CHAPTER 174 Surgery of the Anterior and Middle Cranial Base
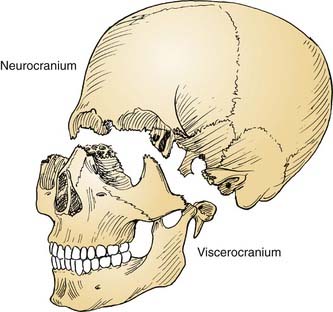
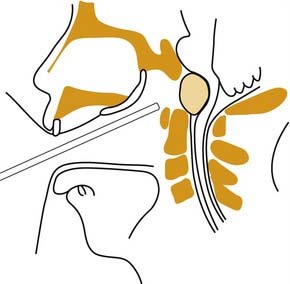
The cranial base often is conceptually divided into three anatomic regions that are named for their intracranial relations to the overlying cranial fossae: anterior, middle, and posterior (Fig. 174-1). From a diagnostic and therapeutic point of view, it is useful to consider the anterior and middle cranial base regions together, for several reasons. They contribute to what is commonly referred to as the craniofacial junction, where the neurocranium and the viscerocranium meet (Fig. 174-2). Both share anatomic relationships with the orbits, the nasal airway, and the paranasal sinuses, and they are therefore affected by similar pathologic processes. The anterior and middle cranial base regions have traditionally been approached using craniofacial disassembly techniques, however, endoscopic approaches to the skull base have assumed a growing role in selected cases while minimizing surgical morbidity. Additionally, robotic-assisted surgery has been investigated and holds promise for the future of anterior and middle cranial base surgery. The posterior and lateral cranial base are clinically regarded as a separate region, often approached with combined neurotologic and neurosurgical approaches (Chapters 127, 173, 176, and 177). This chapter focuses on the surgical management of lesions that affect the anterior and middle cranial base.
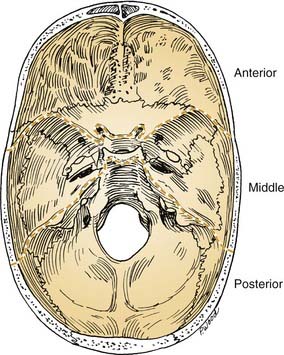
Figure 174-1. Intracranial view of cranial base showing boundaries of anterior, middle, and posterior divisions.
Surgical Anatomy
Anterior Cranial Base
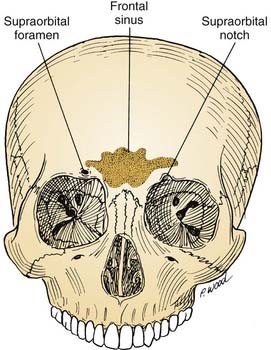
The anterior cranial base can be defined as that portion of the skull base adjacent to the anterior cranial fossa. It is bounded anteriorly by the frontal bone, which contains two surgically important structures: the frontal sinus and the supraorbital foramina (Fig. 174-3). The anatomy of the anterior cranial base is described in detail in Chapter 175, and briefly reviewed here. The supraorbital foramina, which may be incomplete (and therefore referred to as supraorbital notches), transmit the supraorbital nerves and vessels. These vessels supply the galea and the pericranium of the frontal region, and their preservation facilitates subsequent use in reconstructing anterior cranial base defects.
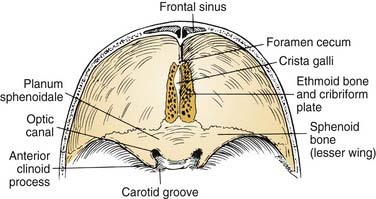
Superiorly, the anterior cranial base is formed by the frontal, ethmoid, and sphenoid bones (Fig. 174-4). An important visible landmark is the foramen cecum, which is the site of a communication between veins of the nasal cavity and the origin of the superior sagittal sinus. The next landmark, the crista galli, protrudes upward from the midline to provide attachment for the falx cerebri. On either side of the crista are the openings of the cribriform plate, through which olfactory nerves are transmitted. Just posterior to the last of these olfactory foramina is a smooth-surfaced area known as the planum sphenoidale; it forms the roof of the sphenoid sinus when the sinus is well pneumatized. The anterior clinoid processes and lesser sphenoid wings delineate the most posterior limit of the anterior cranial base and delineate its boundary with the middle cranial base. Between and slightly below the clinoids are the optic canals and the internal carotid arteries (ICAs).
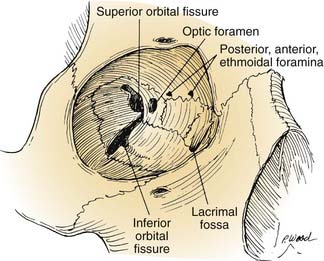
Extracranially, the anterior cranial base is related to the nasal cavity, the ethmoid and sphenoid sinuses, and the orbits (Fig. 174-5). The floor of the anterior cranial fossa is uneven, with the relatively flat orbital roofs sloping downward as they join the roof of the ethmoid sinuses medially. Thus, the ethmoid roof is lower than the orbital roof. The downward slope becomes even more exaggerated as the ethmoid roof joins the cribriform area (the roof of the nasal cavity), near the midline, which usually is the lowest point of the anterior skull base. The relative relationship of the height of the cribriform plate to the roof of the ethmoid is variable and has been classified by Keros into three categories: class 1, 1 to 3 mm; class 2, 4 to 7 mm; and class 3, 8 to 16 mm.1
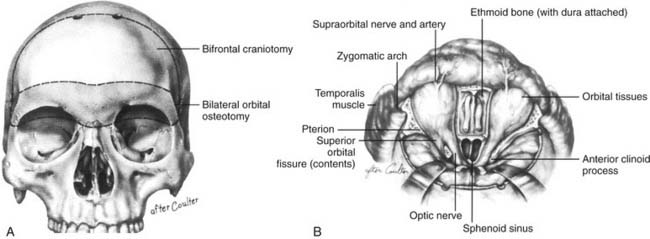
The orbits contain several landmarks that can help surgical orientation during cranial base operations (Fig. 174-6). The superior orbital fissure transmits the oculomotor, trochlear, ophthalmic, and abducens nerves (cranial nerves III, IV, V1, and VI) and the ophthalmic vein, and it communicates with the middle cranial fossa. The inferior orbital fissure contains the maxillary nerve (V2) and communicates primarily with the pterygopalatine fossa; the lateral end of this fissure is an important landmark for the placement of lateral orbital osteotomies. The optic canal transmits the optic nerve and the ophthalmic artery. The anterior and posterior arteries transmitted through ethmoid foramina should be controlled to reduce intraoperative bleeding in the nasal vault region. More important, these ethmoid foramina mark the position of the frontoethmoid suture line, a valuable guide to the level of the ethmoid roof and the anterior fossa floor. The posterior ethmoid foramen is of additional significance because of its consistent relationship with the optic canal, found 4 to 7 mm posteriorly.
Middle Cranial Base
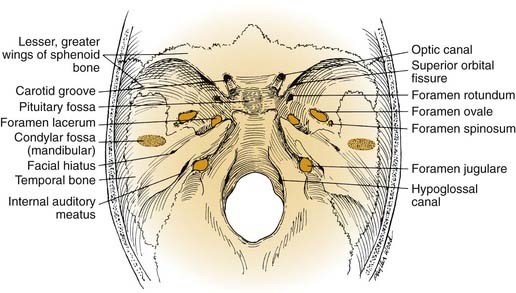
The middle cranial base forms the floor of the middle cranial fossa. From the intracranial perspective (Fig. 174-7), the middle cranial base begins anteriorly at the posterior edge of the lesser sphenoid wing; posteriorly it ends at the posterosuperior edge of the petrous part of the temporal bone. The intracranial surface of the middle cranial base is formed by the greater wing and body of the sphenoid bone and the petrous and squamous portions of the temporal bone. As such, it forms the roof of the infratemporal fossa, middle ear, mastoid, and condylar fossa and the lateral wall of the sphenoid sinus.
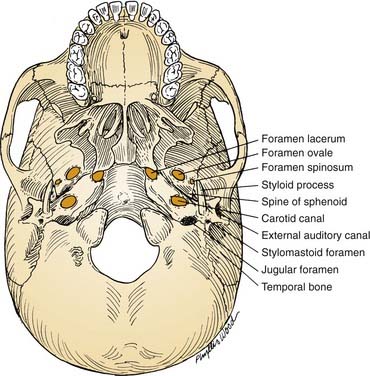
From an extracranial viewpoint, the middle cranial base extends from the posterolateral walls of the maxillary sinuses anteriorly to the petro-occipital sutures posteriorly (Fig. 174-8). It is formed by the greater wing and body of the sphenoid bone and by the temporal bone, including the condylar fossa. As on its intracranial surface, this region also contains numerous openings for major nerves and blood vessels, including the foramina ovale, spinosum, and lacerum and the stylomastoid foramen (cranial nerve VII), the jugular foramen (internal jugular vein, inferior petrosal sinus, cranial nerves IX, X, and XI), and the carotid canal (entrance of ICA into the temporal bone).
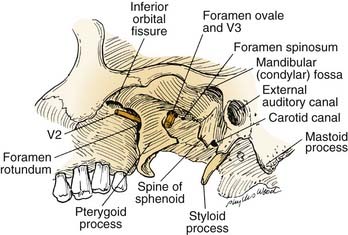
Operative approaches in this region often traverse the infratemporal fossa. Here, the muscles of mastication—the temporalis, masseter, and medial and lateral pterygoids—receive their blood supply from branches of the internal maxillary artery, which should be preserved if, for example, the versatile temporalis muscle is to be used as a flap for reconstruction. Both the lateral and medial pterygoid muscles originate in part from the lateral pterygoid plate (of the sphenoid bone), which serves as an excellent landmark owing to the following features: (1) it is a palpable guide for surgical orientation; (2) it can be easily exposed by dissection carried out medially along the greater sphenoid wing; and (3) it is easily identified on radiographic imaging studies, especially computed tomography (CT). The root of this lateral pterygoid plate is situated immediately posterior to the foramen rotundum and anterior to the foramen ovale. It can be used as an index to the positions of the maxillary (V2) and mandibular (V3) divisions of the trigeminal nerve (Fig. 174-9). After the foramen ovale is identified, the foramen spinosum (transmitting the middle meningeal artery) will be found just posterior to it. This leads to the next critical landmark: the spine of the sphenoid.
The sphenoid spine, which is situated just medial to the condylar fossa, serves as a palpable, radiographically identifiable landmark that is important because of its location immediately lateral to the carotid canal (see Fig. 174-8). It helps the surgeon locate the highest portion of the cervical ICA, which can be followed distally to expose and mobilize the petrous ICA. Because the sphenoid spine is just medial to the condylar fossa, the mandibular condyle often is displaced anteroinferiorly or resected to enhance exposure of the ICA as it enters the cranial base.
Preoperative Considerations
The principles of the clinical evaluation of presenting symptoms and physical findings (Chapter 175) and diagnostic imaging2 (Chapter 135) are considered in detail elsewhere in this book.
Angiography
Angiography also gives anatomic information about the integrity of the circle of Willis, existing collateral circulation to the cerebral hemispheres, and other possible anomalous blood supply patterns that may dictate the approach or prudent use of embolization.3
Angiography alone, however, does not give sufficient physiologic information about the adequacy of the circle of Willis or other collaterals with regard to supplying circulation to the brain.4 To obtain detailed physiologic information, cerebral blood flow studies are performed.
Cerebral Blood Flow Evaluation
If a cranial base lesion involves or impinges on the ICA or if it will be necessary to manipulate the ICA during surgery, cerebral blood flow studies should be considered. These studies give a physiologic index of the adequacy of the circulation to the brain in a quantitative way and are useful for predicting whether a patient can tolerate occlusion of the ICA without major neurologic consequence.5–8
The test begins with a temporary occlusion of the ICA by means of a balloon-tipped catheter inflated within the vessel. The catheter is placed percutaneously through the femoral artery and guided into the cervical portion of the ICA under fluoroscopic control, just as is done for routine cerebral angiography. This is performed with the patient awake, and serial neurologic assessments are done during the 15-minute ICA occlusion. If a neurologic deficit develops during this part of the test, occlusion is immediately discontinued. A patient demonstrating such a deficit is considered to have failed the test and is presumed to be highly dependent on the flow in that ICA; thus, the risk for stroke is increased if the carotid is compromised at surgery. If the patient tolerates 15 minutes of ICA occlusion without the development of neurologic deficit, further study uses a quantitative test in which stable xenon gas is inhaled. The inhaled xenon is distributed throughout the circulation and into the brain, where it is visible on CT scanning; this gives a picture of cerebral blood flow distribution (Fig. 174-10). This xenon-enhanced CT scan is performed with the balloon inflated and deflated in the ICA. The uptake of xenon within both cerebral hemispheres is quantitated using the digitized data from the CT scan (when xenon CT is not available, single-photon emission computed tomography [SPECT] imaging can give similar blood flow imaging, although it is not quantitative).
Preoperative Embolization
Another advantage of preoperative angiography is the possibility for elective embolization of vascular tumor beds to help reduce intraoperative bleeding. In addition, the ICA may be electively embolized using detachable intra-arterial permanent balloons if the decision is made preoperatively that the ICA will have to be sacrificed. This is sometimes done to permit the resection of malignant tumors. These procedures are considered in more detail in Chapter 136.
Other Evaluations
Pathology and Management Planning
Benign tumors and related lesions affecting the skull base have been reviewed by several authors and are summarized in Box 174-1.9–11 In general, benign cranial base neoplasms are managed surgically. The surgical technique often involves “piecemeal” removal, with careful progression from one surgical landmark to the next, to permit the maximal preservation of functionally important structures. For larger tumors or those for which removal carries a high risk of damage to neighboring structures, incomplete resection with postoperative irradiation may be indicated.12
Box 174-1 Benign Lesions of the Skull Base
Modified with permission from Dickins JRE. Approach to diagnosis of skull base lesions. Am J Otol. 1981;3:35.
Malignant skull base lesions are listed in Box 174-2.10 With some notable exceptions (i.e., leukemia, lymphoma, myeloma, metastases), malignant neoplasms are managed surgically, although in most cases surgery will not be used as the sole modality. Adjuvant management with radiation (by external beam, implantation, or brachytherapy) or chemotherapy is usually included in the therapeutic plan. Single-modality radiation therapy, including sterotactic radiotherapy, has been recommended by some investigators for certain smaller lesions.13 Malignant lesions are surgically removed en bloc, with margins of uninvolved tissue after broad circumferential exposure whenever possible.14
Anesthesia
Anesthetic management is crucial for determining the outcome of cranial base operations. Techniques of neuroanesthesia are used, with the primary goals of maximal neuronal preservation and simultaneous facilitation of a controlled surgical environment.15 Maintenance of hemodynamic factors is key in this scheme, because cerebral blood flow cannot be allowed to drop below critical levels for any significant length of time. Thus, close monitoring of arterial pressure, central venous pressure, cardiac function, and urine output is of paramount importance.
Operative Techniques
General Considerations
As the field of skull base surgery has evolved, more and more lesions previously thought to be unreachable or unresectable are now able to be addressed surgically. The contraindications to skull base surgery for malignancy as outlined by Donald16 include anatomic, tumor, and patient factors. Absolute anatomic contraindications include involvement of the brain stem, portions of the cerebrum, superior sagittal sinus, both internal carotid arteries, both cavernous sinuses and certain vital bridging veins. Tumor factors representing contraindications to resection usually include distant metastic disease; although some authors advocate significantly prolonged survival in those with isolated distant metastases after resection of the primary tumor, this has not been well demonstrated in skull base tumors. Certain malignancies typically display aggressive behavior despite treatment, which must be considered before extensive resection. Patients should be well informed and motivated and without medical contraindications to the operation as well.16
Overview of Approaches
Surgical treatment of lesions involving the anterior skull base, nasal cavity, and paranasal sinuses has evolved into a single category of craniofacial approaches. A myriad of surgical approaches have been developed for exposure of the anterior and middle cranial base regions; these range from purely intracranial to purely extracranial. However, most approaches for dealing with lesions of the skull base use combined intracranial and extracranial methods. For anterior cranial base lesions, the most commonly used approaches combine frontal craniotomy with some form of transfacial (transnasal, transmaxillary, or transorbital) exposure. Most commonly, a team of neurosurgeons and otolaryngologists performs this procedure resulting in a bifrontal craniotomy from above and a transfacial approach from below. The transfacial approach often involves midfacial degloving, and facial disassembly, requiring facial incisions and facial osteotomies. Lateral rhinotomy, removal of the frontonasal unit, Le Fort I and II osteotomies, and splitting of the maxilla have been described as means of accessing lesions of the anterior cranial base. Janecka and coworkers17 described the facial translocation approach, which also involves an extensive facial incision and facial disassembly for access to tumors in the anterior cranial base, cavernous sinus, clivus, and infratemporal fossa.
Since the 1990s, endoscopic sinus surgery has virtually replaced the conventional open techniques used by otolaryngologists in treating sinonasal disorders and is discussed in detail in Chapter 175. Use of the endoscope as a supplement to the surgical approaches can alleviate the need for some facial incisions by allowing the surgeon to observe areas hidden from the field of view of the microscope. For those tumors that invade the anterior cranial base, the endoscope may be utilized as an adjunct to the standard bifrontal craniotomy by allowing visualization of paranasal extension into the sphenoid, ethmoid, frontal, and maxillary sinuses. From nasal or maxillary portals, the endoscope allows visualization of all of the paranasal sinuses. As the technology of endoscope optics and video systems improves, the role of endoscopic cranial base surgery will augment and supplement current microsurgical techniques. Intraorbital lesions may be approached by frontal craniotomy or subcranial approaches, often combined with transfacial approaches.18
For middle cranial base lesions, access is most often provided by combining temporal or frontotemporal craniotomy with infratemporal fossa dissection, transfacial exposure, or transtemporal techniques. In addition, endoscope-assisted and image-guided navigational approaches are increasingly being applied (see Chapter 177). In anterior and middle cranial base approaches, craniofacial disassembly techniques have been widely used.
Implicit in the term craniofacial disassembly is the concept of the systematic, stepwise dissection of cranial and facial soft tissues on the basis of the knowledge of regional vascular territories and functional anatomy, followed by osteotomies and dismantling of the craniofacial skeleton. Some of these techniques, which were developed originally by plastic and reconstructive surgeons for the correction of congenital craniofacial deformities, are of major importance in cranial base surgery, because they allow wide exposure of the skull base through temporary displacement of the viscerocranium.19,20 The enhanced exposure of the skull base from below the neuraxis significantly reduces the need for brain retraction and therefore helps minimize postoperative neurologic dysfunction. It also allows the surgeon greater oncologic precision during the extirpative phase, with preservation of the functional and aesthetic units of the face for reconstruction.58
Planning the Operative Approach
Whether the surgery is being performed for a benign tumor, a malignancy, or other indications (e.g., inflammatory or vascular lesions, CSF fistula), the approach should be planned and executed to accomplish the four specific goals that are applicable to all cranial base operations, summarized in Box 174-3.
GOAL 2
Image-guided (stereotactic) computer-assisted surgery has become a very useful alternative—or adjunct—to wide surgical exposure in selected cases.21–26 Such “navigational surgery” employs one of a variety of referencing systems that can assimilate preoperative imaging studies into a computer-based algorithm that allows image reformatting in three dimensions (The preoperative reference images may be CT- or magnetic resonance imaging [MRI]-based, or they may be a “fusion study” that takes advantage of both types of studies). The reformatted images then serve as the basis for intraoperative navigation, which involves the use of an interactive probe that transmits spatial-orientation information back to the computer via transmitters; these can be either electromagnetic or optical. The computer screen then displays the appropriate preoperative images with the location of the probe superimposed, thus “targeting” the area of interest (Figure 174-11). With navigational systems, structures that are anatomically fixed (i.e., orbital walls, carotid canal, optic canal) can be precisely located, and the risk of injury can be minimized.
Reported uses of image-guided surgery include primary and revision endoscopic sinus surgery, osteoplastic frontal sinusotomy, transsphenoidal hypophysectomy, endoscopic cerebrospinal fluid leak and encephalocele or meningocele repair, endoscopic skull base tumor resection, orbital surgery, and endoscopic pterygomaxillary fossa biopsy.27,28
The American Academy of Otolaryngology–Head and Neck Surgery endorses use of computer-aided surgery for the following specific procedures or conditions29:
Intraoperative MRI or CT also has been proposed as a useful adjunct in skull base surgery. Proponents advocate the improved ability to detect residual tumor and evaluate for intraoperative complications, however, limitations of time and cost have prevented its widespread use outside of certain academic centers.30
Although large, multi-institutional prospective studies are yet to be accomplished, smaller studies and anecdotal reports demonstrate the efficacy of endoscope-assisted approaches for the treatment of selected skull base problems, most notably sinonasal neoplasms, pituitary tumors, and CSF leaks.31–37
Stamm38 classifies endoscopic skull base approaches as follows:
The location and suspected pathology dictate the selection of approach.
Endoscopy-based approaches to the nasal cavity, olfactory groove, nasopharynx, ethmoid roof (anterior skull base), orbit and orbital apex, sphenoid sinus and sellar and parasellar areas, clivus, cavernous sinus, optic chiasm, pterygopalatine and infratemporal fossae, parapharyngeal space, craniocervical junction, petrous apex, and jugular foramen all have been described. In short, access to the central skull base from the frontal sinus to C2 and from the sella to the jugular foramen is now possible using the expanded endonasal approach.39–43
The endoscopic approach to the orbital apex and optic canal for orbital decompression in fibrous dysplasia, Graves’ orbitopathy, compressive or traumatic optic neuropathy has been described. Of note, decompression is indicated only in patients with fibrous dysplasia with continuous deterioration of vision or undergoing removal of neighboring bone, but not as a prophylactic procedure.44,45 This approach also is used in the management of clival lesions, anterior skull base tumors, and vascular malformations of the skull base.46,47
Kassam and associates described an approach to identify the petrous portion of the carotid artery following identification and retrograde dissection of the vidian artery. They found this to be a consistent landmark radiographically and intraoperatively.48
Endoscopic approaches to pituitary surgery offer benefits of improved cosmesis, decreased recovery time, and fewer complications. Three approaches exist: transnasal-transethmoid, transnasal-transseptal, and direct transnasal. The direct transnasal approach has been touted because of the advantages of avoiding the ethmoid complex, decreased operative time, and improved visualization.2 Although endoscopic skull base surgery has been more extensively described for the anterior cranial base, endoscopic-assisted approaches to the middle cranial base also have been described.49
The protection of critical structures at the cranial base also is enhanced by the appropriate use of electrophysiologic monitoring, including intraoperative somatosensory evoked potentials, auditory brainstem evoked response, and EMG.14 The injection of low-dose intrathecal fluorescein has been shown to be a useful adjunct intraoperatively to decrease the CSF leak rate and, when appropriate medication is provided, has been proven safe and without significant adverse effects.50
GOAL 3
Such vascularized local flaps are preferable to free flaps or nonvascularized grafts when available, however others have displayed excellent closure rates with materials ranging from acellular dermis to free microvascular transfers.51,52 In general, smaller defects (less than 2 cm) are less likely to require vascularized tissue to achieve closure.53,54
Robotic endoscopic skull base surgery and transoral robotic surgery have been advocated by some researchers for the advantages of improved visualization, access, precise technique, and improved watertight closure following endoscopic skull base surgery. Although promising, more clinical investigation is needed before widespread acceptance of these techniques.55,56
GOAL 4
The choice of operative approach should reflect consideration for functional and aesthetic reconstruction, and it should include the placement of incisions within natural skin lines that respect aesthetic units of the face. The soft tissue closure should follow plastic surgery principles. Excellent skull base exposure usually can be achieved by elective osteotomies and the temporary removal of craniofacial bone segments, which are subsequently replaced, thereby preserving facial contour.17,51,57–59 Even in cases in which aesthetically important segments should be removed for oncologic reasons, acceptable cosmesis can usually be accomplished through the judicious use of bone grafts and soft tissue flaps or through surgical closures designed to support alloplastic or prosthetic materials.
Anterior Cranial Base Approaches
Surgical approaches to the anterior cranial base include methods that are purely extracranial and those that use combined extra- and intracranial exposures. The extracranial techniques—external ethmoidectomy, frontal sinusotomy, and intranasal ethmoidectomy—are suitable only for the management of discrete, well-localized lesions, such as CSF fistulas and some very limited benign anterior cranial base tumors. These procedures are well described elsewhere.60,61
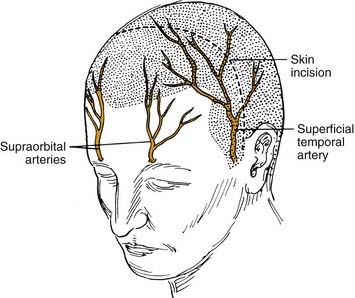
Technical Note: The Bicoronal Incision
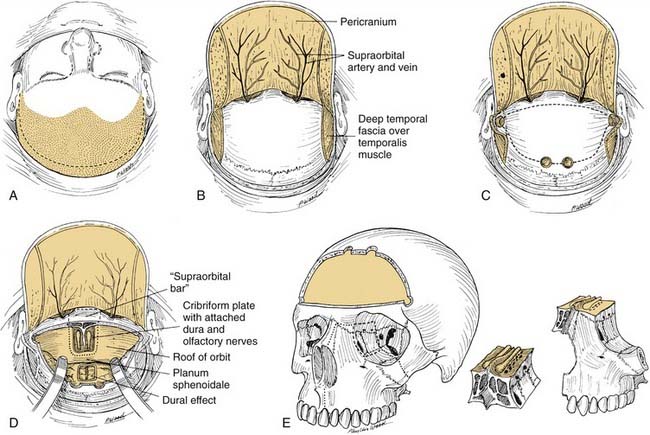
The bicoronal incision should be in the true coronal plane at the level of the top of the helix of the ear or slightly anterior to it (Fig. 174-12). A short, forward-directed, preauricular extension can be made on both sides to enhance scalp flap rotation. This coronal placement of the incision preserves the anterior branches of the superficial temporal artery, enhancing viability of the scalp flap. In addition, it substantially increases the length of vascularized galea and pericranium available for reconstruction as compared with incisions along the anterior hairline, the midforehead crease, or the brow. Additional length can be gained by back-elevating the scalp flap before division and elevation of the pericranial flap.
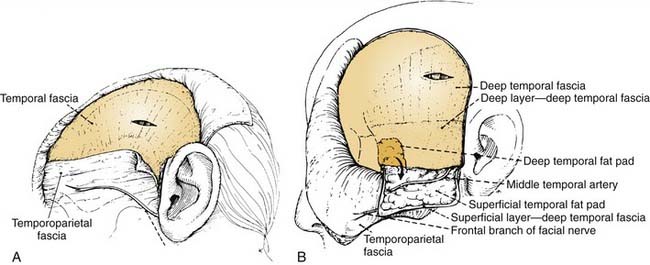
The central portion of the anterior scalp flap (i.e., the portion between the two superior temporal lines) is elevated in the subgaleal plane if a pericranial flap may be used and in the subperiosteal (subpericranial) plane if not. Lateral to the two superior temporal lines, it is elevated in the plane just above the deep temporal fascia. Therefore, at the temporal lines, the pericranium should be sharply incised to separate it from the origin of the deep temporal fascia (see Fig. 174-15). The pericranium is divided far enough posteriorly to provide sufficient length to the flap; then the dissection is carried forward in the subperiosteal plane as described earlier.
This deep temporal fascia begins to split into superficial and deep layers, beginning at approximately the level of the superior orbital rim.62 These fascial layers diverge to envelop the lateral and medial surfaces of the zygomatic arch inferiorly; between the layers is the temporal fat pad. The frontal (temporal) branches of the facial nerve course just superficial to the zygoma along the superficial temporal fascia (temporoparietal fascia) and are prone to injury if the dissection is done at that level (Fig. 174-13A). Often these injuries can be avoided by maintaining the plane of dissection at the level of the deep layer of the deep temporal fascia (i.e., at the surface of the temporalis muscle itself). This deep plane of exposure essentially elevates the fat pad along with the superficial fascia and the superficial layer of the deep temporal fascia, thereby protecting the facial nerve branches (see Fig. 174-13B).63,64 After the dissection reaches the level of the zygomatic arch, the arch is palpated, and its superior surface is directly exposed by sharply incising the fat pad and periosteum.
Further medially, flap elevation proceeds down the frontal area toward the supraorbital rims. Care should be taken to preserve the supraorbital supply of the galea and pericranium. This is best performed with elevation of the supraorbital rim periosteum that begins laterally and proceeds medially. A fine elevator is used first to palpate the supraorbital foramen (or notch) and then to expose its margins. If the pedicle is completely surrounded by bone, a 3-mm osteotome is used to fracture the inferior margin of the foramen, thereby liberating the pedicle and allowing it to be elevated intact, along with the remaining pericranium and underlying periorbita (Fig. 174-14). If the entire scalp flap is difficult to rotate inferiorly, the preauricular parts of the incision can be extended to as low as the tragus (without danger to the facial nerve trunk) or anteriorly (as far as the temporal hairline) to further enhance flap rotation.
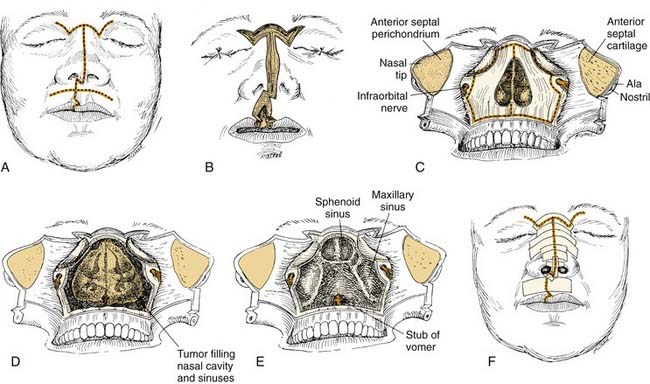
Anterior Craniofacial Resection
Anterior craniofacial resection (Fig. 174-15) combines bifrontal craniotomy with transfacial exposure of the nasal cavity, ethmoid, maxillary, and orbital areas, usually by modifications of lateral rhinotomy, midfacial degloving, or other transfacial approaches.65–67 Anterior craniofacial resection most often is used for removal of neoplasms that originate in the sinonasal tract and invade the anterior cranial fossa floor, such as squamous cell carcinoma, esthesioneuroblastoma, and adenocarcinoma. For cases in which tumor frankly invades the soft tissues of the orbit, the approach is extended to include orbital exenteration.68
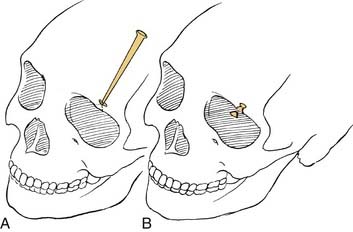
If disease extirpation mandates the removal of the planum, the intracranial portions of the optic nerves should be exposed through the unroofing of the optic canal to protect them from injury at the time of sphenoid osteotomy.14 If the disease is confined to the cribriform area, however, the planum may be entered with a cutting burr or osteotome66 to establish the posterior bony margin without optic nerve decompression. This completes the intracranial portion of the exposure for anterior craniofacial resection.
At this point, a reciprocating saw or cutting drill bit is used to create the osteotomies of the cranial floor. The frontal lobes are retracted or protected from the cutting instrument by inserting an appropriate malleable retractor. The reciprocating saw or cutting drill bit is introduced through the nasal and ethmoid exposures, and, under direct vision, osteotomies are created from the planum sphenoidale, along the ethmoid roof, and forward to the front of the cribriform plate. These osteotomies may or may not include the supraorbital bar of bone, which is a portion of the frontal bone between the supraorbital rims (see Fig. 174-15D). If the tumor extends anteriorly to a significant degree, this supraorbital bar is removed with it as a single specimen, which can be closely inspected. If the supraorbital bar is not actually involved by tumor, it may be detached from the specimen and replaced for reconstruction at the completion of the operation.
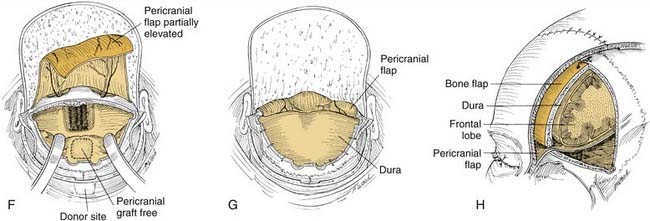
After the resection margins have been verified by frozen section and are negative, reconstruction begins. As mentioned, it is critical that the dura be closed in a watertight fashion. This usually involves the placement of a patch of pericranium, temporal fascia, or fascia lata, followed by the placement of a vascularized pericranial flap over the defect in the floor of the anterior cranial fossa. The pericranial flap is developed by sharp dissection from the previously reflected scalp flap (see Fig. 174-15F).69 Elevation of this flap of pericranium is continued down to the level of the glabella, with care taken to not injure its vascular pedicles. It is then rotated intracranially and positioned to form a bridge of soft tissue across the orbital roofs and back to the planum sphenoidale. If necessary, the distal end of the pericranial flap may be sutured to the dura over the planum for security; this provides a vascularized tissue barrier between the dura above and the nasal cavity below (see Fig. 174-15G and H). Unless a large amount of anterior cranial fossa bone has been resected and concern for brain herniation exists, it is usually not necessary to place a bone graft across the bony defect. Also, it is not necessary to place a skin graft on the undersurface of the pericranium (facing the nasal cavity), because this tissue mucosalizes readily on its nasal surface. After the pericranial flap is in place, the spinal drain is clamped so that no further intraoperative CSF decompression will take place. This will allow the gradual reexpansion of the brain to make contact with the pericranial flap, thereby obliterating any residual dead space. Because the pericranial flap traverses the frontal sinus, it is necessary to obliterate the frontonasal ducts and to remove all mucosa from within the sinus. A small amount of fat or free muscle may be packed into the ducts to obliterate any dead space inferiorly. The flap covers the frontonasal duct orifices and sinus floor. If the sinus is small, it may be obliterated by packing it with abdominal fat. If the sinus is large, however, removing the posterior table completely and allowing the brain and dura to expand and fill the space (cranialization of the frontal sinus) may be advisable.
Basal Subfrontal Approach
The basal subfrontal approach or transbasal approach (Fig. 174-16)14,70 is in many ways similar to the anterior craniofacial resection operation, except that the transfacial exposure is less extensive. Because the target area in this approach is more posterior (sphenoid and clivus) than that in the anterior craniofacial resection (ethmoids and cribriform), the craniotomy bone flap generally is somewhat larger and the orbital bone cuts are broader. This approach is used for lesions that primarily or secondarily involve the bony cranial base in the regions of the sphenoid body and upper clivus (e.g., meningiomas, fibrous dysplasia, chordomas, chondrosarcomas, ossifying fibromas).63
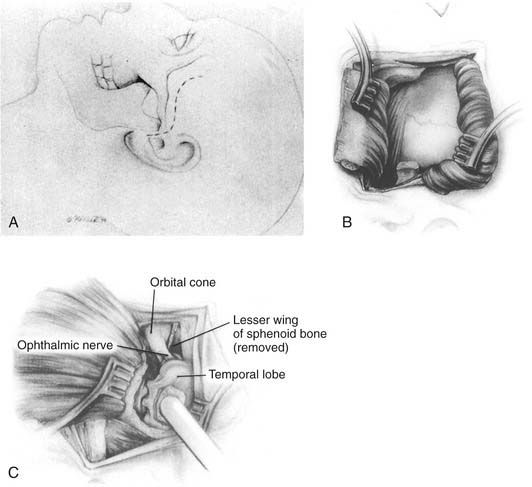
This approach also begins with a bicoronal incision. After exposing the orbital rims, periorbita is elevated from beneath the orbital roofs and medial walls in preparation for osteotomy. The anterior and posterior ethmoid arteries are identified bilaterally as landmarks, but they need not be divided because the axial ethmoid osteotomy can be made just above this level. Bifrontal craniotomy is then performed, and dura is elevated from above the orbital roofs and cribriform areas, as described. Using malleable retractors to protect the orbital contents and the brain, the reciprocating saw is used to create osteotomies that result in the temporary removal of both orbital roofs and the supraorbital bar (see Fig. 174-16A).
The coronal osteotomies along the posterior orbital roof should be made as far posteriorly as possible to simplify reconstruction, to conserve orbital contour, and to prevent postoperative pulsatile exophthalmos. These orbital cuts can be made as far back as the posterior ethmoid foramen but should be made only under direct vision from intra- and extracranial perspectives. With the use of these osteotomies, a very broad and basal exposure of the entire anterior cranial base is achieved (see Fig. 174-16B).
Raveh and colleagues71–73 have described and refined an approach, now generally referred to as the subcranial approach, that offers significant advantages over the traditional anterior craniofacial resection and the basal subfrontal approach while combining elements of each. In the subcranial approach, facial incisions are not used; instead, the bicoronal scalp flap is reflected much further inferiorly to more completely expose both orbits and the nasomaxillary bony framework (Fig. 174-17). Extensive access along the entire anterior cranial base is possible as far back as the sella, the orbital apices, and the upper clivus. Advantages of the subcranial approach include minimal brain retraction, wide exposure, and the absence of facial scars.
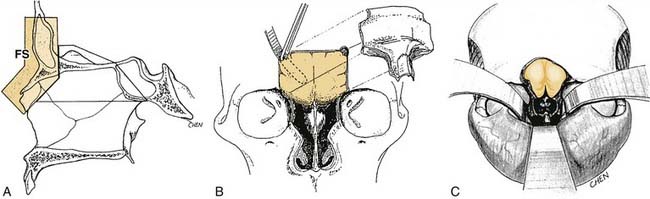
Figure 174-17. Subcranial approach to the anterior cranial base as described by Raveh.71–73 A, Sagittal schematic showing the osteotomized frontonasal segment (FS), which is removed to allow access to deeper structures and then later replaced. B, Initial exposure afforded by the removal of the frontonasal bony segment; note the protection of the dura by a malleable retractor. C, Extent of exposure after lesion extirpation, with sphenoid sinus, sella, and orbital apices visible at the depth of the wound. Note that the bicoronal scalp flap has been elevated quite extensively along the lateral and medial orbital rims and nose to permt the frontonasal osteotomies and to compensate for the very low plane of access to the anterior cranial base while avoiding facial incisions (see text).
(From Raveh J, Laedrach K, Ilzuka T, et al. Subcranial extended anterior approach for skull base tumors: surgical procedure and reconstruction. In: Donald PJ, ed. Surgery of the Skull Base. Philadelphia: Lippincott-Raven; 1998. With permission.)
Middle Cranial Base Approaches
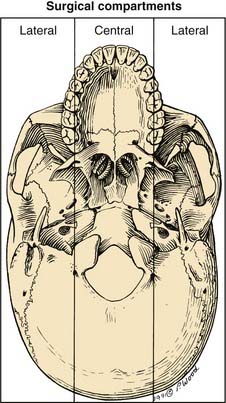
Division into Central and Lateral Compartments
When considering surgical approaches to the middle cranial base, it is helpful to subdivide the region into a single central compartment and paired lateral compartments (Fig. 174-18).74 When viewing the skull from the inferior perspective, the central compartment may be defined as that area between two parasagittal lines drawn from the medial pterygoid plate to the occipital condyle on each side. These lines correspond approximately to the pathways of the ICAs through the skull base. Thus, the central compartment consists of the pituitary fossa, the sphenoid rostrum and lower sphenoid sinus, the nasopharynx, the pterygopalatine fossa, and the lower portion of the clivus. (The lower clivus actually is part of the occipital bone and not technically part of the middle cranial base. However, the surgical approaches to this area and also to the craniovertebral junction and upper cervical spine are essentially extensions of the approaches to the central compartment.)
Approaches to the Central Compartment
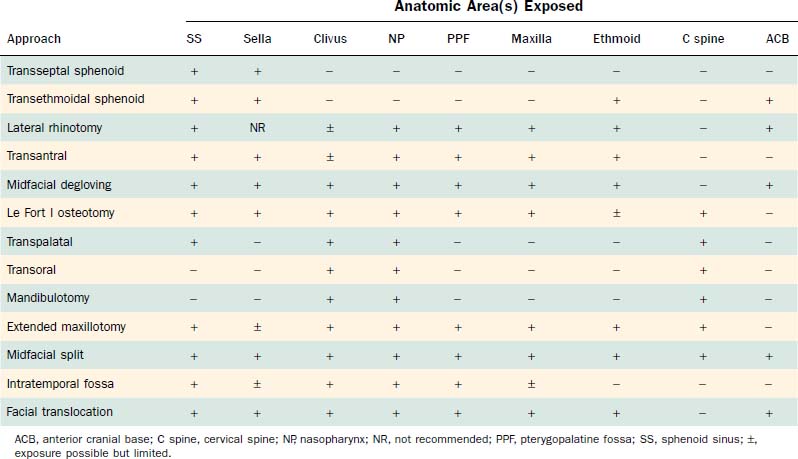
The major surgical approaches to the central compartment of the middle cranial base are listed in Table 174-1, along with the anatomic areas for which they provide useful exposure. Except for pituitary surgery, these approaches are used primarily for the management of extradural lesions along the skull base.
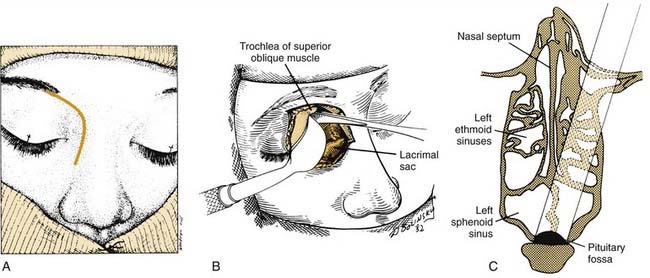
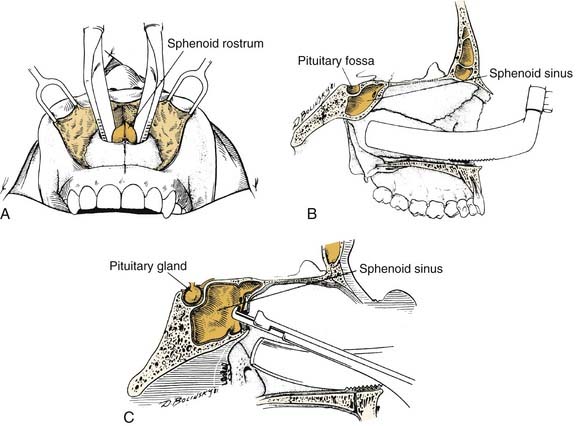
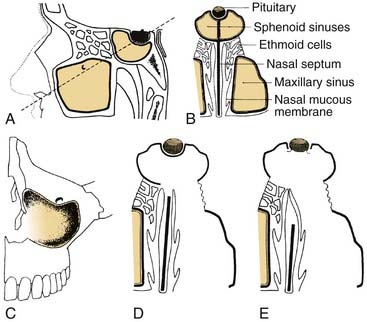
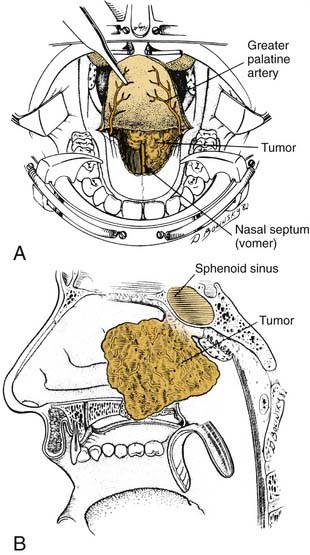
Although most central compartment approaches in the literature can give access to the sphenoid sinus, not all are suitable for pituitary or parasellar surgery because of indirect exposure or oblique angle of entry into the sinus, which increases the risk of injury to adjacent neurovascular structures. When lesions are limited to the sphenoid sinus or the pituitary fossa, the transethmoidal sphenoidotomy (Fig. 174-19)75,76 and the transseptal sphenoidotomy (Fig. 174-20)77,78 are usually satisfactory, safe, and effective. These approaches are useful for obtaining biopsy specimens of more extensive lesions that involve that region. For larger lesions that involve adjacent areas, however, the exposure is inadequate, because the operative field is deep and narrow, thus requiring the use of the operating microscope in most cases.
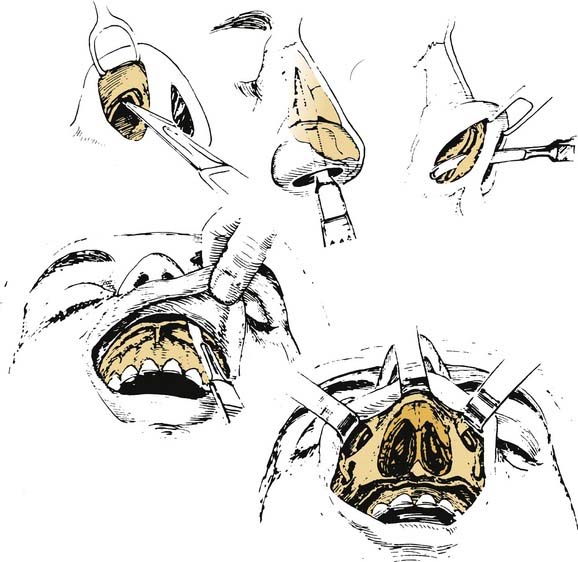
With trends toward less invasive surgical techniques, endoscopic procedures are gaining popularity as primary or adjuvant approaches in cranial base surgery. For tumors of the pituitary gland, endoscopic transnasal-transsphenoidal resection is another valuable option. Proponents of endoscopic pituitary surgery report fewer complications of facial swelling, septal perforations, nasal septal deviation or synechia formation, and numbness of the upper incisors; they also report a shorter hospital stay.79,80 The surgery is performed with the patient under general anesthesia in the supine position with the head elevated 10 degrees. In most cases, the operation can be performed through one nostril, the choice of which depends on the width of the nasal cavity and the laterality of the pituitary tumor. The nose is decongested topically, and further vasoconstriction is achieved by infiltrating the mucosa over the rostrum of the sphenoid, the middle turbinate, and the posterior septum with lidocaine hydrochloride 1% with epinephrine 1 : 100,000.
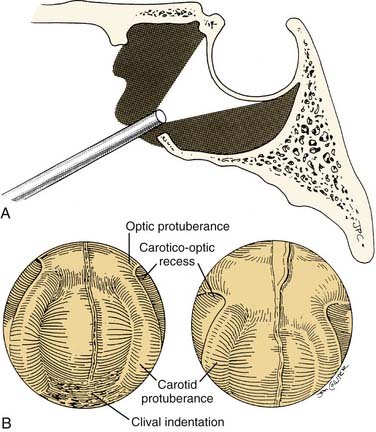
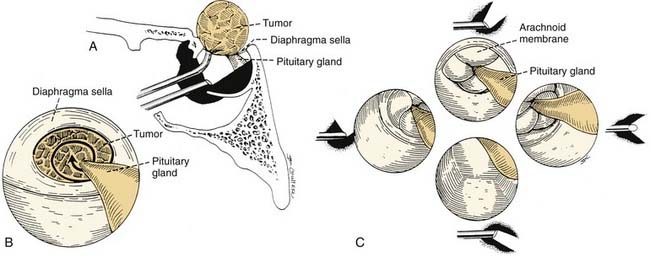
Using a rigid zero-degree endoscope, the sphenoid ostium is identified, and its position may be confirmed by the use of C-arm fluoroscopy or image-guided navigation. The middle turbinate can be outfractured or removed to provide increased exposure to the sphenoid rostrum. The sphenoid ostia are identified and fractured inferomedially. Kerrison rongeurs or Hardy punches are used to widen the sphenoidotomy and to remove the sphenoid rostrum. The posterior septum may be rongeured to provide wide visualization, including that of the contralateral sphenoid sinus cavity. The 30-degree endoscope is inserted into both sides of the sphenoid sinus for identification of the optic and carotid protuberance, the opticocarotid recess, the clival indentation, and the anterior wall of the sella81 (Fig. 174-21). After removal of the intersinus septum, the sella and dura are exposed and entered in the standard fashion. For resection of macroadenomas, insertion of the 30-degree endoscope allows the surgeon to follow intrasellar and extrasellar extension of the tumor and to identify and preserve the arachnoid membrane81 (Fig. 174-22). The ability to directly visualize tumor and healthy pituitary gland through the endoscope is a major advantage over the operating microscope. This increased visualization may allow for more complete tumor resection, while limiting the potential complications associated with blind curettage of the superior and lateral sella contents.
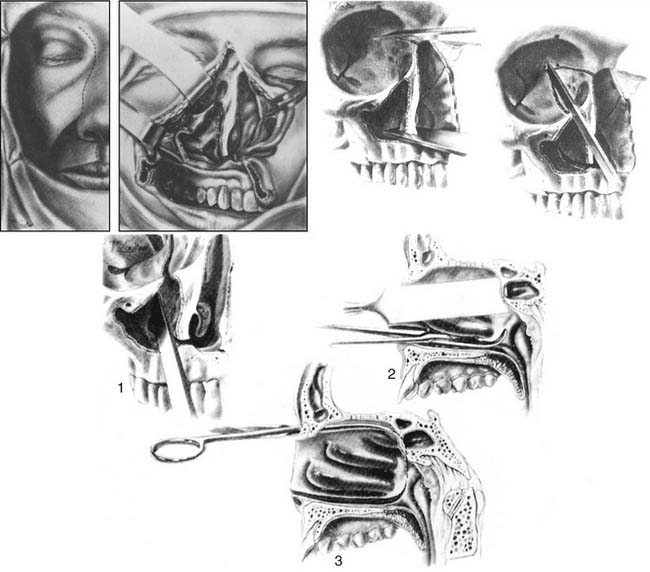
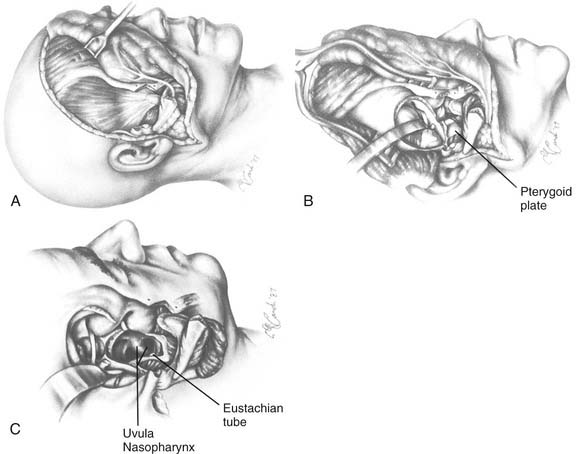
The lateral rhinotomy (Fig. 174-23)82 and transantral (Fig. 174-24)83 procedures afford wider access to the anterior sphenoid and adjacent nasopharynx, the pterygopalatine fossa, the maxilla, and the ethmoid regions. However, they generally are unsatisfactory for extirpation of any but the smallest lesions situated in the sphenoidal-clival area. The midfacial degloving approach (Fig. 174-25)84 is more suitable for dealing with larger central compartment lesions, because it allows for improved midline access through the nose and both maxillary sinuses. Medial maxillectomy and resection of the ascending process of the palatine bone are included, if necessary, giving the surgeon good visualization of the nasopharynx and the adjacent skull base. The Le Fort I osteotomy (Fig. 174-26)85,86 can be an alternative method for reaching these areas from a slightly more inferior angle, or it may be used as an adjunct to midface degloving, offering additional access to the oronasopharynx and clivus by displacing the hard and soft palate inferiorly.
For lesions of the clivus that also involve the upper cervical vertebrae (the craniovertebral junction), mandibulotomy (Fig. 174-27)74,87,88 will afford a paramedian route to reach the lower areas of extension. The transpalatal (Fig. 174-28)89,90 and transoral (Fig. 174-29)91,92 approaches are also used for the management of lesions at the craniovertebral junction and may be used concomitantly with mandibulotomy, if necessary. A significant benefit of such a combined approach is that, with mandibulotomy, it is also possible to expose the parapharyngeal space and therefore to safely dissect along the ICA from the neck up to its entrance into the temporal bone.74,87,88 This advantage is important when removing tumors that arise primarily in the central compartment but that have extended laterally. Conversely, for lesions that originate in the lateral compartment and extend centrally, the infratemporal fossa approach or one of its modifications can be used.93 These approaches and the facial translocation approach,17 which is suitable for lesions in both compartments, will be discussed in the following section.
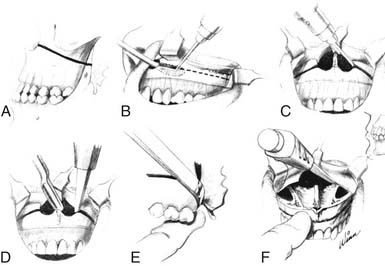
An alternative approach for the removal of extensive lesions of the clivus and craniovertebral junction is the extended maxillotomy operation or its modification, the subtotal maxillectomy (Fig. 174-30).94 In general, these innovative techniques combine the advantages of many approaches. They afford wide exposure of the central compartment by unilaterally displacing or resecting the hemimaxilla.
For very extensive clival and craniovertebral junction tumors, when bilateral transmaxillary access is needed, the midfacial split approach (Fig. 174-31) can be used.51,57 This approach provides access to the entire central skull base in a unified surgical field that can extend vertically from the anterior fossa floor to the level of the second cervical vertebra (and lower, if combined with mandibulotomy) and horizontally from jugular fossa to jugular fossa. This exposure is achieved using craniofacial disassembly to completely displace the midfacial skeleton, including both maxillae, the orbital floors, and the palate. It is especially useful for the management of large tumors that originate in the central compartment and that have also involved the adjacent anterior cranial base, the craniovertebral junction, or the lateral compartment.
Approaches to the Lateral Compartment
Transtemporal Approaches
The transtemporal routes include the transcochlear,95 translabyrinthine,15,96 presigmoid, retrolabyrinthine,2 and combined97 approaches. These are lateral, primarily extradural techniques that traverse the mastoid and petrous portions of the temporal bone to provide exposure of lesions of the petrous apex, the clivus, and the cerebellopontine angle. The anterior limit of purely transtemporal approaches is the intrapetrous course of the ICA, and these approaches therefore give only limited access to the middle cranial base. Accordingly, they more often are used for the management of tumors of the posterior cranial base (e.g., acoustic neuromas, petroclival meningiomas, aggressive cholesteatomas), and they will therefore be discussed in more detail in Chapter 177. With respect to the middle cranial base, the transtemporal approaches are useful mainly as adjunctive techniques in combination with craniotomy, infratemporal, or transfacial approaches. They can be used to enhance exposure of advanced lesions of the middle cranial base that have secondarily extended into or beyond the petroclival region and posterior to the course of the ICA. Postoperative consequences include permanent unilateral deafness with or without disequilibrium caused by functional loss of the middle or inner ear structures and facial paralysis of variable degree and duration caused by seventh nerve decompression or transposition.
Infratemporal Approaches
The infratemporal fossa approach of Fisch and colleagues93,98,99 encompasses three variations for use in specific clinical situations (Fig. 174-32). The type A approach provides exposure between the sigmoid sinus and the condylar fossa, and it is designed to reach to the petrous apex and infralabyrinthine areas. It is most useful for the management of cholesteatomas, meningiomas, and glomus tumors of those regions. The type B approach allows access from the sigmoid sinus to the petrous tip (including exposure of the horizontal ICA and foramen ovale) to reach lesions of the clivus, such as chordomas, meningiomas, and extensive apex cholesteatomas. The type C approach expands this access to include the parasellar region, the cavernous sinus, the foramen rotundum, and the foramen lacerum. Removal of the pterygoid plates in this approach also facilitates access to the nasopharynx. This approach is used in the resection of small nasopharyngeal carcinomas, adenoid cystic carcinomas, and angiofibromas. All three variations of the infratemporal fossa approach involve mastoidectomy, facial nerve dissection (and transposition), and obliteration of the eustachian tube, middle ear, and external auditory canal with resultant permanent conductive hearing deficit. Thus, types A, B, and C infratemporal fossa approaches share some features of the basic transtemporal approaches and yet extend the field of access anteriorly to reach the middle cranial base by virtue of exposing and controlling the petrous ICA (see Chapter 176).
For lesions of the middle cranial base that involve the infratemporal fossa, the upper pterygomaxillary space, the lateral orbit, and the orbital apex, the lateral facial approach100 (Fig. 174-33) and the lateral transtemporal sphenoid approach101 (Fig. 174-34) have been described. These procedures reach the infratemporal fossa from a slightly more superior direction by displacing the zygomatic arch inferiorly and reflecting the temporalis muscle. The greater wing of the sphenoid is followed to the lateral pterygoid plate, which is used as a surgical landmark, as outlined earlier. These approaches also incorporate partial removal of the greater wing of the sphenoid, which means that they include subtemporal craniectomy and extradural dissection. The advantages of these procedures are that they require no facial nerve dissection (the temporal branch of cranial nerve VII is retracted inferiorly with the skin flap), and they allow some access to the cranial base from both intracranial and extracranial perspectives.
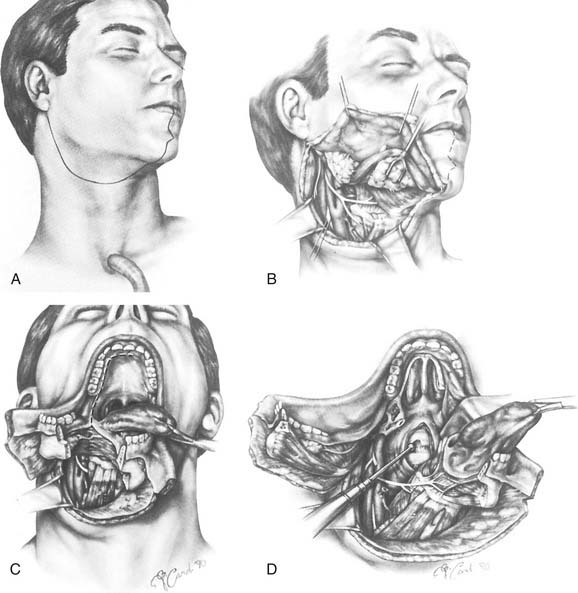
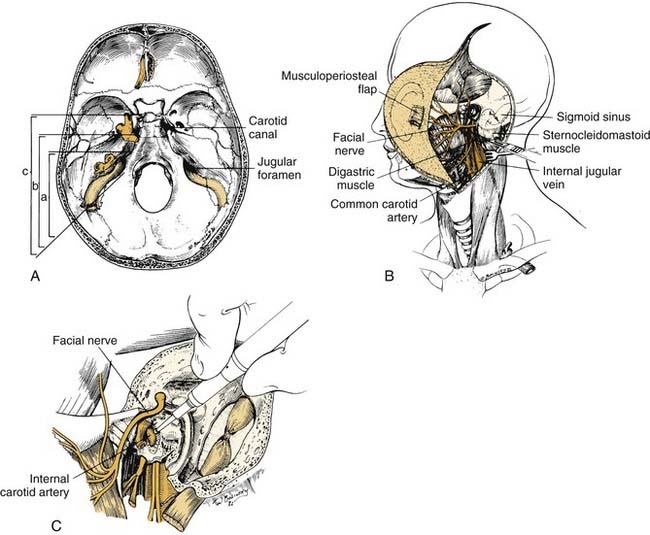
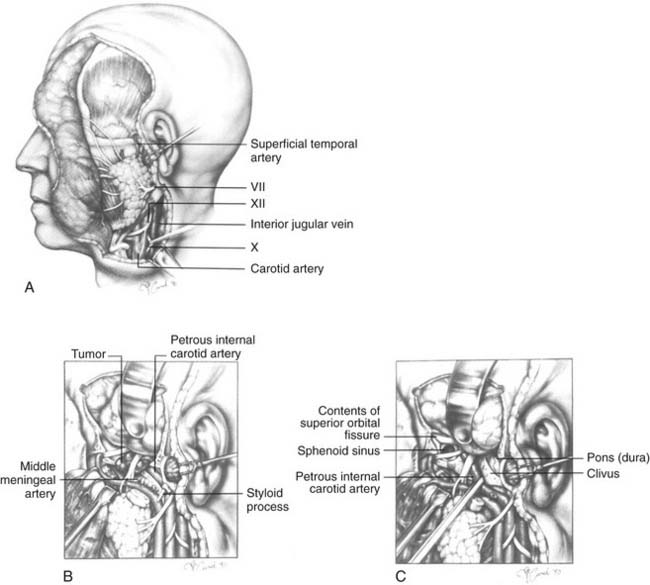
The subtemporal-preauricular infratemporal approach (Fig. 174-35)14,102 is a combination of the transparotid and lateral transtemporal sphenoid procedures in addition to temporal craniotomy. It provides excellent exposure of much of the middle cranial base and allows for improved access for intracranial dissection when necessary. The orientation of the exposure begins laterally and progresses medially so that it is useful for the management of lesions of the greater sphenoid wing, the petrous apex, and the middle and lower clivus. This approach is used extensively for the resection of tumors such as meningiomas, chondrosarcomas, chordomas, and petrous apex cholesteatomas. It illustrates many important techniques used in surgery of the middle cranial base.
The intrapetrous course of the ICA may be exposed, beginning at the infratemporal opening of the carotid canal and extending to just above the foramen lacerum (where the ICA enters the cavernous sinus). This is accomplished with a high-speed drill and a diamond burr using a technique analogous to that used for facial nerve decompression. To facilitate this exposure, the middle meningeal artery and the GSPN should first be divided, and the high cervical ICA with its fibrous periosteal sheath should be rendered accessible by removal of the styloid process and the surrounding bone. The ICA may be displaced forward out of its bony canal; this is useful whenever the lesion involves the clivus medial to the ICA or the petrous part of the temporal bone posterior to the ICA. The inferior aspect of the cavernous sinus may also be approached from this exposure.7
Transfacial Approaches
Transfacial exposure of the cranial base, particularly of the lateral compartment (middle cranial base region), is not a new concept. Operative procedures for the removal of neoplasms from this region were described by several authors in the 1960s.103,104 These early procedures, which were used initially for the radical resection of malignant tumors, resulted in unacceptably high morbidity and mortality rates and considerable deformity, largely because methods to adequately reconstruct major cranial base defects were not yet available. With the introduction of craniofacial disassembly techniques and vascularized reconstructive flaps, it has become possible to dismantle the facial skeleton, to extirpate deep-seated lesions, and to perform functional reconstruction to a greater extent than ever. Accordingly, lesions of the lateral and central compartments are more amenable to surgery.
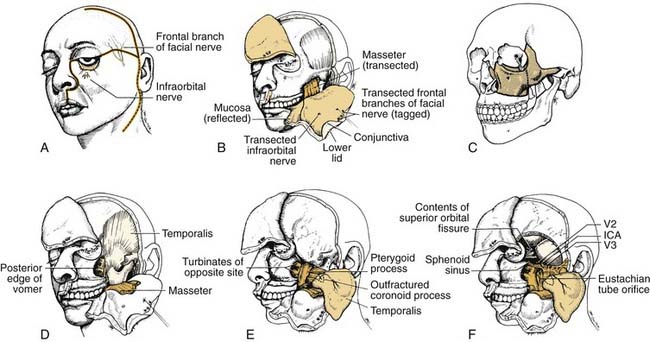
A transfacial approach to this region is desirable because it eliminates the viscerocranial skeleton as an obstacle to exposure, thereby opening up the entire infratemporal fossa and the nasal cavity, the nasopharynx, the pterygopalatine fossa, and the sphenoid region to direct access all at once. The facial translocation approach (Fig. 174-36)17 is such a procedure.
Incisions are made in the face and scalp as shown in Figure 174-36A. The horizontal incision connects a lateral rhinotomy with a hemicoronal incision to create superior and inferior soft tissue flaps. (At this stage, the frontal branches of the facial nerve are identified using evoked EMG, tagged, and divided to be reconnected later on during the procedure. This horizontal incision is a key element in the exposure because it allows a single, unified surgical field that is unhampered by the need to work alternatively from separate cranial and facial incisions that limit the surgeon’s ability to develop three-dimensional exposure at the cranial base.) The soft tissue flaps are then elevated in the subperiosteal plane from the zygoma and maxilla. Periorbita is likewise elevated from the lateral, inferior, and medial orbital rims and walls. A reciprocating saw is used to create osteotomies, and the orbitozygomaticomaxillary skeletal segment is temporarily removed.
Intracranial Approaches
Temporal craniotomy is the traditional, standard neurosurgical method for gaining access to the middle cranial fossa. For intradural lesions within the temporal lobe or for extradural lesions along the lateral convexity of the middle fossa, it is the most direct approach.105 For lesions at the cranial base, however, temporal craniotomy alone is often less than ideal, because it requires (by virtue of its superolateral-to-inferomedial orientation) significant retraction or, in some cases, resection of the brain to reach a target area. Such brain manipulation can result in intraoperative and postoperative cerebral edema; temporal encephalomalacia; deficits of speech, memory, and cognition; and long-term risk of seizure disorder. These risks increase as the location of the lesion becomes more medial and more temporal lobe retraction is required.
The middle fossa approach of House106 involves the use of temporal craniotomy as a preliminary step to reach the IAC by the removal of bone from the superior surface of the petrous portion of the temporal bone. Because most lesions in these areas originate in relation to the seventh and eighth cranial nerves, this approach is considered in more detail in Chapters 171, 176, and 177.
When the skull base is involved only to a minimal extent and the lesion is mostly infracranial, a subtemporal craniectomy may be performed (see Figs. 174-33 and 174-36). In this procedure, only a limited area of cranial bone is removed, generally as a safe means of establishing a superior margin in resecting, for example, the base of the pterygoid plates and surrounding soft tissue. Subtemporal craniectomy may be performed by inserting a Kerrison or a similar rongeur extradurally inside the foramen ovale and gradually removing bone in the direction of the foramen rotundum. The resulting bone window allows for the protection of the middle fossa dura under direct vision while the subjacent lesion is removed.
For many lesions of the lateral compartment of the middle cranial base, temporal craniotomy and subtemporal craniectomy are used in combination with transtemporal, infratemporal, or transfacial exposure. These intracranial approaches provide added control over the neural and vascular structures in the region. As is true for all transcranial operations for cranial base lesions, close cooperation between the otolaryngologist and the neurosurgeon is essential.107
Reconstruction
As stated previously, use of locoregional vascularized flaps is the primary reconstructive method for skull base defects; however, support is growing for microvascular reconstruction of these defects, especially large ones, or in revision surgery, when locoregional flaps may be unavailable.53,54,108
In a retrospective study, Newman and associates found a 95% success rate in reconstructing anterior and middle skull base defects. Despite a 30% overall complication rate, no cases of intracranial complication or CSF leak or deaths were associated with this reconstruction.109 Other authors have experienced higher rates of complications including flap failure and intracranial problems (cerebrovascular accident [CVA], syndrome of inappropriate antidiuretic hormone [SIADH], meningitis, seizure, pneumocephalus, CSF leak), although most reported series have been smaller with a retrospective study design.110
The progression of expanded endoscopic approaches has also necessitated the development of nonvascularized materials to repair skull base defects. Acellular dermis, hydroxyapatite cement, free abdominal fat, autologous cartilage and bone grafts may be used to match each defect. Of importance, hydroxyapatite cement must not be allowed to contact the sinonasal mucosa, to avoid infection and failure.2,111
Postoperative Concerns
Complications
Many of the most serious complications of cranial base surgery are related to the brain,14 and their prevention and management demand neurosurgical expertise. First, brain manipulation, especially by excessive retraction, can lead to cerebral edema, subdural hematoma, and acute brain dysfunction specific to the anatomic site involved. In the long term, it may cause encephalomalacia and deficits of speech, memory, and cognitive and intellectual functions. Brain injury (by contusion) also may predispose the patient to seizures, especially when the temporal lobe is affected. These problems are best prevented by obtaining adequate surgical exposure through basal bone removal and craniofacial disassembly (thereby limiting the need for significant brain manipulation) and by intraoperative brain relaxation through the use of subarachnoid CSF drainage, hyperventilation, and corticosteroids.
CSF fistula is another brain-related complication that is important because of its association with meningitis. It usually manifests as rhinorrhea, but it also may cause the patient to experience a salty taste in the throat. If the nature of nasal discharge is doubtful, the β2-transferrin assay may be used to confirm or rule out the presence of CSF.112 For small-volume leaks, which are the most common, observation and continued spinal drainage (or serial lumbar spinal taps) usually suffice. For high-volume leaks (grossly apparent rhinorrhea) or those that are persistent, careful sinonasal endoscopy or CT cisternography can be used to localize the fistula site (i.e., frontal, ethmoid, sphenoid, eustachian tube, temporal bone), after which operative repair frequently is needed. A CSF fistula may manifest as otorrhea or as wound discharge; the management principles are similar in either case. In some situations, the original repair simply needs to be revised; in others, additional vascularized tissue may be needed to obliterate the fistula site. Such tissues may include temporalis muscle, galeal, or free microvascular flaps (e.g., rectus abdominis, omentum). In selected cases, the closure may be augmented by the judicious use of free autogenous fat grafts or fibrin glue. The risk of reconstructive failure is substantially decreased if the original repair is performed using vascularized local tissue.69
Cranial nerve deficits deserve special consideration because of their profound impact on postoperative recovery and quality of life. The sacrifice of olfactory nerves (cranial nerve I) often is regarded as a minor disability, but it can contribute to significant malnutrition in some patients who, because of anosmia, no longer enjoy eating. Deficits of cranial nerves II, III, IV, and VI lead to visual disability of variable degree, depending on the extent of surgical trauma and nerve involvement by the disease process itself. Dense palsies of the extraocular muscles seldom recover completely. Patients in whom such deficits are predictable should therefore be prepared for the loss of binocular vision before surgery is undertaken. Extraocular muscle surgery by the ophthalmologist can sometimes help to compensate for such deficits. The loss of visual acuity is a potential complication of anterior cranial base surgery, but it is unusual unless the optic nerves are compromised preoperatively. In these patients, recovery of optic nerve function is difficult to predict.63
Many complications of cranial base surgery are not brain related (Table 174-2) and can affect any physiologic system. Despite the relatively less invasive appearance of endoscopic skull base surgery, these procedures carry many of the same risks of more traditional open skull base procedures. CSF leaks remain the most common complication, and additional serious complications including damage to cranial nerves, meninges, blood vessels, and venous sinuses can present in the immediate postoperative period. Delayed complications include meningitis, brain abscess, and tension pneumocephalus, anosmia, and synechia formation. Visual loss or changes may result from damage to the optic nerve or chiasm or orbital contents, including extraocular musculature. Damage to the pituitary stalk also may result with transsphenoidal approaches.38 Postoperative diabetes insipidus rates are reported to be as much as 50% lower in endoscopic cases.113
Table 174-2 Non-neurologic Complications in Cranial Base Surgery
| Type of Complication | Examples |
|---|---|
| Cardiovascular |
Outcomes
Although the many technical advances and multispecialty approaches in cranial base surgery have expanded the ability to achieve complete and safe tumor resection, the question remains regarding the extent that patients benefit from these invasive procedures. A 1995 review of 40 years of cranial base surgery for carcinomas and sarcomas provided some insight into the answer to this question.114 Three general eras of major advancement in the field were analyzed for survival outcomes and trends in morbidity.
From the 1960s to the 1970s, surgical pioneers achieved 3- and 5-year survival rates of 52% and 49%, respectively. The application of skull base approaches was limited, and tumors were designated as inoperable if they extended intracranially or into the pterygopalatine fossa. Morbidity rates were significant, with overall infection rates of 54% and CSF leaks occurring in 31% of cases. From the 1970s to the 1980s, the number of cranial base cases and the number of surgeons performing these cases increased. The major advancement was that patients with tumors previously designated as inoperable underwent attempts at complete tumor resection. Reports included tumors that extended intracranially and intracerebrally and into the pterygopalatine fossa. The 3-year survival rates rose slightly from 57% to 59%, with only limited reports of 5-year survival rates in the range of 49%. Infection rates ranged from 4% to 48%, but the incidence of CSF leaks dropped from 3% to 19%. From the 1980s to the 1990s, a multispecialty approach to cranial base surgery was introduced. Complications dropped significantly, with infection rates ranging from zero to 28% and CSF leaks diminishing from 2% to 4%. Another significant factor is that local disease control improved greatly. The 3-year survival rate increased from 67% to 74%, and the 5-year survival rate increased from 56% to 70%.114 A recent multi-institutional study of patients undergoing craniofacial resection revealed 5-year overall, disease-specific, and recurrence-free survival rates of 48%, 53%, and 45%, respectively. Poor prognostic indicators included positive surgical margins and intracranial and orbital involvement. Melanoma and undifferentiated carcinoma carried the worst outcomes and esthesioneuroblastoma and low-grade sarcoma the best.115
In a large multi-institutional study of craniofacial resection patients, postoperative complications occurred in 36%. Increased risk of postoperative complications was found with the following factors; comorbid illness, previous radiation therapy, and tumor extension. Overall wound complication rates were less than 20%.116
Although reports in the literature are encouraging,117–119 a need for controlled investigations and prospective clinical trials remains. This undertaking will prove to be very difficult. Unlike head and neck cancer, in which most cases are squamous cell carcinoma, cranial base tumors are less common and more varied in histologic type. Compounding the problem of broad histologic variation is the lack of uniformity of location and patterns of invasion of tumors in this complex anatomic region. When these factors are taken into consideration, investigations with adequate patient numbers to yield an accurate reflection of outcomes become nearly impossible. Multi-institutional studies may be required to circumvent these difficulties and to provide important information about the value of cranial base surgery. Such collaborative efforts may help establish the consistent use of primary and adjuvant therapies for tumors of the cranial base.
Anand VK, Schwartz TH. Practical Endoscopic Skull Base Surgery. San Diego: Plural Publishing; 2007.
Barnes L, editor. Surgical Pathology of the Head and Neck, 3rd ed, New York: Informa HealthCare, 2008.
Carrau RL, Snyderman CH, Vescan AD, et al. Cranial base surgery. Section 11. Myers EN, Eibling DE, editors. Operative Otolaryngology–Head and Neck Surgery, 2nd ed, Philadelphia: Elsevier, 2005.
Wenig BM. Atlas of Head and Neck Pathology. Philadelphia: WB Saunders; 1993.
1. Keros P. On the practical value of differences in the level of the lamina cribrosa of the ethmoid. Z Laryngol Rhinol Otol. 1962;41:809-813.
2. Mehta RP, Cueva RA, Brown JD, et al. What’s new in skull base medicine and surgery? Skull Base Committee Report. Otolaryngol Head Neck Surg. 2006;135:620-630.
3. Hayashi N, Kubo M, Tsuboi Y, et al. Impact of anomalous origin of the ophthalmic artery from the middle meningeal artery on selection of surgical approach to skull base meningioma. Surg Neurol. 2007;68:568-571.
4. Janecka IP, Sekhar LN, Horton JA, Yonas H. Cerebral blood flow evaluation. Update II. Cummings C, Fredrickson JM, Harlor LA, et al, editors. Otolaryngology–Head and Neck Surgery. St Louis: Mosby. 1991:54-63.
5. de Vries EJ, Sekhar LN, Janecka IP, et al. Elective resection of the internal carotid artery without reconstruction. Laryngoscope. 1988;98:960-966.
6. Erba SM, Horton JA, Latchaw RE, et al. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533-538.
7. Sekhar LN, Schramm VLJr, Jones NF, et al. Operative exposure and management of the petrous and upper cervical internal carotid artery. Neurosurgery. 1986;19:967-982.
8. Sekhar LN, Sen CN, Jho HD, et al. Surgical treatment of intracavernous neoplasms: a four-year experience. Neurosurgery. 1989;24:18-30.
9. Austin MB, Mills SE. Neoplasms and neoplasm-like lesions involving the skull base. Ear Nose Throat J. 1986;65:57-73.
10. Dickins JR. Approach to diagnosis of skull base lesions. Am J Otol. 1981;3:35-45.
11. Dickins JR, Graham SS. Differential diagnosis of tumors at the cranial base. In: Jackson CG, editor. Surgery of Skull Base Tumors. New York: Churchill Livingstone; 1991:5-9.
12. Elia AE, Shih HA, Loeffler JS. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23:E5.
13. Martin JJ, Niranjan A, Kondziolka D, et al. Radiosurgery for chordomas and chondrosarcomas of the skull base. J Neurosurg. 2007;107:758-764.
14. Sekhar LN, Janecka IP. Intracranial extension of cranial base tumors and combined resection: the neurosurgical perspective. In: Jackson CG, editor. Surgery of Skull Base Tumors. New York: Churchill Livingstone, 1991.
15. Gonzalez RM, et al. Special aesthetic challenges in skull base tumor surgery. In: Jackson CG, editor. Surgery of Skull Base Tumors. New York: Churchill Livingstone; 1991:49-56.
16. Donald PJ. Skull base surgery for malignancy: when not to operate. Eur Arch Otorhinolaryngol. 2007;264:713-717.
17. Janecka IP, Sen CN, Sekhar LN, et al. Facial translocation: a new approach to the cranial base. Otolaryngol Head Neck Surg. 1990;103:413-419.
18. Margalit N, Ezer H, Fliss DM, et al. Orbital tumors treated using transcranial approaches: surgical technique and neuroophthalmogical results in 41 patients. Neurosurg Focus. 2007;23:E11.
19. Jackson IT, Marsh WR, Bite U, et al. Craniofacial osteotomies to facilitate skull base tumour resection. Br J Plast Surg. 1986;39:153-160.
20. Tessier P. Total facial osteotomy. Crouzon’s syndrome, Apert’s syndrome: oxycephaly, scaphocephaly, turricephaly. Ann Chir Plast. 1967;12:273-286.
21. Berry J, O’Malley BWJr, Humphries S, et al. Making image guidance work: understanding control of accuracy. Ann Otol Rhinol Laryngol. 2003;112:689-692.
22. Grunert P, Darabi K, Espinosa J, et al. Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003;26:73-99.
23. Han JK, Hwang PH, Smith TL. Contemporary use of image-guided systems. Curr Opin Otolaryngol Head Neck Surg. 2003;11:33-36.
24. Javer AR, Kuhn FA. Stereotactic computer-assisted navigational sinus surgery: historical perspective and review of the available systems. J Otolaryngol. 2001;30:60-64.
25. Koele W, Stammberger H, Lackner A, et al. Image guided surgery of paranasal sinuses and anterior skull base—five years experience with the InstaTrak-System. Rhinology. 2002;40:1-9.
26. Metson R. Image-guided sinus surgery: lessons learned from the first 1000 cases. Otolaryngol Head Neck Surg. 2003;128:8-13.
27. Citardi MJ, Batra PS. Intraoperative surgical navigation for endoscopic sinus surgery: rationale and indications. Curr Opin Otolaryngol Head Neck Surg. 2007;15:23-27.
28. Schlosser RJ, Bolger WE. Image-guided procedures of the skull base. Otolaryngol Clin North Am. 2005;38:483-490.
29. American Academy of Otolaryngology–Head and Neck Surgery. Intra-operative use of computer aided surgery. http://www.entnet.org/Practice/policyIntraOperativeSurgery.cfm, 2009. Available at
30. Lewin JS, Nour SG, Meyers ML, et al. Intraoperative MRI with a rotating, tiltable surgical table: a time use study and clinical results in 122 patients. AJR Am J Roentgenol. 2007;189:1096-1103.
31. Batra PS, Citardi MJ, Worley S, et al. Resection of anterior skull base tumors: comparison of combined traditional and endoscopic techniques. Am J Rhinol. 2005;19:521-528.
32. Carrau RL, Snyderman CH, Kassam AB, et al. Endoscopic and endoscopic-assisted surgery for juvenile angiofibroma. Laryngoscope. 2001;111:483-487.
33. Chee LW, Sethi DS. The endoscopic management of sinonasal inverted papillomas. Clin Otolaryngol Allied Sci. 1999;24:61-66.
34. Pillay PK, Leo CW, Sethi DS. Computer-aided/image-guided and video-endoscopic resection of pituitary tumors. Stereotact Funct Neurosurg. 2000;74:203-209.
35. Tosun F, Carrau RL, Snyderman CH, et al. Endonasal endoscopic repair of cerebrospinal fluid leaks of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2003;129:576-580.
36. Dave SP, Bared A, Casiano RR. Surgical outcomes and safety of transnasal endoscopic resection for anterior skull tumors. Otolaryngol Head Neck Surg. 2007;136:920-927.
37. Lund V, Howard DJ, Wei WI. Endoscopic resection of malignant tumors of the nose and sinuses. Am J Rhinol. 2007;21:89-94.
38. Stamm AM. Transnasal endoscopy-assisted skull base surgery. Ann Otol Rhinol Laryngol Suppl. 2006;196:45-53.
39. Isaacs SJ, Goyal P. Endoscopic anatomy of the pterygopalatine fossa. Am J Rhinol. 2007;21:644-647.
40. Bassim MK, Senior BA. Endoscopic anatomy of the parasellar region. Am J Rhinol. 2007;21:27-31.
41. Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3.
42. Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19:E4.
43. Kassam AB, Gardner P, Snyderman C, et al. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6.
44. Tan YC, Yu CC, Chang CN, et al. Optic nerve compression in craniofacial fibrous dysplasia: the role and indications for decompression. Plast Reconstr Surg. 2007;120:1957-1962.
45. Pletcher SD, Sindwani R, Metson R. Endoscopic orbital and optic nerve decompression. Otolaryngol Clin North Am. 2006;39:943-958. vi
46. Kassam AB, Thomas AJ, Zimmer LA, et al. Expanded endonasal approach: a fully endoscopic completely transnasal resection of a skull base arteriovenous malformation. Childs Nerv Syst. 2007;23:491-498.
47. Pirris SM, Pollack IF, Snyderman CH, et al. Corridor surgery: the current paradigm for skull base surgery. Childs Nerv Syst. 2007;23:377-384.
48. Kassam AB, Vescan AD, Carrau RL, et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108:177-183.
49. Kabil MS, Shahinian HK. The endoscopic supraorbital approach to tumors of the middle cranial base. Surg Neurol. 2006;66:396-401.
50. Placantonakis DG, Tabaee A, Anand VK, et al. Safety of low-dose intrathecal fluorescein in endoscopic cranial base surgery. Neurosurgery. 2007;61:161-165.
51. Janecka IP, Sekhar LN. Reconstruction of base of skull surgical defects. In: Jackson CJ, editor. Surgery of Skull Base Tumors. New York: Churchill Livingstone; 1991:251-272.
52. Jones NF, Schramm VL, Sekhar LN. Reconstruction of the cranial base following tumour resection. Br J Plast Surg. 1987;40:155-162.
53. Germani RM, Vivero R, Herzallah IR, et al. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol. 2007;21:615-618.
54. Weber SM, Kim JH, Wax MK. Role of free tissue transfer in skull base reconstruction. Otolaryngol Head Neck Surg. 2007;136:914-919.
55. O’Malley BWJr, Weinstein GS. Robotic skull base surgery: preclinical investigations to human clinical application. Arch Otolaryngol Head Neck Surg. 2007;133:1215-1219.
56. Hanna EY, Holsinger C, DeMonte F, et al. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209-1214.
57. Janecka IP, Nuss DW, Sen CN. Midfacial split for access to the central base. Acta Neurochir Suppl (Wien). 1991;53:199-203.
58. Lauritzen C, Vallfors B, Lilja J. Facial disassembly for tumor resection. Scand J Plast Reconstr Surg. 1986;20:201-206.
59. Nuss DW, Janecka IP, Sekhar LN, et al. Craniofacial disassembly in the management of skull-base tumors. Otolaryngol Clin North Am. 1991;24:1465-1497.
60. Calcaterra TC. Extracranial surgical repair of cerebrospinal rhinorrhea. Ann Otol Rhinol Laryngol. 1980;89:108-116.
61. Montgomery WW. Surgery of the frontal sinus. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JB Lippincott, 1984.
62. Abul-Hassan HS, von Drasek Ascher G, Acland RD. Surgical anatomy and blood supply of the fascial layers of the temporal region. Plast Reconstr Surg. 1986;77:17-28.
63. Derome PJ, Visot A. Bony lesions of the anterior and middle cranial fossa. In: Sekhar LN, Sweet WH, editors. Tumors of the Cranial Base: Diagnosis and Treatment. Mount Kisco, NY: Futura Publishing; 1987:321-325.
64. Stuzin JM, Wagstrom L, Kawamoto HK, et al. Anatomy of the frontal branch of the facial nerve: the significance of the temporal fat pad. Plast Reconstr Surg. 1989;83:265-271.
65. Johns ME, Kaplan MJ. Surgical approach to the anterior skull base. Ear Nose Throat J. 1986;65:117-124.
66. Schramm V. Craniofacial resection. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JB Lippincott, 1984.
67. Schramm VLJr, Myers EN, Maroon JC. Anterior skull base surgery for benign and malignant disease. Laryngoscope. 1979;89:1077-1091.
68. Carrau RL, Segas J, Nuss DW, et al. Squamous cell carcinoma of the sinonasal tract invading the orbit. Laryngoscope. 1999;109:230-235.
69. Snyderman CH, Janecka IP, Sekhar LN, et al. Anterior cranial base reconstruction: role of galeal and pericranial flaps. Laryngoscope. 1990;100:607-614.
70. The transbasal approach to tumors invading the base of the skull. In: Schmidek HH, Sweet WH, editors. Current Techniques in Operative Neurosurgery. New York: Grune & Stratton; 1977:92-104.
71. Raveh J, Laedrach K, Ilzuka T, et al. Subcranial extended anterior approach for skull base tumors: surgical procedure and reconstruction. In: Donald PJ, editor. Surgery of the Skull Base. Philadelphia: Lippincott-Raven; 1998:309.
72. Raveh J, Laedrach K, Speiser M, et al. The subcranial approach for fronto-orbital and anteroposterior skull-base tumors. Arch Otolaryngol Head Neck Surg. 1993;119:385-393.
73. Raveh J, Turk JB, Ladrach K, et al. Extended anterior subcranial approach for skull base tumors: long-term results. J Neurosurg. 1995;82:1002-1010.
74. Krespi YP, Sisson GA. Transmandibular exposure of the skull base. Am J Surg. 1984;148:534-538.
75. Kirchner JA. Transethmoidal approach to the sella. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JB Lippincott, 1984.
76. Sasaki CT. Pituitary ablation for carcinoma of the breast: transethmoidal approach to the sella. Otolaryngol Clin North Am. 1981;14:391-403.
77. Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582-594.
78. Sasaki CT. Transseptal-transsphenoidal approach to the sella. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JB Lippincott, 1984.
79. Carrau RL, Jho HD, Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
80. Jankowski R, Auque J, Simon C, et al. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198-202.
81. Jho HD, Carrau RL, Ko Y. Endoscopic pituitary surgery. In: Rengachary SS, Wilkins RH, editors. Neurosurgical Operative Atlas, Vol 5. Park Ridge, Ill: AANS; 1996.
82. Schramm VL, Myers EN. “How I do it”—head and neck. A targeted problem and its solution. Lateral rhinotomy. Laryngoscope. 1978;88:1042-1045.
83. Hamberger CA, Hammer G, Norlen G, et al. Transantrosphenoidal hypophysectomy. Arch Otolaryngol. 1961;74:2-8.
84. Casson PR, Bonanno PC, Converse JM. The midface degloving procedure. Plast Reconstr Surg. 1974;53:102-103.
85. Bell WH. Le Fort I osteotomy for correction of maxillary deformities. J Oral Surg. 1975;33:412-426.
86. Brown DH. The Le Fort I maxillary osteotomy approach to surgery of the skull base. J Otolaryngol. 1989;18:289-292.
87. Biller HF, Lawson W. Anterior mandibular-splitting approach to the skull base. Ear Nose Throat J. 1986;65:134-141.
88. Biller HF, Shugar JM, Krespi YP. A new technique for wide-field exposure of the base of the skull. Arch Otolaryngol. 1981;107:698-702.
89. Jenkins HA, Canalis RF. Transpalatal approach to the skull base. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JP Lippincott, 1984.
90. Kennedy DW, Papel ID, Holliday M. Transpalatal approach to the skull base. Ear Nose Throat J. 1986;65:125. 127-133
91. Crockard HA. The transoral approach to the base of the brain and upper cervical cord. Ann R Coll Surg Engl. 1985;67:321-325.
92. Fang HS, Ong GB. Direct anterior approach of the upper cervical spine. J Bone Joint Surg Am. 1962;44:1588-1604.
93. Fisch U. Infratemporal fossa approach to tumors of the temporal bone and base of skull. In: Silverstein H, editor. Neurological Surgery of the Ear. Birmingham, Ala: Aesculapius Publishing; 1977:34-53.
94. Cocke EWJr, Robertson JH, Robertson JT, et al. The extended maxillotomy and subtotal maxillectomy for excision of skull base tumors. Arch Otolaryngol Head Neck Surg. 1990;116:92-104.
95. House WF, Hitselberger WE. The transcochlear approach to the skull base. Arch Otolaryngol. 1976;102:334-342.
96. Glasscock ME3rd, Hays JW, Jackson CG, et al. A one-stage combined approach for the management of large cerebellopontine angle tumors. Laryngoscope. 1978;88:1563-1576.
97. Jackson CG, Johnson GD, Poe DS. Lateral transtemporal approaches to the skull base. In: Jackson CG, editor. Surgery of Skull Base Tumors. New York: Churchill Livingstone; 1991:95-120.
98. Fisch U, Pillsbury HC, Sasaki CT. Infratemporal approach to the skull base. In: Sasaki CT, McCabe BF, Kirchner JA, editors. Surgery of the Skull Base. Philadelphia: JB Lippincott, 1984.
99. Fisch U, Pillsbury HC. Infratemporal fossa approach to lesions in the temporal bone and base of the skull. Arch Otolaryngol. 1979;105:99-107.
100. Gates GA. The lateral facial approach to the nasopharynx and infratemporal fossa. Otolaryngol Head Neck Surg. 1988;99:321-325.
101. Holliday MJ. Lateral transtemporal-sphenoid approach to the skull base. Ear Nose Throat J. 1986;65:153-162.
102. Sekhar LN, Schramm VLJr, Jones NF. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67:488-499.
103. Terz JJ, Alksne JF, Lawrence WJr. Craniofacial resection for tumors invading the pterygoid fossa. Am J Surg. 1969;118:732-740.
104. Barbosa JF. Surgery of extensive cancer of paranasal sinuses. Presentation of a new technique. Arch Otolaryngol. 1961;73:129-138.
105. Maxwell RE, Chou SN. Convexity meningiomas and general principles of meningioma surgery. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques. 2nd ed. Orlando, Fla: Grune & Stratton; 1988:49-66.
106. House WF. Surgical exposure of the internal auditory canal and its contents through the middle cranial fossa. Laryngoscope. 1961;71:1363-1385.
107. Nuss DW, Rigby PL. Head and neck neoplasms with skull base involvement. In: Arriaga MA, Day J, editors. Neurosurgical Issues in Otolaryngology: Principles and Practice of Collaboration. Philadelphia: Lippincott Williams & Wilkins; 1999:309.
108. Gullane PJ, Lipa JE, Novak CB, et al. Reconstruction of skull base defects. Clin Plast Surg. 2005;32:391-399. vii
109. Newman J, O’Malley BWJr, Chalian A, et al. Microvascular reconstruction of cranial base defects: an evaluation of complication and survival rates to justify the use of this repair. Arch Otolaryngol Head Neck Surg. 2006;132:381-384.
110. Teknos TN, Smith JC, Day TA, et al. Microvascular free tissue transfer in reconstructing skull base defects: lessons learned. Laryngoscope. 2002;112:1871-1876.
111. Verret DJ, Ducic Y, Oxford L, et al. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol Head Neck Surg. 2005;133:897-899.
112. Oberascher G. Cerebrospinal fluid otorrhea—new trends in diagnosis. Am J Otol. 1988;9:102-108.
113. Shah S, Har-El G. Diabetes insipidus after pituitary surgery: incidence after traditional versus endoscopic transsphenoidal approaches. Am J Rhinol. 2001;15:377-379.
114. O’Malley BWJr, Janecka IP. Evolution of outcomes in cranial base surgery. Semin Surg Oncol. 1995;11:221-227.
115. Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: report of an International Collaborative Study. Head Neck. 2005;27:575-584.
116. Ganly I, Patel SG, Singh B, et al. Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study. Head Neck. 2005;27:445-451.
117. Bentz BG, Bilsky MH, Shah JP, et al. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25:515-520.
118. Patel SG, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98:1179-1187.
119. Shah JP, Kraus DH, Bilsky MH, et al. Craniofacial resection for malignant tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123:1312-1317.