Chapter 34 Surgery of the Endolymphatic Sac
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
Meniere’s disease is a clinical syndrome consisting of fluctuating-progressive hearing loss, episodic vertigo lasting 20 minutes to 24 hours, tinnitus, and aural fullness that is diagnosed when other diagnoses have been excluded. Surgical treatment of Meniere’s disease has been controversial and a focus of debate ever since Portmann1 first proposed opening the endolymphatic sac in 1927. Despite controversy, however, surgical manipulation of the endolymphatic sac to alleviate the debilitating symptoms of Meniere’s disease has been a mainstay of surgical treatment when conservative therapy has failed. The mechanism of symptomatic relief from shunting, decompressing, or excising the endolymphatic sac is also controversial.
For the purposes of this chapter, the definition of definite Meniere’s disease published by the American Academy of Otolaryngology–Head and Neck Surgery (AAOHNS) Committee on Hearing and Equilibrium in 19952 is used when referring to Meniere’s disease. The criteria given are as follows:
Meniere’s disease is difficult to study because of its fluctuant nature and the minimum 2-year time course over which results of interventions must be documented.2 The absence of a definitive test for Meniere’s disease necessitates diagnosis based on historical data. Whether or not placebo effect is the cause of success in the endolymphatic sac decompression is also controversial. Before discussing the techniques and outcomes of the endolymphatic sac procedures, we first consider the underlying anatomy and physiology.
ENDOLYMPHATIC ANATOMY AND EMBRYOLOGY
Scarpa (1752-1832) discovered and described endolymph and the membranous labyrinth in 1789,3 72 years before Méniere4 ascribed symptoms of the clinical syndrome to the inner ear. Understanding the embryology of the endolymphatic system may provide some clues to understanding Meniere’s disease. During the fourth week of embryogenesis, three buds of the primordial otocyst appear representing the pars superior, pars inferior, and endolymphatic duct, which eventually develop into the utricle and the semicircular canals, the saccule and the cochlear duct, and the endolymphatic duct and sac.5 The endolymphatic duct leaves the medial vestibule and courses dorsally through the bony vestibular aqueduct to terminate on the posterior surface of the temporal bone, enveloped within dural folds of the posterior fossa. The short, straight endolymphatic duct acquires its mature hook shape configuration by the fourth year of life.6,7 Histologic and functional maturation of sac elements may predate complete anatomic maturity. Potassium-rich endolymph fills the endolymphatic sac and duct, the saccule and utricle, the membranous semicircular canals, and the cochlear duct or scala media. These structures are interconnected by the smaller utricular duct, saccular duct, and ductus reuniens. The membranous endolymphatic structures are surrounded by the sodium-rich perilymph that fills the periotic spaces within the bony labyrinth.8
The position of the endolymphatic sac along the posterior fossa dura is relatively constant, but its size and the amount of bony covering by the operculum are variable.9 In most patients, 50% of the sac lies outside of the temporal bone, and 50% of the sac is intraosseous. Approximately 10% of sacs are completely extraosseous along the posterior fossa dura.10 The sac can extend posterolaterally to cover the lateral sinus.11 The morphology of the sac is also variable. The distal sac has a smooth open lumen within the dura mater. Its cuboidal epithelium contains light and dark cells.12 The lining of the intermediate portion of the sac shows more complex epithelial folds forming papillae and crypts of tall columnar light and dark cells. Cells of the intraosseous proximal rugose sac are intermediate between the taller, more distal cells and the squamous-to-cuboidal cells of the duct. The duct narrows at its isthmus to 0.1 to 0.2 mm in diameter. Luminal folding and transversely oriented tubules make the endolymphatic sac a more complex structure than it otherwise outwardly appears.13
The normal bony vestibular aqueduct is readily apparent on high-resolution computed tomography (CT) scanning of the temporal bone. It is funnel-shaped or tubular with the width of its external aperture averaging 6 mm.14,15 Radiographic observation of the affected ear in patients with Meniere’s disease showed a filiform narrowing of the external aperture averaging 2.2 mm.14 The amount of narrowing of the external aperture was also shown to be correlated with an increasing percentage of positive electrophysiologic measures in the affected ears of patients with Meniere’s disease.15 Statistically significant differences in the percentage of patients with enlarged summating potential-to-action potential (SP:AP) ratios by transtympanic electrocochleography were seen when correlated with the size of the external aperture of the vestibular aqueduct. An increased SP:AP ratio was noticed in 95% of ears with nonvisible external apertures, 91% when the aperture was less than 5 mm, 58% when the aperture was 5 to 7 mm, and 29% when the aperture was greater than 7 mm.
The endolymphatic duct and sac can be seen on high-resolution fast spin echo magnetic resonance imaging (MRI). The endolymphatic sac and duct were seen on MRI of 20 temporal bones in healthy subjects using strongly T2-weighted sequences and postprocessing software.16 A retrospective review of 42 ears with MRI data that underwent endolymphatic sac surgery correlated surgical findings with the ability to image the endolymphatic sac and duct.17 Surgical findings were classified as normoplastic in 17, atrophic in 14, and invisible in 11. Proton density imaging and T2 sequencing positively identified the endolymphatic duct and sac in 14 patients. The endolymphatic sac and duct were shown by proton density imaging alone in 14; neither proton density imaging nor T2 sequencing showed images in the remaining 14 ears. Findings at surgery showed statistically significant correlation with the ability to identify structures on imaging. Normoplastic surgical anatomy was identified on both imaging modalities; however, atrophic sacs were rarely seen on T2 imaging.
In another study using submillimeter MRI, the endolymphatic ducts were visualized in 29% of patients with Meniere’s disease and in 91% of healthy individuals. Temporal bone measurements between the posterior semicircular canal and the subarachnoid space, and the vestibule and the subarachnoid space were shorter in Meniere’s disease patients than in healthy individuals. It was noted anecdotally that endolymphatic shunt surgery was more effective in the few patients with visualized ducts compared with patients with nonvisualized ducts.18 Currently, the practicality of imaging in Meniere’s disease or for endolymphatic surgery lies primarily in ruling out retrocochlear pathology.
From a surgical perspective, the endolymphatic sac is generally approached through the mastoid bone, and is isolated on the lateral surface of the posterior fossa dura. When performing a suboccipital craniotomy, the sac can often be identified on the posterior petrous aspect of the temporal bone. Discussing the location of the endolymphatic sac, Gibson19 noted that the extraosseous portion is difficult to define surgically, appearing only as a thickened area of dura. He also noted that after splitting the layers of the endolymphatic sac no endolymph is seen, and usually no significant electrophysiologic changes occur. Huang20 attributed higher success in endolymphatic sac surgery to definite identification of the sac, with entry into the true sac lumen, and preservation of the sac anatomy. Amiratti and colleagues21 advocated preserving the integrity of the endolymphatic system, suggesting severe audiovestibular disturbances that may follow sac disturbance; however, our experience is that patients tolerate complete excision of the sac without hearing loss, whether incidental in other cranial base procedures, or intentional for complete endolymphatic sac ablation.
Important topographic landmarks for identifying the endolymphatic sac exist from the transmastoid or posterior fossa approach. The transmastoid extradural landmarks for localization of the endolymphatic sac and for preservation of labyrinthine structures include Donaldson’s line, which is an imaginary line drawn posteriorly through the plane of the horizontal semicircular canal (see Fig. 34-1), and measurements delineating the hard angle. The endolymphatic sac is generally found along the posterior fossa dura inferior to Donaldson’s line. Caution must be used when identifying the sac to avoid damage to the facial nerve and the posterior semicircular canal. Anatomic variants of normal temporal bone anatomy have been associated with Meniere’s disease. An understanding of potentially altered anatomy is important for surgical planning of sac procedures.22 Hypoplasia of the mastoid air-cell system, hypocellularity of periaqueductal cells around the endolymphatic duct and sac, reduction of the aditus ad antrum, and hypoplasia of the facial recess all have been described.
Intradural identification of the endolymphatic sac in relation to anatomic structures of the posterior fossa places the sac 10 to 15 mm lateral to the internal auditory meatus, and 11 to 17 mm posterosuperiorly to the eleventh cranial nerve in the jugular foramen.21 Typically, the thickening of the dura and the bony ledge of the operculum pinpoint the location of the sac.
ENDOLYMPHATIC SAC PHYSIOLOGY
Surgical shunting of the endolymphatic sac was proposed soon after the initial anatomic observations of hydrops in the endolymphatic compartments to alleviate inferred dysfunction.1,23 The presumed longitudinal flow of endolymph from the stria vascularis to the endolymphatic sac and the role of the sac as a primary resorptive organ have long been assumed.24 Radial flow of endolymph has also been shown, however.25 Endolymph is produced by dark cells located largely in the stria vascularis, but also in vestibular ampullae, within the maculae of the saccule and utricle, and along the endolymphatic duct. Maintenance of a potassium-rich endolymph produces the endocochlear potential, a DC voltage gradient, to drive the transduction process important in the detection of sound, motion, and position.26,27 This is a pH-sensitive process and is based on an active transport system in the vestibular dark cells by a sodium–hydrogen ion exchange system.27,28 Local production concentrated within strial dark cells and radial movement of endolymph with local chemical exchange throughout its course maintain a chemical balance and gradient that promote physiologic endolymphatic function, and may promote a slow linear flow toward the sac.25
Other theories of endolymphatic fluid homeostasis exist. Salt29 stated that direct measurements of the dispersal of markers in endolymph fail to support dynamic flow theories, and suggested that, in the normal state, there is negligible flow. The ionic component of endolymph is maintained through single cell transport of ions. This local control theory is overridden when endolymph volume is abnormally high or low, with the endolymphatic sac acting as a regulator of bidirectional flow in response to volume needs. The endolymphatic sac functions as the master volume regulator by numerous observed characteristics. In contrast to endolymph throughout the inner ear, a gradient exists along the duct leaving the electrolyte state in the endolymphatic sac high in sodium and low in potassium.30 An active equilibration of ions creates an osmotic potential that may influence the transepithelial flow of fluids. Higher concentrations of Na+,K+-ATPase are seen in the endolymphatic sac, but diminish proximally along the endolymphatic duct.31
Several other findings suggest an active role of the endolymphatic sac on endolymph fluid homeostasis.32–34 Aquaporin 2, vasopressin type 2 receptor, and transient receptor potential channel vanilloid (TRPCV), subfamily type 1 and 4, were found in the epithelial lining of the endolymphatic sac, but not in other extracellular tissues, although TRPCV 1 was seen in the surrounding vasculature. Similar findings are seen within the kidneys, suggesting a parallel role in fluid filtration and resorption.32 A unique protein, saccin, secreted by the endolymphatic sac, acts within the kidney as an endogenous inhibitor of sodium reabsorption. Intracellular morphology of the endolymphatic sac chief cells possesses organelles capable of endocrine function, and cellular ultrastructure consistent with merocrine activity also is observed.33,34
Biochemical and cellular findings within the endolymphatic sac support its functional role in phagocytic, immune, and allergic responses of the inner ear.35–39 Volume may also be regulated by the functional mechanical entity of an extracellular matrix of interstitial cells that support the endolymphatic duct, and take part in the control of inner ear fluid dynamics and endolymph resorption.40 As new techniques are developed to detect and monitor minute fluid volumes and changes, and to evaluate the chemical composition and cellular characteristics of the endolymphatic sac, a better understanding of the physiologic role and pathophysiologic state of the endolymphatic system in Meniere’s disease will be achieved.26,41
ENDOLYMPHATIC HYDROPS: PATHOPHYSIOLOGY
It was not until the histopathologic observation of Hallpike and Cairns in 193842 that a proposed malfunction of the endolymphatic system was correlated with the clinical syndrome. These authors showed dilated endolymphatic spaces in temporal bone specimens from two patients with the clinical symptoms of Meniere’s disease who died after neurotomy of CN VIII. These findings showed end organ changes of the inner ear in patients with hearing and balance symptoms. The hydropic state of the inner ear has been confirmed in other temporal bone studies and described as the primary pathologic correlate of Meniere’s disease.43–45
The underlying cause of the hydropic state is at present unknown, although many theories exist. Hydrops is seen more often in the cochlea and saccule, structures derived from the later developing pars inferior.43 Congenital insults or developmental aberrations later in the course of embryogenesis could presumptively account for this difference. A familial connection is seen in the history of 20% of patients clinically diagnosed with Meniere’s disease.44 This connection, in consideration of the embryologic and anatomic findings associated with Meniere’s disease, suggests a multifactorial predisposition to developing endolymphatic hydrops. Whether this precondition is genetic or related to shared environmental factors or insults is yet to be determined.
The pathophysiologic state of the endolymphatic system has been partially modeled in mice by experimental destruction and obstruction of the sac or duct in attempts to pinpoint the underlying mechanism of the hydropic condition.46,47 Although these experiments were able to reproduce hydrops and audiometric findings similar to Meniere’s disease, vestibular dysfunction or vertigo was noticed only after placing the animals in a head-down position theoretically by inducing additional pressure within inner ear fluids.48
The predominant theories that could explain the symptom complex of Meniere’s disease are based on the observed pathologic and induced experimental evidence of hydrops. The various temporal bone findings are summarized succinctly by Costa and associates,49 and include ruptures of the membranous labyrinth, fistulas of the membranous labyrinth, collapse of the membranous labyrinth, obstruction of longitudinal flow, vestibular fibrosis, sensory lesions, and neural lesions. Schuknecht50 proposed the rupture theory. He reasoned that distention of the endolymphatic space with eventual membrane rupture could cross-contaminate perilymphatic spaces and toxify delicate sensory hair cells with the potassium-rich endolymph. In the distention theory, Paparella51 described decompensation of radial flow as the perilymphatic spaces ebb, leading to largely longitudinal flow along the hydropic membranous pathway with the saccule acting as a reservoir for the excess endolymph. As the dilated saccule encroaches on the confines of the vestibule, mechanical interference of cochlear and vestibular function occurs by inhibition of traveling waves and physical contact with the crista ampullaris.
The drainage theory, as presented by Gibson and Arenberg,10 suggests that obstruction of a narrowed endolymphatic duct divests the sac of endolymph. The endolymphatic sac responds by secreting glycoproteins and saccin. Glycoproteins act osmotically by “pulling” endolymph toward the sac, and saccin stimulates secretion of endolymph from dark cells that distend the endolymphatic spaces “pushing” against the obstruction toward the sac. The obstructing debris ultimately and suddenly passes, and the resultant rapid flow of endolymph purportedly brings on an acute vertiginous episode. Gibson and Arenberg suggested that patients with Meniere’s disease with larger vestibular aqueducts could experience resolution of auditory symptoms as in Lermoyez’s syndrome after clearance of the obstructing debris. Patients with Meniere’s disease have been shown to have widening of the vestibular aqueduct aperture, but, although enticing to establish a mechanism of pathology, patients with Lermoyez’s syndrome have not been shown to have wider vestibular aqueducts than other patients with Meniere’s disease. Gibson and Arenberg also theorized that Tumarkin crises could be the effect of a membrane rupture in the overdistended endolymphatic space.
MENIERE’S DISEASE
Epidemiology
Arenberg and colleagues52 have suggested that incidence and prevalence estimations reported in Meniere’s disease are inaccurately low by not recognizing the early or atypical cases, or cases misdiagnosed by lengthy remissions drawing out the episodic nature. The definition of Meniere’s disease may alter prevalence and incidence numbers. The 1995 AAOHNS Committee on Hearing and Equilibrium recognized that the 1985 diagnostic criteria for Meniere’s disease were rigid to the point of precluding patients who were most likely Meniere’s cases. Current criteria are listed at the beginning of this chapter.2 These gradations may alter the epidemiologic accounting of Meniere’s disease because they allow for inclusion of patients who may be early in the course of their disease, and patients who may have milder symptoms of the disease. Several retrospective reviews have shown the prevalence of Meniere’s disease to be 10 to 20 per 100,000 in various populations around the world.49 Using 1995 committee criteria for diagnosis, a more recent study in the Finnish population showed 43 cases per 100,000 population, and an annual incidence of 4.3 per 100,000.53
Costa and associates49 described in detail the epidemiology of Meniere’s disease. It generally manifests in the fifth decade of life. The incidence in childhood is thought to be 1% to 7% of all Meniere’s cases.54 Of 14 children diagnosed with definite Meniere’s disease, 5 were shown to have secondary disease manifesting 5 to 11 years after a history of Haemophilus influenzae meningitis, mumps, temporal bone fracture, and congenital and embryopathic complications.55 Nine of the 14 children had idiopathic disease. These 14 children represented 1% of the combined Meniere’s population of four neurotologic clinics. There is a slight female preponderance. In women with definite Meniere’s disease who were pregnant, a clear decline in symptoms was associated with delivery.49 No socioeconomic, occupational, or racial effect has been consistently shown, although there are statistically increased numbers in married individuals and anxious individuals, and decreased numbers in obese individuals.
Diagnosis
Electrophysiologic tests, including electrocochleography, cochlear microphonics, and vestibular evoked myogenic potentials, have been used as adjuncts in diagnosis, and to monitor the efficacy of treatment in Meniere’s disease.44,56 In 2002, Ge and Shea57 reported a 10-year experience using transtympanic electrocochleography. Transtympanic electrocochleography was performed in 2421 ears of 2140 patients with Meniere’s disease. These authors concluded that electrocochleography is a reliable test to detect the presence of endolymphatic hydrops in Meniere’s disease using parameters of an enlarged SP:AP ratio greater than 0.4, a broadened action potential waveform (>3 ms), and a prolonged action potential latency (>0.2 ms). Combined click and tone burst responses yielded an enlarged SP:AP ratio in 81.7%, and a prolonged AP latency was found in 62.2% of ears with Meniere’s disease. An enlarged SP:AP ratio significantly correlated with stage and duration of disease. The SP:AP ratio was found to be elevated in 71% of stage 1 Meniere’s disease, 82% of stage 2, 85% of stage 3, and 90% of stage 4. The SP:AP ratio was elevated in 43% of patients during their first year of diagnosis and in 100% of patients with the disease for more than 30 years.
Cochlear microphonics were also used to assess the presence of hair cell survival, and were found to be present in 69% of ears with pure tone averages greater than 40 dB. It has been suggested that large cochlear microphonics in patients with Meniere’s disease indicate hearing loss resulting from altered cochlear mechanics, whereas severe hearing loss with small cochlear microphonics represents a hearing deficit resulting from hair cell loss.58 Electrocochleography has been shown to have a low sensitivity (57%), but is specific for endolymphatic hydrops (94%).44 In patients with an elevated SP:AP ratio in the immediate preoperative period, a statistically significant intraoperative reduction of the SP:AP ratio has been reported after endolymphatic sac incision and drainage.59
Vestibular evoked myogenic potential is used to assess the vestibulocollic or sacculocollic reflex. This test selectively assesses saccular function and integrity of the inferior vestibular nerve. It is being explored in Meniere’s disease for its potential in identifying endolymphatic or saccular hydrops.60 Vestibular evoked myogenic potential testing may aid in identification of active Meniere’s disease,60 and may help identify ears more prone to develop contralateral Meniere’s disease.62,63 Vestibular evoked myogenic potential may also offer information complementary to electronystagmography,64,65 and provide a way to measure severe saccular dysfunction, a finding associated with Tumarkin crisis.66 These tests show promising diagnostic potential. Further testing experience and validation are necessary.
Treatment
Medical Treatment
Dietary measures include reduction or restriction of caffeine, alcohol, and salt. Medical therapy is aimed at affecting fluid dynamics through diuretic therapy. Although many nutritional, vitamin, and medical therapies have been advocated for the treatment of Meniere’s disease, the combination diuretic Dyazide (triamterene and hydrochlorothiazide) is currently the only medical treatment that has shown in a randomized, placebo-controlled trial a statistical decrease in the vestibular symptoms of Meniere’s disease.70 Breakthrough episodes are managed symptomatically with a choice of several options of different classes of vestibulosuppressants. In-depth medical treatment of Meniere’s disease is addressed elsewhere. The work-up and treatment of other processes that secondarily produce Meniere’s syndrome, alone or in conjunction with standard medical treatment of endolymphatic hydrops, are usually successful at alleviating symptoms.
Intratympanic Treatment
Chia and colleagues71 performed a meta-analysis of 980 patients in 27 studies comparing five different delivery methods. Studies were grouped by the delivery method of the gentamicin and fell into five categories: (1) a multiple daily dosing group, (2) a weekly dosing group, (3) a low-dose group, (4) a continuous microcatheter group, and (5) a titration group. Considering all groups together, complete control of vertigo was achieved in 73.6%, with a significantly greater complete vertigo control rate of 81.7% achieved by the titration method. Overall effective vertigo control was achieved in 90.2%, with a significantly higher rate seen in the titration group (96.3%). The low-dosing method showed significantly lower complete and effective vertigo control rates, whereas the remaining methods showed no significant difference in control. Hearing loss was seen in 25.1% of patients, with profound loss occurring in 0.066% overall. The multiple daily dosing regimens showed significantly higher rates of hearing loss (34.7%), and the weekly method trended to lower rates of hearing loss (13.1%), whereas the remaining groups did not show statistically significant differences in hearing loss rates. Intratympanic gentamicin delivery is also an effective therapy for patients who undergo unsuccessful endolymphatic sac procedures, greatly reducing the need for vestibular neurotomy.72,73
Transtympanic instillation of steroids for control of vertigo in Meniere’s disease is receiving considerable attention because it is nonablative, but studies show mixed results.74–76 Surveys of the American Otologic Society and the American Neurotologic Society showed that 80% of respondents list steroid use in their protocols for treating Meniere’s disease. As with transtympanic techniques for gentamicin delivery, a multitude of theoretical applications and dosing methods exist and need to be sorted through to determine legitimate outcomes. Injection or instillation of dexamethasone into proximity with the round window allows effective diffusion of the medicine through the round window membrane into the inner ear. The steroid acts intracellularly, reducing inflammatory or immune-mediated responses, and suppressing destructive cytokines that theoretically may alter endolymph homeostasis. Several prospective randomized controlled studies of intratympanic steroids are under way, although their outcomes are not yet available.
Surgical Treatment
In 1981, Thomsen and coworkers,67 in a well-designed, placebo-controlled surgical trial of endolymphatic sac decompression, attributed the control of Meniere’s disease symptoms to placebo effect. Pillsbury68 and Welling and Nagaraja69 in separate evaluations of the data found fault with the statistical analysis, and noted statistically significant improvements in five key parameters, including control of vertigo, in the actively treated group over the control mastoidectomy group. Despite this long-standing controversy, endolymphatic sac surgery remains the most common primary surgical treatment for medically recalcitrant Meniere’s disease.
Retrospective studies report high efficacy in achieving reliable long-term control of episodic symptoms of vertigo.20,44,69,77–79 The weighted overall control of vertigo achieved by various endolymphatic sac procedures between 1986 and 1996 reached 86%.78 Other surgical procedures that are ablative, such as labyrinthectomy or vestibular nerve sections, have a 90% or greater rate of vertigo resolution; however, the risk of hearing loss and the 25% to 52% risk of developing hydrops in the contralateral ear are of concern. Bilateral vestibular ablation, whether by surgical intent or advanced bilateral Meniere’s disease, is complicated by severely debilitating bobbing oscillopsia or Dandy-Walker syndrome. Until the development and clinical application of vestibular prosthesis, this is a devastating condition. We recommend avoiding destructive surgical treatment of Meniere’s disease as the primary treatment option. According to a more recent survey of members of the American Neurotologic Society and the American Otologic Society, endolymphatic sac surgery was the most commonly employed initial surgical intervention for Meniere’s disease used by 50% of respondents, followed by the primary application of intratympanic gentamicin in 38%.77
Many different manipulations of the endolymphatic sac have been recommended. The sac has been decompressed, destroyed, shunted to the intradural and mastoid cavities, excised, and treated in conjunction with other surgical procedures or medical applications in attempts to enhance resorption, promote drainage, or otherwise favorably alter the homeostasis of the endolymphatic system.19,24,80–88 Adjuncts to surgery, such as exposing the opened sac to gentamicin, steroid, or mitomycin C, have been used in attempts to improve success.20,44,89 A range of success from 77% to 100% class A or class B results from these procedures with a weighted average result of 86% has been reported.78 No clear advantage has been shown so far for any particular procedure to the endolymphatic sac.
ENDOLYMPHATIC SAC SURGERY
Patient Selection
Patients with persistent debilitating vertigo, despite an adequate trial of dietary and medical management, are candidates for endolymphatic sac surgery. Counseling includes a discussion of the natural history of Meniere’s disease, including the possibility of developing bilateral disease, and review of other destructive and nondestructive surgical options. Because of a poor level of medical evidence in the literature, the patient ultimately selects the treatment option most comfortable to him or her. Patients who develop Meniere’s disease in an only hearing ear are not candidates for a destructive or high-risk procedure involving that ear. Endolymphatic sac surgery has been successfully employed to manage these patients.20,44 Preservation of cochlear nerve integrity is important for the rare few patients who end up with nonserviceable hearing as a result of the disease process or its treatment.44 Cochlear implantation has successfully restored hearing for patients in these categories.
Patients who have had endolymphatic sac surgery with initial good results may experience recurrence after several years of quiescent disease. Paparella90 revised 5% of his patients who had good results after primary endolymphatic sac enhancement. Revision endolymphatic sac surgery offers results similar to the good results expected with primary sac procedures, showing significant improvement in 76% to 95% of patients.90–92 Similarly, patients with delayed onset endolymphatic hydrops, or secondary Meniere’s syndrome, have been treated with endolymphatic sac surgery to control intractable symptoms of vertigo and aural pressure. Endolymphatic surgery is not recommended for patients with atypical Meniere’s disease. Endolymphatic shunt surgery may be used for, and is well tolerated by, elderly patients.44
Endolymphatic Shunt Procedure
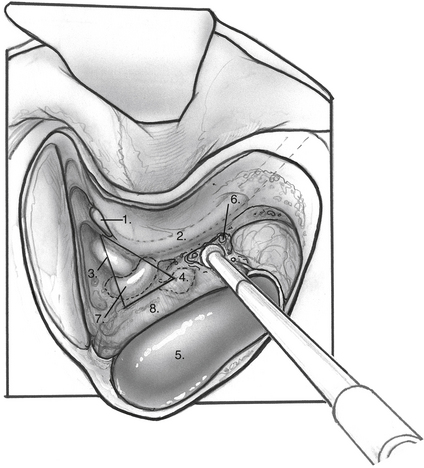
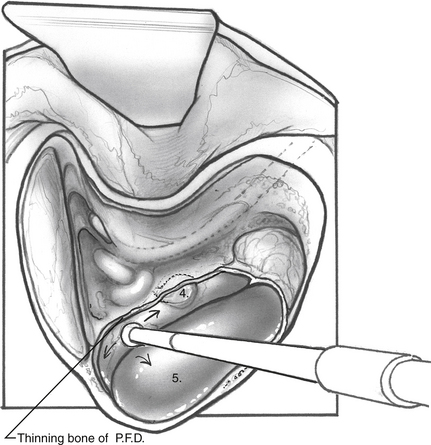
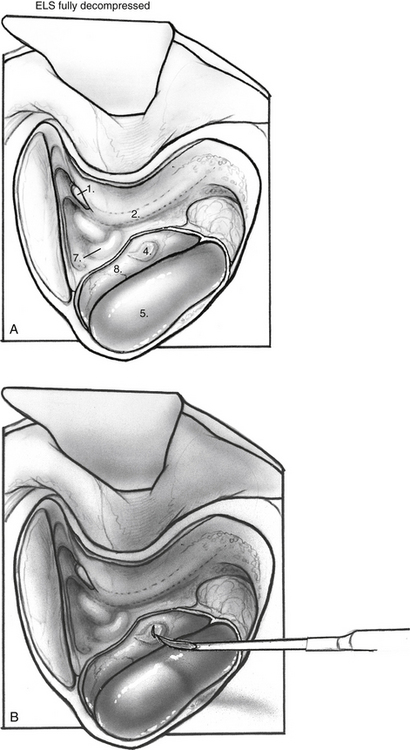
When the mastoid segment of the facial nerve is safely identified, the bone overlying the sigmoid sinus and the posterior fossa dural plate are thinned by removing retrofacial/infralabyrinthine air cells (Fig. 34-1). Bone removal inferior to Donaldson’s line and posteroinferior to the hard angle helps preserve the posterior semicircular canal. Rarely, blue-lining the posterior canal is necessary to identify an atrophic endolymphatic sac and duct. As the bone anterior to the sigmoid sinus is removed, the posterior fossa dura can be elevated from the medial surface of the dural plate. The bony plate overlying the endolymphatic sac is completely decompressed (Fig. 34-2). The endolymphatic sac is seen as a thickening of the posterior fossa dura, and the duct running anterolaterally tents the dura in the direction of the posterior canal (Fig. 34-3A).
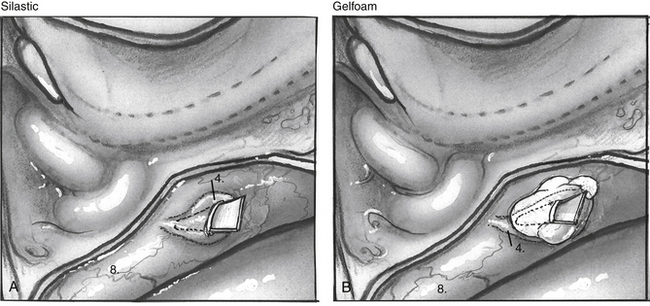
When the endolymphatic sac and duct are exposed, the sac and duct are opened along their posterolateral surface (Fig. 34-3B). The dural leaves are left intact. The duct is probed with a sickle knife feeling for its entry into the posterior aspect of the petrous bone along the operculum. A wedge of thin silicone elastomer (Silastic) is cut to fit along the duct, and this is placed into the sac and slid anterosuperiorly into the duct with a sickle knife (Fig. 34-4). The surgical site is covered with absorbable gelatin sponge (Gelfoam).
COMPLICATIONS
Complications after endolymphatic surgery are rare. Profound hearing loss can occur in 2% of cases, possibly related to labyrinthitis secondary to an exuberant healing process or activation of a latent virus.87,93 When postoperative hearing loss follows an uneventful surgical procedure, oral or transtympanic steroids are considered, although the efficacy of steroids is unknown. Early postoperative vertigo is seen in approximately 10% to 20% of patients. These patients who do not respond to an initial endolymphatic sac operation, as shown by recurrent vertigo, are candidates for other ablative procedures. After an initially successful vestibular response to endolymphatic sac surgery, recurrence of symptoms after several years of quiescence can be seen, and these patients are offered revision endolymphatic sac surgery. They achieve satisfactory results again in 80% of cases in our experience. If symptoms or vestibular testing indicates involvement of the contralateral ear, the possibility of endolymphatic sac surgery for the newly involved side is discussed.
VESTIBULAR OUTCOMES
Because the primary indication for endolymphatic sac surgery is ongoing episodic vertigo despite appropriate medical attempts to control Meniere’s disease, the primary outcomes measure of such surgery is the response of the vestibular system to the surgical manipulation of the sac. As noted earlier, in 1995, the AAOHNS Committee on Hearing and Equilibrium re-established guidelines to standardize reporting of the outcomes of Meniere’s disease interventions (Tables 34-1, 34-2, and 34-3).2 These guidelines were an attempt to obtain some objectivity in a fluctuating disease process.20,44,94
| Numerical Value | Class |
|---|---|
| 0 (complete control) | A |
| 1-40 | B |
| 41-80 | C |
| 81-120 | D |
| >120 | E |
| Secondary treatment initiated owing to disability from vertigo | F |
Numerical value = (X/Y) × 100, rounded to the nearest whole number, where X is the average number of definitive spells per month for the 6 months 18 to 24 months after therapy, and Y is the average number of definitive spells per month for the 6 months before therapy.
TABLE 34-2 Staging of Hearing∗
| Stage | Four-Tone Average (dB) |
|---|---|
| 1 | ≤25 |
| 2 | 26-40 |
| 3 | 41-70 |
| 4 | ≥71 |
∗ Staging is based on the four-tone average (arithmetic mean rounded to the nearest whole number) of the pure tone thresholds at 0.5 kHz, 1 kHz, 2 kHz, and 3 kHz of the worst audiogram during the interval 6 months before treatment. This is the same audiogram that is used as the baseline evaluation to determine hearing outcome from treatment. Staging should be applied only to cases of definite or certain Meniere’s disease.
TABLE 34-3 Functional Level Scale
Retrospective studies following large numbers of patients have collectively shown results with class A and B outcomes in 77% to 100% of patients treated (by the 1995 Committee on Hearing and Equilibrium guidelines).20,44,78,79,84–89 In a study of more than 3000 cases, Huang20 stated that the vestibular results of endolymphatic sac surgery can be expected to yield complete (class A) or substantial (class B) short-term (2 to 3 year) control of vertigo approximately 90% of the time in cases where the sac is definitely delineated, the real sac lumen is entered, the sac’s integrity is preserved, and a sac enlargement technique is employed. Huang20 added that little can likely be done to improve that rate, but that improved technique and earlier treatment may benefit patients’ long-term outcomes.
Other authors report that the effect of surgery on the endolymphatic system is nonspecific, unproven, of no or doubtful value, and perpetuated simply out of emotional ties and training.83,95,96 Pensak and Friedman78 divided reports of endolymphatic sac procedures into three groups: (1) reports of mollified or abolished vertigo in 91% to 100% of cases, (2) reports with a lower percentage (77% to 84%) of class A and B results, and (3) reports dissatisfied with the procedure showing 46% to 67% success.78 Grant and Welling’s analysis97 of the combined results of endolymphatic sac procedures retrospectively reported between 1986 and 1996 collectively showed class A or B outcomes in 86%.
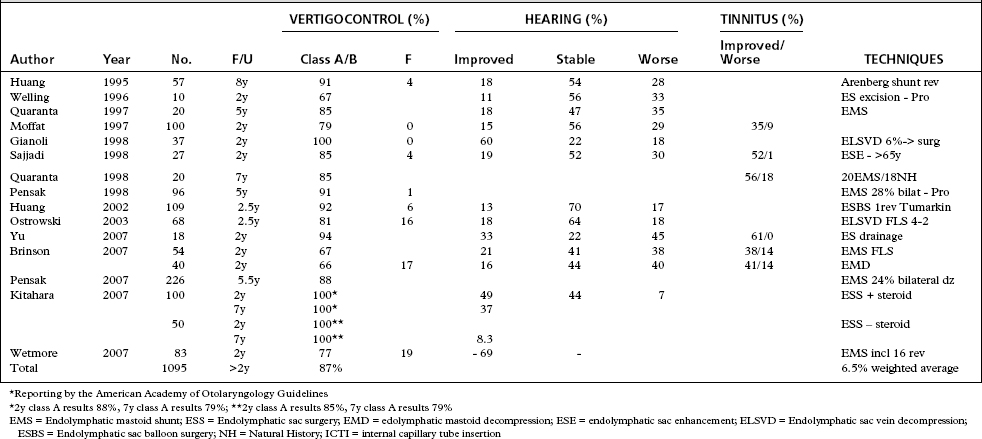
Endolymphatic Sac Procedures 1995 to Present
Since 1995, 33 studies discussing the effects of procedures on the endolymphatic system have been published. Six of these studies were outcomes-based questionnaires looking at the impact of endolymphatic surgery on the quality of life.101–106 Six were reported in foreign language journals, and one dealt solely with immediate postoperative recovery.107–113 The remaining 20 studies attempt to classify results with respect to the 1995 AAOHNS Committee on Hearing and Equilibrium guidelines; however, many still report results in nonstandardized fashions.19,20,24,78,83-85,87,89,91,96,114-122 Three studies used prospective designs.24,78,119
Table 34-4 summarizes studies from 1995 to the present that report according to the 1995 AAOHNS guidelines. Generally, they tell a similar tale—that procedures on the endolymphatic sac offer variable, 53% to 100%, class A and B control of vertigo, but achieve a weighted average of 87% when all studies are considered, and that when outcomes since 1985 are included, the average is consistent, 86%. Studies in which the minimal length of follow-up was less than 2 years, as recommended by the 1995 guidelines, were excluded. Need for revision has been documented in 5% to 7% of larger studies.20,22 Class F outcomes were reported in 7% of cases considering all reports. The ultimate need for neurodestructive procedures is seen in only 7% of surgically treated cases, and less than 2% of all patients with Meniere’s disease.
If the length of the follow-up is not considered, surgical success from reported studies would show greater than 87% class A and B results. Kato and colleagues104 looked at the effect of length of follow-up comparing results of 46 patients assessed at less than 18 months with 119 patients who had greater than 18 months of follow-up. Both groups showed statistically significant improvement in quality of life with the longer follow-up trending to increased improvement. Goldenberg and Justus123 followed a group of 24 patients showing that the 81% class A and B results of endolymphatic sac surgery seen at 1 to 5 years remained (83%) at 7 to 11 years, concluding that shorter follow-up apparently was valid in predicting longer term results. Quaranta and colleagues120 showed that endolymphatic shunts provided a statistically significant improvement over a natural history cohort at 2 and 4 years, but the improvement was not statistically different at 7 years, concluding that intervention hastens the symptomatic recovery that the natural course of Meniere’s disease provides with time.
Several centers have followed large cohorts of patients after endolymphatic surgery for many years. Results over 7 to 12 years remain satisfactory in 63% to 100% of patients.20,22,124–126 Some patients with immediate class A or B results experience recurrent symptoms after several years. These patients also do well after revision sac surgery. Indications for revision sac surgery vary. Revision rates range from 4% to 37%.44,84,87,91 Data comparing revision surgery with primary surgery of the endolymphatic sac show that class A and B outcomes are achieved in similar numbers ranging from 65% to 100%.44,91,125,126 Most authors agree that revision sac surgery is more effective when the response to the initial sac intervention provided positive results.
There is some controversy regarding the minimal length of the symptom-free interval between the primary surgery and the recurrence of symptoms that qualifies as a positive response. Some authors believe that 6 months of symptomatic relief warrant revision sac surgery, and others require 3 years of high functionality to offer revision surgery.124,125 Schwager and colleagues91 indicated that findings of new bone growth in the region of the primary surgery constricting the sac portend favorable revision outcomes when decompressed, although we have not seen such a regrowth in our revision cases. Further evidence-based research regarding timing of revision surgery is needed to provide adequate data to guide practice decisions.
Endolymphatic sac surgery and revision sac surgery have reduced the number of patients with Meniere’s disease who ultimately require ablative surgical procedures. Ablative or titration interventions for failure of endolymphatic sac operations or revisions are undertaken in 0 to 27% of patients, with average 6% of combined reporting.85,119,124 Destructive procedures are validated options for patients with poor hearing and proven unilateral disease, and for patients who fail more conservative endolymphatic sac procedures. Transtympanic gentamicin injections after failed endolymphatic sac surgery are effective.73
Patient’s Perspective—Outcomes Questionnaires
Many retrospective reviews have included functional level scoring based on 1995 AAOHNS Committee on Hearing and Equilibrium guidelines. Besides the functional level scale (see Table 34-3), six studies have directly assessed the effect of treatment on the patient’s perceived disability and resultant quality of life, and function of disease-specific symptoms.101–106 Changes in preoperative and postoperative scoring on the Meniere’s Disease Outcomes Measure Questionnaire (MDOQ), the Medical Outcomes Short-Form 36 health survey (SF-36), the Vertigo Symptom Scale, the Hearing Disability Handicap Scale, the Tinnitus Severity Questionnaire, and the Sense of Coherence Scales were used according to AAOHNS reporting guidelines. In all quality-of-life measures, perception of disability improved after endolymphatic sac surgery in 79% to 100% of patients, and decreased in 0 to 12%.
Tyagi and colleagues102 showed that quality-of-life measures improved most when preoperative functional levels were 4 or greater. This finding coincides with the bias seen in measuring surgical outcomes against natural history cohorts that are offered surgery but opt out, as patients choosing surgery likely have more severe disease. Durland and coworkers86 showed a significantly improved perception of physical health and physical and social functioning after endolymphatic surgery, although subjective assessment of mental health was not altered. Patients scored significantly lower than normal subjects in 6 of 10 categories on the SF-36 preoperatively, but scored below normal subjects only in the category of general health postoperatively. The total number of vertigo spells decreased on average from 8.3 to 2.6 per month. De la Cruz and colleagues103 also showed that although disability and imbalance improved significantly in all patients, imbalance and some vertigo remained. After 2 years of follow-up, their survey of subjects who had undergone endolymphatic sac surgery, vestibular neurectomy, and labyrinthectomy showed that current vertigo characteristics did not differ significantly between surgical groups; however, frequency, severity, and interference of balance did differ, with the endolymphatic sac group having the best ratings and the labyrinthectomy group the poorest. Vertigo was shown to resolve within two months in 75%. Kitahara111 showed symptomatic relief and noted that recovery was quicker in patients with residual vestibular function.
Physician’s Practice Surveys
In 2003, 2005, and 2007, members of the American Neurotologic Society and the American Otologic Society were queried about the way they treat patients with medically recalcitrant Meniere’s disease.77,98,99 Respondents replied at rates of 20% to 68% of all polled. The results of these surveys showed a continued frequent use of endolymphatic sac procedures as a treatment for Meniere’s disease.
The use of intratympanic gentamicin in the 1990s showed a sharp increase, and a report from England showed a decline in the total number of endolymphatic sac surgical procedures performed between 1989 and 2005. Practice habits within the United States show continued predominant use of endolymphatic sac surgery by total numbers, and as a percentage of surgeons’ first-line choice for failed medical treatment.77,98,100 Between 1990 and 1999, 7228 endolymphatic sac procedures were performed followed by 4091 intratympanic gentamicin injections, 3545 vestibular neurectomies, and 2197 labyrinthectomies as reported by the nearly 20% of member surgeon respondents.98 Trends over this period showed a rapid increase in intratympanic gentamicin use, an increase in endolymphatic sac procedures, consistent use of labyrinthectomy, and a decline in the number of nerve sections. Comparing their data on surgical treatment of vertigo, De la Cruz and colleagues103 used the endolymphatic sac operation for 75% of all cases over the last 3 decades. This pattern of use declined in the most recent decade to 62%, and was explained by an increased number of surgeons performing shunts elsewhere.
Fifty percent of member surgeons preferred endolymphatic sac procedures as the first-line treatment of medically refractory Meniere’s disease; this was followed by 34% to 39% who chose intratympanic gentamicin, and 9% who would offer use of a micropressure device such as the Meniette device.80,98 When a patient with active Meniere’s disease in an only hearing ear was considered, the first-choice treatment for Meniere’s disease when medical management failed was a micropressure device for 55 surgeons, intratympanic steroids for 48 surgeons, and endolymphatic mastoid shunt for 33 surgeons.99
Peterson and Isaacson99 noted that practice surveys do not provide objective scientific data. Many factors influence response rate and the value of the data. Surveys are subject to biases held by the authors and the respondents, and the results are based on opinion and memory, not hard data. They define current practice patterns of the respondents only to the best of their recall. With this in mind, approximately 85% of responding members of the American Neurotologic Society and the American Otologic Society continue to perform surgery on the endolymphatic sac. Endolymphatic sac procedures continue to be the most popular first-line surgical choice when medical management has failed in Meniere’s disease, even when contemplating management of an only hearing ear.80,98,99 There was no significant difference related to the duration in practice, or the geographical location of the respondents.
Hearing Outcomes
Hearing status is a main factor in the decision process when selecting a surgical line of treatment. A good hearing outcome is desirable, but is currently a secondary measure behind alleviation of the disabling symptoms of vertigo. Patient satisfaction with procedures is determined more by alleviation of vertigo than preservation of hearing.108,125 When vertigo is adequately treated, disability from hearing loss likely becomes more relevant to patients. Hearing outcomes and quantitative analysis of hearing loss have been less well reported after surgical interventions. The 1995 AAOHNS Committee on Hearing and Equilibrium has standardized reporting of hearing outcomes to improve these observations.
Gianoli and colleagues121 reported improvement in 60% of patients at 2 years using an endolymphatic sac vein decompression technique. Kitahara and associates89 showed 92% improvement in short-term hearing by placing high-dose steroids within the endolymphatic sac. The prospect of hearing improvement is enhanced in patients treated early in the disease process, and there is a higher chance of improving hearing in patients who exhibit a positive dehydration test or fluctuating hearing loss as opposed to progressive and stable hearing deficits.20,44 Huang and colleagues127 followed hearing results of 723 patients who underwent endolymphatic shunt for 12 years and found that hearing of patients with class A results was stable for the duration of the study, whereas patients with class B-D hearing loss progressively lost more hearing slowly over the follow up period. Silverstein and colleagues98 determined that endolymphatic sac surgery resulted in a lower incidence of postoperative hearing loss than either vestibular neurectomy or intratympanic gentamicin. Hearing loss of 0 to 10% was seen in 78% of patients after sac surgery, 11% to 20% in 6% of patients, and greater than 21% in less than 1% of patients. After vestibular neurectomy and intratympanic gentamicin, 28% and 32% of patients had 11% to 30% hearing loss, and 2% and 10% had greater than 40% hearing loss.
Adjunct treatments or patient profiling may lead to better long-term results, or enhanced hearing preservation. The endolymphatic sac and duct system has been shown to be an adequate viaduct to the inner ear for chemical delivery.128 Steroids, gentamicin, mitomycin, and other antibiotics have been used in endolymphatic sac surgery.22,89,116,129 To date, no long-term data regarding the application of mitomycin or gentamicin to the opened endolymphatic system have been reported. Kitahara and associates129 presented the long-term effects of addition of high-dose dexamethasone in conjunction with endolymphatic sac surgery. They showed excellent class A and B control of vertigo (100%), with significant (49%) improvement in hearing at 2 years that decreased only slightly at the 7-year evaluation (37%). These authors had previously reported encouraging short-term effects with significant to complete control of vertigo in 94% of 12 patients, and improved hearing in 92%, and decreased tinnitus in 92%.109
Exposure of the opened sac to 1 mg/mL solution of mitomycin for 5 minutes followed by irrigation with a cephalosporin antibiotic offered 90% class A and B response, similar to 92% response for a similar procedure without mitomycin.94 Hearing improved in 30% of the mitomycin group, however, compared with 12% improvement without mitomycin. Improved outcomes from saturation or perfusion of the opened endolymphatic sac with gentamicin with exposure of the round window to gentamicin for 7 to 10 minutes reportedly improved vestibular response in patients with severe hearing loss.44 In cases with severe hearing loss, a transtympanic injection alone would likely be as beneficial.
1. Portmann G. Vertigo surgical treatment by the opening the saccus endolymphaticus. Arch Otolaryngol. 1927;6:309.
2. Committee on Hearing and Equilibrium. Méniere’s disease: Guidelines for the diagnosis and evaluation of therapy for reporting. Otolaryngol Head Neck Surg. 1995;113:181-185.
3. Canalis R.F., Mira E., Bonandrini L., Hinojosa R. Antonio Scarpa and the discovery of the membranous inner ear. Otol Neurotol. 2001;22:105-112.
4. Méniere P. Maladies de l’oreille interne offrant les symptoms de la congestion cerebrale apoplectiforme. Gaz Med de Paris. 1861;16(88):239-379.
5. Watzke D., Bast T.H. The development and structure of the otic (endolymphatic) sac. Anat Rec. 1950;106:361-379.
6. Fujita S., Sando I. Three-dimensional course of the vestibular aqueduct. Eur Arch Otorhinolaryngol. 2004;253:122-125.
7. Ng M. Postnatal maturation of the human endolymphatic sac. Laryngoscope. 2000;110:1452-1456.
8. Dobie R.A., Snyder J.M., Donaldson J.A. Electronystagmographic and audiologic findings in patients with Méniere’s disease. Acta Otolaryngol (Stockh). 1982;94:19-27.
9. Friberg U., Jansson B., Rask-Anderson H., Bagger-Sjoback D. Variations in surgical anatomy of the endolymphatic sac. Arch Otolaryngol. 1988;114:389-394.
10. Gibson W.P.R., Arenberg K. Pathophysiologic theories in the etiology of Méniere’s disease. Otolaryngol Clin North Am. 1997;30:961.
11. Anson B.J., Donaldson J.A. Surgical Anatomy of the Temporal Bone, 2nd ed. Philadelphia: Saunders; 1973.
12. Lundquist P.G. Aspects of endolymphatic sac morphology and function. Arch Otorhinolaryngol. 1976;212:231-240.
13. Bagger-Sjoback D., Jansson B., Friberg U., Rask Andersen H. Three dimensional anatomy of the human endolymphatic sac. Arch Otolaryngol. 1990;116:345-349.
14. Takeda T., Sawada S., Kakigi A., Saito H. Computed radiographic measurement of the dimensions of the vestibular aqueduct in Méniere’s disease. Acta Otolaryngol Suppl. 1997;528:80-84.
15. Shea J.J.Jr., Ge X., Warner R.M., Orchik D.J. External aperture of the vestibular aqueduct in Méniere’s disease. Am J Otol. 2000;21:351-355.
16. Eberhardt K.E.W., Hollenbach H.P., Deimling M., et al. High-resolution magnetic resonance imaging of the endolymphatic duct and sac. MAGMA. 1995;3:77-81.
17. Kobayashi M., Fukay T., Noda M. The endolymphatic sac in patients with Méniere’s disease: Correlation between MRI and the surgical findings. Acta Otolaryngol. 2000;120:955-959.
18. Welling D.B., Clarkson M.W., Miles B.A., et al. Submillimeter magnetic resonance imaging of the temporal bone in Méniere’s disease. Laryngoscope. 1996;106:1359-1364.
19. Gibson W.P.R. The effect of surgical removal of the extraosseous portion of the endolymphatic sac in patients suffering from Méniere’s disease. J Laryngol Otol. 1996;110:1008-1011.
20. Huang T.S. Endolymphatic sac surgery for Méniere’s disease: Experience with over 3000 cases. Otolaryngol Clin N Am. 2002;35:591-606.
21. Amiratti M., Spallone A., Feghali J., et al. The endolymphatic sac: Microsurgical topographic anatomy. Neurosurgery. 1995;36:416-419.
22. Paparella M.M. Revision of endolymphatic sac surgery for recurrent Méniere’s disease. Otolaryngol Clin North Am. 2002;35:607-619.
23. Guild S. The circulation of endolymph. Am J Anat. 1927;39:57-81.
24. Welling D.B., Pasha R., Roth L.J., Barin K. The effect of endolymphatic sac excision in Méniere’s disease. Am J Otol. 1996;17:278-282.
25. Lawrence M. The flow of endolymph—a unique concept. Otolaryngol Clin North Am. 1980;13:577.
26. Ando M., Takeuchi S., Kakigi A., et al. Acute ischemia causes ‘dark cell’ change of strial marginal cells in gerbil cochlea. Cell Tissue Res. 2002;309:229-335.
27. Marcus D.C., Shipley A.M. Potassium secretion by vestibular dark cell epithelium demonstrated by vibrating probe. Biophys J. 1994;66:1939-1942.
28. Wangemann P., Liu J., Shiga N. Vestibular dark cells contain the Na+/H+ exchanger NHE-1 in the basolateral membrane. Hear Res. 1996;94:94-106.
29. Salt A. Regulation of endolymphatic fluid volume. Ann N Y Acad Sci. 2001;942:306-312.
30. Miyamoto H., Morgenstern C. Potassium level in endolymphatic sac of guinea pigs in vivo. Arch Otorhinolaryngol. 1979;222:77-78.
31. Ichiyama I., Adams J.C., Kimura R.S. Immunolocalization of Na+/K+-ATPase, Ca2+-ATPase, calcium-binding proteins and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngol. 1994;114:167-176.
32. Taguchi D., Takeda T., Kakigi A., et al. Expressions of aquaporin-2, vasopressin type 2 receptor, transient receptor potential channel vanilloid (TRPV)1, and TRPV4 in the human endolymphatic sac. Laryngoscope. 2007;117:695-698.
33. Qvortrup K., Rostgaard J., Holstein-Rathlou N.-H., Bretlau P. The endolymphatic sac: A potential endocrine gland? Acta Otolaryngol. 1999;119:194-199.
34. Rask-Anderson H., Danchkwardt-Lilliestrom N., Linthicum F.H., House W.F. Ultrastructural evidence of a merocrine secretion in the human endolymphatic sac. Ann Otol Rhinol Laryngol. 1991;100:148-156.
35. Fukuzawa K., Sakagami M., Matsunaga T., Fujita H. Endocytic activity of the free floating cells and epithelial cells in the endolymphatic sac: an electron microscopic study. Anat Rec. 1991;230:425-433.
36. Ruckenstein M.J. Immunologic aspects of Méniere’s disease. Am J Otolaryngol. 1999;20:161-165.
37. Welling D.B., Daniels R., Brainard J., et al. Detection of viral DNA in endolymphatic sac tissue from Méniere’s disease patients. Am J Otol. 1994;15:639-643.
38. Derebery J. Allergic management of Méniere’s disease: An outcome study. Otolaryngol Head Neck Surg. 2000;122:174-182.
39. Takumida M., Barbara M., Bagger-Sjoback D., Rask-Andersen H. Lectin detection of carbohydrates in the endolymphatic sac. Eur Arch Otorhinolaryngol. 1989;246:89-93.
40. Hultgard-Ekwall A.H., Couloigner V., Rubin K., Rask-Anderson H. Network organization of interstitial connective tissue cells in the human endolymphatic duct. J Histochem Cytochem. 2003;51:1491-1500.
41. Thalmann I., Hughes I., Tong B.D., et al. Microscale analysis of proteins in inner ear tissues and fluids with emphasis on endolymphatic sac, otoconia, and organ of Corti. Electrophoresis. 2006;27:1598-1608.
42. Hallpike C.S., Cairns H. Observation on pathology of Méniere’s syndrome. J Laryngol Otol. 1938;53:624-654.
43. Okuno T., Sando I. Localization, frequency, and severity of endolymphatic hydrops and the pathology of the labyrinthine membrane in Méniere’s disease. Ann Otol Rhinol Laryngol. 1987;96:438-445.
44. Paparella M.M., Fina M. Endolymphatic sac enhancement: Reversal of pathogenesis. Otolaryngol Clin N Am. 2002;35:621-637.
45. Schuknecht H.F., Ruther A. Blockage of longitudinal flow in endolymphatic hydrops. Eur Arch Otorhinolaryngol. 1991;248:209-217.
46. Kimura R.S. Experimental blockage of the endolymphatic sac and duct and its effect on the inner ear of the guinea pig. Ann Otol Rhinol Laryngol. 1967;76:4664-4687.
47. Yazawa Y., Shea J.J., Kitahara M. Endolymphatic sac in guinea pig after cauterizing the sac with silver nitrate. Arch Otolaryngol. 1985;111:301-304.
48. Andrews J.C., Strelioff D. Modulation of inner ear pressure in experimental endolymphatic hydrops. Otolaryngol Head Neck Surg. 1995;112:78-83.
49. Costa S.S., Sousa L.C.A., Pizza M.R.T. Méniere’s disease: Overview, epidemiology, and natural history. Otolaryngol Clin North Am. 2002;35:455-495.
50. Schuknecht H.F. Correlation of pathology with symptoms of Méniere’s disease. Otolaryngol Clin North Am. 1968;1:433-438.
51. Paparella M.M. Pathology of Méniere’s disease. Ann Otol Rhinolaryngol. 1984;93(Suppl 112):31-35.
52. Arenberg I.K., Balkany T.J., Goldman G., Pillsbury H.C.III. The incidence and prevalence of Méniere’s disease—a statistical analysis of limits. Otolaryngol Clin North Am. 1980;13:597-601.
53. Kotimaki J., Sorri M., Aantaa E., Nuutinen J. Prevalence of Méniere’s disease in Finland. Laryngoscope. 1999;109:748-753.
54. Hance S.E. Méniere’s disease in childhood: Implications for management in the school environment. Language, Speech, and Hearing Services in Schools. 1990;21:132-134.
55. Hausler R., Toupet M., Guidetti G., et al. Méniere’s disease in childhood. Am J Otolaryngol. 1987;8:187-193.
56. Orchik D.J., Shea J.J.Jr., Ge N. Summating potential and action potential ratio in Méniere’s disease before and after treatment. Am J Otol. 1998;19:478-483.
57. Ge X., Shea J.J.Jr. Transtympanic electrocochleography: A 10-year experience. Otol Neurotol. 2002;23:799-805.
58. Ge N.N., Shea J.J.Jr., Orchik D.J. Cochlear microphonics in Méniere’s disease. Am J Otol. 1997;18:58-66.
59. Huang T.S., Hsu J.C., Lee F.P. Electrocochleographic monitoring in endolymphatic sac surgery for Méniere’s disease. Arch Otolaryngol Head Neck Surg. 1994;120:522-529.
60. Young Y.H., Wu C.C., Wu C.H. Augmentation of vestibular evoked myogenic potentials: An indication for distended saccular hydrops. Laryngoscope. 2002;112:509-512.
61. Seo T., Node M., Yukimasa A., Sakagami M. Furosemide loading vestibular evoked myogenic potential for unilateral Méniere’s disease. Otol Neurotol. 2003;24:283-288.
62. Rauch S.D., Zhou G., Kujawa S.G., et al. Vestibular evoked myogenic potentials show altered tuning in patients with Méniere’s disease. Otol Neurotol. 2004;25:333-338.
63. Ribeiro S., Almeida R.R., Cauvilla H.H., Gananca M.M. Vestibular evoked myogenic potentials in affected and asymptomatic ears in unilateral Méniere’s disease. Rev Bras Otorrinolaringol (Engl Ed). 2005;71:60-66.
64. Rauch S.D., Silveira M.B., Zhou G., et al. Vestibular evoked myogenic potentials versus vestibular test battery in patients with Méniere’s disease. Otol Neurotol. 2004;25:981-986.
65. Iwasaki S., Takai Y., Ito K., Murofushi T. Abnormal vestibular evoked myogenic potentials in the presence of normal caloric responses. Otol Neurotol. 2005;26:1196-1199.
66. Timmer F.C., Zhou G., Guinan J.J., et al. Vestibular evoked myogenic potential (VEMP) in patients with Méniere’s disease with drop attacks. Laryngoscope. 2006;116:776-779.
67. Thomsen J., Tos M., Johnson N.J. Placebo effect in surgery for Méniere’s disease. Arch Otolaryngol. 1981;107:271-277.
68. Pillsbury H.C. Endolymphatic sac surgery: The Dannish Sham study: An alternative analysis. Otolaryngol Clin North Am. 1983;16:123-127.
69. Welling D.B., Nagaraja H.N. Endolymphatic mastoid shunt: A reevaluation of efficacy. Otolaryngol Head Neck Surg. 2000;122:340-345.
70. Van Deelen G.W., Huizing E.H. Use of a diuretic (Dyazide) in the treatment of Méniere’s disease: A double-blind cross-over placebo-controlled study. ORL J Otorhinolaryngol Relat Spec. 1986;48:287-292.
71. Chia S.H., Gamst A.C., Anderson J.P., Harris J.P. Intratympanic gentamicin therapy for Méniere’s disease: A meta-analysis. Otol Neurotol. 2004;25:544-552.
72. Marzo S.J., Leonetti J.P. Intratympanic gentamicin therapy for persistent vertigo after endolymphatic sac surgery. Otolaryngol Head Neck Surg. 2002;126:31-33.
73. Gouveris H., Lange G., Mann W.J. Intratympanic gentamicin treatment after endolymphatic sac surgery. Acta Otolaryngol. 2005;125:1180-1183.
74. Arriaga M.A., Goldman S. Hearing results of intratympanic steroid treatment of endolymphatic hydrops. Laryngoscope. 1998;108:1682-1685.
75. Silverstein H., Isaacson J.E., Olds M.J., et al. Dexamethasone inner ear perfusion for the treatment of Méniere’s disease: A prospective, randomized, double-blind, crossover trial. Am J Otol. 1998;19:196-201.
76. Garduno-Anaya M.A., Toledo H.C., Hinojosa-Gonzalez R., et al. Dexamethasone inner ear perfusion by intratympanic injection in unilateral Méniere’s disease: A two-year prospective, placebo-controlled, double-blind, randomized trial. Otolaryngol Head Neck Surg. 2005;133:285-294.
77. Kim H.H., Wiet R.J., Batista R.A. Trends in the diagnosis and the management of Méniere’s disease: Results of a survey. Otolaryngol Head Neck Surg. 2005;132:722-725.
78. Pensak M.L., Friedman R.A. The role of endolymphatic mastoid shunt surgery in the managed care era. Am J Otol. 1998;19:337-340.
79. Kitahara M. Endolymphatic sac surgery for Méniere’s disease: Eighteen years experience with the Kitahara sac operation. Am J Otol. 1987;8:283-286.
80. Welling D.B., Martyn M.D., Miles B.A., et al. Endolymphatic sac occlusion for the enlarged vestibular aqueduct syndrome. Am J Otol. 1998;19:145-151.
81. Huang T.S., Lin C.C. Endolymphatic sac ballooning surgery for Méniere’s disease. Ann Otol Rhinol Laryngol. 1994;103:389-394.
82. Cohen E.J., Mattox D.E. Histologic and ultrastructural features of explanted Arenberg shunts. Arch Otolaryngol Head Neck Surg. 1994;120:326-332.
83. Jackson C.G., Dickins J.R.E., McMenomey S.A., et al. Endolymphatic system shunting: A long-term profile of the Denver inner ear shunt. Am J Otol. 1996;17:85-88.
84. Huang T.S. Three new surgeries for treatment of intractable Méniere’s disease. Am J Otol. 1999;20:233-237.
85. Moffat D.A. Endolymphatic mastoid shunt surgery in unilateral Méniere’s disease. Ear Nose Throat J. 1997;9:642-651.
86. Durland W.F.Jr., Pyle G.M., Conner N.P. Endolymphatic sac decompression as a treatment for Méniere’s disease. Laryngoscope. 2005;115:144-157.
87. Ostrowski V.B., Kartush J.M. Endolymphatic sac-vein decompression for intractable Méniere’s disease: Long term treatment results. Otolaryngol Head Neck Surg. 2003;128:550-559.
88. Luetje C.M. A critical comparison of results of endolymphatic subarachnoid shunt and endolymphatic sac incision operations. Am J Otol. 1988;9:95-101.
89. Kitahara T., Takeda N., Mishiro Y., et al. Effects of exposing the opened endolymphatic sac to large doses of steroids to treat intractable Méniere’s disease. Ann Otol Rhinol Laryngol. 2001;110:109-112.
90. Paparella M.M. Revision of endolymphatic sac surgery for recurrent Méniere’s disease. Otolaryngol Clin North Am. 2002;35:607-619.
91. Schwager K., Baier G., Nour El-Din M., et al. Revision surgery after saccotomy for Méniere’s disease: Does it make sense? Eur Arch Otorhinolaryngol. 2002;259:239-242.
92. Huang T.S., Lin C.C. Revision endolymphatic sac surgery for recurrent Méniere’s disease. Acta Otolaryngol (Stockh) Suppl. 1991;485:131-144.
93. Paparella M.M., Sajjadi H. Endolymphatic sac enhancement principles of diagnosis and treatment. Am J Otol. 1987;8:294-300.
94. Silverstein H., Smouha E., Jones R. Natural history vs. surgery for Méniere’s disease. Otolaryngol Head Neck Surg. 1989;100:6-16.
95. Thomsen J., Kerr A., Bretlau P., et al. Endolymphatic sac surgery: Why we do not do it. The non-specific effect of sac surgery. Clin Otolaryngol Allied Sci. 1996;21:208-211.
96. Kerr A.G. Emotional investments in surgical decision making. J Laryngol Otol. 2002;116:575-579.
97. Grant I.L., Welling D.B. The treatment of hearing loss in Méniere’s disease. Otolaryngol Clin North Am. 1997;30:1123-1144.
98. Silverstein H., Lewis W.B., Jackson L.E., et al. Changing trends in the surgical treatment of Méniere’s disease: Results of a 10-year survey. Ear Nose Throat J. 2003;82:185-194.
99. Peterson W.M., Isaacson J.E. Current management of Méniere’s disease in an only hearing ear. Otol Neurotol. 2007;28:696-699.
100. Hari C.K., Powell R., Weiner G.M. Time trend analysis of otological procedures performed in England, 1989-2005. J Laryngol Otol. 2007;2:1-5.
101. Convert C., Franco-Vidal V., Bebear J.P., Darrouzet V. Outcome-based assessment of endolymphatic sac decompression for Méniere’s disease using the Méniere’s disease outcome questionnaire: A review of 90 patients. Otol Neurotol. 2006;27:687-696.
102. Tyagi I., Goyal A., Syal R. Sac surgery results as a function of preoperative distress level. Otol Neurotol. 2006;27:951-955.
103. De la Cruz A., Teufert K.B., Berliner K.I. Surgical treatment for vertigo, imbalance, and time course for recovery. Otolaryngol Head Neck Surg. 2006;135:541-548.
104. Kato B.M., LaRouere M.J., Bojrab D.I., Michaelides E.M. Evaluating quality of life after endolymphatic sac surgery: The Méniere’s disease outcomes questionnaire. Otol Neurotol. 2004;25:339-344.
105. Soderman A.C.H., Bergenius J., Bagger-Sjoback D., et al. Patients’ subjective evaluations of quality of life related to disease-specific symptoms, sense of coherence, and treatment in Méniere’s disease. Otol Neurotol. 2001;22:526-533.
106. Pal’chun V.T. Levina IuV: Dissection of endolymphatic duct in Méniere’s disease. Vestn Otorinolaringol. 2003;3:4-6.
107. Lu F., Dong R., Zhou W., Lu Y. Comparison of curative effect between decompression and incision of endolymphatic sac. Lin Chuang Er Bi Yan Hou Ke Az Zhi. 2002;16:334-335.
108. Yin S., Ke G., Gu N., et al. Long-term results of surgical treatment of intractable Méniere’s disease for control of vertigo. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 1999;13:291-292.
109. Kitahara T., Takeda N., Kondoh K., et al. Endolymphatic sac drainage and steroid-instillation surgery (EDSS) for intractable Méniere’s disease. Nippon Jibiinkoka Gakkai Kaiho. 2001;104:728-734.
110. Wilschowitz M., Sanchez-Hanke M., Ussmuller J. The value of saccotomy in Méniere disease: A long-term analysis of 42 cases. Head Neck Otolaryngol. 2001;49:180-187.
111. Kitahara T., Takeda N., Mishiro Y., et al. Vestibular symptoms and ENG findings during periods of convalescence after endolymphatic sac drainage and steroid-instillation surgery (EDSS). Nippon Jibiinkoka Gakkai Kaiho. 2000;103:1255-1262.
112. Kitahara T., Kondoh K., Morihana T., et al. Surgical management of special cases of intractable Méniere’s disease: Unilateral cases with intact canals and bilateral cases. Ann Otol Rhinol Laryngol. 2004;113:399-403.
113. Yu Y.P., Yang S.M., Han D.Y., Yang W.Y. Long-term results of endolymphatic sac drainage for Méniere disease. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:173-176.
114. Brinson G.M., Chen D.A., Arriaga M.A. Endolymphatic mastoid shunt versus endolymphatic sac decompression for Méniere’s disease. Otolaryngol Head Neck Surg. 2007;136:415-421.
115. Huang T.S., Lin C.C. A further critical assessment of the efficacy of endolymphatic sac surgery. Acta Otolaryngol Suppl. 1995;520:263-269.
116. Huang T.S. Topical mitomycin C and cephalosporin in endolymphatic sac surgery. Laryngoscope. 2002;112:243-247.
117. Quaranta A., Onofri M., Sallustio V., Iurato S. Comparison of long-term hearing results after vestibular neurectomy, endolymphatic mastoid shunt, and medical therapy. Am J Otol. 1997;18:444-448.
118. Sajjadi H., Paparella M.M., Williams T. Endolymphatic sac enhancement surgery in the elderly patients with Méniere’s disease. Ear Nose Throat J. 1998;77:975-982.
119. Thomsen J., Bonding P., Becker B., et al. The non-specific effect of endolymphatic sac surgery in treatment of Méniere’s disease: A prospective, randomized controlled study comparing “classic” endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane. Acta Otolaryngol. 1998;118:769-773.
120. Quaranta A., Marini F., Sallustio V. Long-term outcome of Méniere’s disease: Endolymphatic mastoid shunt versus natural history. Audiol Neurotol. 1998;3:54-60.
121. Gianoli G.J., Larouere M.J., Kartush J.M., Wayman J. Sac-vein decompression for intractable Méniere’s disease: 2-year treatment results. Otolaryngol Head Neck Surg. 1998;118:22-29.
122. Smith D.R., Pyle G.M. Outcome-based assessment of endolymphatic sac surgery for Méniere’s disease. Laryngoscope. 1997;107:1210-1216.
123. Goldenberg R.A., Justus M.A. Endolymphatic mastoid shunt for Méniere’s disease: Do results change over time? Laryngoscope. 1990;100:141-145.
124. Telischi F.F., Luxford W.M. Long-term efficacy of endolymphatic sac surgery for vertigo in Méniere’s disease. Otolaryngol Head Neck Surg. 1993;109:83-87.
125. Pensak M, Samy R: Contemporary role of endolymphatic mastoid shunt surgery in the era of transtympanic perfusion strategies. Presented at the 26th Politzer Society meeting, Cleveland, OH, 2007.
126. Wetmore S: Endolymphatic sac surgery for Méniere’s disease: Long-term results after primary and revision surgery. Presented at the 26th Politzer Society meeting, Cleveland, OH, 2007.
127. Huang T.S., Lin C.C., Chang Y.L. Endolymphatic sac surgery for Méniere’s disease: A cumulative study of twelve years’ experience. Acta Otolaryngol Suppl. 1991;485:145-154.
128. Yamasoba T., Yagi M., Roessler B.J., et al. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999;10:769-774.
129. Kitahara T, Okumura SI, Kubo T: Effects of intra-endolymphatic sac application of large doses of steroids for intractable Méniere’s disease: A randomized controlled trial. Presented at the 26th Politzer Society meeting, Cleveland, OH, 2007.