Therapy
More sensitive techniques (e.g., PCR) have shown bacterial DNA in middle ear effusions previously thought to be “sterile” or culture negative. Prolonged antibiotic therapy theoretically eradicates the organism and eliminates the chronic source of effusion. Some studies have shown the efficacy of antibiotics in OME.21,56 Despite these studies, theoretical and practical arguments can be made against their use in chronic OME. Clinical experience indicates that the utility of antibiotics is reduced as the number of treatment courses increases. Children receiving four or more courses of antibiotics over a 3 to 4 month period are most likely not going to resolve their effusion with medical management. Other adverse effects of prolonged antimicrobial therapy include development of anaphylaxis and allergic reactions; hematologic disorders; and the emergence of resistant organisms, a serious worldwide problem best shown by the development of resistance to penicillin by S. pneumoniae. Finally, Rosenfeld and Post found through a large meta-analysis of existing studies that the benefit of antimicrobial therapy in chronic OME is slight.
Antihistamines and Decongestant Therapy
Oral antihistamine and decongestant combinations and monotherapy have not been shown to be beneficial in the treatment of chronic OME.56 The AHRQ clinical practice guideline does not recommend these agents for chronic OME.8 A possible exception may be in an adult patient with allergen-induced Eustachian tube dysfunction. Stillwagon and colleagues57 investigated the effects of pharmacotherapy on allergen-induced Eustachian tube dysfunction. In this study, adults with a history of seasonal allergic rhinitis to ragweed pollen received either a combination of antihistamine and decongestants or placebo for 7 days followed by an intranasal challenge with ragweed pollen. Eustachian tube obstruction occurred in fewer patients receiving active treatment than in patients receiving placebo. They concluded that pre-exposure treatment with antihistamines in patients with allergic rhinitis may help decrease the risk of developing Eustachian tube dysfunction. There are no studies to date on a possible role of intranasal antihistamines on treatment of middle ear effusion.
Antileukotrienes
Antileukotrienes have not been well studied for a possible role in the treatment of chronic OME. Combs58 found a significant decrease, however, in the duration of middle ear effusion in otitis-prone children treated with montelukast after AOM compared with a control group. The reader is advised to take into account the paucity of research and the expense of this relatively safe medication when debating its possible merit in treatment.
Corticosteroid Therapy
Steroid therapy for chronic OME has been controversial. Lambert59 found no difference in outcomes between the steroid group and the control group with chronic OME. At this time, the AHRQ guideline does not recommend steroid therapy for chronic OME.8
Treatment for Food Allergy
The younger the child with suspected allergies, the more likely the antigen will be ingested rather than inhaled. Juntti and associates60 found that 34% of children with allergic rhinitis or asthma who also had cow’s milk allergy had recurrent OME compared with 13% of children who had allergic rhinitis and no diagnosed milk allergy.
Immunotherapy
Many studies have suggested that more than 70% of children with chronic OME are considered atopic, based on skin tests or in vitro testing.61–64 Immunotherapy for inhalant allergies has been found to be very efficacious in symptom reduction and in lessening the progression of allergic disease. There have been no studies to date of the same rigor on the possible role of immunotherapy for treatment of allergic middle ear disease. The studies that do report a very high (≥75%) rate of resolution of OME with immunotherapy or dietary elimination virtually never have a sufficient (or any) control group, and have varying definitions of allergy and mode of diagnosis, as opposed to studies that have been published on the role of immunotherapy in treatment for allergic rhinitis.37,61,62,65,66
Surgical Therapy
Armstrong67 introduced ventilation tube placement in 1954 as a treatment for OME. The ventilation tube acts as an artificial Eustachian tube, aerating the middle ear and equilibrating middle ear pressure with atmospheric pressure. The pathophysiology of chronic OME involves Eustachian tube dysfunction and reflux of nasopharyngeal organisms. Ventilation tubes are aimed at correcting Eustachian tube dysfunction. Children with tubes in place can still get otitis media; the acute infection is not painful because the infected effusion is allowed to pass through the tube and out of the middle ear. The effusion also is not associated with hearing loss; correction of hearing loss is one of the most important goals of surgical therapy. Tube insertion with or without adenoidectomy has been shown to improve conductive hearing loss secondary to OME, and to decrease the amount of time spent with middle ear effusion.21 Placement of tubes is often a clinical judgment based on experience and is addressed on a case-by-case basis. Nevertheless, the AHRQ clinical practice guidelines do offer evidence-based recommendations for tympanostomy tube placement (see later).8
Adenoidectomy
Nasopharyngeal reflux of secretions and microorganisms into the middle ear plays a large role in the pathophysiology of chronic OME. Adenoidectomy is designed to remove the source of the infecting microorganisms. Three landmark studies have shown the efficacy and low morbidity associated with adenoidectomy.4,55,68 Adenoidectomy is effective treatment for chronic OME, and significantly reduces its morbidity. Its effect is independent of adenoid size. It is argued that the small, “smoldering” adenoid chronically harbors bacteria and is a major contributor to OME. The decision for adenoidectomy should be based on the severity and persistence of the middle ear disease, not the size of the adenoid. Nasal obstruction with adenoid hypertrophy stands alone as an indication. Given the increased cost and slightly increased risk to the patient, Paradise and Bluestone69 have argued for adenoidectomy only in recurrent cases.
Much of the literature published on the role of adenoidectomy in OME has been in children 4 to 8 years old.8 Nevertheless, adenoidectomy has been shown to be safe in children older than 18 months,5 and may be effective in younger, high-risk children. In the San Antonio study, the effect of adenoidectomy was greater for younger children.4 We recommend adenoidectomy for recurrent cases—cases in which the child (>4 years old) needs a second set of tubes.
PATIENT COUNSELING
Benefits
Hearing improvement and reduction in the number of subsequent episodes of ear infections are the chief benefits of tympanostomy tube placement. Hearing improvement speeds and sharpens language and developmental maturation. If the child develops otitis media, tympanostomy tubes also eliminate pain because the infected fluid is allowed to drain out of the middle ear space. Studies have also shown improvement in vestibular function after tympanostomy tube placement.79–81 Finally, reducing the number of secondary problems of recurrent ear infections means less time lost from work for the parents, fewer (if any) courses of antibiotics, and reduction in the cost to the parents of multiple courses of antibiotics. Surgery has been shown to be a cost-effective treatment for children in whom medical therapy fails.1
SURGICAL TECHNIQUE
Instruments
Tympanostomy Tubes
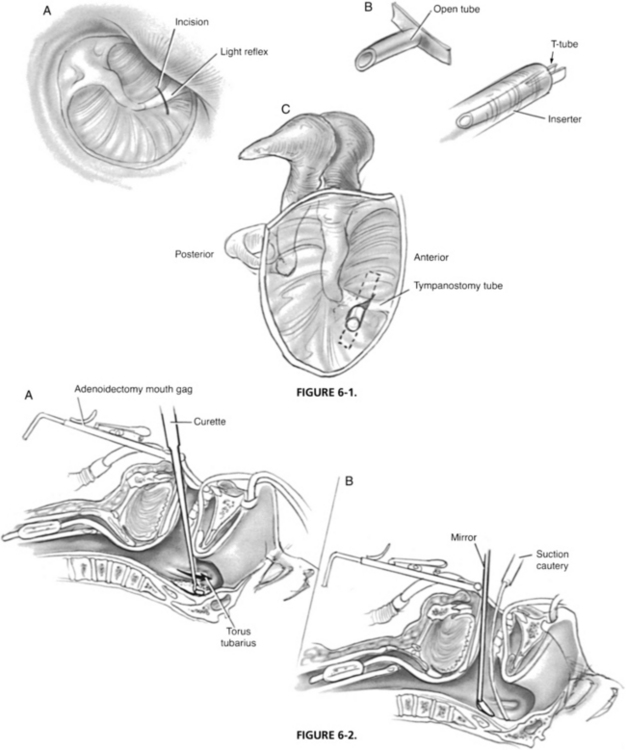
A set of metal specula, cerumen curettes, and several sizes of suction cannulas (Baron Nos. 3, 5, and 7) are necessary. The operating microscope is also essential. Sterile myringotomy knives come in various shapes and angles. It is useful to choose a knife with a blade width the size of the tube to aid in making the correct dimensions of the incision. Tubes are placed with a cup or alligator forceps and positioned with a Rosen needle. Placement of long-stemmed T-tubes is facilitated by the use of an inserter in which the tube is positioned with the short arms of the tube folded forward (Fig. 6-1B). Care is taken to minimize trauma to the external auditory canal, drum, or ossicles.
Technical Details
Tympanostomy Tube Insertion
The ear canal is gently cleaned of all wax and debris. Contact with the anterior bony canal wall is avoided because of risk of bleeding. The tympanic membrane is inspected, and the short process of the malleus is identified. This is a constant landmark and may be the only one available in cases of acute infection. The tympanic membrane is incised anteroinferiorly by using an incision that parallels the fibrous annulus (see Fig. 6-1). Use of a radial incision is satisfactory, but may be limited by an overhanging anterior canal wall. Posterior incisions should be avoided because they place the ossicles at risk. The incision is gently spread open. Care is taken to avoid any major vessel in the tympanic membrane to prevent hemorrhage into the layers of the eardrum. This bleeding into the drum is thought to predispose to tympanosclerosis.
Laser Myringotomy
The laser has become a useful, albeit expensive, tool in the management of chronic OME. Advantages of the laser include office-based application, ease of use, and the ability to place a controlled perforation in the tympanic membrane that stays open for a medium length of time (2 to 6 weeks). Using the CO2 laser at 12 W with a single 100 ms pulse through a 200 mm objective, Goode73 reliably placed 1.5 to 2 mm perforations in the tympanic membranes of 10 subjects. Ten of the 11 ears healed within 6 weeks. Tube placement was avoided.
Marchant and Bisschop74 performed 20 consecutive CO2 laser myringotomies on ears with chronic OME. All myringotomies closed within 4 weeks, with an average closing time of 17 days; 60% of cases of chronic OME were cured after 3 months. CO2 laser myringotomy has application in clinical situations when middle ear ventilation is needed for a medium length of time (weeks) without having to place a ventilation tube. Disadvantages include cost, need for extra machinery that can be bulky, required maintenance, instruction on use and technique, and office space. Local anesthesia (iontophoresis or topical phenol application) is still required. Most otologists prefer simple cold-steel myringotomy with or without tube placement, but CO2 myringotomy is an alternative; only time and experience will tell whether this technique will have widespread application and use.
Adenoidectomy
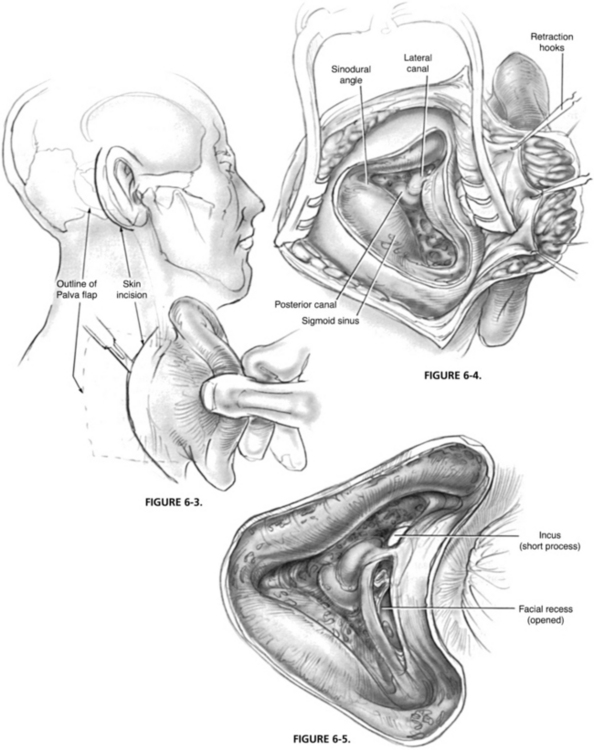
The adenoid is excised with curved curettes of various sizes (Fig. 6-2A). The curette is seated high in the nasopharynx, and the adenoid pad is resected with a down-sweeping motion on the curette. Care must be taken to avoid injury to the prevertebral fascia and muscles, which may cause excessive bleeding. The nasopharynx is palpated for residual adenoid tissue; a second or third pass may be necessary. Curved biting forceps are useful to remove tissue inaccessible by the curette. The mirror is again used to inspect the site. Curettage of the tissue in the fossa of Rosenmüller is not done because it may lead to scar tissue formation and contracture that might result in stenosis or Eustachian tube reflux or both. Direct injury to the Eustachian tube also is avoided. The goal of surgery is the complete removal of the midline adenoid pad to achieve smooth re-epithelialization of the nasopharynx.
Bleeding usually stops quickly; tonsil sponges are used to pack the nasopharynx for hemostasis. The nasal cavities and nasopharynx are irrigated with warm saline. A malleable suction cautery can be used for precise coagulation, but its use is cautioned because of the risk of stenosis (see Fig. 6-2B).
Mastoidectomy
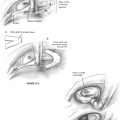
The postauricular incision is based about 1 fingerbreadth behind the postauricular crease, roughly paralleling the free margin of the helix (Fig. 6-3). The further posterior the incision, the greater the ease of inspecting the middle ear and Eustachian tube through the facial recess. The incision is carried slightly anterior in its inferior dimension to allow the ear to be retracted forward easily. In a child, care is taken not to extend the incision beyond the mastoid tip, which is more superior than in an adult. Carrying the incision more inferior or anterior puts the facial nerve at risk. Superiorly, the temporalis fascia is identified, and a piece of fascia is harvested if needed. The fascia identifies the plane of dissection. The ear is held forward with a self-retaining retractor. The linea temporalis is palpated, and an incision is made down to the bone along this line from anterior to posterior. A second incision is made perpendicular to the first in a curvilinear fashion down to the mastoid tip. The Lempert elevator is used to elevate the periosteum to identify the cribriform area and posterior canal wall. A small elevator is next used to elevate the vascular strip out of the canal. The vascular strip is held forward with the ear under the self-retaining retractor. The tympanic membrane is carefully elevated, and the middle ear is inspected.
A large cutting burr and continuous suction-irrigation are used to remove the lateral mastoid cortex. Important landmarks to identify include the posterior bony canal wall anteriorly, the tegmen mastoideum superiorly, the sigmoid sinus posteriorly, and the digastric ridge inferiorly. Care is taken not to expose the dura of the middle cranial fossa or the sigmoid sinus. When these lateral landmarks have been identified, the dissection is continued medially under the microscope. Körner’s septum is opened medially, and the mastoid antrum is identified. This dissection is carried anteriorly to open the aditus ad antrum and attic. The fossa incudis and short process of incus are carefully uncovered. The short process of the incus should be seen refracted through water. The short process marks the level of the facial recess. All air cells of the mastoid cortex should be taken down to reduce the surface area of the system. The bony plates over the posterior and middle fossa dura are skeletonized to form a smooth surface (Fig. 6-4). Care is taken to avoid exposing dura. The mucosa regenerates into a single large cavity.
The facial recess is opened into the middle ear by the use of progressively smaller diamond burrs. The plane of the short process of the incus leads to the facial recess (Fig. 6-5). When the fallopian canal and chorda tympani nerve are identified, the dissection is carried between them medially to open into the middle ear. Coupled with the tympanotomy, all parts of the mesotympanum can be inspected. The ossicular chain is palpated, and any hyperplastic mucosa, granulation tissue, or secretory tissue is removed. If bone of the middle ear/promontory is exposed, a piece of absorbable gelatin film (Gelfilm) is placed through the facial recess and across the promontory toward the Eustachian tube at the end of the procedure to prevent adhesions and to keep the recess open.
RESULTS
Goals of surgery include hearing improvement, reducing the time spent with middle ear effusion, and reducing the number of recurrences of middle ear effusion. Tube insertion with and without adenoidectomy has been shown to improve conductive hearing loss secondary to chronic OME and to decrease the amount of time spent with effusion.21
Gates and colleagues4 assigned 491 children, 4 to 9 years old with OME persisting 60 days or more after repeated medical therapy, to various combinations of myringotomy, tympanostomy tube insertion, and adenoidectomy for chronic OME. They found that time with recurrent middle ear effusion was decreased by 29% in the tympanostomy tubes–only group, by 38% in the adenoidectomy-plus-myringotomy group, and by 47% in the adenoidectomy-plus-tympanostomy tubes group. Hearing was equivalent in all groups except the myringotomy-alone group; hearing in this group was significantly worse. Surgical retreatments were necessary more often in children initially treated with myringotomy alone (36%) or tympanostomy tubes alone (20%) than in children treated by adenoidectomy and myringotomy (10%) or adenoidectomy plus tympanostomy tubes (10%). The number of repeat operations in the two adenoidectomy groups was significantly less than in the two groups without adenoidectomy (P < .001).4
Paradise and associates55 randomly assigned patients who had previously undergone tympanostomy tube placement and had recurrent middle ear disease into either an adenoidectomy or control group. During the first and second years of follow-up, the adenoidectomy group had 47% and 37% less time with otitis media than the control patients.
COMPLICATIONS AND MANAGEMENT
Tympanostomy Tubes
Otorrhea
The most common sequela of tympanostomy tubes is purulent otorrhea. In the Gates study,4 otorrhea occurred one or more times in 22% of the myringotomy-alone group, 29% of the tympanostomy tubes group, 11% of the adenoidectomy-myringotomy group, and 24% of the adenoidectomy-tympanostomy tubes group. Some cases of otorrhea are due to water contamination; others are the result of AOM. Some cases also involve an inflammatory reaction to the tube itself. Treatment is the same: a topical polymicrobial-steroid suspension with or without an oral antibiotic, along with aural hygiene in the office. Most cases clear quickly. In recalcitrant cases, the tube is removed, and cultures are done. Failure to clear persistent otorrhea after maximal medical therapy is an indication for tympanoplasty with mastoidectomy.
Persistent Perforation
Tympanostomy tubes extrude within 1 to 2 years of insertion. Depending on the tube, 1% to 15% of cases result in a persistent perforation. Older children may be able to tolerate attempts to close the perforation in the office with freshening of the edges and placement of a paper patch. Other children are good candidates for a fat plug myringoplasty75 or more formal myringoplasty/tympanoplasty under anesthesia.
Adenoidectomy
Bleeding
The most common complication of adenoidectomy is postoperative bleeding. The incidence is low, however: of 250 cases done by 13 surgeons, only 1 child required operative treatment for bleeding, and none needed transfusion.4 Helmus and colleagues85 noted that only 4 patients in 1000 (0.4%) bled after outpatient adenoidectomy; all instances occurred in the first 6 postoperative hours and were managed without transfusion. Return trip to the operating room for bleeding involves the same positioning as for routine adenoidectomy. Irrigation with suction cautery generally controls bleeding.
ALLERGY TREATMENT
Children with symptomatic food or inhalant allergy deserve therapy whether they have OME or not. Because most patients with OME have had prior nasal infection, and children with nasal allergy have a higher prevalence of infection, allergy evaluation is appropriate for children with OME who also have nasal symptoms. Gates and colleagues4 found a lower incidence of allergy in their subjects with OME, however, than in the general population. Although a cause-and-effect relationship between nasal allergy and OME has not been shown, the surgeon should inquire about allergic symptoms to provide proper therapy to patients with dual problems.
BIOFILMS
It has been known for more than a decade that culture-negative OME is not sterile, or bacteria negative.87-89 Sophisticated techniques including in situ hybridization, PCR, and blotting techniques have shown bacterial DNA, RNA, and proteins in “culture-negative” middle ear fluid.81
Biofilms are implicated in chronic infections of indwelling catheters, including line sepsis. The treatment is removal of the foreign body. In otolaryngology, biofilms have been proposed to cause chronic adenoiditis/tonsillitis, chronic rhinosinusitis, and chronic OME.82
Tympanostomy tube otorrhea has been directly linked to biofilm formation on the tube.83 After the demonstration that biofilms form in the middle ear in an animal model of OME,84 biofilm formation has more recently been shown in the middle ear mucosa of children with chronic OME.85 Resistant to antibiotics, biofilms are a proposed mechanism for chronic OME.
GUIDELINES FOR THE MANAGEMENT OF ACUTE OTITIS MEDIA AND OTITIS MEDIA WITH EFFUSION
In 2004, AHRQ and the American Academies of Pediatrics, Family Medicine, and Otolaryngology–Head and Neck Surgery published clinical practice guidelines for the management of AOM and chronic OME. The guidelines for the management of AOM recommend judicious use of antibiotics and promote observation as a viable management strategy (see Table 6-1).7
The AHRQ and Academies of Pediatrics, Family Medicine, and Otolaryngology–Head and Neck Surgery also published clinical practice guidelines for the management of children 2 months through 12 years with OME.8 These guidelines stress the importance of distinguishing OME from AOM; the role of pneumatic otoscopy in the diagnosis of OME; and the importance of documenting laterality, duration, and presence and severity of associated symptoms at each assessment of a child with OME.
The guidelines recommend that clinicians should distinguish the child with OME who is at risk for speech, language, or learning problems from other children and recognize their need for more prompt intervention. Children not at risk can be managed with watchful waiting for 3 months from the onset of effusion; antihistamines, decongestants, antimicrobials, and steroids do not have long-term efficacy, and are not recommended for the routine treatment of OME. Hearing and language testing are recommended for children with OME that persists longer than 3 months, or at anytime a significant hearing loss or language delay is suspected.8
Children with persistent OME should be observed at 3 to 6 month intervals until the effusion clears, significant hearing loss (>30 dB hearing level in the better hearing ear) is identified, or structural abnormalities of the eardrum or middle ear are suspected. When a child becomes a surgical candidate, tympanostomy tube insertion is the preferred initial procedure. Repeat surgery should include adenoidectomy with myringotomy, with or without tube insertion. No recommendations were made with regard to alternative medicine or allergy management.8 These evidence-based evaluations and recommendations are expected to sharpen the focus on managing AOM and OME, and are likely to result in more careful use of antimicrobial therapy with less bacterial resistance and better patient outcomes.
1. Gates G.A. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996;114:525-530.
2. Schappert S.M. Office visits for otitis media: United States, 1975-90. Adv Data. 1992;214:1-19.
3. Kleinman L.C., Kosecoff J., Dubois R.W., Brook R.H. The medical appropriateness of tympanostomy tubes proposed for children younger than 16 years in the United States. JAMA. 1994;271:1250-1255.
4. Gates G.A., Avery C.A., Prihoda T.J., Cooper J.C. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of otitis media with effusion. N Engl J Med. 1987;317:1444-1451.
5. Gates G.A., Muntz H.R., Gaylis B. Adenoidectomy and otitis media. Ann Otol Rhinol Laryngol. 1992;101:24-32.
6. Stool S.E., Berg A.O., Berman S., et al. Managing Otitis Media with Effusion in Young Children: Quick Reference Guide for Clinicians. AHCPR Publication No. 94-0623. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; July 1994.
7. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media: Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-1465.
8. American Academy of Family Physicians. American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media with Effusion: Otitis media with effusion. Pediatrics. 2004;113:1412-1429.
9. Bluestone C.D. Otitis media: A spectrum of diseases. In: Lalwani A.K., Grundfast K.M., editors. Pediatric Otology and Neurotology. Philadelphia: Lippincott-Raven; 1998:233-240.
10. Casselbrant M.L., Mandel E.M., Kurs-Lasky M., et al. Otitis media in a population of black American and white American infants, 0-2 years of age. Int J Pediatr Otorhinolaryngol. 1995;33:1-16.
11. Teele D.W., Klein J.O., Rosner B.A. Greater Boston Otitis Media Study Group: Epidemiology of otitis media during the first seven years of life in children in Greater Boston: A prospective cohort study. J Infect Dis. 1989;160:83-94.
12. Senturia B.H., Bluestone C.D., Klein J.O., et al. Report of the Ad Hoc Committee on Definition and Classification of Otitis Media and Otitis Media with Effusion. Ann Otol Rhinol Laryngol. 1980;89(Suppl 68):3-4.
13. Kenna M.A. Otitis media with effusion. In: Bailey B.J., editor. Head and Neck Surgery-Otolaryngology. Philadelphia: Lippincott; 1993:1592-1606.
14. Teele D.W., Klein J.O., Rosner B. Greater Boston Otitis Media Study Group: Middle ear disease in the practice of pediatrics: Burden during the first five years of life. JAMA. 1983;249:1026-1029.
15. Teele D.W., Klein J.O., Rosner B. Epidemiology of otitis media in children. Ann Otol Rhinol Laryngol. 1980;89(Suppl 68):5-6.
16. Schwartz R.H., Rodriguez W.J., Grundfast K.M. Duration of middle ear effusion after acute otitis media. Pediatr Infect Dis J. 1984;3:204-207.
17. Shurin P.A., Pelton S.I., Turczyk V.A., et al. Persistence of middle ear effusion after acute otitis media. N Engl J Med. 1979;300:1121-1123.
18. Fireman P. Otitis media and Eustachian tube dysfunction: Connection to allergic rhinitis. J Allergy Clin Immunol. 1997;99:787-797.
19. Henderson F.W., Giebink G.S. Otitis media among children in day care: Epidemiology and pathogenesis. Rev Infect Dis. 1986;8:533-538.
20. Bluestone C.D., Stephenson J.S., Martin L.M. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11(8 Suppl):S7-S11.
21. Bluestone C.D., Klein J.O. Otitis media, atelectasis, and Eustachian tube dysfunction. In: Bluestone C.D., Stool S.E., editors. Pediatric Otolaryngology. 3rd ed. Philadelphia: Saunders; 1996:388-582.
22. Kenna M.A., Bluestone C.D. Microbiology of chronic suppurative otitis media. Pediatr Infect Dis J. 1986;5:223-225.
23. Papastavros T., Giamarellou H., Varlejides S. Role of aerobic and anaerobic microorganisms in chronic suppurative otitis media. Laryngoscope. 1986;96:438-442.
24. Grijalva C.G., Griffin M.R. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Review of Vaccines. 2008;7(1):83-95.
25. Greenberg D., Hoffman S., Leibovitz E., Dagan R. Acute otitis media in children: association with day care centers–antibacterial resistance, treatment, and prevention. Paediatr Drugs. 2008;10(2):75-83.
26. Pichichero M.E., Casey J.R. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 26(10 Suppl), 2007. S12-6
27. Post J.C., Preston R.A., Aul J.J., et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;273:1598-1604.
28. Howie V.M., Ploussard J.H. Simultaneous nasopharyngeal and middle ear exudate cultures in otitis media. Pediatr Digest. 1971;13:31-35.
29. Pillsbury H.C.III, Kveton J.F., Sasaki C.T., Frazier W. Quantitative bacteriology in adenoid tissue. Otolaryngol Head Neck Surg. 1981;89:355-363.
30. Bluestone C.D., Paradise J.L., Beery Q.C. Physiology of the Eustachian tube in the pathogenesis and management of middle ear effusions. Laryngoscope. 1972;82:1654-1670.
31. Liu Y.S., Lang R.W., Lim D.J. Microorganisms in chronic otitis media with effusion. Ann Otol Rhinol Laryngol. 1976;85:245-249.
32. Sade J. The natural history of the secretory otitis media syndrome. In: Sade J., editor. Secretory Otitis Media and Its Sequelae. New York: Churchill Livingstone; 1979:89-101.
33. Tos M. Production of mucus in the middle ear and Eustachian tube: Embryology, anatomy, and pathology of the mucous glands and goblet cells in the Eustachian tube and middle ear. Ann Otol Rhinol Laryngol. 1974;83(Suppl 11):44-58.
34. Gates G.A. Surgery of ventilation and mucosal disease. In: Brackmann D.E., Shelton C., Arriaga M.A., editors. Otologic Surgery. Philadelphia: WB Saunders; 1994:86.
35. Borish L. Allergic rhinitis: Systemic inflammation and implications for management. J Allergy Clin Immunol. 2003;112:1021-1031.
36. Viegas M., Gomez E., Brooks J., Davies R.J., et al. Changes in nasal mast cell numbers in and out of the pollen season. Int Arch All Appl Immunol. 1987;82:275-276.
37. Tomonaga K., Kurono Y., Mogi G. The role of nasal allergy in otitis media with effusion: A clinical study. Acta Otolaryngol Suppl (Stockh). 1988;458:41-47.
38. Baroody F.M. Allergic rhinitis: Broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616-631.
39. Bernstein J.M., et al. Allergic disease and the middle ear. In: Krouse J.H., Chadwick S.J., Gordon B.R., Derebery M.J., et al, editors. Allergy and Immunology. An Otolaryngologic Approach. Philadelphia: Lippincott Williams & Wilkins; 2002:192-200.
40. Doyle W.J., Friedman R., Fireman P., Bluestone C.D. Eustachian tube obstruction after provocative nasal antigen challenge. Arch Otolaryngol. 1984;110:508-511.
41. Hardy S.M., Heavner S.B., White D.R., et al. Late-phase allergy and Eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2001;125:339-345.
42. Pollock H.W., Ebert C.S., Dubin M.G., et al. The role of soluble interleukin-4 receptor and interleukin-5 antibody in preventing late-phase allergy-induced Eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2002;127:169-176.
43. Ebert C.S.Jr., Pollock H.W., Dubin M.G., et al. Effect of intranasal histamine challenge on Eustachian tube function. Int J Pediatr Otorhinolaryngol. 2002;63:189-198.
44. Hurst D.S., Venge P. Levels of eosinophil cationic protein and myeloperoxidase from chronic middle ear effusion in patients with allergy and/or acute infection. Otolaryngol Head Neck Surg. 1996;114:531-544.
45. Hurst D.S., Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000;55:435-441.
46. Sobol S.E., Taha R., Schloss M.D., et al. T(H)2 cytokine expression in atopic children with otitis media with effusion. J Allergy Clin Immunol. 2002;110:125-130.
47. Wright E.D., Hurst D., Miotto D., et al. Increased expression of major basic protein (MBP) and interleukin-5 (IL-5) in middle ear biopsy specimens from atopic patients with persistent otitis media with effusion. Otolaryngol Head Neck Surg. 2000;123:533-538.
48. Bernstein J.M. Role of allergy in Eustachian tube blockage and otitis media with effusion: A review. Otolaryngol Head Neck Surg. 1996;114:562-568.
49. Lazo-Saenz L.G., Galvan-Aguilera A.A., Martinez-Ordaz V.A., et al. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132:626-631.
50. Bernstein J.M., Doyle W.J. Role of IgE-mediated hypersensitivity in otitis media with effusion: Pathophysiologic considerations. Ann Otol Laryngol Suppl. 1994;16:15-19.
51. Braunstahl G.J., Overbeek S.E., Kleinjan A., et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in the upper and lower airways. J Allergy Clin Immunol. 2001;107:469-476.
52. Kovatch A.L., Wald E.R., Michaels R.H. β-Lactamase-producing Branhamella catarrhalis causing otitis media in children. J Pediatr. 1983;102:261-264.
53. McCracken G.H. Management of acute otitis media with effusion. Pediatr Infect Dis J. 1988;7:442-445.
54. Gebhart D.E. Tympanostomy tubes in the otitis mediaprone child. Laryngoscope. 1981;91:849-866.
55. Paradise J.L., Bluestone C.D., Rogers K.D., et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy tube placement. JAMA. 1990;263:2066-2073.
56. Mandel E.M., Rockette H.E., Bluestone C.D., et al. Efficacy of amoxicillin with and without decongestant-antihistamine for otitis media with effusion in children. N Engl J Med. 1987;316:432-437.
57. Stillwagon P.K., Doyle W.J., Fireman P. Effect of an antihistamine/decongestant on nasal and Eustachian tube function following intranasal pollen challenge. Ann Allergy. 1987;58:442-446.
58. Combs J.T. The effect of montelukast sodium on the duration of effusion of otitis media. Clin Pediatr. 2004;43:529-533.
59. Lambert P.R. Oral steroid therapy for chronic middle ear effusion: A double-blind crossover study. Otolaryngol Head Neck Surg. 1986;95:193-199.
60. Juntti H., Tikkanen S., Kokkonen J., et al. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999;119:867-873.
61. Hall L.J., Lukat R.M. Results of allergy treatment on the Eustachian tube in chronic serous otitis media. Am J Otol. 1981;3:116-121.
62. Draper W.L. Secretory otitis media in children: A study of 540 children. Laryngoscope. 1967;77:636-653.
63. Nsouli T.M., Nsouli S.M., Linde R.E., et al. Role of food allergy in serous otitis media. Ann Allergy. 1994;73:215-219.
64. Barenkamp S.J., Kurono Y., Ogra P.L., et al. Recent advances in otitis media, 5: Microbiology and immunology. Ann Otol Rhinol Laryngol Suppl. 2005;194:60-85.
65. Fernandez A.A., McGovern J.P. Secretory otitis media in allergic infants and children. South Med J. 1965;58:581-586.
66. Psifidis A., Hatzistilianou M., Samaras K., et al. Atopy and otitis media in children. In: Ruben R.J., Karma P.H., editors. Proceedings of the Seventh International Congress of Pediatric Otorhinolaryngology. Amsterdam: Elsevier Science; 1998:205.
67. Armstrong B.W. A new treatment for chronic secretory otitis media. Arch Otolaryngol. 1954;69:653-654.
68. Maw A.R. Chronic otitis media with effusion (glue ear) and adenotonsillectomy: A prospective randomized controlled study. BMJ. 1983;287:1586-1588.
69. Paradise J.L., Bluestone C.D. Adenoidectomy and chronic otitis media [Letter]. N Engl J Med. 1988;318:1470-1471.
70. Koyuncu M., Saka M.M., Tanyeri Y., et al. Effects of otitis media with effusion on the vestibular system in children. Otolaryngol Head Neck Surg. 1999;120:117-121.
71. Golz A., Westerman T., Gilbert L.M., et al. Effect of middle ear effusion on the vestibular labyrinth. J Laryngol Otol. 1991;105:987-989.
72. Jones N.S., Radomsky P., Princhard A.N.J., Snashell S.E. Imbalance and chronic secretory otitis media in children: Effect of myringotomy and insertion of ventilation tubes on body sway. Ann Otol Rhinol Laryngol. 1990;99:477-481.
73. Goode R.L. CO2 laser myringotomy. Laryngoscope. 1982;92:420-423.
74. Marchant H., Bisschop P. Value of laser CO2 myringotomy in the treatment of seromucous otitis. Ann Oto-Laryngol Chir Cervico-Fac. 1998;115:347-351.
75. Gross C.G., Bessila M., Lazar R.H., et al. Adipose plug myringoplasty: An alternative to formal myringoplasty techniques in children. Otolaryngol Head Neck Surg. 1989;101:617-620.
76. Helmus C., Grin M., Westfall R. Same-day-stay adenotonsillectomy. Laryngoscope. 1990;100:593-596.
77. Green J.D.Jr., Shelton C., Brackmann D.E. Surgical management of iatrogenic facial nerve injuries. Otolaryngol Head Neck Surg. 1994;111:606-610.
78. Aul J.J., Anderson K.W., Wadowsky R.M., et al. Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Ann Otol Rhinol Laryngol. 1998;107:508-513.
79. Liederman E.M., Post J.C., Aul J.J., et al. Analysis of adult otitis media: Polymerase chain reaction versus culture for bacteria and viruses. Ann Otol Rhinol Laryngol. 1998;107:10-16.
80. Post J.C., Aul J.J., White G.J., et al. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am J Otolaryngol. 1996;17:106-111.
81. Kenna MA: Bacteriology of otorrhea: Polymerase chain reaction versus cultures. In Proceedings of the 6th International Symposium on Recent Advances in Otitis Media. Toronto, BC Decker, 1996, pp 428-430.
82. Post J.C., Stoodley P., Hall-Stoodley L., Ehrlich G.D. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg. 2004;12:185-190.
83. Jang C.H., Cho Y.B., Choi C.H. Structural features of tympanostomy tube biofilm formation in ciprofloxacin-resistant Pseudomonas otorrhea. Int J Pediatr Otorhinolaryngol. 2007;71:591-595.
84. Ehrlich G.D., Veeh R., Wang X., et al. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710-1715.
85. Hall-Stoodley L., Hu F.Z., Gieseke A., et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.