Chapter 46 Surgery for Glomus Tumors and Other Lesions of the Jugular Foramen
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
The therapy for glomus tumors of the temporal bone is controversial. Because the clinical characteristics and growth rates of these tumors vary, the full gamut of management has been recommended from observation alone through radiation therapy and surgical management. Although there are isolated case reports of prolonged survival without treatment, these lesions can be quite deadly. The studies of Rosenwasser,1 Bickerstff and Howell,2 Steinberg and Holz,3 Brown,4 and Spector and colleagues5 have documented mortality rates of 5% to 13% for glomus jugulare tumors. This chapter outlines the diagnostic and preoperative evaluation, surgical techniques, and results and complications in the management of glomus tumors of the temporal bone. The application of these surgical techniques for other lesions of the jugular foramen is also reviewed.
PATIENT SELECTION
Temporal bone glomus tumors are neoplasms of the normal paraganglioma in the temporal bone that principally occur in the adventitia of the dome of the jugular bulb, but are also found in the submucosa of the cochlear promontory within the tympanic plexus. Numerous classification schemes have been proposed for these lesions, primarily by their origin (tympanic plexus versus jugular bulb) and the anatomic extent of lesion. The clinical surgical classification proposed by De La Cruz is particularly useful in planning the clinical management of patients with glomus tumors. The extent of the tumor is described by the involvement of structures of the temporal bone and skull base. A series of operations that correspond to the extent of the tumor is used (Table 46-1). Other classification schemes include the Fisch classification (Table 46-2) and the Glasscock-Jackson classification (Table 46-3).
TABLE 46-1 Modified De La Cruz Glomus Tumor Classification with Associated Surgical Approach
| Classification | Surgical Approach |
|---|---|
| Tympanic | Transcanal |
| Tympanomastoid | Mastoid/extended facial recess |
| Jugular bulb | Mastoid/neck (possible limited facial nerve rerouting) |
| Carotid artery | Infratemporal fossa ± subtemporal |
| Transdural | Infratemporal fossa/intracranial |
| Craniocervical | Transcondylar |
| Vagale | Cervical |
Surgery for Glomus Tumors, Chapter 49 Brackman DE, Arriaga MA in Otologic Surgery, eds Brackmann DE, Shelton C, Arriaga MA WB Saunders, Philadelphia. p579-593
TABLE 46-2 Fisch Classification of Glomus Tumors
| Type A | Tumors limited to middle ear cleft |
| Type B | Tumors limited to tympanomastoid area with no infralabyrinthine compartment involvement |
| Type C1, C2, C3 | Tumors involving infralabyrinthine compartment of temporal bone and extending into petrous apex |
| Type D1 | Tumors with intracranial extension <2 cm in diameter |
| Type D2, D3 | Tumors with intracranial extension >2 cm in diameter |
Fisch U, Infratemporal Fossa Approach for Glomus Tumors of the Temporal Bone. nn Otol Rhinol LAryngol 91–474-479, 1982.
TABLE 46-3 Glasscock-Jackson Glomus Tumor Classification
| Type | Physical Findings |
|---|---|
| GLOMUS TYMPANICUM | |
| I | Small mass limited to promontory |
| II | Tumor completely filling middle ear space |
| III | Tumor filling middle ear and extending into mastoid |
| IV | Tumor filling middle ear, extending into mastoid or through tympanic membrane to fill external auditory canal; may also extend anterior to internal carotid artery |
| GLOMUS JUGULARE | |
| I | Small tumor involving jugulare bulb, middle ear, and mastoid |
| II | Tumor extending under internal auditory canal; may have intracranial extension |
| III | Tumor extending into petrous apex; may have intracranial extension |
| IV | Tumor extending beyond petrous apex into clivus or infratemporal fossa; may have intracranial extension |
Lateral Temporal Approach to the Skull Base, Chapter 8, Jackson G, Johnson GD, Poe DS in Surgery of the Skull Base, ed Jackson CG. New York, Churchill Livingston, 1991 p 141-196.
Glomus Vagale Tumor
Glomus vagale tumors arise from the glomus body along the vagus nerve at the base of the skull. Because glomus vagale tumors do not begin within the temporal bone, they are often larger than glomus tumors of the temporal bone itself because they later produce symptoms of pulsatile tinnitus and hearing loss. Clinically, these lesions produce vocal cord paralysis before the onset of hearing loss or tinnitus or the appearance of a vascular middle ear mass. In contrast, glomus tumors of the temporal bone produce otologic symptoms before the onset of vocal cord paralysis.6
Jugular Foramen Schwannoma
Although schwannomas are the second most frequently occurring tumor of the jugular foramen, they still are extremely rare. They arise from the Schwann cells of CN IX, X, and XI. The most common cranial nerve to be affected is the vagus nerve.7,8 The clinical distinction between glomus vagale tumors and vagale schwannomas is that glomus vagale tumors develop vocal cord paralysis early in their course.9
Presentation of a jugular foramen schwannoma depends on the growth pattern. Kaye and colleagues10 categorized three different patterns: A, B, and C. Type A tumors occur primarily in the posterior fossa. Type A tumors with intracranial extension may manifest with CN IX, X, or XI palsies. Similar to most cerebellopontine angle tumors, hearing loss or imbalance or both may be the only significant presenting symptoms. Type B tumors remain confined to the skull base with extension often into the clivus. Type C tumors begin in the jugular foramen and extend inferiorly into the neck. Type B and C tumors manifest more frequently with lower cranial neuropathies than type A tumors.10
PREOPERATIVE EVALUATION
Magnetic Resonance Imaging
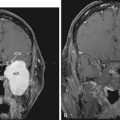
Because bone involvement by tumor is not clearly shown on MRI, this technique provides only adjunctive information regarding the extent of tumor involvement. If the diagnosis is in question, MRI combined with CT provides exquisite preoperative guidance in the differential diagnosis of petrous apex lesions.11 MRI can indicate occlusion of the jugular bulb and vein because the normal flow signals are altered. In intradural tumors, MRI can delineate more clearly the tumor-brain interface and the relationship of the lesion to the intradural structures. MRI must be interpreted cautiously because T1 images of glomus tumors may overestimate the degree of tumor involvement. Marrow-containing bone of the petrous apex is hyperintense and indistinguishable from enhancing tumor in the petrous apex. Magnetic resonance angiography and MR venography offer another diagnostic tool in evaluating glomus tumors. In their present form, however, MR angiography techniques cannot provide adequate imaging resolution to define feeding vessels to the tumor. CT angiography is another promising technology in evaluating vascular temporal bone lesions12; however, the role of these techniques in the preoperative assessment of glomus tumors has not been fully clarified.13,14
Brain Perfusion and Flow Studies
For tumors that abut the ICA, it is necessary to assess the adequacy of the cerebral cross-perfusion from the contralateral ICA. Cross-compression angiography, stump pressure measurements, and clinical evaluation during test occlusion of the involved carotid are basic guides to the risk of stroke in the event that the affected carotid artery must be sacrificed. Xenon blood flow and radioisotope studies offer much more precise quantification of the risk of stroke and the possible need for surgical replacement of the ICA.15 In certain cases with extensive invasion of the carotid artery and acceptable results on the perfusion studies of the contralateral artery, the involved carotid artery may be permanently occluded with a detachable balloon. We generally do not recommend carotid sacrifice. Despite excellent advances in diagnostic flow studies, an appreciable risk (about 5%) of stroke exists with carotid artery sacrifice even in cases with favorable functional and perfusion characteristics or test occlusion studies. If possible, repair or graft replacement of the carotid is recommended if the carotid is injured during tumor removal.
Embolization
Large glomus tumors may result in significant intraoperative blood loss. We have found that preoperative embolization of feeding vessels can significantly reduce such loss.16 The embolization is usually performed with polyvinyl alcohol (Ivalon) and is performed at the time of angiography 1 or 2 days before surgery. A longer interval between embolization and surgery may result in collateral blood flow to the tumor, which may paradoxically increase tumor perfusion and intraoperative blood loss.
SURGICAL APPROACHES
Transcanal Approach
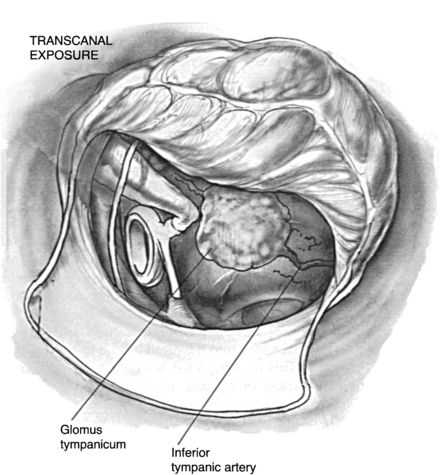
The transcanal approach is used for small glomus tympanicum tumors that are limited to the mesotympanum. Because the entire circumference of the tumor is visible within the middle ear, preoperative imaging studies are unnecessary. If there is any doubt of the possibility of an aberrant ICA, however, preoperative CT scanning should be obtained to exclude this possibility. The tympanomeatal flap is modified with the inferior incision extending more anteriorly so that the inferior aspect of the tympanic membrane can be elevated. The tumor is identified on the promontory (Fig. 46-1).
Tumors with limited hypotympanic extension without posterior involvement on CT scan may be removed by a modified transcanal (hypotympanic) approach.17 After a postauricular incision is performed with transection of the ear canal, a superiorly based tympanomeatal flap is elevated to permit access to the inferior aspect of the tympanic ring. This bone is progressively drilled until the inferior limit of the tumor is identified. The drilling involved with this exposure is usually much less than the drilling required for the transcanal infracochlear drainage procedures for the petrous apex described in Chapter 45.
Mastoid–Extended Facial Recess Approach
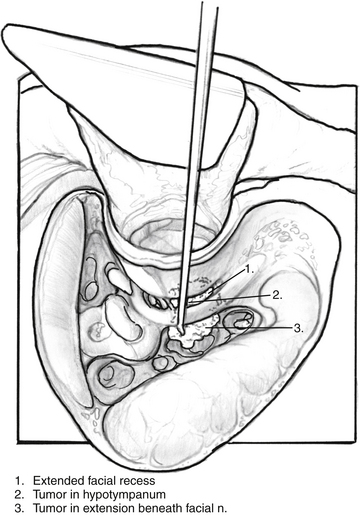
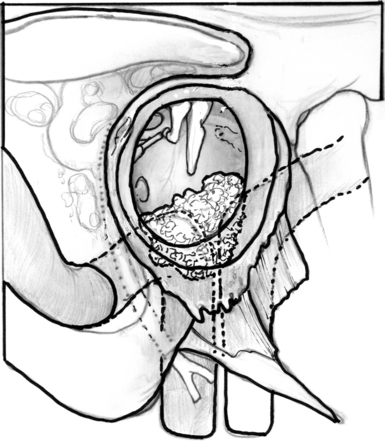
Extension of glomus tympanicum tumors into the retrofacial air cells is managed by direct exposure. A cutting burr is used to remove the air cells inferior to the labyrinth and beneath the facial nerve, leaving the facial nerve suspended with a thin layer of bone to allow the surgeon access to the entire hypotympanum (Fig. 46-2). A small curette is used to remove bits of the tumor from crevices in the hypotympanum. The dome of the jugular bulb can be inspected to ensure that it is free of the tumor.
Mastoid-Neck Approach
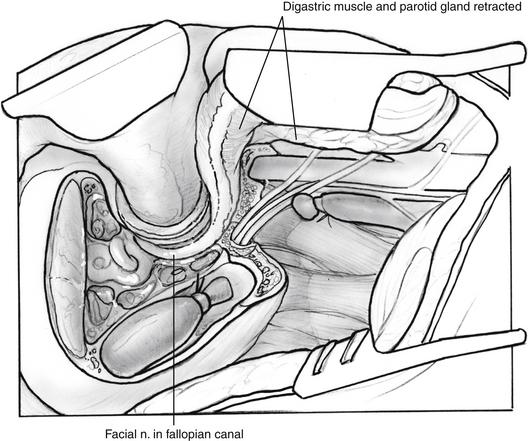
The mastoid-neck approach is used for small glomus jugulare tumors. These tumors involve the jugular bulb, but do not extend onto the ICA or into the neck or posterior fossa. The preoperative evaluation of these patients may include angiography because involvement of the jugular bulb raises the question of possible carotid artery involvement. Continuous intraoperative facial nerve monitoring is used. Additionally, electromyography electrodes in the sternocleidomastoid muscle are useful for monitoring CN XI, electrodes in the lateral pharyngeal wall can monitor CN IX, and electrodes in the vocalis muscle can monitor CN X. Specially designed endotracheal tubes with attached electromyography electrodes are particularly useful for monitoring lower cranial nerve function. The procedure is performed by initially completing the same exposure as that described in the mastoid–extended facial recess approach (Fig. 46-3), then amputating the mastoid tip. The periosteum of the digastric groove is followed anteriorly until it turns abruptly laterally at the stylomastoid foramen. Drilling laterally along the digastric ridge anteriorly and posteriorly frees the entire mastoid tip. The incision is carried into the neck along the anterior border of the sternocleidomastoid muscle. This muscle is freed from the mastoid tip and retracted posteriorly. The mastoid tip can be removed by grasping it with a Kocher clamp and cutting with a curved Mayo scissors along the bone. The posterior belly of the digastric muscle is identified and freed from the digastric groove and retracted anteriorly to allow exposure of the major neurovascular structures of the neck. When the internal jugular vein is identified and dissected free from surrounding tissues, it is occluded with multiple 2-0 silk sutures. The jugular vein is followed over the transverse process of the first cervical vertebra into the base of the skull. In this fashion, CN XI is identified (usually lateral to the vein) and preserved.
The tumor is now ready for resection in continuity with the dome of the jugular bulb. Bipolar cautery helps in hemostasis and shrinkage of the tumor bulk. When the tumor and dome of the jugular bulb are excised, hemostasis is completed with Surgicel packing (see Fig. 46-3). Complete tumor resection is accomplished, and if the tympanic membrane or ossicles are involved, reconstruction may be performed as described earlier. It is usually possible to preserve CN IX, X, and XI with these limited tumors, unless there is preoperative involvement of these structures. Postoperatively, these patients are cared for in the intensive care unit because the possibility of an acute lower cranial neuropathy or major postoperative hemorrhage exists. Usually, minimal morbidity is associated with this approach, and patients are stable and may be discharged from the hospital within a few days. The major pitfall associated with this approach is related to inaccurate preoperative assessment of tumor extent. The mastoid-neck approach is too limited if the tumor extensively involves the carotid artery.
Mastoid-Neck with Limited Facial Nerve Rerouting
A useful modification of the mastoid-neck approach adds a limited facial nerve rerouting to the procedure. Additional exposure in the mastoid-neck approach may be achieved by totally decompressing the facial nerve from the second genu throughout the entire vertical segment. The periosteum of the facial nerve at the stylomastoid foramen is preserved, but the fibrous attachments to the nerve in its vertical portion are sharply transected. The mobilized nerve can be transposed laterally along with the periosteum at the stylomastoid foramen and the attached posterior belly of the digastric muscle. A suture through the stylomastoid periosteum can be used to hold the nerve laterally and prevent traction on this structure. Such transposition of the facial nerve permits further bone removal in the area of the vertical facial canal and retrofacial air cells along the infralabyrinthine air cell tract (Fig. 46-4).
Infratemporal Fossa Approach
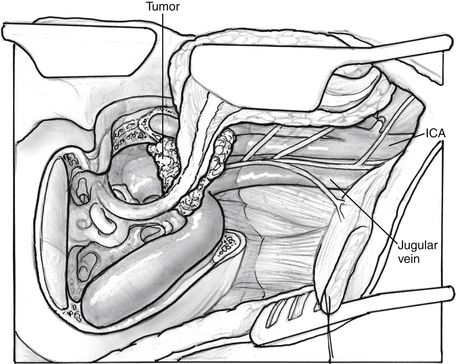
The development of the infratemporal fossa approach by Fisch18 has been a significant advance in the total removal of large glomus jugulare tumors. Previous approaches that did not remove the external auditory canal or reroute the facial nerve did not allow adequate exposure of the tumor or ICA (Fig. 46-5). There are eight distinct steps in the infratemporal fossa exposure: (1) patient preparation, (2) management of the ear canal and tympanic ring, (3) mastoidectomy, (4) initial preparation of the jugular vein and neck exposure, (5) transposition of the facial nerve, (6) completion of the neck exposure and identification of the lower cranial nerves and skull base carotid artery, (7) tumor removal including intracranial extension, and (8) wound closure. Continuous facial nerve monitoring and electromyography monitoring of the lower cranial nerves are employed as previously described.
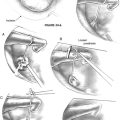
A wide shave is performed, and a large postauricular C-shaped incision is made. The incision is carried anteriorly, and the ear canal is transected slightly medially to the bone-cartilage junction of the ear canal. Cartilage is removed from the ear canal to permit fashioning of the ear canal skin as a cuff that can be everted. The skin of the meatus is closed with 4-0 nylon sutures. The periosteum of the postauricular area is elevated as a flap and sutured behind the opening in the meatus to reinforce the closure further. Next, a mastoidectomy is completed, and the facial recess is opened to allow separation of the incudostapedial joint. The posterior wall of the ear canal can be removed with rongeurs and cutting burrs. The remaining skin of the ear canal and the tympanic membrane, malleus, and incus are removed. The facial nerve is decompressed from the geniculate to the stylomastoid foramen. An eggshell-thin layer of bone is left over the nerve itself. By use of cutting and diamond burrs, the bone of the tympanic ring is progressively removed, the level of the jugular bulb is identified, and the bone over the temporomandibular joint and vertical segment of the petrous carotid artery is removed anteriorly (Fig. 46-6).
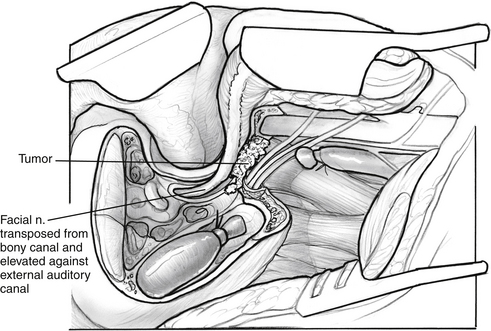
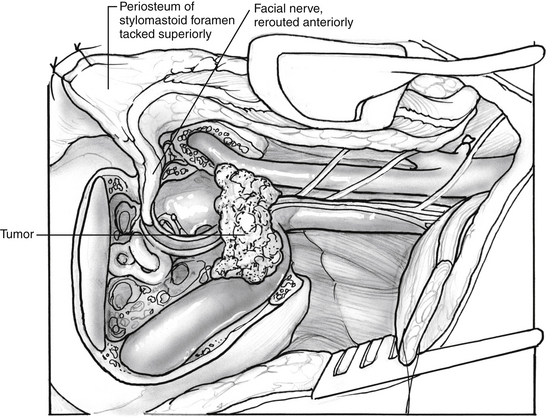
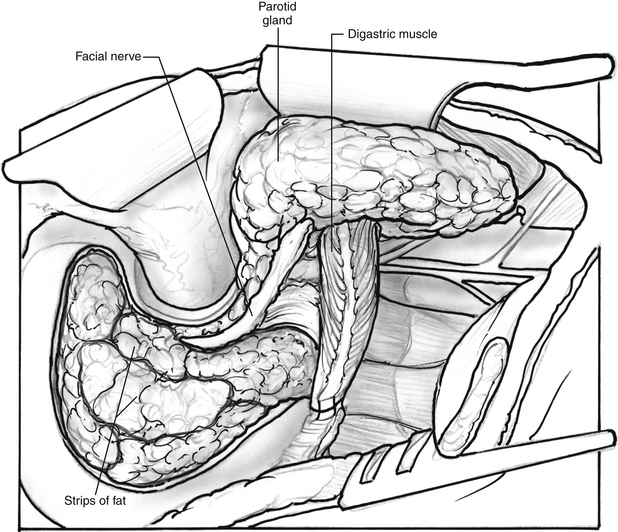
The facial nerve is transposed. The transposition technique originally described by Fisch has been modified because of temporary and sometimes permanent residual facial weakness.19 Rather than exposing the facial nerve in the parotid, the surgeon transposes the nerve with periosteum of the stylomastoid foramen and elevates the entire tail of the parotid.20 After decompressing the nerve, the remaining eggshell-thin bone over the facial nerve is removed with a blunt instrument. The multiple fibrous connections along the descending portion of the nerve are sharply transected. The tympanic portion of the nerve does not have such adhesions, and this section elevates readily. The posterior belly of the digastric muscle is moved anteriorly because the fascia of this muscle contributes to the stylomastoid foramen periosteum. The nerve can be transposed anteriorly along with the tail of the parotid. A large suture is placed through the periosteum of the stylomastoid foramen and attached to the soft tissues in the area of the root of the zygoma (Fig. 46-7), elevating the facial nerve and preventing it from being stretched when retractors are placed. The use of continuous facial nerve monitoring during this maneuver has significantly improved postoperative facial nerve function.21
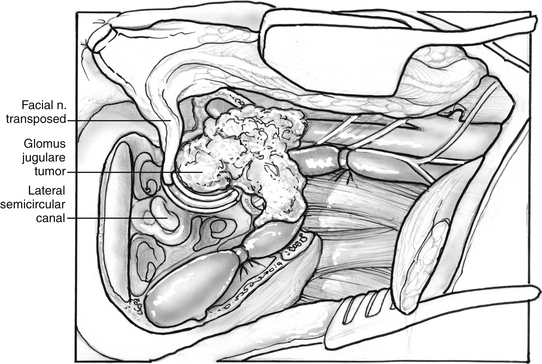
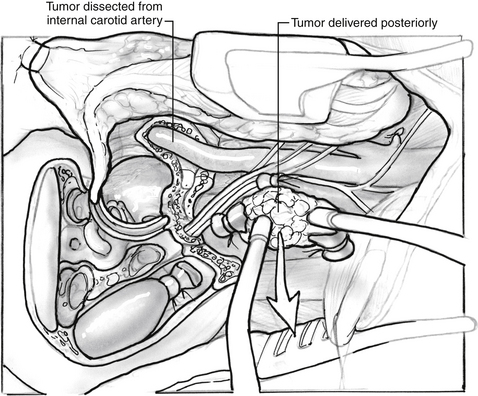
The jugular vein is doubly ligated and transected between ligatures, and the external carotid artery is ligated. If the tumor extends intradurally, the proximal sigmoid sinus is doubly ligated with silk sutures passed through openings in the dura with an aneurysm suture passer (Fig. 46-8). If the tumor is not intradural, the sigmoid can be packed with Surgicel without violating the dura. The jugular vein is elevated, and the tumor in the area of the jugular bulb is freed inferiorly to superiorly following the jugular vein into the jugular bulb. The tumor is freed from the carotid artery anteriorly, and bleeding from the caroticotympanic vessels is controlled with bipolar cautery. If the tumor is adherent to the ICA, it is best to leave a portion of it on the artery and remove the bulk of the tumor. Lower cranial nerve preservation is enhanced if the medial wall of the jugular bulb is left in situ protecting the pathway of the lower cranial nerves through the pars nervosa in the anteromedial portion of the jugular bulb. Tumor hemostasis is continued with bipolar cautery and Surgicel packing of the inferior petrosal sinus; the tumor can be removed in continuity with the dome of the jugular bulb. If the last bit of the tumor from the carotid artery is not removed until the conclusion of the procedure, a small entry into the carotid artery that may occur at the location of the caroticotympanic artery can be repaired directly (Fig. 46-9).
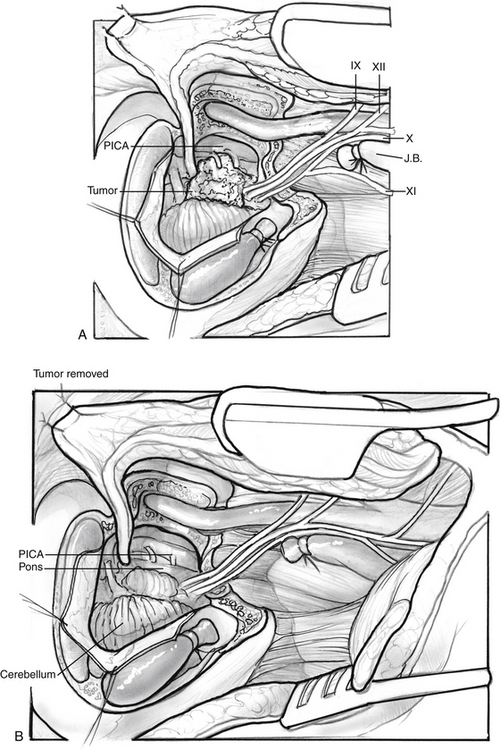
The removal of the intracranial portion of the tumor is often easier than the removal of tumor within the temporal bone. By the time the surgeon is ready for the removal of the intracranial extension, the blood supply has often been controlled. The blood supply to the intracranial portion of the tumor is often discrete and can be controlled with bipolar cautery as with other cerebellopontine angle tumors (Fig. 46-10). If tumor has been left along the ICA, it is now removed. Closure is accomplished by closing the eustachian tube with Surgicel, muscle, and strips of abdominal fat (Fig. 46-11). If cerebrospinal fluid has been encountered, continuous lumbar drainage is used for approximately 5 days until the wound is sealed; however, the use of cranioplasty techniques with hydroxyapatite cement or titanium mesh is effective at controlling cerebrospinal fluid fistulas.22 A drain is left in the neck wound and removed on the first postoperative morning. A pressure dressing is placed for 4 postoperative days.
Fallopian Bridge Technique
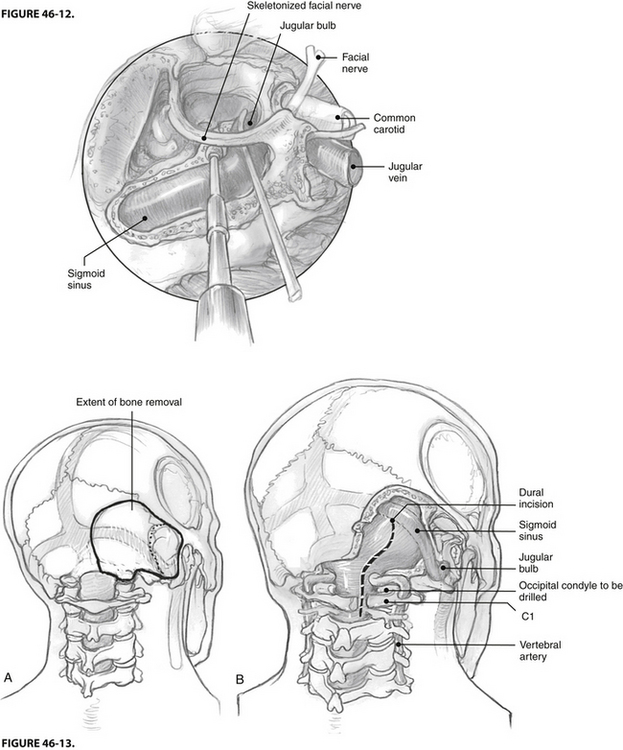
A new strategy for minimizing postoperative facial nerve dysfunction and enhancing preservation of the middle ear in jugular foramen surgery is the fallopian bridge technique (Fig 46-12). In this technique, the facial nerve is left in situ, but the bone surrounding the nerve is almost completely removed.23 This technique can be used with the mastoid-neck approach as an alternative to partial mobilization. It is even applicable with the full infratemporal fossa technique. Although the technique is relatively new, it promises to decrease the number of cases requiring facial nerve translocation, with beneficial outcomes for postoperative facial nerve function and preservation of the conductive hearing mechanism.
Transcondylar Approach
In certain recurrent glomus jugulare tumors, the intracranial tumor may extend significantly toward the foramen magnum. In these situations, a wider exposure of the cranial cervical junction is necessary. Although the transsigmoid, translabyrinthine exposure combined with the infratemporal fossa approach offers a wide view, the cranial cervical junction itself is not fully exposed with this procedure. In this case, the transcondylar exposure is helpful with the infratemporal fossa approach. The steps involved in the transcondylar approach24 are initially extension of the muscular incisions of the posterior superior aspect of the neck to identify the vertebral artery posterior and inferior to the mastoid tip on the transverse process of C1. When this landmark is clearly identified, wide exposure of the occipital condyle and jugular tubercle permits full exposure of the hypoglossal nerve and an unlimited view of the cranial cervical junction (Fig. 46-13). If more than half of the occipital condyle is resected, careful consideration should be given to a simultaneous cervical stabilization procedure. When transcondylar exposure is needed in addition to standard otologic exposure, we find the use of head holding pins in the lateral position helpful for access to the posterior superior skull base.
Complete Carotid Mobilization
In very rare cases of recurring glomus jugulare tumors after previous surgery and radiation, the tumor may completely encase the petrous carotid artery with significant extension into the far anterior infratemporal fossa. In these cases, we have found the combination of standard otologic (posterior) infratemporal fossa approach with the preauricular infratemporal fossa approach quite useful. Specifically, an orbital zygotomy in continuity with the glenoid fossa is performed after a temporal craniotomy. In this fashion, the glenoid can be reconstructed at the conclusion of the procedure. The carotid canal can be followed directly and fully from the base of the skull all the way to the cavernous sinus. In this way, the carotid artery can be mobilized anteriorly and inferiorly if necessary for complete tumor resection. This approach facilitates any carotid reconstruction if necessary. In cases with extensive carotid involvement, carotid injury is a real hazard. One center advocates preoperative stenting as a safety measure.25
RESULTS
Glomus Tympanicum Tumors
O’Leary and colleagues26 reviewed the results of glomus tympanicum surgery at the House Ear Clinic. Between 1957 and 1990, 73 glomus tympanicum tumors (tympanic tumor or tympanomastoid tumor) were managed at the House Ear Clinic. In 80% of these tumors, a mastoid–facial recess approach was required, and in 20%, a transcanal removal was possible. Although significant intraoperative blood loss occurred (average >500 mL), the morbidity was minimal. Hearing levels remained stable: the mean speech reception threshold increased 1 dB postoperatively. Of the five complications, three were residual tympanic membrane perforations requiring a secondary tympanoplasty. One patient developed a cholesteatoma postoperatively. One patient developed a facial nerve weakness requiring re-exploration and facial nerve decompression with an ultimately good outcome (grade II/VI on the House-Brackmann scale). This series showed that the critical feature of glomus tympanicum management is total tumor resection. The three cases in which an incomplete resection was performed resulted in tumor recurrence. Overall, the recurrence rate in this series was less than 5%.
Glomus Jugulare Tumors
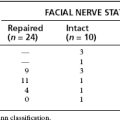
Green and associates27 reviewed the House Ear Clinic experience with glomus jugulare tumors (jugular bulb tumors, carotid artery involvement tumors, and intradural tumors) between 1980 and 1991. During this interval, 52 patients were surgically treated for glomus jugulare tumors who had undergone no prior radiotherapy or surgery. The techniques involved were the infratemporal fossa approach in 83%, the mastoid-neck approach in 7%, and the mastoid-neck with limited facial nerve mobilization in 10%. Complete surgical removal was possible in 85% of the patients. Eight patients required transection of the facial nerve for complete tumor removal. These patients underwent segmental grafting or greater auricular nerve reconstruction and achieved a grade III/VI facial nerve recovery. The mean intraoperative blood loss was 1500 mL. Long-term facial function was good: 95% of patients who had facial nerve rerouting had grade I/VI or II/VI facial function at 1 year follow-up or greater. Nearly 20% of patients required a vocal cord augmentation procedure. No patient required a tracheotomy in the immediate postoperative period. Four patients required prolonged nasogastric tube feeding, and two ultimately required gastrostomy temporarily. No patients required long-term gastrostomy. Of all patients, 85% were able to resume the same activity level as before surgery.
COMPLICATIONS
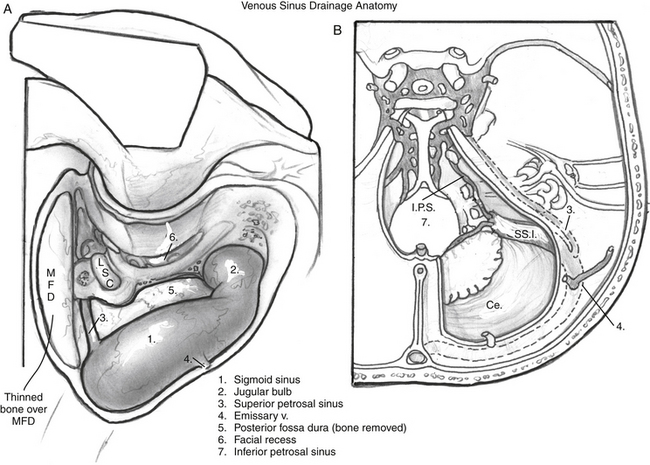
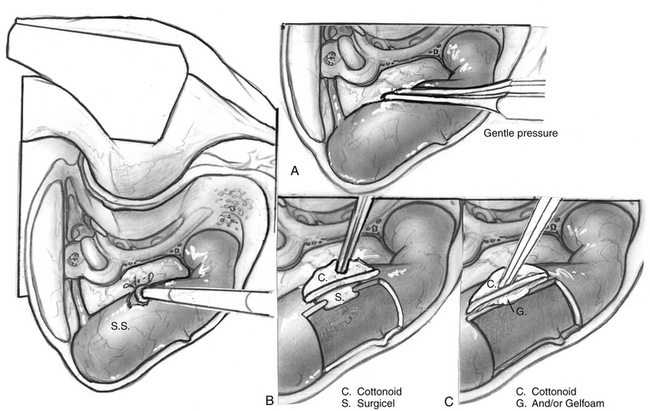
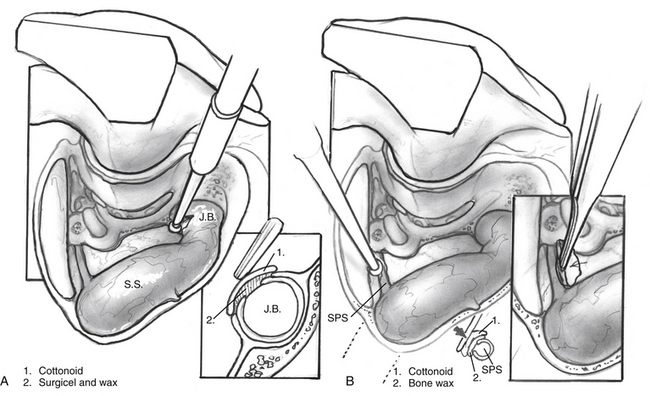
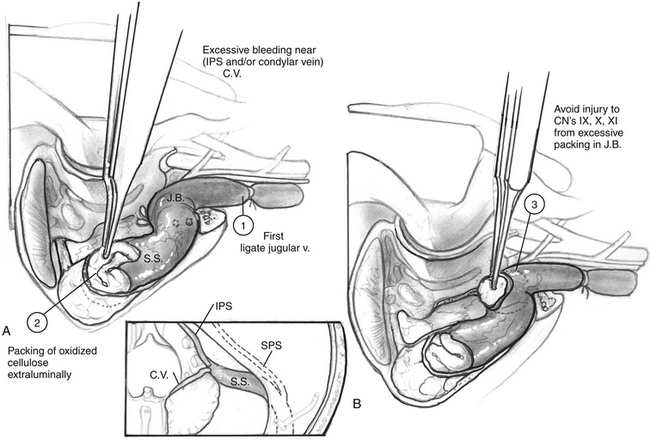
One of the most common issues in glomus surgery or any complex temporal bone surgery is appropriate management of bleeding from the venous sinuses. Figures 46-14 to 46-17 illustrate normal drainage patterns from the venous sinuses, and systematic strategies for managing bleeding or injury from these sinuses.
Glomus Jugulare Tumors
As illustrated in the results section, the categories of morbidity in glomus jugulare surgery include facial nerve injury, lower cranial nerve dysfunction, carotid artery injury, bleeding problems, and intracranial complications. Direct tumor infiltration of the facial nerve necessitates transection of the involved segment and replacement of that segment with a nerve graft using standard techniques. The advent of continuous intraoperative facial nerve monitoring has significantly improved postoperative facial nerve function in infratemporal fossa surgery.21 In our experience, the need for tracheotomy or gastrostomy has been infrequent (<4%). We recommend early vocal cord augmentation for vagal paralysis.28
The possibility of carotid artery injury must be anticipated. Careful preoperative imaging, including CT scans and angiography, is helpful in defining the anatomic relationships of the tumor to the ICA. Preoperative flow studies (i.e., balloon occlusion or xenon or technetium blood flow studies) are helpful in predicting how well a patient would tolerate total occlusion of the ICA. The indications for replacement versus permanent occlusion have been summarized elsewhere.29 The possibility of significant intraoperative blood loss must be anticipated. Autologous blood donation has been helpful in avoiding transfusions of banked blood. In the weeks preceding surgery, the patient donates his or her own blood for possible autologous transfusion intraoperatively. Similarly, the Cell Saver, which recycles the patient’s intraoperative blood loss, may reduce the requirement for banked blood transfusions. Finally, the surgeon must be cognizant of the extent of blood replacement and be certain that the appropriate ratio of platelets and fresh frozen plasma is replaced in addition to the red blood cell products themselves.
RADIATION
The role of radiation therapy in the management of glomus jugulare tumors is still controversial, but increasingly accepted. Histologic studies have shown that the effects of irradiation seem to be on the blood vessels and fibrous elements of the tumor, rather than on the tumor cells themselves.30 With external-beam strategies, these tumors often begin growing after a 10 to 15 year period of control. In addition, the potential for malignant transformation of radiating benign tumors must not be underestimated. Fatal malignant transformations of benign jugular foramen tumors after radiation therapy have been reported.
More recent studies have indicated excellent short-term and intermediate-term control with stereotactic radiosurgery techniques that limit the dose to surrounding tissue, but can effectively shrink paragangliomas in anatomically eloquent areas such as the cavernous sinus.31 The Mayo Clinic series reported greater than 92% control in a “long-term series” of 30 patients with median 13 year follow-up.32 Nonetheless, the longer term results (>15 to 20 years) are unknown, especially for stereotactic radiosurgery; the data can be considered promising, but not yet definitive.
Although we previously recommended radiation therapy only in elderly patients with symptomatic glomus tumors or in patients who otherwise could not withstand a surgical removal, radiation (primarily stereotactic radiosurgery) can be considered as having a role in the modern management of these tumors and should be discussed with patients. Although we generally recommend surgery as definitive therapy for patients who are medically stable enough to undergo an operative procedure because this is the only therapy with the potential for actually curing (eliminating) the tumor, radiation may be part of a strategy to limit cranial neuropathies. Although the patient’s preferences should be discussed before surgery, it may be reasonable to avoid transection and sacrifice of cranial nerves and employ a near-total tumor resection if removing the last bit of tumor would result in cranial nerve morbidity. Similar to glomus tumors, jugular foramen schwannomas show short-term effective control and excellent cranial nerve outcomes with stereotactic radiation therapy.33
1. Rosenwasser H. Carotid body-like tumor of the middle ear and mastoid bone. Arch Otolaryngol. 1945;41:64-67.
2. Bickerstff E.R., Howell J.S. The neurological importance of tumors of the glomus jugulare. Brain. 1953;76:576-693.
3. Steinberg N., Holz W.G. Glomus jugulare tumors. Arch Otolaryngol Head Neck Surg. 1965;82:387-394.
4. Brown J.S. Glomus jugulare tumors revisited: A ten-year statistical follow-up of 231 cases. Laryngoscope. 1985;95:284-285.
5. Spector G.J., Fierstein J., Ogura J.H. A comparison of therapeutic modalities of glomus tumors in the temporal bone. Laryngoscope. 1976;86:690-969.
6. Leonetti J.P., Brackmann D.E. Glomus vagale tumors: The significance of early vocal cord paralysis. Otolaryngol Head Neck Surg. 1989;100:533-537.
7. Horn K.L., Hankinson H. Tumors of the jugular foramen. In: Brackmann D.E., Jackler R.K., editors. Neurotology. Philadelphia: Mosby; 1994:1059-1068.
8. Horn K.L., House W.F., Hitselberger W.E. Schwannomas of the jugular foramen. Laryngoscope. 1985;95:761-765.
9. Leonetti J.P., Brackmann D.E. Glomus vagale tumors: The significance of early vocal cord paralysis. Otolaryngol Head Neck Surg. 1989;100:533-537.
10. Kaye A.H., Hahn J.F., Kinney J.E. Jugular foramen schwannomas. J Neurosurg. 1984;60:1045-1053.
11. Arriaga M.A., Brackmann D.E. Differential diagnosis of primary petrous apex lesions. Am J Otol. 1991;12:470-474.
12. Krishnan A., Mattox D.E., Fountain A.J., Hudgins P.A. CT arteriography and venography in pulsatile tinnitus: Preliminary report. AJNR. Am J Neuroradiol. 2006;27:1635-1639.
13. Arriaga M.A., Lo W.W.M., Brackmann D.E. Imaging case study of the month: Magnetic resonance angiography of synchronized bilateral carotid body paragangliomas and bilateral vagal paragangliomas. Ann Otol Rhinolaryngol. 1992;101:955-957.
14. Rogers G.P., Brackmann D.E., Lo W.W.M. Magnetic resonance angiography, a technique for evaluation of skull base lesions. J Otol. 1993;14:56-62.
15. Janecka I.P., Sekhar L.N., Horton J.A. General blood flow evaluation. In: Cummings C.W., Fredrickson J., Harker L., et al, editors. Otolaryngology Head and Neck Surgery Update II. St. Louis: Mosby-Year Book; 1990:54-63.
16. Murphy T.P., Brackmann D.E. Effects of preoperative embolization on glomus jugulare tumors. Laryngoscope. 1989;99:1244-1247.
17. Farrior J.B. Glomus tumors—postauricular hypotympanotomy. Arch Otolaryngol Head Neck Surg. 1967;86:367-373.
18. Fisch U. Infratemporal fossa approach for glomus tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1982;91:474-479.
19. Fisch U., Faga P., Valvanis A. The infratemporal fossa approach for the lateral skull base. Otolaryngol Clin North Am. 1984;17:513-552.
20. Brackmann D.E. The facial nerve in the infratemporal approach. Otolaryngol Head Neck Surg. 1987;97:15-17.
21. Leonetti J.P., Brackmann D.E., Prass R.C. Improved preservation of facial function in the infratemporal fossa approach to the skull base. Otolaryngol Head Neck Surg. 1989;101:74-78.
22. Arriaga M.A., Chen D.A., Burke E.L. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery—update. Otol Neurotol. 2007;28:538-540.
23. Pensak M.L., Jackler R.K. Removal of jugular foramen tumors. Otolaryngol Head Neck Surg. 1997;117:586-591.
24. Fukushima T. Manual of Skull Base Dissection. Pittsburgh, AF: Neurovideo Inc; 1996.
25. Sanna M., Khrais T., Menozi R., et al. Surgical removal of jugular paraganglioma after stenting of the intratemporal internal carotid artery: A preliminary report. Laryngoscope. 2006;116:742-746.
26. O’Leary M.J., Shelton C., Giddings N., et al. Glomus tympanicum tumors: A clinical perspective. Laryngoscope. 1989;101:74-78.
27. Green J.D., Nguyen C.D., Arriaga M.A., et al. Technical modifications in the surgical management of glomus jugulare tumors. Laryngoscope. 1994;v 104:917-924.
28. Netterville JD: Primary thyroplasty in glomus jugulare surgery. Presented at American Neurotology Society Fall Meeting, Washington DC, September 13, 1992.
29. deVries E.J. A new method to predict safe resection of the internal carotid artery. Laryngoscope. 1990;100:85-89.
30. Brackmann D.E., House W.F., Terry R., et al. Glomus jugulare tumors: Effects of irradiation. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:1423-1431.
31. Gerosa M., Visca A., Rizzo P., et al. Glomus jugulare tumors: The option of gamma knife radiosurgery. Neurosurgery. 2006;59:561-569.
32. Krych A.J., Foote R.L., Brown P.D., et al. Long-term results of irradiation for paraganglioma. Int J Radiat Oncol Biol Phys. 2006;65:1063-1066.
33. Martin J.J., Kondziolka D., Flickinger J.C., et al. Cranial nerve preservation and outcomes after stereotactic radiosurgery for jugular foramen schwannomas. Neurosurgery. 2007;61:76-81.